Submitted:
07 November 2024
Posted:
08 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
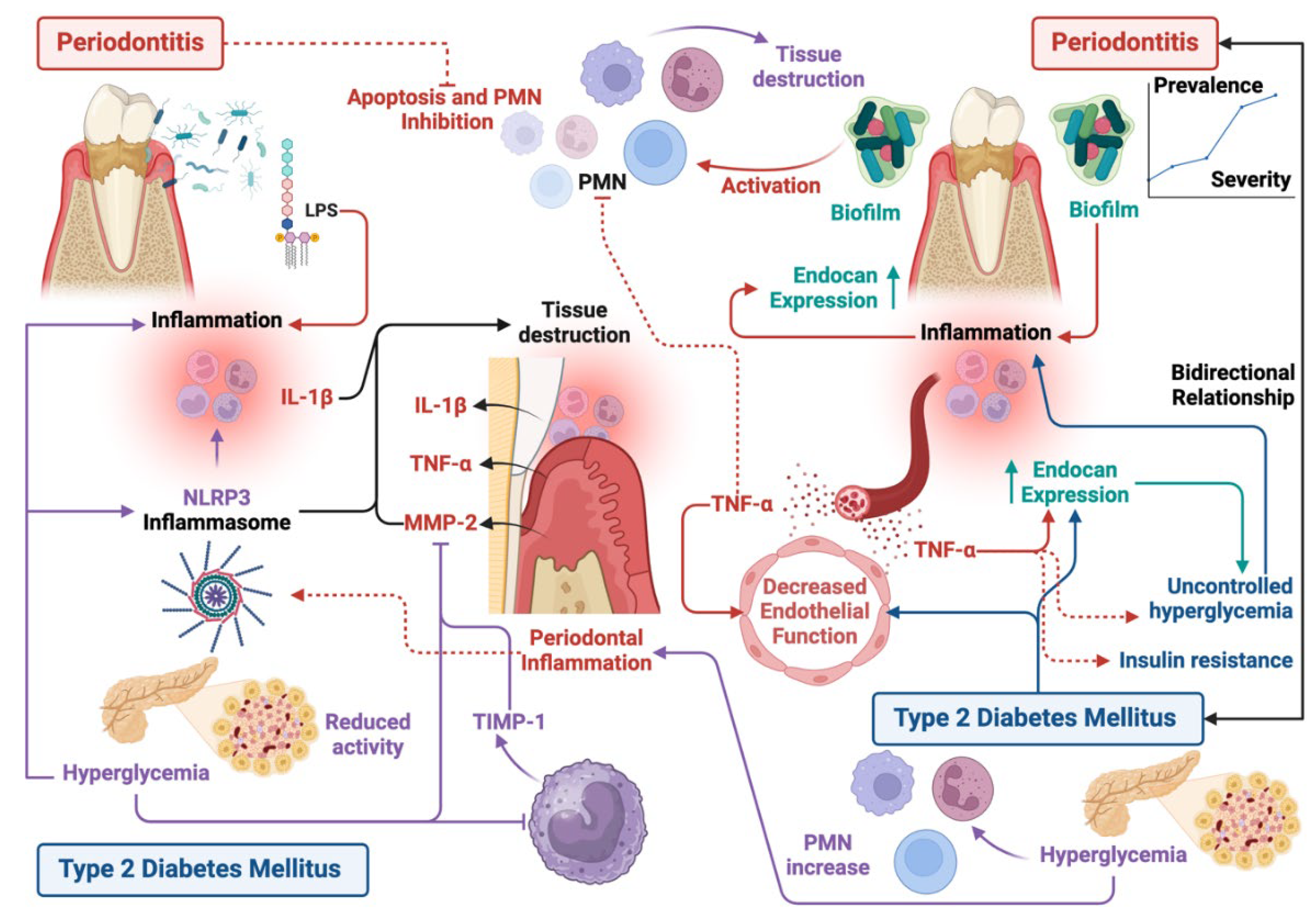
2. Periodontitis and Type 2 Diabetes Mellitus
3. Periodontitis and Type 2 Diabetes Mellitus
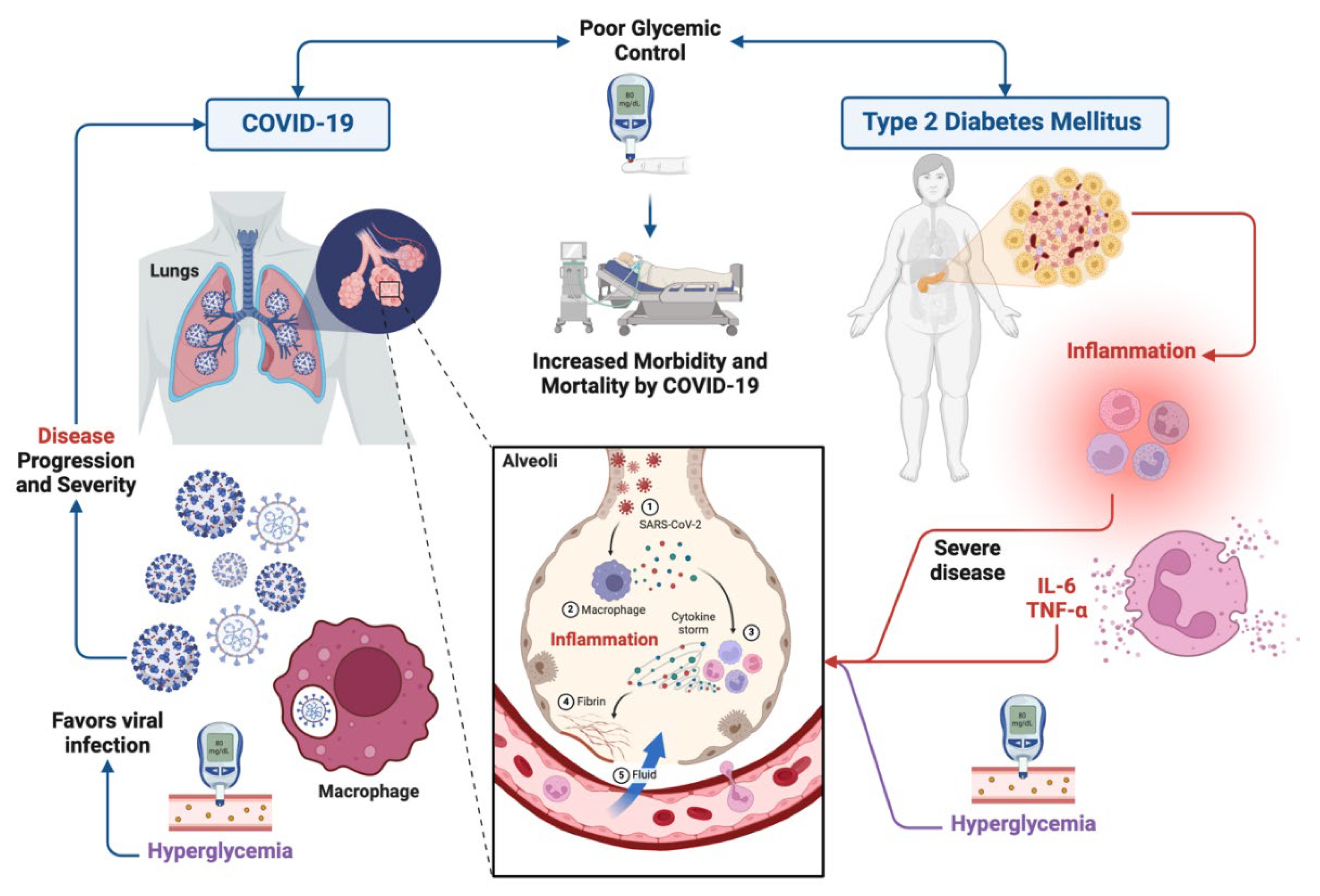
4. Type 2 Diabetes Mellitus and COVID-19
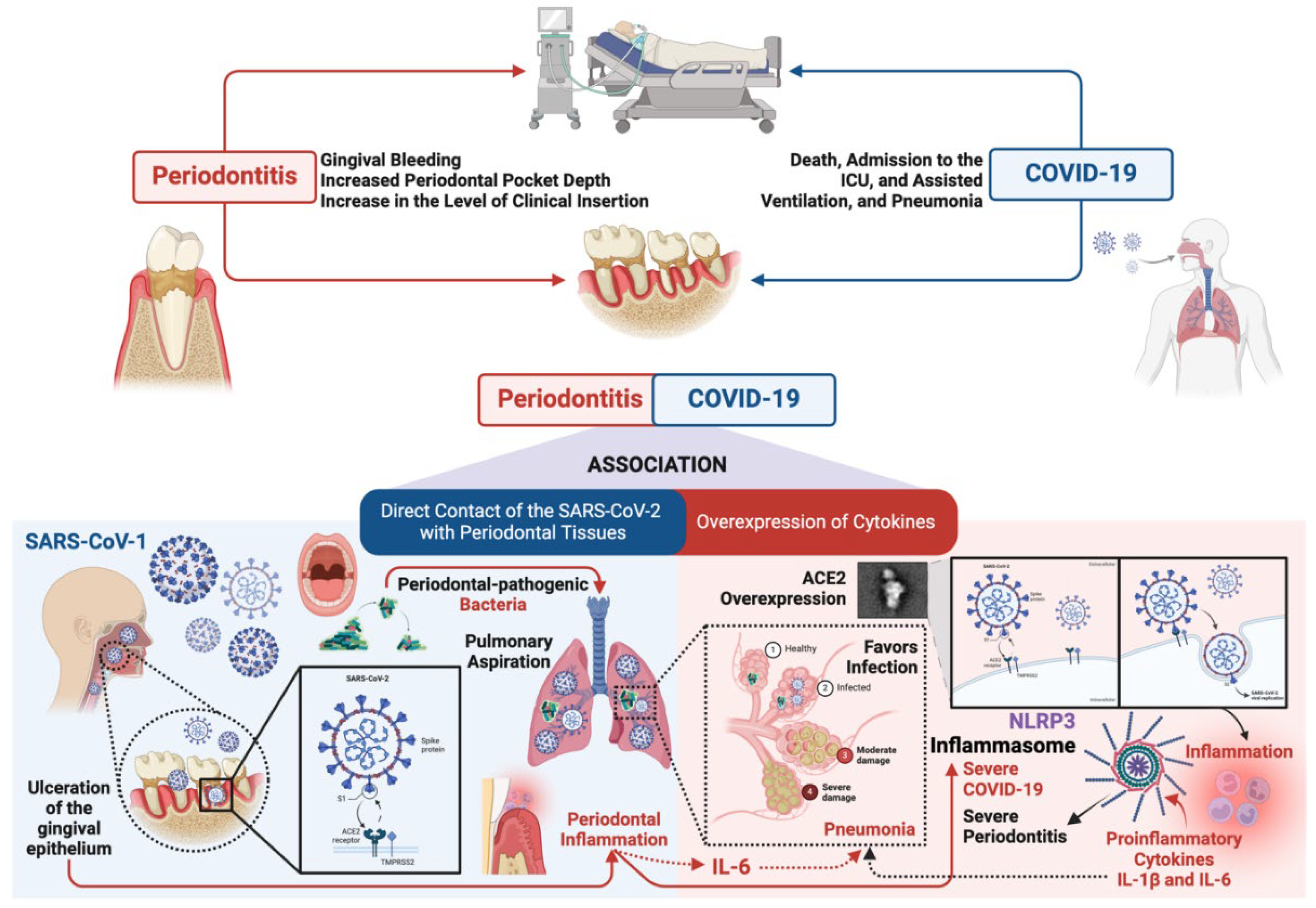
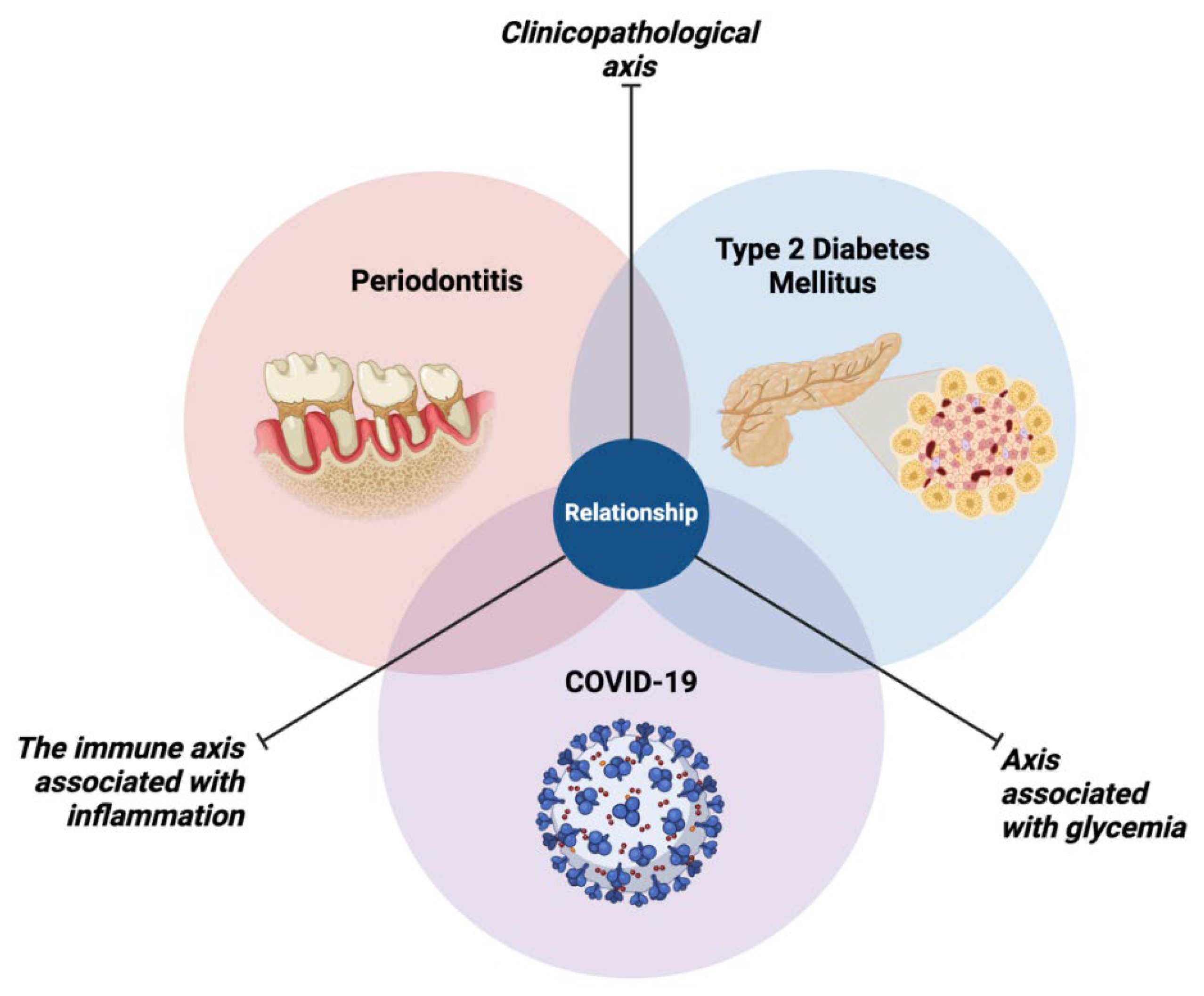
4. Relationship Between Periodontitis, Type 2 Diabetes Mellitus and COVID-19
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl 1), S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Simon-Soro, A.; Curtis, M.A. Role of Microbial Communities in the Pathogenesis of Periodontal Diseases and Caries. J. Clin. Periodontol. 2017, 44 Suppl 18, S23–S38. [Google Scholar] [CrossRef]
- Muñoz-Carrillo, J.L.; Hernández-Reyes, V.E.; García-Huerta, O.E.; Chávez-Ruvalcaba, F.; Chávez-Ruvalcaba, M.I.; Chávez-Ruvalcaba, K.M.; Díaz-Alfaro, L. Pathogenesis of Periodontal Disease. In Diagnostic and Adjunctive Non-surgical Considerations; Nermin, Y., Ed.; IntechOpen: London, 2019; ISBN 978-1-78984-461-0. [Google Scholar]
- Kedlaya, M.N.; Puzhankara, L.; Prasad, R.; Raj, A. Periodontal Disease Pathogens, Pathogenesis, and Therapeutics: The CRISPR-Cas Effect. Cris. J. 2023, 6, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Moreno Caicedo, L.F.; Amaya Sánchez, S.; Cruz Olivo, E.A. Factores de Riesgo Modificables e Inmodificables de La Periodontitis: Revisión Narrativa. Univ. Odontol. 2018, 37. [Google Scholar] [CrossRef]
- Hanson, M.A.; Godfrey, K.M. Genetics: Epigenetic Mechanisms Underlying Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2015, 11, 261–263. [Google Scholar] [CrossRef]
- Stančáková, A.; Laakso, M. Genetics of Type 2 Diabetes. Endocr. Dev. 2016, 31, 203–220. [Google Scholar] [CrossRef]
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr. Diab. Rep. 2018, 18. [Google Scholar] [CrossRef]
- Zhang, H.; Pollin, T.I. Epigenetics Variation and Pathogenesis in Diabetes. Curr. Diab. Rep. 2018, 18. [Google Scholar] [CrossRef]
- Defronzo, R.A. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773. [Google Scholar] [CrossRef]
- Brunton, S. Pathophysiology of Type 2 Diabetes: The Evolution of Our Understanding - PubMed. 2016, 65, 4. [Google Scholar]
- Javeed, N.; Matveyenko, A. V. Circadian Etiology of Type 2 Diabetes Mellitus. Physiology 2018, 33, 138. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.M.L.; Lira, R.; Fischer, R.G.; Santos, A.P.P.; Oliveira, B.H. Systemic Antibiotics in Periodontal Treatment of Diabetic Patients: A Systematic Review. PLoS One 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Turner, C. Diabetes Mellitus and Periodontal Disease: The Profession’s Choices. Br. Dent. J. 2022, 233, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Hayes, C.; Taylor, G.W. Glycemic Control of Type 2 Diabetes and Severe Periodontal Disease in the US Adult Population. Community Dent. Oral Epidemiol. 2002, 30, 182–192. [Google Scholar] [CrossRef]
- Guzman, S.; Karima, M.; Wang, H.-Y.; Dyke, T.E. Van Association between Interleukin-1 Genotype and Periodontal Disease in a Diabetic Population. J. Periodontol. 2003, 74, 1183–1190. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Polak, D.; Shapira, L. An Update on the Evidence for Pathogenic Mechanisms That May Link Periodontitis and Diabetes. J. Clin. Periodontol. 2018, 45, 150–166. [Google Scholar] [CrossRef]
- Salvi, G.E.; Yalda, B.; Collins, J.G.; Jones, B.H.; Smith, F.W.; Arnold, R.R.; Offenbacher, S. Inflammatory Mediator Response as a Potential Risk Marker for Periodontal Diseases in Insulin-Dependent Diabetes Mellitus Patients. J. Periodontol. 1997, 68, 127–135. [Google Scholar] [CrossRef]
- Salvi, G.E.; Beck, J.D.; Offenbacher, S. PGE2, IL-1 Beta, and TNF-Alpha Responses in Diabetics as Modifiers of Periodontal Disease Expression. Ann. Periodontol. 1998, 3, 40–50. [Google Scholar] [CrossRef]
- Domingueti, C.P.; Dusse, L.M.S.A.; Carvalho, M.D.G.; De Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes Mellitus: The Linkage between Oxidative Stress, Inflammation, Hypercoagulability and Vascular Complications. J. Diabetes Complications 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Santos, V.R.; Lima, J.A.; Gonçalves, T.E.D.; Bastos, M.F.; Figueiredo, L.C.; Shibli, J.A.; Duarte, P.M. Receptor Activator of Nuclear Factor-Kappa B Ligand/Osteoprotegerin Ratio in Sites of Chronic Periodontitis of Subjects with Poorly and Well-Controlled Type 2 Diabetes. J. Periodontol. 2010, 81, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Pacios, S.; Andriankaja, O.; Kang, J.; Alnammary, M.; Bae, J.; De Brito Bezerra, B.; Schreiner, H.; Fine, D.H.; Graves, D.T. Bacterial Infection Increases Periodontal Bone Loss in Diabetic Rats through Enhanced Apoptosis. Am. J. Pathol. 2013, 183, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.D.; Choudhury, A.B.; Choudhury, A.B. Relationships between Serum Osteocalcin Levels versus Blood Glucose, Insulin Resistance and Markers of Systemic Inflammation in Central Indian Type 2 Diabetic Patients. Eur Rev Med Pharmacol Sci 2013, 17, 1631–1635. [Google Scholar]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.B.; Thakur, S.; Muddapur, M. V.; Kulkarni, R.D. Cytokine Ratios in Chronic Periodontitis and Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. 2017, 11, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E. Pro-Resolving Mediators in the Regulation of Periodontal Disease. Mol. Aspects Med. 2017, 58, 21–36. [Google Scholar] [CrossRef]
- Andriankaja, O.M.; Galicia, J.; Dong, G.; Xiao, W.; Alawi, F.; Graves, D.T. Gene Expression Dynamics during Diabetic Periodontitis. J. Dent. Res. 2012, 91, 1160–1165. [Google Scholar] [CrossRef]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J. Diabetes Res. 2017, 2017. [Google Scholar] [CrossRef]
- Lim, J.C.; Ko, K.I.; Mattos, M.; Fang, M.; Zhang, C.; Feinberg, D.; Sindi, H.; Li, S.; Alblowi, J.; Kayal, R.A.; et al. TNFα Contributes to Diabetes Impaired Angiogenesis in Fracture Healing. Bone 2017, 99, 26–38. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, S.; Albiero, M.L.; Vieira, G.H.A.; Wang, J.; Feng, J.Q.; Graves, D.T. Diabetes Activates Periodontal Ligament Fibroblasts via NF-ΚB In Vivo. J. Dent. Res. 2018, 97, 580–588. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Sharma, K. Challenging the Dogma of Mitochondrial Reactive Oxygen Species Overproduction in Diabetic Kidney Disease. Kidney Int. 2016, 90, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Shaker, O.; Ghallab, N.A.; Hamdy, E.; Sayed, S. Inducible Nitric Oxide Synthase (INOS) in Gingival Tissues of Chronic Periodontitis with and without Diabetes: Immunohistochemistry and RT-PCR Study. Arch. Oral Biol. 2013, 58, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. ping; Sun, Y. li; Wang, Y. fen; Yazici, D.; Azkur, D.; Ogulur, I.; Azkur, A.K.; Yang, Z. wei; Chen, X. xue; Zhang, A.Z.; et al. Recent Developments in the Immunopathology of COVID-19. Allergy 2023, 78, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Ullah, A.; Gul, A.; Mousavi, T.; Khan, M.W. Novel Coronavirus 2019 (COVID-19) Pandemic Outbreak: A Comprehensive Review of the Current Literature. Vacunas 2021, 22, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, Y.; Wang, Y.; Huang, Z.; Song, B. Chest CT Manifestations of New Coronavirus Disease 2019 (COVID-19): A Pictorial Review. Eur. Radiol. 2020, 30, 4381–4389. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M. V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Muniyappa, R.; Gubbi, S. COVID-19 Pandemic, Coronaviruses, and Diabetes Mellitus. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E736–E741. [Google Scholar] [CrossRef]
- Gurwitz, D. Angiotensin Receptor Blockers as Tentative SARS-CoV-2 Therapeutics. Drug Dev. Res. 2020, 81, 537–540. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the Cardiovascular System. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Wang, R.; Feng, Z.; Zhang, J.; Yang, H.; Tan, Y.; Wang, H.; Wang, C.; Liu, L.; et al. Human Kidney Is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Liu, F.; Long, X.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2128–2130.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet (London, England) 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Abdi, A.; Jalilian, M.; Sarbarzeh, P.A.; Vlaisavljevic, Z. Diabetes and COVID-19: A Systematic Review on the Current Evidences. Diabetes Res. Clin. Pract. 2020, 166, 108347. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, Pathophysiology, Prognosis and Practical Considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 303–310. [Google Scholar] [CrossRef]
- Wysocki, J.; Ye, M.; Soler, M.J.; Gurley, S.B.; Xiao, H.D.; Bernstein, K.E.; Coffman, T.M.; Chen, S.; Batlle, D. ACE and ACE2 Activity in Diabetic Mice. Diabetes 2006, 55, 2132–2139. [Google Scholar] [CrossRef]
- Roca-Ho, H.; Riera, M.; Palau, V.; Pascual, J.; Soler, M.J. Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Rao, S.; Lau, A.; So, H.C. Exploring Diseases/Traits and Blood Proteins Causally Related to Expression of ACE2, the Putative Receptor of SARS-CoV-2: A Mendelian Randomization Analysis Highlights Tentative Relevance of Diabetes-Related Traits. Diabetes Care 2020, 43, 1416–1426. [Google Scholar] [CrossRef]
- Fernandez, C.; Rysä, J.; Almgren, P.; Nilsson, J.; Engström, G.; Orho-Melander, M.; Ruskoaho, H.; Melander, O. Plasma Levels of the Proprotein Convertase Furin and Incidence of Diabetes and Mortality. J. Intern. Med. 2018, 284, 377–387. [Google Scholar] [CrossRef]
- Hodgson, K.; Morris, J.; Bridson, T.; Govan, B.; Rush, C.; Ketheesan, N. Immunological Mechanisms Contributing to the Double Burden of Diabetes and Intracellular Bacterial Infections. Immunology 2015, 144, 171–185. [Google Scholar] [CrossRef]
- Cuschieri, S.; Grech, S. COVID-19 and Diabetes: The Why, the What and the How. J. Diabetes Complications 2020, 34, 107637. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet. Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Z.; Yuan, Y.H.; Liu, H.H.; Li, S.S.; Zhang, B.W.; Chen, W.; An, Z.J.; Chen, S.Y.; Wu, Y.Z.; Han, B.; et al. Epidemiologic Relationship between Periodontitis and Type 2 Diabetes Mellitus. BMC Oral Health 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Monod Nuñez, M.S.; Aransibia, L.V.; Blanco Fernández, M.J.; Hernández Oropesa, T.; Linari, M.A. Frecuencia de Enfermedad Periodontal En Pacientes Adultos Con Diabetes Mellitus Tipo 2 En La Ciudad Autónoma de Buenos Aires y La Provincia de Buenos Aires. Rev. Soc. Argent. Diabetes 2022, 56, 19–30. [Google Scholar] [CrossRef]
- Shamala, A.; Al-Hajri, M.; Ali Al-Wesabi, M.; Shamala De-, A. Risk Factors for Periodontal Diseases among Yemeni Type II Diabetic Patients. A Case-Control Study. J. Oral Res. 2017, 6, 176–181. [Google Scholar] [CrossRef]
- Susanto, H.; Nesse, W.; Dijkstra, P.U.; Agustina, D.; Vissink, A.; Abbas, F. Periodontitis Prevalence and Severity in Indonesians with Type 2 Diabetes. J. Periodontol. 2011, 82, 550–557. [Google Scholar] [CrossRef]
- Trentin, M.S.; de Carli, J.P.; Ferreira, M. de C.; Gambin, D.J.; da Silva, S.O.; Lisboa, H. Prevalence and Severity of Periodontal Disease in Type 2 Diabetes Mellitus Patients: A Cross-Sectional Study. Biosci. j. 2018, 34, 1114–1123. [Google Scholar] [CrossRef]
- Alasqah, M.; Mokeem, S.; Alrahlah, A.; Al-Hamoudi, N.; Abduljabbar, T.; Akram, Z.; Vohra, F.; Javed, F. Periodontal Parameters in Prediabetes, Type 2 Diabetes Mellitus, and Non-Diabetic Patients. Braz. Oral Res. 2018, 32, e81. [Google Scholar] [CrossRef]
- Acharya, A.B.; Thakur, S.; Muddapur, M. V.; Kulkarni, R.D. Cytokine Ratios in Chronic Periodontitis and Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. 2017, 11, 277–278. [Google Scholar] [CrossRef]
- Li, Q.; Ouyang, X.; Lin, J. The Impact of Periodontitis on Vascular Endothelial Dysfunction. Front. Cell. Infect. Microbiol. 2022, 12. [Google Scholar] [CrossRef]
- Janket, S.J.; Jones, J.A.; Meurman, J.H.; Baird, A.E.; Van Dyke, T.E. Oral Infection, Hyperglycemia, and Endothelial Dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 173. [Google Scholar] [CrossRef] [PubMed]
- Gurav, A.N. The Implication of Periodontitis in Vascular Endothelial Dysfunction. Eur. J. Clin. Invest. 2014, 44, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Adam, E.; Lyon, M.; Depontieu, F.; Motte, V.; Landolfi, C.; Lortat-Jacob, H.; Bechard, D.; Lassalle, P.; Delehedde, M. Endocan or Endothelial Cell Specific Molecule-1 (ESM-1): A Potential Novel Endothelial Cell Marker and a New Target for Cancer Therapy. Biochim. Biophys. Acta 2006, 1765, 25–37. [Google Scholar] [CrossRef]
- van Eijk, L.T.; Cox, L.A.E.; Ramakers, B.P.C.; Dorresteijn, M.J.; Gerretsen, J.; Kox, M.; Pickkers, P. 0730. Plasma Endocan Levels Are Associated with Endothelial Dysfunction during Experimental Human Endotoxemia. Intensive Care Med. Exp. 2014, 2. [Google Scholar] [CrossRef]
- Arman, Y.; Akpinar, T.S.; Kose, M.; Emet, S.; Yuruyen, G.; Akarsu, M.; Ozcan, M.; Yegit, O.; Cakmak, R.; Altun, O.; et al. Effect of Glycemic Regulation on Endocan Levels in Patients With Diabetes: A Preliminary Study. Angiology 2016, 67, 239–244. [Google Scholar] [CrossRef]
- Türer, Ç.C.; Durmuş, D.; Balli, U.; Güven, B. Effect of Non-Surgical Periodontal Treatment on Gingival Crevicular Fluid and Serum Endocan, Vascular Endothelial Growth Factor-A, and Tumor Necrosis Factor-Alpha Levels. J. Periodontol. 2017, 88, 493–501. [Google Scholar] [CrossRef]
- Kumar, G.; Ponnaiyan, D.; Parthasarathy, H.; Tadepalli, A.; Veeramani, S. Evaluation of Endocan and Tumor Necrosis Factor-α as Inflammatory Biomarkers in Type 2 Diabetes and Periodontal Disease. Genet. Test. Mol. Biomarkers 2020, 24, 431–435. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Lackey, D.E.; Olefsky, J.M. Regulation of Metabolism by the Innate Immune System. Nat. Rev. Endocrinol. 2016, 12, 15–20. [Google Scholar] [CrossRef]
- Ling, M.R.; Chapple, I.L.C.; Matthews, J.B. Peripheral Blood Neutrophil Cytokine Hyper-Reactivity in Chronic Periodontitis. Innate Immun. 2015, 21, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, G.; Shapira, L. How Has Neutrophil Research Improved Our Understanding of Periodontal Pathogenesis? J. Clin. Periodontol. 2011, 38 Suppl 11, 49–59. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Sonnenschein, S.K.; Groeger, S.E.; Ewald, N.; Arneth, B.; Meyle, J. Refractory Neutrophil Activation in Type 2 Diabetics with Chronic Periodontitis. J. Periodontal Res. 2020, 55, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Manosudprasit, A.; Kantarci, A.; Hasturk, H.; Stephens, D.; Van Dyke, T.E. Spontaneous PMN Apoptosis in Type 2 Diabetes and the Impact of Periodontitis. J. Leukoc. Biol. 2017, 102, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Dhir, S.; Wangnoo, S.; Kumar, V. Impact of Glycemic Levels in Type 2 Diabetes on Periodontitis. Indian J. Endocrinol. Metab. 2018, 22, 672–677. [Google Scholar] [CrossRef]
- Mesia, R.; Gholami, F.; Huang, H.; Clare-Salzler, M.; Aukhil, I.; Wallet, S.M.; Shaddox, L.M. Systemic Inflammatory Responses in Patients with Type 2 Diabetes with Chronic Periodontitis. BMJ open diabetes Res. care 2016, 4. [Google Scholar] [CrossRef]
- Grover, H.; Luthra, S. Molecular Mechanisms Involved in the Bidirectional Relationship between Diabetes Mellitus and Periodontal Disease. J. Indian Soc. Periodontol. 2013, 17, 292–301. [Google Scholar] [CrossRef]
- Sapna, G.; Gokul, S.; Bagri-Manjrekar, K. Matrix Metalloproteinases and Periodontal Diseases. Oral Dis. 2014, 20, 538–550. [Google Scholar] [CrossRef]
- Collazos, J.; Asensi, V.; Martin, G.; Montes, A.H.; Suárez-Zarracina, T.; Valle-Garay, E. The Effect of Gender and Genetic Polymorphisms on Matrix Metalloprotease (MMP) and Tissue Inhibitor (TIMP) Plasma Levels in Different Infectious and Non-Infectious Conditions. Clin. Exp. Immunol. 2015, 182, 213–219. [Google Scholar] [CrossRef]
- Heikkinen, A.M.; Kettunen, K.; Kovanen, L.; Haukka, J.; Elg, J.; Husu, H.; Tervahartiala, T.; Pussinen, P.; Meurman, J.; Sorsa, T. Inflammatory Mediator Polymorphisms Associate with Initial Periodontitis in Adolescents. Clin. Exp. Dent. Res. 2016, 2, 208–215. [Google Scholar] [CrossRef]
- Woessner, J.F. Matrix Metalloproteinases and Their Inhibitors in Connective Tissue Remodeling. FASEB J. 1991, 5, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Bătăiosu, M.; Taisescu, C.I.; Pisoschi, C.G.; Pascu, E.I.; Ţuculină, M.J.; Dăguci, L.; Dăguci, C.; Baniţă, I.M. Effects of Therapy with Two Combinations of Antibiotics on the Imbalance of MMP-2÷TIMP-2 in Chronic Periodontitis. Rom. J. Morphol. Embryol. 2015, 56, 77–83. [Google Scholar] [PubMed]
- Costa Fernandes, C.J. da; Zambuzzi, W.F. Fibroblast-Secreted Trophic Factors Contribute with ECM Remodeling Stimulus and Upmodulate Osteocyte Gene Markers in Osteoblasts. Biochimie 2020, 168, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lira-Junior, R.; Öztürk, V.Ö.; Emingil, G.; Bostanci, N.; Boström, E.A. Salivary and Serum Markers Related to Innate Immunity in Generalized Aggressive Periodontitis. J. Periodontol. 2017, 88, 1339–1347. [Google Scholar] [CrossRef]
- Barreiros, D.; Nelson-Filho, P.; Paula-Silva, F.W.G.; de Oliveira, K.M.H.; Lucisano, M.P.; de Rossi, A.; Silva, L.A.B.; Küchler, E.C.; Silva, R.A.B. MMP2 and MMP9 Are Associated with Apical Periodontitis Progression and Might Be Modulated by TLR2 and MyD88. Braz. Dent. J. 2018, 29, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Arreguin-Cano, J.A.; Ayerdi-Nájera, B.; Tacuba-Saavedra, A.; Navarro-Tito, N.; Dávalos-Martínez, A.; Emigdio-Vargas, A.; Barrera-Rodríguez, E.; Blanco-García, N.; Gutiérrez-Venegas, G.; Ventura-Molina, E.; et al. MMP-2 Salivary Activity in Type 2 Diabetes Mellitus Patients. Diabetol. Metab. Syndr. 2019, 11. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Bostanci, N.; Emingil, G.; Saygan, B.; Turkoglu, O.; Atilla, G.; Curtis, M.A.; Belibasakis, G.N. Expression and Regulation of the NALP3 Inflammasome Complex in Periodontal Diseases. Clin. Exp. Immunol. 2009, 157, 415–422. [Google Scholar] [CrossRef]
- Park, E.; Na, H.S.; Song, Y.R.; Shin, S.Y.; Kim, Y.M.; Chung, J. Activation of NLRP3 and AIM2 Inflammasomes by Porphyromonas Gingivalis Infection. Infect. Immun. 2014, 82, 112–123. [Google Scholar] [CrossRef]
- Jourdan, T.; Godlewski, G.; Cinar, R.; Bertola, A.; Szanda, G.; Liu, J.; Tam, J.; Han, T.; Mukhopadhyay, B.; Skarulis, M.C.; et al. Activation of the Nlrp3 Inflammasome in Infiltrating Macrophages by Endocannabinoids Mediates Beta Cell Loss in Type 2 Diabetes. Nat. Med. 2013, 19, 1132–1140. [Google Scholar] [CrossRef]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 Inflammasome: A Sensor for Metabolic Danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, X.; Ni, J.; Xie, B.; Liu, Y.; Xuan, D.; Zhang, J. Hyperglucose Contributes to Periodontitis: Involvement of the NLRP3 Pathway by Engaging the Innate Immunity of Oral Gingival Epithelium. J. Periodontol. 2015, 86, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Hou, L.T.; Wong, M.Y.; Rossomando, E.F. Relationships between Clinical Parameters, Interleukin 1B and Histopathologic Findings of Gingival Tissue in Periodontitis Patients. Cytokine 1996, 8, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Brock, M.; Bahammam, S.; Sima, C. The Relationships Among Periodontitis, Pneumonia and COVID-19. Front. oral Heal. 2022, 2. [Google Scholar] [CrossRef]
- Gupta, S.; Mohindra, R.; Singla, M.; Khera, S.; Sahni, V.; Kanta, P.; Soni, R.K.; Kumar, A.; Gauba, K.; Goyal, K.; et al. The Clinical Association between Periodontitis and COVID-19. Clin. Oral Investig. 2022, 26, 1361–1374. [Google Scholar] [CrossRef]
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between Periodontitis and Severity of COVID-19 Infection: A Case-Control Study. J. Clin. Periodontol. 2021, 48, 483–491. [Google Scholar] [CrossRef]
- Anand, P.S.; Jadhav, P.; Kamath, K.P.; Kumar, S.R.; Vijayalaxmi, S.; Anil, S. A Case-Control Study on the Association between Periodontitis and Coronavirus Disease (COVID-19). J. Periodontol. 2022, 93, 584–590. [Google Scholar] [CrossRef]
- Kalsi, R.; Ahmad, Z.; Siddharth, M.; Vandana, K.; Arora, S.; Saurav, K. Correlation of COVID-19 with Severity of Periodontitis-A Clinical and Biochemical Study. Indian J. Dent. Res. 2022, 33, 307–312. [Google Scholar] [CrossRef]
- Sánchez Sánchez, R.J.; Sigcho Romero, C.R.; Niño Peña, A. Una Díada de Riesgo: Periodontitis y COVID-19. Gac. méd. espirit 2022, 2420. [Google Scholar]
- Sukumar, K.; Tadepalli, A. Nexus between COVID-19 and Periodontal Disease. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef]
- Campisi, G.; Bizzoca, M.E.; Lo Muzio, L. COVID-19 and Periodontitis: Reflecting on a Possible Association. Head Face Med. 2021, 17. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, F.J.; Márquez-Arrico, C.F. COVID-19 and Periodontitis: A Dangerous Association? Front. Pharmacol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Sun, W.; Wang, K.; Li, W.; Lin, J.; Gong, J.; Wang, L. Periodontitis and COVID-19: Immunological Characteristics, Related Pathways, and Association. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Q.; Lv, C.; Chen, Y.; Zhao, W.; Li, W.; Chen, H.; Wang, H.; Sun, W.; Yuan, H. NLRP3 Regulates Alveolar Bone Loss in Ligature-Induced Periodontitis by Promoting Osteoclastic Differentiation. Cell Prolif. 2021, 54. [Google Scholar] [CrossRef]
- Brodin, P. Immune Determinants of COVID-19 Disease Presentation and Severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 Inflammasome in Cancer and Metabolic Diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Mainas, G.; Nibali, L.; Ide, M.; Mahmeed, W. Al; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; et al. Associations between Periodontitis, COVID-19, and Cardiometabolic Complications: Molecular Mechanisms and Clinical Evidence. Metabolites 2022, 13. [Google Scholar] [CrossRef]
- Ariana Dalys, C.L.; Fabricio Miltom, L. Periodontal Disease and COVID-19: Prognosis and Potential Pathways of Association in Their Pathogenesis. Can J Dent Hyg 2023, 57, 44–51. [Google Scholar]
- Turk Wensveen, T.; Gašparini, D.; Rahelić, D.; Wensveen, F.M. Type 2 Diabetes and Viral Infection; Cause and Effect of Disease. Diabetes Res. Clin. Pract. 2021, 172. [Google Scholar] [CrossRef]
- Shenoy, A.; Ismaily, M.; Bajaj, M. Diabetes and Covid-19: A Global Health Challenge. BMJ open diabetes Res. care 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- You, J.H.; Lee, S.A.; Chun, S.Y.; Song, S.O.; Lee, B.W.; Kim, D.J.; Boyko, E.J. Clinical Outcomes of COVID-19 Patients with Type 2 Diabetes: A Population-Based Study in Korea. Endocrinol. Metab. (Seoul, Korea) 2020, 35, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Rohli, K.E.; Shen, P.; Lu, H.; Liu, Y.; Dou, Q.; Zhang, L.; Kong, X.; Yang, S.; Jia, P. The Epidemiology, Pathophysiological Mechanisms, and Management toward COVID-19 Patients with Type 2 Diabetes: A Systematic Review. Prim. Care Diabetes 2021, 15, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Casillas Santana, M.A.; Arreguín Cano, J.A.; Dib Kanán, A.; Dipp Velázquez, F.A.; Munguía, P.D.C.S.; Martínez Castañón, G.A.; Castillo Silva, B.E.; Sámano Valencia, C.; Salas Orozco, M.F. Should We Be Concerned about the Association of Diabetes Mellitus and Periodontal Disease in the Risk of Infection by SARS-CoV-2? A Systematic Review and Hypothesis. Medicina (Kaunas). 2021, 57. [Google Scholar] [CrossRef]
- Kinane, D.F. Causation and Pathogenesis of Periodontal Disease. Periodontol. 2000 2001, 25, 8–20. [Google Scholar] [CrossRef]
- Torres-Tamayo, M.; Caracas-Portillo, N.A.; Peña-Aparicio, B.; Juárez-Rojas, J.G.; Medina-Urrutia, A.X.; Martínez-Alvarado, M. del R.; Torres-Tamayo, M.; Caracas-Portillo, N.A.; Peña-Aparicio, B.; Juárez-Rojas, J.G.; et al. Infección Por Coronavirus En Pacientes Con Diabetes. Arch. Cardiol. México 2020, 90, 67–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




