1. Introduction
Fibromyalgia (FM) is defined as a long-term disorder expressed widespread pain affecting muscles, bones, and soft tissues, typically accompanied by severe fatigue and sleep disruptions. Additional symptoms may consist of cognitive impairment referred to as “fibro fog”, depression, migraines, irritable bowel syndrome, and pain in the pelvic region, jaw, and bladder [
39]. In ICD-11, FM is diagnosed under chronic widespread pain (MG30.01) and classified as a type of chronic primary pain [
1]. The diagnosis of FM is based on criteria from the American College of Rheumatology, which include a widespread pain index, symptom severity reflecting weariness, poor sleep, and cognitive challenges, a minimum symptom duration of three months, and an unexplained pain origin [
40]. FM symptoms can be mistaken with those of chronic fatigue syndrome, thyroid issues, or polymyalgia rheumatica, hence paraclinical tests and x-rays are employed to rule them out because FM lacks distinct diagnostic indications [
39,
40]. Approximately 5% of the global population was affected by FM, with the majority of cases occurring in America, Europe, and a smaller proportion in Asia [
2]. The disorder primarily impacts women (1:9 ratio) and manifests any ages [
3,
4]. Classified as nociplastic pain, FM is characterized by a lack of definitive neuropathic attributes and an association with altered nociceptive function without clear nociceptor activation [
5].
Education was believed as an initial step toward the practical goal of enhancing the life functioning for FM patients [
6]. Current treatments for alleviating symptoms and improving quality of life and general wellness for patients include pharmacological and non-pharmacological approaches, which are utilized individually and progressively, based on the potential for disability and patient-specific goals [
7]. Nonpharmacological interventions, including cognitive behavioral therapy, exercise, relaxing therapy, electrical stimulation, psychotherapy, meditation, massage, and acupuncture, are recommended as first-line treatments to alleviate FM [
8,
9]. Pharmacological treatments for FM primarily involve antidepressants such as elective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs), and anticonvulsants (pregabalin and gabapentin) [
40]. The FDA has approved several drugs for fibromyalgia treatment, including pregabalin (Lyrica), duloxetine (Cymbalta), and milnacipran (Savella), classified as antiepileptics and antidepressants [
37]. Acupuncture, as a non-pharmacological intervention of FM, has been shown to provide analgesic effects, reduce fatigue and anxiety, and minimize medication side effects, emphasizing the need for a clearer understanding of the pain signaling pathway mechanism [
10].
Acupuncture originated in China several thousand years ago and began with the use of sharp stones and bones, now employs finely crafted, tiny needles for treatment [
38].
Deqi is an essential sign of effective acupuncture, marked by sensations such as soreness, numbness, fullness, and heaviness, indicating successful activation of the meridian system. Electroacupuncture (EA) combines short-term manual acupuncture (MA) for
deqi with extended EA stimulation and encompasses variations in frequency, intensity, wavelength, and duration. EA enhances somatosensory system activation and sciatic nerve blood flow more effectively than MA [
11,
12]. Systemic naloxone (2mg/kg) blocked EA at 2–15 Hz but not at 100 Hz, indicating that low-frequency electrical stimulation relies on the opioid system for analgesia, whereas high-frequency stimulation may utilize alternative mechanisms [
13]. Professor Han firstly provided a groundbreaking explanation for the scientific mechanism of acupuncture-induced release of neurotransmitters [
14]. The European League Against Rheumatism suggests acupuncture as a nonpharmacological remedy for treating FM [
9]. Acupoint selection for FM in Traditional Chinese Medicine (TCM) is guided by the underlying pathophysiology. This includes patterns such as liver-spleen disharmony with symptoms of yin deficiency and empty heat, spleen and kidney yang deficiency, kidney yin and yang deficiency, rising liver fire, as well as conditions involving phlegm and wet heat [
15].
Transient Receptor Potential Vanilloid 1 (TRPV1) is renowned as a ligand-gated non-selective cation channel belonging to the Transient Receptor Potential (TRP) family, which is found in both the peripheral nervous system (PNS) and the central nervous system (CNS). Harmful stimuli such as high temperature (>43 degrees Celcius), pH changes, mechanical stimulation, or permeability can activate TRPV1 expression and sending sensations of pain toward the central region [
16]. TRPV1 gene-deleted mice have recently been studied for their efficacy in suppressing acute and chronic pain. TRPV1 activation triggers extensive hyperphosphorylation of downstream molecules, including phosphoinositide 3-kinases (pPI3K), protein kinase C (pPKC), and protein kinase A (pPKA), followed by mitogen-activated protein kinase (pAkt, pmTOR), mitogen-activated protein kinase (pJNK, pERK, pP38), cAMP-response element binding protein (CREB) and Nuclear factor kappa-light-chain-enhancer of activated B cells (pNF-κB). These proteins are expressed in neuronal cells, microglia, and astrocytes, which are critical for the CNS response to injury and inflammation. Wu et al. discovered that injecting capsaicin, a TRPV1 agonist, into ST36 (
Zusanli) acupoints reproduced the analgesic effect observed with acupuncture [
17].
This study investigates the analgesic effects of EA on FM and its underlying mechanisms via the TRPV1 pain signaling pathway. We examine TRPV1 expression and its downstream molecules in crucial brain structures, including the thalamus, somatosensory cortex (SSC), medial prefrontal cortex (mPFC), hippocampus, and cerebellum (V, VI, VII), as well as the hypothalamus. Importantly, the efficacy of sham EA and real acupuncture is compared in an animal model. By revealing these mechanisms, our research seeks to uncover innovative pain management strategies that enhance the quality of life for those suffering from FM.
2. Materials and Methods
Animal Research and Ethical Approval
The present experiments subjects were 8-12 weeks old female C57/BL6 wild type mice acquired from BioLasc Taiwan Ltd (Yilan, Taiwan), going to weigh 18-20 grams. Female TRPV1 knockout mice from Jackson Laboratory in Bar Harbor, ME, USA, were utilized for the study. Once mice were arrived, they were located in Plexiglas cages with a 12-hours alternating light and dark cycle environment, light day beginning from 6 a.m. to 6 p.m., with a normal room temperature of 25oC and humidity of 60 percent. The researchers were blind to the treatment section during the entire study.
Mice were at random assigned to one of five groups including Control mice (Con), ICS-inducted FM mice (ICS), ICS treated by EA mice (ICS+EA), ICS treated by sham EA mice (ICS+Sham EA), ICS in TRPV1 gene deletion (ICS+KO), each group consisting of six mice.
China Medical University's Institute of Animal Care and USE Committee, Taiwan approved the usage of rodents in this research (Permit number: CMUIACUC-2022-424). In this study, the welfare of the mice was prioritized to minimize their distress. All mice were handled in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Chronic FM Pain Induced by Intermittent Cold Stress (ICS)
This study utilized the Intermittent Cold Stress model in mice, developed by Nishiyori et al. (2008), which induces mechanical allodynia and thermal hyperalgesia lasting over 12 days and can be acutely alleviated by antidepressant treatment [
18,
19].
Four groups of mice were placed in a 4oC environment: ICS, ICS+EA, ICS+Sham EA, and ICS+KO, while the control group was maintained in a regular setting. At 10 a.m. the following day, these four groups were relocated to the outside environment at 25°C for 30 minutes, then back to 4°C for 30 minutes. This process was carried out until 4 p.m for a total of 6 hours, before they were repositioned again overnight from 4:30 p.m. during the first three days. After a week of acclimatization to their new environment, the mice underwent behavioral tests to determine their baseline mechanical and thermal thresholds, followed by the induction of chronic FM pain at 4:30 p.m. on day 0.
Electroacupuncture (EA) Intervention
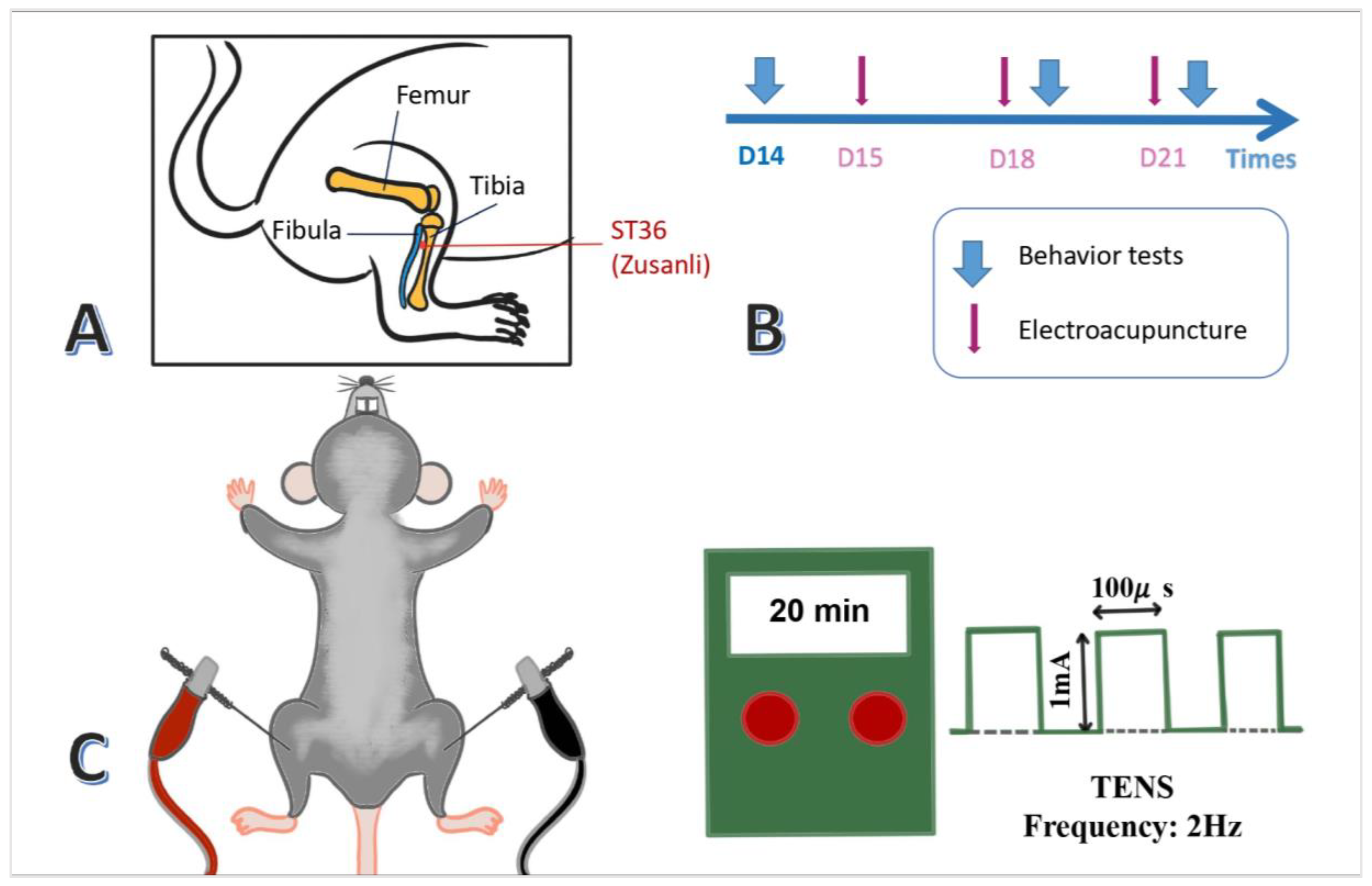
After being anesthetized with 4% isoflurane, the mice were placed in 1% gas via the head-stuffed tube connected to the chamber. Then, a pair of 0.5-inch-long acupuncture needles (32G, Yu Kuang Chem. Ind. Corp., Taiwan) were inserted bilaterally at mice's ST36 acupoint (Zusanli). The murine ST36 was identified 3-4 mm lower than the patella, between the fibula and tibia, and on the anterior side of the anterior tibial muscle. In addition, a depth of 3-4 mm with 1 mA intensity, 2Hz frequency, and 150s width of the constant square pulse was performed for 20 minutes utilizing an electronic source from the machine (Trio 300 stimulator (Ito, Japan)) (
Figure 1A,C). There was a mild twitch near the ST36 acupoint. Only the EA group applied electric current, while the sham EA group only performed sham acupuncture without electrical current. Applications of EA took place on days 15, 18, and 21, prior to the behavioral tests (
Figure 1B).
Mechanical and Thermal Behavior Tests
Both tests were performed seven times on days 0, 4, 7, 10, 14, 18, and 21 at the center of the plantar of the right hind paw. Murine were enclosed in Plexiglas boxes (9.5x11x6.5 cm) above a steel mesh in a dark, noiseless, room-temperature condition to keep themselves calm and adapted to their new surroundings.
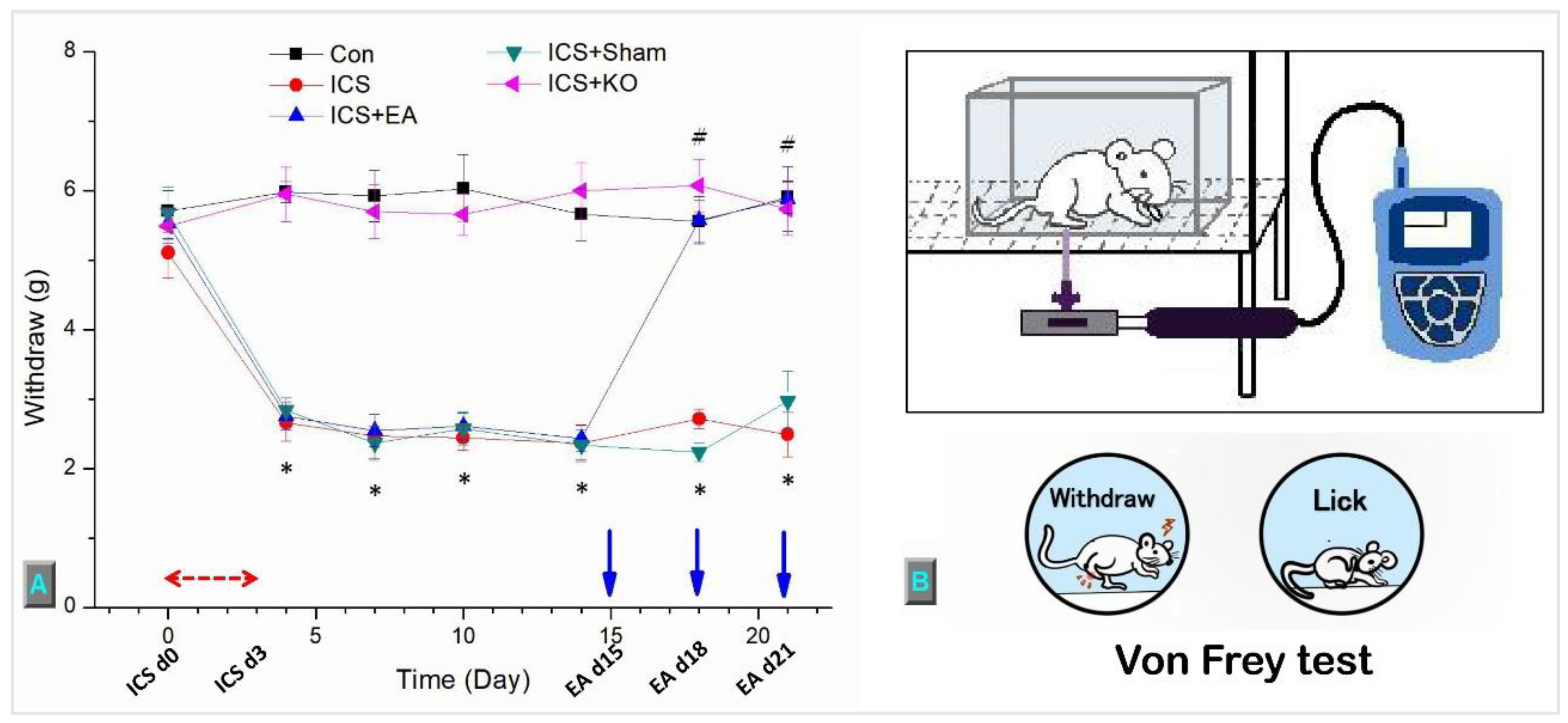
The mechanical pain threshold was evaluated using the von Frey test, where a von Frey plastic tip was gently pressed against the middle of the mouse hindpaw until the paw suddenly withdrew their paw or licked it. The pressure at that moment was recorded as the threshold value, measured in grams. When mice were not sleeping, scratching, or grooming, the von Frey test were performed three times per section, each time 10 minutes apart, to ensure the mouse did not become accustomed to the test plastic tip and tolerate the electronic von Frey filament (IITC Life Science Inc., USA).
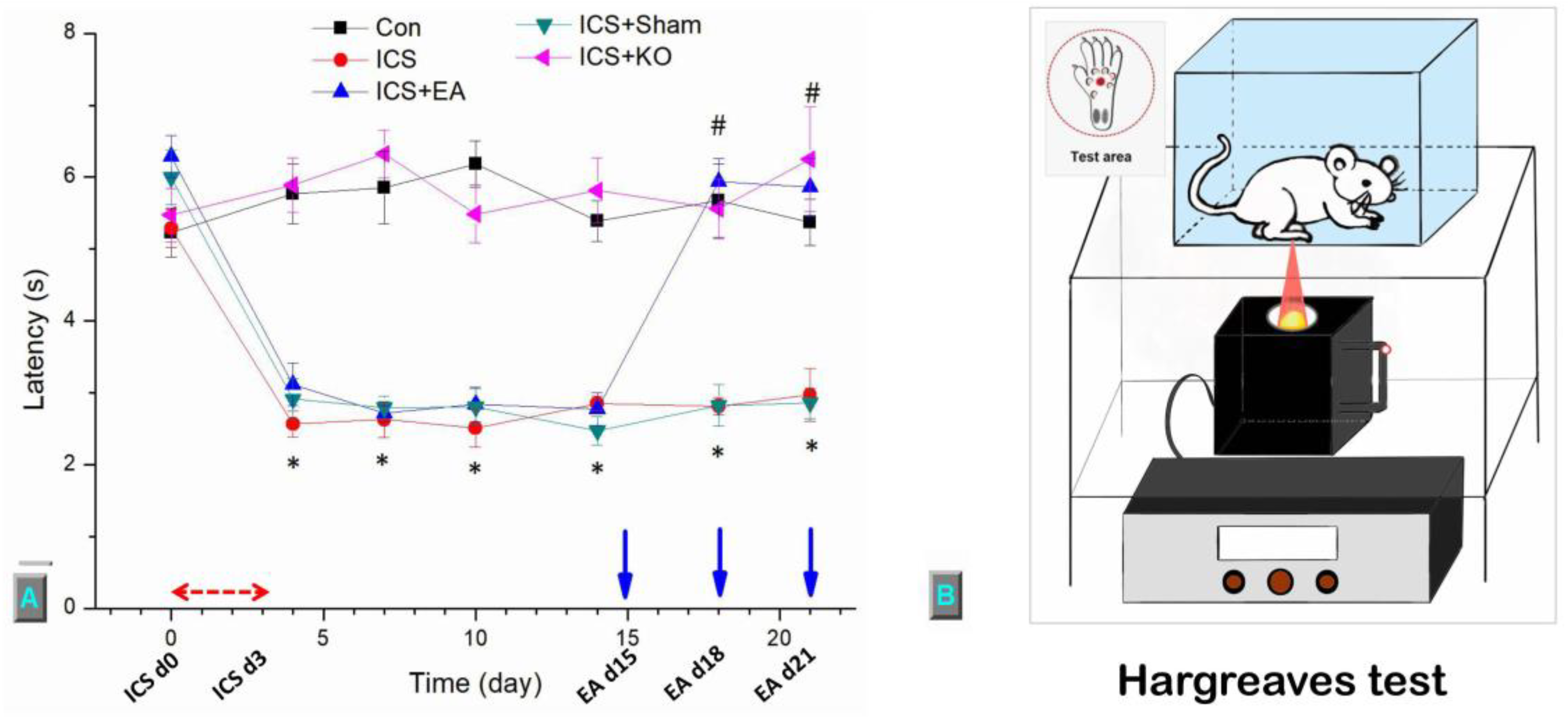
Then, the Hargreaves’ test to measure thermal response of mice was performed. The mice were separated in various plexiglass enclosures to restrict interactions. After 30 minutes of environmental habituation, the examination began. The IITC Plantar Analgesia Meter (IITC Life, Sciences, SERIES8, Model 390G) determined the withdrawal delay of the mice foot through radiant thermal light, which was placed underneath the tempered glass and slid across the surface to the exact middle of the plantar's right hind paw. Each phase was also repeated three times for each rodent. The device was programmed to self-cut at 20 seconds to prevent harming mice's paws. The moment of foot withdrawal of mice under the impact of heat was recorded in the unit of seconds. The outcomes were collected and calculated for mean values and standard errors.
Western Blotting Experiment
After completing behavioral response evaluations, rodents were executed on day 21. Before the procedure, murine subjects were anesthetized via inhalation. In this study, we collected cerebral regions tissues across both halves of the cerebrum involving the thalamus, SSC, mPFC, hippocampus, cerebellum V, VI, VII, and hypothalamus. The tissue was immediately placed on ice and kept at -80 degrees Celsius. During the experiment, manipulations were attentively carried out to minimize their misery.
Initially, the protein homogenization was accomplished using double-distilled water, radioimmunoprecipitation (RIPA) lysis buffer (Fivephoton Biochemicals, RIPA-50), protease inhibitor (Bionovas, FC0070-0001), phosphatase inhibitor (Bionovas, FC0050-0001). Each extracted protein was electrophoretically expelled in an 8% Sodium dodecyl sulfate (SDS)-Tris-glycine gel. A current electrophoresis power supply (PowerPac, Singapore) was used to run gels before delivering the protein on gel onto the Polyvinylidene fluoride (PVDF) membrane utilizing a semi-dry transfer machine (Trans-Blot SD Cell, USA). The transferred membranes were quickly cleaned with phosphate-buffered saline Tween (PBST with 0.05% Tween 20) and blocked with Bovine Serum Albumin.
Subsequently, membranes incubated in primary antibodies (1:1000): anti-TRPV1 (~95 kDa, Alomone, ACC-030), anti-pPI3K (~130 kDa, Abcam, ab154598), anti-pAkt (~65 kDa, Thermo Fisher Scientific, 44-621G), anti-pJNK (~65 kDa, Millipore, 16-293), anti-pmTOR (~180 kDa, Millipore, 09-213), anti-pERK1/2 (~42 kDa, Abcam, ab138482), anti-pP38 (~41 kDa, Thermo Fisher Scientific, 44-684G), anti-pNF-κB (~65 kDa, Abcam, ab86299), anti-pCREB (~43 kDa, Cell Signaling Technology, 9198), anti-pPKC ( ~82 kDa, Santa Cruz Biotechnology, SC-12355), anti-pPKAII (~40 kDa, Santa Cruz Biotechnology, SC-12905), anti-tubulin (~55 kDa, Santa Cruz Biotechnology, SC-5286) in PBST containing bovine serum albumin (BSA) 1% overnight at temperature of 4oC.
After three piles of washing with PBST, secondary antibodies (1:5000) were applied to incubate with the membrane for a period of two hours at 25°C, as Peroxidase-conjugated goat anti-rabbit antibody, and goat anti-mouse antibody (Jackson Immuno Research Laboratory). The protein bands on the membranes were visualized using an enhanced chemiluminescence substrate kit (PIERCE) and by means of Celvin S (Biostep, Germany). The NIH ImageJ software (Bethesda, MD, USA) was employed to determine the image densities for certain protein bands. α-tubulin was used as internal control.
Statistical Analysis
The collected information was statistically analyzed using the Lab Origins program. All statistical data are presented as the mean ± standard error (SEM). Statistically significant differences were calculated via one-way analysis of variance (One-way ANOVA) and a post hoc Tukey’s test to determine the considerable disparity. p<0.05 was regarded as a substantial value.
4. Discussion
Fibromyalgia places a significant burden on patients, negatively affecting their health, productivity, and finances. This often leaves them unable to access treatment, creating a cycle of suffering. The average annual treatment cost for FM in the United States can approach
$40,000, largely due to the cost of prescribed FM medications [
20]. Conversely, acupuncture in the United States is more affordable, a viable alternative for patients, and has the potential to reduce the risk of adverse effects associated with pharmaceutical interventions.
Our current investigation has examined the mechanisms of acupuncture in the ICS model. This model effectively replicates key characteristics of FM, including the induction of mechanical allodynia and thermal hyperalgesia, analogous to the heightened pain responses observed in FM patients following mild stimuli. It elicited chronic pain symptoms in ICS mice that persisted for 21 days, thereby facilitating a thorough investigation into the mechanisms underlying this disease. Furthermore, while behavioral alterations noted through forced swim tests have been referenced in prior studies, they are not addressed in this study to concentrate specifically on pain symptoms.
Research demonstrates that EA is most effective when administered early in neurological and inflammatory conditions, as it enhances recovery and improves quality of life. However, the study categorizes fibromyalgia diagnosis into early (≤2 years), late (>2-7 years), and very late (>7 years) [
21]. As the diagnostic criteria require symptoms to persist for at least 3 months, early treatment or prevention of FM is challenging [
22]. We verified that EA at ST36 reliably suppresses mechanical and thermal hyperalgesia in ICS mice, potentially through the TRPV1 pathway. Additionally, the EA treatment exhibited a specific effect compared to the sham group, indicating its efficacy and confirming that the observed benefits are not attributable to psychological factors or expectations. This indicates that EA is beneficial for mice with fibromyalgia and suggests the potential clinical use of supplementary treatment for fibromyalgia patients.
The brain regions targeted in this experiment are involved in both the ascending and descending pain pathways. The primary regions in the ascending pathway include the thalamus, SSC, and mPFC. The hippocampus is involved in both pathways, while the hypothalamus and cerebellum are primarily involved in the descending modulation of pain. The thalamus, located medially in the cerebrum, serves as a relay station for sensory information and is crucial for perceiving, integrating, and transmitting pain signals to the primary somatosensory cortex. The SSC comprises the primary (SI) and secondary (SII) regions. SI activity increases with more intense painful stimuli while SII is also involved in determining the source of pain [
23]. mPFC plays a regulatory role in modulating pain, dampening pain-induced sympathetic activity, and mitigating facial expressions indicative of pain [
24]. The hippocampus plays a crucial role in memory, emotions, and learning, indirectly influencing pain responses. The CA1 region is central to processing sensory inputs and emotional regulation, relaying this data to CA3 for the formation of chronic pain-associated memories [
25]. The hypothalamus regulates pain through the HPA axis, managing stress hormone release during acute pain. In chronic pain, this axis becomes dysregulated, causing excessive cortisol production, which can lead to fatigue, depression, and impaired immune function [
26]. The cerebellum primarily regulates motor control and enhances sensory processing in relation to pain. The posterior cerebellar lobes, included lobules V, VI, and VII, are involved in sensory processing and motor coordination. Lobule VI is activated by pain and thermal stimuli, while lobule VIIB processes noxious stimuli [
27]. In previous inflammatory pain experiments, protein expression changes were detected in CBV and CBVII on day 3, when animals were sacrificed, with no significant changes in CBVI. In other chronic pain models (5-8 days), notable protein alterations were found in the SSC, mPFC, hippocampus, and posterior cerebellum.
In the current experiment, we applied ICS to induce chronic pain lasting up to 3 weeks in mice, as opposed to continuous cold stress that only produces acute pain. This model meets the criteria of the diagnostic standard [A]. Behavioral assessments showed a significant reduction in mechanical withdrawal threshold and thermal withdrawal latency after just 4 days, persisting until day 21, compared to the control group. Immunoblotting results supported this finding, revealing distinct changes during the chronic phase across studied brain regions. Areas primarily involved in pain processing, including the thalamus, SSC, and mPFC, showed a marked increase in all proteins percentage in the ICS group compared to controls. Conversely, the hippocampus exhibited changes in the density of TRPV1, pPI3K, pJNK, pmTOR, and pNF-κB and cerebellum V in TRPV1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pNF-κB density, while the cerebellum VI displayed no alterations in pPKA, pERK, and pJNK proportion and cerebellum VII in pPKA, pPI3K, pPKC, pP38, pJNK, pmTOR, pNF-κB percentage. Specifically, the hypothalamus, although not a primary pain signaling center, also exhibited alterations in all assessed proteins.
TRPV1 and μ-opioid receptors are involved in the regulation of β-arrestin 2 to regulate inflammatory responses [
28]. Therefore, treatments targeting both the μ-opioid and the TRPV1 receptors are believed to be beneficial in eliminating pain and preventing opioid addiction [
28]. EA reduces the expression of glial cells marker in the PNS and CNS. EA increases adenosine levels while decreasing substance P and inflammatory agents such as IL-1, IL-6, and TNF-α at the ST36 acupoint [
29]. Similarly, EA modulated Nav1.8, COX-2, and pPKCε in animal models via adenosine correction [
30]. The pain signaling pathway of kinases PI3K/Akt plays a crucial role in modulate the pain through the affection to mediated inflammator and reducing cytokines such as TNF-α and IL-17 [
31]. EA inhibited the phosphorylation of PI3K and Akt, which activating the inflamatory, to reduce the hyperalgesia in mice injected Carrageenan [
32] . TRPV1 can stimulate anandamide (AEA) synthesis [
33], which in turn activates PKC [
29]. Flow cytometry experiments have revealed that pERK, p38, and pJNK are co-phosphorylated after proinflammatory injection, implying a role in pain signaling [
34]. Our findings suggest that pJNK and pP38 have been associated with increased sensitivity to toxic stimuli in chronic pain. cAMP (PKA) increases intracellular calcium transients through phosphorylation, triggers neuronal sensitization. Activated PKA (pPKA) can activate various ion channels, including voltage-gated calcium channels such as TRPV1 and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. The activation of these channels facilitates calcium influx into the cell, either directly or indirectly, by promoting the opening of calcium channels, thereby increasing intracellular calcium levels [
35]. The PKA/CREB signaling pathway is closely associated with the mechanisms underlying inflammatory pain.
Our data demonstrated elevated TRPV1 expression and its associated proteins in the thalamus, SSC, mPFC, hippocampus, CB V, VI, VII, and hypothalamus of mice, which was reduced by EA treatment and in TRPV1 KO mice. Previous studies indicate that these brain regions are linked to pain signaling through elevated expression of TRPV1 and other proteins over five days in the model. TRPV1 contributes to both long-term potentiation (LTP) and long-term depression (LTD) at excitatory synapses in the hippocampal dentate gyrus [
36].
This study examined protein expression in eight brain regions using Western blot analysis, providing observational data. The results eliminated the placebo effect by employing a sham EA group without de-qi sensation. Deletion of the TRPV1 gene in mice results in the absence of physical hypersensitivity. Further investigation is needed, particularly focusing on brain regions to determine protein localization. Additionally, its relevance to clinical application remains unaddressed.
Figure 1.
Application of bilateral electroacupuncture (EA) at 2 Hz on the ST36 (Zusanli) acupoint in FM mice. (A) Anatomical location of ST36 in mice. (B) Timeline of EA administration on days 15, 18, and 21. (C) Electric stimulation protocol used in the ICS+EA group.
Figure 1.
Application of bilateral electroacupuncture (EA) at 2 Hz on the ST36 (Zusanli) acupoint in FM mice. (A) Anatomical location of ST36 in mice. (B) Timeline of EA administration on days 15, 18, and 21. (C) Electric stimulation protocol used in the ICS+EA group.
Figure 2.
This diagram depicts the mechanical threshold of mice in five groups. (A) von Frey test measured the withdrawal threshold (gram = g), (B) The figure depicts the process involved in the mechanical test. The study involved five groups: Control (Con), Intermittent Cold Stress-induced Fibromyalgia-like pain (ICS), ICS + Electroacupuncture (ICS + EA), ICS + Sham EA (ICS + Sham), and ICS + TRPV1 knockout (ICS + KO). The ICS model was applied during the first three days (ICS d0, d3), and EA was administered on days 15, 18, and 21, prior to the von Frey test. Each mouse was measured three times, with 10-minute intervals between measurements.* Denotes a significant difference in comparison to the Control group. # denotes significant differences with the ICS group.
Figure 2.
This diagram depicts the mechanical threshold of mice in five groups. (A) von Frey test measured the withdrawal threshold (gram = g), (B) The figure depicts the process involved in the mechanical test. The study involved five groups: Control (Con), Intermittent Cold Stress-induced Fibromyalgia-like pain (ICS), ICS + Electroacupuncture (ICS + EA), ICS + Sham EA (ICS + Sham), and ICS + TRPV1 knockout (ICS + KO). The ICS model was applied during the first three days (ICS d0, d3), and EA was administered on days 15, 18, and 21, prior to the von Frey test. Each mouse was measured three times, with 10-minute intervals between measurements.* Denotes a significant difference in comparison to the Control group. # denotes significant differences with the ICS group.
Figure 3.
This diagram illustrates the thermal sensitivity of mice across five groups. (A) The withdrawal threshold was assessed using the Hargreaves test (second = s), (B) The figure illustrates the procedure of the thermal test. The study involved five groups: Control (Con), Intermittent Cold Stress-induced Fibromyalgia-like pain (ICS), ICS + Electroacupuncture (ICS + EA), ICS + Sham EA (ICS + Sham), and ICS + TRPV1 knockout (ICS + KO). The Intermitten cold stress (ICS) model was applied during the first three days (ICS d0, d3), and EA was administered on days 15, 18, and 21 (EA d15, d18, d21), prior to the von Frey test. Each mouse was measured three times, with 10-minute intervals between measurements.* Denotes a significant difference in comparison to the Control group. # denotes significant differences with the ICS group.
Figure 3.
This diagram illustrates the thermal sensitivity of mice across five groups. (A) The withdrawal threshold was assessed using the Hargreaves test (second = s), (B) The figure illustrates the procedure of the thermal test. The study involved five groups: Control (Con), Intermittent Cold Stress-induced Fibromyalgia-like pain (ICS), ICS + Electroacupuncture (ICS + EA), ICS + Sham EA (ICS + Sham), and ICS + TRPV1 knockout (ICS + KO). The Intermitten cold stress (ICS) model was applied during the first three days (ICS d0, d3), and EA was administered on days 15, 18, and 21 (EA d15, d18, d21), prior to the von Frey test. Each mouse was measured three times, with 10-minute intervals between measurements.* Denotes a significant difference in comparison to the Control group. # denotes significant differences with the ICS group.
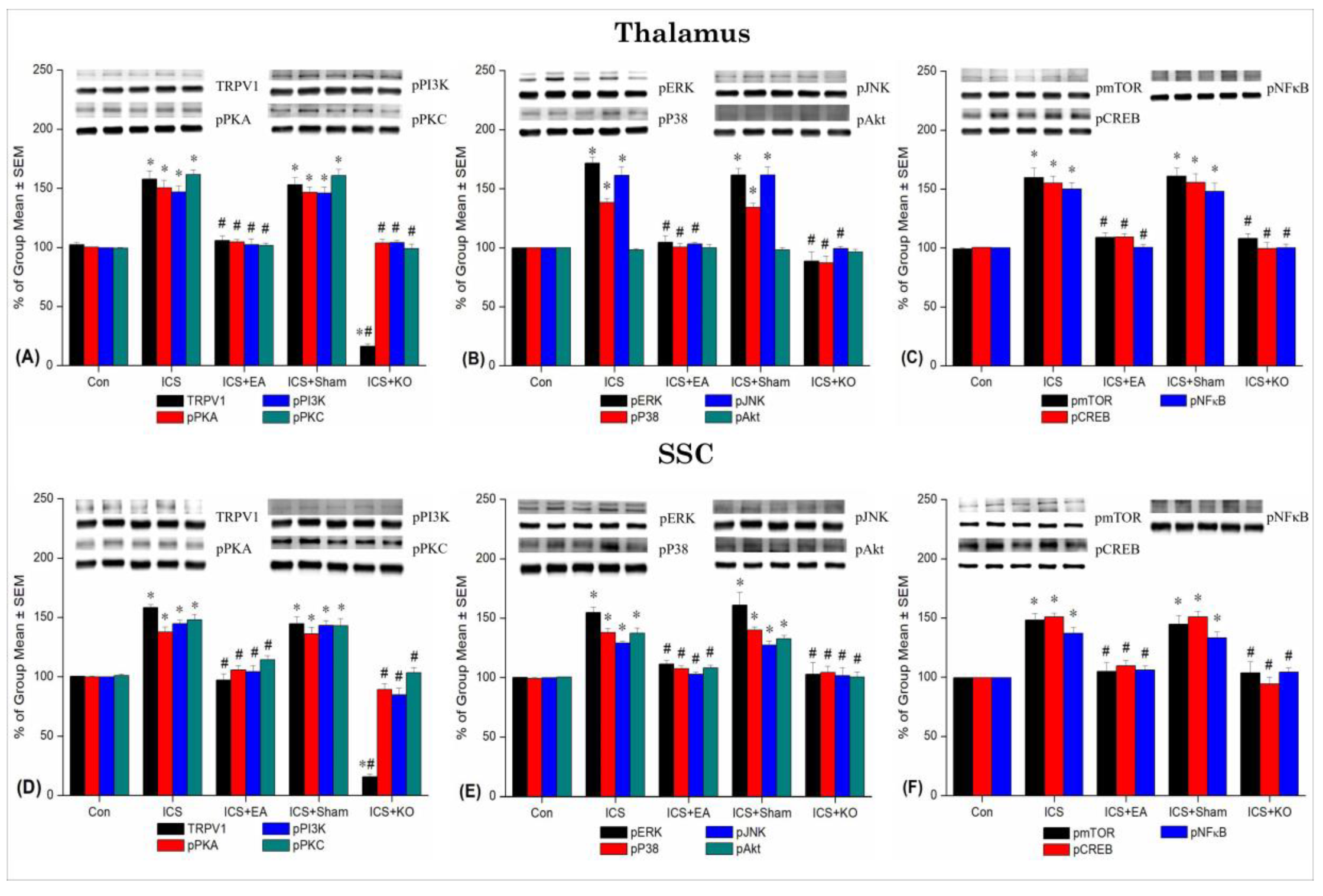
Figure 4.
Percentage changes in TRPV1 and associated molecules in the thalamus and SSC of mice. The Western blotting analysis included five lanes for each protein: Con, ICS, ICS + EA, ICS + Sham, and ICS + KO. The proteins observed were TRPV1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, pP38, pJNK, pCREB, and pNF-κB. Asterisks (*) indicate significant differences between the Con group and the other groups, while hash symbols (#) denote significant differences between the ICS and the other groups. n = 6.
Figure 4.
Percentage changes in TRPV1 and associated molecules in the thalamus and SSC of mice. The Western blotting analysis included five lanes for each protein: Con, ICS, ICS + EA, ICS + Sham, and ICS + KO. The proteins observed were TRPV1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, pP38, pJNK, pCREB, and pNF-κB. Asterisks (*) indicate significant differences between the Con group and the other groups, while hash symbols (#) denote significant differences between the ICS and the other groups. n = 6.
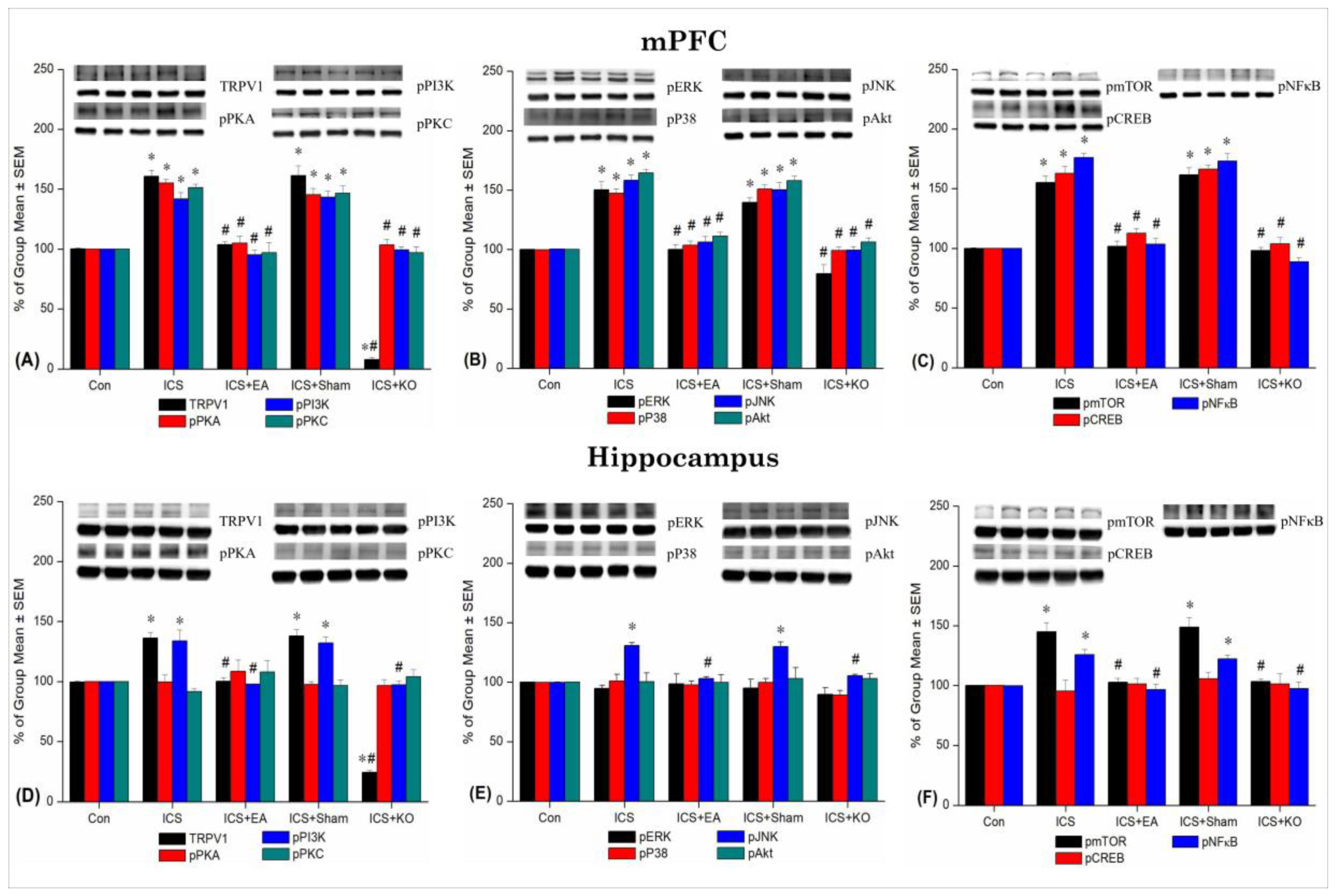
Figure 5.
Percentage changes in TRPV1 and associated molecules in the mPFC and hippocampus of mice. The Western blotting analysis included five lanes for each protein: Con, ICS, ICS + EA, ICS + Sham, and ICS + KO. The proteins observed were TRPV1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, pP38, pJNK, pCREB, and pNF-κB. Asterisks (*) indicate significant differences between the Con group and the other groups, while hash symbols (#) denote significant differences between the ICS and the other groups. n = 6.
Figure 5.
Percentage changes in TRPV1 and associated molecules in the mPFC and hippocampus of mice. The Western blotting analysis included five lanes for each protein: Con, ICS, ICS + EA, ICS + Sham, and ICS + KO. The proteins observed were TRPV1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, pP38, pJNK, pCREB, and pNF-κB. Asterisks (*) indicate significant differences between the Con group and the other groups, while hash symbols (#) denote significant differences between the ICS and the other groups. n = 6.
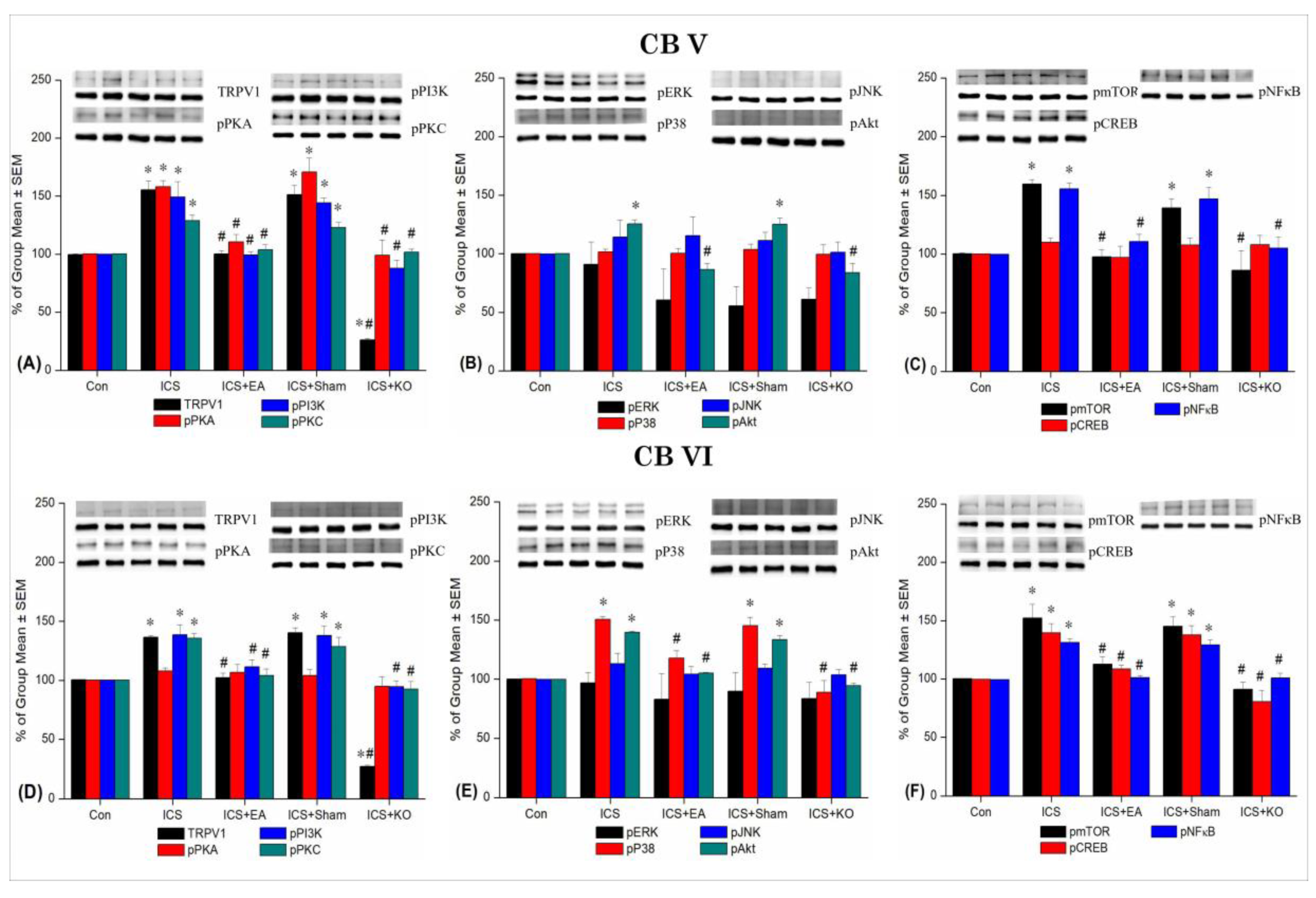
Figure 6.
Percentage changes in TRPV1 and associated molecules in the CBV and CBVI of mice. The Western blotting analysis included five lanes for each protein: Con, ICS, ICS + EA, ICS + Sham, and ICS + KO. The proteins observed were TRPV1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, pP38, pJNK, pCREB, and pNF-κB. Asterisks (*) indicate significant differences between the Con group and the other groups, while hash symbols (#) denote significant differences between the ICS and the other groups. n = 6.
Figure 6.
Percentage changes in TRPV1 and associated molecules in the CBV and CBVI of mice. The Western blotting analysis included five lanes for each protein: Con, ICS, ICS + EA, ICS + Sham, and ICS + KO. The proteins observed were TRPV1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, pP38, pJNK, pCREB, and pNF-κB. Asterisks (*) indicate significant differences between the Con group and the other groups, while hash symbols (#) denote significant differences between the ICS and the other groups. n = 6.
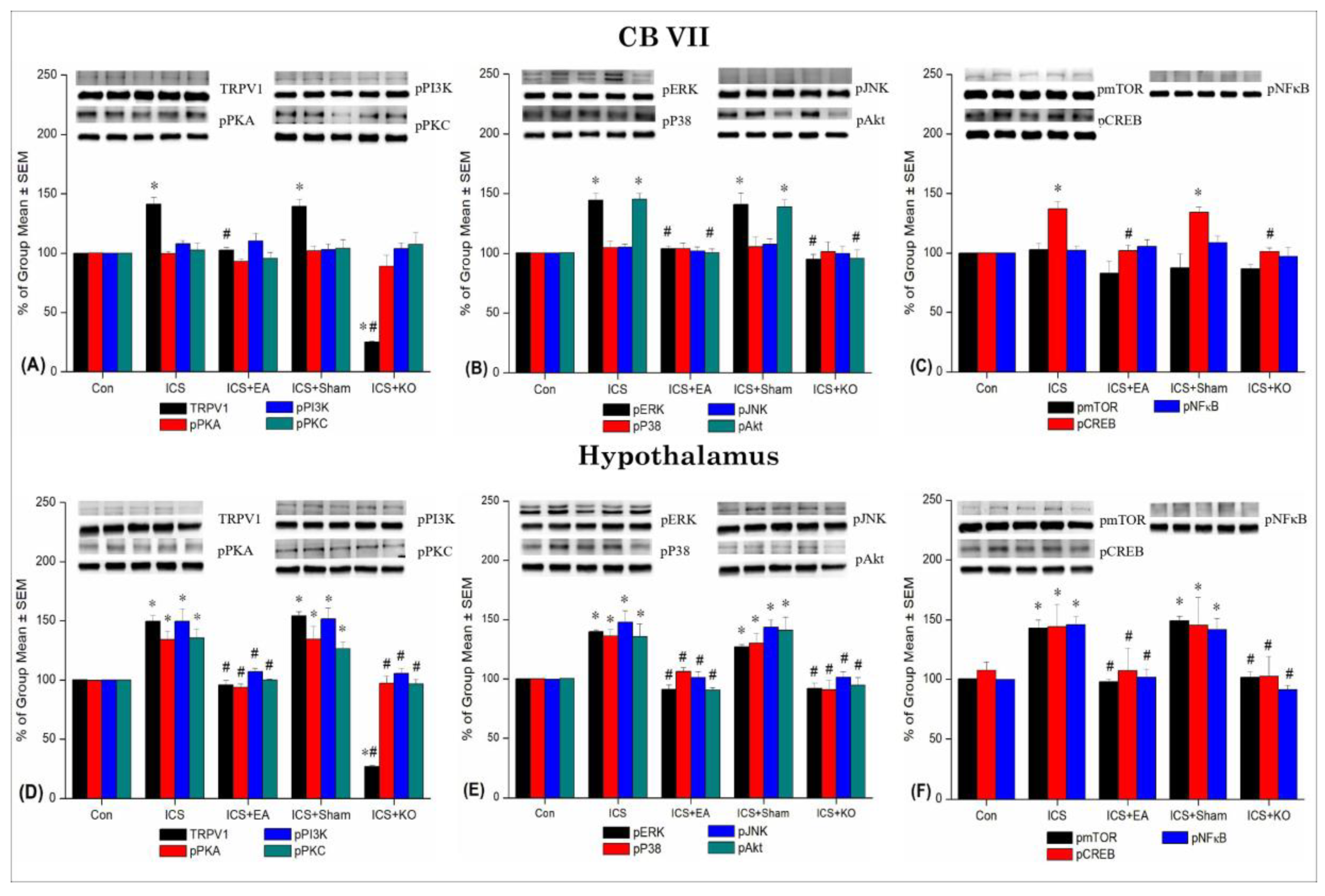
Figure 7.
Percentage changes in TRPV1 and associated molecules in the CB VII and hypothalamus of mice. The Western blotting analysis included five lanes for each protein: Con, ICS, ICS + EA, ICS + Sham, and ICS + KO. The proteins observed were TRPV1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, pP38, pJNK, pCREB, and pNF-κB. Asterisks (*) indicate significant differences between the Con group and the other groups, while hash symbols (#) denote significant differences between the ICS and the other groups. n = 6.
Figure 7.
Percentage changes in TRPV1 and associated molecules in the CB VII and hypothalamus of mice. The Western blotting analysis included five lanes for each protein: Con, ICS, ICS + EA, ICS + Sham, and ICS + KO. The proteins observed were TRPV1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, pP38, pJNK, pCREB, and pNF-κB. Asterisks (*) indicate significant differences between the Con group and the other groups, while hash symbols (#) denote significant differences between the ICS and the other groups. n = 6.
Table 1.
2Hz EA at the Zusanli acupoint significantly suppresses the overexpression of TRPV1 and its associated downstream molecules in the thalamus and SSC after ICS-induced FM. Con: Control, ICS: Intermittent Cold Stress-induced FM, ICS+EA: Electroacupuncture 2Hz at the Zusanli, ICS+Sham: Sham EA, ICS+KO: Transient Receptor Potential Vanilloid 1 knockout gene. TRPV1: Transient Receptor Potential Vanilloid 1, pPKA:phosphorylated Protein Kinase A, pPI3K:phosphorylated Phosphoinositide 3-Kinase, pPKC: phosphorylated Protein Kinase C, pERK: phosphorylated Extracellular Signal-Regulated Kinase, pP38: phosphorylated P38 Mitogen-Activated Protein Kinase, pJNK: phosphorylated c-Jun N-terminal Kinase, pAkt: phosphorylated Protein Kinase B (Akt), pmTOR: phosphorylated Mechanistic Target of Rapamycin, pCREB: phosphorylated cAMP Response Element-Binding Protein, pNFκB: phosphorylated Nuclear Factor Kappa B. *p < 0.05 vs. Con, #p < 0.05 vs. ICS.
Table 1.
2Hz EA at the Zusanli acupoint significantly suppresses the overexpression of TRPV1 and its associated downstream molecules in the thalamus and SSC after ICS-induced FM. Con: Control, ICS: Intermittent Cold Stress-induced FM, ICS+EA: Electroacupuncture 2Hz at the Zusanli, ICS+Sham: Sham EA, ICS+KO: Transient Receptor Potential Vanilloid 1 knockout gene. TRPV1: Transient Receptor Potential Vanilloid 1, pPKA:phosphorylated Protein Kinase A, pPI3K:phosphorylated Phosphoinositide 3-Kinase, pPKC: phosphorylated Protein Kinase C, pERK: phosphorylated Extracellular Signal-Regulated Kinase, pP38: phosphorylated P38 Mitogen-Activated Protein Kinase, pJNK: phosphorylated c-Jun N-terminal Kinase, pAkt: phosphorylated Protein Kinase B (Akt), pmTOR: phosphorylated Mechanistic Target of Rapamycin, pCREB: phosphorylated cAMP Response Element-Binding Protein, pNFκB: phosphorylated Nuclear Factor Kappa B. *p < 0.05 vs. Con, #p < 0.05 vs. ICS.
| Brain region |
Protein |
Con |
ICS |
ICS + EA |
ICS + Sham |
ICS + KO |
| Thalamus |
TRPV1 |
102.15 ± 1.89 |
157.86 ± 6.97* |
105.93 ± 3.88# |
152.95 ± 6.07* |
16.36 ± 1.95*# |
| pPKA |
100.08 ± 0.27 |
150.35 ± 6.62* |
104.55 ± 2.21# |
146.65 ± 4.52* |
103.78 ± 3.22# |
| pPI3K |
99.6 ± 0.29 |
146.81 ± 5.05* |
102.15 ± 4.99# |
146.03 ± 4.89* |
103.93 ± 1.75# |
| pPKC |
99.23 ± 0.57 |
161.5 ± 4.17* |
101.8 ± 2.01# |
160.85 ± 5.11* |
98.98 ± 3.92# |
| pERK |
99.83 ± 0.39 |
171.5 ± 5.47* |
104.63 ± 5.52# |
161.46 ± 5.63* |
88.85 ± 7.54# |
| pP38 |
99.96 ± 0.09 |
138.4 ± 3.43* |
100.45 ± 3.15# |
134.06 ± 3.53* |
87.35 ± 5.57# |
| pJNK |
99.93 ± 0.26 |
161.23 ± 7.35* |
103.16 ± 1.40# |
161.45 ± 6.88* |
99.38 ± 1.66# |
| pAkt |
99.99 ± 0.1 |
98.21 ± 1.07 |
99.89 ± 2.89 |
98.28 ± 1.81 |
96.27 ± 2.46 |
| pmTOR |
99.25 ± 0.79 |
159.91 ± 8.01* |
109.08 ± 3.84# |
161.1 ± 6.55* |
107.98 ± 4.24# |
| pCREB |
100.3 ± 0.47 |
155.05 ± 6.04* |
109.26 ± 2.99# |
155.58 ± 7.33* |
99.46 ± 5.18# |
| pNF-κB |
100.2 ± 0.21 |
150.11 ± 5.26* |
100.36 ± 2.46# |
147.85 ± 7.26* |
100.2 ± 3.07# |
| SSC |
TRPV1 |
100.35 ± 0.48 |
158.3 ± 2.74* |
97.05 ± 5.40# |
144.61 ± 6.07* |
15.66 ± 2.37*# |
| pPKA |
99.93 ± 0.02 |
137.63 ± 4.33* |
105.65 ± 3.51# |
136.2 ± 5.64* |
89.08 ± 5.16# |
| pPI3K |
99.85 ± 0.73 |
144.58 ± 3.20* |
104.41 ± 5.29# |
143.13 ± 4.12* |
84.93 ± 5.33# |
| pPKC |
101.1 ± 1.13 |
147.81 ± 4.85* |
114.28 ± 3.54# |
143.05 ± 5.99* |
103.31 ± 4.45# |
| pERK |
100.05 ± 0.02 |
154.83 ± 4.58* |
111.31 ± 3.22# |
160.88 ± 11.01* |
102.7 ± 10.15# |
| pP38 |
99.18 ± 0.94 |
137.93 ± 3.39* |
107.58 ± 2.18# |
139.9 ± 2.84* |
104.28 ± 5.28# |
| pJNK |
99.85 ± 0.21 |
129.01 ± 1.38* |
102.68 ± 2.24# |
127.35 ± 3.50* |
101.58 ± 6.80# |
| pAkt |
100.33 ± 0.36 |
137.23 ± 4.43* |
108.25 ± 2.20# |
132.66 ± 3.00* |
100.51 ± 4.24# |
| pmTOR |
99.45 ± 0.37 |
148.33 ± 5.58* |
104.78 ± 7.55# |
144.55 ± 7.60* |
103.71 ± 9.28# |
| pCREB |
99.66 ± 0.26 |
151.1 ± 3.05* |
109.63 ± 4.50# |
150.9 ± 4.76* |
94.28 ± 5.82# |
| pNF-κB |
99.51 ± 0.11 |
136.95 ± 5.31* |
105.93 ± 3.71# |
133.23 ± 4.97* |
104.2 ± 3.71# |
Table 2.
The table shows data on the increase of TRPV1, pPKA, pPI3K, pPKC, pERK, pP38, pJNK, pAkt, pmTOR, pCREB, and pNFκB after applying ICS in the mPFC and hippocampus of mice. Con: Control, ICS: Intermittent Cold Stress-induced FM, ICS+EA: Electroacupuncture 2Hz at the Zusanli (ST36), ICS+Sham: Sham EA, ICS+KO: Transient Receptor Potential Vanilloid 1 knockout gene. TRPV1: Transient Receptor Potential Vanilloid 1, pPKA:phosphorylated Protein Kinase A, pPI3K:phosphorylated Phosphoinositide 3-Kinase, pPKC: phosphorylated Protein Kinase C, pERK: phosphorylated Extracellular Signal-Regulated Kinase, pP38: phosphorylated P38 Mitogen-Activated Protein Kinase, pJNK: phosphorylated c-Jun N-terminal Kinase, pAkt: phosphorylated Protein Kinase B (Akt), pmTOR: phosphorylated Mechanistic Target of Rapamycin, pCREB: phosphorylated cAMP Response Element-Binding Protein, pNF-κB: phosphorylated Nuclear Factor Kappa B. *p < 0.05 vs. Con, #p < 0.05 vs. ICS.
Table 2.
The table shows data on the increase of TRPV1, pPKA, pPI3K, pPKC, pERK, pP38, pJNK, pAkt, pmTOR, pCREB, and pNFκB after applying ICS in the mPFC and hippocampus of mice. Con: Control, ICS: Intermittent Cold Stress-induced FM, ICS+EA: Electroacupuncture 2Hz at the Zusanli (ST36), ICS+Sham: Sham EA, ICS+KO: Transient Receptor Potential Vanilloid 1 knockout gene. TRPV1: Transient Receptor Potential Vanilloid 1, pPKA:phosphorylated Protein Kinase A, pPI3K:phosphorylated Phosphoinositide 3-Kinase, pPKC: phosphorylated Protein Kinase C, pERK: phosphorylated Extracellular Signal-Regulated Kinase, pP38: phosphorylated P38 Mitogen-Activated Protein Kinase, pJNK: phosphorylated c-Jun N-terminal Kinase, pAkt: phosphorylated Protein Kinase B (Akt), pmTOR: phosphorylated Mechanistic Target of Rapamycin, pCREB: phosphorylated cAMP Response Element-Binding Protein, pNF-κB: phosphorylated Nuclear Factor Kappa B. *p < 0.05 vs. Con, #p < 0.05 vs. ICS.
| Brain region |
Protein |
Con |
ICS |
ICS + EA |
ICS + Sham |
ICS + KO |
| mPFC |
TRPV1 |
100.1 ± 0.67 |
160.7 ± 4.97* |
103.86 ± 2.27# |
161.23 ± 8.32* |
7.76 ± 1.83*# |
| pPKA |
100.05 ± 0.21 |
155.01 ± 2.92* |
104.98 ± 5.93# |
145.48 ± 5.29* |
103.36 ± 4.87# |
| pPI3K |
99.88 ± 0.17 |
141.9 ± 5.20* |
95.23 ± 4.23# |
143.36 ± 5.24* |
99.28 ± 2.39# |
| pPKC |
99.83 ± 0.12 |
151.26 ± 2.86* |
96.85 ± 8.43# |
146.53 ± 6.26* |
97.03 ± 4.53# |
| pERK |
100.21 ± 0.15 |
150.43 ± 6.95* |
100.06 ± 4.13# |
139.71 ± 3.91* |
79.58 ± 7.68# |
| pP38 |
99.96 ± 0.08 |
147.21 ± 3.82* |
103.76 ± 3.53# |
151 ± 3.45* |
99.36 ± 2.99# |
| pJNK |
100.35 ± 0.36 |
158.16 ± 4.40* |
106.43 ± 4.76# |
150.3 ± 6.06* |
99.43 ± 3.18# |
| pAkt |
100.03 ± 0.26 |
164.43 ± 3.10* |
111.26 ± 3.38# |
158.01 ± 3.88* |
106.21 ± 3.55# |
| pmTOR |
100.03 ± 0.55 |
154.98 ± 5.50* |
101.8 ± 4.39# |
161.55 ± 5.82* |
98.1 ± 2.73# |
| pCREB |
99.88 ± 0.24 |
162.76 ± 6.06* |
112.65 ± 4.11# |
166.33 ± 3.20* |
103.65 ± 5.40# |
| pNF-κB |
99.85 ± 0.26 |
175.91 ± 3.40* |
103.48 ± 4.86# |
172.95 ± 6.59* |
88.71 ± 3.61# |
| Hippo |
TRPV1 |
99.48 ± 0.62 |
136.30 ± 4.76* |
100.17 ± 3.05# |
137.92 ± 5.22* |
24.26 ± 1.46*# |
| pPKA |
99.91 ± 0.21 |
99.52 ± 5.92 |
108.47 ± 9.40 |
97.59 ± 2.25 |
96.79 ± 4.95 |
| pPI3K |
100.04 ± 0.23 |
133.89 ± 9.05* |
97.83 ± 0.41# |
132.04 ± 5.01* |
97.28 ± 2.88# |
| pPKC |
99.82 ± 0.19 |
91.78 ± 2.36 |
107.74 ± 9.82 |
96.65 ± 4.74 |
104.09 ± 5.75 |
| pERK |
100.1 ± 0.17 |
94.84 ± 2.59 |
98.72 ± 8.06 |
94.98 ± 7.46 |
89.71 ± 5.66 |
| pP38 |
99.71 ± 0.10 |
101.03 ± 5.53 |
97.75 ± 3.40 |
99.9 ± 3.30 |
89.29 ± 3.74 |
| pJNK |
99.94 ± 0.31 |
130.89 ± 2.32* |
102.96 ± 1.46# |
129.86 ± 3.90* |
105.41 ± 1.25# |
| pAkt |
100.01 ± 0.14 |
100.31 ± 7.46 |
99.98 ± 6.39 |
103.21 ± 8.92 |
102.95 ± 4.34 |
| pmTOR |
100.22 ± 0.14 |
144.94 ± 7.76* |
102.91 ± 3.18# |
148.85 ± 8.09* |
103.37 ± 2.19# |
| pCREB |
100.11 ± 0.14 |
95.32 ± 9.37 |
101.34 ± 4.78 |
105.71 ± 5.42 |
101.24 ± 8.67 |
| pNF-κB |
99.73 ± 0.07 |
125.95 ± 4.22* |
96.58 ± 4.34# |
122.40 ± 3.17* |
97.44 ± 5.67# |
Table 3.
Statistical data demonstrated the expression levels of TRPV1 and its associated downstream molecules across five groups of mice in cerebellum V and VI (CBV, VI). Con: Control, ICS: Intermittent Cold Stress-induced FM, ICS+EA: Electroacupuncture 2Hz at the Zusanli, ICS+Sham: Sham EA, ICS+KO: Transient Receptor Potential Vanilloid 1 knockout gene. TRPV1: Transient Receptor Potential Vanilloid 1, pPKA:phosphorylated Protein Kinase A, pPI3K:phosphorylated Phosphoinositide 3-Kinase, pPKC: phosphorylated Protein Kinase C, pERK: phosphorylated Extracellular Signal-Regulated Kinase, pP38: phosphorylated P38 Mitogen-Activated Protein Kinase, pJNK: phosphorylated c-Jun N-terminal Kinase, pAkt: phosphorylated Protein Kinase B (Akt), pmTOR: phosphorylated Mechanistic Target of Rapamycin, pCREB: phosphorylated cAMP Response Element-Binding Protein, pNF-κB: phosphorylated Nuclear Factor Kappa B. *p < 0.05 vs. Con, #p < 0.05 vs. ICS.
Table 3.
Statistical data demonstrated the expression levels of TRPV1 and its associated downstream molecules across five groups of mice in cerebellum V and VI (CBV, VI). Con: Control, ICS: Intermittent Cold Stress-induced FM, ICS+EA: Electroacupuncture 2Hz at the Zusanli, ICS+Sham: Sham EA, ICS+KO: Transient Receptor Potential Vanilloid 1 knockout gene. TRPV1: Transient Receptor Potential Vanilloid 1, pPKA:phosphorylated Protein Kinase A, pPI3K:phosphorylated Phosphoinositide 3-Kinase, pPKC: phosphorylated Protein Kinase C, pERK: phosphorylated Extracellular Signal-Regulated Kinase, pP38: phosphorylated P38 Mitogen-Activated Protein Kinase, pJNK: phosphorylated c-Jun N-terminal Kinase, pAkt: phosphorylated Protein Kinase B (Akt), pmTOR: phosphorylated Mechanistic Target of Rapamycin, pCREB: phosphorylated cAMP Response Element-Binding Protein, pNF-κB: phosphorylated Nuclear Factor Kappa B. *p < 0.05 vs. Con, #p < 0.05 vs. ICS.
| Brain region |
Protein |
Con |
ICS |
ICS + EA |
ICS + Sham |
ICS + KO |
| CBV |
TRPV1 |
99.55 ± 0.63 |
155.29 ± 7.73* |
100.27 ± 3.01# |
151.11 ± 8.42* |
26.15 ± 1.14*# |
| pPKA |
100.13 ± 0.18 |
157.92 ± 5.49* |
110.42 ± 6.45# |
170.84 ± 12.04* |
99.08 ± 13.15# |
| pPI3K |
100.06 ± 0.14 |
149.3 ± 13.09* |
99.40 ± 2.94# |
144.25 ± 4.19* |
87.76 ± 7.18# |
| pPKC |
100.16 ± 0.20 |
128.77 ± 4.81* |
103.73 ± 4.51# |
123.02 ± 4.37* |
101.65 ± 2.64# |
| pERK |
100.15 ± 0.14 |
91.12 ± 19.02 |
60.59 ± 26.65 |
55.53 ± 16.47 |
61.16 ± 9.71 |
| pP38 |
100.02 ± 0.05 |
101.72 ± 2.37 |
100.43 ± 4.01 |
103.72 ± 4.75 |
99.41 ± 8.32 |
| pJNK |
99.91 ± 0.16 |
114.17 ± 14.68 |
115.47 ± 16.12 |
111.28 ± 7.17 |
101.21 ± 8.90 |
| pAkt |
100.01 ± 0.10 |
125.44 ± 3.65* |
86.69 ± 5.20# |
125.10 ± 5.47* |
83.84 ± 7.89# |
| pmTOR |
100.39 ± 0.59 |
159.69 ± 3.63* |
97.58 ± 6.11# |
139.32 ± 8.01* |
86.21 ± 16.80# |
| pCREB |
100.03 ± 0.20 |
109.93 ± 3.81 |
97.09 ± 9.79 |
107.53 ± 6.11 |
107.99 ± 7.97 |
| pNF-κB |
99.59 ± 0.23 |
155.50 ± 5.07* |
110.66 ± 6.02# |
146.87 ± 9.93* |
105.09 ± 9.37# |
| CBVI |
TRPV1 |
100.51 ± 0.32 |
136.53 ± 1.39* |
102.45 ± 3.80# |
140.47 ± 3.97* |
27.01 ± 1.33*# |
| pPKA |
100.26 ± 0.08 |
107.88 ± 2.62 |
106.86 ± 6.79 |
104.00 ± 5.16 |
94.94 ± 7.84 |
| pPI3K |
100.25 ± 0.29 |
138.62 ± 8.61* |
111.33 ± 6.13# |
137.87 ± 8.27* |
94.62 ± 4.96# |
| pPKC |
100.16 ± 0.17 |
135.61 ± 4.22* |
104.09 ± 5.54# |
128.54 ± 8.08* |
92.71 ± 6.45# |
| pERK |
100.00 ± 0.11 |
96.98 ± 8.55 |
83.15 ± 21.57 |
89.87 ± 15.50 |
83.60 ± 13.75 |
| pP38 |
100.31 ± 0.41 |
150.73 ± 2.27* |
117.84 ± 6.24# |
145.31 ± 7.01* |
88.91 ± 10.01# |
| pJNK |
99.92 ± 0.11 |
113.02 ± 9.09 |
104.24 ± 6.54 |
109.31 ± 3.45 |
103.61 ± 4.41 |
| pAkt |
99.77 ± 0.28 |
139.54 ± 1.07* |
105.03 ± 0.76# |
133.46 ± 3.68* |
94.61 ± 2.25# |
| pmTOR |
100.27 ± 0.45 |
152.34 ± 11.70* |
112.65 ± 6.11# |
145.04 ± 8.41* |
91.17 ± 6.05# |
| pCREB |
99.65 ± 0.17 |
139.49 ± 7.90* |
108.45 ± 3.28# |
137.89 ± 7.68 |
80.43 ± 9.71# |
| pNF-κB |
99.51 ± 0.30 |
131.25 ± 3.13* |
101.24 ± 1.64# |
129.16 ± 4.32* |
100.84 ± 4.26# |
Table 4.
Western blot data were analyzed showing an increase in pain signaling proteins in CB VII and hypothalamus (Hypo) under intermittent cold stress and the effect of EA in mitigating hyperalgesia consistent with the results of behavioral tests. Con: Control, ICS: Intermittent Cold Stress-induced FM, ICS+EA: Electroacupuncture 2Hz at the Zusanli, ICS+Sham: Sham EA, ICS+KO: Transient Receptor Potential Vanilloid 1 knockout gene. TRPV1: Transient Receptor Potential Vanilloid 1, pPKA:phosphorylated Protein Kinase A, pPI3K:phosphorylated Phosphoinositide 3-Kinase, pPKC: phosphorylated Protein Kinase C, pERK: phosphorylated Extracellular Signal-Regulated Kinase, pP38: phosphorylated P38 Mitogen-Activated Protein Kinase, pJNK: phosphorylated c-Jun N-terminal Kinase, pAkt: phosphorylated Protein Kinase B (Akt), pmTOR: phosphorylated Mechanistic Target of Rapamycin, pCREB: phosphorylated cAMP Response Element-Binding Protein, pNF-κB: phosphorylated Nuclear Factor Kappa B. *p < 0.05 vs. Con, #p < 0.05 vs. ICS.
Table 4.
Western blot data were analyzed showing an increase in pain signaling proteins in CB VII and hypothalamus (Hypo) under intermittent cold stress and the effect of EA in mitigating hyperalgesia consistent with the results of behavioral tests. Con: Control, ICS: Intermittent Cold Stress-induced FM, ICS+EA: Electroacupuncture 2Hz at the Zusanli, ICS+Sham: Sham EA, ICS+KO: Transient Receptor Potential Vanilloid 1 knockout gene. TRPV1: Transient Receptor Potential Vanilloid 1, pPKA:phosphorylated Protein Kinase A, pPI3K:phosphorylated Phosphoinositide 3-Kinase, pPKC: phosphorylated Protein Kinase C, pERK: phosphorylated Extracellular Signal-Regulated Kinase, pP38: phosphorylated P38 Mitogen-Activated Protein Kinase, pJNK: phosphorylated c-Jun N-terminal Kinase, pAkt: phosphorylated Protein Kinase B (Akt), pmTOR: phosphorylated Mechanistic Target of Rapamycin, pCREB: phosphorylated cAMP Response Element-Binding Protein, pNF-κB: phosphorylated Nuclear Factor Kappa B. *p < 0.05 vs. Con, #p < 0.05 vs. ICS.
| Brain region |
Protein |
Con |
ICS |
ICS + EA |
ICS + Sham |
ICS + KO |
| CB VII |
TRPV1 |
99.93 ± 0.17 |
141.34 ± 5.88* |
102.52 ± 2.45# |
139.48 ± 6.30* |
25.09 ± 0.60*# |
| pPKA |
100.33 ± 0.11 |
99.76 ± 1.77 |
93.21 ± 2.25 |
101.93 ± 3.98 |
89.06 ± 9.31 |
| pPI3K |
99.85 ± 0.13 |
107.94 ± 2.46 |
110.17 ± 6.51 |
102.75 ± 5.24 |
103.7 ± 5.14 |
| pPKC |
99.92 ± 0.15 |
102.53 ± 6.12 |
95.54 ± 5.30 |
104.14 ± 7.19 |
107.17 ± 10.55 |
| pERK |
100.38 ± 0.20 |
144.46 ± 5.72* |
104.02 ± 2.33# |
140.84 ± 9.87* |
95.25 ± 4.09# |
| pP38 |
100.10 ± 0.09 |
105.01 ± 5.06 |
104.11 ± 4.47 |
105.45 ± 8.43 |
101.31 ± 8.36 |
| pJNK |
100.08 ± 0.07 |
105.15 ± 2.68 |
102.04 ± 3.59 |
107.55 ± 4.53 |
99.83 ± 6.21 |
| pAkt |
100.48 ± 0.12 |
145.28 ± 4.80* |
100.46 ± 3.19# |
138.80 ± 5.83* |
95.67 ± 7.27# |
| pmTOR |
99.98 ± 0.18 |
102.96 ± 5.18 |
83.08 ± 10.47 |
87.59 ± 11.64 |
86.95 ± 3.43 |
| pCREB |
99.99 ± 0.14 |
137.2 ± 6.15* |
101.94 ± 4.66# |
134.16 ± 4.45* |
101.33 ± 3.11# |
| pNF-κB |
99.94 ± 0.27 |
102.36 ± 3.44 |
105.52 ± 5.81 |
108.63 ± 5.65 |
97.12 ± 7.78 |
| Hypo |
TRPV1 |
100.29 ± 0.30 |
149.64 ± 4.75* |
95.76 ± 3.73# |
154.23 ± 3.62* |
26.63 ± 1.06*# |
| pPKA |
99.66 ± 0.31 |
134.21 ± 6.86* |
93.72 ± 2.82# |
134.42 ± 11.03* |
97.37 ± 5.69# |
| pPI3K |
99.98 ± 0.13 |
149.33 ± 10.77* |
107.09 ± 2.82# |
151.50 ± 9.49* |
105.61 ± 3.86# |
| pPKC |
99.80 ± 0.22 |
135.23 ± 7.79* |
99.99 ± 0.78# |
126.46 ± 5.61* |
96.50 ± 3.89# |
| pERK |
99.94 ± 0.36 |
139.68 ± 1.61* |
91.10 ± 4.01# |
127.05 ± 1.86* |
91.93 ± 4.50# |
| pP38 |
100.09 ± 0.32 |
136.36 ± 5.23* |
106.08 ± 3.30# |
130.19 ± 8.19* |
90.82 ± 7.88# |
| pJNK |
99.77 ± 0.21 |
147.87 ± 9.57* |
101.03 ± 5.02# |
143.79 ± 6.08* |
101.47 ± 4.74# |
| pAkt |
100.20 ± 0.34 |
135.58 ± 10.87* |
90.54 ± 2.48# |
140.86 ± 11.44* |
94.64 ± 6.46# |
| pmTOR |
100.31 ± 0.35 |
143.00 ± 6.88* |
97.94 ± 1.94# |
149.22 ± 3.99* |
101.44 ± 4.92# |
| pCREB |
107.32 ± 7.24 |
144.19 ± 18.97* |
107.18 ± 18.96# |
145.45 ± 23.14* |
102.35 ± 16.66# |
| pNF-κB |
99.84 ± 0.13 |
146.15 ± 6.64* |
101.33 ± 7.22# |
142.00 ± 9.09* |
91.07 ± 3.81# |