1. Introduction
To date, crystals of rare-earth orthoferrites
(
R = La, Pr, ..., Lu, and Y) of almost all available compositions have been synthesized and studied [
1,
2,
3]. The
crystal structure is described by space group
of the orthorhombic system and the unit cell contains four formula units (
Z = 4). This is the distorted structure of a perfect perovskite, in which distortions are mainly caused by oxygen ion displacements leading to rotations of FeO
octahedra (
Figure 1). Briefly, this structure can be described as a frame of vertex-sharing FeO
octahedral with rare-earth element atoms in voids between transition ion octahedra.
Orthoferrites undergo magnetic phase transitions of several types, including the spontaneous spin-reorientation transitions [
4]. The complex magnetic behavior of the
compounds still evokes keen interest of both experimentalist and theoreticians [
3]. Over the past few decades, rare-earth orthoferrites have been in focus of researchers [
5,
6]. The magnetic structure of the high-temperature orthoferrite phase is a canted antiferromagnet. The relatively small canting of the magnetic moments in the
subsystem causes a weak ferromagnetic moment in these compounds [
7]. Orthoferrite crystals have high Néel temperature
, which decreases monotonically as the atomic number of a rare-earth element grows [
8].
Below the Néel temperature, the
orthoferrites undergo a spin-reorientation transition, during which the weak ferromagnetic moment changes its direction by 90
[
9,
10]. The transition temperature varies over a wide range, from units to hundreds of Kelvin, depending on the rare-earth ion type. The highest temperature of the spin-reorientation transition is observed in the
crystal and amounts to 480 K [
11,
12]. Ultrafast switching of the magnetization of domain walls opens up broad prospects for use of orthoferrites in technology [
3]. Therefore, the task of tuning the temperature of this transition is crucial. As was shown previously, a way to control the transition temperature is isovalent substitution in the
subsystem [
13,
14]. This substitution, among other things, affects the Néel temperature. As has been shown recently, changes in the angles and distances in (Fe, Mn)O
oxygen octahedra play an important role, leading to changes in the electric field gradient (EFG) on
ions [
14,
15]. A similar behavior is observed when a rare-earth element in the subsystem is replaced [
16,
17]. In [
14], it was shown that substitution of Jahn–Teller Mn
ions for iron ions in the
crystals induces additional distortions of the (Fe,Mn)O
oxygen octahedron and, consequently, a strong increase in the temperature of the spin-reorientation transition, which can be indicative of the impact of the oxygen octahedron distortions on the magnetic properties of orthoferrites.
In this study, the Mössbauer effect measurements a series of the orthoferrites and theoretical calculations of the EFG tensor were carried out for establishing the effect of substituting manganese ions on the degree of distortion of the immediate environment of an iron ion. For this purpose, the concentration dependences of the quadrupole splitting, EFG tensor components, field asymmetry parameter, and EFG tensor eigenvector directions were found.
2. Materials and Methods
A series of the ( single crystals was synthesized by optical floating zone melting. At the first stage, to obtain the (x = 0, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, and 0.7) samples, powders of the initial , , and oxides (99.9%, Alfa Aesar) were mixed in the desired ratio and annealed at a temperature of 925 C for 18 h. The annealed powders were poured into a rubber mold and pressed in a hydrostatic press under a pressure of ≈100 MPa. The obtained cylindrical samples were then annealed in a vertical furnace at a temperature of 1400 C for 16 h. After annealing, the synthesized polycrystalline ( samples were placed in an FZ-T-4000-H-VIII-VPO-PC optical floating zone furnace (Crystal Systems Corp.) to grow single crystals. The growth occurred in air under normal pressure at a relative rod rotation speed of 30 rpm. The growth rates varied from 3 to 1 mm/h, depending on the manganese concentration in the samples.
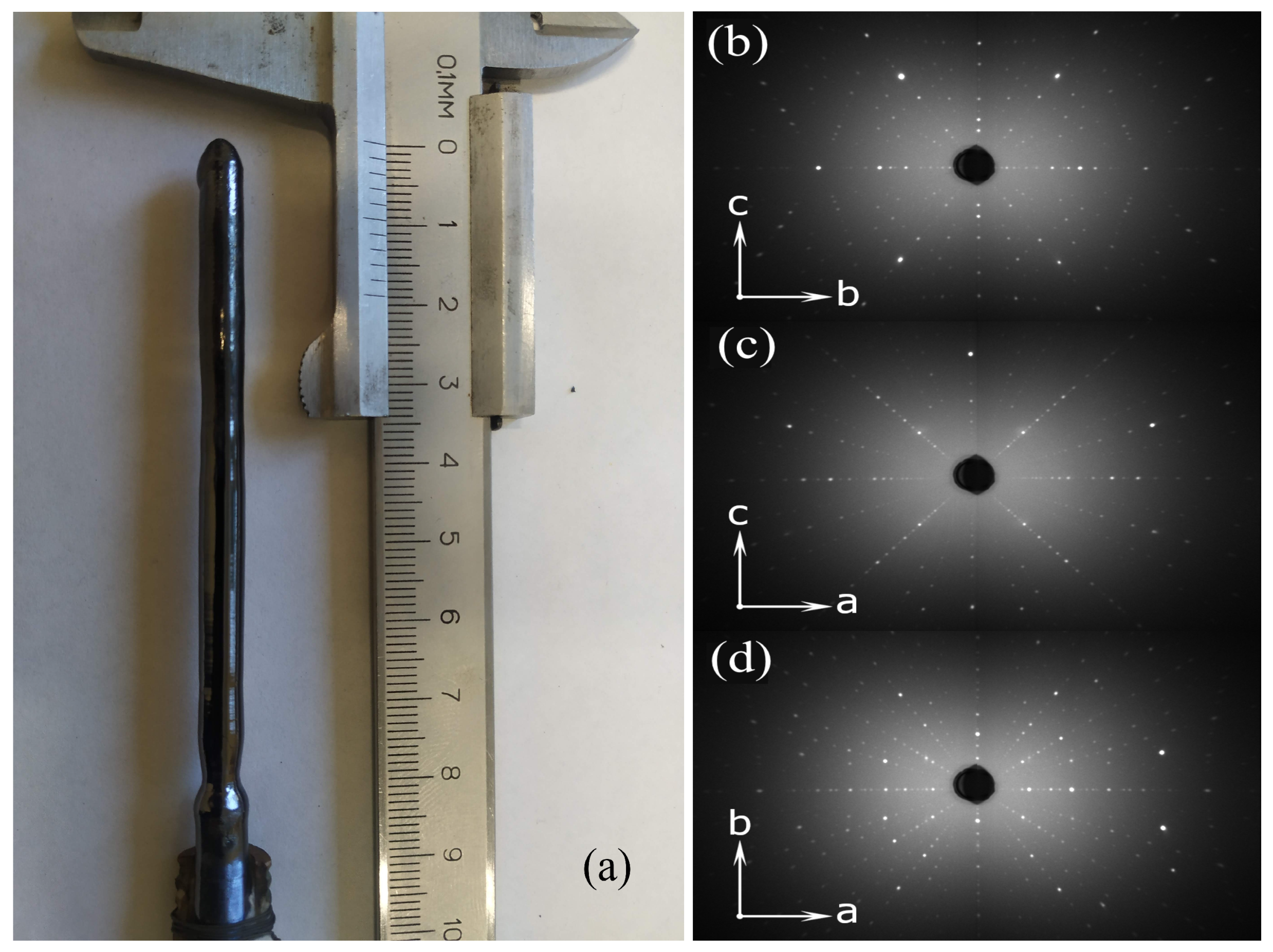
The synthesized rod-shaped
(
single crystals had a diameter of up to 7 mm and a length of up to 10 cm. The quality of the samples of the entire series and their orientation along the three crystallographic axes were tested by X-ray Laue diffraction. As an example,
Figure 2a presents a typical view of a single crystal and Laue patterns for the sample with a manganese concentration of
x = 0.3 along different crystallographic directions (
Figure 2b–d). For all the samples, one can see narrow symmetrical reflections corresponding to space group Pnma (♯62). Our investigations showed that the crystallographic axis direction makes an angle of about 23
with the single crystal growth direction.
Samples for the Mössbauer study were prepared by grinding the
(
single crystals to a powder. For the samples with manganese concentrations above 0.5, the high-temperature Mössbauer measurements require too much time because of the low
concentration and strong absorption by a rare-earth atom. The resulting powder with a
mg/cm
sample by iron content was pressed in aluminum foil 20 mm in diameter. Mössbauer spectra of the investigated samples were obtained on an MS-1104Em spectrometer equipped with an MRP-750K furnace (Research Institute of Physics, Southern Federal University) with a
radioactive source in the transmission geometry at a temperature
T = 700 K, since the measurements in the paramagnetic state yield information about distortions in the close vicinity of the Mössbauer isotope caused by structural factors [
18].
The spectra were processed in two stages. At the first stage, possible nonequivalent states of iron were determined by calculating the quadrupole splitting probability distributions [
19]. Using the results obtained, a preliminary model spectrum was formed. At the next stage, the model spectrum was adjusted to the experimental spectrum by varying the entire set of hyperfine parameters using the least squares method in the linear approximation. The chemical shift values are given relative to metallic iron (
Fe).
The calculations were carried out within the density functional theory using the Perdew–Burke–Ernzerhof exchange-correlation functionals with the generalized gradient approximation (PBE–GGA) implemented in the VASP package [
20,
21]. The number of plane waves was limited by energy of 600 eV. The Monkhorst–Pack grid [
22] was chosen to be
. The calculation used the GGA + U method in the Dudarev approximation [
23], in which the parameter
U for the iron ion was chosen to be 2 eV. Configurations of valence electrons were
, Fe:
for Ho ions and
for O ions. The values of the EFG tensor components were calculated using the technique described in [
24] implemented in the VASP package.
3. Results and Discussion
The room-temperature Mössbauer spectra were described in detail in [
14]. It should be noted that, with increasing concentration of
cations, an increase in the chemical shift of the spectra is observed. In addition, an interplay was suggested between the structural distortions and the magnetic reorientation transition temperature as a result of substitution of the Jahn–Teller
cation for the
cation [
14]. Therefore, to thoroughly examine the direct effect of substitution of the
cation on the degree of distortion of the nearest environment of iron ions, Mössbauer spectra were recorded above the temperature of magnetic ordering of the
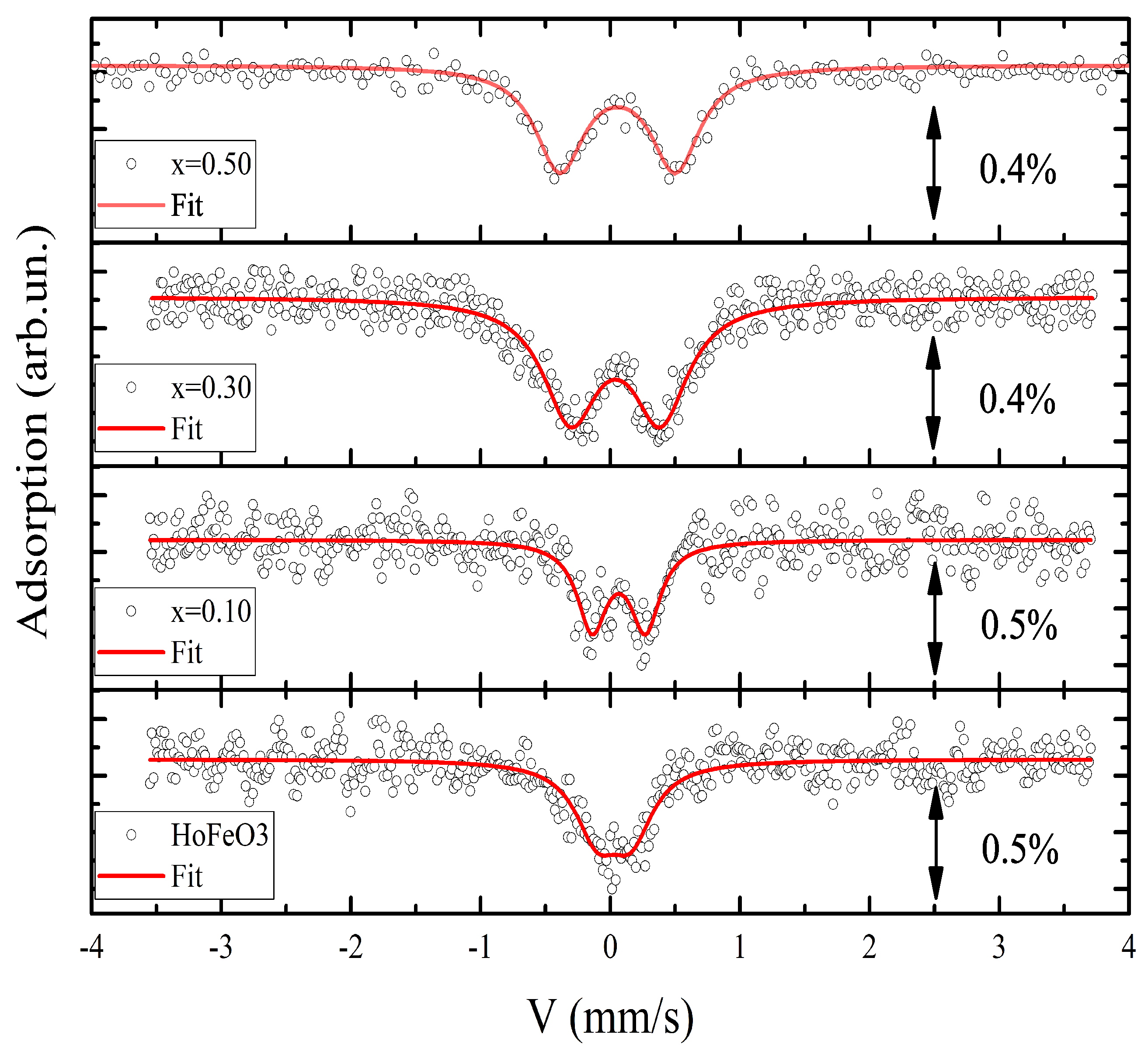
subsystem at 700 K. The obtained spectra are shown in
Figure 3.
At a temperature of 700 K, the spectra for all the samples have the form of quadrupole doublets. Their hyperfine parameters are given in
Table 1. The obtained hyperfine parameters of the
compound at 700 K correspond to those reported previously in [
17]. According to the experimental data obtained here, the quadrupole splitting
has the smallest value for this composition. With an increase in the
concentration, the
value increases in the series
(
). The results obtained show an almost threefold increase in the quadrupole splitting on iron atoms already at
with a tendency for further growth.
A similar situation was observed in the
system [
25], where the observed
growth was attributed to an increase in the degree of distortion of
octahedra. As a result, the crystal field acting on the nucleus of the
atom is significantly changed. Although the nucleus is only sensitive to the second derivative of the crystal field (EFG,
), there is a direct correlation between the parameters of the second-order crystal field splitting and the lattice contribution to the quadrupole splitting [
26]. At the same time, when replacing
by
cations with a spherical
shell in the series of
orthoferrites, the
value barely grows even at a significant degree of substitution (
), despite the difference between ionic radii of the
cations. Thus, one can speak about the decisive role of the cation type in changing the degree of distortion of the nearest environment [
15].
The Mössbauer spectra of orthoferrites in the paramagnetic state allow direct estimation of the effect of cation substitution on the degree of distortions of the FeO octahedron of the orthoferrite structure under the interaction of the quadrupole moment of a nucleus with the EFG on Fe nuclei in the crystal. In the paramagnetic state, the value is only determined by the quadrupole transitions ( and ) of the nucleus of the Mössbauer atom. In general, the quadrupole splitting of the Mössbauer spectrum has two contributions: (i) from charges of the ions surrounding the Mössbauer nuclei and (ii) from intrinsic electrons, in particular, resulting from the covalent admixture.
To identify the main contribution, first-principle calculations of the electric field gradient tensor within the framework of the method [
24] implemented in the VASP package were performed for the iron ion in the
compound (
x = 0, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7). All calculations were performed using the lattice parameters and relative coordinates of atoms obtained from the X-ray diffraction experiment [
14]. Relaxation of the crystal structure of the studied crystals was not carried out. At the same time, calculations for samples with different concentrations of manganese were performed without accounting the manganese ion. That is, in fact, calculations were performed for the
crystal using lattice parameters and atomic coordinates corresponding to
crystals with specific concentrations of manganese. This approach can be justified for the following reasons: 1) this greatly simplifies the calculation procedure and, as a result, significantly saves calculation time; 2) the experiment demonstrates the effect on iron atoms; 3) such an approach allows one to evaluate the contribution of manganese valence electrons to the experimentally observed value of the quadrupole shift by directly comparing the concentration dependences of
obtained from theoretical calculations, where manganese ions were not taken into account, with those obtained from experimental Mössbauer data, where electrons are implemented from both iron ions and manganese ions.
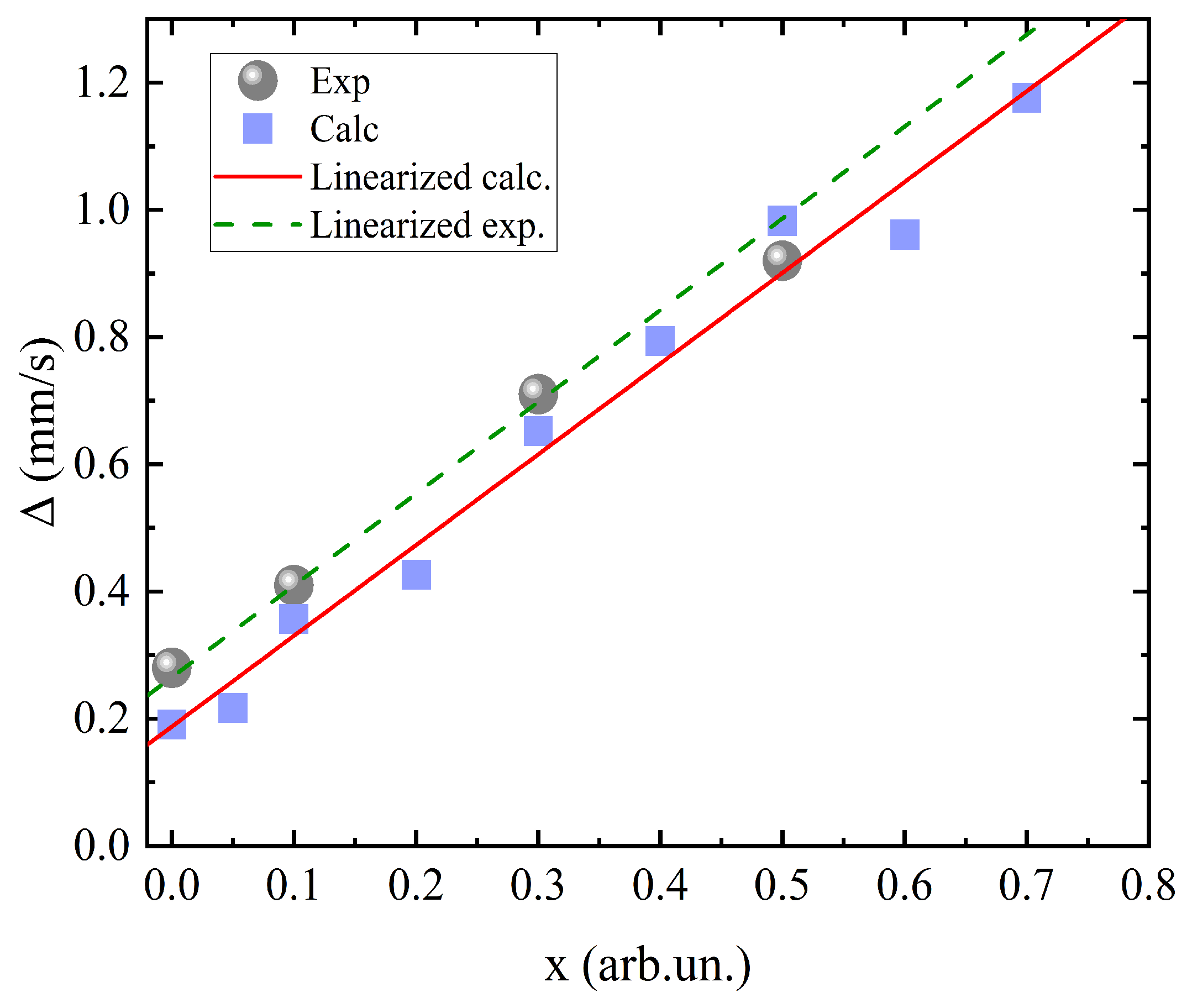
Basing on the data of the theoretical calculation using formula (
1) [
27], the calculated Mössbauer quadrupole splitting values were obtained as a function of the manganese concentration.
where
Q is a nuclear quadrupole moment (
Q = 0.16 b for 57Fe),
is an asymmetry parameter (will be defined below). The results are presented in
Figure 4.
It can be clearly seen that the calculated data are in good agreement with the experiment and the approximation straight lines have the same slope for both the experimental and theoretical values. At all the concentrations, there is a difference only by a constant value. This means that there is no additional contributions depending on the Mn concentration to the shift value.
Good qualitative and quantitative agreement of the
values confirms the appropriateness of the approach used in the calculations described above. Thus, the most important conclusion can be made that Mn
cations affect the Mössbauer spectra mainly due to distortions of the crystal lattice, while there is no admixture of electron density to the iron cations, which is somewhat different from the results of the study [
13].
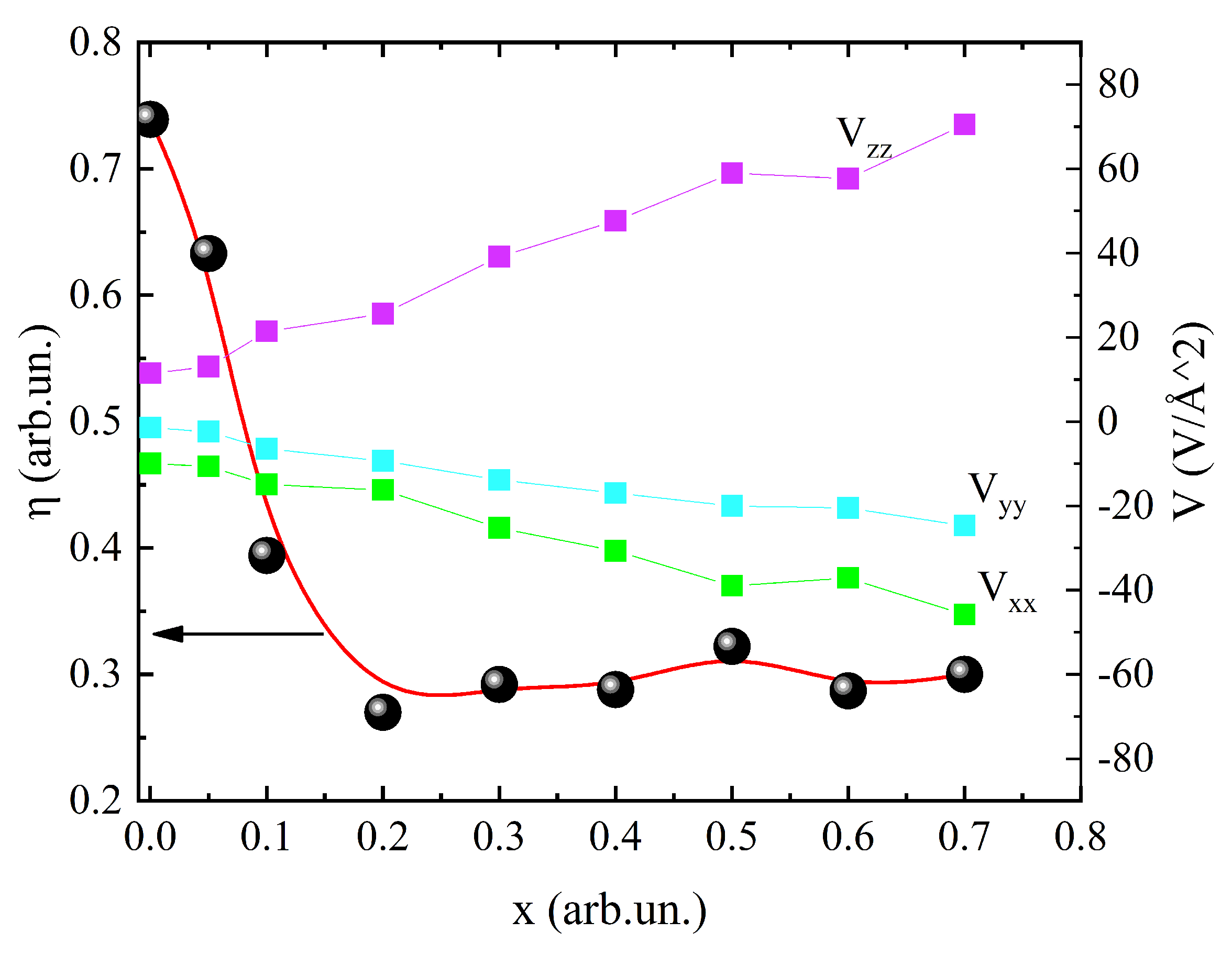
Using the calculated data, concentration dependences of the components
,
, and
(|
|>|
|>|
|) of the diagonalized EFG tensors and the EFG asymmetry parameter
(Eq. (
2)) were plotted [
27]. The plots are shown in
Figure 5. The linear growth of the field components with increasing concentration is seen. The calculation yielded a value of
V/m
for the principal component of the EFG tensor of the
compound, which is consistent with the data reported in [
16] (
V/m
), where the lattice contribution to the EFG in unsubstituted orthoferrite crystals was thoroughly studied by measuring the perturbed angular correlation spectra.
The asymmetry parameter
abruptly drops in the low-concentration region
and has a constant value at
. This follows from the change in the EFG symmetry at
, which becomes closer to axial. The EFG in the unsubstituted sample has no axial symmetry, contrary to the assumption of the previous work [
16].
The relation between and should be closely inspected. The first one is only 1.5 times smaller than the second one for all the considered concentrations of manganese ion. This is a sufficiently large value. Thus, the gradient of the electric field for this series of crystals does not have a pronounced axial symmetry. Therefore, it is necessary to monitor the behavior of the both components and of the field gradient.
The coordinates of the eigenvectors of the components of the electric field gradient were calculated for structures corresponding to concentrations of manganese ion . It should be noted that in the initial compound, due to the orthorhombic structural symmetry, the oxygen octahedron FeO is non-perfect and has some distortions. All the Fe–O bond have different lengths. When manganese is substituted for the iron ion, the degree of distortion of the (Fe,Mn)O octahedron increases, the two axes of the octahedron are decreasing.
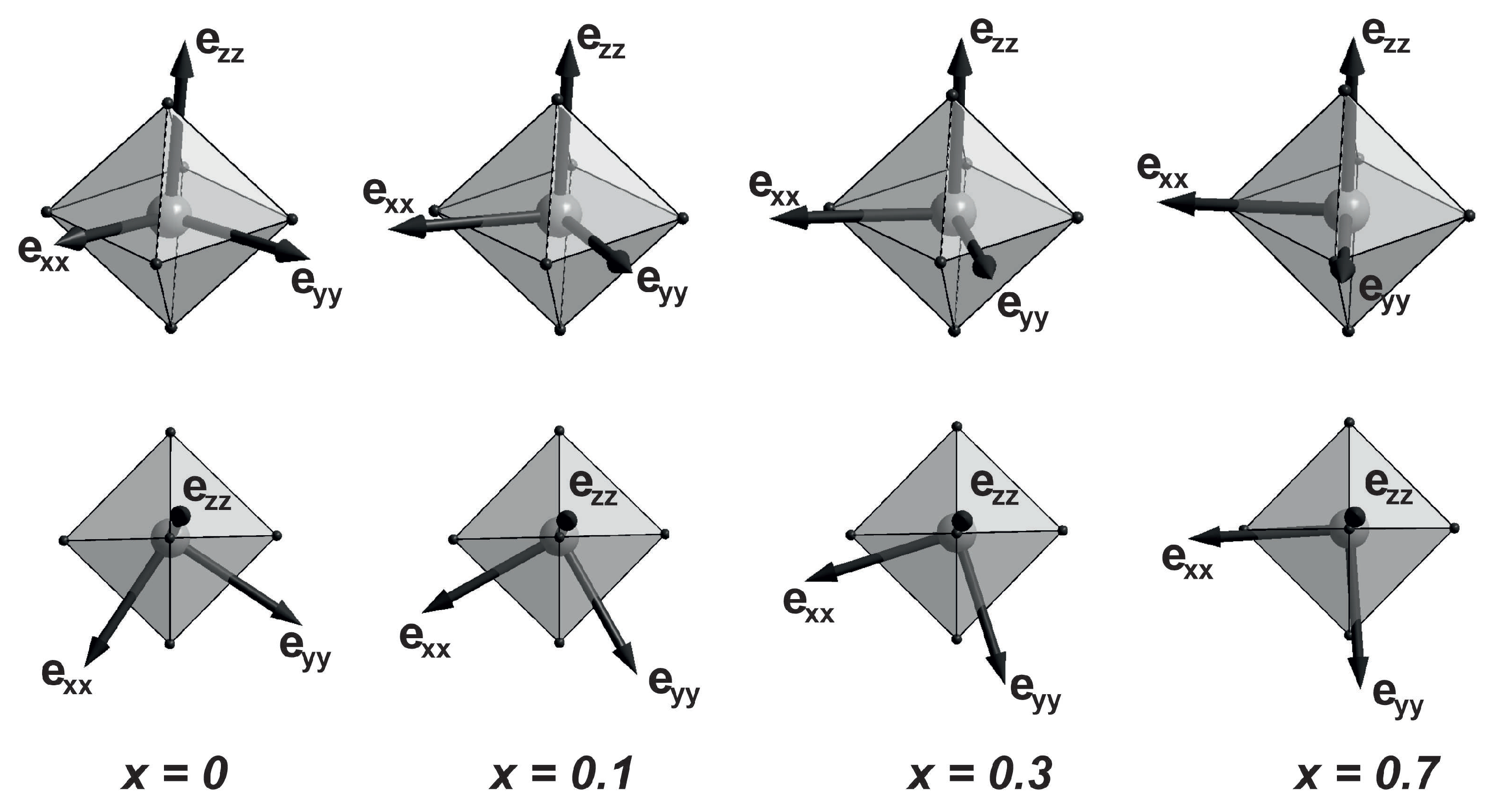
Figure 6 shows the directions of the calculated EFG eigenvectors at concentrations of
x = 0, 0.1, 0.3, and 0.7. The vector
barely change its direction at all the concentrations, which passes close to the longest axis of the FeO
octahedron. A bright feature is the behavior of the vector
. In the initial compound (
x = 0), this vector lies in a plane close to the base of the octahedron, perpendicular to its longest axis, and passes near the middle of the octahedron base edge. At
, the vector of the field component
rotates by 27
in the plane close to the octahedron base, shifting closer to one of oxygen atoms (see
Figure 6). At
x = 0.3, the angle of rotation of the vector
increases to 39
. At
, the angle of rotation of the vector
is 53
. In this case, its direction almost coincides with the shortest axis of the FeO
oxygen octahedron. The direction of the vector
behaves similarly (the field vectors
,
and
are mutually perpendicular).
The obtained features of the behavior of EFG on the Fe ion (a significant increase in the values of the field components and a sharp change in the direction of the field vectors
and
due to the Mn doping), apparently, should lead to a change in the balance of exchange constants in the 3
d subsystem of HoFe
Mn
O
compounds. This is consistent with the results of [
14],where a change in the ground magnetic state at concentrations
, as well as the disappearance of the orientation phase transition at concentrations
were found.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, K.Sh.; methodology, S.S., S.S.; validation, K.Sh.; formal analysis, M.P., Y.K., K.Sh.; investigation, T.B. S.S., S.S., Y.K., M.P.; resources, A.S., D.G.; data curation, M.P., Y.K.; writing—original draft preparation, Y.K., M.P., D.G., K.Sh.; writing—review and editing, Y.K., K.Sh.; visualization, Y.K., M.P.; supervision, K.Sh.; project administration, K.Sh., S.S.; funding acquisition, K.Sh.