1. Introduction
In recent decades, climate-induced factors have affected the crop production, causing the severe food and energy challenges in the world (Wang et al., 2021a). The increasing global demand for nutritious food has led to unsustainable agricultural practices and overuse of natural resources, worsening environmental issues. Open field farming is threatened by pest, drought, and frost stress exacerbated by climate change, prompting the exploration of alternative methods (Keutgen, 2023).
Controlled environment agriculture (CEA) has emerged as a viable solution, creating controlled plant growth environments that minimize external influences (Tan et al., 2021). CEA actively adjusts plant growth conditions to optimize yield and quality, offering a promising approach to evolving agricultural challenges (Lefers et al., 2020). Light is a critical factor in plant development, impacting photosynthesis and growth as the primary source of energy (Leister, 2023). Beyond photosynthesis, light influences the production and quality of various plant macromolecules (Nassarawa et al., 2021).
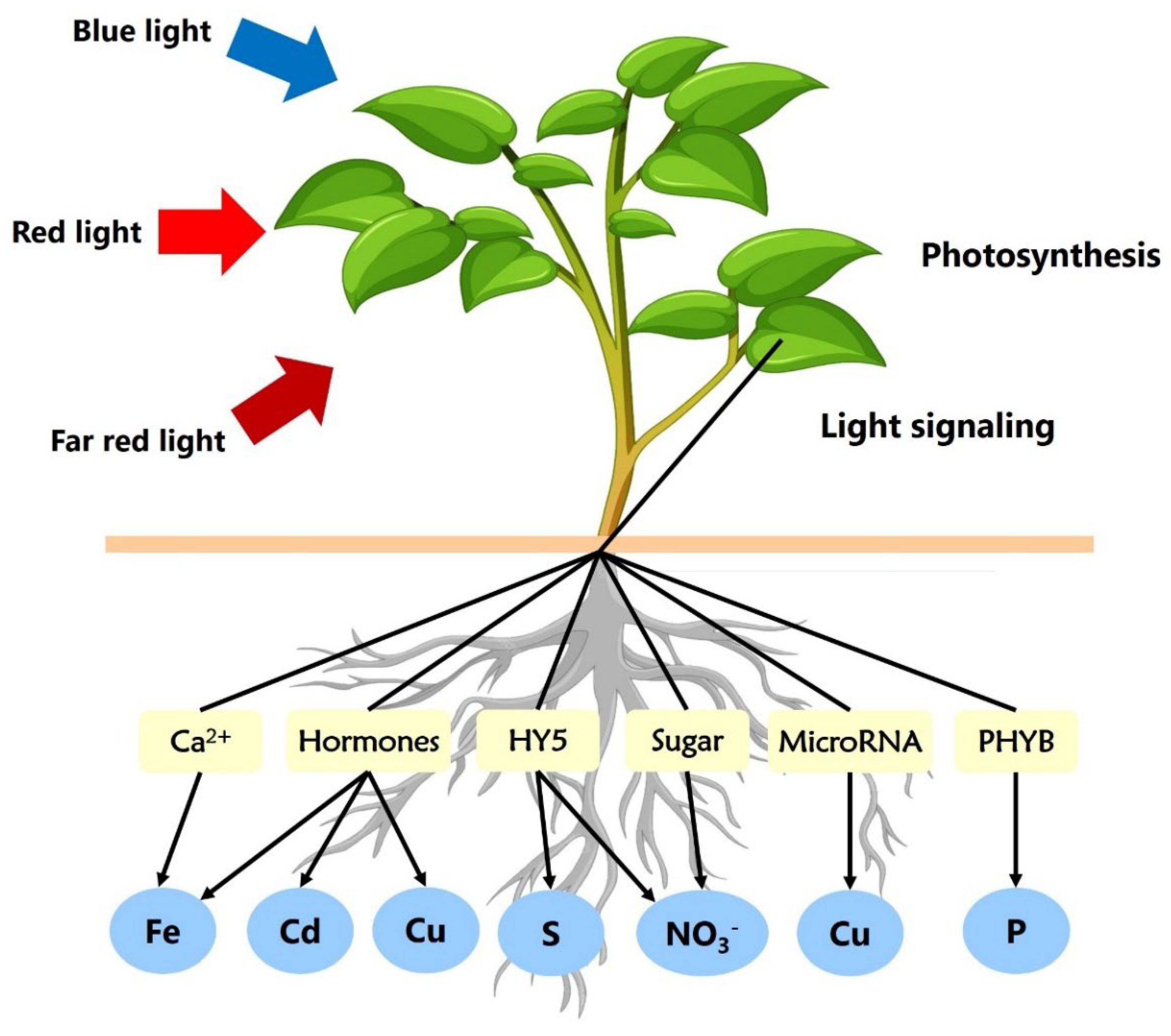
The natural distribution of sunlight is uneven around the world, leading to seasonal fluctuations and varying energy availability (Konapala et al., 2020)., Additional grow lights are used to compensate for insufficient sunlight, especially in indoor farms. The light spectrum, encompassing various wavelengths or light qualities, significantly influences plant growth, morphology, physiological process, and yield (Gawande et al., 2023). Plants rely on 16 essential elements for growth, obtaining carbon (C), hydrogen (H), and oxygen (O) from air and water, while other elements come from soil through roots (Bhatla et al., 2018). Macronutrients like nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulphur (S) are crucial for plant growth and development (Toor et al., 2021). Plants have evolved diverse strategies to optimize the absorption and utilization of these nutrients. They adapt to the changing environment by perceiving light signals and adjusting nutrient absorption accordingly (Ferrante et al., 2017).
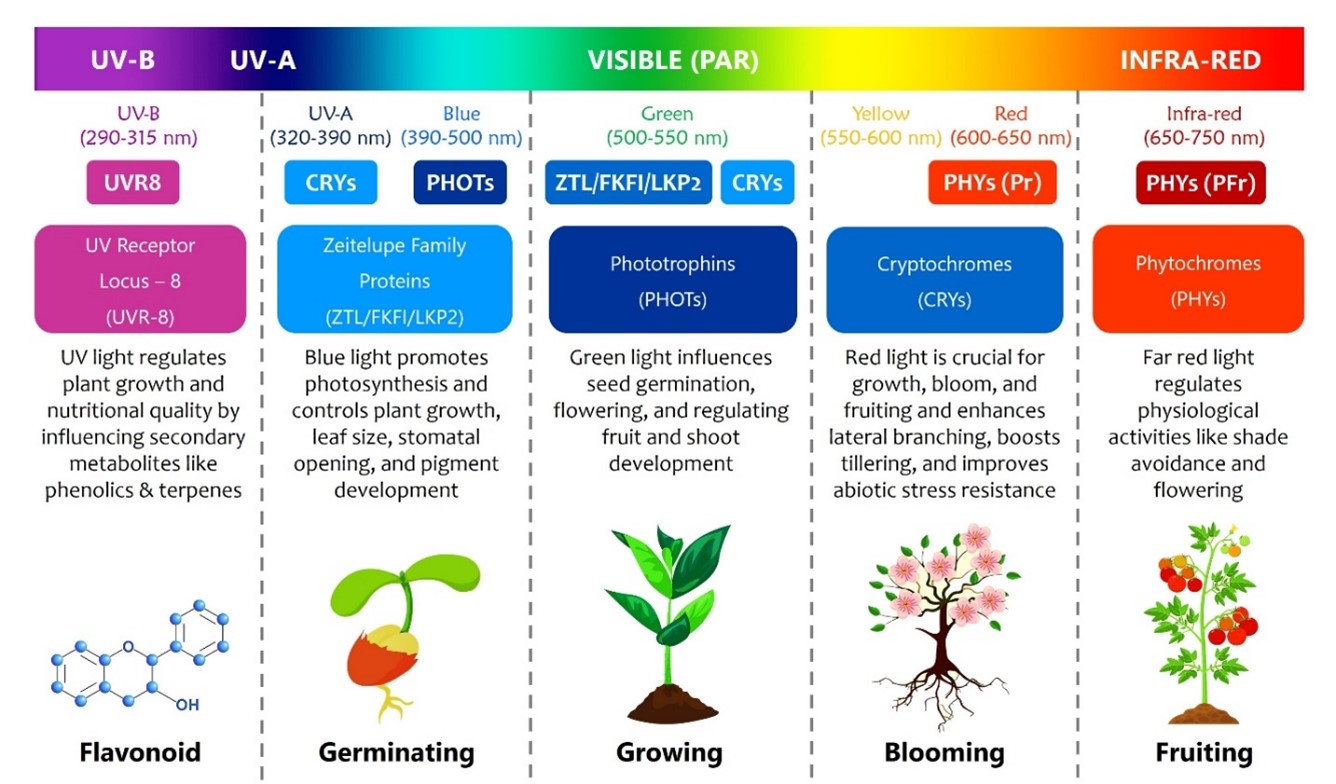
Light, the primary energy source for plants, fluctuates with time, seasons, and circadian rhythms, influencing these processes. Many studies have shown that changes in light quality, intensity, and photoperiod impact the growth, physiology, nutrient uptake, and yield of plants (Tan et al., 2021). Photosynthetically active radiation (PAR), crucial for plant growth, spans 400-700 nm, with red/orange (610-720 nm) and blue/purple (400-510 nm) light accounting for about 85% and 12%, respectively (Han et al., 2020). In contrast, yellow and green light (510-610 nm) are minimally absorbed (Xu et al., 2019). By leveraging plant's response to light, agriculture aims to enhance crop yield and quality through tailored lighting strategies.
Horticulture employs light quality manipulation using photo-selective nets or films to enhance plant productivity, quality, and phytochemical composition (Gawande et al., 2023). These tools shield crops from adverse environmental conditions such as excess sunlight, heat, drought, wind, hail, and pests, ultimately increasing crop yield and quality. Additionally, this approach can extend the shelf life of vegetables and reduce postharvest losses (Xu et al., 2019). Greenhouses are vital for extending the growing season and mitigating the impact of fluctuating weather conditions, addressing the food demands of our growing global population (Keutgen, 2023). However, the expenses related to power grid installation and electricity consumption for climate control, particularly in remote areas, can be prohibitively high (Han et al., 2020). Traditional greenhouse lighting sources including fluorescent, metal halide, high-pressure sodium, and incandescent bulbs often emit excess low-quality wavelengths, which are unsuitable for optimal plant growth (Lefers et al., 2020).
To tackle these challenges, light emitting diode (LED) technology offers a means of modifying plant traits to enhance crop yield and quality in targeted crops. This innovation conserves resources and promotes sustainable food production, particularly in challenging environments such as deserts and interplanetary platforms (Paradiso and Proietti, 2022). LEDs are revolutionizing plant cultivation by efficiently converting electrical energy into light. They offer high luminous efficiency, energy efficiency, low heat production, compactness, long lifespan, eco-friendliness, energy conservation, and easy controllability (Lefers et al., 2020). LEDs shape the light environment for plant growth and find broad applications in tissue culture (Konapala et al., 2020). Considered cutting-edge, they enhance horticultural crop yield under controlled conditions. The function of light signalling in plant growth and development and the transcriptional regulatory network of the light signalling pathways have been summarized. However, the mechanisms of light signalling have not been further explored in terms of nutrient uptake and yield. Here, we review the key lighting technologies in indoor agriculture, focusing on the latest LED advancements. While optimizing light for plant growth is vital, the current review aims to fine-tune the light spectrum to enhance not only growth and yield but also the production of bioactive compounds and secondary metabolites, addressing the challenge of maximizing the nutritional quality of crops.
2. Light Signaling in Plants - A Selective History
The history of light signaling research in plants has fundamentally shaped our understanding of how plants perceive and respond to light. Early research in the 19th century established that light functions not only as an energy source for photosynthesis but also as a signal to regulate growth (Liebers and Pfannschmidt, 2024). In the early 20th century, the discovery of photoperiodism demonstrated that plants could measure day length to time flowering, linking light perception to seasonal transitions in plant life cycles (Gendron and Staiger, 2023). Further advances came with the identification of phytochromes (responsive to red and far-red light), which play pivotal roles in regulating germination, stem elongation and flowering by switching between active and inactive forms (Sharma et al., 2023a). This adaptability led to the discovery of other photoreceptors, such as cryptochromes (responsive to blue light), which regulate growth, circadian rhythms, and stress responses and phototropins. The identification of light-responsive transcription factors, including PHYTOCHROME-INTERACTING FACTORS (PIFs) and ELONGATED HYPOCOTYL 5 (HY5), marked a significant step forward in understanding light signal transduction (Cai and Huq, 2024). PIFs, which are repressed by phytochromes in light, promote compact growth by controlling genes associated with cell elongation and shade avoidance. HY5, on the other hand, integrates signals from multiple photoreceptors to coordinate light-driven growth, thereby acting as a central regulator in photomorphogenic development (Wang et al., 2022).
At the molecular level, the transcriptional regulatory network of light signalling pathways orchestrates growth, survival, and development (Liebers and Pfannschmidt, 2024). The circadian clock, governed by light signalling pathways, plays an essential role in timing the vegetative-to-reproductive transition, aligning flowering with favourable environmental conditions. This clock modulates gene expression in 24-hour cycles, allowing plants to synchronize their growth processes with day-night rhythms (Sharma et al., 2023a). For example, in long-day plants like Arabidopsis, the key gene CONSTANS (CO) activates FLOWERING LOCUS T (FT) in response to light. FT then moves from leaves to the shoot apical meristem, initiating flowering under ideal conditions (Yu et al., 2024). In short-day plants, CO and FT are regulated to delay flowering until shorter days signal optimal seasonal conditions (Cai et al., 2024). Understanding these pathways offers agricultural potential, as adjusting flowering times can optimize crop yields across diverse climates. As a central player in light signaling, HY5 not only integrates photoreceptor signals but also links light conditions to nutrient acquisition (Cai and Huq, 2024). HY5 enhances nitrate uptake by upregulating nitrate transporter genes, increasing nitrogen availability essential for protein synthesis and growth. It similarly affects phosphate transporter genes, promoting phosphorus acquisition for energy transfer and metabolic processes (Wang et al., 2022). These coordinated interactions enable plants to dynamically adapt to their environment, with this regulatory network offering genetic targets to improve crop yields, light efficiency, and resilience in agriculture.
Beyond light, plants must also adapt to temperature changes, and the integration of light and temperature signalling pathways is crucial for coordinating growth and survival in fluctuating environments (Liebers and Pfannschmidt, 2024). In warm conditions, certain phytochromes become less stable, allowing increased PIF activity, which promotes cell elongation and rapid growth to adapt to the season. Conversely, stable phytochromes in cooler temperatures inhibit PIFs, resulting in more compact growth that conserves energy (Wang et al., 2022). In response to abiotic stresses like drought, salinity and temperature extremes, regulatory molecules such as 14-3-3 proteins help plants adapt by modulating key processes. The 14-3-3 proteins interact with H+-ATPases, proton pumps that maintain ion homeostasis and cellular pH (Pertl-Obermeyer et al., 2022). This interaction stabilizes the phosphorylated state of H+-ATPases, facilitating proton pumping into the apoplast. For instance, in drought conditions, the 14-3-3/H+-ATPase complex helps maintain turgor pressure, which is critical when water is scarce (Cai et al., 2024). In saline environments, activated H+-ATPases enable Na+ exclusion through Na+/H+ antiporters, reducing salt toxicity. Furthermore, photoreceptors like phytochrome B (phyB) assist in osmotic adjustment by modulating abscisic acid (ABA) levels, while cryptochrome1 (CRY1) and phototropins mitigate oxidative stress from salt exposure (Yu et al., 2024). Insights into the integration of these pathways deepen our understanding of plant adaptability, showcasing the profound impact of light signalling research on both plant biology and agriculture.
3. Importance of Light in Crop Growth
Light is essential for photosynthesis, which is crucial for healthy plant development. Light quality profoundly impacts plant phenotypic traits through photomorphogenic mechanisms and metabolic regulation (Alsanius et al., 2019). Day length regulates the biological clock, influencing phenological events such as flowering, seed germination, and biomass allocation (Osnato et al., 2022). Alterations in these light factors can trigger transitions in plant development, affecting the distribution of photosynthates among leaves, roots, supporting tissues, and symbiotic partners (Gawande et al., 2023). Initially, Thomas Engelmann's research during 1882 addressed the role of blue light in leaf development and the influence of red light on flowering and fruiting, while green light was considered the least effective due to reflection (Neo et al., 2022). However, recent studies have revealed that green light, ultra violet (UV), and near-infrared radiation also contribute to plant growth (Huché-Thélier et al., 2016). Blue and red light activate photosynthesis mainly on the upper leaf surface, absorbed by chlorophyll a and b pigments, while green light penetrates deeper, aiding carbon fixation in the lower leaf layers facilitated by the accessory pigment β-carotene, which further enhances the photosynthetic process (Simkin et al., 2022).
Photosynthesis relies on the absorption of three primary pigments: chlorophyll a, chlorophyll b, and β-carotene (Neo et al., 2022). Recent researches highlight the importance of light beyond the PAR range, including UV (A and B) and far-red light, which extends the usable light spectrum from 300 to 800 nm (Fortineau et al., 2021). The photosynthetic photon flux (PPF) quantifies the available light for plants, with 1 μmol corresponding to approximately 6.022 x 1017 photons per second (Gorjian et al., 2023). The photosynthetic photon flux density (PPFD) is crucial for comparing different light sources, representing the number of PAR photons landing on a specific surface per unit area (μmol m-2 s-1) (Simkin et al., 2022). Higher PPFD values indicate more accessible PAR photons for photosynthesis per unit area. Comparing different light sources, photon efficacy was measured in μmol J-1, which is the ratio of PAR photons per second (μmol s-1) to the energy consumed by the lighting system per second (Watts or J s-1) (Fortineau et al., 2021). Additionally, the daily light integral (DLI) measures the total PAR photons received by a plant in a day, expressed as μmol m-2 day-1 (Gorjian et al., 2023). This metric is crucial because different plants have varying total light requirements.
3.1. Different Types of Light and Its Effect on Crops
Conflicting findings on the effects of light on plant development arise from variations in study materials, shading intensity, duration, the number of shading days, and ecological factors. Shading affects not only light intensity but also environmental parameters like light quality, air humidity, CO2 levels, and soil temperature (Ferrante et al., 2017). In addition to intensity and duration, light composition such as blue, red, and far-red light also play pivotal roles in plant photomorphogenesis (Nassarawa et al., 2021). Variations in light quality significantly impact crop biomass. Red (R) light reduces internode elongation, enhances lateral branching, and boosts tillering, improving resistance to abiotic stress (Huber et al., 2021). Different red/far-red ratios affect salt and temperature tolerance, coleoptile length, and rootlet quantity in rice (Oryza sativa) (Cao et al., 2018). Red light influences chloroplast, stem, petiole growth, and reproductive activity, while blue (B) light predominantly governs plant growth, leaf size, photomorphogenesis, stomatal opening, photosynthesis, and pigment development (Li et al., 2021). Due to their similarity to photosynthesis spectra, research often focuses on monochromatic or mixed red and blue LED lighting effects.
Research on the effects of white (W), far-red (FR), green (G), yellow (Y), and orange (O) LEDs on plant growth is limited. However, previous studies have shown that monochromatic light can have beneficial effects on root development and physiological metabolism in plants, although these effects vary depending on the plant species (Konapala et al., 2020). For example, red LED light inhibits root growth in Doritaenopsis plants, while blue LED light promotes root formation in Chrysanthemum plants and enhances the rate and number of roots in Achillea millefolium and Vanilla planifolia cultures (Cavallaro et al., 2022). Furthermore, red light can enhance antioxidant activity in pea (Pisum sativum) seedlings, Dendrobium officinale seedlings, and Eleutherococcus senticosus somatic embryos, with enzyme responses differing based on the quality of light (Xu et al., 2019).
Li et al. (2010) demonstrated that red light enhanced root activity, sucrose, and starch levels in Gossypium hirsutum tissue culture seedlings, but adding more blue light to red LEDs resulted in larger, healthier plants with higher biomass. Kulus and Woźny (2020) found that the combination of red and blue light stimulated root growth and increased leaf area in Doritaenopsis tissue culture seedlings. Additionally, carbohydrate synthesis (starch, sucrose, glucose, and fructose) was significantly higher under this light quality compared to monochromatic red, blue, or fluorescent light treatments (Achour et al., 2021).
Green light plays a crucial role in plant growth and development, influencing seed germination, flowering, and regulating fruit and shoot development (Paradiso and Proietti, 2022). In addition, green wavelengths enhance photosynthesis and carbon uptake by penetrating deep into leaf mesophyll and lower canopy layers to benefiting chloroplasts (Gorjian et al., 2023). When combined with red and blue lights, green and orange lights enhance photosynthesis and translocation by impacting source/sink relationships among plants (Huber et al., 2021). These wavelengths also increase water efficiency and overall plant development by accumulating photosynthates in leaves (Konapala et al., 2020). Therefore, incorporating green light into CEA is beneficial alongside red and blue lighting.
Although UV-B radiation is a small part of total solar radiation, it carries high energy and can cause significant biological damage to plant cells even with slight increases (Sharma et al., 2017). Plants are sensitive to elevated UV-B levels as various biological components directly absorb this radiation (Zlatev et al., 2012). Therefore, it is crucial to understand the effects of heightened UV-B exposure on the development and physiology of agricultural crops in greenhouses or open fields. UV radiation spans 100-400 nm, including UV-A (320-400 nm), UV-B (280-320 nm), and UV-C (200-280 nm) (Bosso et al., 2023). UV light serves as both a stressor and regulator, exerting a substantial influence on plant growth and nutritional quality. UV-B radiation is a significant abiotic factor with direct and indirect effects on plants. It impacts the production of secondary metabolites such as phenolics, terpenes, and nitrogen compounds (Cao et al., 2018). Studies indicate that increased UV-B exposure can impair plant physiological processes, including photosynthetic CO2 uptake and efficiency, highlighting its multifaceted impact on plant biology (Sharma et al., 2017).
4. Need and Type of Light Manipulations
Light manipulation in greenhouses offers the potential to increase crop yield, improve crop quality in terms of colour, compactness, and secondary metabolite content, and enhance energy efficiency in greenhouse operations (Paradiso and Proietti, 2022). Microclimate control strategies vary by location, with the need for surplus heat dissipation in sun-drenched tropical regions and heat retention in temperate zones, especially during colder seasons with limited solar radiation (Nwokolo et al., 2023). In greenhouse applications, spectral manipulation is achieved by two main methods: (i) selective transmission of specific spectral components while blocking others via reflection, scattering, or absorption, which can be accomplished using scattering nanoparticles, 1D photonic crystals (1DPCs), or plasmonic nanoparticles; (ii) converting less favourable wavelengths into those more beneficial for photosynthesis, plant growth, development, morphogenic effects, or microclimate control within the greenhouse (Otanicar et al., 2016). This conversion is facilitated by using the phenomena of light down-conversion or up-conversion.
4.1. One-Dimensional Photonic Crystals
Photonic crystals (1DPCs) are engineered materials with a regularly changing refractive index along one dimension. They typically consist of alternating layers with high and low refractive indices, like multilayers (Shen et al., 2016). This periodic variation causes incoming light to undergo successive back reflections at each interface. These structural parameters define a "photonic band gap" (PBG), a range of wavelengths where back reflections align in phase, leading to constructive interference (Zhu et al., 2023). As a result, most incident light is reflected or rejected within the PBG, effectively controlling, and modifying the passage of light through the photonic crystal.
Polymer-based 1DPCs are now gaining attention as lighter, flexible, and cost-effective alternatives, promising efficient near infra-red (NIR) blocking and improved sustainability in greenhouse coverings (Nwokolo et al., 2023). Polymer-based photonic crystals offer advantages such as lower cost and ease of sourcing, but their drawback is limited refractive index contrast between adjacent layers. This impacts band gap width and spectral blocking efficiency, necessitating improvements to match inorganic 1DPCs (Shen et al., 2016). The PBG, which is determined by back reflections' interference in multilayers, varies with the angle of the incident light, an important consideration for adapting 1DPCs to greenhouses. Due to changing orientations, shifting sunlight angles, and diffuse illumination in greenhouses, the precise position and width of the PBG may not remain constant (Otanicar et al., 2016). The true potential of 1DPCs lies in their customizable optical properties, which can be optimized through computer-based design, making them versatile solutions for greenhouse applications (Iezzi et al., 2020). Plasmonic systems, however, harness unwanted wavelengths, offering spectrally selective transmission and absorption, making them versatile in such settings (Shen et al., 2016).
4.2. Plasmonics
Plasmonics hinges on "plasmon resonances," a collective oscillation of free electrons induced by electromagnetic radiation on a plasmonic system. It focuses on localized plasmon resonances, where electron oscillations are confined to the surface of metallic nanoparticles, as opposed to propagating surface plasmons, which are electron plasma waves travelling along a metal-dielectric interface (Kuppe et al., 2020). Gold or silver nanoparticles, rich in free electrons that oscillate in response to incoming light, exemplify such localized plasmon resonances (Yildirim et al., 2021). Resonance wavelength in plasmonic nanoparticles depends on their size, shape, material, and the surrounding medium, allowing for tunable spectral selectivity. Plasmons efficiently convert light to heat due to non-radiative decay of collective oscillations, making them valuable for greenhouse technology, where both light-to-heat conversion and spectral manipulation play crucial roles (Nwokolo et al., 2023).
Computational designs have proposed plasmon-based transparent filters that effectively block UV and NIR light for photo voltaic (PV) cells. However, fabricating precise nanostructures with varying plasmon diameters can be expensive (Thrithamarassery et al., 2017). Recent studies have explored cost-effective solutions, using disordered plasmonic nanostructures made from affordable materials such as Cu and Al embedded in IR-blocking glass (Yildirim et al., 2021). This innovation holds promise for cost-effective passive cooling in buildings and potentially in greenhouses. Plasmonic light-to-heat conversion is a potential solution for low-energy heating in greenhouses in cold-climate (Otanicar et al., 2016). It utilizes wavelength selectivity to absorb less useful solar wavelengths and efficiently convert them into heat.
The primary drawback of plasmon-based technology is its high cost, particularly when utilizing noble metals like Au or Ag (Yildirim et al., 2021). Ongoing research into the plasmonic properties of aluminium, an abundant and affordable metal, and promises enhanced economic feasibility and sustainability for plasmonic systems. However, at high light intensities, significant heating effects at plasmon resonance can cause nanoparticles to melt, altering their shape and size, and thereby affecting the system's spectral characteristics and performance (Thrithamarassery et al., 2017). While plasmon-driven light-to-heat conversion helps save energy in greenhouses, it does not significantly enhance PAR intensity for plant photosynthesis. Spectral conversion through luminescent processes including photon down- and up-conversion offers a more viable approach to boost photosynthetic photon flux density (Kuppe et al., 2020).
4.3. Luminescence
Luminescence denotes the emission of light, which can result from electrical energy, mechanical tension, chemical reactions, or photon absorption, the latter being termed "photoluminescence" (Bai et al., 2015). In greenhouse innovation, we explore two photoluminescent processes: down-conversion and up-conversion. A wealth of publications spanning diverse applications, including fluorescence microscopy, PV technology, and artificial photosynthesis, offer detailed insights into the mechanism, composition, and design of suitable luminescent materials (Liu and Qiu, 2015).
4.3.1. Luminescent Down-Conversion
Luminescent down-converting substances absorb photons of a specific energy and subsequently emit lower-energy photons through internal conversion processes. This process, which includes down-conversion and down-shifting, broadens the absorption and emission spectra around their respective peak wavelengths (Nwokolo et al., 2023). In greenhouses, where blue and red wavelengths are crucial for photosynthesis, UV-to-blue/red or green-to-red spectral down-conversion processes are particularly relevant to optimize light conditions for plant growth (Bai et al., 2015). Lanthanide ions such as neodymium (Nd3+), ytterbium (Yb3+), and europium (Eu3+) can be employed for luminescent down conversion, either alone or in combination with organic molecules (Nalumaga, 2022). However, their limitations include poor absorption coefficients and high cost. Since the 1990s, research on plastic films with fluorescent colours as greenhouse cover for various crops, such as tomatoes and strawberries, has yielded generally satisfactory results (Bai et al., 2015). Recent advances in the commercial production of fluorophores in greenhouse materials claim positive effects on leaf and stem size, fruit size, and yields in various crops including lettuce, strawberries, tomatoes, and cannabis (Mishra et al., 2023).
However, specific studies quantifying photon flux density with and without plastic films have reported intriguing results. Boucher et al. (2023) observed an 8.7% increase in dry weight, an 11% increase in fresh weight, and a 13% increase in lettuce leaf area, despite a 12% reduction in the DLI within the PAR range. Furthermore, they found a 19-22% increase in fresh and dry weight of lettuce, even with a 20% DLI reduction. This effect may be attributed to an elevated red/far-red ratio, known to stimulate developmental activity and regulate stem elongation rates and branching (Liu and Qiu, 2015). Understanding the mechanisms behind yield increases with fluorescent films requires considering two inherent consequences: shading, where transmitted photon flux is efficiently reduced, and diffusion, stemming from isotropic photon emission by fluorophores (Bai et al., 2015). To assess shading's impact, light intensity within and outside the films, measured as photon flux density or DLI, is crucial. These factors can significantly impact production, particularly in high-light environments and for shade-tolerant crops.
A challenge with natural sunlight is to achieve significant photon down conversion to the blue band, since higher-energy wavelength bands are a small fraction of the solar spectrum (Nalumaga, 2022). Red/far-red photons are more accessible through down conversion from the UV, blue, or green wavelengths. While the red band is critical for plant growth and biomass, even slight increases in photon density in the blue and UV regions can impact photomorphogenic characteristics (important for ornamental plants) and secondary metabolites that influence flavour and nutritional content (Mishra et al., 2023). This limitation, which mainly affects spectral down conversion, can be overcome through a phenomenon known as luminescent up-conversion.
4.3.2. Luminescent Up-Conversion
Luminescent up-conversion involves the absorption of multiple low-energy photons and subsequently emitting a higher-energy photon. This process is more complex than down conversion. In a simplified explanation, up conversion occurs when a system absorbs multiple low-energy photons, causing its electrons to reach higher energy levels, and then these electrons release energy by emitting a high-energy photon (Bai et al., 2015). Rare-earth ions like erbium (Er3+), thulium (Tm3+), and holmium (Ho3+) are commonly used activators due to their ladder-like energy levels (Nalumaga, 2022). Ytterbium (Yb3+) serves as a sensitiser due to its broad absorption cross section and efficient energy transfer to activator ions (Wang et al., 2023). In greenhouses, the desired up-conversion could target NIR (λabs) to red/blue/UV (λem) or green (λabs) to red/blue/UV (λem) wavelengths (Liu and Qiu, 2015).
Up-conversion transitions have been observed under low-power artificial lighting but remain far from commercialization for solar radiation in greenhouses. Applications such as bioimaging and medical treatment often demand coherent and powerful laser sources (Otanicar et al., 2016). However, in greenhouses, even slight enhancements in PAR photon flux density through up-conversion can have significant impacts, warranting further proof-of-concept studies (Liu and Qiu, 2015). Modest up-conversion to UV photons can help improve colour, flavour, and nutrient enhancement, while NIR-to-visible photon up-conversion offers both heat control and increased photosynthetic photon flux (Wang et al., 2023). Promising preliminary studies pairing up-converting systems with photosynthetic processes have shown significant benefits. Arabidopsis thaliana exhibited a 12% increase in photosynthetic rate under natural light enriched with UV when utilizing films combining down-converting luminophores and NIR-PAR up-converting luminophores (Nalumaga, 2022). In addition, NIR-PAR up-converting films improved tomato plants by increasing leaf number (12.5%), leaf area (33%), and chlorophyll content under artificial light (Yanykin et al., 2022).
These studies lay the groundwork for materials that can be optimized and assessed for various crops under natural sunlight. Current efforts to enhance up-conversion efficiency under lower light intensities, like Earth's incident solar radiation, involve material parameter experiments, nano structuring, employing photonic structures like dielectric micro lenses for light concentration, and utilizing plasmonic structures to amplify local fields (Nwokolo et al., 2023). These findings represent crucial initial strides toward making up-conversion a viable technology for greenhouses operating under natural sunlight conditions. Organic-based up-conversion systems offer sustainability, safety, larger absorption cross-sections, higher efficiency, and tunability compared to rare-earth-based systems (Yanykin et al., 2022). Organic systems exhibit emission wavelengths from 380-630 nm, while rare-earth-based systems cover 290-800 nm, both promising for enhancing PAR in greenhouses (Mishra et al., 2023).
5. Manipulation of Light and Its Response to Crops
5.1. Manipulation of Light on Crop Growth Changes
Light serves as a pivotal signalling factor in plant growth, influencing plant physiology and development (Bao et al., 2024). Plant photoreceptors, mainly located in leaves, receive and process light signals. This complex system coordinates with various elements including proteins, ions, and hormones to regulate gene expression, metabolic responses, and plant morphology (Kharshiing et al., 2019). Hossain et al. (2022) highlight that the management of reactive oxygen species (ROS) and their interplay with antioxidants is a key process shaping plant development and morphogenesis. Li et al. (2021) conducted a study demonstrating the significant impact of light quality on tomato seedling development. They found that plant height was highest under red (R) light and lowest under purple (P) light. Compared to white (W) light, both monochromatic P and a combination of red and blue (R and B) lights significantly inhibited plant height (p < 0.05). The stem diameter was significantly greater when exposed to the combination of R and B lights, particularly the 3:1 ratio of red to blue light (3R1B treatment), as compared to W, R, or B monochromatic light treatments. In general, shoot dry weight was highest under 3R1B and lowest under P light. Root dry weight patterns were similar across treatments. These results suggest that plant responses to light quality may depend on factors such as species, cultivar, growth time, or light intensity (Li et al., 2021).
Diverse light qualities significantly impacted the rooting rate, mean root number, root length, surface area, volume, and activity of C. lanceolata tissue culture seedlings (p < 0.05) (Xu et al., 2019). The treatment with 8:1:1:1 red, blue, purple, and green light (8R1B1P1G) showed the highest results with a 95.5% rooting rate, 4.63 average root number, 5.95 cm length, 1.92 cm² surface area, and 145.56 ng gh-1 root activity. These values were significantly higher (ranging from 4.2% to 28.9%) than those under the white LED treatment. In general, the combination of red-blue-purple-green light outperformed red-blue and red-blue-purple treatments in promoting root development and activity. This effect is attributed to the complementary interplay of red, blue, and purple light, although plants have a lower demand for this combination (Carvalho and Castillo, 2018). Purple and green photons remain essential for photosynthetic radiation. Incorporating purple and green light alongside red and blue light can potentially enhance photosynthate transport to the roots, thereby promoting root development (Yang et al., 2022).
Blue light plays a role in glycolysis by inhibiting β-ketoglutarate dehydrogenase, activating nitrate reductase, and increasing pyruvate kinase protein production (Bartman et al., 2017). Moreover, the higher energy of blue light photons can provide additional energy for macromolecular synthesis, especially in protein synthesis (Carvalho and Castillo, 2018). Recognizing the influence of various light regimes on crop growth is pivotal for maximizing output in controlled environments, improving resource utilization, and adapting to dynamic environmental shifts.
Table 1 illustrates how light colour affects the growth, yield, and nutritional quality of greenhouse vegetables, providing valuable insights for optimizing cultivation practices.
5.2. Manipulation of Light on Crop Physiological Changes
For more than half a century, research has shown that the conversion of incoming irradiance into chemical energy through photosynthesis is influenced by the wavelength of light. Pioneering studies by McCree (1972), Inada (1976), and Evans (1987) revealed that red light has the highest quantum yield for CO
2 fixation in many species within the photosynthetically active light spectrum. Given the superior photosynthetic quantum yield of red light and the exceptional electrical efficiency of red LEDs, researchers have increasingly utilized high proportions of red light to create optimal conditions for photosynthesis and plant growth (Yang et al., 2018). Hogewoning et al. (2010) quantitatively demonstrated how leaves adjust their photosystem composition in response to diverse light spectra (sun, shade, and blue light). Mixing different wavelengths of light was shown to substantially enhance the quantum yield of for CO
2 fixation, offering valuable insights into photosystem adaptation (Kunz et al., 2020). The intricate light signaling pathways play important role in regulating the absorption and utilization of essential nutrients in plants (
Figure 1).
Plants exhibit dynamic responses and adaptations to their external light conditions. The increased presence of far-red light (FR), often characterized by a low R:FR ratio, in shaded areas beneath dense canopies is due to the wavelength-dependent interactions of leaves with natural light (Lazzarin, 2023). These spectral shifts are not restricted to plant communities but are also observed within individual plants. Li et al. (2021) conducted a 30-day study on tomato seedlings to assess how light quality impacted photosynthetic rates (Pn). The results showed that the leaf Pn of seedlings exposed to red (R) and blue (B) light combinations and monochromatic blue light significantly outperformed those under white (W) light (p < 0.05). The highest Pn was recorded in plants exposed to a 3:1 ratio of red and blue (3R1B) light, while the lowest occurred in those grown under purple (P) light.
Under dye-sensitized solar cell-covered greenhouses, tomato photosynthesis decreased (Ntinas et al., 2019), while a NIR-reducing coating enhanced cucumber photosynthesis (Alsadon et al., 2016). These responses in greenhouse vegetables reflect their adaptation to altered light conditions caused by different cover materials. This adaptation encompasses various processes like light sensing, absorption, and energy conversion (Lazzarin, 2023). The light environment created by these materials has a significant effect on photosynthesis. Pigments, such as chlorophyll and carotenoids, capture light, especially PAR, initiating electron transport and energy conversion (Ramanna et al., 2017). The mobility of chloroplasts and pigments at the cellular level and thylakoid membrane orientation adjustment in response to varying light conditions play essential roles in this complex process (Kirchhoff, 2019).
Photosynthesis relies on light sensing and the activities of photosystem I (PSI) and photosystem II (PSII) (Kunz et al., 2020). Pigments in PSII absorb light, initiating water molecule splitting to create a proton gradient. Optimal photosynthetic conditions vary with plant types and light spectrums (Kaiser et al., 2015). Young tomato plants thrive with 300 mol m-2 s-1 PPFD and at least 8 hours of daily light, while leafy vegetables do well with 200 mol m-2 s-1 (Wojciechowska et al. 2015). For instance, elevating the blue light ratio can enhance leaf photosynthesis, photosynthetic rate, and PSII quantum yield under monochromatic red light (Wang et al. 2018). Understanding these findings on light receptors and acclimation could predict yield under different cover materials.
Cope and Bugbee (2013) highlighted two likely factors restricting the relative quantum efficiency of blue light in photosynthesis: (1) some blue photons are absorbed by non-photosynthetic pigments including anthocyanins, leading to energy loss as heat or fluorescence. (2) Accessory pigments such as carotenoids consume some blue photons and are 10% to 65% less efficient than chlorophyll molecules at transferring light energy to the photosynthetic reaction centre. In many plant species, increased exposure to blue light inhibits cell division and growth, resulting in reduced leaf area, shorter stems, and thicker leaves.
Monochromatic LED lighting can induce physiological anomalies in specific plant species or varieties not typically observed under broad-spectrum light (Kume, 2017). For example, the condition known as intumescence, characterized by abnormal cell outgrowth on plant surfaces due to abiotic stress, was initially associated with a lack of ultraviolet (UV; 300 to 400 nm) and far-red light in the spectrum (Ullah et al., 2019). Studies have shown that UV light can suppress intumescence formation in susceptible tomato (
Solanum lycopersicum) and ornamental sweet potato (
Ipomoea batatas) cultivars (Williams et al., 2016). Intumescence not only disrupts plant physiology but also negatively affects the aesthetic quality of plant products, a significant concern for decorative plants grown under narrowband LED lighting (Anjum et al., 2017). Fine-tuning the spectrum, intensity, and duration of light exposure allows researchers and farmers to modulate plant physiology. This manipulation impacts photosynthesis, transpiration, hormone regulation, and stress responses, altering growth, nutrient uptake, and overall health. Understanding these processes is crucial for optimizing crop production.
Table 2 illustrates the effects of red and blue light on nutrient accumulation in horticultural crops in controlled environments.
5.3. Manipulation of Light on Flowering and Fruiting Changes
The flowering responses to light quality, similar to growth and development, are specific to plant species or cultivars and are primarily influenced by the duration of the uninterrupted darkness period within a day, called the critical night length (Ahn et al., 2015). Plants are typically classified into response groups based on how this critical night length affects their flowering regulation. In the absence of other limiting environmental or cultural factors, day-neutral plants will flower regardless of the photoperiod (Osnato et al., 2022). Short-day (SD) plants flower most rapidly when the continuous dark period surpasses a species-specific required night length (Proietti et al., 2022). Conversely, long-day (LD) plants induce flowering more quickly when the dark periods are shorter than a critical duration (Wang et al., 2021b). In LD plants, when daylight periods are short, extending the days (i.e., reducing the length of the night) by artificial lighting can induce flowering while inhibiting blooming in SD plants may promote vegetative growth (Craig and Runkle, 2016).
Similarly, night interruption (NI) can effectively disrupt the dark phase and enhance the photoperiodic responses of LD. NI is more efficient than day extension in inducing flowering in LD plants (Osnato et al., 2022). Blue light, which is minimally absorbed by phytochromes, has been demonstrated to influence flowering at higher intensities (30 mol m-2 s-1) compared to the typical 2 mol m-2 s-1 required from red and/or far-red light (Proietti et al., 2022). Red and far-red light remain the primary photoreceptors governing flowering in photoperiodic species (Bartucca et al., 2020). To manipulate the flowering and production cycles of plants, low light intensity is typically applied at night. This approach promotes flowering in LD plants, shortening their production cycle, while inhibiting flowering in SD plants, encouraging vegetative growth (Klein et al., 2018). Artificial lighting is employed to extend day length, stimulating vegetative growth, or as a night break to prevent premature flowering. This ensures year-round availability for market demand in the case of SD plants like chrysanthemums and allows for programmed flowering on specific market dates (Craig and Runkle, 2016).
In addition to photoperiod, spectral composition has a profound effect on flowering in both SD and LD plants. Distinct light qualities trigger the transition of flower by orchestrating the transcriptional control of genes that encode flowering activators, such as photoreceptors (Proietti et al., 2022). Multiple photoreceptors play essential roles in wavelength perception and absorption, including phytochromes (absorbing red/far-red light), cryptochromes (absorbing blue/UV-A wavelengths), and phototropins (primarily absorbing UV-B light). For LD plants, blue and far-red light are often used to stimulate flowering (Xu et al., 2021). Blue light accelerates LD flowering, resulting in a higher flowering index, more visible flower buds, and open flowers. This effect is associated with reduced phytochrome activity, also known as a phytochrome photo equilibrium (Wang et al., 2021b).
The impact of green light on photoperiodic flowering has been studied in a few instances. SD plants exposed to green light exhibited delayed or inhibited flowering, akin to responses seen with blue light (Zhao et al., 2020). The effects depend on the species, exposure duration, and light intensity. Meng and Runkle (2014) found that green light fluxes can act as a signal for long-day responses. To achieve optimal flowering responses in petunias, snapdragons, and ageratum floriculture crops required a light spectrum with moderate levels of green light for several hours. In contrast, green light delayed flowering in chrysanthemum and marigold SD plants, underscoring its role in regulating flower induction in photoperiodic species (Kisvarga et al., 2023).
The use of UV light can either promote or delay flowering, with outcomes varying by species, UV spectrum region, and fluence rate (Nazir et al., 2020). Excessive UV radiation can significantly affect flower quality. Phacelia campanularia A. Gray and Salvia splendens Sellow ex Nees plants exposed to high UV doses experienced reduced flowering period and flower numbers, while flower transitions were impeded in Limnanthes alba Hartw. ex Benth. Plants (Trivellini et al., 2023). Conversely, UV-C radiation improved flowering and increased the flower quantity in wild pansy and freesia decorative plants (Chandel et al., 2023). Fukuda et al. (2016) investigated the response of Petunia to light quality in relation to the regulation of gibberellin (GA) content. Their research revealed that blue and red light had contrasting effects on shoot elongation, which appeared to be linked to GA signalling. However, they could not establish a direct connection between light quality and flowering via GA signalling. Dueck et al. (2016) proposed that phytohormones responsible for bud break and inflorescence elongation in Phalaenopsis could be influenced by light rather than temperature. This finding is intriguing, suggesting that light quality could offer alternative signals to substitute for other environmental factors.
The rapid adoption of LEDs replaced incandescent bulbs as photoperiodic lamps due to their longer lifespan and, more importantly, the phasing out of incandescent bulb production in many countries due to their energy inefficiency (Younas et al., 2018). Compact fluorescent lamps are more energy-efficient and longer-lasting than incandescent bulbs, but their spectra have limited influence on flowering regulation (Trivellini et al., 2023). LEDs, with their narrow bandwidths, offer precise control over light quality, enhancing our understanding of how different wavelengths affect flowering. LEDs offer several advantages, including cost savings, accelerated flowering, and prevention of abnormal stem elongation in some plants (Cuong et al., 2019).
Research indicates that not all LED lights are equally effective in regulating flowering; their success depends on their specific spectral composition (Nadeem et al., 2019). Meng and Runkle (2014) found that both cool-white and warm-white LEDs were equally effective in regulating flowering when compared to red or blue plus red LEDs. Limited research has explored the role of green light in photoperiodic flowering control. In the comparison of LED and traditional lamps for their effectiveness in regulating photoperiodic plant flowering, LEDs prove to be as effective as traditional sources with the added advantage of lower overall operational costs (Khurshid et al., 2020). Kohyama et al. (2014) found that combining far-red and red + white light in night interruption enhances flowering in ornamental plants at low DLI (6 mol m-2 d-1) but not at high DLI (12 mol m-2 d-1).
The ripening phase represents the final stage of fruit development, marked by significant physicochemical and sensory changes (Gill et al., 2017). It encompasses processes such as cuticle thinning, cell wall degradation, softening, and hormonal interactions. The ripening mechanisms differ between climacteric and non-climacteric fruits (Kisvarga et al., 2023). The light spectrum plays an important role in regulating fruit growth, impacting cell proliferation, sensory attributes including appearance and taste, and is linked to carotenoid production and chlorophyll loss (Sergejeva et al., 2018). Khan et al. (2022) conducted research using specific wavelengths of green (515-525 nm), blue (464-474 nm), and red (617-627 nm) light to study post-harvest banana properties. LED light exposure promoted ethylene production, along with increased levels of polyphenols, sugars, and ascorbic acid, resulting in accelerated ripening, noticeable changes in peel colour, flesh firmness, and sugar content (Miao et al., 2019).
LED therapy enhanced banana ripening by promoting sugar accumulation and regulating starch-to-sugar conversion through starch-degrading enzymes (Trivellini et al., 2023). Different LED lights had significant effects on banana metabolism, with blue light being the most effective in accelerating banana ripening during storage, followed by red and green lights (Gill et al., 2017). Dhakal and Baek (2014) found that subjecting green tomatoes to blue light with a wavelength of 440-450 nm before placing them in dark conditions significantly accelerated their ripening. Compared to green tomatoes kept in the dark for the same period, those exposed to blue LED treatment showed a slower colour change from green to red over 7 days. In addition, red and dark LED-treated tomatoes were less firm than blue LED-treated tomatoes, with red LED-treated tomatoes showing the lowest firmness even after 21 days. Although blue light-treated tomatoes acquired lycopene at a slower rate, pre-treatment with blue light is an effective method to reduce ripening time and enhance postharvest economic benefits.
Kisvarga et al. (2023) observed an increased ethylene production in the blue light-exposed peaches. Additionally, blue light treatment appeared to sustain higher levels of total soluble solids while reducing the total titratable acid content in peach fruit. Meanwhile, Mashabela et al. (2015) demonstrated that pearl photo-selective nets, which allow increased transmittance of blue and red light relative to far-red light (R/FR and B/FR ratios), could influence ripening processes. In green peppers, higher R/FR photon ratios during development may reduce the activation of phytochrome, subsequently affecting genes related to ripening, including those responsible for colour, β-carotene, and lycopene production (Loconsole et al., 2019).
The influence of cover materials on fruit ripening and shelf life remains unclear. However, light receptors responding to light changes could play a key role in regulating the interactions between fruit maturation and nutrient metabolism (Rouphael et al. 2019). The timing of harvest, cultivar performance, fruit ripening, and nutrient ion metabolism can all be influenced by the intricate interplay between light and the penetration of cover materials into the crop canopy and onto the fruit surface (Fan et al., 2019). By carefully adjusting the spectral composition, intensity, and duration of light exposure enables researchers and growers can influence the timing, duration, and abundance of flowering and fruiting. Specific light cues can trigger or inhibit these processes, affecting plant hormone levels, floral initiation, and fruit development. This knowledge facilitates targeted interventions to enhance reproductive processes, thereby improving crop productivity and economic outcomes.
5.4. Manipulation of Light on Reducing Incidence of Pest and Disease Pressure
Manipulation of light is emerging as a promising strategy for managing pest and disease pressure in plants. Light treatments can disrupt pest behaviour, impede pathogen development, or enhance plant defences, effectively reducing infestation and disease severity (Ahn et al., 2015). As LED technology advances, the integration of light quality into pest management strategies is gaining traction, reducing reliance on environmentally harmful chemicals (Gallé et al., 2021). While studies demonstrate the potential of LEDs to suppress disease and pests in economically significant crops, it is crucial to consider the indirect effects of light-quality treatments on plant growth and development (Loi et al., 2020).
Light not only governs fundamental metabolic processes and plant growth aspects like flowering, stem elongation, and morphology, but it also plays a pivotal role in regulating the intricate system that oversees the production and accumulation of secondary metabolites through the modulation of photosensory signalling pathways led by photoreceptors (Rahman et al., 2010). Secondary metabolites serve multiple functions, including defense against herbivores, communication within or between species, protection against harmful solar radiation, and as cues for pollination or seed dispersal (Murdoch et al., 2013). Manipulating the light spectrum stands as an intriguing strategy to actively and precisely intervene in biosynthetic pathways, augmenting the levels of vital phytochemicals that can be harnessed to enhance growth and the ultimate product quality, ultimately bolstering plant fitness (Gallé et al., 2021).
Table 3 illustrates the effects of UV and LED light on key secondary metabolites in controlled plant cultivation, offering valuable insights into their influence on plant health and resilience.
Illuminating plants with specific LED wavelengths can mitigate insect infestation and reduce plant diseases in agricultural environments, ultimately enhancing production efficiency by limiting crop losses (Kook et al., 2013). Light-triggered signalling pathways that intersect with plant defence mechanisms highlight the importance of light quality in enhancing plant resistance to diseases (Ahn et al., 2015). Light quality affects the accumulation of secondary metabolites, such as flavonoids, which are closely linked to plant immunity, disease susceptibility, and interactions with insects. While numerous studies have highlighted how light-induced secondary metabolites can enhance the nutritional qualities of plant products, there is a paucity of research investigating the degree of plant protection resulting from metabolite-based resistance triggered by light (Kim et al., 2013).
For instance, ultraviolet LEDs, particularly those peaking in the ultraviolet-B (UV-B; 320 to 290 nm) range, have proven effective in suppressing powdery mildew caused by Sphaerotheca aphanis in strawberries and Podosphaera pannosa in roses (Meyer et al., 2021). Blue LEDs have demonstrated the ability to inhibit the development of Botrytis cinerea in detached tomato leaves and reduce the incidence of black leaf mold (Pseudocercospora fuligena) in greenhouse-grown tomatoes (Hui et al., 2017). Green LEDs have been found useful in mitigating strawberry anthracnose (Glomerella cingulata), perilla leaf spot disease (Corynespora cassiicola), cucumber anthracnose (Colletotrichum orbiculare), and gray mold (Botrytis cinerea) (Peralta-Ruiz et al., 2023). Red LEDs, too, have exhibited disease resistance effects, suppressing powdery mildew in roses due to Podosphaera pannosa, Sphaerotheca fuliginea in cucumbers, and downy mildew in basil caused by Peronospora belbahrii (Carvalho and Castillo, 2018).
The incorporation of narrowband LEDs into traditional insect traps has proven to be an interesting method to enhance their attractiveness, specificity, and adaptability (Kim et al., 2013). Adding UV, green, and/or yellow LEDs to insect traps increases their capture efficiency for various pests such as fungus gnats (Bradysia difformis), oriental fruit flies (Bactrocera dorsalis), sweet potato weevils (Euscepes postfasciatus), greenhouse whiteflies (Trialeurodes vaporariorum), red flour beetles (Tribolium castaneum), biting midges (Culicoides brevitarsis), cotton bollworms (Helicoverpa armigera), and others, outperforming traps without LED supplementation (Meyer et al., 2021).
Controlled exposure to low levels of UV-B light for 6 hours effectively reduced powdery mildew infection in densely grown roses in greenhouses, enhancing the production of secondary bioactive compounds and acting as a preventive measure against disease (Ahn et al., 2015). Red LED light has the potential to reduce powdery mildew disease in roses by reducing the amount of conidia produced (Gallé et al., 2021). Blue light and UV radiation have shown promise in reducing B. cinerea growth on harvested vegetables, indicating their potential use for gray mold-sensitive ornamental crops (Hui et al., 2017). The antibacterial properties of LEDs were investigated in fresh pineapple slices exposed to different intensities (254.7, 147.7, and 92 µW cm-2) and temperatures (25℃, 16℃, and 7℃) (Ghate et al. 2017). The research found that the samples exposed to blue light (460 nm) had more antibacterial effects compared to a control group and temperature had a significant impact on antibacterial activity, while irradiation did not affect it.
Photo-selective filtering of sunlight can influence plant pests and diseases, making crops under these shade nets susceptible to infestation (Suthaparan et al., 2010). Despite openings that may permit aphids, whiteflies, and thrips to pass through, their responses differ to various photo-selective shade nets (Peralta-Ruiz et al., 2023). Whiteflies are drawn to yellow nets, while thrips show an affinity for blue ones (Carvalho and Castillo, 2018). Coloured cladding materials hold promise for enhancing agricultural productivity and insect control. They have the potential to enhance crop protection by visually deterring or obstructing pests, reducing the need for pesticides (Kook et al., 2013).
Goren et al. (2011) observed that red peppers grown under 35% pearl and yellow shade nets exhibited significantly improved fruit quality preservation over two consecutive years. This enhancement was evident after 16 days at 7℃ and three days at 20℃, primarily attributed to reduced decay incidence caused by
Alternaria alternata. No significant differences were found in weight loss, hardness, or total soluble solids among fruits grown under differently coloured shade nets. Pearl and yellow shade nets notably reduced the population of
Alternaria spp. in the field, as confirmed by
Alternaria-selective growth media. In contrast, the red shade net showed the highest
Alternaria population (Loi et al., 2020). This innovative approach holds great potential for enhancing crop health, minimizing reliance on chemical interventions, and promoting environmentally friendly agricultural practices.
Table 4 depicts the effects of LED light spectra on induced disease resistance in crops, providing valuable insights into harnessing light to bolster plant defences and mitigate disease impacts.
5.5. Manipulation of Light on Crop Yield
Yield is crucial in assessing production processes, and manipulating light is a potent method for boosting crop productivity. Given light's role in photosynthesis and plant growth, additional lighting is often employed to enhance yields (Paradiso and Proietti, 2022). By adjusting the spectral composition, intensity, and duration of light exposure, researchers and farmers can optimize photosynthesis, nutrient uptake, and plant growth, leading to higher yields. Growers encounter challenges in providing adequate lighting due to factors like shading, cloud cover, and seasonal changes, leading to spatial and temporal light distribution variability (Brazaitytė et al., 2021). Given the diverse light characteristics perceived by plants, it is evident that at least one source of broad-spectrum light, whether from natural lighting, metal halide (MH), High-Pressure Sodium (HPS) lamps, or white and multi-spectral LED arrays, is essential (Jones, 2018). Beyond this requirement, there are further opportunities to tailor the specific light environment for optimal plant growth and increase overall plant production.
Glasshouses have long relied on supplemental overhead lighting to enhance crop productivity during periods of reduced natural light, whether to extend shorter winter days or during inclement weather (Appolloni et al., 2021). In general, a 1% increase in illumination leads to a 1% rise in yield, although interactions with other factors such as temperature and CO2 can complicate this relationship (Brazaitytė et al., 2021). Despite these evident benefits, several studies highlight the diverse responses of different crops to supplemental lighting regimes (Gao, 2015). Therefore, customized lighting regimes for specific crops, considering the local natural lighting conditions, may be more beneficial than a uniform approach.
Under extremely low light intensity, variation in light quality conditions didn't significantly impact yield and yield components. The decrease in rice yield, including low spikelet filling and a drop in 1000-grain weight, was mainly associated with reduced light intensity (Hu, 2018). In contrast, barley seedlings grown under blue light had a significantly higher photosynthetic rate compared to those under red and blue light at the same light intensity. This increase in the blue light fraction notably elevated plant protein content (Jones, 2018).
A pot experiment by Chen et al. (2021) revealed that the effects of light and water treatments on the influence on yield and 2AP (2-acetyl-1-pyrroline) production in two prominent Chinese aromatic rice varieties, Xiangyaxiangzhan and Yuxiangyouzhan. Compared to the control (natural light and adequate water), low light and well-watered conditions (LL-WW) as well as low light with water stress (LL-WS) significantly reduced grain production in Xiangyaxiangzhan by 39.2% and 34.6%, respectively. Compared to the control, grain yield in Yuxiangyouzhan was reduced by 25.4% with LL-WS and 30.8% with LL-WW. These treatments also decreased the proportion of filled grain, with LL-WW and LL-WS experiencing a significant decrease compared to control.
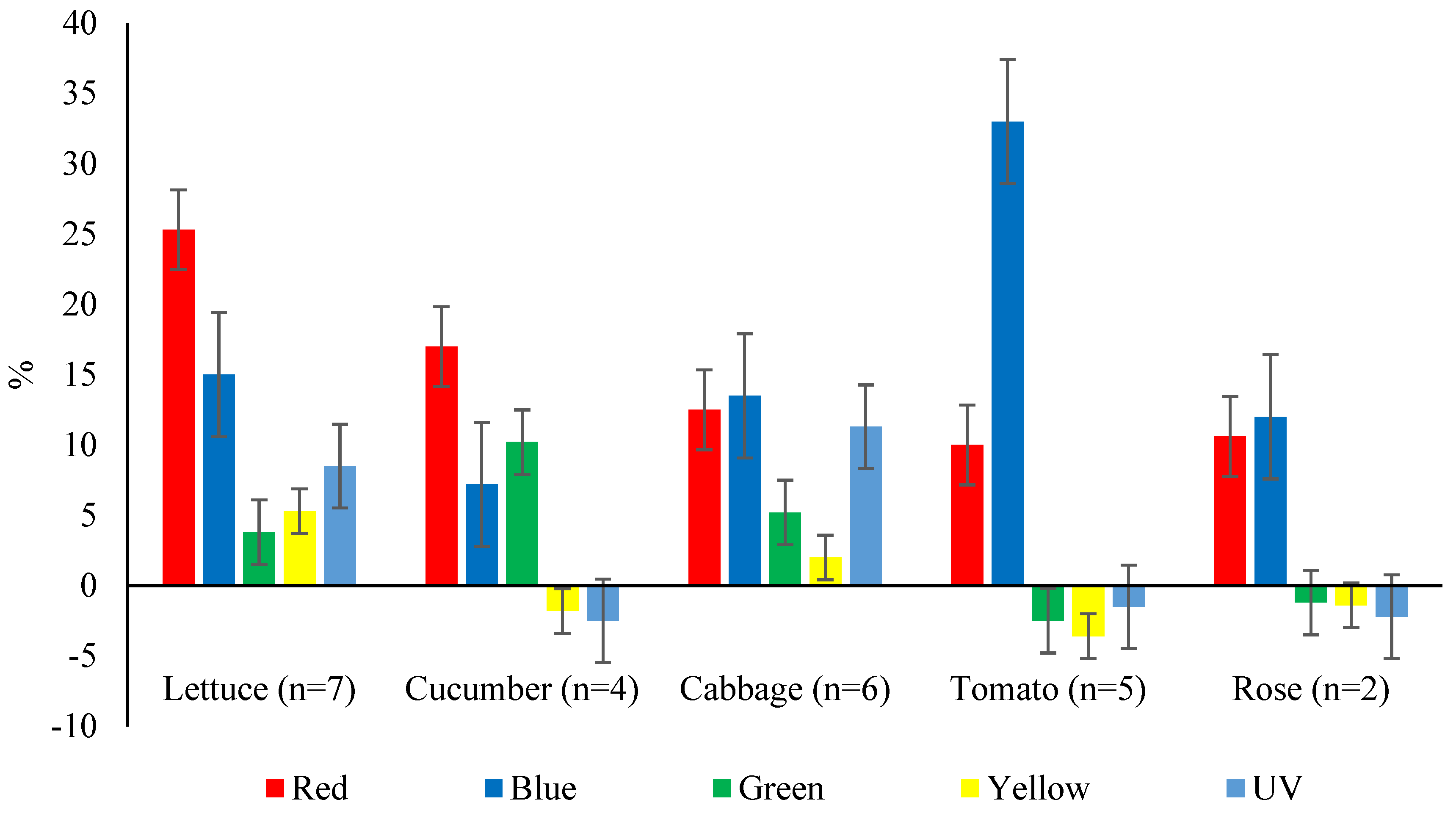
Figure 2 illustrates the impact of diverse light spectra on crop yields in controlled environments, observed across various studies.
6. Unexplored Area of Light Manipulation
The use of artificial lighting in horticulture, whether as the primary source or to complement natural sunlight, is a hot topic. There's a growing interest in exploring lighting strategies to increase crop yield, hasten plant growth, and optimize water and nutrient usage (Rahman et al., 2021). The choice of light source can have diverse effects on plant development, influencing factors such as stem elongation, leaf expansion, root growth, and nutrient levels. These interactions are complex and vary depending on the plant species (Paradiso and Proietti, 2022). An unexplored area of light manipulation for crop development is the optimization of dynamic light settings to mimic natural conditions.
Plants in natural environments experience fluctuating light intensity and spectral composition, affecting various aspects of growth and development. Mimicking these conditions in controlled environments could improve crop production by aligning with natural responses of plants (Schumann et al., 2017). A method to create dynamic light environments is through modern lighting technology, like dynamic LED systems. These can mimic natural light patterns by adjusting intensity, spectral composition, and spatial distribution over time (Chew et al., 2017). Researchers can study plant responses to more realistic light conditions by simulating diurnal and seasonal sunlight fluctuations, potentially uncovering new strategies for increasing agricultural output and resilience (Rahman et al., 2021). Integrating dynamic light with other variables like temperature and humidity could reveal how plants optimize growth and resource use in response to environmental cues.
Recent technological advances in LEDs have revolutionized the horticulture sector, surpassing traditional light sources such as fluorescent lamps. LEDs offer superior energy efficiency, longer lifetime (up to ~50,000 hours), reduced heat generation, and significant user and environmental safety advantages due to their lack of toxic elements like mercury found in fluorescent lamps (Viana et al., 2022). LEDs allow for precise wavelength selection, minimizing energy waste on less crucial spectrum components for plant photomorphogenesis and photosynthesis (Schumann et al., 2017). Exploring the impact of dynamic light conditions on plant-microbe interactions and soil microbiome dynamics holds promise for advancing sustainable farming practices. Light-induced changes in plant physiology and exudate composition can influence rhizosphere microbial communities, impacting nutrient cycling, disease suppression, and soil health (Li et al., 2023). Understanding these interactions may lead to light-based strategies to enhance soil fertility and plant health.
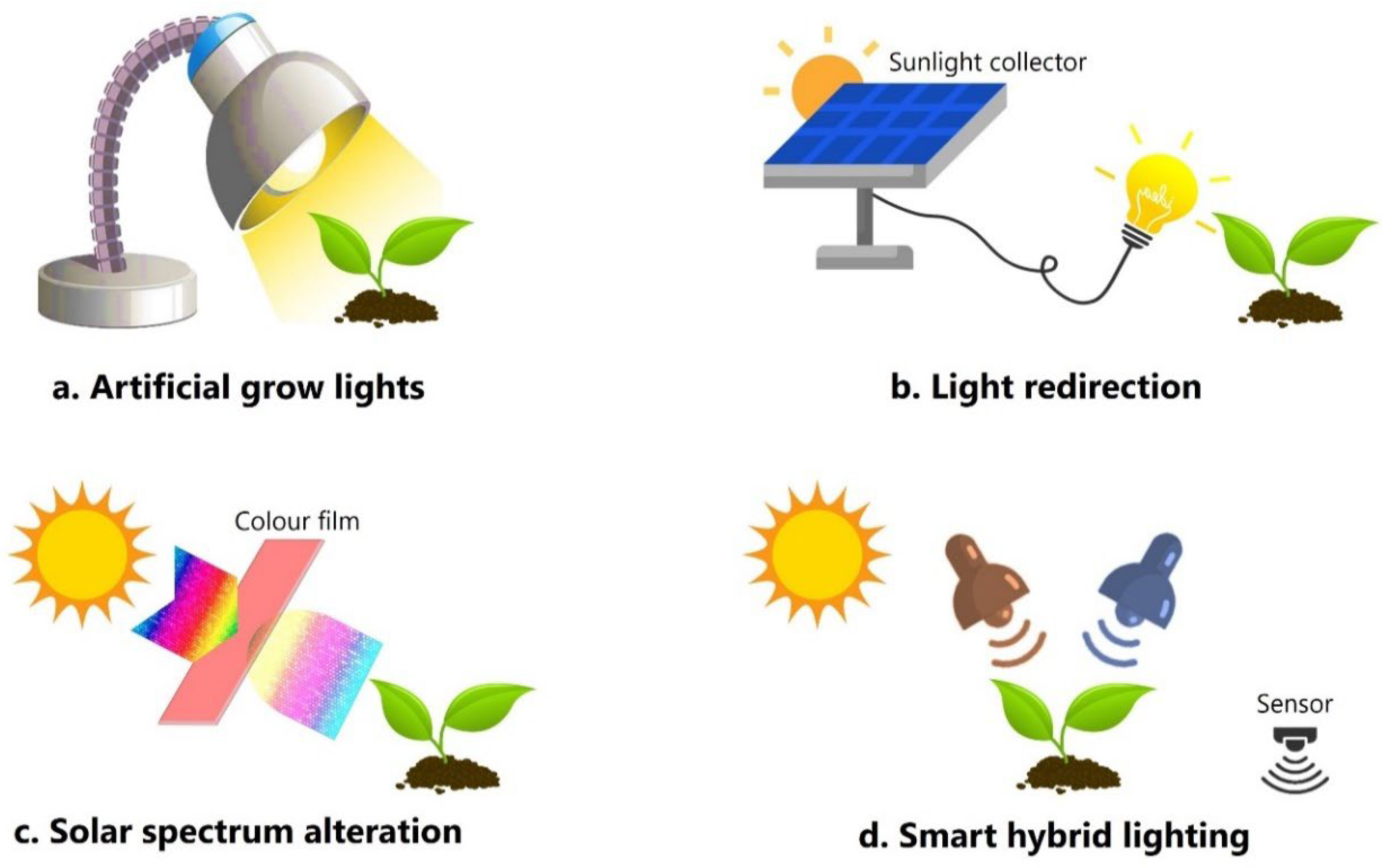
The integration of IoT architectures and distributed monitoring systems provides a practical solution for incorporating sensors, actuators, and wireless connectivity into traditional greenhouses (Rayhana et al., 2020). This integration ensures stable environmental conditions by collecting sensor data for control functions. IoT infrastructures are popular due to their effective balance of data transmission coverage, cost-effectiveness, and low power consumption, finding applications in environmental monitoring and industrial settings (Farooq et al., 2022). While WiFi is widely used, its relatively high-power consumption makes it less ideal. Bluetooth or ZigBee are viable alternatives, but their limited transmission ranges, spanning hundreds of meters, are insufficient for monitoring extensive greenhouse installations encompassing multiple greenhouses over hundreds of meters (Rayhana et al., 2020). The study of dynamic light conditions for optimizing crop development is a promising yet underexplored area. By simulating realistic light conditions and integrating them with environmental factors, researchers may revolutionize sustainable agriculture. Further exploration could lead to innovative approaches addressing global food security challenges. In the cutting-edge lighting systems for smart farming, dynamic LEDs are used to mimic natural light to enhance crop growth (
Figure 3).
7. Prospects
Both conventional and modern lighting systems, such as LEDs, are employed to supplement natural light in greenhouse settings and have been extensively studied across various species, using different combinations to enhance photosynthesis of bioactive phytochemicals and overall production. With the ongoing climate change and environmental stimuli, LED-based lighting solutions have become more popular among horticulturists in recent years. LED illumination has been demonstrated to control various anatomical, morphological, physiological, photosynthetic, developmental, and metabolic aspects in horticultural species. This wealth of data enables breeding companies to focus on the traits and qualities relevant to each crop species, developing LED light-efficient genotypes. It's crucial that researchers fully understand this information while considering how diverse environmental conditions and practices may influence outcomes, determining the suitability of introducing light-use efficient genotypes into commercial practice.
Recent studies have shown that LEDs can impact metabolic and physiological processes in vegetables by altering gene and enzyme expression in biosynthetic pathways. Future studies should investigate the effects of supplemental green (G) LED light combined with basal red (R) and blue (B) light on photosynthesis to directly influence secondary metabolism near the blue light spectrum. While green light is not as strongly absorbed by chlorophylls, it can penetrate deeper into leaf tissue and be absorbed by chloroplasts in lower leaves under a canopy, thereby increasing photosynthesis under the prevailing conditions. An interesting area for future research is the investigation of the phytochemical composition of various edible horticultural species, which are known for their high antioxidant content and used in pharmaceuticals. Significant breakthroughs are expected from interdisciplinary research and development efforts involving close collaboration among LED manufacturers, greenhouse growers, researchers, and government stakeholders.
8. Conclusions
Light is essential for regulating crucial aspects of plant growth and development, impacting flowering, structure, nutrient uptake, secondary metabolite production, and yield. Research into various combinations of light, ranging from monochromatic to polychromatic spectra (including UV to FR), has extensively explored their proportions and effects on different plant species. Key highlights include: a) the critical role of the ratio of red (R) to blue (B) light and R to far-red (FR) light in regulating high-quality plant growth; b) the essential role of blue light in enhancing the production and accumulation of phytochemical compounds, thereby boosting plant antioxidant activity; and c) the potential for exploring the effects of ultraviolet (UV) radiation on plant development and secondary metabolism, despite its limited current use in horticulture. While initial studies focused on red (R) and blue (B) light due to chlorophyll absorption peaks, recent research is expanding to include white (W), green (G), and far-red (FR) light, demonstrating their impact on physiological and metabolic processes. Further research is crucial to validate the optimal wavelength combinations for major plant species and phytochemicals, as well as to explore the effects of less studied wavelengths such as UV, yellow (Y), and green (G).
This report presents a comprehensive study of light manipulation and the effects of LED lighting on crops, covering a wide range of plant species and types. The analysis highlights that red LED lighting (45%) and blue LED illumination (40%) are the most prevalent light spectra for indoor-grown plants. However, far-red (5%) LED light is primarily suited to specific plant applications and is rarely used in greenhouses. Despite its potential benefits, such as compensation for shading, far-red LED illumination has not seen widespread adoption, possibly due to its recent introduction in commercial LED fixtures and the lack of conclusive data on its impact on maximizing plant nutritional quality. These investigations are expected to improve our understanding of the effects of spectral quality on plant development and morphology and highlight the importance of LED lighting in CEA.
Acknowledgments
The authors acknowledge the Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India, for providing library and web source facilities for the review paper. WJ was supported by the China Postdoctoral Foundation under Grant Number 393031.
Competing interests: The authors have no conflict of interest.
References
- Achour:, Y. : Ouammi, A. and Zejli, D., 2021. Technological progresses in modern sustainable greenhouses cultivation as the path towards precision agriculture. Renewable and Sustainable Energy Reviews, 147, p.111251. [CrossRef]
- Ahn, S.Y. , Kim, S.A. and Yun, H.K., 2015. Inhibition of Botrytis cinerea and accumulation of stilbene compounds by light-emitting diodes of grapevine leaves and differential expression of defense-related genes. European journal of plant pathology, 143, pp.753-765. [CrossRef]
- Alrajhi, A.A. , Alsahli, A.S., Alhelal, I.M., Rihan, H.Z., Fuller, M.P., Alsadon, A.A. and Ibrahim, A.A., 2023. The effect of LED light spectra on the growth, yield and nutritional value of red and green lettuce (Lactuca sativa). Plants, 12(3), p.463. [CrossRef]
- Alsadon, A. , Al-Helal, I., Ibrahim, A., Abdel-Ghany, A., Al-Zaharani, S. and Ashour, T., 2016. The effects of plastic greenhouse covering on cucumber (Cucumis sativus L.) growth. Ecological Engineering, 87, pp.305-312. [CrossRef]
- Alsanius, B.W. , Karlsson, M., Rosberg, A.K., Dorais, M., Naznin, M.T., Khalil, S. and Bergstrand, K.J., 2019. Light and microbial lifestyle: The impact of light quality on plant–microbe interactions in horticultural production systems—A review. Horticulturae, 5(2), p.41. [CrossRef]
- Anjum, S. , Abbasi, B.H., Doussot, J., Favre-Réguillon, A. and Hano, C., 2017. Effects of photoperiod regimes and ultraviolet-C radiations on biosynthesis of industrially important lignans and neolignans in cell cultures of Linum usitatissimum L. (Flax). Journal of Photochemistry and Photobiology B: Biology, 167, pp.216-227. [CrossRef]
- Appolloni, E. , Orsini, F., Pennisi, G., Gabarrell Durany, X., Paucek, I. and Gianquinto, G., 2021. Supplemental LED lighting effectively enhances the yield and quality of greenhouse truss tomato production: Results of a meta-analysis. Frontiers in plant science, 12, p.596927. [CrossRef]
- Bai, G. , Tsang, M.K. and Hao, J., 2015. Tuning the luminescence of phosphors: beyond conventional chemical method. Advanced Optical Materials, 3(4), pp.431-462. [CrossRef]
- Bao, Y. , Liu, X., Feng, C.H., Niu, M.X., Liu, C., Wang, H.L., Yin, W. and Xia, X., 2024. Light and Light Signals Regulate Growth and Development in Woody Plants. Forests, 15(3), p.523. [CrossRef]
- Bartman, C.M. , Oyama, Y., Brodsky, K., Khailova, L., Walker, L., Koeppen, M. and Eckle, T., 2017. Intense light-elicited upregulation of miR-21 facilitates glycolysis and cardioprotection through Per2-dependent mechanisms. PloS one, 12(4), p.e0176243. [CrossRef]
- Bartucca, M.L. , Del Buono, D., Ballerini, E., Benincasa, P., Falcinelli, B. and Guiducci, M., 2020. Effect of light spectrum on gas exchange, growth and biochemical characteristics of einkorn seedlings. Agronomy, 10(7), p.1042. [CrossRef]
- Bhatla, S.C., A. Lal, M., Kathpalia, R. and Bhatla, S.C., 2018. Plant mineral nutrition. Plant physiology, development and metabolism, pp.37-81. [CrossRef]
- Bi, X. , Xu, H., Yang, C., Zhang, H., Li, W., Su, W., Zheng, M. and Lei, B., 2024. Investigating the influence of varied ratios of red and far-red light on lettuce (Lactuca sativa): effects on growth, photosynthetic characteristics and chlorophyll fluorescence. Frontiers in Plant Science, 15, p.1430241. [CrossRef]
- Bosso, A. , Tortora, F., Culurciello, R., Di Nardo, I., Pistorio, V., Carraturo, F., Colecchia, A., Di Girolamo, R., Cafaro, V., Notomista, E. and Ingenito, R., 2023. Simultaneous Irradiation with UV-A,-B, and-C Lights Promotes Effective Decontamination of Planktonic and Sessile Bacteria: A Pilot Study. International Journal of Molecular Sciences, 24(16), p.12951. [CrossRef]
- Boucher, L. , Nguyen, T.T.A., Brégard, A., Pepin, S. and Dorais, M., 2023. Optimizing Light Use Efficiency and Quality of Indoor Organically Grown Leafy Greens by Using Different Lighting Strategies. Agronomy, 13(10), p.2582. [CrossRef]
- Brazaitytė, A. , Miliauskienė, J., Vaštakaitė-Kairienė, V., Sutulienė, R., Laužikė, K., Duchovskis, P. and Małek, S., 2021. Effect of different ratios of blue and red led light on brassicaceae microgreens under a controlled environment. Plants, 10(4), p.801. [CrossRef]
- Bucky, A. , Pičmanová, M., Porley, V., Pont, S., Austin, C., Khan, T., McDougall, G., Johnstone, A. and Stewart, D., 2024. Light manipulation as a route to enhancement of antioxidant properties in red amaranth and red lettuce. Frontiers in Nutrition, 11, p.1386988. [CrossRef]
- Budavári, N. , Pék, Z., Helyes, L., Takács, S. and Nemeskéri, E., 2024. An Overview on the Use of Artificial Lighting for Sustainable Lettuce and Microgreens Production in an Indoor Vertical Farming System. Horticulturae, 10(9), p.938. [CrossRef]
- Cai, K. , Zhu, S., Jiang, Z., Xu, K., Sun, X. and Li, X., 2024. Biological macromolecules mediated by environmental signals affect flowering regulation in plants: A comprehensive review. Plant Physiology and Biochemistry, p.108931. [CrossRef]
- Cai, X. and Huq, E., 2024. Shining Light on Plant Growth: Recent Insights into Phytochrome Interacting Factors. Journal of Experimental Botany, p.erae276. [CrossRef]
- Cao, K. , Yu, J., Xu, D., Ai, K., Bao, E. and Zou, Z., 2018. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC plant biology, 18(1), pp.1-12. [CrossRef]
- Carvalho, S.D. and Castillo, J.A., 2018. Influence of light on plant–phyllosphere interaction. Frontiers in plant science, 9, p.1482. [CrossRef]
- Cavallaro, V. , Pellegrino, A., Muleo, R. and Forgione, I., 2022. Light and plant growth regulators on in vitro proliferation. Plants, 11(7), p.844. [CrossRef]
- Chandel, A. , Thakur, M., Singh, G., Dogra, R., Bajad, A., Soni, V. and Bhargava, B., 2023. Flower regulation in floriculture: an agronomic concept and commercial use. Journal of Plant Growth Regulation, 42(4), pp.2136-2161. [CrossRef]
- Chen, J. , Xie, W., Huang, Z., Ashraf, U., Pan, S., Tian, H., Duan, M., Wang, S., Tang, X. and Mo, Z., 2021. Light quality during booting stage modulates fragrance, grain yield and quality in fragrant rice. Journal of Plant Interactions, 16(1), pp.42-52. [CrossRef]
- Chew, I. , Karunatilaka, D., Tan, C.P. and Kalavally, V., 2017. Smart lighting: The way forward? Reviewing the past to shape the future. Energy and Buildings, 149, pp.180-191. [CrossRef]
- Cope, K.R. and Bugbee, B., 2013. Spectral effects of three types of white light-emitting diodes on plant growth and development: absolute versus relative amounts of blue light. HortScience, 48(4), pp.504-509. [CrossRef]
- Craig, D.S. and Runkle, E.S., 2016. An intermediate phytochrome photoequilibria from night-interruption lighting optimally promotes flowering of several long-day plants. Environmental and Experimental Botany, 121, pp.132-138. [CrossRef]
- Cuong, D.M. , Ha, T.W., Park, C.H., Kim, N.S., Yeo, H.J., Chun, S.W., Kim, C. and Park, S.U., 2019. Effects of LED lights on expression of genes involved in phenylpropanoid biosynthesis and accumulation of phenylpropanoids in wheat sprout. Agronomy, 9(6), p.307. [CrossRef]
- d’Aquino, L. , Cozzolino, R., Malorni, L., Bodhuin, T., Gambale, E., Sighicelli, M., Della Mura, B., Matarazzo, C., Piacente, S. and Montoro, P., 2024. Light Flux Density and Photoperiod Affect Growth and Secondary Metabolism in Fully Expanded Basil Plants. Foods, 13(14). [CrossRef]
- Dhakal, R. and Baek, K.H., 2014. Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest biology and Technology, 90, pp.73-77. [CrossRef]
- Dsouza, A. , Dixon, M., Shukla, M. and Graham, T., 2024. Harnessing controlled environment systems for enhanced production of medicinal plants. Journal of Experimental Botany, p.erae248. [CrossRef]
- Dueck, T. , Trouwborst, G., Hogewoning, S.W. and Meinen, E., 2016. Can a high red: Far red ratio replace temperature-induced inflorescence development in Phalaenopsis?. Environmental and Experimental Botany, 121, pp.139-144. [CrossRef]
- Evans, J.R. , 1987. The dependence of quantum yield on wavelength and growth irradiance. Functional Plant Biology, 14(1), pp.69-79. [CrossRef]
- Fan, X.X. , Xue, F., Song, B., Chen, L.Z., Xu, G. and Xu, H., 2019. Effects of blue and red light on growth and nitrate metabolism in pakchoi. Open Chemistry, 17(1), pp.456-464. [CrossRef]
- Farooq, M.S. , Riaz, S., Helou, M.A., Khan, F.S., Abid, A. and Alvi, A., 2022. Internet of Things in Greenhouse Agriculture: A Survey on Enabling Technologies, Applications, and Protocols. IEEE Access, 10, pp.53374-53397. [CrossRef]
- Fayezizadeh, M.R. , Ansari, N.A., Sourestani, M.M., Fujita, M. and Hasanuzzaman, M., 2024. Management of Secondary Metabolite Synthesis and Biomass in Basil (Ocimum basilicum L.) Microgreens Using Different Continuous-Spectrum LED Lights. Plants, 13(10), p.1394. [CrossRef]
- Ferrante, A. , Nocito, F.F., Morgutti, S. and Sacchi, G.A., 2017. Plant breeding for improving nutrient uptake and utilization efficiency. Advances in research on fertilization management of vegetable crops, pp.221-246. [CrossRef]
- Fortineau, A. , Combes, D., Richard-Molard, C., Frak, E. and Jullien, A., 2021. LightCue: An Innovative Far-Red Light Emitter for Locally Modifying the Spectral Cue in Outdoor Conditions with Global Consequences on Plant Architecture. Plants, 10(11), p.2483. [CrossRef]
- Fukuda, N. , Ajima, C., Yukawa, T. and Olsen, J.E., 2016. Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environmental and Experimental Botany, 121, pp.102-111. [CrossRef]
- Gallé, Á. , Czékus, Z., Tóth, L., Galgóczy, L. and Poór, P., 2021. Pest and disease management by red light. Plant, Cell & Environment, 44(10), pp.3197-3210. [CrossRef]
- Gao, B. , 2015. Effects of different light quaility and nutrient solutions on growth, production, quality and photosynthetic characteristics of celery using lighting-emitting diodes (LED) [M. D. Dissertation]. Yangling: Northwest A & F University (in Chinese).
- Gawande, V. , Raut, D., Rai, S., Beese, S., Singh, B.V. and Agnihotri, N., 2023. Artificial Light Spectra and Its Impact on Plant Physiological Processes and Secondary Metabolism. International Journal of Plant & Soil Science, 35(18), pp.2060-2070. [CrossRef]
- Gendron, J.M. and Staiger, D., 2023. New horizons in plant photoperiodism. Annual review of plant biology, 74(1), pp.481-509. [CrossRef]
- Ghate, V. , Kumar, A., Kim, M.J., Bang, W.S., Zhou, W. and Yuk, H.G., 2017. Effect of 460 nm light emitting diode illumination on survival of Salmonella spp. on fresh-cut pineapples at different irradiances and temperatures. Journal of Food Engineering, 196, pp.130-138. [CrossRef]
- Gill, P.P.S. , Jawandha, S.K., Kaur, N. and Singh, N., 2017. Physico-chemical changes during progressive ripening of mango (Mangifera indica L.) cv. Dashehari under different temperature regimes. Journal of food science and technology, 54, pp.1964-1970. [CrossRef]
- Goren, A. , Alkalai-Tuvia, S., Perzelan, Y., Fallik, E. and Aharon, Z., 2011. Photoselective shade nets reduce postharvest decay development in pepper fruits. Photoselective Shade Nets Reduce Postharvest Decay Development in Pepper Fruits, pp.26-31.
- Gorjian, S. , Jamshidian, F.J., Gorjian, A., Faridi, H., Vafaei, M., Zhang, F., Liu, W. and Campana, P.E., 2023. Technological advancements and research prospects of innovative concentrating agrivoltaics. Applied Energy, 337, p.120799. [CrossRef]
- Han, J. , Guo, C., Ye, S., Zhang, L., Li, S., Wang, H. and Yu, G., 2020. Effects of diffuse photosynthetically active radiation on gross primary productivity in a subtropical coniferous plantation vary in different timescales. Ecological Indicators, 115, p.106403. [CrossRef]
- Hashim, M. , Ahmad, B., Drouet, S., Hano, C., Abbasi, B.H. and Anjum, S., 2021. Comparative effects of different light sources on the production of key secondary metabolites in plants in vitro cultures. Plants, 10(8), p.1521. [CrossRef]
- He, R. , Gao, M., Shi, R., Song, S., Zhang, Y., Su, W. and Liu, H., 2020. The combination of selenium and LED light quality affects growth and nutritional properties of broccoli sprouts. Molecules, 25(20), p.4788. [CrossRef]
- Hogewoning, S.W. , Trouwborst, G., Maljaars, H., Poorter, H., van Ieperen, W. and Harbinson, J., 2010. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. Journal of experimental botany, 61(11), pp.3107-3117. [CrossRef]
- Hong, J. , Meng, K., Thomas, H.R., Yang, Y., Williams, B., Kang, H. and Zhou, Y., 2024. Reframing agriculture by light: The role of light-mediated jasmonates/salicylic acid regulation in plant defense, development and beyond. Vegetable Research, 4(1). [CrossRef]
- Hossain, A. , Pamanick, B., Venugopalan, V.K., Ibrahimova, U., Rahman, M.A., Siyal, A.L., Maitra, S., Chatterjee, S. and Aftab, T., 2022. Emerging roles of plant growth regulators for plants adaptation to abiotic stress–induced oxidative stress. In Emerging plant growth regulators in agriculture (pp. 1-72). Academic Press. [CrossRef]
- Hu, J.W. , 2018. Effects of different proportions of red and blue light on the growth and physiological characteristics of mulberry seedlings. Acta Agric Boreali-Occident Sin, 33: 160–169 in Chinese.
- Huber, M. , Nieuwendijk, N.M., Pantazopoulou, C.K. and Pierik, R., 2021. Light signalling shapes plant–plant interactions in dense canopies. Plant, Cell & Environment, 44(4), pp.1014-1029. [CrossRef]
- Huché-Thélier, L. , Crespel, L., Le Gourrierec, J., Morel, P., Sakr, S. and Leduc, N., 2016. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environmental and Experimental Botany, 121, pp.22-38. [CrossRef]
- Hui, X.U. , FU, Y.N., LI, T.L. and Rui, W.A.N.G., 2017. Effects of different LED light wavelengths on the resistance of tomato against Botrytis cinerea and the corresponding physiological mechanisms. Journal of Integrative Agriculture, 16(1), pp.106-114. [CrossRef]
- Iezzi, B. , Afkhami, Z., Sanvordenker, S., Hoelzle, D., Barton, K. and Shtein, M., 2020. Electrohydrodynamic jet printing of 1d photonic crystals: Part ii—optical design and reflectance characteristics. Advanced Materials Technologies, 5(10), p.2000431. [CrossRef]
- Inada, K. , 1976. Action spectra for photosynthesis in higher plants. Plant and Cell Physiology, 17(2), pp.355-365. [CrossRef]
- Jones, M.A. , 2018. Using light to improve commercial value. Horticulture Research, 5. [CrossRef]
- Kaiser, E. , Morales, A., Harbinson, J., Kromdijk, J., Heuvelink, E. and Marcelis, L.F., 2015. Dynamic photosynthesis in different environmental conditions. Journal of Experimental Botany, 66(9), pp.2415-2426. [CrossRef]
- Keutgen, A.J. , 2023, May. Climate change: challenges and limitations in agriculture. In IOP Conference Series: Earth and Environmental Science (Vol. 1183, No. 1, p. 012069). IOP Publishing. [CrossRef]
- Khan, S. , Dar, A.H., Shams, R., Aga, M.B., Siddiqui, M.W., Mir, S.A., Rizvi, Q.U.E.H., Khan, S.A. and Altaf, A., 2022. Applications of ultraviolet light–emitting diode technology in horticultural produce: A systematic review and meta-analysis. Food and Bioprocess Technology, pp.1-11. [CrossRef]
- Kharshiing, E. , Sreelakshmi, Y. and Sharma, R., 2019. The light awakens! Sensing light and darkness. Sensory biology of plants, pp.21-57. [CrossRef]
- Khurshid, R. , Ullah, M.A., Tungmunnithum, D., Drouet, S., Shah, M., Zaeem, A., Hameed, S., Hano, C. and Abbasi, B.H., 2020. Lights triggered differential accumulation of antioxidant and antidiabetic secondary metabolites in callus culture of Eclipta alba L. Plos one, 15(6), p.e0233963. [CrossRef]
- Kim, K. , Kook, H., Jang, Y., Lee, W., Kamala-Kannan, S., Chae, J. and Lee, K., 2013. The effect of blue-light-emitting diodes on antioxidant properties and resistance to Botrytis cinerea in tomato. Journal of Plant Pathology and Microbiology, 4(9).
- Kirchhoff, H. , 2019. Chloroplast ultrastructure in plants. New Phytologist, 223(2), pp.565-574. [CrossRef]
- Kisvarga, S. , Horotán, K., Wani, M.A. and Orlóci, L., 2023. Plant responses to global climate change and urbanization: Implications for sustainable urban landscapes. Horticulturae, 9(9), p.1051. [CrossRef]
- Klein, F.R.S. , Reis, A., Kleinowski, A.M., Telles, R.T., Amarante, L.D., Peters, J.A. and Braga, E.J.B., 2018. UV-B radiation as an elicitor of secondary metabolite production in plants of the genus Alternanthera. Acta Botanica Brasilica, 32, pp.615-623. [CrossRef]
- Kohyama, F. , Whitman, C. and Runkle, E.S., 2014. Comparing flowering responses of long-day plants under incandescent and two commercial light-emitting diode lamps. HortTechnology, 24(4), pp.490-495. [CrossRef]
- Konapala, G. , Mishra, A.K., Wada, Y. and Mann, M.E., 2020. Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nature communications, 11(1), p.3044. [CrossRef]
- Kook, H.S. , Park, S.H., Jang, Y.J., Lee, G.W., Kim, J.S., Kim, H.M., Oh, B.T., Chae, J.C. and Lee, K.J., 2013. Blue LED (light-emitting diodes)-mediated growth promotion and control of Botrytis disease in lettuce. Acta Agriculturae Scandinavica, Section B–Soil & Plant Science, 63(3), pp.271-277. [CrossRef]
- Kukri, A. , Czékus, Z., Gallé, Á., Nagy, G., Zsindely, N., Bodai, L., Galgóczy, L., Hamow, K.Á., Szalai, G., Ördög, A. and Poór, P., 2024. Exploring the effects of red light night break on the defence mechanisms of tomato against fungal pathogen Botrytis cinerea. Physiologia Plantarum, 176(4), p.e14504. [CrossRef]
- Kulus, D. and Woźny, A., 2020. Influence of light conditions on the morphogenetic and biochemical response of selected ornamental plant species under in vitro conditions: A mini-review. BioTechnologia. Journal of Biotechnology Computational Biology and Bionanotechnology, 101(1), pp.75-83. [CrossRef]
- Kume, A. , 2017. Importance of the green color, absorption gradient, and spectral absorption of chloroplasts for the radiative energy balance of leaves. Journal of plant research, 130, pp.501-514. [CrossRef]
- Kunz, L.Y. , Redekop, P., Ort, D.R., Grossman, A.R., Cargnello, M. and Majumdar, A., 2020. A phytophotonic approach to enhanced photosynthesis. Energy & Environmental Science, 13(12), pp.4794-4807. [CrossRef]
- Kuppe, C. , Rusimova, K.R., Ohnoutek, L., Slavov, D. and Valev, V.K., 2020. “Hot” in plasmonics: temperature-related concepts and applications of metal nanostructures. Advanced Optical Materials, 8(1), p.1901166. [CrossRef]
- Lazzarin, M. , 2023. The role of far-red light in plant photosynthesis and photoprotection under artificial solar irradiance (Doctoral dissertation, Wageningen University and Research).
- Lee, M. , Rivard, C., Pliakoni, E., Wang, W. and Rajashekar, C.B., 2021. Supplemental UV-A and UV-B affect the nutritional quality of lettuce and tomato: Health-promoting phytochemicals and essential nutrients. American Journal of Plant Sciences, 12(01), p.104. http://www.scirp.org/journal/Paperabs.aspx? 1068. [Google Scholar]
- Lefers, R.M. , Tester, M. and Lauersen, K.J., 2020. Emerging technologies to enable sustainable controlled environment agriculture in the extreme environments of Middle East-North Africa coastal regions. Frontiers in Plant Science, 11, p.801. [CrossRef]
- Leister, D. , 2023. Enhancing the light reactions of photosynthesis: Strategies, controversies, and perspectives. Molecular plant, 16(1), pp.4-22. [CrossRef]
- Li, H. , Xu, Z. and Tang, C., 2010. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell, Tissue and Organ Culture (PCTOC), 103, pp.155-163. [CrossRef]
- Li, X.M. , Li, S., Huang, F.Y., Wang, Z., Zhang, Z.Y., Chen, S.C. and Zhu, Y.G., 2023. Artificial light at night triggers negative impacts on nutrients cycling and plant health regulated by soil microbiome in urban ecosystems. Geoderma, 436, p.116547. [CrossRef]
- Li, Y. , Liu, Z., Shi, Q., Yang, F. and Wei, M., 2021. Mixed red and blue light promotes tomato seedlings growth by influencing leaf anatomy, photosynthesis, CO2 assimilation and endogenous hormones. Scientia Horticulturae, 290, p.110500. [CrossRef]
- Liebers, M. and Pfannschmidt, T., 2024. New horizons in light control of plant photomorphogenesis and development. Frontiers in Photobiology, 1, p.1346705. [CrossRef]
- Lin, K.H. , Huang, M.Y. and Hsu, M.H., 2021. Morphological and physiological response in green and purple basil plants (Ocimum basilicum) under different proportions of red, green, and blue LED lightings. Scientia Horticulturae, 275, p.109677. [CrossRef]
- Liu, X. and Qiu, J., 2015. Recent advances in energy transfer in bulk and nanoscale luminescent materials: from spectroscopy to applications. Chemical Society Reviews, 44(23), pp.8714-8746. [CrossRef]
- Liu, X. , Shi, R., Gao, M., He, R., Li, Y. and Liu, H., 2023. Growth of tomato and cucumber seedlings under different light environments and their development after transplanting. Frontiers in Plant Science, 14, p.1164768. [CrossRef]
- Liu, Y. , Xue, J., Che, G., Guo, L. and Tang, J., 2022. Effects of blue light on physiological and biochemical changes in germinating cabbage sprouts. Agricultural and Food Science, 31(2), pp.78-88. [CrossRef]
- Loconsole, D. , Cocetta, G., Santoro, P. and Ferrante, A., 2019. Optimization of LED lighting and quality evaluation of romaine lettuce grown in an innovative indoor cultivation system. Sustainability, 11(3), p.841. [CrossRef]
- Loi, M. , Villani, A., Paciolla, F., Mulè, G. and Paciolla, C., 2020. Challenges and opportunities of light-emitting diode (Led) as key to modulate antioxidant compounds in plants. a review. Antioxidants, 10(1), p.42. [CrossRef]
- López, L.E.O. , Rodríguez-Fuentes, H., Luna-Maldonado, A.I., Márquez-Reyes, J.M. and Rojas, R., 2022. Effect of LED Light in Biomass Production and Presence of Metabolites in Medicinal Plants. In Biocontrol Systems and Plant Physiology in Modern Agriculture (pp. 201-220). Apple Academic Press.
- Mashabela, M.N. , Selahle, K.M., Soundy, P., Crosby, K.M. and Sivakumar, D., 2015. Bioactive compounds and fruit quality of green sweet pepper grown under different colored shade netting during postharvest storage. Journal of Food Science, 80(11), pp.H2612-H2618. [CrossRef]
- McCree, K.J. , 1972. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agricultural meteorology, 10, pp.443-453. [CrossRef]
- Meng, L. , Song, J., Ni, D. and Kamaruddin, M.A., 2024. Effects of far-red light on growth, endogenous hormones, antioxidant capacity and quality of Lettuce. Food Production, Processing and Nutrition, 6(1), p.25. [CrossRef]
- Meng, Q. and Runkle, E.S., 2014. Controlling flowering of photoperiodic ornamental crops with light-emitting diode lamps: A coordinated grower trial. HortTechnology, 24(6), pp.702-711. [CrossRef]
- Meyer, P. , Van de Poel, B. and De Coninck, B., 2021. UV-B light and its application potential to reduce disease and pest incidence in crops. Horticulture Research, 8. [CrossRef]
- Miao, Y. , Chen, Q., Qu, M., Gao, L. and Hou, L., 2019. Blue light alleviates ‘red light syndrome’by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Scientia Horticulturae, 257, p.108680. [CrossRef]
- Mishra, K. , Stanghellini, C. and Hemming, S., 2023. Technology and Materials for Passive Manipulation of the Solar Spectrum in Greenhouses. Advanced Sustainable Systems, 7(5), p.2200503. [CrossRef]
- Modarelli, G.C. , Paradiso, R., Arena, C., De Pascale, S. and Van Labeke, M.C., 2022. High light intensity from blue-red LEDs enhance photosynthetic performance, plant growth, and optical properties of red lettuce in controlled environment. Horticulturae, 8(2), p.114. [CrossRef]
- Murdoch, L.E. , McKenzie, K., Maclean, M., Macgregor, S.J. and Anderson, J.G., 2013. Lethal effects of high-intensity violet 405-nm light on Saccharomyces cerevisiae, Candida albicans, and on dormant and germinating spores of Aspergillus niger. Fungal Biology, 117(7-8), pp.519-527. [CrossRef]
- Nadeem, M. , Abbasi, B.H., Younas, M., Ahmad, W., Zahir, A. and Hano, C., 2019. LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. Journal of Photochemistry and Photobiology B: Biology, 190, pp.172-178. [CrossRef]
- Nalumaga, H. , 2022. The Luminescence of Lanthanide-doped NaMgF3 Nanoparticles: Radioluminescence, Radiophotoluminescence and Optically Stimulated Luminescence (Doctoral dissertation, Open Access Te Herenga Waka-Victoria University of Wellington).
- Nassarawa, S.S. , Abdelshafy, A.M., Xu, Y., Li, L. and Luo, Z., 2021. Effect of light-emitting diodes (LEDs) on the quality of fruits and vegetables during postharvest period: A review. Food and Bioprocess Technology, 14, pp.388-414. [CrossRef]
- Nazir, M. , Asad Ullah, M., Mumtaz, S., Siddiquah, A., Shah, M., Drouet, S., Hano, C. and Abbasi, B.H., 2020. Interactive effect of melatonin and UV-C on phenylpropanoid metabolite production and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Molecules, 25(5), p.1072. [CrossRef]
- Neo, D.C.J. , Ong, M.M.X., Lee, Y.Y., Teo, E.J., Ong, Q., Tanoto, H., Xu, J., Ong, K.S. and Suresh, V., 2022. Shaping and tuning lighting conditions in controlled environment agriculture: A review. ACS Agricultural Science & Technology, 2(1), pp.3-16. [CrossRef]
- Ntinas, G.K. , Kadoglidou, K., Tsivelika, N., Krommydas, K., Kalivas, A., Ralli, P. and Irakli, M., 2019. Performance and hydroponic tomato crop quality characteristics in a novel greenhouse using dye-sensitized solar cell technology for covering material. Horticulturae, 5(2), p.42. [CrossRef]
- Nwokolo, S.C. , Obiwulu, A.U. and Ogbulezie, J.C., 2023. Machine learning and analytical model hybridization to assess the impact of climate change on solar PV energy production. Physics and Chemistry of the Earth, Parts A/B/C, 130, p.103389. [CrossRef]
- Osnato, M. , Cota, I., Nebhnani, P., Cereijo, U. and Pelaz, S., 2022. Photoperiod control of plant growth: Flowering time genes beyond flowering. Frontiers in plant science, 12, p.805635. [CrossRef]
- Otanicar, T.P. , DeJarnette, D., Hewakuruppu, Y. and Taylor, R.A., 2016. Filtering light with nanoparticles: a review of optically selective particles and applications. Advances in Optics and Photonics, 8(3), pp.541-585. [CrossRef]
- Paradiso, R. and Proietti, S., 2022. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. Journal of Plant Growth Regulation, 41(2), pp.742-780. [CrossRef]
- Peralta-Ruiz, Y. , Rossi, C., Grande-Tovar, C.D. and Chaves-López, C., 2023. Green Management of Postharvest Anthracnose Caused by Colletotrichum gloeosporioides. Journal of Fungi, 9(6), p.623. [CrossRef]
- Pertl-Obermeyer, H. , Gimeno, A., Kuchler, V., Servili, E., Huang, S., Fang, H., Lang, V., Sydow, K., Pöckl, M., Schulze, W.X. and Obermeyer, G., 2022. pH modulates interaction of 14-3-3 proteins with pollen plasma membrane H+ ATPases independently from phosphorylation. Journal of Experimental Botany, 73(1), pp.168-181. [CrossRef]
- Proietti, S. , Scariot, V., De Pascale, S. and Paradiso, R., 2022. Flowering mechanisms and environmental stimuli for flower transition: Bases for production scheduling in greenhouse floriculture. Plants, 11(3), p.432. [CrossRef]
- Rahman, M.M. , Field, D.L., Ahmed, S.M., Hasan, M.T., Basher, M.K. and Alameh, K., 2021. LED illumination for high-quality high-yield crop growth in protected cropping environments. Plants, 10(11), p.2470. [CrossRef]
- Rahman, M.Z. , Khanam, H., Ueno, M., Kihara, J., Honda, Y. and Arase, S., 2010. Suppression by red light irradiation of Corynespora leaf spot of cucumber caused by Corynespora cassiicola. Journal of Phytopathology, 158(5), pp.378-381. [CrossRef]
- Ramanna, L. , Rawat, I. and Bux, F., 2017. Light enhancement strategies improve microalgal biomass productivity. Renewable and Sustainable Energy Reviews, 80, pp.765-773. [CrossRef]
- Rayhana, R. , Xiao, G. and Liu, Z., 2020. Internet of things empowered smart greenhouse farming. IEEE Journal of Radio Frequency Identification, 4(3), pp.195-211. [CrossRef]
- Razzak, M.A. , Asaduzzaman, M., Tanaka, H. and Asao, T., 2022. Effects of supplementing green light to red and blue light on the growth and yield of lettuce in plant factories. Scientia Horticulturae, 305, p.111429. [CrossRef]
- Rezaei, S. , Zarei, H., Nikbakht, A. and Sabzalian, M.R., 2024. Supplementary Top LED Lighting Improved Physiological Parameters and the Postharvest Life of Cut Rose Flowers. Journal of Plant Growth Regulation, 43(1), pp.122-134. [CrossRef]
- Rouphael, Y. , Petropoulos, S.A., El-Nakhel, C., Pannico, A., Kyriacou, M.C., Giordano, M., Troise, A.D., Vitaglione, P. and De Pascale, S., 2019. Reducing energy requirements in future Bioregenerative Life Support Systems (BLSSs): Performance and bioactive composition of diverse lettuce genotypes grown under optimal and suboptimal light conditions. Frontiers in Plant Science, 10, p.1305. [CrossRef]
- Schumann, T. , Paul, S., Melzer, M., Dörmann, P. and Jahns, P., 2017. Plant growth under natural light conditions provides highly flexible short-term acclimation properties toward high light stress. Frontiers in Plant Science, 8, p.681. [CrossRef]
- Sergejeva, D. , Alsina, I., Duma, M., Dubova, L., Augspole, I., Erdberga, I. and Berzina, K., 2018. Evaluation of different lighting sources on the growth and chemical composition of lettuce. [CrossRef]
- Sharma, A. , Hazarika, M., Heisnam, P., Pandey, H., Devadas, V.S., Wangsu, M. and Kartha, B.D., 2023b. Factors affecting production, nutrient translocation mechanisms, and LED emitted light in growth of microgreen plants in soilless culture. ACS Agricultural Science & Technology, 3(9), pp.701-719. [CrossRef]
- Sharma, A. , Samtani, H., Sahu, K., Sharma, A.K., Khurana, J.P. and Khurana, P., 2023a. Functions of Phytochrome-Interacting Factors (PIFs) in the regulation of plant growth and development: A comprehensive review. International Journal of Biological Macromolecules, 244, p.125234. [CrossRef]
- Sharma, S. , Chatterjee, S., Kataria, S., Joshi, J., Datta, S., Vairale, M.G. and Veer, V., 2017. A review on responses of plants to UV-B radiation related stress. UV-B Radiation: From Environmental Stressor to Regulator of Plant Growth, pp.75-97. [CrossRef]
- Shen, H. , Wang, Z., Wu, Y. and Yang, B., 2016. One-dimensional photonic crystals: fabrication, responsiveness and emerging applications in 3D construction. RSC advances, 6(6), pp.4505-4520. [CrossRef]
- Shmarev, A. , Vereshagin, M., Pashkovskiy, P., Kreslavski, V.D. and Allakhverdiev, S.I., 2024. Influence of additional far-red light on photosynthetic and growth parameters of lettuce plants and the resistance of the photosynthetic apparatus to high irradiance. Photosynthetica, 62(2), pp.180-186. [CrossRef]
- Simkin, A.J. , Kapoor, L., Doss, C.G.P., Hofmann, T.A., Lawson, T. and Ramamoorthy, S., 2022. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynthesis Research, 152(1), pp.23-42. [CrossRef]
- Singh, C. , Joshi, N.U., Kumar, R., Neha and Kumar, A., 2024. Ultraviolet Rays in Food Processing. Nonthermal Food Engineering Operations, pp.435-485. [CrossRef]
- Singh, Y.K. , Topno, S.E., Bahadur, V. and Shrivastava, P., 2021. Effect of Light Intensity and Different Levels of Nitrogen on Growth, Yield and Photosynthetic Characteristics of Giant Mustard (Brassica juncea var. Wong Bok). In Biological Forum (pp. 461-466).
- Sipos, L. , Balázs, L., Székely, G., Jung, A., Sárosi, S., Radácsi, P. and Csambalik, L., 2021. Optimization of basil (Ocimum basilicum L.) production in LED light environments–a review. Scientia Horticulturae, 289, p.110486. [CrossRef]
- Smith, B.J. , Rezazadeh, A., Stafne, E.T. and Sakhanokho, H.F., 2022. Effect of Light-emitting Diodes, Ultraviolet-B, and Fluorescent Supplemental Greenhouse Lights on Strawberry Plant Growth and Response to Infection by the Anthracnose Pathogen Colletotrichum gloeosporioides. HortScience, 57(8), pp.856-863. [CrossRef]
- Suthaparan, A. , Torre, S., Stensvand, A., Herrero, M.L., Pettersen, R.I., Gadoury, D.M. and Gislerød, H.R., 2010. Specific light-emitting diodes can suppress sporulation of Podosphaera pannosa on greenhouse roses. Plant disease, 94(9), pp.1105-1110. [CrossRef]
- Tan, B. , Li, Y., Liu, T., Tan, X., He, Y., You, X., Leong, K.H., Liu, C. and Li, L., 2021. Response of plant rhizosphere microenvironment to water management in soil-and substrate-based controlled environment agriculture (CEA) systems: A review. Frontiers in Plant Science, 12, p.691651. [CrossRef]
- Thrithamarassery, G.D. , Xu, Z., Liu, Y., Izquierdo, R. and Ma, D., 2017. Recent advancements in plasmon-enhanced promising third-generation solar cells. Nanophotonics, 6(1), pp.153-175. [CrossRef]
- Toor, M.D. , Adnan, M., Rehman, F.U., Tahir, R., Saeed, M.S., Khan, A.U. and Pareek, V., 2021. Nutrients and their importance in agriculture crop production; A review. Ind. J. Pure App. Biosci, 9(1), pp.1-6. [CrossRef]
- Trivellini, A. , Toscano, S., Romano, D. and Ferrante, A., 2023. LED lighting to produce high-quality ornamental plants. Plants, 12(8), p.1667. [CrossRef]
- Ullah, M.A. , Tungmunnithum, D., Garros, L., Drouet, S., Hano, C. and Abbasi, B.H., 2019. Effect of ultraviolet-C radiation and melatonin stress on biosynthesis of antioxidant and antidiabetic metabolites produced in in vitro callus cultures of Lepidium sativum L. International journal of molecular sciences, 20(7), p.1787. [CrossRef]
- Vaštakaitė-Kairienė, V. , Brazaitytė, A., Miliauskienė, J. and Runkle, E.S., 2022. Red to blue light ratio and iron nutrition influence growth, metabolic response, and mineral nutrients of spinach grown indoors. Sustainability, 14(19), p.12564. [CrossRef]
- Viana, L.N. , Soares, A.P.S., Guimarães, D.L., Rojano, W.J.S. and Saint'Pierre, T.D., 2022. Fluorescent lamps: A review on environmental concerns and current recycling perspectives highlighting Hg and rare earth elements. Journal of Environmental Chemical Engineering, p.108915. [CrossRef]
- Wang, D. , Jenkins, K., Forstenhäusler, N., Lei, T., Price, J., Warren, R., Jenkins, R. and Guan, D., 2021a. Economic impacts of climate-induced crop yield changes: evidence from agri-food industries in six countries. Climatic Change, 166(3-4), p.30. [CrossRef]
- Wang, P. , Abid, M.A., Qanmber, G., Askari, M., Zhou, L., Song, Y., Liang, C., Meng, Z., Malik, W., Wei, Y. and Wang, Y., 2022. Photomorphogenesis in plants: The central role of phytochrome interacting factors (PIFs). Environmental and Experimental Botany, 194, p.104704. [CrossRef]
- Wang, S. , Fang, H., Xie, J., Wu, Y., Tang, Z., Liu, Z., Lv, J. and Yu, J., 2021b. Physiological responses of cucumber seedlings to different supplemental light duration of red and blue LED. Frontiers in Plant Science, 12, p.709313. [CrossRef]
- Wang, W. , Su, M., Li, H., Zeng, B., Chang, Q. and Lai, Z., 2018. Effects of supplemental lighting with different light qualities on growth and secondary metabolite content of Anoectochilus roxburghii. PeerJ, 6, p.e5274. [CrossRef]
- Wang, X. , ping Li, X., Zhang, Y. and Chen, B., 2023. Optical transition and near-infrared upconversion luminescence properties of YNbO4: Er3+, Yb3+ phosphors. Journal of Luminescence, 263, p.120055. [CrossRef]
- Williams, K.A. , Miller, C.T. and Craver, J.K., 2016. Light quality effects on intumescence (oedema) on plant leaves. LED lighting for urban agriculture, pp.275-286. [CrossRef]
- Wojciechowska, R. , Długosz-Grochowska, O., Kołton, A. and Żupnik, M., 2015. Effects of LED supplemental lighting on yield and some quality parameters of lamb's lettuce grown in two winter cycles. Scientia Horticulturae, 187, pp.80-86. [CrossRef]
- Wong, H.M. , Hofmann, R.W. and Reichman, S.M., 2023. The interactions of iron nutrition, salinity and ultraviolet-B radiation on the physiological responses of wheat (Triticum aestivum L.). Environmental and Experimental Botany, 207, p.105201. [CrossRef]
- Xu, J. , Guo, Z., Jiang, X., Ahammed, G.J. and Zhou, Y., 2021. Light regulation of horticultural crop nutrient uptake and utilization. Horticultural Plant Journal, 7(5), pp.367-379. [CrossRef]
- Xu, Y. , Liang, Y. and Yang, M., 2019. Effects of composite LED light on root growth and antioxidant capacity of Cunninghamia lanceolata tissue culture seedlings. Scientific reports, 9(1), p.9766. [CrossRef]
- Yang, J. , Song, J. and Jeong, B.R., 2022. Low-intensity blue light supplemented during photoperiod in controlled environment induces flowering and antioxidant production in kalanchoe. Antioxidants, 11(5), p.811. [CrossRef]
- Yang, X. , Xu, H., Shao, L., Li, T., Wang, Y. and Wang, R., 2018. Response of photosynthetic capacity of tomato leaves to different LED light wavelength. Environmental and Experimental Botany, 150, pp.161-171. [CrossRef]
- Yanykin, D.V. , Burmistrov, D.E., Simakin, A.V., Ermakova, J.A. and Gudkov, S.V., 2022. Effect of up-converting luminescent nanoparticles with increased quantum yield incorporated into the fluoropolymer matrix on Solanum lycopersicum growth. Agronomy, 12(1), p.108. [CrossRef]
- Yildirim, D.U. , Ghobadi, A. and Ozbay, E., 2021. Nanosensors based on localized surface plasmon resonance. Plasmonic Sensors and Their Applications, pp.23-54. [CrossRef]
- Younas, M. , Drouet, S., Nadeem, M., Giglioli-Guivarc'h, N., Hano, C. and Abbasi, B.H., 2018. Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several spectres of monochromatic lights. Journal of Photochemistry and Photobiology B: Biology, 184, pp.61-70. [CrossRef]
- Yu, B. , Hu, Y. and Hou, X., 2024. More than flowering: CONSTANS plays multifaceted roles in plant development and stress responses. Journal of Integrative Plant Biology. [CrossRef]
- Zare Mehrjerdi, M. , Safari, N., Kharrazi, M., Khadem, A. and Sharifi, A., 2024. The Effect of Different Qualities of LED Light on the Morphophysiological Indicators of Cucumis sativus L. var. Officer. Journal Of Horticultural Science, 37(4), pp.1029-1041. [CrossRef]
- Zhang, L. , Wang, G., Wang, G. and Cao, F., 2021. Ginkgo biloba L. responds to red and blue light: via phenylpropanoid and flavonoid biosynthesis pathway. Forests, 12(8), p.1079. [CrossRef]
- Zhang, R. , Yang, W., Pan, Q., Zeng, Q., Yan, C., Bai, X., Liu, Y., Zhang, L. and Li, B., 2023. Effects of long-term blue light irradiation on carotenoid biosynthesis and antioxidant activities in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Food Research International, 174, p.113661. [CrossRef]
- Zhao, B. , Wang, L., Pang, S., Jia, Z., Wang, L., Li, W. and Jin, B., 2020. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Industrial crops and products, 151, p.112483. [CrossRef]
- Zhong, L. , Hao, S., Zhai, T., Yang, Y., Lin, H., Lin, B., Shen, B., Liu, S., Hu, Y. and Chen, X., 2024. The role of LED supplementary lighting in promoting graft necrotic layer formation in pumpkin-cucumber grafts. Scientia Horticulturae, 330, p.112953. [CrossRef]
- Zhu, P. , Chen, R., Yang, X., Fan, Y., Lian, H. and Wang, Z.Y., 2023. Formation of photonic band gaps by direct destructive interference. Optics Express, 31(26), pp.43390-43400. [CrossRef]
- Zhu, Y. , Singh, J., Patil, B.S. and Zhen, S., 2024. End-of-production supplemental blue light intensity and duration co-regulate growth, anthocyanin, and ascorbic acid production in red leaf lettuce. Scientia Horticulturae, 335, p.113333. [CrossRef]
- Zlatev, Z.S. , Lidon, F.J. and Kaimakanova, M., 2012. Plant physiological responses to UV-B radiation. Emirates Journal of Food and Agriculture, pp.481-501. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).