1. Introduction
Uncontrolled bleeding may lead to a series of severe morbidities, including hypotension, organ failure, and even death from shock[
1,
2]. Rapid and effective hemostasis intervention is substantial to saving the lives of excessive bleeding patients before professional treatment[
3]. Currently, surgical sutures and staples as the “gold standards” are often used in clinical practice to close wounds and stop bleeding[
4]. Although surgical suture treatment has enhanced wound and surgical care to a certain extent, but its application in numerous cases entails considerable complications[
5]. For instance, preoperative preparation, strict surgical requirements, and long suturing times all make it only able to operate in the operating room, it cannot meet the requirements of first aid for the wounded in an emergency[
6]. Additionally, surgical suturing is a complex and time-consuming procedure that demands high surgical proficiency[
5,
7]. Sutures and staples need to be removed 7-10 days after the surgery to prevent foreign body infection, which not only causes secondary pain to the patient but also prolongs the wound healing time[
8,
9]. Currently, there are many commercially available hemostatic materials such as gauze, sponge, hydrogel, and powder[
10,
11]. Gauze and sponges were used to control bleeding by mechanical pressure and promoting blood cell coagulation[
12]. However, the lack of function in adapting to different wound shapes and the adhesion of biological tissues requires external extrusion to fix the material to the damaged tissue, which is not suitable for irregularly shaped wounds and incompressible wounds in vivo[
13,
14]. Hydrogel is a three-dimensional network of polymers prepared by physical or chemical cross-linking[
15]. They can be used as tissue sealants and adhesives to form a physical hemostatic barrier at the Bleeding site to control bleeding[
16,
17]. However, blood flowing persistently from the damaged site would weaken the adhesion between the hydrogel and wound, resulting in compromised hemostatic abilities[
18,
19]. The hemostatic powder, such as Quik Clot® and Celox® can be applied directly to fill irregular wounds of various shapes and absorb the liquid component of the blood to effectively control bleeding[
20,
21]. However, powder-based hemostatic materials fail to maintain a moist environment and lack of the mechanical strength to form a physical barrier at bleeding tissue[
10]. In addition, some powders are often used with safety concerns such as exothermic, potential metabolic toxicity and even the formation of blood clots[
22]. Therefore, it is essential to develop hemostatic powders with rapid hemostasis and good biosafety.
Recent studies have demonstrated that the self-gelling powder could rapidly absorb interfacial fluid to form hydrogel in situ owing to physical cross-linked interactions among self-gelling powders such as hydrogen bonding and electrostatic interaction and quickly seal damaged tissue despite tissue surface irregularities, making it a potential application in effective hemostasis[
23,
24]. For example, a self-gelling hemostatic powder developed by Tan et al[
12], consist of polyacrylic acid/polyacrylamide/quaternate chitosan (PAA/PAM/QCS). When the self-gelling hemostatic powders in contact with water, the PAA/PAM/QCS can fuse and rapidly form a stable hydrogel in a short time, and can rapidly adsorb lots of blood, aggregate blood cells and platelets. Peng et al.[
1] prepared a self-gelling hemostatic powder base on polyethyleneimine (PEI), polyacrylic acid (PAA), and quaternate chitosan (QCS), which can accelerate hemostasis by absorbing a large amount of blood to concentrate coagulation factors.
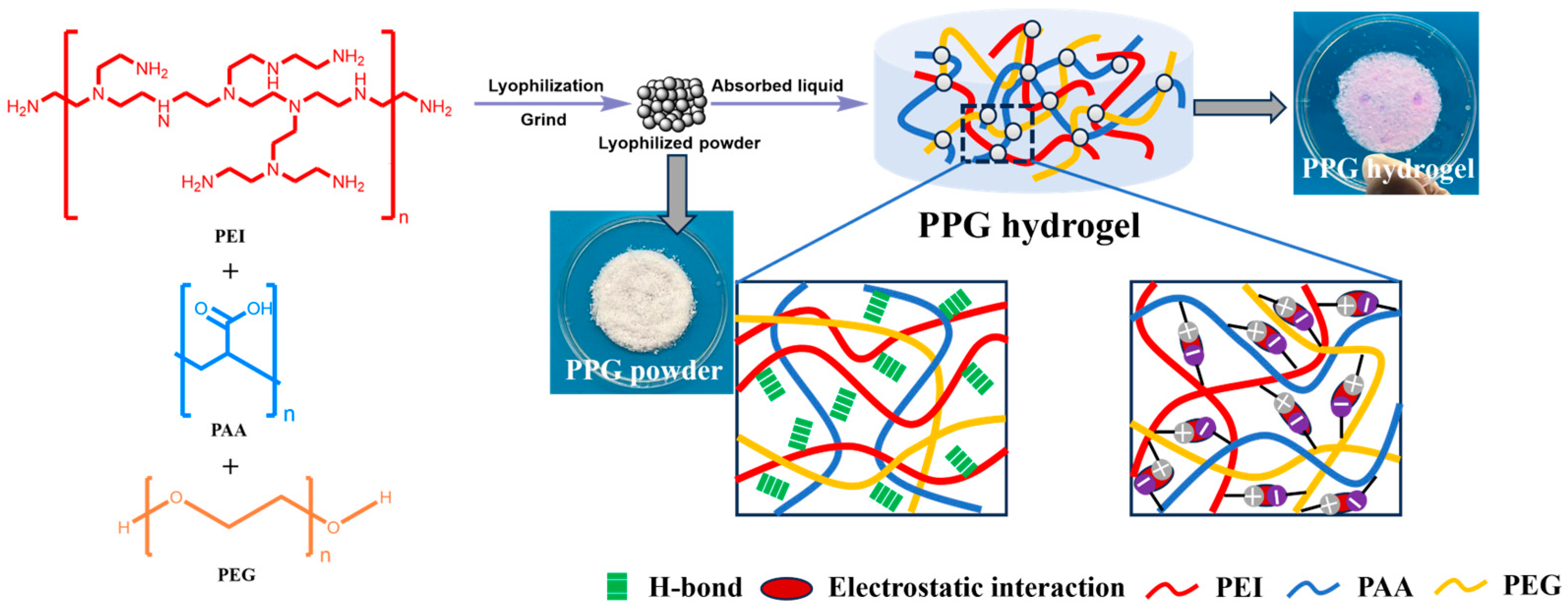
Herin, PAA/PEI/PEG(PPG) self-gelling powder with different PEG content were designed and developed by freeze-drying and grinding a mixture of PAA, PEI and PEG. Subsequently, the physicochemical and biological properties of PPG self-gelling powders were systematically investigated, including that water absorption capacity, tissue adhesion and hemocompatibility. Based on these results, the PPG4 self-gelling powder were preferred. We further measured the antibacterial ability in vitro of PPG4 self-gelling powders by the inhibition rate against both Gram-positive (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli), and the hemostatic effects in vivo using four bleeding models in mice and rabbit (the rat liver bleeding model, rat tail bleeding model and rabbit ear vein and artery bleeding models). The results of antibacterial performance showed that , compared to the control group, PPG4 self-gelling power groups exhibited an excellent bacteriostasis rate against S. aureus (97.0% ± 1.7%) and E. coli (97.6% ± 1.5%). In vivo hemostasis test results exhibited that the PPG4 self-gelling powders can show effective hemostasis, especially for non-compressible wounds, unknown bleeding points and irregularly shaped wounds. Thus, it holds wide application promise as a potential biomaterial for treating acute tissue bleeding.
2. Materials and Methods
2.1. Materials
Polyethyleneimine (PEI, Mw≈70000) aqueous solution (50wt%), was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China), polyacrylic acid (PAA, Mw ≈5000) aqueous solution (50wt%), were purchased from Shanghai Macklin Biochemical Technology Co., Ltd (Shanghai, China). Polyethylene glycol (PEG, Mw = 1000), was purchased from Shanghai yuanye Bio-Technology Co., Ltd (Shanghai, China). Gauze was purchased from Nanchang Zhenfeng Medical Equipment Co., Ltd (Nanchang, China).
2.2. Preparation of PPG self-gelling powder
Briefly, 10 g of PEG was dissolved in 90 g of deionized (DI) water to form a mass fraction of 10wt%. 50wt% aqueous solutions of PEI and PAA were diluted to 10wt% with DI water. Subsequently, 10wt%PEI, 10wt%PAA and 10wt%PEG precursor solution were evenly mixed in different volume ratio, the detailed compositions of PPG self-gelling powder are shown in
Table 1. Next, the obtained mixtures were immediately immersed in liquid nitrogen for about 20 min, then transferred to a vacuum freeze dryer and freeze-dried for 72 h. Finally, the obtained freeze-dried samples were ground with a mortar to obtain PPG powders (
Figure 1). The PPG self-gelling powder were named PPGX, where the number after G represent the volume of PEG in the mixture.
2.3. Characterization of PPG Self-Gelling Powder
We studied the self-gelation time of the PPG self-gelling powder using the inverted tube method.
The Chemical structural and their interactions of self-gelling powder PPG were analyzed by Fourier transform infrared spectroscopy(FTIR, Nicolet 6700, Thermo Fisher, USA) in the wavenumber region of 4000 cm−1 to 500 cm-1.
Seat drop method were utilized by contact angle tester (SDC 350KS, Kunshan Shengding, China) to study the contact angle of the PPG self-gelling powder.
The microstructures and surface morphology of PPG self-gelling powder were characterized using scanning electron microscopy(SEM, Nova NanoSEM450, FEI, USA) under an accelerating voltage of 20 kV after spraying with gold.
The dynamic rheological experiment was tested to study the viscoelasticity of PPG self-gelling powder-derived hydrogel by rheometer (MCR 302, Anton Paar, Graz, Austria) with a 20 mm parallel plate . Briefly, 0.2 g of the PPG self-gelling powders was placed on parallel plates preheated to 37 ◦C, Next, PBS was added dropwise to the PPG self-gelling powders. After the powder were evenly immersed with PBS to prepare PPG self-gelling powder-derived hydrogel. The storage modulus G′ and loss modulus G′′ tests were conducted at 37℃ within the frequency range of 0.1–10 Hz and a strain of 1%.
2.4. The Water Absorption Capacity of the PPG Self-Gelling Powders
The water absorption capacity of PPG self-gelling powder with different composites were tested by immersing the powders in PBS at room temperature until they reached equilibrium. Briefly, the lyophilized sample was weighed as W
0 and were immersed in 25 ml PBS for specific time( 1 min, 2 min, 3 min, 5 min, 8 min, 10 min, 15 min, 20 min, 30 min, 50 min, 80 min, 120 min, 150 min). At a set period of soaking , the sample was removed from solution, and the surface water of these samples was dried with fflter paperas and the weighed as W
1. The swelling ratio of PPG self-gelling powders was calculated according to the Eq. (1):
2.5 Tissue Adhesion Experiments
The adhesion performance of the PPG self-gelling powder on porcine skin were tested by lap shear experiments using a universal electromechanical tester (WDW-05, Si Pai Inc., China). For adhesion test, Fresh porcine skin (50 × 20 mm) was polished and washed with DI water and ethanol before being utilized. Then, the PPG self-gelling powders with different ratios were placed evenly over a 20 × 20 mm square area(S) on the skin tissue, hydrated by a certain amount of PBS onto the PPG self-gelling powders. Subsequently, another piece of porcine skin was quickly pressed onto the powder-coated porcine and pinned down at room temperature for 60min, and then peeled at 180° with a fixed pulling speed of 10 mm/min on a universal electromechanical tester until the hydrogel was completely peeled off from the porcine skin, and the maximum peeling force (F
max) exerted during the stretching process was recorded. The adhesion stress was calculated by Eq. (2):
2.6. In Vitro Hemolysis Assay
The in vitro hemolysis assay was conducted using freshly collected whole blood samples from rabbits to measure the biocompatibility of the PPG self-gelling powder. First, the whole blood samples from rabbits were centrifuged at 3000 rpm for 25 min at room temperature and washed three times with a certain amount of PBS solution to collect precipitated red blood cells (RBCs). Then, the obtained RBCs were diluted in adequate volume of PBS to obtain 2% erythrocyte suspension. Subsequently, 50mg PPG self-gelling powders with different composites were selected as the experimental groups and were added to the prepared RBCs suspension, respectively, and then incubated at 37 °C for 2 h. Besides, PBS and deionized water were set as negative and positive controls. These mixtures were incubated under the same conditions. Finally, all samples were centrifuged for 10 min to obtain the supernatant. The absorbance of the supernatant was investigated three times with a microplate reader at 540 nm. The hemolysis ratio of all samples were calculated in terms of the following Eq(3).
Where OD sample, OD negative, and OD positive are absorbance value of sample, negative control (PBS) and positive control (DI water), respectively.
2.7. Antibacterial Activity Test
The antibacterial abilities of the PPG self-gelling powders were evaluated by the inhibition rate against Escherichia coli (
E. coli, CMCC44102, Gram-negative) and Staphylococcus aureus (
S. aureus, CMCC26003, Gram-positive). The coated plate method was used to measure the antimicrobial properties of the PPG self-gelling powders. Firstly, PPG4 self-gelling powder and 10wt% solution of PEI was added into the 1mL of
E. coli and 1 mL of
S. aureus (1 × 10
7 CFU/ mL) bacterial stock suspensions, respectively. The same volume of E. coli and S. aureus bacterial stock suspensions without adding any substance were used as the positive control groups. Then, they were incubated at 37℃ for 12 h. The resulting bacterial stock suspension were evenly plated onto lysogeny broth (LB) medium after being diluted to a concentration of 10
4 CFU/mL, and then the bacteria and their colonies that formed on each LB medium were photographed and counted after incubation at 37 ℃ for 12 h in a bacterial incubator. For each group, the test was repeated three times, and the ratio was calculated by Eq (4):
Where AB is the number of survivor bacterial of the blank control group, AD is the number of survivor bacterial of determined sample group.
2.8. In Vivo Hemostatic Assay
The in vivo hemostatic effect was measured by four models of the liver bleeding model, tail bleeding model, ear vein and artery bleeding models. All animal experiments were executed according to the Guidelines for Care and Use of Laboratory Animals of Hubei University of science and technology and approved by the Animal Ethics Committee of Hubei University of science and technology, and all animal procedures were conducted in terms of the Guidelines for the Care and Use of Laboratory Animal of Hubei University of science and technology. For the rat liver and tail vein bleeding model, 15 SD rats (8 weeks, female, 180–200 g, Beijing HFK Bio-Technology Co., Ltd, Beijing, China) were randomly divided into 3 groups including control, Gauze and PPG4. In the rat liver hemostasis model, the SD rat was anesthetized with 2% pentobarbital and fixed on the surgical operating table. Subsequently, the left lobe of the rat liver was exposed through a longitudinal abdominal incision and then punctured with a 22 G needle. Next, immediately Gauze, and PPG4 self-gelling powders were applied on the bleeding areas and the duration of the bleeding process was recorded using a digital camera at certain times. At the same time, the untreated bleeding liver model was used as the control. After the bleeding stops, the amount of blood loss and the hemostatic time of different treatment methods were evaluated (n=5). For the bleeding model of rat tail amputation, the part about 2-3 cm away from the tail tip of the rat was cut off, and then the Gauze and PPG4 powder were used on the wound surface, while the untreated wound was set as the control. The hemostasis process was similar to that of the liver bleeding model. The amount of blood lost and the hemostatic time was recorded (n = 5). For the ear vein and artery bleeding models of rabbit. The experiments were operated on male rabbits (1.8–2 kg, Beijing HFK Bio-Technology Co., Ltd, Beijing, China). All rabbits were anesthetized with 2wt% pentobarbital and fixed on the surgical operating table. Subsequently, the ear vein and artery of rabbit was punctured by using a 22 G medical syringe needle to induce bleeding, and then the PPG4 self-gelling powders were put on the bleeding site for treatment(n=5).
2.9. Statistical Analysis
Each experimental data was expressed as the mean ± standard deviations (SD) from at least triplicate independent experiments (n≥3). Significant differences were assessed using one-way ANOVA. The statistical significance was treated as ***p < 0.001, **p < 0.01, *p < 0.05, ns means no significant difference.
3. Results and Discussion
3.1. Gelation Time of the PPG Self-Gelling
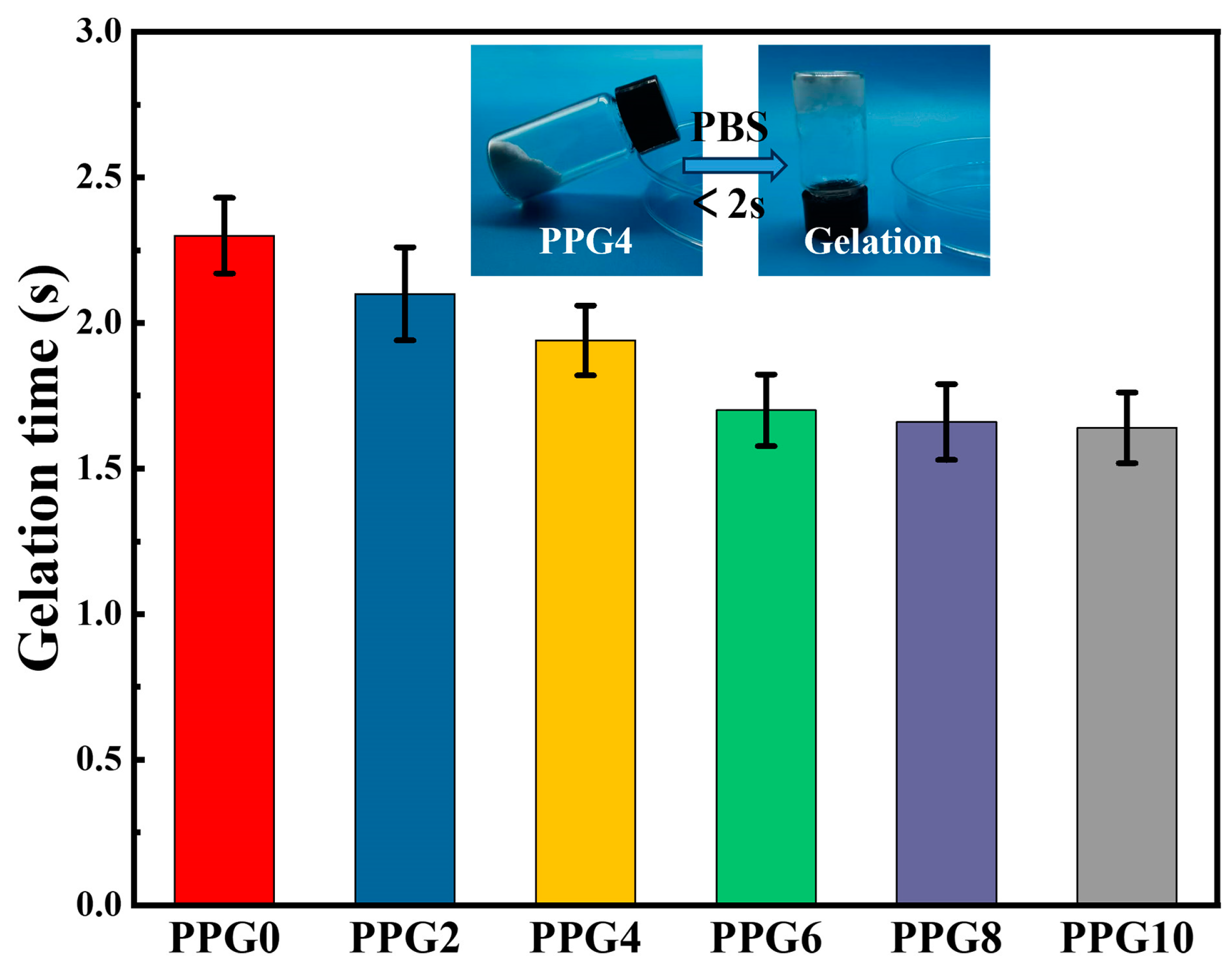
PPG powders were prepared by lyophilization and grinding their complexes. Meanwhile, they can absorb liquid quickly and form hydrogels (
Figure 2). In addition, with increasing mass ratios of PEG, the PEG powder shows a shortened self-gelation time from 2.30 ± 0.13s to 1.64 ± 0.12s (
Figure 2). The possible reason for this phenomenon was that the presence of hydrophilic PEG component obviously enhanced the surface wettability and liquid absorption capacity of PPG self-gelling powder, which can be confirmed by the contact angle data later.
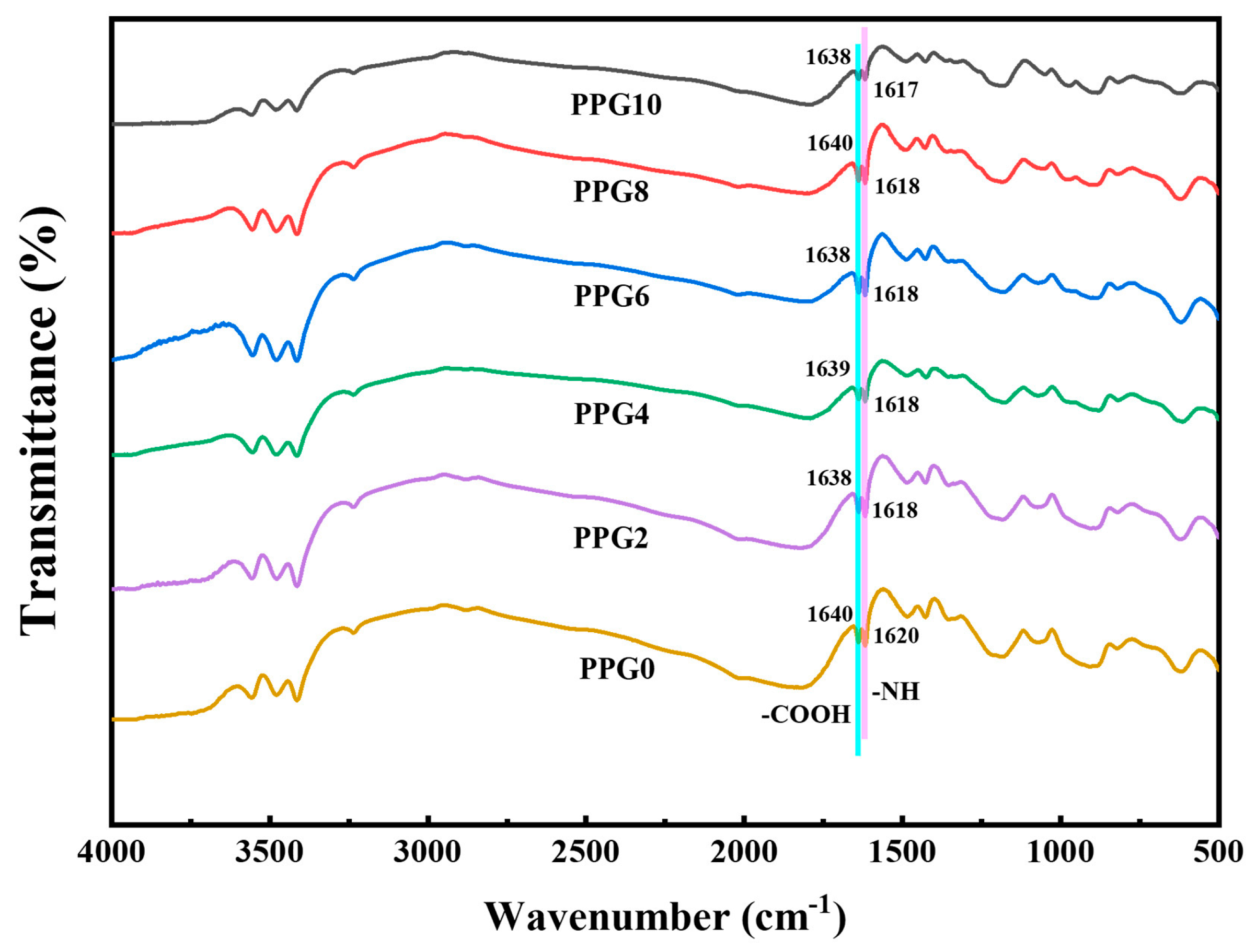
3.2. FTIR Analysis
FTIR spectroscopy was performed to analyze the chemical structural and their interactions of self-gelling powder PPG with different composites. As show in
Figure 3, comparing the FTIR spectra of PPG self-gelling powders with different composites , it can be seen that there was no significant differenc, and the slight shifting of the characteristic carboxylic acid(-COOH) and amine group peaks(-NH or -NH
2) in the PPG self-gelling powders with different composites without generating new characteristic peaks showed that PEI, PAA, and PEG are crosslinked through physical interactions such as hydrogen bonding and electrostatic interaction instead of covalent bonds[
24].
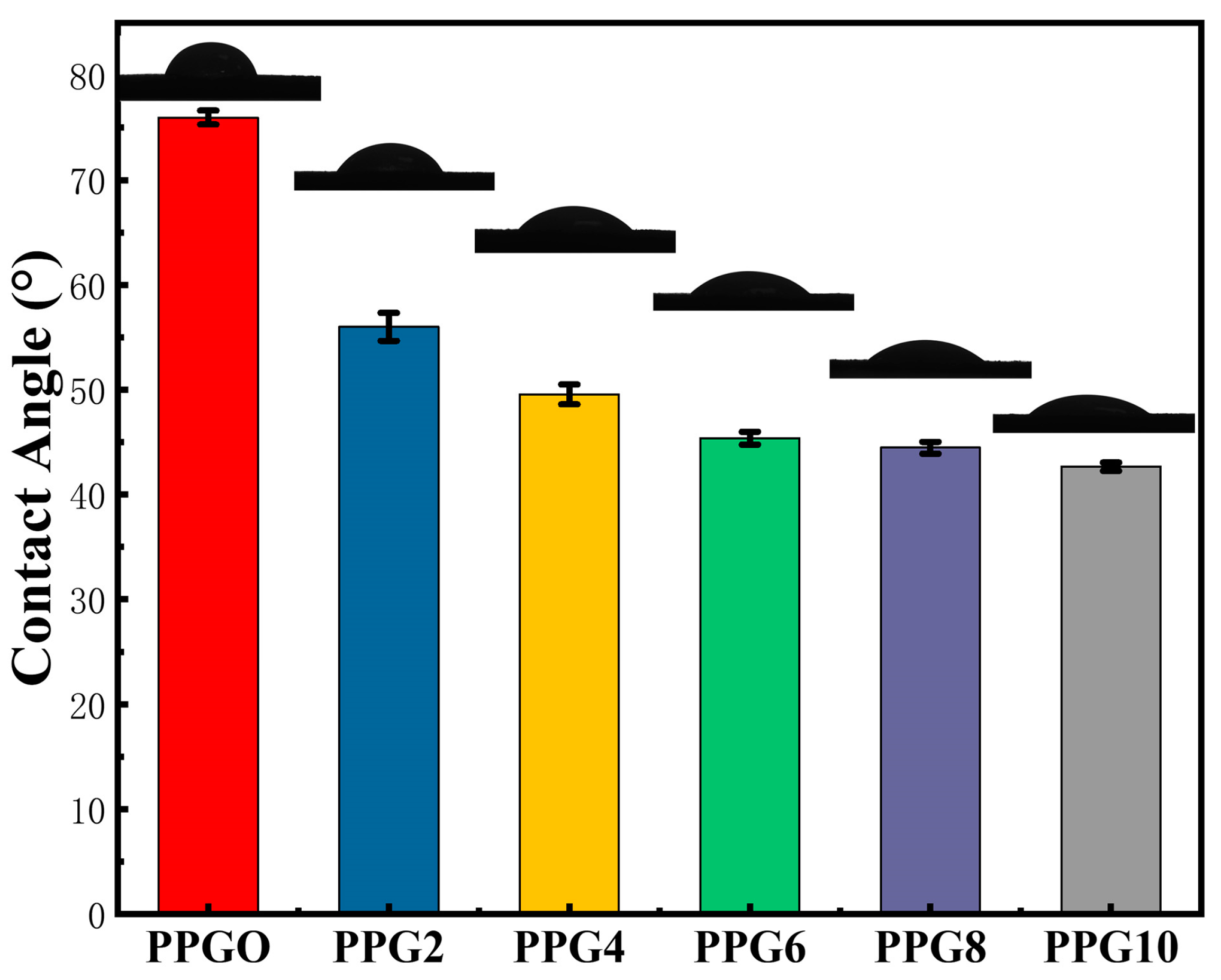
3.3. Contact Angle (θH2O) of PPG Self-Gelling Powder
Water contact angle (
θH2O) has been commonly used to evaluate the hydrophilicity and hydrophobicity of the materials. The water contact angle of the PPG self-gelling powder with different composites was investigated to characterize their interfacial hydrophilicity and showed a trend toward lower angles at higher PEG content. As show in
Figure 4, the water contact angle of the PPG0, PPG2, PPG4, PPG6, PPG8, and PPG10 self-gelling powder was 75.94 ± 0.67°, 56.00 ± 1.34°, 49.56 ± 0.95°, 45.38 ± 0.61°, 44.46 ± 0.55°, 42.66 ± 0.40°, respectively, implying that the presence of hydrophilic PEG content significantly enhanced the surface wettability and water absorption capacity of the PPG powder.
3.4. SEM Analysis
The microstructures and surface morphology of PPG self-gelling powder were characterized using SEM. When the PPG self-gelling powder particles (
Figure 5a) became a PPG powder-derived hydrogel after absorbing the liquid, the self-gel exhibited a three-dimensional porous structure (
Figure 5b). and there were no significant cracks on the surface of the formed gels (
Figure 5c). The three-dimensional porous structure can rapidly absorb body liquid exuded from damaged tissue, thereby promoting hemostasis.
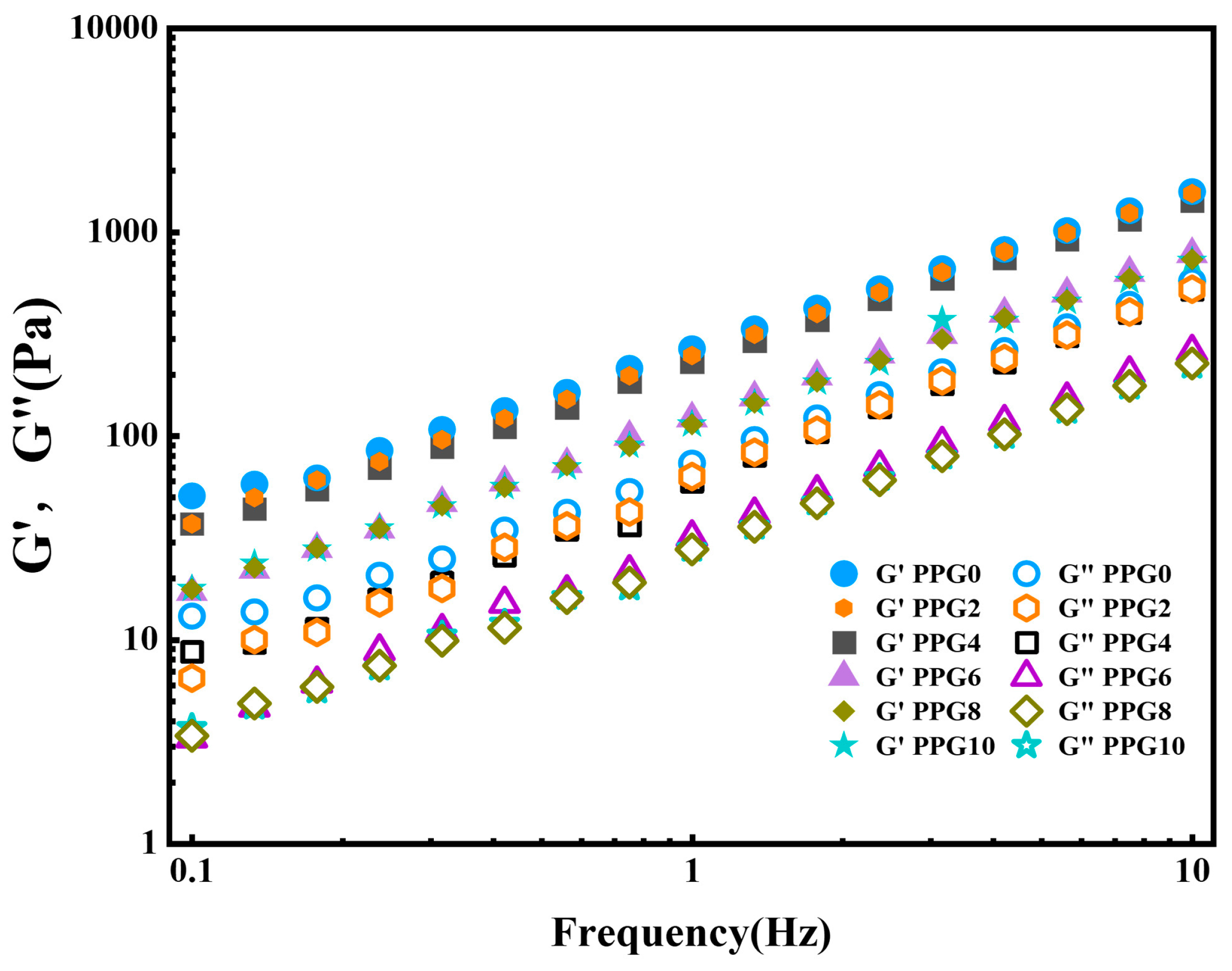
3.5. Rheological Analysis
The rheological characteristics of the hydrogel formed by the PPG self-gelling powder were analyzed to measure its viscoelastic behavior. As show in
Figure 6, for all samples, the storage modulus (G′) was consistently higher than the loss modulus (G″), indicating that the PPG self-gelling powder could form gel with a stable elastic state[
25,
26].
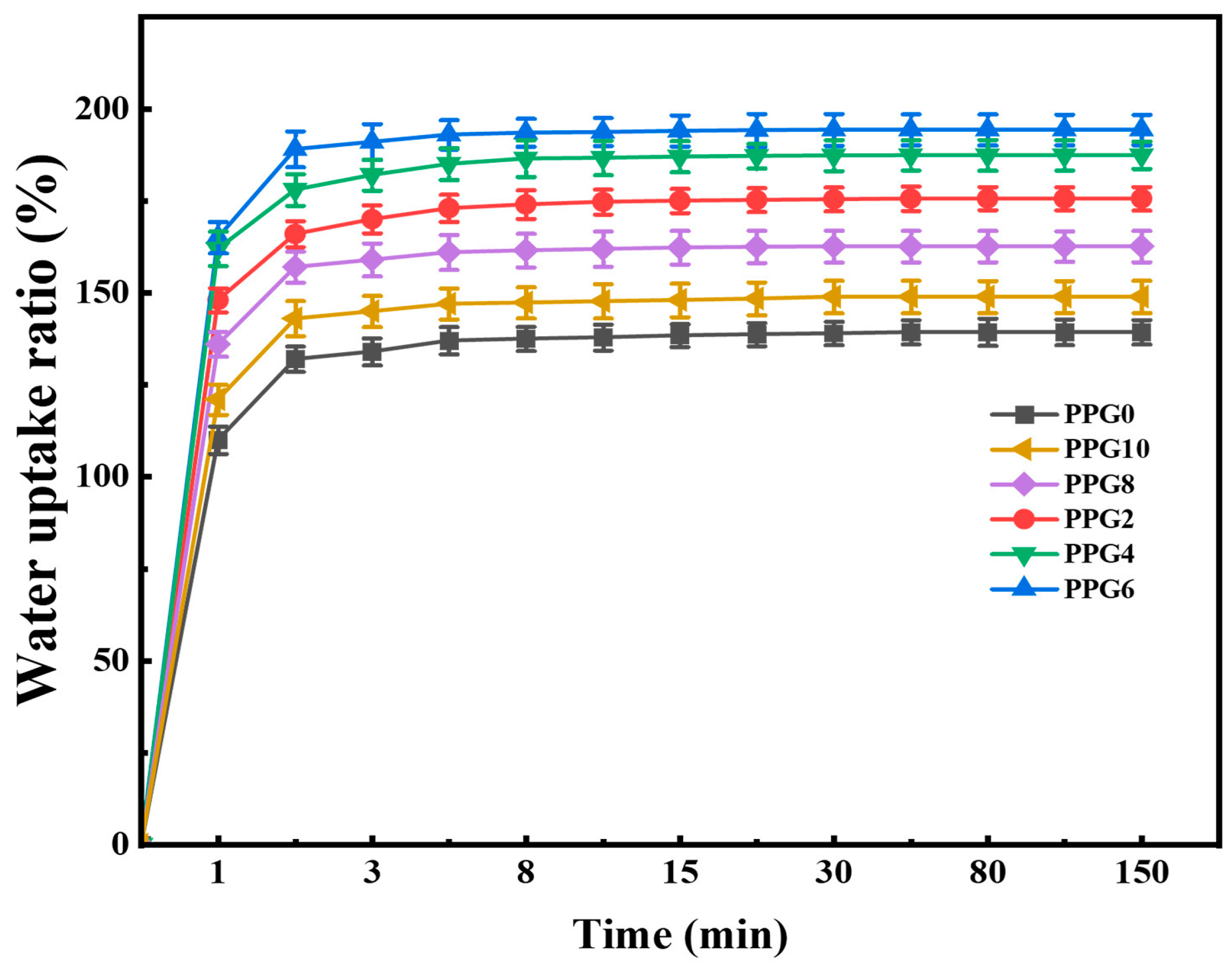
3.6. Water Absorption Capacity of PPG Self-Gelling Powder
The water absorption capacity of the hemostatic powders plays an important role in increasing the concentration of coagulation factors and activating the cascade reaction at the bleeding tissue[
12]. Thus, we performed a swelling experiment to investigate the absorption effect of the PPG self-gelling powder of with different composites. As show in
Figure 7, All powders rapidly absorbed lots of water, eventually reaching an equilibrium state within 60 min, and the content of PEG is the main factor regulating the water absorption capacity of the PPG self-gelling powder with different composites. Detailly, the PPG0 powder showed a limited water absorption capacity with a maximum value of 139.3%± 3.31%.Meanwhile, as the content of PEG increased, PPG2 powder, PPG4 powder and PPG6 powder shows a growing water absorption ratio of 175.6% ± 3.16%, 187.4 ± 3.71%, and 194.3 ± 4.11%, respectively. It has been reported that the presence of hydrophilic PEG component significantly enhanced the surface wettability and water absorption capacity of the material[
27], in accord with the above results. However, it was worth noting that the PPG8 and PPG10 self-gelling powder, despite having the highest content of PEG, did not exhibit the highest swelling ratio, the reason might be that the introduction of excess PEG disrupts the electrostatic cross-linking network structure between PEI and PAA, further restricting the absorption and retention of water molecules during the swelling process, resulting in reduced water absorption capacity.
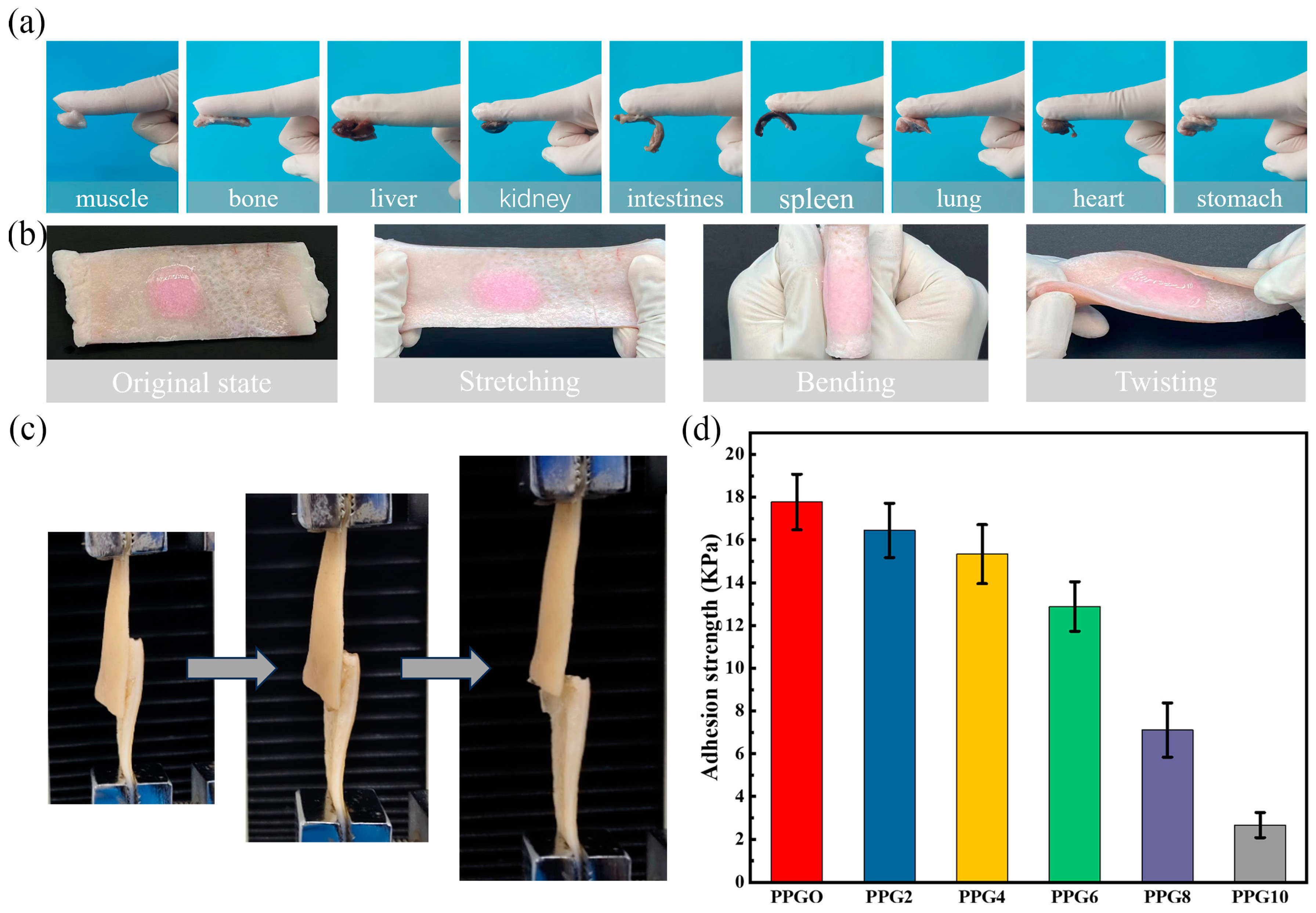
3.7. Adhesion Performance Test of PPG Self-Gelling Powder-Derived Hydrogel
Adhesion capacity with the bleeding tissue is the main parameter to enhance the hemostasis effect of the powder. The adhesion abilities were tested by using the tissue attachment method and lap shear test as described above. After contact with water, the PPG powders can form a stable hydrogel and adhere to the tissue surface. As show in
Figure 8a, the PPG4 self-gelling powder-derived hydrogel could steadily adhere to different tissue surfaces, including the muscle, bone, liver, kidney, intestines, spleen, lung, heart, and stomach, and
Figure 8b show that when thePPG4 self-gelling powder-derived hydrogel adhered in situ to porcine skin, it could firmly accommodate complex deformations, such as stretching, bending, and twisting (the hydrogel was dyed with rhodamine B for better visualization). Above result indicating that the PPG self-gelling powders could form gels with robust adhesion performance to the surface of the biological tissue. The adhesion performance was also measured using the lap shear test (
Figure 8c). As show in
Figure 8d, as the content of PEG increased, the adhesion strength of the PPG self-gelling powder-derived hydrogel decreased from 17.7 ± 1.3 kPa to 2.6 ± 0.59 kPa. One possible explanation for this phenomenon is that the introduction of PEG attenuates the electrostatic interaction between PEI and PAA. Although the adhesion strength of PPG self-gelling powder-derived hydrogel decreased obviously with increasing content of PEG, the PPG0, PPG2, PPG4, PPG6 self-gelling powder-derived hydrogel still had better adhesion performance compared with other hemostasis materials[
12,
28].
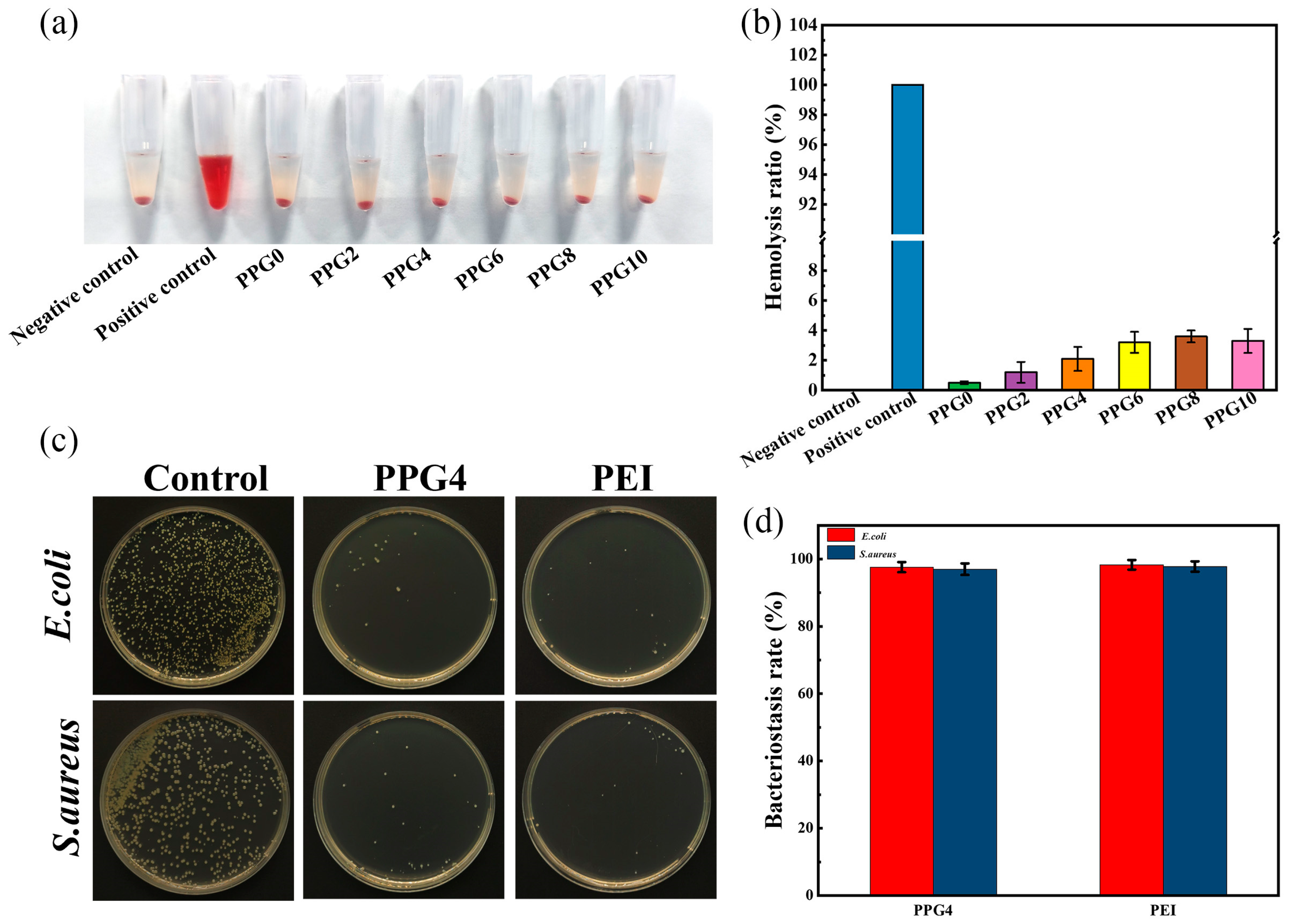
3.8. In Vitro Hemolysis Assay
The biosafety is crucial element for materials applied in the biomedical field. To evaluate the hemocompatibility of PPG self-gelling powders with different composites, an in vitro hemolysis test is performed. The hemolysis test was conducted using DI water as a positive control and PBS as a negative control., as shown in
Figure 9a. The results showed that the hemolysis rates of PPG self-gelling powders with different composites were all below 5%(
Figure 9b), which indicated all PPG self-gelling powder had excellent hematological compatibility.
3.9. Antibacterial Capability of PPG Self-Gelling Powder
To balance the physicochemical and biological properties, the PPG4 self-gelling powder were selected for subsequent experiments.
The antibacterial ability of PPG4 self-gelling powders against S. aureus and E. coli was conducted by coated plate method. The bacterial suspensions were incubated with the PPG4 self-gelling powder and 10wt% solution of PEI at 37℃ for 12h, respectively. As shown in
Figure 9c, d, The results showed that
, compared to the control group, PPG4 self-gelling powder groups exhibited an excellent bacteriostasis rate against S. aureus (97% ±1.7%) and E. coli (97.6% ± 1.5%), and its bactericidal ratio was similar to the 10wt% solution of PEI (97.8% ± 1.54% and 98.3% ± 1.42% against S. aureus and E. coli respectively). These result show that the antibacterial activity mainly because cationic amine groups of polyethyleneimine (PEI) are able to capture and kill the bacteria with a negatively charged cell membrane on contact as shown in the previous reports[
29].
Figure 9.
In vitro hemolysis assay and antibacterial performance. (a) digital images of the hemocompatibility of PPG self-gelling powders with different composites. (b) the hemolysis ratios of PPG self-gelling powders with different composites. (c) bacteriostatic effect of different samples on S. aureus and E. coli. (d) bacterial inhibition rates of different samples against S. aureus and E. coli.
Figure 9.
In vitro hemolysis assay and antibacterial performance. (a) digital images of the hemocompatibility of PPG self-gelling powders with different composites. (b) the hemolysis ratios of PPG self-gelling powders with different composites. (c) bacteriostatic effect of different samples on S. aureus and E. coli. (d) bacterial inhibition rates of different samples against S. aureus and E. coli.
3.10. In Vivo Hemostatic Assay
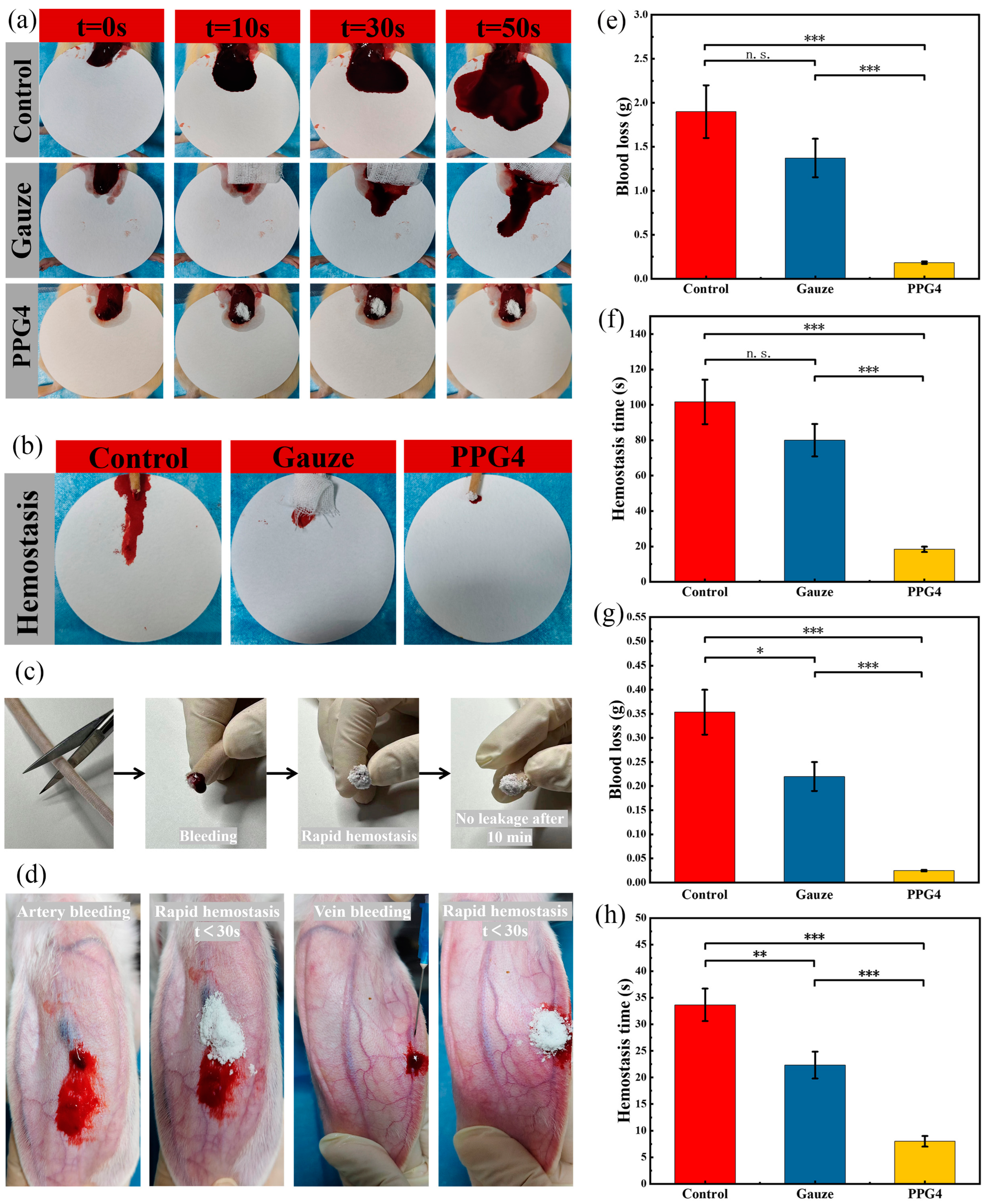
The hemostatic ability of different hemostatic materials in vivo was measured using the liver bleeding model, tail bleeding model, ear vein and artery bleeding models. In the rat liver hemorrhage model(
Figure 10a), As shown in
Figure 10 e, f , the PPG4 self-gelling powder significantly decreased the blood loss to 183.4 ± 13.5 mg and the bleeding time was shortened to 18.3±1.52s, and the hemostatic effect was obviously better than the control group(1900.0 ± 310mg, 101.6 ± 12.5s) (***p < 0.001), Gauze(1373 ± 218.8mg, 80 ± 9.1s) (***p <0.001), because the PPG4 self-gelling powder can absorb a significant amount of blood on the bleeding tissue surface and form a physical hemostatic barrier, but Gauze cannot. Besides, the hydrophilic PEG component can obviously improve the interfacial liquids uptake and surface wettability of PPG self-gelling powder, as show in
Figure 2a and
Figure 4, which was the main parameter to regulate the hemostatic performance and quickly swell into a hemostatic micro-gels network of PPG self-gelling powders.
The PPG4 self-gelling powder also showed excellent hemostatic activity for the bleeding model of rat tail amputation (
Figure 10b, c, g & h). As shown in
Figure 10g, h, the PPG4 self-gelling powder obviously decreased the blood loss to 24.6.4 ± 1.5 mg and the bleeding time was shortened to 8.3 ± 1.12s, and the hemostatic effect was obviously better than the control group (353.3 ± 46.5mg, 33.6 ± 3.05s) (***p < 0.001), Gauze (220.0 ± 30.0mg, 22.3 ± 2.52s) (***p < 0.001).
We further demonstrated the capacity of PPG4 self-gelling powders to control bleeding in terms of the ear vein and artery bleeding experiments in rabbit. After placing the PPG4 self-gelling powders on the damaged tissue surface, the powders can form a physical hemostatic barrier by absorbing interfacial blood and quickly control bleeding (ear vein bleeding < 20s, ear artery bleeding< 30s) As shown in
Figure 10d.
4. Conclusion
In summary, we successfully synthesized a self-gelling powder via a simple preparation method that can quickly absorb blood and tissue fluid and rapidly form a stable robust hydrogel as physical barrier to achieve effective hemostasis even for irregularly shaped and noncompressible wounds. The high adhesion of this PPG self-gelling powder-derived hydrogel can allow the hydrogel to be applied on wounds with broken and bleeding blood vessels to resist the pressure of strong blood flow. Furthermore, the biosafety was evaluated in terms of an in vitro hemolysis test. In addition, the PPG self-gelling powder can inhibit bacteria, showing inhibition to both Gram-negative and Gram-positive bacteria. Moreover, the effect of the powder in promoting hemostasis was conformed through the rat liver bleeding model, rat tail bleeding model, rabbit ear vein and artery bleeding models, demonstrating its ability to effectively stop bleeding. Attributed to rapid and effective hemostatic performance, adaptability to satisfy various complex bleeding wounds and non-compressible sites, excellent antimicrobial properties and hemocompatibility, easy usage and low cost, we believe that the PPG self-gelling powder is a promising biomedical material with wide application prospects in the field of medicine.
Author Contributions
Conceptualization, S.L. and A.Z.; methodology, C.W.; software, J.L.; validation, A.Z., X.J. and L.L.; formal analysis, J.L.; investigation, L.L.; resources, M.Z.; data curation, C.W.; writing—original draft preparation, J.L.; writing—review and editing, M.Z.; visualization, L.L.; supervision, M.Z.; project administration, C.W..; funding acquisition, C.W., J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hubei University of Science and Technology Doc toral Start-up Fund(BK202201、BK202307); Hubei Provincial Natural Science Foundation Youth Project(2022CFB755、2023AFB538); Hubei Provincial Department of Education Scientific Research Program for Young and Middle-aged Talents(Q20222804); National Natural Science Foundation of China Youth Program (52303182)
Institutional Review Board Statement
The animal study protocol was approved in accordance with the guidelines of the Laboratory Animal Center of Hubei University of science and technology (Xianning, China) and approved by the Laboratory Animal Ethics Committee of Hubei University of science and technology, approval number 2021-01-010.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peng, X.; Xu, X.; Deng, Y.; Xie, X.; Xu, L.; Xu, X.; Yuan, W.; Yang, B.; Yang, X.; Xia, X.; et al. Ultrafast Self-Gelling and Wet Adhesive Powder for Acute Hemostasis and Wound Healing. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Du Y; Chen, X. ; Li, L.; Zheng, H.; Yang, A.; Li, H.; Lv, G. Benzeneboronic-alginate/quaternized chitosan-catechol powder with rapid self-gelation, wet adhesion, biodegradation and antibacterial activity for non-compressible hemorrhage control. Carbohydr. Polym. 2023, 318, 121049. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shariati, K.; Ghovvati, M.; Vo, S.; Origer, N.; Imahori, T.; Kaneko, N.; Annabi, N. Hemostatic patch with ultra-strengthened mechanical properties for efficient adhesion to wet surfaces. Biomaterials 2023, 301, 122240. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, J.; Lai, J.; Zheng, X.; Wang, H.; Lu, B.; Huang, R.; Zhang, L. M. Novel Natural Polymer-Based Hydrogel Patches with Janus Asymmetric-Adhesion for Emergency Hemostasis and Wound Healing. Adv. Funct. Mater. 2024, 34, 2401030. [Google Scholar] [CrossRef]
- Wu, S. J.; Zhao, X. Bioadhesive Technology Platforms. Chem. Rev. 2023, 123, 14084–14118. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, J.; Sun, F.; Dou, X.; Liu, J.; Wang, Y.; Li, M.; Gao, J.; Liu, X.; Wang, X.; et al. A Super Tough, Rapidly Biodegradable, Ultrafast Hemostatic Bioglue. Adv. Mater. 2023, 35, 2208622. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, J.; Huang, Y.; Mu, L.; Chen, J.; Liang, Z.; Yin, Z.; Chu, D.; Han, Y.; Guo, B. Injectable Antiswelling and High-Strength Bioactive Hydrogels with a Wet Adhesion and Rapid Gelling Process to Promote Sutureless Wound Closure and Scar-free Repair of Infectious Wounds. ACS Nano 2023, 17, 22015–22034. [Google Scholar] [CrossRef]
- Yang, W.; Kang, X.; Gao, X.; Zhuang, Y.; Fan, C.; Shen, H.; Chen, Y.; Dai, J. Biomimetic Natural Biopolymer-Based Wet-Tissue Adhesive for Tough Adhesion, Seamless Sealed, Emergency/Nonpressing Hemostasis, and Promoted Wound Healing. Adv. Funct. Mater. 2023, 33, 2211340. [Google Scholar] [CrossRef]
- Pan, G.; Li, F.; He, S.; Li, W.; Wu, Q.; He, J.; Ruan, R.; Xiao, Z.; Zhang, J.; Yang, H. Mussel- and Barnacle Cement Proteins-Inspired Dual-Bionic Bioadhesive with Repeatable Wet-Tissue Adhesion, Multimodal Self-Healing, and Antibacterial Capability for Nonpressing Hemostasis and Promoted Wound Healing. Adv. Funct. Mater. 2022, 32, 2200908. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Zhang, W.; Huang, W.; Liu, Z.; Shi, R.; Wang, S.; Liu, S.; Shi, W.; Li, Y.; et al. A contact-polymerizable hemostatic powder for rapid hemostasis. Biomater. Sci. 2023, 11, 3616–3628. [Google Scholar] [CrossRef]
- Qiao, Z.; Lv, X.; He, S.; Bai, S.; Liu, X.; Hou, L.; He, J.; Tong, D.; Ruan, R.; Zhang, J.; et al. A mussel-inspired supramolecular hydrogel with robust tissue anchor for rapid hemostasis of arterial and visceral bleedings. Bioact. Mater. 2021, 6, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Li, X.; Yu, C.; Yao, M.; Zhao, Z.; Guo, B.; Liang, L.; Wei, Y.; Yao, F.; Zhang, H.; et al. A self-gelling powder based on polyacrylic acid/polyacrylamide/quaternate chitosan for rapid hemostasis. Int. J. Biol. Macromol. 2023, 232, 123449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sun, W.; Wang, W.; Zhang, Y.; Ma, Q. Robust and Wet Adhesive Self-Gelling Powders for Rapid Hemostasis and Efficient Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 6756–6771. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Z.; Xing, J. Rapid hemostatic antibacterial self-gelling powder based on methacryloylsulfonyl betaine and quaternized carboxymethyl chitosan. J. Mech. Behav. Biomed. Mater. 2023, 146, 106079. [Google Scholar] [CrossRef]
- Huang, H.; Dong, Z.; Ren, X.; Jia, B.; Li, G.; Zhou, S.; Zhao, X.; Wang, W. High-strength hydrogels: Fabrication, reinforcement mechanisms, and applications. Nano Res. 2023, 16, 3475–3515. [Google Scholar] [CrossRef]
- Li, J.; Su, J.; Liang, J.; Zhang, K.; Xie, M.; Cai, B.; Li, J. A hyaluronic acid / chitosan composite functionalized hydrogel based on enzyme-catalyzed and Schiff base reaction for promoting wound healing. Int. J. Biol. Macromol. 2024, 255, 128284. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Cai, P.; Huang, X.; Huang, Y.; Liu, R.; Zhang, M.; Song, J.; Chen, X.; Yang, H. A silk-based sealant with tough adhesion for instant hemostasis of bleeding tissues. Nanoscale Horiz 2019, 4, 1333–1341. [Google Scholar] [CrossRef]
- Cui, C.; Liu, W. Recent advances in wet adhesives: Adhesion mechanism, design principle and applications. Prog. Polym. Sci. 2021, 116, 101388. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Wang, L.; Cai, H.; Wang, Q.; Wang, W.; Shao, J.; Dong, X. Underwater Adhesion and Anti-Swelling Hydrogels. Adv. Mater. Technol. 2023, 8, 2201477. [Google Scholar] [CrossRef]
- Lv, C.; Zhou, X.; Wang, P.; Wu, Z.; Jiao, Z.; Guo, M.; Wang, Z.; Wang, Y.; Wang, L.; Zhang, P. Antibacterial microspheres with a bionic red-blood-cell like hollow structure and superior swelling recovery capacity for efficient traumatic hemostasis. Appl. Mater. Today 2022, 29, 101559. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Meng, Z.; Yang, Z.; Fan, S.; Ye, T.; Yang, L.; Li, T.; Gu, R.; Wu, Z.; et al. Preparation and characterization of tranexamic acid modified porous starch and its application as a hemostatic agent. Int. J. Biol. Macromol. 2022, 200, 273–284. [Google Scholar] [CrossRef]
- Anthis, A. H. C.; Hu, X.; Matter, M. T.; Neuer, A. L.; Wei, K.; Schlegel, A. A.; Starsich, F. H. L.; Herrmann, I. K. Chemically Stable, Strongly Adhesive Sealant Patch for Intestinal Anastomotic Leakage Prevention. Adv. Funct. Mater. 2021, 31, 2007099. [Google Scholar] [CrossRef]
- Sekhar, K. P. C.; Zhang, X.; Geng, H.; Yu, Q.; Zhang, P.; Cui, J. Biomimetic Hemostatic Powder Derived from Coacervate-Immobilized Thermogelling Copolymers. Biomacromolecules 2023, 24, 5394–5402. [Google Scholar] [CrossRef]
- Ni, P.; Ye, S.; Xiong, S.; Zhong, M.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.; Zhang, X. A chitosan-optimized polyethyleneimine/polyacrylic acid multifunctional hydrogel for reducing the risk of ulcerative arterial bleeding. J. Mat. Chem. B 2023, 11, 5207–5222. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.; Zhao, Z.; Li, D.; Dong, W.; Lu, Y.; Jin, B.; Li, H.; Liu, Q.; Deng, B. Quarternized chitosan/quercetin/polyacrylamide semi-interpenetrating network hydrogel with recoverability, toughness and antibacterial properties for wound healing. Int. J. Biol. Macromol. 2023, 228, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, Y.; Shen, Z.; Ni, M.; Xu, J.; Wang, T. Enhancing Wound Recovery: A Self-Gelling Powder for Improved Hemostasis and Healing. Polymers 2024, 16, 1795. [Google Scholar] [CrossRef]
- Geng, A.; Luo, Y.; Zheng, M.; Zheng, J.; Zhu, R.; Bai, S. Silk fibroin-based hemostatic powders with instant and robust adhesion performance for sutureless sealing of gastrointestinal defects. J. Mat. Chem. B 2024, 12, 5439–5454. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Li, S.; Zheng, X.; Zhang, Q.; Liu, J.; Liang, C.; Duan, K.; Ye, J.; Yin, Y.; Chen, X. Superwettable calcium ion exchanged carboxymethyl cellulose powder with self-gelation, tissue adhesion and bioabsorption for effective hemorrhage control. Chem. Eng. J. 2024, 481, 148770. [Google Scholar] [CrossRef]
- Hao, Y.; Yuan, C.; Deng, J.; Zheng, W.; Ji, Y.; Zhou, Q. Injectable Self-Healing First-Aid Tissue Adhesives with Outstanding Hemostatic and Antibacterial Performances for Trauma Emergency Care. ACS Appl. Mater. Interfaces 2022, 14, 16006–16017. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).