Submitted:
05 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Triple-Negative Breast Cancer
2.1. Clinical Characteristics of TNBC
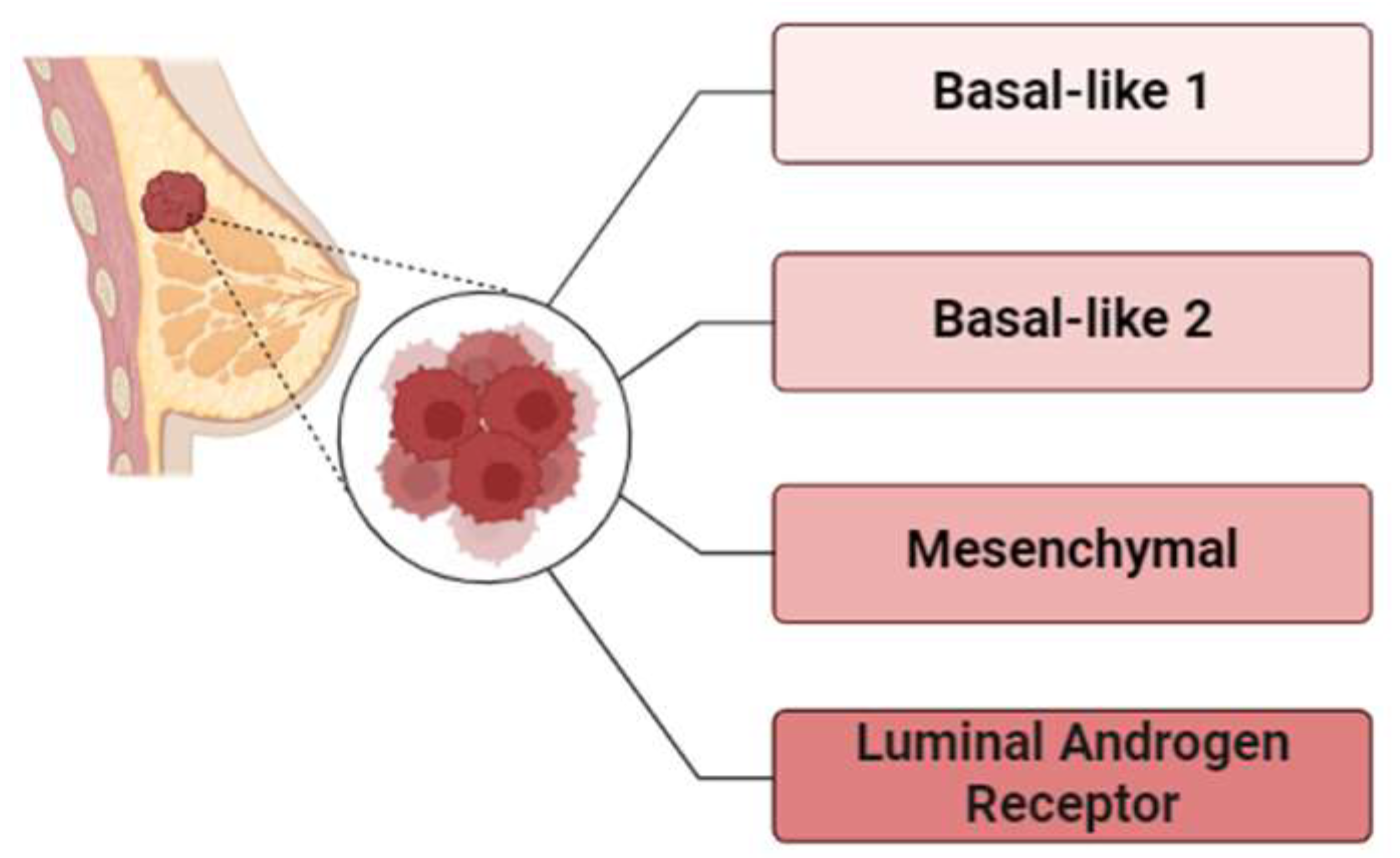
2.2. Molecular Subtypes and Heterogeneity of TNBC
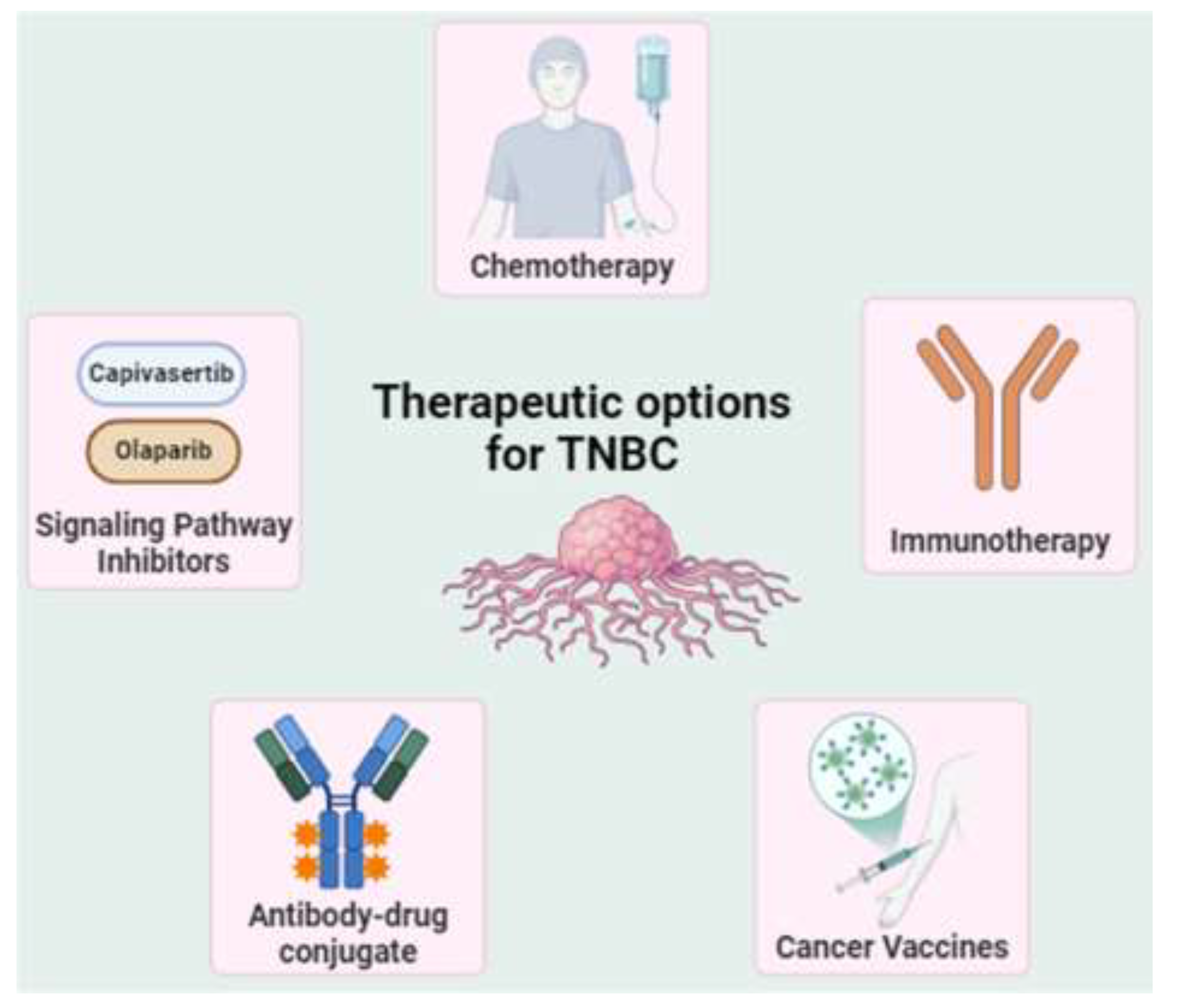
2.3. Treatment Challenges
2.3.1. Neoadjuvant Treatments
2.3.3. AKT Inhibitors
2.3.4. Cancer Vaccines
2.3.5. Immunotherapy
2.3.6. Antibody-Drug Conjugates (ADCs)
3. Sacituzumab govitecan, a Trop-2–directed ADC
3.1. The Discovery and Development of Sacituzumab Govitecan
3.1.1. Trop-2 and Its Expression Pattern
3.1.2. RS7 Monoclonal Antibody
3.1.3. SN-38, a Camptothecin Derivative
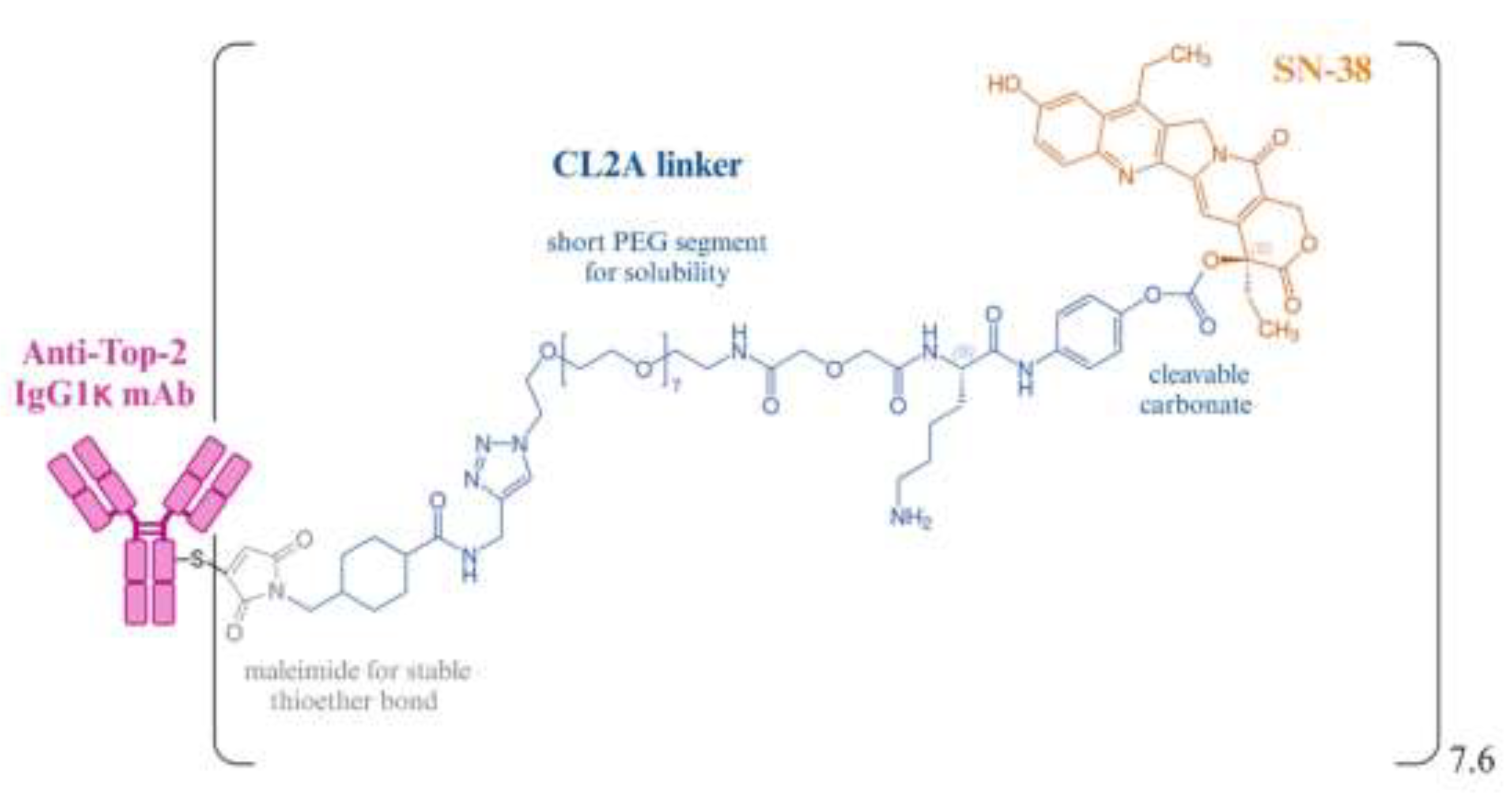
3.1.4. Conjugation of RS7 with SN-38
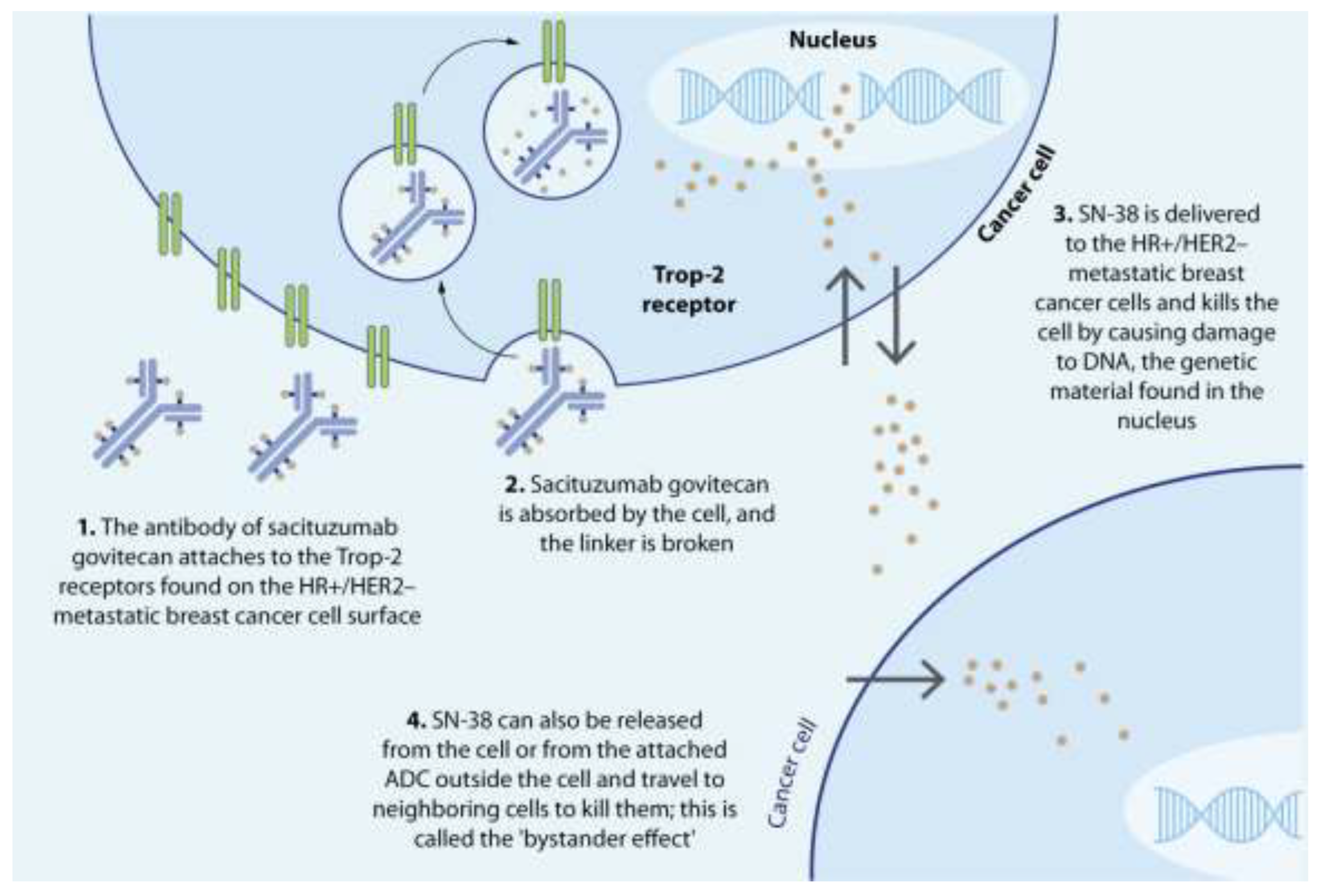
3.2. Mode of Action of Sacituzumab Govitecan
3.3. Dosing and Administration
3.3. Pharmacokinetics (PK) profile of Sacituzumab govitecan
3.4. Clinical Efficacy of Sacituzumab Govitecan
3.5. Safety Profile of Sacituzumab Govitecan
3.6. Cost evaluation of Sacituzumab Govitecan
3.6. Drug resistance to Sacituzumab Govitecan
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Aversano, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kong, D.; Liu, J.; et al. Breast cancer heterogeneity and its implication in personalized precision therapy. Exp Hematol Oncol 2023, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Huppert, L.A.; Gumusay, O.; Rugo, H.S. Emerging treatment strategies for metastatic triple-negative breast cancer. Ther Adv Med Oncol. 2022, 14, 17588359221086916. [Google Scholar] [CrossRef]
- Li, C.H.; Karantza, V.; Aktan, G.; Lala, M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res. 2019, 21, 143. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs. 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- Won, K.A.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives. Int J Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J Clin Oncol. 2023, 41, 1809–1815. [Google Scholar] [CrossRef]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget. 2017, 8, 1913–1924. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Kalari, K.R.; Suman, V.J.; Moyer, A.M.; Yu, J.; Visscher, D.W.; Dockter, T.J.; Vedell, P.T.; Sinnwell, J.P.; Tang, X.; et al. Tumor sequencing and patient-derived xenografts in the neoadjuvant treatment of breast cancer. J Natl Cancer Inst 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.A.; Chen, Y.H.; Solzak, J.P.; Ahmad, M.N.; Wedge, D.C.; Brinza, D.; Scafe, C.; Veitch, J.; Gottimukkala, R.; Short, W.; et al. Profiling molecular regulators of recurrence in chemorefractory triple-negative breast cancers. Breast Cancer Res. 2019, 21, 87. [Google Scholar] [CrossRef]
- Wu, Q.; Siddharth, S.; Sharma, D. Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs. Cancers (Basel). 2021, 13, 3697. [Google Scholar] [CrossRef]
- Dent, R.; et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Reddy, S.M.; et al. Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease-free at 5 years and relationship with low hormone receptor positivity. Br. J. Cancer 2018, 118, 17–23. [Google Scholar] [CrossRef]
- Lin, N.U.; et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive. Cancer Netw. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of triple-negative breast cancer. Annu Rev Pathol. 2022, 17, 181. [Google Scholar] [CrossRef]
- Keam, B.; Im, S.A.; Lee, K.H.; Han, S.W.; Oh, D.Y.; Kim, J.H.; Lee, S.H.; Han, W.; Kim, D.W.; Kim, T.Y.; et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011, 13, R22. [Google Scholar] [CrossRef]
- Sharma, P.; Klemp, J.R.; Kimler, B.F.; et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: Implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treatment 2014, 145, 707–714. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Timms, K.; Liu, M.S.; et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res 2011, 17, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Garza, C.; Weitzel, J.N.; Llacuachaqui, M.; et al. The prevalence of BRCA1 and BRCA2 mutations among young Mexican women with triple-negative breast cancer. Breast Cancer Res Treatment 2015, 150, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, D.H. Distribution of BRCA1 and BRCA2 mutations in asian patients with breast cancer. J Breast Cancer 2013, 16, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Espinosa Fernandez, J.R.; Eckhardt, B.L.; Lee, J.; et al. Identification of triple-negative breast cancer cell lines classified under the same molecular subtype using different molecular characterization techniques: Implications for translational research. PLoS ONE. 2020, 15, e0231953. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; et al. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS One. 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef]
- Manjunath, M.; Choudhary, B. Triple-negative breast cancer: A run-through of features, classification and current therapies. Oncol Lett. 2021, 22, 1–21. [Google Scholar] [CrossRef]
- So, J.Y.; Ohm, J.; Lipkowitz, S.; Yang, L. Triple negative breast cancer (TNBC): Non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol Ther. 2022, 237, 108253. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Wu, H.; Yu, Z. Advancements and challenges in triple-negative breast cancer: a comprehensive review of therapeutic and diagnostic strategies. Front Oncol. 2024, 14, 1405491. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Turati, A.; Rosato, A.; Carpanese, D. Sacituzumab govitecan in triple-negative breast cancer: from bench to bedside, and back. Front. Immunol. 2024, 15, 1447280. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the Benefits of Therapy for Early-Stage Breast Cancer: The St. Gallen International Consensus Guidelines for the Primary Therapy of Early Breast Cancer 2019. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef]
- Pusztai, L.; Foldi, J.; Dhawan, A.; DiGiovanna, M.P.; Mamounas, E.P. Changing Frameworks in Treatment Sequencing of Triple-Negative and HER2-Positive, Early-Stage Breast Cancers. Lancet Oncol. 2019, 20, e390–e396. [Google Scholar] [CrossRef]
- Holanek, M.; Selingerova, I.; Bilek, O.; Kazda, T.; Fabian, P.; Foretova, L.; et al. Neoadjuvant Chemotherapy of Triple-Negative Breast Cancer: Evaluation of Early Clinical Response, Pathological Complete Response Rates, and Addition of Platinum Salts Benefit Based on Real-World Evidence. Cancers (Basel). 2021, 13, 1586. [Google Scholar] [CrossRef]
- LeVasseur, N.; Sun, J.; Gondara, L.; Diocee, R.; Speers, C.; Lohrisch, C.; Chia, S. Impact of pathologic complete response on survival after neoadjuvant chemotherapy in early-stage breast cancer: a population-based analysis. J Cancer Res Clin Oncol. 2020, 146, 529–536. [Google Scholar] [CrossRef]
- Huang, M.; Qi, C.Z.; Ramsey, S.; Briggs, A.; Zhao, J.; Haiderali, A.; et al. Evaluation of pathological complete response as a trial-level surrogate for long-term survival outcomes among triple-negative breast cancer patients receiving neoadjuvant therapy. Ann Oncol. 2019, 30, iii34–iii35. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care. 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Lee, J.S.; Yost, S.E.; Yuan, Y. Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges. Cancers (Basel). 2020, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yee, D. I-SPY 2: a Neoadjuvant Adaptive Clinical Trial Designed to Improve Outcomes in High-Risk Breast Cancer. Curr Breast Cancer Rep. 2019, 11, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.; Abuhadra, N.; Sun, R.; Adrada, B.E.; Ding, Q.Q.; White, J.B.; et al. Molecular Characterization and Prospective Evaluation of Pathologic Response and Outcomes with Neoadjuvant Therapy in Metaplastic Triple-Negative Breast Cancer. Clin Cancer Res. 2022, 28, 2878–2889. [Google Scholar] [CrossRef]
- Yam, C.; Yen, E.Y.; Chang, J.T.; Bassett, R.L.; Alatrash, G.; Garber, H.; et al. Immune Phenotype and Response to Neoadjuvant Therapy in Triple-Negative Breast Cancer. Clin Cancer Res. 2021, 27, 5365–5375. [Google Scholar] [CrossRef]
- Mandapati, A.; Lukong, K.E. Triple negative breast cancer: approved treatment options and their mechanisms of action. J Cancer Res Clin Oncol 2023, 149, 3701–3719. [Google Scholar] [CrossRef]
- McCrea, C.; Hettle, R.; Gulati, P.; Taneja, A.; Rajora, P. Indirect treatment comparison of olaparib and talazoparib in germline BRCA-mutated HER2-negative metastatic breast cancer. J Comp Eff Res. 2021, 10, 1021–1030. [Google Scholar] [CrossRef]
- Eikesdal, H.P.; et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann Oncol 2021, 32, 240–249. [Google Scholar] [CrossRef]
- McCann, K.E. Advances in the use of PARP inhibitors for BRCA1/2-associated breast cancer: talazoparib. Future Oncol 2019, 15, 1707–1715. [Google Scholar] [CrossRef]
- Zhang, Hp. , Jiang, Ry. , Zhu, Jy. et al. PI3K/AKT/mTOR signaling pathway: an important driver and therapeutic target in triple-negative breast cancer. Breast Cancer 2024, 31, 539–551. [Google Scholar] [CrossRef]
- Schmid, P.; Abraham, J.; Chan, S.; Wheatley, D.; Brunt, A.M.; Nemsadze, G.; et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. JCO. 2020, 38, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Abraham, J.; Chan, S.; Brunt, A.M.; Nemsadze, G.; Baird, R.D.; et al. Abstract PD1-11: mature survival update of the double-blind placebo-controlled randomised phase II PAKT trial of first-line capivasertib plus paclitaxel for metastatic triple-negative breast cancer. Cancer Res. 2021, 81, D1–D11. [Google Scholar] [CrossRef]
- Kim, S.-B.; Dent, R.; Im, S.-A.; Espié, M.; Blau, S.; Tan, A.R.; et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Oliveira, M.; Isakoff, S.J.; Im, S.-A.; Espié, M.; Blau, S.; et al. Final results of the double-blind placebo-controlled randomized phase 2 LOTUS trial of first-line ipatasertib plus paclitaxel for inoperable locally advanced/metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2021, 189, 377–386. [Google Scholar] [CrossRef]
- Dent, R.; Kim, S.-B.; Oliveira, M.; Barrios, C.; O’Shaughnessy, J.; Isakoff, S.J.; et al. Abstract GS3-04: double-blind placebo (PBO)-controlled randomized phase III trial evaluating first-line ipatasertib (IPAT) combined with paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered locally advanced unresectable or metastatic triple-negative breast cancer (aTNBC): primary results from IPATunity130 Cohort, A. Cancer Res. 2021, 81, GS3–04. [Google Scholar]
- Clifton, G.T.; Peoples, G.E.; Mittendorf, E.A. The development and use of the E75 (HER2 369–377) peptide vaccine. Future Oncol. 2016, 12, 1321–1329. [Google Scholar] [CrossRef]
- Clive, K.S.; Tyler, J.A.; Clifton, G.T.; Holmes, J.P.; Ponniah, S.; Peoples, G.E.; Mittendorf, EA. The GP2 peptide: a HER2/neu-based breast cancer vaccine. J Surg Oncol. 2012, 105, 452–458. [Google Scholar] [CrossRef]
- Brown, T.A.; Mittendorf, E.A.; Hale, D.F.; Myers, J.W.; Peace, K.M.; Jackson, D.O.; et al. Prospective, randomized, single-blinded, multi-center phase II trial of two HER2 peptide vaccines, GP2 and AE37, in breast cancer patients to prevent recurrence. Breast Cancer Res Treat. 2020, 181, 391–401. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Ardavanis, A.; Symanowski, J.; Murray, J.L.; Shumway, N.M.; Litton, J.K.; et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol. 2016, 27, 1241–1248. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Clifton, G.T.; Holmes, J.P.; Schneble, E.; Van Echo, D.; Ponniah, S.; et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014, 25, 1735–1742. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Zhou, J.; Wang, J.; Jin, G.; Wang, X. Breast Cancer Vaccine containing a Novel toll-like receptor 7 agonist and an aluminum adjuvant exerts Antitumor effects. IJMS. 2022, 23, 15130. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M. , Butti, R., Panda, V.K. et al. Modulation of the tumor microenvironment and mechanism of immunotherapy-based drug resistance in breast cancer. Mol Cancer 2024, 23, 92. [Google Scholar] [CrossRef]
- Katz, H.; Alsharedi, M. Immunotherapy in triple-negative breast cancer. Med Oncol 2017, 35, 13. [Google Scholar] [CrossRef] [PubMed]
- Pembrolizumab (Keytruda): CADTH Reimbursement Review: Therapeutic area: Triple-negative breast cancer [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2023. Clinical Review. Available from: https://www.ncbi.nlm.nih.gov/books/NBK596330.
- Preziosi, A.J.; Priefer, R. Oncology's trial and error: Analysis of the FDA withdrawn accelerated approvals. Life Sci. 2024, 346, 122615. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P. , Cortes, J., Pusztai, L., McArthur, H., Kümmel, S., Bergh, J., et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Schmid, P. , Salgado, R., Park, Y.H., Muñoz-Couselo, E., Kim, S.B., Sohn, J., Im, S.-A., Foukakis, T., Kuemmel, S., Dent, R., et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. [CrossRef]
- Lan, H.R.; Chen, M.; Yao, S.Y.; Chen, J.X.; Jin, K.T. Novel immunotherapies for breast cancer: Focus on 2023 findings. Int Immunopharmacol. 2024, 128, 111549. [Google Scholar] [CrossRef]
- Liu, Y. , Hu, Y. , Xue, J. et al. Advances in immunotherapy for triple-negative breast cancer. Mol Cancer 2023, 22, 145. [Google Scholar] [CrossRef]
- Padzińska-Pruszyńska, I.; Kucharzewska, P.; Matejuk, A.; Górczak, M.; Kubiak, M.; Taciak, B.; Król, M. Macrophages: Key Players in the Battle against Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2024, 25, 10781. [Google Scholar] [CrossRef]
- Ren, X. , Cheng, Z., He, J. et al. Inhibition of glycolysis-driven immunosuppression with a nano-assembly enhances response to immune checkpoint blockade therapy in triple negative breast cancer. Nat Commun 2023, 14, 7021. [Google Scholar] [CrossRef]
- Moon, S.-J. , Govindan, S.V.; Cardillo, T.M.; D’Souza, C.A.; Hansen, H.J.; Goldenberg, D.M. Antibody conjugates of 7-ethyl-10-hydroxycamptothecin (SN-38) for targeted cancer chemotherapy. J Med Chem. 2008, 51, 6916–6926. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumabgovitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.; Parks, D.R.; Rouse, R.V.; Herzenberg, L.A. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci USA. 1981, 78, 5147–5150. [Google Scholar] [CrossRef] [PubMed]
- Cubas, R.; Li, M.; Chen, C.; Yao, Q. Trop2: A possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009, 1796, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015, 6, 84–105. [Google Scholar] [CrossRef]
- Lin, H.; Huang, J.F.; Qiu, J.R.; Zhang, H.L.; Tang, X.J.; Li, H.; et al. Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasiveductal breast cancer. Exp Mol Pathol. 2013, 94, 73–78. [Google Scholar] [CrossRef]
- Zaman, S.; Jadid, H.; Denson, A.C.; Gray, J.E. Targeting Trop-2 in solid tumors: future prospects. Onco Targets Ther 2019, 12, 1781–1790. [Google Scholar] [CrossRef]
- Jeon, Y.; Jo, U.; Hong, J.; Gong, G.; Lee, H.J. Trophoblast cell-surface antigen 2 (TROP2) expression in triple-negative breast cancer. BMC Cancer. 2022, 22, 1014. [Google Scholar] [CrossRef]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; et al. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021, 20, 2329–2340. [Google Scholar] [CrossRef]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, GA; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, R.; Ou, Z.; Ye, Y.; Zeng, Q.; Wang, Y.; et al. TROP2 is highly expressed in triple-negative breast cancer CTCs and is a potential marker for epithelial mesenchymal CTCs. Mol Therapy: Oncol. 2024, 32, 200762. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Kalinsky, K. Sacituzumab Govitecan-hziy in Triple-Negative Breast Cancer. Reply. N Engl J Med. 2019, 380, 2382. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2019, 380, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; et al.; ASCENT Clinical Trial Investigators Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A. , Hurvitz, S.A., Rugo, H.S., Brufsky, A., Cortes, J., Loibl, S., et al (2021d). A Plain Language Summary of the ASCENT Study: Sacituzumab Govitecan for Metastatic Triple-Negative Breast Cancer. Future Oncology, 17(30), 3911–3924. [CrossRef]
- Bardia, A.; Rugo, H.S.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; et al. Final results from the randomized phase III ASCENT clinical trial in metastatic triple-negative breast cancer and association of outcomes by human epidermal growth factor receptor 2 and trophoblast cell surface antigen 2 expression. J Clin Oncol. (2024)42:1738–44. [CrossRef]
- D’Arienzo, A.; Verrazzo, A.; Pagliuca, M.; Napolitano, F.; Parola, S.; Viggiani, M.; et al. Toxicity profile of antibody-drug conjugates in breast cancer: practical considerations. eClinical Medicine. (2023) 62:102113. [CrossRef]
- Dum, D.; Taherpour, N.; Menz, A.; Höflmayer, D.; Völkel, C.; Hinsch, A.; et al. Trophoblast cell surface antigen 2 expression in human tumors: A tissue microarray study on 18,563 tumors. Pathobiology. (2022) 89:245–58. [CrossRef]
- Kong, D.; Hughes, C.J.; Ford, H.L. Cellular plasticity in breast cancer progression and therapy. Front Mol Biosci. (2020) 7. [CrossRef]
- Hsu, E.C.; Rice, M.A.; Bermudez, A.; Jose, F.; Marques, G.; Aslan, M.; et al. Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc Natl Acad Sci USA. (2020) 117(4):2032–42. [CrossRef]
- Liu, X.; Deng, J.; Yuan, Y.; Chen, W.; Sun, W.; Wang, Y.; et al. Advances in Trop2- targeted therapy: Novel agents and opportunities beyond breast cancer. Pharmacol Ther. (2022) 239:108296. [CrossRef]
- Bessede, A.; Peyraud, F.; Besse, B.; Cousin, S.; Cabart, M.; Chomy, F.; et al. TROP2 is associated with primary resistance to immune checkpoint inhibition in patients with advanced non-small cell lung cancer. Clin Cancer Res. (2024) 30:779–85. [CrossRef]
- Redlich, N.; Robinson, A.M.; Nickel, K.P.; Stein, A.P.; Wheeler, D.L.; Adkins, D.R.; et al. Anti-Trop2 blockade enhances the therapeutic efficacy of ErbB3 inhibition in head and neck squamous cell carcinoma. Cell Death Dis. (2018) 9(1):5. [CrossRef]
- Stein, R.; Basu, A.; Chen, S.; Shih, L.B.; Goldenberg, D.M. Specificity and properties of MAb RS7-3G11 and the antigen defined by this pancarcinoma monoclonal antibody. Int J Cancer. 1993, 55, 938–946. [Google Scholar] [CrossRef]
- Stein, R.; Basu, A.; Goldenberg, D.M.; Lloyd, K.O.; Mattes, M.J. Characterization of cluster 13: the epithelial/carcinoma antigen recognized by MAb RS7. Int J Cancer Suppl. 1994, 8, 98–102. [Google Scholar] [CrossRef]
- Raji, R. , et al. , Uterine and ovarian carcinosarcomas overexpressing Trop-2 are sensitive to hRS7, a humanized anti-Trop-2 antibody, J. Exp. Clin. Cancer Res. 2011, 30, 106. [Google Scholar]
- De Leij, L.; Helrich, W.; Stein, R.; Mattes, M.J. SCLC-cluster-2 antibodies detect the pancarcinoma/epithelial glycoprotein EGP-2. Int J Cancer Suppl. 1994, 8, 60–63. [Google Scholar] [CrossRef]
- Basu, A.; Goldenberg, D.M.; Stein, R. The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7-3G11, is phosphorylated on serine 303. Int J Cancer. 1995, 62, 472–479. [Google Scholar] [CrossRef]
- Leung, K. 111In-Diethylenetriamine pentaacetic acid-anti-epithelial glycoprotein-1 hRS7 humanized monoclonal antibody. 2012 Jan 12 [Updated 2012 Apr 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK92276/.
- Rothenberg, M.L. Topoisomerase I inhibitors: Review and update. Ann Oncol. 1997, 8, 837–855. [Google Scholar] [CrossRef]
- Hayashi, H.; Tsurutani, J.; Satoh, T.; et al. Phase II study of bi-weekly irinotecan for patients with previously treated HER2-negative metastatic breast cancer: KMBOG0610B. Breast Cancer. 2013, 20, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.; Zhong, X.; He, P.; Zheng, H.; Tian, T. ; Yan X and Luo T (2021) A Retrospective Analysis of the Effect of Irinotecan-Based Regimens in Patients With Metastatic Breast Cancer Previously Treated With Anthracyclines and Taxanes. Front. Oncol. 11:654974. [CrossRef]
- Innocenti, F.; Iyer, L.; Ratain, M.J. Pharmacogenetics of anticancer agents: Lessons from amonafide and irinotecan. Drug Metab Dispos. 2001, 29, 596–600. [Google Scholar]
- Goldenberg, D.M.; Sharkey, R.M. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: a case study of anti-TROP-2 sacituzumabgovitecan. MAbs 2019, 11, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Mathijssen, R.H.; van Alphen, R.J.; Verweij, J.; et al. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 2001, 7, 2182–2194. [Google Scholar]
- Gilead Sciences, Inc. 2023. Trodelvy® (sacituzumab govitecan-hziy) Impact of UGT1A1 Status on Safety Profile in Patients With mUC.
- Govindan, S.V.; Cardillo, T.M.; Moon, S.J.; Hansen, H.J.; Goldenberg, D.M. CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates. Clin Cancer Res. 2009, 15, 6052–6061. [Google Scholar] [CrossRef]
- Cardillo, T.M.; Govindan, S.V.; Sharkey, R.M.; Trisal, P.; Goldenberg, D.M. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res. 2011, 17, 3157–3169. [Google Scholar] [CrossRef]
- Bravaccini, S.; Maltoni, R. Trop-2 therapy in metastatic triple negative breast cancer in Italy: clinical opportunity and regulatory pitfalls. J Pers Med 2021, 11, 1211. [Google Scholar] [CrossRef]
- Nagayama A et al (2020) Novel antibody–drug conjugates for triple negative breast cancer. Thera Adv Med Oncol 12:1758835920915980.
- Gilead Sciences Inc. Trodelvy 200 mg powder for concentrate for solution for infusion: EU summary of product characteristics. 2023. https:// www. ema. europa. eu. Accessed 18 Oct 2024.
- Kalinsky, K.; Diamond, J.R.; Vahdat, L.T.; Tolaney, S.M.; Juric, D.; O'Shaughnessy, J.; et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020, 31, 1709–1718. [Google Scholar] [CrossRef]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Sharkey, R.M.; McBride, W.J.; Cardillo, T.M.; et al. Enhanced delivery of SN-38 to human tumor xenografts with an anti-Trop-2-SN-38 antibody conjugate (sacituzumabgovitecan). Clin Cancer Res 2015, 21, 5131–5138. [Google Scholar] [CrossRef]
- Takeba, Y.; et al. Irinotecan activates p53 with its active metabolite, resulting in hepatocellular carcinoma apoptosis. J Pharmacol Sci 2007, 104, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Ocean, A.J.; Starodub, A.N.; Bardia, A.; et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2- SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics. Cancer. 2017, 123, 3843–3854. [Google Scholar] [CrossRef] [PubMed]
- Immunomedics Inc. (2021). Trodelvy (sacituzumab govitecan-hziy) package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761115s000lbl.
- Cardillo, T.M.; Govindan, S.V.; Sharkey, R.M.; et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem 2015, 26, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Sathe, A.G.; Singh, P.; et al. Pharmacokinetics of Sacituzumab Govitecan, a Trop-2-Directed Antibody-Topoisomerase I Inhibitor SN-38 Drug Conjugate, in Patients With Advanced Solid Tumors [Poster Abstract P-161]. Paper presented at: American Society for Clinical Pharmacology and Therapeutics (ASCPT) 2022 Annual Meeting; 16-18 March, 2022; Virtual.
- Sathe, A.G.; Singh, P.; Singh, I.; et al. Impact of UGT1A1 Polymorphisms on the Pharmacokinetics of Sacituzumab Govitecan [Poster Abstract P-151]. Paper presented at: American Society for Clinical Pharmacology and Therapeutics (ASCPT) 2022 Annual Meeting; 16-18 March, 2022; Virtual.
- Sathe, A.G.; Singh, I.; Jones, A.; et al. Population Pharmacokinetics of Sacituzumab Govitecan in Patients With Locally Advanced or Metastatic Breast Cancer or Other Solid Tumors [Poster PII-082]. Paper presented at: American Society for Clinical Pharmacology & Therapeutics,; March 22-24, 2023; Atlanta, GA.
- Singh, I.; Sathe, G.K.; Diderichsen, P.M.; et al. Exposure-Response Analyses of Sacituzumab Govitecan Efficacy and Safety in Patients With Metastatic Breast Cancer [Poster PO1-04-06]. Paper Paper Presented at: San Antonio Breast Cancer Symposium (SABCS); December 5-9, 2023; San Antonio, TX, USA.
- Seligson, J.M.; Patron, A.M.; Berger, M.J.; Harvey, R.D.; Seligson, N.D. Sacituzumab Govitecan-hziy: An Antibody-Drug Conjugate for the Treatment of Refractory, Metastatic, Triple-Negative Breast Cancer. Ann Pharmacother. 2021, 55, 921–931. [Google Scholar] [CrossRef]
- Wahby, S.; Fashoyin-Aje, L.; Osgood, C.L.; Cheng, J.; Fiero, M.H.; Zhang, L.; et al. FDA Approval Summary: Accelerated Approval of Sacituzumab Govitecan-hziy for Third-line Treatment of Metastatic Triple-negative Breast Cancer. Clin Cancer Res. 2021, 27, 1850–1854. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J Clin Oncol. 2017, 35, 2141–2148. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023, 402, 1423–1433. [Google Scholar] [CrossRef]
- Xu, B.; Ma, F.; Wang, T.; Wang, S.; Tong, Z.; Li, W.; et al. A Phase IIb, single arm, multicenter trial of sacituzumab govitecan in Chinese patients with metastatic triple-negative breast cancer who received at least two prior treatments. Int J Cancer. 2023, 152, 2134–2144. [Google Scholar] [CrossRef]
- Spring, L.M.; Tolaney, S.M.; Fell, G.; Bossuyt, V.; Abelman, R.O.; et al. Response-guided neoadjuvant sacituzumab govitecan for localized triple-negative breast cancer: results from the NeoSTAR trial. Ann Oncol. 2024, 35, 293–301. [Google Scholar] [CrossRef]
- O'Shaughnessy, J.; Brufsky, A.; Rugo, H.S.; Tolaney, S.M.; Punie, K.; Sardesai, S.; et al. Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the phase 3 randomized ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2022, 195, 127–139. [Google Scholar] [CrossRef]
- Fenn, K.M.; Kalinsky, K. Sacituzumab govitecan: antibody-drug conjugate in triple-negative breast cancer and other solid tumors. Drugs Today (Barc). 2019, 55, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Nakajima, E.; Hutchinson, J.; Viscosi, E.; Blouin, G.; Weekes, C.; Rugo, H.; Moy, B.; Bardia, A. Sacituzumab Govitecan for Metastatic Triple-Negative Breast Cancer: Clinical Overview and Management of Potential Toxicities. Oncologist. 2021, 26, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Sacituzumab Govitecan (Trodelvy): CADTH Reimbursement Recommendation: Indication: For the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer who have received 2 or more prior therapies, with at least 1 of them for metastatic disease [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2022 Feb. Table 4, Cost and Cost-Effectiveness. Available from: https://www.ncbi.nlm.nih.gov/books/NBK595400/table/t04/.
- Xie, J., Li, S., Li, Y. et al. Cost-effectiveness of sacituzumab govitecan versus chemotherapy in patients with relapsed or refractory metastatic triple-negative breast cancer. BMC Health Serv Res 2023, 23, 706. [CrossRef]
- Khoury, R.; Saleh, K.; Khalife, N.; Saleh, M.; Chahine, C.; Ibrahim, R.; Lecesne, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Int J Mol Sci. 2023, 24, 9674. [Google Scholar] [CrossRef]
- Hafeez, U. , Parakh, S., Gan, H.K., Scott, A.M., Antibody-drug conjugates for cancer therapy, Molecules (2020). 25, 4764. [CrossRef]
- Coates, J.T. , Sun, S., Leshchiner, I., Thimmiah, N., Martin, E.E., McLoughlin, D., et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer Discov. 2021, 11, 2436–2445. [Google Scholar] [CrossRef]
- Zhu, J. , Wu, W., Togashi, Y., Nihira, N.T., Johmura, Y., Zhu, D., Nakanishi, M., Miyoshi, Y., Ohta, T. Alteration of Trop-2 expression in breast cancer cells by clinically used therapeutic agents and acquired tamoxifen resistance. Breast Cancer. 2022, 29, 1076–1087. [Google Scholar] [CrossRef]
- Lussier, D.M., et al., Radiation-induced neoantigens broaden the immunotherapeutic window of cancers with low mutational loads, Proc. Natl. Acad. Sci. USA. (2021). 118(24):e2102611118. [CrossRef]
- Abelman, R.O. , Spring, L., Fell, G.G., Ryan, P., Vidula, N., Medford, A.J., Shin, J., Abraham, E., Wander, S.A., Isakoff, S.J., et al. Sequential use of antibody-drug conjugate after antibody-drug conjugate for patients with metastatic breast cancer: ADC after ADC (A3) study. J. Clin. Oncol. 2023, 41, 1022. [Google Scholar] [CrossRef]
- Chang, C.H.; Wang, Y.; Zalath, M.; Liu, D.; Cardillo, T.M.; Goldenberg, D.M. Combining ABCG2 Inhibitors with IMMU-132, an Anti-Trop-2 Antibody Conjugate of SN-38, Overcomes Resistance to SN-38 in Breast and Gastric Cancers. Mol Cancer Ther. 2016, 15, 1910–1919. [Google Scholar] [CrossRef]
- Schmid, P. , Loi, S., De la Cruz Merino, L. et al. 181O Interim analysis (IA) of the atezolizumab (atezo) + sacituzumab govitecan (SG) arm in patients (pts) with triple-negative breast cancer (TNBC) in MORPHEUS-pan BC: A phase Ib/II study of multiple treatment (tx) combinations in pts with locally advanced/metastatic BC (LA/mBC). ESMO Open, (2024) 9, 103203. [CrossRef]
- Cardillo, T.M.; Sharkey, R.M.; Rossi, D.L.; et al. Synthetic lethality exploitation by an Anti-Trop-2-SN-38 antibody-drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2-wild-type triple-negative breast cancer. Clin Cancer Res. 2017, 23, 3405–3415. [Google Scholar] [CrossRef]
- Bardia, A.; Coates, J.T., Spring L et al; Abstract 2638: Sacituzumab Govitecan, combination with PARP inhibitor, Talazoparib, in metastatic triple-negative breast cancer (TNBC): Translational investigation. Cancer Res 2022; 82 (12_Supplement): 2638. [CrossRef]
- Liu, D.; Cardillo, T.M.; Wang, Y.; Rossi, E.A.; Goldenberg, D.M.; Chang, C.H. Trop-2-targeting tetrakis-ranpirnase has potent antitumor activity against triple-negative breast cancer. Mol Cancer. 2014, 13, 53. [Google Scholar] [CrossRef]
- Liu, X.; Ma, L.; Li, J.; Sun, L.; Yang, Y.; Liu, T.; Xing, D.; Yan, S.; Zhang, M. Trop2-targeted therapies in solid tumors: advances and future directions. Theranostics. 2024, 14, 3674–3692. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.S.; Seligson, N.D.; Bottiglieri, S.; Carballido, E.; Cueto, A.D.; Imanirad, I.; Levine, R.; Parker, A.S.; Swain, S.M.; Tillman, E.M.; Hicks, J.K. UGT1A1 Guided Cancer Therapy: Review of the Evidence and Considerations for Clinical Implementation. Cancers (Basel). 2021, 13, 1566. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, M.; Guerra, E.; Ali, Z.; et al. Trop-2 cleavage by ADAM10 is an activator switch for cancer growth and metastasis. Neoplasia 2021, 23, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Trerotola, M.; Relli, V.; et al. Trop-2 induces ADAM10-mediated cleavage of E-cadherin and drives EMT-less metastasis in colon cancer. Neoplasia 2021, 23, 898–911. [Google Scholar] [CrossRef]
| Parameters | Key features |
|---|---|
| Drug class | Antibody drug conjugate |
| Brand name | Trodelvy |
| Generic name | Sacituzumab govitecan-hziy |
| Company | Gilead Sciences Inc. |
| Dosage form | Injection |
| Color | Off-white powder |
| Reconstitution | Sterile normal saline |
| Mode of action | Antibody targets the Trop2 receptor on the cancer cell membrane SN-38 is released intracellularly as well as extracellularly SN-38 binds to and stabilizes topoisomerase IB |
| Indication | Advanced TNBC, after at least two lines of systemic treatment (including taxane), one in the metastatic setting Advanced HR+/HER2– breast cancer resistant to endocrine therapy, with at least two prior lines of treatments in the metastatic setting |
| FDA Approval date | April 22, 2020 for metastatic TNBC with at least two prior therapies February 3, 2023 for HR+/HER2– breast cancer |
| Dosage | 10 mg/kg intravenously |
| Maximum tolerated dose | 10 mg/kg/day, if weight changes by more than 10% from baseline, recalculate the dose |
| Administration | Premedicate with antipyretics and histamine antagonists Antiemetics for moderately emetogenic drugs on days 1, 8 every 21 days. Continue till progressive disease or dose-limiting toxicity |
| Toxicities % | Neutropenia (boxed warning): 61% Diarrhea (boxed warning): 65% Hypersensitivity: 37% |
| Molecular predictors of response |
No molecular predictors have been validated Testing for Trop2 expression is not needed |
| Special considerations | To consider UGT1A1 polymorphisms in patients with unusually severe or rapid onset adverse effects |
| Cost | $12,478 for a single 50-mL, 180 mg vial |
| Parameter | SG | SN-38 |
|---|---|---|
| Cmax, mean (CV%), (ng/mL) | 239,000 (11%) | 98 (45%) |
| AUC 0–168, mean (CV%), (ng.h/mL) | 5,640,000 (22%) | 3696 (56%) |
| Vd (L) | 3.6 | - |
| t½ (hours) | 16 | 18 |
| CLa, L/h | 149 | 270,000 |
| Vssa,L | 2820 | 6900 |
| Systemic clearance time (hrs) | 11-14 | 10-20 |
| Mean clearance rate, L/h | 0.13 | 11.2 |
| Median elimination time (hrs) | 23.4 | 17.6 |
| Clinical Trial | Design | Intervention | Patient population |
No. of Pts. |
Response rates | Median PFS (months) | Median OS (months) | Grade ≥ 3 AEs | FDA approval |
|---|---|---|---|---|---|---|---|---|---|
| ASCENT (NCT02574455) [85] |
Phase III RCT |
SG vs. single agent CT | Metastatic TNBC refractory to two prior lines (including taxanes) | 529 | 31.1 % vs 4.2 % | 4.8 vs. 1.7 | 11.8 vs 6.9 |
Neutropenia (51%) Leukopenia (10%) Diarrhea (10%) Anemia (8%) |
Regulatory approval (April 7, 2021) |
| TROPiCS-02 (NCT03901339) [128] |
Phase III RCT | SG vs. single-agent CT | HR+/HER2- mBC prior taxane, CDK4/6 inhibitor and 2-4 prior CTs | 543 | 57 % vs 38 % |
5.5 vs. 4.0 | 13.9 vs 12.3 | Neutropenia (51%) Diarrhea (10%) |
Regulatory approval (February 3, 2023) |
| Clinical Trial | Sponsor | Drugs | Phase | Enrollment | Status | Population | Primary Endpoints |
|---|---|---|---|---|---|---|---|
| NCT04039230 (ASSET) |
Massachusetts General Hospital |
SG + talazoparib | Ib/II | 75 | Recruiting | Metastatic TNBC | DLT, PFS, ORR |
| NCT04434040 (ASPRIA) |
Dana-Farber Cancer Institute | SG + atezolizumab | II | 40 | Active, not recruiting | Residual invasive disease in TNBC following NAC | Undetectable ctDNA |
| NCT04468061 (Saci-IO TNBC) | Dana-Farber Cancer Institute | SG with or without pembrolizumab | II | 110 | Recruiting | First-line PD-L1- mTNBC | PFS |
| NCT05633654 (ASCENT-05/AFT-65 OptimICE-RD/NSABP B-63) |
Gilead Sciences | SG + pembrolizumab vs. capecitabine | III | 1514 | Recruiting | TNBC with residual invasive disease after surgery and NAC | IDFS |
| NCT05382286 (ASCENT-04) |
Gilead Sciences | SG + pembrolizumab vs. TPC | III | 443 | Active, not recruiting | First-line metastatic TNBC whose tumors express PD-L1 | PFS |
| NCT03971409 (InCITe) | University of California, San Francisco |
SG + immunotherapy | II | 150 | Recruiting | Metastatic TNBC | ORR |
| NCT05143229 (ASSET) | University of Kansas Medical Center | SG + aleplisib | I | 18 | Recruiting | 2+L HER2+ mBC | RP2D |
| NCT03424005 (Morpheus-panBC) |
Hoffmann-La Roche | SG + Atezolizumab | I/II | 580 | Recruiting | Metastatic or Locally Advanced BC | ORR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





