1. Introduction
The Affordable Care Act (ACA), passed in 2010, aimed to enhance healthcare accessibility by increasing the number of patients with health insurance and expanding health insurance coverage for low-income populations [
1]. The ACA includes a provision for the Medicaid expansion program, providing states with the option to offer Medicaid coverage to non-elderly adults (ages 19-64), with incomes at or below 138% of the federal poverty level. While six states participated in the Medicaid expansion in 2010, most states implemented the Medicaid expansion in 2014, improving access to care and utilization of healthcare services for previously uninsured, low-income citizens. These individuals are now more likely to receive preventative care and numerous studies have shown increased cancer screening rates and healthcare utilization in Medicaid expansion states in colorectal, breast, and cervical cancer [
2,
3,
4,
5].
Additionally, research studies have indicated that following the expansion, there was increased detection of early-stage disease in patients with various cancers, including breast, colorectal cancer (CRC), head and neck, and lung cancer in Medicaid expansion states and potentially improved survival rates compared to those in non-expansion states [
6,
7,
8]. Melanoma ranks as the fifth most common cancer diagnosis in the United States, with its incidence rising both domestically and globally and disproportionately affecting patients of lower socioeconomic status who are at higher risk for delayed diagnosis and worse outcomes [
9,
10]. For localized melanoma, the standard treatment based on the National Comprehensive Cancer Network (NCCN) guidelines is wide local excision of the primary lesion and sentinel lymph node biopsy (SLNB) if indicated in patients with T1b or higher stages at high risk of nodal metastases [
11]. Following primary surgery, patients may undergo additional treatments with radiation therapy, immunotherapy, targeted therapy, or chemotherapy as indicated.
The ACA, including the Medicaid expansion provision, was designed to improve overall healthcare accessibility, however the extent to which Medicaid expansion has impacted tumor staging of melanoma at presentation and melanoma care remains under-explored. This study aims to evaluate the impact of Medicaid expansion on the primary outcome of interest and melanoma tumor stage, as well as primary surgery, use of immunotherapy, and 3-year mortality, shedding light on the potential benefits of expanded healthcare coverage in low-income communities.
2. Materials and Methods
A retrospective cohort study was performed using the National Cancer Database (NCDB), a collaborative project between the American Cancer Society (ACS) and the American College of Surgeons Commission on Cancer (ACS CoC) which is a facility-based, nationwide dataset on more than 70% of new cancer diagnoses in the U.S. and includes collected data on approximately 40 million records from hospital cancer registries in the U.S.
The NCDB was used to identify 12,667 non-elderly patients (age range, 40-64) who were newly diagnosed with melanoma from 2010 to 2020. The flow diagram in
Figure 1 features the study inclusion and exclusion criteria used to define the study cohort. Patients of less than 40 years of age were excluded as the NCDB does not report expansion status information for this age range, and patients older than 64 were excluded as they are eligible for Medicare. Additionally, patients with private insurance and other types of government or unknown primary insurance were not included in the study. The analytic stage is assigned by the NCDB based on the reported pathologic stage; however, if this was unavailable, the clinical stage was used. The sub-stage groups were consolidated into their corresponding general stage designations of I to IV. Patients with unknown analytic stage were excluded from the study. This study analysis was exempt by the Institutional Review Board and all deidentified data in compliance with the Health Insurance Portability and Accountability Act.
A ꭕ2 test for categorical variables and t-test for means were performed to evaluate the clinical and demographic characteristics of the study population. Year-to-year trend analysis was used to examine trends on melanoma stage and effect of expansion on insurance status over time between MES and non-MES states from 2010 to 2020.
Difference-in-difference (DID) analysis was performed to analyze tumor staging at presentation between Medicaid expansion states (MES) and non-Medicaid expansion states (non-MES) before the expansion (2010-2013) and after the expansion (2014-2020). Non-expansion states consisted of TN, NC, ID, GA, FL, MO, AL, MS, KS, TX, WI, UT, SC, SD, VA, OK, NE, WY, ME. The interaction between time periods (2010–2013 and 2014–2020) and expansion status (MES and non-MES) were defined for the DID estimate and a multivariable linear probability model was used. The multivariable analysis was adjusted for age, sex, race, comorbidity, income, education, population density, facility type, tumor histology.
The primary outcome of interest was melanoma tumor stage at presentation and secondary outcomes include primary surgery, use of immunotherapy, and 3-year mortality.
Early-expansion states (WA, CA, NJ, MN, DC, CT) from 2010 to 2013 were excluded from the DID analysis for sensitivity analysis. The parallel trend assumption ensures internal validity of DID models and this was validated by visual inspection of the graph between the exposure group (MES) and control group (non-MES) before the Medicaid expansion time period between 2010 to 2013.
All tests were two sided with an alpha of 0.05. Data processing and statistical analyses were performed with SAS software (version 9.1.3; SAS Institute Inc) and STATA MP (version 13.1; StataCorp).
3. Results
3.1. Study Population
In the study population, there were a total of 12,667 melanoma patients (56% male, 95% white) meeting the inclusion criteria. In the pre-expansion time period (2010 to 2013), there were 2,307 patients (18.2%) residing in MES and 1,804 (14.2%) residing in non-MES. In the post-expansion time period (2014 to 2020), there were 5,571 patients (43.9%) residing in MES and 2,985 (23.6%) residing in non-MES.
A greater proportion of patients with Medicaid resided in MES compared to non-MES (pre-expansion 51% vs 32%, post-expansion 82% vs 40%, p<0.001). Following expansion, the percent of patients with Medicaid increased by approximately 30% in MES states (
Table 1).
Median income was overall lower in non-MES compared to MES both before and after expansion. Before expansion, there were more low-income patients making less than $40,227 in non-MES compared to MES (pre-expansion 25% vs. 13%, post-expansion 26% vs 16%, p <0.001).
Education status was also lower in non-MES compared to MES. In the lowest education group categorized as 17.6% or more without high school, non-MES had a greater proportion compared to MES (25% vs 16%, p<0.001), and this was overall unchanged between pre- and post-expansion. As shown in
Table 1, MES and non-MES patients have various differences in several demographic characteristics.
3.2. Change in Insurance Coverage Over Time
In this cohort study, over 80% of patients’ insurance status consisted of private/managed care. In MES, uninsured patients decreased from 3.5% in 2010 to 1.7% in 2020 with the greatest decrease occurring between 2013 to 2014 when more widespread Medicaid expansion took effect. In contrast, in non-MES the percentage of uninsured patients decreased only by 0.6% (6.3% in 2010 to 5.7% in 2020). The proportion of patients with Medicaid increased in MES from 3.4% to 8.9% in the study period. In contrast, patients with Medicaid increased only by 0.4% from 3.5% to 3.9% in non-MES.
When accounting for patients without insurance and Medicaid only, there is a clear increase of Medicaid status and, inversely, decrease of uninsured patients (
Figure 2). The proportion of patients with Medicaid increased greatly from 49% in 2010 to 84% in 2020. The proportion of uninsured patients decreased greatly from 51% to 16% in the same period.
3.3. Trend Analysis
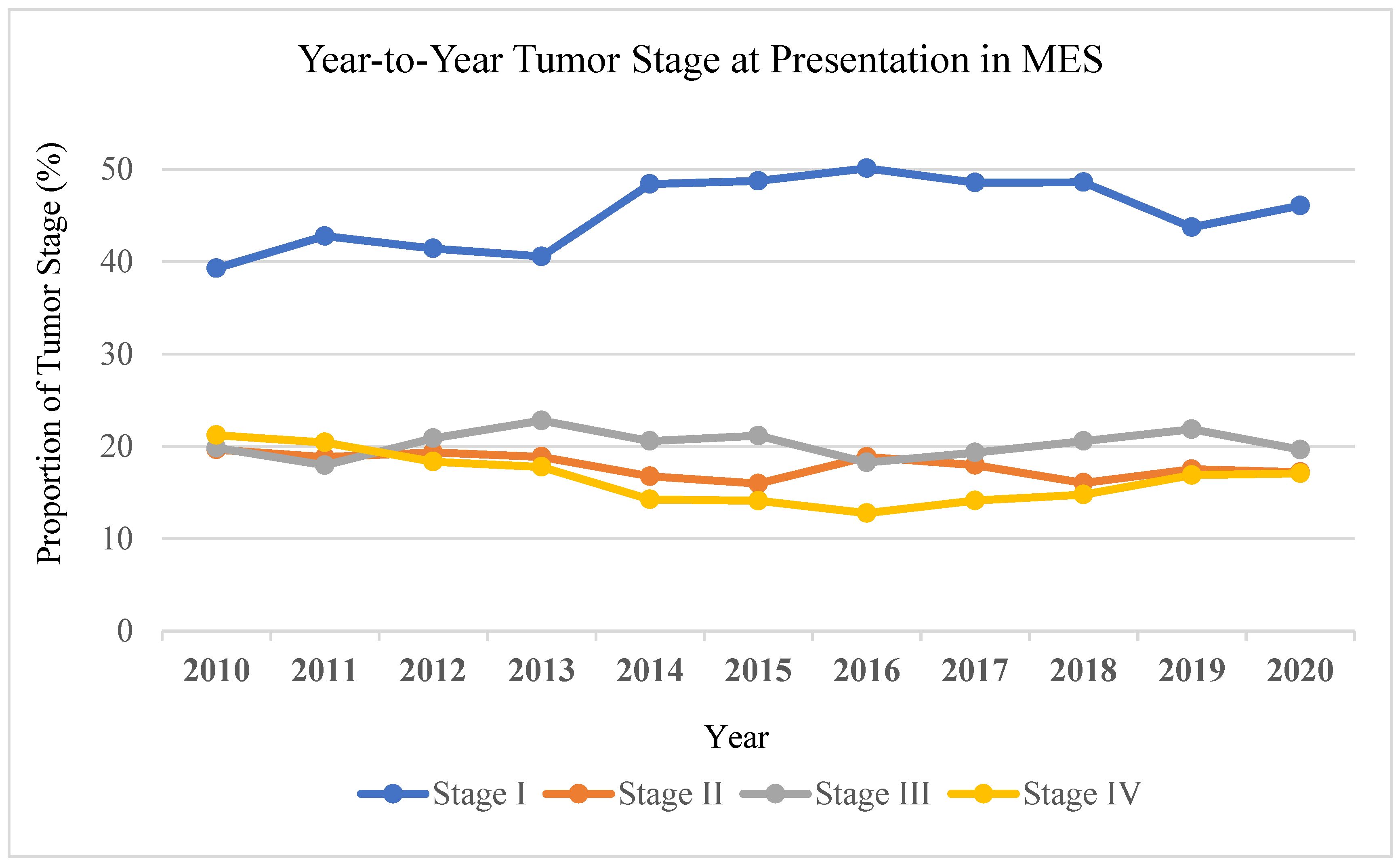
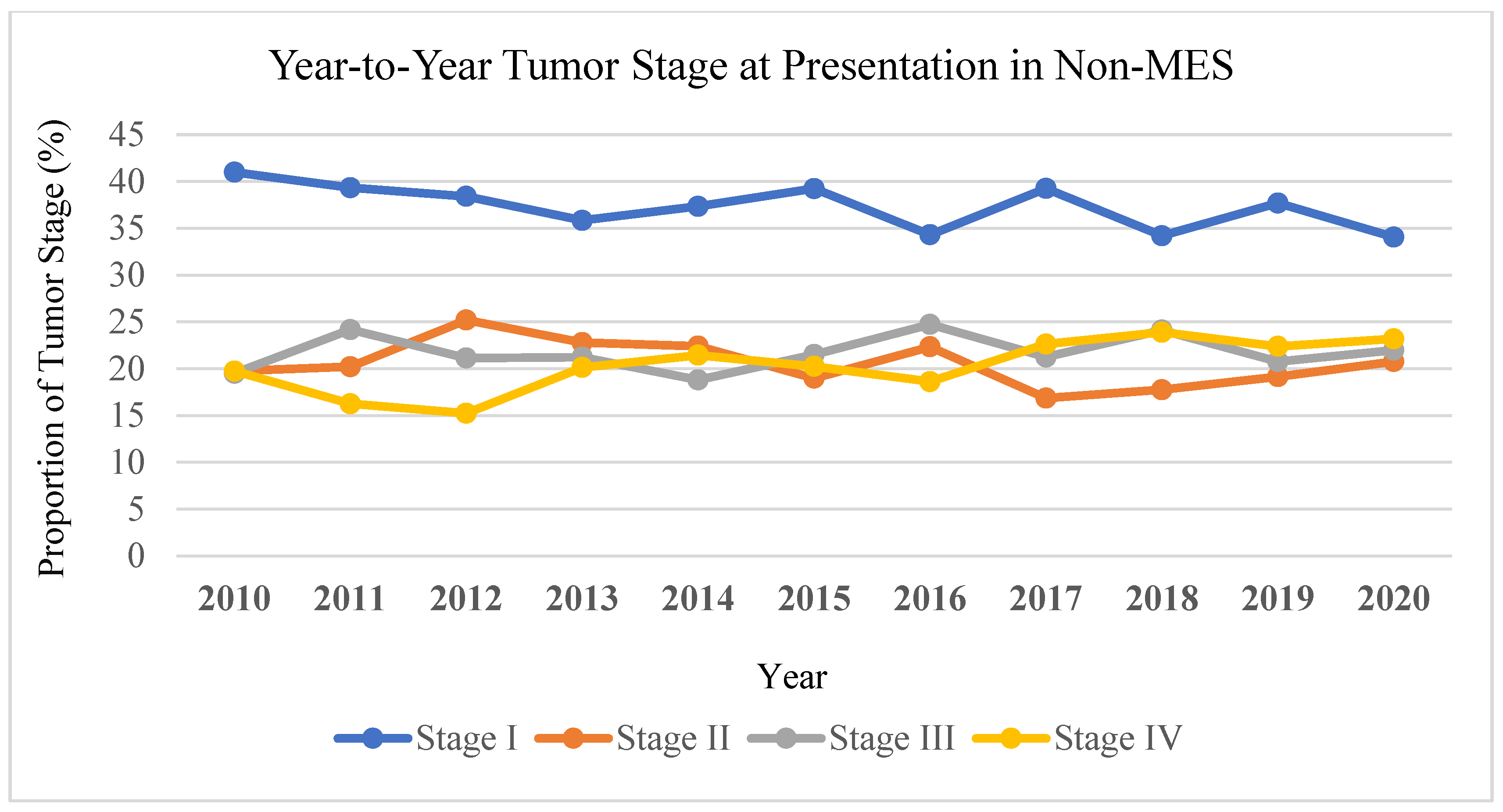
Trend analysis revealed the proportion of stage IV melanoma at presentation in MES decreased from 21% in 2010 to 17% in 2020. In contrast, stage IV melanoma at presentation in non-MES increased from 20% to 23% over the same period of time (
Figure 3). In MES, the proportion of stage I melanoma increased from 39% to 46% from 2010 to 2020, whereas in non-MES stage I melanoma decreased from 41% to 34%.
3.4. DID Analysis
3.4.1. Tumor Stage
Before Medicaid expansion, the adjusted tumor stage of non-MES and MES were approximately the same at 1.71 and 1.77 respectively (difference 0.06). After Medicaid expansion, the adjusted tumor stage in non-MES increased to 1.85, whereas the tumor stage in MES decreased to 1.68 (difference -0.17). DID analysis revealed a statistically significant decrease in stage IV melanoma (DID -0.222, p <0.001) between MES and non-MES before and after Medicaid expansion (
Table 2).
3.4.2. Definitive Surgery
Prior to Medicaid expansion, the occurrence of definitive surgery after diagnosis in stage I-III was the same at 0.85 in non-MES and MES. The occurrence of definitive surgery in stage IV was also similar between non-MES and MES at 0.44 and 0.49 respectively prior to expansion.
After Medicaid expansion, the occurrence of definitive surgery in stage I-III was 0.86 in non-MES and 0.88 in MES (difference 0.02). After expansion, in stage IV, the occurrence of primary surgery was 0.42 in non-MES and 0.44 (difference 0.02). The DID analysis results were not statistically significant for primary surgery between MES and non-MES before and after expansion
3.4.3. Use of Systemic Immunotherapy
Before Medicaid expansion, the proportion of patients receiving immunotherapy was 0.17 in non-MES and 0.20 in MES (difference 0.03). After expansion, the proportion of patients receiving immunotherapy increased to 0.41 in non-MES and 0.47 in MES (difference 0.06). The results demonstrated the use of immunotherapy in MES was significantly higher than non-MES after expansion (p<0.001), although DID analysis did not reveal a statistically significant difference in the use of immunotherapy between MES and non-MES before and after Medicaid expansion.
3.4.4. 3-Year Mortality
The 3-year mortality in non-MES was 0.21 and in MES was 0.24 prior to the Medicaid expansion (difference 0.03). After Medicaid expansion, the 3-year mortality in non-MES decreased to 0.14 and in MES decreased to 0.12 (difference -0.02). DID analysis showed a statistically significant decrease in 3-year mortality (DID -0.05, p 0.001) between MES and non-MES before and after Medicaid expansion (
Table 2).
4. Discussion
In this study involving 12,667 non-elderly melanoma patients, Medicaid expansion was associated with greater insurance coverage in MES compared to non-MES. Overall, the number of uninsured patients decreased by 1.8% in MES, whereas the number of uninsured patients decreased only slightly by 0.6% in non-MES. When only accessing Medicaid and uninsured patients and removing patients with private, government, or unknown insurance status, the percentage of uninsured patients decreases from 51% in 2010 to 16% in 2020.
Although there have been several studies utilizing the NCDB to evaluate the impact of the ACA Medicaid expansion on the staging of various types of cancer, its effect on melanoma is under-studied. This study indicated a positive impact of Medicaid expansion on melanoma stage at presentation. Trend analysis revealed that stage IV melanoma decreased in MES whereas it increased in non-MES from 2010 to 2020. DID analysis additionally supported these results of decreased tumor stage in MES and increased tumor stage in non-MES. The decrease in tumor stage between MES and non-MES before and after expansion was statistically significant. These findings are in accordance with the findings of prior research studies which measured changes in insurance and cancer staging after Medicaid expansion with most studies indicating similar results of greater insurance coverage and earlier tumor staging [
5,
6,
7,
8]. The results suggest that improved access to healthcare services from the Medicaid expansion can help facilitate the diagnosis of early-stage melanoma for patients of low socioeconomic status due to greater accessibility to healthcare services. Prior literature has indicated that greater screening through regular self-skin examinations and dermatology discussions have the potential to improve melanoma care [
12,
13]. Additionally, studies have revealed an association between dermatologist skin examinations with earlier melanoma diagnosis, supporting that increased accessibility to healthcare providers and screening examinations with the Medicaid expansion can facilitate earlier diagnosis of melanomas in low-income populations [
14,
15,
16,
17]. Overall, the Medicaid expansion appears to provide early diagnosis of melanomas in marginalized communities that are disproportionately affected by melanoma, indicating evidence of the intended benefit of the ACA to provide better care to vulnerable low-income populations.
To our knowledge, our study is the first to examine the association of melanoma patient outcomes with 3-year mortality, use of immunotherapy, and primary surgery after implementation of the ACA’s Medicaid expansion. Our analysis showed that the decrease in 3-year mortality was statistically significant which suggests that Medicaid expansion positively impacts melanoma patient outcomes. Several studies have indicated that early diagnosis provides more curative treatment options and better prognosis for various cancers, including early-stage melanoma which is concordant to this study’s findings in decreased 3-year mortality [
18,
19,
20,
21,
22]. These findings suggest that Medicaid expansion has the potential to improve the outcomes of melanoma patients by promoting earlier diagnosis.
The use of immunotherapy in MES was significantly higher than non-MES after expansion (47% vs 41%, p<0.001); however, DID analysis did not show a statistically significant difference in the use of immunotherapy in MES and non-MES before and after expansion. There was also no association of significant difference in primary surgery noted on the DID analysis. The lack of statistically significant difference in use of immunotherapy and primary surgery can be attributed to various factors: patients may face additional barriers to receiving melanoma care, such as transportation issues, lack of health education and awareness of treatment options, harmful health-seeking behaviors/attitudes of newly insured patients, or further socioeconomic barriers [
23,
24]. Moreover, the capacity of healthcare facilities and availability of specialists to perform melanoma surgeries or provide immunotherapy may not have increased proportionally with the expanded Medicaid coverage which may contribute to the lack of significance in the use of immunotherapy and primary surgery in our results. Lastly, after expansion, tumor staging may have changed where immunotherapy or surgery is not indicated, contributing to the lack of significance in immunotherapy and primary surgery before and after Medicaid expansion. Further investigation is required to evaluate the factors impacting the care of melanoma after Medicaid expansion.
Several limitations should be considered in this study. Firstly, this was a retrospective observational study. Although the DID model was adjusted for several confounders, all potential confounding variables could not be controlled which may affect the observed associations between variables and outcomes in undefined ways. Additionally, the NCDB is facility-based and only includes data from accredited cancer centers, as such it may not be representative of the entire population treated in non-accredited or smaller hospitals; thus, prevalence or incidence rates of the general population cannot be determined. The NCDB also does not monitor continuity or disruption of insurance coverage; therefore, the effects of insurance interruption on melanoma stage at presentation cannot be assessed. Additionally, the effects of Medicaid expansion on the young-adult population are unknown as the NCDB did not provide information on the expansion status of patients less than 40 years old. Lastly, it is important to consider other healthcare policies enacted during the study period and their potential impact on melanoma tumor stage and care. Policies mandating private insurance carriers to fully cover preventive services or various state-specific policies may have contributed to the earlier melanoma diagnoses observed in this study [
25,
26]. While these factors cannot be controlled, it is unlikely that they are solely responsible for the melanoma staging trends and should not deter from the major findings of this study.
5. Conclusions
This study revealed the positive impact of the ACA’s Medicaid expansion on melanoma stage at presentation and suggest improved access to healthcare services can facilitate the diagnosis of early-stage melanoma and decrease mortality rate. The findings illustrate the impact of public health policies in addressing the health disparities in vulnerable low socioeconomic populations through greater accessibility to healthcare services and increased insurance coverage. Despite these findings, there are still 16% of patients without insurance in 2020, indicating that continued effort is needed to improve the outcomes of these uninsured patients.
Author Contributions
Conceptualization, software, methodology, formal analysis, resources, data curation, supervision, and project administration, Y.X.; validation and investigation, R.M., H.T.W. and Y.X.; writing—original draft preparation, R.M., H.T.W., S.W. and Y.X.; writing—review and editing, All authors; visualization, R.M., H.T.W., S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the use of de-identified, publicly available data from the National Cancer Database, which does not involve direct interaction with human subjects.
Informed Consent Statement
Patient consent was waived due to the use of de-identified data from the National Cancer Database, which does not involve identifiable patient information or direct interaction with human subjects.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, U.; Koroukian, S.; Statler, A.; Rose, J. The Effect of Medicaid Expansion among Adults from Low-income Communities on Stage at Diagnosis in Those with Screening-amenable Cancers. Cancer 2020, 126, 4209–4219. [CrossRef]
- Fedewa, S.A.; Yabroff, K.R.; Smith, R.A.; Goding Sauer, A.; Han, X.; Jemal, A. Changes in Breast and Colorectal Cancer Screening After Medicaid Expansion Under the Affordable Care Act. Am. J. Prev. Med. 2019, 57, 3–12. [CrossRef]
- Lee, G.; Dee, E.C.; Orav, E.J.; Kim, D.W.; Nguyen, P.L.; Wright, A.A.; Lam, M.B. Association of Medicaid Expansion and Insurance Status, Cancer Stage, Treatment and Mortality among Patients with Cervical Cancer. Cancer Rep. 2021, 4, e1407. [CrossRef]
- Hendryx, M.; Luo, J. Increased Cancer Screening for Low-Income Adults Under the Affordable Care Act Medicaid Expansion. Med. Care 2018, 56, 944–949. [CrossRef]
- Zhao, J.; Mao, Z.; Fedewa, S.A.; Nogueira, L.; Yabroff, K.R.; Jemal, A.; Han, X. The Affordable Care Act and Access to Care across the Cancer Control Continuum: A Review at 10 Years. CA. Cancer J. Clin. 2020, 70, 165–181. [CrossRef]
- Snyder, R.A.; Hu, C.; DiBrito, S.R.; Chang, G.J. Association of Medicaid Expansion with Racial Disparities in Cancer Stage at Presentation. Cancer 2022, 128, 3340–3351. [CrossRef]
- Osazuwa-Peters, N.; Barnes, J.M.; Megwalu, U.; Adjei Boakye, E.; Johnston, K.J.; Gaubatz, M.E.; Johnson, K.J.; Panth, N.; Sethi, R.K.V.; Varvares, M.A. State Medicaid Expansion Status, Insurance Coverage and Stage at Diagnosis in Head and Neck Cancer Patients. Oral Oncol. 2020, 110, 104870. [CrossRef]
- Hoehn, R.S.; Rieser, C.J.; Phelos, H.; Sabik, L.M.; Nassour, I.; Paniccia, A.; Zureikat, A.H.; Tohme, S.T. Association Between Medicaid Expansion and Diagnosis and Management of Colon Cancer. J. Am. Coll. Surg. 2021, 232, 146-156e1. [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA. Cancer J. Clin. 2019, 69, 7–34. [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. 2024.
- Breitbart, E.W.; Waldmann, A.; Nolte, S.; Capellaro, M.; Greinert, R.; Volkmer, B.; Katalinic, A. Systematic Skin Cancer Screening in Northern Germany. J. Am. Acad. Dermatol. 2012, 66, 201–211. [CrossRef]
- Brunssen, A.; Waldmann, A.; Eisemann, N.; Katalinic, A. Impact of Skin Cancer Screening and Secondary Prevention Campaigns on Skin Cancer Incidence and Mortality: A Systematic Review. J. Am. Acad. Dermatol. 2017, 76, 129-139.e10. [CrossRef]
- Kovalyshyn, I. The Impact of Physician Screening on Melanoma Detection. Arch. Dermatol. 2011, 147, 1269. [CrossRef]
- Swetter, S.M.; Pollitt, R.A.; Johnson, T.M.; Brooks, D.R.; Geller, A.C. Behavioral Determinants of Successful Early Melanoma Detection: Role of Self and Physician Skin Examination. Cancer 2012, 118, 3725–3734. [CrossRef]
- Ercia, A. The Impact of the Affordable Care Act on Patient Coverage and Access to Care: Perspectives from FQHC Administrators in Arizona, California and Texas. BMC Health Serv. Res. 2021, 21, 920. [CrossRef]
- Marchetti, M.A.; Adamson, A.S.; Halpern, A.C. Melanoma and Racial Health Disparities in Black Individuals—Facts, Fallacies, and Fixes. JAMA Dermatol. 2021, 157, 1031. [CrossRef]
- Weinstock, M.A. Early Detection of Melanoma. JAMA 2000, 284, 886. [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma Staging: Evidence-based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA. Cancer J. Clin. 2017, 67, 472–492. [CrossRef]
- Buccafusca, G.; Proserpio, I.; Tralongo, A.C.; Rametta Giuliano, S.; Tralongo, P. Early Colorectal Cancer: Diagnosis, Treatment and Survivorship Care. Crit. Rev. Oncol. Hematol. 2019, 136, 20–30. [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early Detection of Cancer. Science 2022, 375, eaay9040. [CrossRef]
- Henrikson, N.B.; Ivlev, I.; Blasi, P.R.; Nguyen, M.B.; Senger, C.A.; Perdue, L.A.; Lin, J.S. Skin Cancer Screening: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2023, 329, 1296. [CrossRef]
- Zheng, D.X.; Cwalina, T.B.; Mulligan, K.M.; Levoska, M.A.; Scott, J.F.; Mostaghimi, A. Prevalence and Predictors of Transportation Barriers to Health Care among US Adults with a History of Skin Cancer. J. Am. Acad. Dermatol. 2023, 88, 201–203. [CrossRef]
- Cortez, J.L.; Vasquez, J.; Wei, M.L. The Impact of Demographics, Socioeconomics, and Health Care Access on Melanoma Outcomes. J. Am. Acad. Dermatol. 2021, 84, 1677–1683. [CrossRef]
- Obama, B. United States Health Care Reform: Progress to Date and Next Steps. JAMA 2016, 316, 525. [CrossRef]
- Levine, D.M.; Chalasani, R.; Linder, J.A.; Landon, B.E. Association of the Patient Protection and Affordable Care Act With Ambulatory Quality, Patient Experience, Utilization, and Cost, 2014-2016. JAMA Netw. Open 2022, 5, e2218167. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).