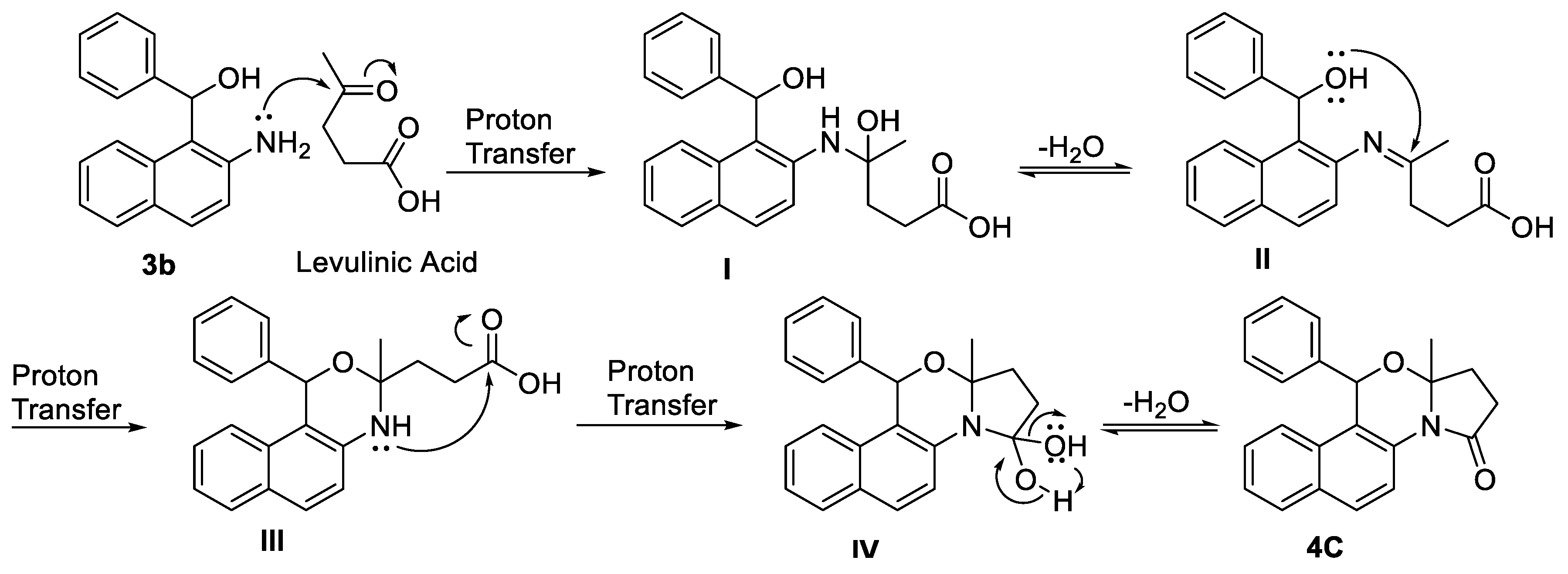
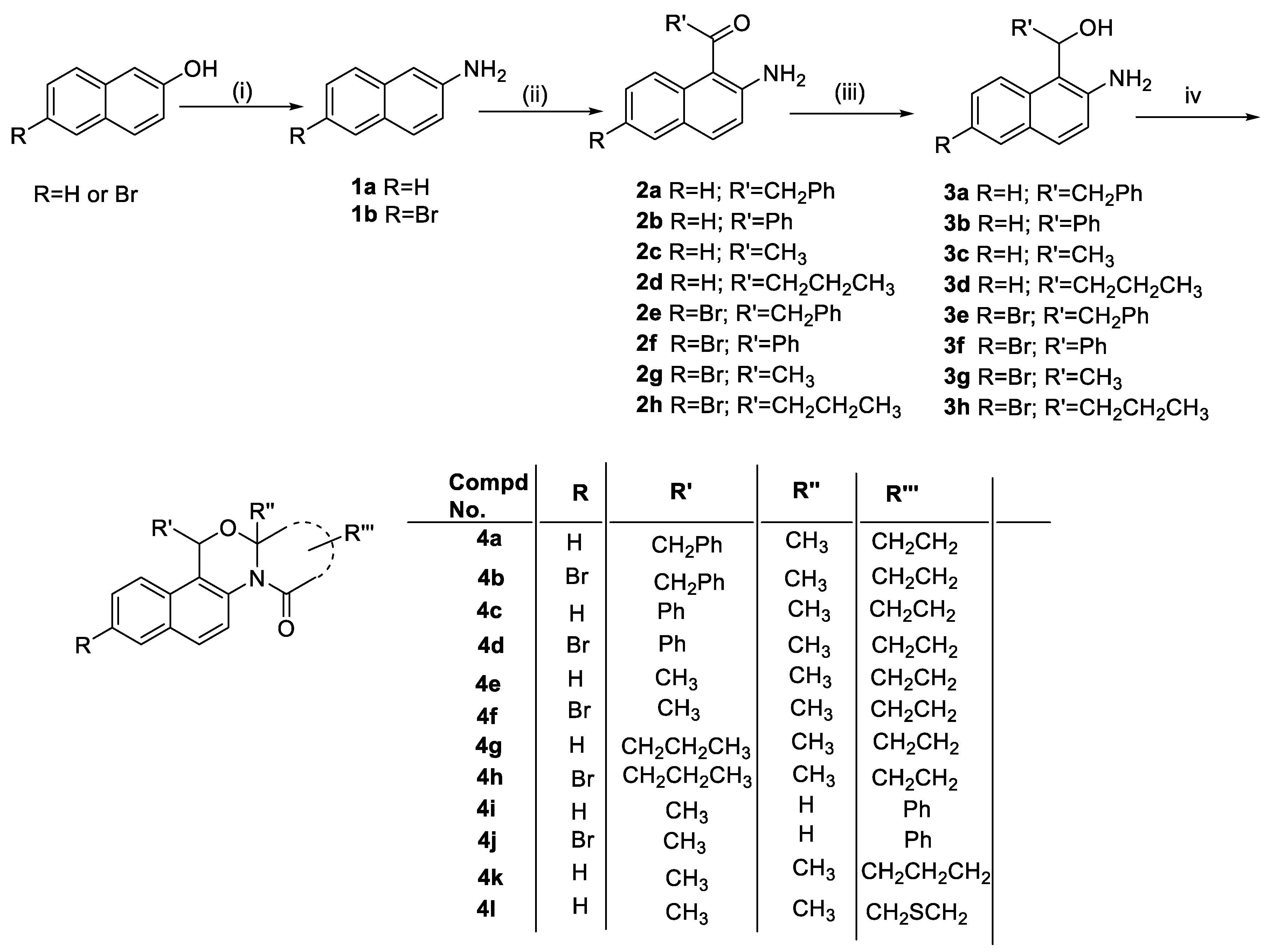4. Materials and Methods
General: All the chemicals and solvents used for these syntheses were obtained from commercial sources and were used without modification, except for toluene and methanol. Toluene was dried with sodium metal overnight and distilled before reactions. Methanol was dried with molecular sieves overnight before use. Among the various keto acids used for our synthesis, levulinic acid, 2-carboxybenzaldehyde and 4-acetylbutyric acids were obtained from A.K. Scientific, Inc. USA. (2-Oxopropyl sulfanyl)-acetic acid was synthesized by following a literature procedure [
20] starting from mercaptoacetic acid (obtained from Sigma Aldrich). TLCs were performed on pre-coated Merck silica gel 60F254 plates with the spots detected under UV light. The silica gel used in column chromatography was 230-400 mesh. NMR spectra were recorded using a Bruker AC-300 Avance spectrometer at 300 MHz for
1H NMR and 75 MHz for
13C{
1H} NMR. The
1H and
13C{
1H} NMR spectra were recorded in either CDCl
3 or DMSO-
d6 solvents.
1H NMR spectra were reported relative to CHCl
3 (δ: 7.26) or DMSO (δ: 2.50).
13C{
1H} NMR spectra were reported relative to CDCl
3 (δ: 77.16) or DMSO-d
6 (δ: 39.52). HRMS (ESI) spectra were recorded using Orbitrap Q-exactive analyser. In X-ray crystallography, the crystal chosen was attached to the tip of a MicroLoop with Paratone-N oil. Measurements were made on a Bruker D8 VENTURE diffractometer equipped with a PHOTON III CMOS detector using monochromated Cu Kα radiation (λ = 1.54178 Å) from an Incoatec micro-focus sealed tube at 100 K. Among the various keto acids used for our synthesis, levulinic acid, 2-carboxybenzaldehyde and 4-acetylbutyric acids were obtained from a commercial supplier, A.K. Scientific, Inc. USA. (2-Oxopropyl sulfanyl)-acetic acid was synthesized by following a literature procedure starting from mercaptoacetic acid [
20], which in turn was obtained from Sigma Aldrich, USA.
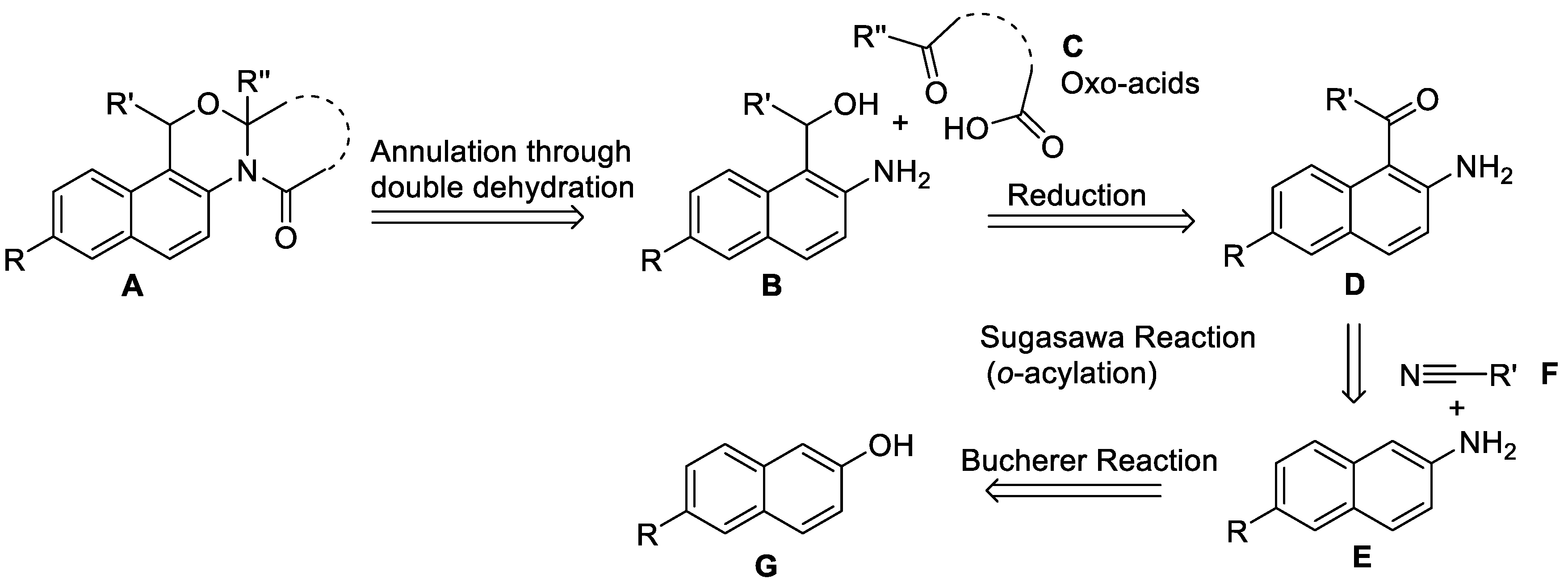
General procedure for the synthesis of 1-acyl-2-aminonaphthalenes (2a-h): In a N2-flushed 250 mL round bottom flask, 2-naphthylamines (2.00 g, 1a: 13.96 mmol; 1b: 9.01 mmol, 1 eq.) and AlCl3 (1.2 eq.) were added. The flask was flushed again with N2. Toluene was added to the reaction mixture followed by BCl3 heptane solution (1.0 M, 1.2 eq.). The R′CN (acetonitrile/butyronitrile/benzonitrile/benzyl nitrile, 6.5 eq) was then added to the reaction mixture. The mixture was refluxed and was monitored with TLC (2a-b: 3.5 h, 2c-d: 2 h, 2e-f: 4 h, 2g-h: 2.5 h). After cooling to room temperature, aq. HCl (1.0 M, 1.2 eq., 2b and 2f: 12.0 M, 500 eq.) was added to the resultant mixture forming a yellow-orange mixture, which was heated at 80 °C for 30 mins (4 hrs for 2b and 2f). During the work up, the reaction mixture was cooled to room temperature and the yellow-orange mixture was poured into ice-water (ca. 50 mL). This was followed by the addition of aq. NaOH (5.0 M) until pH > 13. More ice and NaOH pellets were used as needed with 2b and 2f, to bring the pH >13. The mixture was extracted with EtOAc (3 × 50 mL). The organic layers were added together and washed with brine (50 mL) and dried with anhydrous Na2SO4. The solvent was removed in vacuo to form the crude product. The crude product was purified by column chromatography to yield 2a-h.
1-(2-Aminonaphthalen-1-yl)-2-phenylethanone (2a): Dark orange solid; 50% yield (1.81 g); m.p. 72-73 °C; 1H NMR (CDCl3, 300 MHz): δ 7.85 (d, J = 9.0 Hz, 1H), 7.75 (d, J = 9.0 Hz, 1H), 7.70 (d, J = 9.0 Hz, 1H), 7.51 (t, J = 7.5 Hz, 1H), 7.39-7.28 (m, 6H), 6.83 (d, J = 9.0 Hz, 1H), 5.48 (brs, 2H), 4.36 (s, 2H); 13C{1H} NMR (CDCl3, 75 MHz): δ 203.6, 146.0, 135.6, 133.4, 129.4, 128.7, 128.4, 127.4, 126.6, 124.1, 122.6, 122.6, 119.2, 115.4, 50.0; ESI-HRMS: calcd. C18H16NO: 262.1226 [M+H]+; found 262.1227.
(2-Aminonaphthalen-1-yl)(phenyl)methanone (2b): Yellow crystals; 60% yield (2.06 g); m.p. 166-167 °C. 1H NMR (DMSO, 300 MHz): δ 7.78 (d, J = 9.0 Hz, 1H), 7.71 (d, J = 6.0 Hz, 1H), 7.66 (d, J = 6.0 Hz, 2H), 7.59 (t, J = 6.0 Hz, 1H), 7.46 (t, J = 7.5 Hz, 2H), 7.17-7.06 (m, 4H), 5.93 (brs, 2H); 13C{1H} NMR (DMSO, 75 MHz): δ 197.8, 145.7, 138.6, 132.8, 132.2, 131.4, 129.0, 128.5, 128.0, 126.3, 125.9, 123.3, 121.2, 118.9, 112.4; ESI-HRMS: calcd. C17H14NO: 248.1070 [M+H]; found 248.1070.
1-Acetyl-2-aminonaphthalene (2c): Orange solid; 63% yield (1.62 g); m.p. 107-108 °C. 1H NMR (CDCl3, 300 MHz): δ 7.82 (d, J = 9.0 Hz, 1H), 7.68 (d, J = 6.0 Hz, 1H), 7.65 (d, J = 6.0 Hz, 1H), 7.46 (t, J = 9.0 Hz, 1H), 7.27 (t, J = 9.0 Hz, 1H), 6.85 (d, J = 9.0 Hz, 1H), 5.80 (brs, 2H), 2.70 (s, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 202.9, 146.4, 133.8, 132.6, 128.7, 127.7, 127.4, 124.4, 122.6, 119.5, 115.2, 32.3.
1-(2-Aminonaphalen-1-yl)butan-1-one (2d): Orange oil; 50% yield (1.33 g); 1H NMR (CDCl3, 300 MHz): δ 7.74-7.68 (m, 2H), 7.66 (d, J = 9.0 Hz, 1H), 7.46 (t, J = 9.0 Hz, 1H), 7.28 (t, J = 7.5 Hz, 1H), 6.87 (d, J = 9.0 Hz, 1H), 5.17 (brs, 2H), 2.99 (t, J = 7.5 Hz, 2H), 1.86 (sx, J = 7.8 Hz, 2H), 0.97 (t, J = 6.0 Hz, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 207.7, 144.8, 132.8, 131.2, 128.6, 127.6, 127.2, 124.0, 122.5, 119.6, 116.4, 46.2, 19.3, 13.8; ESI-HRMS: calcd. C14H16NO: 214.1226 [M+H]+; found 214.1231.
1-(2-Amino-6-bromonaphthalen-1-yl)-2-phenylethanone (2e): Dark purple solid; 75% yield (2.29 g); m.p. 101-102 °C; 1H NMR (CDCl3, 300 MHz): δ 7.84 (s, 1H), 7.65 (d, J = 9.0 Hz, 1H), 7.55 (m, 2H), 7.34-7.26 (m, 5H), 6.83 (d, J = 12.0 Hz, 1H), 5.46 (brs, 2H), 4.26 (s, 2H); 13C{1H} NMR (CDCl3, 75 MHz): δ 203.2, 146.0, 135.3, 132.3, 130.6, 130.4, 129.3, 128.7, 128.5, 126.8, 125.7, 120.3, 115.9, 50.1; ESI-HRMS: calcd. C18H15BrNO: 340.0332 [M+H]+; found 340.0335.
(2-Amino-6-bromonaphthalen-1-yl)(phenyl)methanone (2f): Yellow crystals; 65% yield (1.90 g); m.p. 120-121 °C; 1H NMR (DMSO, 300 MHz): δ 7.97 (d, J = 1.5 Hz, 1H), 7.77 (d, J = 12.0 Hz, 1H), 7.64 (d, J = 6.0 Hz, 2H), 7.60 (d, J = 6.0 Hz, 1H), 7.46 (t, J = 7.5 Hz, 2H), 7.27 (d, J = 6.0 Hz, 1H), 7.16 (d, J = 9.0 Hz, 1H), 7.00 (d, J = 9.0 Hz, 1H), 6.03 (s, 2H); 13C{1H} NMR (DMSO, 75 MHz): δ 197.6, 146.4, 138.6, 133.3, 131.1, 130.9, 129.9, 129.3, 128.9, 127.4, 125.6, 120.4, 113.9, 112.3; ESI-HRMS: calcd. C17H13BrNO: 326.0175 [M+H]+; found 326.0184.
1-Acetyl-2-amino-6-bromonaphthalene (2g): Orange solid; 95% yield (2.25 g); m.p. 135-137 °C. 1H NMR (CDCl3, 300 MHz): δ 7.80 (s, 1H), 7.68 (d, J = 9.0 Hz, 1H), 7.55 (d, J = 9.0 Hz, 2H), 6.84 (d, J = 9.0 Hz, 1H), 5.94 (brs, 2H), 2.66 (s, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 202.3, 147.0, 132.7, 131.2, 130.6, 130.4, 130.0, 128.8, 128.2, 125.9, 120.6, 32.2.
1-(2-Amino-6-bromonaphalen-1-yl)butan-1-one (2h): Brown solid; 60% yield (1.57 g); m.p. 42-43 °C; 1H NMR (CDCl3, 300 MHz): δ 7.81 (s, 1H), 7.59-7.47 (m, 3H), 6.86 (d, J = 9.0 Hz, 1H), 5.02 (brs, 2H), 2.92 (t, J = 7.5 Hz, 2H), 1.79 (q, J = 9.0 Hz, 2H), 0.95 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 207.1, 145.1, 131.8, 130.7, 130.5, 130.2, 128.7, 125.6, 120.3, 116.1, 115.7, 46.2, 19.3, 13.8; ESI-HRMS: calcd. C14H15BrNO: 292.0332 [M+H]; found 292.0337.
General procedure for the synthesis of 1-(1-hydroxyalkyl)-2-aminonaphthalenes (3a-h): In a 50 mL flask, 2a-h (500 mg, 1 eq.) were dissolved in a 1:1 mixture of methanol and THF solution (10 mL). The reaction flask was flushed with N2 gas and cooled to 0 °C in an ice bath. While stirring, NaBH4 powder (6 eq., except 2b & 2f: 12 eq) was added in small batches. The reaction was stirred in a round bottom flask with a N2 balloon for 5 mins in the ice bath, and further stirred at room temperature until no starting material was observed on the TLC (ca. 1 hr). The solvent was removed in vacuo leaving a solid. Distilled water (ca. 5 mL) was added and the products were extracted with EtOAc (2 × 25 mL) and washed with distilled water (2 × 10 mL). The organic layer was dried with anhydrous Na2SO4 and the solvent was removed in vacuo yielding the products in pure form.
1-(2-Amimonaphthalen-1-yl)-2-phenylethanol (3a): Orange solid; 93% yield (467 mg); m.p. 129-131 °C; 1H NMR (CDCl3, 300 MHz): δ 7.82 (d, J = 6.0 Hz, 1H), 7.73 (d, J = 6.0 Hz, 1H), 7.61 (d, J = 9.0 Hz, 1H), 7.44 (t, J = 7.5 Hz, 1H), 7.39-7.25 (m, 6H), 6.89 (d, J = 9.0, 1H), 5.85 (dd, J = 9.0 Hz, 3.0 Hz, 1H), 3.81 (brs, 3H), 3.39 (dd, J = 15.0 Hz, 9.0 Hz,1H), 3.12 (dd, J = 12.0 Hz, 3.0 Hz, 1H); 13C{1H} NMR (CDCl3, 75 MHz): δ 143.1, 138.6, 131.8, 129.5, 129.1, 128.8, 128.5, 128.1, 126.6, 126.6, 121.8, 120.6, 120.3, 115.9, 72.0, 40.7; ESI-HRMS: calcd. C18H18NO: 264.1383 [M+H]; found 264.1383.
(2-Aminonaphthalen-1-yl)(phenyl)methanol (3b): Pale orange solid; 94% yield (474 mg); m.p. 131-133 °C; 1H NMR (DMSO, 300 MHz): δ 7.97 (d, J = 9.0 Hz, 1H), 7.67 (d, J = 9.0 Hz, 1H), 7.59 (d, J = 9.0 Hz, 1H), 7.38 (d, J = 6.0 Hz, 2H), 7.31-7.23 (m, 3H), 7.19-7.09 (m, 2H), 7.02 (d, J = 9.0 Hz, 1H), 6.62 (s, 1H), 6.16 (s, 1H), 5.61 (brs, 2H); 13C{1H} NMR (DMSO, 75 MHz): δ 144.7, 144.4, 132.8, 128.4, 128.3, 127.8, 127.0, 126.3, 126.0, 125.9, 122.1, 120.6, 119.9, 116.0, 68.5; ESI-HRMS: calcd. C17H16NO: 250.1226 [M+H]; found 250.1214.
1-(1-Hydroxyethyl)-2-aminonaphthalene (3c): Colourless solid; 98% yield (499 mg); m.p. 89.9-91.2 °C. 1H NMR (CDCl3, 300 MHz): δ 7.78 (d, J = 9.0 Hz, 1H), 7.70 (d, J = 9.0 Hz, 1H), 7.58 (d, J = 9.0 Hz, 1H), 7.41 (t, J = 6.0 Hz, 1H), 7.23 (t, J = 6.0 Hz, 1H), 6.87 (d, J = 9.0 Hz, 1H), 5.91 (q, J = 7.5 Hz, 1H), 4.82 (brs, 1H), 3.33 (brs, 2H), 1.65 (d, J = 7.5 Hz, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 142.8, 131.6, 128.8, 128.7, 127.9, 126.6, 121.8, 120.6, 120.2, 117.4, 67.0, 20.2. ESI-HRMS: calcd. for C12H14NO: 188.1070 [M+H]+; found 188.1068.
1-(2-Aminonaphthalen-1-yl)butan-1-ol (3d): Orange solid; 95% yield (480 mg); m.p. 86-88 °C; 1H NMR (DMSO, 300 MHz): δ 7.87 (d, J = 6.0 Hz, 1H), 7.64 (d, J = 9.0 Hz, 1H), 7.52 (d, J = 9.0 Hz, 1H), 7.32 (t, J = 7.5 Hz, 1H), 7.10 (t, J = 7.5 Hz, 1H), 6.97 (d, J = 9.0, 1H), 5.64 (brs, 2H), 5.53 (brs, 2H), 2.01-1.89 (m, 1H), 1.73-1.62 (m, 1H), 1.57-1.46 (m, 1H), 1.34-1.23 (m, 1H), 0.89 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (DMSO, 75 MHz): δ 144.5, 132.2, 128.4, 127.7, 126.9, 121.2, 120.5, 120.0, 116.5, 68.1, 37.8, 19.1, 14.1; ESI-HRMS: calcd. C14H18NO: 216.1383 [M+H]+; found 216.1386.
1-(2-Amimo-6-bromonaphthalen-1-yl)-2-phenylethanol (3e): Purple solid; 93% yield (468 mg); m.p. 144-146 °C; 1H NMR (DMSO, 300 MHz): 7.86 (s, 1H), 7.54 (d, J = 9.0 Hz, 1H), 7.35 (d, J = 9.0 Hz, 1H), 7.25-7.21 (m, 4H), 7.18-7.12 (m, 2H), 7.06 (d, J = 9.0, 1H), 5.80 (brs, 2H), 5.61 (brs, 1H), δ 5.69 (s, 1H), 3.20 (dd, J = 15.0 Hz, 9.0 Hz, 1H), 2.96 (dd, J = 12.0 Hz, 6.0 Hz, 1H); 13C{1H} NMR (DMSO, 75 MHz): δ 145.1, 140.5, 139.3, 130.7, 129.9, 129.5, 128.4, 128.2, 127.9, 127.3, 125.8, 121.1, 115.9, 113.0, 69.6, 40.4; ESI-HRMS: calcd. C18H17BrNO: 342.0488 [M+H]+; found 342.0495.
(2-Amino-6-bromonaphthalen-1-yl)(phenyl)methanol (3f): Very pale orange solid; 94% yield (474 mg); m.p. 171-172 °C; 1H NMR (DMSO, 300 MHz): δ 7.94-7.89 (m, 2H), 7.58 (d, J = 9.0 Hz, 1H), 7.34 (d, J = 9.0 Hz, 3H), 7.25 (t, J = 7.5 Hz, 2H), 7.16 (t, J = 6.0 Hz, 1H), 7.06 (d, J = 9.0 Hz, 1H), 6.54 (s, 1H), 6.17 (s, 1H), 5.72 (brs, 2H); 13C{1H} NMR (DMSO, 75 MHz): δ 145.2, 144.2, 131.4, 129.8, 128.4, 127.8, 127.7, 126.3, 125.9, 124.9, 120.9, 116.1, 113.1, 68.2; ESI-HRMS: calcd. C17H15BrNO: 328.0332 [M+H]; found 328.0319.
1-(1-Hydroxyethyl)-2-amino-6-bromonaphthalene (3g): Colourless solid; 98% yield (495 mg); m.p. 120-123 °C. 1H NMR (DMSO-d6, 300 MHz): δ 7.90 (s, 1H), 7.87 (d, J = 3.0 Hz, 1H), 7.50 (d, J = 9.0 Hz, 1H), 7.39 (d, J = 3.0 Hz, 1H), 6.99 (d, J = 9.0 Hz, 1H), 5.74 (brs, 2H), 5.59 (q, J = 6.0 Hz, 1H), 5.52 (brs, 1H), 1.41 (d, J = 6.0 Hz, 3H); 13C{1H} NMR (DMSO-d6, 75 MHz): δ 144.6, 130.3, 129.9, 128.4, 128.2, 126.9, 123.7, 121.0, 117.1, 112.9, 64.4, 21.1; ESI-HRMS: calcd. for C12H13BrNO: 266.0175 [M+H]+; found 266.0173.
1-(2-Amino-6-bromonaphthalen-1-yl)butan-1-ol (3h): Brown solid; 98% yield (493 mg); m.p. 138-140 °C; 1H NMR (DMSO, 300 MHz): δ 7.87 (d, J = 1.5 Hz, 2H), 7.52 (d, J = 9.0 Hz, 1H), 7.40 (d, J = 9.0 Hz, 1H), 7.01 (d, J = 9.0, 1H), 5.80 (brs, 2H), 5.61 (brs, 1H), 5.42 (t, J = 6.0 Hz, 1H), 1.96-1.84 (m, 1H), 1.69-1.56 (m, 1H), 1.54-1.42 (m, 1H), 1.30-1.18 (m, 1H), 0.87 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (DMSO, 75 MHz): δ 144.7, 130.8, 129.9, 128.4, 127.0, 121.1, 116.9, 113.0, 68.0, 36.9, 19.1, 14.1; ESI-HRMS: calcd. C14H17BrNO: 294.0488 [M+H]+; found 294.0490.
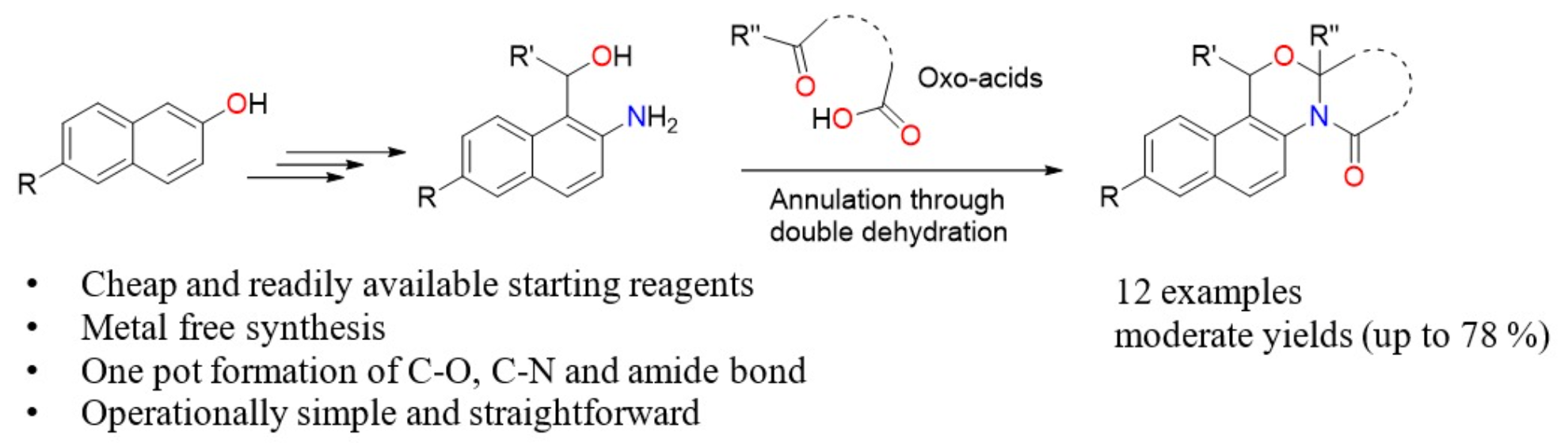
General procedure for the synthesis of 14-aza-12-oxasteroid analogs (4a-l): In a 50 mL round bottom flask with a stir bar, 3a-h (200 mg, 1 eq.) were dissolved in toluene (ca. 5 mL), and then appropriate keto acid [levulinic acid/2-carboxybenzaldehyde/4-acetylbutyric acid/(2-oxopropyl sulfanyl)-acetic acid, 1.5 eq.] was added. The reaction mixture was refluxed for 2 hrs with a Dean–Stark apparatus, with dry toluene filled in the vertical column. The solvent was removed from the reaction mixture in vacuo and the crude product was purified using column chromatography to yield respective 14-aza-12-oxasteroid analogs.
11-Benzyl-12a-methyl-11,12a-dihydro-1H-naphtho[2,1-d]pyrrolo[2,1-b][1,3]oxazin-3(2H)-one (4a): Orange solid; 40% yield (104 mg); m.p. 124-126 °C; 1H NMR (CDCl3, 300 MHz): δ 8.16 (d, J = 9.0 Hz, 1H), 7.93 (t, J = 7.5 Hz, 2H), 7.84 (d, J = 9.0 Hz, 1H), 7.62 (t, J = 7.5 Hz, 1H), 7.52 (t, J = 7.5 Hz, 1H), 7.27-7.22 (m, 3H), 7.07 (d, J = 3.0, 2H), 5.85 (d, J = 3.0 Hz, 1H), 3.43 (d, J = 12.0 Hz, 1H), 3.06 (dd, J = 15.0 Hz, 6.0 Hz, 1H), 2.58 (t, J = 7.5 Hz, 2H), 2.37-2.20 (m, 2H), 1.42 (s, 3H); 13C{1H} NMR (DMSO, 75 MHz): δ 172.1, 137.7, 131.4, 129.5, 129.1, 128.4, 127.8, 126.5, 126.5, 124.9, 122.6, 121.8, 120.7, 89.3, 72.1, 42.8, 32.6, 29.8, 22.4; ESI-HRMS: calcd. C23H22NO2: 344.1645 [M+H]+; found 344.1644.
11-Benzyl-8-bromo-12a-methyl-11,12a-dihydro-1H-naphtho[2,1-d]pyrrolo[2,1-b][1,3]oxazin-3(2H)-one (4b): Purple solid; 33% yield (82 mg); m.p. 106-108 °C; 1H NMR (CDCl3, 300 MHz): δ 8.16 (d, J = 9.0 Hz, 1H), 8.05 (d, J = 1.5 Hz, 1H), 7.79 (d, J = 9.0 Hz, 1H), 7.72 (d, J = 9.0 Hz, 1H), 7.66 (d, J = 9.0 Hz, 1H), 7.23-7.19 (m, 3H), 7.00-6.99 (m, 2H), 5.79 (dd, J =6.0 Hz, 3.0 Hz, 1H), 3.36 (dd, J = 15.0 Hz, 3.0 Hz, 1H), 3.04 (dd, J = 15.0 Hz, 6.0 Hz, 1H), 2.56 (t, J = 7.5 Hz, 2H), 2.18-2.34 (m, 2H), 1.40 (s, 3H); 13C{1H} NMR (DMSO, 75 MHz): δ 172.0, 137.2, 132.3, 131.7, 131.0, 129.8, 129.4, 127.8, 127.5, 126.6, 124.4, 122.0, 121.8, 118.8, 71.9, 89.3, 42.8, 32.6, 29.8, 22.3; ESI-HRMS: calcd. C23H21BrNO2: 421.0677 [M+H]+; found 421.0740.
12a-Methyl-11-phenyl-11,12a-dihydro-1H-naphtho[2,1-d]pyrrolo[2,1-b][1,3]oxazin-3(2H)-one (4c): Yellow-orange powder; 45% yield (119 mg); m.p. 189-191 °C; 1H NMR (CDCl3, 300 MHz): δ 8.46 (d, J = 9.0 Hz, 1H), 7.90 (d, J = 9.0 Hz, 1H), 7.83 (d, J = 9.0 Hz, 1H), 7.48 (d, J = 9.0 Hz, 1H), 7.36 (t, J = 6.0 Hz, 1H), 7.32-7.26 (m, 6H), 6.45 (s, 1H), 2.71-2.66 (m, 2H), 2.29 (t, J = 9.0 Hz, 2H), 1.64 (s, 3H); 13C{1H} NMR (DMSO, 75 MHz): δ 172.6, 141.6, 132.3, 131.5, 129.7, 129.5, 129.2, 129.0, 129.0, 128.8, 126.6, 125.2, 124.6, 120.7, 120.6, 90.6, 75.8, 33.1, 30.6, 22.0; ESI-HRMS: calcd. C22H20NO2: 330.1489 [M+H]+; found 330.1489.
8-Bromo-12a-methyl-11-phenyl-11,12a-dihydro-1H-naphtho[2,1-d]pyrrolo[2,1-b][1,3]oxazin-3(2H)-one (4d): Purple-brown solid; 30% yield (75 mg); m.p. 203-205 °C; 1H NMR (CDCl3, 300 MHz): δ 8.49 (d, J = 9.0 Hz, 1H), 7.97 (s, 1H), 7.79 (d, J = 9.0 Hz, 1H), 7.31-7.27 (m, 5H), 7.23-7.21 (m, 2H), 6.39 (s, 1H), 2.70-2.65 (m, 2H), 2.29 (t, J = 6.0 Hz, 2H), 1.64 (s, 3H); 13C{1H} NMR (DMSO, 75 MHz): δ 172.2, 140.9, 132.3, 132.2, 130.6, 129.5, 128.9, 128.5, 128.1, 127.8, 125.9, 121.4, 120.3, 118.8, 90.2, 75.2, 32.7, 30.1, 21.6; ESI-HRMS: calcd. C22H19BrNO2: 408.0594 [M+H]+; found 408.0584.
11,12a-Dimethyl-11,12a-dihydro-1H-naphtho[2,1-d]pyrrolo[2,1-b][1,3]oxazin-3(2H)-one (4e): Viscous orange liquid; 34% yield (96 mg); 1H NMR (CDCl3, 300 MHz): δ 8.35 (d, J = 1.5 Hz, 1H), 7.87-7.78 (m, 3H), 7.53-7.43 (m, 2H), 5.66 (q, J = 6.0 Hz, 1H), 2.67 (t, J = 9.0 Hz, 2H), 2.30 (t, J = 9.0 Hz, 2H), 1.65 (d, J = 6.0 Hz, 3H), 1.47 (s, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 172.1, 131.2, 130.2, 129.2, 128.9, 128.2, 126.2, 124.8, 123.3, 123.1, 120.4, 89.3, 68.0, 32.8, 30.1, 23.1, 21.5; ESI-HRMS: calcd. for C17H18NO2: 268.1332 [M+H]+; found 268.1328.
8-Bromo-11,12a-dimethyl-11,12a-dihydro-1H-naphtho[2,1-d]pyrrolo[2,1-b][1,3]-oxazin-3(2H)-one (4f): Viscous orange liquid; 35% yield (92 mg); 1H NMR (CDCl3, 300 MHz): δ 8.35 (d, J =1.5 Hz, 1H), 7.98 (s, 1H), 7.69-7.39 (m, 3H), 5.58 (q, J = 6.0 Hz, 1H), 2.65 (t, J = 7.5 Hz, 2H), 2.27 (t, J = 7.5 Hz, 2H), 1.59 (d, J = 6.0 Hz, 3H), 1.43 (s, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 172.2, 132.3, 130.7, 129.4, 127.7, 127.2, 124.8, 123.5, 121.4, 119.0, 118.7, 89.3, 67.7, 32.7, 30.0, 23.1, 21.4; ESI-HRMS: calcd. for C17H17BrNO2: 346.0437 [M+H]+; found 346.0433.
12a-Methyl-11-propyl-11,12a-dihydro-1H-naphtho[2,1-d]pyrrolo[2,1-b][1,3]oxazin-3(2H)-one (4g): Orange solid; yield 45% (123 mg); m.p. 123-125 °C; 1H NMR (CDCl3, 300 MHz): δ 8.27 (d, J = 9.0 Hz, 1H), 7.87-7.76 (m, 3H), 7.52 (t, J = 9.0 Hz, 1H), 7.45 (t, J = 9.0 Hz, 1H), 5.58 (dd, J = 6.0 Hz, 1.5 Hz, 1H), 2.67 (t, J = 7.5 Hz, 2H), 2.30 (t, J = 7.5 Hz, 2H), 2.12-2.01 (m, 1H), 1.87-1.75 (m, 1H), 1.55-1.44 (m, 1H), 1.44 (s, 3H), 1.32-1.21 (m, 1H), 0.87 (t, J = 6.0 Hz, 3H). 13C{1H} NMR (DMSO, 75 MHz): δ 172.3, 131.2, 131.0, 129.2, 128.9, 128.1, 126.2, 124.8, 122.9, 122.6, 120.5, 89.3, 71.3, 38.8, 32.8, 30.1, 21.9, 18.0, 13.9; ESI-HRMS: calcd. C19H22NO2: 296.1645 [M+H]+; found 296.1631.
8-Bromo-12a-methyl-11-propyl-11,12a-dihydro-1H-naphtho[2,1-d]pyrrolo[2,1-b][1,3]-oxazin-3(2H)-one (4h): Purple oil; 40% yield (102 mg); 1H NMR (CDCl3, 300 MHz): δ 8.29 (d, J = 9.0 Hz, 1H), 7.99 (d, J = 1.5 Hz, 1H), 7.70-7.62 (m, 2H), 7.56 (d, J = 9.0 Hz, 1H), 5.53 (dd, J = 6.0 Hz, 3.0 Hz, 1H), 2.66 (t, J = 7.5 Hz, 2H), 2.29 (t, J = 7.5 Hz, 2H), 2.06-1.95 (m, 1H), 1.82-1.70 (m, 1H), 1.51-1.39 (m, 1H), 1.43 (s, 3H), 1.23-1.15 (m, 1H), 0.85 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (DMSO, 75 MHz): δ 172.3, 132.4, 131.3, 130.8, 129.4, 127.6, 127.2, 124.6, 122.7, 121.5, 118.7, 89.3, 71.1, 38.8, 32.8, 30.0, 21.8, 17.9, 13.8; ESI-HRMS: calcd. C19H21BrNO2: 374.0750 [M+H]+; found 374.0739.
7-Methyl-7H-naphtho[2',1':4,5][1,3]oxazino[2,3-a]isoindol-13(8aH)-one (4i): Viscous orange liquid; 78% yield (249 mg). Mixture of two diastereomers (6:4). Data for the major diastereomer are presented here; 1H NMR (CDCl3, 300 MHz): δ 8.56 (d, J = 1.5 Hz, 1H), 7.94-7.43 (m, 9H), 5.95 (q, J = 9.0 Hz, 1H), 5.79 (s, 1H), 1.72 (d, J = 9.0 Hz, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 164.8 140.2, 132.7, 130.2, 128.9, 128.5, 126.6, 126.4, 124.7, 124.6, 124.0, 123.5, 123.2, 123.1, 122.4, 120.8, 119.0, 83.1, 72.4, 23.3; ESI-HRMS: calcd. for C20H16NO2: 302.1176 [M+H]+; found 302.1173.
4-Bromo-7-methyl-7H-naphtho[2',1':4,5][1,3]oxazino[2,3-a]isoindol-13(8aH)-one (4j): Colourless solid; 40% yield (112 mg); m.p. 230-233 °C. Mixture of two diastereomers (8:2). Data for the major diastereomer are presented here; 1H NMR (CDCl3, 300 MHz): δ 8.58 (d, J = 9.0 Hz, 1H), 8.03 (s, 1H), 7.95 (d, J = 9.0 Hz, 1H), 8.03-7.59 (m, 6H), 5.94 (q, J = 6.0 Hz, 1H), 5.87 (s, 1H), 1.70 (d, J = 6.0 Hz, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 164.9, 140.2, 132.9, 132.2, 131.0, 130.5, 130.0, 129.7, 128.2, 127.7, 124.9, 124.2, 123.6, 122.5, 120.3, 118.6, 83.2, 72.3, 23.4; ESI-HRMS: calcd. for C20H15BrNO2: 380.0281 [M+H]+; found 380.0284.
12,13a-Dimethyl-1,2,3,13a-tetrahydronaphtho[2,1-d]pyrido[2,1-b][1,3]oxazin-4(12H)-one (4k): Viscous orange liquid; 30% yield (91 mg); 1H NMR (CDCl3, 300 MHz): δ 7.87-7.70 (m, 4H), 7.55-7.46 (m, 2H), 5.65 (q, J = 6.0 Hz, 1H), 2.63 (t, J = 9.0 Hz, 2H), 2.13 (t, J = 9.0 Hz, 2H), 1.63 (d, J = 6.0 Hz, 3H), 1.38 (s, 3H), 0.83 (t, J = 9.0 Hz, 2H); 13C{1H} NMR (CDCl3, 75 MHz): δ 170.1, 132.8, 131.6, 128.9, 128.8, 127.6, 127.1, 126.2, 125.2, 122.5, 86.1, 67.3, 37.0, 34.1, 25.0, 23.5, 17.0.
12,13a-Dimethyl-12,13a-dihydro-1H-naphtho[2,1-d][1,4]thiazino[3,4-b][1,3]oxazin-4(3H)-one (4l): Viscous orange liquid; 30% yield (92 mg); 1H NMR (CDCl3, 300 MHz): δ 7.87-7.66 (m, 4H), 7.56-7.47 (m, 2H), 5.65 (q, J = 7.5 Hz, 1H), 3.60 (m, 2H), 3.06 (s, 2H), 1.68 (d, J = 7.5 Hz, 3H), 1.54 (s, 3H); 13C{1H} NMR (CDCl3, 75 MHz): δ 165.6, 132.1, 131.6, 128.8, 128.7, 127.2, 126.3, 125.5, 124.6, 122.5, 87.7, 67.5, 39.0, 33.8, 23.4, 23.3; ESI-HRMS: calcd. for C17H18NO2S: 300.1053 [M+H]+; found 300.1047.