Submitted:
07 November 2024
Posted:
08 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Scaling Procedure
3. Resulting Scaling Factors
4. Illustrative Cases
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MW | microwave |
| MMW | millimeter wave |
| THZ | terahertz |
| QC | quantum-chemical |
| DFT | density functional theory |
| rRMSD | relative root-mean-square deviation |
| MAD | mean absolute deviation |
References
- Morino, Y.; Hirota, E. Microwave Spectroscopy. Annual Review of Physical Chemistry 1969, 20, 139–166. [Google Scholar] [CrossRef]
- Park, G.B.; Field, R.W. Perspective: The first ten years of broadband chirped pulse Fourier transform microwave spectroscopy. The Journal of Chemical Physics 2016, 144, 200901. [Google Scholar] [CrossRef] [PubMed]
- Prozument, K.; Barratt Park, G.; Shaver, R.G.; Vasiliou, A.K.; Oldham, J.M.; David, D.E.; Muenter, J.S.; Stanton, J.F.; Suits, A.G.; Barney Ellison, G.; Field, R.W. Chirped-pulse millimeter-wave spectroscopy for dynamics and kinetics studies of pyrolysis reactions. Phys. Chem. Chem. Phys. 2014, 16, 15739–15751. [Google Scholar] [CrossRef] [PubMed]
- Cuisset, A.; Hindle, F.; Mouret, G.; Bocquet, R.; Bruckhuisen, J.; Decker, J.; Pienkina, A.; Bray, C.; Fertein, E.; Boudon, V. Terahertz Rotational Spectroscopy of Greenhouse Gases Using Long Interaction Path-Lengths. Applied Sciences 2021, 11. [Google Scholar] [CrossRef]
- Swearer, D.F.; Gottheim, S.; Simmons, J.G.; Phillips, D.J.; Kale, M.J.; McClain, M.J.; Christopher, P.; Halas, N.J.; Everitt, H.O. Monitoring Chemical Reactions with Terahertz Rotational Spectroscopy. ACS Photonics 2018, 5, 3097–3106. [Google Scholar] [CrossRef]
- Giesen, T.; Brünken, S.; Caris, M.; Neubauer-Guenther, P.; Fuchs, U.; Lewen, F. Terahertz Rotational Spectroscopy. 2005, Vol. 231, pp. 87–96. [CrossRef]
- Pinacho, P.; Quesada-Moreno, M.M.; Schnell, M. Conformations of borneol and isoborneol in the gas phase: Their monomers and microsolvation clusters. The Journal of Chemical Physics 2023, 159, 194305. [Google Scholar] [CrossRef]
- Loru, Donatella.; Cabezas, Carlos.; Cernicharo, José.; Schnell, Melanie.; Steber, Amanda L.. Detection of ethynylbenzene in TMC-1 and the interstellar search for 1,2-diethynylbenzene. A&A 2023, 677, A166. [CrossRef]
- Xie, F.; Sun, W.; Hartwig, B.; Obenchain, D.A.; Schnell, M. Hydrogen-Atom Tunneling in a Homochiral Environment. Angewandte Chemie International Edition 2023, 62, e202308273. [Google Scholar] [CrossRef]
- Loru, D.; Steber, A.L.; Pérez, C.; Obenchain, D.A.; Temelso, B.; López, J.C.; Schnell, M. Quantum Tunneling Facilitates Water Motion across the Surface of Phenanthrene. Journal of the American Chemical Society 2023, 145, 17201–17210. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tikhonov, D.S.; Schnell, M. Double Proton Transfer Across a Table: The Formic Acid Dimer–Fluorobenzene Complex. Angewandte Chemie International Edition 2021, 60, 25674–25679. [Google Scholar] [CrossRef]
- Nguyen, H.V.L.; Caminati, W.; Grabow, J.U. The LAM of the Rings: Large Amplitude Motions in Aromatic Molecules Studied by Microwave Spectroscopy. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, M.; Trombetti, A.; Velino, B.; Vrbancich, J. The rotation-inversion spectrum of 15NH3. Journal of Molecular Spectroscopy 1980, 83, 401–407. [Google Scholar] [CrossRef]
- Krin, A.; Quesada Moreno, M.M.; Pérez, C.; Schnell, M. A Scent of Peppermint—A Microwave Spectroscopy Analysis on the Composition of Peppermint Oil. Symmetry 2022, 14. [Google Scholar] [CrossRef]
- Sun, W.; Pinacho, P.; Obenchain, D.A.; Schnell, M. Gas-Phase Characterization of Adipic Acid, 6-Hydroxycaproic Acid, and Their Thermal Decomposition Products by Rotational Spectroscopy. The Journal of Physical Chemistry Letters 2024, 15, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Tretyakov, M. Spectroscopy underlying microwave remote sensing of atmospheric water vapor. Journal of Molecular Spectroscopy 2016, 328, 7–26. [Google Scholar] [CrossRef]
- Muhleman, D.O.; Clancy, R.T. Microwave spectroscopy of the Mars atmosphere. Appl. Opt. 1995, 34, 6067–6080. [Google Scholar] [CrossRef]
- Guélin, M.; Cernicharo, J. Organic Molecules in Interstellar Space: Latest Advances. Frontiers in Astronomy and Space Sciences 2022, 9. [Google Scholar] [CrossRef]
- Gordy, W.; Cook, R. Microwave molecular spectra, 3rd ed. ed.; Wiley New York, 1984.
- Atkins, P.; Friedman, R. Molecular Quantum Mechanics; OUP Oxford, 2011.
- Cristina Puzzarini, J.F.S.; Gauss, J. Quantum-chemical calculation of spectroscopic parameters for rotational spectroscopy. International Reviews in Physical Chemistry 2010, 29, 273–367. [Google Scholar] [CrossRef]
- Gottschalk, H.C.; Poblotzki, A.; Fatima, M.; Obenchain, D.A.; Pérez, C.; Antony, J.; Auer, A.A.; Baptista, L.; Benoit, D.M.; Bistoni, G.; Bohle, F.; Dahmani, R.; Firaha, D.; Grimme, S.; Hansen, A.; Harding, M.E.; Hochlaf, M.; Holzer, C.; Jansen, G.; Klopper, W.; Kopp, W.A.; Krasowska, M.; Kröger, L.C.; Leonhard, K.; Mogren Al-Mogren, M.; Mouhib, H.; Neese, F.; Pereira, M.N.; Prakash, M.; Ulusoy, I.S.; Mata, R.A.; Suhm, M.A.; Schnell, M. The first microsolvation step for furans: New experiments and benchmarking strategies. The Journal of Chemical Physics 2020, 152, 164303. [CrossRef]
- Tikhonov, D.S.; Vishnevskiy, Y.V. Describing nuclear quantum effects in vibrational properties using molecular dynamics with Wigner sampling. Phys. Chem. Chem. Phys. 2023, 25, 18406–18423. [Google Scholar] [CrossRef]
- Mata, R.A.; Suhm, M.A. Benchmarking Quantum Chemical Methods: Are We Heading in the Right Direction? Angewandte Chemie International Edition 2017, 56, 11011–11018. [Google Scholar] [CrossRef] [PubMed]
- Pulay, P.; Fogarasi, G.; Pongor, G.; Boggs, J.E.; Vargha, A. Combination of theoretical ab initio and experimental information to obtain reliable harmonic force constants. Scaled quantum mechanical (QM) force fields for glyoxal, acrolein, butadiene, formaldehyde, and ethylene. Journal of the American Chemical Society 1983, 105, 7037–7047. [Google Scholar] [CrossRef]
- Irikura, K.K.; Johnson, R.D.; Kacker, R.N. Uncertainties in Scaling Factors for ab Initio Vibrational Frequencies. The Journal of Physical Chemistry A 2005, 109, 8430–8437. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, M.K.; Brauer, B.; Martin, J.M.L. Frequency and Zero-Point Vibrational Energy Scale Factors for Double-Hybrid Density Functionals (and Other Selected Methods): Can Anharmonic Force Fields Be Avoided? The Journal of Physical Chemistry A 2015, 119, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Alecu, I.M.; Zheng, J.; Zhao, Y.; Truhlar, D.G. Computational Thermochemistry: Scale Factor Databases and Scale Factors for Vibrational Frequencies Obtained from Electronic Model Chemistries. Journal of Chemical Theory and Computation 2010, 6, 2872–2887. [Google Scholar] [CrossRef] [PubMed]
- Laury, M.L.; Carlson, M.J.; Wilson, A.K. Vibrational frequency scale factors for density functional theory and the polarization consistent basis sets. Journal of Computational Chemistry 2012, 33, 2380–2387. [Google Scholar] [CrossRef]
- Merrick, J.P.; Moran, D.; Radom, L. An Evaluation of Harmonic Vibrational Frequency Scale Factors. The Journal of Physical Chemistry A 2007, 111, 11683–11700. [Google Scholar] [CrossRef] [PubMed]
- Pople, J.A.; Scott, A.P.; Wong, M.W.; Radom, L. Scaling Factors for Obtaining Fundamental Vibrational Frequencies and Zero-Point Energies from HF/6–31G* and MP2/6–31G* Harmonic Frequencies. Israel Journal of Chemistry 1993, 33, 345–350. [Google Scholar] [CrossRef]
- Khaikin, L.S.; Grikina, O.E.; Vogt, N.; Stepanov, N.F. Interpreting the vibrational spectra of uracil molecules and their deuterated isotopomers using a scaled quantum-chemical quadratic force field. Russian Journal of Physical Chemistry A 2012, 86, 1855–1861. [Google Scholar] [CrossRef]
- Tikhonov, D.S.; Gordiy, I.; Iakovlev, D.A.; Gorislav, A.A.; Kalinin, M.A.; Nikolenko, S.A.; Malaskeevich, K.M.; Yureva, K.; Matsokin, N.A.; Schnell, M. Harmonic scale factors of fundamental transitions for dispersion-corrected quantum chemical methods. ChemPhysChem, n/a, e202400547, [https://chemistry-europe.onlinelibrary.wiley.com/doi/pdf/10.1002/cphc.202400547]. [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. The Journal of Chemical Physics 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Physical Review A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. The Journal of Chemical Physics 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. Journal of Computational Chemistry 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A generally applicable atomic-charge dependent London dispersion correction. The Journal of Chemical Physics 2019, 150, 154122. [Google Scholar] [CrossRef]
- Grimme, S.; Brandenburg, J.G.; Bannwarth, C.; Hansen, A. Consistent structures and interactions by density functional theory with small atomic orbital basis sets. The Journal of Chemical Physics 2015, 143, 054107. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. The Journal of Chemical Physics 2021, 154, 064103. [Google Scholar] [CrossRef]
- Fokin, A.A.; Zhuk, T.S.; Blomeyer, S.; Pérez, C.; Chernish, L.V.; Pashenko, A.E.; Antony, J.; Vishnevskiy, Y.V.; Berger, R.J.F.; Grimme, S.; Logemann, C.; Schnell, M.; Mitzel, N.W.; Schreiner, P.R. Intramolecular London Dispersion Interaction Effects on Gas-Phase and Solid-State Structures of Diamondoid Dimers. Journal of the American Chemical Society 2017, 139, 16696–16707. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. The Journal of Chemical Physics 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Computational Molecular Science 2022, 12, e1606. [Google Scholar] [CrossRef]
- Vishnevskiy, Y.V. UNEX version 1.6, https://unex.vishnevskiy.group (accessed Fri Oct 13, 2023).
- Arenas, B.E.; Batra, G.; Steber, A.L.; Bizzocchi, L.; Pietropolli Charmet, A.; Giuliano, B.M.; Caselli, P.; Harris, B.J.; Pate, B.H.; Guillemin, J.C.; Schnell, M. Rotational spectroscopy of imidazole: Accurate spectroscopic information for three vibrationally excited states and the heavy-atom isotopologues up to 295 GHz. Journal of Molecular Spectroscopy 2021, 378, 111452. [Google Scholar] [CrossRef]
- Kisiel, Z.; Białkowska-Jaworska, E.; Pszczółkowski, L.; Milet, A.; Struniewicz, C.; Moszynski, R.; Sadlej, J. Structure and properties of the weakly bound trimer (H2O)2HCl observed by rotational spectroscopy. The Journal of Chemical Physics 2000, 112, 5767–5776. [Google Scholar] [CrossRef]
- Xie, F.; Tikhonov, D.S.; Schnell, M. Electric nuclear quadrupole coupling reveals dissociation of HCl with a few water molecules. Science 2024, 384, 1435–1440. [Google Scholar] [CrossRef]
- Winnewisser, G.; Maki, A.G.; Johnson, D.R. Rotational constants for HCN and DCN. Journal of Molecular Spectroscopy 1971, 39, 149–158. [Google Scholar] [CrossRef]
- Creswell, R.; Winnewisser, G.; Gerry, M. Rotational spectra of the 13C and 15N isotopic species of cyanoacetylene. Journal of Molecular Spectroscopy 1977, 65, 420–429. [Google Scholar] [CrossRef]
| Method | rRMSD(s) | ||||
| DF | Dn | Basis | |||
| D3(BJ) | def2-SVP | 2.748 | 2.645 | ||
| def2-TZVP | 2.225 | 2.225 | |||
| D4 | def2-SVP | 2.765 | 2.656 | ||
| B3LYP | def2-TZVP | 2.244 | 2.244 | ||
| D3(BJ) | def2-SVP | 2.633 | 2.579 | ||
| def2-TZVP | 2.530 | 2.202 | |||
| D4 | def2-SVP | 2.637 | 2.583 | ||
| PBE0 | def2-TZVP | 2.535 | 2.206 | ||
| PBEh-3c | 3.092 | 2.778 | |||
| SCAN-3c | 2.689 | 2.577 | |||
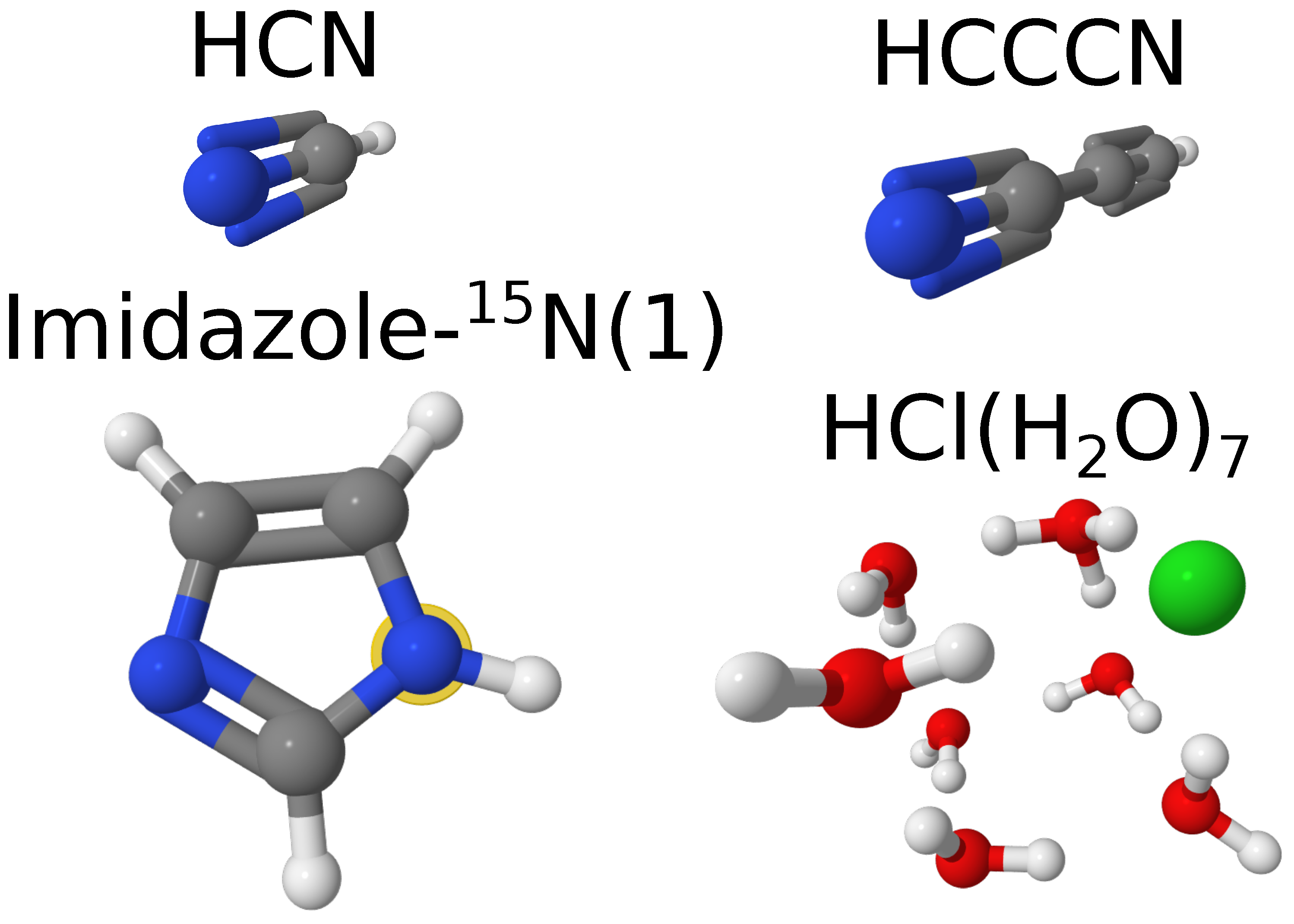
| Molecular system | Rotational constant value [MHz] | ||||
| Experimental | Theoretical | ||||
| B3LYP | PBE0 | ||||
| HCN [50] | B | 44316 | 44941 | 44969 | 44415 |
| HCCCN [51] | B | 4549 | 4591 | 4593 | 4537 |
| N(1) [47] | A | 9695 | 9756 | 9850 | 9729 |
| B | 9188 | 9218 | 9271 | 9157 | |
| C | 4716 | 4740 | 4776 | 4717 | |
| HCl(O)7 [49] | A | 914 | 935 | 947 | 935 |
| B | 737 | 739 | 750 | 740 | |
| C | 689 | 709 | 720 | 711 | |
| Dataset | rRMSD(s) | MAD(s) [MHz] | |||
| Linear molecules | 1.0 | 0.3 | 33 | 16 | |
| Isotopologues | 1.4 | 0.4 | 110 | 26 | |
| HCl(O)n | 7.9 | 6.8 | 132 | 115 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





