1. Introduction
Epilepsy is a common disease that affects people of all ages and genders. Patients struggling with epilepsy are at risk of seizures, which often occur for no apparent reason. The cause of epilepsy might be genetic, metabolic (with a genetic basis or acquired deficiencies), or as a result of brain disease or damage, such as infections, immune reactions, and structural abnormalities (stroke, trauma, etc.), however, in about 50% the cause remains unknown. Epilepsy is manifested by seizures of various motor and non-motor neurological signs and loss of consciousness [
1]. Unfortunately, despite the intensive development of pharmacological treatment, many patients fail to completely reduce seizures; it is estimated that this applies to approximately 30% of patients [
2].
There is growing interest in searching for serum biomarkers of epileptic seizures that could help in the correct diagnosis of seizures (epileptic vs non-epileptic), assessment of seizure risk, and prediction of treatment outcome. MMP-9 and S100B, the proteins involved in blood-brain barrier integrity, are particularly interesting because they are increased in epilepsy patients in the interictal period [
3] and are triggered by the seizures themselves. [
4,
5]
Recently, several studies have suggested the involvement of the blood-brain barrier (BBB) in the pathogenesis of both epilepsy and seizure induction. An increase in the permeability of the BBB seems to be the cause of increased neuronal excitability. On the other hand, seizures, and even epileptic discharges, increase BBB permeability and activation of endothelial cells. This was shown in experimental studies [
6] as well as in brain samples taken from epileptic foci in human [
7]. The serum biomarkers of BBB were shown to be increased in epileptic patients after seizures [
8] and in the interictal period when compared to age/sex matched control group [
3]. These observations indicate chronic BBB disruption in epileptic patients probably due to chronic inflammation, and also BBB involvement in seizures inducement. Additionally, higher levels of MMP-2 and CCL-2 in the interictal period were associated with lower risk of seizures, whereas a higher level of MMP-9 was associated with higher risk of seizure in 1-6 month period [
9].
Looking for reliable BBB biomarkers has however limitations since most of them are not specific for the brain and might originate from peripheral vasculature and peripheral tissues. Many of them, for example, MMP-9, P-selectin, ICAM-1 participate also in brain inflammatory responses and may be involved in many brain pathologies. In epilepsy, muscle contraction during focal motor or tonic-clonic seizures may also increase BBB serum biomarkers due to muscle vessel dilatation and injury. Levels of some vascular biomarkers, such as MMP-9, were altered after exercise, indicating that muscle contractions during seizures may cause them to change [
10].
MMP-9 is a metalloproteinase highly expressed in the adult brain and has been shown to increase in many brain pathologies. Serum MMP-9 level depends mainly on leukocyte and endothelial secretion, and augments significantly in many conditions (trauma, stroke for example) [
11]. MMP-9 contributes to a wide variety of brain disorders, including epilepsy, schizophrenia, autism spectrum disorders, brain damage, stroke, neurodegeneration, pain, brain tumors, and more [
12]. It activates various cytokines and chemokines, participates in breaking the blood-brain barrier, and facilitates the passage of leukocytes across the BBB into the brain parenchyma.
S100B protein is physiologically produced and released predominantly by astrocytes in the central nervous system [
13]. It plays a role in many cellular processes, including cell proliferation, migration, apoptosis, and differentiation. S100B has been shown to increase in cerebrospinal fluid (CSF) and serum after various neurological diseases (trauma, stroke, inflammation, bacterial and viral meningitis, for example) and it has been postulated that S100B could serve as a serum marker for brain damage.
It has been shown that in epilepsy, the concentration of MMP-9 and S100B increased after tonic-clonic seizures, with the highest level occurring approximately 6 hours after the onset of the seizure, and the increase persisted for up to 24 hours [
3,
4,
5,
8,
9,
10]. There it is possible that the release of some biomarkers into the serum after seizures was caused by muscle contraction rather than originating from the cerebrovasculature.
This study aimed to check the changes in metallopeptidase 9 (MMP-9) and S100B protein levels after simulated physical effort in healthy subjects. The physical activity planned for the study had similar characteristics to muscle activity (type of muscle contractions) in bilateral tonic-clonic seizures. The purpose of the study was to evaluate if similar effort in the healthy subjects’ group would result in a change of MMP-9 and S100B levels in venous blood samples.
2. Results
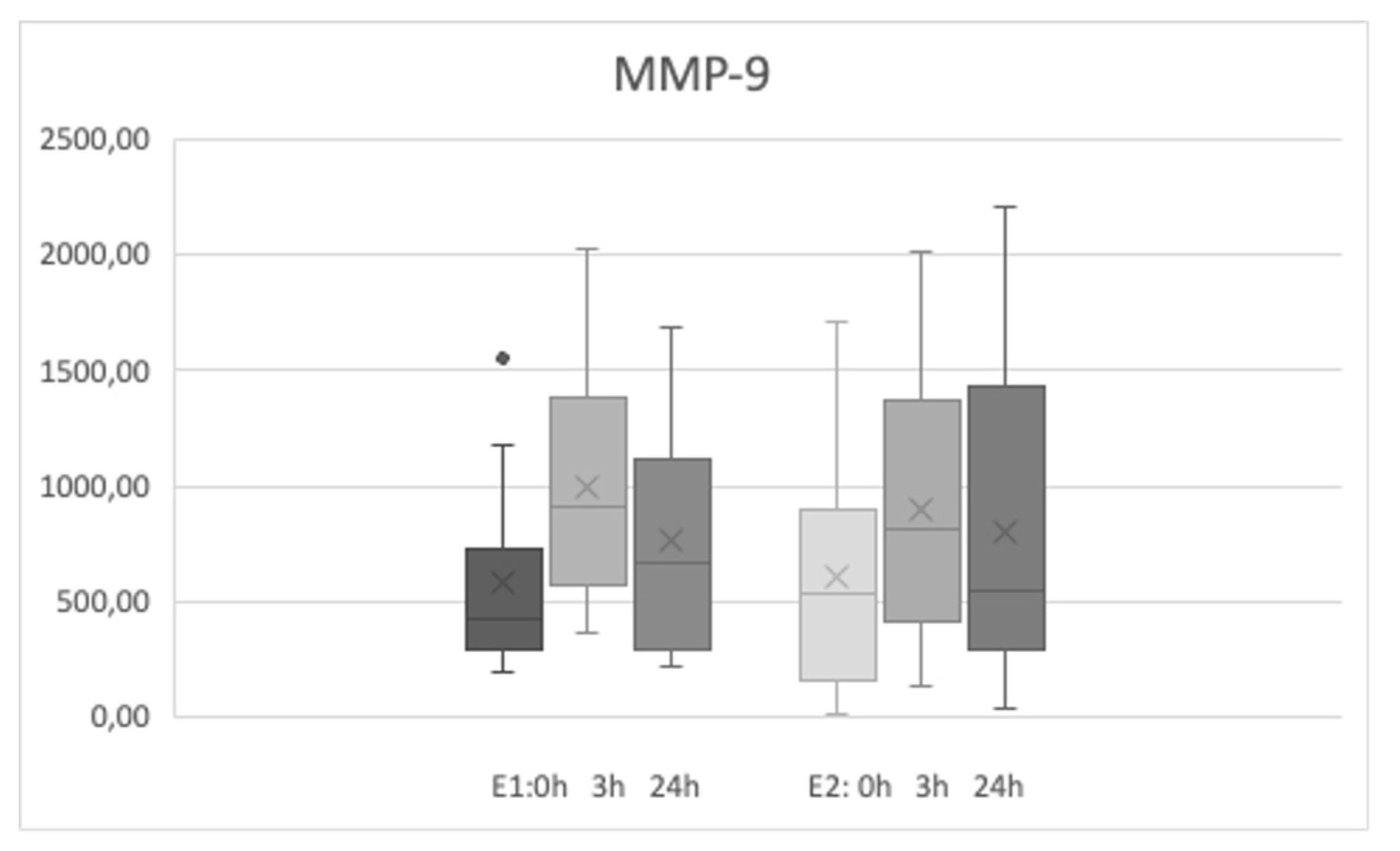
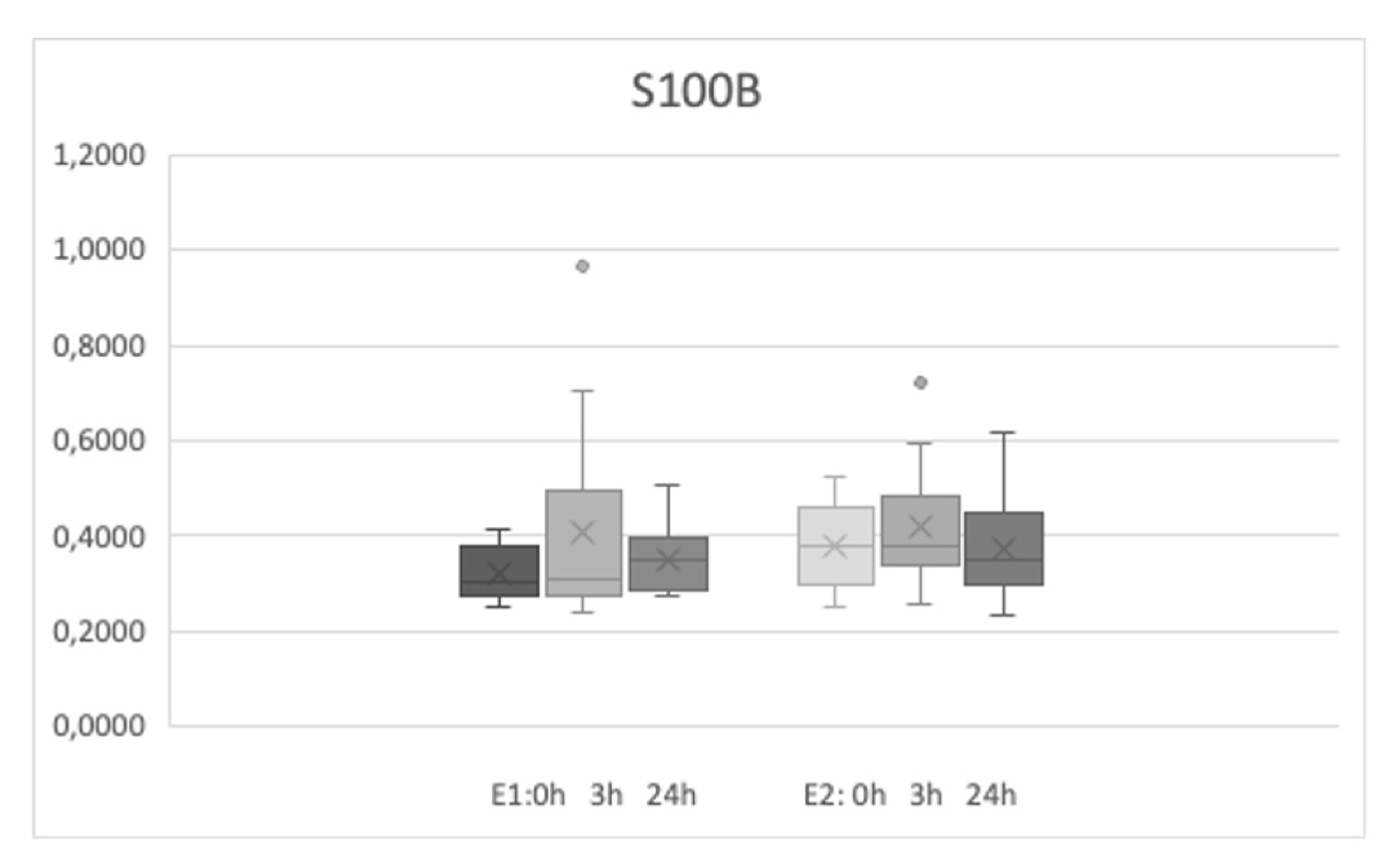
Sixteen participants (4 men and 12 women) took part in the study. The mean age was 32.37±9.23 years, and the range of age was from 22 to 55 years old. Both sets of exercises (E1 and E2) were performed for all the group of participants approximately 4 months apart from each other. The basic levels of MMP-9 were 583.45±385.26 ng/ml and 603.33±478 ng/ml before first and second physical efforts respectively (no statistical difference was noted). The basic levels of S100B were 32.28 ±5.35 and 38.06±9.05 pg/ml before the second effort (E2) and both values were slightly different (p<0.025).
The level of MMP-9 increased significantly after the E1 and E2 sets of exercises and was higher 3 and 24 hours after the efforts (
Table 1,
Figure 1). S100B levels did not increase after the efforts regardless the type of exercise. The levels of MMP-9 during both E1 and E2 physical efforts did not differ at the same time points - the increases after 3 hours and after 24 hours after exercises were comparable for both exercise type (p2 from
Table 1 describes the comparison of E1 and E2 biomarkers levels at the same time points). The level of S100B were slightly different only on the basic level (time points - 0h) between E1 and E2 measurements.
The groups were not numerous, and no correlation of sex or age were noted. The levels of MMP-9 were associated, and higher basic level of MMP-9 were strongly correlated with higher level 3 and 24 hours after effort in both E1 an E2 exercise sets. S100B level did not correlate with MMP-9 at any time point.
3. Discussion
Our study showed that short but intense exercise increases serum MMP-9 levels, which persist for 24 hours. Serum S100B levels did not change. These observations clearly demonstrated that serum MMP-9 levels are sensitive to physical activity and it should be taken into account when evaluating pathological conditions, especially associated with physical activity such as motor seizures.
Current evidence supports the effect of intense exercise on MMP-9 serum levels [
14,
15,
16], although some studies report no or only a slight effect [
15,
16]. Factors such as the type and duration of exercise, muscle and tissue damage, and underlying health conditions may influence these outcomes [
17,
18]. Short-term exercise tends to increase MMP-9 levels, whereas prolonged training may decrease baseline serum levels of MMP-9, observed in both healthy volunteers and athletes [
14,
15,
16,
19]. In individuals with chronic conditions that elevate MMP-9, physical activity might reduce its levels [
17,
18,
20].
In epilepsy, MMP-9 level is higher compared to age and sex- matched control group [
3] and also increases after tonic-clonic seizures reaching the level about 2 times higher than age matched control [
4,
5]. Epileptic seizures are suggested to induce inflammation and blood-brain barrier leakage which may be the cause of the increase in MMP-9 and S100B, along with other biomarkers in the serum [
10]. However, some biomarkers elevation may be caused by muscle contraction. Our study aimed to develop exercises that mimic bilateral tonic-clonic seizures and confirmed that these exercises are responsible for an increase in MMP-9 but not in S100B. Thus, S100B is a more reliable biomarker for epilepsy with motor seizures than MMP-9.
The mechanism that causes the increase in MMP-9 concentration in serum after exercise could be related to several factors. Matrix metalloproteinase-9 (MMP-9) is an enzyme involved in the breakdown of extracellular matrix proteins, playing a role in tissue remodeling and repair. Physical exercises, especially intense or prolonged activities, can induce inflammation and tissue damage. MMP-9 is produced and released by various cells, including neutrophils and macrophages. Physical exercise can stimulate the mobilization and activation of these cells, leading to increased MMP-9 release. To support this hypothesis Reihmane et al. [
21] conducted a study among professional male hockey players. The effort led participants to maximum fatigue and the highest possible level of oxygen uptake. Authors showed that short 2 minutes exercise induced leucocytosis and increased IL-6, myeloperoxidase (MPO) and MMP-9 concentrations. Since increased MPO (marker of neutrophils degranulation) correlated with increase of IL-6 and MMP-9 concentrations, neutrophils could be the main source of these inflammatory proteins during maximal effort exercise. Given that creatine kinase activity did not change, it was suggested that the increase was not due to physiological damage to muscle fibers and connective tissue caused by physical exercise [
11]. In our study we also did not show differences in MMP-9 levels depending on the type of exercise and whether or not muscle tissue was damaged. Both types of exercise resulted in similar increases in MMP-9, while one of the motor activities (E2) caused muscle failure and other (E1) did not. Other authors also showed that MMP-9 level increased after exercise but did not depend on the extent of tis-sue damage and the rate of recovery [
22].
Another study of Reihmane’s [
14] showed that MMP-9 level depended on the duration of high-intensity physical activity. They compared MMP-9 levels after long-distance running. MMP-9 levels were significantly higher after running a marathon than a half-marathon and dropped after next 28 hours to the basic level.
On the other hand, various types of systematic training reduce MMP-9 level. It is very well known that regular physical exercise contributes to reducing inflammation through multiple pathways, including the reduction of adipose tissue, improved insulin sensitivity, increased production of anti-inflammatory cytokines, enhanced immune regulation, reduced oxidative stress, and regulation of stress hormones. This mechanism may lead to MMP-9 decrease after long training both in healthy people and in patients with inflammatory states such as diabetes [
17,
18] or cancer [
20] and may be responsible for good effect of training on the outcome of these diseases.
S100B has also been studied as one of the markers during sports activity. The S100B protein is mainly related to damage to the central nervous system since it is expressed in astrocytes and certain neuronal populations (however also in many other cells, for example Schwann cells, melanocytes, chondrocytes, adipocytes, skeletal myofibers, and lymphocytes) [
23,
24]. The transient increase of S100B was shown after intensive aerobic efforts such as a long-distance swimming race [
25] and also after high-intensity intermittent exercise or moderate continuous exercise on bicycle ergometer [
26]. A swimming race over a distance of 7,600 meters and exercises on bicycle ergometers were primarily aerobic efforts, where muscle fibers are not intensely stimulated in a short time. The mechanism behind the increase in S100B levels in serum was likely different from that of nervous system damage. It is possible that intense blood flow through the cerebral vascular bed may cause the release of S100B. Similarly, Reichel et al. [
27] used endurance training to assess concentrations of S100B, MMP-9, and various other markers. Their protocol involved assessing maximal oxygen consumption (VO2 max) on a treadmill. Both MMP-9 and S100B levels increased after exercise.
In our study, relatively short and highly anaerobic stimulation with strength-type exercise did not result in an increase in S100B. It is possible that the characteristics of muscle work used in the exercise may be the cause of different results. Cardiorespiratory exercises may induce an increase in S100B, unlike the type of exercise used in our study. On the other hand, the time point of blood collection may also be the cause, because in every above mentioned studies, blood was collected just after the exercise (minutes) [
23,
27] and the S100B level might return to the initial level very quickly. In one of the study, blood taken one hour after effort did not shown any significant differences [
25]. Briefly elevated S100B levels following intense exercise may not necessarily indicate pathological brain damage but rather a temporary physiological response to exercise stress. On the other hand, numerous studies have shown a correlation between S100B levels and various types of concussion [
28]. Similarly to MMP-9, the S100B protein level decrease after prolonged, systematic training process [
24].
In epilepsy studies of blood-brain barrier biomarkers, both MMP-9 and S100B levels were increased for a longer period after seizures. We showed elevated MMP-9 and S100B levels in serum, 1 hour, 24 and 72 hours after seizures [
4,
8]. Nass et al. [
5] also showed MMP-9 increased for 6 hours and S100B only just after seizures. These observations indicate that levels of S100B might be better indicator of seizure than MMP-9 which are more dependent on motor activity during tonic-clonic muscle contractions.
4. Methods
4.1. Participants
The study was approved by the Committee for Ethics in Human Research at the Institute of Psychiatry and Neurology (Warsaw, Poland) and was performed in the 2nd Department of Neurology, Institute of Psychiatry and Neurology in Warsaw.
The participants were recruited among healthy volunteers. The participants were examined to exclude contraindications to intense physical exercise. Two types of activity were designed to be similar to the muscle work seen in patients during tonic-clonic seizures. The first was intended to induce delayed onset muscle soreness, such as those experienced by epileptic patients and the second activity was invented to be more similar to the intensity of exertion found in epileptic seizures.
The following inclusion criteria were used in the study group: age over 18 and signed written informed consent.
The exclusion criteria were as follows: active infection, any neurological disorder, immunosuppressive or immunomodulatory treatment in the last six months, surgery or significant trauma within the last two months, hepatic or renal insufficiency, pregnancy, clinical and laboratory symptoms of infection, muscle tear in the last four weeks, bone fractures, severe cardiorespiratory disease, active strength training, active delayed onset muscle soreness, or a high-intensity training session in the last week.
The first physical effort (E1) involved maintaining a plank position for up to one minute, followed by tapping the lower limbs in a fast rhythm for 5 minutes while staying in the push-up position throughout the second phase. For test subjects who were unable to sustain continuous effort, an alternative was provided: changing the position during the second phase to a back support position with increased hip flexion. Tapping intensity and frequency remained the same in the easier option.
The second effort (E2) focused more on replicating the muscle contraction conditions observed during seizures. Healthy subjects were asked to perform a wall sit position until muscular failure (minimum of one minute) and were verbally motivated to achieve maximum possible motor unit recruitment, approaching the intensity of MVC (maximum voluntary contraction). Immediately after the inability to maintain the isometric position, subjects were instructed to perform bodyweight squats for a minimum of 5 minutes or until muscle failure.
Both sets of exercises were performed by the same group of participants with a 4-month interval. Additionally, each participant was asked to avoid intensive exercise 24 hours before the test and until the last blood draw.
4.2. Blood Sampling
Venous blood samples were taken from each subject three times:: before activity on the fasting state in the morning, 3 hours and 24 hours after the exercises. Venous whole blood samples (10 mL) were immediately centrifuged, and serum was collected and frozen at -80 °C. All participants had their blood examined for blood count and CRP with standard laboratory tests.
4.3. Biomarkers Evaluation
Serum level of MMP-9 and S100B were measured using sandwich-type ELISA in accordance with the manufacturer’s instructions. For MMP-9 we used kit from R&D Systems, (Minneapolis, MN, USA), and for S100B from Merck Millipore (Darmstadt, Germany). The absorbance at 450 nm was measured using a Multiscan Go spectrophotometre (Thermo Scientific, Waltham, MA, USA). The protein concentrations were calculated according to the manufacturer’s instructions.
4.4. Statistical Analysis
Statistica for Windows (version 13.3) was used to analyze data and compare groups. Results were considered significant when p values were less than 0.05 (p < 0.05). One-way analysis of variance was used to analyze data including participants' characteristic and biomarker concentration levels. The Newman-Keuls test was used for individual post hoc comparisons. Student's t-test was used to compare means between groups. The Pearson correlation test was used to assess the correlation between age and biomarker levels at different time points.
5. Conclusions
In our study, we demonstrated that single, short, several-minute physical exercises mimicking those occurring during a motor epileptic seizure—engaging many muscle groups simultaneously, as well as exercises engaging only selected muscle groups of the lower limbs—resulted in an increase in MMP-9 but not in S100B serum levels 3 hours after the effort. Additionally, the levels of biomarkers were similar, independently of effort type. Our study supports observations that MMP-9 is secreted during various forms of exercise, and is independent from the muscle injury.
The increase in MMP-9, but not S100B, following physical activity differs from the pattern observed after bilateral motor seizures [
4,
5,
8]. This suggests that additional mechanisms are responsible for the elevation of these biomarkers during seizures. While part of the MMP-9 increase may be attributed to physical exertion, the elevation of both MMP-9 and S100B is more closely associated with seizure activity and potential disruption of the blood-brain barrier.
S100B may change in a very short time after physical efforts [
25,
26,
27,
28] but was not detectable 3 and 24 hours after physical effort in our study. It might be a good biomarker for distinguishing epileptic seizures from other factors that may influence its secretions, but it needs more research to describe these relationships more precisely. On the other hand, a prolonged increase in S100B following physical activity (if detected) and after seizures may indicate blood-brain barrier leakage and potential brain damage [
28]. Thus, the "old" biomarker S100B might eventually serve as a marker for brain injury and a useful indicator of seizures.
Author Contributions
Conceptualization, I.K-J., and M.K. and J.S. and A.C.; methodology, I.K-J., and M.K. and J.S. and A.C.; software, A.C and I.K.-J.; validation, A.C..; formal analysis, I.K-J., and M.K. and J.S. .; investigation, I.K.-J. ; data curation, J.S. and A.C.; writing—original draft preparation, J.S. and I.K.-J. and M.K. and A.C.; writing—review and editing, I.K-J., and M.K. and J.S . ; visualization, I.K.-J.; supervision, I.K.-J.; project administration, I.K.-J..; All authors have read and agreed to the published version of the manuscript.”.
Funding
The analyses performed for this study were financed by Institute of Psychiatry and Neurology within statutory activity. No additional external funding and support was received for this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Institute of Psychiatry and Neurology in Warsaw, Poland.
Informed Consent Statement
All participants signed a written informed consent form before participating in the study.
Data Availability Statement
Data are available from the author of the publication on request ; email: jsielczak@ipin.edu.pl.
Acknowledgments
The authors are grateful to all colleagues from the neurology clinic team who took part in the study and performed the required demanding physical activity.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia. 2018, 59, 2179–2193. [Google Scholar] [CrossRef] [PubMed]
- Bronisz, E. , Cudna, A., Wierzbicka, A., & Kurkowska-Jastrzębska, I. (). Blood-Brain Barrier-Associated Proteins Are Elevated in Serum of Epilepsy Patients. Cells 2023, 12, 368. [Google Scholar] [PubMed]
- Cudna A, Jopowicz A, Mierzejewski P, Kurkowska-Jastrzębska I. Serum metalloproteinase 9 levels increase after generalized tonic-clonic seizures. Epilepsy Res. 2017, 129, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Nass RD, M. Wagner, R. Surges, S. Holdenrieder Time courses of HMGB1 and other inflammatory markers after generalized convulsive seizures. Epilepsy Res. 2020, 162, 106301. [Google Scholar] [CrossRef]
- Fabene, P.F.; Mora, G.N.; Martinello, M.; Rossi, B.; Ottoboni, L.; Bach, S.; Angiari, S.; Benati, D.; Zanetti, L.; Schio, F.; et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 2008, 14, 1377–1383. [Google Scholar] [CrossRef]
- Li, S.; Yu, S.; Zhang, C.; Shu, H.; Liu, S.; An, N.; Yang, M.; Yin, Q.; Yang, H. Increased expression of matrix metalloproteinase 9 in cortical lesions from patients with focal cortical dysplasia type IIb and tuberous sclerosis complex. Brain Res. 2012, 1453, 46–55. [Google Scholar] [CrossRef]
- Cudna, A. , Bronisz, E., Jopowicz, A., & Kurkowska-Jastrzębska, I. Changes in serum blood-brain barrier markers after bilateral tonic-clonic seizures. Seizure.
- Bronisz E; Cudna, A. ; Wierzbicka, A.; Kurkowska-Jastrz˛ebska, I. Serum Proteins Associated with Blood–Brain Barrier as Potential Biomarkers for Seizure Prediction. Int. J. Mol. Sci. 2022, 23, 14712. [Google Scholar] [CrossRef]
- Reihmane D, Jurka A, Tretjakovs P, Dela F. Increase in IL-6, TNF-α, and MMP-9, but not sICAM-1, concentrations depends on exercise duration. Eur J Appl Physiol. 2013, 113, 851–858. [Google Scholar] [CrossRef]
- Zhong C, Yang J, Xu T, Xu T, Peng Y, Wang A, Wang J, Peng H, Li Q, Ju Z, Geng D, Zhang Y, He J; CATIS Investigators. Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology. 2017, 89, 805–812. [Google Scholar] [CrossRef]
- Vafadari B, Salamian A, Kaczmarek L. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem. 2016, 139 (Suppl 2), 91–114. [Google Scholar] [CrossRef] [PubMed]
- Michetti F, Clementi ME, Di Liddo R, Valeriani F, Ria F, Rende M, Di Sante G, Romano Spica V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int J Mol Sci. 2023, 24, 9605. [Google Scholar] [CrossRef] [PubMed]
- Reihmane D, Jurka A, Tretjakovs P, Dela F. Increase in IL-6, TNF-α, and MMP-9, but not sICAM-1, concentrations depends on exercise duration. Eur J Appl Physiol. 2013, 113, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Koskinen SO, Höyhtyä M, Turpeenniemi-Hujanen T, Martikkala V, Mäkinen TT, Oksa J, Rintamäki H, Löfberg M, Somer H, Takala TE. Serum concentrations of collagen degrading enzymes and their inhibitors after downhill running. Scand J Med Sci Sports. 2001, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Rullman E, Olsson K, Wågsäter D, Gustafsson T. Circulating MMP-9 during exercise in humans. Eur J Appl Physiol. 2013, 113, 1249–1255. [Google Scholar] [CrossRef]
- Lucotti P, Monti LD, Setola E, Galluccio E, Gatti R, Bosi E, Piatti P. Aerobic and resistance training effects compared to aerobic training alone in obese type2 diabetic patients on diet treatment. Diabetes Res Clin Pract. 2011, 94, 395–403. [Google Scholar] [CrossRef]
- Kadoglou NP, Vrabas IS, Sailer N, Kapelouzou A, Fotiadis G, Noussios G, Karayannacos PE, Angelopoulou A. Exercise ameliorates serum MMP-9 and TIMP-2 levels in patients with type 2 diabetes. Diabetes Metab. 2010, 36, 144–151. [Google Scholar] [CrossRef]
- Urso ML, Pierce JR, Alemany JA, Harman EA, Nindl BC. Effects of exercise training on the matrix metalloprotease response to acute exercise. Eur J Appl Physiol. 2009, 106, 655–663. [Google Scholar] [CrossRef]
- Giganti MG, Tresoldi I, Sorge R, Melchiorri G, Triossi T, Masuelli L, Lido P, Albonici L, Foti C, Modesti A, Bei R. Physical exercise modulates the level of serum MMP-2 and MMP-9 in patients with breast cancer. Oncol Lett. 2016, 12, 2119–2126. [Google Scholar] [CrossRef]
- Reihmane D, Jurka A, Tretjakovs P. The relationship between maximal exercise-induced increases in serum IL-6, MPO and MMP-9 concentrations. Scand J Immunol. 2012, 76, 188–192. [Google Scholar] [CrossRef]
- Kim J, Lee J. Plasma MMP-9, TIMP-1, and TGF-β1 Responses to Exercise-Induced Muscle Injury. Int J Environ Res Public Health. 2020, 17, 566. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A. P. , Moes, K. A., Shiu, M. Y., Hutchison, M. G., Churchill, N., Thomas, S. G., & Rhind, S. G. High-intensity interval training is associated with alterations in blood biomarkers related to brain injury. Frontiers in physiology 2018, 9, 1367. [Google Scholar]
- Bessa, A. L. , Oliveira, V. N., Agostini, G. G., Oliveira, R. J., Oliveira, A. C., White, G. E et al.Exercise intensity and recovery: biomarkers of injury, inflammation, and oxidative stress. The Journal of Strength & Conditioning Research 2016, 30, 311–319. [Google Scholar]
- Dietrich MO, Tort AB, Schaf DV, Farina M, Gonçalves CA, Souza DO, Portela LV. Increase in serum S100B protein level after a swimming race. Can J Appl Physiol. 2003, 28, 710–716.
- Buzdagli Y, Ozan M, Baygutalp N, Oget F, Karayigit R, Yuce N, Kan E, Baygutalp F, Ucar H, Buzdağlı Y. The effect of high-intensity intermittent and moderate-intensity continuous exercises on neurobiological markers and cognitive performance. BMC Sports Sci Med Rehabil. 2024, 16, 39. [Google Scholar]
- Reichel T, Held S, Schwarz A, Hacker S, Wesemann F, Donath L, Krüger K. Acute response of biomarkers in plasma from capillary blood after a strenuous endurance exercise bout. Eur J Appl Physiol. 2023, 123, 179–189. [Google Scholar] [CrossRef]
- Kiechle, K. , Bazarian, J. J., Merchant-Borna, K., Stoecklein, V., Rozen, E., Blyth, B.,... & Biberthaler, P. (). Subject-specific increases in serum S-100B distinguish sports-related concussion from sports-related exertion. PloS one 2014, 9, e84977. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).