1. Introduction
Climate change caused by greenhouse gases has catalyzed scientific research in many fields and has driven researchers to develop technologies for a more sustainable world [
1,
2,
3,
4,
5]. Climate change causes reduction of arable land leading to food security concerns [
1,
6,
7,
8]. In Food Technology, the demand to reduce the carbon dioxide footprint and thus minimize climate change while increasing food security, requires the reduction of food waste by developing new preservation technologies that can extend the shelf-life of foods [
9,
10,
11,
12,
13]. In the direction of a more sustainable world, researchers have replaced chemical additives used as preservation agents with natural bioactive compounds such as essential oils (EOs) and their derivatives, as well as natural extract preservatives [
14,
15,
16,
17]. In the food packaging sector, these natural preservatives are incorporated into packages by developing active packaging [
18,
19,
20]. To that end, nanotechnology provides new approaches for the nanoencapsulation of such bioactive compounds in active packaging films that minimize their loss and increase their antioxidant and antibacterial activity [
12,
21,
22,
23,
24]. One of the most used nanoencapsulation technologies of EOs and their derivatives is r adsorption on nanocarriers such as nanoclays, natural zeolites, silicas and activated carbons [
25,
26,
27,
28,
29,
30,
31] and the incorporation of such hybrids in packaging films which results in slow and controlled release of EO’s [
32,
33,
34,
35,
36,
37]. Among natural nanoclays halloysite nanotubes (HNT) and montmorillonite (Mt) are the most common. HNT has an advantage because of its tubular shape which leads to the slow release of the adsorbed EOs and/or their derivatives, as well as for the mechanical reinforcement of the obtained polymer/HNT nanocomposite matrix [
33,
38,
39]. On the other hand, Mt has the advantage that it can be easily organically modified (OrgMt) and, thus adsorb higher amounts of EOs and their derivatives than pure Mt [
40,
41]. Recently Laponite nanoclay (Lap) was studied as an ideal nanocarrier for drug delivery, biomedical and essential oil adsorption applications [
42,
43,
44,
45,
46]. Lap is a synthetic layered smectite clay mineral and its crystalline structure is very similar to that of montmorillonite, where an octahedral layer of magnesium cations is bonded with oxygen and hydroxyl groups, is sandwiched by two tetrahedral silicate layers, referred to as the 2:1 structure[
42,
43,
47]. Lap has a higher external surface area and higher d-spacing than Mt, while its particles have a discoidal shape with a diameter ranging from 25 to 30 nm [
42,
43,
48]. Recently, Ferreira et al. [
43] directly mixed Lavender, tea tree, and rosemary oil with Lap. Thermogravimetric analysis showed that the EO amount absorbed into Lap was more than 240 mg∙g-1 for all EOs used. X-Ray diffraction analysis showed an increase in the Lap interlayer d-spacing, indicating that EOs molecules were successfully intercalated, and forming larger grain aggregates [
43]. In contrast, EOs adsorption on Mt is usually limited to the external surfaces of Mt and no intercalation takes place [
25,
29,
40].
Eugenol (EG) is a hydroxyphenyl propene, naturally occurring in the essential oils of plants such as clove oil and is largely used in both foods and cosmetics as a flavoring agent [
49]. A large body of recent scientific studies suggest that EG exerts beneficial effects on human health as an antioxidant and anti-inflammatory agent. It also shows excellent antimicrobial activity against fungi and a wide range of gram-negative and gram-positive bacteria [
49,
50,
51]. Even though in the last few years there have been many studies on the adsorption of EOs derivatives such as thymol (TO) and carvacrol (CV) in nanoclays to develop antibacterial nanohybrids to the best of our knowledge none has focused on EG alone [
52,
53,
54,
55]. Recently, Saadat et al. [
56] incorporated clove oil which contains EG into HNT and the developed clove oil-HNT nanohybrid was further combined with chitosan for the formulation of active food packaging films used organically modified Mt and HNT nanoclays with TWEEN-80 surfactant to incorporate TO, CV and EG. The obtained nanohybrids were incorporated into linear low-density polyethylene films to obtain nanocomposite active films with strong antibacterial activity against pathogenic bacteria, and controlled release properties of thymol, carvacrol and eugenol [
57]. However, no study has focused on the incorporation of pure EG in either unmodified Mt or Lap nanoclays.
Low-density polyethylene (LDPE) is widely used in flexible food packaging films because of its high water barrier properties, good tensile properties, and tear resistance up to -60°C [
58,
59,
60]. Because of its excellent thermomechanical properties, LDPE can be used in the production of flexible packaging films via hot extrusion and hot-blowing processes [
61]. It is quite resistant to acids, bases, salt solutions and usual organic solvents up to a temperature of 60°C [
60]. The ability to produce bio-polyethylene (bio-PE) which is polyethylene made from ethylene derived from non-fossil fuels such as bioethanol makes LDPE a material that can be used in the future in food packaging [
62,
63].
This study presents the development and characterization of novel EG@Lap and EG@Mt nanohybrids and novel LDPE/xEG@Lap and LDPE/xEG@Mt hybrid (where x= 5, 10 and 15 wt.%) active films. The two hybrids are studied and their performance in active packaging films compared and discussed.
2. Materials and Methods
2.1. Materials
The LDPE used was offered by the Mantzaris plastic film industry (Tsakiri, Zevgolatio, Korinthia, 200 06, Greece). Eugenol, 2-Methoxy-4-(2-propenyl)phenol, 4-Allyl-2-methoxyphenol, 4-Allylguaiacol with CAS No.: 97-53-0 was purchased from Sigma-Aüldrich (Darmstadt, Germany). Lap (Laponite) with a cation exchange capacity (CEC) of 50 meq per 100 g was purchased from Southern Clay Products Inc. Mt with CAS Number:1318-93-0 30 and CEC 30 meq/100g was purchased from Sigma-Aüldrich (Darmstadt, Germany). 2,2-diphenyl-1-picrylhydrazyl (DPPH) (CAS Number:1898-66-4 was purchased also from Sigma-Aüldrich (Darmstadt, Germany).
2.2. Preparation of EG@Lap and EG@Mt Nanohybrids
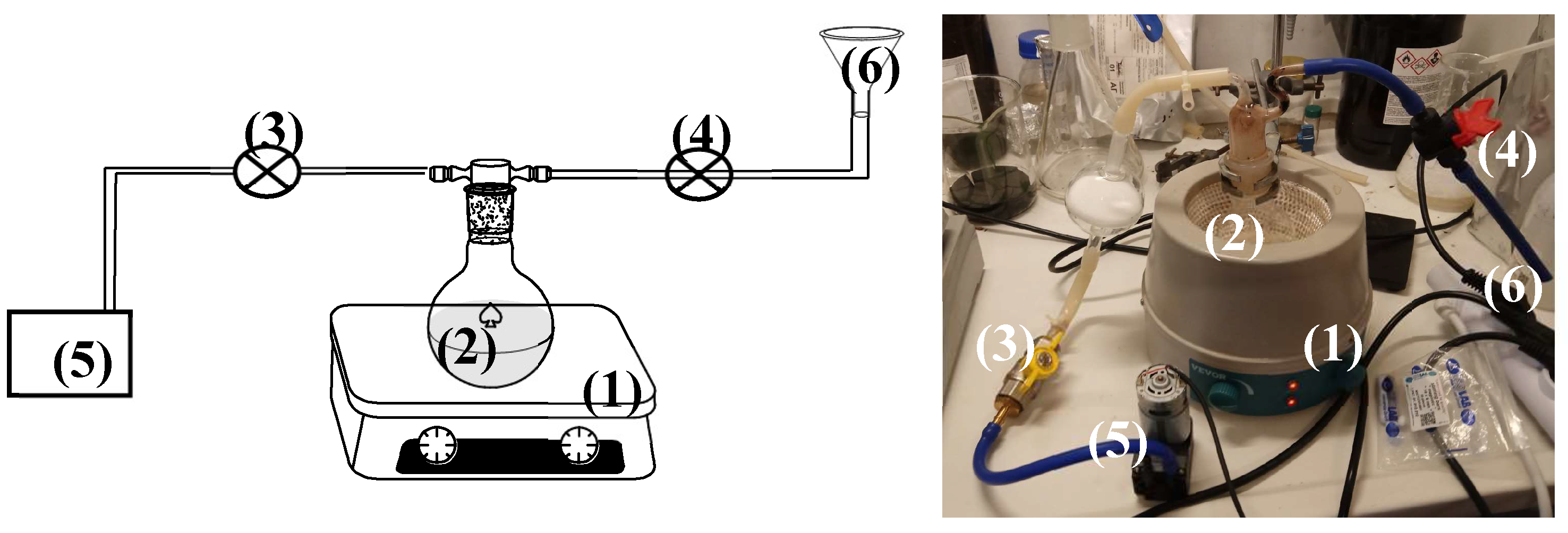
For the preparation of both EG@Lap and EG@Mt nanohybrids the vacuum-assisted adsorption process was followed. Specifically, 3 g of pure Lap or Mt were placed in a spherical glass flask and heated for 15 min at 100 °C under 3 bar of vacuum (see
Figure 1). Under these conditions, all the adsorbed water was removed from the clay. After the adsorbed water was removed, Laponite turned its colour from light white to grey while Mt turned from light beige to grey-beige. After the end of the drying process, the security valve of the pump was closed, and the safety valve of the EG tank was opened to allow the EG to be adsorbed dropwise and under-stirring into the glass spherical flask (see
Figure 1). The EG@Lap and EG@Mt nanohybrids were removed from the glass flask and kept at 25 oC at 50%RH for further characterization and use.
2.3. Preparation of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
LDPE/xEG@Lap and LDPE/xEG@Mt active films were made using a twin screw mini lab extruder (Haake Mini Lab II, Thermo Scientific, ANTISEL, S.A., Athens, Greece). The twin screw extruder rotation was adjusted at 100 rpm, while the temperature was kept at 200 oC; the extrusion time was 3 min. Appropriate amounts aof LDPE pellets were mixed with 0.25, 0.50 and 0.75 g of EG@Lap or EG@Mt to achieve a final EG@Lap or EG@Mt nominal content of 5, 10 and 15 wt.%, respectively. For comparison pure LDPE was also extruded under the same conditions and referred to as “blank” sample. The extruded pellets (LDPE/xEG@Lap and LDPE/xEG@Mt) were converted to LDPE/xEG@Lap and LDPE/xEG@Mt active films with 10 cm average diameter and 0.1 mm average thickness by heat pressing the pellets at 110 oC with 1 tn pressure for 2 min (Specac Atlas™ Series Heated Platens, Specac, Orpinghton, UK). The sample names LDPE, EG@Lap, and the EG@Mt as well as the twin extruder operating condition employed for the preparation of all LDPE/xEG@Lap and LDPE/xEG@Mt active films are listed in
Table 1 for comparison.
2.4. Physicochemical Characterization of EG@Lap and EG@Mt Nanohybrids
EG@Lap and EG@Mt nanohybrids as well as pure Lap and Mt were characterized with X-Ray Diffraction (XRD) analysis using a Brüker XRD D8 Advance diffractometer (Brüker, Analytical Instruments, S.A., Athens, Greece). FTIR spectra were recorded using an FT/IR-6000 JASCO Fourier-transform spectrometer (JASCO, Interlab, S.A., Athens, Greece).
To determine the total EG amount adsorbed on Lap or Mt, desorption kinetic experiments were carried out for both EG@Lap and EG@Mt nanohybrids, using a moisture analyzer AXIS AS-60 (AXIS Sp. z o.o. ul. Kartuska 375b, 80–125 Gdańsk, Poland). Approximately 100 mg of each nanohybrid was placed in the moisture analyzer and its weight was recorded as a function of time (m
t). Measurements were done at 70, 90, and 110 °C. The desorption kinetic experiments were repeated thrice. From the mt vs t measurements, the normalized values of the fraction q
t=(1−m
t/m
0) were calculated and plotted as a function of time. The plots were fitted using the well-known pseudo-second-order adsorption-desorption equation [
64,
65]. For process order, n=2 the overall normalized mass balance is given by:
where k
2 is the rate constant of the pseudo-second-order kinetic model (s−1), qt is the desorbed fraction capacity at time t, qe=(1-me/m0) is the maximum desorbed fraction capacity at equilibrium, m0 is the initial EOs loading into the nanohybrid, and mt is the EOs amount remaining in the nanohybrid at time t. By integrating equation (1) we achieve the pseudo-second-order kinetic model:
The initial release rate can be computed via the equation (1) and for t=0 (i.e., q
t=0). Thus:
From the best-fitted plots, the k
2 and q
e values were calculated. Using the estimated k
2 parameter the ln(k
2) term was calculated and plotted as a function of (1/T) to determine the desorption energy (Ε
0des) according to the Arrhenius equation and the theory presented in detail in [
66,
67,
68]:
and its linear transformed type:
where k
2 is the rate constant of the pseudo-second order kinetic model (s
−1), Ε
0des is the desorption activation energy, and A is the Arrhenius constant.
2.5. Physicochemical Characterization of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
The obtained LDPE/xEG@Lap and LDPE/xEG@Mt active films as well as pure LDPE film characterized with XRD analysis by using a a Brüker XRD D8 Advance diffractometer (Brüker, Analytical Instruments, S.A., Athens, Greece) and FTIR analysis by using an FT/IR-6000 JASCO Fourier-transform spectrometer (JASCO, Interlab, S.A., Athens, Greece).
2.6. Morphological Characterization of EG@Lap and EG@Mt Nanohybrids and LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
Scanning electron microscopy (SEM) images EG@Lap and EG@Mt nanohybrids as well as pure Lap and Mt nanoclays were acquired using a Zeiss Gemini 500 SEM at a low accelerating voltage of 3 kV to reduce the excitation volume and enhance resolution. The images were captured using an in-lens secondary electron detector, and the powders were mounted on an aluminum holder with double-sided adhesive carbon tape. In case of LDPE/xEG@Lap and LDPE/xEG@Mt active films as well as pure LDPE film the SEM study was conducted at a low accelerating voltage of 1.5 kV to prevent films degradation.
2.7. Tensile Properties of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
Tensile properties of active films as well as neat LDPE film were determined according to the ASTM D638 method by employing a Simantzü AX-G 5kNt instrument (Simantzü. Asteriadis, S.A., Athens, Greece). Three to five dog bone-shaped samples were tensioned, and the stress–strain values were recorded. By using the applicable software (TrapeziumX version 1.5.6, Simantzü, Asteriadis, S.A., Athens, Greece) the mean elastic modulus (E), ultimate strength (σuts), and % elongation at break (%ε) were calculated.
2.8. Water/Oxygen Barrier Properties of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
2.8.1. Water Barrier Properties
Water barrier properties of active films as well as neat LDPE film were measured with a handmade apparatus according to the ASTM E96/E 96M-05 method at 38 °C and 95% RH by following the methodology described in detail elsewhere [
69]. The Water Vapor Transmission Rate (WVTR) values were transformed to water vapor diffusion coefficient (D
wv) by using Fick’s law and the following equation:
where WVTR [g/(cm
2.s)] is the water vapor transmission rate, Dx (cm) is the film thickness, and DC (g/cm
3) is the humidity concentration gradient on the two opposite sides of the film.
2.8.2. Oxygen Barrier Properties
Oxygen barrier properties were determined according to ASTM D 3985 at 23
oC and 0%RH using an oxygen permeation analyzer (O.P.A., 8001, Systech Illinois Instruments Co., Johnsburg, IL, USA). Three disk shape films with an average diameter of 10 cm were placed in the sample area of the analyzer and the oxygen transmission rate (OTR cc/m
2/day) values were measured. The average thickness of each sample was measured after the end of OTR measurament by measuring the thickness in fiftheen different points of the film. The oxygen diffusion coefficient value for each sample was calculated using the methodology described in detail elsewhere [
69].
2.9. In Vitro Antioxidant Activity Determination of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
For all experiments a 250 ml of 2.16 mM (mmol/L) DPPH radical methanolic standard solution was used. For the calibration curve five DPPH radical methanolic solution with 10, 20, 30, 40, and 50 mg/L were prepared by diluting the appropriate amounts of DPPH radical standard solution and their absorbance measured at 517 nm using a SHIMADZU UV-1280 UV/VIS Spectrometer. The calibration curve of absorbance (y) versus the concentration (x) of [DPPH
•] free radical was expressed by the following equation:
For the determination of the concentration required to obtain a 50% antioxidant effect (EC
50) from all films, 10, 20, 30, 40, and 50 mg of granule film were placed in dark vials in triplicates. 3 mL of DPPH radical methanolic solution and 2 mL of acetate buffer 100 mM (pH = 7.10) were added to each vial, and the absorbance of the reaction mixture was measured at 517 nm after 8 h. For a blank sample, we used a vial containing 3 mL of DPPH radical methanolic solution and 2 mL of acetate buffer without the addition of any granule film. The % inhibition of DPPH radical was calculated using the following equation:
2.10. EG Release Kinetics of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
Release kinetics of EG from all samples were determined by using a moisture analyzer AXIS AS-60 (AXIS Sp. z o.o. ul. Kartuska 375b, 80-125 Gdańsk, Poland). For each film three to five samples 500 to 700 mg of each film was placed inside the moisture analyzer, and its mass was monitored by heating at 70 °C for 1 h. From the mass loss values (mt) as a function of time (t), the mean values of initial EG release rate (RREG,initial) were calculated. Finallly the rate constant of the pseudo-second order kinetic model k2 (s−1), and the desorption capacity at equilibrium qe s were calculated by fiiting the obtained mt and t values with the pseudo-second order kinetic equation described above (2).
2.11. Packaging Preservation Test of Fresh Minced Pork Wrapped with LDPE/15EG@Lap, LDPE/15EG@Mt Active Films and Pure LDPE Film
2.11.1. Packaging Preservation Test of Minced Pork Meat
For the packaging preservation test of minced pork meat LDPE/15EG@Lap, LDPE/15EG@Mt films were selected as the optimum to test. Minced pork meat was offered by the Aifantis meat procesing company within one hour of slaughter. The minced pork meat was from the hind pork’s leg. The minced pork meat was seperated in portions of approximately 80–100 g each. Its portion was aseptically wrapped between two disk shaped films of LDPE/15EG@Lap and LDPE/15EG@Mt and placed inside the Aifantis company’s commercial wrapping paper without the inner film. As a control, a portion of the minced pork meat was aseptically wrapped with two neat LDPE films and placed inside the Aifantis company’s commercial wrapping paper without the inner film. For all tests, samples for the second, forth, sixth, eighth, and tenth day of preservation were prepared and stored under dark refrigerator conditions at 4 ± 1 °C (LG GC-151SA, Weybridge, UK). Then, total viable count, sensory analysis, pH analysis, and colorimetry analysis were measured periodically during the ten days of storage.
2.11.2. Total Viable Count (TVC) of Minced Pork Meat
For the determination of total viable count (TVC) for all samples a recently described methodology was followed [
26].
2.11.3. Sensory Analysis of Minced Pork Meat
Meat color, odor, and texture during the 10 days of storage was evaluated periodically via the sensory analysis and ranked from 0 (lowest degree of each characteristic in the tested samples) to 5 (highest degree of each characteristic in the tested samples) by seven experienced panelist/members of the Department of Food Science and Technology experienced in meat sensory evaluation using the conventional descriptive analysis [
70,
71,
72].
2.11.4. pH Analysis of Minced Pork Meat
The pH values of the minced pork meat was measured using a portable pH meter fitted with a penetration electrode and a temperature sensor (pH-Star, Matthäus GmbH, Poettmes, Germany) by following the procedure described in detail recently [
24]. To ensure accuracy and reliability the procedure was conducted in triplicate, and for each treatment group, ten separate pH readings were taken.
2.11.5. Lab* Analysis
The alterations in the CIELAB color parameters (L*, a*, and b*) of the minced pork meat over a period of 10 days of storage were assessed using a LS171 colorimeter from Linshang Company. Color evaluations were conducted directly on the surface of the minced pork meat, with each treatment group comprising three separate portions. For each of these portions, five discrete readings were taken to capture a robust assessment of the color. The total color differences (ΔE) were calculated using the following equation:
In this equation, L*0, a*0, b*0 denote the initial color parameters of the minced pork meat at day 0 post-treatment. L*, a*, b* represent the respective color parameters at different time points during the 10 days of storage at 4 °C.
2.12. Statistical Analysis
All data acquired from structural and mechanical properties measurements, along with, antioxidant activity, total viable count, sensory analysis, pH analysis, and colorimetry analysis were subjected to statistical analysis to indicate any statistical differences. The non-parametric statistical test method Kruskal-Wallis was chosen to evaluate the significance of difference between the properties’ mean values. Assuming a significance level of p < 0.05, all measurements were conducted using three separate samples of each LDPE, LDPE/xEG@Lap, and LDPE/xEG@Mt film. Statistical analysis was done using SPSS software (v. 28.0, IBM, Armonk, NY, USA).
3. Results
3.1. Physicochemical Characterization of EG@Lap and EG@Mt Nanohybrids
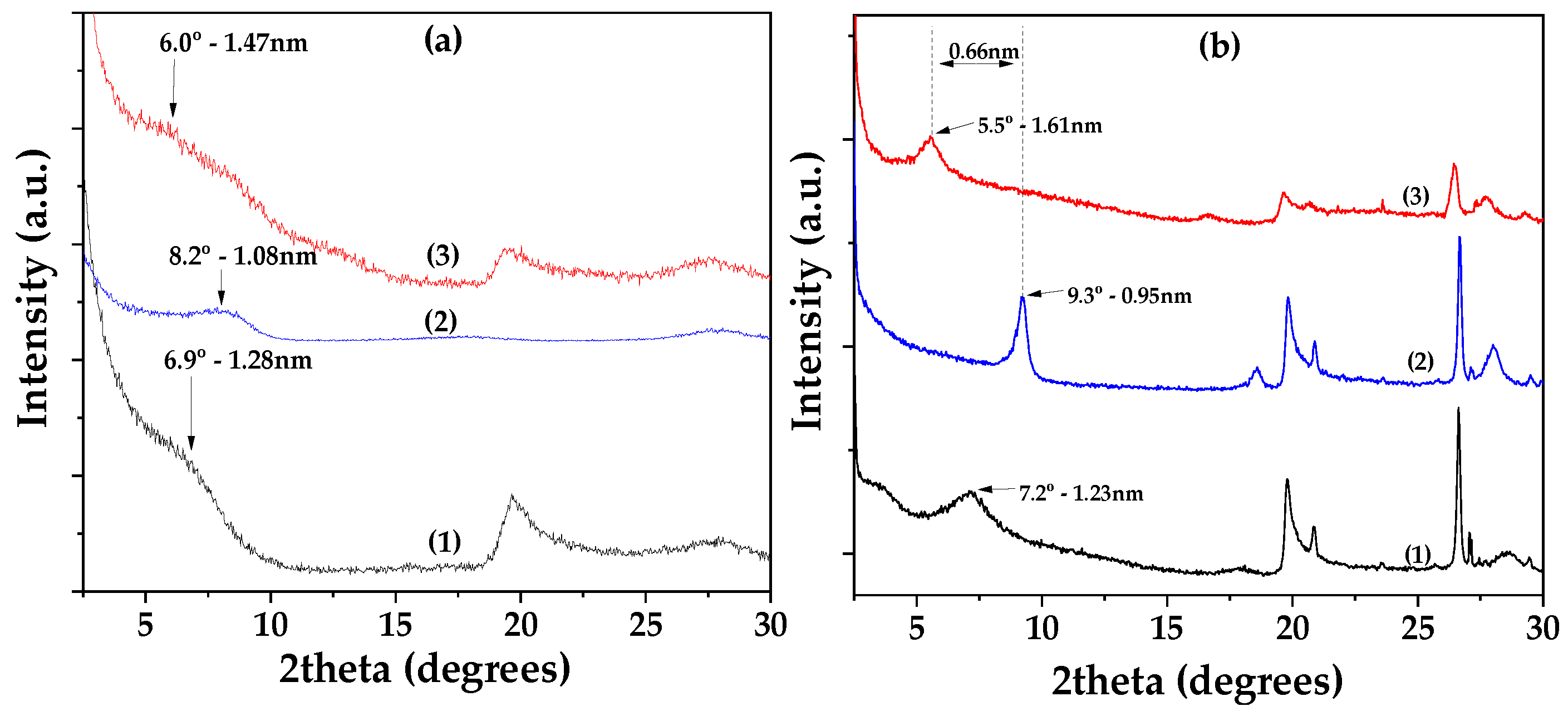
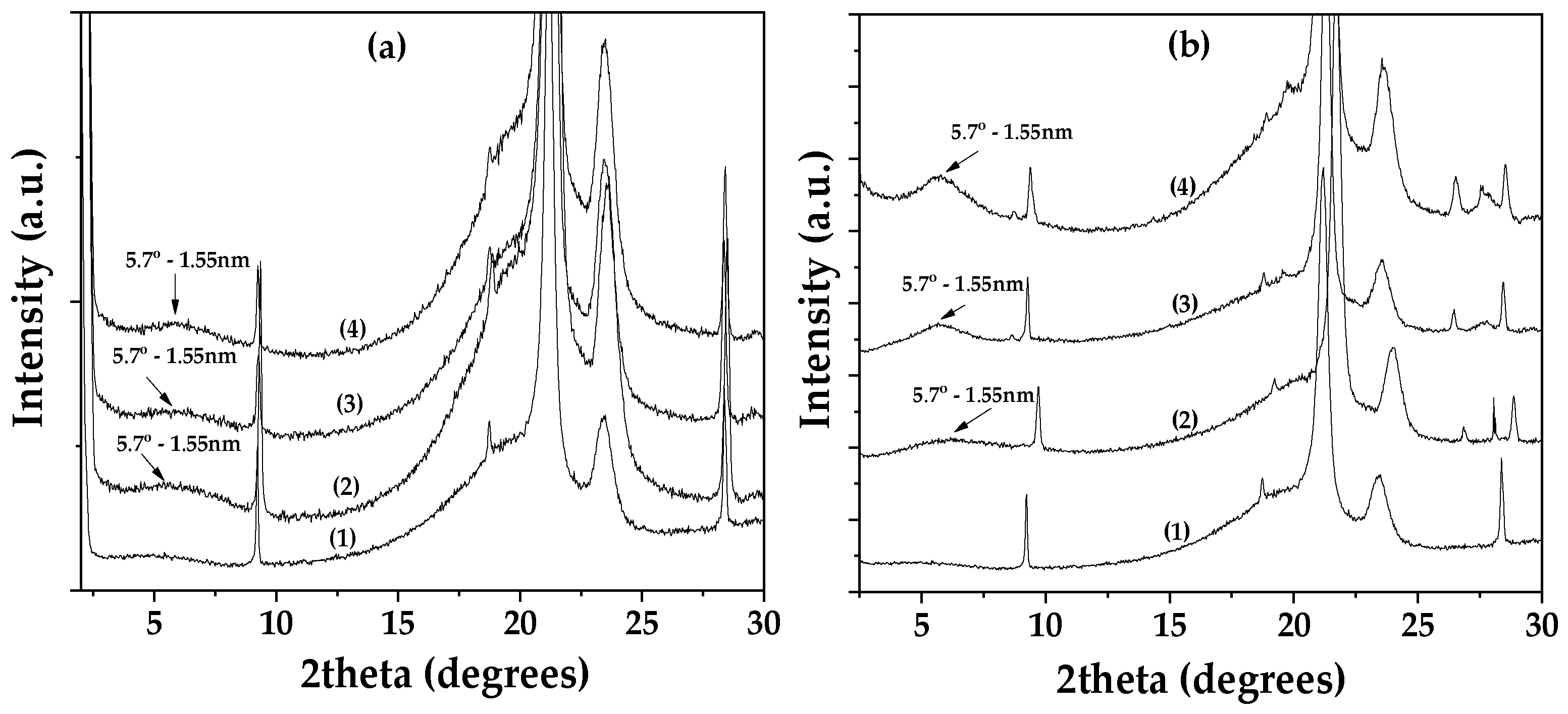
Figure 1(a) shows the XRD patterns of Lap as received (bottom), dried Lap (middle), and modified EG@Lap nanohybrid (top). The XRD pattern of the as received Lap shows the characteristic reflection of Lap’s basal space at 2theta=6.9
o which corresponds to a d-spacing of 1.28 nm. After the vacuum desorption process the d-spacing of dried Lap decreases to 1.08 nm. The decrease is consistent with the removal of water molecules from the interlayer of laponite. In EG@Lap nanohybrid a broad shoulder can be seen at ~6.0
o. While it is difficult to assign a single d-spacing from this broad peak it is clear that it is higher from the dry or even the pristine laponite and consistent with the intercalation of EG in the galleries of Lap.
Figure 1(b) shows the corresponding patterns for montmorillonite. The XRD pattern of the as received Mt shows a d-spacing of 1.23 nm which decreases to 0.93 nm after drying. The latter corresponds to collapsed layers suggesting that vacuum assisted drying removes successfully all the adsorbed water molecules. In the modified EG@Mt nanohybrid the characteristic reflection of Mt’s basal space is at 2theta=5.5
o corresponding to 1.61nm interlayer space and consistent with a nanohybrid with intercalated EG molecules.
Thus, both EG@Lap and EG@Mt nanohybrids display intercalated EG molecules in the interlayer spaces. The relatively small particle size of laponite tends to lead to a less structured, turbostratic morphology with a less well-defined d-spacing. Considering that the size of EG molecule lying flat between the layers is equal to that of phenol (0.4 nm) the observed spacings are consistent with such potential configuration.
Consistent with the above the FTIR spectra (see
Figure S1 in supplementary materials) of EG@Lap and EG@Mt (top) show the characteristic peaks of both EG (bottom) and either Lap or Mt (middle) suggesting that the intercalations is the result of simple physisorption with no chemical interactions between EG molecules and either clay.
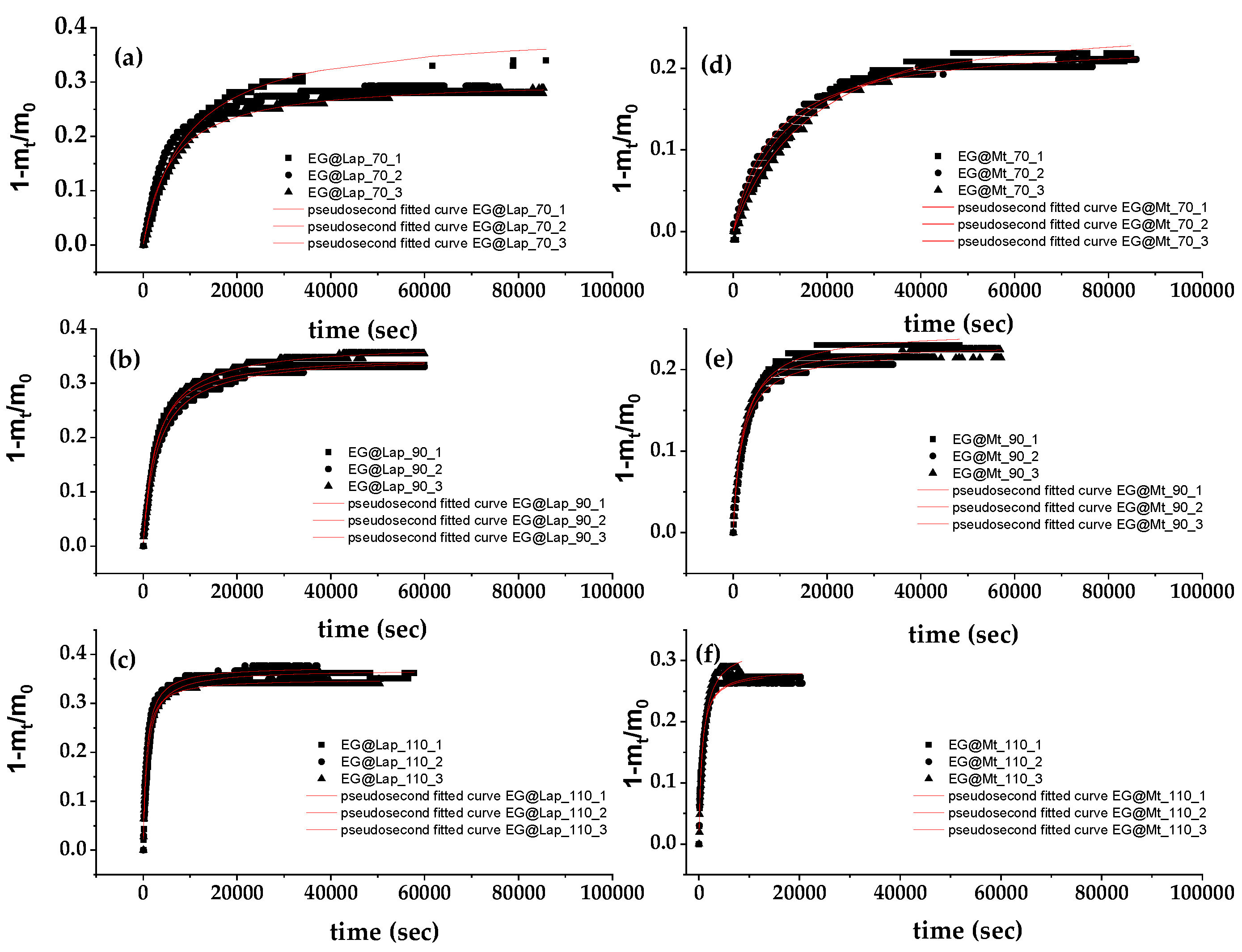
Figure 2 shows the recorded values of (1-m
t/m
0) as a function of time (t) for EG@Lap and EG@Mt nanohybrids (in triplicate) at 70, 90 and 110
oC.
These plots were fitted with pseudo second order kinetic to calculate the k
2 and q
e values according to equation (1) which are listed in
Table 1.
As seen in
Table 1 the calculated k
2, q
e mean values of EG@Lap nanohybrid are higher than the calculated k
2, q
e mean values for EG@Mt nanohybrid. Increasing the temperature leads to higher mean values for k
2, and q
e for both EG@Lap and EG@Mt nanohybrids. . The amount of desorbed EG at 70, 90 and 110
oC is 33.8, 35.6, and 36.5% respectively for EG@Lap. The corresponding values are 23.5, 26.4, and 29.9% for EG@Mt. The higher q
e mean values for EG@Lap compared to EG@Mt suggest that the desorbed EG amount is higher in the former. On the other hand, higher k
2 values mean that the EG molecules are released faster. Overall, EG@Lap nanohybrid desorbs higher EG amounts with higher initial and total rates than EG@Mt nanohybrid. This is expected because a significant amount of EG molecules is adsorbed on the external surfaces of Lap. The latter is consistent with the less well defined XRD pattern showing a turbostratic morphology [
43,
44].
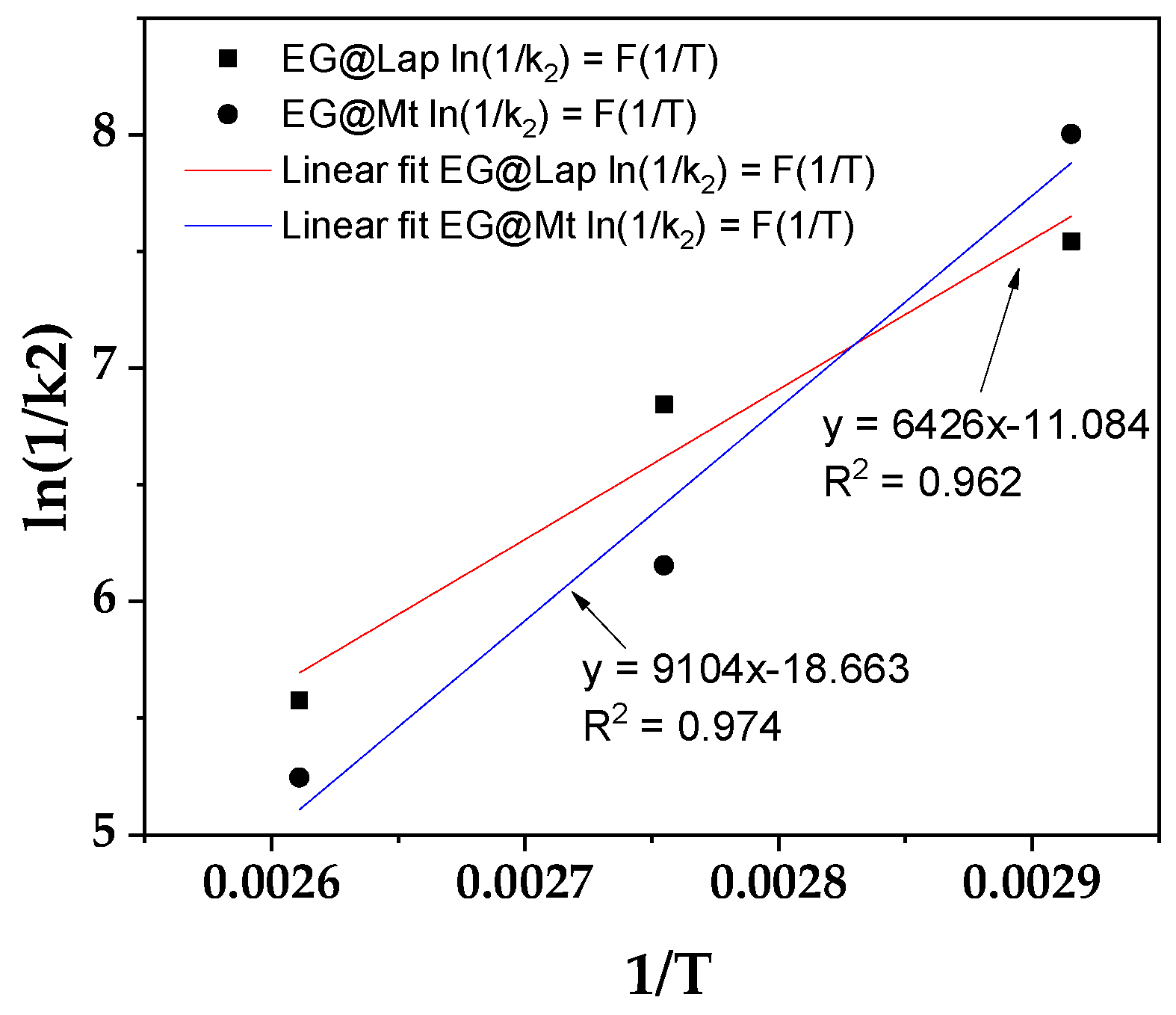
From the calculated k
2 values the ln(1/k
2) values were plotted as a function of (1/T) for both EG@Lap and EG@Mt nanohybrids (
Figure 3).
From the linear fitted plots in
Figure 3 the calculated slope was used with the equations (2) and (3) to determine the EG desorption energy (E
0des). The values are 12.6 and 17.8 Kcal/mol for EG@Lap and EG@Mt, respectively. The values are consistent with the results above. In the case of EG@Lap the EG molecules tend to be more on the external surfaces of the clay and therefore a lower desorption activation energy value is seen.
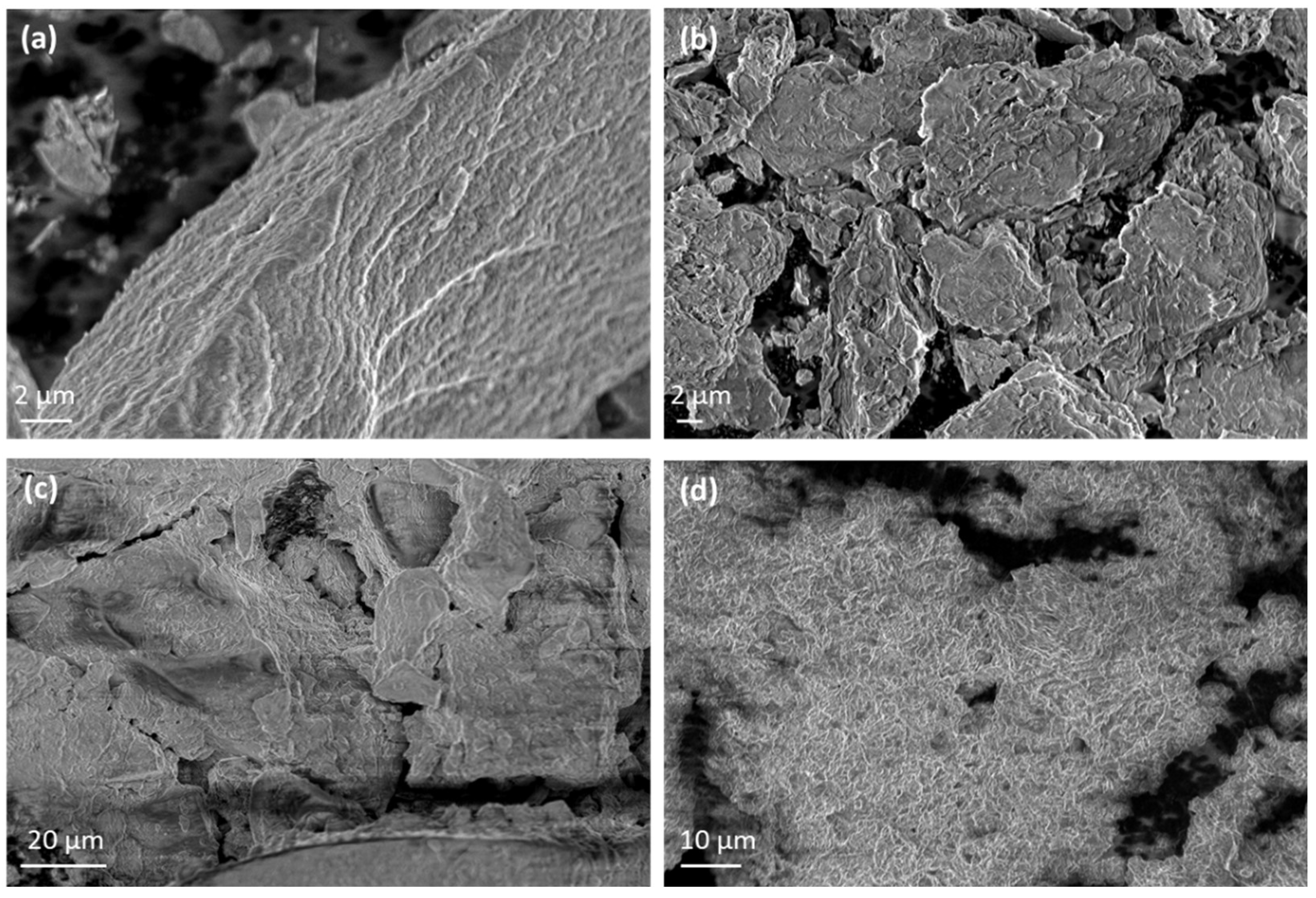
The morphological characteristics of pure Lap and Mt along with the corresponding nanohybrids are presented in
Figure 4. Representative images of pure Lap and Mt (
Figure 4a and
Figure 4b, respectively) reveal an irregular disc-like or platelet morphology with obvious lamellar structure for both cases, which is typical feature of clays [
73,
74]. Larger platelets or clusters of Lap and Mt are presented revealing their tendency to stack or overlap due to interlayer forces and particle-particle interactions.
The SEM images of EG-modified nanohybrids revealed significant changes in morphology compared to the unmodified clays. The surface of clay nanoplatelets is more compact and smoother due to the intercalation of EG molecules within the clay layers. The characteristic flaky or plate-like structure of the Lap and Mt is less pronounced, with a tendency toward a denser packing of clay flakes. The EG intercalation leads to a more uniform appearance, with fewer visible pores indicating successful adsorption between eugenol and the clay particles.
3.2. Physicochemical Characterization of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
Figure 5 shows the XRD patterns for LDPE/xEG@Lap active films (top left) and LDPE/xEG@Mt active films (top right). The corresponding FTIR spectra are on bottom left and bottom right, respectively.
The d-spacing of the films is more or less similar to the EG intercalated hybrids suggesting that no intercalation of the LDPE polymer chains inside the EG@Lap or EG@Mt nanohybrids takes place and that both EG@Lap and EG@Mt nanohybrids are dispersed in the LDPE matrix.
Figure S2 shows the FTIR spectra of pure LDPE film (bottom spectrum in the series) and those of LDPE/xEG@Lap and LDPE/xEG@Mt active films containing varying amounts of EG@clay hybrids. In FTIR spectrum of pure LDPE film, the bands centered at 2850-2950 can be attributed to the CH
2 symmetric and asymmetric stretching, respectively while the peaks located at 1460 and 715 cm
−1 are ascribed to the bending and rocking deformation of the CH
3 and CH
2 groups of LDPE [82]. For all the films consisting of either Lap or Mt the broad peak at 1050-1100 cm
−1 is attributed to the Si-O-Si stretching vibration due to the presence of clay particles.
In both LDPE/xEG@Lap and LDPE/xEG@Mt active films no shifting of the LDPE characteristic peaks is seen implying good compatibility but no specific interactions of both EG@Lap and EG@Mt nanohybrids with the LDPE chains. The results agree with previous reports were EO modified halloysite, natural zeolite, activated carbon and mesoporous silica SBA-15 dispersed in LDPE [
27,
28,
55,
75].
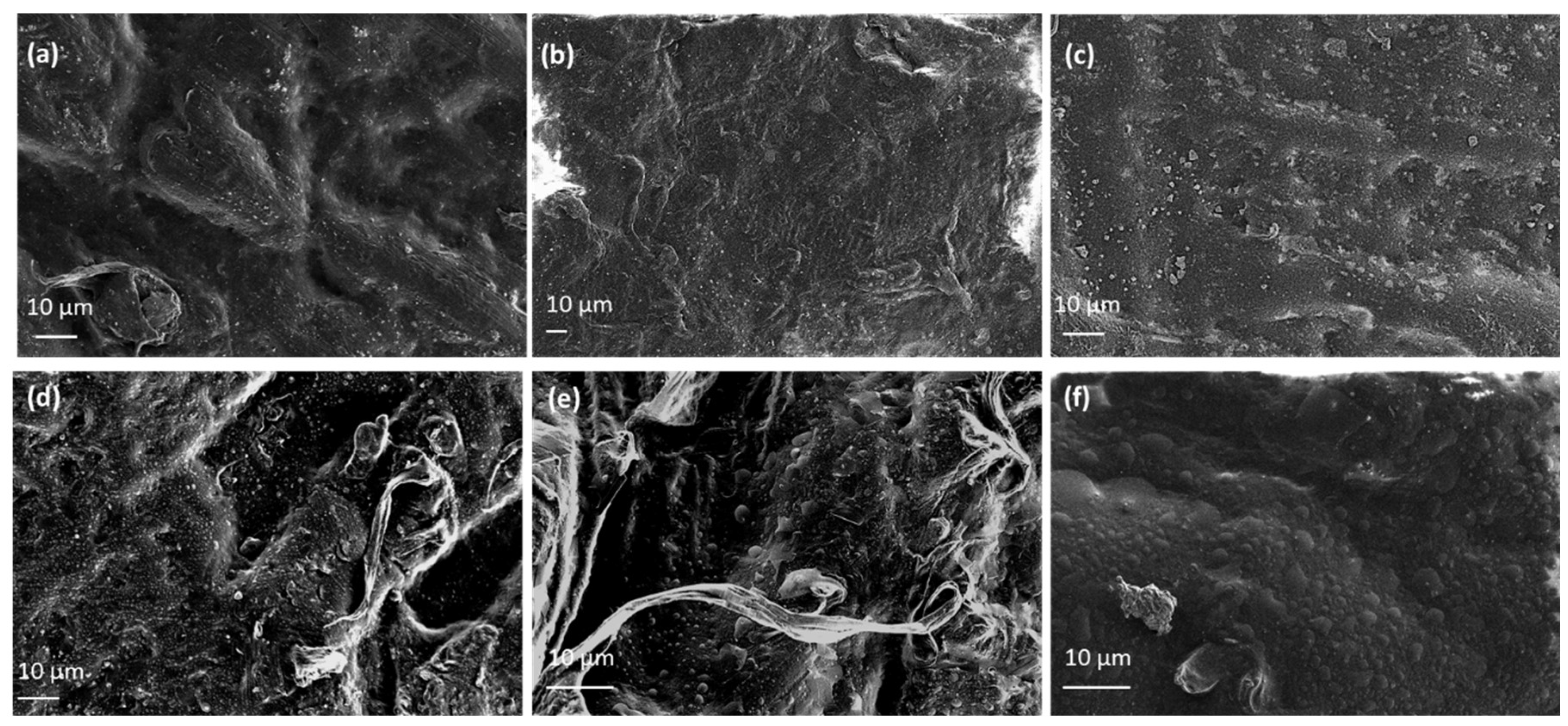
Representative SEM images of EG@Lap incorporated into LDPE shows homogeneous dispersion of clay platelets within the polymer matrix (
Figure 6, a-c). The eugenol-modified laponite platelets can be seen embedded within the LDPE film, resulting in relatively smooth surfaces. In the case of EG@Lap a rougher surface compared to laponite nanohybrid is present (
Figure 6, d-f). Overall, for both cases, as EG@clay content increases larger particles are observed implying particle agglomeration. We also note that the SEM images show a more compact morphology, indicating effective integration of the 2 types of clays and
EG within the LDPE matrix. These features are advantageous for applications in packaging, where enhanced barrier properties and antimicrobial activity are desired.
3.3. Tensile Properties of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
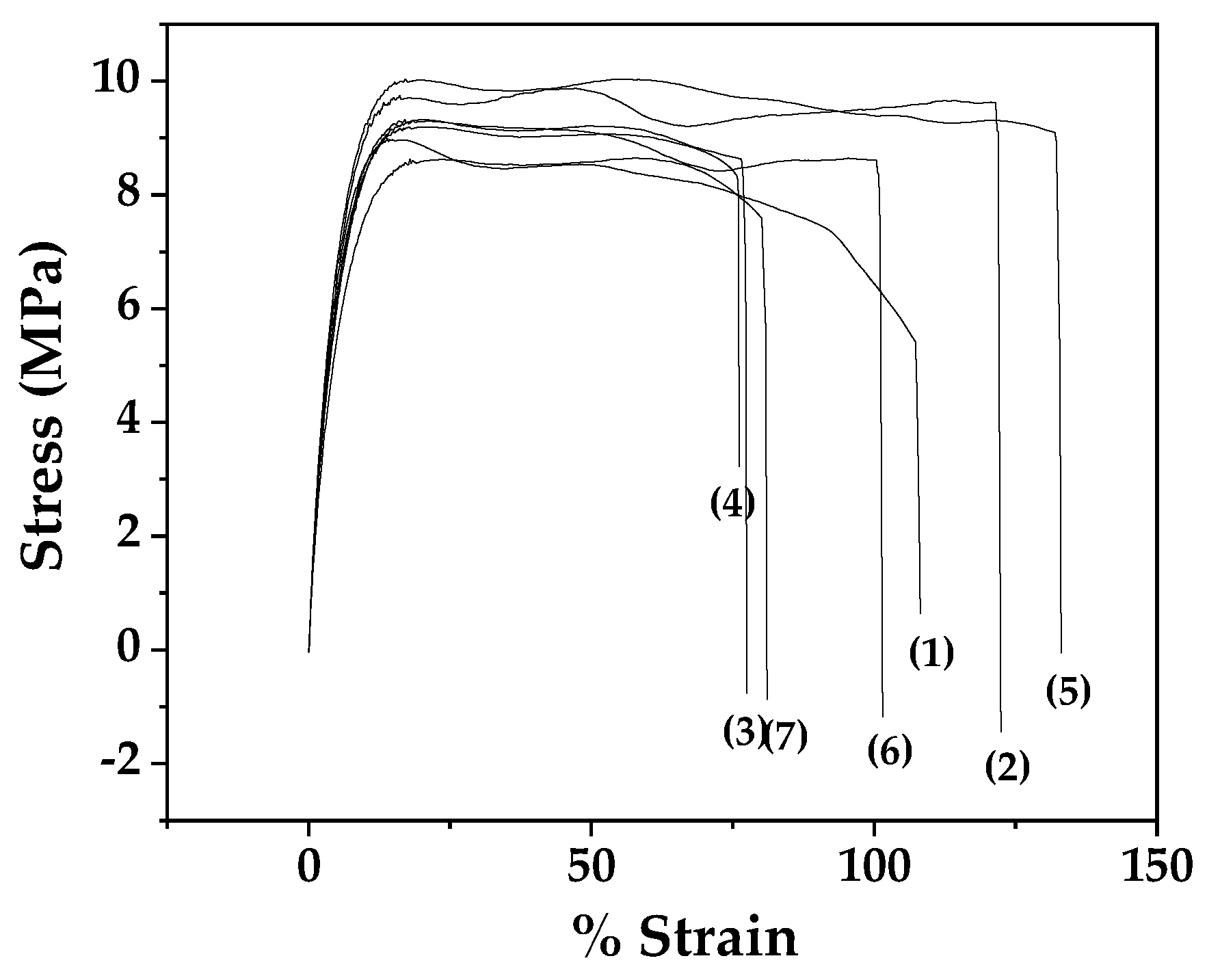
Figure 7 shows the stress-strain curves for all LDPE/xEG@Lap and LDPE/xEG@Mt active films as well as for pure LDPE film.
From the stress-strain curves of
Figure 7 the Elastic Modulus E (MPa), ultimate strength σ
uts (MPa), and % elongation at break values were calculated and are listed in
Table 2 for comparison.
Addition of EG@clay nanohybrids in LDPE leads to a softening of the films compared to the neat polymer. Interestingly, the softer films containing 5% clay led to increased ductility (higher % elongation at break). However, when the clay content is increased further the elongation at break decreased probably due to the introduction of more defects with the more agglomerated clay particles. Consistent with the above, the ultimate strength of the 5% containing clay also increased somewhat (perhaps not statistically significant) compared to the neat polymer.
3.4. Water/Oxygen Barrier Properties of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
Table 3, summarizes the water vapor transmission rate (WVTR) and oxygen transmission rate (OTR) mean values as well as the calculated water vapor diffusion coefficient (D
wv), and oxygen permeability Pe
O2 mean values of all tested of LDPE/xEG@Lap and LDPE/xEG@Mt active films as well as pure LDPE film.
It is obvious that both EG@Lap and EG@Mt nanohybrids enhance both the water and oxygen barrier properties compared to pure LDPE. The result agrees with previous reports of EO modified halloysite, natural zeolite, activated carbon and mesoporous silica SBA-15 dispersed in LDPE showed o decreased the water and oxygen permeability [
27,
28,
55,
75]
. The decrease in barrier properties is the result of the incorporation of the platy EG@Lap and EG@Mt nanohybrids [
76,
77,
78]. Calculated D
wv values are not statistically changed between LDPE/xEG@Lap and LDPE/xEG@Mt active films while the lowest P
eO2 values are obtained for LDPE films loaded with the lowest EG@Lap and EG@Mt nanohybrids content (LDPE/5EG@Lap and LDPE/5EG@Mt).
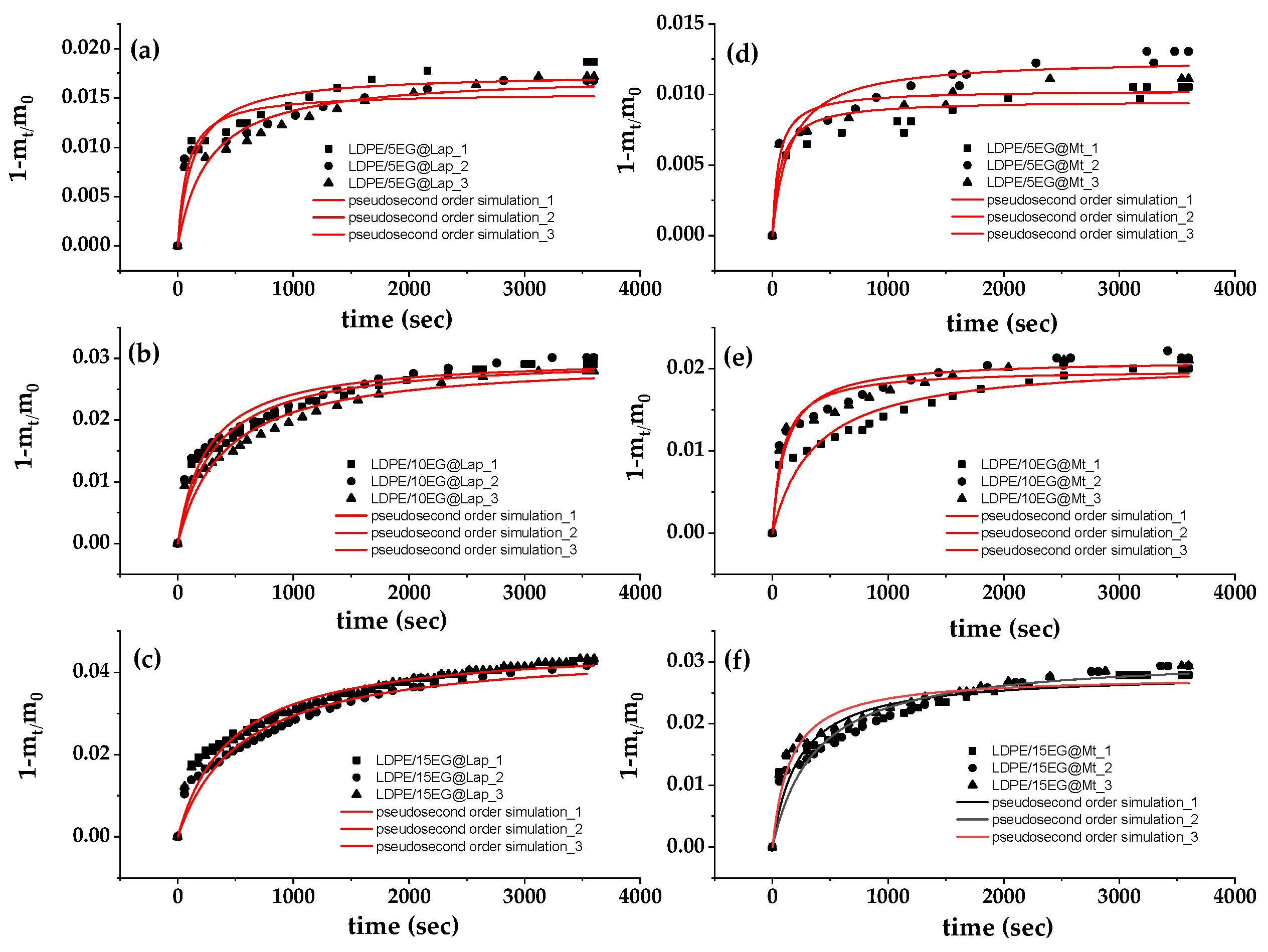
3.5. EG release Kinetics from LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
Figure 8 shows the (1-m
t/m
0) values as a function of time (t) for all LDPE/xEG@Lap and all LDPE/xEG@Mt active films (in triplicate) at 70,
oC. The data is fitted with pseudo second order kinetic to calculate the k
2 and q
e values by using equation (1). The resulted k
2, and q
e mean values are listed in
Table 4 for all LDPE/xEG@Lap and all LDPE/xEG@Mt active films.
As the EG@Lap and EG@Mt %wt. content increased the k
2 values decreased and q
e mean values increased for all LDPE/xEG@Lap and all LDPE/xEG@Mt active films. This means that as the EG@Lap and EG@Mt %wt. content increased the EG desorbed amount increased and the EG release rate decreased. This is because the dispersion of higher EG@Lap and EG@Mt amounts in LDPE matrix decreases the diffusivity and mobility of EG molecules inside the LDPE matrix in accordance with previous reports [
27,
75,
79]. Finally, all LDPE/xEG@Lap active films release higher amounts of EG with lower release rates than all LDPE/xEG@Mt active films. This is expected because of the adsorption of EG mostly on the external surfaces of Lap than Mt as it was shown above.
3.6. Antioxidant Activity of LDPE/xEG@Lap and LDPE/xEG@Mt Active Films
The EC
50,DPPH mean values for all LDPE/xEG@Lap and LDPE/xEG@Mt active films are listed in
Table 4 for comparison. As the amount of EG@Lap and EG@Mt nanohybrids in LDPE active films increased the EC
50,DPPH values decreased which means that antioxidant activity increases. In addition, the LDPE/xEG@Lap active films achieved lower EC
50,DPPH than the LDPE/xEG@Mt active films suggesting higher antioxidant activity for the former. This result agrees with higher release rates and higher % EG loaded amounts as described above for all LDPE/xEG@Lap compared to LDPE/xEG@Mt active films.
3.7. Packaging Preservation Test of Fresh Minced Pork Wrapped with LDPE/15EG@Lap, LDPE/15EG@Mt Active Films and Pure LDPE Film
3.7.1. Total Viable Counts (TVC)
Table 5 presents the results of TVC in minced meat Samples coated with the examined films across the days of storage, allowing comparison of microbial growth between the different coatings.
The results underline that the LDPE/15EG@Lap and LDPE/15EG@Mt films are more effective in controlling microbial growth throughout the storage period compared to the other samples. Specifically, by Day 6, minced meat covered with LDPE film surpassed the 7 log CFU/g threshold, while the other two samples only reached this limit by Day 8, demonstrating the superior performance of both films. To conclude, the LDPE/15EG@Lap film achieved a 1log reduction in TVC compared to LDPE by day 6, giving a shelf-life extension of 2 days more which is also a statistically significant result (see
supplementary material).
3.7.2. Sensory Analysis Results
Table 6 presents the results of the impact of the tested films on the sensory attributes of minced meat, specifically aroma, color, and texture, throughout a 10-day storage period.
The results from the table above show that both LDPE/15EG@Lap and LDPE/15EG@Mt films exhibited the highest efficacy in preserving the aroma, color, and texture of minced meat throughout the storage period compared to LDPE. In terms of aroma and color, minced meat covered with LDPE exhibited a significant decline from day 2 to day 10. In contrast, both LDPE/15EG@Lap and LDPE/15EG@Mt films-maintained meat’s sensory attributes stable, with not significant decline. Texture followed a similar trend, with LDPE showing significant deterioration between day 2 and day 10, while LDPE/15EG@Lap and LDPE/15EG@Mt remained more consistent over the storage period.
3.7.3. pH Analysis
Table 7 lists the pH evolution of the coated minced meat by the three examined samples over 10 days.
As can be clearly seen from the results above. LDPE/15EG@Lap film seems to be the most effective in controlling pH over time without statistically significant fluctuations reaching 5.39. Moreover, minced meat coated with LDPE/15EG@Mt film shows high stability over the passage of storage days. In contrary LDPE film was observed exhibited a significant pH decline starting from Day 4, reaching the lowest value of 5.37 by Day 10.
3.7.4. Lab* Colorimetry Analysis
Table 8 illustrates the changes in Lab* colorimetry parameters of coated mined meat over a 10day storage period, underlining the impact of the coating on color stability throughout the examined period.
4. Discussion
While the adsorption of essential oils (EOs) and their derivatives onto nanoclays is well established, it is known that these compounds typically adhere to the external surfaces of nanoclays rather than intercalating into their interlayer spaces [
25,
35,
80]. In non-intercalated EO@nanoclay hybrids, the interlayer space remains hydrophilic, making it incompatible with the hydrophobic chains of low-density polyethylene (LDPE), a commonly used packaging material. Achieving intercalation of EOs and their derivatives within the nanoclay interlayers could improve the controlled release properties of EO@nanoclay hybrids and enhance their functionality at higher loadings in polymer matrices like LDPE, thus enhancing the active packaging capabilities of these nanocomposite films [
81].
In this study, we demonstrate for the first time successful intercalation of eugenol (EG) molecules into the interlayer spaces of Montmorillonite (Mt) and Laponite (Lap) nanoclays using a vacuum-assisted adsorption method, as confirmed by XRD, FTIR, and SEM analyses of the resulting EG@Mt and EG@Lap nanohybrids. The vacuum-assisted adsorption method also led to higher EG loading on Mt than previously reported. Desorption kinetics experiments showed that EG@Lap desorbs more EG and at a faster rate than EG@Mt, likely due to the greater adsorption of EG on the external surfaces of Lap.
The EG@Lap and EG@Mt nanohybrids were successfully incorporated into the LDPE matrix at low (5 wt.%), medium (10 wt.%), and high (15 wt.%) concentrations via extrusion, resulting in LDPE/EG@Lap and LDPE/EG@Mt active packaging films. XRD analysis of the LDPE/xEG@Lap and LDPE/xEG@Mt films confirmed that there was no intercalation of LDPE chains into the nanohybrids, with both EG@Lap and EG@Mt dispersed uniformly throughout the LDPE matrix. FTIR analysis indicated good compatibility between the nanohybrids and LDPE without specific interactions, while SEM morphology studies revealed a compact structure that supports the effective integration of the nanoclays and EG, enhancing barrier and antimicrobial properties desirable for packaging applications.
The addition of EG@clay nanohybrids to LDPE resulted in a softening of the films compared to pure LDPE. However, as the clay content increased, elongation at break decreased, likely due to defect formation associated with clay particle agglomeration. Both EG@Lap and EG@Mt nanohybrids improved the water and oxygen barrier properties of LDPE, consistent with previous studies.
Increasing the EG@Lap and EG@Mt content in the films enhanced their antioxidant activity. Higher loadings also led to lower EG release rates (k) and higher desorbed EG contents (qe) across all LDPE/xEG@Lap and LDPE/xEG@Mt films, likely due to reduced diffusivity and mobility of EG molecules within the LDPE matrix.
Overall, LDPE/15EG@Lap and LDPE/15EG@Mt active films showed mechanical strengths comparable to pure LDPE, but with reduced water and oxygen permeability, the highest antioxidant activity, and the most favorable release characteristics (highest qe and lowest k2 values). Testing these optimized films as wrapping materials for fresh minced pork indicated that both LDPE/15EG@Lap and LDPE/15EG@Mt extended the meat’s shelf life, with LDPE/15EG@Lap providing an approximately two-day longer shelf-life extension than LDPE/15EG@Mt. This enhanced performance of LDPE/15EG@Lap is attributed to its higher EG content and faster EG release rate.
As shown in
Table 1 the total EO released fraction per gram of nanohybrid (q
e) ) for EG@Lap is consistently higher than for EG@Mt (approximately 35 % and 25 %, respectively). This indicates a greater degree of intercalation and stronger bonding between the clay’s internal surface and the EO molecules in EG@Mt. Additionally, the k
2 values indicate that below 70°C, EG@Lap releases EO at a faster rate than EG@Mt likely due to differences in bonding. However, at higher temperatures (90°C and 110°C), EG@Mt shows an increased EO release rate, possibly due to enhanced diffusion. This trend is consistent with the desorption activation energy values (E
des0) derived from the Arrhenius plots in
Figure 3. The desorption energy for EG@Mt is 17.8 kcal/mol, which is higher than the 12.6 kcal/mol observed for EG@Lap, indicating that EG@Mt interactions more closely resemble chemisorption, while EG@Lap bonds are closer to physisorption. The activation energies fall within 10-20 kcal/mol range, suggesting a combination of both physisorption and chemisorption mechanisms, supporting the pseudo- second order kinetics assumption that these two phenomena coexist.
The diffusion coefficient values suggest that adding the EG nanohybrids enhances the water vapor barrier properties of the films while the oxygen permeability coefficient remains nearly constant, exhibiting a slight improvement. This effect maybe due to the hydrophobic nature of EO. Comparing diffusion coefficients for LDPE/xEG@Lap and LDPE/xEG@Mt, it is evident that EG@Mt nanohybrids reduce these values more effectively, consistent with the lower EO release rate observed for the EG@Mt materials. Combined with the higher EO release (k2) of EG@Lap at temperatures below 70°C, these findings align with the results of the minced meat storage experiments where the LDPE/15EG@Lap film demonstrated superior preservation performance.
5. Conclusions
This study demonstrates the effectiveness of Lap and Mt as nanocarriers for EG in LDPE active packaging films, aimed at improving shelf-life and quality preservation of perishable food items. The research shows that both EG@Lap and EG@Mt nanohybrids enhance the barrier properties and antimicrobial effectiveness of LDPE films, though with distinct behaviors due to the different intercalation capacities and surface areas of Lap and Mt. The EG@Lap nanohybrids showed higher initial release rates and total desorbed eugenol quantities, attributed to Lap’s discoidal structure, which supports a more substantial eugenol loading and release profile compared to Mt.
In packaging trials with minced pork, the LDPE/15EG@Lap films extended shelf life by approximately two days longer than LDPE/15EG@Mt films. This finding highlights the potential of EG@Lap nanohybrids in active packaging applications where high release rates and enhanced food preservation are critical. Overall, this study underscores the advantages of using nanoclays, particularly Laponite, for controlled-release active packaging systems. Future studies could focus on scaling up production processes and testing additional natural antimicrobials in combination with EG@Lap to further expand the applications of this active packaging material.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Figure S1: (a) FTIR plots of (1) pure EG, (2) pure Lap and (3) EG@Lap nanohybrid, (b) FTIR plots of (1) pure EG, (2) pure Mt and (3) EG@Mt nanohybrid; Figure S2: (a) FTIR plots of (1) pure LDPE film, (2) LDPE/5EG@Lap active film, (3) LDPE/10EG@Lap active film, and (4) LDPE/15EG@Lap active film (b) FTIR plots of (1) pure LDPE film, (2) LDPE/5EG@Mt active film, (3) LDPE/10EG@Mt active film, and (4) LDPE/15EG@Mt active film; statistical analysis results.
Author Contributions
Conceptualization— C.E.S., and A.E.G.; data curation—A.K., N.C., A.L; A.E.G., and C.E.S.; formal analysis—A.K., N.C., E.P.G., A.E.G., and C.E.S.; investigation—N.C., A.L., M.A.K., and E.P.G.; methodology—A.K., M.A.K., C.E.S., and A.E.G,; project administration—E.P.G., C.E.S. and A.E.G.; resources—A.K., A.L., and N.C.; software—A.K., A.L., N.C., C.E.S., and A.E.G.; supervision—E.P.G., C.E.S., and A.E.G.; validation—A.K., A.L., N.C., M.A.K., and E.P.G.; visualization— M.A.K., E.P.G., C.E.S., and A.E.G.; writing–original draft— A.L., N.C., E.P.G., C.E.S., and A.E.G.; writing–review and editing—N.C., E.P.G., C.E.S., and A.E.G.; all authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Acknowledgments
The authors want to acknowledge Mantzaris plastic film industry (Tsakiri, Zevgolatio, Korinthia, 200 06, Greece) and especially to Mr Pantelis Kiliaris, (CEO Assistant - Alpha Pack Technical Manager) and Mr Alkiviadis Kalaras (R&D Department) for offering the LDPE pellets used for the development of films.
References
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ Sci Pollut Res 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Donovan, J.; Topple, C. Achieving Sustainability in Food Manufacturing Operations and Their Supply Chains: Key Insights from a Systematic Literature Review. Sustainable Production and Consumption 2021, 28, 1491–1499. [Google Scholar] [CrossRef]
- Ananno, A.A.; Masud, M.H.; Chowdhury, S.A.; Dabnichki, P.; Ahmed, N.; Arefin, A.Md.E. Sustainable Food Waste Management Model for Bangladesh. Sustainable Production and Consumption 2021, 27, 35–51. [Google Scholar] [CrossRef]
- Takacs, B.; Borrion, A. The Use of Life Cycle-Based Approaches in the Food Service Sector to Improve Sustainability: A Systematic Review. Sustainability 2020, 12, 3504. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Ares, G.; Thøgersen, J.; Monteleone, E. A Sense of Sustainability? – How Sensory Consumer Science Can Contribute to Sustainable Development of the Food Sector. Trends in Food Science & Technology 2019, 90, 180–186. [Google Scholar] [CrossRef]
- Branca, G.; Lipper, L.; McCarthy, N.; Jolejole, M.C. Food Security, Climate Change, and Sustainable Land Management. A Review. Agron. Sustain. Dev. 2013, 33, 635–650. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ Chem Lett 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Jain, P.C. Greenhouse Effect and Climate Change: Scientific Basis and Overview. Renewable Energy 1993, 3, 403–420. [Google Scholar] [CrossRef]
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.-P.; Marchesseau, S. Potentialities and Limits of Some Non-Thermal Technologies to Improve Sustainability of Food Processing. Front. Nutr. 2019, 5. [Google Scholar] [CrossRef]
- Rahman, M.S. Food Preservation: An Overview. In Handbook of Food Preservation; CRC Press, 2020 ISBN 978-0-429-09148-3.
- En, H.; Gupta, R.K.; Lou, F.; Moon, S.H. Role of Microbes in Environmental Sustainability and Food Preservation. In Microbes for Sustainable Development and Bioremediation; CRC Press, 2020 ISBN 978-0-429-27587-6.
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food Preservation Techniques and Nanotechnology for Increased Shelf Life of Fruits, Vegetables, Beverages and Spices: A Review. Environ Chem Lett 2021, 19, 1715–1735. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innovative Food Science & Emerging Technologies 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Giannakas, A.E. Plant Extracts-Based Food Packaging Films. In Natural Materials for Food Packaging Application; John Wiley & Sons, Ltd, 2023; pp. 23–49 ISBN 978-3-527-83730-4.
- Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; Al-Ansi, W.; Wei, M.; Yanyu, Z.; Phyo, H.M.; Galeboe, O.; Yao, W. Application of Essential Oils as Preservatives in Food Systems: Challenges and Future Prospectives – a Review. Phytochem Rev 2022, 21, 1209–1246. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Ahmed, Md.W.; Haque, Md.A.; Mohibbullah, Md.; Khan, Md.S.I.; Islam, M.A.; Mondal, Md.H.T.; Ahmmed, R. A Review on Active Packaging for Quality and Safety of Foods: Current Trends, Applications, Prospects and Challenges. Food Packaging and Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Chacha, J.S.; Ofoedu, C.E.; Xiao, K. Essential Oil-Based Active Polymer-Based Packaging System: A Review of Its Effect on the Antimicrobial, Antioxidant, and Sensory Properties of Beef and Chicken Meat. Journal of Food Processing and Preservation 2022, 46, e16933. [Google Scholar] [CrossRef]
- Aouada, F.A.; de Moura, M.R. Nanotechnology Applied in Agriculture: Controlled Release of Agrochemicals. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer International Publishing: Cham, 2015; pp. 103–118. ISBN 978-3-319-14024-7. [Google Scholar]
- de Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in Packaging for Food Industry: Past, Present, and Future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Zaharioudakis, K.; Kollia, E.; Leontiou, A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Ragkava, E.; Kehayias, G.; Proestos, C.; et al. Carvacrol Microemulsion vs. Nanoemulsion as Novel Pork Minced Meat Active Coatings. Nanomaterials 2023, 13, 3161. [Google Scholar] [CrossRef]
- Zaharioudakis, K.; Salmas, C.E.; Andritsos, N.D.; Kollia, E.; Leontiou, A.; Karabagias, V.K.; Karydis-Messinis, A.; Moschovas, D.; Zafeiropoulos, N.E.; Avgeropoulos, A.; et al. Carvacrol, Citral, Eugenol and Cinnamaldehyde Casein Based Edible Nanoemulsions as Novel Sustainable Active Coatings for Fresh Pork Tenderloin Meat Preservation. Front. Food. Sci. Technol. 2024, 4. [Google Scholar] [CrossRef]
- de Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with Essential Oils as Antimicrobial Agents, Packaging, Repellents, and Insecticides: An Overview. Colloids and Surfaces B: Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Leontiou, A.A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Development of Carvacrol@natural Zeolite Nanohybrid and Poly-Lactide Acid / Triethyl Citrate / Carvacrol@natural Zeolite Self-Healable Active Packaging Films for Minced Pork Shelf-Life Extension 2024.
- Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Leontiou, A.; Karabagias, I.K.; Georgopoulos, S.; Karydis-Messinis, A.; Zaharioudakis, K.; Andritsos, N.; Kehayias, G.; et al. Thymol@activated Carbon Nanohybrid for Low-Density Polyethylene-Based Active Packaging Films for Pork Fillets’ Shelf-Life Extension. Foods 2023, 12, 2590. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Baikousi, M.; Karabagias, V.K.; Karageorgou, I.; Iordanidis, G.; Emmanouil-Konstantinos, C.; Leontiou, A.; Karydis-Messinis, A.; Zafeiropoulos, N.E.; Kehayias, G.; et al. Low-Density Polyethylene-Based Novel Active Packaging Film for Food Shelf-Life Extension via Thyme-Oil Control Release from SBA-15 Nanocarrier. Nanomaterials 2024, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, D.; Majdoub, H.; Darder, M. An Overview of Clay-Polymer Nanocomposites Containing Bioactive Compounds for Food Packaging Applications. Applied Clay Science 2022, 216, 106335. [Google Scholar] [CrossRef]
- Villa, C.C.; Valencia, G.A.; López Córdoba, A.; Ortega-Toro, R.; Ahmed, S.; Gutiérrez, T.J. Zeolites for Food Applications: A Review. Food Bioscience 2022, 46, 101577. [Google Scholar] [CrossRef]
- Chaemsanit, S.; Matan, N.; Matan, N. Activated Carbon for Food Packaging Application: Review. Walailak Journal of Science and Technology (WJST) 2018, 15, 255–271. [Google Scholar] [CrossRef]
- Giannakas, A.E. 7 - Bionanocomposites with Hybrid Nanomaterials for Food Packaging Applications. In Advances in Biocomposites and their Applications; Karak, N., Ed.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing, 2024; pp. 201–225 ISBN 978-0-443-19074-2.
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled Release of Essential Oils Using Laminar Nanoclay and Porous Halloysite / Essential Oil Composites in a Multilayer Film Reservoir. Microporous and Mesoporous Materials 2021, 316, 110882. [Google Scholar] [CrossRef]
- Saad, H.; Ayed, A.; Srasra, M.; Attia, S.; Srasra, E.; Bouhtoury, F.C.-E.; Tabbene, O.; Saad, H.; Ayed, A.; Srasra, M.; et al. New Trends in Clay-Based Nanohybrid Applications: Essential Oil Encapsulation Strategies to Improve Their Biological Activity. In Nanoclay - Recent Advances, New Perspectives and Applications; IntechOpen, 2022 ISBN 978-1-80356-558-3.
- Essifi, K.; Hammani, A.; Berraaouan, D.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A. Montmorillonite Nanoclay Based Formulation for Controlled and Selective Release of Volatile Essential Oil Compounds. Materials Chemistry and Physics 2022, 277, 125569. [Google Scholar] [CrossRef]
- Uddin, Md.N.; Hossain, Md.T.; Mahmud, N.; Alam, S.; Jobaer, M.; Mahedi, S.I.; Ali, A. Research and Applications of Nanoclays: A Review. SPE Polymers n/a. [CrossRef]
- Frontiers | Active Food Packaging Based on Biopolymers and Aroma Compounds: How to Design and Control the Release Available online:. Available online: https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2019.00398/full (accessed on 3 August 2024).
- Biddeci, G.; Spinelli, G.; Colomba, P.; Di Blasi, F. Nanomaterials: A Review about Halloysite Nanotubes, Properties, and Application in the Biological Field. International Journal of Molecular Sciences 2022, 23, 11518. [Google Scholar] [CrossRef]
- Fahimizadeh, M.; Wong, L.W.; Baifa, Z.; Sadjadi, S.; Auckloo, S.A.B.; Palaniandy, K.; Pasbakhsh, P.; Tan, J.B.L.; Singh, R.K.R.; Yuan, P. Halloysite Clay Nanotubes: Innovative Applications by Smart Systems. Applied Clay Science 2024, 251, 107319. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Applied Clay Science 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Oliveira, L.H.; de Lima, I.S.; da, S. Neta, E.R.; de Lima, S.G.; Trigueiro, P.; Osajima, J.A.; da Silva-Filho, E.C.; Jaber, M.; Fonseca, M.G. Essential Oil in Bentonite: Effect of Organofunctionalization on Antibacterial Activities. Applied Clay Science 2023, 245, 107158. [Google Scholar] [CrossRef]
- Das, S.S.; Neelam; Hussain, K.; Singh, S.; Hussain, A.; Faruk, A.; Tebyetekerwa, M. Laponite-Based Nanomaterials for Biomedical Applications: A Review. Current Pharmaceutical Design 25, 424–443. [CrossRef]
- Ferreira, C.M.; da Silva, G.J. Absorption of Essential Oils in Laponite: Stability Enhancement and Structural Characteristics. Applied Clay Science 2023, 238, 106936. [Google Scholar] [CrossRef]
- Kiaee, G.; Dimitrakakis, N.; Sharifzadeh, S.; Kim, H.-J.; Avery, R.K.; Moghaddam, K.M.; Haghiniaz, R.; Yalcintas, E.P.; Barros, N.; Karamikamkar, S.; et al. Laponite-Based Nanomaterials For Drug Delivery. Adv Healthc Mater 2022, 11, e2102054. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, W.; Zhang, Y.; Wu, X.; Huang, J.; Bo, R.; Liu, M.; Yu, J.; Li, J. Laponite/Lactoferrin Hydrogel Loaded with Eugenol for Methicillin-Resistant Staphylococcus Aureus-Infected Chronic Skin Wound Healing. Journal of Tissue Viability 2024, 33, 487–503. [Google Scholar] [CrossRef]
- Kinninmonth, M.A.; Liauw, C.M.; Verran, J.; Taylor, R.; Edwards-Jones, V.; Shaw, D.; Webb, M. Investigation into the Suitability of Layered Silicates as Adsorption Media for Essential Oils Using FTIR and GC–MS. Applied Clay Science 2013, 83–84, 415–425. [Google Scholar] [CrossRef]
- Ruzicka, B.; Zaccarelli, E. A Fresh Look at the Laponite Phase Diagram. Soft Matter 2011, 7, 1268–1286. [Google Scholar] [CrossRef]
- Jr, J.T.; Bohor, B.F. Surface Area of Montmorillonite from the Dynamic Sorption of Nitrogen and Carbon Dioxide. Clays and Clay Minerals 1968, 16, 83–91. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit Rev Microbiol 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Elbestawy, M.K.M.; El-Sherbiny, G.M.; Moghannem, S.A. Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter Pylori. Molecules 2023, 28, 2448. [Google Scholar] [CrossRef]
- Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field - Hu - 2018 - Journal of Food Science - Wiley Online Library Available online:. Available online: https://ift.onlinelibrary.wiley.com/doi/full/10.1111/1750-3841.14180 (accessed on 4 August 2024).
- Wrona, M.; Manso, S.; Silva, F.; Cardoso, L.; Salafranca, J.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. New Active Packaging Based on Encapsulated Carvacrol, with Emphasis on Its Odour Masking Strategies. Food Packaging and Shelf Life 2023, 40, 101177. [Google Scholar] [CrossRef]
- Rojas, A.; Misic, D.; de Dicastillo, C.L.; Zizovic, I.; Velásquez, E.; Gutiérrez, D.; Aguila, G.; Vidal, C.P.; Guarda, A.; Galotto, M.J. A Review on Thymol-Based Bioactive Materials for Food Packaging. Industrial Crops and Products 2023, 202, 116977. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Alonso, J.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Modifications Induced by the Addition of a Nanoclay in the Functional and Active Properties of an EVOH Film Containing Carvacrol for Food Packaging. Journal of Membrane Science 2012, 423–424, 247–256. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Karabagias, V.K.; Karabagias, I.K.; Baikousi, M.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Zafeiropoulos, N.E.; et al. Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers 2023, 15, 282. [Google Scholar] [CrossRef]
- Saadat, S.; Rawtani, D.; Rao, P.K. Antibacterial Activity of Chitosan Film Containing Syzygium Aromaticum (Clove) Oil Encapsulated Halloysite Nanotubes against Foodborne Pathogenic Bacterial Strains. Materials Today Communications 2022, 32, 104132. [Google Scholar] [CrossRef]
- Tornuk, F.; Sagdic, O.; Hancer, M.; Yetim, H. Development of LLDPE Based Active Nanocomposite Films with Nanoclays Impregnated with Volatile Compounds. Food Research International 2018, 107, 337–345. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S.; et al. Active Flexible Films for Food Packaging: A Review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef]
- Bamps, B.; Buntinx, M.; Peeters, R. Seal Materials in Flexible Plastic Food Packaging: A Review. Packaging Technology and Science 2023, 36, 507–532. [Google Scholar] [CrossRef]
- Giannakas, A.E. 7 - Extrusion of Biopolymers for Food Applications. In Advances in Biopolymers for Food Science and Technology; Pal, K., Sarkar, P., Cerqueira, M.Â., Eds.; Elsevier, 2024; pp. 137–169 ISBN 978-0-443-19005-6.
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Burelo, M.; Hernández-Varela, J.D.; Medina, D.I.; Treviño-Quintanilla, C.D. Recent Developments in Bio-Based Polyethylene: Degradation Studies, Waste Management and Recycling. Heliyon 2023, 9, e21374. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A. Chapter 3 - Kinetic Models and Thermodynamics of Adsorption Processes: Classification. In Interface Science and Technology; Saleh, T.A., Ed.; Surface Science of Adsorbents and Nanoadsorbents; Elsevier, 2022; Vol. 34, pp. 65–97.
- Asimakopoulos, G.; Baikousi, M.; Salmas, C.; Bourlinos, A.B.; Zboril, R.; Karakassides, M.A. Advanced Cr(VI) Sorption Properties of Activated Carbon Produced via Pyrolysis of the “<i>Posidonia Oceanica”</i> Seagrass. Journal of Hazardous Materials 2021, 405, 124274. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, J. Theorie der Adsorption und verwandter Erscheinungen. Z. Physik 1924, 26, 117–138. [Google Scholar] [CrossRef]
- Knopf, D.A.; Ammann, M. Technical Note: Adsorption and Desorption Equilibria from Statistical Thermodynamics and Rates from Transition State Theory. Atmospheric Chemistry and Physics 2021, 21, 15725–15753. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Zeitschrift für Physikalische Chemie 1889, 4U, 96–116. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- ISO 11035:1994(En), Sensory Analysis — Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach Available online:. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:11035:ed-1:v1:en (accessed on 6 June 2024).
- Heymann, H.; Machado, B.; Torri, L.; Robinson, A. l. How Many Judges Should One Use for Sensory Descriptive Analysis? Journal of Sensory Studies 2012, 27, 111–122. [Google Scholar] [CrossRef]
- Assanti, E.; Karabagias, V.K.; Karabagias, I.K.; Badeka, A.; Kontominas, M.G. Shelf Life Evaluation of Fresh Chicken Burgers Based on the Combination of Chitosan Dip and Vacuum Packaging under Refrigerated Storage. J Food Sci Technol 2021, 58, 870–883. [Google Scholar] [CrossRef]
- Chalmpes, N.; Kouloumpis, A.; Zygouri, P.; Karouta, N.; Spyrou, K.; Stathi, P.; Tsoufis, T.; Georgakilas, V.; Gournis, D.; Rudolf, P. Layer-by-Layer Assembly of Clay–Carbon Nanotube Hybrid Superstructures. ACS Omega 2019, 4, 18100–18107. [Google Scholar] [CrossRef]
- Enotiadis, A.; Tsokaridou, M.; Chalmpes, N.; Sakavitsi, V.; Spyrou, K.; Gournis, D. Synthesis and Characterization of Porous Clay-Organic Heterostructures. J Sol-Gel Sci Technol 2019, 91, 295–301. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.M.; Chang, B.P.; Mekonnen, T.H. The Barrier Properties of Sustainable Multiphase and Multicomponent Packaging Materials: A Review. Progress in Materials Science 2023, 133, 101071. [Google Scholar] [CrossRef]
- Tyagi, P.; Salem, K.S.; Hubbe, M.A.; Pal, L. Advances in Barrier Coatings and Film Technologies for Achieving Sustainable Packaging of Food Products – A Review. Trends in Food Science & Technology 2021, 115, 461–485. [Google Scholar] [CrossRef]
- Huang, H.-D.; Ren, P.-G.; Zhong, G.-J.; Olah, A.; Li, Z.-M.; Baer, E.; Zhu, L. Promising Strategies and New Opportunities for High Barrier Polymer Packaging Films. Progress in Polymer Science 2023, 144, 101722. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Leontiou, A.A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Shelf Life of Minced Pork in Vacuum-Adsorbed Carvacrol@Natural Zeolite Nanohybrids and Poly-Lactic Acid/Triethyl Citrate/Carvacrol@Natural Zeolite Self-Healable Active Packaging Films. Antioxidants 2024, 13, 776. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Kumar, L.; Gaikwad, K.K. Halloysite Nanotubes for Food Packaging Application: A Review. Applied Clay Science 2023, 234, 106856. [Google Scholar] [CrossRef]
- Giannakas Na-Montmorillonite, Vs. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef]
Figure 1.
Schematic presentation (left part) and image (right part) of handmade apparatus used for the preparation of the EG@Lap and EG@Mt nanohybrids: (1) stirrer with a heating plate, (2) spherical glass flask, (3) security valve of the pump, (4) security valve of the CV tank, (5) air vacuum pump, and (6) CV tank. CV: carvacrol and NZ: natural zeolite.
Figure 1.
Schematic presentation (left part) and image (right part) of handmade apparatus used for the preparation of the EG@Lap and EG@Mt nanohybrids: (1) stirrer with a heating plate, (2) spherical glass flask, (3) security valve of the pump, (4) security valve of the CV tank, (5) air vacuum pump, and (6) CV tank. CV: carvacrol and NZ: natural zeolite.
Figure 1.
(a) XRD plots of (1) pure Lap, (2) dried Lap and (3) EG@Lap nanohybrid, (b) XRD plots of (1) pure Mt, (2) dried Mt and (3) EG@Mt nanohybrid.
Figure 1.
(a) XRD plots of (1) pure Lap, (2) dried Lap and (3) EG@Lap nanohybrid, (b) XRD plots of (1) pure Mt, (2) dried Mt and (3) EG@Mt nanohybrid.
Figure 2.
EG desorption isotherm kinetic plots (in triplicates) for EG@Lap (left part (a), (b), and (c) plots) and EG@Mt (right part (d), (e), and (f) plots) nanohybrids at 70 oC ((a) and (d) plots), 90 oC ((b) and (e) plots), and 110 oC ((c) and (f) plots). With a red line plot the simulation plots according to the pseudo second-order kinetic model.
Figure 2.
EG desorption isotherm kinetic plots (in triplicates) for EG@Lap (left part (a), (b), and (c) plots) and EG@Mt (right part (d), (e), and (f) plots) nanohybrids at 70 oC ((a) and (d) plots), 90 oC ((b) and (e) plots), and 110 oC ((c) and (f) plots). With a red line plot the simulation plots according to the pseudo second-order kinetic model.
Figure 3.
Plot of ln(1/k2) values as a function of (1/T) for both EG@Lap and EG@Mt nanohybrids.
Figure 3.
Plot of ln(1/k2) values as a function of (1/T) for both EG@Lap and EG@Mt nanohybrids.
Figure 4.
Representative SEM images of (a) pure Lap, (b) pure Mt, (c) EG@Lap, and (d) EG@Mt.
Figure 4.
Representative SEM images of (a) pure Lap, (b) pure Mt, (c) EG@Lap, and (d) EG@Mt.
Figure 5.
(a) XRD plots of (1) pure LDPE film, (2) LDPE/5EG@Lap active film, (3) LDPE/10EG@Lap active film, and (4) LDPE/15EG@Lap active film (b) XRD plots of (1) pure LDPE film, (2) LDPE/5EG@Mt active film, (3) LDPE/10EG@Mt active film, and (4) LDPE/15EG@Mt active film,.
Figure 5.
(a) XRD plots of (1) pure LDPE film, (2) LDPE/5EG@Lap active film, (3) LDPE/10EG@Lap active film, and (4) LDPE/15EG@Lap active film (b) XRD plots of (1) pure LDPE film, (2) LDPE/5EG@Mt active film, (3) LDPE/10EG@Mt active film, and (4) LDPE/15EG@Mt active film,.
Figure 6.
SEM images of (a) LDPE/5EG@Lap, (b) LDPE/10EG@Lap, (c) LDPE/15EG@Lap, (d) LDPE/5EG@Mt, (e) LDPE/10EG@Mt, and (f) LDPE/15EG@Mt active films.
Figure 6.
SEM images of (a) LDPE/5EG@Lap, (b) LDPE/10EG@Lap, (c) LDPE/15EG@Lap, (d) LDPE/5EG@Mt, (e) LDPE/10EG@Mt, and (f) LDPE/15EG@Mt active films.
Figure 7.
Stress-strain curves for (1) LDPE, (2) LDPE/5EG@Lap, (3) LDPE/10EG@Lap, (4) LDPE/15EG@Lap, (5) LDPE/5EG@Mt, (6) LDPE/10EG@Mt and (7) LDPE/15EG@Mt.
Figure 7.
Stress-strain curves for (1) LDPE, (2) LDPE/5EG@Lap, (3) LDPE/10EG@Lap, (4) LDPE/15EG@Lap, (5) LDPE/5EG@Mt, (6) LDPE/10EG@Mt and (7) LDPE/15EG@Mt.
Figure 8.
EG desorption isotherm kinetic plots (in triplicates) for all LDPE/xEG@Lap (left part (a), (b), and (c) plots) and all LDPE/xEG@Mt (right part (d), (e), and (f) plots) nanohybrids at 70 oC. With a red line plot the simulation plots according to the pseudo second-order kinetic model.
Figure 8.
EG desorption isotherm kinetic plots (in triplicates) for all LDPE/xEG@Lap (left part (a), (b), and (c) plots) and all LDPE/xEG@Mt (right part (d), (e), and (f) plots) nanohybrids at 70 oC. With a red line plot the simulation plots according to the pseudo second-order kinetic model.
Table 1.
Sample names, contents of LDPE, EG@Lap, and the EG@Mt nanohybrid, and the twin extruder operating conditions (temperature, rotation speed and time) used for the development of all LDPE/xEG@Lap and LDPE/xEG@Mt active films.
Table 1.
Sample names, contents of LDPE, EG@Lap, and the EG@Mt nanohybrid, and the twin extruder operating conditions (temperature, rotation speed and time) used for the development of all LDPE/xEG@Lap and LDPE/xEG@Mt active films.
| Sample Name |
LDPE
(g) |
EG@Lap
(g- wt.%) |
EG@Mt
(g-wt.%) |
Twin Extruder Operating Conditions |
| T (°C) |
Speed (rpm) |
Time (min) |
| LDPE |
5 |
- |
- |
200 |
100 |
3 |
| LDPE/5EG@Lap |
4.75 |
0.25-5 |
- |
200 |
100 |
3 |
| LDPE/10EG@Lap |
4.50 |
0.50-10 |
- |
200 |
100 |
3 |
| LDPE/15EG@Lap |
4.25 |
0.75-15 |
- |
200 |
100 |
5 |
| LDPE/5EG@Mt |
4.75 |
- |
0.25-5 |
200 |
100 |
3 |
| LDPE/10EG@Mt |
4.50 |
- |
0.50-10 |
200 |
100 |
3 |
| LDPE/15EG@Mt |
4.25 |
- |
0.75-15 |
200 |
100 |
3 |
Table 1.
Calculated k2, qe, and RREG,initial mean values from EG desorption kinetic plots for both EG@Lap and EG@Mt nanohybrids.
Table 1.
Calculated k2, qe, and RREG,initial mean values from EG desorption kinetic plots for both EG@Lap and EG@Mt nanohybrids.
| 70 oC |
EG@Lap |
EG@Mt |
| k2 (s-1) |
5.29x10-4±0.24x10-4
|
3.34x10-4±1.7x10-4
|
| qe |
0.338±0.053 |
0.235±0.031 |
| 90 oC |
EG@Lap |
EG@Mt |
| k2 (s-1) |
1.07x10-3±0.12x10-3
|
2.12x10-3±0.31x10-3
|
| qe |
0.356±0.013 |
0.264±0.012 |
| 110 oC |
EG@Lap |
EG@Mt |
| k2 (s-1) |
3.79x10-3±0.8x10-3
|
5.27x10-3±1.47x10-3
|
| qe |
0.365±0.014 |
0.299±0.025 |
Table 2.
Elastic Modulus E (MPa), ultimate strength σuts (MPa), and % elongation at break values were calculated for all LDPE/xEG@Lap, and LDPE/xEG@Mt active films as well as pure LDPE film.
Table 2.
Elastic Modulus E (MPa), ultimate strength σuts (MPa), and % elongation at break values were calculated for all LDPE/xEG@Lap, and LDPE/xEG@Mt active films as well as pure LDPE film.
| Sample Name |
E (Mpa) |
σuts (MPa) |
%ε |
| LDPE |
206.8 ± 16.2a
|
9.5 ± 1.0a,b
|
106.5 ± 3.7a,b
|
| LDPE/5EG@Lap |
186.7 ± 13.9a,b
|
10.2 ± 0.4a
|
122.5 ± 4.6a,d
|
| LDPE/10EG@Lap |
162.9 ± 13.4b,c
|
9.5 ± 0.3a,b
|
76.2 ± 3.4b,c
|
| LDPE/15EG@Lap |
140.4 ± 3.2c
|
8.7 ± 0.2b
|
74.8 ± 3.0c
|
| LDPE/5EG@Mt |
182.8 ± 4.6a,b
|
10.0 ± 0.4a
|
132.6 ± 4.7a
|
| LDPE/10EG@Mt |
161.6 ± 3.1b,c
|
9.5 ± 0.4a.b
|
100.6 ± 4.5a,c
|
| LDPE/15EG@Mt |
159.5 ± 2.0b,c
|
9.4 ± 0.4a,b
|
85.0 ± 4.3b,c,d
|
Table 3.
Water vapor transmission rate (WVTR) and oxygen transmission rate (O.T.R.) mean values as well as the calculated water vapor diffusion coefficient (Dwv), and oxygen permeability PeO2 mean values of all tested films.
Table 3.
Water vapor transmission rate (WVTR) and oxygen transmission rate (O.T.R.) mean values as well as the calculated water vapor diffusion coefficient (Dwv), and oxygen permeability PeO2 mean values of all tested films.
| |
Dwv (cm2.s-1) x10-5
|
PeO2 (cm2.s-1)x10-8
|
| LDPE |
48.7±9.5a
|
2.61±0.013a
|
| LDPE/5EG@Lap |
9.3±3.4b
|
1.77±0.343b
|
| LDPE/10EG@Lap |
11.1±6.8b
|
2.34±0.082a,c
|
| LDPE/15EG@Lap |
11.7±4.4a,b
|
2.03±0.136b,c,d
|
| LDPE/5EG@Mt |
12.9±6.2a,b
|
1.84±0.026b,d
|
| LDPE/10EG@Mt |
8.8±2.1b
|
2.08±0.012b,c
|
| LDPE/15EG@Mt |
6.4±4.1b
|
2.52±0.265a,c
|
Table 4.
Calculated k2, qe, and EC50,DPPH mean values for all LDPE/xEG@Lap and LDPE/xEG@Mt active films.
Table 4.
Calculated k2, qe, and EC50,DPPH mean values for all LDPE/xEG@Lap and LDPE/xEG@Mt active films.
| |
k2 (s-1) |
qe |
EC50,DPPH (mg/L) |
| LDPE |
- |
- |
n.d. |
| LDPE/5EG@Lap |
0.480±0.090 |
0.017±0.001 |
12.1±0.3 |
| LDPE/10EG@Lap |
0.110±0.023 |
0.030±0.002 |
0.33±0.02 |
| LDPE/15EG@Lap |
0.040±0.004 |
0.046±0.003 |
0.11±0.01 |
| LDPE/5EG@Mt |
1.280±0.271 |
0.011±0.002 |
18.14±2.3 |
| LDPE/10EG@Mt |
0.370±0.016 |
0.021±0.001 |
3.57±0.54 |
| LDPE/15EG@Mt |
0.160±0.072 |
0.029±0.001 |
1.29±0.23 |
Table 5.
Total Viable Counts (TVC) in Minced Meat Samples Coated with the films examined over the examined storage period.
Table 5.
Total Viable Counts (TVC) in Minced Meat Samples Coated with the films examined over the examined storage period.
| Samples |
Day 0 |
Day 2 |
Day 4 |
Day 6 |
Day 8 |
Day 10 |
| LDPE |
4.68Aa
|
6.20Bb
|
6.44Bc
|
7.14Cd
|
8.13De
|
8.27De
|
| LDPE/15EG@Lap |
4.68Aa
|
5.49Bb
|
5.7Bc
|
6.08Cd
|
7.09Ce
|
7.78Df
|
| LDPE/15EG@Mt |
4.68Aa
|
6.09Bb
|
5.72Bc
|
6.30Cd
|
7.54Ce
|
8.09Df
|
Table 6.
Sensory attributes change of coated minced meat over 10 days of storage.
Table 6.
Sensory attributes change of coated minced meat over 10 days of storage.
| Parameters |
Sample |
Day 0 |
Day 2 |
Day 4 |
Day 6 |
Day 8 |
Day 10 |
| |
LDPE |
5.00Aa
|
3.97Bb
|
3.40Cc
|
3.27Cd
|
2.77De
|
2.10Ef
|
| Aroma |
LDPE/15EG@Mt |
5.00Aa
|
4.53Bb
|
3.87Bc
|
3.50Cd
|
3.30Ce
|
2.53Df
|
| |
LDPE/15EG@Lap |
5.00Aa
|
4.47Bb
|
3.93Bc
|
3.40Cd
|
3.33Ce
|
2.73Df
|
| Color |
LDPE |
5.00 Aa
|
4.10Bb
|
4.10Bb
|
3.63Cc
|
3.53Cd
|
2.17Df
|
| |
LDPE/15EG@Mt |
5.00 Aa
|
4.37Bb
|
3.80Bc
|
3.43Cd
|
3.23Ce
|
2.43Df
|
| |
LDPE/15EG@Lap |
5.00 Aa
|
4.57Bb
|
3.90Bc
|
3.43Cd
|
3.27Ce
|
2.57Df
|
| Texture |
LDPE |
5.00 Aa
|
4.03Bb
|
3.73Cc
|
3.80Bd
|
3.67Be
|
2.57Cf
|
| |
LDPE/15EG@Mt |
5.00Aa
|
4.57Bb
|
3.87Bc
|
3.63Bd
|
3.57Be
|
2.73Cf
|
| |
LDPE/15EG@Lap |
5.00 Aa
|
4.57Bb
|
3.93Bc
|
3.87Bc
|
3.77Bd
|
2.87Ce
|
Table 7.
pH changes in minced meat samples over 10 days.
Table 7.
pH changes in minced meat samples over 10 days.
| Samples |
Day 0 |
Day 2 |
Day 4 |
Day 6 |
Day 8 |
Day 10 |
| LDPE |
5.66 (±0.04)Aa
|
5.57 (±0.04)Aa
|
5.47 (±0.04)Bb
|
5.50 (±0.04)Ab
|
5.46 (±0.04)Ab
|
5.37 (±0.04)Ac
|
| LDPE/15EG@Mt |
5.66 (±0.04)Aa
|
5.53 (±0.04)Aa
|
5.54 (±0.04)Ab
|
5.58 (±0.04)Bc
|
5.52 (±0.04)Ac
|
5.44 (±0.04)Bd
|
| LDPE/15EG@Lap |
5.66 (±0.04)Aa
|
5.53 (±0.04)Aa
|
5.52 (±0.04)Ab
|
5.55 (±0.04)Ab
|
5.52 (±0.04)Bc
|
5.39 (±0.04)Ad
|
Table 8.
Variation in Lab colorimetry parameters of coated minced meat during 10 days of storage.
Table 8.
Variation in Lab colorimetry parameters of coated minced meat during 10 days of storage.
| Sample |
Days |
L* |
a* |
b* |
ΔL (±std) |
Δa (±std) |
Δb (±std) |
ΔE (±std) |
| LDPE |
Day 0 |
56.27 (±0.52)Aa
|
16.96 (±0.64)Aa
|
10.37 (±0.19)Aa
|
— |
— |
— |
— |
| LDPE/15EG@Mt |
Day 0 |
56.27 (±0.52)Aa
|
16.96 (±0.64)Aa
|
10.37 (±0.19)Aa
|
— |
— |
— |
— |
| LDPE/15EG@Lap |
Day 0 |
56.27 (±0.52)Aa
|
16.96 (±0.64)Aa
|
10.37 (±0.19)Aa
|
— |
— |
— |
— |
| LDPE |
Day 2 |
53.34 (±0.38)Ba
|
12.49 (±0.53)Bb
|
12.19 (±0.86)Ba
|
-2.93 (±0.38) |
-4.47 (±0.53) |
1.82 (±0.86) |
5.65 (±0.38) |
| LDPE/15EG@Mt |
Day 2 |
55.34 (±0.39)Aa
|
16.33 (±0.35)Aa
|
10.53 (±0.45)Aa
|
-0.93 (±0.39) |
-0.63 (±0.35) |
0.16 (±0.45) |
1.13 (±0.39) |
| LDPE/15EG@Lap |
Day 2 |
56.09 (±0.86)Aa
|
16.06 (±0.26)Aa
|
10.41 (±0.36)Aa
|
-0.18 (±0.86) |
-0.9 (±0.26) |
0.04 (±0.36) |
0.92 (±0.86) |
| LDPE |
Day 4 |
50.75 (±0.47)Ca
|
10.24 (±0.68)Cb
|
12.92 (±0.91)Bb
|
-2.59 (±0.47) |
-2.25 (±0.68) |
0.73 (±0.91) |
3.51 (±0.47) |
| LDPE/15EG@Mt |
Day 4 |
53.18 (±0.54)Ba
|
14.19 (±0.76)Ba
|
12.03 (±0.41)Ab
|
-2.16 (±0.54) |
-2.14 (±0.76) |
1.5 (±0.41) |
3.39 (±0.54) |
| LDPE/15EG@Lap |
Day 4 |
53.00 (±0.53)Ba
|
14.75 (±0.48)Bb
|
11.86 (±0.45)Ab
|
-3.09 (±0.53) |
-1.31 (±0.48) |
1.45 (±0.45) |
3.66 (±0.53) |
| LDPE |
Day 6 |
47.23 (±0.46)Db
|
13.35 (±0.59)Db
|
13.79 (±1.04)Bb
|
-3.52 (±0.46) |
3.11 (±0.59) |
0.87 (±1.04) |
4.78 (±0.46) |
| LDPE/15EG@Mt |
Day 6 |
51.05 (±0.52)Ca
|
13.62 (±0.73)Ca
|
12.51 (±0.42)Ab
|
-2.13 (±0.52) |
-0.57 (±0.73) |
0.48 (±0.42) |
2.26 (±0.52) |
| LDPE/15EG@Lap |
Day 6 |
50.35 (±0.50)Ca
|
13.87 (±0.45)Cb
|
12.57 (±0.48)Ab
|
-2.65 (±0.50) |
-0.88 (±0.45) |
0.71 (±0.48) |
2.88 (±0.50) |
| LDPE |
Day 8 |
41.06 (±0.38)Ec
|
11.48 (±0.61)Ec
|
15.62 (±1.10)Cc
|
-6.17 (±0.38) |
-1.87 (±0.61) |
1.83 (±1.10) |
6.7 (±0.38) |
| LDPE/15EG@Mt |
Day 8 |
49.01 (±0.50)Cb
|
13.08 (±0.70)Ca
|
13.01 (±0.44)Ab
|
-2.04 (±0.50) |
-0.54 (±0.70) |
0.5 (±0.44) |
2.17 (±0.50) |
| LDPE/15EG@Lap |
Day 8 |
47.83 (±0.47)Db
|
13.03 (±0.43)Cb
|
13.33 (±0.51)Ab
|
-2.52 (±0.47) |
-0.84 (±0.43) |
0.76 (±0.51) |
2.76 (±0.47) |
| LDPE |
Day 10 |
35.72 (±0.33)Fd
|
9.99 (±0.53)Fd
|
17.65 (±1.24)Cd
|
-5.34 (±0.33) |
-1.49 (±0.53) |
2.03 (±1.24) |
5.9 (±0.33) |
| LDPE/15EG@Mt |
Day 10 |
47.05 (±0.48)Cb
|
12.55 (±0.67)Ca
|
13.53 (±0.46)Ab
|
-1.96 (±0.48) |
-0.53 (±0.67) |
0.52 (±0.46) |
2.1 (±0.48) |
| LDPE/15EG@Lap |
Day 10 |
45.44 (±0.45)Db
|
12.25 (±0.40)Cb
|
14.13 (±0.54)Bd
|
-2.39 (±0.45) |
-0.78 (±0.40) |
0.8 (±0.54) |
2.64 (±0.45) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).