Introduction
Maize is a vital staple food crop globally. All components of the maize plant have significant commercial value, including kernels, leaves, stems, tassels, and ears, which are utilized in producing both food-related and not-food-related products. Maize is a primary supply of sustenance for people, a source of nourishment for animals, and a fundamental ingredient for industrial applications (Morris, 2001). Maize is a significant source of daily calories in Africa, contributing about 20-30% of the total intake. The grains of maize are abundant in vitamins C and E, carbohydrates, important minerals, and include 9% protein (Krivanek et al., 2014; Shiferaw et al., 2011).
The excessive reliance of millions of Africans on white maize-based diets, which lacks sufficient quantities of essential micro-nutrients such as vitamin A, can have detrimental effects on the quality of their lives throughout their lifespan (Bailey et al., 2015; Nuss and Tanumihardjo, 2010; Gebremeskel et al., 2017). Vitamin A deficiency (VAD) is associated with multiple health consequences, including permanent vision loss, stunted growth, weakened immune function, higher early childhood mortality, more vulnerability to infection, rough and itchy skin, and impaired formation of gums and bones (Dhliwayo et al., 2014).
Notwithstanding the implementation of several supplementations and food fortification initiatives with Vitamin A, affects over 190 million children and 19 million pregnant women, causing nearly 800,000 fatalities annually, in low-income countries of Southeast Asia and Americas (UNICEF, 2018). Every child is expected to have the option of eating a diverse nutritious diet that includes a wide range of vegetables and fruits. Nevertheless, the diversity of diets is sometimes limited by factors such as the yearly availability of crops, price, and the minimal consumption of carotenoids found in green leafy plants (West et al., 2002). Direct vitamin supplementation has not been widely used because of poor infrastructure in poorer nations. The strategy of biofortification, in which staple crops are specifically bred for higher nutrient concentration (Fraser et al., 2004; Graham et al., 2001), or the creation of essential micronutrient-dense crop varieties through plant breeding, is considered the most feasible method to eradicate mortality and illness caused by malnutrition (Ortiz-Monasterio et al., 2007). Maize is a suitable candidate for improving nutrition, including micronutrient deficiencies due to its high genetic diversity (Chandrasekharan et al., 2022). The edible maize endosperm is considered a perfect target for biofortification because it naturally accumulates carotenoid compounds, both provitamin A carotenoids and non-provitamin carotenoids in its kernel (Burt et al., 2011; Menkir et al., 2008).
HarvestPlus, a global program that aims to improve food nutrition by developing and promoting biofortified crops has set a target to achieve the PVA concentration of 15 μg/g in maize (Bouis & Welch, 2010). Maazou et al. (2021), Gebremeskel et al. (2018), and Pixley et al. (2013) reported maize breeding lines with provitamin A levels of 51.7 μg/g, 22.30 μg/g, and 30 μg/g, respectively. Significant progress has been made in enhancing the levels of provitamin A carotenoids in cultivated varieties of maize in Sub-Saharan Africa where over 40 varieties that are high in provitamin A have already been released (Andersson et al., 2017; Listman et al., 2019). However, the provitamin A concentration found in most of the released varieties varies from 6 to 10 μg/g (Andersson et al., 2017) which does not meet the desired 15 μg/g threshold. Consequently, there is the need to further improve the concentrations of provitamin A in the kernels of hybrids with desirable agronomic performance for cultivation in Sub-Saharan Africa.
Breeding that integrates molecular markers is capital intensive. To curtail genotyping expenses and expediting enhancement in carotenoid content in maize, the International Maize and Wheat Improvement Center (CIMMYT) identified seven Kompetitive Allele-Specific PCR (KASP) Single Nucleotide Polymorphisms (SNPs) markers linked to the crtRB1 gene on the chromosome 10 to identify genotypes with favorable provitamin A alleles (Gowda et al., 2017). Obeng-bio et al. (2019) and Maazou et al. (2021) highlighted that the KASP SNP, zm0015 and zm0016 markers associated with crtRB1 are suitable for rapid selection of provitamin A enriched maize. KASP markers are a type of genotyping assay used to detect SNPs and insertions/deletions (INDELs) in DNA samples (Intertek Group Plc., Sweden, unpublished). In comparison to gel-based genotyping methods, KASP markers proved to be cost-effective in handling large sample sizes owing to their scalability and multiplexing features. This multiplexing capability not only saves time and reduces costs but also ensures a swift turnaround time for the analysis of genetic variations.
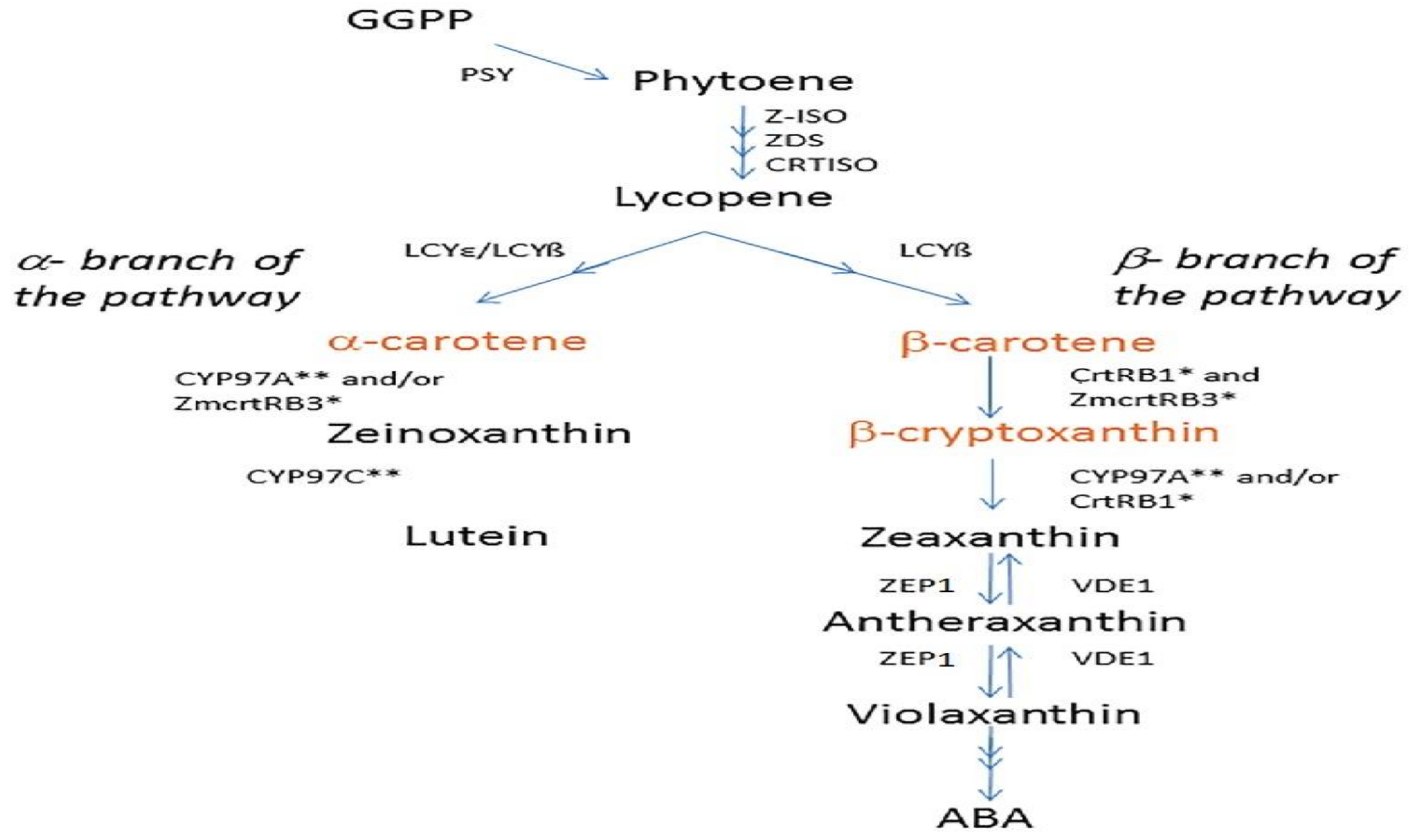
The plant carotenoid biosynthetic pathway has been extensively studied, and the major genes involved in essential stages have been identified (Giuliano et al., 2008; Wurtzel et al., 2012) (
Figure 1). Phytoene synthase1 (Psy1) catalyzes the first committed step in the pathway, facilitating the conversion of geranylgeranyl diphosphate into phytoene, which is primarily responsible for the transition from white to yellow in maize grain endosperm (Li et al., 2010). LCYe and other related genes catalyze the conversion of phytoene to α-carotene through zeta-carotene. CrtRB1 has been shown to regulate the accumulation of provitamin A compounds. CrtRB1, a hydroxylase gene, converts β-carotene (BC) into β-cryptoxanthin (BCX), whose provitamin A activity is theoretically only half that of BC. Recently, natural genetic variation in CrtRB1 has been discovered, leading to greater retention of BC in maize endosperm (Yan et al., 2010; Babu et al., 2013). Kandianis et al. (2013) and Owens et al. (2014) have identified additional genes within the carotenoid biosynthetic pathway, including
ZEP1 with association to carotenoid accumulation.
ZEP1 appears promising for improving total carotenoid levels in future breeding programs by selecting genotypes that harbor non-functional
ZEP1 alleles. This strategy preserves zeaxanthin, a carotenoid that contributes to the total carotenoid content. The
ZEP1 gene converts the β-rings of zeaxanthin into violaxanthin via antheraxanthin (
Figure 1)
Owen et al. (2014) undertook a Genome-Wide Association Study (GWAS) using a group of 281 inbred lines that varied from bright yellow to deep orange. They found zeaxanthin epoxidase 1 (ZEP1) as a key gene that influences the carotenoid content in seeds with potential significant impact on increasing provitamin A carotenoid levels in maize through selection of alleles that increase overall metabolic flow towards carotenoid production or hinder carotenoid breakdown in the pathway. These discoveries present new opportunities for focused breeding techniques aiming at further improving the provitamin A concentration in maize. Including the favorable alleles of the ZEP1 gene with Carotenoid Cleavage Dioxygenase 1 (crtRB1) can raise provitamin A carotenoids to levels exceeding 15 ug/g in the kernels of maize varieties. The present study was thus conducted to (i) determine the carotenoid content of 147 inbred lines, (ii) identify regions within the ZEP1 gene with variation and their association with variations in carotenoid content, and (iii) evaluate the efficacy of crtRB1 KASP SNP zm0015 and zm0016 markers in lines selected for varying carotenoid levels.
Materials and Methods
Plant Material
A total of 147 maize inbred lines were used in the present study (
Supplementary Table S1). These lines were derived from 28 backcrosses. Twelve yellow and orange maize inbred lines of tropical and temperate origin (CML328, A619, CI7, DE3, KVI11, KVI13, M162W, NC298, NC350, NC354, NC358, and SC55) well characterized with β-carotene content ranging from 5.2 to 13.6 l μg/g, identified at the University of Illinois as potential donors of high levels of provitamin A and other carotenoids (Liu et al., 2003; Islam et al., 2004) were introduced and crossed to adapted yellow and orange maize inbred lines showing moderate levels of provitamin A in previous studies (Menkir et al., 2008). Each F
1 was crossed to the same or different elite inbred line to generate 28 backcrosses. Each backcross was grown in a single 5 m row containing 22 plants, which were self-pollinated to produce bulk seeds after harvest. The bulk seeds from each backcross were then planted in 10 rows of 5 m length spaced 0.75 m apart with 0.25 m spacing between hills in which more than 160 plants were self-pollinated. At the S
1 to S
3 stages of inbreeding, ear-to-row planting was made at Ikenne (3º42´E, 6º54´N, altitude 30 m) and Saminaka (8º39'E, 10º34'N, altitude 760 m) in Nigeria where individual plants showing synchronous pollen shed and silking, low ear placement, good standability, and resistance to diseases, including
Puccinia polysora rust,
Bipolaris maydis blight, and
Curvularia lunata leaf spot were self-pollinated. The breeding approach leveraged on to develop inbred lines from these backcrosses encompassed the selection of ears showing bright yellow to orange kernel colors with semi-flint to flint kernel texture among the self-pollinated plants alongside selection based on carotenoid content quantified by HPLC for further inbreeding. At the S4 and subsequent stages, similar plants were self-pollinated in each line to form bulk seeds for further inbreeding. A total of 147 S5 to S7 lines derived from the 28 backcrosses containing exotic germplasm, were chosen for the present study.
Field Evaluation and DNA Extraction
In the current study, 147 sub-tropical maize inbred lines were evaluated in IITA’s research station (7°29′11.99″N, 3°54′2.88″E, and altitude 190 m), Ibadan, Nigeria in 2023 cropping season. The lines were arranged in a 21 x 7 alpha lattice design with two replications. Each maize line was planted in a single 5m row plot, with 0.75 m between rows and 0.25 m distance between plants. The standard cultural practices for maize production were utilized. In order to create seed samples for carotenoid analyses, each row's minimum of 4 representative plants were self-pollinated. The self-pollinated ears in every row were collected, dried, and threshed. One hundred and twenty kernels were taken from the seed samples for carotenoid analysis. For PCR based genotyping of the functional ZEP1 DNA marker, leaf samples were collected from four to seven plants of each inbred line 21 days after planting. The leaves were lyophilized at -80°C and subsequently used for DNA extraction. The extraction of whole genomic DNA was performed using an adjusted Cetyl-trimethyl ammonium bromide (CTAB) procedure, as outlined by Azmach et al. (2013). DNA purity and amount was quantified using a NanoDrop ND-800 Spectrophotometer.
Primer Design for ZEP 1 (Zeaxanthin Epoxidase) Gene
The QIAGEN CLC Genomic Workbench, a primer design software was used to generate the primers targeting two SNP positions namely 44448432(C/T) and 44448438(T/G). The SNP positions coordinates are from the Chromosome 2 and version 5 of the B73 reference genome. The primer length ranges from {18bp---19bp} with GC content lower than 60%. The primer melting temperature varied from {600C---680C}. The designed primers were blasted using NCBI-BLAST (National Center for Biotechnology Information (NCBI)-Best Local Alignment Search Tool) to test the specificity. CATCGATTGGCTTGAGCA was used as the forward primer, while GTACGCCCCATATCCCTTC was used as the reverse primer.
Amplification and Visualization
The PCR reaction mix for the ZEP1 gene was prepared using 5 μl of 10x NH4 PCR buffer, 2 μl of each primer, 2 μl of 50 mM MgCl2, 0.2 μl of BIOTAQ™ polymerase, 4 μl of DNA and 3 μl of Dimethyl Sulfoxide (DMSO), and ultra-pure water making up to 50 μl final volume. Of the total volume, 45 μl was purified and used for sequencing, the remaining 5 μl volume was used for gel-electrophoresis. Gel electrophoresis was conducted to visualize the amplified DNA fragments of the samples. A 2% agarose gel was prepared using sodium borate buffer and ethidium bromide for DNA staining. After electrophoresis, the gel was removed, and the DNA bands were visualized using a gel documentation system (Enduro GDS, Labnet International Inc.)
Sequencing and SNP Identification
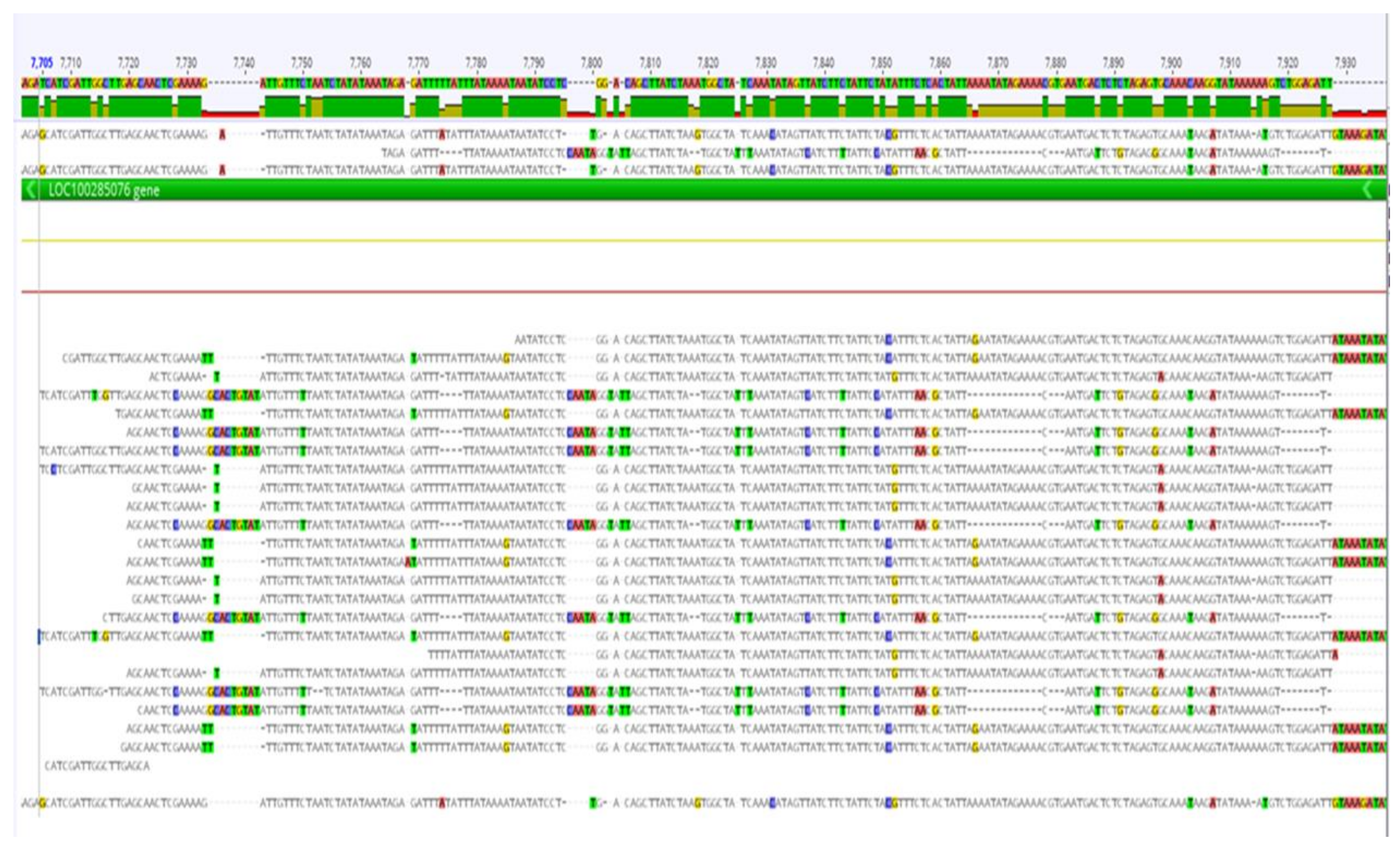
The PCR products of ZEP1 for the 24 selected lines with contrasting carotenoid levels were sequenced at IITA Bioscience Center. The sequencing was carried out bidirectionally, utilizing both forward and reverse primers. The analysis of SNPs and InDels was done by aligning the sequences using CodonCode Aligner, as illustrated in Figure 4.
KASP Genotyping
For the KASP genotyping reaction, Genomic DNA isolated from the leaf tissue of the 147 maize inbred lines was used as template. The DNA concentration was adjusted to a working concentration of 50 ng/uL. Each KASP reaction was performed in a volume of 10 uL, which consisted of 5 uL template DNA, and 5 uL of the prepared genotyping mix (2x KASP master mix and primer mix). Two crtRB1 SNP assays purchased from LGC Genomics, UK, was used for genotyping. Amplification was performed using the Roche Light Cycler 480 II (LC480 II) System (Roche-Life Science, USA) at the Bioscience Centre of the International Institute of Tropical Agriculture (IITA) Ibadan, Nigeria. Amplification condition was as follow: 1 cycle of KASP special Taq activation at 94°C for 15 min, followed by 36 cycles of denaturation at 94°C for 20 s, and annealing and elongation at 61°C (dropping 0.6°C per cycle) for 1 min. Endpoint detection of the fluorescence signal was acquired for 1 min at 30°C using the same instrument. Allele calls for all SNPs were done using KlusterCaller software (LGC Group), as homozygous for FAM or HEX allele, or heterozygous for both alleles.
Data Analysis
Analyses of variance for carotenoids were computed with PROC MIXED procedure in SAS version 9.4 (SAS Institute, 2020). Repeatability values for each carotenoid was estimated using PROC MIXED procedure in SAS (SAS Institute 2020) as described by Holland et al. (2003). Pearson phenotypic correlation coefficients for carotenoid concentrations were calculated using inbred line means. Descriptive statistics were computed with the pastecs package in R Statistical Software (v4.3.2; R Core Team, 2023). Additionally, R Statistical Software (v4.3.2; R Core Team, 2023) psych package was employed to compute the correlation between the two KASP markers and carotenoids. Statistical significance was defined as p < 0.05.
Results
Phenotypic Analysis
The 147 lines exhibited marked differences in provitamin A and other carotenoids (
Table 1). The zeaxanthin concentration varied from 1.25 to 32.44 µg/g followed by β-carotene ranging from 1.71 to 32.35 µg/g, and α-carotene varying from 0.22 and 5.07 µg/g. The concentration of total carotenoid level reached a maximum of 65.62 µg/g due to high concentrations of zeaxanthin, β-carotene and lutein. The concentration of provitamin A varied from 2.24 to 34.20 µg/g with a mean value of 12.11 µg/g (
Table 1).
Correlation Among Carotenoids
The phenotypic correlations between distinct carotenoids followed their established relationships in the carotenoid biosynthesis pathway in this study (Farré et al., 2010). The correlation among the distinct carotenoids exhibited significant positive or negative relationship. Lutein located in the α-branch of the carotenoid biosynthesis pathway was not significantly correlated with β-cryptoxanthin and β-carotene, which are located in the β branch (
Table 3). The correlation between lutein and zeaxanthin was very small (r = -0.05) indicating the possibility of simultaneous improvement of these beneficial carotenoids. The correlation between β-cryptoxanthin and zeaxanthin (r = 0.43, p< 0.001) was significant and positive, suggesting that substantial conversion occurred from β-cryptoxanthin to zeaxanthin in the β-branch of the pathway. Β-cryptoxanthin had a positive correlation with both α-carotene and provitamin A but a negative correlation with β-carotene (
Table 3). As expected, the major component of provitamin A, which is β-carotene has strong and positive correlation with provitamin A (r=0.98, p<0.001), indicating the possibility of increasing provitamin A to much higher levels through redirecting the metabolic flow from the α to the β branch of the carotenoid biosynthetic pathway, or through increasing the supply of precursors at the beginning of the pathway and decreasing the activity farther down.
Nucleotide Variation
Twenty-four lines with contrasting carotenoid content determined by HPLC were selected for sequencing. Amongst these lines, 16 had medium-to-high carotenoid concentration while the remaining 8 lines had low levels of carotenoids (
Table 2). The profiles of the inbred lines, specifically the values for individual carotenoids measured in this study such as zeaxanthin, lutein, alpha-carotene, beta-carotene, provitamin A, and total carotenoids, were arranged in increasing order. For each carotenoid, four inbred lines were selected: two lines with the lowest values and the other two lines with the highest values resulting in 24 inbred lines. Sequencing was carried out for the discovery of genetic modification, including SNPs, insertions, deletions, and structural variants in the lines and the precise order of the nucleotides (A, T, C, and G). Alignment of the 24 lines sequence results using Codon Code aligner software (
Figure 4) found 8 allele groups (
Table 4). Amongst the 8 allele groups, two showed significant relationships with the carotenoid content of the 24 selected lines (
Table 5). Group 1 showed a significant association with zeaxanthin content, with samples in the group carrying allele “BB” having significantly reduced zeaxanthin content while those carrying allele “AA” had significantly high zeaxanthin content (
Table 5). Although allele group 8 also showed a significant relationship with zeaxanthin content, samples having allele “BB” had a significantly higher carotenoid content than the samples with allele “AA” (p = 0.002) (
Table 5). The variant of
ZEP1 (
ZEP1_7852) present in allele group 8 was significantly correlated with both zeaxanthin (p=0.00) and total carotenoid content (p=0.006). The inbred lines with allele “AA” had higher zeaxanthin and total carotenoid content followed by lines having allele “GG” (
Table 5).
Table 2.
The 24 selected maize inbred lines with contrasting carotenoid content determined by high-performance liquid chromatography (HPLC) used for sequencing.
Table 2.
The 24 selected maize inbred lines with contrasting carotenoid content determined by high-performance liquid chromatography (HPLC) used for sequencing.
| Category |
Entry |
Line |
Zeaxanthin (μg/g) |
βetacryptoxanthin (μg/g) |
Alpha-carotene
(μg/g) |
Beta-carotene
(μg/g) |
Provitamin A (μg/g) |
Total-carotenoids
(μg/g) |
| L |
27 |
TZMI2015-3 |
16.83 |
3.08 |
0.98 |
3.60 |
5.66 |
26.14 |
| L |
37 |
TZMI2019 |
1.25 |
3.84 |
1.42 |
15.49 |
18.17 |
25.30 |
| L |
40 |
TZMI2023-5-1 |
29.48 |
5.96 |
2.43 |
9.00 |
13.21 |
60.91 |
| L |
41 |
TZMI2023-5-2 |
32.44 |
4.81 |
2.07 |
7.97 |
11.47 |
57.19 |
| L |
53 |
TZMI2027-2 |
2.25 |
1.82 |
0.59 |
4.57 |
5.72 |
14.01 |
| L |
57 |
TZMI2028-2-2 |
2.02 |
1.97 |
0.67 |
4.05 |
5.45 |
12.99 |
| L |
61 |
TZMI2028-5-1 |
2.09 |
1.67 |
0.48 |
4.09 |
5.24 |
12.99 |
| L |
75 |
TZMI2056 |
14.08 |
3.47 |
1.03 |
2.88 |
5.05 |
29.10 |
| M-H |
76 |
TZMI2058 |
28.82 |
4.68 |
1.66 |
10.12 |
13.20 |
58.87 |
| M-H |
83 |
TZMI2078 |
32.25 |
10.48 |
2.55 |
7.92 |
14.40 |
65.62 |
| M-H |
99 |
TZMI2043-2 |
2.59 |
2.41 |
0.75 |
5.42 |
6.99 |
14.54 |
| M-H |
110 |
TZMI2113 |
25.77 |
6.87 |
2.34 |
11.50 |
16.12 |
52.04 |
| M-H |
118 |
TZMI2032-1-2 |
3.13 |
3.65 |
5.07 |
11.06 |
15.55 |
30.58 |
| M-H |
131 |
TZMI2050 |
21.37 |
8.24 |
1.81 |
9.67 |
14.69 |
43.72 |
| M-H |
138 |
TZMI2075 |
31.14 |
8.72 |
2.27 |
7.94 |
13.42 |
55.07 |
| M-H |
151 |
TZMI2104 |
1.67 |
3.00 |
1.25 |
10.49 |
12.64 |
23.94 |
| M-H |
161 |
TZMI2110 |
4.56 |
3.05 |
0.57 |
7.83 |
9.56 |
19.24 |
| M-H |
171 |
TZMI2067-1-1 |
11.10 |
1.29 |
1.30 |
22.05 |
25.30 |
38.56 |
| M-H |
172 |
TZMI2067-1-2 |
9.62 |
1.73 |
0.87 |
27.52 |
28.79 |
42.84 |
| M-H |
174 |
TZMI2114 |
10.03 |
2.21 |
1.31 |
32.35 |
34.20 |
51.37 |
| M-H |
175 |
TZMI2119 |
7.91 |
2.10 |
1.69 |
30.44 |
32.35 |
46.74 |
| M-H |
176 |
TZMI2120 |
10.47 |
2.00 |
1.55 |
24.98 |
26.74 |
44.80 |
| M-H |
182 |
KU1409 |
11.72 |
0.84 |
0.22 |
1.71 |
2.24 |
21.09 |
| M-H |
183 |
TZI2354 |
13.93 |
3.39 |
0.69 |
2.57 |
4.63 |
23.55 |
| |
|
Mean |
13.60 |
3.80 |
1.48 |
11.46 |
14.2 |
36.3 |
| |
|
SD |
11.03 |
2.54 |
1.02 |
9.16 |
9.20 |
17.01 |
| |
|
CV |
0.81 |
0.66 |
0.68 |
0.79 |
0.64 |
0.47 |
Table 3.
Pearson’s correlation coefficients among mean values of carotenoid concentrations of the 147 maize inbred lines.
Table 3.
Pearson’s correlation coefficients among mean values of carotenoid concentrations of the 147 maize inbred lines.
| |
Lutein |
Zeaxanthin |
β-cryptoxanthin |
α-carotene |
β-carotene |
|
| Zeaxanthin |
-0.05 |
|
|
|
|
|
| β-cryptoxanthin |
-0.051 |
0.43*** |
|
|
|
|
| α-carotene |
0.14 |
0.17* |
0.56*** |
|
|
|
| β-carotene |
-0.013 |
-0.21* |
-0.041 |
0.27*** |
|
|
| Provitamin A |
-0.019 |
-0.13 |
0.14 |
0.40*** |
0.98*** |
|
Table 4.
A categorization of the observed loci into groups based on similarities in the allelic pattern across the 24 selected maize inbred lines of the loci.
Table 4.
A categorization of the observed loci into groups based on similarities in the allelic pattern across the 24 selected maize inbred lines of the loci.
| Group 1 |
Group 2 |
Group 3 |
Group 4 |
Group 5 |
Group 6 |
Group 7 |
Group 8 |
|
ZEP18018 |
ZEP18032 |
ZEP1798 |
ZEP18281 |
ZEP18282-8284 |
ZEP18318 |
ZEP18324 |
ZEP17852 |
|
ZEP17852 |
ZEP17803 |
ZEP17785 |
ZEP18254-8259 |
|
|
|
ZEP17914 |
|
ZEP17914 |
ZEP17805 |
ZEP17851 |
|
|
|
|
ZEP17819 |
|
ZEP17819 |
ZEP1780 |
ZEP17769 |
|
|
|
|
ZEP18005 |
|
ZEP18005 |
ZEP17825 |
ZEP17851 |
|
|
|
|
ZEP18016 |
|
ZEP18016 |
ZEP17846 |
ZEP18020 |
|
|
|
|
ZEP18081 |
|
ZEP18081 |
ZEP17856 |
ZEP18028 |
|
|
|
|
ZEP18138-8145 |
|
ZEP18092 |
ZEP17859 |
ZEP18302 |
|
|
|
|
|
|
ZEP17733-34 |
ZEP17849 |
ZEP1794-7944 |
|
|
|
|
|
|
ZEP17735-43 |
ZEP17827 |
ZEP18034-8051 |
|
|
|
|
|
|
ZEP17865-7882 |
ZEP17837 |
|
|
|
|
|
|
|
ZEP1787-793 |
ZEP17843 |
|
|
|
|
|
|
|
ZEP18138-8145 |
ZEP17849 |
|
|
|
|
|
|
| |
ZEP17887 |
|
|
|
|
|
|
| |
ZEP17891 |
|
|
|
|
|
|
| |
ZEP17897 |
|
|
|
|
|
|
| |
ZEP17898 |
|
|
|
|
|
|
| |
ZEP17993 |
|
|
|
|
|
|
| |
ZEP18009 |
|
|
|
|
|
|
| |
ZEP18011 |
|
|
|
|
|
|
| |
ZEP18089 |
|
|
|
|
|
|
| |
ZEP18100 |
|
|
|
|
|
|
| |
ZEP18106 |
|
|
|
|
|
|
| |
ZEP18108 |
|
|
|
|
|
|
| |
ZEP181202 |
|
|
|
|
|
|
| |
ZEP18184 |
|
|
|
|
|
|
| |
ZEP18190 |
|
|
|
|
|
|
| |
ZEP18245 |
|
|
|
|
|
|
| |
ZEP18305 |
|
|
|
|
|
|
| |
ZEP17727 |
|
|
|
|
|
|
| |
ZEP17750 |
|
|
|
|
|
|
| |
ZEP17796-7800 |
|
|
|
|
|
|
Table 5.
The 3 loci groups of the 24 selected maize inbred lines with significant association with carotenoid content.
Table 5.
The 3 loci groups of the 24 selected maize inbred lines with significant association with carotenoid content.
| Group 1 |
|
Group 8 |
|
ZEP1_7852 |
| Alleles |
Mean |
StDev |
|
Alleles |
Mean |
StDev |
|
Allele |
Mean |
StDev |
| AA |
19.591 |
10.001 |
|
AA |
4.501 |
3.774 |
|
AA |
19.233 |
10.088 |
| BB |
4.501 |
3.774 |
|
BB |
18.157 |
10.654 |
|
GG |
4.226 |
3.626 |
| CC |
16.723 |
11.769 |
|
|
|
|
|
|
|
|
Screening Inbred Lines with Favorable Alleles of crtRB1
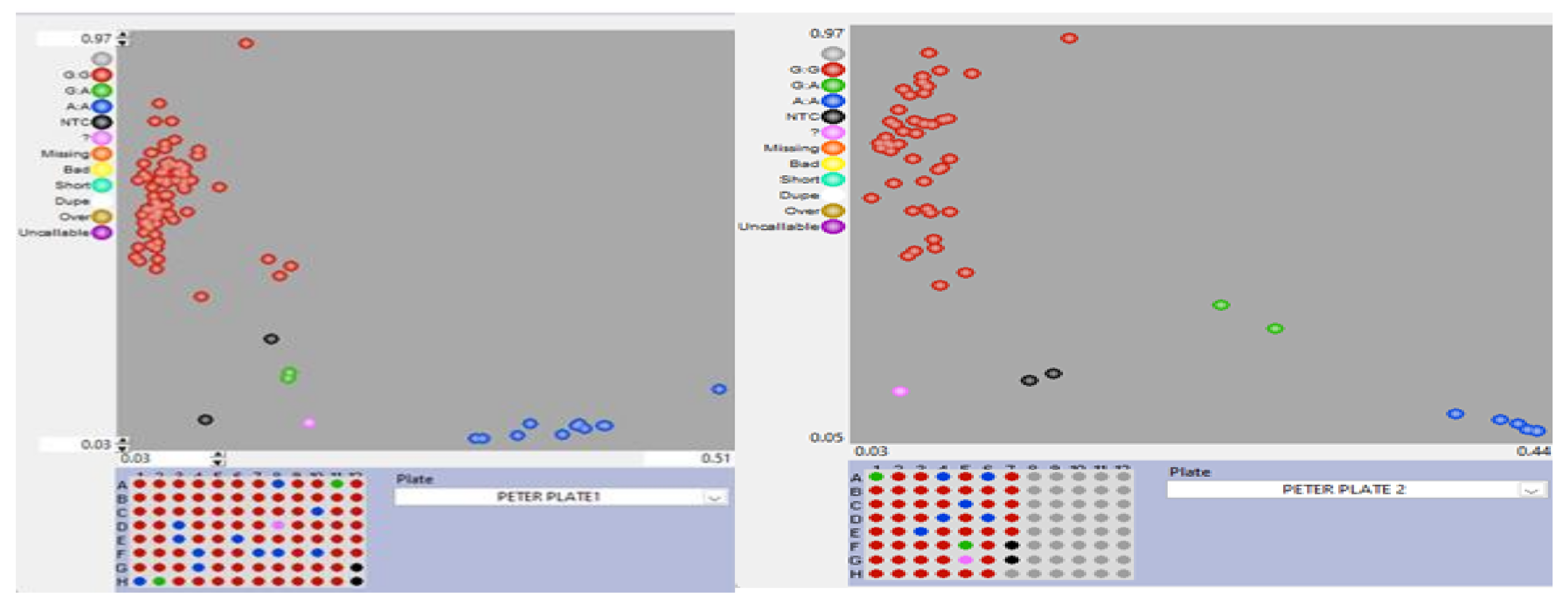
Genotyping of the 147 lines with KASP SNP markers zm0015 and zm0016 found the effectiveness of KASP SNP zm0016 in successfully differentiating the favorable homozygous and heterozygous alleles for the crtRB1 gene (
Figure 2). Amongst the 147 lines, 132 exhibited heterozygote allele (G: A [green]), with only one inbred line showing no amplification. The remaining 14 inbred lines carried the favorable allele (G: G [red]) (
Table 6). The 5 non-template controls (NTC) were used to verify the amplification and effectiveness of the KASP SNP zm0016. As shown in
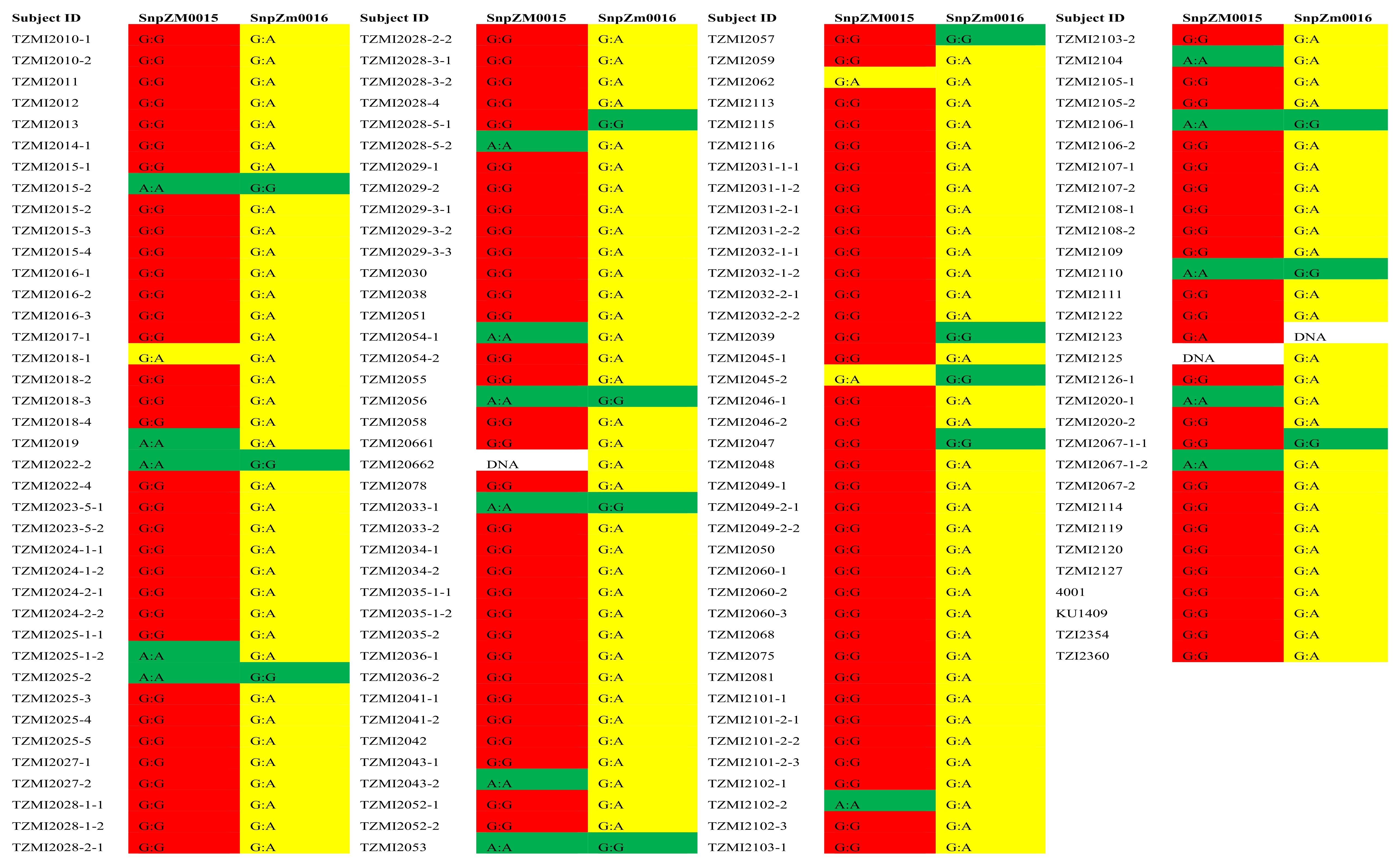
Figure 2, these controls formed a distinct cluster, consistent with the findings of, Maazou et al. (2021). It is worth noting that most of the inbred lines with the unfavorable crtRB1 alleles for KASP SNP zm0015 (
Figure 5) displayed increased levels of provitamin A carotenoids. These findings suggest that genes other than
crtRB1, like
LCYE and
LUT1 might have contributed to the accumulation of provitamin A carotenoids in these specific inbred lines (Owens et al., 2014). Inbreds TZMI2015-2, TZMI2022-2, TZMI2025-2, TZMI2056, TZMI2033-1, TZMI2053, TZMI2106-1, and TZMI2110 carried homozygous favorable alleles of the two KASP SNPs markers (
Figure 5). The two markers (zm0016 and zm0015) were not significantly associated with either high or low content carotenoid content of the inbred lines (data not shown). Analysis of pedigrees of the inbred lines resulted in grouping of the lines based on their source population. As the groups exhibited marked differences in their carotenoid content, this could lead to the absence of a significant association between carotenoid content and the two KASP SNP markers (zm0015 and zm0016). The inbred lines belonging to group 1 (TZMI1017/SW 1 (S) C14-7-1-1-B)-57-1-1-2-B) displayed the highest lutein content among all the groups, with. In contrast, the lines in group 26 (TZMI1029/SW 5 (S) C6-18-2-1-B)-18-4-1-1-B) had the lowest lutein content (
Supplementary Table S5). Lines belonging to the 28 pedigree groups did not exhibit discernible relationship with α-carotene and β-cryptoxanthin (data not shown). In contrast, lines belonging to group 1, 26, 31, 43, and 34 (TZMI1029/SW 5 (S) C6-18-2-1-B)-21-2-2-1-B) exhibited significant difference in zeaxanthin. Amongst these, group 26 displayed the highest zeaxanthin content among the inbred lines (
Supplementary Table S6). Lines belonging to group 43 (TZMI1046/SW 5 (S) C6-18-3-1-B)-35-1-1-1-B) displayed significant difference in β-carotene and provitamin A content. Similarly, lines in group 38 (TZMI1029/SW 5 (S) C6-18-2-1-B)-32-4-1-1-B) exhibited striking differences in its β-carotene and provitamin A content (
Supplementary Table S2 and S4). Furthermore, the total carotenoid content of lines in groups 1, 43, 28, and 17 (TZMI1029/SW 5 (S) C6-18-2-1-B)-15-4-5-2-B) showed significant differences in total carotenoid content. Specifically, lines in group 1 exhibited the highest total carotenoid content, while those in group 43 (
Supplementary Table S3) showing the second-highest total carotenoid content.
Figure 2.
Genotype plot for 147 maize inbred lines genotyped using crtRB1-KASP marker zm0016. Red = Favourable alleles; Green= Heterozygous; Pink = No amplification; Black = no template controls.
Figure 2.
Genotype plot for 147 maize inbred lines genotyped using crtRB1-KASP marker zm0016. Red = Favourable alleles; Green= Heterozygous; Pink = No amplification; Black = no template controls.
Figure 3.
Genotype plot for 147 maize inbred lines genotyped using crtRB1-KASP marker snpz0015. Red = Unfavorable alleles; Blue= Favourable alleles; Green= Heterozygous; Pink = No amplification; Black = no template controls.
Figure 3.
Genotype plot for 147 maize inbred lines genotyped using crtRB1-KASP marker snpz0015. Red = Unfavorable alleles; Blue= Favourable alleles; Green= Heterozygous; Pink = No amplification; Black = no template controls.
Figure 4.
Multiple sequence alignment of selected 24 maize lines for variance identification, leveraging on ClustalW sequence alignment algorithm in Geneious software.
Figure 4.
Multiple sequence alignment of selected 24 maize lines for variance identification, leveraging on ClustalW sequence alignment algorithm in Geneious software.
Figure 5.
Genotypes of 147 Provitamin A maize inbred lines with the different alleles of crtRB1-KASP SNP markers. Genotypes highlighted GREEN, RED, and YELLOW have the favourable, unfavorable, and heterozygous alleles respectively.
Figure 5.
Genotypes of 147 Provitamin A maize inbred lines with the different alleles of crtRB1-KASP SNP markers. Genotypes highlighted GREEN, RED, and YELLOW have the favourable, unfavorable, and heterozygous alleles respectively.
Discussion
The major carotenoids found in yellow/orange maize grain are lutein, zeaxanthin and β-carotene, with lesser amounts of β-cryptoxanthin and α-carotene (USDA National Nutrient Database, ndb.nal.usda.gov), which is consistent with the pattern observed in the present study. The highest level of provitamin A carotenoids found in our study was β-carotene. Among the 147 assayed inbred lines, 25 had provitamin A content close to or exceeding 15 μg/g dry weight. An inbred line TZMI2114 had provitamin A concentration of 34.20 μg/g dry weight, which can be used as parents of source populations and hybrids to boost provitamin A carotenoids in sub-tropical maize.
Provitamin A carotenoids including α-carotene, β-cryptoxanthin except β-carotene had lower average values than zeaxanthin and lutein in our study, consistent with findings in other studies (Harjes et al., 2008; Vallabhaneni & Wurtzel, 2009; Gebremeskel et al., 2018; Obeng-bio et al., 2019). In our study, β-carotene made a greater contribution to provitamin A content compared to β-cryptoxanthin, consistent with the findings of Senete et al. (2011) and Maazou et al. (2021), but differed from the findings of Egesel et al. (2003), Menkir et al. (2008), and Suwarno et al. (2014) that reported higher β-cryptoxanthin relative to β-carotene. Such differences could arise from the genetic makeup of the lines used in the current (Menkir et al., 2021). Most of the maize varieties developed and released globally contain provitamin A concentrations ranging from 6 to 10 μg/g (Anderson et al., 2017). The results of our study and others (Obeng-bio et al., 2019; Harjes et al., 2008; Azmach et al., 2013) reported much high β-carotene levels, showing the potential to breed maize hybrids with much higher concentrations of provitamin A.
The development of markers that can detect single nucleotide substitution using sequencing was among the eventual objectives of this study. Primers were designed for two SNP positions of the ZEP1 gene namely ZEP1SNP (432) and ZEP1SNP (438). The marker-trait association study using the presence or absence of the sequence of these markers in 24 selected yellow to orange maize inbred lines in our study found a significant association of the markers with zeaxanthin and total carotenoids, consistent with the results of Owen et al. (2014). Previous studies did not demonstrate the impact of ZEP1 on grain carotenoid composition in association studies. Vallabhaneni and Wurtzel (2009) examined the ZEP1 gene related to carotenoid accumulation and found that zeaxanthin epoxidase (ZEP1) exhausts the carotenoid reservoir during the process of conversion into abscisic acid. Their investigations revealed a negative correlation between ZEP1 transcript levels and the accumulation of carotenoids.
Conclusions
Enhancing the nutritional content of staple crops through biofortification emerges as a cost-effective and efficient strategy to combat VAD in impoverished nations heavily reliant on staple foods. Ongoing investment in traditional food enrichment methods may not be financially viable in such contexts. The use of marker-assisted breeding offers a resource-efficient approach to developing provitamin A-rich lines, reducing associated costs. In this present study, 25 inbred lines demonstrated provitamin A levels surpassing the 15 μg/g dry weight target set by HarvestPlus. These lines can play a crucial role in expediting the development of maize hybrids with elevated provitamin A content for cultivation in Sub-Saharan Africa to mitigate the adverse effects of vitamin A deficiency.
The ZEP1 gene regulates the transformation of zeaxanthin into abscisic acid, leading to a reduction in carotenoid levels in seeds. If the ZEP1 gene is non-functional or has a weak allele, it is likely to result in greater carotenoid content that may reduce abscisic acid synthesis. Based on our results, it appears that ZEP1 should be regarded as equally important as crtRB1 gene. It should be noted that these results are based on a limited set of materials examined in a single environment as carotenoid levels are known to be sensitive to environmental conditions. Additional research is required to analyze the genetic diversity at these specific locations and to create molecular markers that are easy to use and can differentiate between the functional alleles in breeding programs. In future studies, it is imperative to consider exploration of alternative alleles or markers that exhibit a more widespread distribution across the genome. Moreover, focusing on markers that are present within a more homogenous population can mitigate potential confounding factors and achieve a higher level of accuracy and reliability in genetic analyses. Expanding exploration and incorporation of additional KASP SNP markers such as zm0013, zm0014, zm0017, zm0018 and zm0019 will undoubtedly enrich our understanding and enable us to capture a more comprehensive picture of the genetic variations present in maize.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
PA executed both field and laboratory experiments, performed statistical analyses, and crafted the initial manuscript. OJI and NU were actively involved in the laboratory experiments and statistical analyses. AM played a pivotal role in project conception, provided maize inbred lines developed by AM and contributed to critical manuscript revisions. NU and OJI closely supervised laboratory work at IITA and revised the manuscript. VOA contributed to project conception and participated in manuscript revisions. Additionally, VOA, and AM collectively guided PA in MSc supervision. The final manuscript was reviewed and approved by all authors.
Funding
Pan African University Institute of Life and Earth Science (Including Health and Agriculture) with grant number PAU020110MB (PAN African Scholarship).
Data Availability Statement
The original contributions presented in the study are included in the article/
Supplementary data, further inquiries can be directed to the corresponding author.
Acknowledgments
This research constitutes a crucial segment of an MSc project, generously supported by the benevolence of the African Union through the prestigious Pan African University and the International Institute of Tropical Agriculture (IITA). The authors extend heartfelt gratitude to the immense contributions of the Bioscience Center of IITA and Dr Melaku Gedil.
Conflicts of Interest
The authors affirm that the study was conducted devoid of any conflicts of interest.
References
- Andersson, M.S., Saltzman, A., Virk, P. S., & Pfeiffer, W. H. (2017). Progress update: Crop development and biofortified staple food crops under HarvestPlus. African Journal Food and Agriculture Nutrition, 2, 11905–11935.
- Azmach, G., Gedil, M., Menkir, A., Spillane, C. (2013). Marker-trait association analysis of functional gene markers for provitamin A levels across diverse tropical yellow maize inbred lines. BMC Plant Biol 13: 227.
- Babu, R., N. Palacios Rojas, S. Gao, J. Yan, and K. Pixley. (2013). Validation of the effects of molecular marker polymorphisms in LcyE and CrtRB1 on provitamin A concentrations for 26 tropical maize populations. Theor. Appl. Genet. 126:389–399. [CrossRef]
- Bailey, R.L., West, K.P., J.r., Black, R.E. (2015). The epidemiology of global micronutrient deficiencies.Ann. Nutr. Metab., 66, 22–33.
- Burt, A.J., Grainger CM, Smid M.P., Shelp, B.J., Lee, E.A. (2011). Allele mining of exotic maize germplasm to enhance macular carotenoids. Crop Sci, 51:991–1004.
- Bouis, H. E., & Welch, R. M. (2010). Biofortification-A Sustainable Agricultural Strategy for Reducing Micronutrient Malnutrition in the Global South. Crop Science, 50, S-20-S-32. [CrossRef]
- Chandrasekharan, N., Ramanathan, N., Pukalenthy, B., Chandran, S., Manickam, D., Adhimoolam, K., Nalliappan, G. K., Manickam, S., Rajasekaran, R., Sampathrajan, V., Muthusamy, V., Hossain, F., Gupta, H. S., & Natesan, S. (2022). Development of β-carotene, lysine, and tryptophan-rich maize (Zea mays) inbreds through marker-assisted gene pyramiding. Scientific Reports, 12(1), 1–11. [CrossRef]
- Dhliwayo, T., Palacios-Rojas, N., Crossa, J., & Pixley, K. V. (2014). Effects of S1 recurrent selection for provitamin a carotenoid content for three open-pollinated maize cultivars. Crop Science, 54(6), 2449–2460. [CrossRef]
- Egesel, C. O., Wong, J. C., Lambert, R. J., and Rocheford, T. R. (2003). Combining ability Of maize inbreds for carotenoids and tocopherols. Crop Sci. 43, 818–823. [CrossRef]
- Farré, G., Sanahuja, G., Naqvi, S., Bai, C., Capell, T., Zhu, C., Christou, P. (2010). Travel advice on the road to carotenoids in plants. Plant Sci 179:28–48. [CrossRef]
- Fraser, P.D., Bramley, P.M. (2004). The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res, 43: 228–265.
- Gebremeskel, S., Garcia-oliveira, A. L., Menkir, A., Adetimirin, V., & Gedil, M. (2017). Pan African University Life and Earth Science (PAULESI), Nigeria. Journal of Cereal Science. [CrossRef]
- Giuliano, G., Tavazza, R., Diretto, G., Beyer, P., Taylor, M. (2008). Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotechnol 26:139–145.
- Gowda, M., Worku, M., Nair, S. K., Palacios-Rojas, N., & Prasanna, B. M. (2017). Quality Assurance/Quality Control (QA/QC) in Maize Breeding and Seed Production: Theory and Practice. CIMMYT, Nairobi.
- Graham, R.D., Welch, R.M., & Bouis, H.E. (2001). Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Advances in Agronomy 70, 77–142.
- Harjes, C. E., Rocheford, T. R., Bai, L., Brutnell, T. P., Kandianis, C. B., Sowinski, S. G., Stapleton, A.E., Vallabhaneni, R., Williams, M., Wurtzel, E. T., Yan, J., & Buckler, E. S. (2008). Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science, 319(5861), 330–333. [CrossRef]
- Howe JA, Tanumihardjo SA. (2006). Carotenoid-biofortified maize maintains adequate vitamin a status in mongolian gerbils. J Nutr, 136:2562–2567.
- Holland, B., Carolina, N., Nyquist, W., E., Cervantes-Martinez CT. (2003). Estimating and Interpreting Heritability for Plant Breeding : An Update.
- Islam, S., N., Paul, C., Buckler, E.,S., Rochford, T. (2004). Genetic and diversity studies on carotenoids (provitamin A) in maize. Pp. 77-107. In Fortieth Annual Illinois Corn Breeders’ School. University of Illinois at Urban-Champaign.
- Kandianis, C.B., R., Stevens, W., Liu, N., Palacios, K., Montgomery, K., Pixley, W.S., White, and T., Rocheford. (2013). Genetic architecture controlling variation in grain carotenoid composition and concentrations in two maize populations. Theor. Appl. Genet. 126:2879–2895. [CrossRef]
- Krivanek, A. F., Groote, H. De, Gunaratna, N. S., & Diallo, A. O. (2014). Breeding and disseminating quality protein maize (QPM) for Africa. November.
- Listman, G. M., Guzmán, C., Palacios-Rojas, N., Pfeiffer, W. H., Vicente, F. S., & Govindan, V. (2019).
- Improving Nutrition through Biofortification: Preharvest and Postharvest Technologies. Cereal Foods World, 64(3). [CrossRef]
- Li, Q., Farre, G., Naqvi, S., Breitenbach, J., Sanahuja, G., Bai, C., Sandmann, G., Capell, T., Christou, P., Zhu, C. (2010). Cloning and functional characterization of the maize carotenoid isomerase and bcarotene hydroxylase genes and their regulation during endosperm maturation. Transgenic Res 19:1053–1068.
- Liu, K., Goodman, M., Muse, S.,J., Smith, J.,S., Buckler, E., Doebley, J. (2003). Genetic structure and diversity among maize inbred lines as inferred from DNA microsatellites. Genetics 165:2117–2128.
- Maazou, A. R. S., Gedil, M., Adetimirin, V. O., Meseka, S., Mengesha, W., Babalola, D., Offornedo, Q. N., & Menkir, A. (2021). Comparative assessment of the effectiveness of alternative genotyping assays for characterizing carotenoid accumulation in tropical maize inbred lines. Agronomy, 11(10). [CrossRef]
- Menkir, A., Liu, W., White, W.S., Maziya-Dixon, B., Rocheford, T. (2008). Carotenoid diversity in tropical adapted yellow maize inbred lines. Food Chem; 109(3): 521–529.
- Menkir, A., Dieng, I., Mengesha, W., Meseka, S., & Maziya-dixon, B. (2021). Unravelling the Effect of Provitamin A Enrichment on Agronomic Performance of Tropical Maize Hybrids. 1–19.
- Morris, M.L. (2001). Assessing the Benefits of International Maize Breeding Research: An Overview of the Global Maize Impacts Study; in Pingali, P.L. (Ed). CIMMYT 1999-2000 World Maize Facts and Trends. Meeting World Maize Needs Technological Opportunities and Priorities for the Public Sector. Mexico, D.F.: CIMMYT.25-27.
- Matthews, P.D., Luo, R., Wurtzel, ET. (2003). Maize phytoene desaturase and zeta carotene desaturase catalyzes a poly Z desaturation pathway: implications for genetic engineering of carotenoid content among cereal crops. J Exp Bot 54: 2215- 2230.
- Nuss, E.T., and Tanumihardjo, S.A. (2010). Maize: A Paramount Staple Crop in the Context of Global Nutrition. Comprehensive Reviews in Food Science and Food Safety, 9, 417-436. [CrossRef]
- Obeng-bio, E., Beatrice, B. B., Ifie, E., Danquah, A., Blay, E. T., & Abu, M. (2019). Phenotypic characterization and validation of provitamin A functional genes in early maturing provitamin A quality protein maize (Zea mays) inbred lines. November, 1–14. [CrossRef]
- Ortiz-Monasterio, J.I., Palacios-Rojas, N., Meng, E., Pixley, K., Trethowan, R., and Pena, R.J. (2007). Enhancing the mineral and vitamin content of wheat and maize through plant breeding. Journal of Cereal Science.,46: 293-307.
- Owens, B.F., Lipka, A.E., Magallanes-Lundback, M., Tiede, T., Diepenbrock, C.H., Kandianis, C.B., Kim, E., Cepela, J., Mateos- Hernandez, M., Buell, C.R. (2014). A foundation for provitamin A biofortification of maize: genome-wide association and genomic prediction models of carotenoid levels. Genetics 198: 1699–1716.
- Pixley, K., Rojas, N. P., Babu, R., Mutale, R., Surles, R., & Simpungwe, E. (2013). Biofortification of maize with provitamin A carotenoids. In Carotenoids and Human Health (pp. 271–292). Humana Press Inc. [CrossRef]
- R Core Team (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/.
- Senete, C. T., De Oliveira Guimarães, P. E., Paes, M. C. D., and De Souza, J. C. (2011). Diallel analysis of maize inbred lines for carotenoids and grain yield. Euphytica 182, 395–404. [CrossRef]
- Shiferaw, B., Prasanna, B., M., Hellin, J., and Bänziger, M. (2011). Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Security 3:307-327.
- Suwarno, W.B., Pixley, K.V., Palacios-Rojas, N., Kaeppler, S.M., Babu, R. (2015). Genome-wide association analysis reveals new targets for carotenoid biofortification in maize. Theor Appl Genet 128:851–864.
- United Nations International Children’s Fund, Coverage at a Crossroads. (2018). New directions for vitamin A supplementation programmes, UNICEF, New York.
- US Institute of Medicine (2001). Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: The National Academies Press.
- Vallabhaneni, R., and E.T., Wurtzel. (2009). Timing and biosynthetic potential for carotenoid accumulation in genetically diverse germplasm of maize. Plant Physiol. 150:562–572. [CrossRef]
- West, C.E., Eilander, A., van, Lieshout, M. (2002). Consequences of revised estimates of carotenoid bioefficacy for dietary control of vitamin A deficiency in developing countries. J Nutr132:2920S2926S.
- Wurtzel, E., Cuttris, A., Vallabhaneni, R. (2012). Maize provitamin A carotenoids, current resources, and future metabolic engineering challenges. Frontiers Plant Sci 3:1–12.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).