1. Introduction
C-C chemokine receptor type 7 (CCR7), also called CD197, is a receptor that facilitates the homing of immune cells to lymph nodes [
1,
2]. The interactions between CCR7 and its ligands, CC-chemokine ligand (CCL) 19 and CCL21, promote the migration of CCR7-expressing cells to secondary lymphoid organs, such as the lymph nodes, thymus, and spleen [
1,
2,
3,
4,
5]. CCL19 and CCL21 are constitutively expressed on the high endothelial venules of lymph nodes [
6]. These chemokines recruit CCR7-expressing cells into the lymph nodes to maintain the immune system.
Lymph node metastasis is an important parameter to determine the prognosis of patients [
4,
7]. The CCR7-CCL19 and CCR7-CCL21 axes have been shown to promote lymph node metastasis in CCR7-expressing breast cancer cells [
8,
9]. Furthermore, the elevated expression of CCR7 correlates with lymph node metastasis in various solid cancers, such as prostate [
9], colorectal [
10], esophageal [
11], gastric [
12], pancreatic [
13], oral [
14], thyroid [
15], and non-melanoma skin cancers [
16]. In addition, CCR7 is recognized as a therapeutic target in hematologic malignancies, such as T-cell prolymphocytic leukemia [
17], B-cell chronic lymphocytic leukemia [
18], and non-Hodgkin’s lymphoma [
19].
Blocking (non-activating) monoclonal antibodies (mAbs) that target CCR7 or its ligands have demonstrated high antitumor efficacy in preclinical models of hematologic malignancies, such as B-cell acute lymphoblastic leukemia [
20], chronic lymphocytic leukemia [
21,
22,
23], mantle cell lymphoma [
24], T-cell acute lymphoblastic leukemia [
20,
25], and T-cell prolymphocytic leukemia [
17]. In addition, targeting CCR7 with an antibody-drug conjugate has been reported as a promising therapeutic strategy for lymphoid malignancies [
26].
CCL21 and CCL19 expressed in several stromal cells [
27] may exhibit antitumor activities at primary tumor sites because these chemokines induce the recruitment of CCR7-expressing activated dendritic cells to the tumor site [
28,
29,
30,
31]. In this context, anti-mouse CCR7 (mCCR7) mAbs that specifically bind to the endogenous receptor are needed to evaluate efficacy and safety in animal models, thereby accelerating the development of CCR7-targeting therapeutics.
We have developed various mAbs against membrane proteins using the Cell-Based Immunization and Screening (CBIS) method [
32,
33,
34,
35,
36,
37,
38,
39]. This method effectively produces mAbs that recognize conformational structures. The obtained mAbs are suitable for flow cytometry because the target molecule is expressed as an antigen on the surface of immunized cells. Furthermore, some of the obtained mAbs are also suitable for western blot and immunohistochemistry. This allows simultaneous contributions to the development of therapeutic and diagnostic applications.
Among the anti-mCCR7 mAbs developed to date, 4B12 is frequently utilized to detect the intact structure of mCCR7 [
28], while E75 (rabbit IgG) is used for western blot [
40] and EPR23192-57 (rabbit IgG) is used for immunohistochemistry [
41]. In addition, anti-human CCR7 mAbs, such as 3D12 (rat IgG
2a, kappa [
42]) and ARC0231 (rabbit IgG), have been reported to cross-react with mCCR7. However, no anti-mCCR7 mAbs are currently available for use in all three applications, such as flow cytometry, western blot, and immunohistochemistry. In this study, we employed the CBIS method to generate a highly versatile anti-mCCR7 mAb.
2. Materials and Methods
2.1. Cell Lines
Chinese hamster ovary (CHO)-K1, mouse myeloma P3X63Ag8.U1 (P3U1), and human glioblastoma LN229 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). LN229 cells were maintained in DMEM supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B (Nacalai Tesque, Inc., Kyoto, Japan), and 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA, USA). CHO-K1 and P3U1 cells were maintained in RRPMI-1640 medium (Nacalai Tesque) with the same antibiotics described above and 10% heat-inactivated FBS. All cells were cultured in a humidified incubator at 37°C with 5% CO2.
2.2. Plasmid Construction and Establishment of Stable Transfectants
The synthesized DNA (Eurofins Genomics KK, Tokyo, Japan) encoding mouse CCR7 (Accession No.: NM_007719) was subcloned into pCAG-Ble-PAcH vector (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) using the In-Fusion HD Cloning Kit (Takara Bio, Inc., Shiga, Japan). The constructed vector was designated pCAG-mCCR7-PA. Using the Neon transfection system, the plasmid was transfected into CHO-K1 and LN229 cells (Thermo Fisher Scientific, Inc.). Transfectants expressing the target gene were detected using the anti-mCCR7 mAb 4B12 (rat IgG2a, kappa, BioLegend, San Diego, CA, USA). Stable transfectants were isolated by cell sorting (SH800 Cell Sorter, Sony Corporation, Tokyo, Japan), and cell lines were established by introducing pCAG-mCCR7-PA into CHO-K1 cells (CHO/mCCR7 cells) and LN229 cells (LN229/mCCR7 cells). These cell lines were maintained in medium containing 0.5 mg/mL Zeocin (InvivoGen, San Diego, CA, USA).
2.3. Development of Hybridomas
A 5-week-old female Sprague-Dawley rat (Jcl: SD rat, CLEA Japan, Tokyo, Japan) was housed under specific pathogen-free conditions. The rat was immunized intraperitoneally with LN229/mCCR7 cells (1×10⁹ cells/injection) with Alhydrogel adjuvant 2% (InvivoGen). Following three weekly immunizations, a booster injection was administered two days prior to the harvesting of spleen cells. Hybridoma production was performed as previously described [
43].
2.4. Flow Cytometric Analysis
Cells were detached using 1 mM ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.) to prevent enzymatic degradation of surface proteins. The cells were washed with 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) (blocking buffer) and incubated with mAbs at 4°C for 30 minutes. After washing, the cells were incubated with anti-rat IgG (H+L)-Alexa Fluor 488 conjugate (1:2,000 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C for 30 minutes. Data were collected using the SA3800 Cell Analyzer and analyzed using FlowJo software (BD Biosciences, Franklin Lakes, NJ, USA).
2.5. Determination of Dissociation Constant (KD) by Flow Cytometry
CHO/mCCR7 cells were treated with serial dilutions of C7Mab-7 and 4B12 (10 to 0.005 μg/mL). The cells were stained with anti-rat IgG (H+L)-Alexa Fluor 488 conjugate (1:200 dilution) at 4°C for 30 minutes. Data were collected using the SA3800 Cell Analyzer and analyzed using FlowJo software. GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA) was used to fit the binding isotherms and determine dissociation constant (KD) values using built-in one-site binding models.
2.6. Western Blot Analysis
Whole-cell lysates (10 μg of protein per lane) were separated using 5–20% polyacrylamide gels (FUJIFILM Wako Pure Chemical Corporation). The separated proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Merck KGaA, Darmstadt, Germany). The membranes were blocked with 4% skim milk (Nacalai Tesque, Inc.) in PBST. Subsequently, the membranes were incubated with 1 μg/mL of C7Mab-7, 4B12, or an anti-β-actin mAb (AC-15; Sigma-Aldrich Corporation), followed by incubation with rabbit anti-rat IgG conjugated with horseradish peroxidase (1:20,000 dilution; Merck KGaA) or rabbit anti-mouse immunoglobulins conjugated with horseradish peroxidase (1:2,000 dilution; Agilent Technologies, Inc., Santa Clara, CA, USA). Secondary antibodies were matched to the host species of each primary antibody. Chemiluminescence signals were developed using the ImmunoStar LD (FUJIFILM Wako Pure Chemical Corporation) or Pierce ECL Plus Western Blotting Substrate (Thermo Fisher Scientific, Inc.) and detected with a Sayaca-Imager (DRC Co., Ltd., Tokyo, Japan).
2.7. Immunohistochemistry
Cell blocks were prepared using iPGell (Genostaff Co., Ltd., Tokyo, Japan) and fixed in a 4% paraformaldehyde phosphate buffer solution (FUJIFILM Wako Pure Chemical Corporation). The blocks were processed to create four-micrometer-thick paraffin-embedded cell sections. The sections were autoclaved in citrate buffer (pH 6.0; Nichirei Biosciences, Inc., Tokyo, Japan) for 20 minutes. These sections were blocked with SuperBlock T20 Blocking Buffer (Thermo Fisher Scientific Inc.), incubated with C7Mab-7 (1 μg/mL) at room temperature for 1 hour, and subsequently treated with Histofine Simple Stain Mouse MAX PO (Rat) (Nichirei Biosciences, Inc.) for 30 minutes at room temperature. Color development was achieved using 3,3’-diaminobenzidine tetrahydrochloride (DAB; Agilent Technologies Inc.), and counterstained with hematoxylin (Merck KGaA).
3. Results
3.1. Development of Anti-Mouse CCR7 mAbs
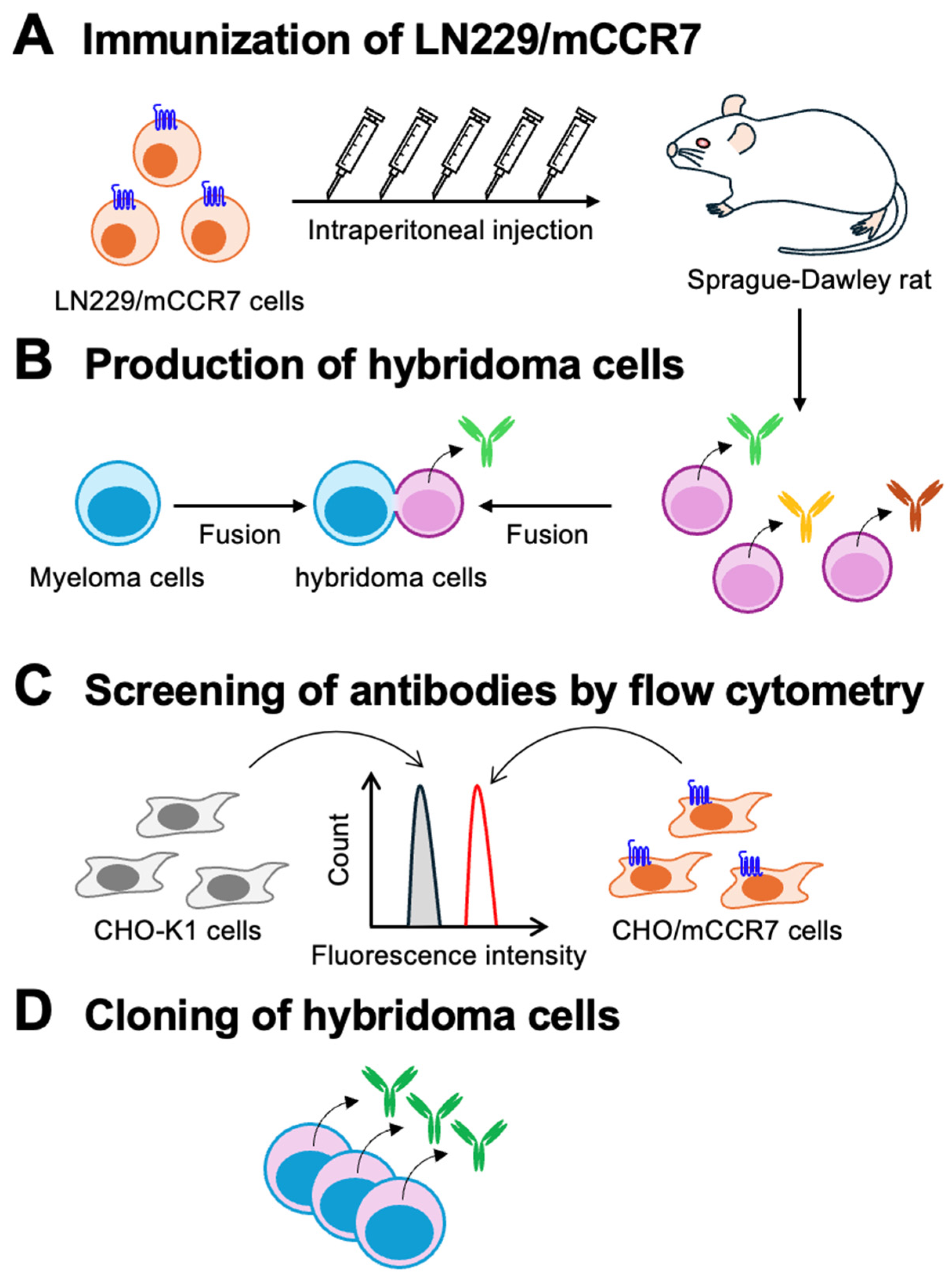
A Jcl: SD rat was immunized with LN229/mCCR7 cells (
Figure 1A). Spleen cells were harvested from the immunized rat, and hybridomas were produced by fusion with P3U1 cells (
Figure 1B). These hybridomas were seeded into 96-well plates. After colony formation, supernatants were collected and analyzed using a flow cytometry-based high-throughput screening to identify supernatants that were positive for CHO/mCCR7 cells but negative for CHO-K1 cells (
Figure 1C). Anti-mCCR7 mAb-producing hybridomas were subsequently cloned by limiting dilution, and C
7Mab-7 (rat IgG
1, kappa) was finally established (
Figure 1D).
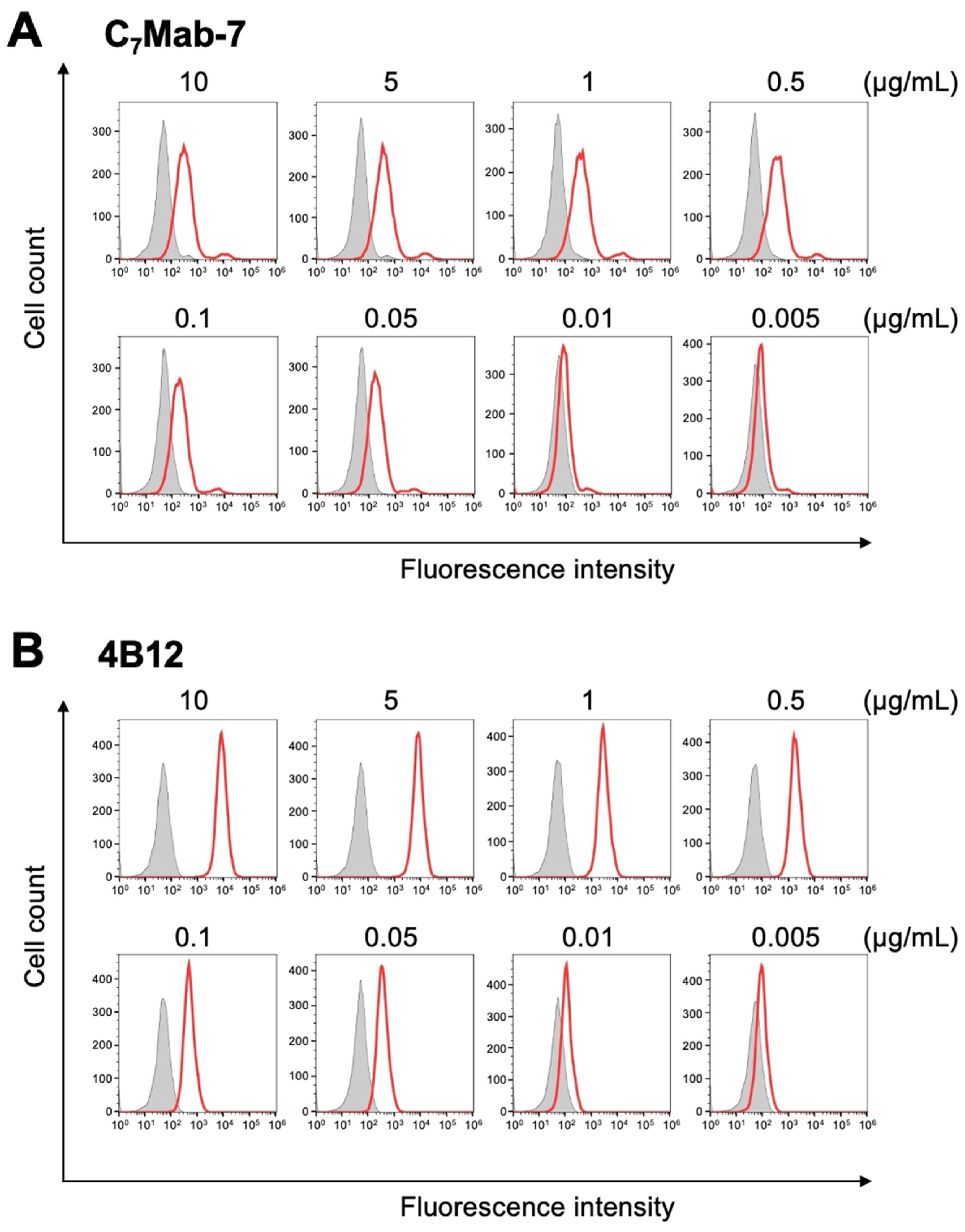
3.2. Flow Cytometry Using C7Mab-7 and 4B12
The binding of purified C
7Mab-7 to CHO/mCCR7 and CHO-K1 cells was analyzed using flow cytometry. C
7Mab-7 exhibited dose-dependent reactivity with CHO/mCCR7 cells at concentrations ranging from 10 to 0.005 μg/mL but did not bind to CHO-K1 cells at any concentration (
Figure 2A). Additionally, a commercially available anti-mCCR7 mAb (4B12) showed higher fluorescence intensity against CHO/mCCR7 cells at concentrations ranging from 0.1 to 10 μg/mL compared to C
7Mab-7 (
Figure 2B) although the fluorescence intensities of C
7Mab-7 and 4B12 were comparable at lower concentrations (0.005 to 0.05 μg/mL). These results indicate that C
7Mab-7 specifically recognized mCCR7 on the cell surface although the fluorescence intensity saturates at mAb concentrations above 0.5 μg/mL in flow cytometry.
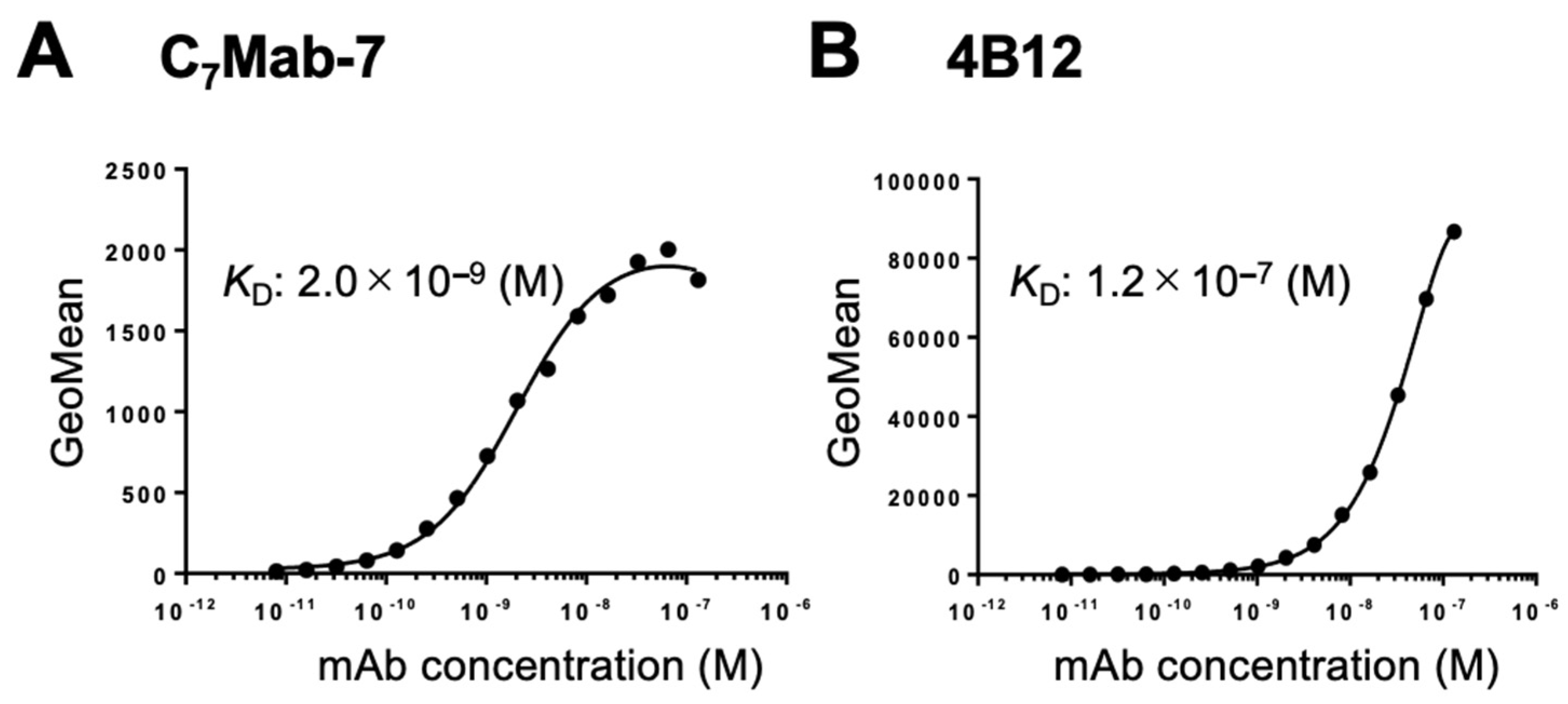
3.3. Determination of KD Values of C7Mab-7 and 4B12 by Flow Cytometry
The binding affinity of C
7Mab-7 and 4B12 was evaluated using flow cytometry. The
KD value of C
7Mab-7 for CHO/mCCR7 cells was 2.0 × 10⁻⁹ M (
Figure 3), while that of 4B12 for CHO/mCCR7 cells was 1.2 × 10⁻⁷ M. The lower affinity of 4B12 is thought to be due to the vigorous fluorescence intensity observed at high concentrations of mAb, which rapidly decreased with lower concentrations of mAb.
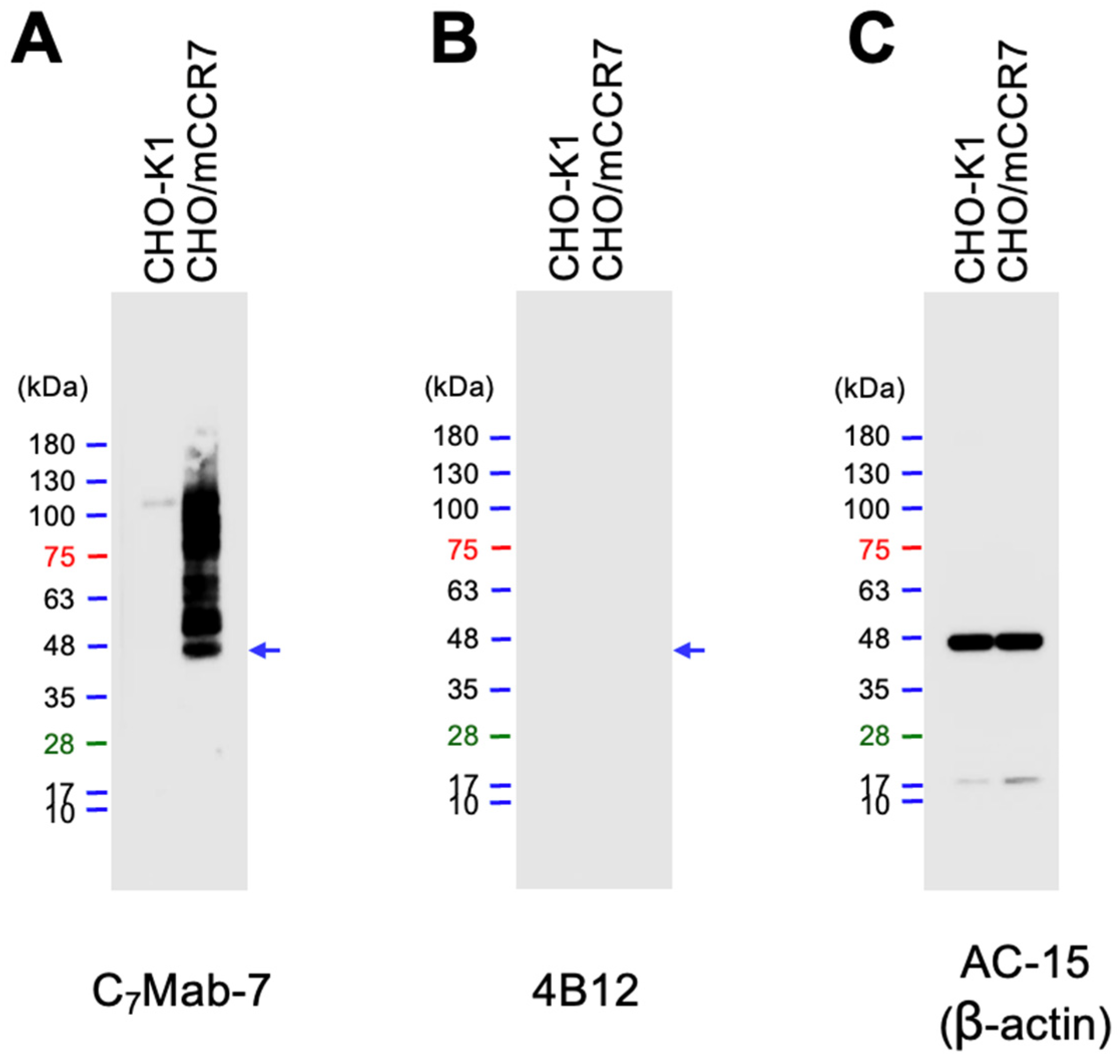
3.4. Western Blot Analysis
The availability of C
7Mab-7 for western blot analysis was evaluated using whole cell lysates of CHO-K1 and CHO/mCCR7 cells. C
7Mab-7 exhibited strong reactivity with mCCR7 at an estimated molecular weight of 42.9 kDa and higher molecular weight positions (
Figure 4A). In contrast, 4B12 did not exhibit any reactivity with mCCR7 (
Figure 4B). These results indicate that C
7Mab-7 is available not only for flow cytometry but also for western blot analysis.
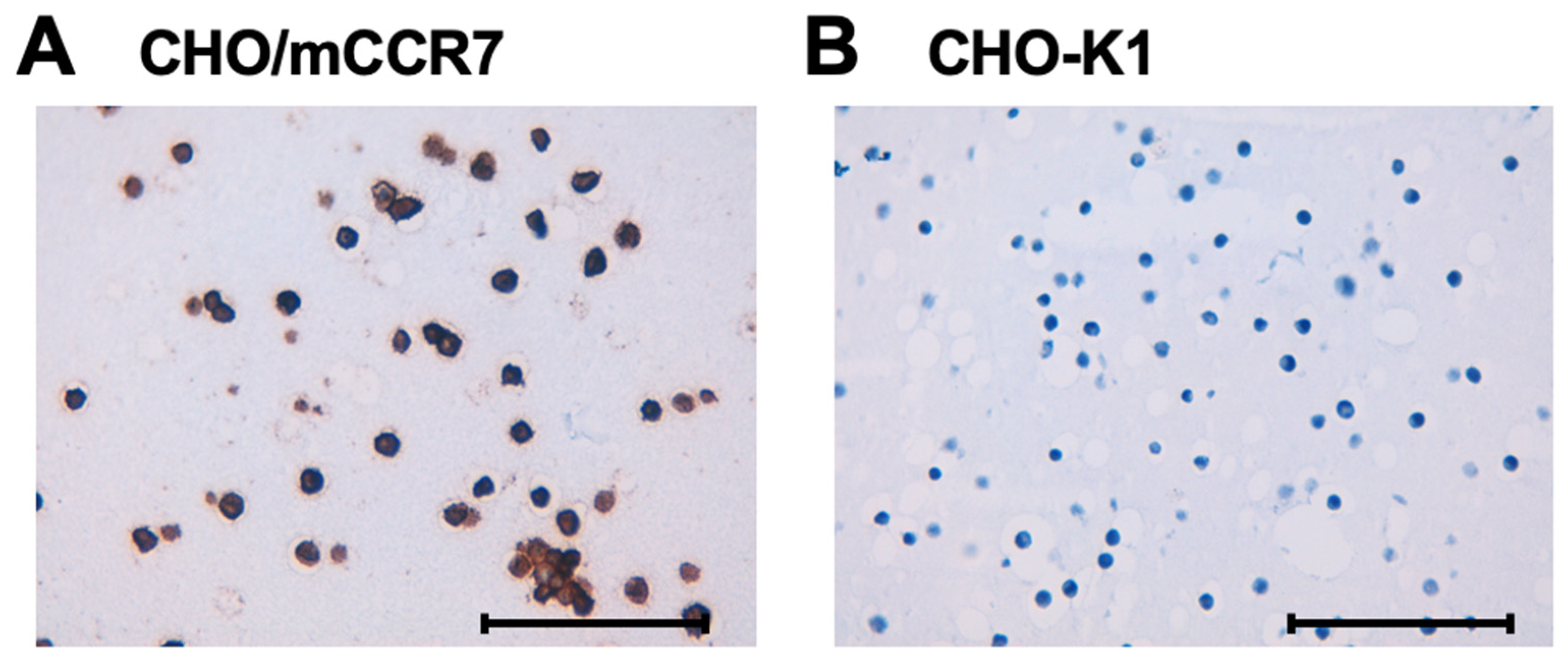
3.5. Immunohistochemistry Using C7Mab-7 in Mouse CCR7-Overexpressed CHO-K1 Cells
To evaluate the suitability of C
7Mab-7 for immunohistochemistry in formalin-fixed paraffin-embedded (FFPE) samples, paraffin-embedded sections of CHO/mCCR7 and CHO-K1 cells were stained with C
7Mab-7. The cytoplasmic and membranous staining of mCCR7 was observed in CHO/mCCR7 cells (
Figure 5A), whereas no staining was detected in CHO-K1 cells (
Figure 5B).
4. Discussion
We developed a mAb against mCCR7 using the CBIS method. The obtained mAb, C
7Mab-7, is available for flow cytometry, western blot, and immunohistochemistry (Figs. 2–5). The successful establishment of C
7Mab-7 suggests the presence of an epitope with a stable structure, regardless of the state of mCCR7. This is evidenced by the fact that the epitope is recognized in its native conformation in flow cytometry and is detected in a denatured form in western blot. Antibodies that recognize such epitopes have the potential for susceptible detection in immunohistochemistry. Indeed, C
7Mab-7 is suitable for immunohistochemistry (
Figure 5). Therefore, identifying the epitope of C
7Mab-7 will facilitate the development of more sensitive anti-CCR7 mAbs, thereby contributing to the advancement of mAb-based therapies and diagnostics.
Western blot analysis of C
7Mab-7 using CHO/mCCR7 cells showed a band at the estimated molecular weight of 42.9 kDa and bands at higher molecular weight positions (
Figure 4A). A similar band pattern of the western blot was observed in human CCR7 overexpressed HEK293 cells due to the constitutive polyubiquitylation. The ubiquitylation regulates the basal trafficking of CCR7 in the absence of ligands [
44]. As shown in
Figure 5A, mCCR7 was detected in both cytoplasm and plasma membrane in immunohistochemistry. Therefore, overexpressed mCCR7 is thought to receive the basal trafficking by ubiquitylation in CHO/mCCR7 cells. C
7Mab-7 could contribute to the study of the membrane-to-cytoplasm trafficking of mCCR7 through immunofluorescence or antibody-induced receptor internalization studies.
Anti-CCR7 mAbs have already been developed as therapeutic agents for hematologic malignancies [
3,
26]. Expanding their application to solid cancers requires the efficacy and safety of these mAbs against metastatic cancers in mouse models [
45,
46]. To target the mCCR7-positive cancer cells using C
7Mab-7 (rat IgG
1), generating a class-switched mouse IgG2a mAb from rat IgG
1 is necessary. Furthermore, generating defucosylated IgG
2a-type mAbs is also effective for evaluating antibody-dependent cellular cytotoxicity and the
in vivo antitumor effect in mouse xenograft models [
47,
48].
In a syngeneic mouse model of oral squamous cell carcinoma, the tumor growth rate was significantly low in mCCR7-knockout mice compared with the wild-type mice [
49]. Single-cell RNA sequence and bioinformatics analyses revealed that the proportion of M2 macrophages in the knockout group was lower than that in the wild-type group [
49].
In vitro studies showed that mCCR7 can promote M2 macrophage polarization, which promotes the proliferation, invasion, and migration of tumor cells [
49]. Therefore, the depletion of mCCR7-positive cells by anti-mCCR7 mAbs like defucosylated IgG
2a-type C
7Mab-7 could inhibit tumor growth.
In the unilateral ureteral obstruction model in mice, mCCR7-expressing circulating fibrocytes infiltrate the kidney and contribute to renal fibrosis [
50]. The blockade of CCL21/mCCR7 signaling by anti-CCL21 mAbs reduced the renal fibrosis [
51]. Therefore, anti-mCCR7 mAbs that block mCCR7 signaling could suppress renal fibrosis. Further studies are required to investigate the neutralizing activity of C
7Mab-7.
In conclusion, C7Mab-7 is a highly sensitive and versatile mAb for basic research and is anticipated to obtain proof-of-concept in preclinical models for the development of antibody therapies.
Author Contributions
Hiroyuki Satofuka: Investigation, Funding acquisition, Writing – original draft. Hiroyuki Suzuki: Investigation, Funding acquisition. Tomohiro Tanaka: Investigation, Funding acquisition. Rena Ubukata: Investigation. Miu Hirose: Investigation. Haruto Yamamoto: Investigation. Yu Kaneko: Investigation. Shiori Fujisawa: Investigation. Guanjie Li: Investigation. Mika K. Kaneko: Conceptualization, Funding acquisition. Yukinari Kato: Conceptualization, Funding acquisition, Project administration, Writing – review and editing. All authors have read and agreed to the published version of the manuscript
Funding Information
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Numbers: JP24am0521010 (to Y.Kato), JP24ama121008 (to Y.Kato), JP24ama221339 (to Y.Kato), JP23am0401013 (to Y.Kato), JP24ama221339 (to Y.Kato), JP24bm1123027 (to Y.Kato), and JP24ck0106730 (to Y.Kato), and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant nos. 24K11652 (to H.Satofuka), 22K06995 (to H.Suzuki), 21K20789 (to T.T.), 21K07168 (to M.K.K.), and 22K07224 (to Y.Kato).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest involving this article.
References
- Forster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 2008;8(5): 362-371. [CrossRef]
- Brandum, E.P.; Jorgensen, A.S.; Rosenkilde, M.M.; Hjorto, G.M. Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. Int J Mol Sci 2021;22(15). [CrossRef]
- Cuesta-Mateos, C.; Terron, F.; Herling, M. CCR7 in Blood Cancers - Review of Its Pathophysiological Roles and the Potential as a Therapeutic Target. Front Oncol 2021;11: 736758. [CrossRef]
- Bill, C.A.; Allen, C.M.; Vines, C.M. C-C Chemokine Receptor 7 in Cancer. Cells 2022;11(4). [CrossRef]
- Alrumaihi, F. The Multi-Functional Roles of CCR7 in Human Immunology and as a Promising Therapeutic Target for Cancer Therapeutics. Front Mol Biosci 2022;9: 834149. [CrossRef]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol 2004;22: 891-928. [CrossRef]
- Ji, H.; Hu, C.; Yang, X.; et al. Lymph node metastasis in cancer progression: molecular mechanisms, clinical significance and therapeutic interventions. Signal Transduct Target Ther 2023;8(1): 367. [CrossRef]
- Muller, A.; Homey, B.; Soto, H.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410(6824): 50-56. [CrossRef]
- Cabioglu, N.; Yazici, M.S.; Arun, B.; et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res 2005;11(16): 5686-5693. [CrossRef]
- Shi, W.; Zou, R.; Yang, M.; et al. Analysis of Genes Involved in Ulcerative Colitis Activity and Tumorigenesis Through Systematic Mining of Gene Co-expression Networks. Front Physiol 2019;10: 662. [CrossRef]
- Irino, T.; Takeuchi, H.; Matsuda, S.; et al. CC-Chemokine receptor CCR7: a key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC Cancer 2014;14: 291. [CrossRef]
- Mashino, K.; Sadanaga, N.; Yamaguchi, H.; et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res 2002;62(10): 2937-2941.
- Li, K.; Xu, B.; Xu, G.; Liu, R. CCR7 regulates Twist to induce the epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma. Tumour Biol 2016;37(1): 419-424. [CrossRef]
- Tsuzuki, H.; Takahashi, N.; Kojima, A.; et al. Oral and oropharyngeal squamous cell carcinomas expressing CCR7 have poor prognoses. Auris Nasus Larynx 2006;33(1): 37-42. [CrossRef]
- Wagner, P.L.; Moo, T.A.; Arora, N.; et al. The chemokine receptors CXCR4 and CCR7 are associated with tumor size and pathologic indicators of tumor aggressiveness in papillary thyroid carcinoma. Ann Surg Oncol 2008;15(10): 2833-2841. [CrossRef]
- Basile, J.; Thiers, B.; Maize, J., Sr.; Lathers, D.M. Chemokine receptor expression in non-melanoma skin cancer. J Cutan Pathol 2008;35(7): 623-629. [CrossRef]
- Cuesta-Mateos, C.; Fuentes, P.; Schrader, A.; et al. CCR7 as a novel therapeutic target in t-cell PROLYMPHOCYTIC leukemia. Biomark Res 2020;8: 54. [CrossRef]
- Lopez-Giral, S.; Quintana, N.E.; Cabrerizo, M.; et al. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol 2004;76(2): 462-471. [CrossRef]
- Yang, J.; Wang, S.; Zhao, G.; Sun, B. Effect of chemokine receptors CCR7 on disseminated behavior of human T cell lymphoma: clinical and experimental study. J Exp Clin Cancer Res 2011;30(1): 51. [CrossRef]
- Alsadeq, A.; Fedders, H.; Vokuhl, C.; et al. The role of ZAP70 kinase in acute lymphoblastic leukemia infiltration into the central nervous system. Haematologica 2017;102(2): 346-355. [CrossRef]
- Alfonso-Perez, M.; Lopez-Giral, S.; Quintana, N.E.; et al. Anti-CCR7 monoclonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J Leukoc Biol 2006;79(6): 1157-1165. [CrossRef]
- Cuesta-Mateos, C.; Lopez-Giral, S.; Alfonso-Perez, M.; et al. Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia. Exp Hematol 2010;38(9): 756-764, 764 e751-754. [CrossRef]
- Cuesta-Mateos, C.; Loscertales, J.; Kreutzman, A.; et al. Preclinical activity of anti-CCR7 immunotherapy in patients with high-risk chronic lymphocytic leukemia. Cancer Immunol Immunother 2015;64(6): 665-676. [CrossRef]
- Somovilla-Crespo, B.; Alfonso-Perez, M.; Cuesta-Mateos, C.; et al. Anti-CCR7 therapy exerts a potent anti-tumor activity in a xenograft model of human mantle cell lymphoma. J Hematol Oncol 2013;6: 89. [CrossRef]
- Buonamici, S.; Trimarchi, T.; Ruocco, M.G.; et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 2009;459(7249): 1000-1004. [CrossRef]
- Abrisqueta, P.; Marks, R.; Avivi, I.; et al. A phase 1 study of JBH492, an anti C-C chemokine receptor 7 antibodydrug conjugate (anti-CCR7 ADC), assessing safety and efficacy in lymphoid malignancies. Cancer Research 2024;84(7). [CrossRef]
- Rizeq, B.; Malki, M.I. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers (Basel) 2020;12(4). [CrossRef]
- Clatworthy, M.R.; Aronin, C.E.; Mathews, R.J.; et al. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nat Med 2014;20(12): 1458-1463. [CrossRef]
- Lee, C.Y.C.; Kennedy, B.C.; Richoz, N.; et al. Tumour-retained activated CCR7(+) dendritic cells are heterogeneous and regulate local anti-tumour cytolytic activity. Nat Commun 2024;15(1): 682. [CrossRef]
- Ben-Baruch, A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis 2008;25(4): 345-356. [CrossRef]
- Ben-Baruch, A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev 2006;25(3): 357-371. [CrossRef]
- Asano, T.; Nanamiya, R.; Takei, J.; et al. Development of Anti-Mouse CC Chemokine Receptor 3 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021;40(3): 107-112. [CrossRef]
- Nanamiya, R.; Takei, J.; Asano, T.; et al. Development of Anti-Human CC Chemokine Receptor 9 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021;40(3): 101-106. [CrossRef]
- Nanamiya, R.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Development of an Anti-EphB4 Monoclonal Antibody for Multiple Applications Against Breast Cancers. Monoclon Antib Immunodiagn Immunother 2023;42(5): 166-177. [CrossRef]
- Saito, M.; Suzuki, H.; Tanaka, T.; et al. Development of an Anti-Mouse CCR8 Monoclonal Antibody (C. Monoclon Antib Immunodiagn Immunother 2022;41(6): 333-338. [CrossRef]
- Suzuki, H.; Tanaka, T.; Li, G.; et al. Development of a Sensitive Anti-Mouse CCR5 Monoclonal Antibody for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2024;43(4): 96-100. [CrossRef]
- Tanaka, T.; Nanamiya, R.; Takei, J.; et al. Development of Anti-Mouse CC Chemokine Receptor 8 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021;40(2): 65-70. [CrossRef]
- Tateyama, N.; Asano, T.; Suzuki, H.; et al. Epitope Mapping of Anti-Mouse CCR3 Monoclonal Antibodies Using Flow Cytometry. Antibodies (Basel) 2022;11(4). [CrossRef]
- Yoshida, S.; Kato, T.; Kanno, N.; et al. Cell type-specific localization of Ephs pairing with ephrin-B2 in the rat postnatal pituitary gland. Cell Tissue Res 2017;370(1): 99-112. [CrossRef]
- Lu, M.; Xu, C.; Zhang, Q.; et al. Inhibition of p21-activated kinase 1 attenuates the cardinal features of asthma through suppressing the lymph node homing of dendritic cells. Biochem Pharmacol 2018;154: 464-473. [CrossRef]
- Zha, Z.; Hong, Y.; Tang, Z.; et al. FCGR3A: A new biomarker with potential prognostic value for prostate cancer. Front Oncol 2022;12: 1014888. [CrossRef]
- Wijewardana, V.; Kristoff, J.; Xu, C.; et al. Kinetics of myeloid dendritic cell trafficking and activation: impact on progressive, nonprogressive and controlled SIV infections. PLoS Pathog 2013;9(10): e1003600. [CrossRef]
- Kobayashi, H.; Asano, T.; Suzuki, H.; et al. Establishment of a Sensitive Monoclonal Antibody Against Mouse CCR9 (C(9)Mab-24) for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2023;42(1): 15-21. [CrossRef]
- Schaeuble, K.; Hauser, M.A.; Rippl, A.V.; et al. Ubiquitylation of the chemokine receptor CCR7 enables efficient receptor recycling and cell migration. J Cell Sci 2012;125(Pt 19): 4463-4474. [CrossRef]
- Hart, I.R. Role of integrins in tumor invasion and metastasis. Exp Dermatol 2004;13(10): 663. [CrossRef]
- Trusheim, M.R.; Berndt, E.R. The clinical benefits, ethics, and economics of stratified medicine and companion diagnostics. Drug Discov Today 2015;20(12): 1439-1450. [CrossRef]
- Ishikawa, K.; Suzuki, H.; Ohishi, T.; et al. Antitumor activities of anti-CD44 monoclonal antibodies in mouse xenograft models of esophageal cancer. Oncol Rep 2024;52(5). [CrossRef]
- Ishikawa, K.; Suzuki, H.; Ohishi, T.; et al. Anti-CD44 Variant 10 Monoclonal Antibody Exerts Antitumor Activity in Mouse Xenograft Models of Oral Squamous Cell Carcinomas. Int J Mol Sci 2024;25(17). [CrossRef]
- Wang, Z.; Kirkwood, K.L.; Wang, Y.; et al. Analysis of the effect of CCR7 on the microenvironment of mouse oral squamous cell carcinoma by single-cell RNA sequencing technology. J Exp Clin Cancer Res 2024;43(1): 94. [CrossRef]
- Wada, T.; Sakai, N.; Matsushima, K.; Kaneko, S. Fibrocytes: a new insight into kidney fibrosis. Kidney Int 2007;72(3): 269-273. [CrossRef]
- Sakai, N.; Wada, T.; Yokoyama, H.; et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A 2006;103(38): 14098-14103. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).