1. Introduction
Carotenoids are a group of natural pigments widely used in the food industry. The importance of this component has recently increased due to its high provitamin-A content, coloring properties and antioxidant effect [
1]. They are preventive agents especially for skin, liver and some other types of cancer. It also reduces the risk of heart disease [
2]. Carotenoids are natural pigments that occur in bacteria, fruits, plants and fungi. They cannot be synthesized within human metabolism. They must be obtained from food sources. One of the most important sources of carotenoids for humans is plants. The compounds found in relatively high concentrations in fruits and vegetables are lycopene, β-carotene, lutein, zeaxanthin, β-cryptoxanthin and α-carotene [
3].
ß-Carotene is a significant carotenoid, and it is also the most common carotenoid found in the plants [
4]. It is an organic compound, which has red-orange color, that is found in plants, fruits and fungi [
5]. ß-Carotene is best known for being precursor to vitamin A, which is essential for vision, immune functions and skin health. Some of the most common uses of ß-Carotene include vitamin A supplementation, skin health and protection, antioxidant support, vision health, immune system support, dietary supplement, cancer prevention research [
6,
7,
8,
9], digestive health [
10,
11], food colorant, cosmetics and hair products.
ß-Carotene is isolated from plants and fruits, which have ample amounts of carotenoids, by utilization of column chromatography. Aside from that, it is industrially manufactured by chemical synthesis or by extraction from biological sources such as vegetables rich in ß-Carotene and microalgae (especially
Dunaliella salina). In order to separate ß-Carotene among the mixture of carotenoids, polarity of the compound needs to be taken into account. ß-Carotene is a non-polar hydrocarbon based (C
40H
56) compound. Therefore, it is separated via utilization of non-polar solvents such as hexane. The other environmentally non-friendly solvent choices could be tetrahydrofurane, methyltert-butylether, benzene and other halogenated solvents due to their specific advantage over other non-polar solvents in terms of solubility of ß-Carotene [
12]. None of these solvents are attractive due to their negative effects on the environment and human health (especially benzene is suspected to cause cancer [
13] and considered to be class-I (very dangerous for human health) solvent [
14] in the pharmaceutical industry).
Food waste is a hot topic. In 2022 the globally wasted amount of food was 1.052 billion tons [
15]. And this number keeps increasing over time. Pumpkin and spinach have also place in this food loss and waste. Especially one of the major sources of pumpkin waste is originated from Halloween decorations. In 2016, more than 1.3 billion pounds (~589,670 metric tons) of pumpkins were discarded as waste after Halloween in United States alone [
16]. Loss of spinach is also considered in the context of vegetables. Being a highly perishable leafy green vegetable itself, loss of spinach is also contributing to vegetable waste. In 2020 approximately 31.0 million tonnes of spinach manufactured globally and 6.5% of this spinach (over 2 million tonnes) was estimated to be lost during retail phase and approximately 35% (~10.85 million tonnes) was lost in the house hold consumption [
17]. Solely, in the UK, 24.8% of the spinach is wasted within the farm, grading, storage, packaging and retail before reaching to households, which corresponds to ~15,384 metic tonnes [
18].
Considering the importance of ß-Carotene, and the pumpkin and spinach that go to the waste, a need for environmentally safer “green” solvents arises for the extraction of ß-Carotene (which is a valuable commodity) from vegetables and fruits (such as pumpkin and spinach leaves). With regards to ß-Carotene extraction, some of the following studies could be given as examples. Sebdani and Abbasi had used ultrasound-assisted extraction method with using sunflower oil as the green solvent in 2023 [
19]. Stupar
et.al. had used several natural deep eutectic solvents to extract ß-Carotene from pumpkin (NADES) with the support of ultrasonic power in 2021 [
20]. Shi
et.al. extracted carotenoids from big sized pumpkins via utilization of supercritical CO
2 in 2013 [
21].
In this regard, utilization of Deep Eutectic Solvents (DESs) could come into the picture as a solution. A Deep Eutectic Solvent (DES) is a mixture of two or more compounds that has lower melting point than the compounds makes it up. One of these compounds is typically a hydrogen bond donor (HBD) and the other one is a hydrogen bond acceptor (HBA). Combining an HBD and HBA forms a eutectic mixture, which has a melting point considerably lower than that of the individual components. The DESs are often used in green chemistry due to their adjustable properties, their lower toxicity, and environmental beneficial factors.
The aim of this study is to extract ß-Carotene from both pumpkin (Curcubita moschata) and spinach (Spinacia oleracea) by using L-menthol and carboxylic acid based natural DESs with the assistance of mechanical mixing and homogenization, and constructing mathematical models to determine the parameters of maximum efficiency for each extraction method. The natural DESs are obtained by coupling L-menthol as the hydrogen bond acceptor (HBA) and Acetic Acid, Propionic Acid and Butyric Acid as the hydrogen bond donors (HBD). Menthol is an organic compound which naturally occurs in the oils of several plants in the mint family, such as peppermint and corn mint. Menthol is a cheap and natural compound to form a DES, where it will be used as the hydrogen bond acceptor (HBA). The hydrogen bond donors (HBD) are selected among Acetic Acid, Propionic Acid and Butyric Acid, where all these acids are naturally found in the foods and plants (i.e., vinegar, potatoes, butter…etc.). Experimental results of MMAE and HAE of ß-Caroten from pumpkin and spinach with these DESs were modeled, and formulae were created for each extraction setup. Optimum conditions were obtained depending on the formulae.
2. Materials and Methods
2.1. Materials
The pumpkins (Curcubita moschata) were obtained in its raw form from local a farm located in Ordu/Türkiye. The spinach (Spinacia oleracea) leaves were obtained as leaves in their raw form from a market located in Istanbul/Türkiye. The pumpkins were peeled and cut into pieces, where the pieces were sized around ≤2 mm and used as the pumpkin samples. The spinach leaves were washed, cleaned and dried, then chopped into small pieces (also ≤2mm) and used as the spinach samples. The baselines were prepared with the reference material, ß-Carotene (≥97.0%) Sigma Aldrich Chemie GmbH (Albuch/Germany). L-Menthol flakes (≥99.7%) were purchased from BASF SE (Ludwigshafen/Germany). DES preparation compounds, acetic acid (≥99.5%) was purchased from Merck (Darmstadt/Germany), propionic acid (≥99.0%) and butyric acid (≥99.0%), were purchased from Merck Schuchardt OHG (Hohenbrunn/Germany). The 0.45 μm RC filters were purchased from Sartorius Türkiye (Istanbul/Türkiye).
2.2. Mechanical Mixing Assisted Extraction (MMAE) of ß-Carotene
The pumpkin and spinach samples were dispensed and put into 10-20 ml Deep Eutectic Solvents (DESs). The sample amounts were determined in order to read absorbance values not more than the meaningful upper limit of UV Spectrophotometer (1.000). After putting samples into the DESs, the items were stirred with mechanical mixer (Daihan Scientific Co., Ltd., model: MSH-20A, South Korea) at 500 rpm speed at the room temperature (298.15 K) under atmospheric pressure. The samples were mechanically stirred for 15, 30, 45 and 60 minutes.
2.3. Homogenization Assisted Extraction (HAE) of ß-Carotene
The pumpkin and spinach samples were dispensed and put into 7-10 ml DESs. The sample amounts were determined in order to read absorbance values not more than the meaningful upper limit of UV Spectrophotometer (1.000). After putting samples into the DESs, the items are treated with homogenizer (IKA T25 Ultra Turrax) at 7000 rpm, 10500 rpm and 14000 rpm speeds at the room temperature (298.15 K) under atmospheric pressure. The samples were treated for 30, 60, 90 and 120 seconds.
2.4. Analysis of samples
After the mixing and homogenization, the mixtures were filtered through 0.45 μm RC filters and the UV absorbance measurements were collected. UV-Visible spectrophotometer (PG Instruments, T60/Leicestershire, England) was used. Each sample was measured 3 times with glass/quartz UV cuvettes at λ=450 nm wavelength [
22] against the placebo (the relevant DES itself without any solute in it). The quantitative results were given in micrograms of ß-Carotene per gram of pumpkin (μg-ß-Carotene/gr-pumpkin) and spinach (μg-ß-Carotene/gr-spinach) samples.
2.5. Statistical Design of Experiments
In order to design the experimental plan; mathematical modelling and optimization of parameters, a statistical method called Response Surface Methodology (RSM) was used. The response surface methodology is a statistical and mathematical method which creates a relationship between a dependent variable (response), which is denoted as “y” and one or more independent variables, which could be denoted as “x
1, x
2,…,x
n” [
23]. In this research work, in order to evaluate the obtained data and the affecting factors, the necessary calculations and data processing algorithms were performed by utilization of Stat-Ease 360
® software. Prior to beginning of the study, the appropriate experimental design type for quadratic response surfaces had to be selected [
24]. Within the several design classes (3-level factorial design, Doehlert design, Box-Behnken and central composite designs), central composite design (CCD) was selected. It is also the most commonly applied design type [
23]. The number of experiment (N) is calculated by equation (1):
k= Number of process parameter
Cp= Number of replicate at centre points.
3. Results and Discussion
3.1. Preparation of Deep Eutectic Solvents (DESs)
The DESs used in the extraction ß-Carotene were prepared by compounding L-menthol flakes, used as the hydrogen bond acceptor (HBA) and acetic acid, propionic acid and butyric acid as hydrogen bond donors (HBD). The amounts to be mixed are dispensed by using and analytical balance (Shimadzu Corporation, Type: ATX224, Kyoto/Japan) with an accuracy of ± 0.0001 gr according to the required molar ratio as shown in
Table 1.
Afterwards, HBA and HBD at predetermined ratios were mixed and heated on a hotplate stirrer (Daihan Scientific Co., Ltd., model: MSH-20D, South Korea) up to 353.15 K and kept there until a homogeneous transparent liquid was obtained. The pH and the densities of the obtained DESs were measured with Mettler-Toledo, SevenCompact pH/Ion S220 and Mettler-Toledo, DM40, respectively. The pH and density results that were obtained are provided in
Table S1 along with the physical state observation at temperatures 278.15 K and 255.15 K.
3.2. Baseline (Calibration Line) Study for ß-Carotene in Deep Eutectic Solvents (DESs)
To establish the baselines (calibration lines) for determination of ß-Carotene concentration in the extraction samples, the ß-Carotene is dissolved in each of the obtained DESs with concentrations of 20 ppm, 40 ppm, 60 ppm, 80 ppm and 100 ppm at each measurement. The prepared samples were analyzed in the UV Spectrophotometer (PG Instruments Limited, model: T60 U) under wavelength of λ=450 nm. Based on the results obtained from UV Spectrophotometer, ß-Carotene baseline (calibration line) is calculated for each and every DES used in this study. The baseline equations of ß-Carotene for each DES are given in
Table S2.
3.3. Mechanical Mixing Assisted Extraction (MMAE) and Homogenization Assisted Extraction (HAE) of ß-Carotene
The pumpkin samples were dispensed and put into 10 ml DESs. The sample amounts varied between 100.1 mg and 344.9 mg with an average of 214.3 mg. Similarly, the spinach samples were dispensed and put into 7-20 ml DESs. The sample amounts varied between 15.4 mg and 217.9 mg with an average of 61.4 mg. After putting samples into the DESs, the items were mixed with mechanical mixer and homogenized with homogenizer as defined in sections 2.2 and 2.3, and then analyzed in accordance with the method defined in section 2.4. The respective calibration lines (equations in
Table S2) were used to calculate the ß-Carotene extracted amounts with each DES. The results are tabulated in
Table 2 and
Table 3 for MMAE and HAE of ß-Carotene, respectively.
3.4. Modelling and Optimization Studies
Based on the obtained data, the second-order models for the MMAE and HAE of ß-Carotene have been derived and shown in
Table 4. Considering the fact that the R
2 values of these equations derived by the relevant design method are generally ≥0.7000 shows that the equations calculated for responses are satisfactory to provide explanation to the relationship between dependent and independent variables [
25].
The response surfaces formed by the equations provided in
Table 4 are shown in the three-dimensional (3D) graphs through
Figure 1,
Figure 2 and
Figure 3. Within these figures, effects of parameters and their corresponding interactions on the μg-ß-Carotene/gr-pumpkin and μg-ß-Carotene/gr-spinach are also displayed visually.
To determine the effects of each parameter, a suitable mathematical model, which fits the polynomial equation (2), was selected and statistical analysis of variance (ANOVA) was applied.
The ANOVA analyses results of the model equations derived for μg-ß-Carotene/gr-pumpkin results of MMAE and HAE of ß-Carotene from pumpkin samples by using DESs are given in
Table S3,
Table S4,
Table S8 and
Table S9. The ANOVA analyses results of the model equations derived for μg-ß-Carotene/gr-spinach results of MMAE and HAE of ß-Carotene from spinach samples by using DESs are given in
Table S5,
Table S6,
Table S7,
Table S10,
Table S11 and
Table S12. The high F values and P values <0.05 indicate that the model equations are suitable and statistically significant for the current experimental data sets.
When the Menthol:Acetic acid DESs considered in the MMAE of ß-Carotene from pumpkin samples, the most effective parameters (P<0.0001) were observed to be both acetic acid molar ratio and its quadratic power (
Table S3). The models were found to be not significant for MMAE and HAE of ß-Carotene from spinach samples via utilization Menthol:Acetic acid DESs (
Table S5 and S10). Even though these models were found not significant, the acetic acid molar ratio was the most effective parameter in the HAE of ß-Carotene from spinach (P<0.05). For the HAE of ß-Carotene from pumpkin samples via utilization Menthol:Acetic acid DESs, again the acetic acid molar ratio and its quadratic power were found to be the most effective parameters (P<0.0001), which were followed by homogenization speed and homogenization time (P<0.05). Additionally, the interaction effect between acetic acid molar ratio and homogenization speed was found to be significant (
Table S8).
When the Menthol:Propionic acid DESs considered in the MMAE of ß-Carotene from pumpkin samples, the most effective parameter was observed to be the second-order power of propionic acid molar ratio (P<0.0001), followed by propionic acid molar ratio and mixing time. Additionally, the interaction effect between propionic acid molar ratio and mixing time was also found to be significant in MMAE of ß-Carotene from pumpkin (
Table S4). When the Menthol:Propionic acid DESs considered in the MMAE of ß-Carotene from spinach samples, the most effective parameter was observed to be the propionic acid molar ratio (P<0.05), followed by its quadratic power. No interaction effect between parameters was observed (
Table S6). For the HAE of ß-Carotene from pumpkin samples via utilization Menthol:Propionic acid DESs, the homogenization time was found to be the most effective parameter (P<0.0001), which was followed by propionic acid molar ratio and its second-order power and homogenization speed (P<0.05). Additionally, the interaction effect between propionic acid molar ratio and homogenization speed was found to be significant (
Table S9). With regards to the HAE of ß-Carotene from spinach samples via utilization Menthol:Propionic acid DESs, the most effective parameter was observed to be the propionic acid molar ratio (P<0.0001), followed by its quadratic power (P<0.05). No interaction effect between parameters was observed (
Table S11).
The only effective parameter (P<0.05) was observed to be the mixing time, when the Menthol:Butyric acid DESs are taken into account in the MMAE of ß-Carotene from spinach samples. No interaction effect between parameters was observed (
Table S7). For the HAE of ß-Carotene from spinach samples via utilization Menthol:Butyric acid DESs, the butyric acid molar ratio was found to be the most effective parameter (P<0.05), which was followed by homogenization time and homogenization speed (P<0.05). Additionally, the interaction effect between homogenization time and homogenization speed was found to be significant (
Table S12).
3.5. Validation Study and Discussion
The optimum extraction conditions calculated using RSM approach and the highest μg-ß-Carotene/gr-pumpkin values observed under these conditions are presented in
Table 5. The best ß-Carotene extraction value from pumpkin was obtained via MMAE with Menthol:Propionic acid (1:2) DES. This was followed by Menthol:Propionic acid (1:2) DES for HAE process. The polarizability parameter (π*) provides a measure of solvent’s dipolarity and polarizability [
26]. The μg-ß-Carotene/gr-pumpkin extracted via Menthol:Acetic acid DESs both by MMAE and HAE were considerably low when compared to Menthol:Propionic acid DESs. The partition coefficient (logP) measures how hydrophilic or hydrophobic a chemical substance is. The higher the logP value, the more hydrophobic the compound. If logP>0, the the compound is generally considered as apolar and if logP<0 the compound is generally considered as polar. The logP values of acetic acid and propionic acid are -0.17 and 0.25 respectively [
27,
28]. This corresponds to the fact that acetic is not only more water soluble compound than propionic acid but also giving it more affinity to dissolve or be dissolved in polar substances due to having a logP<0 (solubility in water > solubility in oily substances/mixtures). The DESs produced by acetic acid, then, would have more polar structure when compared to DESs produced by propionic acid. Since ß-Caroten is a hydrocarbon and highly apolar (logP=14.764 [
29]), the extracted amount of ß-Caroten via Menthol:Propionic acid DESs was found considerable higher than Menthol:Acetic acid DESs.
The optimum extraction conditions calculated using RSM approach and the highest μg-ß-Carotene/gr-spinach values observed under these conditions are presented in
Table 6. With the HAE process; the best ß-Carotene value for extraction from spinach was obtained via HAE with Menthol:Propionic acid (1:4) DES. This was followed by Menthol:Acetic acid (1:2) DES and Menthol:Butyric acid (1:4) DES.
The polarizability parameter (π*) value found to be decreasing with the increase of the alkyl chain of the HBD [
26]. As a result; the increase of the non-polar part of the HBD decreases the overall polarizability of the DESs [
26]. In other words, the amount of ß-Carotene (an apolar compound) extracted is also expected to decrease with the increase of the alkyl chain of the HBD. Hence; the μg-ß-Carotene/gr-spinach extracted via Menthol:Propionic acid (1:4) DES was found relatively higher than extracted amount via Menthol:Butyric acid (1:4) DES. Even though acetic acid’s alkyl chain is smaller than propionic acid, the μg-ß-Carotene/gr-spinach extracted via Menthol:Acetic acid (1:2) DES was still observed to be less when compared to Menthol:Propionic acid (1:4) DES. This is, as explained above, considered to be due to the hydrophilic nature of acetic acid with logP<0 and hydrophobic nature of the extract, ß-Carotene. The optimum extraction conditions calculated using RSM approach and the highest μg-ß-Carotene/gr-spinach values observed under these conditions are presented in
Table 6. With the HAE process; the best ß-Carotene value for extraction from spinach was obtained via HAE with Menthol:Propionic acid (1:4) DES. This was followed by Menthol:Acetic acid (1:4) DES and Menthol:Butyric acid (1:4) DES.
4. Conclusions
Deep eutectic solvents (DESs) are still considered to be a relatively young chemical technology. They have a history of slightly over 20 years [
30]. Synthesizing DESs with natural and green solvents not only provides affordable solution for extraction but also it is an environmentally safe approach. In this research work, ß-Carotene was extracted from pumpkin and spinach via utilization of the synthesized DESs for the first time.
HBD molar ratio is the most important parameter that can affect the process in this method. This study has also emphasized the significance of that phenomenon. While determining the DES to be synthesized, the interaction of the HBA and HBD, the solubility of the ß-Carotene in the DESs and selecting HBA and HBD within natural compounds were taken into consideration. Being apolar and natural compound, L-Menthol, was selected as the HBA within the scope of this study due to it ability to form DESs that have high capacity to donate and accept protons when compared to common solvents [
26]. Acetic acid, propionic acid and butyric acid were selected as the HBD within the scope of this study due to having carboxylic acids which possess alkyl chains and being naturally found in food and plants.
For each type of DES and applied extraction method (MMAE and HAE), experimental design was applied using Stat-Ease 360® software (Central Composite Design via Response Surface Method). It has been observed that Menthol:Propionic Acid DESs, which has the smallest alkyl chain with having logP>0, has had the best effect on the extraction of ß-Carotene (11.528 μg-ß-Carotene/gr-pumpkin for MMAE, 8.966 μg-ß-Carotene/gr-pumpkin for HAE, 16.924 μg-ß-Carotene/gr-spinach for MMAE and 18.870 μg-ß-Carotene/gr-spinach for HAE) under the optimal conditions (HBD molar ratio: 0.6452, mixing time: 15 min. for MMAE of ß-Carotene from pumpkin, HBD molar ratio: 0.6198, homogenization time: 30.0 sec., homogenization speed: 7061 rpm for HAE of ß-Carotene from pumpkin, HBD molar ratio: 0.8000, mixing time: 60 min. for MMAE of ß-Carotene from spinach, HBD molar ratio: 0.8000, homogenization time: 120.0 sec., homogenization speed: 7000 rpm for HAE of ß-Carotene from spinach).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: pH and Density measurements of the DESs used in this study; Table S2: Equations and correlation coefficients of calibration lines for UV Spectrophotometer analysis of ß-Carotene dissolved in DESs used in this study. y=ß-Carotene concentration (ppm) and x=UV Absorbance reading at λ=450nm; Table S3: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-pumpkin results of MMAE of ß-Carotene from pumpkin samples by using M1ACA1, M1ACA2, M1ACA3 and M1ACA4; Table S4: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-pumpkin results of MMAE of ß-Carotene from pumpkin samples by using M1PRA1, M1PRA2, M1PRA3 and M1PRA4; Table S5: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-spinach results of MMAE of ß-Carotene from spinach samples by using M1ACA1, M1ACA2, M1ACA3 and M1ACA4; Table S6: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-spinach results of MMAE of ß-Carotene from spinach samples by using M1PRA1, M1PRA2, M1PRA3 and M1PRA4; Table S7: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-spinach results of MMAE of ß-Carotene from spinach samples by using M1BTA1, M1BTA2, M1BTA3 and M1BTA4; Table S8: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-pumpkin results of HAE of ß-Carotene from pumpkin samples by using M1ACA1, M1ACA2, M1ACA3 and M1ACA4; Table S9: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-pumpkin results of HAE of ß-Carotene from pumpkin samples by using M1PRA1, M1PRA2, M1PRA3 and M1PRA4; Table S10: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-spinach results of HAE of ß-Carotene from spinach samples by using M1ACA1, M1ACA2, M1ACA3 and M1ACA4; Table S11: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-spinach results of HAE of ß-Carotene from spinach samples by using M1PRA1, M1PRA2, M1PRA3 and M1PRA4; Table S12: The ANOVA evaluation of second-order model equation derived for μg-ß-Carotene/gr-spinach results of HAE of ß-Carotene from spinach samples by using M1BTA1, M1BTA2, M1BTA3 and M1BTA4.
Author Contributions
Conceptualization, K.T.; methodology, K.T. and S.Ş.S.; software, K.T. and İ.T.Y.; validation, K.T..; formal analysis, K.T.; investigation, K.T.; resources, K.T., M.B., S.Ş.S. and E.K.Ş.; data curation, K.T.; writing—original draft preparation, K.T.; writing—review and editing, K.T., M.B. and S.Ş.S.; visualization, K.T. and S.Ş.S.; supervision, K.T., M.B. and S.Ş.S.; project administration, K.T.; funding acquisition, K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Acknowledgments
The authors gratefully acknowledge BASF SE, Abdi İbrahim İlaç San. ve Tic A.Ş., World Medicine İlaç San. ve Tic. A.Ş. and Neutec İlaç San. Ve Tic A.Ş. companies for freely supplying some of the laboratory materials used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Şanal, İ.S.; Güvenç, A.; Salgın, U.; Mehmetoğlu, Ü.; Çalımlı, A. Recycling of apricot pomace by supercritical CO2 extraction. J. Supercrit. Fluids 2004, 32, 221–230. [Google Scholar] [CrossRef]
- Vega, P.J.; Balaban, M.O.; Sims, C.A.; O’Keefe, S.F.; Cornell, J.A. Supercritical carbon dioxide extraction efficiency for carotenes from carrots by RSM. J. Food Sci. 1996, 61, 757–759. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- α-Carotene, β-Carotene, β-Cryptoxanthin, Lycopene, Lutein, and Zeaxanthin. Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/carotenoids#introduction (accessed on 11 November 2024).
- Lee, S.C.; Ristaino, J.B.; Heitman, J. Parallels in intercellular communication in oomycete and fungal pathogens of plants and humans. PLOS Pathog. 2012, 8, e1003028. [Google Scholar] [CrossRef]
- The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Eng. J. Med 1994, 330, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Beson, J.; Curtin, K.; Ma, K.-N.; Schaffer, D.; Potter, J.D. Carotenoids and colorectal cancer. Am. J. Clin. Nutr. 2000, 71, 575–582. [Google Scholar] [CrossRef]
- Kirsh, V.A.; Hayes, R.B.; Mayne, S.T.; Chatterjee, N.; Subar, A.F.; Dixon, L.B.; Albanes, D.; Andriole, G.L.; Urban, D.A.; Peters, U. Supplemental and dietary vitamin E, ß-Carotene, and vitamin C intakes and prostate cancer risk. J. Natl. Cancer Inst. 2006, 98, 245. [Google Scholar] [CrossRef]
- Stahl, W.; Krutmann, J. Systemische photoprotektion druch karotinoide. Hautarzt 2006, 57, 281–285. [Google Scholar] [CrossRef]
- Blot, W.J.; Li, J.-Y.; Taylor, P.R.; Guo, W.; Dawsey, S.; Wang, G.-Q.; Yang, C.S.; Zheng, S.-F.; Gail, M.; Li, G.-Y.; et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and desease-specific mortality in the general population. J. Natl. Cancer Inst. 1993, 85, 1483–1993. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Craft, N.E.; Soares, J.H., Jr. Relative Solubility, Stability, and absorptivity of lutein and β-Carotene in organic solvents. J. Agric. Food. Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]
- Huff, J. Benzene-induced cancers: Abridged history and occupational health impact. Int. J. Occup. Environ. Health. 2007, 13, 213–221. [Google Scholar] [CrossRef] [PubMed]
- ICH guideline Q3C (R9) on impurities: Guideline for residual solvents (EMA/CHMP/ICH/82260/2006). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q3c-r9-guideline-impurities-guideline-residual-solvents-step-5_en.pdf, (accessed on 11th November 2024).
- United Nations Environment Programme. Food Waste Index Report 2024. Think Eat Save: Tracking Progress to Halve Global Food Waste; Knowledge Repository - UNEP. UNEP.: Nairobi, Kenya, 2024; p. 12. [Google Scholar]
- Oh my gourd! 1.3B pounds of pumpkins reach landfills every year. Available online: https://www.wastedive.com/news/oh-my-gourd-13b-pounds-of-pumpkins-reach-landfills-every-year/408341/, (accessed on 8th November 2024).
- Lin, H.; Black, M.J.; Walsh, L.; Giordano, F.S.; Borrion, A. Life cycle assessment of baby leaf spinach: Reduction of waste through interventions in growing treatments and packaging. J. Clean. Prod. 2024, 449, 141723. [Google Scholar] [CrossRef]
- Frankowska, A.; Jeswani, H.K.; Azapagic, A. Environmental impacts of vegetables consumption in the UK, Science of the Total Environment. Sci. Total Environ. 2019, 682, 80–105. [Google Scholar] [CrossRef]
- Sebdani, M.M.; Abbasi, H. Green extraction of carotenoids from pumpkin with ultrasound-assisted method; optimization using response surface methodology. Microchem. J. 2023, 193, 109092. [Google Scholar] [CrossRef]
- Stupar, A.; Šeregelj, V.; Ribeiro, B.D.; Pezo, L.; Cvetanović, A.; Mišan, A.; Marrucho, I. Recovery of β-carotene from pumpkin using switchable natural deep eutectic solvents. Ultrason. Sonochem. 2021, 76, 105638. [Google Scholar] [CrossRef]
- Shi, X.; Wu, H.; Shi, J.; Xue, S.J.; Wang, D.; Wang, W.; Cheng, A.; Gong, Z.; Chen, X.; Wang, C. Effect of modifier on the composition and antioxidant activity of carotenoid extracts from pumpkin (Cucurbita maxima) by supercritical CO2. LWT 2013, 51, 433–440. [Google Scholar] [CrossRef]
- The United States Pharmacopoeia Beta Carotene monograph. Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/beta_carotene.pdf (accessed on 12th November 2024).
- Moradi, M.; Fazlzadehdavil, M.; Pirsaheb, M.; Mansouri, Y.; Khosravi, T.; Sharafi, K. Response surface methodology (RSM) and its application for optimization of ammonium ions removal from aqueous solutions by pumice as a natural and low cost adsorbent. Arch. Environ. Prot. 2016, 42, 33–43. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Bhat, B.; Vaid, S.; Habib, B.; Bajaj, B.K. Design of experiments for enhanced production of bioactive exopolysaccharides from indigenous probiotic lactic acid bacteria. Indian J. Biochem. Biophys. 2020, 57, 539–551. [Google Scholar]
- Florindo, C.; McIntosh, A.J.S.; Welton, T.; Branco, L.C.; Marrucho, I.M. A closer look into deep eutectic solvents: Exploring intermolecular interactions using solvatochromic probes. Phys. Chem. Chem. Phys. 2018, 20, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Acetic acid (glacial) 100% SDS. Available online: https://www.merckmillipore.com/DE/en/product/msds/MDA_CHEM-100056 (accessed on 13th November 2024).
- Propionic acid for synthesis SDS. Available online: https://www.merckmillipore.com/DE/en/product/msds/MDA_CHEM-800605?Origin=PDP (accessed on 13th November 2024).
- Kwan, Y.H.; Tung, Y.K.; Kochhar, J.S.; Li, H.; Poh, A.L.; Kang, L. 6-Esstial Monographs. In Handbook of Cosmeceutical Excipients and their Safeties, 1st ed.; Woodhead Publishing: Kidlington, UK, 2014; p. 105. [Google Scholar]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2023, 70–71. [Google Scholar]
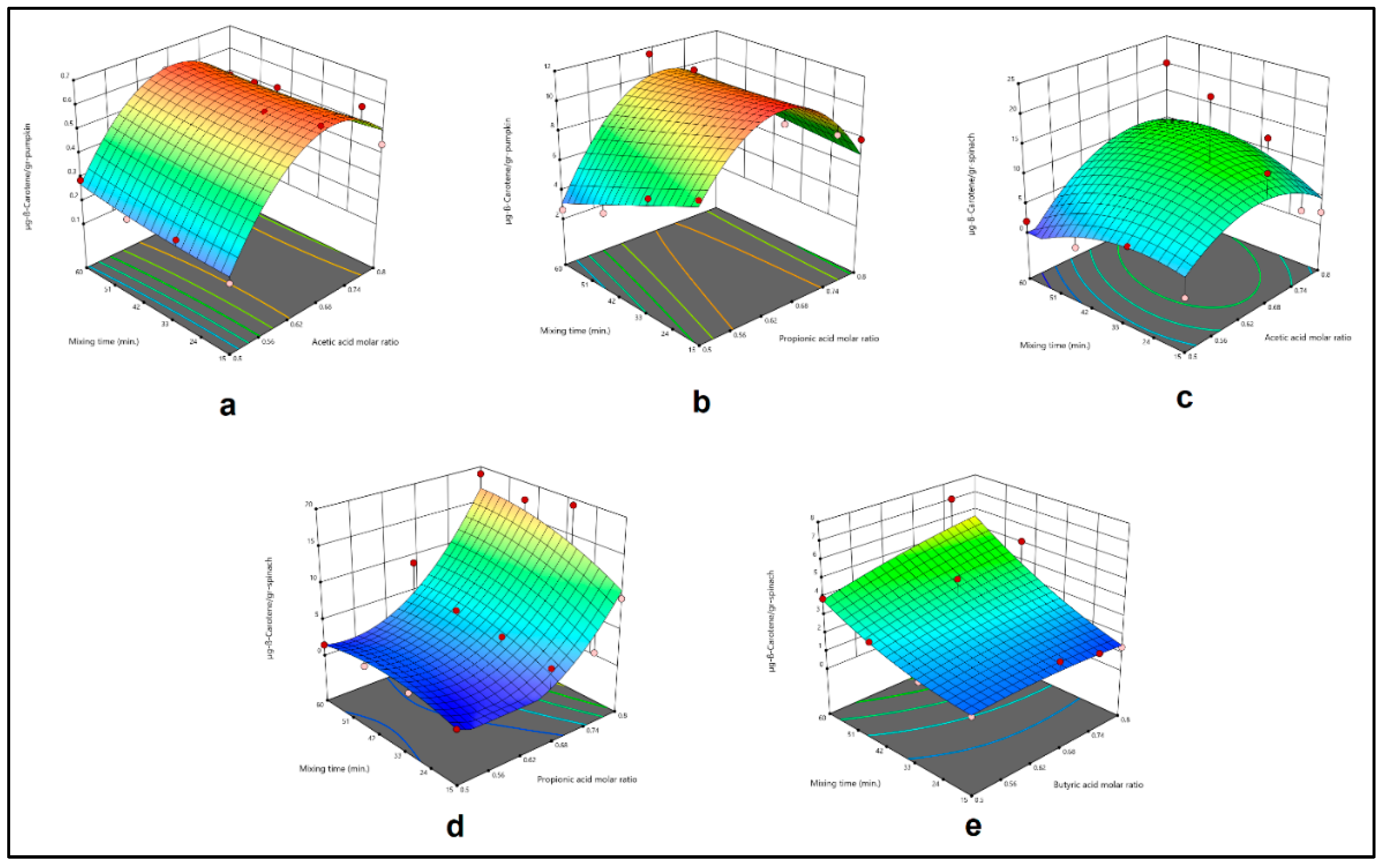
Figure 1.
Effect of acetic acid molar ratio and mixing time (a), propionic acid molar ratio and mixing time (b) on β-Carotene extract obtained by MMAE from pumpkin, and effect of acetic acid molar ratio and mixing time (c), propionic acid molar ratio and mixing time (d), butyric acid molar ratio and mixing time (e) on β-Carotene extract obtained by MMAE from spinach.
Figure 1.
Effect of acetic acid molar ratio and mixing time (a), propionic acid molar ratio and mixing time (b) on β-Carotene extract obtained by MMAE from pumpkin, and effect of acetic acid molar ratio and mixing time (c), propionic acid molar ratio and mixing time (d), butyric acid molar ratio and mixing time (e) on β-Carotene extract obtained by MMAE from spinach.
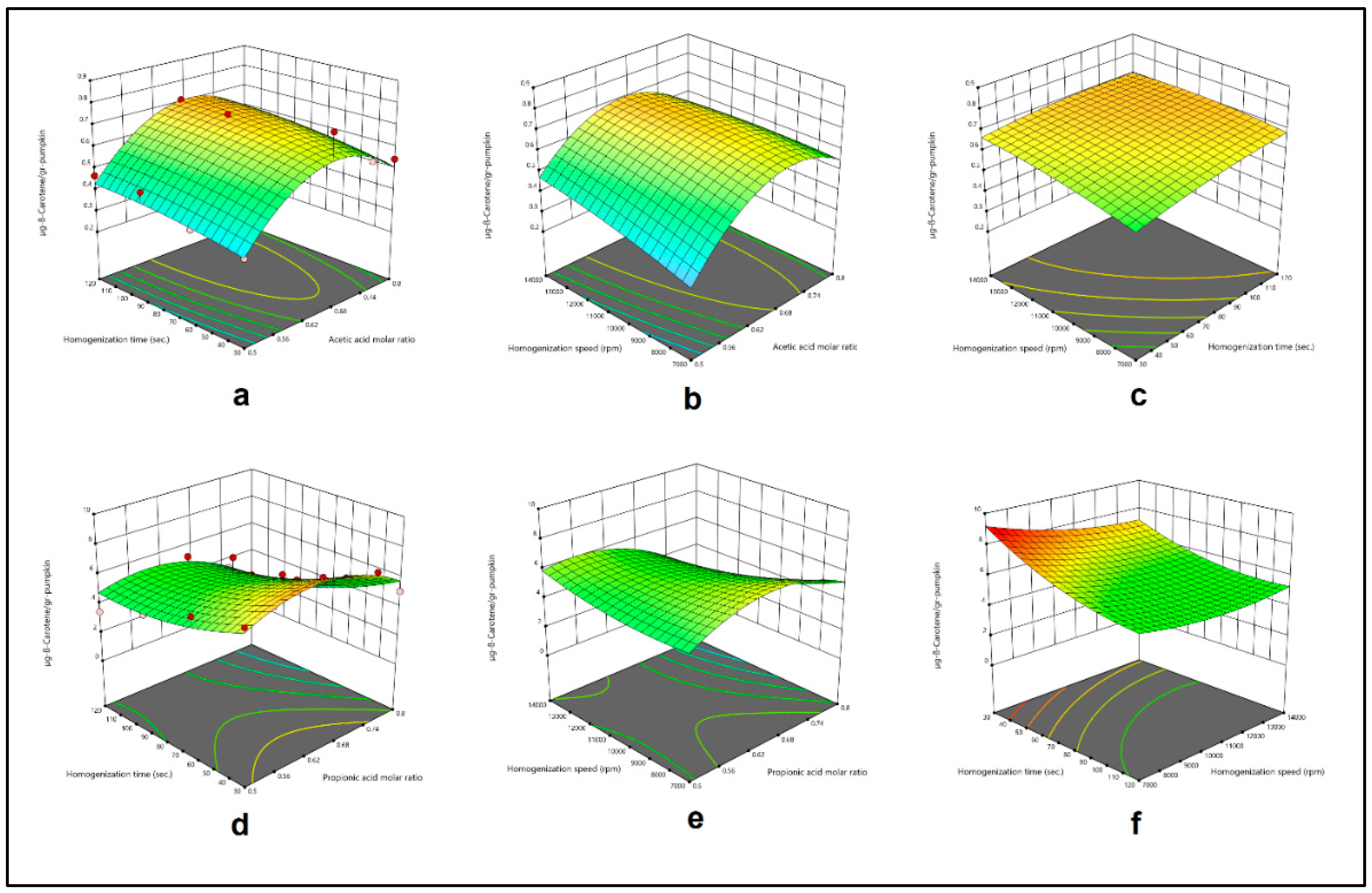
Figure 2.
Effect of acetic acid molar ratio and homogenization time (a), acetic acid molar ratio and homogenization speed (b), homogenization time and homogenization speed (c), and effect of propionic acid molar ratio and homogenization time (d), propionic acid molar ratio and homogenization speed (e), homogenization time and homogenization speed (f) on β-Carotene extract obtained by HAE from pumpkin.
Figure 2.
Effect of acetic acid molar ratio and homogenization time (a), acetic acid molar ratio and homogenization speed (b), homogenization time and homogenization speed (c), and effect of propionic acid molar ratio and homogenization time (d), propionic acid molar ratio and homogenization speed (e), homogenization time and homogenization speed (f) on β-Carotene extract obtained by HAE from pumpkin.
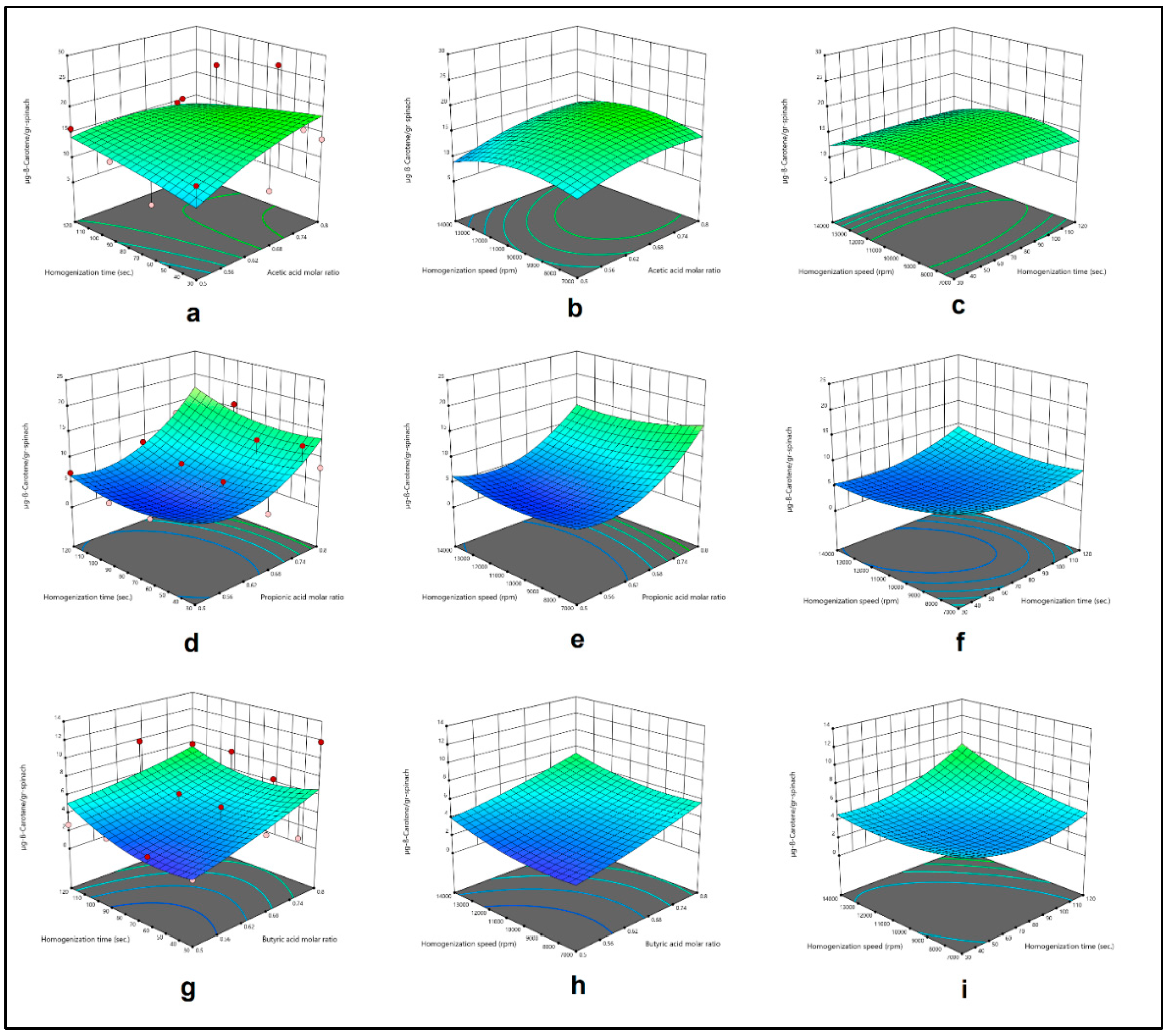
Figure 3.
Effect of acetic acid molar ratio and homogenization time (a), acetic acid molar ratio and homogenization speed (b), homogenization time and homogenization speed (c), and effect of propionic acid molar ratio and homogenization time (d), propionic acid molar ratio and homogenization speed (e), homogenization time and homogenization speed (f), effect of butyric acid molar ratio and homogenization time (g), butyric acid molar ratio and homogenization speed (h), homogenization time and homogenization speed (i), on β-Carotene extract obtained by HAE from spinach.
Figure 3.
Effect of acetic acid molar ratio and homogenization time (a), acetic acid molar ratio and homogenization speed (b), homogenization time and homogenization speed (c), and effect of propionic acid molar ratio and homogenization time (d), propionic acid molar ratio and homogenization speed (e), homogenization time and homogenization speed (f), effect of butyric acid molar ratio and homogenization time (g), butyric acid molar ratio and homogenization speed (h), homogenization time and homogenization speed (i), on β-Carotene extract obtained by HAE from spinach.
Table 1.
HBA, HBD, molar ratios and the abbreviations of DESs used in this study.
Table 1.
HBA, HBD, molar ratios and the abbreviations of DESs used in this study.
| Hydrogen bond acceptor (HBA) |
Hydrogen bond donor (HBD) |
Molar Ratio
(HBA:HBD) |
Abbreviations |
| L-Menthol |
Acetic acid |
1:1 |
M1ACA1 |
| L-Menthol |
Acetic acid |
1:2 |
M1ACA2 |
| L-Menthol |
Acetic acid |
1:3 |
M1ACA3 |
| L-Menthol |
Acetic acid |
1:4 |
M1ACA4 |
| L-Menthol |
Propionic acid |
1:1 |
M1PRA1 |
| L-Menthol |
Propionic acid |
1:2 |
M1PRA2 |
| L-Menthol |
Propionic acid |
1:3 |
M1PRA3 |
| L-Menthol |
Propionic acid |
1:4 |
M1PRA4 |
| L-Menthol |
Butyric acid |
1:1 |
M1BTA1 |
| L-Menthol |
Butyric acid |
1:2 |
M1BTA2 |
| L-Menthol |
Butyric acid |
1:3 |
M1BTA3 |
| L-Menthol |
Butyric acid |
1:4 |
M1BTA4 |
Table 2.
The experimental results of ß-Carotene extraction from pumpkin and spinach samples by using DESs with mechanical mixing assistance.
Table 2.
The experimental results of ß-Carotene extraction from pumpkin and spinach samples by using DESs with mechanical mixing assistance.
| DES |
Mixing Time (min.) |
μg-β-Carotene
/gr-Pumpkin 1
|
μg-β-Carotene
/gr-Spinach 1
|
| M1ACA1 |
15 |
0.196 ± 0.043 |
0.714 ± 0.025 |
| M1ACA1 |
30 |
0.254 ± 0.011 |
5.093 ± 0.032 |
| M1ACA1 |
45 |
0.229 ± 0.012 |
1.083 ± 0.016 |
| M1ACA1 |
60 |
0.290 ± 0.026 |
1.959 ± 0.031 |
| M1ACA2 |
15 |
0.637 ± 0.011 |
13.867 ± 0.069 |
| M1ACA2 |
30 |
0.603 ± 0.009 |
23.238 ± 0.075 |
| M1ACA2 |
45 |
0.566 ± 0.006 |
9.348 ± 0.108 |
| M1ACA2 |
60 |
0.610 ± 0.004 |
6.186 ± 0.026 |
| M1ACA3 |
15 |
0.639 ± 0.020 |
4.447 ± 0.029 |
| M1ACA3 |
30 |
0.558 ± 0.012 |
10.994 ± 0.029 |
| M1ACA3 |
45 |
0.572 ± 0.010 |
6.863 ± 0.040 |
| M1ACA3 |
60 |
0.561 ± 0.003 |
5.058 ± 0.028 |
| M1ACA4 |
15 |
0.442 ± 0.008 |
2.202 ± 0.039 |
| M1ACA4 |
30 |
0.483 ± 0.010 |
12.089 ± 0.035 |
| M1ACA4 |
45 |
0.510 ± 0.007 |
7.820 ± 0.069 |
| M1ACA4 |
60 |
0.486 ± 0.009 |
20.168 ± 0.044 |
| M1PRA1 |
15 |
7.873 ± 0.023 |
0.866 ± 0.019 |
| M1PRA1 |
30 |
6.452 ± 0.040 |
1.803 ± 0.009 |
| M1PRA1 |
45 |
3.960 ± 0.019 |
1.869 ± 0.009 |
| M1PRA1 |
60 |
2.631 ± 0.014 |
1.476 ± 0.005 |
| M1PRA2 |
15 |
10.816 ± 0.044 |
3.567 ± 0.020 |
| M1PRA2 |
30 |
10.585 ± 0.045 |
4.508 ± 0.026 |
| M1PRA2 |
45 |
11.542 ± 0.034 |
5.044 ± 0.027 |
| M1PRA2 |
60 |
11.566 ± 0.044 |
8.986 ± 0.033 |
| M1PRA3 |
15 |
8.796 ± 0.051 |
3.256 ± 0.011 |
| M1PRA3 |
30 |
8.234 ± 0.042 |
5.889 ± 0.019 |
| M1PRA3 |
45 |
9.264 ± 0.023 |
6.944 ± 0.017 |
| M1PRA3 |
60 |
8.939 ± 0.072 |
5.915 ± 0.023 |
| M1PRA4 |
15 |
7.917 ± 0.041 |
9.312 ± 0.049 |
| M1PRA4 |
30 |
8.063 ± 0.031 |
19.156 ± 0.122 |
| M1PRA4 |
45 |
7.757 ± 0.047 |
17.580 ± 0.043 |
| M1PRA4 |
60 |
7.465 ± 0.043 |
18.990 ± 0.057 |
| M1BTA1 |
15 |
|
1.552 ± 0.045 |
| M1BTA1 |
30 |
|
1.944 ± 0.039 |
| M1BTA1 |
45 |
|
2.774 ± 0.031 |
| M1BTA1 |
60 |
|
3.925 ± 0.024 |
| M1BTA2 |
15 |
|
2.193 ± 0.040 |
| M1BTA2 |
30 |
|
1.718 ± 0.044 |
| M1BTA2 |
45 |
|
4.321 ± 0.036 |
| M1BTA2 |
60 |
|
4.142 ± 0.029 |
| M1BTA3 |
15 |
|
1.593 ± 0.032 |
| M1BTA3 |
30 |
|
2.280 ± 0.026 |
| M1BTA3 |
45 |
|
2.720 ± 0.021 |
| M1BTA3 |
60 |
|
7.036 ± 0.032 |
| M1BTA4 |
15 |
|
1.319 ± 0.024 |
| M1BTA4 |
30 |
|
2.226 ± 0.028 |
| M1BTA4 |
45 |
|
5.109 ± 0.029 |
| M1BTA4 |
60 |
|
4.468 ± 0.017 |
Table 3.
The experimental results of ß-Carotene extraction from pumpkin and spinach samples by using DESs with homogenization assistance.
Table 3.
The experimental results of ß-Carotene extraction from pumpkin and spinach samples by using DESs with homogenization assistance.
| DES |
Homogenization Time (sec.) |
Homogenization Speed (rpm) |
μg-β-Carotene/gr-Pumpkin 1
|
μg-β-Carotene/gr-Spinach 1
|
| M1ACA1 |
30 |
7000 |
0.287 ± 0.002 |
8.830 ± 0.027 |
| M1ACA1 |
60 |
7000 |
0.295 ± 0.002 |
13.459 ± 0.084 |
| M1ACA1 |
90 |
7000 |
0.241 ± 0.005 |
12.948 ± 0.056 |
| M1ACA1 |
120 |
7000 |
0.291 ± 0.003 |
11.703 ± 0.012 |
| M1ACA1 |
30 |
10500 |
0.359 ± 0.003 |
14.116 ± 0.054 |
| M1ACA1 |
60 |
10500 |
0.390 ± 0.002 |
7.225 ± 0.021 |
| M1ACA1 |
90 |
10500 |
0.471 ± 0.005 |
12.260 ± 0.047 |
| M1ACA1 |
120 |
10500 |
0.467 ± 0.004 |
15.822 ± 0.044 |
| M1ACA1 |
30 |
14000 |
0.464 ± 0.002 |
7.731 ± 0.078 |
| M1ACA1 |
60 |
14000 |
0.449 ± 0.002 |
7.511 ± 0.070 |
| M1ACA1 |
90 |
14000 |
0.441 ± 0.003 |
7.239 ± 0.020 |
| M1ACA1 |
120 |
14000 |
0.506 ± 0.002 |
9.139 ± 0.106 |
| M1ACA2 |
30 |
7000 |
0.553 ± 0.039 |
19.093 ± 0.074 |
| M1ACA2 |
60 |
7000 |
0.666 ± 0.019 |
13.158 ± 0.067 |
| M1ACA2 |
90 |
7000 |
0.637 ± 0.028 |
9.728 ± 0.056 |
| M1ACA2 |
120 |
7000 |
0.811 ± 0.032 |
12.469 ± 0.056 |
| M1ACA2 |
30 |
10500 |
0.762 ± 0.044 |
7.622 ± 0.014 |
| M1ACA2 |
60 |
10500 |
0.652 ± 0.041 |
15.598 ± 0.053 |
| M1ACA2 |
90 |
10500 |
0.707 ± 0.008 |
20.129 ± 0.041 |
| M1ACA2 |
120 |
10500 |
0.711 ± 0.002 |
14.714 ± 0.029 |
| M1ACA2 |
30 |
14000 |
0.562 ± 0.038 |
18.474 ± 0.017 |
| M1ACA2 |
60 |
14000 |
0.632 ± 0.020 |
9.334 ± 0.016 |
| M1ACA2 |
90 |
14000 |
0.771 ± 0.006 |
11.925 ± 0.081 |
| M1ACA2 |
120 |
14000 |
0.670 ± 0.006 |
17.460 ± 0.107 |
| M1ACA3 |
30 |
7000 |
0.434 ± 0.003 |
6.767 ± 0.027 |
| M1ACA3 |
60 |
7000 |
0.639 ± 0.005 |
8.553 ± 0.031 |
| M1ACA3 |
90 |
7000 |
0.651 ± 0.054 |
16.257 ± 0.048 |
| M1ACA3 |
120 |
7000 |
0.640 ± 0.000 |
15.457 ± 0.098 |
| M1ACA3 |
30 |
10500 |
0.571 ± 0.002 |
16.982 ± 0.024 |
| M1ACA3 |
60 |
10500 |
0.563 ± 0.010 |
15.152 ± 0.015 |
| M1ACA3 |
90 |
10500 |
0.645 ± 0.003 |
24.930 ± 0.057 |
| M1ACA3 |
120 |
10500 |
0.588 ± 0.002 |
14.775 ± 0.042 |
| M1ACA3 |
30 |
14000 |
0.606 ± 0.018 |
13.767 ± 0.053 |
| M1ACA3 |
60 |
14000 |
0.611 ± 0.002 |
11.293 ± 0.028 |
| M1ACA3 |
90 |
14000 |
0.689 ± 0.000 |
15.814 ± 0.039 |
| M1ACA3 |
120 |
14000 |
0.644 ± 0.000 |
13.564 ± 0.027 |
| M1ACA4 |
30 |
7000 |
0.449 ± 0.012 |
22.831 ± 0.056 |
| M1ACA4 |
60 |
7000 |
0.548 ± 0.020 |
20.749 ± 0.062 |
| M1ACA4 |
90 |
7000 |
0.629 ± 0.029 |
12.540 ± 0.014 |
| M1ACA4 |
120 |
7000 |
0.579 ± 0.005 |
7.834 ± 0.041 |
| M1ACA4 |
30 |
10500 |
0.545 ± 0.004 |
13.751 ± 0.063 |
| M1ACA4 |
60 |
10500 |
0.534 ± 0.008 |
26.006 ± 0.092 |
| M1ACA4 |
90 |
10500 |
0.572 ± 0.005 |
11.626 ± 0.014 |
| M1ACA4 |
120 |
10500 |
0.554 ± 0.011 |
9.568 ± 0.038 |
| M1ACA4 |
30 |
14000 |
0.581 ± 0.003 |
18.883 ± 0.055 |
| M1ACA4 |
60 |
14000 |
0.525 ± 0.017 |
14.477 ± 0.080 |
| M1ACA4 |
90 |
14000 |
0.581 ± 0.050 |
7.578 ± 0.053 |
| M1ACA4 |
120 |
14000 |
0.543 ± 0.006 |
7.993 ± 0.069 |
| M1PRA1 |
30 |
7000 |
7.675 ± 0.030 |
6.081 ± 0.039 |
| M1PRA1 |
60 |
7000 |
5.030 ± 0.023 |
7.450 ± 0.020 |
| M1PRA1 |
90 |
7000 |
3.769 ± 0.013 |
5.234 ± 0.014 |
| M1PRA1 |
120 |
7000 |
4.698 ± 0.023 |
8.447 ± 0.034 |
| M1PRA1 |
30 |
10500 |
6.896 ± 0.013 |
11.252 ± 0.027 |
| M1PRA1 |
60 |
10500 |
6.088 ± 0.021 |
4.241 ± 0.019 |
| M1PRA1 |
90 |
10500 |
4.712 ± 0.019 |
4.010 ± 0.019 |
| M1PRA1 |
120 |
10500 |
3.456 ± 0.014 |
6.955 ± 0.013 |
| M1PRA1 |
30 |
14000 |
6.120 ± 0.024 |
8.539 ± 0.020 |
| M1PRA1 |
60 |
14000 |
7.147 ± 0.013 |
3.704 ± 0.020 |
| M1PRA1 |
90 |
14000 |
6.487 ± 0.023 |
6.080 ± 0.012 |
| M1PRA1 |
120 |
14000 |
5.340 ± 0.020 |
8.139 ± 0.028 |
| M1PRA2 |
30 |
7000 |
8.762 ± 0.029 |
4.731 ± 0.082 |
| M1PRA2 |
60 |
7000 |
6.586 ± 0.037 |
5.323 ± 0.013 |
| M1PRA2 |
90 |
7000 |
8.579 ± 0.043 |
6.669 ± 0.021 |
| M1PRA2 |
120 |
7000 |
6.472 ± 0.039 |
11.232 ± 0.013 |
| M1PRA2 |
30 |
10500 |
7.316 ± 0.045 |
2.958 ± 0.033 |
| M1PRA2 |
60 |
10500 |
6.561 ± 0.038 |
6.132 ± 0.011 |
| M1PRA2 |
90 |
10500 |
6.411 ± 0.043 |
6.950 ± 0.058 |
| M1PRA2 |
120 |
10500 |
5.201 ± 0.032 |
8.706 ± 0.013 |
| M1PRA2 |
30 |
14000 |
6.935 ± 0.040 |
8.774 ± 0.032 |
| M1PRA2 |
60 |
14000 |
5.880 ± 0.031 |
10.494 ± 0.053 |
| M1PRA2 |
90 |
14000 |
4.935 ± 0.039 |
4.587 ± 0.048 |
| M1PRA2 |
120 |
14000 |
6.839 ± 0.017 |
7.195 ± 0.035 |
| M1PRA3 |
30 |
7000 |
8.596 ± 0.021 |
14.763 ± 0.034 |
| M1PRA3 |
60 |
7000 |
5.541 ± 0.045 |
10.024 ± 0.044 |
| M1PRA3 |
90 |
7000 |
3.802 ± 0.043 |
17.005 ± 0.031 |
| M1PRA3 |
120 |
7000 |
4.888 ± 0.039 |
14.631 ± 0.048 |
| M1PRA3 |
30 |
10500 |
6.981 ± 0.041 |
13.676 ± 0.052 |
| M1PRA3 |
60 |
10500 |
5.321 ± 0.039 |
12.177 ± 0.034 |
| M1PRA3 |
90 |
10500 |
1.803 ± 0.033 |
9.143 ± 0.035 |
| M1PRA3 |
120 |
10500 |
3.367 ± 0.039 |
12.853 ± 0.035 |
| M1PRA3 |
30 |
14000 |
5.253 ± 0.041 |
10.178 ± 0.065 |
| M1PRA3 |
60 |
14000 |
2.010 ± 0.032 |
5.838 ± 0.052 |
| M1PRA3 |
90 |
14000 |
0.631 ± 0.038 |
11.197 ± 0.045 |
| M1PRA3 |
120 |
14000 |
3.596 ± 0.043 |
13.982 ± 0.049 |
| M1PRA4 |
30 |
7000 |
8.898 ± 0.031 |
24.598 ± 0.126 |
| M1PRA4 |
60 |
7000 |
6.158 ± 0.027 |
19.838 ± 0.113 |
| M1PRA4 |
90 |
7000 |
7.714 ± 0.031 |
15.597 ± 0.069 |
| M1PRA4 |
120 |
7000 |
1.773 ± 0.045 |
12.177 ± 0.118 |
| M1PRA4 |
30 |
10500 |
5.064 ± 0.028 |
7.972 ± 0.091 |
| M1PRA4 |
60 |
10500 |
4.638 ± 0.037 |
10.282 ± 0.065 |
| M1PRA4 |
90 |
10500 |
3.145 ± 0.030 |
15.900 ± 0.056 |
| M1PRA4 |
120 |
10500 |
2.184 ± 0.025 |
16.399 ± 0.057 |
| M1PRA4 |
30 |
14000 |
5.653 ± 0.026 |
8.387 ± 0.078 |
| M1PRA4 |
60 |
14000 |
3.880 ± 0.034 |
14.157 ± 0.115 |
| M1PRA4 |
90 |
14000 |
2.029 ± 0.040 |
13.669 ± 0.100 |
| M1PRA4 |
120 |
14000 |
1.318 ± 0.025 |
24.910 ± 0.071 |
| M1BTA1 |
30 |
7000 |
|
2.563 ± 0.033 |
| M1BTA1 |
60 |
7000 |
|
3.240 ± 0.032 |
| M1BTA1 |
90 |
7000 |
|
3.129 ± 0.033 |
| M1BTA1 |
120 |
7000 |
|
4.082 ± 0.035 |
| M1BTA1 |
30 |
10500 |
|
2.244 ± 0.023 |
| M1BTA1 |
60 |
10500 |
|
2.768 ± 0.032 |
| M1BTA1 |
90 |
10500 |
|
2.889 ± 0.039 |
| M1BTA1 |
120 |
10500 |
|
2.701 ± 0.021 |
| M1BTA1 |
30 |
14000 |
|
2.979 ± 0.022 |
| M1BTA1 |
60 |
14000 |
|
4.285 ± 0.029 |
| M1BTA1 |
90 |
14000 |
|
3.862 ± 0.050 |
| M1BTA1 |
120 |
14000 |
|
6.460 ± 0.038 |
| M1BTA2 |
30 |
7000 |
|
4.171 ± 0.046 |
| M1BTA2 |
60 |
7000 |
|
4.609 ± 0.038 |
| M1BTA2 |
90 |
7000 |
|
4.188 ± 0.027 |
| M1BTA2 |
120 |
7000 |
|
6.461 ± 0.026 |
| M1BTA2 |
30 |
10500 |
|
3.844 ± 0.042 |
| M1BTA2 |
60 |
10500 |
|
5.263 ± 0.042 |
| M1BTA2 |
90 |
10500 |
|
5.191 ± 0.031 |
| M1BTA2 |
120 |
10500 |
|
9.820 ± 0.077 |
| M1BTA2 |
30 |
14000 |
|
5.457 ± 0.045 |
| M1BTA2 |
60 |
14000 |
|
7.097 ± 0.050 |
| M1BTA2 |
90 |
14000 |
|
6.782 ± 0.034 |
| M1BTA2 |
120 |
14000 |
|
13.221 ± 0.044 |
| M1BTA3 |
30 |
7000 |
|
5.708 ± 0.045 |
| M1BTA3 |
60 |
7000 |
|
4.283 ± 0.020 |
| M1BTA3 |
90 |
7000 |
|
3.736 ± 0.032 |
| M1BTA3 |
120 |
7000 |
|
3.884 ± 0.020 |
| M1BTA3 |
30 |
10500 |
|
2.020 ± 0.038 |
| M1BTA3 |
60 |
10500 |
|
2.917 ± 0.021 |
| M1BTA3 |
90 |
10500 |
|
3.239 ± 0.030 |
| M1BTA3 |
120 |
10500 |
|
3.332 ± 0.027 |
| M1BTA3 |
30 |
14000 |
|
5.543 ± 0.037 |
| M1BTA3 |
60 |
14000 |
|
4.595 ± 0.029 |
| M1BTA3 |
90 |
14000 |
|
2.968 ± 0.033 |
| M1BTA3 |
120 |
14000 |
|
8.684 ± 0.027 |
| M1BTA4 |
30 |
7000 |
|
8.584 ± 0.028 |
| M1BTA4 |
60 |
7000 |
|
6.231 ± 0.037 |
| M1BTA4 |
90 |
7000 |
|
9.738 ± 0.049 |
| M1BTA4 |
120 |
7000 |
|
4.637 ± 0.037 |
| M1BTA4 |
30 |
10500 |
|
11.840 ± 0.043 |
| M1BTA4 |
60 |
10500 |
|
6.343 ± 0.024 |
| M1BTA4 |
90 |
10500 |
|
8.197 ± 0.039 |
| M1BTA4 |
120 |
10500 |
|
7.814 ± 0.046 |
| M1BTA4 |
30 |
14000 |
|
8.211 ± 0.025 |
| M1BTA4 |
60 |
14000 |
|
5.023 ± 0.033 |
| M1BTA4 |
90 |
14000 |
|
7.401 ± 0.046 |
| M1BTA4 |
120 |
14000 |
|
12.534 ± 0.023 |
Table 4.
Model equations and their compatibility indicators derived from CCD through RSM in MMAE and HAE of ß-Carotene from pumpkin and spinach.
Table 4.
Model equations and their compatibility indicators derived from CCD through RSM in MMAE and HAE of ß-Carotene from pumpkin and spinach.
| Extraction setup |
Independent Variables |
Dependent Variable |
Model Equation |
R2
|
Sample: Pumkin
Method: MMAE &
Menthol:Acetic acid DESs |
X1:Acetic acid molar ratio
X2:Mixing time (min.) |
μg-β-Carotene/
gr-Pumpkin |
-4.45150+14.57986X1+0.001912X2-0.005262X1X2
-10.44080X12+0.000024X22
|
0.9642 |
Sample: Pumkin
Method: MMAE &
Menthol:Propionic acid DESs |
X1:Propionic acid molar ratio
X2:Mixing time (min.) |
μg-β-Carotene/
gr-Pumpkin |
-63.76949+241.35123X1-0.301289X2+0.401802X1X2
-191.69872X12+0.000041X22
|
0.9006 |
Sample: Spinach
Method: MMAE &
Menthol:Acetic acid DESs |
X1:Acetic acid molar ratio
X2:Mixing time (min.) |
μg-β-Carotene/
gr-Spinach |
-87.29436+291.71772X1-0.080113X2+0.815406X1X2
-232.20477X12-0.006091X22
|
0.3941 |
Sample: Spinach
Method: MMAE &
Menthol:Propionic acid DESs |
X1:Propionic acid molar ratio
X2:Mixing time (min.) |
μg-β-Carotene/
gr-Spinach |
81.41602-286.71718X1+0.011693X2
+0.437977X1X2+239.97280 X12-0.002896X22
|
0.7835 |
Sample: Spinach
Method: MMAE &
Menthol:Butyric acid DESs |
X1:Butyric acid molar ratio
X2:Mixing time (min.) |
μg-β-Carotene/
gr-Spinach |
0.019209+7.15429X1-0.089691X2+0.147515X1X2
-7.71617X12+0.000871X22
|
0.7688 |
Sample: Pumkin
Method: HAE &
Menthol:Acetic acid DESs |
X1:Acetic acid molar ratio
X2:Homogenization time (sec.)
X3:Homogenization speed (rpm) |
μg-β-Carotene/
gr-Pumpkin |
-4.30138+12.54252X1+0.003089X2+0.000103X3
+0.000543X1X2-0.000081X1X3-1.29643*10-7X2X3
-8.63510X12-8.37963*10-6X22-1.42092*10-9X32
|
0.8418
|
Sample: Pumkin
Method: HAE &
Menthol:Propionic acid DESs |
X1:Propionic acid molar ratio
X2:Homogenization time (sec.)
X3:Homogenization speed (rpm) |
μg-β-Carotene/
gr-Pumpkin |
-24.31624+109.68892X1-0.063963X2+0.000120X3
-0.068021X1X2-0.001957X1X3+2.65619*10-6X2X3
-69.07260X12+0.000334X22+3.75153*10-8X32
|
0.7052
|
Sample: Spinach
Method: HAE &
Menthol:Acetic acid DESs |
X1:Acetic acid molar ratio
X2:Homogenization time (sec.)
X3:Homogenization speed (rpm) |
μg-β-Carotene/
gr-Spinach |
-46.56104+104.57014X1+0.229675X2+0.003205X3
-0.333622X1X2+0.000977X1X3+2.93333*10-7X2X3
-59.45170X12-0.000142X22-1.93862*10-7X32
|
0.2725
|
Sample: Spinach
Method: HAE &
Menthol:Propionic acid DESs |
X1:Propionic acid molar ratio
X2:Homogenization time (sec.)
X3:Homogenization speed (rpm) |
μg-β-Carotene/
gr-Spinach |
88.81933-218.95677X1-0.304985X2-0.002093X3
+0.146208X1X2-0.001599X1X3+7.80619*10-6X2X3
+195.82812X12+0.000968X22+1.13311*10-7X32
|
0.6540
|
Sample: Spinach
Method: HAE &
Menthol:Butyric acid DESs |
X1:Butyric acid molar ratio
X2:Homogenization time (sec.)
X3:Homogenization speed (rpm) |
μg-β-Carotene/
gr-Spinach |
9.20900+1.75614X1-0.106600X2-0.001464X3
-0.073053X1X2-0.000142X1X3+7.09500*10-6X2X3
+12.70142X12+0.000667X22+5.99541*10-8X32
|
0.4718 |
Table 5.
Optimum ß-Carotene extraction conditions and maximum response values (predicted vs. actual*) obtained under these conditions calculated by CCD for DESs in MMAE and HAE of ß-Carotene from pumpkin samples.
Table 5.
Optimum ß-Carotene extraction conditions and maximum response values (predicted vs. actual*) obtained under these conditions calculated by CCD for DESs in MMAE and HAE of ß-Carotene from pumpkin samples.
| Extraction method |
DESs (HBA:HBD) |
Optimum extraction conditions |
μg-β-Carotene/gr-Pumpkin |
| Predicted |
Actual 1
|
| MMAE |
Menthol:Acetic acid |
HBD Molar Ratio: 0.6831
Mixing Time: 60.0 min. |
0.621 |
0.610 |
| Menthol:Propionic acid |
HBD Molar Ratio: 0.6452
Mixing Time: 15.0 min. |
11.528 |
10.816 |
| HAE |
Menthol:Acetic acid |
HBD Molar Ratio: 0.6757
Homogenization Time: 116.3 sec.
Homogenization Speed: 11623 rpm |
0.712 |
0.711 |
| Menthol:Propionic acid |
HBD Molar Ratio: 0.6198
Homogenization Time: 30.0 sec.
Homogenization Speed: 7061 rpm |
8.966 |
8.762 |
Table 6.
Optimum ß-Carotene extraction conditions and maximum response values (predicted vs. actual 1) obtained under these conditions calculated by CCD for DESs in MMAE and HAE of ß-Carotene from spinach samples.
Table 6.
Optimum ß-Carotene extraction conditions and maximum response values (predicted vs. actual 1) obtained under these conditions calculated by CCD for DESs in MMAE and HAE of ß-Carotene from spinach samples.
| Extraction method |
DESs (HBA:HBD) |
Optimum extraction conditions |
μg-β-Carotene/gr-spinach |
| Predicted |
Actual 1
|
| MMAE |
Menthol:Acetic acid |
HBD Molar Ratio: 0.6987
Mixing Time: 40.2 min. |
13.010 |
9.348 |
| Menthol:Propionic acid |
HBD Molar Ratio: 0.8000
Mixing Time: 60.0 min. |
16.924 |
18.990 |
| Menthol:Butyric acid |
HBD Molar Ratio: 0.8000
Mixing Time: 60.0 min. |
5.640 |
4.468 |
| HAE |
Menthol:Acetic acid |
HBD Molar Ratio: 0.8000
Homogenization Time: 30.0 sec.
Homogenization Speed: 10311 rpm |
18.390 |
13.751 |
| Menthol:Propionic acid |
HBD Molar Ratio: 0.8000
Homogenization Time: 120.0 sec.
Homogenization Speed: 7000 rpm |
18.870 |
12.177 |
| Menthol:Butyric acid |
HBD Molar Ratio: 0.8000
Homogenization Time: 120.0sec.
Homogenization Speed: 14000 rpm |
10.131 |
12.534 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).