Submitted:
04 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
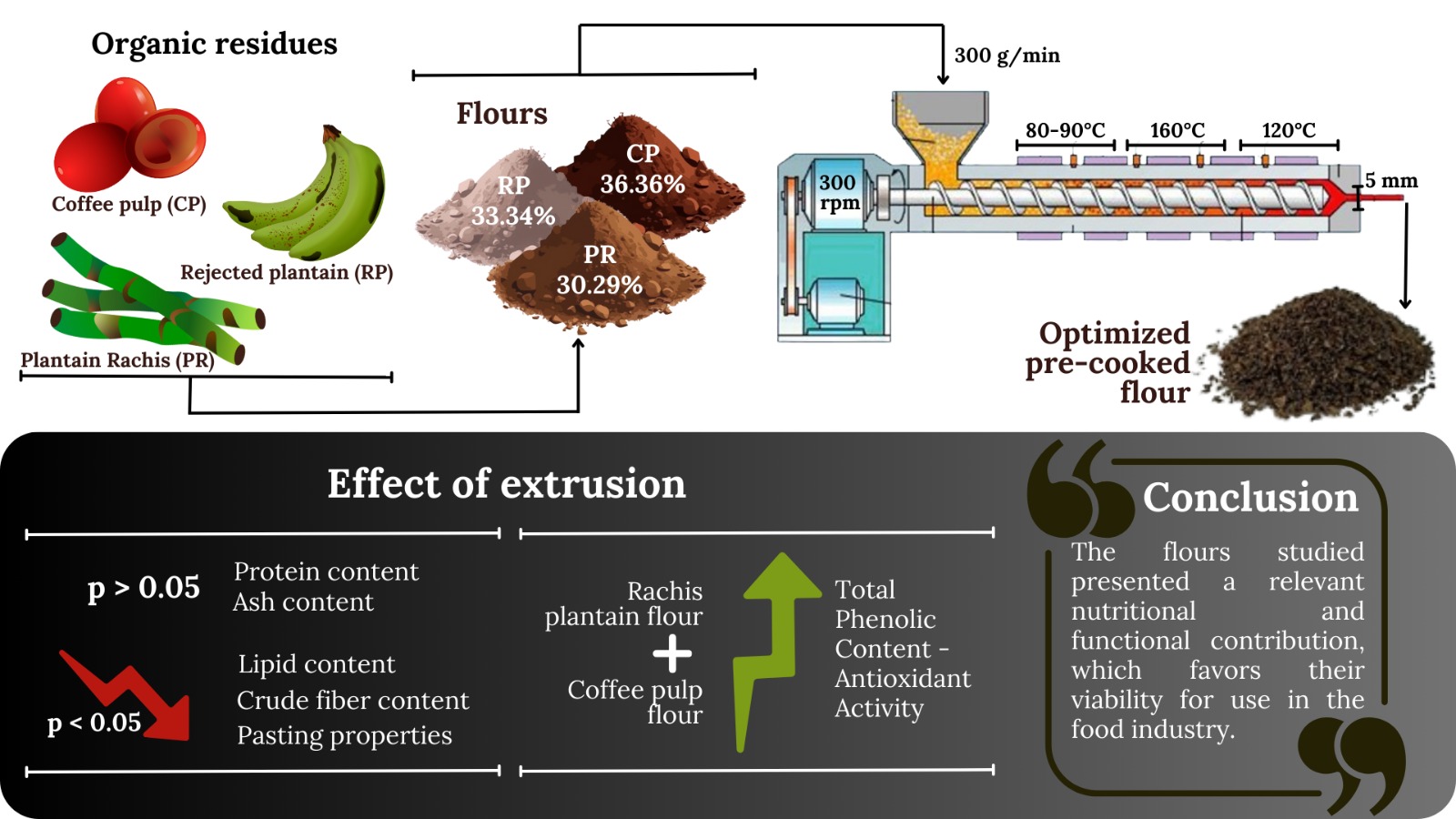
Agroindustrial Byproducts
Experimental Design and Statistical Analysis
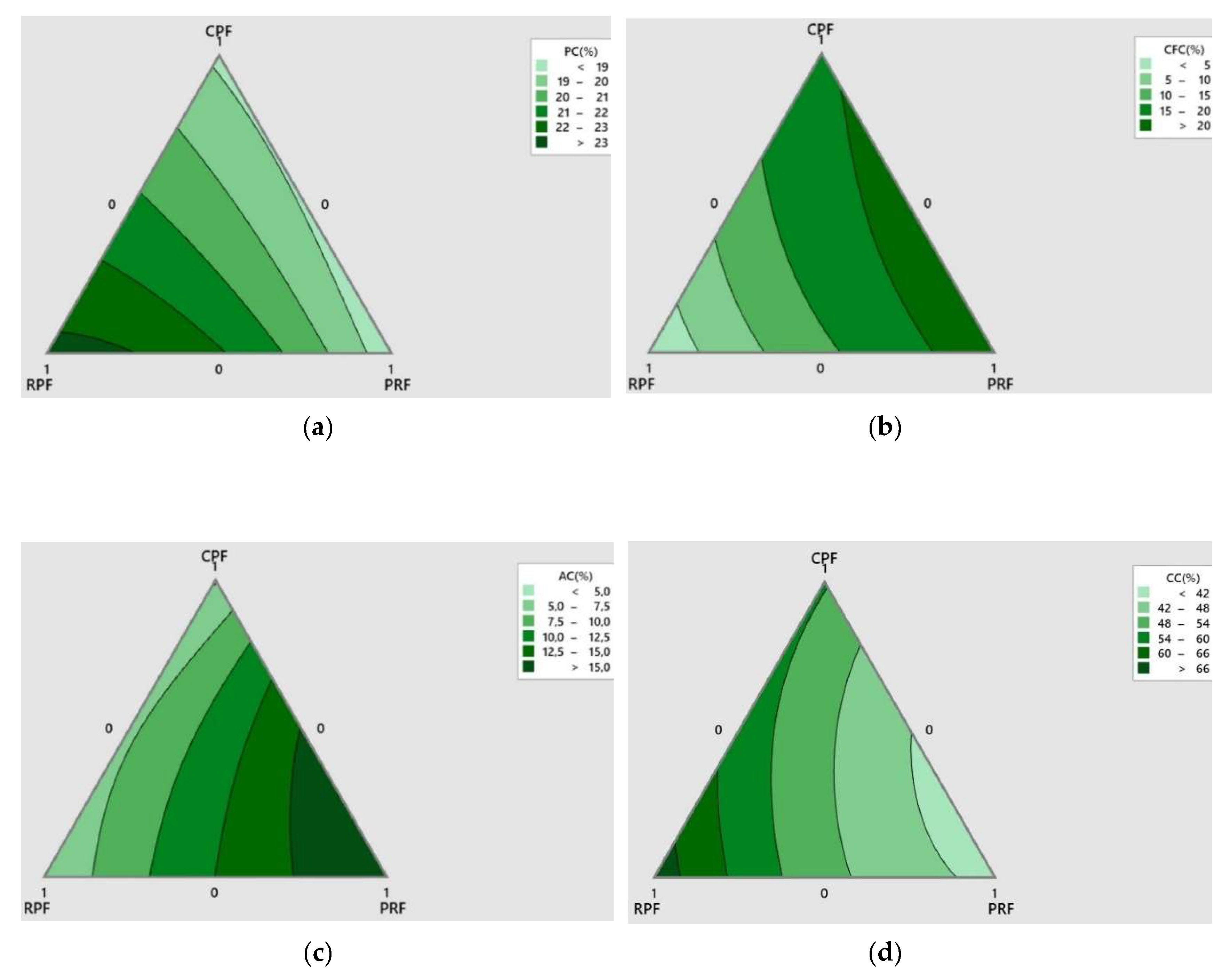
Optimization
Extrusion
Physicochemical properties
Nutritional composition
Antioxidant Activity and Total Phenolic Content
Extraction procedure
Antioxidant Assays
Pasting properties
Results
Physicochemical Properties
| Run | Independent variables | pH | pH* | Water Activity |
Water Activity* |
Water Absorption Capacity (g water/g) |
Water Absorption Capacity* (g water/g) |
Oil Absorption Capacity (g oil/g ) |
Oil Absorption Capacity* (g oil/g ) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CPF | RPF | PRF | |||||||||
| 1 | 1,00 | 0,00 | 0,00 | 4,24 ± 0,01 | 4,17 ± 0,01 | 0,4851 ± 0,003 | 0,5485 ± 0,001 | 3,9164 ± 0,089 | 3,3124 ± 0,053 | 1,3121 ± 0,053 | 1,0903 ± 0,103 |
| 2 | 0,00 | 1,00 | 0,00 | 5,92 ± 0,03 | 6,10 ± 0,06 | 0,4081 ± 0,007 | 0,5480 ± 0,003 | 1,6044 ± 0,044 | 4,2627 ± 0,025 | 1,0431 ± 0,023 | 0,9915 ± 0,017 |
| 3 | 0,00 | 0,00 | 1,00 | 5,96 ± 0,01 | 5,20 ± 0,01 | 0,3081 ± 0,004 | 0,5236 ± 0,003 | 6,6615 ± 0,097 | 3,0204 ± 0,058 | 1,9160 ± 0,051 | 1,2779 ± 0,015 |
| 4 | 0,67 | 0,00 | 0,33 | 4,69 ± 0,04 | 4,66 ± 0,02 | 0,4125 ± 0,002 | 0,5415 ± 0,002 | 5,6088 ± 0,092 | 3,2244 ± 0,003 | 1,6073 ± 0,047 | 1,0633 ± 0,001 |
| 5 | 0,33 | 0,00 | 0,67 | 5,12 ± 0,02 | 5,15 ± 0,01 | 0,3773 ± 0,002 | 0,5462 ± 0,002 | 6,1680 ± 0,1393 | 3,1037 ± 0,092 | 1,8121 ± 0,023 | 1,1851 ± 0,034 |
| 6 | 0,67 | 0,33 | 0,00 | 4,55± 0,03 | 4,66 ± 0,02 | 0,4563 ± 0,003 | 0,5760 ± 0,002 | 3,3887 ± 0,065 | 3,1331 ± 0,148 | 1,2040 ± 0,030 | 1,0553 ± 0,022 |
| 7 | 0,33 | 0,67 | 0,00 | 5,02 ± 0,02 | 5,05 ± 0,01 | 0,4357 ± 0,002 | 0,5952 ± 0,004 | 2,6518 ± 0,252 | 3,2797 ± 0,042 | 1,0803 ± 0,048 | 1,0860 ± 0,001 |
| 8 | 0,17 | 0,67 | 0,17 | 5,46 ± 0,01 | 5,59 ± 0,01 | 0,3931 ± 0,001 | 0,6190 ± 0,003 | 3,0824 ± 0,019 | 3,3633 ± 0,040 | 1,1392 ± 0,066 | 1,1506 ± 0,024 |
| 9 | 0,00 | 0,33 | 0,67 | 5,96 ± 0,01 | 5,79 ± 0,01 | 0,3419 ± 0,001 | 0,5768 ± 0,002 | 4,8451 ± 0,250 | 3,4160 ± 0,237 | 1,5726 ± 0,034 | 1,4075 ± 0,001 |
| 10 | 0,17 | 0,17 | 0,67 | 5,45 ± 0,01 | 5,21 ± 0,01 | 0,3549 ± 0,002 | 0,5610 ± 0,003 | 5,6020 ± 0,298 | 3,2285 ± 0,024 | 1,5949 ± 0,040 | 1,2122 ± 0,016 |
| 11 | 0,00 | 0,67 | 0,33 | 5,99 ± 0,01 | 6,06 ± 0,01 | 0,3915 ± 0,003 | 0,5917 ± 0,005 | 3,4146 ± 0,085 | 3,6963 ± 0,028 | 1,2644 ± 0,081 | 1,2062 ± 0,017 |
| 12 | 0,67 | 0,17 | 0,17 | 4,64 ± 0,02 | 4,69 ± 0,02 | 0,4378 ± 0,001 | 0,6160 ± 0,002 | 4,5940 ± 0,097 | 3,3806 ± 0,049 | 1,3292 ± 0,039 | 1,1719 ± 0,060 |
| 13 | 0,33 | 0,33 | 0,33 | 5,11 ± 0,02 | 5,23 ± 0,02 | 0,3944 ± 0,003 | 0,5852 ± 0,003 | 4,5562 ± 0,079 | 2,7325 ± 0,059 | 1,4086 ± 0,026 | 1,1998 ± 0,041 |
| 14 | 0,33 | 0,33 | 0,33 | 5,21 ± 0,03 | 5,20 ± 0,01 | 0,4031 ± 0,002 | 0,5798 ± 0,003 | 4,5678 ± 0,090 | 3,0612 ± 0,002 | 1,3517 ± 0,085 | 1,1702 ± 0,009 |
| 15 | 0,33 | 0,33 | 0,33 | 5,24 ± 0,01 | 5,21 ± 0,01 | 0,3989 ± 0,003 | 0,5886 ± 0,003 | 4,3311 ± 0,092 | 3,7046 ± 0,010 | 1,3755 ± 0,021 | 1,3926 ± 0,020 |
| 16 | 0,33 | 0,33 | 0,33 | 5,16 ± 0,02 | 5,18 ± 0,01 | 0,3998 ± 0,002 | 0,5816 ± 0,0003 | 4,3319 ± 0,084 | 3,3750 ± 0,045 | 1,3623 ± 0,028 | 1,2675 ± 0,028 |
| 17 | 0,33 | 0,33 | 0,33 | 5,17 ± 0,03 | 5,19 ± 0,01 | 0,3894 ± 0,001 | 0,5930 ± 0,001 | 4,2916 ± 0,185 | 3,1875 ± 0,073 | 1,3503 ± 0,044 | 1,0538 ± 0,104 |
Nutritional Composition
Antioxidant Activity and Total Phenolic Content
Statistical Analysis


Optimization and validation
Pasting properties
Discussion
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayana, P.; Wani, K.M. Unlocking the Green Potential: Sustainable Extraction of Bioactives from Orange Peel Waste for Environmental and Health Benefits. Journal of Food Measurement and Characterization 2024. [CrossRef]
- Ozcan, B.E.; Tetik, N.; Aloglu, H.S. Polysaccharides from Fruit and Vegetable Wastes and Their Food Applications: A Review. Int J Biol Macromol 2024, 276, 134007. [CrossRef]
- Noor, A.; Moyle, P.M.; Malik, A.; Ziora, Z.M.; Pant, K.K. Transformative Upcycling of Fruit-Vegetable Waste for Nutraceutical and Pharmaceutical Breakthroughs and Circular Economy Evolution. Process Safety and Environmental Protection 2024, 187, 1022–1036. [CrossRef]
- Panda, J.; Mishra, A.K.; Mohanta, Y.K.; Patowary, K.; Rauta, P.R.; Mishra, B. Exploring Biopolymer for Food and Pharmaceuticals Application in the Circular Bioeconomy: An Agro-Food Waste-to-Wealth Approach. Waste Biomass Valorization 2024. [CrossRef]
- Cortés-Macías, E.T.; López, C.F.; Gentile, P.; Girón-Hernández, J.; López, A.F. Impact of Post-Harvest Treatments on Physicochemical and Sensory Characteristics of Coffee Beans in Huila, Colombia. Postharvest Biol Technol 2022, 187, 111852. [CrossRef]
- Rubio-Jovel, K. Coffee Production Networks in Costa Rica and Colombia: A Systems Analysis on Voluntary Sustainability Standards and Impacts at the Local Level. J Clean Prod 2024, 445, 141196. [CrossRef]
- Rojas-Ospina, A.; Zuñiga-Collazos, A.; Castillo-Palacio, M. Factors Influencing Environmental Sustainability Performance: A Study Applied to Coffee Crops in Colombia. Journal of Open Innovation: Technology, Market, and Complexity 2024, 10, 100361. [CrossRef]
- Thai, L.Q.; Niwat, C.; Qin, S.; Konsue, N. Supercritical Carbon Dioxide and Ethanol-Assisted Extraction of Bioactive Compounds from Bourbon, Catimor, and Caturra Coffee Pulp for Maximized Antioxidant and Therapeutic Properties. Future Foods 2024, 9, 100381. [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Socha, K.; Zujko, M.E. Coffee Brews: Are They a Source of Macroelements in Human Nutrition? Foods 2021, 10.
- Hu, S.; Gil-Ramírez, A.; Martín-Trueba, M.; Benítez, V.; Aguilera, Y.; Martín-Cabrejas, M.A. Valorization of Coffee Pulp as Bioactive Food Ingredient by Sustainable Extraction Methodologies. Curr Res Food Sci 2023, 6, 100475. [CrossRef]
- Herrán, O.F. Usual Portions of Foods Consumed in Colombia and Their Contributions to Total Energy Intake/Day: Results of a Nationally Representative Survey (ENSIN-2015). Food and Humanity 2024, 3, 100358. [CrossRef]
- Gómez, J.A.; Matallana, L.G.; Teixeira, J.A.; Sánchez, Ó.J. A Framework for the Design of Sustainable Multi-Input Second-Generation Biorefineries through Process Simulation: A Case Study for the Valorization of Lignocellulosic and Starchy Waste from the Plantain Agro-Industry. Chemical Engineering Research and Design 2023, 195, 551–571. [CrossRef]
- Gómez, J.A.; Nobre, C.; Teixeira, J.A.; Sánchez, Ó.J. Towards a Biorefinery Processing Waste from Plantain Agro-Industry: Assessment of the Production of Dairy Cattle Feed through Process Simulation. Biosyst Eng 2022, 217, 131–149. [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Supramolecular Solvent Extraction of Bioactives from Coffee Cherry Pulp. J Food Eng 2020, 278, 109933. [CrossRef]
- Biratu, G.; Woldemariam, H.W.; Gonfa, G. Optimization of Pectin Yield Extracted from Coffee Arabica Pulp Using Response Surface Methodology. Heliyon 2024, 10, e29636. [CrossRef]
- Biratu, G.; Woldemariam, H.W.; Gonfa, G. Development of Active Edible Films from Coffee Pulp Pectin, Propolis, and Honey with Improved Mechanical, Functional, Antioxidant, and Antimicrobial Properties. Carbohydrate Polymer Technologies and Applications 2024, 8, 100557. [CrossRef]
- Hu, D.; Yang, G.; Liu, X.; Qin, Y.; Zhang, F.; Sun, Z.; Wang, X. Comparison of Different Drying Technologies for Coffee Pulp Tea: Changes in Color, Taste, Bioactive and Aroma Components. LWT 2024, 200, 116193. [CrossRef]
- Reza Rizkiansyah, R.; Mardiyati, Y.; Hariyanto, A.; Steven, S.; Dirgantara, T. Non-Wood Paper from Coffee Pulp Waste: How Its Performance as Coffee Filter. Cleaner Materials 2024, 12, 100241. [CrossRef]
- Sommano, S.R.; Jantrawut, P.; Sangta, J.; Chanabodeechalermrung, B.; Sunanta, P.; Bakshani, C.; Willats, W. Utilization of Coffee Pulp for the Production of Sustainable Cellulosic Composite and Plant-Based Hydrogel as a Potential Human Wound Dressing. Food Structure 2023, 37, 100347. [CrossRef]
- Nweke, C.N.; Onu, C.E.; Nwabanne, J.T.; Ohale, P.E.; Madiebo, E.M.; Chukwu, M.M. Optimal Pretreatment of Plantain Peel Waste Valorization for Biogas Production: Insights into Neural Network Modeling and Kinetic Analysis. Heliyon 2023, 9, e21995. [CrossRef]
- Gómez, J.A.; Matallana, L.G.; Teixeira, J.A.; Sánchez, Ó.J. A Framework for the Design of Sustainable Multi-Input Second-Generation Biorefineries through Process Simulation: A Case Study for the Valorization of Lignocellulosic and Starchy Waste from the Plantain Agro-Industry. Chemical Engineering Research and Design 2023, 195, 551–571. [CrossRef]
- Giwa, A.S.; Sheng, M.; Maurice, N.J.; Liu, X.; Wang, Z.; Chang, F.; Huang, B.; Wang, K. Biofuel Recovery from Plantain and Banana Plant Wastes: Integration of Biochemical and Thermochemical Approach. J Renew Mater 2023, 11, 2593–2629. [CrossRef]
- Hernández-Carmona, F.; Morales-Matos, Y.; Lambis-Miranda, H.; Pasqualino, J. Starch Extraction Potential from Plantain Peel Wastes. J Environ Chem Eng 2017, 5, 4980–4985. [CrossRef]
- Mohammed, A.R.; Attiogbe, F.; Emahi, I. Improved Greywater Quality after Biofiltration with a Fibre-Biofilter Derived from Plantain Pseudo Stem. Sci Afr 2024, 25, e02293. [CrossRef]
- Xie, J.; Zhang, Y.; Klomklao, S.; Simpson, B.K. Pectin from Plantain Peels: Green Recovery for Transformation into Reinforced Packaging Films. Waste Management 2023, 161, 225–233. [CrossRef]
- Yakoubi, S.; Kobayashi, I.; Uemura, K.; Tounsi, M.S.; Nakajima, M.; Hiroko, I.; Neves, M.A. Enhancing Plantain Epicarp Active Edible Coating Performance through Investigation of Optimal Spray Coating Conditions. Colloids Surf A Physicochem Eng Asp 2023, 678, 132474. [CrossRef]
- Patiño-Rodríguez, O.; Bello-Pérez, L.A.; Flores-Silva, P.C.; Sánchez-Rivera, M.M.; Romero-Bastida, C.A. Physicochemical Properties and Metabolomic Profile of Gluten-Free Spaghetti Prepared with Unripe Plantain Flours. LWT 2018, 90, 297–302. [CrossRef]
- Alonso-Gómez, L.A.; Solarte-Toro, J.C.; Bello-Pérez, L.A.; Cardona-Alzate, C.A. Performance Evaluation and Economic Analysis of the Bioethanol and Flour Production Using Rejected Unripe Plantain Fruits (Musa Paradisiaca L.) as Raw Material. Food and Bioproducts Processing 2020, 121, 29–42. [CrossRef]
- Pérez-Viveros, D.J.; Díaz-Batalla, L.; Navarro-Cortez, R.O.; Palma-Rodríguez, H.M.; Hernández-Uribe, J.P. Effect of Extrusion on Physicochemical and Functional Properties of Cladode Flour from Opuntia Cochenillifera and Opuntia Ficus-Indica. Applied Food Research 2024, 4, 100568. [CrossRef]
- Zhang, X.; Gao, Y.; Wang, R.; Zhang, G.; Sun, Y.; Li, X.; Liang, J. Effects of Adding Blueberry Residue Powder and Extrusion Processing on Microstructure and in Vitro Digestibility of Indica Rice Flour. Bioactive Carbohydrates and Dietary Fibre 2024, 32, 100435. [CrossRef]
- Yan, Y.; Fang, J.; Zhu, X.; Ji, X.; Shi, M.; Niu, B. Effect of Extrusion Using Plasma-Activated Water on the Structural, Physicochemical, Antioxidant and in Vitro Digestive Properties of Yam Flour. Food Chem 2024, 460, 140687. [CrossRef]
- Wang, Y.; Zhao, R.; Liu, W.; Zhao, R.; Liu, Q.; Hu, H. Effect of Twin-Screw Extrusion Pretreatment on Starch Structure, Rheological Properties and 3D Printing Accuracy of Whole Potato Flour and Its Application in Dysphagia Diets. Int J Biol Macromol 2024, 278, 134796. [CrossRef]
- Zhang, Z.; Zhu, M.; Xing, B.; Liang, Y.; Zou, L.; Li, M.; Fan, X.; Ren, G.; Zhang, L.; Qin, P. Effects of Extrusion on Structural Properties, Physicochemical Properties and in Vitro Starch Digestibility of Tartary Buckwheat Flour. Food Hydrocoll 2023, 135, 108197. [CrossRef]
- Del Castillo, E.; Montgomery, D.C.; McCarville, D.R. Modified Desirability Functions for Multiple Response Optimization. Journal of Quality Technology 1996, 28, 337–345. [CrossRef]
- Carlos de Sousa, W.; Alves Morais, R.; Damian Giraldo Zuniga, A. Buriti (Mauritia Flexuosa) Shell Flour: Nutritional Composition, Chemical Profile, and Antioxidant Potential as a Strategy for Valuing Waste from Native Brazilian Fruits. Food Research International 2024, 190, 114578. [CrossRef]
- Ekeledo, E.; Abass, A.; Müller, J. Effect of Packaging and Storage Conditions on the Pasting and Functional Properties of Pretreated Yellow-Fleshed Cassava Flour. Applied Food Research 2024, 4, 100467. [CrossRef]
- Horwitz, William. Official Methods of Analysis of AOAC International; AOAC International, 2006; ISBN 0935584773.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic Biol Med 1999, 26, 1231–1237. [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Science and Technology 1995, 28, 25–30. [CrossRef]
- Granato, D.; Karnopp, A.R.; van Ruth, S.M. Characterization and Comparison of Phenolic Composition, Antioxidant Capacity and Instrumental Taste Profile of Juices from Different Botanical Origins. J Sci Food Agric 2015, 95, 1997–2006. [CrossRef]
- Kumar, P.S.; Saravanan, A.; Sheeba, N.; Uma, S. Structural, Functional Characterization and Physicochemical Properties of Green Banana Flour from Dessert and Plantain Bananas (Musa Spp.). LWT 2019, 116, 108524. [CrossRef]
- Jiménez-Ochoa, J.P.; Barrios-Rodríguez, Y.F.; Bahamón-Monje, A.F.; Gutiérrez-Gúzman, N. Physicochemical and Sensory Characteristics of Dehydrated Coffee Pulp in Function of Drying Temperature. Revista Brasileira de Engenharia Agrícola e Ambiental 2022, 26.
- Chakraborty, R.; Sabruna, S.; Roy, R.; Majumdar, S.; Roy, S. Banana Pseudostem Substitution in Wheat Flour Biscuits Enriches the Nutritional and Antioxidative Properties with Considerable Acceptability. SN Appl Sci 2021, 3. [CrossRef]
- Sarawong, C.; Schoenlechner, R.; Sekiguchi, K.; Berghofer, E.; Ng, P.K.W. Effect of Extrusion Cooking on the Physicochemical Properties, Resistant Starch, Phenolic Content and Antioxidant Capacities of Green Banana Flour. Food Chem 2014, 143, 33–39. [CrossRef]
- Qin, S.; Hu, F.; Yang, N.; Li, L.; Yang, H.; Suo, Y.; He, F. Influence of Native Coffee Yeast Fermentation on Phenolic Content, Organic Acids, and Volatile Compounds in Cascara. LWT 2024, 210, 116860. [CrossRef]
- Karim, M.D.; Abuhena, M.; Hossain, M.D.; Billah, M.M. Assessment and Comparison of Cooking Qualities and Physio-Chemical Properties of Seven Rice Varieties in Terms of Amylose Content. Food Physics 2024, 1, 100014. [CrossRef]
- Yang, Z.; Zhou, Y.; Xing, J.-J.; Guo, X.-N.; Zhu, K.-X. Influence of Extrusion on Storage Quality of Dried Oat Noodles: Lipid Degradation and off-Flavours. J Cereal Sci 2021, 101, 103316. [CrossRef]
- Garcia-Valle, D.E.; Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; Tovar, J. Extruded Unripe Plantain Flour as an Indigestible Carbohydrate-Rich Ingredient. Front Nutr 2019, 6. [CrossRef]
- Omojokun, A.O.; Jokoh, A.O. Effects of Fermentation and Extrusion on the Mineral and Antinutrient Composition of Plantain-Cowpea Flour Blends. 2020. [CrossRef]
- Wang, Q.; Li, L.; Wang, T.; Zheng, X. A Review of Extrusion-Modified Underutilized Cereal Flour: Chemical Composition, Functionality, and Its Modulation on Starchy Food Quality. Food Chem 2022, 370, 131361. [CrossRef]
- Pismag, R.Y.; Polo, M.P.; Hoyos, J.L.; Bravo, J.E.; Roa, D.F. Effect of Extrusion Cooking on the Chemical and Nutritional Properties of Instant Flours: A Review. F1000Res 2023, 12, 1356. [CrossRef]
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M.G.; Martinez, M.M. Manufacturing the Ultimate Green Banana Flour: Impact of Drying and Extrusion on Phenolic Profile and Starch Bioaccessibility. Food Chem 2019, 297, 124990. [CrossRef]
- Li, N.; Taylor, L.; Ferruzzi, M.; Mauer, L. Kinetic Study of Catechin Stability: Effects of PH, Concentration, and Temperature. J Agric Food Chem 2012, 60. [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Polyphenolic Composition and Antioxidant Capacity of Extruded Cranberry Pomace. J Agric Food Chem 2010, 58, 4037–4042. [CrossRef]
- Pandey, S.; Kumar, A.; Rao, P.S. Optimization, Modeling, and Characterization Study for the Physicochemical Properties of Raw Banana and Defatted Soy Composite Extrudates. Food Chem 2021, 339, 127865. [CrossRef]
- Sommano, S.R.; Jantrawut, P.; Sangta, J.; Chanabodeechalermrung, B.; Sunanta, P.; Bakshani, C.; Willats, W. Utilization of Coffee Pulp for the Production of Sustainable Cellulosic Composite and Plant-Based Hydrogel as a Potential Human Wound Dressing. Food Structure 2023, 37, 100347. [CrossRef]
- Torres-Vargas, O.L.; Gaytan-Martinez, M.; Fernanda, C.-C.; Millán-Malo, B.M.; Rodriguez-Garcia, M.E. Changes in the Physicochemical Properties of Isolated Starch and Plantain (Musa AAB Simmonds) Flours for Early Maturity Stage. Heliyon 2023, 9, e18939. [CrossRef]

| Run | Water C. | Water C.* | Protein C. | Protein C.* | Lipid C. | Lipid C.* | Crude Fiber C. | Crude Fiber C.* | Carbo. C. | Carbo. C.* | Ash C. | Ash C.* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3,50 ± 0,02 | 5,27 ± 0,17 | 18,95 ± 0,12 | 18,56 ± 0,10 | 2,80 ± 0,05 | 2,26 ± 0,04 | 25,02 ± 0,05 | 18,59 ± 0,07 | 47,33 ± 0,12 | 55,73 ± 0,13 | 5,91 ± 0,20 | 4,86 ± 0,02 |
| 2 | 3,11 ± 0,01 | 3,70 ± 0,15 | 23,71 ± 0,11 | 23,28 ± 0,11 | 1,44 ± 0,02 | 1,05 ± 0,01 | 3,22 ± 0,03 | 1,77 ± 0,07 | 67,19 ± 0,11 | 68,72 ± 0,16 | 4,44 ± 0,06 | 5,18 ± 0,03 |
| 3 | 1,70 ± 0,06 | 4,40 ± 0,13 | 20,40 ± 0,12 | 18,34 ± 0,08 | 2,34 ± 0,06 | 1,42 ± 0,01 | 39,90 ± 0,39 | 22,27 ± 0,62 | 24,59 ± 0,10 | 40,57 ± 0,12 | 21,77 ± 0,11 | 17,40 ± 0,15 |
| 4 | 2,90 ± 0,03 | 5,74 ± 0,14 | 19,44 ± 0,12 | 18,63 ± 0,05 | 2,64 ± 0,05 | 2,93 ± 0,04 | 27,00 ± 0,16 | 21,41 ± 0,38 | 39,66 ± 0,11 | 44,74 ± 0,12 | 11,26 ± 0,17 | 12,29 ± 0,08 |
| 5 | 2,30 ± 0,05 | 5,50 ± 0,20 | 19,93 ± 0,12 | 18,57 ± 0,19 | 2,49 ± 0,06 | 2,02 ± 0,02 | 28,96 ± 0,28 | 20,40 ± 0,23 | 32,08 ± 0,12 | 41,19 ± 0,10 | 16,55 ± 0,14 | 17,82 ± 0,12 |
| 6 | 3,37 ± 0,02 | 6,40 ± 0,20 | 20,54 ± 0,11 | 20,51 ± 0,05 | 2,35 ± 0,04 | 2,02 ± 0,04 | 17,73 ± 0,04 | 17,14 ± 0,40 | 53,96 ± 0,11 | 52,70 ± 0,10 | 5,42 ± 0,15 | 7,63 ± 0,09 |
| 7 | 3,24 ± 0,01 | 4,22 ± 0,24 | 22,13 ± 0,11 | 21,72 ± 0,07 | 1,89 ± 0,03 | 1,46 ± 0,01 | 10,47 ± 0,03 | 7,73 ± 0,03 | 60,59 ± 0,12 | 63,13 ± 0,12 | 4,93 ± 0,10 | 5,96 ± 0,05 |
| 8 | 2,94 ± 0,02 | 6,00 ± 0,07 | 22,36 ± 0,11 | 22,13 ± 0,12 | 1,82 ± 0,03 | 0,82 ± 0,02 | 11,51 ± 0,09 | 8,04 ± 0,25 | 56,70 ± 0,10 | 59,87 ± 0,10 | 7,61 ± 0,09 | 9,14 ± 0,04 |
| 9 | 2,17 ± 0,04 | 6,30 ± 0,00 | 21,49 ± 0,11 | 20,45 ± 0,17 | 2,04 ± 0,05 | 0,89 ± 0,01 | 21,76 ± 0,27 | 16,89 ± 0,19 | 38,65 ± 0,10 | 47,10 ± 0,14 | 16,05 ± 0,09 | 14,67 ± 0,23 |
| 10 | 2,24 ± 0,04 | 11,33 ± 0,11 | 20,71 ± 0,12 | 20,70 ± 0,04 | 2,27 ± 0,05 | 1,16 ± 0,02 | 25,36 ± 0,28 | 22,92 ± 0,65 | 35,36 ± 0,12 | 41,87 ± 0,14 | 16,30 ± 0,11 | 13,35 ± 0,05 |
| 11 | 2,64 ± 0,02 | 11,35 ± 0,02 | 22,59 ± 0,12 | 22,45 ± 0,25 | 1,75 ± 0,03 | 1,97 ± 0,05 | 12,53 ± 0,15 | 10,14 ± 0,18 | 52,86 ± 0,11 | 54,10 ± 0,15 | 10,27 ± 0,07 | 10,34 ± 0,05 |
| 12 | 3,14 ± 0,02 | 11,54 ± 0,25 | 19,99 ± 0,11 | 20,78 ± 0,37 | 2,50 ± 0,04 | 1,48 ± 0,03 | 22,38 ± 0,10 | 19,15 ± 0,17 | 46,79 ± 0,10 | 50,41 ± 0,13 | 8,35 ± 0,16 | 8,18 ± 0,01 |
| 13 | 2,77 ± 0,03 | 5,30 ± 0,04 | 20,98 ± 0,11 | 20,25 ± 0,02 | 2,19 ± 0,04 | 2,16 ± 0,03 | 19,76 ± 0,11 | 14,29 ± 0,07 | 46,31 ± 0,12 | 52,25 ± 0,14 | 10,76 ± 0,12 | 11,05 ± 0,07 |
| 14 | 2,77 ± 0,03 | 6,30 ± 0,17 | 21,11 ± 0,11 | 20,46 ± 0,05 | 2,11 ± 0,04 | 2,16 ± 0,03 | 20,01 ± 0,14 | 17,19 ± 0,56 | 45,34 ± 0,11 | 47,48 ± 0,11 | 11,43 ± 0,12 | 12,71 ± 0,11 |
| 15 | 2,78 ± 0,03 | 5,70 ± 0,16 | 21,02 ± 0,11 | 20,33 ± 0,04 | 2,24 ± 0,04 | 2,01 ± 0,01 | 18,11 ± 0,12 | 17,62 ± 0,65 | 47,12 ± 0,11 | 48,11 ± 0,15 | 11,54 ± 0,12 | 11,93 ± 0,01 |
| 16 | 2,80 ± 0,03 | 6,50 ± 0,09 | 20,87 ± 0,11 | 20,51 ± 0,10 | 2,34 ± 0,04 | 1,04 ± 0,02 | 18,98 ± 0,11 | 17,03 ± 0,19 | 47,70 ± 0,11 | 49,03 ± 0,11 | 10,11 ± 0,12 | 12,39 ± 0,03 |
| 17 | 2,75 ± 0,03 | 10,45 ± 0,38 | 21,10 ± 0,11 | 21,41 ± 0,20 | 2,01 ± 0,04 | 1,17 ± 0,03 | 20,12 ± 0,16 | 19,17 ± 0,21 | 46,46 ± 0,10 | 46,56 ± 0,12 | 10,31 ± 0,12 | 11,69 ± 0,04 |
| Run | TPC (mg GAE/ g db) |
TPC* (mg GAE/ g db) |
DPPH (mM TEAC/g db) |
DPPH* (mM TEAC/g db) |
ABTS (mM TEAC/g db) |
ABTS* (mM TEAC/g db) |
|---|---|---|---|---|---|---|
| 1 | 6,051 ± 0,205 | 5,285 ± 0,115 | 0,110 ± 0,01142 | 1,013 ± 0,08914 | 1,784 ± 0,305 | 1,459 ± 0,086 |
| 2 | 2,404 ± 0,129 | 2,550 ± 0,149 | 0,513 ± 0,26823 | 0,210 ± 0,01964 | 0,749 ± 0,037 | 1,009 ± 0,008 |
| 3 | 6,525 ± 0,923 | 8,497 ± 0,379 | 0,499 ± 0,00907 | 0,763 ± 0,08914 | 2,425 ± 0,089 | 2,060 ± 0,175 |
| 4 | 4,797 ± 0,011 | 7,999 ± 0,424 | 0,817 ± 0,20389 | 1,015 ± 0,04215 | 1,291 ± 0,147 | 2,030 ± 0,092 |
| 5 | 6,312 ± 0,759 | 8,734 ± 0,288 | 0,659 ± 0,28465 | 0,755 ± 0,14750 | 2,145 ± 0,126 | 2,349 ± 0,099 |
| 6 | 4,616 ± 0,871 | 5,291 ± 0,269 | 0,786 ± 0,36725 | 0,719 ± 0,03238 | 1,157 ± 0,619 | 1,140 ± 0,134 |
| 7 | 3,471 ± 0,333 | 3,300 ± 0,065 | 0,658 ± 0,10612 | 0,558 ± 0,12192 | 0,913 ± 0,066 | 0,784 ± 0,013 |
| 8 | 3,417 ± 0,061 | 3,394 ± 0,183 | 0,483 ± 0,03034 | 0,202 ± 0,06201 | 0,807 ± 0,018 | 0,875 ± 0,031 |
| 9 | 5,138 ± 0,547 | 4,813 ± 0,545 | 0,344 ± 0,03703 | 0,238 ± 0,10652 | 1,816 ± 0,056 | 1,276 ± 0,100 |
| 10 | 5,598 ± 0,174 | 5,200 ± 0,439 | 0,567 ± 0,03703 | 0,706 ± 0,08552 | 2,299 ± 0,091 | 1,969 ± 0,004 |
| 11 | 3,711 ± 0,155 | 3,221 ± 0,228 | 0,290 ± 0,01392 | 0,149 ± 0,01811 | 1,204 ± 0,177 | 0,975 ± 0,277 |
| 12 | 5,397 ± 0,255 | 4,149 ± 0,083 | 0,837 ± 0,09273 | 0,529 ± 0,11073 | 1,289 ± 0,071 | 0,793 ± 0,016 |
| 13 | 4,884 ± 0,231 | 4,658 ± 0,184 | 0,603 ± 0,00292 | 0,677 ± 0,08041 | 1,676 ± 0,046 | 1,607 ± 0,162 |
| 14 | 4,772 ± 0,066 | 4,868 ± 0,042 | 0,601 ± 0,09382 | 0,507 ± 0,05072 | 1,515 ± 0,194 | 1,503 ± 0,214 |
| 15 | 5,454 ± 0,108 | 5,817 ± 0,093 | 0,602 ± 0,09043 | 0,507 ± 0,07383 | 1,476 ± 0,199 | 1,640 ± 0,046 |
| 16 | 4,447 ± 0,011 | 6,174 ± 0,254 | 0,522 ± 0,00318 | 0,659 ± 0,05232 | 1,287 ± 0,144 | 1,717 ± 0,200 |
| 17 | 4,887 ± 0,352 | 4,820 ± 0,420 | 0,619 ± 0,07278 | 0,524 ± 0,07259 | 1,524 ± 0,240 | 1,458 ± 0,171 |
| Response variables | Source | ||||
|---|---|---|---|---|---|
| SSR | p-value | SSE | Lack of fit (p-value) |
R2 | |
| pH | 3,91494 | 0,000 | 0,06545 | 0,300 | 98,36 |
| WA | 0,008359 | 0,014 | 0,002850 | 0,900 | 74,58 |
| WAC (g water/g) | 1,18624 | 0,054 | 0,63001 | 0,983 | 65,31 |
| OAC (g oil/g) | 0,137309 | 0,062 | 0,077204 | 0,976 | 64,01 |
| Water Content % | 44,089 | 0,673 | 108,793 | 0,213 | 28,84 |
| Protein Content % | 33,1882 | 0,000 | 3,9344 | 0,218 | 89,40 |
| Lipid Content % | 2,67695 | 0,287 | 2,94649 | 0,92 | 47,60 |
| Crude Fiber Content % | 507,757 | 0,000 | 20,994 | 0,477 | 93,82 |
| Ash Content % | 223,338 | 0,000 | 11,158 | 0,107 | 95,24 |
| Carbohydrates Content % | 916,947 | 0,000 | 46,065 | 0,551 | 95,22 |
| TPC (mg GAE/ g) | 45,0672 | 0,001 | 6,7000 | 0,214 | 87,06 |
| DPPH (mM TEAC/g db) | 0,92663 | 0,001 | 0,1634 | 0,151 | 85,01 |
| ABTS (mM TEAC/g db) | 2,98816 | 0,003 | 0,68521 | 0,168 | 81,35 |
| Response variable | Lower | Upper | Target |
|---|---|---|---|
| Protein Content (% db) | 18,30 | 23,45 | Maximize |
| Crude Fiber Content (% db) | 1,70 | 22,92 | In range |
| Carbohydrates Content (% db) | 40,57 | 68,72 | In range |
| Total Phenolic Content (mg GAE/g) | 2,55 | 8,73 | Maximize |
| DPPH (mM TEAC/g db) | 0,15 | 1,02 | Maximize |
| ABTS (mM TEAC/g db) | 0,78 | 2,00 | Maximize |
| EF | 1 | 5 | Maximize |
| Response variable | Predicted response | Validated response | RMSE |
|---|---|---|---|
| Protein Content (% db) | 20,72 | 20,04 ± 0,67 | 0,8806 |
| Crude Fiber Content (% db) | 17,09 | 19,39 ± 0,52 | 2,3319 |
| Carbohydrates Content (% db) | 49,28 | 48,69 ± 0,93 | 0,9662 |
| Total Phenolic Content (mg GAE/g) | 4,86 | 6,30 ± 0,28 | 1,4588 |
| DPPH (mM TEAC /g db) | 0,56 | 0,59 ± 0,20 | 0,4179 |
| ABTS (mM TEAC/g db) | 1,48 | 1,62 ± 0,16 | 0,1997 |
| Sample | Peak viscosity (Pas) | Trough viscosity (Pas) | Breakdown viscosity (Pas) | Final Viscosity (Pas) | Setback Viscosity (Pas) | Pasting time (min) | Pasting temperature (°C) |
|---|---|---|---|---|---|---|---|
| CPF-NE | 0,019e ± 0,007 | 0,019e ± 0,007 | 0,0000g ± 0,000 | 0,0404e ± 0,003 | 0,0211f ± 0,004 | 4,25b ± 0,494 | 90,7a ± 0,919 |
| CPF-E | 0,031e ± 0,000 | 0,024e ± 0,000 | 0,0062g ± 0,000 | 0,0440e ± 0,001 | 0,0191f ± 0,001 | 2,99d ± 0,256 | 71,8e ± 2,247 |
| RPF-NE | 2,597b ± 0,029 | 2,196b ± 0,043 | 0,4005b ± 0,013 | 5,0075b ± 0,267 | 2,8110b ± 0,311 | 2,95d ± 0,000 | 79,2c ± 0,000 |
| RPF-E | 0,183 ± 0,003 | 0,124d ± 0,001 | 0,0596d ± 0,001 | 0,3698 ± 0,027 | 0,2455d ± 0,027 | 1,75e ± 0,070 | 60,2f ± 0,919 |
| PRF-NE | 6,783a ± 0,521 | 5,061a ± 0,911 | 1,7223a ± 0,570 | 11,250a ± 0,940 | 6,1886a ± 1,606 | 3,86c ± 0,230 | 86,2b ± 0,378 |
| PRF-E | 0,061e ± 0,002 | 0,045e ± 0,001 | 0,0163e ± 0,000 | 0,0922d ± 0,005 | 0,0468e ± 0,003 | 2,97d ± 0,098 | 81,1c ± 1,060 |
| ONEB | 0,599c ± 0,031 | 0,599c ± 0,031 | 0,0000g ± 0,000 | 1,3880c ± 0,002 | 0,7880c ± 0,008 | 5,77a ± 0,106 | 75,2d ± 1,060 |
| OEB | 0,195d ± 0,231 | 0,038e ± 0,002 | 0,1577c ± 0,232 | 0,0945d ± 0,009 | 0,0564e ± 0,007 | 4,01b ± 0,246 | 72,3e ± 3,437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





