1. Introduction
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors have emerged as a cornerstone of treatment for hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer. By selectively targeting the cyclin D-CDK4/6-retinoblastoma protein pathway, these agents effectively halt cancer cell proliferation, enhancing the efficacy of endocrine therapy [
2,
3]. Three CDK4/6 inhibitors, palbociclib, ribociclib, and abemaciclib, have been approved for use in combination with endocrine therapy, and their inclusion in treatment regimens has significantly improved patient outcomes [
4,
5,
6].
Pivotal randomized clinical trials, including PALOMA-2, MONALEESA-2, and MONARCH 2, have demonstrated the superiority of CDK4/6 inhibitors combined with endocrine therapy over endocrine therapy alone in terms of progression-free survival in both the first-line and subsequent treatment settings [
7,
8]. In 2022 and 2023, median OS data from these trials were published showing a difference between CDK4/6 inhibitors. Palbociclib had a median survival of approximately 54 months and no statistically significant OS difference to endocrine therapy alone. In contrast, both ribociclib and abemaciclib demonstrated a statistically significant median survival greater than 60 months. While these trials have established the clinical benefit of CDK4/6 inhibitors, direct comparisons between these agents are lacking, and the generalizability of the trial results to real-world clinical practice remains uncertain.
In this study, we aimed to assess and compare the real-world PFS and OS outcomes associated with the use of palbociclib and ribociclib in combination with endocrine therapy in patients with HR+, HER2- advanced breast cancer at our cancer center. Our objective was to expand upon the knowledge gained from the pivotal clinical trials and enhance the understanding of the relative effectiveness of these CDK4/6 inhibitors in real-world settings.
2.. Materials and Methods
2.1. Study Design
A retrospective chart review was conducted for patients ≥18 years old with advanced metastatic HR+/HER2- breast cancer at a single academic center diagnosed between January 1, 2015 and December 1, 2022 and treated with first-line palliative AI and CDK4/6 inhibitors. Relevant demographic and clinical variables were extracted and median OS and PFS for each CDK4/6 inhibitor was estimated.
2.2. Statistical Analysis
Survival outcomes, including progression-free survival and overall survival, were analyzed using the Kaplan-Meier method to estimate survival functions. The log-rank test was used to compare survival distributions between treatment groups. Median survival times were reported where at least 50% of patients had experienced the event of interest; otherwise, survival was described as "not reached."
To adjust for potential confounding factors, multivariable Cox proportional hazards regression models were constructed for both PFS and OS. The models included treatment group, age, PR status, and menopausal status as covariates. Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported to quantify the association between variables and survival outcomes.
All statistical analyses were performed using RStudio (version 2023.12.0+369). The Kaplan-Meier analyses and log-rank tests were conducted using the survival and survminer packages. The Cox proportional hazards models were constructed using the coxph function in the survival package, and survival plots were generated using ggsurvplot to display Kaplan-Meier curves.
2.3. Ethics Approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the institutional ethics review board. As this was a retrospective study, informed consent was waived.
3. Results
Of 80 patient charts reviewed, 75 were included in the analysis. Five patients were excluded: one was prescribed abemaciclib, and four did not meet eligibility criteria due to insufficient survival to initiate treatment or a diagnosis of triple-negative breast cancer. Among the included patients, 98.7% (n = 74) were female, with a median age of 63.9 years. The majority were postmenopausal (n = 58, 77.3%), while 14.7% (n = 11) were premenopausal, and 6.7% (n = 5) had unknown menopausal status. Of the cohort, 52.0% (n = 39) had de novo stage IV disease, 72.0% (n = 54) were progesterone receptor-positive, and 65.3% (n = 49) were HER2-negative, with a third (33.3%, n = 25) classified as HER2-low. Nearly half (42.7%, n = 32) had received adjuvant or neoadjuvant chemotherapy. The most prescribed aromatase inhibitor was letrozole (n = 64, 85.3%), followed by anastrozole (n = 9, 12.0%) and exemestane (n = 2, 2.7%).
Ribociclib was prescribed to 19 patients (25.3%), while 56 patients (74.7%) received palbociclib. The ribociclib group was significantly younger (mean age 57.6 vs. 67.5 years, p= 0.013) and more likely to be premenopausal (42.1% vs. 5.4%, p < 0.001) compared with the palbociclib group. Baseline characteristics are summarized in
Table 1.
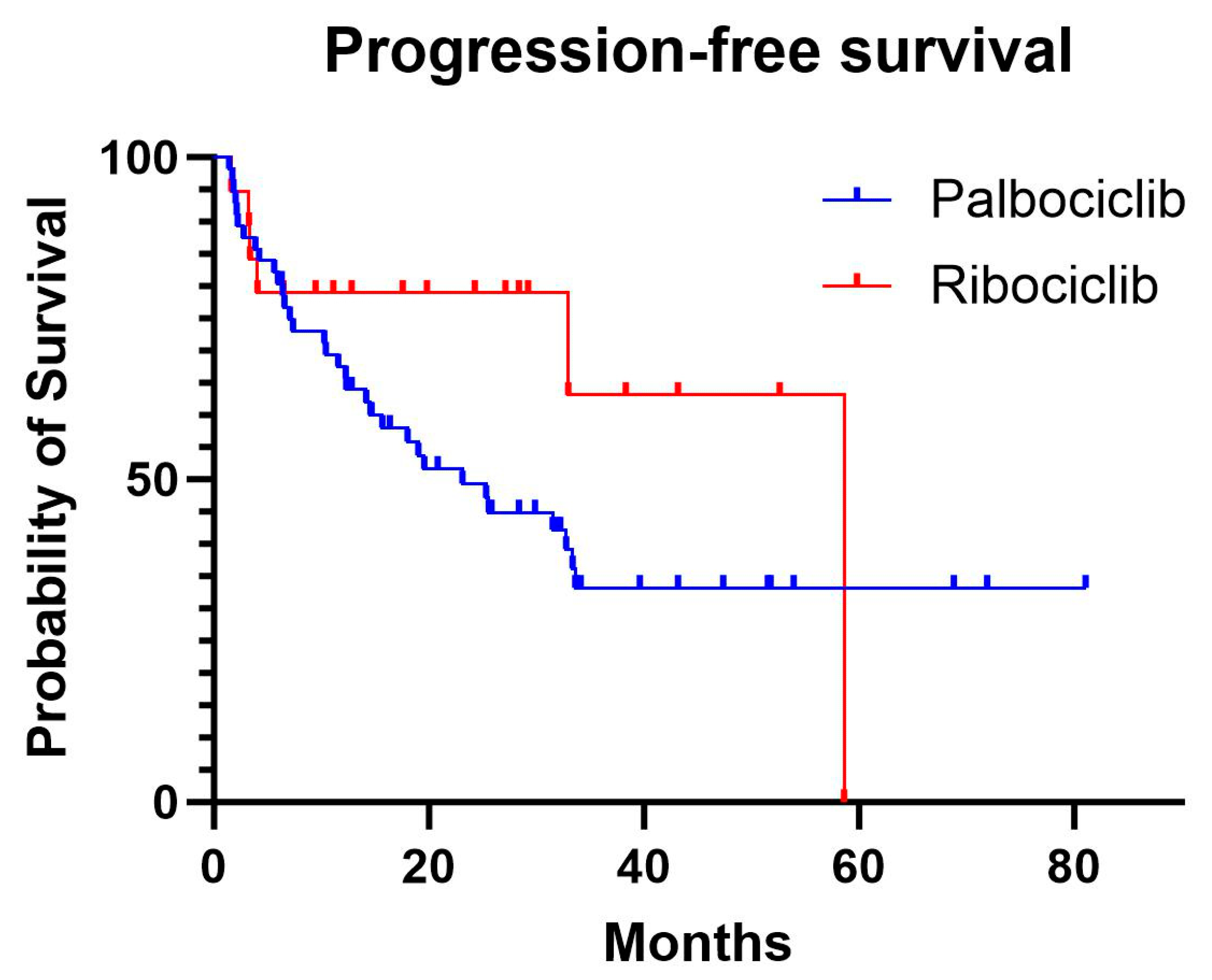
The median progression-free survival was 20.3 months in the palbociclib group, while the median was not reached in the ribociclib group, as fewer than 50% of patients experienced progression. In the Cox proportional hazards model, adjusting for age and menopausal status, there was no significant difference in PFS between ribociclib and palbociclib (HR, 0.92; 95% confidence interval [CI], 0.35–2.37; p=0.86). Increasing age showed a non-significant trend towards higher progression risk (HR, 1.03; 95% CI, 0.99–1.07; p=0.096), while premenopausal status had the opposite effect on progression (HR, 0.09; 95% CI, 0.01–1.01; p=0.051).
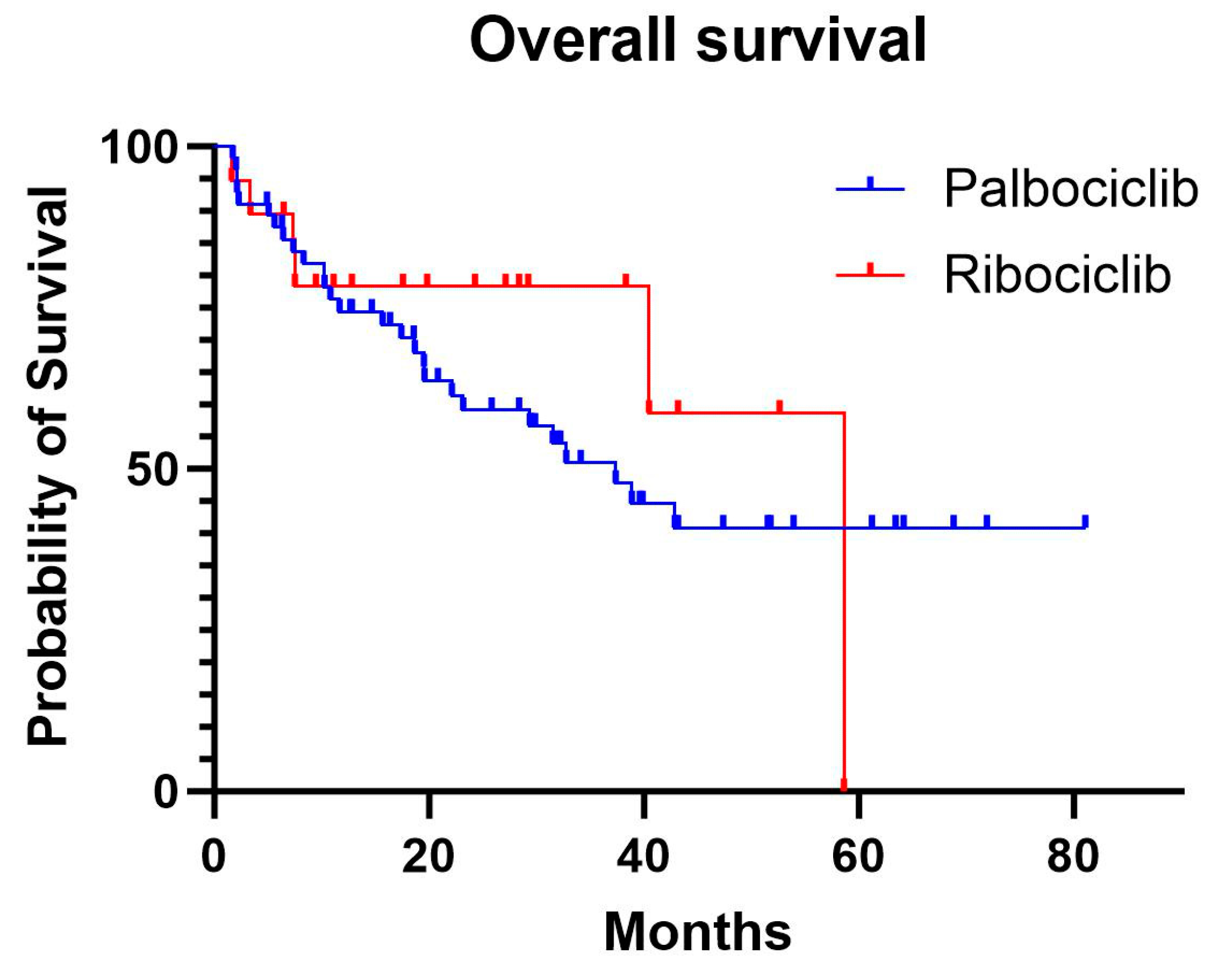
The median overall survival was 37.2 months in the palbociclib group, while the median was not reached in the ribociclib group due to insufficient events. In the adjusted Cox model, there was no significant difference in OS between the treatments (HR, 0.95; 95% CI, 0.32–2.80; p= 0.92). Age was significantly associated with worse OS (HR, 1.05; 95% CI, 1.01–1.09; p=0.02). Building on the observed trends in outcomes with ribociclib and palbociclib, further analysis explored the potential impact of progesterone receptor (PR) status on survival. Among all patients, PR-positive status was associated with a significantly reduced risk of progression compared with PR-negative patients (HR, 0.52; 95% CI, 0.28–0.99; p=0.045). For OS, PR-positive status showed a trend toward reduced mortality risk compared with PR-negative patients (HR, 0.61; 95% CI, 0.30–1.24), though this did not reach statistical significance (p=0.173).
Among the palbociclib group, 4 (7.14%) patients discontinued the drug due to adverse events. The reasons for discontinuation were: neutropenia (2 [3.57%]), liver toxicity (1 [1.79%]), and renal toxicity (1 [1.79%]. Among the ribociclib group, 2 (10.53%) patients discontinued the agent due to unknown adverse side effects (
Table 2).
4. Discussion
The introduction of CDK4/6 inhibitors has transformed the treatment paradigm for advanced hormone receptor-positive, HER2-negative breast cancer, establishing them as a cornerstone of first-line therapy. Despite robust evidence from pivotal trials demonstrating their efficacy, direct head-to-head comparisons between these agents in real-world settings remain limited. As a result, the choice of a specific CDK4/6 inhibitor in clinical practice often hinges on considerations such as toxicity profiles, patient comorbidities, and individual tolerability.
In our study, the real-world median progression-free survival for palbociclib was 20.3 months (95% CI: 14.8–46), closely mirroring the 24.8 months (95% CI: 22.1–NR) reported in the PALOMA-2 trial. However, the real-world median overall survival for palbociclib was 37.2 months (95% CI: 20.3–NR), which is notably shorter than the 53.9 months (95% CI: 49.8–60.8) observed in the trial's final analysis [
2,
13]. This discrepancy highlights the challenges of translating trial results into real-world settings, where patients often present with more complex baseline characteristics, a broader spectrum of comorbidities, and less stringent follow-up protocols compared to trial participants.
For ribociclib, the real-world median PFS and OS were not reached due to insufficient events. This reflects the smaller sample size of ribociclib-treated patients in our cohort, a consequence of its more recent introduction compared to palbociclib [
14]. Palbociclib’s earlier market approval allowed for greater adoption and a more mature dataset, resulting in a higher number of events for analysis. However, recent trends indicate increasing utilization of ribociclib, suggesting that future studies will provide a more balanced dataset for comparison.
Although statistical significance was not achieved for differences in PFS and OS between the two agents, ribociclib exhibited a non-significant trend toward improved outcomes. This aligns with data from the MONALEESA-2 trial, where ribociclib demonstrated a median OS of 63.9 months (95% CI: 52.4–71.0), outperforming the 53.9 months (95% CI: 49.8–60.8) observed for palbociclib in the PALOMA-2 trial [
16]. While these findings suggest a possible advantage for ribociclib, cross-trial comparisons must be interpreted cautiously due to differences in trial designs, populations, and endpoints.
In our study, there were demographic differences between the two treatment groups. Ribociclib-treated patients were significantly younger (mean age 57.6 vs. 67.5 years; p=0.013) and more likely to be premenopausal (42.1% vs. 5.4%; p<0.001) compared with palbociclib-treated patients. Younger age and premenopausal status are associated with better resilience and improved survival outcomes in breast cancer [17,18]. This age disparity reflects clinical practice at our center, where medical oncologists tended to prescribe palbociclib for older patients, prioritizing tolerability over perceived efficacy.
The rates of treatment discontinuation due to adverse events in our cohort were consistent with those reported in the PALOMA-2 and MONALEESA-2 trials. For palbociclib, the real-world discontinuation rate was 7.14%, closely aligning with the 9.7% observed in PALOMA-2 [
2]. The primary reason for discontinuation was neutropenia, mirroring the trial findings. For ribociclib, the real-world discontinuation rate was 10.53%, slightly higher than the 7.5% reported in MONALEESA-2 [
3]. While this suggests comparable tolerability profiles between the two agents, the limited number of ribociclib-treated patients in our cohort precludes definitive conclusions.
This study highlights the need for further real-world research to better delineate the relative effectiveness of ribociclib and palbociclib. Larger, multicenter datasets with balanced cohorts and longer follow-up are essential to validate these findings and address the limitations of smaller sample sizes and demographic heterogeneity.
Figure 1.
Progression-free survival curves for palbociclib and ribociclib.
Figure 1.
Progression-free survival curves for palbociclib and ribociclib.
Figure 2.
Overall survival curves for palbociclib and ribociclib .
Figure 2.
Overall survival curves for palbociclib and ribociclib .
Conflicts of Interest/Funding Information
A.N: none directly related to this work; unrelated: honoraria or advisory board or consulting fees from Pfizer, Novartis, AstraZeneca, and Merck. Other authors report no conflict of interest.
References
- Patel, R. , & Nasser, A. Real-world experience with CDK4/6 inhibitors in the first-line palliative setting for HR+/HER2- Advanced Breast Cancer. Journal of Clinical Oncology 2024, 42. [Google Scholar] [CrossRef]
- Finn RS, Martin, M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016, 375, 1925–1936. [CrossRef] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016, 375, 1738–1748. [CrossRef] [PubMed]
- Goetz MP, Toi, M, Campone M, et al. MONARCH 3: Abemaciclib as Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017, 35, 3638–3646. [CrossRef] [PubMed]
- Im S-A, Lu Y-S, Bardia A, et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med. 2019, 381, 307–316. [CrossRef] [PubMed]
- Sledge GW Jr, Toi, M, Neven, P., et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [CrossRef] [PubMed]
- Turner NC, Slamon DJ, Ro, J, et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2018, 379, 1926–1936. [CrossRef] [PubMed]
- O'Leary B, Hrebien S, Morden JP, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018, 9, 896. [CrossRef] [PubMed]
- Howie LJ, Scherle PA, Horne EA, et al. Comparison of progression-free survival in clinical trials of CDK4/6 inhibitors in combination with endocrine therapy. Ann Oncol. 2018, 29 (Suppl. 8), viii719. [CrossRef]
- Spring LM, Wander SA, Zangardi M, et al. CDK 4/6 Inhibitors in Breast Cancer: Current Controversies and Future Directions. Curr Oncol Rep. 2019, 21, 25. [CrossRef] [PubMed]
- Cristofanilli M, Turner NC, Bondarenko, I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [CrossRef]
- Stephen, R.D.; Johnston, Enhancing Endocrine Therapy for Hormone Receptor–Positive Advanced Breast Cancer: Cotargeting Signaling Pathways. JNCI: Journal of the National Cancer Institute, 2015; 107, djv212. [CrossRef]
- Slamon, D.J. , Diéras, V., Rugo, H.S., Harbeck, N., Im, S.-A., Gelmon, K.A., Lipatov, O.N., Walshe, J.M., Martin, M., Chavez-MacGregor, M., Bananis, E., Gauthier, E., Lu, D.R., Kim, S., & Finn, R.S. Overall survival with palbociclib plus letrozole in Advanced breast cancer. Journal of Clinical Oncology 2024, 42, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research. (n.d.). Palbociclib (Ibrance capsules), U.S. Food and Drug Administration; https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance-capsules.
- Desnoyers, A. , Nadler, M.B., Kumar, V., Saleh, R., & Amir, E. Comparison of treatment-related adverse events of different cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis. Cancer Treatment Reviews 2020, 90, 102086. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N. , Stemmer, S.M., Burris, H.A., Yap, Y.-S., Sonke, G.S., Hart, L., Campone, M., Petrakova, K., Winer, E.P., Janni, W., Conte, P., Cameron, D.A., André, F., Arteaga, C.L., Zarate, J.P., Chakravartty, A., Taran, T., Le Gac, F., Serra, P., & O’Shaughnessy, J. Overall survival with ribociclib plus letrozole in Advanced breast cancer. New England Journal of Medicine 2022, 386, 942–950. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Patient characteristics and aromatase inhibitor use.
Table 1.
Patient characteristics and aromatase inhibitor use.
| |
Palbociclib (N=56) |
Ribociclib (N=19) |
| Female |
55 (98.21%) |
19 (100%) |
| Median age (IQR) |
66.69 (59.78–75.18) |
52.14 (49.12–67.02) |
| Postmenopausal |
50 (89.29%) |
8 (42.11%) |
| Premenopausal |
3 (5.36%) |
8 (42.11%) |
| De novo presentation |
31 (55.36%) |
11 (57.89%) |
| Grade I |
11 (19.64%) |
5 (26.32%) |
| Grade II |
28 (50.00%) |
7 (36.84%) |
| Grade III |
12 (21.43%) |
6 (31.58%) |
| Positive PR status |
38 (67.86%) |
16 (84.21%) |
| Negative PR status |
17 (30.36%) |
3 (15.79%) |
| HER2 negative (IHC 0) |
36 (64.29%) |
13 (68.42%) |
| HER2 low (IHC 1+/2+, negative FISH) |
19 (33.93%) |
6 (31.58%) |
| Received chemotherapy in past |
24 (42.86%) |
8 (42.11%) |
| Letrozole |
49 (87.50%) |
15 (78.90%) |
| Anastrozole |
5 (8.90%) |
4 (21.1%) |
| Exemestane |
2 (3.60%) |
0 (0.00%) |
Table 2.
Rates of discontinuation for palbociclib and ribociclib due to adverse side effects.
Table 2.
Rates of discontinuation for palbociclib and ribociclib due to adverse side effects.
| |
Palbociclib (N=56) |
Ribociclib (N=19) |
| Overall |
4 (7.14%) |
2 (10.53%) |
| Neutropenia |
2 (3.57%) |
0 |
| Hepatic toxicity |
1 (1.79%) |
0 |
| Renal toxicity |
1 (1.79%) |
0 |
| Unknown side effects |
0 |
2 (10.53%) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).