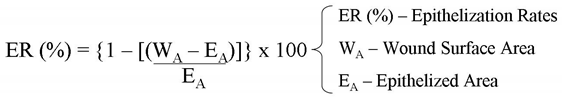1. Introduction
Wound healing is a dynamic and complex biological process that restores tissue integrity after injury. This process involves several phases: inflammation, cell proliferation, granulation tissue formation, and remodeling (Diegelmann & Evans, 2004; Akubugwo et al., 2022). The search for new healing agents, especially those derived from natural sources, has intensified recently, focusing on medicinal plants with a long tradition of widespread use (Biswas & Mukherjee, 2003; Chabane et al., 2021).
Libidibia ferrea var. ferrea (Mart. ex Tul.) LP Queiroz (synonym of Caesalpinia ferrea), popularly known as jucá, is a plant widely distributed in the Amazon region and northeastern Brazil, traditionally used in the treatment of various diseases, including diabetes, infections, and inflammations (Drumond et al., 2000; Barbosa et al., 2007). Scientific studies have shown that this plant's fruits, bark, and leaves have a wide range of pharmacological properties, such as anti-inflammatory, antimicrobial, and antioxidant activity, essential for healing. (Carvalho et al., 1996; Menezes et al., 2007).
Several bioactive compounds have been isolated from L. ferrea, such as gallic acid and methyl gallate, which have shown antitumor potential in vitro and in vivo assays, inhibiting early activation of the Epstein-Barr antigen, as well as other models of carcinogenesis (Nakamura et al., 2002; Ueda et al., 2001). Furthermore, the aqueous extract of L. ferrea has demonstrated beneficial effects in protecting against gastric ulcers in experimental models, reducing the volume of gastric secretion and promoting the regeneration of injured tissues (Bacchi & Sertie, 1994; Bacchi et al., 1995).
The wound-healing activity of medicinal plants is of particular interest in the development of topical pharmaceutical forms, such as gels and creams, which can accelerate the healing of skin wounds and reduce the occurrence of complications, such as infections (Kilic, 2005; Biswas & Mukherjee, 2003). In previous studies, the crude extract of L. ferrea demonstrated a potent anti-inflammatory action in models of carrageenan-induced edema and analgesic properties, suggesting its use in managing pain associated with the inflammatory process (Carvalho et al., 1996). Adding to the antimicrobial activity against oral pathogens such as Candida albicans and Streptococcus mutans, these effects indicate that L. ferrea may effectively prevent infections during healing (Sampaio et al., 2009). A study on the literature review of the species L. ferrea reports the need for studies on its healing action (Gonçalves et al., 2023).
Furthermore, L. ferrea has shown positive effects on regulating the immune system, particularly in the stimulation of myelopoiesis in infection and tumor models, which may improve the immune response during wound healing (Queiroz et al., 2001). This is relevant for the clinical use of the plant since an adequate immune response is essential for tissue regeneration and the prevention of complications during the healing process (Diegelmann & Evans, 2004).
The present study aims to evaluate the healing action of two topical preparations of Libidibia ferrea fruits - an infusion (InLf) and a gel containing glycolic extract (GLf) - in cutaneous wounds induced in Wistar rats. Previous studies indicate that the plant has great potential for wound use due to its anti-inflammatory, antimicrobial, and immunomodulatory properties (Carvalho et al., 1996; Menezes et al., 2007; Gonçalves et al., 2023). The evaluation of these pharmaceutical forms aims to explore the therapeutic potential of L. ferrea in treating wounds and contribute to developing new natural healing agents.
2. Results
2.1. Phytochemical Assay and Evaluation of Wound Contraction
Phytochemical analyses (
Table 1) indicated that the glycolic extract of the fruits of Libidibia ferrea presented a tannin content of 23% and flavonoids of 0.25%, while the infusion had 21% tannins and 0.32% flavonoids. The gel prepared from the glycolic extract presented the lowest contents, with 18% tannins and 0.14% flavonoids.
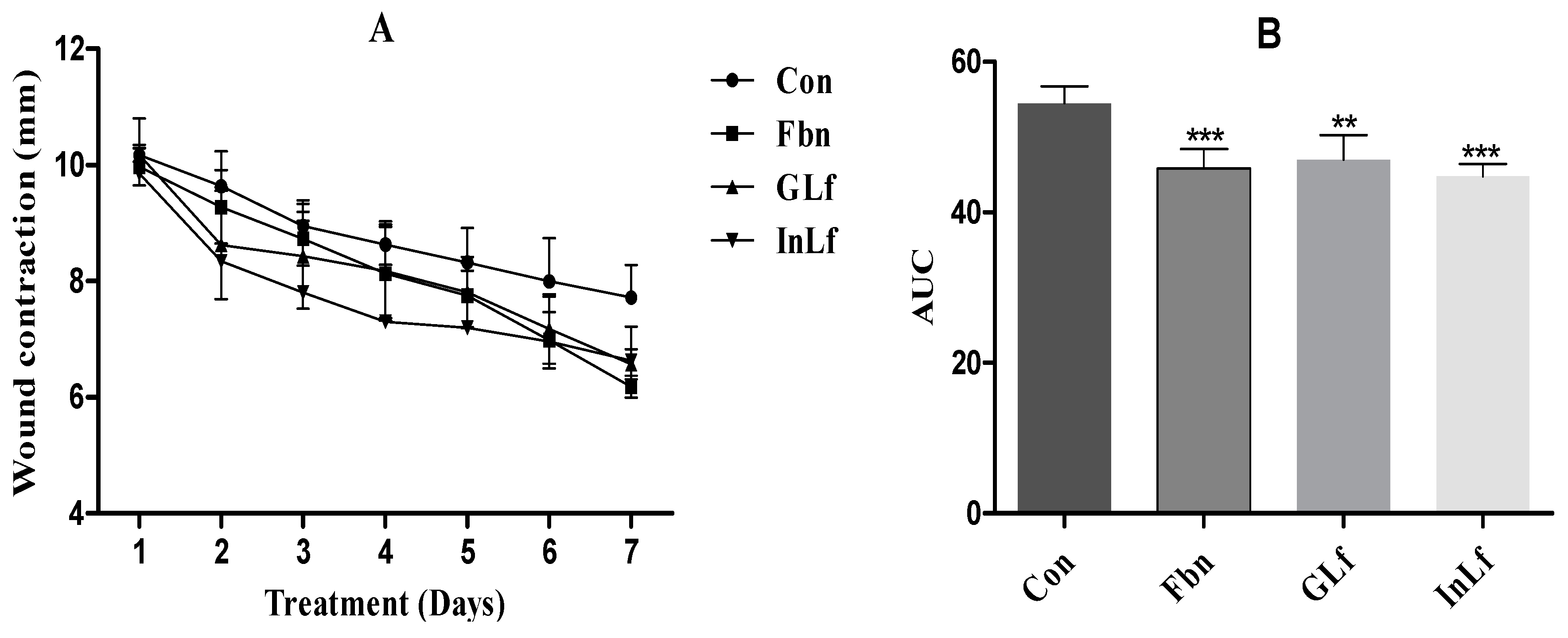
The results of topical treatments on wound healing (
Figure 1) demonstrated that both the infusion (InLf) and the gel (GLf) of Libidibia ferrea significantly reduced (p < 0.001) wound contraction in all measured periods, proving that the treatments were able to accelerate tissue repair. The area under the curve (AUC) data demonstrated that the treatments presented a significant difference compared to the control group (Con). The difference was observed mainly in the last days of treatment, where the difference to the control group was visibly more significant.
2.2. Histopathological Evaluation of Wounds
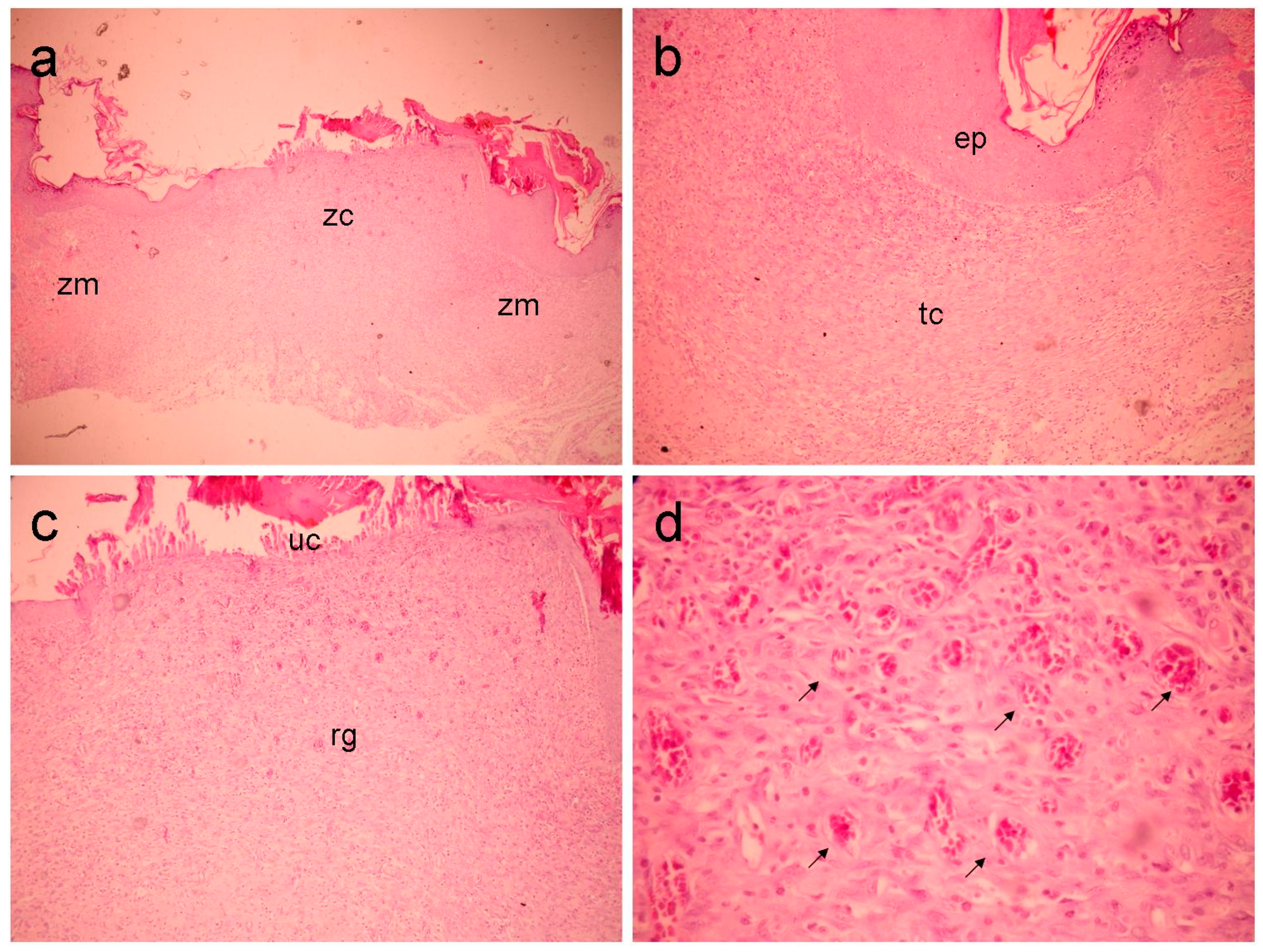
GLf group (
Figure 2), moderate inflammatory infiltrate was observed in the marginal portion of the wound, predominantly lymphocytic, as well as intense proliferation of voluminous fibroblasts, organized in parallel, associated with the deposit of rectilinear and delicate bundles of collagen, exhibiting varied thickness. The vascular component was represented by capillary vessels, some of them congested. The surface of the wound was covered by acanthotic ortho-keratinized squamous epithelium. The most central portion of the wound exhibits a superficial third, representing an exuberant and abundantly vascular granulation reaction. The deepest third presents a mature fibrocellular granulation reaction with moderate lymphocytic infiltration, fibroblastic proliferation, and collagen deposition like the pattern at the margins.
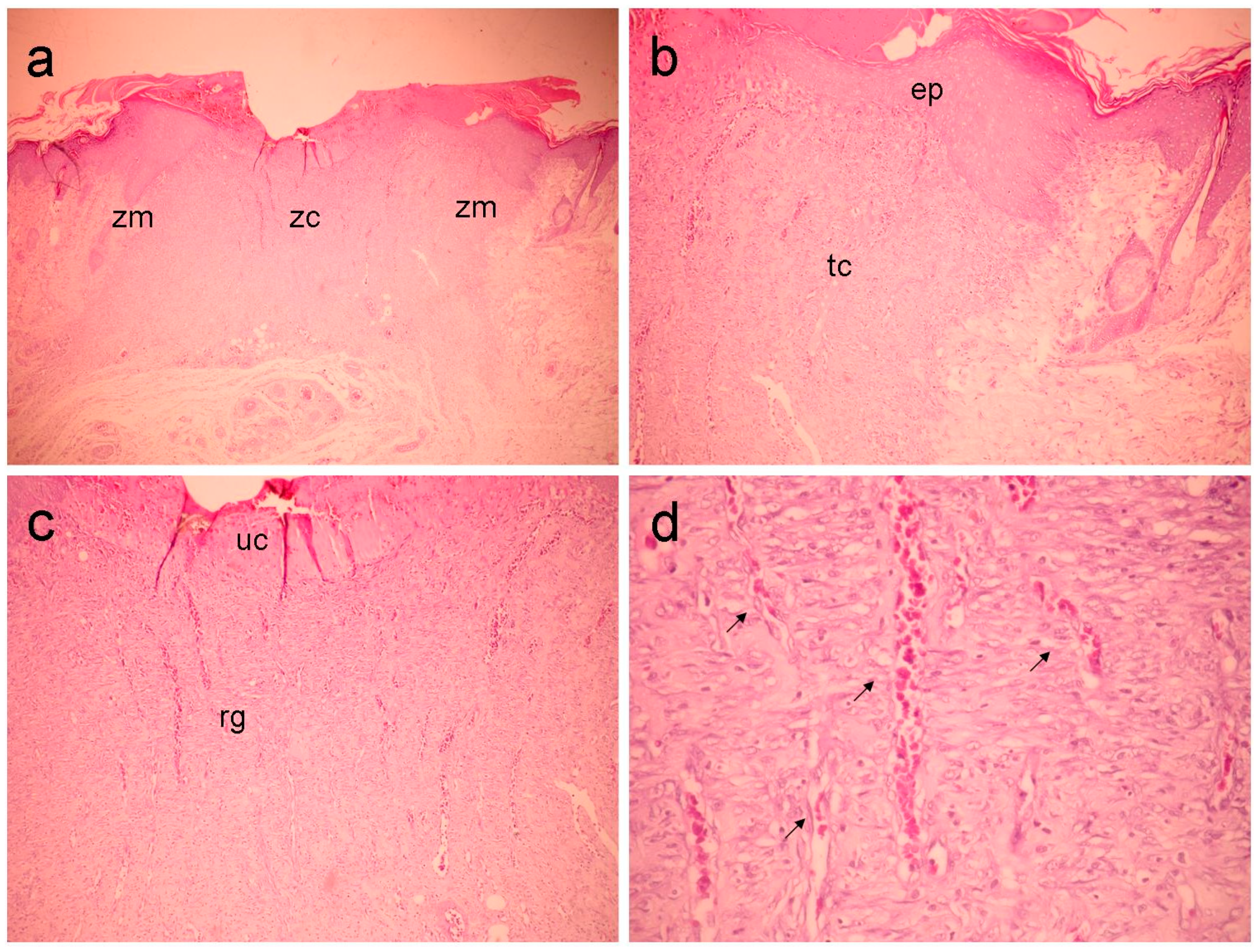
In the marginal portion, the Fbn group (
Figure 3) shows moderate inflammatory infiltration and a granulation reaction showing a less significant vascular-capillary component. The connective tissue appears denser and is formed by thin, short fibrils with intense proliferation of fibroblasts. There is clear epithelialization of the wound edges. In the central region, a fibrovascular granulation reaction is observed. It should be noted that the connective tissue is represented by a highly loose matrix formed by thin, delicate and very short collagen fibrils. Peripheral epithelialization is visible.
InLf Group (
Figure 4), moderate-intensity inflammatory infiltrate, lymphohistiocytic, was observed, as well as intense proliferation of voluminous fibroblasts, arranged parallel to the surface, associated with the deposition of straight and delicate collagen bundles, exhibiting varied thickness, well-marked marginal epithelialization, with visible acanthosis of the newly formed epithelium. In the central portion, an immature granulation reaction (eminently fibrovascular) projects from the surface to the depth.
Con group (
Figure 5), the marginal portion exhibits a lymphohistiocytic inflammatory reaction, a highly significant vascular component of the granulation reaction. However, it is still represented by small, poorly formed capillaries with irregular morphology (“slit-like” vessels), in addition to moderate proliferation of fibroblasts in association with deposition of short, delicate fibrillar collagen, with a widely spaced parallel distribution, characterizing a highly immature connective tissue. In the central portion, a richly vascularized granulation reaction extending from the surface to the depth and moderate interstitial edema are evident. Persistent superficial neutrophilic infiltration and moderate interstitial edema occur in all specimens.
2.3. Assessment of Epithelialization, Angiogenesis, and Inflammatory Cells
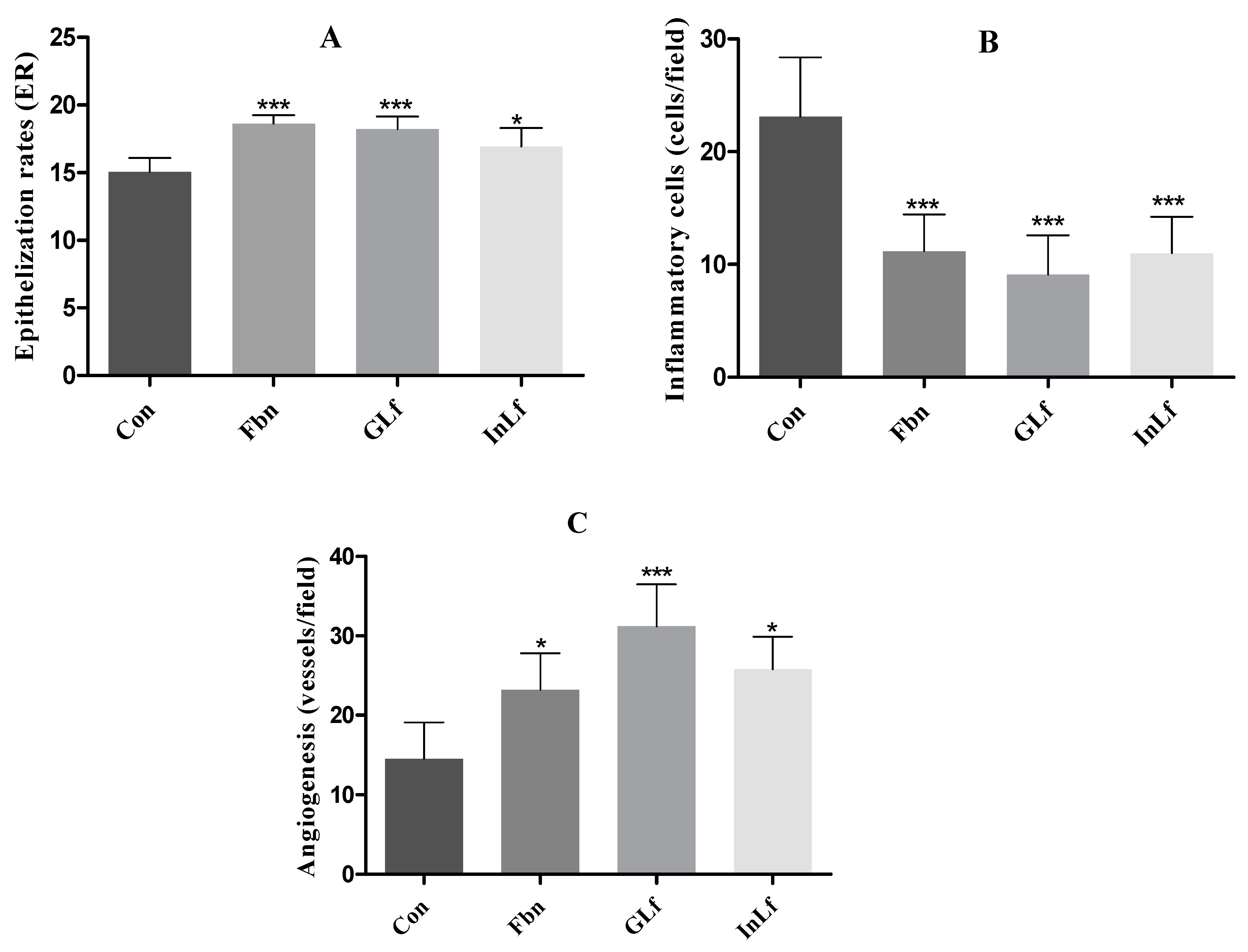
Epithelialization index (
Figure 6.A) in the groups treated with GLf and Fbn showed significantly higher results (p < 0.001) when compared to the Con group. The group treated with InLf also showed a significant difference (p < 0.05).
In the microscopic analysis with 400x magnification, regarding the presence of inflammatory cells (
Figure 6.B), the mean number of cells in the Con group was significantly higher than the other groups. In the groups treated with GLf , InLf, and Fbn, significantly lower values were observed with p < 0.001 and means of 9.1±4.48, 11±3.22, and 11.2±3.28, respectively.
Regarding angiogenesis (
Figure 6.C), there was an extremely significant difference in the mean values of the groups compared to the Con group. For example, in the group treated with GLf, a significant increase (p < 0.001) was observed in the mean value of vessels per field (31.2 ± 5.27). In the groups treated with Fbn and InLf, values with significance levels of p<0.05 were observed when compared to the Con group, being 23.2±4.62 and 25.8±4.08, respectively.
2.4. Quantification of Fibroblasts and Analysis of Collagen Deposition
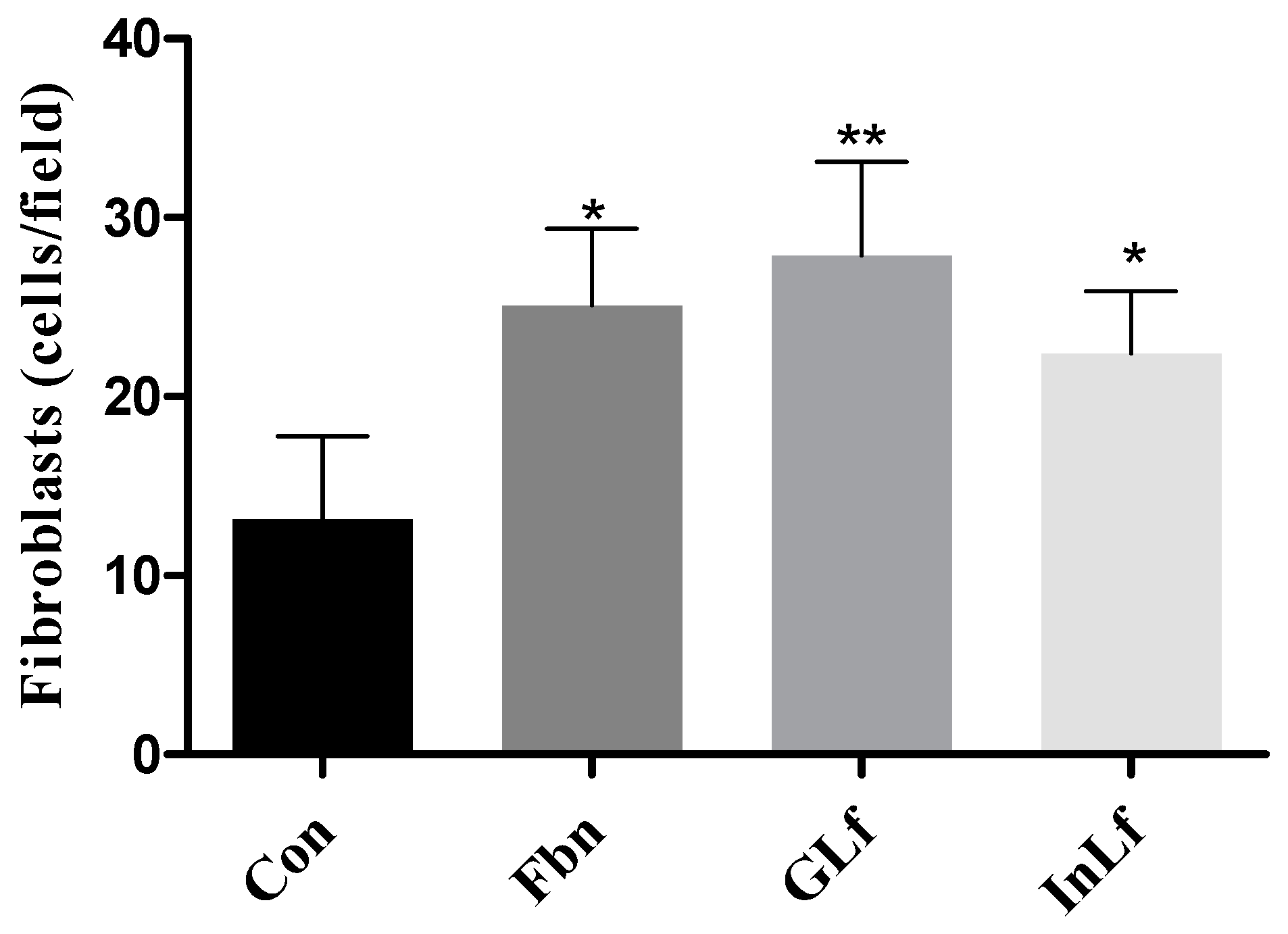
Regarding the quantification of fibroblasts in the histopathological slides (
Figure 7), a significant increase (p < 0.01) was observed in the average number of new fibroblasts in the group treated with GLf, with a value of 27.88 ± 5.24 cells/field. In the groups treated with InLF and Fbn, significant values (p < 0.05) were also observed compared to the Con group. The Con group presented significantly reduced values in the proliferation of new fibroblast cells, with an average value of 13.2 ± 4.6 cells/field.
Regarding the analysis of collagen deposition in histopathological sections stained with picrosirius and examined under polarized light microscopy (
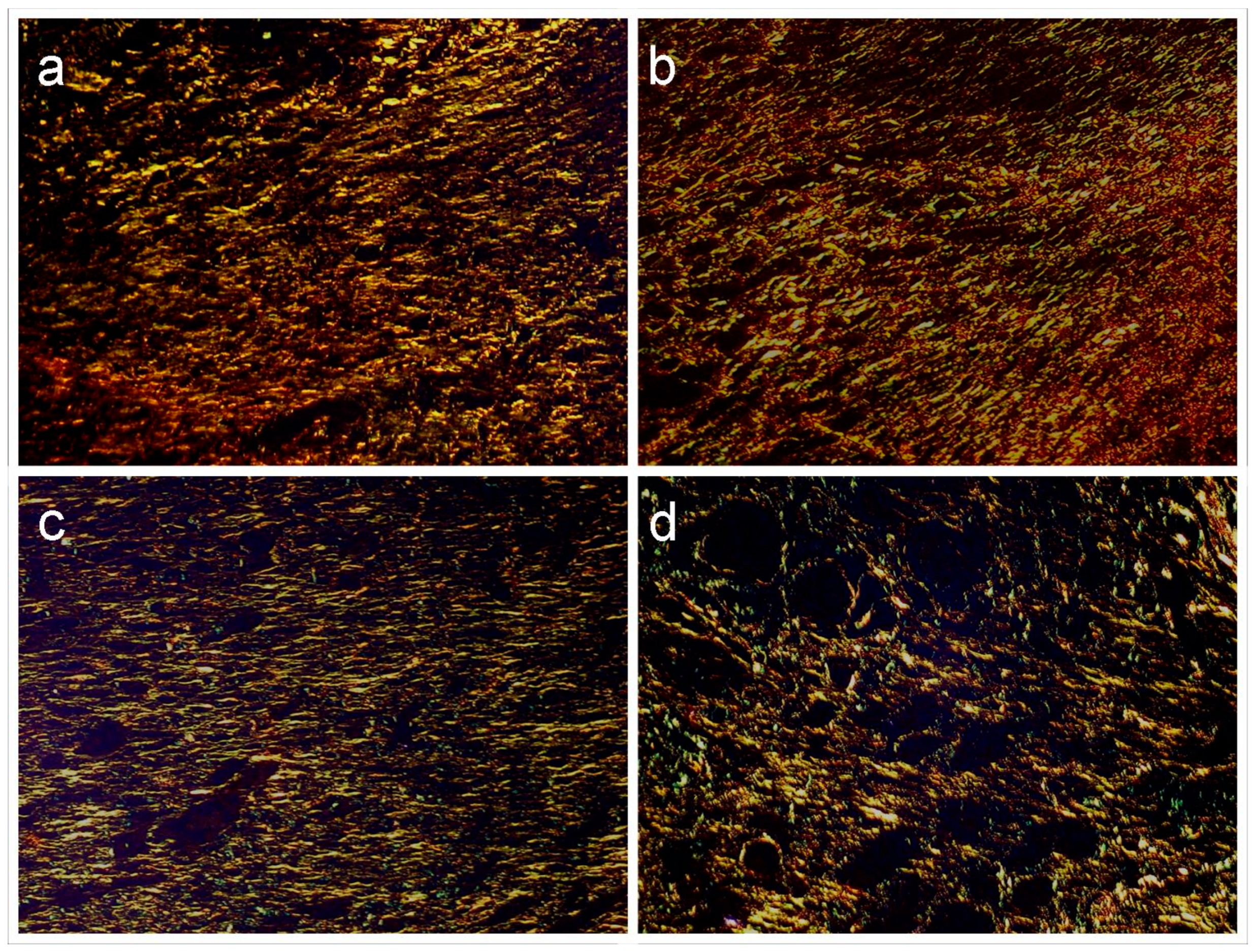
Figure 8). In the GLf and Fbn groups, the presence of short, thin, and delicate fibrous bundles with a slightly wavy appearance, irregular arrangement, and greenish and yellow-greenish birefringence was observed, broadly consistent with deposition of reticular collagen (type III). Deeper in the wound and at the edges of the wound, the bundles were more abundant, and it was possible to demonstrate the formation of fibrils with yellow-orange birefringence compatible with type I collagen. The interfibrillar spaces were plentiful. The pattern observed in the InLf group was similar, but no deposition of type I collagen was evidenced; even in the marginal zones, this type of collagen was scarce. In G4, in turn, only type III collagen was evidenced along the entire extension of the wound, however the bundles were quite thin and assumed a reticular arrangement.
3. Discussion
Wound healing is a complex process involving the interaction of several phases, including inflammation, granulation tissue formation, and collagen remodeling. This study investigated the effects of topical application of products containing Libidibia ferrea fruit derivatives on the healing process in cutaneous wounds, correlating the histopathological findings with the phytochemical composition of the formulations, specifically the tannin and flavonoid contents.
Phytochemical analyses indicated that the formulations had high tannin content ranging from 23 to 18% and flavonoid content ranging from 0.25 to 0.14%. Tannins and flavonoids are widely known for their antioxidant and anti-inflammatory properties, which suggests that formulations richer in these compounds may favor the healing process due to their ability to modulate inflammation and protect cells from oxidative stress (Barbosa et al., 2007; Biswas & Mukherjee, 2003).
Libidibia ferrea formulations could significantly reduce wound size and promote lesion contraction in rats in an optimized manner compared to the control group. Wound contraction is an important indicator of healing; it is the phase responsible for the closure of the lesion itself since it involves re-epithelialization, movement of epithelial cells, fibroplasia, and angiogenesis, which make up the so-called granulation tissue responsible for the occupation of the injured tissue approximately four days after the injury (Mendonça, Coutinho-Netto, 2009; Barros et al., 2017).
In the histopathological evaluation, on the seventh day, it was observed that the inflammatory reaction was intense in all groups, which is expected in wounds by secondary intention, where inflammation is a necessary step for the elimination of harmful agents and the beginning of the repair process (Diegelmann & Evans, 2004). However, there was a clear difference in the pattern of inflammation between the groups. In the groups treated with Libidibia ferrea (GLf and InLf) and with Fbn, inflammation was reduced throughout the treatment and favored wound closure, suggesting a rapid transition from acute to controlled inflammation and decreased inflammatory cells in the scar tissue. In contrast, in the control group (Con), inflammation remained predominantly acute, with a significant presence of neutrophils. This may indicate a prolonged inflammatory response and a factor that delays healing (Ribeiro, 2006; Ozay et al., 2018).
Libidibia ferrea formulations (Barreto, 2007). The high concentration of tannins in GLf and InLf may have contributed to improving the inflammatory condition, reducing the need for continuous recruitment of neutrophils, and promoting a faster transition to the proliferative phase and, consequently, wound closure (Isaac et al., 2010).
In the proliferative phase, granulation tissue is characterized by the presence of fibroblasts and newly formed vessels; this phase was best observed in the groups treated with GLf, Fbn, and InLf, which presented higher rates of epithelialization and increased angiogenesis compared to the Con group. Angiogenesis is essential for wound healing, as the new blood vessels formed to provide the oxygen and nutrients necessary for wound repair (Oudega, 2012).
The epithelialization process, which involves the proliferation and migration of epithelial cells to cover the injured area, was more advanced in the GLf, Fbn, and InLf groups compared to the Con group. These findings suggest that formulations rich in tannins and flavonoids may have accelerated the epithelialization process, possibly due to their ability to modulate the inflammatory response and protect epithelial cells from oxidative damage (McDougall et al., 2006; Akubugwo et al., 2022).
This pattern is consistent with more advanced healing, as it is observed that groups with higher epithelialization rates also present more significant amounts of tissue fibroblasts, indicating a greater tendency to transition from the proliferative phase to the remodeling phase (Balbino, Pedreira & Curi, 2005).
In the remodeling phase, it is observed that the wound tissue begins to reorganize and strengthen from the collagen produced and realigned to increase the strength and elasticity of the wound (Isaac et al., 2010). The deposition of type I collagen, which confers greater resistance to scar tissue, was observed more prominently in the GLf and Fbn groups, while type III collagen, which is more common in the early phases of repair, predominated in the Con group. The replacement of type III collagen by type I collagen is an important indicator of scar tissue maturation, suggesting that treatments with Libidibia ferrea based formulations contributed to and accelerated this process (Fleischmajer et al., 1990; Dantas Filho et al., 2007).
4. Materials and Methods
4.1. Plant Material
The fruits of
Libidia ferrea were collected at the Medicinal Plant Garden of the Institute of Studies and Research of the State of Amapá (IEPA), from a specimen previously identified and cataloged in the Herbarium of the Agronomic Institute of the North (IAN185211 - FABACEAE Libidibia ferrea var. ferrea (Mart. ex Tul.) LP Queiroz) (
Figure 9).
Libidia ferrea species were used to obtain the infusion, the glycolic extract, and the gel. The methods performed were those described in the Phytotherapeutic Formulary of the Brazilian Pharmacopoeia (ANVISA, 2nd Edition, 2021) and, for the dosage of tannins and flavonoids of the preparations, the method described by Gonzalez (2005) was used.
4.2. Animals Used in the Study
The Research Ethics Committee of UNIFAP approved the study under protocol no. 008/2017 of 08/23/2017. It used twenty male Wistar rats, albino variety, with body weight varying between 300 ± 40 g, acquired from the Multidisciplinary Center for Biological Research in Laboratory Animals Science – CEMIB, University of Campinas, UNICAMP. The rats were 21 days old and were acclimatized in polyethylene cages under controlled temperature conditions (22 ± 3°C), with free access to food and water ad libitum, under 12/12 hours of light/dark cycle. After the acclimation period, the animals were individually placed in metabolic cages.
Each experimental group consisted of five animals in polyethylene cages in an air-conditioned room at 25° ± 2°C. The animals were deprived of food for 24 hours before the experiment but remained with access to water ad libitum. They received standardized food (Labina®) and water throughout the experiment, remaining in a 12-hour light/dark cycle for seven days.
4.3. Experimental Groups for Healing Activity
The animals were distributed into four experimental groups, containing five animals each, individually identified, so that all groups had the same weight, as follows:
The first group was treated with GLf at a concentration of 5% topically, with a dose of 188 mg/kg;
The second group, defined as positive control, was treated topically with fibrinolysin (Fbn) (Cristália - Prod. Quím. Farm. Ltda., Itapira, São Paulo), with a dose of 31 mg/kg;
The third group (G3) was treated with InLf at a concentration of 5% with a dose of 182 mg/kg;
The fourth group (G4), defined as negative control (Con), was treated with 0.5 mL of saline solution (NaCl) at a concentration of 0.9%.
4.4. Surgical Procedure and Morphometric Evaluation
All surgical procedures were performed under general anesthesia, using 45 mg/kg of sodium thiopental (Cristália Co., São Paulo, São Paulo) administered intraperitoneally and following the method described by Barros et al. (2017). Immediately after hemostasis, treatment of the animals began, and they were treated once a day, at standardized times (12:00 h - 13:00 h), for seven days, with the wounds remaining without dressings throughout the experiment.
The wounds were measured in a standardized manner in the craniocaudal direction, from the 1st day (immediately after surgery) to the 7th day postoperatively, with the aid of a digital caliper, and the speed of wound healing was analyzed. Based on the values of the measurements obtained, the contraction of the wound was evaluated using the following formula (Agren et al., 1997):
Digital images of the wounds were obtained in a standardized manner immediately after the surgical procedure (1st day) until the 7th postoperative day. For this purpose, a camera (Sonic 7.0, Brazil) was used, attached to a support fixed perpendicularly to the horizontal plane, and kept at a distance of 08 cm from this plane, providing adequate focusing of the ulcer. The digitalized images were stored in Windows Bitmap (BMP) format and analyzed in ImageLab.
The animals were euthanized on the 7th day after the surgical procedure and were previously anesthetized and placed in the CO2 chamber. After sacrifice, the wound was excised with a 0.5 mm margin of intact skin around the lesion, deep to the muscle fascia, under aseptic conditions, making a median cut, and the piece was fixed in 10% formalin.
4.5. Histopathological Evaluation of Wounds
The histopathological evaluation was based on the method described by Barros et al. (2017). For this purpose, the skin fragments fixed in 10% formalin were dehydrated and embedded in paraffin. They were then cut 5 m thick and stained with hematoxylin-eosin and picrosirius. The quantitative and qualitative analyses of the effect of both GLf and InLf included counting fibroblasts, inflammatory cells, and angiogenesis (blood vessels) present in the histopathological slides analyzed at 400x.
Determination of epithelization rates (ER). Using histological sections stained in HE, the extent of epithelization of the wounds was evaluated by measuring the epidermal migration from the average wound margin to the point where the migrating epithelium stopped processing. ER represented the percentage of the new epithelium present in the total wound, determined in four high-power fields (x400) on each slide by using software for morphometry (Imagetool®), according to the equation as follows:
Assessment of the collagen deposition. Histological sections stained in picrosirius and analyzed under polarized light were used for the descriptive analysis of the collagen deposition. Collagen fibers were analyzed according to their birefringence pattern (greenish/yellow-greenish or orange, orange-reddish), morphological appearance (wavy or stretched, thin or thick, short or long), and disposition (parallel-arranged or interlaced).
4.6. Statistical Analysis
All experimental quantitative results were expressed as mean ± standard deviation for statistical analysis, and one-way analysis of variance (ANOVA) was used, followed by Tukey's test for multiple comparisons. Results with significance levels of p < 0.05 were considered statistically significant. The statistical program used GraphPad Prism (version 5.03).
5. Conclusions
The integration of phytochemical data with histopathological results demonstrates that formulations containing high levels of tannins and flavonoids from Libidibia ferrea fruits presented beneficial effects on the wound healing process by secondary intention, modulating the inflammatory response, promoting the formation of granulation tissue and accelerating epithelialization and collagen remodeling. These results reinforce the therapeutic healing potential of pharmaceutical formulations of Libidibia ferrea in treating wounds.
Author Contributions
A.K.L.A.C., F.P.S.P., C.R.S.F., S.G.L.B., L.P. N.O. Conceptualization; methodology and investigation; H.O.C., validation, formal analysis, resources, and data curation. R.L.C.A. was responsible for the histopathological study and J.C.T.C. writing—review and editing, visualization, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES (1723/2018-00 PROCAD-AMAZONIA), CNPq - MAI-DAI Program (Proc. 421808/2022-5), and INCT North-Northeast Network of Phytoproducts – CNPq.
Institutional Review Board Statement
The Research Ethics Committee of UNIFAP approved the animal study protocol under protocol no. 008/2017 of 08/23/2017.
Informed Consent Statement
Not applicable.
Acknowledgments
The CAPES funded this study - PROCAD AMAZONIA, CNPq - MAI-DAI Program, and INCT North-Northeast Network of Phytoproducts - CNPq.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Agência Nacional de Vigilância Sanitária. (2021). Formulário de Fitoterápicos da Farmacopeia Brasileira (2ª ed.). Brasília, DF: ANVISA.
- Alves Barros, A. S., Carvalho, H. O., Santos, I. V. F., Taglialegna, T., Sampaio, T. I. S., Duarte, J. L., Fernandes, C. P., & Carvalho, J. C. T. (2017). Study of the non-clinical healing activities of the extract and gel of Portulaca pilosa L. in skin wounds in Wistar rats: A preliminary study. Biomedicine & Pharmacotherapy, 96, 182-190. [CrossRef]
- Akubugwo, E.I.; Emmanuel, O.; Ekweogu, C.N.; Ugbogu, O.C.; Onuorah, T.R.; Egeduzu, O.G.; Ugbogu, E.A. GC-MS Analysis of the Phytochemical Constituents, Safety Assessment, Wound Healing and Anti-Inflammatory Activities of Cucurbita pepo Leaf Extract in Rats. Sci. Pharm. 2022, 90, 64. [CrossRef]
- Azulay, R. D., & Azulay, D. R. (1999). Dermatologia (2ª ed.). Rio de Janeiro: Guanabara Koogan.
- Bacchi, E. M., & Sertie, J. A. (1994). Antiulcer action of Styrax camporum and Caesalpinia ferrea in rats. Planta Med, 60(2), 118-120.
- Bacchi, E. M., Sertie, J. A., Villa, N., & Katz, H. (1995). Antiulcer action and toxicity of Styrax camporum and Caesalpinia ferrea. Planta Med, 61(3), 204-207.
- Balbino, C. A., Pedreira, L. M., & Curi, R. (2005). Mecanismos envolvidos na cicatrização: uma revisão. Rev Bras Ciênc Farm, 41(1), 27-51.
- Barbosa, R., Costa, E., Ribeiro, R. M., & Santos, L. (2007). Estudo do efeito anti-inflamatório do extrato aquoso de Caesalpinia ferrea em modelos experimentais. Revista Brasileira de Plantas Medicinais, 10(2), 102-109.
- Barreto, A. C. S. (2007). Avaliação da atividade antimicrobiana de extratos vegetais de Caesalpinia ferrea. Tese de Doutorado. Universidade Federal de Pernambuco.
- Biswas, T. K., & Mukherjee, B. (2003). Plant medicines of Indian origin for wound healing activity: A review. International Journal of Lower Extremity Wounds, 2(1), 25-39. [CrossRef]
- Brito, N. M. B., Simões, M. J., Gomes, P. O., Pessoa, A. F., & Melo, M. C. F. (2001). Aspectos microscópicos da cicatrização de feridas abertas tratadas com óleo de copaíba em ratos. Revista Paraense de Medicina, 13(1), 12-17.
- Brito, N. M. B., Silva, P. R. F., Silva, G. C. F., Casella, S. F. M., Sampaio, A. R. S., & Carvalho, R. A. (2001). Avaliação macroscópica de feridas cutâneas abertas em ratos tratadas com óleo de andiroba. Revista Paraense de Medicina, 15(2), 17-22.
- Carneiro, C. G., Sennes, L. U., Saldiva, P. H. N., Tsuji, D. H., & Ximenes Filho, J. A. (2005). Avaliação da deposição de colágeno após implante de fáscia lata e de gordura na prega vocal de coelho: estudo histomorfométrico. Rev Bras Otorrinolaringol, 71(6), 798-802.
- Carvalho, J. C., Teixeira, J. R., Souza, P. J., Bastos, J. K., Santos Filho, D. S., & Sarti, S. J. (1996). Preliminary studies of analgesic and anti-inflammatory properties of Caesalpinia ferrea crude extract. Journal of Ethnopharmacology, 53(3), 175-178. [CrossRef]
- Chabane, S.; Boudjelal, A.; Keller, M.; Doubakh, S.; Potterat, O. Teucrium polium—Wound healing potential, toxicity and polyphenolic profile. S. Afr. J. Bot. 2021, 137, 228–235.
- Dantas Filho, A. M., Aguiar, J. L. A., Rocha, L. R. M., Azevedo, I. M., Ramalho, E., & Medeiros, A. C. (2007). Effects of the basic fibroblast growth factor and its anti-factor in the healing and collagen maturation of infected skin wound. Acta Cir Bras, 22(S1), 64-71. [CrossRef]
- Desmouliere, A., Redard, M., Darby, I., & Gabbiani, G. (1995). Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol, 146(1), 56-66.
- Diegelmann, R. F., & Evans, C. M. (2004). Wound healing: An overview of acute, fibrotic, and delayed healing. Front Biosci, 9(1), 283-289.
- Drumond, D. C., Nunes, R. F., & Santos, P. F. (2000). Efeito da Caesalpinia ferrea no tratamento de doenças respiratórias e infecciosas. Jornal de Medicina Natural, 8(2), 34-40.
- Fleischmajer, R., Perlish, J. S., Burgeson, R. E., Shaikh-Bahai, F., & Timpl, R. (1990). Type I and type III interactions during fibrillogenesis. Am NY Acad Sci, 580(1), 161-175.
- Fonseca, V. R. C. D. (2003). Quantificação da deposição de colágeno de submucosa de pregas vocais suinas após exerese de fragmento de mucosa a frio e uso de Mitomicina-C tópica (Dissertação de Mestrado, IPEM, Curitiba, PR, Brasil).
- Friedman, D. W., Boyd, C. D., Mackenzie, J. W., Norton, P., Olson, R. M., & Deak, S. B. (1993). Regulation of collagen gene expression in keloids and hypertrophic scars. J Surg Res, 55(2), 214-222. [CrossRef]
- Ghadially, F. N. (1997). Extracellular matrix, Ultrastructural Pathology of the cell and matrix (4ª ed., pp. 1307-1399).
- Gonçalves, R.; Nathalia de Aguiar Pereira; Pereira Alves, M. H. .; Mendes Silva, H. H. .; Leão Araújo, J. . Ação cicatrizante da Libidibia férrea M.: uma revisão integrativa. Revista de Casos e Consultoria, 14, 1, p. e30969, 2023.
- Gonzalez, F. G. (2005). Estudo farmacognóstico e farmacológico de Caesalpinia ferrea Martius (Tese de doutorado, Faculdade de Ciências Farmacêuticas, Universidade de São Paulo).
- Gopinath, D., Ahmed, M. R., Gomathi, K., Chitra, K., Sehgal, P. K., & Jayakumar, R. (2004). Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials, 25(10), 1911-1917. [CrossRef]
- Helary, C., Ovtracht, L., Coulomb, B., Godeau, G., & Giraud-Guille, M. M. (2006). Dense fibrillar collagen matrices: A model to study myofibroblast behaviour during wound healing. Biomaterials, 27(25), 4443-4452. [CrossRef]
- Isaac C, Ladeira PRS, Rego FMP, Aldunate JCB, Ferreira MC. Processo de cura das feridas: cicatrização fisiológica. Rev Med (São Paulo). 2010;89(3/4):125-31.
- Kilic, T. (2005). Investigating the wound healing properties of traditional medicinal plants. Journal of Herbal Medicine, 6(1), 23-31.
- Mandelbaum, S. H., Di Santis, E. P., & Mandelbaum, M. H. S. (2003). Cicatrização: conceitos atuais e recursos auxiliares - Parte I. An Bras Dermatol, 78(4), 393-408.
- McDougall, S., Dallon, J., Sherratt, J., & Maini, P. (2006). Fibroblast migration and collagen deposition during dermal wound healing: Mathematical modeling and clinical implications. Philos Transact A Math Phys Eng Sci, 364(1843), 1385-1405. [CrossRef]
- Menezes, I. A., Moreira, I. J., Carvalho, A. A., Antoniolli, A. R., & Santos, M. R. (2007). Cardiovascular effects of the aqueous extract from Caesalpinia ferrea: Involvement of ATP-sensitive potassium channels. Vascul Pharmacol, 47(1), 41-47. [CrossRef]
- Mendonça R. J., Coutinho-Netto, J. Aspectos celulares da cicatrização. Anais Brasileiro de Dermatologia. 2009;84(3):257-62.
- Nakamura, E. S., Kurosaki, F., Arisawa, M., Mukainaka, T., Okuda, M., Tokuda, H., Nishino, H., & Pastore, F. (2002). Cancer chemopreventive effects of constituents of Caesalpinia ferrea and related compounds. Cancer Letters, 177(2), 119-124. [CrossRef]
- Orsolic, N., & Basic, I. (2003). Immunomodulation by water-soluble derivative of propolis: A factor of antitumor reactivity. J Ethnopharmacol, 84(2-3), 265-273. [CrossRef]
- Ostrovskii, A. A., & Syatrova, V. O. (1992). Development of interfollicular epidermis on surface of collagen skeleton of the dermis: Experimental study. Bull Exp Biol Med, 113(5), 734-738. [CrossRef]
- Oudega, M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Resarch.2012, 349: 269. [CrossRef]
- Ozay, Y.; Oliveira, S.; Erdogdu, I.H.; Yildirim, Z.; Pehlivanoglu, B.; Aydın Turk, B.; Darcan, S. Avaliação das propriedades cicatrizantes de pomadas de luteolina em modelos de feridas de excisão e incisão em ratos diabéticos e não diabéticos. Registros Nat. Prod. 2018, 12, 350–366.
- Queiroz, M. L., Justo, G. Z., Valadares, M. C., & Pereira-da-Silva, F. R. (2001). Evaluation of Caesalpinia ferrea extract on bone marrow hematopoiesis in the murine models of listeriosis and Ehrlich ascites tumor. Immunopharmacol Immunotoxicol, 23(3), 367-382.
- Rabau, M. Y., & Dayan, D. (1994). Polarization microscopy of picrosirius red stained sections: A useful method for qualitative evaluation of intestinal wall collagen. Histol Histopathol, 9(3), 525-528.
- Reynolds, J. E., & Dweck, A. C. (1999). Plant-based ingredients for wound healing. International Journal of Cosmetic Science, 21(5), 353-364.
- Ribeiro, F. A. Q. (2006). Processo inflamatório e resposta imune no reparo cicatricial. São Paulo: Editora Universitária.
- Ribeiro, F. A. Q., Borges, J. P., Zaccbi, F. F. S., & Guaraldo, L. (2004). Clinical and histological healing of surgical wounds treated with mitomycin C. Laryngoscope, 114(1), 148-152. [CrossRef]
- Ribeiro, M. A., Albuquerque, R. L., Ramalho, L. M., Pinheiro, A. L., Bonjardim, L. R., & Da Cunha, S. S. (2009). Immunohistochemical assessment of myofibroblasts and lymphoid cells during wound healing in rats subjected to laser photobiomodulation at 660 nm. Photomed Laser Surg, 27(1), 49-55. [CrossRef]
- Rich, L., & Whitaker, P. (2005). Collagen and picrosirius red staining: A polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci, 22(2), 97-104.
- Sampaio, F. C., Pereira, M. S. V., Dias, C. S., Costa, V. C. O., Conde, N. C. O., & Buzalaf, M. A. R. (2009). In vitro antimicrobial activity of Caesalpinia ferrea Mart. fruits against oral pathogens. Journal of Ethnopharmacology, 124(2), 289-294.
- Sanches Neto, R., Barone, B., Teves, D. C., Simões, M. J., Novo, N. F., & Juliano, Y. (1993). Aspectos morfológicos e morfométricos da reparação tecidual de feridas cutâneas em ratos com e sem tratamento com solução de papaína a 2%. Acta Cir Bras, 8, 18-23.
- Semenoff Segundo, A., Bosco, A. F., Da Maia, D., Ribeiro, R. V., De Aguiar, E. B. H., Rocatto, G. E. G. D., & Maia, D. A. (2007). Influência do aloe vera e própolis na contração de feridas em dorso de ratos. Periodontia, 17(01), 5-10.
- Srivastava, S., Gorham, S. D., French, D. A., Shivas, A. A., & Courtney, J. M. (1990). In vivo evaluation and comparison of collagen, acetylated collagen and collagen/glycosaminoglycan composite films and sponges as candidate biomaterials. Biomaterials, 11(3), 155-161. [CrossRef]
- Teves, D. C., Cabral, A. C. V., Simões, M. J., & Kulay, J. R. L. (1986). Biologia da reparação tecidual. J. Bras. Med, 50, 39-44.
- Ueda, H., Tachibana, Y., Moriyasu, M., & Kawanishi, K. (2001). Aldose reductase inhibitors from the fruits of Caesalpinia ferrea Mart. Phytomedicine, 8(5), 377-381.
Figure 1.
Effect of topical administration of GLf (188 mg/kg), InLf (182 mg/kg), and Fbn (31 mg/kg) on cutaneous wound healing. (A) shows the evolution of wound contraction throughout treatment. (B) shows the area under the curve (AUC) of the results for each group. Values show the mean and standard deviation. *** represents a statistically significant result against the Con group. Tukey's post-test followed one-way ANOVA.
Figure 1.
Effect of topical administration of GLf (188 mg/kg), InLf (182 mg/kg), and Fbn (31 mg/kg) on cutaneous wound healing. (A) shows the evolution of wound contraction throughout treatment. (B) shows the area under the curve (AUC) of the results for each group. Values show the mean and standard deviation. *** represents a statistically significant result against the Con group. Tukey's post-test followed one-way ANOVA.
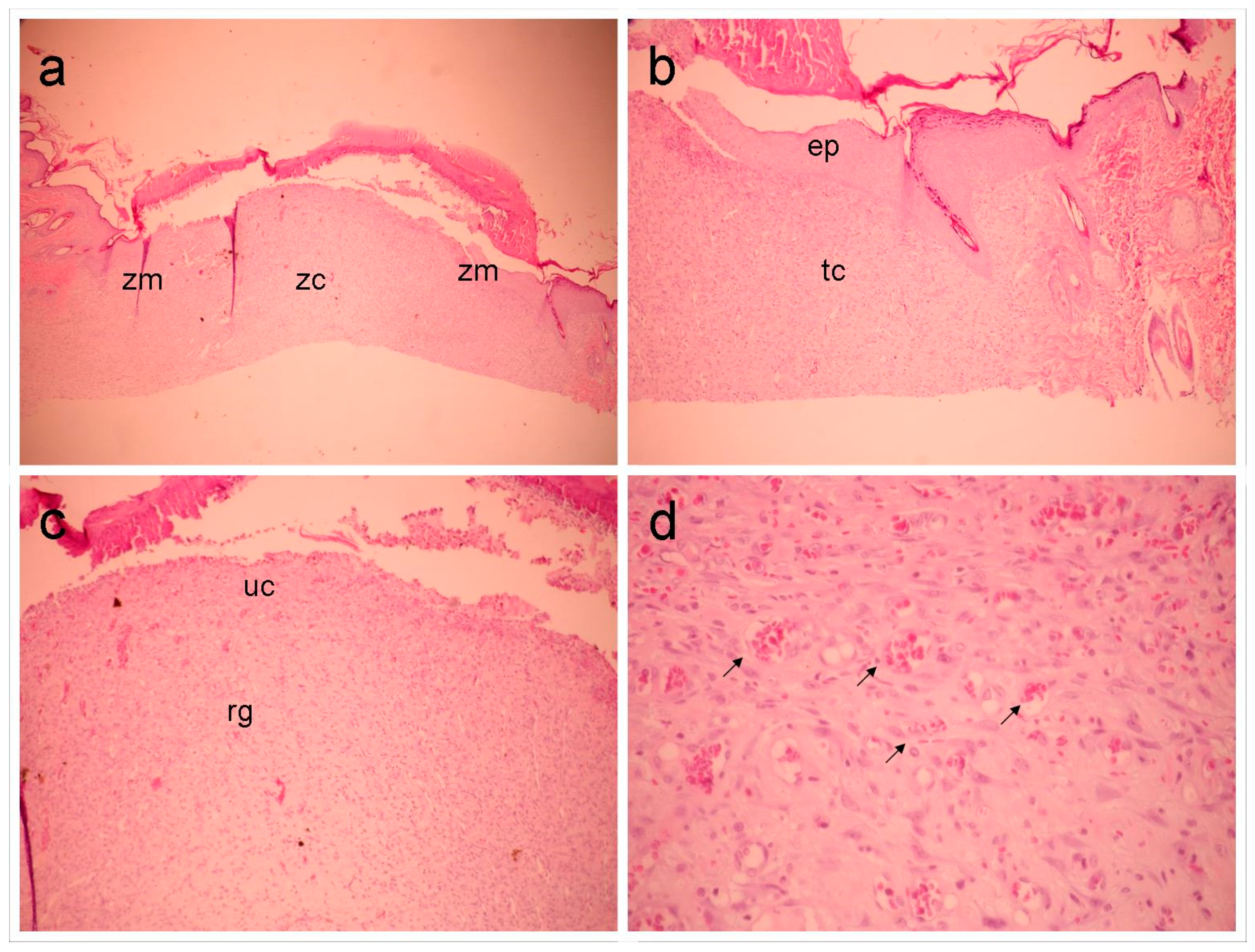
Figure 2.
The GLf group (188 mg/kg), shows (a) an overall view of the wound, highlighting the marginal (Zn) and central (zc) zones (40x magnification). In (b), the marginal zone is highlighted, where an area of epithelialization (ep) and underlying, inflamed connective tissue (tc) can be observed (100x magnification). In (c), the central portion of the wound is seen (100x magnification), highlighting the granulation reaction (rg) and ulcerated zone (without epithelial lining) (UC). In (d), detail can be seen in the superficial region of the previous figure, highlighting the vascular component (arrows) (400x magnification).
Figure 2.
The GLf group (188 mg/kg), shows (a) an overall view of the wound, highlighting the marginal (Zn) and central (zc) zones (40x magnification). In (b), the marginal zone is highlighted, where an area of epithelialization (ep) and underlying, inflamed connective tissue (tc) can be observed (100x magnification). In (c), the central portion of the wound is seen (100x magnification), highlighting the granulation reaction (rg) and ulcerated zone (without epithelial lining) (UC). In (d), detail can be seen in the superficial region of the previous figure, highlighting the vascular component (arrows) (400x magnification).
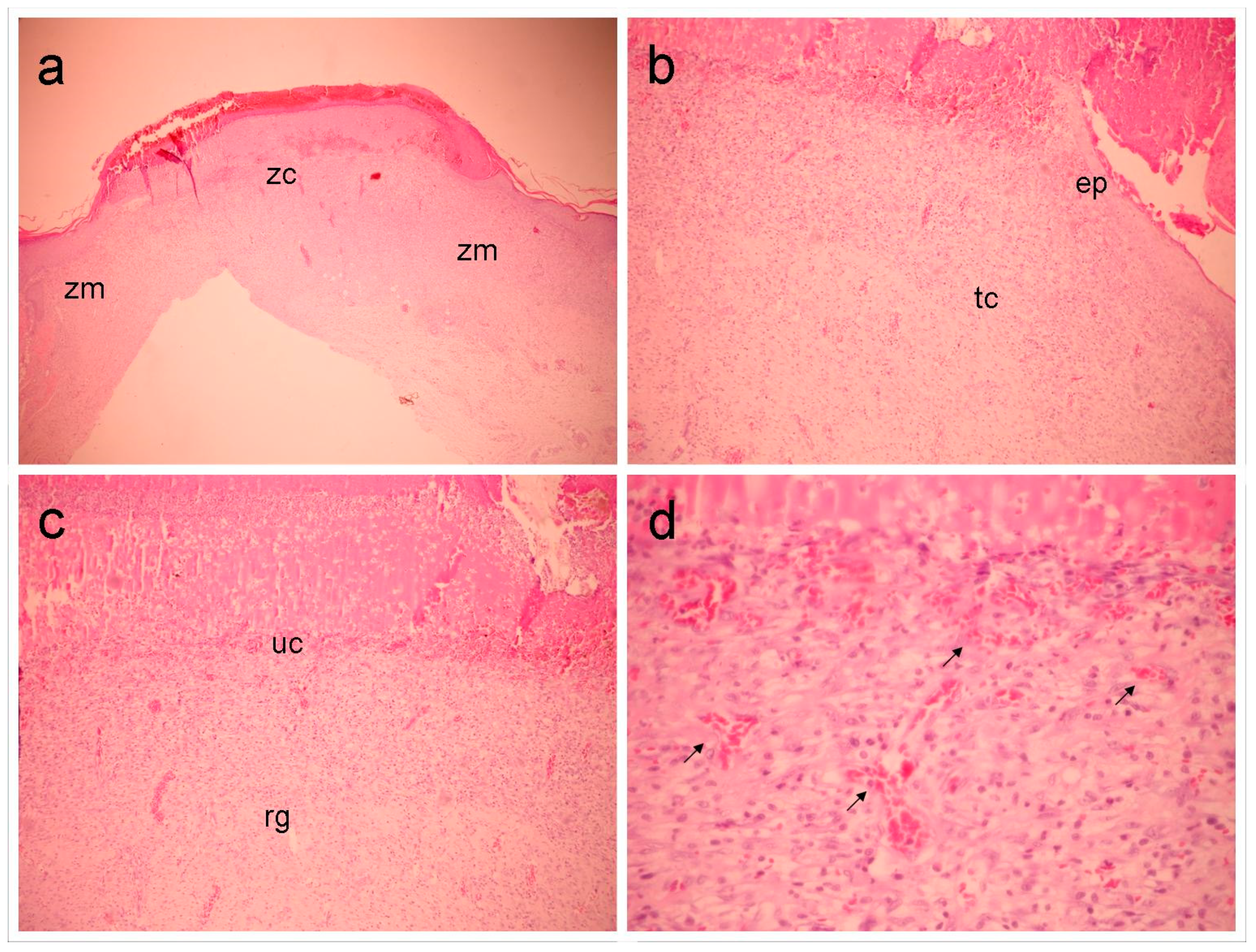
Figure 3.
Fbn group (31 mg/kg) showing in (a) an overall view of the wound, highlighting the marginal (Zn) and central (zc) zones (40x magnification). In (b), the marginal zone is highlighted, where an area of epithelialization (ep) and underlying, inflamed connective tissue (tc) can be observed (100x magnification). In (c), the central portion of the wound is seen (100x magnification), highlighting the granulation reaction (rg) and ulcerated zone (without epithelial lining) (UC). In (d), detail can be seen in the superficial region of the previous figure, highlighting the vascular component (arrows) (400x magnification).
Figure 3.
Fbn group (31 mg/kg) showing in (a) an overall view of the wound, highlighting the marginal (Zn) and central (zc) zones (40x magnification). In (b), the marginal zone is highlighted, where an area of epithelialization (ep) and underlying, inflamed connective tissue (tc) can be observed (100x magnification). In (c), the central portion of the wound is seen (100x magnification), highlighting the granulation reaction (rg) and ulcerated zone (without epithelial lining) (UC). In (d), detail can be seen in the superficial region of the previous figure, highlighting the vascular component (arrows) (400x magnification).
Figure 4.
InLf group (182 mg/kg) showing in (a) an overall view of the wound, highlighting the marginal (Zn) and central (zc) zones (40x magnification). In (b), the marginal zone is highlighted, where an area of epithelialization (ep) and underlying, inflamed connective tissue (tc) can be observed (100x magnification). In (c), the central portion of the wound is seen (100x magnification), highlighting the granulation reaction (rg) and ulcerated zone (without epithelial lining) (uc). In (d), detail can be seen in the superficial region of the previous figure, highlighting the vascular component (arrows) (400x magnification).
Figure 4.
InLf group (182 mg/kg) showing in (a) an overall view of the wound, highlighting the marginal (Zn) and central (zc) zones (40x magnification). In (b), the marginal zone is highlighted, where an area of epithelialization (ep) and underlying, inflamed connective tissue (tc) can be observed (100x magnification). In (c), the central portion of the wound is seen (100x magnification), highlighting the granulation reaction (rg) and ulcerated zone (without epithelial lining) (uc). In (d), detail can be seen in the superficial region of the previous figure, highlighting the vascular component (arrows) (400x magnification).
Figure 5.
Group Con shows in (a) an overall view of the wound, highlighting the marginal (Zn) and central (zc) zones (40x magnification). In (b), the marginal zone is highlighted, where an area of epithelialization (ep) and underlying, inflamed connective tissue (tc) can be observed (100x magnification). In (c), the central portion of the wound is seen (100x magnification), highlighting the granulation reaction (rg) and ulcerated zone (without epithelial lining) (uc). In (d), detail can be seen in the superficial region of the previous figure, highlighting the vascular component (arrows) (400x magnification).
Figure 5.
Group Con shows in (a) an overall view of the wound, highlighting the marginal (Zn) and central (zc) zones (40x magnification). In (b), the marginal zone is highlighted, where an area of epithelialization (ep) and underlying, inflamed connective tissue (tc) can be observed (100x magnification). In (c), the central portion of the wound is seen (100x magnification), highlighting the granulation reaction (rg) and ulcerated zone (without epithelial lining) (uc). In (d), detail can be seen in the superficial region of the previous figure, highlighting the vascular component (arrows) (400x magnification).
Figure 6.
(A) Epithelialization rate of the GLf (188 mg/kg), InLf (182 mg/kg), and Fbn (31 mg/kg) treated groups. (B) Shows the number of inflammatory cells (cells/field). (C) Represents the Angiogenesis values (vessels/field) of the treated groups. Where ***(p < 0.001), *(p < 0.05) represents a statistically significant result compared to the Con group. One-way ANOVA test followed by Tukey's test.
Figure 6.
(A) Epithelialization rate of the GLf (188 mg/kg), InLf (182 mg/kg), and Fbn (31 mg/kg) treated groups. (B) Shows the number of inflammatory cells (cells/field). (C) Represents the Angiogenesis values (vessels/field) of the treated groups. Where ***(p < 0.001), *(p < 0.05) represents a statistically significant result compared to the Con group. One-way ANOVA test followed by Tukey's test.
Figure 7.
It presents the values of fibroblast proliferation (cells/field at 400x) of the GLf (188 mg/kg), InLf (182 mg/kg), and Fbn (31 mg/kg) treated groups. Where **(p < 0.01) and *(p < 0.05) represent statistically significant results compared to the Com group. One-way ANOVA test followed by Tukey's test.
Figure 7.
It presents the values of fibroblast proliferation (cells/field at 400x) of the GLf (188 mg/kg), InLf (182 mg/kg), and Fbn (31 mg/kg) treated groups. Where **(p < 0.01) and *(p < 0.05) represent statistically significant results compared to the Com group. One-way ANOVA test followed by Tukey's test.
Figure 8.
Groups GLf (188 mg/kg), (a), and Fbn (31 mg/kg) (b) exhibit a predominance of type III collagen fibers (greenish-yellow) but with apparent deposition of type I collagen fibers (orange) in the depth of the lamina. Also, note the parallel arrangement of the fibers. In InLf (182 mg/kg), (c) and Con groups (d), exclusively type III collagen fibers are observed in a parallel arrangement in the first and reticular in the second (Picrossirius-polarization, 100x).
Figure 8.
Groups GLf (188 mg/kg), (a), and Fbn (31 mg/kg) (b) exhibit a predominance of type III collagen fibers (greenish-yellow) but with apparent deposition of type I collagen fibers (orange) in the depth of the lamina. Also, note the parallel arrangement of the fibers. In InLf (182 mg/kg), (c) and Con groups (d), exclusively type III collagen fibers are observed in a parallel arrangement in the first and reticular in the second (Picrossirius-polarization, 100x).
Figure 9.
Photograph of the specimen collected in the Medicinal Plant Garden of the Institute of Studies and Research of the State of Amapá (IEPA), previously identified and cataloged in the Herbarium of the Agronomic Institute of the North (IAN185211 - Fabaceae - Libidibia ferrea var. ferrea (Mart. ex Tul.) LP Queiroz).
Figure 9.
Photograph of the specimen collected in the Medicinal Plant Garden of the Institute of Studies and Research of the State of Amapá (IEPA), previously identified and cataloged in the Herbarium of the Agronomic Institute of the North (IAN185211 - Fabaceae - Libidibia ferrea var. ferrea (Mart. ex Tul.) LP Queiroz).
Table 1.
Tannin and flavonoid content in glycolic extract, infusion, and gel of Libidibia ferrea fruits.
Table 1.
Tannin and flavonoid content in glycolic extract, infusion, and gel of Libidibia ferrea fruits.
| Product |
Tannin % |
Flavonoid % |
| Glycolic Extract |
23 |
0,25 |
| InLf |
21 |
0,32 |
| GLf |
18 |
0,14 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).