1. Introduction
Environmental and economic issues together with reduction of petroleum oil reserves have determined both academia and industry to seek replacements derived from natural and sustainable resources (lignin, rosin acids, vegetable oils, chitosan, fats) [
1,
2,
3,
4,
5]. Epoxides are one of the most important classes of resins used in obtaining different thermosets for various application fields. Epoxy resins account for 70% of the commercial markets and have a wide range of applications, from adhesive and coatings to aerospace [
6,
7]. Cured epoxy matrices exhibit excellent abrasive, chemical resistance and thermal, mechanical and electrical behavior, being well known as excellent insulators [
8,
9].
However, most epoxy resins are synthesized from the petrochemical environmentally hazardous epoxy monomer diglycidyl ether of bisphenol A (BPA). BPA is an endocrine–disrupting compound which also affects the central and immune system [
10,
11,
12]. Furthermore, a thermosetting formulation may be comprised of the epoxy resin, curing agent, reactive diluent, filler, activator, catalyst etc. Hence, the BPA based epoxies are cured with low molecular weight petroleum based hardeners, such as amines, anhydrides, polyacids, Lewis acids, known to be toxic agents to both human health and the environment [
13,
14,
15,
16,
17,
18]. Moreover, most of them increase system viscosity. Epoxy resins are known for their high viscosity at room temperature [
15,
16], and poor toughening [
17], these aspects leading to difficulties in processing and applications overcome by solvent addition of which most are toxic. Reactive diluents may surpass these impediments. Jagtap and More [
19] have recently reviewed reactive diluents for epoxy resins. Of these, the acrylate–based multifunctional monomer trimethylolpropane triacrylate (TMPTMA) significantly enhanced the crosslinking density of epoxy acrylates without adding toxicity.
It is therefore that all the mentioned risk factors compelled the need to develop sustainable epoxy systems from renewable resources [
20], following one of the green chemistry’s principles stated by Anastas and Eghbali [
21]. Among these resources, during the last two decades vegetable oils, especially soy–bean and linseed, have received increasing attention due to their availability, low cost, chemical functionality and facile processing [
22,
23]. Due to their very low reactivity during polymerization, the double bonds in vegetable oils need to be converted into reactive functional groups. Vegetable oils can undergo epoxidation and, cured with an adequate hardener, produce a bio–based sustainable epoxy resin [
24,
25]. Due to its versatility, the epoxide ring can be further converted into a wide palette of functional groups through different reactions, such as with acids (e.g. acrylic), anhydrides or hydroxyl (alcohols, thiols, diols) [
26]. Therefore, the development of soy–based epoxy resins for engineering applications remains a challenge for the polymer composite industries. Hence, both academic and industrial research is greatly expanding in exploring the feasibility of producing polymer composites based on epoxidized soybean oil. Such bio–based compounds may constitute raw materials to obtain polymeric formulations for micro– and nanocomposites (e.g. with metal oxides) for protection of different substrates (wood, metal, plastic) [
27,
28,
29,
30,
31]. One example of metal oxide micro– and nanoparticles is TiO
2 which are very good semiconductors, have high chemical resistance, low toxicity and excellent UV photoactivity [
32]. Other examples include Ag nanoparticles or their combined systems (Ag–TiO
2) which are biocompatible and have excellent antibacterial properties. ZnO nanoparticles offer excellent UV and antibacterial protection. CeO nanoparticles are excellent insulators due to their high chemical resistance. TMPTMA may replace traditional curing agents in different materials: (i) macroporous poly (glycidyl methacrylate–co–trimethylolpropane trimethacrylate)s with fine controlled porosity; (ii) poly(hydroxyethyl methacrylate) based nanocomposites applied in dentistry; (iii) organic column obtained through controlled or free–radical polymerization used as stationary phase in the technique of capillary liquid chromatography, and (iv) silicone rubber [
19].
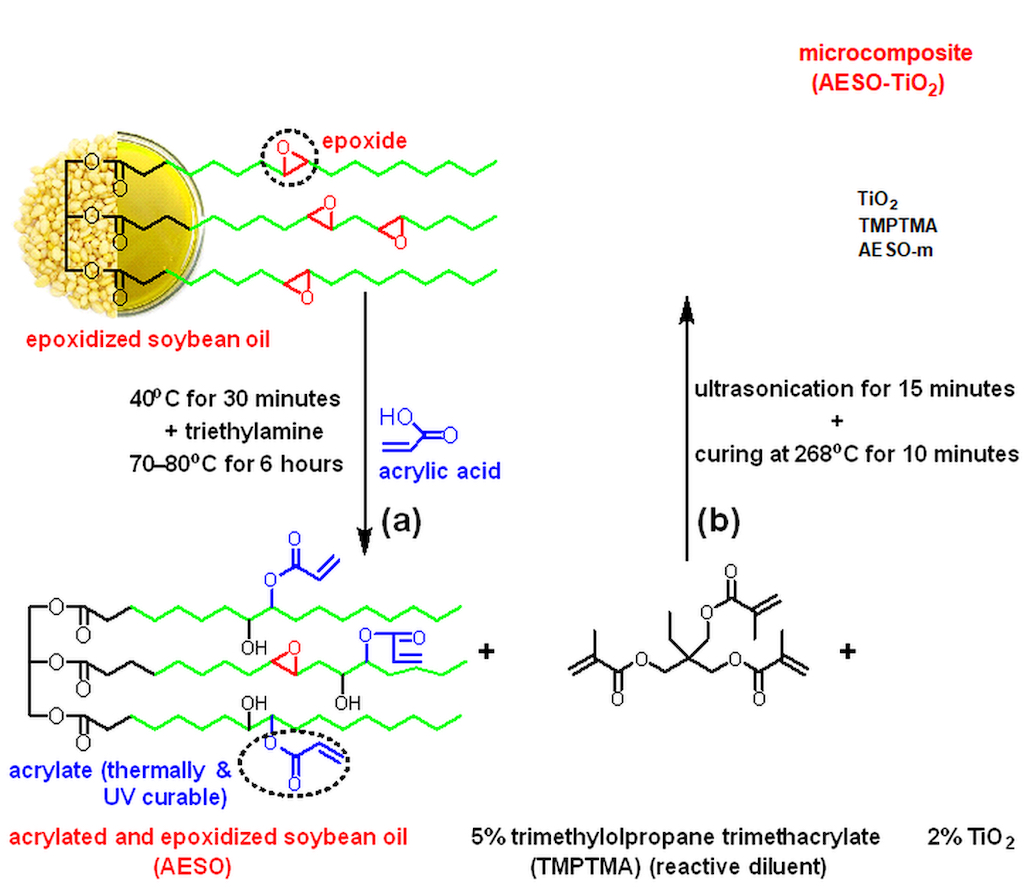
This is the first report the obtaining and characterization of a thermo–curable formulation based on epoxidized and acrylated soybean oil (AESO) containing TMPTMA as reactive diluent. This formulation was used as matrix in obtaining a bio–based composite with TiO2 microparticles filler (AESO–TiO2). The scope of this research is: (i) to reduce system viscosity; (ii) to simplify the system and its synthesis towards a green approach by eliminating the use of environmental and health hazardous solvent and curing agent in the matrix formulation for engineering sustainable microcomposites.
2. Experimental Section
2.1. Materials
The epoxidized soybean oil is a product under the commercial name LankroflexTM E2307 from Valtris Speciality Chemicals (Manchester, UK) (molar mass 975.399 g mol–1; density 0.997 g mL–1; acid value 0.7 mg KOH g–1; viscosity 325 mPa·s at 25oC; boiling point 150oC at 0.5 kPa; flash point 310oC). According to the manufacturer, Lankroflex™ E2307 is a low odor epoxy resin, certified safe for food contact and with medical approval. It is used in all types of PVC formulations in combination with metal soap stabilizers. It is also used as plasticizer for coatings applications, being an excellent water, oils and solvents repellent. Acrylic acid (98 % purity) was purchased from Acros Organics, Germany, and used as received. Methylhydroquinone was a product of Aldrich Chemistry, China. Triethylamine was a product of Sigma–Aldrich, Germany. Trimethylolpropane trimethacrylate (TMPTMA) was purchased from Sigma–Aldrich, USA and used as received. The TiO2 (IV) microparticles (99%) (d = 1–1.3 µm) were purchased from abcr GmbH, Germany.
2.2. Synthesis of AESO
100 g (0.102 mol) epoxidized soybean oil, 34.3 g acrylic acid (0.465 mol) and 0.04 g (3.2·10
–4 mol) methylhydroquinone, as thermal polymerization inhibitor, were mixed in a glass flask with a round bottom (500 mL capacity) provided with three necks, water cooler and mechanical stirrer. The temperature required for the reaction was ensured with a silicon oil bath equipped with a thermostat. The reaction mixture was preheated to 40
oC for 30 minutes followed by the adding of 0.15 mL triethylamine. To complete the chemical reaction, the mechanical stirring was continued for another 6 hours at 70–80
oC. After the completion of the reaction the AESO was washed a few times in a funnel with saline solution (28 g 100 mL
–1) to remove the unreacted acrylic acid and dried on anhydrous sodium sulfate. After filtration, 267 g of AESO were obtained with a reaction yield of 98.8%. The final product was of yellow–orange coloring and oily texture. The characterization of AESO by
1H–NMR technique is detailed in the
supplementary materials (Text S1, Figure S1).
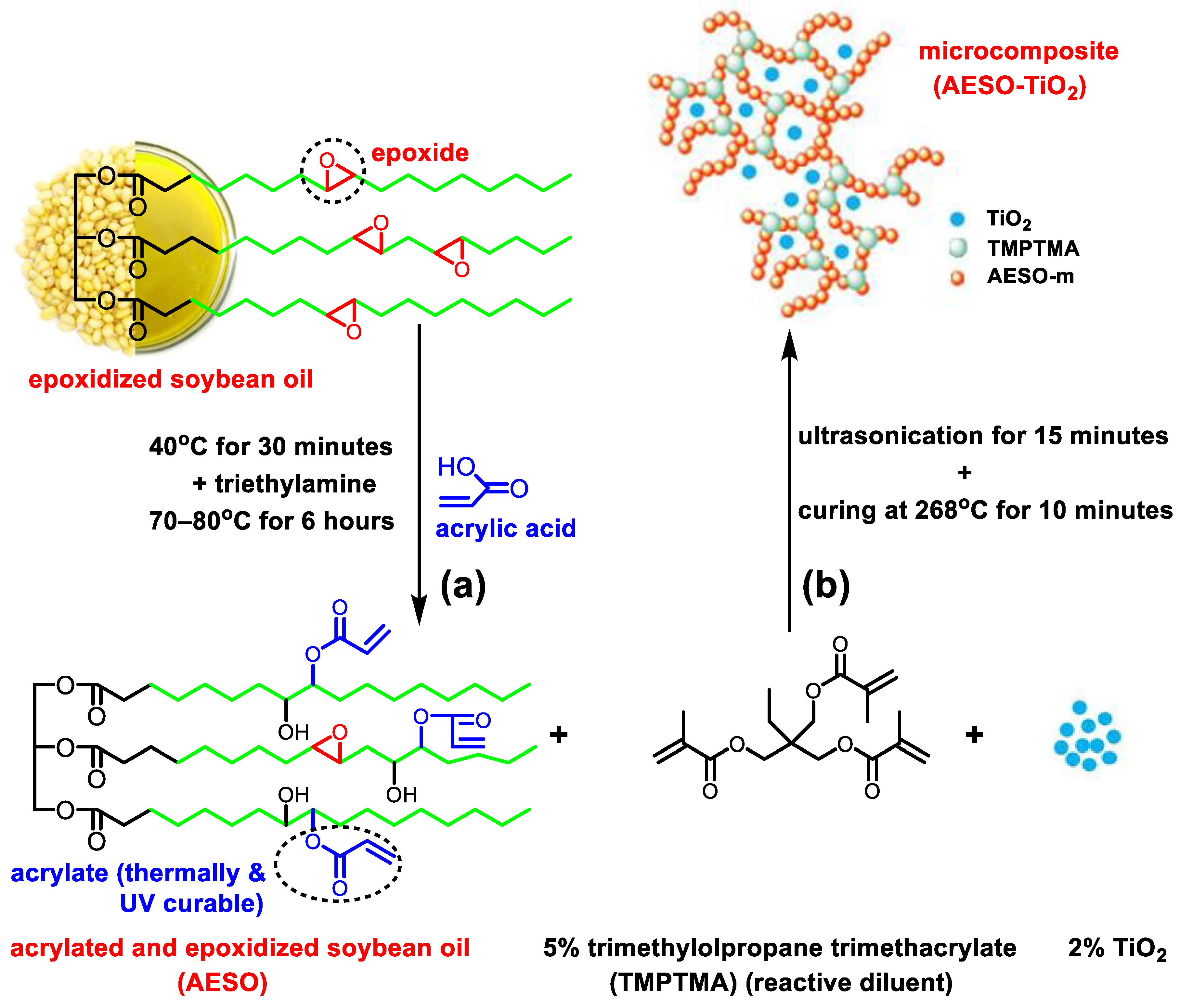
2.3. Obtaining of the AESO–TiO2 Microcomposite
44.75 g AESO and 2.521 g (5.63%) TMPTMA were stirred until the homogeneous acrylated epoxy formulation for the matrix (AESO–m) was obtained. Afterwards the AESO–m formulation was mixed with 0.89 g (2%) TiO
2 microparticles in an ultrasonication bath (S 15 Elmasonic from Elma Schimdbauer GmbH, Singen, Germany) for 15 minutes and the AESO–TiO
2 formulation was obtained. The thermally curable AESO–TiO
2 liquid formulation was of a white pasty texture. In order to obtain the AESO–TiO
2 composite the experimental thermal conditions needed to be established. In this sense, 10 mg of composite formulation was first placed in a pierced and sealed shut aluminum crucible and a differential scanning calorimetry (DSC) heating curve was recorded from room temperature to 300
oC with a 10
oC min
–1. A wide exothermal peak was observed at 268
oC, describing the thermal curing process, shown in the
supplementary materials (Figure S2). Then the rest of the composite formulation was heated in an oven (Vulcan 3–130 from Dentsply Ceramco, York, PA, USA) in the same conditions and held 10 minutes at 268
oC to obtain the solid white and hard AESO–TiO
2 microcomposite (
Figure 1b).
2.4. Methods
2.4.1. Proton Nuclear Magnetic Resonance (1H–NMR)
The 1H–NMR spectrum of AESO was recorded on an Advance DRX 400 (Brüker, Germany) in CDCl3 as solvent. The device was equipped with a 5 mm direct detection z–gradient probe.
2.4.2. Fourier–Transform Infrared Spectroscopy (FT–IR)
The FT–IR spectra were registered on a Vertex 70 apparatus (Brüker, Germany) equipped with a MIRacleTM ATR accessory provided with diamond crystal plate with a 1.8 mm diameter. The liquid formulations were measured within KBr pellets and the solid cured matrix and composite using the ATR module.
2.4.3. Thermogravimetric Analysis (TGA)
The thermogravimetric curves were recorded on a STA 449 F1 Jupiter apparatus (Netzsch–Gerätebau GmbH, Selb, Germany). Approximately 14 mg of sample was placed and heated in alumina crucibles. A heating rate of 10 oC min–1 was applied in nitrogen atmosphere (flow rate 50 mL min–1) up to 700 oC.
2.4.4. Differential Scanning Calorimetry (DSC)
The differential scanning calorimetry curves were recorded on a DSC 200 F3 Maia apparatus (Netzsch–Gerätebau GmbH, Germany). Approximately 10 mg of sample was placed heated in aluminum crucibles which were sealed shut with pierced lids. Experiments were conducted in nitrogen atmosphere (flow rate of 50 mL min–1) and with a heating rate of 10 oC min–1. Calibrations were made with standard indium.
2.4.5. Scanning Electron Microscopy (SEM)
The scanning electron microscopy micrographs were recorded on a Verios G4 UC scanning electron microscope (Thermo Fisher Scientific, Brno–Cernovice, Czech Republic). The samples were fixed onto aluminum stubs using double adhesive carbon tape and afterwards coated with 6 nm platinum with a Leica EM ACE200 sputter coater (Leica, Vienna, Austria) for providing electrical conductivity and preventing charge accumulation during the electron beam exposure. There was used a secondary electron detector (ETD detector – Everhart–Thornley detector) in order to highlight the size and shape of the particles at an acceleration voltage of 5 kV and a spot size of 0.4 nA.
2.4.6. Broadband Dielectric Spectroscopy
The broadband dielectric spectroscopy measurements were undertaken on a Broadband Dielectric Spectrometer (Novocontrol Technologies, Germany). The dielectric parameters were recorded isothermally, in a frequency window between 1 Hz and 1 kHz and at temperatures between –50 oC and 250 oC.
2.4.7. Contact Angle Measurements
Static contact angle measurements were performed on a CAM–200 instrument (KSV Instruments, Helsinki, Finland) with sessile drop profile analysis. The measurements were undertaken at room temperature by placing a 1 μL drop of liquid on the sample surface. Each contact angle value was a mean of five measurements taken on different surface sites.
3. Results and Discussion
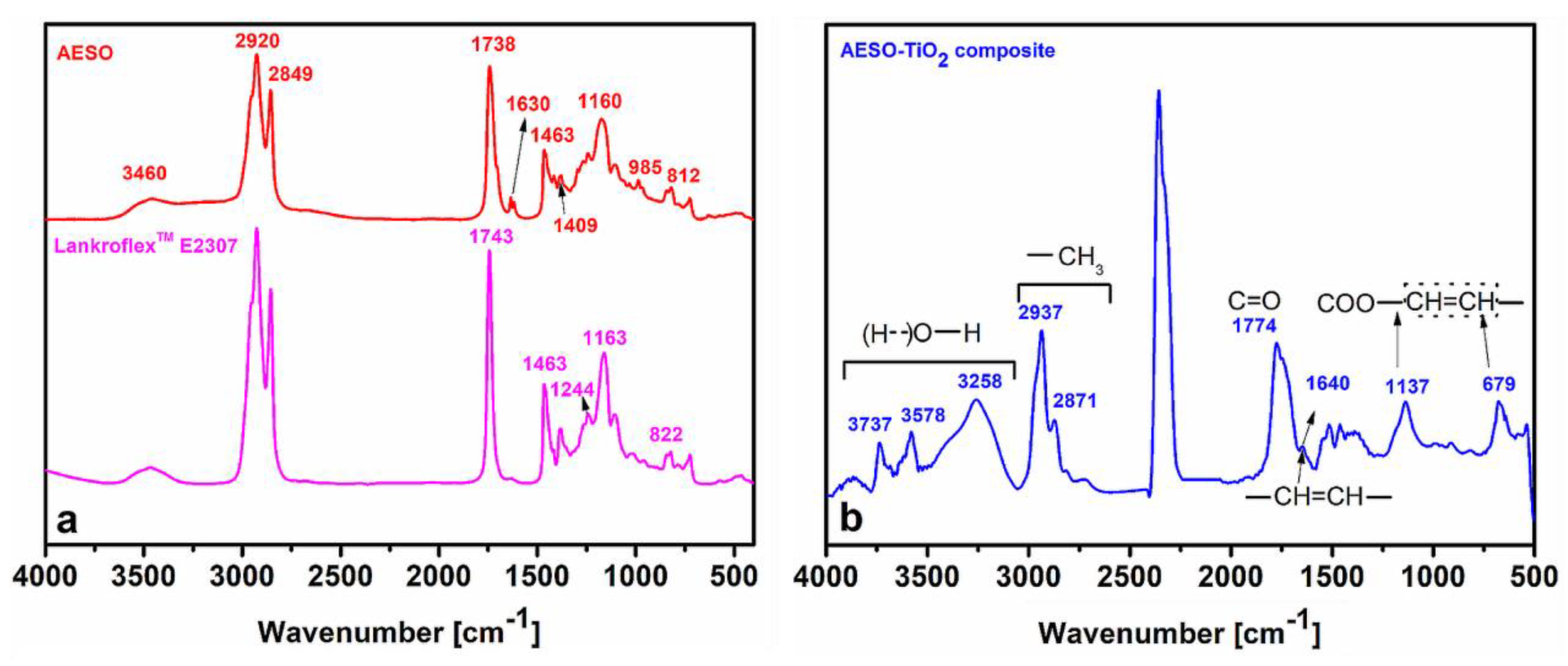
3.1. Structural Characterization by FT–IR
Figure 2a shows the comparative FT–IR spectra of Lankroflex
TM E2307 and the AESO formulation. The signals at 822 cm
–1 and 1244 cm
–1 in the FT–IR spectrum of Lankroflex
TM E2307 demonstrate the presence of the epoxy rings [
33]. The FTIR spectrum in AESO confirms the synthesis of its structure via the formation of the peaks at 3460 cm
–1 corresponding to the –OH moiety from epoxy ring opening and associated with intermolecular polymeric hydrogen bonding [
34]. The wider peak at 1738 cm
–1 is due to the appearance of supplementary carbonyl (C=O) entities. The vibration of the –CH=CH
2 in acrylate is depicted by the signals at 1409 cm
–1, 985 cm
–1 and 812 cm
–1. The –CH=CH– stretching vibration is represented by the peak at 1630 cm
–1 [
34].
Figure 2b shows the FTIR spectrum of the microcomposite AESO–TiO
2. Compared to the FT–IR spectra in
Figure 2a, the complex FT–IR spectrum of the composite AESO–TiO
2 contains more signals originating also from the bulky TMPTMA structure and its curing with and within the AESO matrix [
35]. According to the literature, the peaks in the range 3000–3750 cm
–1 and the one at 3258 cm
–1 may correspond to the stretching vibrations of different hydrogen bonds between –OH entities. The peak at 1640 cm
–1 corresponds to the terminal C=C bonds of TMPTMA and its very low intensity indicates their implication in curing reactions. Due to their bulky nature, the TMPTMA molecules become sterically hindered, restricting mobility in the three–dimensional composite network and leaving some unreacted double bonds in the system [
36]. The peak at 1774 cm
–1 could be attributed to some new carbonyl structures, such as conjugated anhydrides with 5 members and/or cyclic aldehydes [
37].
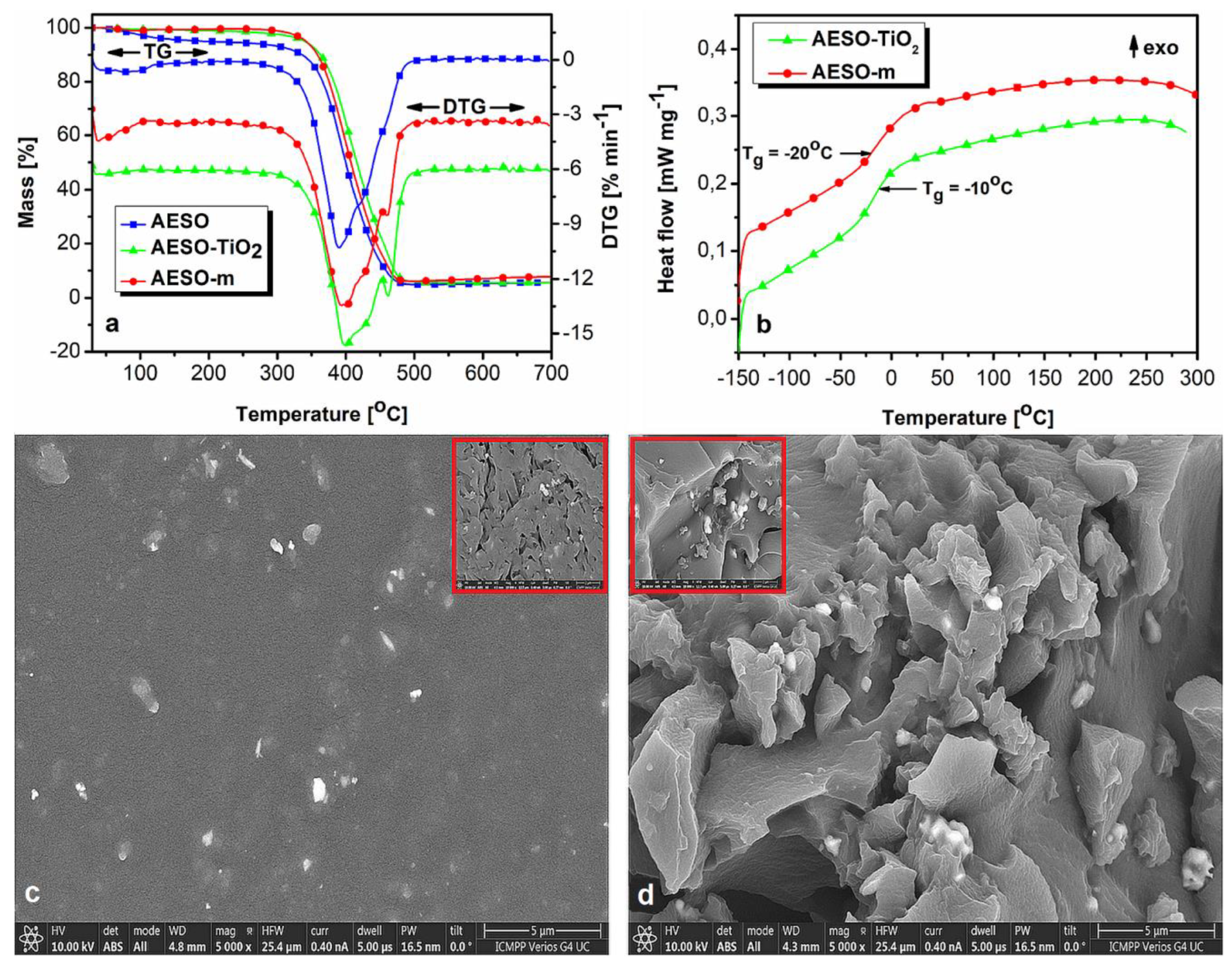
3.2. Thermal and Morphological Characterization
Figure 3a, b show the thermogravimetric curves, with their corresponding first derivatives (DTG) curves, respectively.
Table S1 in the supplementary material lists the data extracted from the thermal analyses: the static heat resistant index (
Ts), calculated with Equation (1) [
38], as criterion to assess thermal stability; the temperature at 5 % (
T5%) mass loss; the temperature at 30 % (
T30%) mass loss; the temperature at maximum rate of degradation (
Tpeak); mass loss (%) of each thermal degradation stage (
M); the residual mass (%) at the end of the thermal degradation process (700
oC) (
R) and the glass transition temperature (
Tg).
The thermogravimetric curves (
Figure 3a) show a significant increase in the thermal stability of the materials after curing, from
Ts = 150
oC for AESO to
Ts = 180
oC and 183
oC for the cured matrix (AESO–m) and composite (AESO–TiO
2), respectively. The DTG curves indicate a thermal degradation profile in three overlapping stages, almost indiscernible in the TGA curves. The
Tpeak in the DTG curves shifts toward higher values with curing and TiO
2 addition. Also, the mass loss (
M) decreases for thermal degradation stages I and II and increases for stage III of AESO–m and AESO–TiO
2 compared to AESO (
Table S1). The three overlapping thermal degradation stages are due to initial random chain scission followed by almost simultaneous branching and crosslinking phenomena in AESO [
39]. Stage III of thermal degradation (440
oC–490
oC), where the mass loss increases (
Table S1), is better evidenced for AESO–m and composite AESO–TiO
2 where the crosslinks in the matrix decompose (DTG curve peak
Tmax in
Table S1 and
Figure 3a).
The DSC second heating curves of the studied materials (
Figure 3b) show the presence of a single neat T
g, demonstrating the formation of a fully thermally cured composite, a confirmation of a good miscibility of the components and a uniform distribution of the TiO
2 microparticles within the composite matrix. These aspects also led to the increasing the
Tg of the composite from –20
oC (AESO–m) to –10
oC (AESO–TiO
2), as confirmed by the SEM measurements in
Figure 3c, d.
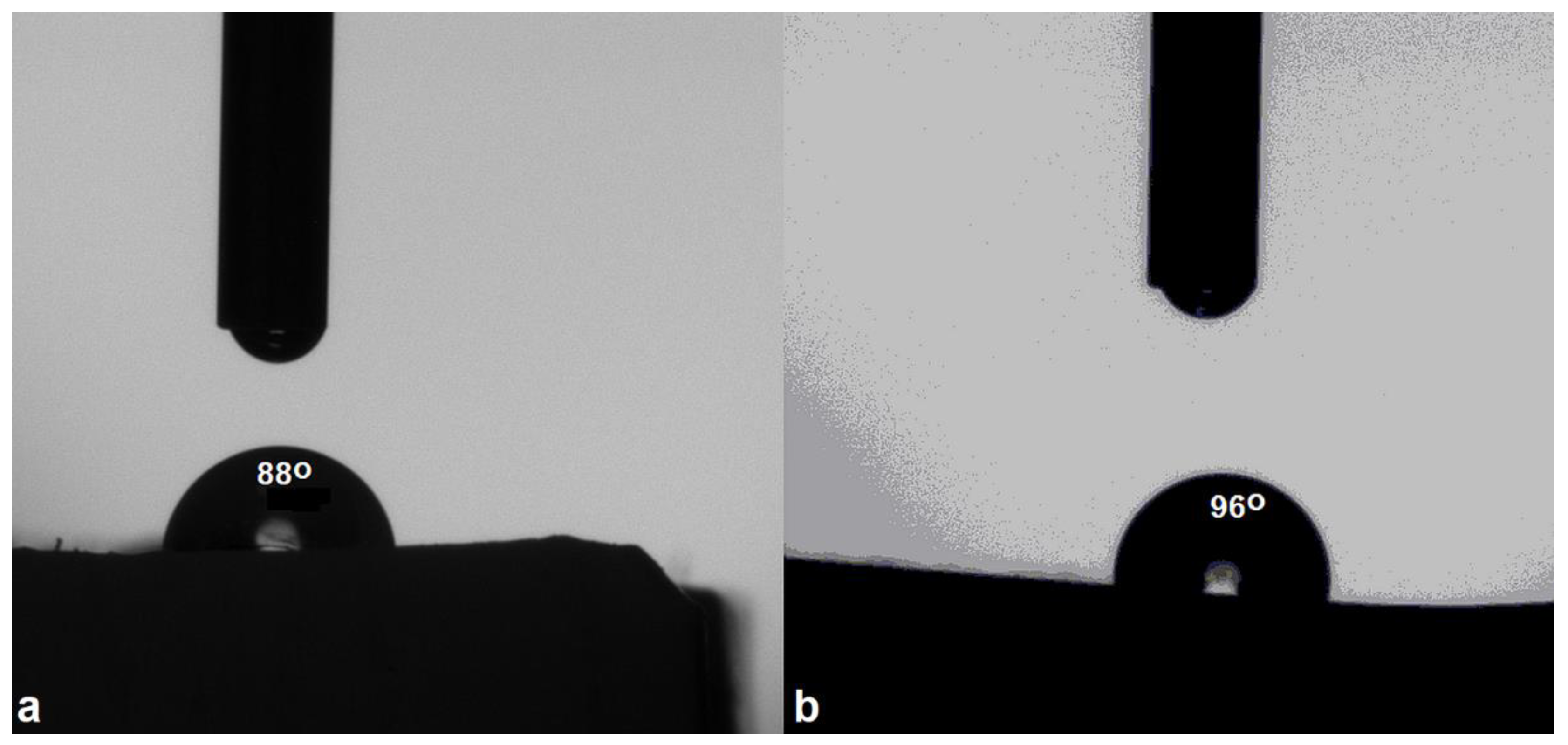
3.3. Wettability
For materials to be used in applications domains such as anti–weathering protective coatings and in microelectronics it is also crucial for them to possess hydrophobicity [
40]. The water contact angle of the matrix and composite was measured. It was observed that compared to the matrix, with a contact angle value below 90
o, the microcomposite possesseed a contact angle value above 90
o. This aspect implies that the incorporation of TiO
2 microparticles renders the composite hydrophobic. The contact value of the matrix was 88
o, indicating a hydrophilic behavior. The micro composite manifested hydrophobic character, displaying a contact angle value of 96
o. An explanation could reside in the microparticles mobility, as demonstrated by the increased permittivity in the dielectric studies discussed in the next section, allowing them to migrate in the polymer matrix, leading to a higher packing degree and forming a microstructured compact texture. This phenomenon not only increases surface hydrophobicity, but at the same time generates a good compatibility with the polymer matrix, as previously shown by DSC and SEM results. Moreover, the anchoring of TiO
2 microparticles may expose alkyl moieties at the solid−air interface, which, together with the suitable morphology, also increases the contact angle value of the microcomposite [
41].
Figure 4.
(a) Water contact angle of the cured epoxy matrix and (b) of the microcomposite.
Figure 4.
(a) Water contact angle of the cured epoxy matrix and (b) of the microcomposite.
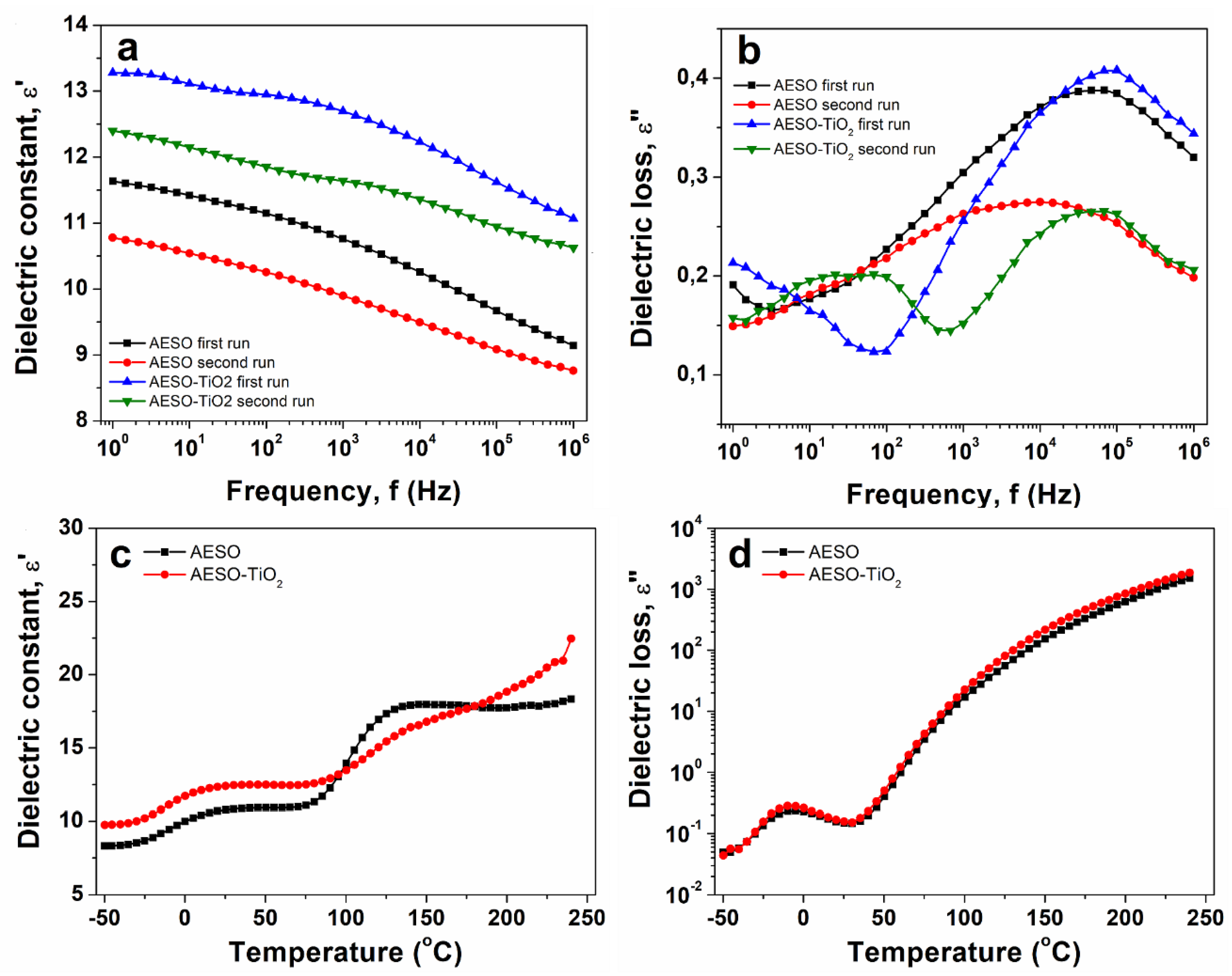
3.4. Broadband Dielectric Spectroscopy
The dielectric properties play a crucial role in assessing the use of epoxy resins as electrical insulators, and are greatly influenced by their molecular structure [
42]. The behaviors of dielectric constant, ε’, and dielectric loss, ε”, parameters with frequency are presented in
Figure 5. The isothermal plots are selected at room temperature. The ε’ parameter is related with the dipolar activity of a material. Following the
Figure 5a, ε’ decreases gradually towards increasing frequency, as generally observed for polymer materials [
43]. The magnitude of ε’ of the composite is higher than that of the cured matrix. The enhanced magnitude of ε’ for the composite may be due the incorporation of TiO
2 filler in the matrix that induces supplementary polarizable units [
44]. Increased permittivity values of the matrix and the composite at lower frequencies may be associated either with the Maxwell–Wagner effect, when the alternating current and applied potential are in phase, or direct current conductivity, defined as the result of increased ion mobility, or both, detailed in the literature [
45,
46]. The dielectric loss encompasses the dissipation energy required to align the polarizable units in the direction of an external electrical field. Similar with ε’(f) dependences, the magnitude of ε” (
Figure 5b) decreases considerably, due to the vegetable oil entities.
The isochronal plots of ε’ and ε” vs. temperature of the studied materials are comparatively displayed in
Figure 5c, d. The spectra are selected at 1 Hz. At temperatures lower than 0
oC, a clear relaxation process is retrieved as a step–increase in the dielectric spectra of ε’, higher for the composite than for the matrix (
Figure 5c), and as a well–defined dielectric peak in the spectra of ε” (
Figure 5d). According with the DSC data, the relaxation process is connected with the glass transition temperature of the materials, found in both ε’(T) and ε”(T) profiles of the two materials. At temperatures between 100
oC and 200
oC, a secondary step increase is detected in the ε’(T) profiles of both samples. This step–increase may suggest hindering or cleavage of physical bonds [
47]. In the ε”(T) profiles of the structures, no relaxation process is detected between 100
oC and 200
oC. Due to its enhanced dielectric properties compared to the cured matrix, the microcomposite may also be recommended in applications such as optoelectronic devices and as dielectric layer in thin–film transistors [
48].
4. Conclusions
This is the first report on a composite from solvent and hardener–free bio–based acrylated and epoxidized soybean oil matrix with 2% TiO2 microparticle filler. The material possessed reduced initial matrix formulation viscosity and was obtained through thermal curing. The components showed excellent miscibility within the cured matrix. Here we demonstrated that the microcomposite exhibited superior thermal stability, glass transition temperature, hydrophobicity and dielectric behavior to those of the pristine cured matrix. All results recommend the microcomposite for optoelectronic devices and as layer in thin–film transistors.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org., Text S1: Characterization of The Acrylated and Epoxidized Soybean Oil (AESO); Figure S1: The
1H–NMR spectrum of AESO; Figure S2: The first DSC heating curve of AESO–TiO
2 formulation; Table S1: Thermal analyses data.
Author Contributions
Conceptualization, C.-D.V. and D.R.; methodology, C.-D.V., L.R. and D.R., investigation C.-D.V., L.R., D.R. and M.A.; writing-original draft preparation, C.-D.V. and M.A.; writing–review and editing, C.-D.V., D.R.; project administration, C.-D.V and D.R. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This article is dedicated to the memory of Acad. Bogdan C. Simionescu (1948–2024).
Conflicts of Interest
The authors claimed no conflicts of interest.
References
- Mora AS, Tayouo R, Boutevin B, David G, Caillol S (2018) Vanillin-derived amines for bio-based thermosets. Green Chem 20:4075-4084. [CrossRef]
- Li R, Zhang P, Liu T, Muhunthan B, Xin JN, Zhang JW (2018) Use of hempseed-oil-derived polyacid and rosin-derived anhydride acid as cocuring agents for epoxy materials. ACS Sustainable Chem Eng 6:4016-4025. [CrossRef]
- Xin JN, Li M, Li R, Wolcott MP, Zhang JW (2016) Green epoxy resin system based on lignin and tung oil and its application in epoxy asphalt. ACS Sustainable Chem Eng 4:2754-2761. [CrossRef]
- Ng F, Couture G, Philippe C, Boutevin B, Caillol S (2017) Bio-based aromatic epoxy monomers for thermoset materials. Molecules 22:149-197. [CrossRef]
- Roudsari GM Mohanty AK, Misra M (2014) Study of the curing kinetics of epoxy resins with biobased hardener and epoxidized soybean oil. ACS Sustainable Chem Eng 2:2111-2116. [CrossRef]
- Maksym P, Tarnacka M, Dzienia A, Matuszek K, Chrobok A, Kaminski K, Paluch M (2017) Enhanced polymerization rate and conductivity of ionic liquid-based epoxy resin. Macromol 50:3262–3272. [CrossRef]
- Zhou J, Xu K, Xie M, Wu H, Hua Z, Wang Z (2019) Two strategies to precisely tune the mechanical properties of plant oil-derived epoxy resins. Compos Part B Eng 173:106885. [CrossRef]
- Petrie EM (2006) Epoxy adhesive formulations. McGraw Hill, New York.
- Visakh PM, Rosu D (2016) Photochemical behavior of multicomponent polymeric-based materials. Springer International Publishing Switzerland, Cham.
- Liang X, Yin N, Liang S, Yang R, Liu S, Lu Y, Jiang L, Zhou Q, Jiang G, Faiola F (2020) Bisphenol A and several derivatives exert neural toxicity in human neuron-like cells by decreasing neurite length. Food Chem Toxicol 135:111015. [CrossRef]
- Qiu W, Zhan H, Hu J, Zhang T, Xu H, Wong M, Xu B, Zheng C (2019) The occurrence, potential toxicity, and toxicity mechanism of bisphenol S, a substitute of bisphenol A: a critical review of recent progress. Ecotoxicol Environ Saf 173:192-202. [CrossRef]
- Godiya CB, Park BJ (2022) Removal of bisphenol A from wastewater by physical, chemical and biological remediation techniques. A review. Environ Chem Lett 20:1801-1837. [CrossRef]
- Di Mauro C, Tran TN, Mija A (2021) One-pot terpolymerization synthesis of high carbon biocontent recyclable epoxy thermosets and their composites with flax woven fibers. ACS Sustainable Chem Eng 9:8526-8538. [CrossRef]
- Mustata F, Tudorachi N, Bicu I (2016) Curing kinetics, thermal and morphological characterization of the biobased thermosets from epoxy resin/epoxidized hemp oil. J Anal Appl Pyrolysis 122:191-201. [CrossRef]
- Rosu D, Mustata F, Tudorachi N, Musteata VE, Rosu L, Varganici CD (2015) Novel bio-based flexible epoxy resin from diglycidyl ether of bisphenol A cured with castor oil maleate. RSC Adv 5:45679-45687. [CrossRef]
- Mustata F, Tudorachi N, Bicu I (2014) The curing reaction of epoxidized methyl esters of corn oil with Diels-Alder adducts of resin acids. The kinetic study and thermal characterization of crosslinked products. J Anal Appl Pyrolysis 108:254-264. [CrossRef]
- Worzakowska M (2007) The kinetic study of the curing reaction of mono- and diepoxides obtained during the reaction of divinylbenzene and hydrogen peroxide with acid anhydrides. Polymer 48:1148-1154. [CrossRef]
- Yang X, Wang C, Li S, Huang K, Li M, Mao W, Cao S, Xia J (2017) Study on the synthesis of bio-based epoxy curing agent derived from myrcene and castor oil and the properties of the cured products. RSC Adv 7:238-247. [CrossRef]
- Jagtap AR, More A (2022) Developments in reactive diluents: a review. Polym Bull 79:5667-5708. [CrossRef]
- Chen L, Zhang Y, Chen Z et al (2024) Biomaterials technology and policies in the building sector: a review. Environ Chem Lett 22:715-750. [CrossRef]
- Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301-312. [CrossRef]
- Chong KL, Lai JC, Rahman RA, Adrus N, Al-Saffar ZH (2021) Self-healable biobased epoxy resin from epoxidized palm oil. Chem Eng Trans 89:379-384. [CrossRef]
- Zhang C, Garrison TF, Madbouly SA, Kessler MR (2017) Recent advances in vegetable oil-based polymers and their composites. Prog Polym Sci 71:91-143. [CrossRef]
- Kadam A, Pawar M, Yemul O, Thamke V, Kodam K (2015) Biodegradable biobased epoxy resin from karanja oil. Polymer 72:82-92. [CrossRef]
- Tsujimoto T, Takeshita K, Uyama H (2016) Bio-based epoxy resins from epoxidized plant oils and their shape memory behaviors. JAOCS, J Am Oil Chemists’ Soc 93:1663-1669. [CrossRef]
- Varganici CD, Rosu L, Rosu D, Mustata F, Rusu T (2021) Sustainable wood coatings made of epoxidized vegetable oils for ultraviolet protection. Environ Chem Lett 19:307-308. [CrossRef]
- Rosu L, Mustata F, Rosu D, Varganici CD, Rosca I, Rusu T (2021) Bio–based coatings from epoxy resins crosslinked with a rosin acid derivative for wood thermal and anti-fungal protection. Prog Org Coat 151:106008. [CrossRef]
- Salarbashi D, Bazeli J, Tafaghodi M (2019) Environment-friendly green composites based on soluble soybean polysaccharide: a review. Int J Biol Macromol 122:216-223. [CrossRef]
- Pin JM, Sbirrazzuoli N, Mija A (2015) From epoxidized linseed oil to bioresin: an overall approach of epoxy/anhydride cross-linking. ChemSusChem 8:1232-1243. [CrossRef]
- Mustata F, Tudorachi N (2016) Thermosets based on castor oil modified with Diels-Alder adduct of levopimaric acid and diglycidyl ether of bisphenol A. The kinetic analysis of the curing reactions and thermal behavior of the cured products. Compos Part B Eng 97:263-273. [CrossRef]
- Zia KM, Noreen A, Zuber M, Tabasum S, Mujahid M (2016) Recent developments and future prospects on bio-based polyesters derived from renewable resources: a review. Int J Biol Macromol 82:1028-1040. [CrossRef]
- Gu H, Ma C, Gu J, Yan X, Huang J, Zhang Q, Guo Z (2016) An overview of multifunctional epoxy nanocomposites. J Mater Chem C 4:5890-5906. [CrossRef]
- Rana A, Evitts RW (2015) Synthesis and characterization of acrylated epoxidized flaxseed oil for biopolymeric applications. Int Polym Proc 30:331-336. [CrossRef]
- Balaban AT, Banciu M, Pogany I (1983) Applications of physical methods in organic chemistry. Encyclopedic and Scientific Publishing, Bucharest.
- Lai W, Li X, Liu H, Han L, Zhao Y, Li X (2014) Interfacial polycondensation synthesis of optically sensitive polyurea microcapsule. J Chem 2014:597578. [CrossRef]
- Gunewardena A, Gilbert M (2008) Peroxide crosslinking of rigid poly(vinyl chloride). J Vinyl Addit Technol 14:92-98. [CrossRef]
- Silverstein RM, Webster FS, Kiemle DJ (2005) Spectrometric identification of organic compounds. John Wiley & Sons, Hoboken.
- Mustata F, Rosu D, Varganici CD, Rosu L, Rosca I, Tudorachi N (2022) Assessing the thermal and fungal behavior of eco–friendly epoxy thermosets derived from vegetable oils for wood protective coatings. Prog Org Coat 163:106612. [CrossRef]
- Behera D, Banthia AK (2008) Synthesis, characterization, and kinetics study of thermal decomposition of epoxidized soybean oil acrylate. J Appl Polym Sci 109:2583-2590. [CrossRef]
- Zou C, Fothergill JC, Rowe S (2008) The effect of water absorption on the dielectric properties of epoxy nanocomposites. IEEE Trans Dielectr Electr Insul 15:106-117. [CrossRef]
- Passaro J, Bifulco A, Calabrese E, Imparato C, Raimondo M, Pantani R, Aronne A, Guadagno L (2023) Hybrid Hemp Particles as Functional Fillers for the Manufacturing of Hydrophobic and Anti-icing Epoxy Composite Coatings. ACS Omega 8:23596-23606. [CrossRef]
- Li C, Fan H, Aziz T, Bittencourt C, Wu L, Wang DY, Dubios P (2018) ACS Sustainable Chem Eng 6:8856-8867. [CrossRef]
- Kremer F, Schönhals A (2003) Broadband dielectric spectroscopy. Springer, Berlin/Heidelberg.
- Chen S, Yao K, Tay FEH, Liow CL (2007) Ferroelectric poly (vinylidene fluoride) thin films on Si substrate with the β phase promoted by hydrated magnesium nitrate. J Appl Phys 102:104108. [CrossRef]
- Saad ALG, Hassan AM, Youssif MA, Ahmed MGM (1997) Studies of electrical properties of some fire-retarding poly (vinyl chloride) compositions. J Appl Polym Sci 65:27-35. [CrossRef]
- Abd–El–Messieh S, Naguib H (2005) Electrical conductivity and dielectric behavior of some poly(alkyl methacrylate) s/polyvinylpyrrolidone blends. Polym–Plast Technol Eng 44:1591-1606. [CrossRef]
- Zhang D, Runt J (2004) Segmental dynamics and ionic conduction in poly(vinyl methyl ether)-lithium perchlorate complexes. J Phys Chem B 108:6295-6302. [CrossRef]
- Shubha A, Manohara SR, Angadi B (2022) Influence of TiO2 nanoparticles on structural, optical, dielectric and electrical properties of bio–compatible PEOX–PVP–TiO2 nanocomposites. Polym Bull 79:7117-7135. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).