1. Introduction
The human microbiota represents a very diverse microbial community that inhabits most if not all, body sites; it plays a fundamental role in maintaining health and influencing disease processes. Comprised of trillions of bacteria, viruses, fungi, and archaea, it participates in basic physiological processes from metabolism and immune modulation to maintaining homeostasis [
1,
2]. Especially, the gut microbiota has become widely studied for elaborating such a complex interaction with its host that it will have implications for gastrointestinal health and extend into systemic processes, including the brain [
3].
Accumulating evidence has illustrated the role of the gut-brain axis (GBA), a bidirectional communicatory network between the gut microbiota and brain function [
3,
4]. The ability of microbiota to influence neuroinflammation, cognitive function, and emotional well-being was mediated through several means, namely metabolic production, immune modulation, and alteration of the gut barrier [
5,
6]. Currently, it is now recognised that dysbiosis, or imbalance of microbial composition, has been implicated in a wide range of clinical neuropsychiatric conditions such as depression, anxiety, and even autism spectrum disorders [
7,
8].
With the increased interest in microbiota and their involvement in neurological health, it has become very timely to research its relevance to cerebral small vessel disease (CSVD). CSVD is a prevalent cerebrovascular disorder with changes in the small, penetrating arteries supplying blood to the brain. It is associated with several adverse outcomes such as cognitive impairment, vascular dementia, and stroke [
9]. Several recent publications have shown that chronic inflammation and endothelial dysfunction, the hallmarks of CSVD, are both modulated by the gut microbiota, making it a new avenue to reconsider the pathophysiology [
10,
11].
Recently, another critical player that helps to clear metabolic waste from the brain through a network has come into play: the glymphatic system [
12]. The impaired glymphatic function (or glymphopathy) might be linked to various neurodegenerative diseases and cognitive decline [
13,
14]. Of note, recent findings suggest that the microbiota may modulate this very process of glymphatic activity indirectly through systemic inflammation and circadian rhythm regulation, further supporting a complex association between gut health and brain function [
15,
16,
17].
Moreover, circulating cell-derived microparticles (MPs), or extracellular vesicles, are small membrane-bound particles released from various cell types during their activation or apoptosis [
18]. More recently, they have gained significant interest due to their mediation role in intercellular communication and inflammation [
18]. In the context of CSVD, MPs may thus facilitate the transfer of pro-inflammatory signals from the gut to the brain, thereby potentiating neuroinflammation and endothelial dysfunction [
19,
20]. Furthermore, there is evidence that gut dysbiosis may be associated with the increased release of MPs, thus directly linking gut health and cerebrovascular pathology [
21,
22,
23].
Thus, in this narrative review, we hope to discuss the complex relationship between human microbiota and neurological health with an emphasis on cerebral small vessel disease and the glymphatic system. By integrating current literature on microbial dynamics, neuroinflammation, and vascular health, we aspire to explain a potential therapeutic avenue through which one can leverage the microbiota to enhance brain health and diminish the impact of cerebral small vessel disease.
2. The Human Microbiota: An Overview
Human microbiota is the collective community of microorganisms, including bacteria, archaea, viruses, fungi, and protozoa, inhabiting different body sites, such as the gut, oral cavity, skin, and other mucosa. Among these, the gut microbiota is one of the most studied; thus, trillions of microorganisms bear important functions related to digestion, metabolic activity, and immune response [
1,
2]. Each human has his or her microbiota signature, shaped by unique individual genetic makeup, environmental exposures, and lifestyle behaviours [
24,
25].
Moreover, the composition of the microbiota varies significantly among different anatomical sites. According to research from the Human Microbiome Project (HMP) [
26] and the Metagenomics of the Human Intestinal Tract (MetaHIT) consortium [
27], the human gut is home to 2766 microbial species. Bacteria from the phyla Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes account for more than 90% of the gut microbiome [
27,
28]. The bulk of gut bacteria are Firmicutes, which include Gram-positive
Lactobacillus species and Gram-negative
Bacteroides species [
29].
Fusobacteria species and
Verrucomicrobia species account for the remaining 10% of the gut microbiome [
30]. In turn, the skin microbiota is dominated by Actinobacteria, especially
Propionibacterium and
Corynebacterium species [
31]. This diversity and abundance are critical for their functional capacity and resilience against perturbations.
Several factors affect the composition and diversity of human microbiota: geographical location, hygiene practices, and exposure to antibiotics capable of tremendous changes in microbial populations are among the leading ones. According to Ridaura et al. [
32], such factors take leading positions among others. Genetic predisposition may be taken into consideration as well, whereby genetic factors can influence the establishment and maintenance of gut microbiota [
33,
34]. Moreover, the dietary pattern is among the critical determinants of microbiota composition. Whereby diets high in fibre, for example, allow for the proliferation of beneficial microbes that can produce short-chain fatty acids (SCFAs) contributing to colonic health and regulation of the immune system [
35]. Finally, lifestyle factors such as physical activity and stress will also modulate the microbiota to promote health or disease [
36].
Furthermore, the human microbiota not only allows for homeostasis and regulates the immune system of the host, but they also help in the digestion of complex carbohydrates and synthesize essential nutrients such as vitamins B and K [
37]. Besides, the microbiota is crucial for the normal development of the immune system and functioning. It helps the immune system differentiate between injurious pathogens and benign ones, hence avoiding inappropriate inflammatory responses [
2,
11,
21]. However, dysbiosis, characterised by loss of microbial diversity and imbalance of microbial populations, has been linked to various diseases that include autoimmune disorders, allergies, and metabolic syndrome [
5,
38].
Hence, it is worth noting that through various signalling pathways, the microbiota communicates with host cells and modulates inflammation in both systemic and immune responses. Metabolite production, which encompasses SCFAs, can do more than just give energy sources to colonocytes, as they exert anti-inflammatory actions that are believed to contribute toward maintaining gut and systemic health [
38]. In brief, the human microbiota represents an elaborated and dynamic ecosystem playing a core role in maintaining health, regulating the immune system, and preventing disease. The factors which shape the composition and function of microbiota are of exceptional importance to be understood to develop strategies that will lead to promoting health and mitigating disease. These complex interactions point to the necessity of studying the interrelations of the microbiota with neurological health first and foremost through the GBA.
3. Microbiota and the Brain: The Gut-Brain Axis (GBA)
The GBA is a term for the bidirectional communication network between gut microbiota and the central nervous system (CNS). According to Cryan et al. [
3], this bidirectional interaction is of utmost importance in maintaining homeostasis and modulates other physiological processes, including emotional regulation, cognition, and immune response. The GBA includes neural pathways, endocrine, and immune systems – what a great influence that microbiota has far beyond the borders of the gastrointestinal tract.
The are multiple mechanisms that help in the communication within the GBA. These are mainly through microbial metabolites, especially SCFAs derived from the fermentation of dietary fibres by gut bacteria. The most common forms of SCFAs, such as acetate, propionate, and butyrate, have been demonstrated to cross the blood-brain barrier (BBB) and affect brain function in influencing neuroinflammation, neurotransmission, and neurogenesis [
39]. Moreover, some gut bacteria are capable of modulating the synthesis and functioning of neurotransmitters such as gamma-aminobutyric acid (GABA) and serotonin, which directly influence mood and behaviour [
40,
41].
On the other hand, the immune system is the other crucial player in the GBA. The gut microbiota plays a very important role in the development and regulation of the immune system, influencing systemic inflammation and the integrity of the BBB [
5,
6,
39]. It has been demonstrated that dysbiosis increases intestinal permeability, leading to systemic inflammation, which may contribute to neuroinflammatory disorders such as depression and anxiety [
4,
7,
41]. For example, some intestinal bacteria can induce the production of cytokines, interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α), which may easily cross the BBB and negatively affect neuroinflammation processes [
42].
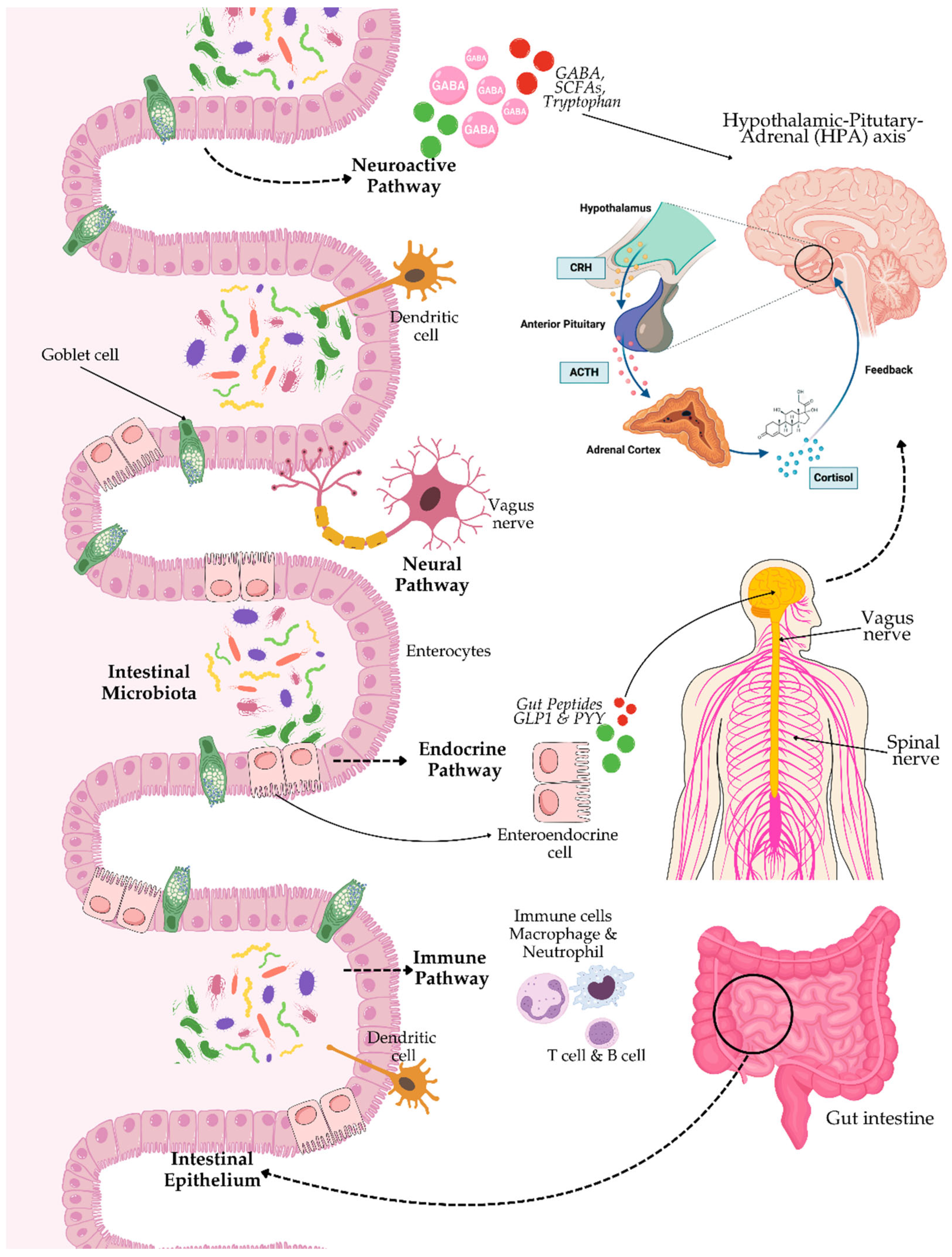
Additionally, the neural pathway is another critical GBA communication factor, which involves the enteric nervous system (ENS), vagus nerve, and spinal nerves. The ENS has even been known as the "second brain", with thousands of neurons coating the wall of the gut, and capable of regulating gastrointestinal activities independently [
43,
44]. Besides, the vagus nerve is one of the primary conduits between the gut and the brain. The vagus nerve mediates signals from the gastrointestinal tract to the brain, consequently playing a role in the regulation of autonomic responses and the response to stress in the organism [
45,
46]. Apart from that, the endocrine pathway also plays an important role in the GBA, particularly through the hypothalamic-pituitary-adrenal (HPA) axis (see
Figure 1). The HPA axis is excited by stress, whereby neurons of the hypothalamus release corticotropin-releasing hormone (CRH) into the portal circulation, or directly into the brain. This release starts a chain reaction with the synthesis of adrenocorticotropic hormone (ACTH) from the pituitary and subsequent cortisol production and release from the adrenals [
47] (see
Figure 1). Cortisol, as a major stress hormone, represents a modulatory hormone of neuroimmune signalling reactions in as much as it can modulate immune responses and neuronal activity. Overall, these very elaborately developed communication pathways make the interaction between gut microbiota and the brain quite complex, as microbial signalling can overwhelm neurobiology and behaviour.
The influence of dysbiosis is thus profound in brain health. Emergent research alludes to the fact that an imbalance of gut microbiota can exacerbate neurodegenerative diseases, affect cognitive functions, and influence mood disorders. For example, such studies have identified that individuals with depression show altered gut microbiota profiles compared to healthy controls, thus pointing to an association between microbial composition and mood regulation [
48]. Besides that, animal models of neurodegenerative diseases have shown that dysbiosis might exacerbate neuroinflammation and accelerate cognitive decline, indicating the importance of microbial health in maintaining brain function [
49].
Moreover, interventions aimed at restoring a microbial balance, for example, through the application of probiotics and dietary modification also taken the front seat in helping improve mental health outcomes. In some instances, clinical trials have suggested that certain types of probiotics might reduce symptoms of anxiety and depression, perhaps through changes within the GBA itself [
50]. Essentially, the GBA is a very dynamic process between the gut microbiota and the CNS, involved in almost every physiological and psychological process. Understanding how this relationship works will be important to inform new therapeutic approaches that promote brain health and mitigate the challenges posed by dysbiosis. Therefore, with the wide effects of gut microbiota in the brain, it's so important to look into their possible roles in certain neurological conditions, such as CSVD, due to dysbiosis acting through microbial interaction contributing to neuroinflammation and vascular pathology.
4. Cerebral Small Vessel Disease and Microbiota
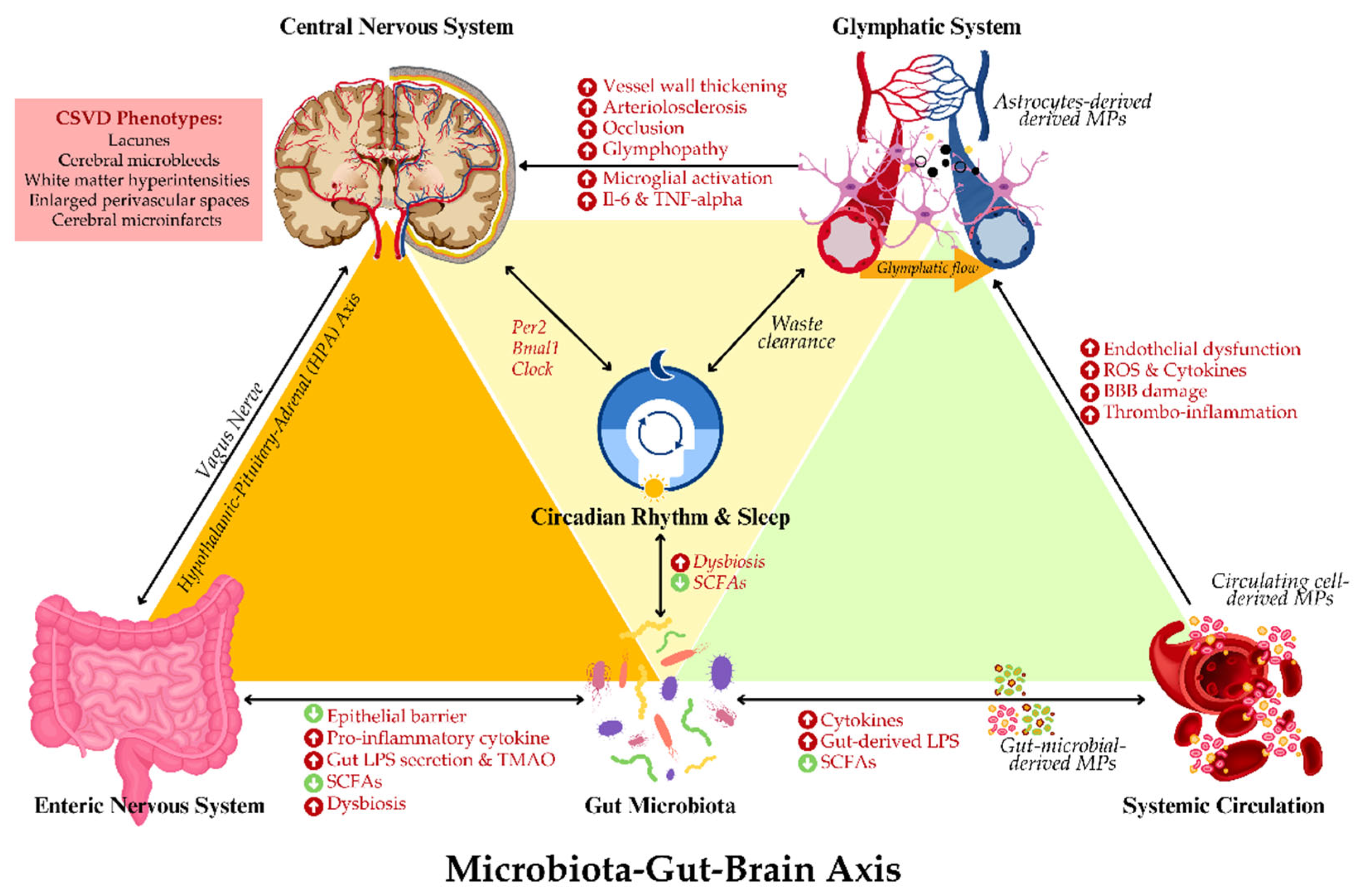
Cerebral small vessel disease (CSVD) is considered the leading cause of stroke and dementia, responsible for approximately 25% of ischemic strokes, and a large proportion of vascular dementia [
51]. CSVD involves small arteries, arterioles, capillaries, and brain venules, causing various pathological changes including cerebral microbleeds, white matter hyperintensities (WMHs), lacunes, and cerebral microinfarcts [
52]. These structural abnormalities often lead to clinical manifestations encompassing the impairment of cognition, disturbances in gait, and mood disorders, leading to diminished quality of life in the elderly [
53].
Multiple overlapping factors make the underlying pathophysiology of CSVD multifactorial, which includes chronic endothelial dysfunction, disturbed cerebral autoregulation, thrombo-inflammation, oxidative stress, and disruption of the BBB [
54]. It is considered that damage to the endothelium starts a cascade of pathological events that include increased permeability of the BBB, leakage of plasma proteins, and perivascular inflammation [
55]. Moreover, chronic low-grade inflammation contributes to vascular stiffness, and impairment in cerebral microcirculation leading to subsequent ischemia, white matter degeneration, and ultimately cognitive impairment [
56,
57]. Furthermore, oxidative stress, through the overproduction of reactive oxygen species (ROS), exacerbates endothelial injury and promotes neurodegenerative processes that further deteriorate CSVD outcomes [
56,
57].
Potential Links Between Gut Microbiota and CSVD
Recent evidence points to the potential involvement of gut microbiota dysbiosis, or imbalance in microbial homeostasis, in CSVD pathogenesis. Gut dysbiosis is characterized by increased intestinal permeability, enabling microbial endotoxins, such as lipopolysaccharides (LPS), to translocate into the systemic circulation. These endotoxins induce systemic inflammation, which is capable of crossing the BBB, thereby contributing to neuroinflammation-a key factor in the development of CSVD [
3].
There is also gut dysbiosis-related inflammation that contributes to the development of endothelial dysfunction and impairs the integrity of the cerebrovasculature. Systemic high levels of pro-inflammatory cytokines, for instance, TNF-α and IL-6 predispose one toward vascular stiffness, defective vasodilation, and increased vascular resistance, contributing to the small vessel injury characteristic of CSVD [
58]. Besides, gut-derived metabolites, such as trimethylamine N-oxide (TMAO), have been associated with increased atherosclerotic burden and vascular inflammation, thus perhaps exacerbating cerebrovascular damage in CSVD [
59].
Furthermore, gut dysbiosis also influences another significant factor in CSVD such as oxidative stress. The imbalance in the microbiome can facilitate the excessive generation of ROS, which then acts on the oxidative destruction of cerebral vasculature, impairs the regulation of cerebral blood flow, and increases white matter damage [
56,
57]. Additionally, some gut-derived metabolites, including SCFAs, especially butyrate, exert protective functions against disturbance in endothelial function or neuroinflammation [
36,
39].
Figure 2 illustrates the putative concept of microbiota-GBA concerning CSVD. Thus, it is assumed that modulation of gut microbiota might be a promising therapeutic strategy for preventing or minimizing features of CSVD.
The accumulating evidence has pointed out that the gut microbiota influences not only the vasculature per se but also the brain's waste clearance system i.e., the glymphatic system. The glymphatic system plays an important role in metabolic waste removal, including amyloid beta (Aβ), from the brain, processes in which disturbances have been recorded in both CSVD and neurodegenerative diseases [
12]. Disruptions in the gut microbiota have been associated with impaired glymphatic function or glymphopathy, which may further promote the accumulation of neurotoxic proteins and contribute to the cognitive decline of CSVD patients [
60].
Recent studies have suggested that the use of gut microbiota-targeting interventions, including probiotics, prebiotics, and dietary approaches, may improve cerebrovascular health. Indeed, certain strains of probiotics have been shown to dampen systemic inflammation and improve endothelial function, suggesting that targeting microbiota may represent a novel therapeutic approach in CSVD treatment and prevention [
50]. In summary, CSVD is a leading cause of stroke and cognitive impairment, increasingly being recognized to be influenced by gut microbiota, through mechanisms that include inflammation, oxidative stress, and vascular dysfunction. Understanding the GBA and its role in cerebrovascular health opens new avenues for therapeutic interventions aimed at the modulation of microbiota in the prevention or mitigation of the progression of CSVD.
5. Overview of the Glymphatic System and Its Role in Brain Waste Clearance
The glymphatic system is a specialized waste-clearing system of the brain that is responsible for removing metabolic by-products including Aβ, tau proteins, and other neurotoxic substances. It functions through perivascular channels particularly the aquaporin-4 (AQP4) water channels polarized on the astrocytic feet, which enable the movement of cerebrospinal fluid (CSF) into the brain parenchyma for exchange with interstitial fluid and subsequent clearance of waste products [
12]. This is a system that is activated during rest, especially during slow-wave non-rapid eye movement (non-REM) sleep, in which the interstitial space of the brain is expanded to improve the flow of CSF and the removal of wastes [
61].
Alarmingly, if the integrity of the cerebrovasculature is disrupted, as it is in CSVD, this glymphatic dysfunction (or glymphopathy) may further promote the accumulation of metabolic waste products in the brain [
14,
62,
63]. The vascular stiffness, endothelial damage, and impaired cerebral autoregulation accompanying CSVD may disorganize the clearance routes of the glymphatic system, allowing the accumulation of neurotoxic substances such as Aβ-associated with cognitive decline and neurodegeneration [
62]. Therefore, glymphopathy may thus be a contributing aspect of the disease process for both CSVD and associated neurodegenerative conditions, such as vascular dementia, Parkinsonism and Alzheimer's disease (AD) [
62,
63].
5.1. Glymphopathy as a Catalyst for Neuroinflammation and Endothelial Damage in CSVD
Emerging evidence points to the fact that glymphopathy makes significant contributions to neuropathology and endothelial damage, two important pathologies in CSVD. Glymphopathy results in neurotoxic protein deposition within the interstitial space of the brain. For instance, Aβ has been reported to cause chronic neuroinflammation by activating microglia into the secretion of pro-inflammatory cytokines, such as IL-6 and TNF-α, which directly injure the cerebral endothelial cells. Evidence from recent studies identifies that even a 20% reduction in the rate of glymphatic clearance, i.e., ventricular CSF clearance, can induce a statistically significant temporal increase in cerebral amyloid levels, and thus the risk of cognitive decline and neurodegeneration [
64].
Moreover, the persistence of metabolic waste in glymphatic-affected regions appears to perpetuate endothelial oxidative stress, a critical factor in vascular injury. Amyloid accumulation impairs mitochondrial function in endothelial cells, leading to excessive ROS production. Increased levels of ROS promote BBB permeability, facilitating the diffusion of inflammatory cells and cytokines into brain parenchyma and further amplifying the inflammatory milieu within CSVD [
56,
57]. Furthermore, impaired glymphatic activity compromises interstitial fluid dynamics, influencing cerebral perfusion and promoting local hypoxia [
62]. The hypoxic environment stimulates the release of vascular endothelial growth factor, which, though having a neuroprotective effect in an acute way, becomes perniciously angiogenic after chronic exposure and destabilizes endothelial integrity. A recent study further correlates glymphopathy with the significant upregulation of VEGF expression in ischemic stroke patients, further associating impaired glymphatic function with endothelial dysfunction [
65].
Finally, glymphopathy promotes a pro-inflammatory state that perpetuates vessel wall thickening, arteriolosclerosis, and vessel occlusion, the characteristic features of CSVD. Indeed, the chronic inflammatory burden from impaired glymphatic flow has been linked to an increased 15% risk for white matter hyperintensities and cognitive impairment in CSVD patients independently of comorbidities such as hypertension and diabetes [
66]. In summary, glymphopathy is one of the critical drivers of neuroinflammation and endothelial damage in CSVD, presenting new therapeutic targets aimed at improving glymphatic flow and dampening inflammatory cascades. Further studies into modulators of glymphatic efficiency may lead to leaps in management that are nothing short of quantum.
5.2. Role of Microbiota In Regulating Glymphatic Function
Recent evidence suggests that gut microbiota might influence glymphatic function through the regulation of systemic inflammation and circadian rhythm [
17,
67]. The neuroinflammatory consequences of dysbiosis-induced inflammation, especially the release of circulating LPS and pro-inflammatory cytokines, could perturb astrocytic regulation of CSF flow and promote neuroinflammation. Both factors facilitate glymphopathy [
68]. Moreover, such inflammatory cytokines weaken the integrity of the BBB and hinder the glymphatic system from efficiently clearing metabolic waste.
Besides that, gut microbiota controls the circadian rhythms of the host, which work in direct relation with the activity of the glymphatic system. The most active state of the glymphatic system occurs during sleep; disturbance in circadian rhythm regulation or sleep-wake cycles has had negative effects on glymphatic system activities [
67]. Intestinal bacteria control the circadian rhythm by producing necessary metabolites, including SCFAs and neurotransmitters like serotonin, which act to modulate sleep-wake cycles and promote clearance via the glymphatic system during sleep [
7,
68]. Dysbiosis could thus lead to disturbed sleep and, subsequently, impaired functioning of the glymphatic system, leading to the accumulation of wastes in the brain that could contribute to the lower cognitive scores seen in CSVD [
69].
5.3. The Connection Between Sleep Quality, Glymphatic Clearance, and Microbiota
The correlation between sleep quality and glymphatic function with gut microbiota composition is becoming increasingly convincing. Poor sleep quality, sleep fragmentation, or sleep deprivation may lead to impaired glymphatic clearance of neurotoxic substances determining the accumulation of Aβ and tau proteins implicated in neurodegenerative diseases and cognitive impairment [
70]. Sleep disturbances are associated with an increased risk of CSVD, thus underlining the importance of sleep and glymphatic function in maintaining cerebrovascular health [
71,
72].
In addition, gut microbiota composition also plays a role in sleep modulation [
73]. Sleep disturbance has been related to reduced diversity and enrichment of pro-inflammatory bacterial species that may foster systemic and neuroinflammation. Besides, a diverse and healthy microbiota, especially the abundance of SCFA-producing bacteria, is associated with better sleep quality and higher efficiency in glymphatic clearance [
73,
74]. These findings suggest that gut microbiota modulation-based intervention strategies may promote sleep quality and glymphatic function, possibly providing a potential therapeutic benefit in CSVD prevention and treatment.
In summary, CSVD, a significant cause of stroke and cognitive impairment, is increasingly recognised as being influenced by the GBA, with the gut microbiota playing a pivotal role in its pathogenesis. Inflammation, oxidative stress, and vascular dysfunction—key drivers of CSVD—are modulated by gut microbiota dysbiosis. Furthermore, emerging evidence highlights the critical role of the glymphatic system in brain waste clearance, which is closely linked to both sleep quality and gut microbiota composition. Understanding these intricate connections opens new therapeutic avenues targeting the gut microbiota to improve glymphatic function, cerebrovascular health, and cognitive outcomes in CSVD.
6. The Impact of Sleep and Diet on the Microbiota
6.1. Sleep Disruption and Its Influence on Microbiota Diversity
Sleep is one of the crucial biological processes, and its disturbance affects several physiological systems, including gut microbiota. Disturbances in the pattern of the sleep-wake cycle, fragmented sleep, or disruption of circadian rhythm affect the diversity and composition of microbiota gravely as discussed. More than this, as few as one night of sleep deprivation reportedly causes low microbial diversity, i.e., increased
Firmicutes to
Bacteroidetes ratio, higher abundances of the families
Coriobacteriaceae and
Erysipelotrichaceae, and lower abundance of
Tenericutes [
70,
75]. Further, it causes enhanced replication of pathogenic bacteria with a reduced abundance of beneficial species [
75]. That to which it will contribute will be the changes in gut microbiota, which might induce systemic inflammation and metabolic dysregulation-insulin resistance, a factor closely related to the pathogenesis of cerebrovascular diseases including CSVD.
Some evidence has suggested that sleep deprivation can disturb the production of microbial metabolites, including SCFAs, which are important for intestinal barrier integrity and immune response modulation [
76]. SCFAs, like butyrate, decrease gut permeability, which prohibits the translocation into the systemic circulation of bacterial endotoxins such as LPS. Besides, lack of sleep also affects metabolism and food intake, leading to dietary changes that influence the gut microbiome. For example, people with disrupted sleep patterns often consume more sugary or high-fat foods, promoting the growth of certain bacterial species like Firmicutes over Bacteroidetes, and impacting metabolic health [
77,
78]. Additionally, sleep disturbances could increase cortisol levels via the activation of the HPA axis; thus, further disturbing gut microbiota by facilitating the growth of bacterial strains associated with inflammation [
79]. Thus, increased circulating LPS, diet-related sleep disruption and altered HPA axis are associated with increased systemic inflammation, oxidative stress, and neuroinflammation impeding CSVD pathophysiology.
6.2. Sleep and Gut-Brain Homeostasis: Implications for CSVD and Glymphatic Function
The role of sleep, particularly in maintaining gut-brain homeostasis is closely tied to the bidirectional communication within the GBA. Whereby, healthy sleep patterns promote the growth of beneficial gut bacteria, which support gut integrity, nutrient absorption, and immune responses. Conversely, poor sleep quality disrupts CNS and gastrointestinal health regulation i.e., dysbiosis, characterized by the overgrowth of pro-inflammatory bacteria and loss of protective species like
Lactobacillus and
Bifidobacterium [
70,
75]. This imbalance affects gut-brain communication, potentially increasing neuroinflammation and oxidative stress [
79].
The glymphatic system, which is responsible for the removal of neurotoxic waste products from the brain, such as Aβ, becomes most active during deep sleep; this increases the interstitial space in the brain, thus enabling more efficient removal of these harmful substances from the brain. Poor sleep interferes with this, and concurrently decreases the regulation of gut microbiota, which can further enhance neuroinflammatory processes.
Moreover, during sleep, vagal activity (parasympathetic tone) increases, promoting gut motility and the regulation of inflammation, meanwhile, poor sleep or sleep deprivation dampens vagal tone, contributing to impaired gut-brain communication and systemic inflammation, which could accelerate cerebrovascular changes [
80]. This is in line with the fact that sleep deprivation may weaken the BBB and impair endothelial function, important in the pathogenesis of CSVD [
70]. Apart from that, poor sleep has also been separately associated with a reduction in the production of SCFAs, further compromising the integrity of the cerebrovasculature and enhancing neuroinflammation [
77,
78]. The effects of sleep disturbance on the integrity of the cerebrovasculature and neuroinflammation might worsen the outcomes of CSVD by increasing stroke risk, white matter damage, and cognitive impairment. This interrelationship may be viewed as a synergistic relationship that emphasizes sleep disturbance treatment as integral to the prevention of CSVD in at-risk populations.
6.3. Circadian Regulation by Gut Microbiota and Its Impact on Glymphatic Clearance in CSVD
The emerging evidence outlines that gut microbiota may modulate circadian rhythms, which in turn influence the efficiency of the glymphatic clearances, especially during sleep. Gut dysbiosis-coupled circadian rhythm disturbances impede the functions of the glymphatic system, hence leading to an increased risk for CSVD due to the build-up of neurotoxic wastes and increased neuroinflammation. Moreover, the gut microbiota communicates with the host circadian system through microbial metabolites, such as SCFAs and polyamines, whose concentrations oscillate over a day. This in turn modifies the expression of host circadian genes that regulate sleep-wake cycles, neuroinflammation, and metabolism [
81]. For instance, it has been demonstrated that butyrate is an SCFA produced by intestinal microbiota, which enhances circadian clock gene expression, such as
Per2,
Bmal1 and
Clock, both in the gut and brain, promoting physiological sleep patterns that allow for normal glymphatic activity [
82]. Additionally, animal studies also indicate that two
Clostridium sporogenes-derived metabolites such as 3-(4-hydroxyphenyl)propionic acid (4-OH-PPA) and 3-phenyl propionic acid (PPA) are involved in the regulation of circadian oscillation of
Per2 and
Bmal1 clock genes in the host’s peripheral and central clock machinery, whereby treatment of 4-OH-PPA increased the amplitude and lengthened the period of PER2 oscillation in the suprachiasmatic nucleus and other tissues [
83].
Moreover, the glymphatic system, responsible for the removal of waste from brain metabolic processes, including Aβ, is also optimally active during slow-wave sleep. Microbial dysbiosis contributes to the disrupted production of SCFAs and impairs host circadian entrainment to further contribute to perturbations in sleep and reductions in glymphatic clearance. In one recent study, gut dysbiosis resulted in a 25% reduction in SWS, concomitant with a 15% reduction in glymphatic efficiency [
84]. This decrease weakens the elimination of neurotoxic waste, creating an environment that results in enhanced neuroinflammation. Enhanced neuroinflammation has been recorded to be a contributor to CSVD pathology. A few microbial metabolites, such as precursors of serotonin and melatonin, have been shown to influence sleep and glymphatic flow. Gut-derived serotonin also contributes to regulating sleep architecture, which in turn affects the process of glymphatic indirectly. It has been hypothesised that microbial modulation of serotonin may be responsible for the improvements in sleep quality, enhancing functions of the glial lymphatic system and thus potentially reducing CSVD risk [
17,
85].
Therefore, the modulation of gut microbiota for the restoration of circadian rhythm and improvement of glymphatic function opens new perspectives in the prevention of CSVD. In this regard, probiotic interventions promoting SCFA production or serotonin pathways may improve sleep quality, reduce neuroinflammation, and enhance glymphatic clearance. However, specific strains and dosages are still under investigation as to how they restore circadian alignment in patients with dysbiosis. In short, gut microbiota indirectly yet significantly contributes to the maintenance of glymphatic health through circadian regulation, sleep modulation, and the production of metabolites. Disruption to the system by dysbiosis may disturb glymphatic clearances and increase the risk for CSVD, thus justifying gut-targeted approaches in managing neurovascular health.
6.4. Diet's Influence on Microbiota Composition and Brain Health
Diet on the other hand is one of the most important modulators of gut microbiota composition. Food, in general, from fibre and polyphenols to fats and other nutrients, reaches the colon and directly influences microbial diversity, the production of bioactive metabolites, and the overall inflammatory status of the host. For example, high-fibre diets tend to feed bacteria capable of producing SCFA, including
Bifidobacteria and
Faecalibacterium prausnitzii, leading to an anti-inflammatory response and thus promoting vascular and brain health [
86]. For example, SCFAs, including butyrate, have been shown to prevent endothelial dysfunction, lower neuroinflammation, and enhance the integrity of the BBB, thus exerting protective effects against CSVD [
87].
By contrast, diets high in saturated fat and added sugars are typified by a pro-inflammatory signature of the gut microbiome: reduced gut microbial diversity and greater abundance of pathogenic bacteria capable of producing pro-inflammatory metabolites, including TMAO. Because TMAO has been strongly associated with atherosclerosis development, vascular dysfunction, and cognitive decline, it turns out to be an excellent biomarker of disease risk [
59]. Poor dietary habits could promote cerebrovascular damage through inflammation and oxidative stress, thus exacerbating the course of CSVD and impairing the efficiency of brain waste clearance via the glymphatic system, further linking diet to cognitive decline and neurodegeneration [
19,
88].
Besides macronutrient intake, there is an aspect of polyphenols as plant-derived compounds found in fruits, vegetables, and tea that have been recognized to exert beneficial effects on microbiota composition. A diet rich in polyphenols increases microbial diversity and the abundance of beneficial bacteria, including
Lactobacillus and
Bifidobacterium. This helps in improving metabolic health, anti-inflammatory effects, and enhanced cognitive function [
89]. Interestingly, dietary polyphenols may act as a potential therapeutic candidate to enhance glymphatic function and reduce neuroinflammation potential dietary strategy to mitigate the features of CSVD.
6.5. The Interplay Between Diet, Sleep, and Gut Microbiota in CSVD
Sleep and diet are two parameters that interact in maintaining gut microbiota and, thus, would affect the risk of CSVD. A poor sleeping habit in combination with a diet characterized by a high intake of processed foods and a low fibre intake acts synergistically to promote gut dysbiosis, systemic inflammation, and vascular health impairment [
90]. Conversely, the interventions on improving sleep quality and dietary changes by adding fibre and anti-inflammatory nutrients showed restoration in microbial balance, reduced systemic inflammation, and promoted good brain health.
Therefore, improving sleep and diet is one of the most promising treatment approaches to prevent and manage CSVD. Indeed, dietary intervention, especially with high fibre and polyphenol intake, has been suggested to have a positive effect on gut microbiota modulation and enhanced glymphatic function to slow down CSVD progression and cognitive decline in support of such intervention [
73,
77,
86]. Additionally, therapeutic interventions that promote sleep, including cognitive behavioural therapy for insomnia (CBT-I) and regulation of the circadian rhythm, will further support gut-brain homeostasis and improve cerebrovascular health [
91].
Table 1 summarises the interplay between sleep and diets and their impact on gut microbiota and cerebrovascular health.
The intricate connections between sleep, diet, and gut microbiota underline the importance of integrated therapeutic strategies for managing CSVD. Targeting these modifiable lifestyle factors may offer new avenues for preventing and slowing the progression of cerebrovascular disease. Moreover, given the critical role of inflammation in both CSVD and gut microbiota dysbiosis, it is important to explore the role of circulating cell-derived microparticles (MPs) in mediating inflammation and their interactions with the microbiota, as these extracellular vesicles are emerging as key players in the regulation of immune responses and vascular health.
7. Role of Microparticles in Inflammation and Microbiota Interaction
7.1. Microparticles: Definition and Significance in Cerebrovascular Health
Circulating cell-derived microparticles (MPs) are small membrane-bound vesicles (100–1,000 nm in diameter) released by various cell types, including endothelial cells, platelets, and immune cells, in response to cellular activation or apoptosis. MPs play a pivotal role in intercellular communication by transferring bioactive molecules such as proteins, lipids, mRNA, and microRNA between cells and tissues. Their involvement in systemic and cerebral inflammation has garnered significant attention, especially in the context of cerebrovascular diseases like CSVD [
92,
93]. Elevated levels of MPs have been found in a variety of inflammatory and vascular conditions, making them key contributors to disease progression by promoting oxidative stress, endothelial dysfunction, and thrombogenesis [
93].
In CSVD, MPs can directly contribute to cerebrovascular injury by inducing endothelial cell dysfunction, exacerbating BBB disruption, and promoting neuroinflammation. MPs derived from damaged endothelial cells are particularly relevant in CSVD, as they carry pro-inflammatory and pro-coagulant signals that further propagate vascular damage [
93]. The clinical relevance of MPs in CSVD is underscored by their presence in higher concentrations in patients with cerebrovascular pathology compared to healthy individuals, making them potential biomarkers for early detection of vascular damage and disease progression [
94].
The impact of MPs on cerebrovascular health is multifaceted, particularly when considering their role in inflammation, coagulation, and endothelial function. Recent findings suggest that MPs derived from both endothelial cells and circulating immune cells play a pivotal role in the development and progression of CSVD. For instance, elevated levels of MPs specifically platelet-derived MP (CD62P) have been detected in individuals with increased risk factors for CSVD, including hypertension, diabetes, and chronic inflammation [
94]. MPs contribute to vascular dysfunction by promoting oxidative stress and vascular stiffening, both of which are hallmark features of CSVD [
93]. Additionally, MPs carrying pro-coagulant signals can exacerbate microthrombi formation in small cerebral vessels, further contributing to ischemic damage and white matter lesions [
95].
Furthermore, despite limited evidence of the role of MPs in glymphatic system function, recent findings on the glymphatic system particularly in regulating the clearance of neurotoxic waste products such as Aβ are promising. It is suggested that MPs may influence the activity of astrocytes and the permeability of the BBB, both of which are crucial for glymphatic clearance [
13]. Whereby a study by Ruhela et al., reported that astrocyte-derived MPs expressing thrombospondin-1 establish a feed-forward neuroinflammatory cycle involving endothelial CD36-to-astrocyte nuclear factor-kappa beta (NF-κB) crosstalk [
96]. This supports the notion that the dysregulation of MP production or function in the setting of systemic inflammation or sleep disruption may impair glymphatic clearance, contributing to neurodegeneration and cognitive decline, both of which are closely linked to CSVD.
Interestingly, the gut microbiota appears to be a significant modulator of MP-mediated inflammation. In conditions of gut dysbiosis, gut-microbial-derived MPs can exacerbate systemic inflammation, promoting a pro-inflammatory milieu conducive to vascular damage [
97,
98,
99]. Experimental studies have demonstrated that restoring gut microbiota balance through prebiotic or probiotic interventions can reduce the production of pro-inflammatory MPs and improve vascular health [
100]. For example, MPs produced by the probiotic
Propionibacterium freudenreichii CIRM-BIA 129 have been reported to reduce inflammation by modulating the NF-κB pathway [
101]. These findings highlight the intricate interplay between gut health, MPs, and cerebrovascular integrity.
7.2. Putative Roles of MPs as Mediators of Gut-Brain Communication
Recent research has highlighted the emerging role of MPs in mediating communication between the gut microbiota and the vascular endothelium, impacting both systemic and neuroinflammation. Gut-microbial-derived MPs, particularly those released in response to microbial dysbiosis, can influence vascular health and inflammation by transporting microbial components like LPS and bacterial metabolites into the systemic circulation [
102]. This may promote a pro-inflammatory cascade that can exacerbate vascular damage and contribute to the pathophysiology of CSVD.
Gut-microbial-derived MPs have been shown to increase the permeability of the intestinal barrier, facilitating the translocation of LPS into the bloodstream, a process termed metabolic endotoxemia [
103]. Elevated levels of circulating LPS and associated MPs trigger systemic inflammatory responses, characterized by the release of cytokines like IL-6 and TNF-α, which can also affect cerebral vasculature by enhancing endothelial activation, oxidative stress, and BBB disruption [
104]. These interactions are especially relevant to the GBA, where gut-microbial-derived MPs can serve as intermediaries linking gut microbiota dysbiosis to neuroinflammatory processes and cerebrovascular dysfunction.
MPs are also key players in vascular endothelial homeostasis, a crucial factor in maintaining the integrity of cerebral microcirculation. In a dysbiotic state, MPs carrying inflammatory signals may contribute to endothelial dysfunction and impaired glymphatic clearance, further exacerbating CSVD [
19]. Dysregulation in the production or clearance of MPs, particularly in inflammatory conditions, has been implicated in the development of neurovascular pathologies, highlighting their potential as both biomarkers and therapeutic targets in diseases like CSVD [
105].
MPs act as key intermediaries between gut-microbial-derived inflammation and neurovascular health, particularly through their role in amplifying immune responses and promoting endothelial dysfunction. In CSVD, where vascular integrity is already compromised, MPs may serve as both markers of disease progression and potential therapeutic targets. Studies have shown that interventions aimed at reducing MP levels, either through anti-inflammatory therapies or microbiota-targeted treatments, can improve vascular health and reduce the risk of neuroinflammation and cerebrovascular damage [
100,
101,
106].
Table 2 summarizes the multifaceted roles of MPs in cerebrovascular disease, linking gut microbiota dysbiosis, systemic inflammation, and CSVD progression.
These findings underscore the significance of MPs as both contributors and mediators in the pathogenesis of CSVD. Their role in facilitating gut-brain communication, particularly in the context of inflammation, highlights their potential as therapeutic targets for mitigating the effects of gut dysbiosis and cerebrovascular dysfunction. Therefore, given the significant role of MPs in modulating inflammation and vascular health, it is critical to explore therapeutic strategies that aim to modulate the microbiota to improve neurological health.
Figure 3 summarises the putative interrelationship between GBA-microbiota, MPs and glymphopathy. The next section will delve into the potential of microbiota-targeted interventions as a novel therapeutic approach for managing CSVD and neuroinflammation.
8. Therapeutic Potential: Modulating Microbiota for Neurological Health
This new link between gut microbiota and cerebrovascular integrity through neuroinflammation opens a window of opportunity for therapeutic intervention. Current strategies range from the use of probiotics, prebiotics, dietary manipulations, and FMT as an effective way to modulate gut microbiota to reduce inflammation, enhance vascular health, and improve glymphatic clearance. More recently, attention has been paid to the reduction of MPs' burden participating in systemic and cerebrovascular in-flammation.
8.1. Probiotics, Prebiotics, and Dietary Interventions
Probiotics are living microorganisms that, when administered in adequate amounts, provide health benefits to the host by enhancing microbial balance. Some probiotics, such as
Lactobacillus rhamnosus,
Bifidobacterium longum, and
Akkermansia muciniphila, show neuroprotective effects via immunomodulatory response, gut barrier integrity, and reduction of systemic inflammation [
107,
108]. They were effective in animal models of CSVD, improving the cognitive outcomes, blunting vascular dysfunction by the production of SCFAs and anti-inflammatory cytokines [
109].
L. rhamnosus and
B. longum are two of the most well-recognized and highly studied probiotic species with neuroprotective effects. Recent publications present that
L. rhamnosus by modulating neuroinflammation via GBA resulted in significant reduction in systemic markers of inflammation, represented by IL-6 and TNF-α, in LPS-induced
Escherichia coli-driven inflammation in C57BL/6 mice [
110].
B. longum in turn is observed to promote endothelial health by increasing nitric oxide bioavailability, enhancing vascular dilation, thereby decreasing blood pressure-a feature particularly pertinent to the management of CSVD [
111]. The translation of such findings into human populations has been variable, as microbial composition and host factors, including age or diet, influence the efficacy with which such probiotics function. To this date, inconsistent clinical trial results have emerged, with only approximately 60% of patients showing detectable neuroinflammation reductions [
112].
Probiotic supplementation has been associated with reduced circulating levels of TNF-α and IL-6, and CRP [
113], all of which are increased in CSVD phenotypes. By improving gut barrier integrity and reducing pro-inflammatory cytokine production, probiotics may prevent the translocation of microbial endotoxins, which are linked to neuroinflammation and endothelial dysfunction. Of particular note,
Akkermansia muciniphila has emerged as a novel probiotic with demonstrated benefits for endothelial function via enhancement of gut barrier integrity and reduction of systemic endotoxemia. Animal studies have postulated that it reduces neuroinflammatory cytokines by more than 20%, further reducing vascular inflammation markers such as IL-6 [
114]. However, the strain’s clinical efficacy in humans remains limited by its low natural abundance in Western diets, requiring high doses for measurable effects. Other roles of SCFAs relate to the modulation of cerebral blood flow and glymphatic clearance, which is very important in view of the fact that SCFA-producing bacteria such as
Butyrivibrio fibrisolvens take part in maintaining the integrity of cerebral vasculature [
115].
There is one study investigating the relationship between gut microbiota, sleep quality, and cognitive flexibility in older adults in order to shed light on how these factors interact in age-related cognitive resilience [
116]. The stool samples of 37 healthy older adults were analysed along with the assessment of sleep quality and cognitive flexibility by the Stroop test. Results indicated that higher sleep quality related to better Stroop performance and to higher relative abundance of the gut microbial phyla Verrucomicrobia and Lentisphaerae. Interestingly, the relation of cognitive flexibility with Lentisphaerae was partly mediated by sleep quality [
116]. This may suggest that sleep is also an important factor through which the gut-brain axis modulates cognitive flexibility. The influence of Verrucomicrobia was primarily linked to sleep quality. Consequently, it is still unknown whether Verrucomicrobia directly impacts cognitive measures. These findings underlined the potential of gut microbiota as one such contributor to cognitive flexibility in older adults, influenced largely by sleep quality. Further studies should investigate whether strategies aimed at improving gut microbiome health might protect against sleep-related cognitive decline. The findings open a way for exploring microbiome-modulating treatments; however, experimental approaches are also needed to verify these preliminary results and clinically test potential therapeutic interventions for aging populations.
Conversely, prebiotics are indigestible food ingredients that stimulate the growth of beneficial bacteria and also contribute to an important role in modulating GBA. Prebiotic fibres such as inulin and fructo-oligosaccharides stimulate the proliferation of SCFAs-producing bacteria including
Faecalibacterium prausnitzii, which plays a role in immune modulation and endothelial health and enhance glymphatic clearance and reduce oxidative stress in the brain [
117]. Yet, despite these promising effects, prebiotics induce gastrointestinal discomfort-such as bloating or diarrhoea-which certainly limits the compliance and long-term use of these substrates in the clinical setting. Intriguingly, there is emerging evidence that prebiotics exert an improvement in endothelial function and reduction in vascular stiffness [
118], an important aspect in the management of CSVD.
Moreover, diets rich in fibre, polyphenols, and omega-3 fatty acids can be considered as dietary intervention targeting the microbiota. High-fibre diets increase the production of SCFAs, whereas the polyphenolic compounds such as flavonoids and resveratrol have been reported to affect gut microbiota composition in order to lower oxidative stress and improve vascular health [
119]. Besides, omega-3 fatty acids, especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), may act directly on gut microbiota composition, increasing the abundance of anti-inflammatory bacterial strains while decreasing vascular inflammation [
120,
121], i.e., the risk for CSVD. Therefore,
Table 3 summarises the potential Therapeutic approaches to modulate gut microbiota for CSVD and glymphatic function
Large heterogeneity in gut microbiota among individuals prevents the extrapolation of those findings into the clinical setting due to different individual responses against the administration of probiotics and prebiotics. Further, the absence of standard probiotic strains and dosing makes it even more difficult to reproduce the same effect in different studies. Only very limited data are present on long-term safety, especially for newer probiotics like A. muciniphila, and its cautious application needs to be defined in CSVD patients. While some are promising, including certain probiotics and prebiotics for neuroinflammation and vascular health, it is variability from host responses that places limitations on the current clinical translatability due to logistical issues related to dosing and tolerability. Further research into strain-specific effects is needed, with the development of uniform formulations that would contribute to the optimization of such interventions in the management of CSVD.
8.2. Faecal Microbiota Transplantation (FMT) and Emerging Therapies
The FMT is one of those rising stars that has gained increasing attention and has been turned into an intervention for restoration of microbial diversity and decreasing inflammation in disrupted gut microbiota. FMT was successfully applied to patients with recurrent
Clostridium difficile infections [
122], while in the case of application for neurological diseases, including CSVD, interest had been growing [
123]. Indeed, various pre-clinical and human studies have shown the potential of FMT to dampen neuroinflammation and improve cognitive function, even to the restoration of glymphatic clearance of toxic proteins such as amyloid-β [
124,
125].
One of the main mechanisms through which FMT would confer benefit in subjects with CSVD would be through restitution of a healthy microbiota that may increase production of SCFAs and decrease systemic inflammation. FMT also directly restored the integrity of the intestinal barrier, preventing translocation into the systemic circulation of proinflammatory bacterial products, including LPS, a process intimately linked to neuroinflammation and endothelial dysfunction [
122]. Further newer microbiota-based therapies such as postbiotics and synbiotics have also been explored for neuroprotective potential [
126]. The non-viable bacterial products or metabolic byproducts, for example SCFAs, referred to as postbiotics, have demonstrated efficacy in lowering systemic inflammation and promoting vascular health [
127]. Synbiotics are a combination of probiotics and prebiotics that have been studied in relation to their synergetic effects on GBA functioning, with initial positive results in lessened cognitive decline in animal models with vascular dementia [
126].
8.3. Strategies to Control the Levels of MPs
Anti-inflammatory interventions directed to the GBA or direct inhibition of the formation of MPs by pharmacological agents, including statins and angiotensin-converting enzymes (ACE) inhibitors, represent potential therapeutic strategies to reduce MPs [
93]. Reduction of circulating MPs has indeed been associated with an improvement in vascular function and reduction of ischemic stroke risk [
128], in particular in patients affected by CSVD. Furthermore, novel anti-inflammatory therapies target major pathways involved in neurovascular inflammation, such MPs, especially in CSVD. This includes therapies directed at the blockage of pro-inflammatory cytokine activity, such as TNF-α, IL-1β, and IL-6, and inhibition of inflammatory mediators such as cyclooxygenase-2 (COX-2). For example, statins are renowned not only for cholesterol-lowering but also for anti-inflammatory action. It has also been reported to reduce vascular inflammation and oxidative stress [
129]. Therefore, it brings about the controlling effect of MPs on vasculature and thus, improves endothelial function in CSVD patients.
ACE inhibitors and angiotensin II receptor blockers (ARBs) represent another pharmacological strategy aimed at reducing vascular inflammation by inhibiting the renin-angiotensin system, implicated in both hypertension and vascular dysfunction [
130], i.e. risk factors for MPs formations (and vice versa) and common features of CSVD. Specific probiotics have been proven to modulate MPs release by reducing endothelial activation and inflammation, indicating a possible microbiota-target strategy to control MP-related vascular damage [
100,
101,
106].
While such microbiota-targeting therapies do indeed herald encouraging prospects, challenges and limitations exist in the path towards these therapies. First, the gut microbiota is greatly heterogeneous among individuals, yielding variability in therapeutic responses [
131]. Personalized approaches that would take into consideration individual microbiota profiles and genetic backgrounds are likely to be needed for optimizing therapeutic benefits. However, standardization of probiotics, prebiotics, and FMT remains one of the main challenges in their clinical use. The fact that probiotic strains differ from one another and various effects on microbiota and the immune response makes generalization difficult among studies. In the same way, the use of FMT in neurological diseases has not yet been established for long-term safety or efficacy, under concerns of possible adverse effects and durability of microbiota changes.
Future multi-modal treatments will include combinations of microbiota-targeted therapies with other anti-inflammatory or neuroprotective compounds that could bring synergistic benefits in patients with CSVD. Future improvements in metagenomic analysis and profiling technologies will also allow more precise identification of microbial species and metabolites implicated in GBA and enable more selective therapeutic strategies [
132]. While the knowledge of gut microbiota interaction with neuroinflammation and vascular health becomes more advanced, so do microbiota-based therapies in the prevention and treatment of CSVD and other neurovascular disorders. Consequently, this will lead to a comprehension of how combining microbiota modulation with newly developed anti-inflammatory strategies will further improve neurovascular health and enhance clinical outcomes in patients with CSVD.
Such a combination of microbiota modulation with emerging anti-inflammatory strategies might therefore lead to a synergistic approach that will greatly enhance neurovascular health towards the improvement of clinical outcomes in CSVD patients. This approach leverages the GBA, where the gut microbiota plays a key role in the regulation of systemic inflammation-including that mediated by MPs-vascular health, and neurological function. The clinicians would thus be in a position to address the multifactorial pathogenesis of CSVD, involving vascular dysfunction, endothelial damage, oxidative stress, and neuroinflammation, by targeting both the microbiota and inflammation.
8.4. Synergistic Effects: Microbiota Modulation Combined with Anti-Inflammatory Therapy
Firstly, is the improved SCFA production and immune modulation. Microbiota-targeted interventions, including the use of probiotics and prebiotics, increase SCFA production, which in turn helps in reducing systemic inflammation and oxidative stress. SCFAs directly influence the immune system by enhancing anti-inflammatory cytokine production, such as IL-10, and Treg cells considered to modulate inflammation both locally in the gut and systemically [
133]. When these approaches are combined with anti-inflammatory strategies, either statins or COX-2 inhibitors, the total inflammatory burden would be profoundly decreased, with actions of both stabilizing endothelial function and reducing the neurovascular damage common in CSVD.
The second mechanism is through the reduction of circulating MPs. As discussed CSVD patients have reported increased levels of circulating MPs, released after vascular and endothelial injury, closely associated with increased inflammation and vascular dysfunction. Targeting the microbiota with probiotics and prebiotics reduces MPs release by modulating endothelial cell activation. Coupling of the latter approach with anti-inflammatory agents such as statins, known to reduce MPs levels may improve therapeutic efficacy by reducing ischemic events and improving vascular health [
129].
Next is the gut barrier integrity and BBB protection. Gut dysbiosis and the resulting increased intestinal permeability (leaky gut) drive systemic inflammation by allowing bacterial components, such as LPS, to leak into the bloodstream and thus induce neuroinflammation [
103]. Pure anti-inflammatory therapies might not suffice to address the origin of the inflammation. However, the restoration of gut barrier function by microbiota modulation may prevent the translatability of harmful bacterial products across the barrier and reduce the systemic inflammatory burden. This may exert a dual effect-for instance, reduction of gut-derived inflammation can directly inhibit the pro-inflammatory pathways in the brain either through drugs such as inhibitors of IL-6 or blockers of TNF-α, and therefore, this combined strategy could enhance the integrity of BBB and protect against further damage to the brain.
Modulation of the oxidative stress and function of the glymphatic system has also been referred to as synergistic effects. The oxidative stress acts as a key player in endothelial dysfunction and cerebral microvascular damage due to CSVD. Markers of oxidative stress are reduced by probiotics, strains of
Lactobacillus and
Bifidobacterium [
134], and anti-inflammatory drugs, such as statins, exhibit antioxidant actions through their effects on nuclear factor erythroid 2-related factor 2 (Nrf2) [
135]. Such a double-edged strategy may improve not only vascular function but also glymphatic elimination of noxious waste products, including Aβ accumulating in the neurodegenerative disorders associated with CSVD.
Finally, there is the circadian rhythm and the glymphatic regulation. Gut microbiota plays a critical role in regulating the circadian rhythm, which is important for glymphatic clearance, especially during sleep. A disruption in this circadian rhythm is associated with glymphopathy and increased neurovascular damage. Anti-inflammatory therapies that reduce sleep disturbances and enhance vascular health may, therefore, act synergistically with microbiota-targeted interventions that improve circadian regulation and glymphatic function. Such may be the case, for example, with probiotics targeting melatonin production or sleep quality acting in concert with anti-inflammatory drugs that target neurovascular inflammation [
136,
137].
9. Clinical Implications and Future Directions
A combined approach of microbiota modulation with anti-inflammatory therapies may therefore confer significant benefits in improving the clinical outcome in CSVD patients. Although positive results have emerged from both preclinical studies and early clinical trials, much larger and well-designed randomised control trials (RCTs) must be conducted to confirm such findings. One of the important challenges in that respect concerns the heterogeneity within the gut microbiota across individuals, and thus future therapies may have to be personalized depending on the individual's microbiome profile, genetic makeup, and disease status. Long-term safety and efficacy need to be established for microbiota-targeted therapies, especially for FMT and postbiotics before wide clinical acceptance of these approaches can be achieved.
Moreover, the establishment of biomarkers that monitor the effect of both microbiota and anti-inflammatory treatments in real-time will improve the precision of treatment. Such biomarkers might include microbial metabolites like SCFAs, inflammatory cytokines, and circulating MPs that give insight into the efficacy of applied treatments and enable early intervention in CSVD patients.
In brief, the integration of microbiota modulation with new anti-inflammatory strategies thus provides a more holistic approach toward the management of CSVD. Such an integrated strategy may better address inflammation and microbial contributors to neurovascular dysfunction and, as such, may potentially reduce disease progression and improve glymphatic clearance in patients with CSVD.
10. Conclusions
The complex interaction between gut microbiota, cerebrovascular health, and neurological function makes microbiota an important modulator of CSVD. Dysbiosis promotes systemic inflammation, neuroinflammation, and vascular dysfunction that accelerates CSVD. Emerging evidence underlines the role of the GBA in modulating neurovascular integrity, in particular, through the interactions with the glymphatic system and the circulating MPs. Target modulation by probiotics, prebiotics, and dietary interventions presents a promising therapeutic strategy for restoring gut-brain homeostasis, enhancing waste clearance, and reducing neurovascular damage. This might be usefully combined into anti-inflammatory treatments, opening a new avenue for mitigating CSVD and improving cognitive outcomes. As research continues, personalized microbiome-based interventions may offer novel strategies for the prevention and treatment of neurovascular disorders, potentially leading to better long-term brain health. Future studies should be directed at the validation of such therapeutic approaches and the exploration of their clinical benefit in the management of CSVD.
Author Contributions
Conceptualization, C.M.N, C.M.N.; resources, C.M.N, C.M.N.; M.D.C.R and Z.M.H; data curation, C.M.N, C.M.N.; writing—original draft preparation, C.M.N, C.M.N.; writing—review and editing, C.M.N, C.M.N.; M.D.C.R.; U.J.; M.M.G.; H.A.H.; M.Z.H. and Z.M.H; visualization, C.M.N, C.M.N.; funding acquisition, Z.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Ajman University, United Arab Emirates.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, N.; Gupta, V.; Gaur, M.; Sharma, V.; Puri, A.; Singh, Y.; Dubey, G.P.; Lal, R. Interplay of human gut microbiome in health and wellness. Indian J. Microbiol. 2020, 60, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O'Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef]
- Anand, N.; Gorantla, V.R.; Chidambaram, S.B. The role of gut dysbiosis in the pathophysiology of neuropsychiatric disorders. Cells 2022, 12, 54. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From Gut Dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Staals, J.; Makin, S.D.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total mri brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer's disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Cardiovascular disease may be triggered by gut microbiota, microbial metabolites, gut wall reactions, and inflammation. Int. J. Mol. Sci. 2024, 25, 10634. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Lee, H.; Yu, M.; Feng, T.; Logan, J.; Nedergaard, M.; Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Investig. 2013, 123, 1299–1309. [Google Scholar] [CrossRef]
- Ringstad, G.; Valnes, L.M.; Dale, A.M.; Pripp, A.H.; Vatnehol, S.S.; Emblem, K.E.; Mardal, K.A.; Eide, P.K. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 2018, 3, e121537. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hamid, H.; Hambali, A.; Okon, U.; Che Mohd Nassir, C.M.N.; Mehat, M.Z.; Norazit, A.; Mustapha, M. Is cerebral small vessel disease a central nervous system interstitial fluidopathy? IBRO Neurosci. Rep. 2023, 16, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Y.; Leng, S.; Ma, Y.; Jiang, Q.; Wen, Q.; Ju, S.; Hu, J. The relationship between inflammation, impaired glymphatic system, and neurodegenerative disorders: A vicious cycle. Neurobiol. Dis. 2024, 192, 106426. [Google Scholar] [CrossRef]
- Camberos-Barraza, J.; Guadrón-Llanos, A.M.; De la Herrán-Arita, A.K. The gut microbiome-neuroglia axis: Implications for brain health, inflammation, and disease. Neuroglia 2024, 5, 254–273. [Google Scholar] [CrossRef]
- Stampouloglou, P. K. , Siasos, G., Bletsa, E., Oikonomou, E., Vogiatzi, G., Kalogeras, K., Katsianos, E., Vavuranakis, M. A., Souvaliotis, N., Vavuranakis, M. The role of cell-derived microparticles in cardiovascular diseases: Current concepts. Curr. Pharm. Des. 2022, 28, 1745–1757. [Google Scholar] [CrossRef]
- Che Mohd Nassir, C. M. N. , Ghazali, M. M., Hashim, S., Idris, N. S., Yuen, L. S., Hui, W. J., Norman, H. H., Gau, C. H., Jayabalan, N., Na, Y., et al (2021). Diets and cellular-derived microparticles: Weighing a plausible link with cerebral small vessel disease. Front. Cardiovasc. Med. 2021, 8, 632131. [Google Scholar] [CrossRef]
- Puddu, P.; Puddu, G.M.; Cravero, E.; Muscari, S.; Muscari, A. The involvement of circulating microparticles in inflammation, coagulation, and cardiovascular diseases. Can. J. Cardiol. 2010, 26, 140–145. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E. The multifactorial etiopathogeneses interplay of inflammatory bowel disease: An overview. Gastrointest. Disord. 2019, 1, 75–105. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Li, J. , Jia, H., Cai, X., Zhong, H., Feng, Q., Sunagawa, S., Arumugam, M., Kultima, J. R., Prifti, E., Nielsen, T., et al. An Integrated Catalog of Reference Genes in the Human Gut Microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Bilen, M.; Dufour, J.-C.; Lagier, J.-C.; Cadoret, F.; Daoud, Z.; Dubourg, G.; Raoult, D. The contribution of culturomics to the repertoire of isolated human bacterial and Archaeal species. Microbiome 2018, 6, 94. [Google Scholar] [CrossRef]
- Giovannini, M.; Lana, D.; Traini, C.; Vannucchi, M. The microbiota–gut-brain axis and Alzheimer disease: From dysbiosis to neurodegeneration: focus on the central nervous system glial cells. J. Clin. Med. 2021, 10, 2358. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genomics Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, T.; Ubaldi, F.; Volpini, V.; Valeriani, F.; Romano Spica, V. The role of gut microbiota in different types of physical activity and their intensity: Systematic review and meta-analysis. Sports 2024, 12, 221. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Lin, K.; Zhu, L.; Yang, L. Gut and obesity/metabolic disease: Focus on microbiota metabolites. MedComm 2022, 3, e171. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut bacteria and neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Mhanna, A.; Martini, N.; Hmaydoosh, G.; Hamwi, G.; Jarjanazi, M.; Zaifah, G.; Kazzazo, R.; Haji Mohamad, A.; Alshehabi, Z. The correlation between gut microbiota and both neurotransmitters and mental disorders: A narrative review. Medicine 2024, 103, e37114. [Google Scholar] [CrossRef]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: Focus on the available therapeutic approaches and gut microbiome. J. Cell Commun. Signal. 2023, 17, 55–74. [Google Scholar] [CrossRef]
- Marano, G.; Mazza, M.; Lisci, F.M.; Ciliberto, M.; Traversi, G.; Kotzalidis, G.D.; De Berardis, D.; Laterza, L.; Sani, G.; Gasbarrini, A.; Gaetani, E. The microbiota-gut-brain axis: Psychoneuroimmunological insights. Nutrients 2023, 15, 1496. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 2019, 101, 246–259e6. [Google Scholar] [CrossRef]
- Yu, C.D.; Xu, Q.J.; Chang, R.B. Vagal sensory neurons and gut-brain signaling. Curr. Opin. Neurobiol. 2020, 62, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, X.; Chen, J.; Zhang, N.; Liu, Z. The Vagus nerve mediates the stomach-brain coherence in rats. NeuroImage 2022, 263, 119628. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 2014, 5, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Jianguo, L.; Xueyang, J.; Cui, W.; Changxin, W.; Xuemei, Q. Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2019, 9, 40. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, L.; Chen, Q.; Jiao, C.; Wang, Y.; Li, H.; Xie, J.; Zhu, F.; Wang, J.; Zhang, W.; et al. Gut microbiota dysbiosis contributes to depression-like behaviors via hippocampal NLRP3-mediated neuroinflammation in a postpartum depression mouse model. Brain Behav. Immun. 2024, 119, 220–235. [Google Scholar] [CrossRef]
- Merkouris, E.; Mavroudi, T.; Miliotas, D.; Tsiptsios, D.; Serdari, A.; Christidi, F.; Doskas, T.K.; Mueller, C.; Tsamakis, K. Probiotics' effects in the treatment of anxiety and depression: A comprehensive review of 2014-2023 clinical trials. Microorganisms 2024, 12, 411. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral Small Vessel Disease: From Pathogenesis and Clinical Characteristics to Therapeutic Challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of Sporadic Cerebral Small Vessel Disease: Insights from Neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Cannistraro, R.J.; Badi, M.; Eidelman, B.H.; Dickson, D.W.; Middlebrooks, E.H.; Meschia, J.F. CNS Small Vessel Disease: A Clinical Review. Neurology 2019, 92, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Koueik, J.; Wesley, U.V.; Dempsey, R.J. Pathophysiology, Cellular and Molecular Mechanisms of Large and Small Vessel Diseases. Neurochem. Int. 2023, 164, 105499. [Google Scholar] [CrossRef]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Csiszar, A.; Ungvari, A.; Patai, R.; Gulej, R.; Yabluchanskiy, A.; Benyo, Z.; Kovacs, I.; Sotonyi, P.; Kirkpartrick, A.C.; Prodan, C.I.; et al. Atherosclerotic Burden and Cerebral Small Vessel Disease: Exploring the Link through Microvascular Aging and Cerebral Microhemorrhages. GeroScience 2024, 46, 5103–5132. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.M.C.S.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef]
- van Veluw, S.J.; Shih, A.Y.; Smith, E.E.; Chen, C.; Schneider, J.A.; Wardlaw, J.M.; Greenberg, S.M.; Biessels, G.J. Detection, Risk Factors, and Functional Consequences of Cerebral Microinfarcts. Lancet Neurol. 2017, 16, 730–740. [Google Scholar] [CrossRef]
- Zhen, J.; Zhou, Z.; He, M.; Han, H.X.; Lv, E.H.; Wen, P.B.; Liu, X.; Wang, Y.T.; Cai, X.C.; Tian, J.Q.; et al. The Gut Microbial Metabolite Trimethylamine N-Oxide and Cardiovascular Diseases. Front. Endocrinol. 2023, 14, 1085041. [Google Scholar] [CrossRef]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019, 65, 106–119. [Google Scholar] [CrossRef]
- Reddy, O.C.; van der Werf, Y.D. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Zul Ramli, S.M.A.; Abdul Hamid, H.; Che Mohd Nassir, C.M.N.; A Rahaman, S.N.; Mehat, M.Z.; Kumar, J.; Lee, S.Y.; Mustapha, M. Aberrant Blood-Brain Barrier Dynamics in Cerebral Small Vessel Disease - A Review of Associations, Pathomechanisms and Therapeutic Potentials. Vessel Plus 2024, 8, 30. [Google Scholar] [CrossRef]
- Che Mohd Nassir, C.M.N.; Damodaran, T.; Yusof, S.R.; Norazit, A.; Chilla, G.; Huen, I.; K. N., B.P.; Mohamed Ibrahim, N.; Mustapha, M. Aberrant Neurogliovascular Unit Dynamics in Cerebral Small Vessel Disease: A Rheological Clue to Vascular Parkinsonism. Pharmaceutics 2021, 13, 1207. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rusinek, H.; Butler, T.; Glodzik, L.; Pirraglia, E.; Babich, J.; Mozley, P.D.; Nehmeh, S.; Pahlajani, S.; Wang, X.; et al. Decreased CSF Clearance and Increased Brain Amyloid in Alzheimer’s Disease. Fluids Barriers CNS 2022, 19, 21. [Google Scholar] [CrossRef]
- Keuters, M.H.; Antila, S.; Immonen, R.; Plotnikova, L.; Wojciechowski, S.; Lehtonen, S.; Alitalo, K.; Koistinaho, J.; Dhungana, H. The Impact of VEGF-C-Induced Dural Lymphatic Vessel Growth on Ischemic Stroke Pathology. Transl. Stroke Res. Advance Online Publication. [CrossRef]
- Ke, Z.; Mo, Y.; Li, J.; Yang, D.; Huang, L.; Yang, Z.; Qin, R.; Mao, C.; Lv, W.; Huang, Y.; et al. Glymphatic Dysfunction Mediates the Influence of White Matter Hyperintensities on Episodic Memory in Cerebral Small Vessel Disease. Brain Sci. 2022, 12, 1611. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Ho, Y.S.; Chang, R.C. Linking Circadian Rhythms to Microbiome-Gut-Brain Axis in Aging-Associated Neurodegenerative Diseases. Ageing Res. Rev. 2022, 78, 101620. [Google Scholar] [CrossRef]
- Fung, T.C. The Microbiota-Immune Axis as a Central Mediator of Gut-Brain Communication. Neurobiol. Dis. 2020, 136, 104714. [Google Scholar] [CrossRef]
- Boespflug, E.L.; Iliff, J.J. The Emerging Relationship between Interstitial Fluid-Cerebrospinal Fluid Exchange, Amyloid-β, and Sleep. Biol. Psychiatry 2018, 83, 328–336. [Google Scholar] [CrossRef]
- Shokri-Kojori, E.; Wang, G.-J.; Wiers, C.E.; Volkow, N.D. β-Amyloid Accumulation in the Human Brain after One Night of Sleep Deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef]
- Li, X.; Qin, R.R.; Chen, J.; Jiang, H.F.; Tang, P.; Wang, Y.J.; Xu, D.W.; Xu, T.; Yuan, T.F. Neuropsychiatric Symptoms and Altered Sleep Quality in Cerebral Small Vessel Disease. Front. Psychiatry 2022, 13, 882922. [Google Scholar] [CrossRef]
- Lloret, A.; Esteve, D.; Lloret, M.A.; Monllor, P.; López, B.; León, J.L.; Cervera-Ferri, A. Is Oxidative Stress the Link Between Cerebral Small Vessel Disease, Sleep Disruption, and Oligodendrocyte Dysfunction in the Onset of Alzheimer’s Disease? Front. Physiol. 2021, 12, 708061. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S. Roles of Diet-Associated Gut Microbial Metabolites on Brain Health: Cell-to-Cell Interactions between Gut Bacteria and the Central Nervous System. Adv. Nutr. 2024, 15, 100136. [Google Scholar] [CrossRef]
- Horn, J.; Mayer, D.E.; Chen, S.; Mayer, E.A. Role of Diet and Its Effects on the Gut Microbiome in the Pathophysiology of Mental Disorders. Transl. Psychiatry 2022, 12, 164. [Google Scholar] [CrossRef]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schürmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef]
- Pala, B.; Pennazzi, L.; Nardoianni, G.; Fogacci, F.; Cicero, A.F.G.; Di Renzo, L.; Barbato, E.; Tocci, G. Gut Microbiota Dysbiosis and Sleep Disorders: Culprit in Cardiovascular Diseases. J Clin Med. 2024, 13, 3254. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Dietary Fats and the Gut Microbiota: Their impacts on lipid-induced metabolic syndrome. J Funct Foods 2022, 91, 105026. [Google Scholar] [CrossRef]
- Siddhu, N.; T M, V.; Venkatesh, T. Impact of Food Intake and Sleep Disturbances on Gut Microbiota. Cureus 2024, 16, e70846. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: the gut microbiota and hypothalamic-pituitary-adrenal axis. Front Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Ikeda, Y.; Fukuda, K.; Tsuruta, H.; Shibata, S. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci Rep. 2018, 8, 1395. [Google Scholar] [CrossRef]
- Firoozi, D.; Masoumi, S.J.; Hosseini Asl, S.M.K.; Labbe, A.; Razeghian-Jahromi, I.; Fararouei, M.; Khorramdelazad, H.; Fararouei, F.; Moallem, S.A.; Hajigholami, M. Effects of short-chain fatty acid-butyrate supplementation on expression of circadian-clock genes, sleep quality, and inflammation in patients with active ulcerative colitis: a double-blind randomized controlled trial. Lipids Health Dis. 2024, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.; Park, I.; Kim, D.; Kim, J.; Jang, S.; Choi, M.; Ryu, D.; Yoo, J.; Shin, Y.; Lee, H. Gut Microbial Metabolites Induce Changes in Circadian Oscillation of Clock Gene Expression in the Mouse Embryonic Fibroblasts. Mol Cells 2020, 43, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, H.; Liu, Z.; Li, Y.; Zhao, Y.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med Rev. 2022, 65, 101691. [Google Scholar] [CrossRef] [PubMed]
- Deyang, T.; Baig, M.A.I.; Dolkar, P.; Hediyal, T.A.; Rathipriya, A.G.; Bhaskaran, M.; Manivasagam, T.; Essa, M.M.; Selvaraj, S.; Suvitha, S.; et al. Sleep apnoea, gut dysbiosis and cognitive dysfunction. FEBS J. 2024, 291, 2519–2544. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, S.; Tian, J.; Liu, X. Significance of Gut Microbiota and Short-Chain Fatty Acids in Heart Failure. Nutrients 2022, 14, 3758. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Bendlin, B.B.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer's disease. Alzheimers Res Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Bishehsari, F.; Voigt, R.M.; Keshavarzian, A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol. 2020, 16, 731–739. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Zhang, L.; Li, Y.; Shen, M.; Han, X.; Liu, W.; Wu, Y.; He, Y.; et al. Gut liver brain axis in diseases: the implications for therapeutic interventions. Sig Transduct Target Ther. 2023, 8, 443. [Google Scholar] [CrossRef]
- Voukalis, C.; Shantsila, E.; Lip, G.Y.H. Microparticles and cardiovascular diseases. Ann Med. 2019, 51, 193–223. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Gavidia, L.M.; Burger, D.; Matthews, V.B.; Nolde, J.M.; Galindo Kiuchi, M.; Carnagarin, R.; Schlaich, M.P.; Lambert, G.W.; Thompson, S.C.; McDonald, D.; et al. Role of Microparticles in Cardiovascular Disease: Implications for Endothelial Dysfunction, Thrombosis, and Inflammation. Hypertension 2021, 77, 1825–1844. [Google Scholar] [CrossRef] [PubMed]
- Che Mohd Nassir, C.M.N.; Mohamad Ghazali, M.; Ahmad Safri, A.; Jaffer, U.; Abdullah, W.Z.; Idris, N.S.; Muzaimi, M. Elevated Circulating Microparticle Subpopulations in Incidental Cerebral White Matter Hyperintensities: A Multimodal Study. Brain Sci. 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Zifkos, K.; Dubois, C.; Schäfer, K. Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 9317. [Google Scholar] [CrossRef] [PubMed]
- Ruhela, D.; Bhopale, V.M.; Kalakonda, S.; Thom, S.R. Astrocyte-Derived Microparticles Initiate a Neuroinflammatory Cycle Due to Carbon Monoxide Poisoning. Brain Behav. Immun. Health 2021, 18, 100398. [Google Scholar] [CrossRef]
- Mishra, S.; Tejesvi, M.V.; Hekkala, J.; Turunen, J.; Kandikanti, N.; Kaisanlahti, A.; Suokas, M.; Leppä, S.; Vihinen, P.; Kuitunen, H.; et al. Gut Microbiome-Derived Bacterial Extracellular Vesicles in Patients with Solid Tumours. J. Adv. Res. 2024, S2090-1232, (24)00090-0, Advance Online Publication. [Google Scholar] [CrossRef]
- Choi, Y.; Kwon, Y.; Kim, D.K.; et al. Gut Microbe-Derived Extracellular Vesicles Induce Insulin Resistance, Thereby Impairing Glucose Metabolism in Skeletal Muscle. Sci Rep. 2015, 5, 15878. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.; Jiang, M.; Peng, D.; Dou, X.; Song, Y.; Shi, J. The Potential Role of Gut Microbial-Derived Exosomes in Metabolic-Associated Fatty Liver Disease: Implications for Treatment. Front. Immunol. 2022, 13, 893617. [Google Scholar] [CrossRef]
- Domínguez Rubio, A.P.; D'Antoni, C.L.; Piuri, M.; Pérez, O.E. Probiotics, Their Extracellular Vesicles and Infectious Diseases. Front. Microbiol. 2022, 13, 864720. [Google Scholar] [CrossRef]
- Rodovalho, V.R.; da Luz, B.S.R.; Rabah, H.; do Carmo, F.L.R.; Folador, E.L.; Nicolas, A.; Jardin, J.; Briard-Bion, V.; Blottière, H.; Lapaque, N.; et al. Extracellular Vesicles Produced by the Probiotic Propionibacterium freudenreichii CIRM-BIA 129 Mitigate Inflammation by Modulating the NF-κB Pathway. Front. Microbiol. 2020, 11, 1544. [Google Scholar] [CrossRef]
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D'Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, C.M.; Guerri, C.; Ureña, J.; Pascual, M. Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication. Int. J. Mol. Sci. 2021, 22, 4235. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Tiwari, P.; Dwivedi, R.; Bansal, M.; Tripathi, M.; Dada, R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J. Clin. Med. 2023, 12, 1650. [Google Scholar] [CrossRef]
- Filidou, E.; Kandilogiannakis, L.; Shrewsbury, A.; Kolios, G.; Kotzampassi, K. Probiotics: Shaping the gut immunological responses. World J. Gastroenterol. 2024, 30, 2096–2108. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017, 23, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef]
- Olimpio, F.; da Silva, J.R.M.; Vieira, R.P.; Oliveira, C.R.; Aimbire, F. Lacticaseibacillus rhamnosus modulates the inflammatory response and the subsequent lung damage in a murine model of acute lung inflammation. Clinics 2022, 77, 100021. [Google Scholar] [CrossRef]
- Tang, J.; Wei, Y.; Pi, C.; Zheng, W.; Zuo, Y.; Shi, P.; Chen, J.; Xiong, L.; Chen, T.; Liu, H.; et al. The therapeutic value of bifidobacteria in cardiovascular disease. NPJ Biofilms Microbiomes 2023, 9, 82. [Google Scholar] [CrossRef]
- Forssten, S.D.; Ouwehand, A.C.; Griffin, S.M.; Patterson, E. One Giant Leap from Mouse to Man: The Microbiota–Gut–Brain Axis in Mood Disorders and Translational Challenges Moving towards Human Clinical Trials. Nutrients 2022, 14, 568. [Google Scholar] [CrossRef] [PubMed]
- Noshadi, N.; Heidari, M.; Naemi Kermanshahi, M.; Zarezadeh, M.; Sanaie, S.; Ebrahimi-Mameghani, M. Effects of Probiotics Supplementation on CRP, IL-6, and Length of ICU Stay in Traumatic Brain Injuries and Multiple Trauma Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid.-Based Complement. Altern. Med. 2022, 2022, 4674000. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shen, J.; Feng, S.; Huang, C.; Wang, H.; Huo, F.; Liu, H. Akkermansia muciniphila, which is enriched in the gut microbiota by metformin, improves cognitive function in aged mice by reducing the proinflammatory cytokine interleukin-6. Microbiome 2023, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.R.; Carroll, I.; Azcarate-Peril, M.A.; Rochette, A.D.; Heinberg, L.J.; Peat, C.; Steffen, K.; Manderino, L.M.; Mitchell, J.; Gunstad, J. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 2017, 38, 104–107. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Vasquez, E.C.; Pereira, T.M.C.; Peotta, V.A.; Baldo, M.P.; Campos-Toimil, M. Probiotics as Beneficial Dietary Supplements to Prevent and Treat Cardiovascular Diseases: Uncovering Their Impact on Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 3086270. [Google Scholar] [CrossRef]
- Vauzour, D.; Martinsen, A.; Layé, S. Neuroinflammatory processes in cognitive disorders: Is there a role for flavonoids and n-3 polyunsaturated fatty acids in counteracting their detrimental effects? Neurochem. Int. 2015, 89, 63–74. [Google Scholar] [CrossRef]
- Feng, R.; Ma, L.J.; Wang, M.; Liu, C.; Yang, R.; Su, H.; Yang, Y.; Wan, J.B. Oxidation of fish oil exacerbates alcoholic liver disease by enhancing intestinal dysbiosis in mice. Commun. Biol. 2020, 3, 1. [Google Scholar] [CrossRef]
- Kumar, M.; Pal, N.; Sharma, P.; Kumawat, M.; Sarma, D.K.; Nabi, B.; Verma, V.; Tiwari, R.R.; Shubham, S.; Arjmandi, B.; et al. Omega-3 Fatty Acids and Their Interaction with the Gut Microbiome in the Prevention and Amelioration of Type-2 Diabetes. Nutrients 2022, 14, 1723. [Google Scholar] [CrossRef]
- Gupta, K.; Tappiti, M.; Nazir, A.M.; Koganti, B.; Memon, M.S.; Aslam Zahid, M.B.; Shantha Kumar, V.; Mostafa, J.A. Fecal Microbiota Transplant in Recurrent Clostridium difficile Infections: A Systematic Review. Cureus 2022, 14, e24754. [Google Scholar] [CrossRef] [PubMed]
- Vendrik, K.E.W.; Ooijevaar, R.E.; de Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gu, Y.; Xu, C.; Du, K.; Zhao, C.; Zhao, Y.; Liu, X. Transplantation of fecal microbiota from APP/PS1 mice and Alzheimer’s disease patients enhanced endoplasmic reticulum stress in the cerebral cortex of wild-type mice. Front. Aging Neurosci. 2022, 14, 858130. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef]
- Cani, P.D.; Moens de Hase, E.; Van Hul, M. Gut Microbiota and Host Metabolism: From Proof of Concept to Therapeutic Intervention. Microorganisms 2021, 9, 1302. [Google Scholar] [CrossRef]
- Wang, B.; Cai, W.; Zhang, Z.; Zhang, H.; Tang, K.; Zhang, Q.; Wang, X. Circulating microparticles in patients after ischemic stroke: a systematic review and meta-analysis. Rev. Neurosci. 2021, 32(1), 1–10. [Google Scholar] [CrossRef]
- Feingold, K.R. Cholesterol Lowering Drugs. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Eds.; MDText.com, Inc.: South Dartmouth (MA), 2000. [Google Scholar]
- Alcocer, L.A.; Bryce, A.; De Padua Brasil, D.; Lara, J.; Cortes, J.M.; Quesada, D.; Rodriguez, P. The Pivotal Role of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers in Hypertension Management and Cardiovascular and Renal Protection: A Critical Appraisal and Comparison of International Guidelines. Am. J. Cardiovasc. Drugs 2023, 23(6), 663–682. [Google Scholar] [CrossRef] [PubMed]
- Wilmanski, T.; Kornilov, S.A.; Diener, C.; Conomos, M.P.; Lovejoy, J.C.; Sebastiani, P.; Orwoll, E.S.; Hood, L.; Price, N.D.; Rappaport, N.; Magis, A.T.; Gibbons, S.M. Heterogeneity in statin responses explained by variation in the human gut microbiome. Med (N.Y.). [CrossRef]
- Pérez-Cobas, A.E.; Gomez-Valero, L.; Buchrieser, C. Metagenomic approaches in microbial ecology: An update on whole-genome and marker gene sequencing analyses. Microb. Genom. 2020, 6. [Google Scholar] [CrossRef]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12(1), 1801944. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Reiner, Ž.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Bagheri Ekta, M.; Sahebkar, A. Antioxidant effects of statins by modulating Nrf2 and Nrf2/HO-1 signaling in different diseases. J. Clin. Med. 2022, 11(5), 1313. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Lu, J.; Qiao, G.; Mao, X.; Zhao, J.; Wang, G.; Tian, P.; Chen, W. Bifidobacterium breve CCFM1025 improves sleep quality via regulating the activity of the HPA axis: A randomized clinical trial. Nutrients 2023, 15(21), 4700. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Tan, H.T.T.; Groeger, D.; Andrews, M.; Buckley, M.; Murphy, E. F.; Groeger, J. A. Bifidobacterium longum 1714 improves sleep quality and aspects of well-being in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Sci. Rep. 2024, 14, 3725. [Google Scholar] [CrossRef]
Figure 1.
Schematic diagram of gut-brain-microbiota axis. The brain and gut microbes communicate via many pathways. The central nervous system (CNS) interacts with gut microbes through a variety of direct and indirect gut-brain axis (GBA) pathways. They include the immune pathway (including cytokines), short-chain fatty acids (SCFAs), and microbial metabolites; the neuroactive pathway (including neurotransmitters and neuroactive metabolites); the neural pathway (including the enteric nervous system, vagus nerve, and spinal nerves); and the endocrine pathway i.e., the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis response involves hypothalamic neurons that release hormones such as corticotropin receptor hormone (CRH) into the portal circulation or the brain, resulting in the release of the hormone adrenocorticotropic hormone (ACTH), which initiates cortisol production and release. Cortisol regulates the neuroimmune signaling reactions.
Figure 1.
Schematic diagram of gut-brain-microbiota axis. The brain and gut microbes communicate via many pathways. The central nervous system (CNS) interacts with gut microbes through a variety of direct and indirect gut-brain axis (GBA) pathways. They include the immune pathway (including cytokines), short-chain fatty acids (SCFAs), and microbial metabolites; the neuroactive pathway (including neurotransmitters and neuroactive metabolites); the neural pathway (including the enteric nervous system, vagus nerve, and spinal nerves); and the endocrine pathway i.e., the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis response involves hypothalamic neurons that release hormones such as corticotropin receptor hormone (CRH) into the portal circulation or the brain, resulting in the release of the hormone adrenocorticotropic hormone (ACTH), which initiates cortisol production and release. Cortisol regulates the neuroimmune signaling reactions.
Figure 2.
Schematic representation linking the microbiota-gut-brain axis and their potential impact on cerebral small vessel disease (CSVD). An imbalance in gut microbiota, also known as dysbiosis, contributes to decreased short-chain fatty acids (SCFAs) activity, increased secretion of lipopolysaccharides (LPS), trimethylamine-N-oxide (TMAO), and pro-inflammatory cytokines. These alterations can weaken the gut epithelial barrier and promote a systemic inflammatory response and vice versa. Dysbiosis-related molecules such as cytokines, and gut-derived LPS enter the systemic circulation, activating the peripheral immune system and leading to further inflammation. Through vagal signalling and systemic inflammation, these circulating factors influence the central nervous system. In the brain, they contribute to disrupted microcirculation i.e., endothelial dysfunction, increased production of reactive oxygen species (ROS), elevated cytokine levels, and blood-brain barrier (BBB) damage. This cascade of events ultimately exacerbates CSVD phenotypes by promoting neuroinflammation, vascular impairment, and potential cognitive decline.
Figure 2.
Schematic representation linking the microbiota-gut-brain axis and their potential impact on cerebral small vessel disease (CSVD). An imbalance in gut microbiota, also known as dysbiosis, contributes to decreased short-chain fatty acids (SCFAs) activity, increased secretion of lipopolysaccharides (LPS), trimethylamine-N-oxide (TMAO), and pro-inflammatory cytokines. These alterations can weaken the gut epithelial barrier and promote a systemic inflammatory response and vice versa. Dysbiosis-related molecules such as cytokines, and gut-derived LPS enter the systemic circulation, activating the peripheral immune system and leading to further inflammation. Through vagal signalling and systemic inflammation, these circulating factors influence the central nervous system. In the brain, they contribute to disrupted microcirculation i.e., endothelial dysfunction, increased production of reactive oxygen species (ROS), elevated cytokine levels, and blood-brain barrier (BBB) damage. This cascade of events ultimately exacerbates CSVD phenotypes by promoting neuroinflammation, vascular impairment, and potential cognitive decline.
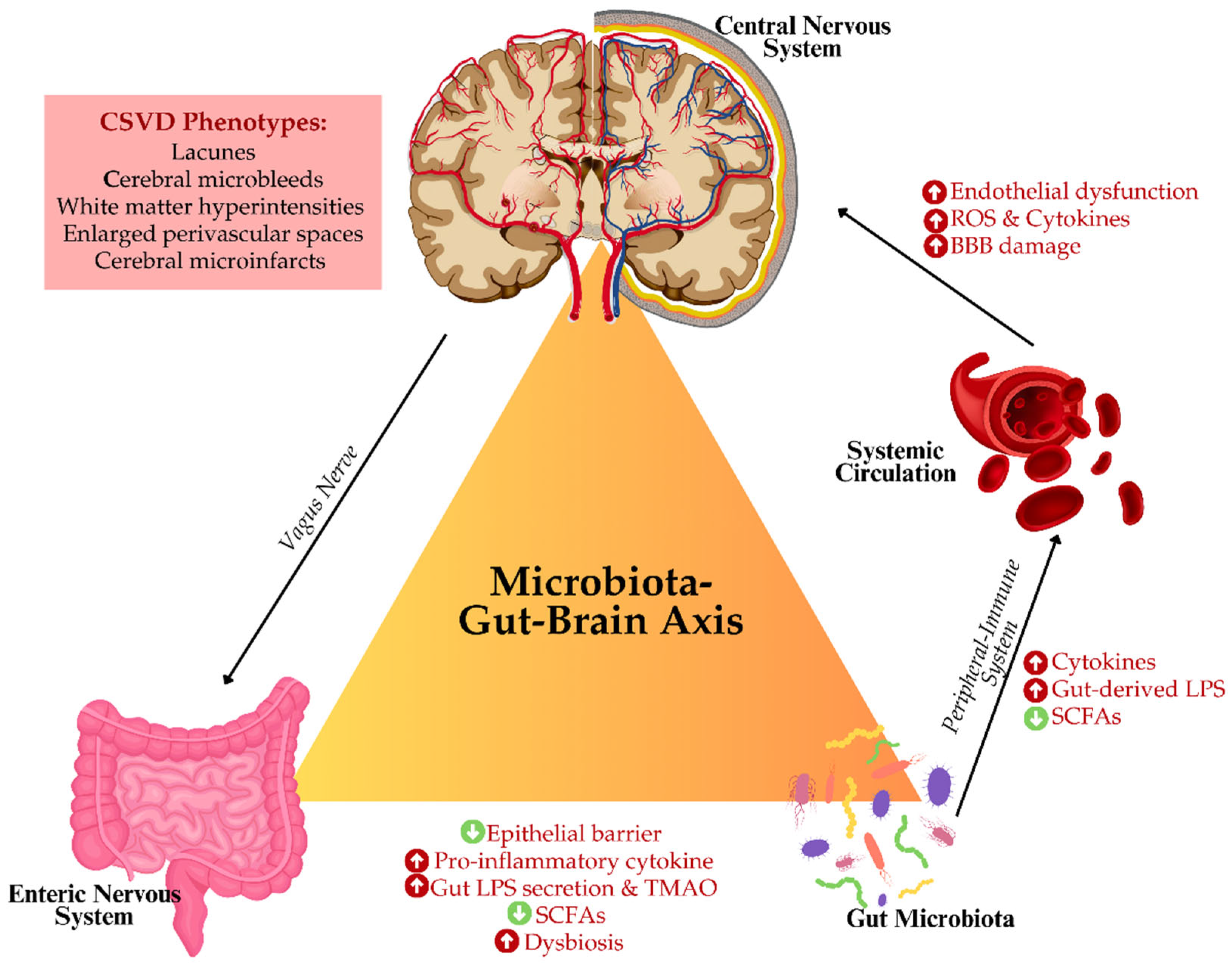
Figure 3.
This schematic illustration of the complex interplay between the microbiota-gut-brain axis, cerebral small vessel disease (CSVD), sleep, and the glymphatic system. Dysbiosis in gut microbiota triggers inflammatory responses, producing cytokines, lipopolysaccharides (LPS), and reducing short-chain fatty acids (SCFAs) that affect the central nervous system (CNS). Disruptions in circadian rhythm impair the glymphatic waste clearance, impacting glymphatic flow and microglial activity. Gut-microbial-, circulating cell- and astrocyte-derived microparticles (MPs) and inflammatory mediators contribute to blood-brain barrier (BBB) damage and thrombo-inflammation. These interactions promote CSVD phenotypes, including microbleeds and white matter changes, emphasizing the role of systemic inflammation and neuroimmune dysfunction in CSVD progression.
Figure 3.
This schematic illustration of the complex interplay between the microbiota-gut-brain axis, cerebral small vessel disease (CSVD), sleep, and the glymphatic system. Dysbiosis in gut microbiota triggers inflammatory responses, producing cytokines, lipopolysaccharides (LPS), and reducing short-chain fatty acids (SCFAs) that affect the central nervous system (CNS). Disruptions in circadian rhythm impair the glymphatic waste clearance, impacting glymphatic flow and microglial activity. Gut-microbial-, circulating cell- and astrocyte-derived microparticles (MPs) and inflammatory mediators contribute to blood-brain barrier (BBB) damage and thrombo-inflammation. These interactions promote CSVD phenotypes, including microbleeds and white matter changes, emphasizing the role of systemic inflammation and neuroimmune dysfunction in CSVD progression.
Table 1.
Impact of sleep and diet on gut microbiota and cerebrovascular health.
Table 1.
Impact of sleep and diet on gut microbiota and cerebrovascular health.
| Factors |
Effects on Gut Microbiota |
Impact on Cerebrovascular Health |
| Sleep deprivation |
|
Impaired glymphatic clearance Increased neuroinflammation Weakened blood-brain barrier |
| High-fibre diet |
|
|
| High-fat diet |
|
|
| Poor sleep and diet |
|
|
| Polyphenol-rich diet |
|
|
Table 2.
Microparticles (MPs) in Gut-Brain Interaction and Cerebrovascular Health.
Table 2.
Microparticles (MPs) in Gut-Brain Interaction and Cerebrovascular Health.
| MPs Source |
Mechanism of Action |
Impact on CSVD |
| Circulating cell-derived MPs (e.g., platelets and endothelial cells-derived) |
Promote oxidative stress Increase coagulation |
|
| Gut-microbial-derived MPs |
|
|
| Immune cell-derived MPs |
|
Neuroinflammation BBB disruption |
| Probiotic-regulated MPs |
|
Reduced inflammation Improved vascular health |
Table 3.
Therapeutic approaches to modulate gut microbiota for CSVD and glymphatic function.
Table 3.
Therapeutic approaches to modulate gut microbiota for CSVD and glymphatic function.
| Therapy |
Mechanism |
Potential Impact on Glymphatic System & CSVD |
| Probiotics |
|
|
| Prebiotics |
|
|
| Omega-3 Fatty Acids |
Modulate gut microbiota Reduce oxidative stress |
|
| Polyphenol-Rich Diet |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).