1. Introduction
Prenatal diagnosis holds a very important place in modern obstetric care since it allows to monitor the health status of the mother and fetus at different stages of pregnancy. In general, prenatal diagnosis is used in the detection of defects in the fetus and to develop a plan of treatment before or after birth. It also helps in identifying the causes that may be harmful to future pregnancies [
1]. Several invasive and non-invasive methods are employed at specific stages in the pregnancy to foresee an exact diagnosis. Ultrasonography has conventionally remained a fundamental, encompassing imaging methodology widely used to visualize the fetus to gain real-time information related to fetal anatomy, growth, and development. Thus far, it is popular for the assessment of morphology and vascularity and lacks the wide use of advanced imaging biomarkers it may provide.
Sonoelastography is one of the new ultrasound-based modalities, which measures the stiffness of tissues in vivo. It promises to advance the capabilities of conventional ultrasound by measuring the elasticity or stiffness of tissues through its deformation upon mechanically or acoustically induced pressure [
2]. It is based on the pressure force applied to the targeted area and monitors the tissue displacement. The degree of displacement reflects the elasticity or stiffness of the tissue, where softer tissue deforms more easily. These differences are presented as color images or even as quantitative data that also enables clinicians to establish tissues’ biochemical properties. There are three major types of sonoelastography: shear wave elastography (SWE), strain elastography (SE), and transient elastography (TE) [
2]. Strain elastography evaluates the degree of tissue deformation in response to an applied physical force by the operator, while SWE measures the speed at which transverse waves, induced via acoustic pulses, travel through tissue, with faster wave propagation indicating stiffer tissues. Both methods offer real-time, dynamic information, which is particularly valuable for evaluating soft tissues that are difficult to assess with standard imaging techniques. Meanwhile, TE, a non-imaging method, is employed when a motor vibrates the skin, causing a passing distortion in the tissue, and as that distortion moves deeper into the body, a quantitative one-dimensional image of tissue stiffness is obtained [
2]. The latter type is popular in hepatological tissue assessment and seldom used in gynecological or obstetric assessment. The role of sonoelastography has been widely established for the assessment of breast, liver and thyroid. However, the literature in maternal and fetal assessment is relatively smaller, albeit growing. Hence, it is significant to review the current status of the clinical applications of sonoelastography to understand and determine the direction for future prenatal diagnostics.
The purpose of this review is to discuss the rapidly increasing use of sonoelastography in prenatal diagnosis and to elucidate its potential role in assessing maternal and fetal health. Current studies have great promise regarding sonoelastography in various fields related to prenatal care, such as uterine and cervical stiffness measurement, abnormal placentas, fetal organs, and soft tissues [
3,
4,
5]. Sonoelastography may sensitize the early detection of complications like preterm labor, placental insufficiency, and fetal growth restrictions due to its ability to detect even slight changes in tissue elasticity. This review sheds light on the elastic properties of various related organs and tissues. It also discusses how this advanced imaging technique may improve maternal and fetal outcomes
.
2. Cervix Assessment
Cervix Elastic Properties
The cervix plays a very important role in conception, maintaining the pregnancy, and timely delivery of the fetus by acting as a structural barrier that holds the fetus in place within the uterus [
6]. It is composed of a complex mixture of smooth muscle fibers, collagen, and extracellular matrix proteins, all of which contribute to its biomechanical properties [
7]. During pregnancy, the cervix undergoes a sequence of four distinct stages, each marked by specific alterations in the alignment of collagen layers: (a) an initial softening phase; (b) a phase of shortening and pronounced softening known as ‘ripening’; (c) active dilation; and (d) recovery post-delivery [
8]. The initial softening is a gradual process, typically unfolding over days to weeks, while the ripening phase accelerates, occurring over several hours to days. These progressive changes are essential for enabling the fetus’s passage through the cervical canal.
Traditional methods for assessing the cervix, such as internal examination (e.g., Bishop score) and transvaginal ultrasonography, provide limited information on the tissue’s biomechanical properties. While ultrasound can measure cervical length and detect some degree of cervical softening, it does not assess tissue elasticity with high precision. This is where sonoelastography presents a significant advantage by examining biomechanical cervical changes in real-time.
Recently, Thomsen et al. proposed specific recommendations for strain elastography of the uterine cervix, emphasizing factors like probe placement and region of interest (ROI) selection [
9]. They found that variations in probe-to-ROI distance and angle affected strain values, with posterior cervical assessments yielding consistently lower values due to tissue interference. These guidelines aim to standardize elastography practices, enhancing measurement reliability and thereby improving its clinical relevance for assessing cervical integrity.
Predictive Value of Cervical Elastography for Preterm Birth
Cervical elastography has emerged as a promising technique in assessing the risk of preterm birth (PTB), offering a quantitative means to evaluate cervical stiffness, a factor central to cervical insufficiency and premature delivery. Across various studies, elastographic measurements, specifically strain values and strain ratios, have consistently demonstrated potential as predictors of PTB, complementing or even enhancing traditional methods like cervical length measurement.
A foundational study in 2014 demonstrated that quantitative elastography could distinguish changes in cervical stiffness, showing that elevated strain values correlated significantly with PTB. In this study, a strain threshold was identified, where values exceeding 0.89 were associated with a higher likelihood of PTB, with a specificity of 86% and moderate sensitivity of 59% [
10]. This finding supported the value of elastography as a PTB predictor and motivated further research to refine and expand its clinical application.
Subsequent studies highlighted the value of elastography in both high-risk and low-risk pregnancies. For patients with short cervical lengths, elastographic assessments at the internal os indicated that softer cervical tissue predicted higher PTB risk. Sensitivity and specificity reached 82.2% and 75%, respectively, suggesting that elastography, when used alongside cervical length, provides a more robust prediction model than cervical length alone [
11]. For low-risk pregnancies, elastography was shown to improve prediction capabilities, particularly during the second trimester. The area under the curve (AUC) for elastographic measurements at the internal os in this group was 0.730, underscoring elastography’s role in identifying potential PTB in cases where traditional risk factors may not be evident [
12].
Further advancing this field, a recent study by Natarajan et al. incorporated multiple parameters, including cervical length, anterior uterocervical angle, and cervical strain values. This integrative approach showed that combining cervical strain with other parameters enhanced the predictive accuracy of PTB, capturing risk factors that might be missed if only a single parameter, such as cervical length, were used [
13]. Another study underscored the reliability of cervical strain ratios, with findings indicating that higher strain at the internal and external os corresponded with increased PTB likelihood [
14]. This study supported the notion that cervical softness, as quantified by strain ratios, could serve as an early indicator of premature cervical ripening, a key contributor to PTB.
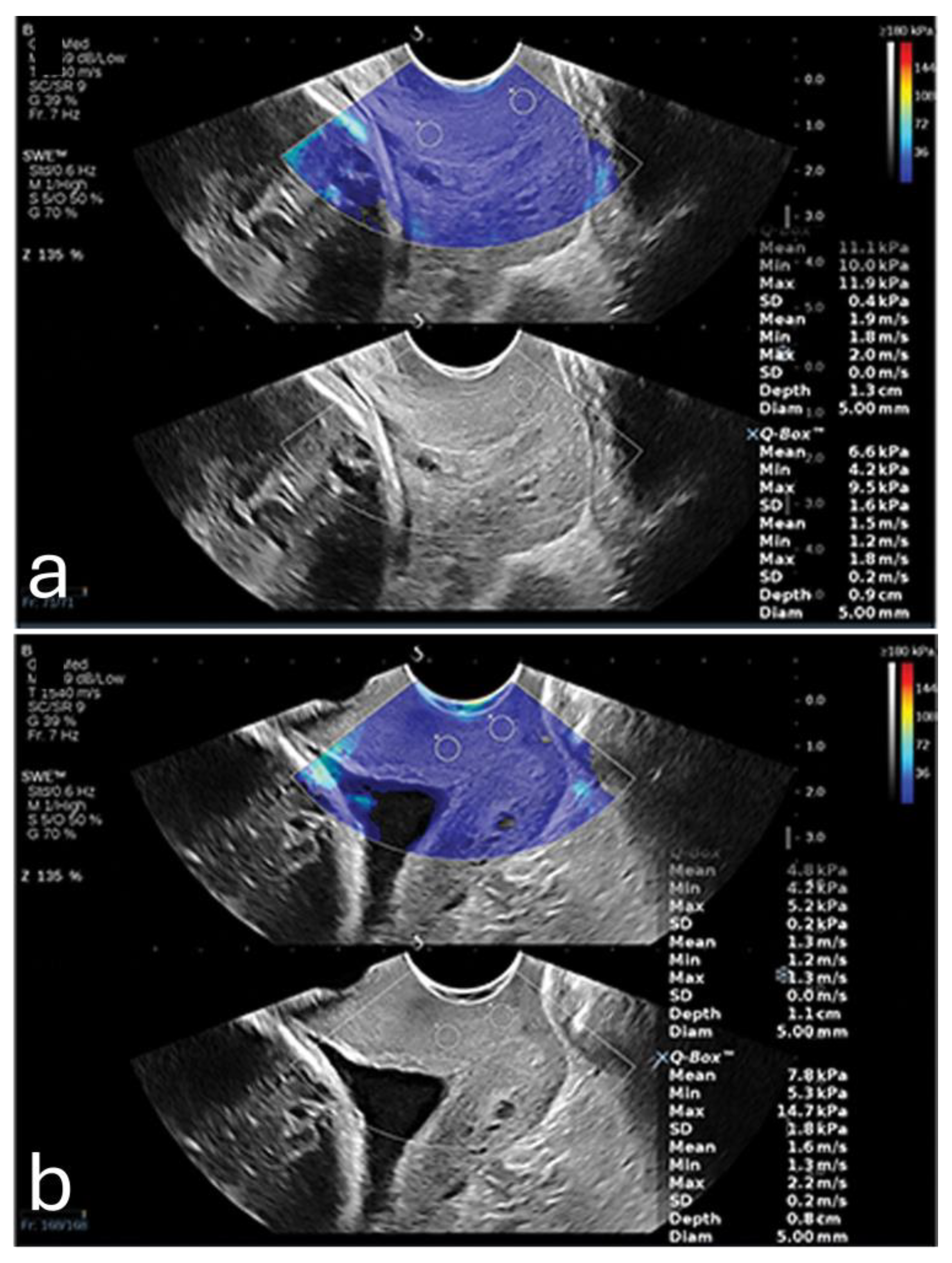
Furthermore, a 2024 study demonstrated that SWE could precisely measure tissue elasticity at specific cervical points, such as the inner and outer cervical os, yielding predictive values higher than cervical length alone. When incorporated into a multifactorial model that included cervical length and pregnancy-related comorbidities, SWE achieved an AUC of 0.892, with a sensitivity of 86.7% and specificity of 79.2%. It also showed that SWE values were lower in PTB (8.76 kPa ± 3.04 kPa) in comparison to full-term pregnancies (14.95 kPa ± 8.21 kPa). All of these positive findings were only valid when the samples where acquired from the anterior or posterior lip of the internal os. An example is shown in
Figure 1. This combined approach shows promise for developing a more comprehensive PTB risk model that integrates both elastographic data and clinical factors [
15].
Predicting Successful Labor Induction
In predicting labor induction success, studies on cervical elastography reveal mixed results on its utility and accuracy compared to traditional assessment tools like the Bishop score and cervical length.
Pereira et al. assessed the potential of elastographic scoring and the angle of progression (AOP) as predictors for labor induction outcomes [
16]. They found significant correlations between cervical length, AOP, and elastography; however, only cervical length and parity proved to be strong predictors of vaginal delivery success and induction-to-delivery intervals. This study suggests that elastographic scores, specifically at the internal os, may have limited predictive power compared to cervical length, which remained a primary independent predictor of successful induction outcomes.
Fruscalzo et al. examined the role of quantitative elastography, comparing its predictive accuracy against the Bishop score and cervical length [
17]. Their findings indicated that cervical tissue strain (TS) was a modest predictor of labor induction failure. Although elastography slightly improved the accuracy of predicting an early response to labor induction, it was not significantly better than the Bishop score or cervical length alone. These findings imply that elastography might serve as an adjunctive tool rather than a primary method for assessing induction readiness.
Londero et al. conducted a systematic review and meta-analysis, comparing cervical elastography, cervical length, and the Bishop score [
18]. The analysis revealed that both cervical elastography and cervical length had a higher diagnostic odds ratio (DOR) for predicting successful vaginal delivery compared to the Bishop score, suggesting that elastography provides a similar level of predictive reliability as cervical length. This reinforces the notion that elastography can be a valuable, objective measure, particularly in cases where cervical length alone is insufficient.
Lu et al. demonstrated that inner cervical SWE values were independent predictors of overall cesarean section likelihood and specifically predicted cases where labor failed to reach the active phase [
19]. Models incorporating SWE and cervical length yielded significantly higher predictive accuracy than models based solely on the Bishop score, marking SWE as a promising technique for enhancing induction success assessments.
These findings collectively suggest that while cervical elastography may not replace existing methods like cervical length and the Bishop score, it has value as an adjunct tool, particularly in cases where objective cervical stiffness measurements could provide further insights.
Assessing Cervical Elasticity and Stiffness Across Gestational Ages
In evaluating cervical elasticity and stiffness across gestational ages, several studies utilized elastography techniques to track changes in cervical softening throughout pregnancy, with significant emphasis on both feasibility and correlation with gestational age.
One of the early studies by Fruscalzo et al. confirmed the reliability of cervical elastography in the late first and second trimesters, demonstrating a high interobserver agreement, which suggests elastography’s reproducibility during early and mid-pregnancy [
20]. A later study by the same group revealed that cervical tissue stiffness is inversely correlated with gestational age [
21]. This correlation was particularly strong in the third trimester, indicating elastography’s potential to non-invasively monitor cervical readiness for labor.
Hernandez-Andrade et al. observed that cervical strain measurements showed significant variation across different gestational ages and positions within the cervix, suggesting a progressive softening that likely correlates with cervical length changes, particularly as pregnancy advances [
22]. Ono et al. found significant gestational age-related decreases in SWE stiffness at specific cervical points, emphasizing that the upper part of the cervix might serve as a more sensitive indicator of cervical softening compared to the lower part [
23]. Duan et al. corroborated these findings with SWE, reporting that the cervical stiffness gradient from the inner to the external parts correlated weakly but positively with cervical length, and decreased stiffness was particularly noticeable in multiparous women during later trimesters [
24]. Further, Woo et al. confirmed these observations, showing that SWE reliably measures cervical stiffness across all trimesters, with the internal os providing the most consistent readings, reinforcing its suitability for longitudinal monitoring [
25].
Nguyen-Hoang et al. conducted a longitudinal analysis, revealing that cervical softening generally precedes cervical shortening, particularly in the first trimester, thus identifying SWE as a promising method for early prediction of cervical changes that may indicate labor onset [
26]. Hu et al. introduced a quantitative cervical elastography system, which combines stress measurement with traditional ultrasound, finding that it can produce consistent elasticity measures across sessions, potentially improving the clinical applicability of cervical elastography [
27].
Evaluating Cervical Integrity and Cervical Competence
Several studies demonstrated promising results for sonoelastography for cervical insufficiency and competence [
28,
29,
30,
31,
32]. Chen et al. investigated cervical elastography in women with prior cervical insufficiency during the first trimester. They observed that the anterior cervical lip in these women had significantly higher strain rates, indicating softer tissue, compared to those without such a history [
32]. Additionally, cervical length and endocervical canal width were both notable predictors, with shorter lengths and wider canals associated with insufficiency. These findings suggest that early elastographic assessments may help in identifying women at higher risk for cervical incompetence, potentially guiding early interventions.
Mlodawski et al. focused on the repeatability and reproducibility of quantitative elastography in the third trimester using proprietary ‘E-Cervix’ software [
31]. Their findings underscored good intra- and interobserver reliability for elastographic parameters, including elasticity index and strain at the internal and external os. This high reproducibility highlights E-Cervix as a practical tool for consistently evaluating cervical integrity, which may be valuable in longitudinal assessments across gestation.
Qu et al. performed a systematic review and meta-analysis on gray-scale ultrasound combined with SWE for evaluating cervical integrity in primiparous women [
33]. They found that SWE reliably measured tissue elasticity in both anterior and posterior cervical lips, although posterior lip values were generally lower due to anatomical variances. This comprehensive analysis reinforced SWE’s capacity to provide consistent, quantitative insights into cervical stiffness, which could support broader clinical applications in predicting cervical competency.
3. Placental Assessment
Plcental Elastic Properties
The placenta’s biomechanical properties, specifically its elasticity, are rooted in its unique anatomical and physiological structure, which evolves throughout gestation to support fetal development [
34]. The elasticity of placental tissue largely reflects the organization and integrity of its core structural components, including the chorionic villi and the extensive vascular network. These structures facilitate the placenta’s role in nutrient and gas exchange, requiring flexibility and adaptability to meet the growing demands of the fetus over time [
35].
One of the primary factors influencing placental elasticity is the layered arrangement of the chorionic villi, which are densely populated with capillaries and responsible for mediating maternal-fetal nutrient transfer. As trophoblastic cells invade and remodel maternal spiral arteries early in pregnancy, the placental vascular network expands, allowing the tissue to maintain elasticity and accommodate increased blood flow. This vascular adaptation not only supports fetal needs but also underpins the biomechanical softness that elastographic assessments detect in healthy placentas [
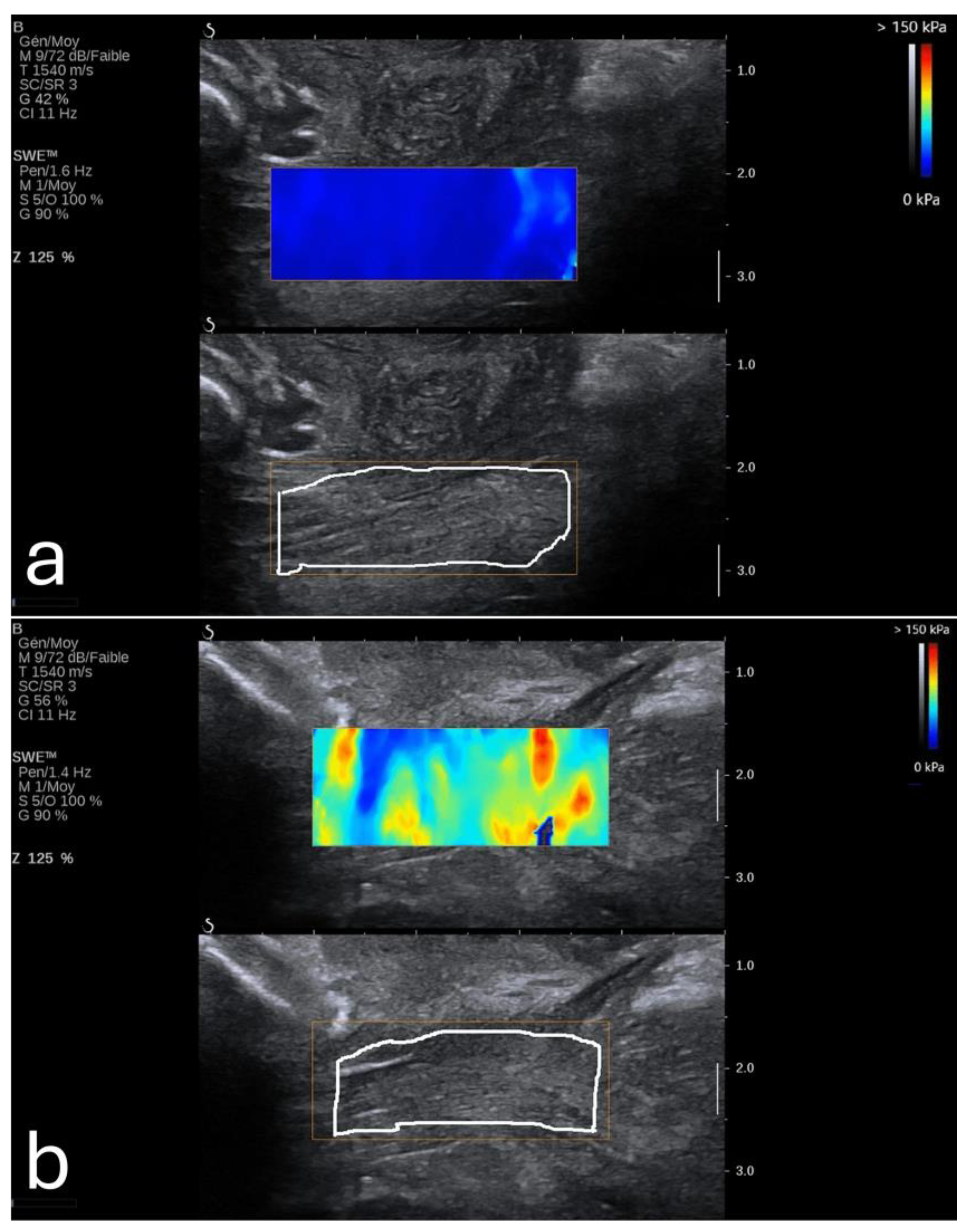
36]. This softness becomes altered in cases of placenta percreta, where the placenta becomes significantly stiffer (
Figure 2) [
37].
Research also shows that placental elasticity naturally declines with advancing gestation, a process associated with maturation of the vascular and stromal components. Studies highlight that this softening results from the structural evolution of the placental tissue, wherein the density and arrangement of the villi and blood vessels continue to shift to optimize efficiency [
36]. This progressive change is essential for maintaining placental functionality, as it ensures adequate resilience and flexibility to sustain fetal growth and health.
In normal pregnancies, these elastic properties are well-regulated, enabling a balance between structural stability and the adaptability necessary for dynamic blood flow adjustments. By understanding the baseline elasticity in healthy placental tissue, deviations observed in elastographic measurements can be more accurately interpreted, providing valuable insight into potential complications such as preeclampsia or intrauterine growth restriction [
38].
Predicting and Monitoring Preeclampsia
Multiple studies demonstrate that placental stiffness values are significantly higher in pregnancies complicated by preeclampsia, with predictive power noted from the first trimester [
39,
40,
41,
42,
43].
Killic et al. were one of the first researchers that found that SWE measurements could effectively distinguish between preeclamptic and normal placentas, reporting a significant increase in stiffness across all regions in preeclamptic cases [
41]. The highest differences appeared at the fetal-facing central placental region, where SWE values reached a median of 21 kPa in preeclamptic cases versus 4 kPa in controls. The authors identified a diagnostic cutoff of 7.35 kPa, achieving 88% sensitivity and specificity, suggesting SWE’s utility in differentiating at-risk cases as early as the second trimester.
Cimsit et al. found no statistically significant variations in the elastic modulus values between areas, although they did find connections concerning increases in the placental modulus values of respective PE groups [
44]. Later, Fujita et al. conducted a study to evaluate whether SWE could serve as a marker in the first trimester [
43]. They found that SWE measurements in high-risk patients were significantly elevated, with an optimal cutoff of 1.188 m/s for detecting preeclampsia risk. This study highlighted that increased placental stiffness might correlate with early pathophysiological changes associated with the condition, supporting SWE’s potential for early detection in routine screenings.
Further support for SWE’s use in early detection comes from Sirinoglu et al. , [
42] who explored SWE in low-risk pregnancies during the first trimester. They identified an SWE cutoff of 7.43 kPa, achieving 88% sensitivity and 78% specificity, suggesting that SWE can reliably predict preeclampsia even in the absence of traditional risk factors. This study suggests that SWE might be advantageous as a primary screening tool, reducing reliance on other biochemical markers that require lab processing.
In a recent study by Tian et al. where SWE was combined with 3-dimensional power Doppler indices (3D-PDI) to test a potential improved predictive accuracy [
39]. They found that combining SWE with vascularization indices (e.g., flow index and vascularization flow index) improved the sensitivity and specificity for preeclampsia prediction compared to SWE alone. Another recent study by Singh et al. assessed SWE in high-risk pregnancies at mid-gestation, revealing significantly higher elasticity values in preeclamptic placentas compared to controls, especially at the central and peripheral placental regions [
40]. Their study suggested a diagnostic threshold of 13.1 kPa, achieving a high sensitivity of 95.2% and specificity of 92.8%, which indicates SWE’s potential as an adjunctive screening measure for high-risk pregnancies.
Detecting Fetal Growth Restriction
For detecting fetal growth restriction (FGR) through placental elasticity, few studies showcased sonoelastography as an emerging, promising approach [
45,
46,
47]. Each contributes nuanced insights into placental stiffness as a marker for FGR, emphasizing elasticity differences between FGR and healthy pregnancies, and the correlation with perinatal outcomes.
Habibi et al. utilized SWE to assess in vivo placental elasticity, identifying significantly higher stiffness values in FGR cases. This increase in placental stiffness—recorded at both central and peripheral regions—correlated with adverse outcomes, including lower birth weights and Apgar scores [
46]. This study highlights SWE’s diagnostic capacity, suggesting its role as a supplementary tool to traditional Doppler measures, offering quantitative insights into placental health linked to FGR.
Akbas et al. conduced a case-control study where they revealed a distinct stiffness increase in FGR cases versus healthy controls, with stiffness values showing a strong correlation with Doppler indices and perinatal risks [
47]. Notably, this study underscores that elevated placental elasticity values align with compromised placental function, reinforcing the value of SWE as a potential early diagnostic tool for FGR when standard ultrasound measures might miss early-stage abnormalities.
In a broader recent study, Ansar et al. examined SWE’s utility not only for detecting FGR but also for staging its severity, noting progressive stiffness elevations from mild to severe cases [
45]. This study reinforces that increased stiffness mirrors worsening placental pathology, suggesting that SWE may be capable of staging FGR and enabling tailored monitoring for higher-risk pregnancies. This study showed that SWE could offer a more direct assessment of placental tissue alterations rather than indirect signs alone.
4. Pelvic Floor Muscles Assessment
Elastic Properties of the Pelvic Floor Muscles
The pelvic floor muscles (PFM), particularly the levator ani muscle (LAM) and the external anal sphincter (EAS), are essential for maintaining pelvic support and enabling control over movement within the pelvic region. These muscles exhibit a unique combination of elasticity and viscoelasticity, which allows them to withstand and adapt to various physiological forces, including those exerted during pregnancy, childbirth, and daily physical activities [
48].
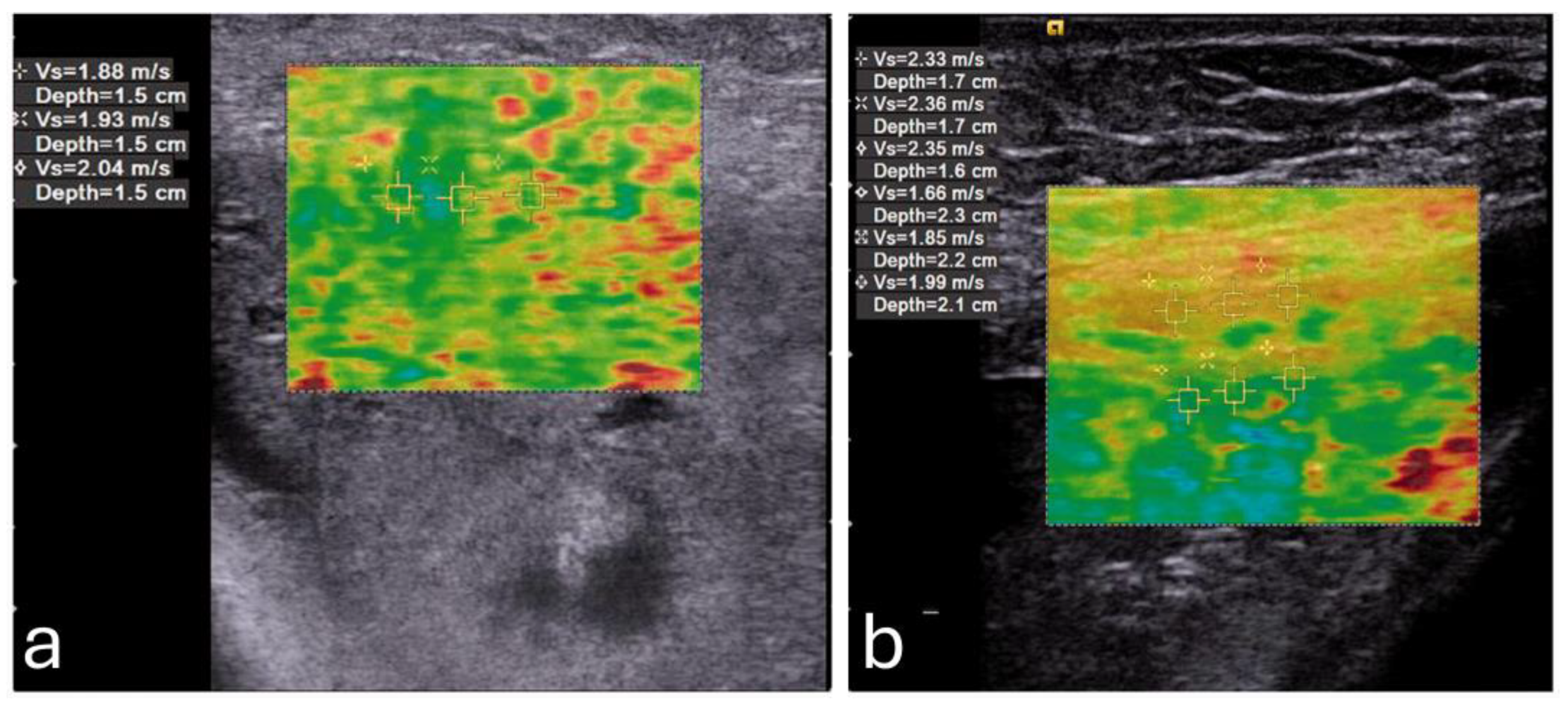
Elasticity in the PFM enables them to respond dynamically to increased abdominal pressure and other physical stresses. For instance, during actions such as the Valsalva maneuver or maximal contraction, the LAM and EAS demonstrate considerable elasticity, which provides resilience against deformation (
Figure 3) [
49]. This quality is particularly critical for maintaining the integrity of the pelvic organs and facilitating controlled movement.
The viscoelastic properties of these muscles also play a crucial role. Viscoelasticity allows the muscles to exhibit both solid-like and fluid-like behavior under stress, meaning they can absorb and dissipate energy effectively. This dual response is vital for protecting the pelvic structures from injury and supporting the body’s functional demands over time [
50]. The PFM’s ability to adjust stiffness levels across different states—such as resting, contracting, or stretching—also highlights their adaptability, which is influenced by factors like hormonal changes during pregnancy and biomechanical demands [
51].
It is crucial, however, to note that examining muscles using sonoelastography should be performed using the correct technique due to the anisotropic nature of these muscles. In such instances, the probe should be oriented along the muscle fibers to ensure correct dissipation of the transverse shear waves [
52].
Clinical Applications
Studies utilizing SWE have demonstrated that quantifying PFM elasticity, particularly in LAM and EAS, provides clinicians with valuable insights into muscle integrity, readiness for childbirth, and postpartum recovery.
A key application of PFM elasticity assessment lies in predicting childbirth-related injuries. Research has shown that increased stiffness in the EAS is associated with a lower risk of perineal tears and obstetric anal sphincter injuries during vaginal delivery. For instance, Gachon et al. conducted a study indicating that women with a stiffer EAS in late pregnancy had a reduced likelihood of experiencing perineal tears [
54]. This finding suggests that SWE measurements can serve as early indicators of trauma risk, guiding clinicians in implementing preventative strategies or opting for modified delivery management techniques to reduce injury [
54,
55].
Postpartum recovery assessment is another significant application of SWE in clinical practice. Okada et al. demonstrated that elasticity in the LAM differed significantly between women who underwent vaginal delivery and those who had cesarean sections [
55]. Their study revealed that postpartum women who delivered vaginally exhibited lower LAM elasticity, with an average elasticity difference of 28.2 kPa (p = 0.0036), potentially indicating muscle relaxation or injury. This measurable reduction in elasticity serves as a reliable metric for evaluating postpartum pelvic health and identifying cases where pelvic floor rehabilitation may be warranted to prevent conditions like pelvic organ prolapse and urinary incontinence [
56].
For patients with symptoms of pelvic floor dysfunction, including incontinence and prolapse, SWE assessments provided an objective means of quantifying muscle elasticity loss, often a characteristic of such disorders. Davidson et al., for example, found that the active force and stiffness of the LAM varied significantly during pregnancy and postpartum (p = 0.002). This highlights SWE’s usefulness in monitoring muscle function over time. Such tailored rehabilitation may accelerate recovery and improve the efficacy of physical therapy interventions for those with pelvic floor dysfunction.
SWE’s capability extends to monitoring PFM functionality across various conditions. The technology allows elasticity to be measured under different states—such as at rest, during Valsalva, or under maximal contraction—providing a comprehensive view of muscle adaptability. This feature is particularly valuable in cases where gradual muscle weakening or functional impairments are suspected, allowing clinicians to track changes in muscle strength and elasticity that might necessitate early intervention.
In addition to its practical applications, SWE-based assessments of PFM elasticity are valuable in research, advancing our understanding of pelvic floor biomechanics. Studies on elasticity provide a foundational understanding of how pelvic floor muscle characteristics impact obstetric outcomes, inform prevention strategies, and support therapeutic approaches in maternal health. Davidson et al.’s work contributes to this body of knowledge, showing that active force and elasticity measurements could enhance the understanding of how PFM resilience is impacted by the demands of pregnancy and childbirth [
57].
5. Fetal Assessment
The assessment of fetal tissue elasticity using sonoelastography may provide a unique window into fetal development, offering both quantitative insights into tissue properties and valuable clinical markers for identifying developmental progress and potential complications. By measuring the elastic properties of key organs such as the lungs, liver, and brain, clinicians could potentially evaluate organ maturity, detect potential anomalies, and make informed decisions regarding perinatal care [
58,
59,
60,
61,
62,
63,
64,
65,
66].
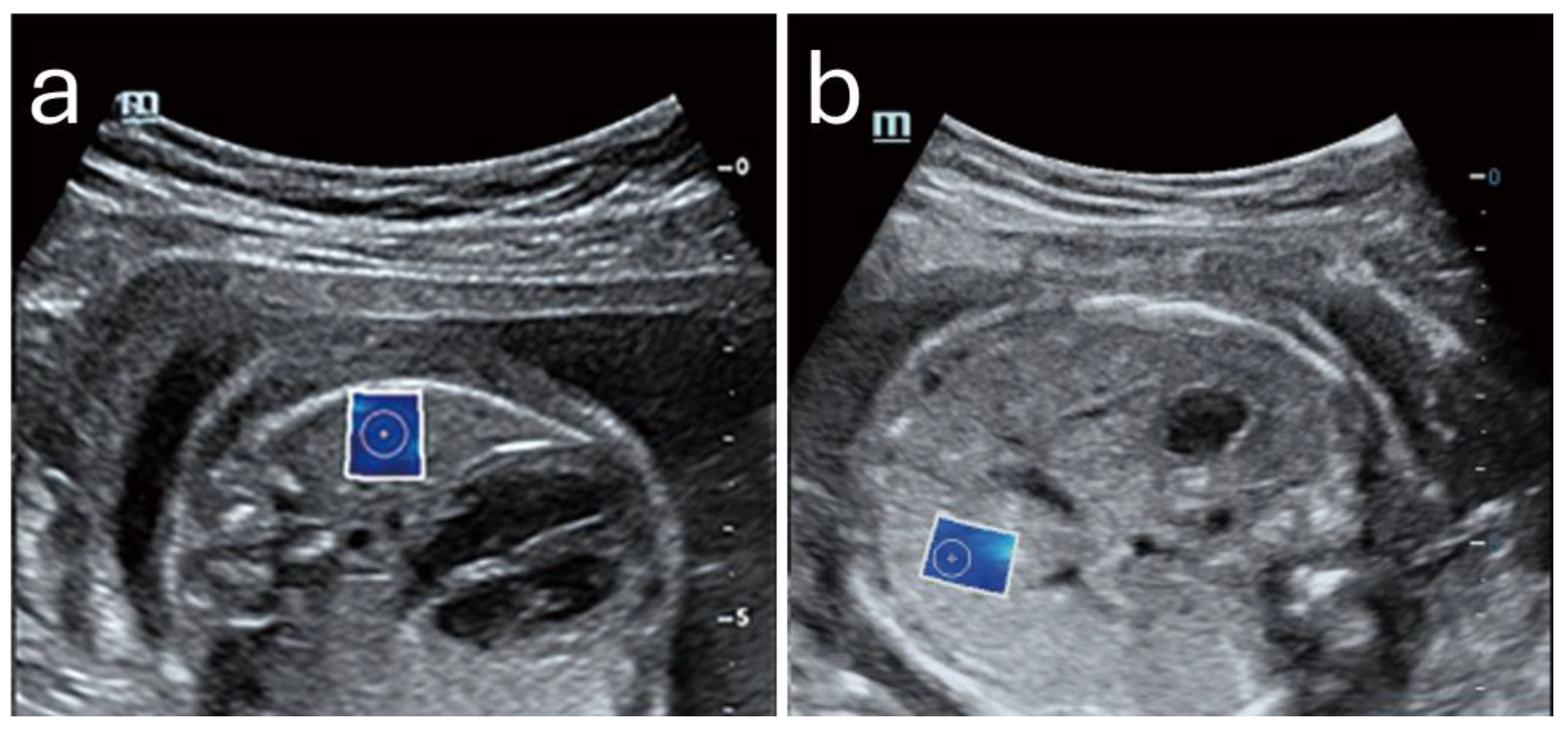
Figure 4 shows an example of normal lung and liver in a fetus.
Lung Elasticity and Maturity Assessment
Fetal lung stiffness measured through SWE has been correlated with maturity markers, notably in assessing the risk of neonatal respiratory distress syndrome (RDS). Research by Nallet et al. has documented that lung stiffness typically increases from the second trimester, peaking around 32 weeks of gestation, which corresponds with the development of alveolar structures and the production of surfactant necessary for respiratory function [
58]. Beyond 32 weeks, lung elasticity values tend to decrease slightly, which may indicate that the tissue is reaching the final stages of maturation in preparation for the demands of postnatal breathing [
58,
59]. The typical values of lung stiffness ranges from around 4 kPa in early gestation to slightly over 5 kPa at their peak, then tapering as full-term approaches [
63].
An important metric derived from fetal elastography is the lung-to-liver elasticity (LLE) ratio, which has emerged as a non-invasive indicator of lung maturity [
63,
64]. This ratio, remaining consistently between 0.8 and 0.9 in healthy pregnancies, allows clinicians to contrast lung development with the more gradually maturing liver. The LLE ratio could especially be helpful in cases where premature delivery is a concern [
64].
Liver Elasticity as a Developmental Benchmark
The fetal liver’s elasticity shows a steady, linear increase throughout gestation, reflecting its progressive functional and metabolic maturation. Unlike the lung, which exhibits peak and subsequent reduction in stiffness, liver elasticity increases consistently, typically ranging from about 3.86 kPa at 24 weeks to approximately 4.45 kPa by 39 weeks of gestation [
58]. This gradual increase aligns with the liver’s role in metabolic preparation for postnatal life and is used as a reference value when examining the lung-to-liver elasticity ratio [
63,
64] .
The stability and predictable progression of liver elasticity also offer a reliable baseline against which abnormalities in other tissues, particularly the lungs, can be detected. By comparing liver stiffness to lung stiffness, clinicians gain insight into whether lung development is progressing within expected limits, which is particularly useful in assessing fetuses at risk of developmental delays or other complications [
58].
Brain Elasticity and Structural Maturation
The brain is another focus for elastographic assessment, with specific regions such as the cerebral parenchyma and choroid plexus showing elasticity changes that correlate with brain development stages. Studies, including those by Zheng et al., reveal that elasticity in cerebral regions increases with gestational age, likely reflecting the progressive formation of neural structures and the complexity of brain tissue [
65]. The elasticity of the cerebral parenchyma, for instance, tends to be higher than other regions like the choroid plexus, suggesting its dense cellular organization and critical role in neural activity.
Clinical applications of brain elasticity assessment extend to detecting structural abnormalities and monitoring development in high-risk pregnancies. In one case study exploring the elasticity of a fetal brain affected by atypical choroid plexus papilloma, the elasticity values provided additional information on the lesion’s characteristics, aiding in diagnosis and management [
66].
Safety in Fetal Assessment
The safety of fetal sonoelastography, specifically SWE, has been investigated due to concerns over the potential bioeffects of the high-intensity acoustic radiation force used to generate shear waves. The primary areas of concern are thermal effects, mechanical impacts on tissue, and possible teratogenicity.
Thermal effects, due to the absorption of acoustic energy, are measured by the thermal index (TI). Studies such as Issaoui et al. [
60] suggest that SWE’s thermal impact is like that of pulsed Doppler ultrasound, which is widely accepted in obstetrics. However, SWE involves brief energy peaks that could lead to localized heating, especially at bone-tissue interfaces, where thermal buildup might approach safety thresholds.
Mechanical effects include tissue displacement caused by the acoustic radiation force, measured by the mechanical index (MI). Although no direct cavitation or significant displacement effects have been documented in clinical settings, in silico studies raise caution about potential impacts on delicate fetal structures, such as the cochlea, which may be vulnerable to high-intensity shear vibrations. Preliminary findings by Massó et al. [
67] in newborns showed no increase in hypoacusis or adverse birth outcomes following prenatal SWE, though further research is recommended to substantiate these results across larger populations [
67,
68].
Overall, current evidence supports SWE as safe within regulated TI and MI limits, but the findings highlight a need for cautious application, adhering to ALARA (As Low As Reasonably Achievable) principles to mitigate potential risks associated with acoustic peaks. Further studies are encouraged to expand understanding of SWE’s long-term bioeffects on fetal tissues.
7. Conclusions
Sonoelastography has shown tremendous potential in advancing prenatal diagnostics, offering a non-invasive means of assessing tissue elasticity and stiffness in maternal and fetal organs. This imaging modality bridges a critical gap in traditional ultrasound, which primarily captures anatomical structure but lacks quantitative data on tissue biomechanical properties. By providing insight into tissue stiffness, sonoelastography enables early identification of complications such as cervical insufficiency, placental insufficiency, and fetal growth restriction. Its applications in evaluating cervical stiffness for predicting preterm birth, assessing placental elasticity in cases of preeclampsia and intrauterine growth restriction, and monitoring fetal organ development underscore its versatility and clinical value.
The ability of sonoelastography to quantify cervical elasticity holds particular promise for managing preterm birth risks. Studies indicate that combining cervical elastography with traditional markers, such as cervical length and uterocervical angle, enhances the accuracy of preterm birth predictions. Similarly, placental elastography measurements have proven effective in detecting early signs of preeclampsia and fetal growth restriction, often before structural anomalies become apparent. This early detection capability facilitates timely interventions that could improve maternal and fetal outcomes significantly. In fetal assessments, measuring the elasticity of organs such as the lungs, liver, and brain provides developmental benchmarks that help assess organ maturity and detect anomalies in utero, offering a unique opportunity to monitor fetal health dynamically.
Despite these promising applications, sonoelastography’s use in obstetrics must be tempered with considerations of safety. Preliminary studies suggest that SWE is safe for fetal assessments when used within regulated parameters, but adherence to the ALARA principle remains critical. Further research into the long-term bioeffects on fetal tissues is essential to ensure that its diagnostic benefits outweigh any potential risks.
In summary, sonoelastography represents a valuable tool in prenatal diagnostics, complementing conventional ultrasound by providing real-time, quantitative insights into tissue elasticity. Its ability to detect subtle changes in tissue stiffness offers a new dimension in monitoring maternal and fetal health, positioning it as an impactful modality for the future of obstetric care. Continued research and clinical trials will further define its role, optimize its protocols, and potentially expand its application across various facets of prenatal health management.
8. Future Directions
As the clinical utility of sonoelastography in prenatal diagnosis continues to expand, several future directions stand out for advancing its role in obstetric care. One critical area is the development of standardized protocols and reference ranges specific to pregnancy stages and individual organ systems. Establishing gestational age-adjusted norms for tissue elasticity across various maternal and fetal tissues will enhance the precision and reliability of sonoelastography as a diagnostic tool, allowing clinicians to differentiate between normal variability and pathological changes with greater confidence.
Further technological advancements in sonoelastography, particularly in shear wave elastography (SWE), could improve image resolution and reduce artifacts, making it possible to capture more detailed biomechanical properties in challenging anatomical regions, such as the cervix and deeper fetal organs. Integrating machine learning and artificial intelligence into sonoelastographic imaging analysis holds promise for automating elasticity assessments, potentially enhancing diagnostic accuracy, consistency, and efficiency in busy clinical settings.
Research into the long-term safety of SWE in fetal applications is also essential, especially with regard to cumulative effects in repeated examinations. Additional studies evaluating the impact of SWE on fetal development and newborn health outcomes will help establish definitive safety guidelines and optimize the technique for prenatal care.
Exploring combined applications of sonoelastography with other diagnostic tools, such as Doppler ultrasound and biochemical markers, represents a promising avenue for comprehensive risk assessment. For instance, integrating elasticity metrics with vascular indices in placental evaluation or with cervical biomarkers in preterm birth prediction could yield valuable insights and improve the ability to stratify risks and personalize care. It should, however, be emphasized that advancing this research area requires collaboration between the radiology and obstetric disciplines. Sonoelastography can be refined and adapted to maximize its potential, paving the way for it to become a mainstay in prenatal diagnostics and maternal-fetal medicine.
Funding
This research was funded by Prince Sattam bin Abdulaziz University, grant number PSAU/2024/01/78904.
Conflicts of Interest
The author declare no conflicts of interest
References
- Okoror, C.E.M.; Arora, S. Prenatal Diagnosis after High Chance Non-Invasive Prenatal Testing for Trisomies 21, 18 and 13, Chorionic Villus Sampling or Amniocentesis? – Experience at a District General Hospital in the United Kingdom. Eur J Obstet Gynecol Reprod Biol X 2023, 19. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, F.; Uhrik, P.; Uhrikova, Z. Ultrasound Elastography: Review of Techniques, Clinical Application, Technical Limitations, and Safety Considerations in Neonatology. Acta Medica Martiniana 2020, 20, 72–79. [Google Scholar] [CrossRef]
- Mottet, N.; Cochet, C.; Vidal, C.; Metz, J.P.; Aubry, S.; Bourtembourg, A.; Eckman-Lacroix, A.; Riethmuller, D.; Pazart, L.; Ramanah, R. Feasibility of Two-Dimensional Ultrasound Shear Wave Elastography of Human Fetal Lungs and Liver: A Pilot Study. Diagn Interv Imaging 2020, 101, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Cimsit, C.; Yoldemir, T.; Akpinar, I.N. Strain Elastography in Placental Dysfunction: Placental Elasticity Differences in Normal and Preeclamptic Pregnancies in the Second Trimester. Arch Gynecol Obstet 2015, 291, 811–817. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Mazza, E.; Feltovich, H.; Schmitz, R. Cervical Elastography during Pregnancy: A Critical Review of Current Approaches with a Focus on Controversies and Limitations. J Med Ultrason (2001) 2016, 43, 493–504. [Google Scholar] [CrossRef]
- Nott, J.P.; Bonney, E.A.; Pickering, J.D.; Simpson, N.A.B. The Structure and Function of the Cervix during Pregnancy. Translational Research in Anatomy 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Abdallah, Y.E.E.D.M.; Mostafa, Y.H.A.; Gaafar, H.M.I.; Hegazy, R.M.A. Value of Cervical Strain in Ultrasound Elastography as a Predictor of Spontaneous Preterm Delivery. Egyptian Journal of Radiology and Nuclear Medicine 2023, 54, 1–16. [Google Scholar] [CrossRef]
- Carlson, L.C.; Feltovich, H.; Palmeri, M.L.; Dahl, J.J.; del Rio, A.M.; Hall, T.J. Shear Wave Speed Estimation in the Human Uterine Cervix. Ultrasound Obstet Gynecol 2014, 43, 452. [Google Scholar] [CrossRef]
- Thomsen, C.R.; Jensen, M.S.S.; Bor, P.; Hinge, M.; Sandager, P.; Uldbjerg, N. Recommendations for Strain Elastography of the Uterine Cervix. Arch Gynecol Obstet 2024, 310, 2023–2033. [Google Scholar] [CrossRef]
- Köbbing, K.; Fruscalzo, A.; Hammer, K.; Möllers, M.; Falkenberg, M.; Kwiecien, R.; Klockenbusch, W.; Schmitz, R. Quantitative Elastography of the Uterine Cervix as a Predictor of Preterm Delivery. Journal of Perinatology 2014, 34, 774–780. [Google Scholar] [CrossRef]
- Woźniak, S.; Czuczwar, P.; Szkodziak, P.; Wrona, W.; Paszkowski, T. Elastography for Predicting Preterm Delivery in Patients with Short Cervical Length at 18-22 Weeks of Gestation: A Prospective Observational Study. Ginekol Pol 2015, 86, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, L.H.; Zheng, Q.; Xie, H.N.; Gu, Y.J.; Lin, M.F.; Wu, L.H. Evaluation of Cervical Elastography for Prediction of Spontaneous Preterm Birth in Low-Risk Women: A Prospective Study. Journal of Ultrasound in Medicine 2020, 39, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Alavandar, E.; Kashyap, R.; Rajan, S.; Muthusamy, D.; Subramani, A. Correlation of Cervical Length, Anterior Uterocervical Angle, and Cervical Elastography with the Incidence of Preterm Labor. Journal of Fetal Medicine 2023, 10, 016–022. [Google Scholar] [CrossRef]
- Abdallah, Y.E.E.D.M.; Mostafa, Y.H.A.; Gaafar, H.M.I.; Hegazy, R.M.A. Value of Cervical Strain in Ultrasound Elastography as a Predictor of Spontaneous Preterm Delivery. Egyptian Journal of Radiology and Nuclear Medicine 2023, 54. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Yang, F.; Wu, D.; Qi, J.; Ji, Y.; Hu, M. The Value of Real-Time Shear Wave Elastography in Spontaneous Preterm Birth. Medicine (United States) 2024, 103, e39288. [Google Scholar] [CrossRef]
- Pereira, S.; Frick, A.P.; Poon, L.C.; Zamprakou, A.; Nicolaides, K.H. Successful Induction of Labor: Prediction by Preinduction Cervical Length, Angle of Progression and Cervical Elastography. Ultrasound in Obstetrics and Gynecology 2014, 44, 468–475. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Londero, A.P.; Fröhlich, C.; Meyer-Wittkopf, M.; Schmitz, R. Quantitative Elastography of the Cervix for Predicting Labor Induction Success. Ultraschall in der Medizin 2015, 36, 65–73. [Google Scholar] [CrossRef]
- Londero, A.P.; Schmitz, R.; Bertozzi, S.; Driul, L.; Fruscalzo, A. Diagnostic Accuracy of Cervical Elastography in Predicting Labor Induction Success: A Systematic Review and Meta-Analysis. J Perinat Med 2016, 44, 167–178. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, Y.K.Y.; Ho, S.Y.S.; Sahota, D.S.; Hui, L.L.; Poon, L.C.; Leung, T.Y. The Predictive Value of Cervical Shear Wave Elastography in the Outcome of Labor Induction. Acta Obstet Gynecol Scand 2020, 99, 59–68. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Schmitz, R.; Klockenbusch, W.; Steinhard, J. Reliability of Cervix Elastography in the Late First and Second Trimester of Pregnancy. Ultraschall in der Medizin 2012, 33. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Londero, A.P.; Fröhlich, C.; Möllmann, U.; Schmitz, R. Quantitative Elastography for Cervical Stiffness Assessment during Pregnancy. Biomed Res Int 2014, 2014. [Google Scholar] [CrossRef]
- Hernandez-Andrade, E.; Hassan, S.S.; Ahn, H.; Korzeniewski, S.J.; Yeo, L.; Chaiworapongsa, T.; Romero, R. Evaluation of Cervical Stiffness during Pregnancy Using Semiquantitative Ultrasound Elastography. Ultrasound in Obstetrics and Gynecology 2013, 41, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Katsura, D.; Yamada, K.; Hayashi, K.; Ishiko, A.; Tsuji, S.; Kimura, F.; Takahashi, K.; Murakami, T. Use of Ultrasound Shear-Wave Elastography to Evaluate Change in Cervical Stiffness during Pregnancy. Journal of Obstetrics and Gynaecology Research 2017, 43, 1405–1410. [Google Scholar] [CrossRef]
- Duan, H.; Chaemsaithong, P.; Ju, X.; Ho, S.Y.S.; Sun, Q.; Tai, Y. yun; Leung, T.Y.; Poon, L.C. Shear-Wave Sonoelastographic Assessment of Cervix in Pregnancy. Acta Obstet Gynecol Scand 2020, 99, 1458–1468. [Google Scholar] [CrossRef]
- Woo, J.; Ge, W.; Mancheri, J.; Hyett, J.; Mogra, R. Shear Wave Elastography: The Relationship of the Cervical Stiffness with Gestational Age and Cervical Length- a Feasibility Study. Journal of Maternal-Fetal and Neonatal Medicine 2022, 35, 9684–9693. [Google Scholar] [CrossRef]
- Nguyen-Hoang, L.; Chaemsaithong, P.; Cheng, Y.K.Y.; Feng, Q.; Fung, J.; Duan, H.; Chong, M.K.C.; Leung, T.Y.; Poon, L.C. Longitudinal Evaluation of Cervical Length and Shear Wave Elastography in Women with Spontaneous Preterm Birth. Ultrasound in Obstetrics and Gynecology 2024, 63, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, P.; Qu, Y.; Maslov, K.; Chubiz, J.; Tuuli, M.G.; Stout, M.J.; Wang, L. V. Quantification of Cervical Elasticity During Pregnancy Based on Transvaginal Ultrasound Imaging and Stress Measurement. IEEE Trans Biomed Eng 2024. [Google Scholar] [CrossRef]
- Hernandez-Andrade, E.; Maymon, E.; Luewan, S.; Bhatti, G.; Mehrmohammadi, M.; Erez, O.; Pacora, P.; Done, B.; Hassan, S.S.; Romero, R. A Soft Cervix, Categorized by Shear-Wave Elastography, in Women with Short or with Normal Cervical Length at 18-24 Weeks Is Associated with a Higher Prevalence of Spontaneous Preterm Delivery. J Perinat Med 2018, 46, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Patberg, E.T.; Wells, M.; Vahanian, S.A.; Zavala, J.; Bhattacharya, S.; Richmond, D.; Akerman, M.; Demishev, M.; Kinzler, W.L.; Chavez, M.R.; et al. Use of Cervical Elastography at 18 to 22 Weeks’ Gestation in the Prediction of Spontaneous Preterm Birth. Am J Obstet Gynecol 2021, 225, 525.e1–525.e9. [Google Scholar] [CrossRef]
- Feng, Q.; Chaemsaithong, P.; Duan, H.; Ju, X.; Appiah, K.; Shen, L.; Wang, X.; Tai, Y.; Leung, T.Y.; Poon, L.C. Screening for Spontaneous Preterm Birth by Cervical Length and Shear-Wave Elastography in the First Trimester of Pregnancy. Am J Obstet Gynecol 2022, 227, 500.e1–500.e14. [Google Scholar] [CrossRef]
- Mlodawski, J.; Mlodawska, M.; Plusajska, J.; Detka, K.; Michalska, A.; Swiercz, G.; Sikorski, M. Repeatability and Reproducibility of Quantitative Cervical Strain Elastography (E-Cervix) in Pregnancy. Sci Rep 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.P.; Sun, F.J. Assessment of the Cervix in Pregnant Women with a History of Cervical Insufficiency during the First Trimester Using Elastography. Acta Obstet Gynecol Scand 2020, 99, 1497–1503. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, H.; Chen, J.; Bai, J.; Liu, Y.; You, Y. Systematic Review and Meta-Analysis: Gray-Scale Ultrasound and Shear Wave Elastography in the Diagnosis of Primipara Pregnancy and Delivery. Ann Palliat Med 2021, 10, 11664–11677. [Google Scholar] [CrossRef] [PubMed]
- Kilic, F.; Kayadibi, Y.; Yuksel, M.A.; Adaletli, I.; Ustabasioglu, F.E.; Oncul, M.; Madazli, R.; Yilmaz, M.H.; Mihmanli, I.; Kantarci, F. Shear Wave Elastography of Placenta: In Vivo Quantitation of Placental Elasticity in Preeclampsia. Diagnostic and Interventional Radiology 2015, 21, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Nan, R.; Li, Y.; Cui, X.; Liang, X.; Zhao, Y. Measurement of Elasticity of Normal Placenta Using the Virtual Touch Quantification Technique. Ultrasonography 2016, 35, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Cavanagh, E.; Kumar, S.; Clifton, V.L.; Borg, D.J.; Priddle, J.; Wille, M.L.; Drovandi, C.; Fontanarosa, D. Changes in Placental Elastography in the Third Trimester - Analysis Using a Linear Mixed Effect Model. Placenta 2021, 114, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Dokumaci, D. Sen; Uyanikoglu, H. Shear-Wave Elastography for Detection of Placenta Percreta: A Case-Controlled Study. Acta radiol 2022, 63, 424–430. [Google Scholar] [CrossRef]
- Fujita, Y.; Nakanishi, T.O.; Sugitani, M.; Kato, K. Placental Elasticity as a New Non-Invasive Predictive Marker of Pre-Eclampsia. Ultrasound Med Biol 2019, 45, 93–97. [Google Scholar] [CrossRef]
- Tian, F.; Dou, L.F.; Tang, L.W.; Gao, Q.M.; Li, B.W. Predictive Value of Placental Real-Time Shear Wave Elastography Combined with 3-Dimensional Power Doppler Index for Preeclampsia. Medicine (United States) 2024, 103, E37372. [Google Scholar] [CrossRef]
- Singh, T.; Roy Choudhury, S.; Singh, M.; Singla, V.; Jain, V. Role of Shear Wave Elastography of Placenta in Prediction of Preeclampsia in High-Risk Pregnancy. Ultrasound Q 2024, 40, 119–125. [Google Scholar] [CrossRef]
- Kılıç, F.; Kayadibi, Y.; Yüksel, M.A.; Adaletli, İ.; Ustabaşıoğlu, F.E.; Öncül, M.; Madazlı, R.; Yılmaz, M.H.; Mihmanlı, İ.; Kantarcı, F. Shear Wave Elastography of Placenta: In Vivo Quantitation of Placental Elasticity in Preeclampsia. Diagnostic and Interventional Radiology 2015, 21, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Sirinoglu, H.A.; Uysal, G.; Nazik, H.; Cingillioglu, B.; Genc, S.; Pekin, O. Efficacy of Shear Wave Elastography in Predicting Preeclampsia in the First Trimester. Rev Assoc Med Bras 2021, 67, 1558–1563. [Google Scholar] [CrossRef]
- Fujita, Y.; Nakanishi, T.O.; Sugitani, M.; Kato, K. Placental Elasticity as a New Non-Invasive Predictive Marker of Pre-Eclampsia. Ultrasound Med Biol 2019, 45, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Cimsit, C.; Yoldemir, T.; Akpinar, I.N. Strain Elastography in Placental Dysfunction: Placental Elasticity Differences in Normal and Preeclamptic Pregnancies in the Second Trimester. Arch Gynecol Obstet 2015, 291, 811–817. [Google Scholar] [CrossRef]
- Ansar, M.; Ali, M.A.; Ali, N.; Haider, Z.; Latif, A.; Tazeen, A.; Fatima, Z.; Anjum, M.N. Ultrasound Shear Wave Elastography of the Placenta: A Potential Tool for Early Detection of Fetal Growth Restriction. Clin Imaging 2024, 116. [Google Scholar] [CrossRef]
- Arioz Habibi, H.; Alici Davutoglu, E.; Kandemirli, S.G.; Aslan, M.; Ozel, A.; Kalyoncu Ucar, A.; Zeytun, P.; Madazli, R.; Adaletli, I. In Vivo Assessment of Placental Elasticity in Intrauterine Growth Restriction by Shear-Wave Elastography. Eur J Radiol 2017, 97, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Akbas, M.; Koyuncu, F.M.; Artunç-Ulkumen, B. Placental Elasticity Assessment by Point Shear Wave Elastography in Pregnancies with Intrauterine Growth Restriction. J Perinat Med 2019, 47, 841–846. [Google Scholar] [CrossRef]
- Odofin, F.M. Examination of Predisposing Risk Factors among Primiparous Women at Risk of Developing Pelvic Organ Prolapse within a Year of Childbirth. Journal of Pelvic, Obstetric and Gynaecological Physiotherapy 2020. [Google Scholar]
- Sobhgol, S.S.; Smith, C.A.; Thomson, R.; Dahlen, H.G. The Effect of Antenatal Pelvic Floor Muscle Exercise on Sexual Function and Labour and Birth Outcomes: A Randomised Controlled Trial. Women and Birth 2022, 35, e607–e614. [Google Scholar] [CrossRef]
- Lamers, E.P.; Soltys, J.C.; Scherpereel, K.L.; Yang, A.J.; Zelik, K.E. Low-Profile Elastic Exosuit Reduces Back Muscle Fatigue. Sci Rep 2020, 10, 15958. [Google Scholar] [CrossRef]
- Sigurdardottir, Thorgerdur. Postpartum Pelvic Floor Symptoms and Early Physical Therapy Intervention. 2020.
- Alfuraih, A.M.; Tan, A.L.; O’Connor, P.; Emery, P.; Mackie, S.; Wakefield, R.J. Reduction in Stiffness of Proximal Leg Muscles during the First 6 Months of Glucocorticoid Therapy for Giant Cell Arteritis: A Pilot Study Using Shear Wave Elastography. Int J Rheum Dis 2019, 22, 1891–1899. [Google Scholar] [CrossRef]
- Gachon, B.; Fritel, X.; Pierre, F.; Nordez, A. Transperineal Ultrasound Shear-Wave Elastography Is a Reliable Tool for Assessment of the Elastic Properties of the Levator Ani Muscle in Women. Sci Rep 2021, 11. [Google Scholar] [CrossRef]
- Gachon, B.; Fritel, X.; Pierre, F.; Nordez, A. In Vivo Measurement of the Elastic Properties of Pelvic Floor Muscles in Pregnancy Using Shear Wave Elastography. Arch Gynecol Obstet 2024, 309, 2623–2631. [Google Scholar] [CrossRef]
- Okada, Y.; Nakagawa, C.; Shigeta, M.; Nomura, Y.; Inoue, E.; Ichizuka, K.; Yoshimura, Y. Evaluation of Levator Ani Muscle Elasticity after Vaginal Delivery and Cesarean Section Using Shear Wave Elastography. Journal of Medical Ultrasonics 2024, 51, 95–101. [Google Scholar] [CrossRef]
- Gachon, B.; Clergue, O.; Fritel, X.; Pierre, F.; Nordez, A. In Vivo Assessment of the Elastic Properties of the External Anal Sphincter in Term Pregnant Women Using Shear Wave Elastography. Int Urogynecol J 2023, 34, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.J.; Nielsen, P.M.F.; Taberner, A.J.; Kruger, J.A. Change in Levator Ani Muscle Stiffness and Active Force during Pregnancy and Post-Partum. Int Urogynecol J 2020, 31, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Nallet, C.; Pazart, L.; Cochet, C.; Vidal, C.; Metz, J.-P.; Jacquet, E.; Gorincour, G.; Mottet, N. Prenatal Quantification of Human Foetal Lung and Liver Elasticities between 24 and 39 Weeks of Gestation Using 2D Shear Wave Elastography. Eur Radiol 2022, 32, 5559–5567. [Google Scholar] [CrossRef]
- Mottet, N.; Metz, J.P.; Chaussy, Y. Evolution of Fetal Lung Stiffness during Gestation in Two Different Congenital Malformations. Journal of Obstetrics and Gynaecology Research 2019, 45, 931–934. [Google Scholar] [CrossRef]
- Issaoui, M.; Debost-Legrand, A.; Skerl, K.; Chauveau, B.; Magnin, B.; Delabaere, A.; Boyer, L.; Sauvant-Rochat, M.P.; Lémery, D. Shear Wave Elastography Safety in Fetus: A Quantitative Health Risk Assessment. Diagn Interv Imaging 2018, 99, 519–524. [Google Scholar] [CrossRef]
- Rodrigues Simões, A.P.; Rossi Feliciano, M.A.; Maronezi, M.C.; Uscategui, R.A.R.; Bartlewski, P.M.; de Almeida, V.T.; Oh, D.; do Espírito Santo Silva, P.; da Silva, L.C.G.; Russiano Vicente, W.R. Elastographic and Echotextural Characteristics of Foetal Lungs and Liver during the Final 5 Days of Intrauterine Development in Dogs. Anim Reprod Sci 2018, 197, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Diguisto, C.; Simon, E.G.; Callé, S.; Ternifi, R.; Remeniéras, J.P.; Hervé, P.; Perrotin, F. Ultrasonic Elastography Exploration of the Foetal Brain: A Case of Atypical Choroid Plexus Papilloma. J Obstet Gynaecol (Lahore) 2017, 37, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Cochet, C.; Vidal, C.; Metz, J.P.; Aubry, S.; Bourtembourg, A.; Eckman-Lacroix, A.; Riethmuller, D.; Pazart, L.; Ramanah, R. Feasibility of Two-Dimensional Ultrasound Shear Wave Elastography of Human Fetal Lungs and Liver: A Pilot Study. Diagn Interv Imaging 2020, 101, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, Q.; Xu, Z.; Li, L.; Lyu, G. Evaluating Fetal Lung Development at Various Gestational Weeks Using Two-Dimensional Shear Wave Elastography. Quant Imaging Med Surg 2024, 14, 5373–5384. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Z.; Wu, J.; Tan, X.Y. A Novel Approach to Assessing Fetal Tissue Stiffness Using Virtual Touch Tissue Quantification. Med Ultrason 2016, 18, 70–74. [Google Scholar] [CrossRef]
- Kim, H.G.; Park, M.S.; Lee, J.D.; Park, S.Y. Ultrasound Elastography of the Neonatal Brain: Preliminary Study. Journal of Ultrasound in Medicine 2017, 36, 1313–1319. [Google Scholar] [CrossRef]
- Massó, P.; Melchor, J.; Rus, G.; Molina, F.S. A Preliminary Study on the Safety of Elastography during Pregnancy: Hypoacusia, Anthropometry, and Apgar Score in Newborns. Diagnostics 2020, 10. [Google Scholar] [CrossRef]
- Massó, P.; Rus, G.; Molina, F.S. Safety of Elastography in Fetal Medicine: Preliminary Study on Hypoacusis. Ultrasound in Obstetrics and Gynecology 2017, 50, 660–661. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).