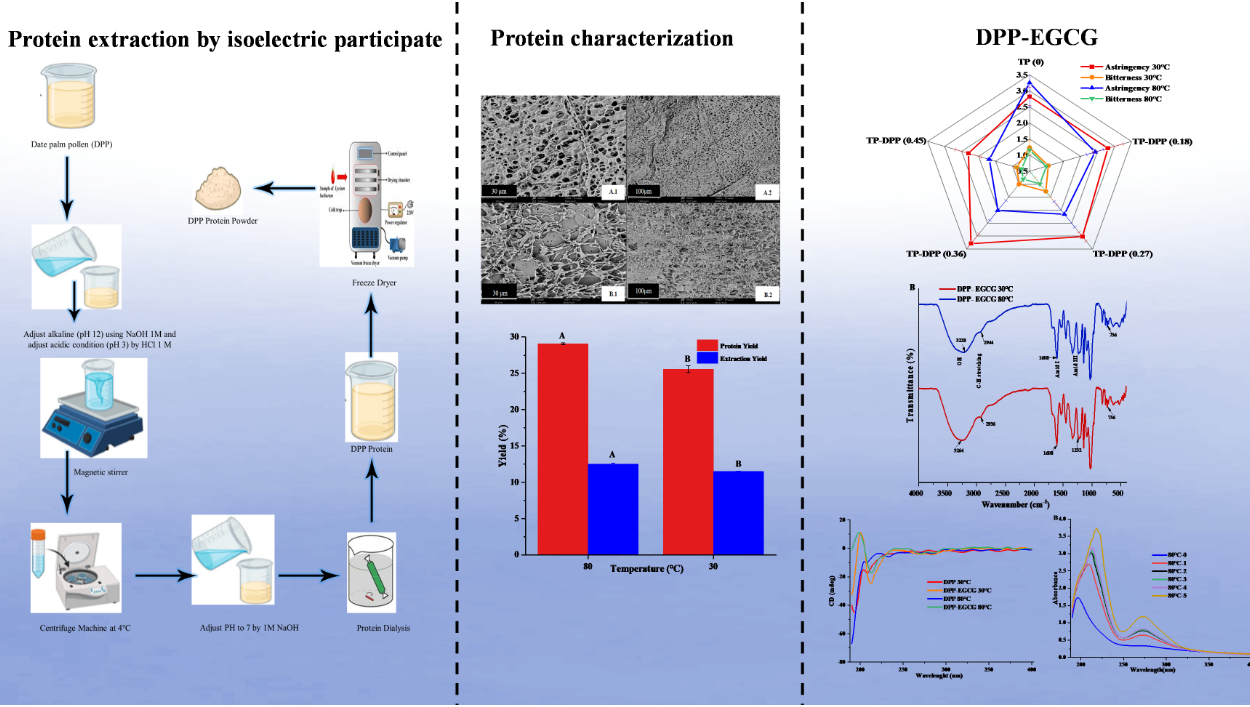
1. Introduction
The complex astringency in tea is affected by the interaction of salivary proteins and different oral components, which makes the tea feel dry and rough to the touch [
1]. In the past, astringency was linked to insoluble precipitates that were made when polyphenols bonded to salivary proteins. However, newer theories say that astringent compounds directly affect taste receptors or trigeminal nerves.
Despite its numerous health benefits, the enduring aversion to the astringent and bitter qualities of tea, particularly green tea, presents a challenge. The bitter and astringent attributes of tea are attributed to its polyphenol-rich composition, including flavonoids, phenolic acids, and alkaloids [
2]. Catechins, particularly EGCG, prevalent in tea leaves significantly impact the taste profile, with caffeine intensifying their bitter and astringent properties in a synergistic manner [
3].
Techniques involving physical or chemical modifications of tea components are employed to alleviate its unpleasant taste, with a focus on reducing astringency and enhancing palatability. The assessment of tea’s bitterness and astringency through quantitative descriptive analysis is imperative in the quest to expand the utility of tea and its derivatives in the realm of bakery foods and functional food industries [
3]. Therefore, the mitigation of bitterness and astringency in tea and its derivatives holds substantial importance in broadening their application and appeal [
4].
Proteins are commonly used in the manufacture of food because they are high in nutrients and have a wealth of functional activities. Numerous studies have examined the relationship between Tea Polyphenols (TPs) and proteins [
5]. According to [
6], TPs have been observed to interact with proteins that include a large concentration of alkaline amino acids, which can cause peptide tertiary structure to loosen and expose hydrophobic groups. Because of their abundance in hydroxyl groups, catechins can create hydrogen bonds with proteins’ nitrogen and oxygen [
7,
8]. According to most theories, there are three steps to the interaction between TPs like EGCG and proteins [
9]. First, a loose, randomly coiled conformation protein binds to several sites on polyphenols, causing the protein to shrink physically and take on a tighter, spherical structure. The polyphenol complex binds to the protein surface in the second step as the concentration of polyphenols increases. Protein molecules dimerize one another, which lowers their solubility. The final stage involves the dimers’ spontaneous assembly, which results in a significant quantity of particle precipitation [
10]. This suggests that, in order to avoid unnecessary precipitation, the ratio of polyphenols to proteins should be considered in practical commercial production.
Processing factors like heat treatment can influence the interactions between polyphenols and proteins, as well as the structure of proteins and the biological activities of polyphenols. Several authors have reported that high-temperature thermal treatments may increase the strength of the polyphenol - protein binding [
11].
The pollen of the date palm tree (Phoenix dactylifera L.), a member of the Aceraceae family, has been widely utilized in the Middle East and was originally utilized as a medicinal remedy by the ancient Egyptians and Chinese. Within palm flowers, it serves as the male reproductive cells. Due to its impressive protein, vitamin, mineral, carbohydrate, lipid, organic acid, sterol, nucleic acid, enzyme, and cofactor content, it is considered a natural and beneficial dietary supplement. The bioactive volatile unsaturated fatty acids and phenolic components, such as flavonoids, are especially interesting because they are strong antioxidants and have been shown to fight cancer, especially breast cancer [
12]. A previous study found that dried male date palm flowers have a lot of protein. This means that they could be used as a by-product and have a lot of nutritional value and useful properties that make them good for use in food formulations [
13,
14]. This led to an intriguing exploration of the proteic fraction and an investigation into the influence of extraction temperature on its diverse characteristics. Moreover, the dried male date palm flowers are extensively employed in the Arabian region for the creation of a tonic beverage with reported antidiabetic and antioxidant effects [
15].
The objective of this study is to explore the influence of heat treatment (at 30 and 80 °C) on the quality of DPP protein based on its physio-chemical properties. Furthermore, the aim is to analyse the interactions between tea polyphenols (specifically EGCG) and DPP protein and to assess their potential application in the food industry as a functional beverage with a novel flavour profile. The determination of binding kinetics was carried out using particle size and ultraviolet-visible (UV-Vis) spectroscopy, while circular dichroism and FTIR were employed to measure the impact of protein structure on binding. Additionally, sensory evaluation (QDA) was utilized to investigate how this interaction influenced the astringent and bitter flavours of the tea. Ultimately, the findings from this study may serve as a valuable foundation for developing plant-based alternatives for popular food products that possess similar physical and chemical attributes to their traditional counterparts.
2. Materials and Methods
2.1. Materials
The date palm pollen powder was procured from Xi’an Xuanqing Import & Export Co., Ltd. and maintained at 4 °C until required. EGCG was provided by Shanghai Darui Fine Chemistry Co., Ltd. and subsequently stored at 4°C for future use. All remaining chemicals utilized were of analytical grade, unless specified otherwise.
2.2. Proximate Composition and Bioactive Compounds of Date Palm Pollen
2.2.1. Chemical Composition Analysis
Was conducted to determine the proximate composition using the official method AOAC [
16]. This included the assessment of moisture, crude fiber, protein (micro-Kjeldahl), crude fat, ash, and total nonfibrous carbohydrates. The calculation for total nonfibrous carbohydrates was derived using the equation: 100 − [moisture + crude fat + crude protein + ash + crude fiber].
2.2.2. Polyphenol Extraction
A total of 2 grams of DPP were combined with 20 milliliters of 50% acetone and vigorously stirred for 2 hours at 25 degrees Celsius before being centrifuged for 20 minutes at 4500 revolutions per minute. To enhance the extraction of polyphenols and flavonoids, the process was repeated twice [
17].
The Folin-Ciocâlteu method was utilized to determine the total phenolic content. A combination of 500 µL of the extract and 2.5 mL of a 1:10 Folin-Ciocâlteu solution in water was prepared, followed by the addition of a two-ml solution of 7.5% (w/v) sodium carbonate. Subsequently, the tubes were left at room temperature for fifteen minutes, and the absorbance was measured at 765 nm, with distilled water used as the blank sample [
18]. The total polyphenol content was quantified in mg/100 DPP and expressed as gallic acid equivalents (GAE). A standard curve using gallic acid with concentrations ranging from 0 to 50 mg/L was established [
17].
2.2.3. Flavonoids
The process involved transferring the previously mentioned extract (250 µL) into a tube with 1 mL of distilled water and 150 µL of NaNO2 15% (w/v). After six minutes, 75 µL of AlCl3 10% (w/v) were introduced. Subsequently, 1 mL of 1 mol/L NaOH was added, and the final volume was adjusted to 2.5 mL with distilled water. Following a 15-minute incubation, the absorbance was gauged at 510 nm against distilled water as the blank sample. The content of flavonoids was denoted as quercetin equivalents (EQ) in mg/100 g DPP [
19].
2.3. Protein Extraction (Isoelectric Participate)
A mixture of 1:10 (w/v) DPP combined with distilled water and adjusted to a pH of 12 using 1 mol/L NaOH was subjected to magnetic stirring for two hours at temperatures of 30°C and 80°C. Afterward, a 30-minute centrifugation at 10,000 g at 4°C was carried out to obtain pollen protein concentrate through the isoelectric precipitation method. To ensure maximum protein recovery, the pellet underwent double extraction, and the resulting supernatants were collected for subsequent precipitation. Then lowering the pH of the supernatant to 3 with 1 mol/L HCl and incubating it at 4°C overnight. Protein concentration was achieved by centrifugation at 10,000 g for 30 minutes at 4°C. The resulting pollen protein concentrate was neutralized, dialyzed against ultra-pure water for 7 days (molecular weight cutoff: 10 KDa), and freeze-dried. The samples were then stored at -80°C until use [
20].
2.4. Determination of Extraction and Protein Yields
The quantification of extraction yield for each sample involves a mathematical representation of the amount of product acquired from the initial amount of DPP. The determination of protein yield was derived from the protein content and the weight of the extract, taking into account the protein content and the initial weight of DPP.
2.5. Scanning Electronic Microscopy (SEM)
Scanning Electron Microscopy (SEM) is widely considered an unparalleled imaging technique of interest. This device operates by scanning a beam of electrons onto a specimen, causing it to view fine surface details of the specimens. The DPP protein sample preparation for SEM was spread on a conductive adhesive carbon strip that was fastened to the stub and covered with a thin coating of gold. In order to effectively produce images through SEM (JSM-5400, Jeol, Japan), the microstructure was examined at an accelerating voltage of 15 kV and a magnification of 500 and 2000.
2.6. Differential Scanning Calorimetry (DSC)
The application of Differential Scanning Calorimetry (DSC) involves the utilization of an isothermal analysis instrument (TA DSC, USA). A quantity of 2 mg of DPP protein samples was placed into sealed aluminum pans, while an empty pan of equal mass was used as a reference. Subsequently, the heat flow rate was controlled within the range of 50 to 250 °C at a scan rate of 5 °C/min under a nitrogen environment. This heat flow rate is equivalent to the rate of temperature change, any heat flow resistance present within the sample, and the rate at which the sample generates or absorbs heat. The experiment was conducted in triplicate.
2.7. Surface Tension
The capillary rise method serves as a renowned technique for surface tension measurement. In the dynamic mode, the surface tension was measured using the automated drop volume tensiometer (TVT1, Germany). To ascertain the surface tension of DPP, two concentrations (0.5 and 1% of protein/100 ml) of each sample were examined. The solutions were prepared at pH 7 the day before the experiment. The syringe volume was almost 3 mL. All measurements were conducted in triplicate at room temperature.
2.8. Sensory Evaluation
The QDA technique was utilized to examine the impact of DPP (30; 80°C) inclusion on the astringency and bitterness of the TP solution. Trained evaluators, consisting of 2 males and 8 females aged 20–30, from the School of Food Science at Southwest University (Chongqing, China), performed the sensory assessment. Prior to participating, each individual provided written informed consent. For the evaluation samples, the tea polyphenol concentration remained constant at 0.18 g/l, while the DPP concentrations at 30 and 80 °C were 0.18, 0.27, 0.36, and 0.45 g/l. The samples were dissolved at room temperature and filtered through Whatman No. 1 filter paper. Each evaluator received nine sequentially labeled cups of sample solution, each approximately 10 mL. Before tasting each sample, the evaluators rinsed their mouths with pure water, then swirled the sample in their mouth for 8–10s, rating the astringency and bitterness before spitting out the solution. After scoring each sample, the evaluators were instructed to rinse their mouths with pure water. A 6-minute interval was observed between each sample test to minimize sensory fatigue and carry-over effects. Evaluators were asked to rate the astringency and bitterness taste intensities of each solution using a five-point scale, ranging from “extremely strong” to “extremely weak.” Tannin served as the standard reference for astringency, while quinine monohydrochloride dehydrate was the standard reference for bitterness. Each standard solution was presented in various concentrations corresponding to different points on the scale. Two one-hour sessions were conducted, with nine samples tested in each session, and each sample repeated twice.
2.9. Preparation of Protein Solution and Tea Polyphenol Solution
Particle Size Measurements
Different amounts of EGCG were amalgamated with DPP to formulate combinations with EGCG concentrations of 100, 200, 300, and 400 μM while sustaining the DPP content at 10 μM. Using the technique described by Yang et al. [
21], the mixture was kept at 25 °C for two hours, and the development of haze was tracked using DLS. Utilizing dynamic light scattering (DLS) techniques is a prevalent method for determining the size of particles in a given sample. We used the Zetasizer Nano-ZS90 machine in Worcestershire, UK, to measure the size and spread of DPP-EGCG complexes by checking the amount of light that was scattered at a 90-degree angle using dynamic light scattering (DLS). The results were represented in terms of the polydispersity index (PdI) for size distribution and the cumulative mean diameter (nm) for particle size. To ensure precision, this experiment was repeated three times.
2.10. Fourier Transform Infrared (FTIR) Spectroscopy
FTIR spectroscopy instruments quantitatively evaluated samples using infrared light, following the method of Yang et al. (2014) with minor modifications. Prior to the scan, DPP-EGCG complexes were prepared in a 1:1 molar ratio of DPP to EGCG. Subsequently, they were freeze-dried and combined with potassium bromide (KBr). The FTIR spectroscopy of DPP protein and the DPP-EGCG complex was conducted using a Spectrum 100 FTIR instrument (Perkin-Elmer, UK) within the 400-4000 cm−1 range, at a resolution of 4 cm−1. Twenty scans were conducted for each measurement. The analysis was conducted utilizing Origin 8.0 software.
2.11. Circular Dichroism (CD) Measurement
Circular dichroism (CD) spectra were obtained using a spectropolarimeter (MOS-500, Cecine Parisse, France) for a DPP protein sample at a concentration of 0.5 g/L, following its mixing with EGCG. Measurements were conducted with a light source spanning 190-400 nm wavelength at 25 °C, utilizing a 1 mm path-length cell. The scanning speed was established at 100 nm/min, with a bandwidth of 1.0 nm. The analysis of the sample was conducted using Origin 8.0 software.
2.12. UV–vis Absorption Spectra
The DPP-EGCG aqueous extract was prepared at a fixed concentration of 4.0 × 10−5 mol/L for DPP, while EGCG was introduced in increasing concentrations of 0, 2.0, 4.0, 6.0, 8.0, and 10.0 × 10−5 mol/L. Measurements were conducted using a UV-visible spectrophotometer (Model UV-2450 Shimadzu, Tokyo, Japan) over a wavelength range of 190-400 nm at 25℃, utilizing a 1 cm quartz cell [
22].
2.13. Statistical Analysis
The analytical values were obtained from three separate measurements. The results were presented as mean values ± standard error from three separate measurements. The statistical analysis, data processing, and mapping were performed utilizing the software programs Origin and Minitab to compute variance (ANOVA) and conduct Tukey multiple comparisons.
3. Results and Discussion
3.1. Chemical Composition and Bio Active Components
3.1.1. Chemical Composition
This study involved an analysis of moisture, Fiber, ash, crude fat, total protein, and carbohydrate content for palm pollen powder.
Table 1 presents the detailed chemical composition of DPP powder. Our analysis indicated that protein, carbs, and fat constituted 34%, 32%, and 20% of the total, respectively. [
14,
23,
24] These researchers presented analogous findings. Compared to date fruit (1.1-3.0 g/100 g) and seeds (2.29–7.08 g/100 g), DPP demonstrated a superior protein concentration (15.8–38.18 g/100 g) [
25]. Hassan [
14] identified palmitic (C16:0), linoleic (C18:2), and myristic (C14:0) acids as the predominant fatty acids in date palm pollen grains. Our data unequivocally demonstrates that DPP functions as a significant source of proteins employed in the agri-food and medicinal industries.
3.1.2. Bio-Active Components of DPP
According to the findings in
Table 1, the phenol content of DPP was ascertained to be 227.46 mg GAE/100 g, which is inferior to the polyphenol content of date palm pollen documented by [
17] (909.30 mg GAE/100 g) and [
26] (213.36-197.62 mg GAE/g). This finding confirms that DPP is a good source of bioactive chemicals that are found naturally and can effectively stop oxidation in a wide range of food products. Also, the amount of flavonoids in DPP was measured to be 6.37 mg QE/100 g. This is similar to the value found by [
17] (4.31 mg EQ/100 g), but much lower than the 266 mg EQ/100 g found in date palm pollen by [
27] and the 168 mg EQ/100 g found by [
13]. The overall concentration of flavonoids and phenolic chemicals may vary depending on a variety of factors, including storage, drying methods, and geographical origin. Also, the choice of extraction solvents is likely to have a big effect on how well the phenolic component is recovered [
17].
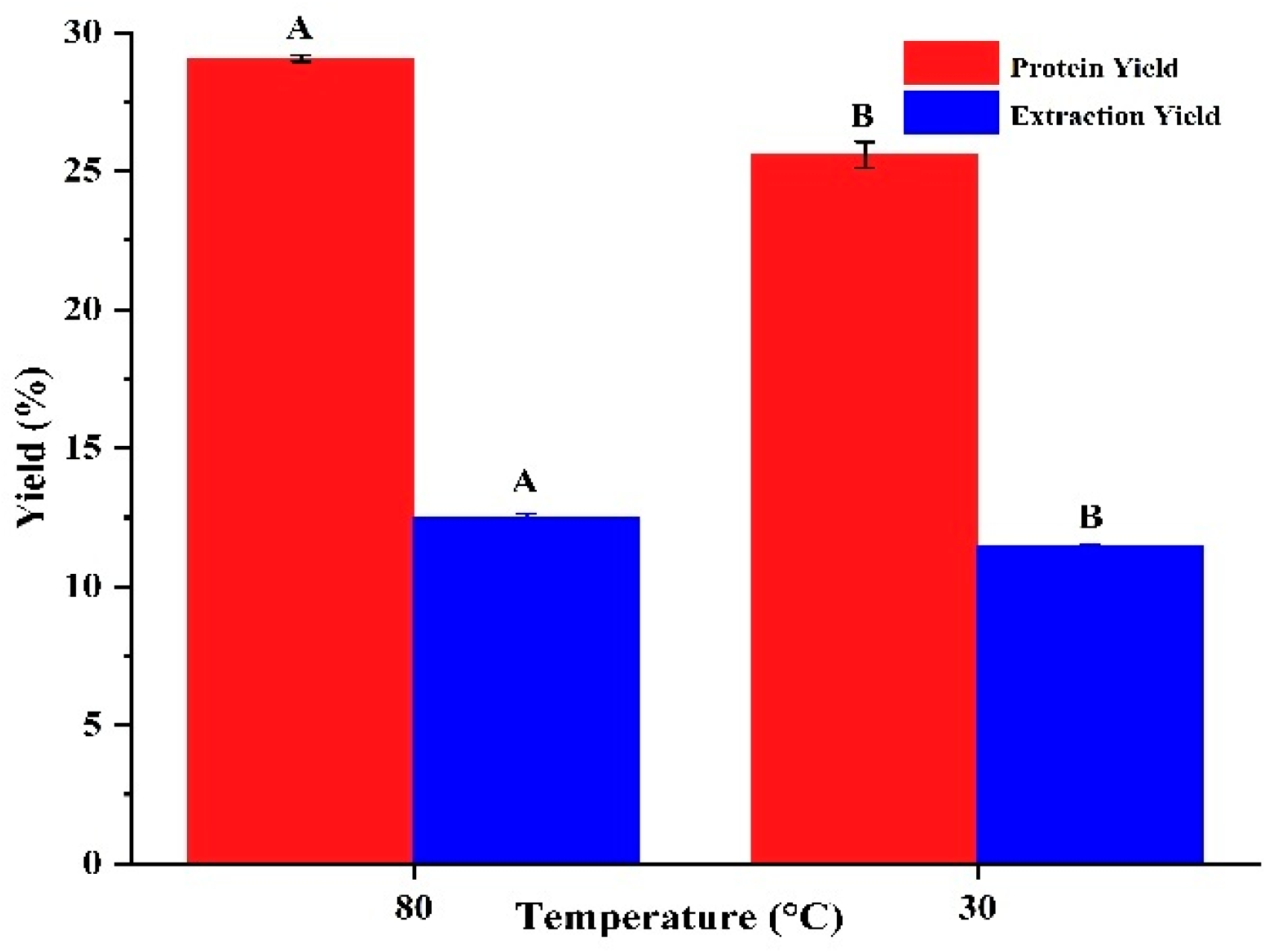
3.2. Effect of Temperature on Protein Extraction and Yield from DPP
Figure 1. shows the protein yield varied significantly (P < 0.05) by heat treatment during the isoelectric precipitation procedure of DPP at 30°C and 80°C. Temperature at 80°C significantly improved protein yields and extraction. As can be shown, for DPP 30°C and DPP 80°C, respectively, the protein yield increased from 25.58 to 29.07%, and the extraction yield increased slightly from 11.5 to 12.5%. This occurred because rising temperatures cause disruptions in the cell wall, which facilitate the release of various compounds, [
20,
28] reported a similar finding. The weight obtained for each concentrate was mainly responsible for this difference.
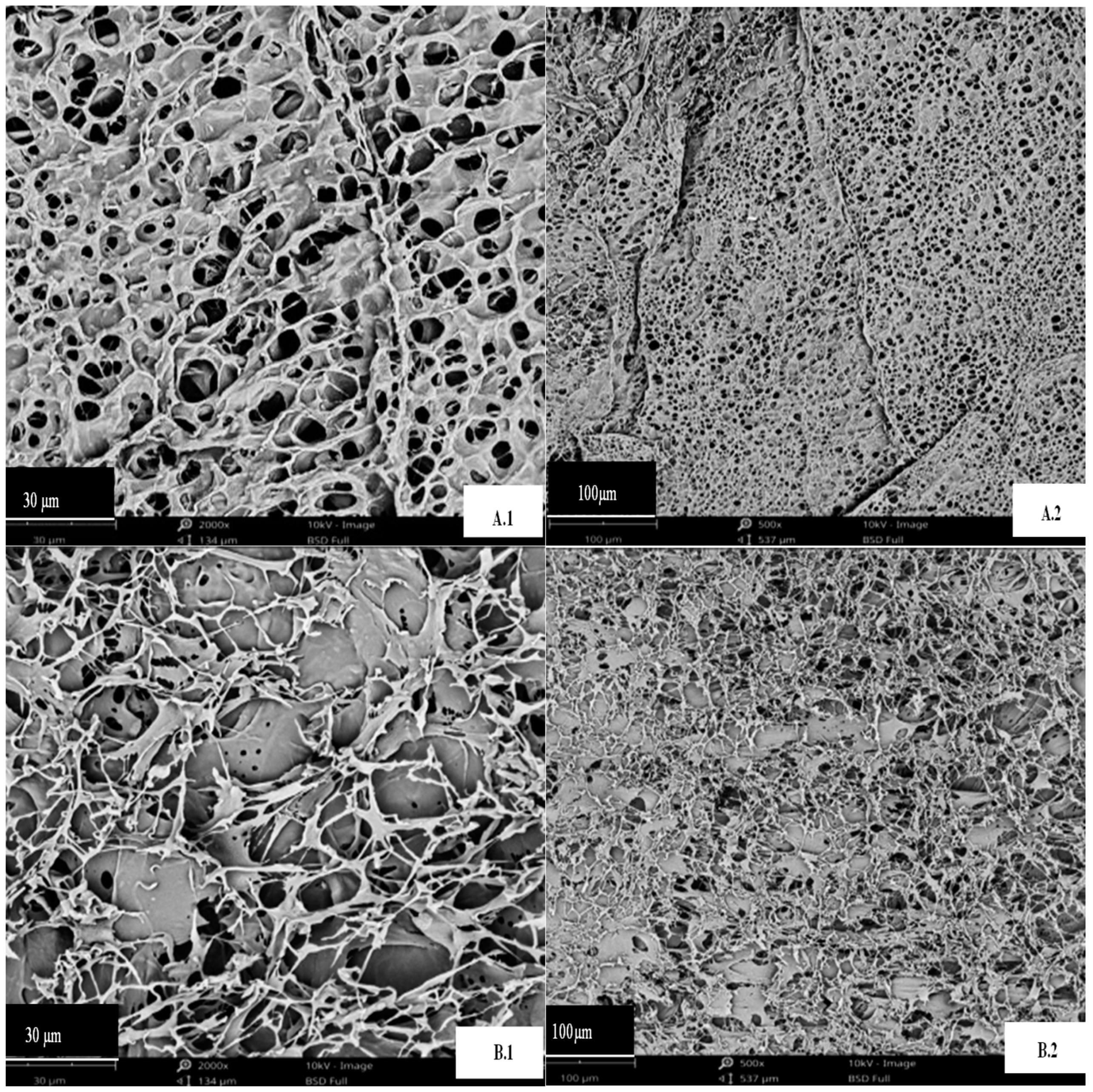
3.3. Scanning Electron Microscopy
SEM analysis was employed to investigate the microstructure of the freeze-dried extracts to elucidate the effect of temperature on DPP protein.
Figure 2 illustrates the variations in the protein structure of DPP at 30°C and 80°C. At magnifications of 500× and 2000×, the aggregate sizes (about 100 and 30 μm) exhibited morphological variations in the protein bodies of the temperature isolates.
The detected entities exhibited various sizes, occasionally round and at other times oval, characterized by a smooth surface and many indentations. The comparison of DPP at 30°C (
Figure 2 A2) with DPP at 80°C indicated that DPP at 30°C exhibited several smaller entities encircling the bigger ones, but DPP at 80°C had predominantly larger entities, as illustrated in
Figure 2 B2. Samples treated at 30 °C demonstrated enhanced homogeneity, resulting in the formation of a protein framework. Temperature influences the dimensions of the final product’s entities; higher temperatures lead to augmented charges and the development of larger clusters during freeze-drying [
28]. Treatment at 80 °C produces more irregular fragments and disordered structures. These findings corroborate the concept that thermal treatment can modify protein structure [
20].
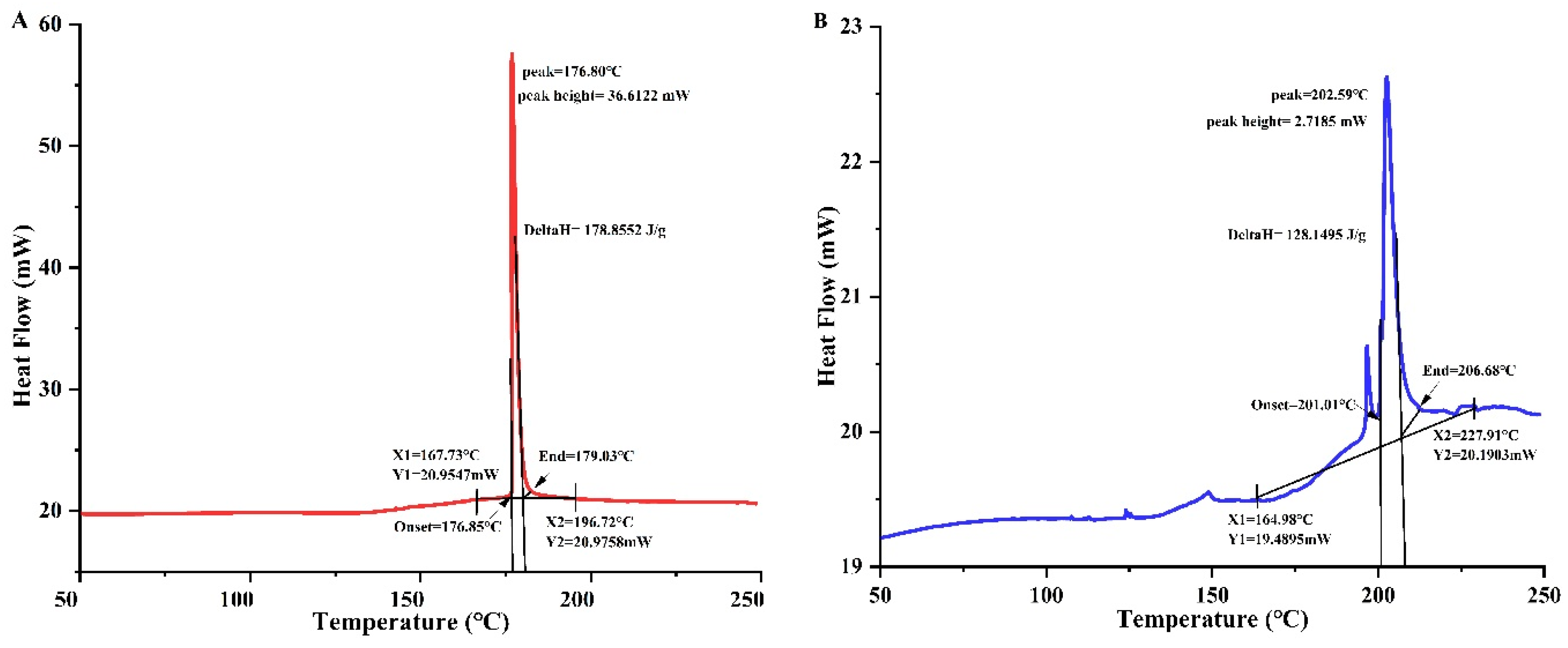
3.4. Differential Scanning Calorimetry (DSC)
A differential scanning calorimeter is a useful tool for checking how stable proteins are at high temperatures. It makes it simple to quickly and accurately check the heat resistance of any extract. It provides critical information regarding the denaturation enthalpy (ΔH) and denaturation temperature (Td) of the samples. Td is a measure of thermal stability, while ΔH is the quantity of energy needed to denaturize a protein.
Figure 3 (A and B) demonstrated that both extracts (30°C and 80°C) displayed distinct endothermic peaks. The addition of heat caused the Td for DPP 30°C and DPP 80°C to rise from 176.8°C to 202.5°C, respectively, and the H to drop from 178.86 J/g to 128.15 J/g. These findings indicate the considerable heat resilience of both extracts. Karra et al. [
28] observed similar findings for the protein concentrations in date palm pollen. The observed discrepancies (P < 0.05) between the two samples can be attributed to the differing compositions of the concentrates. A protein with very little water changes its Td value and enthalpy because a higher ΔH value means a more ordered structure [
29]. When the water content drops to less than 20%, heating stabilizes the protein. According to the discussion in the section on scanning electron microscopy, we can conclude that in our case, heating probably caused a modification in the structure of the pollen protein. More research has shown that the treated sample had broken intermolecular bonds, which led to the release of its hidden negative charges and a drop in ΔH [
20].
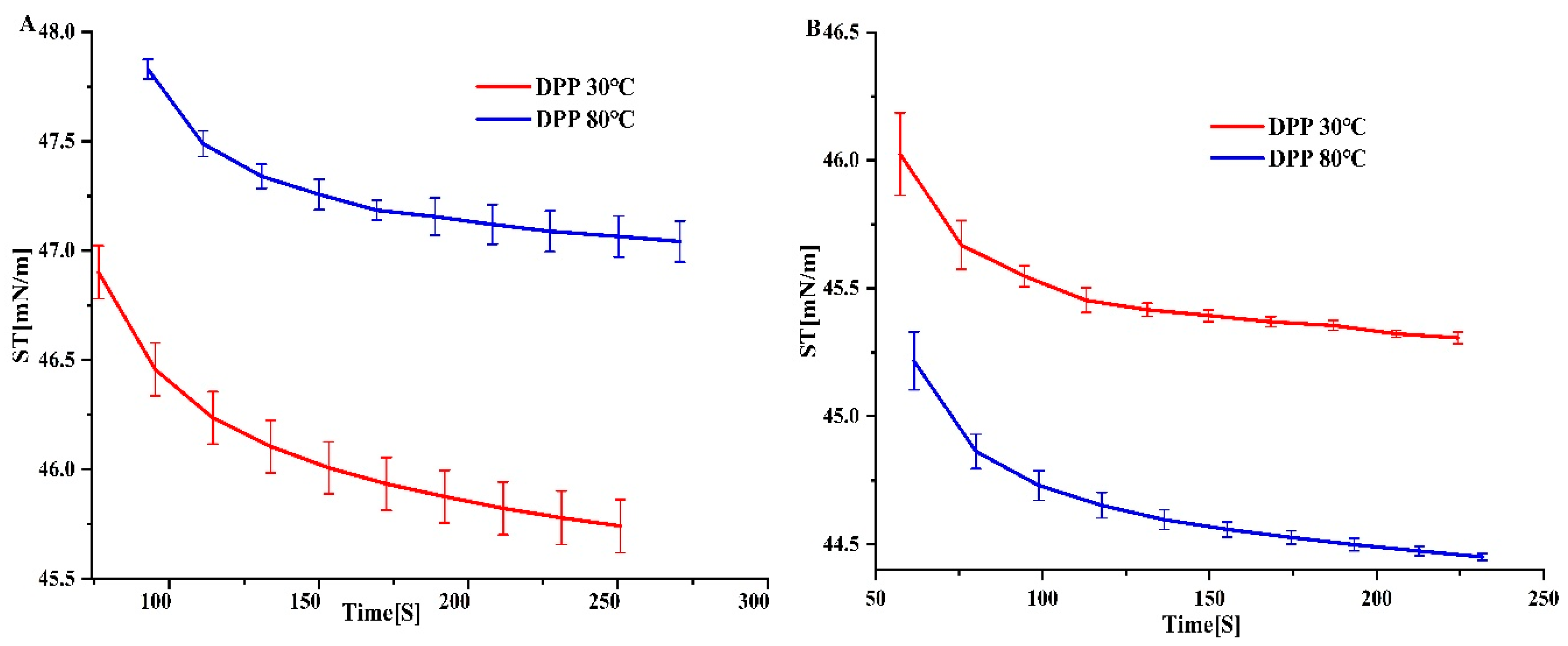
3.5. Surface Tension
We evaluated the surfactant efficacy of an extract by measuring its surface tension.
Figure 4 depicts the behaviour of DPP solutions at 30°C and 80°C with protein concentrations of 0.5 g/100 mL and 1 g/100 mL, respectively. We observed variations irrespective of the concentration. DPP at 80 °C demonstrated considerably (P < 0.05) enhanced surface activity at a concentration of 1 g/100 mL in comparison to DPP at 30 °C. Also, the surface tension of pollen protein concentrations changed as the protein content in dispersions went from 0.5 to 1 g/100 mL while keeping the pH the same. The measurements of the equilibrium surface tension ranged from 44.4 to 47.6 mN/m. These results were similar to those found for biosurfactants by [
28]. DPP at 30°C exhibited comparable values for 0.5 and 1 g/100 mL protein dispersions, in contrast to DPP at 80°C. Several parameters, including concentrations and pH, influence the dispersion’s ability to reduce surface tension, as all surface tension measurements demonstrate. DPP at 80 °C exhibited enhanced surfactant characteristics, perhaps because of the participation of other agents beyond proteins. Therefore, pollen protein concentrates could serve as natural surfactants in the agricultural and food sectors, potentially enhancing the sensory qualities of food systems [
20].
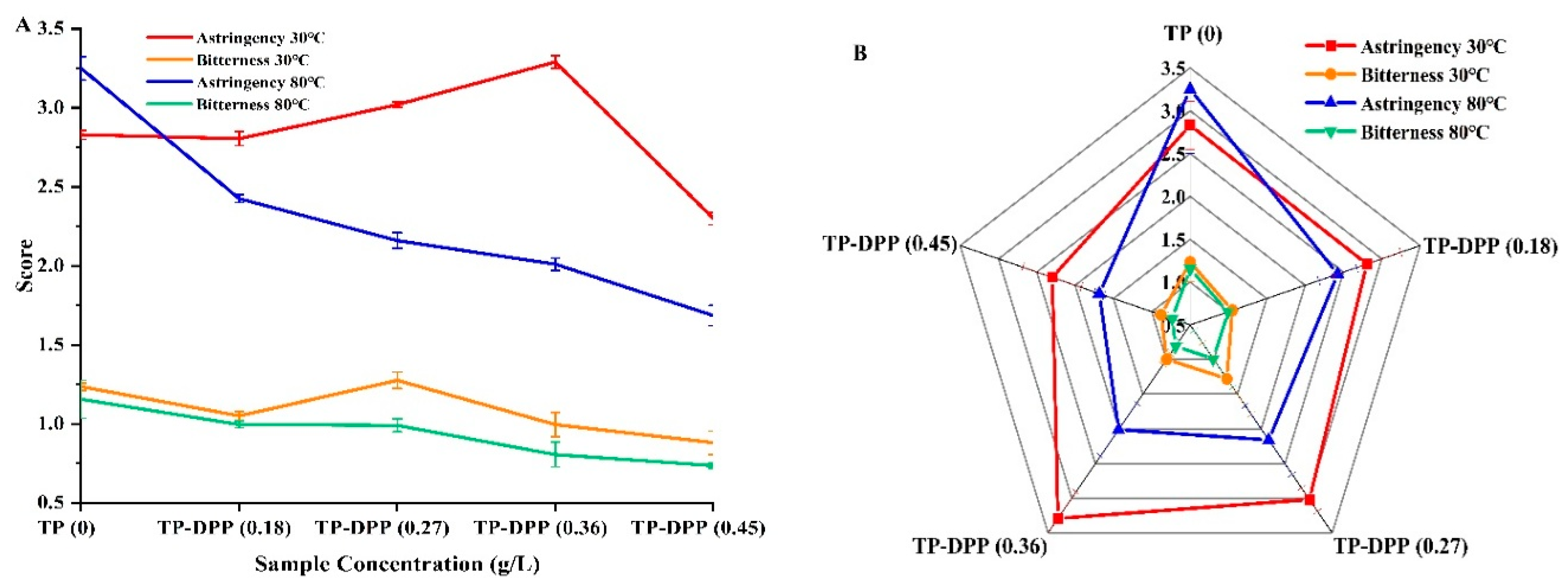
3.6. Descriptive Sensory Evaluation
The aim of the sensory QDA assessment was to identify the tea with DPP addition that exhibited the lowest astringency and would attract the typical consumer.
Figure 5 depicts the sensory assessments of the tea polyphenol with TP and DPP additions regarding astringency and bitterness, evaluated by 10 panelists. A standardized solution was employed to evaluate the astringency and bitterness of the samples on a scale of 8 to 10, 6 to 8, 4 to 6, 2 to 4, and 0 to 2, denoting “extremely strong,” “strong,” “neutral,” “weak,” and “extremely weak,” respectively.
The astringency scores were considerably affected (p < 0.5) by the incorporation of different quantities of DPP at 30 and 80 °C to TP; however, there was no significant alteration in the bitterness values (p > 0.05).
Figure 5A illustrates the sensory score results for bitterness and astringency in TP-DPP at 30 and 80 °C at several concentrations (0.18, 0.27, 0.36, and 0.45). The graph demonstrates that at 80°C concentrations, astringency had a negative correlation, whereas at 30°C, it showed variability. The radar plot (
Figure 5B) indicated that sample TP-DPP (0.18) at 30°C had a high similarity to tea polyphenol regarding astringency. Furthermore, sample TP-DPP (0.45) at 80°C attained the best acceptance rating for astringency mitigation.
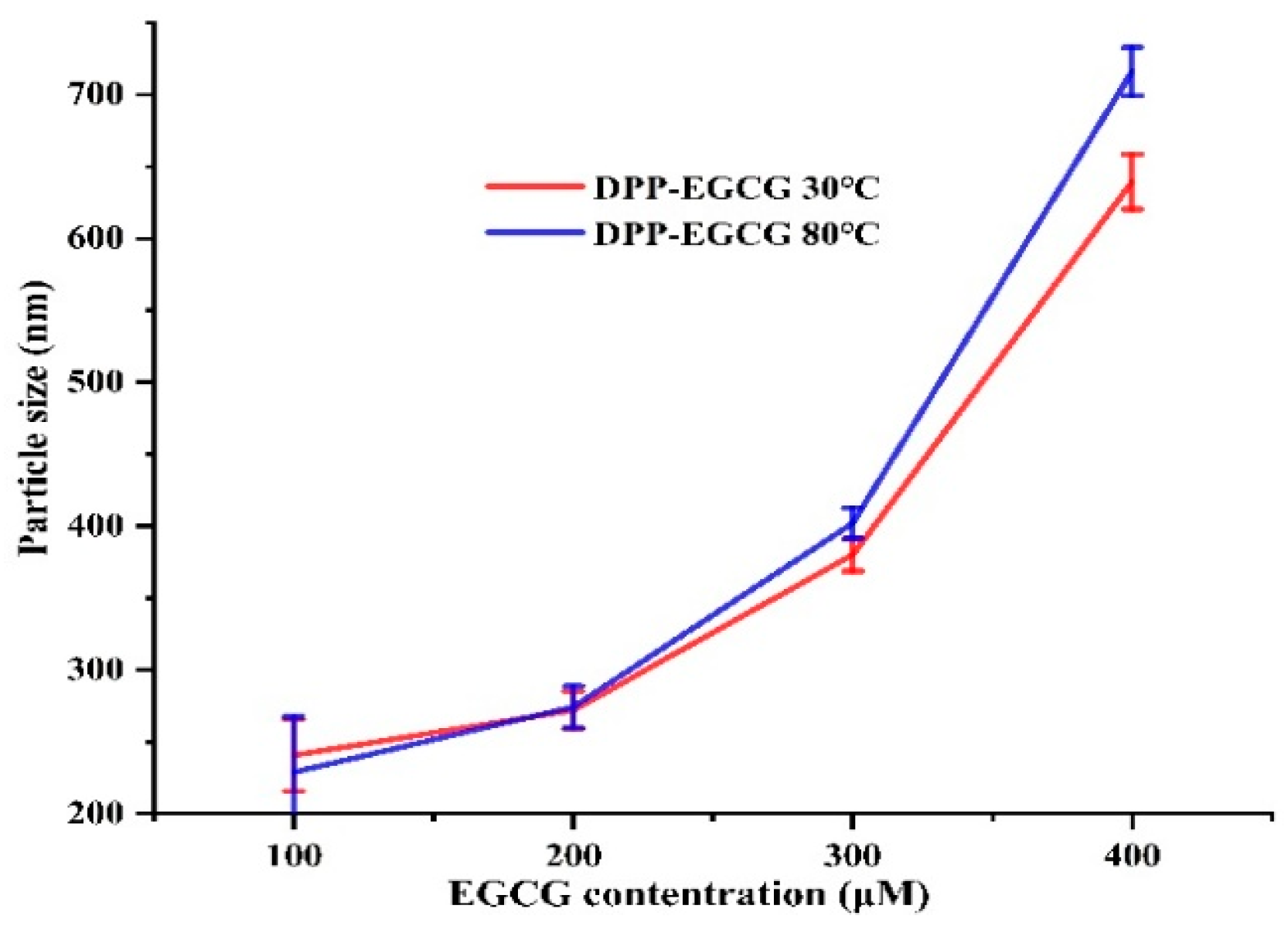
3.7. Analysis of the Interaction between DPP and EGCG
According to Yang et al. [
14], light scattering measurements using DLS could provide useful information about the change in quaternary structure in proteins. Therefore, at temperatures of 30 and 80 °C, DLS can evaluate the complexes formed by the direct interaction of EGCG with DPP. The DLS data reveal that the compaction of DPP molecules occurs within the first minute after mixing and then stabilizes. The results show an anticipated initial shrinkage at a low EGCG ratio, then an increase in particle size due to aggregation. We used DLS to measure the average size and polydispersity of the DPP EGCG complexes at 30 and 80 °C (
Figure 6). We noted that the size of the complexes remained unchanged when the EGCG concentration was less than 0.2 mM. Nevertheless, when the EGCG concentration was above 0.2 mM, it significantly (P < 0.05) enlarged the size of the complexes, especially in the instance of DPP at 80 °C. Our study showed that we could only look at soluble DPP-EGCG complexes when the EGCG level dropped below 0.2 mM. This means that the molar ratio of EGCG to DPP was 1:1. The results showed that date palm pollen protein extracted at 80 °C may be more susceptible to EGCG cross-linking, which could cause it to aggregate and grow in particle size. Cheng et al. [
30] found that a high concentration of polyphenols in tea could cause myofibrillar proteins (MP) in pork to aggregate, which is in agreement with this result.
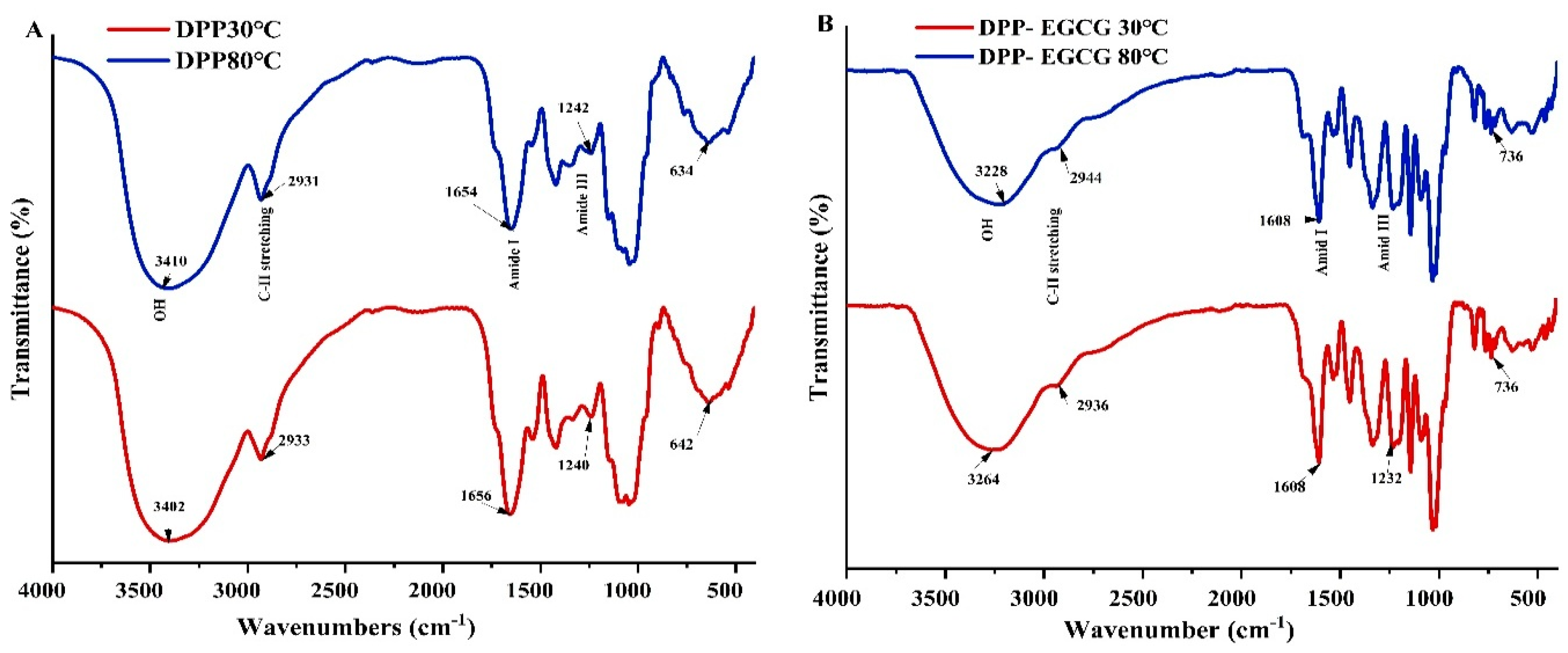
3.8. FTIR-ATR Analysis
The FTIR spectra of freeze-dried DPP protein (30, 80 °C) and the complex to EGCG (1:1) were compared, revealed peaks that originated in the 4000–400 cm
-1 region. These peaks are shown in
Figure 7. (a, b). The DPP30℃and DPP80℃ spectra are nearly the same, with a few peaks changing. In each spectrum, a major peak corresponding to the hydroxyl group bands O-H which range from 3402 for 30℃to 3410 for 80℃ cm
-1 and EGCG/DPP complex the peak at 3264 cm
-1 was shifted to 3228 cm
-1 at 30 and 80℃ respectively, indicating the heating treatment and EGCG addition involved intermolecular hydrogen bonds in DPP protein [
31]. The C-H stretching vibrations correspond to the band between 3000 and 2800 cm
-1 and the changes in this region showed the hydrophobic interaction between EGCG/ DPP protein (30, 80℃). The C=O stretching vibrations of amide I (α-helical structure) were found to occur in the wide range (80%) between 1660 and 1540 cm
-1 for pure DPP protein at 30, 80°C, but in EGCG/ DPP (30, 80℃) the peak of amide I band decreased slightly and ranged between 1620-1520 cm
-1, Thus, the decreased peak indicated the formation of complex. This band is widely used in protein secondary structural analysis. Because the amide II band (β-sheet) is mostly formed from N-H (20%) bending vibration that is strongly related to C-N stretching, the amide II band of nearly 1550 cm
-1 is very helpful. While peak regions between 1350 and 1200 cm
-1 displayed amide III’s C-H stretching vibrations and N-H deformation. Carbohydrates mainly occupy the spectral range of 1200 and 900 cm
-1 [
32]. The fingerprint zones of both extracts 30 and 80℃, ranging from 1500 to 500 cm
-1, showed differences in protein structure between DPP protein alone and DPP/EGCG, where both peaks have disappeared 642 and 634 found in DPP at 30 and 80℃ respectively that confirmed the formation of complexes. This might be because the high levels of certain amino acids in DPP protein or the high temperatures used for extraction break up the bonds between molecules [
17]. These amino acids are known to disrupt the regular structures of proteins, effectively forming turns and hydrophobic regions that align with the FTIR results. This means that the highly polymerized polyphenols in tea are more likely to interact with the hydrophobic parts of proteins because they have more conjugated and galloylated structures [
33]. Yu et al. [
34] documented a comparable observation.
3.9. Circular Dichroism Analysis (CD)
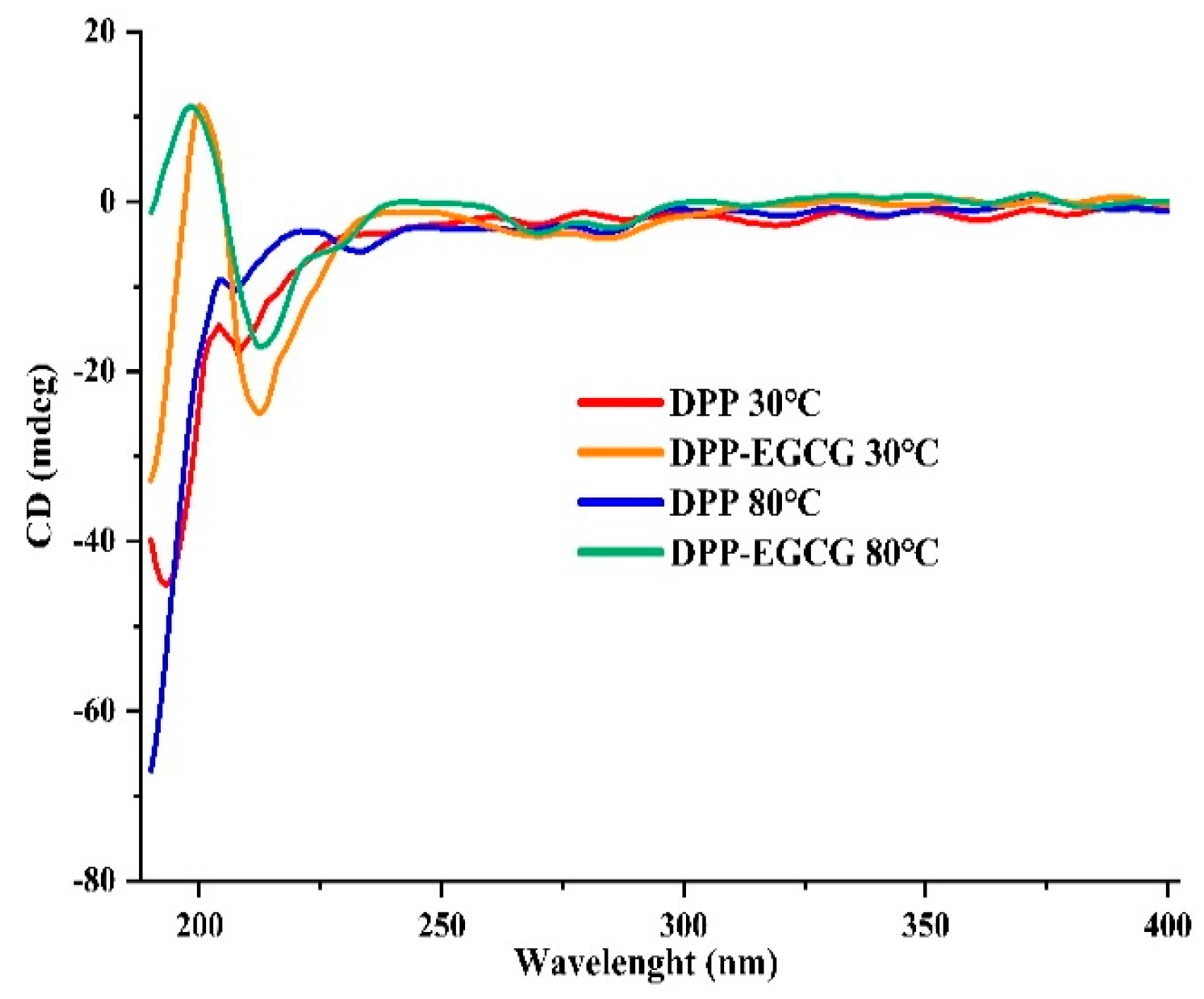
Far-UV CD spectroscopy was employed to evaluate the effect of DPP/EGCG complex formation on the secondary structure of DPP at temperatures of 30 and 80 °C.
Figure 8 illustrates that the distinctive α-helix conformation of the protein generated discernible negative peaks at 208 and 207 nm for DPP at 30 and 80 °C, respectively, and at 212 nm for EGCG with DPP at the same temperatures. The interaction between EGCG with DPP led to a minor decrease in band intensity, suggesting that EGCG modified the composition of the protein’s helical structure, encompassing the α-helix, β-sheet, and random coil configurations. Prior work indicates that a predominant α-helix conformation fosters a compact protein structure, whereas a significant random coil conformation may result in a more relaxed protein structure [
35]. The results of this study indicate that EGCG may influence the secondary structure of DPP, resulting in a more relaxed protein conformation. Nevertheless, another study [
36,
37] has demonstrated that the inclusion of polyphenols enhances the α-helix content of proteins, but the precise mechanism underlying this enhancement is still to be elucidated. The variability in secondary structural alterations can be ascribed to the distinct interferences arising from the composition and binding mechanism of EGCG in the establishment of hydrogen and disulfide bonds within proteins. This observation is consistent with the findings of [
38,
39] in their studies on pork and chicken protein.
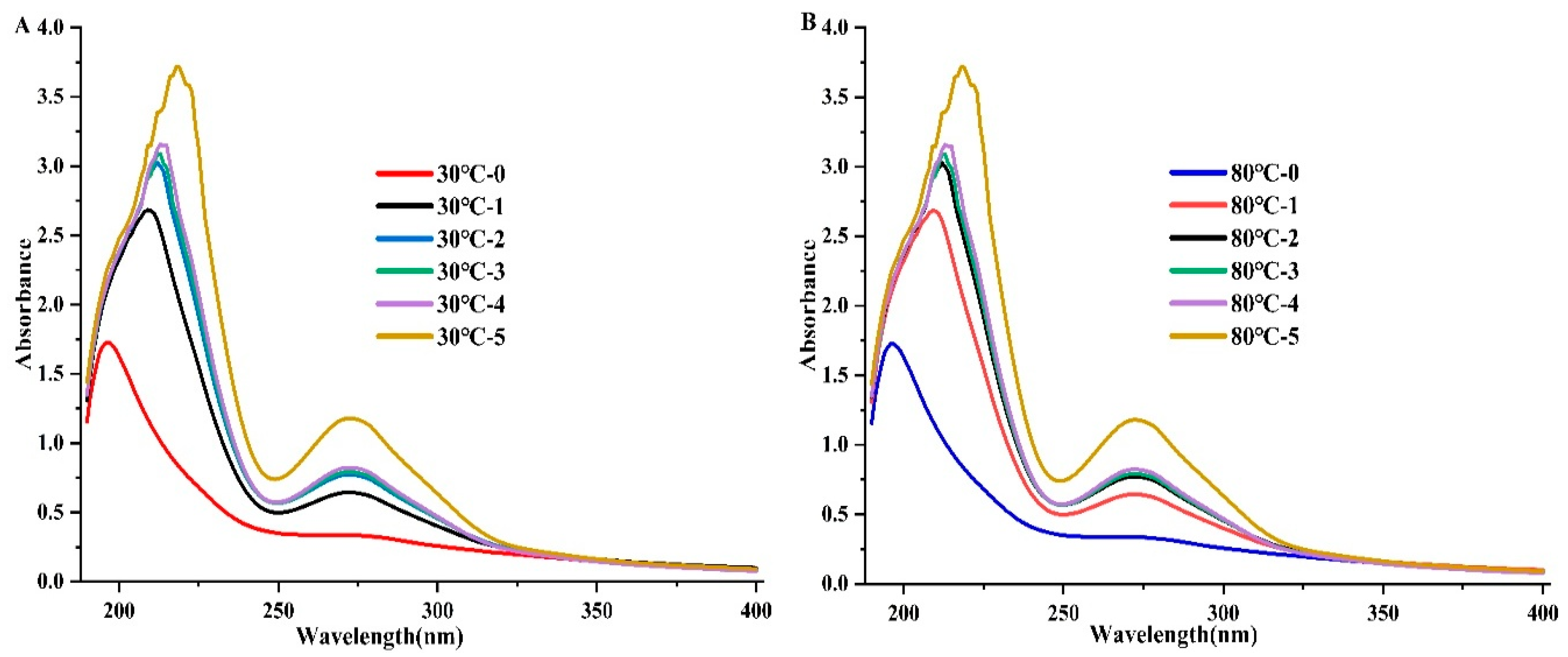
3.10. Ultraviolet–Visible (UV–VIS) Spectrophotometry
Utilized a UV-visible spectrum to study the protein structure and how it interacts with ligands. It’s a quick, easy, and useful way to find changes in structure and the stage of complex formation [
40]. The EGCG/DPP protein complexes can absorb UV light because of changes in electrons in their molecular orbitals (MOs), mostly in their aromatic rings. [
41] primarily attribute the absorption peaks around 280 nm in the UV-visible spectra to aromatic amino acids.
Figure 9 illustrates the DPP absorption spectra at 30 and 80 °C, both in the presence and absence of EGCG. According to the results, there was a blue shift and an increase in the absorption peak as the EGCG concentration rose. The findings suggest that EGCG might make DPP’s peptide chain longer, which would reveal its aromatic hydrophobic groups and change the shape of DPP. Haslam et al. [
42] stated that the increased absorption of UV light by EGCG indicated the formation of hydrogen bonds between its phenolic hydroxyl and the amide group of these proteins.
4. Conclusions
In this work, the isoelectric precipitation method was used to extract protein from a unique vegetal product called date palm pollen (DPP), and a freeze-dryer was used to turn the DPP into powder. The study examined the impact of varying extraction temperatures (30; 80 °C) on the surface, structural, thermal, and physicochemical characteristics of protein extracts obtained from DPP powder. According to this study, extracting the protein at 80°C increased its yield but also caused structural alterations in the pollen protein, such as the dissociation of some intermolecular bonds and a decrease in enthalpy. Although all cited properties were changed, the pollen protein at 80°C was more surface active than that obtained at 30°C; therefore, it can be used as a natural surfactant in food formulation. Tea polyphenol (EGCG) and DPP protein were utilized to bind in order to reduce the astringency of tea because of the good physiochemical results of DPP protein. Tea polyphenols (EGCG) treatment altered or enhanced the DPP protein’s functional characteristics by revealing more of the protein’s hidden functional groups and relaxing the polypeptide chains. The particle size experiment revealed that it promoted the binding of DPP protein to EGCG at a molar ratio of 1:1. The results of the FTIR, CD, and UV absorption spectra showed that EGCG had a major impact on the secondary structure of the DPP protein that was extracted at 30 and 80°C. This result lends credence to the theory that heating DPP increases the binding affinity of EGCG to DPP. This study’s conclusions are useful for lowering the astringency and bitterness of tea and enhancing the sensory experiences of tea beverages made with DPP protein at 80℃. The results of this study were encouraging for the creation of food formulations that use DPP as an efficient carrier in the context of food items.
Author Contributions
Conceptualization, Z.L. and L.L.; methodology, R.M., X.J., and W.F.; formal analysis, R.M.; data curation, X.J., and W.F.; investigation, R.M.; resources, L.L.; writing—original draft: R.M.; writing—review and editing, R.M., L.W.; supervision, L.W. and Z.L.; project administration, L.L.; funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Chongqing Technical Innovation and Application Development Special Project (CSTB2023TIAD-KPX0033 and CSTB2022TIAD-KPX0094), and the Project of the Ministry of Science and Technology (2022YFD1600800), National Natural Science Foundation of China funded project (32172627), Fundamental Research Funds for the Central Universities (SWU-XDJH202316), Chongqing Modern Agricultural Industrial Technology System Innovation Team (CQMAITS202308), Integration and Demonstration of Continuous Pest Control and Product Targeted Efficiency Enhancement Technology for Yibin Earliest Tea (YBYJY20221402).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Soares, S.; Soares, S.; Brandão, E.; Guerreiro, C.; Mateus, N.; de Freitas, V. Oral interactions between a green tea flavanol extract and red wine anthocyanin extract using a new cell-based model: Insights on the effect of different oral epithelia. Sci. Reports 2020, 10, 12638. [Google Scholar] [CrossRef] [PubMed]
- Fan, F. Y.; Huang, C. S.; Tong, Y. L.; Guo, H. W.; Zhou, S. J.; Ye, J. H.; et al. Widely targeted metabolomics analysis of white peony teas with different storage time and association with sensory attributes. Food Chem. 2021, 362, 130257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Q. Q.; Granato, D.; Xu, Y. Q.; Ho, C. T. Association between chemistry and taste of tea: A review. Trends in Food Sci. & Tech. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Ye, J. H.; Ye, Y.; Yin, J. F.; Jin, J.; Liang, Y. R.; Liu, R. Y.; Tang, P.; Xu, Y. Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends in Food Sci. & Tech. 2022, 123, 130–143. [Google Scholar] [CrossRef]
- Yi, H. D.; Jia, R.W.; Xiao, Q. C. Interactions between tea polyphenols and nutrients in food. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3130–3150. [Google Scholar] [CrossRef]
- Dong, Z. B.; Lu, J. L.; Sun, Q. L.; Dong, J. J.; Liang, Y. R. Advances on research of haze-active proteins in beverage. Food and Ferm. Ind. 2009, 35, 136–139. [Google Scholar]
- Buitimea-Cantúa, N. E.; Gutiérrez-Uribe, J. A.; Serna-Saldívar, S. O. Phenolic–protein interactions: Effects on food properties and health benefits. J of Med. Food 2017, 21(2), 188–198. [Google Scholar] [CrossRef]
- Yildirim-Elikoglu, S.; Erdem, Y. K. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes, and the future trends in the dairy industry. Food Rev. Int. 2017, 34(7), 665–697. [Google Scholar] [CrossRef]
- Jöbstl, E.; O’Connell, J.; Fairclough, J. P. A.; Williamson, M. P. Molecular model for astringency produced by polyphenol/protein interactions. Biomacromolecules 2004, 5, 942–949. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Ghosh, A. K.; Ghosh, C. Recent developments on polyphenol–protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food & Func. 2012, 3(6), 592–605. [CrossRef]
- Ge, G.; Zhao, J.; Zheng, J.; Zhou, X.; Zhao, M.; Sun, W. Green tea polyphenols bind to soy proteins and decrease the activity of soybean trypsin inhibitors (STIs) in heated soymilk. Food Func. 2022, 13, 6726–6736. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; Soliman, T.N.; Darwish, A.M.G. Evaluation of date palm pollen (Phoenix dactylifera L.) encapsulation, impact on the nutritional and functional properties of fortified yoghurt. PLoS. One 2019, 14(10), e0222789. [CrossRef]
- Karra, S.; Sebii, H.; Jardak, M.; Bouaziz, M. A.; Hamadi Attia, H.; Blecker, C.; Besbes, S. Male date palm flowers: Valuable nutritional food ingredients and alternative antioxidant source and antimicrobial agent. South Afri. J. of Bot. 2020, 131, 181- 187. [CrossRef]
- Hassan, H. Chemical composition and nutritional value of palm pollen grains. Global J of Biochem. & Biotech. 2011, 6, 1–7. [Google Scholar]
- Hamed, A.I.; Ben Said, R.; Al-Ayed, A.S.; Moldoch, J.; Mahalel, U.A.; Mahmoud, A.M.; Elgebaly, H.A.; Perez, A.J.; Stochmal, A. Fingerprinting of strong spermatogenesis steroidal saponins in male flower of Phoenix dactylifera (Date Palm) by LC-ESI-MS. Nat. Prod. Res. 2017, 31, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis of AOAC Int. 2023, (22nd Edition). Washington DC.
- Sebii, H.; Karra, S.; Bchir, B.; Ghribi, A. M.; Danthine, S.; Blecker, C.; Attia, H.; Besbes, S. Physico-chemical, surface and thermal properties of date palm pollen as a novel nutritive ingredient. Adv. Food Tech. Nutri. Sci. Open J. 2019, 5(3), 84–91. [Google Scholar] [CrossRef]
- Serea, C.; Barna, O. Phenolic content and antioxidant activity in oat. Anal. Food Sci. & Tech. 2011, 12, 164–168. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Sebii, H.; Karra, S.; Bchir, B.; Ghribi, A. M.; Danthine, S.; Blecker, C.; Besbes, S. Effect of sonication pretreatment on physico-chemical, surface and thermal properties of date palm pollen protein concentrate. LWT. 2019, 106, 128–136. [Google Scholar] [CrossRef]
- Yang, W.; Liu, F.; Xu, C.; Yuan, F.; Gao, Y. Molecular interaction between (−)-epigallocatechin-3-gallate and bovine lactoferrin using multi-spectroscopic method and isothermal titration calorimetry. Food Res. Int. 2014, 64, 141–149. [Google Scholar] [CrossRef]
- Zhong, S.; Luo, L.; Pittia, P.; Xie, J.; Wen, H.; Luo, W.; Zeng, L. Studies on the effects of preheated β-lactoglobulin on the physicochemical properties of theaflavin-3,3′-digallate and the interaction mechanism. Food Hydrocolloids 2024, 154, 110087. [Google Scholar] [CrossRef]
- Shahin, f. M. I. (2014). Utilization of date palm pollen as natural source for producing function bakery product. Egypt. J. Agric. Res. 2014, 92(4), 1457–1470. [Google Scholar] [CrossRef]
- Abdel-Shaheed, M.M.; Abdalla, E.S.; Khalil, A.F.; El-Hadidy, E.M. Effect of Egyptian Date Palm Pollen (Phoenix dactylifera L.) and Its Hydroethanolic Extracts on Serum Glucose and Lipid Profiles in Induced Diabetic Rats. Food and Nut. Sci. 2021, 12, 147–161. [Google Scholar] [CrossRef]
- Salomón-Torres, R.; Krueger, R.; García-Vázquez, J.P.; Villa-Angulo, R.; Villa-Angulo, C.; Ortiz-Uribe, N.; Sol-Uribe, J.A.; Samaniego-Sandoval, L. Date Palm Pollen: Features, Production, Extraction and Pollination Methods. Agronomy 2021, 11, 504. [Google Scholar] [CrossRef]
- Daoud, A.; Malika, D.; Bakari, S.; Hfaiedh, N.; Mnafgui, K.; Kadri, A.; Gharsallah, N. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of Date Palm Pollen (DPP) from two Tunisian cultivars. Arab. J. of Chem. 2019, 12, 3075–3086. [Google Scholar] [CrossRef]
- LeBlanc, B.W.; Davis, O. K.; Boue, S.; DeLucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Karra, S.; Sebii, H.; Bouaziz, A.M.; Blecker, C.; Danthine, S.; Attia, H.; Besbes, S. Effect of sonication pre-treatment on physico-chemical, surface, thermal and functional properties of fibro proteic extracts from male date palm flowers. J. of Food Proc. and Pres. 2020, 44, 14963. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultra. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Cheng, J. R.; Xu, L.; Xiang, R.; Liu, X. M.; Zhu, M. J. Effects of mulberry polyphenols on oxidation stability of sarcoplasmic and myofibrillar proteins in dried minced pork slices during processing and storage. Meat Sci. 2020, 160, 107973. [Google Scholar] [CrossRef]
- Kasprzyk, I.; Depciuch, J.; Grabek-Lejko, D.; Parlinska-Wojtan, M. FTIR-ATR spectroscopy of pollen and honey as a tool for unifloral honey authentication. The case study of rape honey. Food Cont. 2018, 84, 33–40. [Google Scholar] [CrossRef]
- Andrade, J.; Pereira, C. G.; Almeida Junior, J. C.d.; Viana, C. C. R.; Neves, L. N.d. O.; Silva, P. H. F.d.; Anjos, V.d. C. D. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT- Food Sci. & Tech. 2019, 99, 166–172. [CrossRef]
- Ye, J.; Fan, F.; Xu, X.; Liang, Y. Interactions of black and green tea polyphenols with whole milk. Food Res. Inter. 2013, 53, 449–45. [Google Scholar] [CrossRef]
- Yu, X.; Cai, X.; Li, S.; Luo, L.; Wang, J.; Wang, M.; Zeng, L. Studies on the interactions of theaflavin-3,3’-digallate with bovine serum albumin: Multispectroscopic analysis and molecular docking. Food Chem. 2022, 366, Article 130422. [CrossRef]
- Qiu, C. J.; Xia, W. S.; Jiang, Q. X. (2014). Pressure-induced changes of silver carp (Hypophthalmichthys molitrix) myofibrillar protein structure. Euro. Food Res. and Tech. 2014, 238, 753–761. [Google Scholar] [CrossRef]
- Kanakis, C.; Hasni, I.; Bourassa, P.; Tarantilis, P.; Polissiou, M.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef]
- Wu, X.; He, W.; Yao, L.; Zhang, H.; Liu, Z.; Wang, W.; Ye, Y.; Cao, J. Characterization of binding interactions of (−)-epigallocatechin-3-gallate from green tea and lipase. J. of Agri. and Food Chem. 2013, 61(37), 8829–8835. [Google Scholar] [CrossRef] [PubMed]
- Chong Tan, C.; Qian-Da Xu, Q.; Nan Chen, N.; Qiang He, Q.; Qun Sun, Q.; Zeng, W. Cross-linking effects of EGCG on myofibrillar protein from common carp (Cyprinus carpio) and the action mechanism. J. Food Biochem. 2022, 46, e14416. [Google Scholar] [CrossRef]
- Cong, J.; Cui, J.; Zhang, H.; Dzah, C. S.; He, Y.; Duan, Y. Binding affinity, antioxidative capacity and in vitro digestion of complexes of grape seed procyanidins and pork, chicken and fish protein. Food Res. Int. 2020, 136, 109530. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, K.; Ince, C.; Condict, L.; Hung, A.; Kasapis, S. Combined spectroscopic and molecular docking study on the pH dependence of molecular interactions between β-lactoglobulin and ferulic acid. Food Hydrocolloids 2020, 101. [Google Scholar] [CrossRef]
- You, Y.; Yang, L.; Chen, H.; Xiong, L.; Yang, F. Effects of (−)-Epigallocatechin-3-gallate on the Functional and Structural Properties of Soybean Protein Isolate. J. Agric. Food Chem. 2021, 69, 2306−2315. [CrossRef]
- Haslam, E.; Williamson, M. P.; Baxter, N. J.; Charlton, A. J. Astringency and polyphenol protein interactions. Rec. Advan. in Phytochem. 1999, 33, 289. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).