Submitted:
17 November 2024
Posted:
19 November 2024
Read the latest preprint version here
Abstract
Coconut oil (CNO) is often characterized as an “artery clogging fat” because it is a predominantly saturated fat that ostensibly raises total cholesterol (TChol) and LDL-cholesterol (LDL-C). Whereas previous analyses assessed CNO based on relative effects on lipid parameters against other fats and oils, this analysis focuses on the effects of CNO itself. Here, we review the literature on CNO and analyze 984 lipid profile data sets from 26 CNO studies conducted over the past 40 years. This analysis found considerable heterogeneity among CNO studies regarding participant selection, amount consumed, and study duration. The analysis reveals that, overall, CNO consumption gives variable TChol and LDL-C values, but that HDL-cholesterol (HDL-C) values increase and triglycerides (TG) decrease. This holistic lipid assessment, together with consideration of lipid ratios, shows that CNO does not pose a health risk for heart disease. Because the predominantly medium-chain fatty acid profile of CNO is significantly different from lard and palm oil, studies using these as reference materials do not apply to CNO. This paper concludes that the recommendation to avoid consuming coconut oil due to the risk of heart disease is not justified.
Keywords:
Introduction
1. Review of the Major Issues Regarding Coconut Oil and Saturated Fat
1.1. “Saturated Fats” Are a Heterogenous Group
- Only CNO can be considered a saturated fat, having a total saturated fatty acid (SFA) content of 83 gm/100 gm. In comparison, the total SFA content of palm oil, tallow, and butter is less than 50 gm/100 gm.
- CNO has a MCFA composition of 54 gm/100 gm. In contrast, none of the other members of this group has a significant amount of MCFA.
- CNO contains 41.8 gm lauric acid (C12:0) per 100 gm, while butter, which is the next closest member, only has 2.6 gm C12:0 per 100 gm.
- The plant oils – CNO and palm oil – have 0 mg of cholesterol/100 gm, while the animal fats have high amounts of cholesterol. As will be discussed later, because the presence of cholesterol in animal fat may have metabolic effects on the gut microflora, plant oils and animal fats should not be directly compared regarding their metabolic effects.
- CNO and palm oil, which are assumed to be similar, have very different MCFA, saturated LCFA, MUFA, and PUFA compositions.
1.2. Effects of Dietary Cholesterol
1.3. Medium-Chain Fatty Acids And Long-Chain Fatty Acids Are Metabolically Different
1.4. Trans-Fat and Saturated Fat
1.5. The Problem of Intra-Individual and Inter-Individual Differences in Cholesterol Measurements
1.6. Changes in Lipid Profile Values from Addition of Dietary Fat May Be Transient
1.7. Reviews of Prospective Studies Comparing Coconut Oil with Other Dietary Oils and Fats
1.8. Reviews of Interventional Studies Comparing Coconut Oil to Other Oils and Fats
1.9. Studies of Populations That Consume Coconut Oil as a Staple
1.10. Studies on High Omega-6 Diets
2. Analysis of 26 Coconut Oil Studies
2.1. Objective
2.2. Search for Studies
2.3. Calculations
3. Results of the Analysis
3.1. Heterogeneity of the Studies
3.2. Coconut Studies Data Available for Analysis
3.3. Results
3.3.1. Overview of Results
- HDL-C increased in all time periods;
- TG decreased in all time periods;
- TChol and LDL-C showed both increases and decreases in different time periods. The wide variability of these two lipid values makes TChol and LDL-C less reliable measures.
3.3.2. All Studies Combined
3.3.3. Short-Duration Studies (3 to 7 Weeks)
3.3.4. Medium-Duration Studies (8 to 24 Weeks)
3.3.5. Long-Duration Study (1 to 2 Years)
3.4. Wide Range of Average Lipid Profile Values and Standard Deviations Between Studies
3.5. Similar Small Studies Can Have Different Results
3.6. Lipid Ratios
3.7. Total Cholesterol, LDL-Cholesterol, and Triglyceride Values Did Not Change Significantly over Time for Coconut Oil or Sunflower Oil in a Two-Year Study of People with Cardiovascular Disease
4. Discussion
4.1. Coconut Oil Raises HDL-Cholesterol and Improves Lipid Ratios in Short-, Medium- and Long-Duration Studies
- For all studies combined, the average differences for TChol, LDL-C, and TG were below baseline. For long-duration studies, average differences for TChol and TG were below baseline, and LDL-C was less than 1% above baseline. For medium-duration studies, average differences for TChol, LDL-C, and TG were below baseline. For short-duration studies, the average difference for TChol was less than 1% above baseline, and for LDL-C and TG were below baseline. Therefore, the average difference for TChol was slightly above baseline only for studies lasting 3 to 7 weeks which was likely due to higher HDL-C, since the average difference for LDL-C was below baseline.
- HDL-C values were above the baseline values for the combined studies, as well as for all study durations;
- The lipid ratios TChol/HDL-C, LDL-C/HDL-C, and TG/HDL-C improved in all groups combined, as well as for all study durations;
- Large SDs for differences in all lipid values reported by the authors in most studies suggest that there is marked individual variability in the response to introducing CNO into the diet, highlighting the complexity of cholesterol metabolism.
4.2. Strengths and Limitations
4.2.1. Similar Limitations Are a Problem for Other Reviews and Meta-Analyses
4.2.2. Limitations Due to Calculated Rather Than Direct Measurement of LDL-Cholesterol
4.3. How an Oil Is Processed Might Explain Inconsistent Results Between Similar Studies
4.4. Large Standard Deviations Question the Applicability of the p-Value to the Real World
4.5. Cardiac Risk Factors Beyond the Lipid Profile
4.6. Impact of Individual MCFAs on Lipid Parameters in Humans
5. Summary and Conclusions
- Coconut oil is sui generis. It has the highest amount of MCFA (C6:0 to C12:0) among all the common dietary oils and fats, whereas the other commonly used oils and fats are mainly LCFA, both saturated and unsaturated (> C14). CNO is unique in that it is predominantly lauric acid (C12:0).
- MCFAs, in particular C12:0, have different metabolic properties than saturated LCFAs, such as C16:0. High intake of C16:0 may cause hepatic inflammation when consumed in high amounts; this is not observed with C12:0. Studies using lard, palm oil, and C16:0 as comparators are not applicable to CNO and C12:0.
- Measurements of lipid parameters with CNO intake appear to depend on the duration of the study. Analyses of studies on CNO show that there is no consistency in the levels of TChol and LDL, while TG levels tend to decrease and HDL-C levels tend to increase regardless of duration.
- The large SDs and ranges of results reported in most studies indicate that there is marked variability in the lipid profile responses of individuals to adding a new oil to the diet.
- There is no evidence from interventional studies that consuming CNO increases the incidence of adverse fatal or nonfatal cardiovascular events. Likewise, studies of populations consuming CNO and the coconut diet as a staple have found no evidence of an increase in the incidence of CVD.
- Unlike many other studies that draw conclusions based on comparisons between CNO and PUFA oils based on TChol and LDL-C only, this study shows that CNO has a strong tendency to raise HDL-C regardless of study duration, and that calculation of the lipid ratios – TChol/HDL-C, LDL-C/HDL-C, and TG/HDL-C – give healthy ratios and indicate that CNO is a heart healthy oil. And most importantly, there are no studies that show that CNO increases the incidence of cardiovascular disease.
- Conclusions of studies on the health effects of “saturated fat”, including animal studies and clinical trials, should specify what fat or oil or what specific saturated fatty acids were studied.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Page, I.H.; Allen, E.V.; Chamberlain, F.L.; Keys, A.; Stamler, J.; Stare, F.J. Dietary fat and its relation to heart attacks and strokes. Circulation 1961, 23, 133–136. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, Editions 1 to 9; U.S. Department of Health and Human Services: Washington, DC, USA, 1980–2020. Available at: https://www.dietaryguidelines.gov. Accessed 28 October 2024.
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Newport, M.T.; Dayrit, F.M. The Lipid–Heart Hypothesis and the Keys Equation Defined the Dietary Guidelines but Ignored the Impact of Trans-Fat and High Linoleic Acid Consumption. Nutrients 2024, 16, 1447. [Google Scholar] [CrossRef]
- James, A.T.; Martin, J.P. Gas-liquid Partition Chromatography: The Separation and Micro-estimation of Volatile Fatty Acids from Formic Acid to Dodecanoic Acid. Biochem. J. 1952, 50, 679–690. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Han, X.; Zhou, F.; Guo, J.; Huang, W.; Zhan, J.; You, Y. Coconut oil and medium-chain fatty acids attenuate high-fat diet-induced obesity in mice through increased thermogenesis by activating brown adipose tissue. Front. Nutr. 2022, 9, 896021. [Google Scholar] [CrossRef]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef]
- Hashim, S.A.; VanItallie, T.B. Ketone body therapy: From the ketogenic diet to the oral administration of ketone ester. J. Lipid Res. 2014, 55, 1818–1826. [Google Scholar] [CrossRef]
- Watanabe, S.; Tsujino, S. Applications of medium-chain triglycerides in foods. Front. Nutr. 2022, 9, 802805. [Google Scholar] [CrossRef]
- Toleikis, A.; Trumbeckaite, S.; Liobikas, J.; Pauziene, N.; Kursvietiene, L.; Kopustinskiene, D.M. Fatty acid oxidation and mitochondrial morphology changes as key modulators of the affinity for ADP in rat heart mitochondria. Cells 2020, 9, 340. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. FoodData Central: https://ndb.nal.usda.gov.
- Codex Alimentarius. Standard for Named Vegetable Oils, Codex Stan 210-1999. Food and Agriculture Organization; 2015.Accessed 25 March 2019. Available at: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/it/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B210-1999%252FCXS_210e.pdf.
- Keys, A.; Mickelsen, O.; Miller, E.v.O.; Hayes, E.R.; Todd, R.L. The Concentration of Cholesterol in the Blood Serum of Norman Man and its Relation to Age. J. Clin. Invest. 1950, 29, 1347–1353. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L. Dietary cholesterol and cardiovascular risk: A science advisory from the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Murillo, A.G. Is There a Correlation between Dietary and Blood Cholesterol? Evidence from Epidemiological Data and Clinical Interventions. Nutrients 2022, 14, 2168. [Google Scholar] [CrossRef]
- Kübeck, R.; Bonet-Ripoll, C.; Hoffmann, C.; Walker, A.; Müller, V.M.; Schüppel, V.L.; Lagkouvardos, I.; Scholz, B.; Engel, K.-H.; Daniel, H.; et al. Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Molec. Metab. 2016, 5, 1162–1174. [Google Scholar] [CrossRef]
- Deng, C.; Pan, J.; Zhu, H.; Chen, Z.-Y. Effect of gut microbiota on blood cholesterol: A Review on Mechanisms. Foods 2023, 12, 4308. [Google Scholar] [CrossRef]
- Fisher, E.A.; Blum, C.B.; Zannis, V.I.; Breslow, J.L. Independent effects of dietary saturated fat and cholesterol on plasma lipids, lipoproteins, and apolipoprotein E. J. Lipid Res. 1983, 24, 1039–1048. [Google Scholar] [CrossRef]
- Newport, M.T. Ketogenic strategies for Alzheimer’s disease and other memory impairments: History, rationale, and 288 caregiver case reports. Med. Res. Arch. 2024, 12. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Du, Q.; Xu, J.; Wang, D.; Chen, L.; Dong, K.; Chen, Z.; Yang, J. The different mechanisms of lipid accumulation in hepatocytes induced by oleic acid/palmitic acid and high-fat diet. Molecules 2023, 28, 6714. [Google Scholar] [CrossRef]
- Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.A.; Desouza, C. Lauric acid versus palmitic acid: Effects on adipose tissue inflammation, insulin resistance, and non-alcoholic fatty liver disease in obesity. Biology 2020, 9, 346. [Google Scholar] [CrossRef]
- Sergi, D.; Luscombe-Marsh, N.; Naumovski, N.; Abeywardena, M.; O’Callaghan, N. Palmitic acid, but not lauric acid, induces metabolic inflammation, mitochondrial fragmentation, and a drop in mitochondrial membrane potential in human primary myotubes. Front. Nutr. 2021, 8, 663838. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. The effect of inflammation and infection on lipids and lipoproteins. In Feingold, K.R.; Anawalt, B.; Boyce, A.; et al. editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000.
- Johnston, P.V.; Johnson, O.C.; Kummerow, F.A. Occurrence of trans fatty acids in human tissue. Science 1957, 126, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Enig, M.G.; Atal, S.; Keeney, M.; Sampugna, J. Isomeric trans fatty acids in the U.S. diet. J. Am. Coll. Nutr. 1990, 9, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Satchithanandam, S.; Oles, C.J.; Spease, C.J.; Brandt, M.M.; Yurawecz, M.P.; Rader, J.I. Trans, saturated, and unsaturated fat in foods in the United States prior to mandatory trans-fat labeling. Lipids 2004, 39, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kummerow, F.A. The negative effects of hydrogenated trans fats and what to do about them. Atherosclerosis 2009, 205, 458–465. [Google Scholar] [CrossRef]
- Amico, A.; Wootan, M.G.; Jacobson, M.F.; Leung, C.; Willett, W. The demise of artificial trans-fat: A history of a public health achievement. The Milbank Quarterly 2021, 99, 746–770. [Google Scholar] [CrossRef]
- Keys, A. Prediction and possible prevention of coronary artery disease. Am. J. Public Health 1953, 43, 1399–1407. [Google Scholar] [CrossRef]
- Keys, A. The Diet and the Development of Coronary Heart Disease. J. Chron. Dis. 1965, 4, 364–380. [Google Scholar] [CrossRef]
- Jensen, T. The Consumption of Fats in Denmark 1900–2000. Anthropol Food, 2012, S7. Available at: https://journals.openedition.org/aof/7100. Accessed 4 April 2024. [CrossRef]
- Kris-Etherton, P.M. Trans fatty acids and coronary heart disease risk. Expert panel on trans fatty acids and coronary heart disease. Am. J. Clin. Nutr. 1995, 62, 655S–708S. [Google Scholar]
- FDA. Direct Final Rule to Revoke Use of Partially Hydrogenated Oils in Foods - Docket No. FDA- FDA-2019-N-4750. Food and Drug Administration, 2019, available at: https://www.fda.gov/media/170845/download Accessed on October 30, 2024.
- NDHS. National Diet Heart Study Final Report. American Heart Association, Inc.: New York, NY, USA, 1968.
- Vijayakumar, M.; Vasudevan, D.M.; Sundaram, K.R.; Krishnan, S.; Vaidyanathan, K.; Nandakumar, S.; Chandrasekhar, R.; Mathew, N. A randomized study of coconut oil versus sunflower oil on cardiovascular risk factors in patients with stable coronary heart disease. Indian Heart J. 2016, 68, 498–506. [Google Scholar] [CrossRef]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.; Hassan, K.; Lim, J.B.; Lye, M.S.; Ishak, R. Nonhypercholesterolemic effects of a palm-oil diet in Malaysian volunteers. Am. J. Clin. Nutr. 1991, 53 (Suppl. S4), 1015S–1020S. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.; Hayes, K.C.; DeWitt, G.F.; Jegathesan, M.; Satgunasingam, N.; Ong, A.S.; Tan, D. Dietary palmitic and oleic acids exert similar effects on serum cholesterol and lipoprotein profiles in normocholesterolemic men and women. J. Am. Coll. Nutr. 1992, 11, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Bogensberger, B.; Benčič, A.; Knüppel, S.; Boeing, H.; Hoffmann, G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J. Lipid Res. 2018, 59, 1771–1782. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schlesinger, S. Coconut oil and cardiovascular disease risk. Curr. Atheroscl. Rep. 2023, 25, 231–236. [Google Scholar] [CrossRef]
- Unhapipatpong, C.; Shantavasinkul, P.C.; Kasemsup, V.; Siriyotha, S.; Warodomwichit, D.; Maneesuwannarat, S.; Vathesatogkit, P.; Sritara, P.; Thakkinstian, A. Tropical oil consumption and cardiovascular disease: An umbrella review of systematic reviews and meta-analyses. Nutrients 2021, 13, 1549. [Google Scholar] [CrossRef]
- Neelakantan, N.; Seah, J.Y.H.; van Dam, R.M. The effect of coconut oil consumption on cardiovascular risk factors: A systematic review and meta-analysis of clinical trials. Circulation 2020, 141, 803–814. [Google Scholar] [CrossRef]
- Johansson, A.K.; Korte, H.; Yang, B.; Stanley, J.C.; Kallio, H.P. Sea buckthorn berry oil inhibits platelet aggregation. J. Nutr. Biochem. 2000, 11, 491–495. [Google Scholar] [CrossRef]
- Oliveira-de-Lira, L.; Santos, E.M.C.; de Souza, R.F.; Matos, R.J.B.; Silva, M.C.D.; Oliveira, L.D.S.; Nascimento, T.G.D.; Schemly, P.; Souza, S.L. Supplementation-dependent effects of vegetable oils with varying fatty acid compositions on anthropometric and biochemical parameters in obese women. Nutrients, 2018, 20, E932. [Google Scholar] [CrossRef]
- McKenney, J.M.; Proctor, J.D.; Wright, J.T., Jr.; Kolinski, R.J.; Elswick, R.K., Jr.; Coaker, J.S. The effect of supplemental dietary fat on plasma cholesterol levels in lovastatin-treated hypercholesterolemic patients. Pharmacotherapy 1995, 15, 565–572. [Google Scholar] [CrossRef]
- Assunção, M.L.; Ferreira, H.S.; dos Santos, A.F.; Cabral, C.R., Jr.; Florêncio, T.M. Effects of dietary coconut oil on the biochemical and anthropometric profiles of women presenting abdominal obesity. Lipids 2009, 44, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Voon, P.T.; Ng, T.K.; Lee, V.K.; Nesaretnam, K. Diets high in palmitic acid (16:0), lauric and myristic acids (12:0 + 14:0), or oleic acid (18:1) do not alter postprandial or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am. J. Clin. Nutr. 2011, 94, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.T.; Sharp, S.J.; Finikarides, L.; Afzal, I.; Lentjes, M.; Luben, R.; Forouhi, N.G. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open 2018, 8, e020167. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Hutchins, A.; Fryda, L. The impact of virgin coconut oil and high oleic safflower oil on body composition, lipids, and inflammatory markers in postmenopausal women. J. Med. Food 2017, 20, 345–351. [Google Scholar] [CrossRef]
- Lu, Z.; Hendrich, S.; Shen, N.; White, P.J.; Cook, L.R. Low linolenate and commercial soybean oils diminish serum HDL cholesterol in young free-living adult females. J. Am. Coll. Nutr. 1997, 16, 562–569. [Google Scholar]
- Reiser, R.; Probstfield, J.L.; Silvers, A.; Scott, L.W.; Shorney, M.L.; Wood, R.D.; O’Brien, B.C.; Gotto, A.M., Jr.; Insull, W., Jr. Plasma lipid and lipoprotein response of humans to beef fat, coconut oil and safflower oil. Am. J. Clin. Nutr. 1985, 42, 190–197. [Google Scholar] [CrossRef]
- Heber, D.; Ashley, J.M.; Solares, M.E.; Wang, H.J.; Alfin-Slater, R.B. The effects of a palm-oil enriched diet on plasma lipids and lipoproteins in healthy young men. Nutr. Res. 1992, 12, S53–S59. [Google Scholar] [CrossRef]
- Korrapati, D.; Jeyakumar, S.M.; Putcha, U.K.; Mendu, V.R.; Ponday, L.R.; Acharya, V.; Koppala, S.R.; Vajreswari, A. Coconut oil consumption improves fat-free mass, plasma HDL-cholesterol and insulin sensitivity in healthy men with normal BMI compared to peanut oil. Clin. Nutr., 2019, 38, 2889–2899. [Google Scholar] [CrossRef]
- Sabitha, P.; Vaidyanathan, K.; Vasudevan, D.M.; Kamath, P. Comparison of lipid profile and antioxidant enzymes among south Indian men consuming coconut oil and sunflower oil. Indian J. Clin. Biochem. 2009, 24, 76–81. [Google Scholar] [CrossRef]
- Palazhy, S.; Kamath, P.; Vasudevan, D.M. Dietary fats and oxidative stress: A cross-sectional study among coronary artery disease subjects consuming coconut oil/sunflower oil. Indian J. Clin. Biochem. 2018, 33, 69–74. [Google Scholar] [CrossRef]
- Palazhy, S.; Kamath, P.; Rajesh, P.C.; Vaidyanathan, K.; Nair, S.K.; Vasudevan, D.M. Composition of plasma and atheromatous plaque among coronary artery disease subjects consuming coconut oil or sunflower oil as the cooking medium. J. Am. Coll. Nutr. 2012, 31, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.R.; Suchindran, C.M.; Ringel, A.; Davis, J.M.; Hibbeln, J.R. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013, 346, e8707. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.R.; Broste, S.K.; Frantz, R.P.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968–1973). BMJ 2016, 353, i1246. [Google Scholar] [CrossRef] [PubMed]
- Frantz, I.D., Jr.; Dawson, E.A.; Ashman, P.L.; Gatewood, L.C.; Bartsch, G.E.; Kuba, K.; Brewer, E.R. Test of effect of lipid lowering by diet on cardiovascular risk. Arterioscler. 1989, 9, 129–135. [Google Scholar] [CrossRef]
- Dayton, S.; Pearce, M.L.; Hashimoto, S.; Dixon, W.J.; Tomiyasu, U. A controlled clinical trial of a diet high in unsaturated fat in preventing complications of atherosclerosis. Circulation 1969, 40, II-1–II-63. [Google Scholar] [CrossRef]
- Stehbens, W.E. Coronary heart disease, hypercholesterolemia, and atherosclerosis. I. False premises. Exp. Mol. Pathol. 2001, 70, 103–119. [Google Scholar] [CrossRef]
- Cox, C.; Mann, J.; Sutherland, W.; Chisholm, A.; Skeaff, M. Effects of coconut oil, butter, and safflower oil on lipids and lipoproteins in persons with moderately elevated cholesterol levels. J. Lipid Res. 1995, 36, 1787–1795. [Google Scholar] [CrossRef]
- Swarnamali, H.; Ranasinghe, P.; Jayawardena, R. Changes in serum lipids following consumption of coconut oil and palm olein oil: A sequential feeding crossover clinical trial. Diabetes Metab. Syndr. 2024, 18, 103070. [Google Scholar] [CrossRef]
- International Coconut Community. Virgin Coconut Oil. https://coconutcommunity.org/products-detail/virgin-coconut-oil. Downloaded on November 1, 2024.
- Cox, C.; Sutherland, W.; Mann, J.; de Jong, S.; Chisholm, A.; Skeaff, M. Effects of dietary coconut oil, butter, and safflower oil on plasma lipids, lipoproteins, and lathosterol levels. Eur. J. Clin. Nutr. 1998, 52, 650–654. [Google Scholar] [CrossRef]
- Maki, K.C.; Hasse, W.; Dicklin, M.R.; Bell, M.; Buggia, M.A.; Cassens, M.E.; Eren, F. Corn oil lowers plasma cholesterol compared with coconut oil in adults with above-desirable levels of cholesterol in a randomized crossover trial. J. Nutr. 2018, 148, 1556–1563. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultra-centrifuge. Clin. Chem. 1972, 18, 499–552. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Blaha, M.J.; Elshazly, M.B.; Toth, P.P.; Kwiterovich, P.O.; Blumenthal, R.S.; Jones, S.R. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013, 310, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Chinwong, S.; Chinwong, D.; Mangklabruks, A. Daily consumption of virgin coconut oil increases high-density lipoprotein cholesterol levels in healthy volunteers: A randomized crossover trial. Evid.- Based Complement. Altern. Med. 2017, 7251562. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, S.M.; Damayanti, K.; Rajkumar Ponday, L.; Acharya, V.; Koppala, S.R.; Putcha, U.K.; Nagalla, B.; Vajreswari, A. Assessment of virgin coconut oil in a balanced diet on indicators of cardiovascular health in non-obese volunteers: A human metabolic study. Diabetes Metab. Syndr. 2023, 17, 102844. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.É.; Crovesy, L.; Rosado, E.L.; Soares-Mota, M. Effect of coconut oil on weight loss and metabolic parameters in men with obesity: A randomized controlled clinical trial. Food Funct. 2020, 11, 6588–6594. [Google Scholar] [CrossRef]
- Cardoso, G.M.; Raggio Luiz, R.; Rosa, G. A coconut extra virgin oil-rich diet increases HDL cholesterol and decreases waist circumference and body mass in coronary artery disease patients. Nutricion Hospitalaria, 2015, 32, 2144–2152. [Google Scholar] [CrossRef]
- Nikooei, P.; Hosseinzadeh-Attar, M.J.; Asghari, S.; Norouzy, A.; Yaseri, M.; Vasheghani-Farahani, A. Effects of virgin coconut oil consumption on metabolic syndrome components and asymmetric dimethylarginine: A randomized controlled clinical trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 939–949. [Google Scholar] [CrossRef]
- Setyawati, A.; Sangkala, M.S.; Malasari, S.; Jafar, N.; Sjattar, E.L.; Syahrul, S.; Rasyid, H. Virgin coconut oil: A dietary intervention for dyslipidaemia in patients with diabetes mellitus. Nutrients, 2023, 15, 564. [Google Scholar] [CrossRef]
- Warnick, G.R.; Myers, G.L.; Cooper, G.R.; Rifai, N. Impact of the third cholesterol report from the Adult Treatment Panel of the National Cholesterol Education Program on the clinical laboratory. Clinical Chemistry, 2002, 48, 11–17. [Google Scholar] [CrossRef]
- Ekroos, K.; Lavrynenko, O.; Titz, B.; Pater, C.; Hoeng, J.; Ivanov, N.V. Lipid-based biomarkers for CVD, COPD, and aging – A translational perspective. Prog. Lipid Res. 2020, 78, 101030. [Google Scholar] [CrossRef]
- Mohammadshahi, J.; Ghobadi, H.; Matinfar, G.; Boskabady, M.H.; Aslani, M.R. Role of lipid profile and its relative ratios (Cholesterol/HDL-C, Triglyceride/HDL-C, LDL-C/HDL-C, WBC/HDL-C, and FBG/HDL-C) on admission predicts in-hospital mortality COVID-19. J. Lipids, 2023, 1, 6329873. [Google Scholar] [CrossRef] [PubMed]
- Kip, K.E.; Diamond, D.; Mulukutla, S.; Marroquin, O.C. Is LDL cholesterol associated with long-term mortality among primary prevention adults? A retrospective cohort study from a large healthcare system. BMJ Open 2024, 14, e077949. [Google Scholar] [CrossRef] [PubMed]
- Quispe, R.; Elshazly, M.B.; Zhao, D.; Toth, P.P.; Puri, R.; Virani, S.S.; Blumenthal, R.S.; Martin, S.S.; Jones, S.R.; Michos, E.D. Total cholesterol/HDL-cholesterol ratio discordance with LDL-cholesterol and non-HDL-cholesterol and incidence of atherosclerotic cardiovascular disease in primary prevention: The ARIC study. Eur. J. Preven. Cardiol. 2020, 27, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic. Cholesterol ratio or non-HDL cholesterol: Which is most important? Downloaded from: https://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/expert-answers/cholesterol-ratio/faq-20058006. Accessed 24 September 2024.
- Park, B.; Jung, D.H.; Lee, H.S.; Lee, Y.J. Triglyceride to HDL-cholesterol ratio and the incident risk of ischemic heart disease among Koreans without diabetes: A longitudinal study using national health insurance data. Front. Cardiovasc. Med. 2021, 8, 716698. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The triglyceride/high-density lipoprotein Cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and cardiovascular disease. Diagnostics, 2023, 13, 929. [Google Scholar] [CrossRef]
- Sun, T.; Chen, M.; Shen, H.; Yin, P.; Fan, L.; Chen, X.; Wu, J.; Xu, Z.; Zhang, J. Predictive value of LDL/HDL ratio in coronary atherosclerotic heart disease. BMC Cardiovasc. Disord. 2022, 22, 273. [Google Scholar] [CrossRef]
- Teng, K.T.; Loganathan, R.; Chew, B.H.; Khang, T.F. Diverse impacts of red palm olein, extra virgin coconut oil and extra virgin olive oil on cardiometabolic risk markers in individuals with central obesity: A randomised trial. Eur. J. Nutr. 2024, 63, 1225–1239. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; Stone, N.J.; Van Horn, L.V. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Mensink, R.P. Effects of saturated fatty acids on serum lipids and lipoproteins: A systematic review and regression analysis. Geneva, Switzerland: World Health Organization, 2016. Available at: https://iris.who.int/handle/10665/246104. Accessed 29 October 2024.
- Faridi, K.F.; Kris-Etherton, P.M.; Aspry, K.E. (letter to the editor) Putting saturated fat in context—Replacement and total intake matter. Eur. J. Prevent. Cardiol. 2023, 30, e58. [Google Scholar] [CrossRef]
- Clarke, R.; Frost, C.; Collins, R.; Appleby, P.; Peto, R. Dietary lipids and blood cholesterol: Quantitative meta-analysis of metabolic ward studies. BMJ 1997, 314, 112. [Google Scholar] [CrossRef]
- Zararsız, G.E.; Bolat, S.; Cephe, A.; Kochan, N.; Yerlitaş, S.İ.; Doğan, H.O.; Zararsız, G. Validation of Friedewald, Martin-Hopkins and Sampson low-density lipoprotein cholesterol equations. PLoS ONE, 2022, 17, e0263860. [Google Scholar] [CrossRef]
- Arunima, S.; Rajamohan, T. Influence of virgin coconut oil-enriched diet on the transcriptional regulation of fatty acid synthesis and oxidation in rats: A comparative study. Br. J. Nutr. 2014, 111, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cheng, M.; Kothapalli, K.S.D.; Wang, Z.; Mendralla, E.; Park, H.G.; Block, R.C.; Wang, X.; Brenna, J.T. Glycerol derived process contaminants in refined coconut oil induce cholesterol synthesis in HepG2 cells. Food Chem. Toxicol. 2019, 127, 135–142. [Google Scholar] [CrossRef]
- Eisenreich, A.; Monien, B.H.; Götz, M.E.; Buhrke, T.; Oberemm, A.; Schultrich, K.; Abraham, K.; Braeuning, A.; Schäfer, B. 3-MCPD as contaminant in processed foods: State of knowledge and remaining challenges. Food Chem. 2023, 403, 134332. [Google Scholar] [CrossRef]
- Mendis, S.; Kumarasunderam, R. The effect of daily consumption of coconut fat and soya-bean fat on plasma lipids and lipoproteins of young normolipidaemic men. Br. J. Nutr. 1990, 63, 541–552. [Google Scholar] [CrossRef]
- Smith, S.J.; Cooper, G.R.; Myers, G.L.; Sampson, E.J. Biological variability in concentrations of serum lipids: Sources of variation among results from published studies and composite predicted values. Clin. Chem. 1993, 39, 1012–1022. [Google Scholar] [CrossRef]
- Bautista, L.E.; Rueda-Ochoa, O.L. Methodological challenges in studies of the role of blood lipids variability in the incidence of cardiovascular disease. Lipids Health Dis. 2011, 20, 51. [Google Scholar] [CrossRef]
- Mogadam, M.; Ahmed, S.W.; Mensch, A.H.; Godwin, I.D. Within-person fluctuations of serum cholesterol and lipoproteins. Arch. Intern. Med. 1990, 150, 1645–1648. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; et al. ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar]
- Tholstrup, T.; Ehnholm, C.; Jauhiainen, M.; Petersen, M.; Høy, C.-E.; Lund, P.; Sandström, B. Effects of medium-chain fatty acids and oleic acid on blood lipids, lipoproteins, glucose, insulin, and lipid transfer protein activities. Am. J. Clin. Nutr. 2004, 79, 564–569. [Google Scholar] [CrossRef]
- McKenzie, K.M.; Lee, C.M.Y.; Mijatovic, J.; Haghighi, M.M.; Skilton, M.R. Medium-Chain Triglyceride Oil and Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Trials. J. Nutr. 2021, 151, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.M.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- De la Rubia Ortí, J.E.; Sánchez Álvarez, C.; Selvi Sabater, P.; Bueno Cayo, A.M.; Sancho Castillo, S.; Rochina, M.J.; Yang, I.H. Influencia del aceite de coco en enfermos de alzhéimer a nivel cognitive [article in Spanish: How does coconut oil affect cognitive performance in alzheimer patients?]. Nutr. Hosp. 2017, 34, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.G.; Silva, R.; Binosha Fernando, W.M.A.D.; de Silva, H.A.; Wickremashinghe, A.R.; Dissanayake, A.S.; Soharbi, H.R.; Martins, R.N.; Williams, S.S. Effect of Virgin Coconut Oil Supplementation on Cognition of Individuals with Mild-to-Moderate Alzheimer’s Disease in Sri Lanka (VCO-AD Study): A Randomized Placebo-Controlled Trial. J. Alz. Dis. 2023, 96, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.A.; Sagher, F.A. Antiviral and antibacterial lipids in human milk and infant formula. Arch. Dis. Child. 1991, 66, 272–273. [Google Scholar] [CrossRef]
- Dayrit, F.M. The properties of lauric acid and their significance in coconut oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Schwab, U.S.; Niskanen, L.K.; Maliranta, H.M.; Savolainen, M.J.; Kesäniemi, Y.A.; Uusitupa, M.I. Lauric and palmitic acid-enriched diets have minimal impact on serum lipid and lipoprotein concentrations and glucose metabolism in healthy young women. J. Nutr. 1995, 125, 46–73. [Google Scholar]
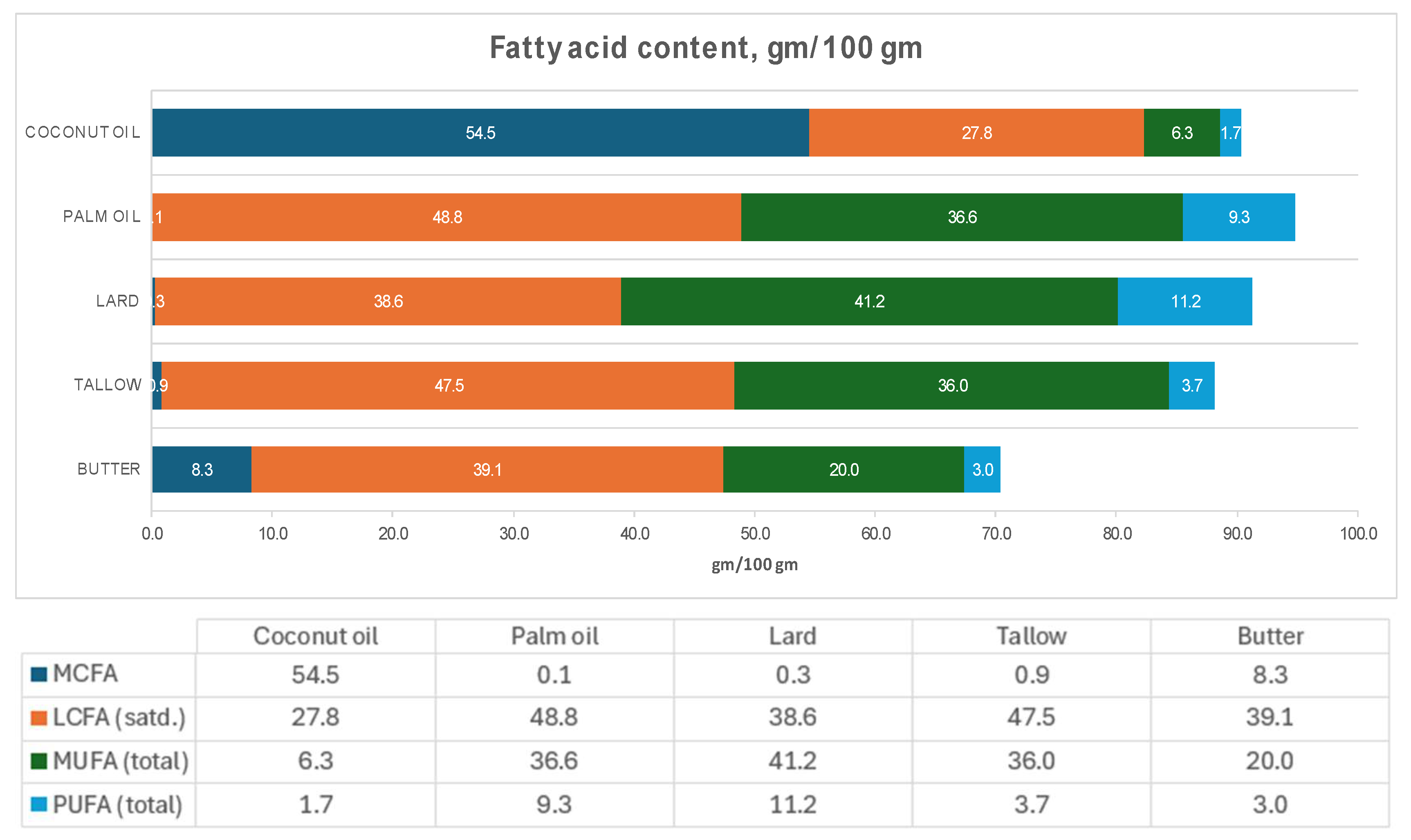




| Constituent | Plant oils | Animal fat | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CNO | Corn | Olive | Palm | SaffO* | SBO | SunO | Lard | Tallow | Butter | |
| Fatty acids | gm/100 gm | |||||||||
| C6:0 Caproic acid |
0.5 | - | 0.0 | 0.0 | ND | - | 0.0 | 0.0 | 0.0 | 2.0 |
| C8:0 Caprylic acid |
6.8 | - | 0.0 | 0.0 | ND | - | 0.0 | 0.0 | 0.0 | 1.2 |
| C10:0 Capric acid |
5.4 | - | 0.0 | 0.0 | ND | - | 0.0 | 0.1 | 0.0 | 2.5 |
| C12:0 Lauric acid |
41.8 | - | 0.0 | 0.1 | ND | - | 0.0 | 0.2 | 0.9 | 2.6 |
| C14:0 Myristic acid |
16.7 | 0.0 | 0.0 | 1.0 | 0.09 | 0.1 | 0.0 | 1.3 | 3.7 | 7.4 |
| C16:0 Palmitic acid |
8.6 | 11.1 | 11.3 | 43.5 | 6.1 | 10.3 | 4.5 | 23.8 | 24.9 | 21.7 |
| C18:0 Stearic acid |
2.5 | 1.6 | 2.0 | 4.3 | 2.3 | 3.7 | 3.0 | 13.5 | 18.9 | 10.0 |
| C18:1 Oleic acid |
6.3 | 27.2 | 71.3 | 36.6 | 13.6 | 21.4 | 62.9 | 41.2 | 36.0 | 20.0 |
| C18:2 Linoleic acid |
1.7 | 51.9 | 9.8 | 9.1 | 69.1 | 50.9 | 20.6 | 10.2 | 3.1 | 2.7 |
| C18:3 Linolenic acid |
0.02 | 1.0 | 0.8 | 0.2 | 0.5 | 6.6 | 0.2 | 1.0 | 0.6 | 0.3 |
| MCFA |
54.5 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.3 | 0.9 | 8.3 |
| LCFA (satd) |
27.8 | 12.7 | 13.3 | 48.8 | 8.5 | 14.1 | 7.5 | 38.6 | 47.5 | 39.1 |
| MUFA |
6.3 |
27.2 | 71.3 | 36.6 | 13.6 | 21.4 | 62.9 | 41.2 | 36.0 | 20.0 |
| PUFA |
1.7 |
52.9 | 10.5 | 9.3 | 69.6 | 57.5 | 20.8 | 11.2 | 3.7 | 3.0 |
| Total FA | 90.3 | 92.9 | 95.1 | 94.8 | 91.5 | 93.0 | 91.2 | 91.3 | 88.1 | 70.4 |
| mg/100 gm | ||||||||||
|
Cholesterol |
0 |
0 | 0 | 0 | 0 | 0 | 0 | 95 | 109 | 215 |
| Group | n= | TChol mg/dL |
LDL-C mg/dL |
HDL-C mg/dL |
TG mg/dL |
|---|---|---|---|---|---|
| CNO, healthy | 35 | 161.3 ± 30.7 | 78.3 ± 24.2 | 47.8 ± 10.0 | 136.5 ± 44.0 |
| SunO, healthy | 35 | 157.1 ± 28.0 | 82.6 ± 26.9 | 44.3 ± 8.5 | 125.2 ± 38.3 |
| CNO, diabetes | 35 | 172.4 ± 35.9 | 108.2 ± 35.4 | 43.8 ± 10.3 | 162.1 ± 47.9 |
| SunO, diabetes | 35 | 179.1 ± 32.2 | 121.7 ± 34.9 | 39.9 ± 9.8 | 151.2 ± 37.4 |
| Test oil | n= | TChol mg/dL |
LDL-C mg/dL |
HDL-C mg/dL |
TG mg/dL |
|---|---|---|---|---|---|
| CNO | 73 | 148.2 ± 36.5 | 96.7 ± 30.6 | 41.3 ± 18.9 | 119.2 ± 49.5 |
| SunO | 80 | 147.2 ± 26.7 | 93.1 ± 22.1 | 37.1 ± 9.8 | 107.8 ± 44.1 |
| Duration | # Data Groups | # Data Sets | Study | Comments |
|---|---|---|---|---|
|
Long 1 - 2 years |
2 | 192 | 1. Vijayakumar 2016 [36], 1 year 2. Vijayakumar 2016 [36], 2 years |
India, 2 years, results at 3 months, 1 year, 2 years, n=96, CNO 15% energy (33-40 gm) |
|
Medium 8 - 24 weeks |
11 | 442 | 1. Mendis 1990 [94] | Sri Lanka, 8 weeks, n=25, CNO 21% of energy (55 gm) |
| 2. Assunção 2009 [47] | Brazil, 12 weeks, n=20, CNO 30 mL daily | |||
| 3. Cardoso 2015 [73] | Brazil, 3 months, n=92, VCO 13 mL daily | |||
| 4. Vijayakumar 2016 [36], 3 months | India, 2 years, results at 3 months, 1 year, 2 years, n=96, CNO 15% energy (33-40 gm) | |||
| 5. Chinwong 2017 [70] | Thailand, 8 weeks, n=32, VCO 30 mL daily | |||
| 6. Oliveira-de-Lira 2018 [45] | Brazil, 8 weeks, n=18, weight loss diet, CNO 6 gm daily | |||
| 7. Korrapati 2019 [54] | India, 8 weeks, n=9, CNO 35 gm daily | |||
| 8. Fernando 2023 [103] | Sri Lanka, 24 weeks, n=43, VCO 30 mL daily | |||
| 9. Jeyakumar 2023 [71] | India, 8 weeks, n= 22, VCO 35 gm daily | |||
| 10. Swarnamali 2024 [64] | Sri Lanka, 8 weeks, n=37, CNO 10% of energy (17 gm) | |||
| 11. Teng 2024 [85] | Malaysia, 12 weeks, n=48, VCO 53 gm daily | |||
|
Short 3 - 7 weeks |
16 | 350 | 1. Reiser 1985 [52] | USA, 5 weeks, n=16, CNO 21% of energy (80-117 gm) |
| 2. Heber 1992 [53] | USA, 3 weeks, n=9, CNO, CNO 17.5% of energy (41 gm) | |||
| 3. Cox 1995 [63], males 4. Cox 1995 [63], females |
New Zealand, 6 weeks, CNO 39 gm, results for males (n=13) and females (n=15) reported separately | |||
| 5. Schwab 1995 [106] | Finland, 4 weeks, n=15, lauric acid 4% of total energy, CNO varied from 16 to 26 gm daily | |||
| 6. McKenney 1995 [46] (Study 1) | USA, 6 weeks, n= 11, CNO 42 gm daily | |||
| 7. McKenney 1995 [46] (Study 2) | USA, 6 weeks, n=17, lovastatin 6 weeks, then CNO 42 gm for 6 more weeks | |||
| 8. Lu 1997 [51] | USA, 3 weeks, n=15, CNO 10% of energy (30 gm) | |||
| 9. Cox 1998 [66] | New Zealand, 4 weeks, n=37, CNO 39 gm daily, male and female results combined | |||
| 10. Voon 2011 [48] | Malaysia, 5 weeks, n=45, CNO 44 gm daily | |||
| 11. Khaw 2017 [49] | UK, 4 weeks, n=28, VCO 50 mL daily | |||
| 12. Harris 2017 [50] | USA, 4 weeks, n=12, VCO 30 mL daily | |||
| 13. Maki 2018 [67] | USA, 4 weeks, n=12, CNO 54 gm daily | |||
| 14. Vogel 2020 [72] | Brazil, 45 days, n= 15, VCO 12 mL daily | |||
| 15. Nikooei 2021 [74] | Iran, 4 weeks, n=22, VCO 30 mL daily | |||
| 16. Setyawati 2023 [75] | Indonesia, 30 days, n=68, VCO 1.2 mL/kg/day |
| Duration | # Data Groups | # Data Sets | Average | TChol | LDL-C | HDL-C | TG |
|---|---|---|---|---|---|---|---|
|
All groups combined, 3 weeks to 2 years |
29 | 984* | Baseline (mg/dL) | 186.2 | 117.7 | 45.4 | 125.3 |
| Final (mg/dL) | 185.9 | 115.5 | 48.1 | 121.7 | |||
| Difference (mg/dL) | -0.3 | -2.2 | 2.7 | -3.6 | |||
| Percent (%) | -0.13 | -1.8 | 5.8 | -2.9 | |||
|
Long, 1 to 2 years |
2 | 192 | Baseline (mg/dL) | 149.8 | 90.3 | 40.8 | 115.0 |
| Final (mg/dL) | 146.9 | 91.0 | 42.8 | 110.7 | |||
| Difference (mg/dL) | -2.9 | 0.7 | 2.0 | -4.3 | |||
| Percent (%) | -2.0 | 0.8 | 4.9 | -3.7 | |||
|
Medium, 8 to 24 weeks |
11 | 442 | Baseline (mg/dL) | 182.8 | 115.5 | 44.3 | 122.8 |
| Final (mg/dL) | 182.2 | 112.9 | 45.3 | 121.2 | |||
| Difference (mg/dL) | -0.6 | -2.6 | 1.0 | -1.6 | |||
| Percent (%) | -0.31 | -2.3 | 2.3 | -1.4 | |||
|
Short, 3 to 7 weeks |
16 | 350 | Baseline (mg/dL) | 210.4 | 135.5 | 49.5 | 134.1 |
| Final (mg/dL) | 212.0 | 132.3 | 54.4 | 128.4 | |||
| Difference (mg/dL) | 1.6 | -3.2 | 4.9 | -5.7 | |||
| Percent (%) | 0.8 | -2.3 | 10.0 | -4.3 |
| Study Group | Duration | Amount of CNO/day | n = | TChol % Diff |
LDL-C % Diff | HDL-C % Diff | TG % Diff |
|---|---|---|---|---|---|---|---|
| Long duration (1 to 2 years) | |||||||
| Vijayakumar 2016 [36] | 1 year | 33-40 gm | 96 | -3.5 | 0.8 | 3.9 | -2.6 |
| Vijayakumar 2016 [36] | 2 years | 33-40 gm | 96 | -0.4 | 0.8 | 5.9 | -4.9 |
| Medium duration (8 to 24 weeks) | |||||||
| Mendis 1990 [94] | 8 weeks | 55 gm | 25 | -0.6 | -3.7 | 3.6 | 2.1 |
| Oliveira-de-Lira 2018 [45] | 8 weeks | 6 gm | 18 | -8.1 | -10.4 | 5.0 | -24.9 |
| Korrapati 2019 [54] | 8 weeks | 35 gm | 9 | 0.0 | -2.4 | 10.4 | -6.6 |
| Chinwong 2017 [70] | 8 weeks | 30 mL | 32 | -1.4 | -5.2 | 6.5 | -4.6 |
| Jeyakumar 2023 [71] | 8 weeks | 35 gm | 22 | 8.1 | 11.5 | -0.3 | 6.0 |
| Swarnamali 2024 [64] | 8 weeks | 17 gm | 37 | 4.0 | -1.5 | 1.2 | 0.9 |
| Assuncao 2009 [47] | 12 weeks | 30 mL | 20 | 2.9 | 3.5 | 7.0 | 4.0 |
| Teng 2024 [85] | 12 weeks | 53 gm | 48 | -9.3 | -11.0 | -9.1 | -0.8 |
| Cardoso 2015 [73] | 3 months | 13 mL | 92 | 3.3 | 3.7 | 8.3 | -1.3 |
| Vijayakumar 2016 [36] | 3 months | 33-40 gm | 96 | 0.9 | -1.1 | 0.0 | -3.2 |
| Fernando 2023 [103] | 24 weeks | 30 mL | 43 | -3.3 | -7.4 | 4.9 | 4.1 |
| Short duration (3 to 7 weeks) | |||||||
| Heber 1992 [53] § | 3 weeks | 41 gm | 9 | 18.2 | 24.0 | 5.0 | 18.3 |
| Lu 1997 [51] § | 3 weeks | 30 gm | 15 | -5.7 | -2.6 | -6.6 | -14.3 |
| Schwab 1995 [106] § | 4 weeks | 16-26 gm | 15 | 0.4 | -1.7 | -3.9 | -4.4 |
| Cox 1998 [66] § | 4 weeks | 39 gm | 37 | -0.6 | 6.5 | 10.0 | -9.0 |
| Harris 2017 [50] § | 4 weeks | 30 mL | 12 | 8.3 | 10.9 | 10.3 | -8.3 |
| Khaw 2017 [49] § | 4 weeks | 50 mL | 28 | 3.7 | -2.6 | 14.0 | 7.9 |
| Maki 2018 [67] § | 4 weeks | 54 gm | 12 | 7.1 | 4.6 | 6.5 | 5.9 |
| Nikooei 2021 [74] § | 4 weeks | 30 mL | 22 | 15.8 | 19.5 | 18.8 | -20.5 |
| Setyawati 2023 [75] | 30 days | 1.2 mL/kg | 68 | -8.5 | -21.2 | 23.5 | -4.8 |
| Reiser 1985 [52] | 5 weeks | 80-117 gm | 16 | 6.3 | 14.6 | 2.2 | -2.5 |
| Voon 2011 [48] | 5 weeks | 44 gm | 45 | 5.1 | 7.8 | 11.4 | -6.2 |
| Cox 1995 [63], Males | 6 weeks | 39 gm | 13 | 1.5 | 2.8 | -3.0 | 13.4 |
| Cox 1995 [63], Females | 6 weeks | 39 gm | 15 | 1.4 | 1.3 | -2.3 | -7.3 |
| McKenney 1995 [46] #1 | 6 weeks | 42 gm | 11 | 4.9 | 4.3 | 8.2 | 2.5 |
| McKenney 1995 [46] #2 | 6 weeks | 42 gm | 17 | -2.7 | -9.2 | 10.2 | 7.8 |
| Vogel 2020 [72] | 45 days | 12 mL | 15 | -4.8 | -10.2 | 9.3 | -1.6 |
| TChol mg/dL |
LDL-C mg/dL |
HDL-C mg/dL |
TG mg/dL |
|
|---|---|---|---|---|
| Average baseline values | 186.2 | 117.7 | 45.4 | 125.3 |
| Range of average baseline values between groups | 149.8 to 251.4 | 90.1 to 179.6 | 35 to 77.3 | 67.8 to 216.5 |
| Average final values | 185.9 | 115.5 | 48.1 | 121.7 |
| Range of average final values between groups | 144.6 to 255 | 87.8 to 171 | 34.9 to 88.2 | 64.7 to 231 |
| Average difference | -0.3 (-0.13%) | -2.2 (-1.8%) | 2.7 (5.8%) | -3.6 (-2.9%) |
| Range of differences between studies | -21.1 to +32.6 | -38.2 to +25.0 | -5.0 to +10.8 | -44.6 to +27.3 |
| Range of SDs in final values | ±3.0 to ±53.6 | ±4.1 to ±41.5 | ±1.1 to ±18.8 | ±3.6 to ±114.6 |
| Duration | TChol | LDL-C | HDL-C | TG |
|---|---|---|---|---|
|
Short 3 to 7 weeks |
-21 to +33 -32% to +16% SD ±3 to ±53.6 |
-38 to +25 -21% to +21% SD ±4.1 to ±37.2 |
-3.5 to +10.8 -6.6% to +14% SD ±1.1 to ±18.3 |
-44 to +27 -21% to +13% SD ±3.6 to ±114.6 |
|
Medium 8 to 24 weeks |
-19 to +14 -9% to +8.1% SD ±6.0 to ±44.7 |
-15 to +13 -10% to +12% SD ±2.2 to ±41.5 |
-5.0 to +4.8 -9.1% to +10.4% SD ±2.4 to ±11.5 |
-33 to +6.9 -25% to +4% SD ±12.3 to ±93.7 |
|
Long 1 to 2 years |
-5.3 to -0.6 -3.5% to -0.4% SD ±28.6 to ±30.9 |
+0.7 to +0.8 +0.8% to +0.8% SD ±20.7 to ±21.8 |
+1.6 to +2.4 +3.9% to +5.9% SD ±9.5 to ±10.8 |
-5.6 to -3.0 -4.9% to -2.6% SD ±47.1 to ±50.2 |
| Study, Duration, # Subjects |
TChol mg/dL |
LDL-C mg/dL |
HDL-C mg/dL |
TG mg/dL |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | Diff | SD (+) | Base | Diff | SD (+) | Base | Diff | SD (+) | Base | Diff | SD (+) | |
| Harris 2017 4 weeks n=12 |
220 | +18.2 +8.3% | 24 | 124 | +13.5 +10.9% | 27 | 64 | +6.6 +10.3% | 18 | 117 | -9.7 -8.3% |
81 |
| Khaw 2017 4 weeks n=28 |
228 | +8.5 +3.7% | 21 | 135 | -3.5 -2.6% |
19 | 77 | +10.8 +14.0% | 11 | 79 | +6.2 +7.9% |
51 |
| Combined n=40 |
226 | +11.4 +5.0% | - | 132 | +1.6 +1.2% |
- | 73 | +9.6 +13.0% | - | 90 | +1.43 +1.6% |
- |
| Duration | # Data Groups |
# Data Sets |
TChol/ HDL-C |
LDL-C/ HDL-C |
TG/ HDL-C |
|
|---|---|---|---|---|---|---|
|
All groups 3 weeks to 2 years |
29 | 984 | Baseline ratio | 4.1 | 2.6 | 2.8 |
| Final ratio | 3.9 | 2.4 | 2.5 | |||
| Difference (Baseline - Final) | -0.2 | -0.2 | -0.3 | |||
|
Long 1 to 2 years |
2 | 192 | Baseline ratio | 3.7 | 2.2 | 2.8 |
| Final ratio | 3.4 | 2.1 | 2.5 | |||
| Difference (Baseline - Final) | -0.3 | -0.1 | -0.3 | |||
|
Medium 8 to 24 weeks |
11 | 442 | Baseline ratio | 4.1 | 2.6 | 2.8 |
| Final ratio | 4.0 | 2.5 | 2.7 | |||
| Difference (Baseline - Final) | -0.1 | -0.1 | -0.1 | |||
|
Short 3 to 7 weeks |
16 | 350 | Baseline ratio | 4.3 | 2.7 | 2.7 |
| Final ratio | 3.9 | 2.4 | 2.4 | |||
| Difference (Baseline - Final) | -0.4 | -0.3 | -0.3 |
| Oil, time point |
TChol mg/dL | LDL-C mg/dL | HDL-C mg/dL | TG mg/dL (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | Diff | % | Base | Diff | % | Base | Diff | % | Base | Diff | % | |
| Difference between baseline and 3-month values | ||||||||||||
| CNO | 150 | 1.4 | 0.9 | 90 | -1.0 | -1.1 | 41 | 0.02 | 0.0 | 115 | -3.7 | -3.2 |
| SunO | 147 | -3.4 | -2.3 | 86 | -2.0 | -2.3 | 41 | -1.2 | -2.9 | 111 | -2.3 | -2.0 |
| Difference between baseline and 1-year values | ||||||||||||
| CNO | 150 | -5.2 | -3.5 | 90 | 0.7 | 0.8 | 41 | 1.6 | 3.9 | 115 | -3.0 | -2.6 |
| SunO | 147 | -7.0 | -4.8 | 86 | 1.5 | 1.7 | 41 | -0.6 | -1.6 | 111 | 3.4 | 3.0 |
| Difference between baseline and 2-year values | ||||||||||||
| CNO | 150 | -0.6 | -0.4 | 90 | 0.8 | 0.8 | 41 | 2.4 | 5.9 | 115 | -5.6 | -4.9 |
| SunO | 147 | 4.8 | 3.3 | 86 | 3.5 | 4.1 | 41 | 3.6 | 8.9 | 111 | 1.0 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





