1. Introduction
The movement of the microworld particle, always described as the matter wave, which is developed by de Broglie, is an old and recurring topic in quantum mechanics. The double-slit experiment is an archetypal demonstration of the matter waves, which is also called wave-particle duality. Traditionally, one often interprets the wave-particle duality as the statement of observing either the wave feature or the particle feature, but not both simultaneously, which has to be explained by Bohr’s complementarity principle [
1]. However, “The paradoxes of the dualism between wave picture and particle picture were not solved; they were hidden somehow in the mathematical scheme” [
2]. In other hands, the practical application of quantum mechanics has been extraordinarily successful and has penetrated many areas of modern science and technology. So, no one questions what the theory predicts; only what it means [
3].
The Copenhagen interpretation is always seen as traditional explanations of quantum mechanics, which is based on the steady-state transition, uncertainty principles[
4], and the probability explanation of wave functions. These concepts are far from classical mechanics and are increasingly questioned by the community. There are three groups that have attempted to criticize the Copenhagen interpretation and replaced it with one that is more in line with the concepts of classical physics or materialistic philosophy [
5]. The first group does not want to change the Copenhagen interpretation, but tries changing the language of this interpretation to obtain a closer resemblance to classical physics. The second group believes that the Copenhagen interpretation is the only adequate and tries changing the quantum theory to some extent at certain critical points. The third group rather expresses its general dissatisfaction with the results of the Copenhagen interpretation and especially with its philosophical conclusions, without making any definite counterproposals [
5]. In this paper, we will find a new way to interpret the quantum mechanics.
2. The Electron Movement in the Hydrogen Atoms
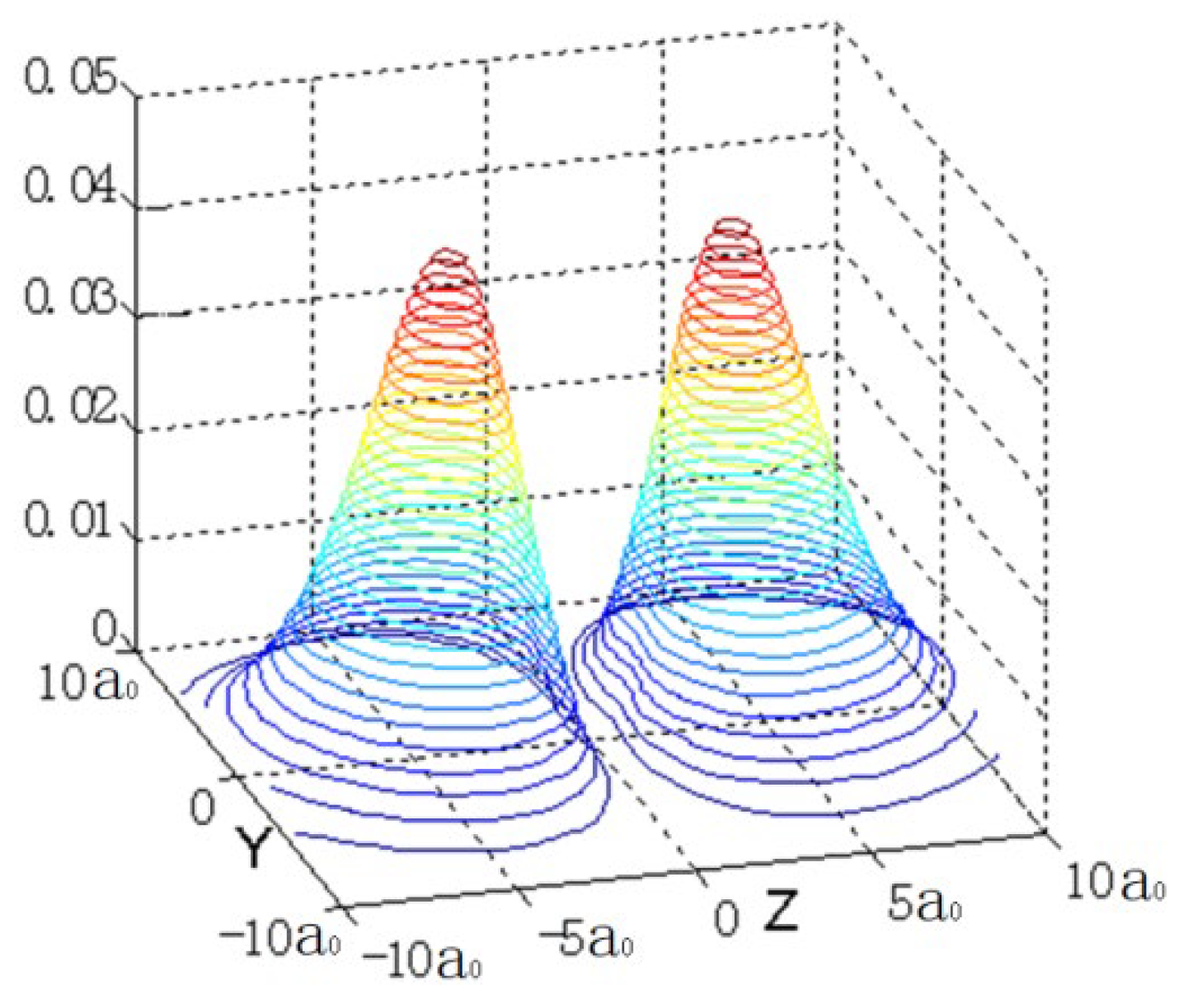
We first consider the hydrogen atom, which can be strictly solved through the Schrödinger equation. The stationary wave function of 2P
z is the product of the radial wave function and the spherical harmonics function. The shape of the electron cloud (which is the electron probability density, given by the square of the amplitude of the stationary wave function) is similar to two symmetrical spherical caps [
6,
7].
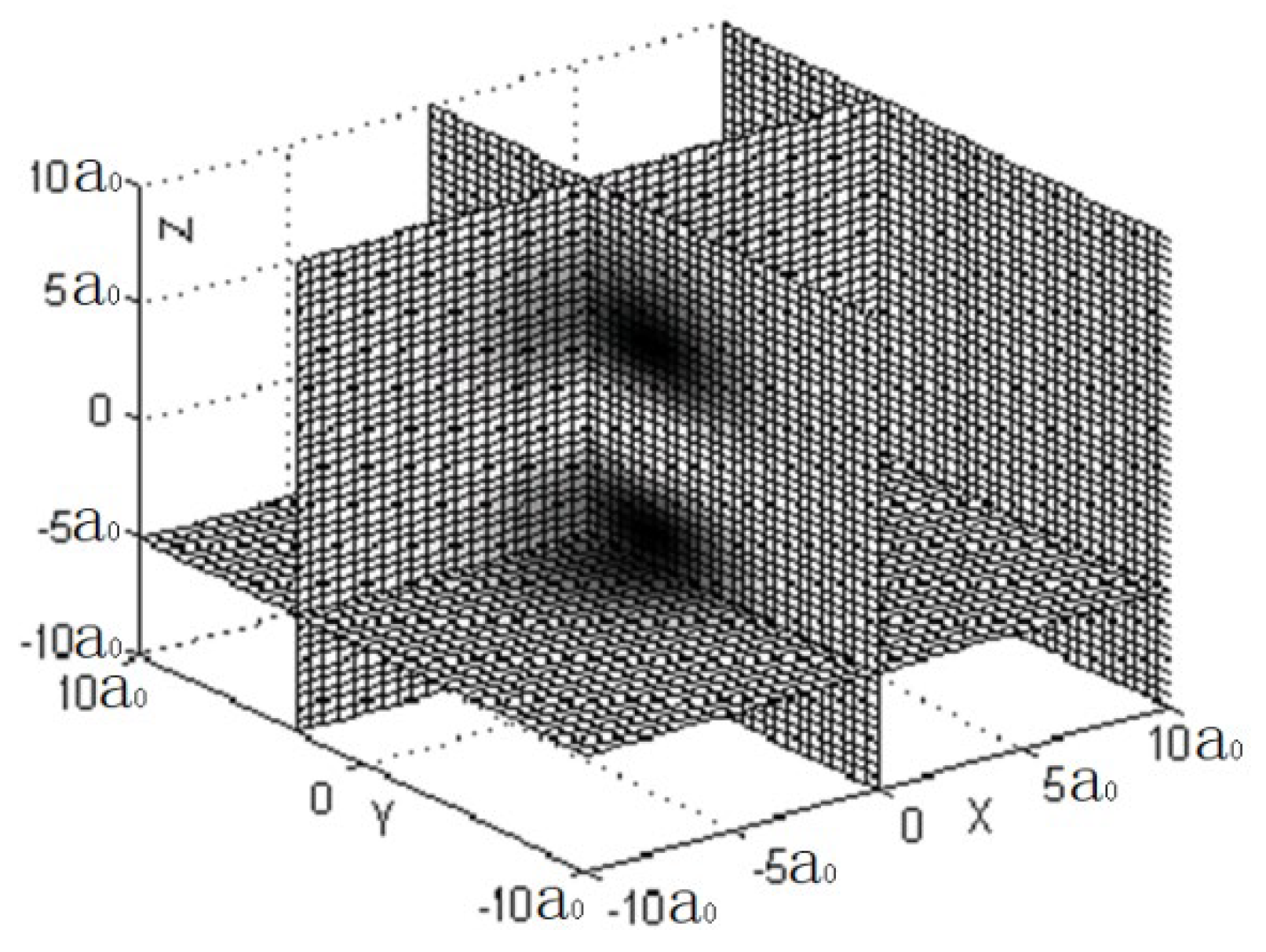
Figure 1 shows three sectional views of X=0, Y=3, Z=-5.
Figure 2 shows a sectional view of X=0.
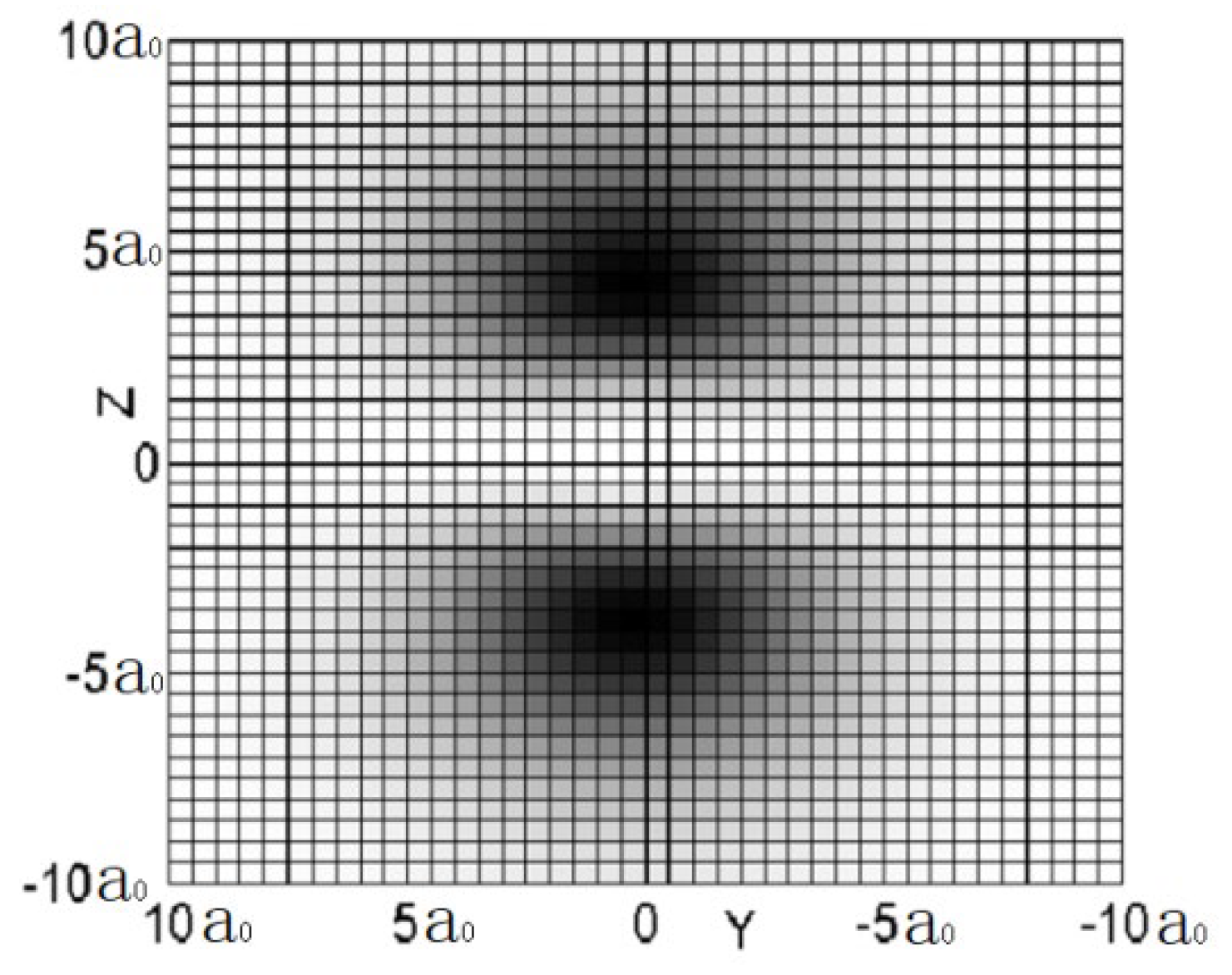
Figure 3 is a contour diagram of the sectional probability density shown in
Figure 2. The a
0 in these figures is the Bohr radius.
We can discover from the three figures, especially in
Figure 3, the probability density of appearing electron at Z=0 is zero. This indicates that no electron travels through the plane of Z=0, due to the fact that the spherical harmonics function of 2P
Z is zero when θ is 90-degree.
Now, the question is how to explain the electron motion in a steady state. In quantum mechanics, many people believe that the observation of the electron in hydrogen atoms is the collapse of the wave function, which can introduce disturbance and induce the uncertainty principle. As we know, the wave function is the exact solution of the Schrödinger equation, and the disturbance is not taken into account, which means that the electron probability density is what it should be, rather than the result of measurement disturbance.
If the electron movement is spatially continuous, it is impossible that the probability density of electron at Z=0 is zero. As we know, there is only one electron in the hydrogen atom, and it is impossible for the electron to appear only in the space of Z>0 or Z<0. Therefore, there must be a transportation between the space of Z>0 and Z<0. And then, the electron must pass through the plane of Z=0. The faster the electron moves, the smaller the electron probability density. However, the electron probability density is not zero, even if the electron velocity equals the speed of light when passing through the plane of Z=0. Thus, there is a contradiction between the wave function and the spatially continuous motion.
As we know, the wave function is the exact solution and can be proved in many experiments, so it is impossible to deny. However, the spatially continuous movement of the electron may be our illusion coming from our daily experiments. If the movement is discontinuous in space, the conflict can be resolved perfectly. Since only when the motion in space is discontinuous, it is possible that the electron probability density at Z=0 is zero. We can call this type of movement mode “annihilation- generation movement” (AGM).
The annihilation in AGM is different from that in the quantum electrodynamics (QED). The annihilation in AGM is a type of intrinsic motion, and the annihilation in QED is a result of particle interaction. An electron may leave place A and arrive at place B, but it is impossible to ascribe a precise trajectory linking the two [
3]. This statement is absolutely right, because there is no trajectory between one point where the electron annihilates and another point where the electron generates. We can say that the procedure of AGM is dominated by the Schrödinger equation. In other words, the AGM is described through the wave function. So the role of the wave function in quantum mechanics is equivalent to that of the motion trajectory of the mass center in Newtonian mechanics.
3. An Experiment for Verifying AGM
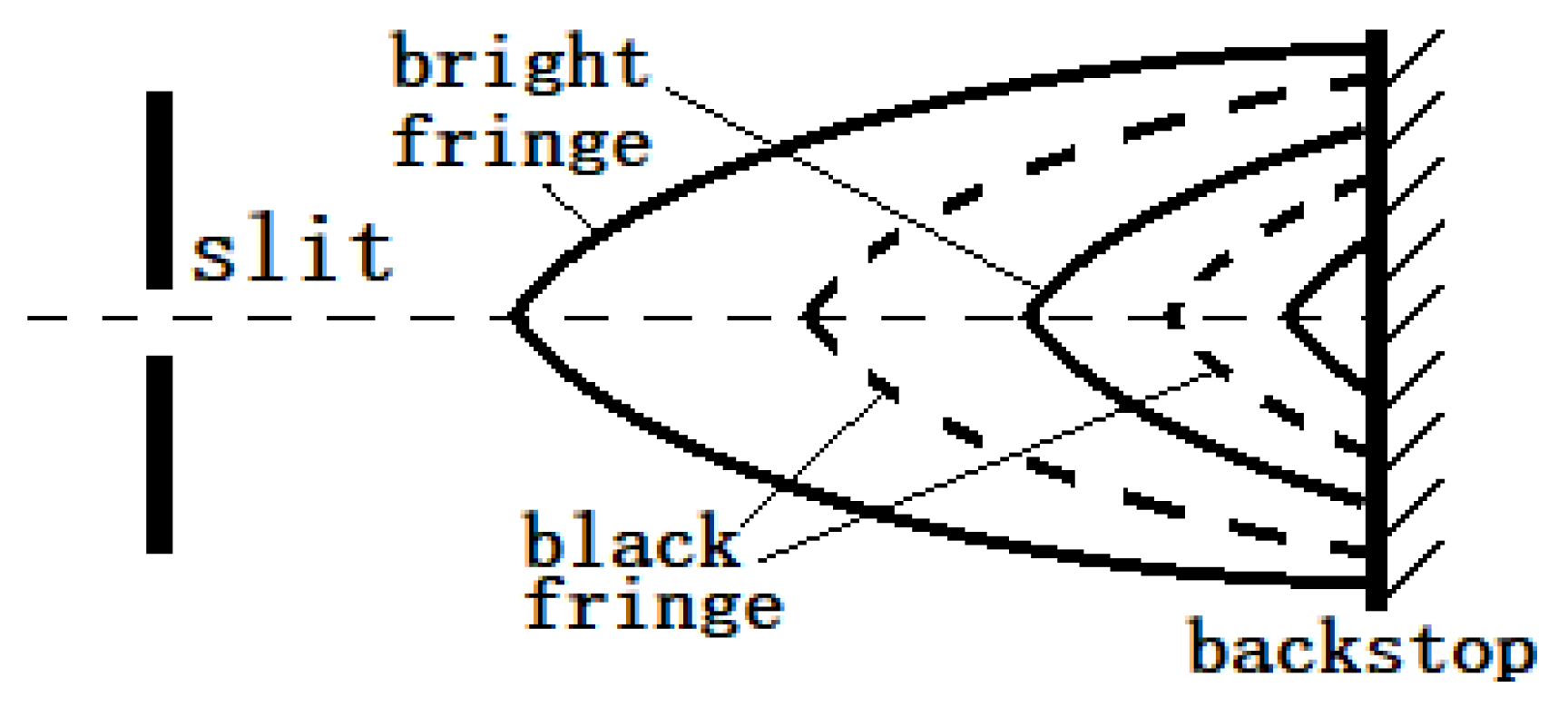
If we delve into the single-slit diffraction, we will obtain a very interesting result. As we know, fringes can be obtained at the backstop after the electron passes through a single silt [
8]. When you move the backstop back and forth, the bright and dark fringes in the center of the backstop will appear sequentially, as shown in
Figure 4. Also, we can find that the spatial distribution of the dark and bright fringes in
Figure 4 is a set of curved surfaces.
If we place a curved surface to fit the dark fringe that is distributed in 3-dimensional space, as shown in
Figure 4, there will be no electrons viewed on the curved surface. The curved surface can be large enough to separate completely the moving space of the electron into two parts, which means that the electron cannot be detected in the whole space when the curved surface is used to separate the space into two parts. Therefore, the only explanation is that the electron undergoes annihilation during its movement.
Although the set of the curved surface is obtained by moving the backstop back and forth, it can also be obtained by the superposition of the wavefront of the electron within the single slit. Therefore, the curved surface is a mathematical result, and it is not the result of measurement.
In the process of AGM, there is no collapse of wave function when the electron is under detection, the wave function always exists whether the electron is detected or not. This is the reason of quantum entanglement..
4. The Wave Function of a Free Electron
As we know, the wave function of a free electron with a certain energy can be written as ψ=
ei(ωt-kz), if the electron moves along the z direction [
8]. Thus, the probability density of finding the electron is the same at all positions, because of ψψ*=1. If we suppose the electron appears at point A at a certain time, the total probability of finding the electron at the next time between point A and point A+δz is given by
. Here, δz can be any value, indicating that the total probability can change with different δz. However, this is not physical, since the probability should be always 1.
To resolve this problem through the AGM, it is reasonable to suppose that the integral interval of the total probability of the electron at a certain moment should be λ/2, where λ is the wavelength of the matter wave of the electron. Because in the one-dimensional infinite deep potential well, the wave function of the ground state is the half-wavelength of the sine function, the free electron should appear once in the half-wavelength of the matter wave. Therefore, there should be a normalizing coefficient in the wave function as follows:
And then total probability of finding electron should be given by
According to Equation (1), the electron can only appear in a small space of λ/2 at a certain moment, instead of the whole space. At the next time, the electron will appear at the next λ/2. In this procedure, the position of electron generation is random, which is decided by the wave function. So, the essence of the wave-particle duality and the uncertainty principles comes from AGM. The wave function can be used to describe the single electron moment.
Because Equation (2) is always correct at any time, the procedure of AGM is continuous in time, which means that when the electron annihilates at one point, it should be generated at another point at the same time. The electron has no translational movement in the interval of λ/2 and will stay for a piece of time at one point, and then stay for another piece of time at another point. If the electron exist the translational motion during the AGM, there will be no plane with zero probability density of electron in the hydrogen atom.
5. The Double Slit Experiment
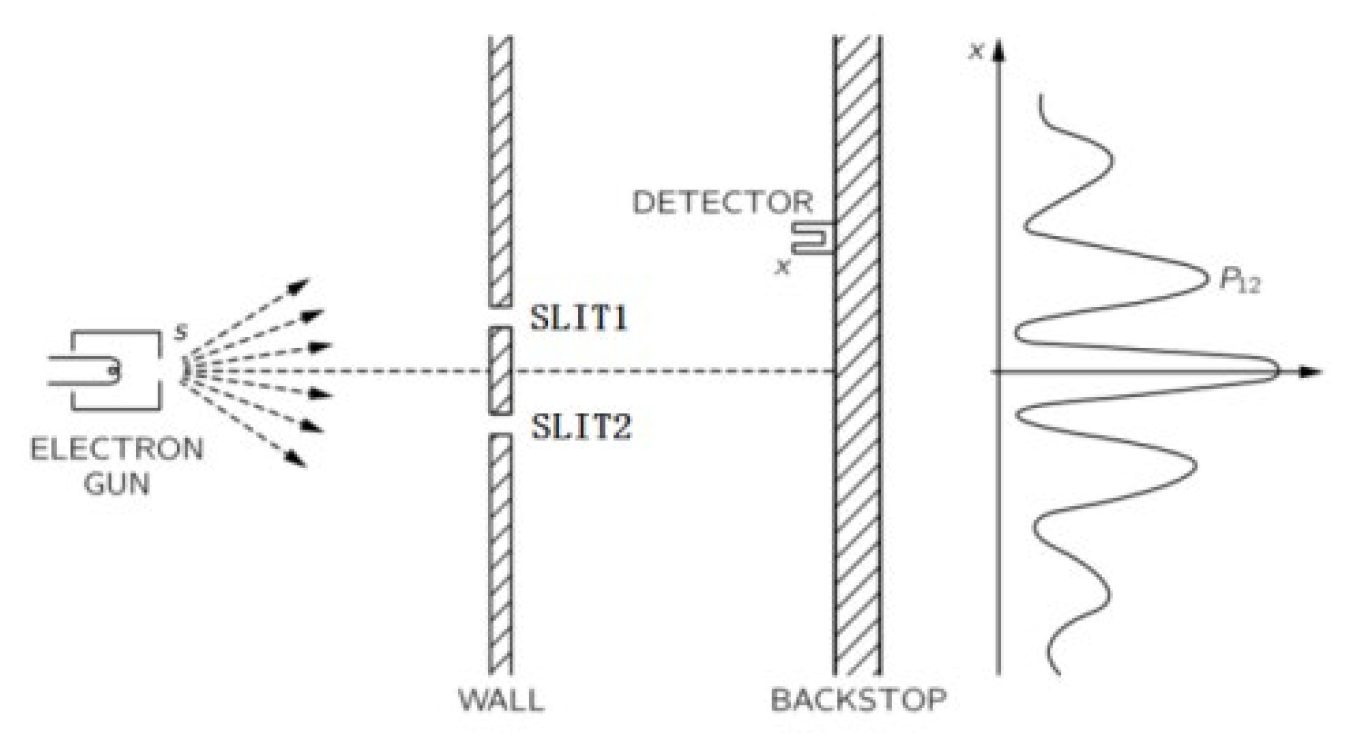
The double-slit experiment is an important experiment for studying the wave-particle duality, which can be explained on the basis of the AGM. Many people always ask which slit the electron passes through in the double-slit experiment as shown in
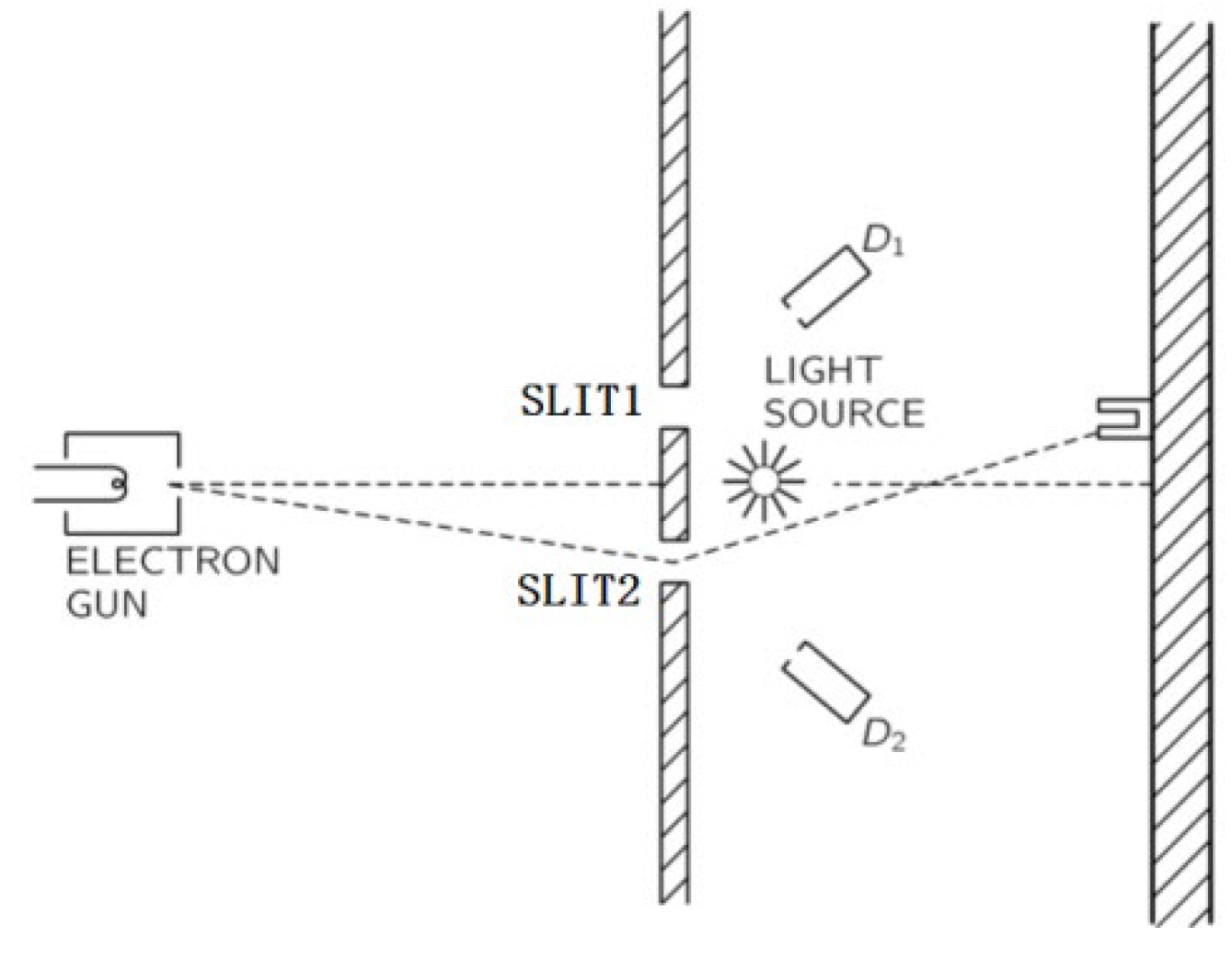
Figure 5, and the experiment with adding a light source behind the two slits was done as shown in
Figure 6. However, if the electron can be detected by the light source, D1, D2, the interference fringes will disappear at the backstop [
9].
If Equation (1) is used to describe a large number of electrons moving along the z direction with different transverse positions of x and y, the electron can appear at any transverse position. For the movement of one electron, the electron cannot appear at any transverse position, and then the coordinates of x and y should be included in Equation (1). Thus, the wave function of one electron can be combined with the Gaussian function:
In Equation (3), the electron always appears in the field of 2σ in the transverse direction. σ is related to the matter wave
. A shorter
will give a smaller σ. This can be demonstrated by the double-slit experiment, such as the distance between two slits is about 1um for 50keV electron [
10].
As we know, the electron can interfere with itself when the electron is emitted from an electron gun one by one. This phenomenon can be explained on the basis of AGM that the electron is always generated in the field of 2σ in the transverse direction, and then there is a certain probability to go through each slit. These probabilities can only be determined on the basis of the wave function of Equation (3), The wave function passes through two slits at the same time and interferes with itself. When the distance between two slits is greater than 2σ, the single electron can pass through only one slit for a certain probability, and then, the double-slit interference disappears.
For the experiment in
Figure 6, a common perspective is that the reason for the disappearance of interference is the disturbance of the light [
9]. However, if we explain this disappearance based on AGM, the problem of which slit the electron passes through may be solved. When we succeeded in identifying which slit the electron passes through, the probability of passing through the other slit is zero, so there is no possibility of interference.
In the macroworld, the distance of electron movement is much greater than σ, and then the AGM of free electron can be treated as straight line movement. Therefore, AGM is the link between micro-movement and macro-movement.
6. The Explanation of Heisenberg’s Uncertainty Principle and the Steady-State Transition
It is difficult to understand the wave-particle duality in classical physics, because the two pictures are mutually exclusive. In other words, “a certain thing cannot at the same time be a particle (i.e., substance confined to a very small volume) and a wave (i.e., a field spread out over a large space)” [
11]. Therefore, Bohr had to develop the complementarity principle to incorporate apparently contradictory properties. “One experiment may reveal the wave nature of the electron, another the particle nature. Both cannot be manifested at once; it is up to the experimenter to decide which facet to expose by his choice of experiment.”[
3] However, when the double-slit experiment is carried out with the electron passing through the slit one by one, the interference fringes will be obtained after a sufficiently long time. Simultaneously, each electron can be detected within a small volume, and half of the electron can never be detected. In other words, the wave nature and the particle nature can be manifested at the same time.
From the view of the AGM, there is no contradiction between the wave picture and the particle picture. During the procedure of AGM, the electron annihilates and it is regenerated as a particle. After the electron annihilates, it will generate at a certain position with a certain probability in a region spreading out over a large space. So, the electron as a particle moves like a wave. In other words, the wave picture and the particle picture are not complementary but are two side views of the AGM at the same time.
The Heisenberg’s uncertainty principle is another law distinct from classical physics, and it can be proved in mathematics
without considering the measurement disturbance. Einstein maintained that there must exist a deeper level of hidden dynamical variables that affect the system and bestow upon it merely an apparent indeterminism and unpredictability[
3]. However, in the process of annihilation and generation, it is difficult to accurately predict the electron position, because of the random position of the generation. It is also difficult to accurately define the electron velocity according to some laws. In other words, the chance element is inherent in the nature of the quantum system and not merely imposed by our limited grasp of all variables that affect the system [
3]. Therefore, there is no “hidden dynamical variables” in quantum mechanics to remove indeterminism and unpredictability, and the Heisenberg’s uncertainty principle is the natural result of AGM.
In classical physics, one would get a probability distribution for the value of coordinates and velocities during measurements, but these probabilities are different from those in quantum mechanics, because the probability of classical physics comes from measurement errors. In theory, errors in classical physics can be reduced to any desired degree of accuracy by improving the measuring instrument. However, the probability of quantum mechanics is not only a mathematical expression deduced from the observation, and it should indeed occur during microscopic particle movement. This is the reason why we cannot describe what happens between this observation and the next [
12]. The probability of quantum mechanics does not come from observation, so there is no subjective element in probability.
The steady-state transition is developed by Bohr[
13]. It is difficult to understand in traditional physics because it takes no time during the steady-state transition. If the electron of different steady state run in different classical orbits, it will take some time during the steady-state transition. However, the electron appears almost all of the space in the same atom space in every steady state, and just the appearance probability is different for the different steady state. So the change of different probability between the different steady state takes no time according to the AGM mode, and then it takes no time during the steady-state transition.
7. The Physical and Philosophic Meaning of AGM
In Newton mechanics, velocity and acceleration are the differentiation of the position vector of the center of mass with respect to time. For AGM, the differential operation of the position vector is impossible. Therefore, the velocity and acceleration of the microscopic particles are not the fundamental physical concepts in quantum mechanics. Then, the laws of conservation of energy and momentum defined by the wavelength of the matter wave are also different from those defined in the macroworld.
The Copenhagen interpretation of quantum mechanics rejects the objective reality of the quantum microworld, because it denies that an electron has a well-defined position and well-defined momentum in the absence of actual observation of either its position or momentum [
3]. According to the view of AGM, because of the random position of the generation, it is impossible to have an actual observation of either a well-defined position or well-defined momentum. But the electron is an indeed objective reality, because we can detect it with a detector. The objective reality of the electron is not based on the detection of the exact position or momentum, but on the detectability of the electron at any time.
There are three reasons for that the wave function exists objectively, but the wave function is not reality. The first reason is that the wave function is the result of the Schrödinger equation. Therefore, the wave function is like the motion trajectory in Newtonian mechanics, and the trajectory is not reality but objectively. The second reason is that the wave function can always change with different potential energy functions in the Schrödinger equation, just like the trajectory in Newtonian mechanics. However, the particle does not change with different potential energy functions. The third reason is that the wave function cannot be detected directly like the electron. We always detect the wave function by the fringe of the interference and the diffraction, and the fringe should be detected by the statistical measurement of many electrons; moreover, we cannot directly detect the wave function of one electron.
If the AGM exists, it must be the form of the particle being. That is, any particle exists in the successive AGM, which means that the particle movement is definite. The particle energy is lower; the wavelength of matter wave is longer. In other words, temperature cannot be reduced infinitely, and absolute zero degree is nonexistent.
This mode of movement denies our classical concept of existence. That is, the electron will annihilate and generate without excuse and keep going like this. The electron motion we observed in the macroworld view is just a combination of a series of annihilation-generation processes, which are like playing a movie. This mode of movement has its rationality, that is, if there is a Big Bang start-up, in which the generation process of the particle should be preserved today, as one phase of the annihilation-generation process. Moreover, the process of annihilation of the positive and negative electrons to generate photons is likely connected with AGM. That is, after annihilating, thanks to overlapping and interactive perturbations during generation, the positive and negative electrons evolve into photons.
8. Conclusion
As a conclusion, the AGM is a perfect physical image to solve the puzzles of quantum mechanics. Although Bopp had ever considered the creation or the annihilation of a particle as the fundamental process of quantum theory [
14], the particle’s AGM cannot be accepted in classical western philosophy, which compelled the western physicists to launch a philosophical revolution. However, the AGM is consistent with the ideas of Eastern philosophy. Laozi said in the Tao and Teh: “The nameless is the origin of heaven and earth; the named is the mother of all things”. “Being and non-being interdependent in growth.” Buddha also said: “Form does not differ from emptiness; emptiness does not differ from form. Form itself is emptiness; emptiness itself is form. “ These philosophy thoughts have provided the foundation for the particle’s AGM.
In summary, the AGM model is proposed here, which is in consistent with the probability concept of Copenhagen interpretation, but the physical philosophy is resembled to the Eastern philosophy. Therefore, this can be seen as the fourth group that has attempted to criticize the Copenhagen interpretation and replace it with concepts of classical philosophy, and the observer can be independent of the interpretation of quantum theory.
Acknowledgments
This work was supposed by the doctor foundation of East China University of Technology. The author wants to thank Dr. Wenlong Zhang and Youheng Qu for his help of writing the article.
References
- Bohr, N. The quantum postulate and the recent development of atomic theory, Nature, 1928, 121, 580-590.
- Heisenberg, W. Physics and Philosophy, Penguin Books, London, 2000, 10.
- Heisenberg,W. Physics and Philosophy, Penguin Books, London, 2000, Introduction.
- Heisenberg, W. Über quantentheoretische Umdeutung kinematischer und mechanischer Beziehungen, Z. Phys., 1925, 33, 879-893. [CrossRef]
- Heisenberg, W. Physics and Philosophy, Penguin Books, London, 2000, 82.
- Li, J.H. An analysis of the probability density distribution in hydrogen atom. College Physics, 23, 13-17(2004).
- Feynman, R.P. Leighton, R.B. Sands, M. The Feynman Lectures on Physics, The New Millenium Edition: Vol.3 Quantum Mechanics, California Institute of Technology, New York, 2010, 19-12.
- Feynman, R.P. Leighton, R.B. Sands, M. The Feynman Lectures on Physics, The New Millenium Edition: Vol.3 Quantum Mechanics, California Institute of Technology, New York, 2010, 2-2.
- Feynman, R.P. Leighton, R.B. Sands, M. The Feynman Lectures on Physics, The New Millenium Edition: Vol.3 Quantum Mechanics, California Institute of Technology, New York, 2010, 1-7.
- Jönsson, C. Elektroneninterferenzen an mehreren künstlich hergestellten Feinspalten. Zeitschrift für Physik, 161, 454-474. [CrossRef]
- Heisenberg, W. Physics and Philosophy, Penguin Books, London, 2000, 18.
- Heisenberg, W. Physics and Philosophy, Penguin Books, London, 2000, 19.
- French, A.P., Kenndy, P.J., Niels Bohr – A Centenary Volume, 1985, Cambridge: Harvard U.P., 80-90.
- Heisenberg, W. Physics and Philosophy, Penguin Books, London, 2000, 86.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).