Introduction
The evolution and ecology of smallness, a phenomenon known as miniaturization, is of great interest to the scientific community [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16]. Small size can be advantageous in a variety of ecological niches, permitting organisms to exploit resources efficiently, evade predators, and adapt to diverse environmental conditions [
1]. From a physiological perspective, small organisms exhibit high surface-to-volume ratios, facilitating efficient exchange of nutrients and gases, factors that are crucial for survival in resource limited environments [
2]. Smallness also permits rapid growth and exploitation of microhabitats such as leaf litter, crevices, or small pools [
3] and confers several other advantages, such as increased agility and reduced resource requirements [
4]. Miniaturization can facilitate rapid population growth and colonization of new habitats, contributing to speciation events and biodiversity patterns [
3,
4]. However, being small also imposes constraints, such as limited energy reserves, heightened vulnerability to environmental fluctuations, and challenges in maintaining physiological functions [
4]. Despite these trade-offs, miniaturization has evolved repeatedly in response to various selective pressures, highlighting its adaptive significance [
1]. Studying the ecology and evolution of smallness provides valuable insights into the interplay between biological traits, ecological interactions, and evolutionary processes [
5]. The author now presents the finding of miniaturized squamates colonizing a trace fossil from Hamblen County, Tennessee.
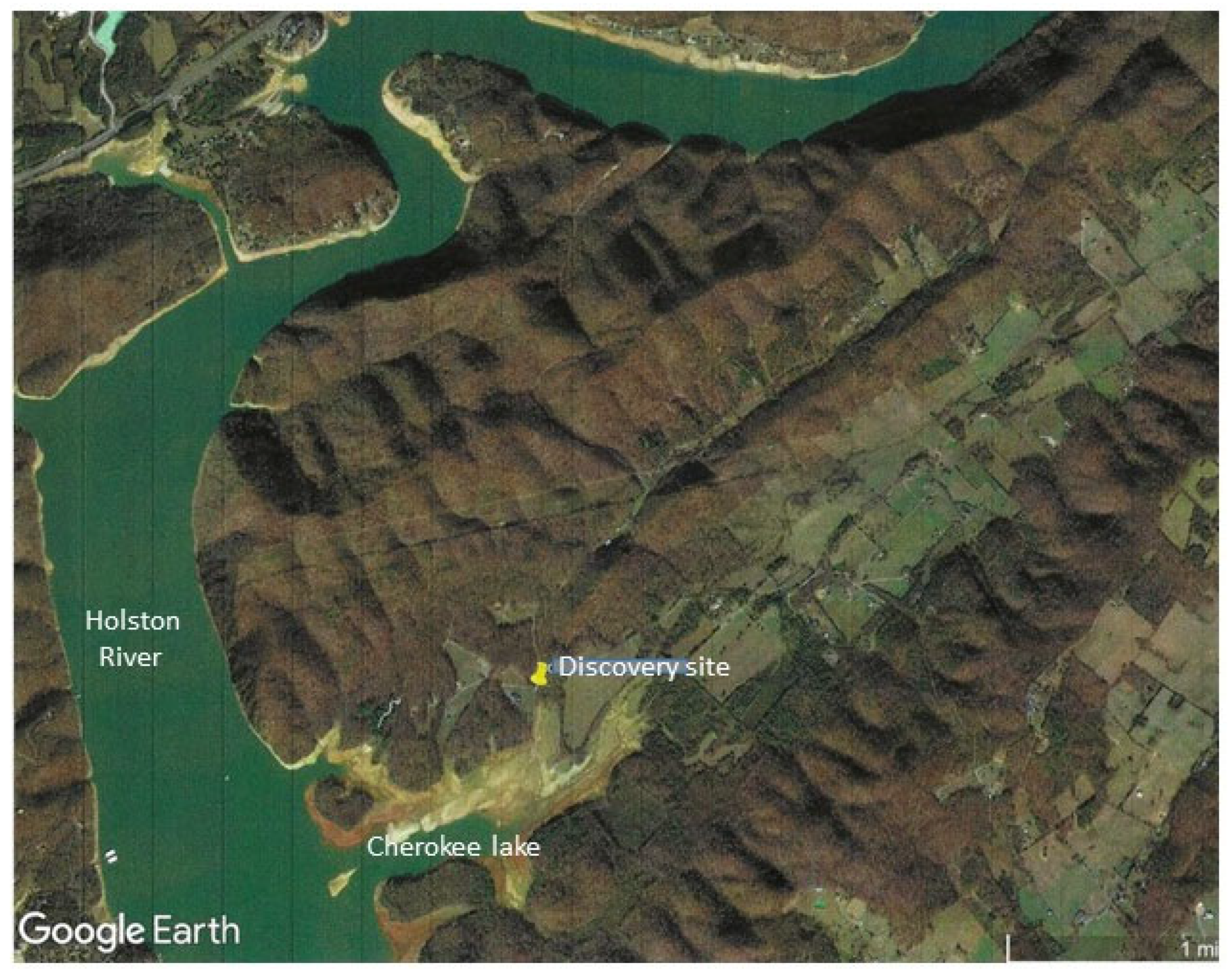
Discovery Site
The trace fossil was found at 36°19’44.39” N, 83°9’46.72°W, on the western margin of a 1,865 acre Boy Scout camp located in Hamblen County, Tennessee, a mountainous region of northeast Tennessee with elevations ranging from 330 to 931 meters above sea level. The geology of Hamblen County consists of Cambrian (541-485.4 ma), Ordovician (485.5-443.8 ma), and Cambrian to Ordovician rocks [
17]. The sedimentary cover is composed mainly of Cambrian to Lower Ordovician rocks deposited in a passive margin setting, as well as Middle Ordovician to Pennsylvanian rocks deposited on the Appalachian foreland during the Taconic and Alleghenian orogenic phases. Basement rocks are thought to date back to the Grenville age, although the position of the Grenville front in the region is unknown [
17,
18] (
Figure 1).
Trace Fossil Description
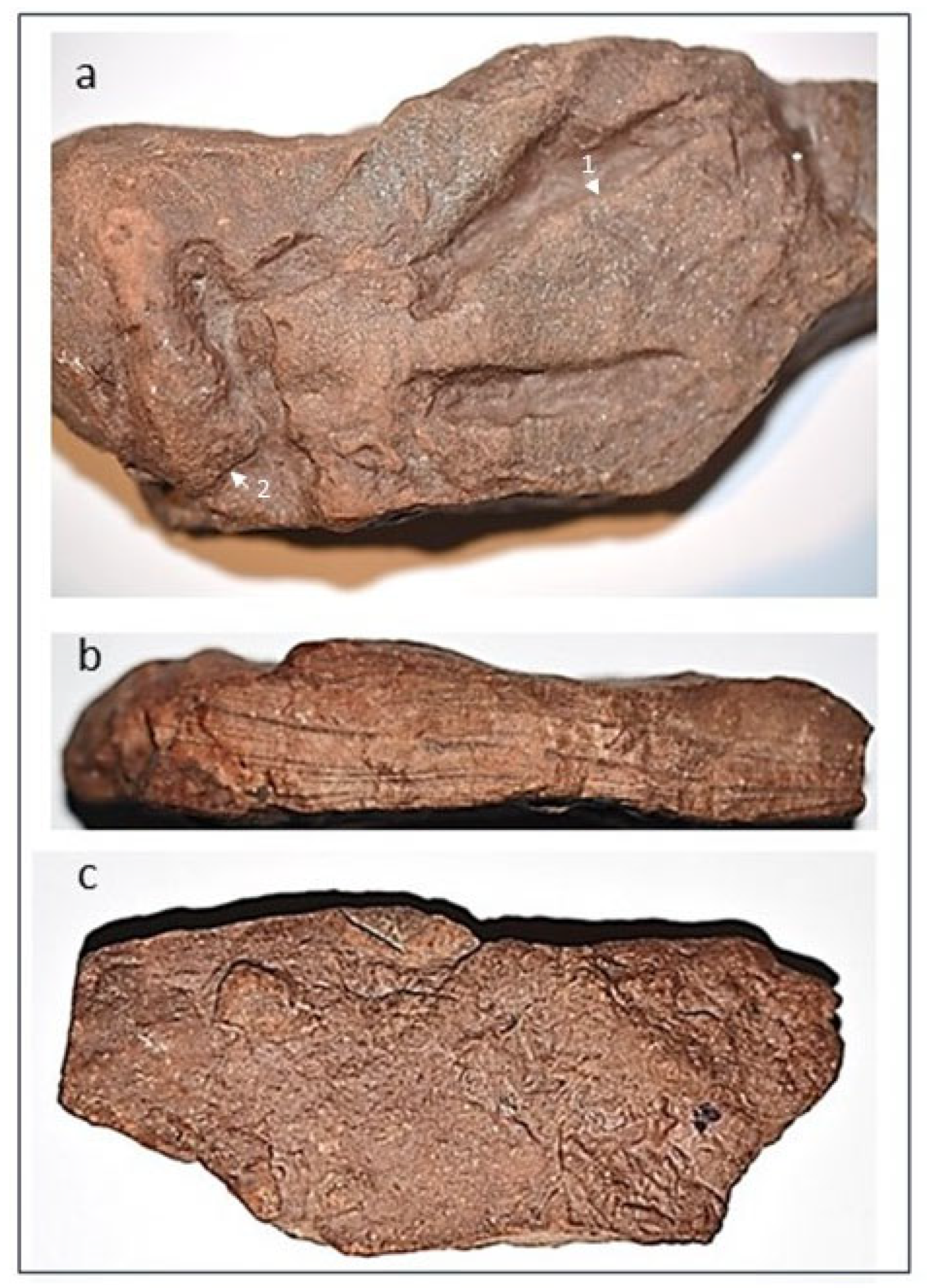
The fossil is identified as a sedimentary rock of siliciclastic mudstone with a maximum length of 119 mm, a maximum width of 53 mm, and a depth ranging between 19 and 25 mm. The dorsal surface of the fossil has eight concave epireliefs ≤ 2.1 mm deep and 3-5 mm wide thought to have been made by burrowing worm-like organisms or small crustaceans. There is one convex epirelief of unknown origin (
Figure 2a). Visible on the lateral side of the Trace Fossil are multiple alternating layers of sandstone and mudstone (
Figure 2b). Evident on the ventral side of the trace fossil are multiple convex epireliefs (
Figure 2c).
Materials and Methods
The trace fossil was examined using a ADSM302 digital platform microscope (Andonstar Tech Co., LTD, Shenzhen, Guangdong, China). Image size was calibrated using a 0.5 mm-interval stainless steel ruler suspended over the focal point of the microscope. Images were projected onto a 50-inch Vizio television via an HDMI cable . Photographs of the images were made using a Nikon digital camera and a Nikon DX SWM Micro 1:1 lens and stored for future viewing and processing on 265 GB microchips. Images were enlarged and processed using Power Point’s Picture Format (Microsoft Corporation, Redmond, Washington, USA). Microfossil measurements were confirmed using a Mitutoyo IP 65 coolant proof micrometer with an accuracy range of 0.001 mm and/or a Kynu digital caliper with a resolution of 0.1 mm and an accuracy of +/- 0.2 mm.
Results
Dorsal Surface Microfossil Morphology and Morphometrics
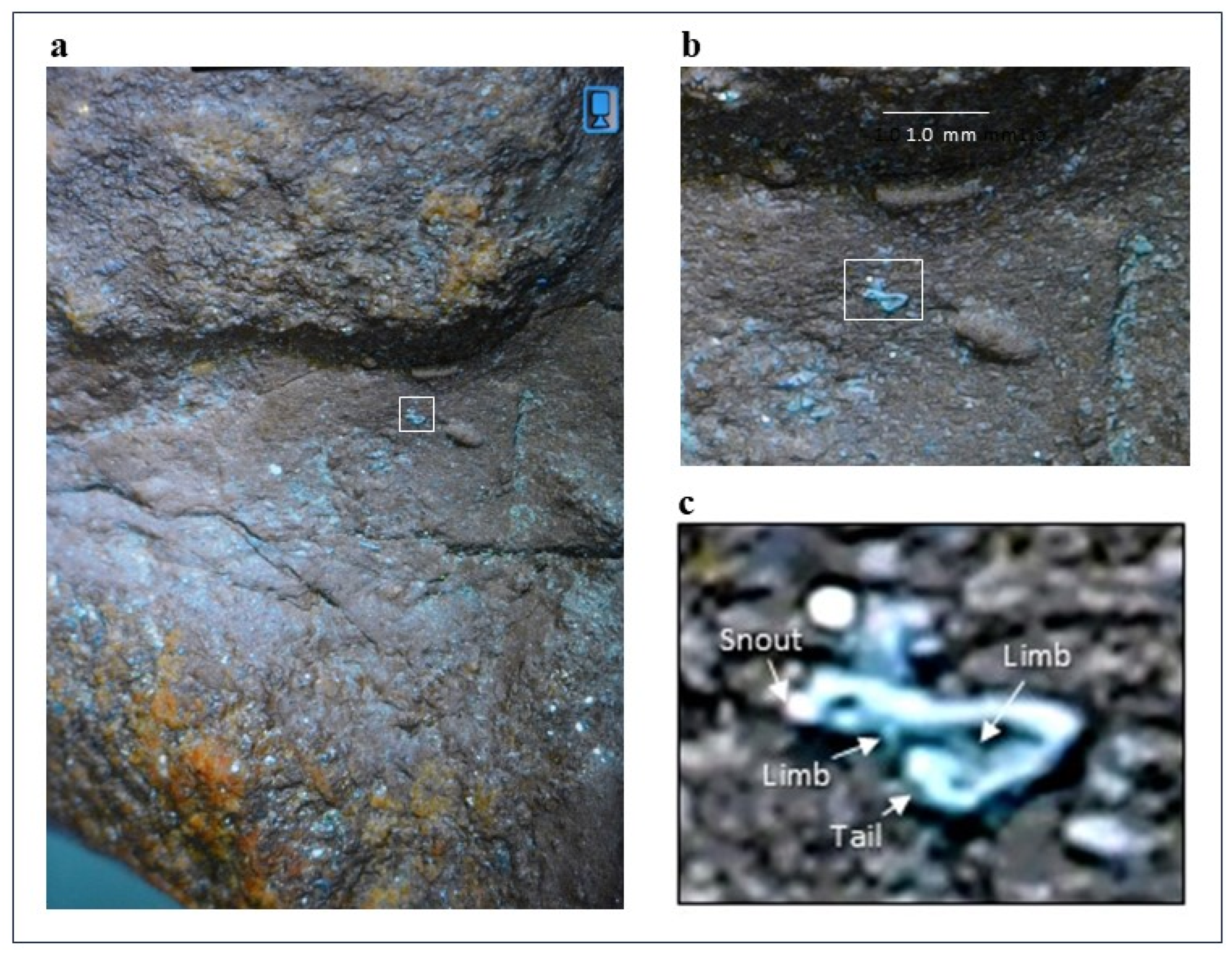
Found on imprint 2 of the trace fossil is a ~0.5 – 0.6 mm long limbed squamate with a spade-like tail and features that otherwise resemble those of extant Geckos (
Figure 3a–c).
Also found on imprint 2 of the trace fossil are three ~0.4-0.6 mm long squamates with saurian-like crania, elongated bodies, tails, and the absence or near absence of limbs suggesting they were saurians transitioning into more snake-like forms (a–c)
Found on imprint 1 of the trace fossil is a snake-like squamate with a snout-to-vent length of~1.0 mm and a tail length of ≥ 6 mm (
Figure 5).
Discussion
Trace Fossil Geology and Taphonomy
The dimensions and orientations of the trace fossil’s dorsal surface traces are in keeping with the likelihood that they are burrows of worm-like organisms or small crustaceans made during the Cambrian, Ordovician or Cambrian to Ordovician when the sedimentary rocks of Hamblen County were formed and when the area that is now Tennessee was covered by a shallow inland sea
, [
16,
17]. These animals are posited to have burrowed into sediments to feed on organic matter and microorganisms and to seek shelter from predation and from adverse environmental conditions [
18]. The trace fossil’s multiple alternating layers of sandstone and mudstone indicate that it was formed in an environment of fluctuating wave energies, sediment supply, and/or sea levels. These are characteristics of a coastal plain, delta, or estuary where rivers carry both sandy and muddy sediments [
19,
20,
21,
22,
23]. In this regard, Foote noted that areas that generate evolutionary novelties tend to originate preferentially in near shore environments [
12]. The obvious ecological advantage of this environment is the ready availability of water in species whose small body size enhances evaporative water loss. In addition, a variety of food sources for miniaturized squamates can thrive in swampy conditions including some collembolan species, insect larvae, homopterans, spiders, fungi, and bacteria [
24].
Hence, the trace fossil is parsimoniously identified as belonging to the genus
Planolites, a genus of trace fossil characterized by unbranched horizontal burrows that are typically found in sandy or muddy sedimentary rocks. These burrows are thought to have been made by worm-like organisms possibly similar to modern polychaete worms which inhabited shallow marine environments during the Cambrian and Ordovician periods [
18,
21,
22,
23].
Miniaturization
The smallest known multicellular animals are myxoans - a parasitic species that measure 8 to 10 micrometers (0.008 to 0.01 mm) in length. In comparison, the trace fossil saurian-like squamates measure 0.4 to 0.6 mm in length and the snake-like squamate measures 1.0 mm snout to vent and 7 mm snout to tail.
The smallest known saurian reported to date is Oculudentavis nanus a tiny lizard-like animal from the Late Cretaceous (100.5 to 66 Ma) which measure ~5 cm in length [ ]. The smallest known snake is Tetrapodophis amplectus from the early Cretaceous period (145 to 100.5 Ma) which measures ~20 cm in length.
The factors contributing to the unprecedented miniaturization of the trace fossil squamates are a matter of conjecture. The potential advantages of miniaturization include an ability to explore new ecological niches and habitats that are inaccessible to larger-bodied species [
1]. Miniature species have enhanced agility and maneuverability and lower energy requirements than larger species [
5]. They can survive on smaller prey items, occupy smaller home ranges, and tolerate lack of resources more efficiently. And they may have a reduced predation risk since they can hide in narrow crevices, leaf litter or vegetation making it more difficult for predators to detect and capture them [
1,
6]. Miniaturization may provide reproduction benefits since smaller-bodied squamates tend to have shorter generation times, mature earlier, and may produce more offspring per reproductive event, allowing for more rapid population growth and adaptation to changing environments [
3]. In contrast, the costs of larger body size may include an increase in the mortality of juveniles due to their long development time and/or fast growth requirements; decreased viability in adults and juveniles due to predation, parasitism, or starvation because of reduced agility, increased detectability, higher energy requirements, heat stress, and/or intrinsic costs of reproduction. Other factors include a decreased in mating success of large males due to reduced agility and/or high energy requirements, and a decrease in reproductive success of large females and males due a relative increase in the time of reproduction [
15].
In lizards, miniaturization has been observed in several lineages, including some members of the families Gekkonidae and Scincidae. One of the primary drivers of miniaturization in lizards is adaptation to specific habitats, such as small islands or dense forests, where smaller body size may confer advantages in locomotion, foraging, and predator avoidance [
26]. Miniaturization in lizards can also be linked to ecological factors such as resource limitation, competition, and the colonization of novel environments [
27]. The North American Craton, a stable continental core encompassing a significant portion of North America, has been a pivotal region for understanding the evolutionary history of reptiles, including lizards. Fossil evidence suggests that lizards likely originated during the Late Jurassic to Early Cretaceous periods, approximately 160 to 120 million years ago. During this time, the North American Craton experienced diverse environmental conditions, including tropical forests, coastal plains, and inland seas. These varied habitats likely provided suitable ecological niches for the early diversification of reptiles. Additionally, geological processes such as tectonic activity and climate fluctuations influenced landscape dynamics, potentially driving speciation and dispersal patterns among lizard populations [
28,
29].
Similarly, miniaturization has occurred independently in multiple lineages of snakes, resulting in the evolution of species commonly referred to as "dwarf" or "miniature" snakes. Miniaturization in snakes has been associated with adaptation to varying habitats, such as leaf litter, undergrowth, or rocky crevices, where smaller body size facilitates maneuverability and prey capture. Additionally, miniaturization in snakes may enable exploitation of unique ecological niches and reduce competition with larger predators or prey [
30].
Overall, miniaturization in lizards and snakes reflects a complex interplay between evolutionary processes, ecological interactions, and environmental factors.
Lizard-to-Snake Transition
The transition of lizard-to-snake is thought to have occurred at least 25 times during the late Jurassic/early Cretaceous periods. In their review of limb reduction in squamates, Camaiti et. al. noted that, as was true in the trace fossil saurians, limb regression starts in the distal elements and progresses proximately [
40]. In his studies on the ontogeny of limb-reduced lizards and snakes, Raynaud noted that the reduction in limb buds resulted from the early onset of necrosis at the level of the apical ectodermal ridge (AER) [
41]. Cohn and Tickle found that axial de-regionalization observed in limbless squamates was explained by the expansion of homeobox (
Hox) gene domains along the body axis and by the inhibition of fibroblast growth factor 8 (FGF8) and sonic hedgehog (SHH) expression at the level of the AER and the zone of polarizing activity (ZPA), respectively [
42]. These changes are thought to have arisen in response to environments in which the existence of limbs would be an impediment to undulating locomotion in, for example, underground burrows and crevices [
40]. Evidence suggests that adaptation to life underground in limbless squamates is associated with the shortening of tails whereas surface-dwelling in limbless squamates is associated with elongation of tails [43].
The trace fossil findings provide evidence of a post-embryonic lizard to snake transformation in which the stem snake is a tiny four legged saurian, perhaps a member of the family Gekkonidae which is known to host some of the World’s smallest lizards. At present, approximately 16% of the more than 900 species of extant lizards exceed the minimum body mass threshold of the smallest mammals and birds currently known. Gekkonidae have the largest number of miniaturized species (178) followed by Sphaerodactylidae (154), Iguania (34) and Lacertoidea (23) [44]. Using data on 99% of the World’s lizard species, Meiri found that small body size in lizard families is associated with high genera richness and that smallness is associated with a decreased risk of extinction. In this study, which used SVL as a measure of size, the smallest lizard was Sphaerodactylus elasmorhynchus (maximum SVL 17 mm) [45].
In their study of leaf litter geckos residing in the tropical rainforests of Brazil and Nicaragua, Vitt and coauthors report the ecological effects of smallness in four species of closely related geckos whose sizes range from 20.7 +/- 0.2 to 32.5 +/- 1.5 mm (SVL). They note that their tiny size put the geckos at risk of hypothermia, desiccation, and predation by other terrestrial vertebrates and birds while providing a diet of readily accessible collembolans, insect larvae, homopterans and spiders as well as the protection of the leaf litter per se [
24]. It is reasonable to assume that similar ecological challenges existed for the trace fossil saurians.
Author Contributions
John K. Smith examined the trace fossil and identified, measured, and photographed the microfossils therein. He did the research and wrote the article.
Data Availability Statement
Supplementary information is available for this article. Correspondence and requests for materials should be addressed to smithj@etsu.edu.
Acknowledgments
The author is grateful to the Department of Medical Education for it’s support and to David Smith who discovered the trace fossil. He is grateful to Greg Leitch who gave his permission to examine the fossil and publish the results of the examination.
Conflicts of Interest
The author declares no competing interest.
References
- Hanken, J.; Wake, D.B. Miniaturization of Body Size: Organismal Consequences and Evolutionary Significance. Annu. Rev. Ecol. Syst. 1993, 24, 501–519. [Google Scholar] [CrossRef]
- Gould, S.J. On the Scaling of Tooth Size in Mammals. Am. Zoöl. 1975, 15, 353–362. [Google Scholar] [CrossRef]
- Peters, R. H. The Ecological Implications of Body Size. (Cambridge Univ. Press, 1983).
- McMahon, T. A. , Bonner J. T. On size and life, 1983. [Google Scholar]
- Woodward, G.; Ebenman, B.; Emmerson, M.; Montoya, J.M.; Olesen, J.M.; Valido, A.; Warren, P.H. Body size in ecological networks. 20. [CrossRef]
- Blanckenhorn, W.U. Small? Quart. Rev. Biol, 2000. [Google Scholar]
- Voje, K.L.; Holen. H.; Liow, L.H.; Stenseth, N.C. The role of biotic forces in driving macroevolution: Beyond the Red Queen. Proc. R. Soc. B: Biol. Sci. 2015, 282, 20150186–20150186. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.B.; Clancey, E.; Roches, S.D.; Eastman, J.M.; Gentry, L.; Godsoe, W.; Hagey, T.J.; Jochimsen, D.; Oswald, B.P.; Robertson, J.; et al. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 2010, 23, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Haloin, J.R.; Strauss, S.Y. Interplay between ecological communities and evolution: Review of feedbacks from microevolutionary to macroevolutionary scales. Ann. N.Y. Acad. Sci. 2008, 1133, 87–125. [Google Scholar] [CrossRef] [PubMed]
- Foote, M. The Evolution of Morphological Diversity. Annu. Rev. Ecol. Syst. 1997, 28, 129–152. [Google Scholar] [CrossRef]
- Kozak, K.H.; Wiens, J.J. Phylogeny, ecology, and the origins of climate-richness relationships. Ecology 2012, 93, S167–S181. [Google Scholar] [CrossRef]
- Kozak, K.H.; Wiens, J.J. What explains patterns of species richness? The relative importance of climate-niche evolution, morphological evolution, and ecological limits in salamanders. Ecology and evolution. 2016, 6, 5940–5949. [Google Scholar] [CrossRef] [PubMed]
- Blanckenhorn, W.U. The evolution of body size: What keeps organisms small? The Quarterly Review of Biology. 2000, 75, 385–407. [Google Scholar] [CrossRef] [PubMed]
- Geologic units in Hamblen County, Tennessee - USGS. Available online: https://www.usgs.gov/geology/Tennessee/Hamblen/county/ (accessed on 4 December 22).
- Geology of the Appalachians. Wikipedia. Available online: https://en.wikipedia.org/wiki/geology of the Appalachians (accessed on 4 December 22).
- Seilacher, A. Trace Fossil Analysis (Springer Science & Business Media) (2007).
- Dalrymple, R.W.; Boyd, R.; Zaitlin, B.A. Estuarine facies models: Conceptual basis and stratigraphic implications. Journal of Sedimentary Petrology 1992, 62, 1130–1146. [Google Scholar] [CrossRef]
- Davis, R.A.; Dalrymple, R.W. Principles of tidal sedimentology. Springer Science & Business Media.
- Pemberton, S.G.; Frey, R.W.; Bromley, R.G. The ichnology of shallow marine and nonmarine environments. Society for Sedimentary Geology ( 1992.
- Buatois, L.A.; Mángano, M.G. Decoupling of body-plan diversification and ecological structuring during the Ediacaran–Cambrian transition: Evolutionary and geobiological feedbacks. Proc. R. Soc. B Biol. Sci. 2011, 278, 3266–3275. [Google Scholar]
- Mángano, M.G.; Droser, M.L. The ichnologic record of the Ordovician radiation. Chapter 34 in The great Ordovician biodiversification event. B. Webby, F. Paris, M. Droser, I. Percival eds. Columbia University Press, NY, NY pp 369-381 (2004).
- Vitt, L.J.; Sartorius, S.S.; Avila-Pires TC, S.; Zani, P.A.; Esposito, M.C. Small in a big world: Ecology of leaf-litter geckos in new world tropical forests. Herpetological Monographs 2005, 19, 137–152. [Google Scholar] [CrossRef]
- Evans, S.E. At the feet of dinosaurs: The early history and radiation of lizards. Biol Rev Camb Philos Soc. 2003, 78, 513–51. [Google Scholar] [CrossRef] [PubMed]
- Simões, T.R.; Caldwell, M.W.; Talanda, M.; Bernardi, M.; Palci, A.; Vernygora, O.; Bernardini, F.; Mancini, L.; Nydam, R.L. The origin of squamates revealed by a middle Triassic lizard from the Italian alps. Nature. 2018, 557, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.J. Hyperthermal-driven mass extinctions: Killing models during the Permian–Triassic mass extinction. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2018, 376, 20170076. [Google Scholar] [CrossRef] [PubMed]
- Matthew, M.J.; Jones, M.; Jones, B.B.; Selby, D.; Jacobson AD, S.J.; Batenburg, L. ; Riquier; et al. MCE. Abrupt episode of mid-Cretaceous ocean acidification triggered by massive volcanism. Nature Geoscience 2023, 16, 169–174. [Google Scholar]
- Meiri, S. Evolution and ecology of lizard body sizes. Global Ecol. Biogeogr. 2008, 17, 724–734. [Google Scholar] [CrossRef]
- Pianka, E.R.; Vitt, L.J. Lizards: Windows to the evolution of diversity (University of California Press, 2003).
- Schoener, T.W.; Schoener, A. Ecological and evolutionary traps. Trends in ecology & evolution 2004, 19, 474–480. [Google Scholar]
- Henderson, R.W.; Powell, R. Natural history of west Indian reptiles and amphibians (University Press of Florida, 2009).
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC evolutionary biology 2008, 8, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, X. Anchiorus huxley. Nature Ecology and Evolution 2024, 8, 1200. [Google Scholar] [CrossRef] [PubMed]
- Padian, K. In retrospect. 25th anniversary of first feathered-dinosaur finds. Nature 2023, 613, 251–52. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Zhou, Z.; Wang, X. The smallest known non-avian theropod dinosaur. Nature 2000, 408, 705–708. [Google Scholar]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartman, K.; Moores, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Camaiti, M.; Evans, A.R.; Hipsley, C.A.; Chapple, D.G. A farewell to arms and legs: A review of limb reduction in squamates. Biol. Rev. 2021, 96, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, A. Developmental mechanism involved in the embryonic reduction of limbs in reptiles. Intern. J. Develop. Biol. 2003, 34, 233–243. [Google Scholar]
- Cohn, M.J.; Tickle, C. Developmental basis of limblessness and axial patterning in snakes. Nature 1999, 399, 174–479. [Google Scholar] [CrossRef]
- Wiens, J.J.; Slingluff JU, L. How lizards turn into snakes: A phylogenetic analysis of body-form evolution in anguid lizards. Evolution 2001, 55, 2303–2318. [Google Scholar] [PubMed]
- Perez-Martinez, C.A.; Leal, M. Lizards as models to explore the ecological and neuroanatomical correlates of miniaturization. Behaviour 2021, 158, 1121–1168. [Google Scholar] [CrossRef]
- Caldwell, M.W. The Origin of Snakes (CRC Press, Boca Raton, 2020, pp 57-58).
- Strong, C.C.; Scherz, S.; Caldwell, W. Convergence, divergence, and macroevolutionary constraint as revealed by anatomical network analysis of the squamate skull, with an emphasis on snake. Scientific Reports 2022, 12, 14469. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).