Submitted:
23 November 2024
Posted:
26 November 2024
You are already at the latest version
Abstract
Vanilla planifolia Jacks. ex Andrews pods can lose up to 50% of their vanillin during curing. One explanation for this is the transformation of vanillin due to enzyme action, such as peroxidases, which generate the formation of dimers like divanillin. Therefore, in this work, a simultaneous high-performance liquid chromatography method (HPLC-DAD) was developed and validated for the separation and quantification of the main compounds present in Vanilla planifolia Jacks. ex Andrews and divanillin. The separation of 9 compounds of interest was achieved within 15 minutes using a Zorbax Eclipse XDB-C18 column (250 mm x 4.6 mm i.d., 5 μm particle size). The variables optimized included the mobile phase (water as solvent A, methanol as solvent B, and acidified water, 10–2 M H3PO4, as solvent C), the separation gradient, and the column tempera-ture (40-60 °C). The maximum divanillin content was 0.02 g/100 g d.w. in a sample from Papantla de Olarte. Chromatographic performance evaluation revealed excellent resolution, retention factor, and selectivity. The method was successfully validated in terms of limits of detection and quantification, linearity, and precision, as well as its application to cured pods, with evidence of divanillin presence in all analyzed sample.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Preparation of Standard Solutions
2.3. Raw Material
2.4. Sample Preparation
2.5. Instrument and Development of Chromatographic Conditions
2.6. Validation of the Chromatographic Method
2.6.1. Linearity
2.6.2. Detection and Quantification Limits
2.6.3. Precision
2.6.4. Robustness
3. Results and Discussion
3.1. Development of the HPLC Method
3.1.1. Optimization of the Separation Gradient
3.1.2. Study of the Effect of Column Temperature
3.1.3. Study of the Effect of Flow Rate
3.2. Characteristics of the Developed Method
3.2.1. Linearity
3.2.2. Detection and Quantification Limits
3.2.3. Precision
3.2.4. Robustness
3.3. Application to Real Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odoux, E. Changes in Vanillin and Glucovanillin Concentrations during the Various Stages of the Process Traditionally Used for Curing Vanilla fragrans Beans in Reunion. Fruits 2000, 55, 119–125. [Google Scholar] [CrossRef]
- Dunphy, P.; Bala, K. Vanilla Curing. Perfum. flavorist 2009, 34, 34–40. [Google Scholar]
- Pérez Silva, A.; Gunata, Z.; Lepoutre, J.P.; Odoux, E. New Insight on the Genesis and Fate of Odor-Active Compounds in Vanilla Beans (Vanilla Planifolia G. Jackson ) during Traditional Curing. Food Res. Int. 2011, 44, 2930–2937. [Google Scholar] [CrossRef]
- Januszewska, R.; Giret, E.; Clement, F.; Van Leuven, I.; Goncalves, C.; Vladislavleva, E.; Pradal, P.; Nåbo, R.; Landuyt, A.; D’Heer, G.; et al. Impact of Vanilla Origins on Sensory Characteristics of Chocolate. Food Res. Int. 2020, 137, 109313. [Google Scholar] [CrossRef]
- Singletary, K.W. Vanilla: Potential Health Benefits. Nutr. Today 2020, 55, 186–196. [Google Scholar] [CrossRef]
- Odoux, E.; Escoute, J.; Verdeil, J.L. The Relation between Glucovanillin, β-D-Glucosidase Activity and Cellular Compartmentation during the Senescence, Freezing and Traditional Curing of Vanilla Beans. Ann. Appl. Biol. 2006, 149, 43–52. [Google Scholar] [CrossRef]
- Brillouet, J.M.; Odoux, E.; Conejero, G. A Set of Data on Green, Ripening and Senescent Vanilla Pod (Vanilla Planifolia ; Orchidaceae): Anatomy, Enzymes, Phenolics and Lipids. Fruits 2010, 65, 221–235. [Google Scholar] [CrossRef]
- Khoyratty, S.; Kodja, H.; Verpoorte, R. Vanilla Flavor Production Methods: A Review. Ind. Crops Prod. 2018, 125, 433–442. [Google Scholar] [CrossRef]
- Odoux, E. Glucosylated Aroma Precursors and Glucosidase(s) in Vanilla Bean (Vanilla Planifolia G. Jackson). Fruits 2006, 60, 171–183. [Google Scholar] [CrossRef]
- Dunphy, P.; Bala, K. Review: A Flavor of Vanilla. Perfum. flavorist 2010, 35, 42–42. [Google Scholar]
- Buitimea-Cantúa, G. V.; Chávez-Leal, V.; Soto-Caballero, M.C.; Tellez-Medina, D.I.; Welti-Chanes, J.; Escobedo-Avellaneda, Z. Enzymatic Activity and Its Relationships with the Total Phenolic Content and Color Change in the High Hydrostatic Pressure-Assisted Curing of Vanilla Bean (Vanilla Planifolia). Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Ranadive, A. Vanilla- inside Look: Chemistry and Biochemistry of Vanilla Flavor. Perfum. Flavourist 2006, 31, 38–44. [Google Scholar]
- Pardío, V.T.; Flores, A.; López, K.M.; Martínez, D.I.; Márquez, O.; Waliszewski, K.N. Effect of Endogenous and Exogenous Enzymatic Treatment of Green Vanilla Beans on Extraction of Vanillin and Main Aromatic Compounds. J. Food Sci. Technol. 2018, 55, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Gatfield, I.; Rei, I.; Krammer, G.; Schmidt, C.O.; Kindel, G.; Bertram, H. Divainillin Novel Taste-Active Component during the Traditional Curing Process. Perfum. flavorist 2006, 31, 18–20. [Google Scholar]
- Hernández-Ramos, D. Estudio de La Degradación Química de La Vainillina. Tesis de Maestría en Ciencias en Alimentos. Instituto Tecnológico de Tuxtepec. Oaxaca, México. 2009.
- Peña-Mojica, E. Estudio Del Efecto Enzimático, Térmico y Fotoquímico En La Transformación de La Vainilla, Tesis de Maestría en Ciencias en Alimentos. Instituto Tecnológico Tuxtepec. Oaxaca, México., 2019.
- Frenkel, C.; Havkin-Frenkel, D. Material Properties: The Physics and Chemistry of Vanillin. Perfum. Flavourist 2006, 31, 28–36. [Google Scholar]
- Li, Z.; Jiang, Y.; Wust, K.; Callari, M.; Stenzel, M.H. Crosslinking of Self-Assembled Protein-Polymer Conjugates with Divanillin. Aust. J. Chem. 2020, 73, 1034–1041. [Google Scholar] [CrossRef]
- Anklam, E.; Gaglione, S.; Müller, A. Oxidation Behaviour of Vanillin in Dairy Products. Food Chem. 1997, 60, 43–51. [Google Scholar] [CrossRef]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Adams, T.B. GRAS Flavoring Substances 22. Food Technol. 2005, 59, 24–62. [Google Scholar]
- Reiss, I.; Gatfield, I.-L.; Krammer, G.; Clerc, A.; Kindel, G. Use of Divanillin as a Flavouring Agent (U.S. Patent Application No. 10/547,981.) 2006.
- Krings, U.; Esparan, V.; Berger, R.G. The Taste Enhancer Divanillin: A Review on Sources and Enzymatic Generation. Flavour Fragr. J. 2015, 30, 362–365. [Google Scholar] [CrossRef]
- Schwarz, B.; Hofmann, T. Identification of Novel Orosensory Active Molecules in Cured Vanilla Beans (Vanilla Planifolia ). J. Agric. Food Chem. 2009, 57, 3729–3737. [Google Scholar] [CrossRef]
- Oliveira, G.S.N. de; Furlaneto, C.G.; Tokuhara, C.K.; Ventura, T.M.O.; Pessôa, A. de S.; Fakhoury, V.S.; Pagnan, A.L.; Inacio, K.K.; Rovis Sanches, M.L.; Buzalaf, M.; et al. Antitumor Effect of Vanillin and Divanillin on Murine Osteosarcoma Cells. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Jantaree, P.; Lirdprapamongkol, K.; Kaewsri, W.; Thongsornkleeb, C.; Choowongkomon, K.; Atjanasuppat, K.; Ruchirawat, S.; Svasti, J. Homodimers of Vanillin and Apocynin Decrease the Metastatic Potential of Human Cancer Cells by Inhibiting the FAK/PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2017, 65, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Silva, A.; Odoux, E.; Brat, P.; Ribeyre, F.; Rodriguez-Jimenes, G.; Robles-Olvera, V.; García-Alvarado, M.A.; Günata, Z. GC-MS and GC-Olfactometry Analysis of Aroma Compounds in a Representative Organic Aroma Extract from Cured Vanilla (Vanilla Planifolia G. Jackson) Beans. Food Chem. 2006, 99, 728–735. [Google Scholar] [CrossRef]
- Brunschwig, C.; Collard, F.X.; Bianchini, J. P. Raharivelomanana, P. Evaluation of Chemical Variability of Cured Vanilla Beans (Vanilla Tahitensis and Vanilla Planifolia). Nat. Prod. Commun. 2009, 4, 1393–1400. [Google Scholar] [CrossRef]
- Peña-Barrientos, A.; Perea-Flores, M. de J.; Martínez-Gutiérrez, H.; Patrón-Soberano, O.A.; González-Jiménez, F.E.; Vega-Cuellar, M.; Dávila-Ortiz, G. Physicochemical, Microbiological, and Structural Relationship of Vanilla Beans (Vanilla Planifolia Andrews) during Traditional Curing Process and Use of Its Waste. J. Appl. Res. Med. Aromat. Plants 2023, 32. [Google Scholar] [CrossRef]
- Gassenmeier, K.; Riesen, B.; Magyar, B.; Barzalona, M.; Casanova, J. Commercial Quality and Analytical Parameters of Cured Vanilla Beans (Vanilla Planifolia) from Different Origins from the 2006--2007 Crop. Flavour Fragr. J. 2008, 23, 194–201. [Google Scholar] [CrossRef]
- Zhang, S.; Mueller, C. Comparative Analysis of Volatiles in Traditionally Cured Bourbon and Ugandan Vanilla Bean (Vanilla Planifolia) Extracts. J. Agric. Food Chem. 2012, 60, 10433–10444. [Google Scholar] [CrossRef]
- Pérez-Silva, A.; Nicolás-García, M.; Petit, T.; Dijoux, J.B.; Vivar-Vera, M. de los Á.; Besse, P.; Grisoni, M.; de los Ángeles Vivar-Vera, M.; Besse, P.; Grisoni, M. Quantification of the Aromatic Potential of Ripe Fruit of Vanilla Planifolia (Orchidaceae) and Several of Its Closely and Distantly Related Species and Hybrids. Eur. Food Res. Technol. 2021, 247, 1489–1499. [Google Scholar] [CrossRef]
- Da Silva Oliveira, J.P.; Garrett, R.; Bello Koblitz, M.G.; Furtado Macedo, A. Vanilla Flavor: Species from the Atlantic Forest as Natural Alternatives. Food Chem. 2021, 375, 131891. [Google Scholar] [CrossRef]
- NF-ISO-5565-2. Vanille[Vanilla Fragans (Salisbury) Ames]— Partie 2:Méthodes d’essai. 1999.
- NMX-FF-074-SCFI-2009. Norma Mexicana “Productos No Industrializados Para Uso Humano-Vainilla (Vanilla fragrans (Salisbury) Ames) Especificaciones y Métodos de Prueba” 2009.
- NOM-182-SCFI-2011. Norma Oficial Mexicana, Vainilla de Papantla, Extractos y Derivados. Especificaciones, Información Comercial y Métodos de Ensayo (Prueba). 2011.
- De Jager, L.S.; Perfetti, G.A.; Diachenko, G.W. Comparison of Headspace-SPME-GC-MS and LC-MS for the Detection and Quantification of Coumarin, Vanillin, and Ethyl Vanillin in Vanilla Extract Products. Food Chem. 2008, 107, 1701–1709. [Google Scholar] [CrossRef]
- Sabik, H.; Pérez-Silva, A.; Bélanger, D.; Vivar-Vera, M.D.L.Á.; Nicolás-García, M.; Reyes López, D. Identification of Volatile Compounds in Cured Mexican Vanilla (Vanilla Planifolia G. Jackson) Beans Using Headspace Solid-Phase Microextraction with Gas Chromatography-Mass Spectrometry. Fruits 2016, 71, 407–418. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Konteles, S.; Kalogeropoulos, N.; Karathanos, V.T. Thermal Oxidation of Vanillin Affects Its Antioxidant and Antimicrobial Properties. Food Chem. 2009, 114, 791–797. [Google Scholar] [CrossRef]
- Zheng, J.; Polyakova, Y.; Row, K.H. Retention Factors and Resolutions of Amino Benzoic Acid Isomers with Some Lonic Liquids. Biotechnol. Bioprocess Eng. 2006, 11, 477–483. [Google Scholar] [CrossRef]
- Snyder, L.R.; Dolan, J.W. High-Performance Gradient Elution the Practical Application of the Linear-Solvent-Strength Model.; 1th ed.; John Wiley & Sons. New Jersey, USA, 2006. [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A. V.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Simultaneous Determination by UHPLC-PDA of Major Capsaicinoids and Capsinoids Contents in Peppers. Food Chem. 2021, 356, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ich ICH Topic Q2 (R1) Validation of Analytical Procedures : Text and Methodology (Vol. 1994). Geneva. 2005.
- Sharma, A.; Verma, S.C.; Saxena, N.; Chadda, N.; Singh, N.P.; Sinha, A.K. Microwave- and Ultrasound-Assisted Extraction of Vanillin and Its Quantification by High-Performance Liquid Chromatography in Vanilla Planifolia. J. Sep. Sci. 2006, 29, 613–619. [Google Scholar] [CrossRef]
- Ferreira, M.; Schwanz, M.; Cosenza, G.; Henriques, A.; Soares, L.A.L. Analysis of Vanillin by TLC and HPLC-PDA in Herbal Material and Tincture from Vanilla Planifolia Jacks Ex. Andrews. Drug Anal. Res. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Sinha, A.K.; Verma, S.C.; Sharma, U.K. Development and Validationof an RP-HPLC Method for Quantitative Determination of Vanillin and Related Phenolic Compunds in Vanilla Planifolia. J. Sep. Sci. 2007, 30, 15–20. [Google Scholar] [CrossRef]
- Cicchetti, E.; Chaintreau, A. Quantitation of the Main Constituents of Vanilla by Reverse Phase HPLC and Ultra-High-Pressure-Liquid-Chromatography with UV Detection: Method Validation and Performance Comparison. J. Sep. Sci. 2009, 32, 3043–3052. [Google Scholar] [CrossRef]
- Takahashi, M.; Sakamaki, S.; Fujita, A. Simultaneous Analysis of Guaiacol and Vanillin in a Vanilla Extract by Using High-Performance Liquid Chromatography with Electrochemical Detection. Biosci. Biotechnol. Biochem. 2013, 77, 595–600. [Google Scholar] [CrossRef]
- Maruenda, H.; Vico, M.D.L.; Householder, J.E.; Janovec, J.P.; Cañari, C.; Naka, A.; Gonzalez, A.E. Exploration of Vanilla Pompona from the Peruvian Amazon as a Potential Source of Vanilla Essence: Quantification of Phenolics by HPLC-DAD. Food Chem. 2013, 138, 161–167. [Google Scholar] [CrossRef]
- AOAC AOAC Peer Verified Methods Program, Manual on Policies and Procedures. (AOAC Inter). Maryland (USA). 2012.
- Shabir, G.A. Validation of High-Performance Liquid Chromatography Methods for Pharmaceutical Analysis. J. Chromatogr. A 2003, 987, 57–66. [Google Scholar] [CrossRef]
- Adedeji, J.; Hartman, T.G.; Ho, C.T. Flavor Characterization of Different Varieties of Vanilla Beans. Perfum. Flavorist 1993, 18, 25–33. [Google Scholar]
- Ehlers, D.; Pfister, M. Compounds of Vanillons (Vanilla Pompona Schiede). J. Essent. Oil Res. 1997, 9, 427–431. [Google Scholar] [CrossRef]
- Brunschwig, C.; Senger-Emonnot, P.; Aubanel, M.L.; Pierrat, A.; George, G.; Rochard, S.; Raharivelomanana, P. Odor-Active Compounds of Tahitian Vanilla Flavor. Food Res. Int. 2012, 46, 148–157. [Google Scholar] [CrossRef]
- Brunschwig, C.; Rochard, S.; Pierrat, A.; Rouger, A.; Senger-Emonnot, P.; George, G.; Raharivelomanana, P. Volatile Composition and Sensory Properties of Vanilla ×tahitensis Bring New Insights for Vanilla Quality Control. J. Sci. Food Agric. 2015, 96, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Tavares de Oliveira, R.; da Silva Oliveira, J.P.; Furtado Macedo, A. Vanilla beyond Vanilla Planifolia and Vanilla × tahitensis: Taxonomy and Historical Notes, Reproductive Biology, and Metabolites. Plants 2022, 11. [Google Scholar] [CrossRef]
- Ranadive, A.S. Vanilla-Cultivation, Curing, Chemistry, Technology and Commercial Products. In Spices, herbs and edible fungi; Charalambous, G., Ed.; Elsevier Science: Amsterdam Netherlands, 1994; pp. 517–576. ISBN 9780444817617. [Google Scholar]
- Saltron, F.; Langella, C.; Guerere, M. Évaluation de La Qualité de La Vanille Malgache. Ann. des Falsif. l’expertise Chim. Toxicol. 2002, 95, 79–105. [Google Scholar]
- Homayouni, A.; Javadi, M.; Ansari, F.; Pourjafar, H.; Jafarzadeh, M.; Barzegar, A. Advanced Methods in Ice Cream Analysis: A Review. Food Anal. Methods 2018, 11, 3224–3234. [Google Scholar] [CrossRef]
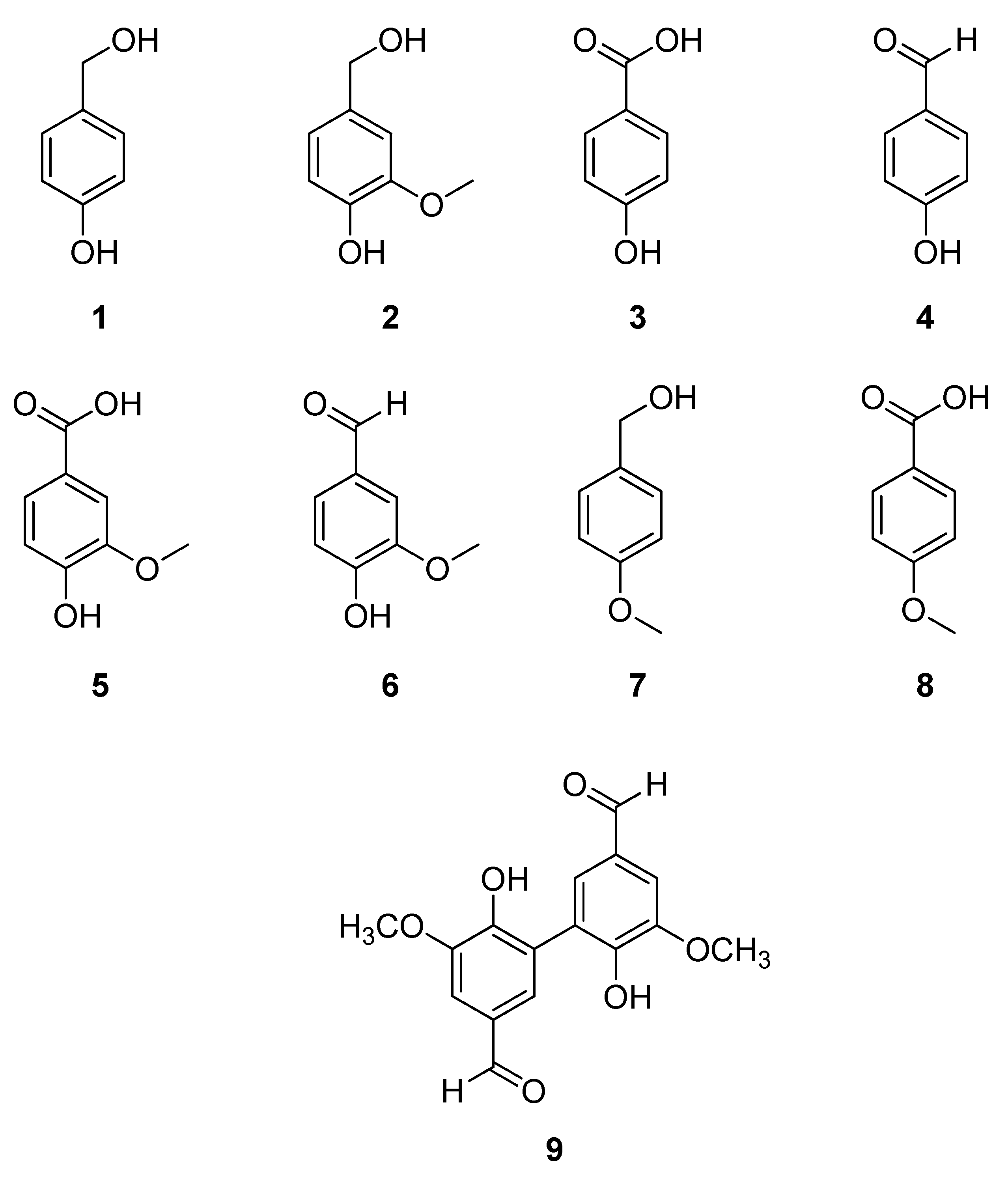
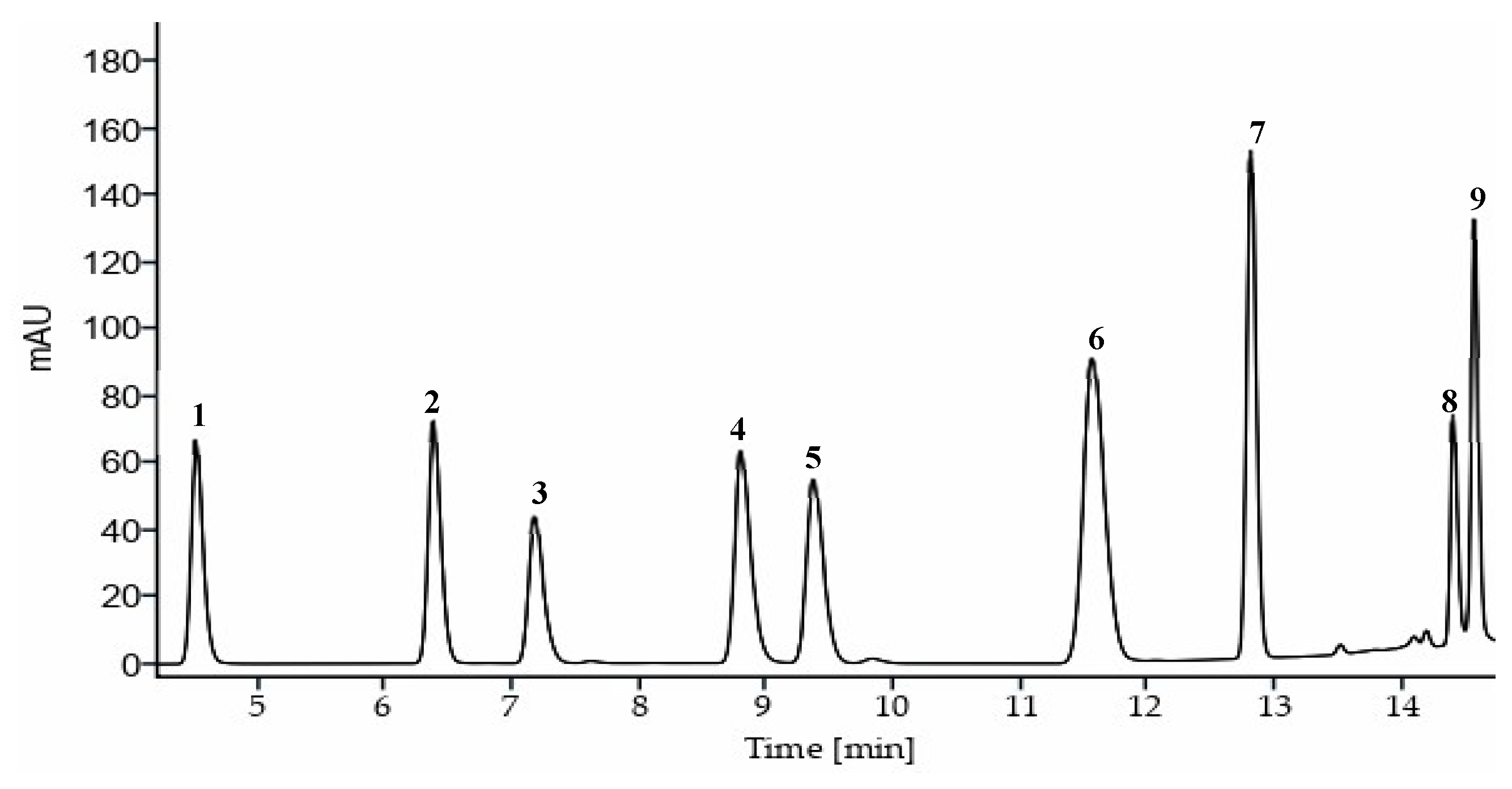
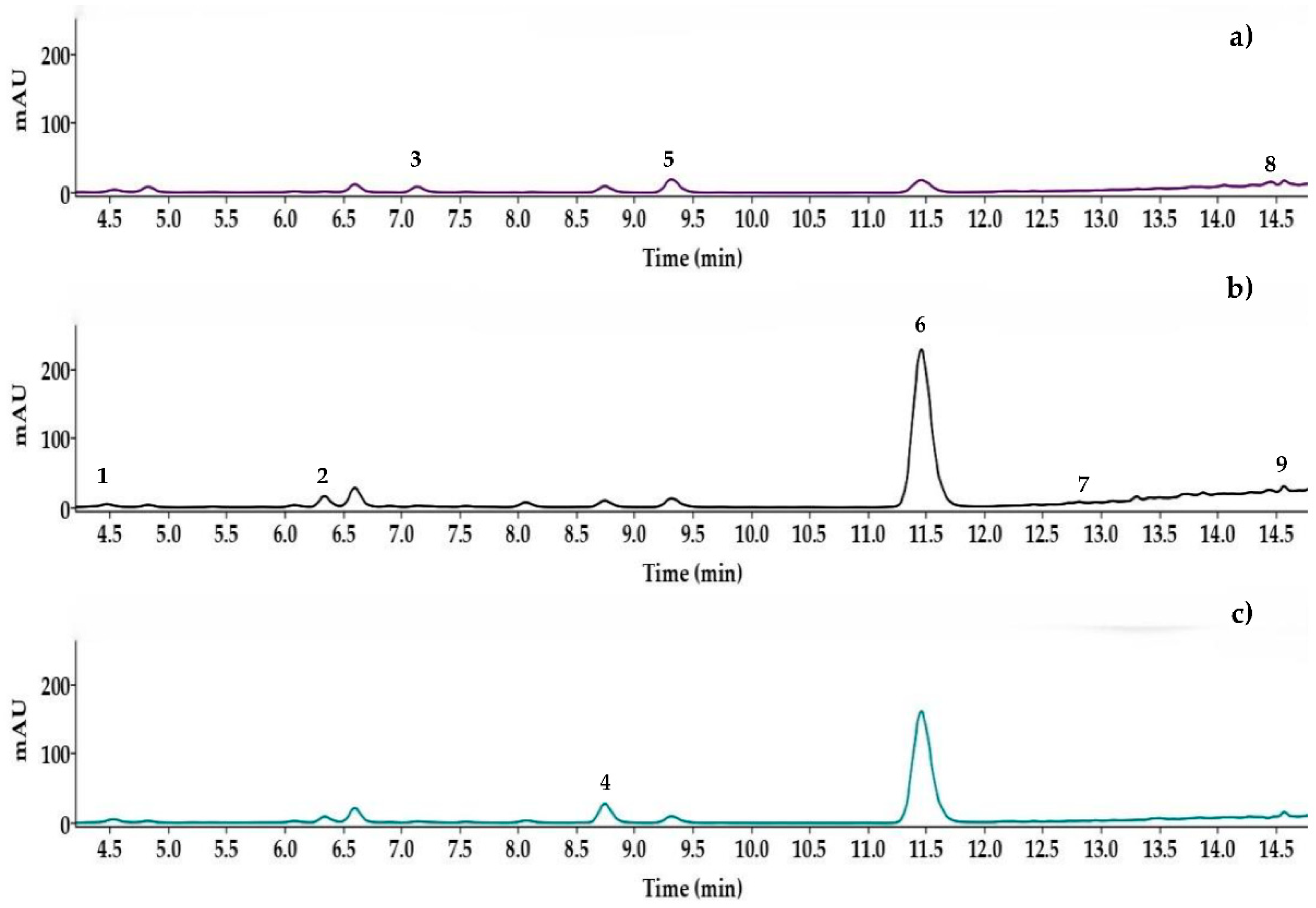
| # | Compound | Retention time (min) | Width (seg) | Retention factor (k) | Selectivity (α) | Resolution (Rs) |
|---|---|---|---|---|---|---|
| 1 | p-hydroxybencyl alcohol | 4.45 | 0.27 | 3.86 | 1.53 | 6.51 |
| 2 | Vanillyl alcohol | 6.31 | 0.30 | 5.89 | 1.14 | 2.50 |
| 3 | p-hydroxybenzoic acid | 7.09 | 0.34 | 6.74 | 1.26 | 4.54 |
| 4 | p-hydroxybenzaldehyde | 8.71 | 0.37 | 8.50 | 1.07 | 1.49 |
| 5 | Vanillinic acid | 9.27 | 0.38 | 9.11 | 1.26 | 4.72 |
| 6 | Vanillin | 11.41 | 0.53 | 11.45 | 1.13 | 3.58 |
| 7 | Anisyl alcohol | 12.75 | 0.22 | 12.91 | 1.14 | 8.83 |
| 8 | Anisic acid | 14.38 | 0.15 | 14.69 | 1.01 | 1.08 |
| 9 | Divanillin | 14.54 | 0.17 | 14.87 | - | - |
| # | Compound | Retention time (min) | ||||||
|---|---|---|---|---|---|---|---|---|
| This work | [43] | [45] | [46] | [47] | [48] | [44] | ||
| 1 | p-hydroxybencyl alcohol | 4.45 | - | 5.9 | - | - | 5.34 | - |
| 2 | Vanillyl alcohol | 6.31 | - | 8.05 | - | - | 7.41 | - |
| 3 | p-hydroxybenzoic acid | 7.09 | - | 10.27 | 7.4 | - | 8.17 | - |
| 4 | p-hydroxybenzaldehyde | 8.71 | - | 12.33 | 9.9 | - | 9.93 | - |
| 5 | Vanillinic acid | 9.27 | - | 11.33 | 9.1 | - | 11.43 | - |
| 6 | Vanillin | 11.41 | 9.90 | 13.9 | 11.8 | 12.5 | 14.29 | 13.0 |
| 7 | Anisyl alcohol | 12.75 | - | - | - | - | 15.10 | - |
| 8 | Anisic acid | 14.38 | - | - | - | - | - | - |
| 9 | Divanillin | 14.54 | - | - | - | - | - | - |
| # | Compound | Calibration curve | R2 | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|
| 1 | p-hydroxybencyl alcohol | y = 8.838x + 4.2881 | 0.9995 | 0.0387 | 0.1291 |
| 2 | Vanillyl alcohol | y = 9.6997x + 1.0834 | 0.9998 | 0.0371 | 0.1238 |
| 3 | p-hydroxybenzoic acid | y = 27.764x + 1.4178 | 1 | 0.0131 | 0.0436 |
| 4 | p-hydroxybenzaldehyde | y = 30.9288x - 1.4969 | 0.9999 | 0.0119 | 0.0397 |
| 5 | Vanillinic acid | y = 14.9978x + 0.3961 | 0.9999 | 0.0243 | 0.0810 |
| 6 | Vanillin | y = 24.3273x - 11.1028 | 0.9992 | 0.0161 | 0.0535 |
| 7 | Anisyl alcohol | y = 17.515x - 0.2164 | 1 | 0.0209 | 0.0698 |
| 8 | Anisic acid | y = 27.6438x + 2.1590 | 0.9997 | 0.0131 | 0.0436 |
| 9 | Divanillin | y = 8.6675x + 0.568 | 0.9999 | 0.0418 | 0.1395 |
| # | Compound | Retention time | Peak area | Peak resolution | |||
|---|---|---|---|---|---|---|---|
| Repe.a | IPb | Repe.a | IPb | Repe.a | IPb | ||
| 1 | p-hydroxybencyl alcohol | 0.04 | 0.47 | 3.83 | 4.16 | 4.09 | 4.56 |
| 2 | Vanillyl alcohol | 0.03 | 1.02 | 4.78 | 5.32 | 3.74 | 4.49 |
| 3 | p-hydroxybenzoic acid | 0.03 | 0.42 | 4.38 | 4.89 | 3.76 | 4.23 |
| 4 | p-hydroxybenzaldehyde | 0.02 | 0.32 | 5.13 | 5.52 | 3.00 | 4.59 |
| 5 | Vanillinic acid | 0.02 | 0.41 | 3.66 | 4.27 | 2.62 | 3.60 |
| 6 | Vanillin | 0.02 | 0.42 | 4.11 | 4.53 | 5.08 | 5.53 |
| 7 | Anisyl alcohol | 0.01 | 0.13 | 5.50 | 5.74 | 3.58 | 4.23 |
| 8 | Anisic acid | 0.01 | 0.01 | 3.20 | 5.12 | 1.95 | 5.98 |
| 9 | Divanillin | 0.01 | 0.03 | 3.68 | 4.37 | - | - |
| # Compound | Temperature (°C) | Flow rate (mL/min) | Injection volume (μL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 42 | 45 | 48 | 2.15 | 2.25 | 2.35 | 9.5 | 10 | 10.5 | |||
| Retention time (min) | 1 | p-hydroxybencyl alcohol | 4.65a | 4.51b | 4.38c | 4.71a | 4.53b | 4.35c | 4.54a | 4.50a | 4.54a |
| 2 | Vanillyl alcohol | 6.53a | 6.38b | 6.23c | 6.60a | 6.40b | 6.20c | 6.41a | 6.41a | 6.40a | |
| 3 | p-hydroxybenzoic acid | 7.44a | 7.21b | 6.98c | 7.48a | 7.23b | 7.01c | 7.25a | 7.26a | 7.25a | |
| 4 | p-hydroxybenzaldehyde | 9.05a | 8.81b | 8.59c | 9.11a | 8.83b | 8.58c | 8.85a | 8.86a | 8.85a | |
| 5 | Vanillinic acid | 9.68a | 9.40b | 9.15c | 9.71a | 9.43b | 9.17c | 9.45a | 9.46a | 9.45a | |
| 6 | Vanillin | 11.87a | 11.59b | 11.25c | 11.92a | 11.61b | 11.26c | 11.63a | 11.64a | 11.63a | |
| 7 | Anisyl alcohol | 12.91a | 12.81b | 12.69c | 13.01a | 12.81b | 12.63c | 12.82a | 12.83a | 12.84a | |
| 8 | Anisic acid | 14.16a | 14.40b | 14.33c | 14.53a | 14.40b | 14.28c | 14.41a | 14.41a | 14.41a | |
| 9 | Divanillin | 14.61a | 14.56b | 14.51c | 14.67a | 14.56b | 14.47c | 14.56a | 14.56a | 14.57a | |
| Peak area | 1 | p-hydroxybencyl alcohol | 868.32a | 866.74a | 866.98a | 908.24a | 869.09b | 831.72c | 825.80c | 870.78b | 912.76a |
| 2 | Vanillyl alcohol | 975.67a | 973.17b | 971.51b | 1019.89a | 973.46b | 933.88c | 925.89c | 975.69b | 1023.98a | |
| 3 | p-hydroxybenzoic acid | 2855.06a | 2850.69a | 2847.27a | 2986.38a | 2854.19b | 2735.78c | 2713.27c | 2860.93b | 3001.60a | |
| 4 | p-hydroxybenzaldehyde | 3314.75a | 3307.27a | 3306.63a | 3466.72a | 3312.86b | 3177.17c | 3148.89c | 3317.92b | 3481.35a | |
| 5 | Vanillinic acid | 1573.53a | 1573.36a | 1573.58a | 16447.76a | 1573.89b | 1510.19c | 1498.03c | 1578.22b | 1656.57a | |
| 6 | Vanillin | 2217.02a | 2211.84ab | 2207.45b | 2317.35a | 2214.87b | 2114.51c | 2104.80c | 2218.34b | 2327.29a | |
| 7 | Anisyl alcohol | 1687.10a | 1685.42a | 1683.46a | 1761.03a | 1685.47b | 1615.21c | 1603.78c | 1688.79b | 1771.34a | |
| 8 | Anisic acid | 2644.18a | 2636.90ab | 2631.36b | 2757.75a | 2638.10b | 2523.55c | 2507.05c | 2642.36b | 2771.19a | |
| 9 | Divanillin | 1056.07b | 1053.30c | 1089.0a | 1128.90a | 1053.28b | 1036.16c | 998.23c | 1052.72b | 1108.32a | |
| Peak resolution |
1 | p-hydroxybencyl alcohol | 6.20a | 6.56a | 6.56a | 6.37a | 6.29a | 6.33a | 6.13a | 6.31a | 6.21a |
| 2 | Vanillyl alcohol | 2.67a | 2.45b | 2.41b | 2.58a | 2.41ab | 2.38b | 2.34a | 2.42a | 2.44a | |
| 3 | p-hydroxybenzoic acid | 4.06a | 3.96a | 4.25a | 3.89a | 3.78a | 3.87a | 3.80a | 3.83a | 3.80a | |
| 4 | p-hydroxybenzaldehyde | 1.59a | 1.49ab | 1.47b | 1.44a | 1.44a | 1.46a | 1.45a | 1.48a | 1.47a | |
| 5 | Vanillinic acid | 5.23a | 4.58b | 4.83ab | 5.58a | 4.90b | 4.67b | 4.79a | 4.69 a | 4.63a | |
| 6 | Vanillin | 3.06b | 2.95b | 3.77a | 3.34a | 3.28a | 3.47a | 3.15a | 2.97a | 3.07a | |
| 7 | Anisyl alcohol | 7.56b | 7.73ab | 8.26a | 8.07a | 7.65a | 7.46a | 7.79a | 7.57a | 7.41a | |
| 8 | Anisic acid | 0.98ab | 1.02a | 0.95b | 0.98ab | 1.06a | 0.96b | 1.04a | 1.07a | 1.05a | |
| Sample | Compounds (g/100 g d.w.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| p-hydroxybencyl alcohol | Vanillyl alcohol | p-hydroxybenzoic acid | p-hydroxybenzaldehyde | Vanillic acid, | Vainillin | Anisyl alcohol | Anisic acid | Divainillin | |
| VAZN02 | 0.09 ± 0.00c | 0.05 ± 0.01c | 0.02 ± 0.00d | 0.12 ± 0.01b | 0.15 ± 0.01b | 2.74 ± 0.08d | 0.02 ± 0.00ab | 0.003± 0.00b | 0.002 ± 0.01c |
| VAZN03 | 0.06 ±0.00d | 0.05 ± 0.00c | 0.04 ± 0.00b | 0.09 ± 0.00c | 0.16 ± 0.01b | 3.37 ± 0.01b | 0.004 ± 0.00b | 0.003 ± 0.00ab | 0.01 ± 0.00b |
| VMST01G | 0.15 ± 0.00a | 0.12 ± 0.00a | 0.04 ± 0.00b | 0.08 ± 0.00c | 0.17 ± 0.01ab | 1.41 ± 0.02f | 0.02 ± 0.01ab | 0.01 ± 0.00ab | 0.005 ± 0.00c |
| VPAP02G | 0.03 ± 0.00e | 0.09 ± 0.02abc | 0.01 ± 0.00e | 0.07 ± 0.00d | 0.19 ± 0.01a | 3.57 ± 0.01a | 0.01 ± 0.00ab | 0.01 ± 0.01ab | 0.003 ± 0.00c |
| VPAP05 | 0.05± 0.01de | 0.07± 0.01bc | 0.02 ± 0.00d | 0.06 ± 0.00e | 0.09 ± 0.01c | 0.93 ± 0.03g | 0.004 ± 0.00b | 0.01 ± 0.00a | 0.02 ± 0.00a |
| VTEC01 | 0.14 ± 0.00a | 0.07 ± 0.01bc | 0.02 ± 0.00c | 0.13 ± 0.00a | 0.19 ± 0.01a | 3.08 ± 0.00c | 0.03 ± 0.00a | N.D. | 0.002± 0.00c |
| VZGL01 | 0.12 ± 0.01b | 0.1 ± 0.01ab | 0.04 ± 0.00a | 0.12 ± 0.00b | 0.16 ± 0.01b | 2.5 ± 0.01e | 0.02 ± 0.01a | 0.01 ± 0.00ab | 0.01 ± 0.00c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





