1. Introduction
Cell-based therapy using mesenchymal stem cells (MSCs) represents a promising approach for the treatment of several diseases due to their anti-inflammatory, immunomodulatory, and anti-apoptotic properties [
1,
2].
The therapeutic effects of MSCs are primarily mediated through direct cell-to-cell interactions and the release of soluble factors and extracellular vesicles via paracrine mechanisms [
3]. MSCs interact with several immune cells, including T lymphocytes, natural killer cells, neutrophils, and macrophages, thereby modulating the immune response [
3,
4].
MSCs can be derived from various tissues, including adipose tissue, bone marrow, and fetal tissues such as amniotic fluid, placenta, and umbilical cord [
5]. In horses, adipose tissue is considered a reliable and easily accessible source of MSCs [
6]. Importantly, studies in large animal models, including horses, not only address species-specific health and welfare needs but also as a translational model for human diseases, given the shared pathophysiological similarities [
7].
To optimize the therapeutic properties of MSCs, various priming strategies have been developed. Priming involves exposing MSCs to specific stimuli to enhance their biological functions, such as paracrine activity, regenerative potential, and immunomodulatory capacity [
8,
9]. For instance, MSC priming with pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ), has been shown to enhance their immunoregulatory functions [10-13]. Additional strategies include genetic modification [
14] and the use of optimized culture conditions [
15,
16].
In this context, Cannabidiol (CBD), a non-psychoactive phytocannabinoid derived from Cannabis sativa, has recently garnered attention for its anti-inflammatory and immunomodulatory properties in both human and veterinary medicine [17-19]. Based on these effects, CBD could be used as a priming agent to enhance the therapeutic potential of MSCs.
Previous studies have demonstrated that CBD modulate MSCs proliferation and differentiation [
20,
21], while dowregulating the expression of genes associated with inflammation, immune response, and apoptosis in human MSCs [
22]. Furthermore, CBD potentiates the signaling of type 1 (CB1) and type 2 (CB2) endocannabinoid receptors, key components of the endocannabinoid system [
23]. This system plays pivotal roles in regulating a wide range of physiological functions, making it a potential target for the development of new treatments [
24].
Thus, this study aims to evaluate the in vitro immunomodulatory potential of EqAT-MSCs following priming with a CBD-rich cannabis extract. The findings from this study may contribute to the development of more effective cell-based therapies and provide novel insights into the application of CBD as a priming agent.
2. Results
2.1. Morphological changes
No differences in morphology were observed between the groups primed with CBD-rich cannabis extract and the control group after 24 hours of priming (
Figure 1).
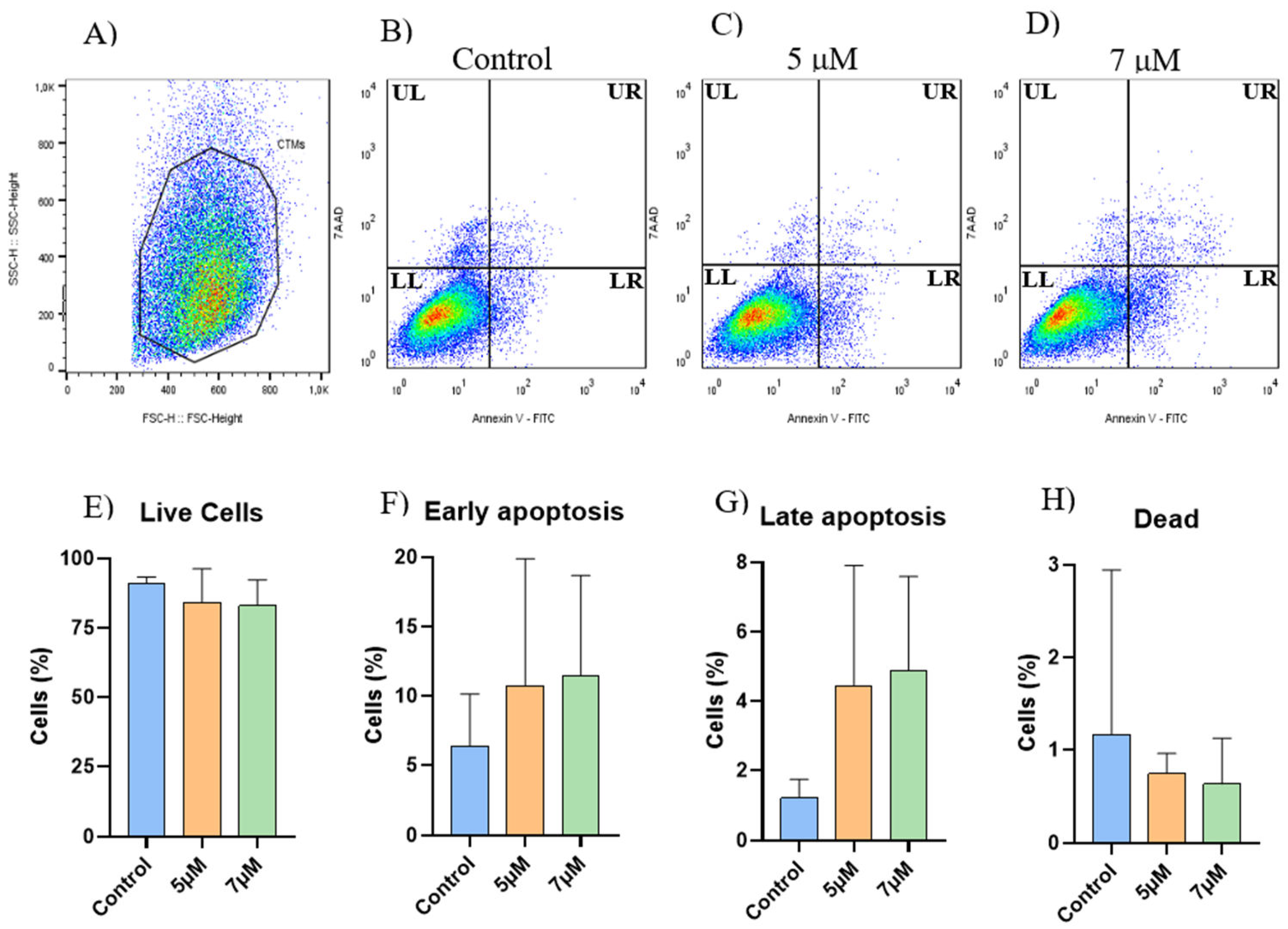
2.2. Apoptosis Assay
Apoptosis assessment was performed on three samples of EqAT-MSCs, and no significant changes were observed when comparing the groups in this experiment (
Figure 2).
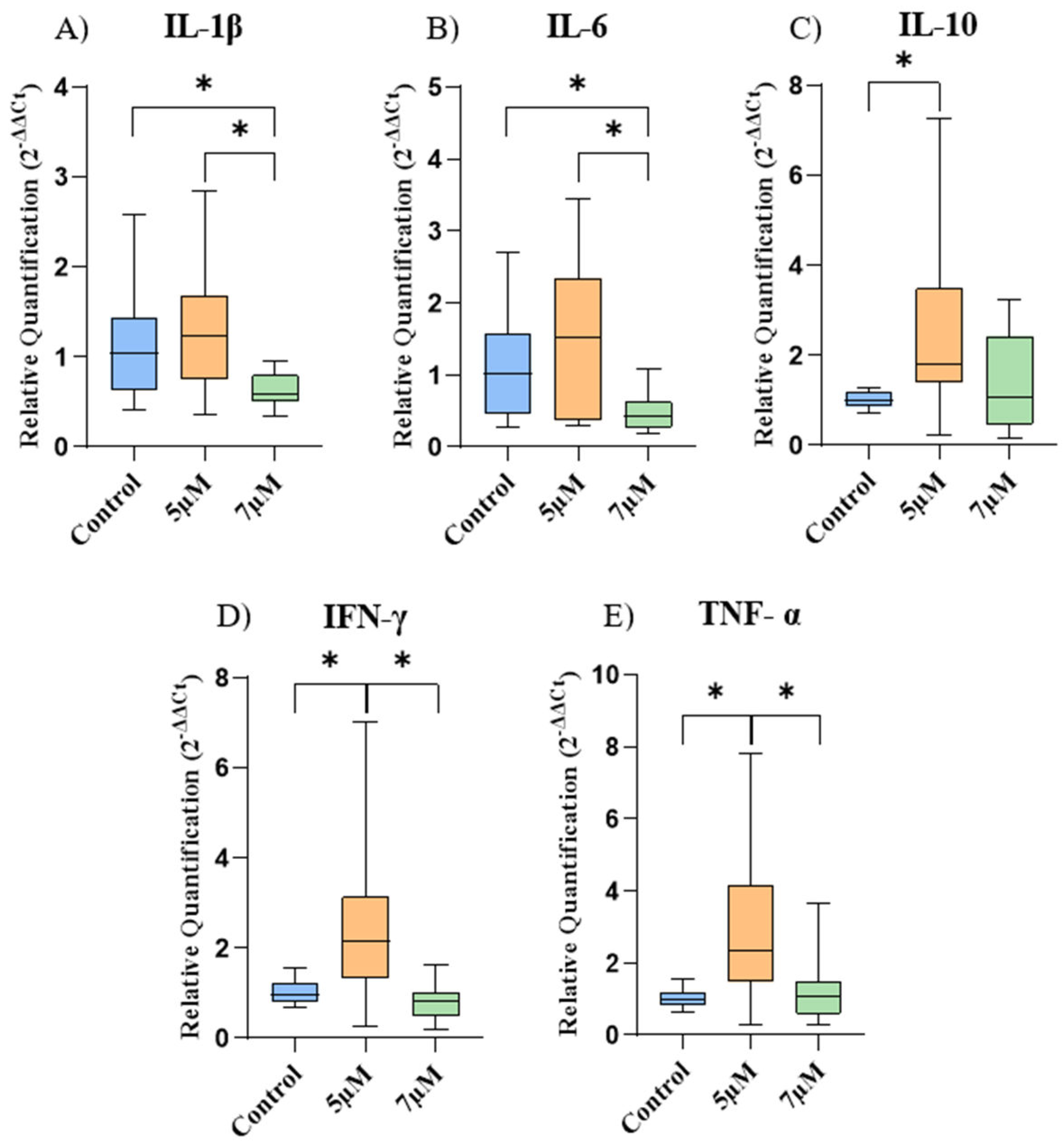
2.3. Gene Expression
The gene expression of the cytokines is represented in
Figure 3. In EqAT-MSCs, there was a significant decrease in the gene expression of IL-1β and IL-6 after priming with a concentration of 7 μM, compared to the control group. Regarding IL-10, IFN-γ, and TNF-α, a considerable increase was observed after priming with a concentration of 5 μM compared to the control group.
Furthermore, when comparing cells among the different concentrations of CBD-rich cannabis extract used in this study, it was noticed a significant increase in the expression of IL-1β, IL-6, IFN-γ and TNF-α in the concentration of 5 μM compared to the concentration of 7 μM.
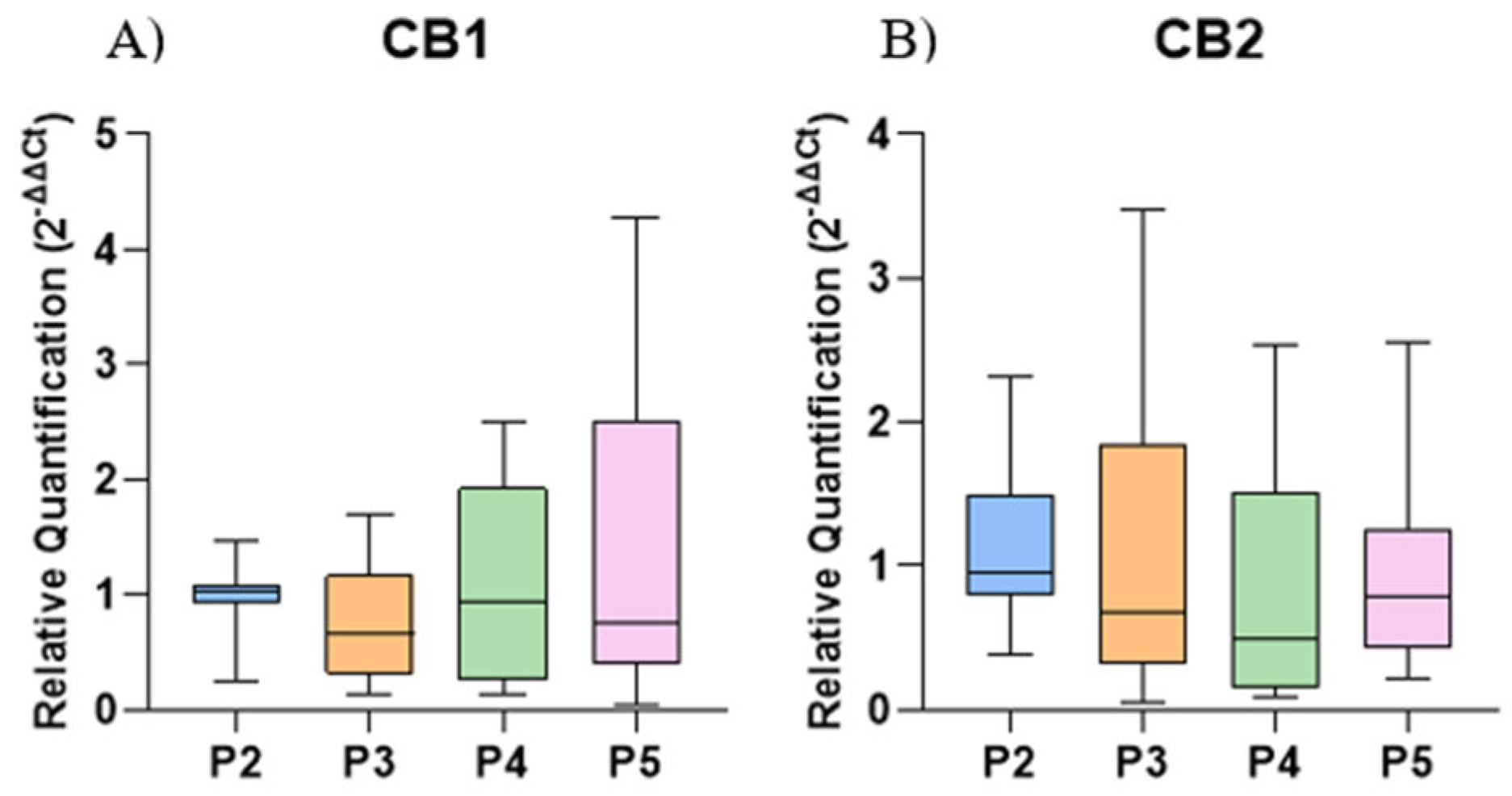
There was no significant difference in the gene expression of CB1 and CB2 among the different culture passages compared to P2 (
Figure 4).
3. Discussion
Mesenchymal stem cells (MSCs) possess significant therapeutic potential; however, challenges such as limited clinical efficacy, functional quiescence post-transplantation, poor engraftment, and cellular senescence continue to hinder their clinical application [
25]. To address these limitations, various priming strategies have been developed to enhance MSC functionality and improve therapeutic outcomes. These include 3D culture, growth factor stimulation, hypoxic conditions, electrical and thermal stimulation, the use of chemical agents and drugs, as well as genetic modifications [
8,
26].
In this context, phytocannabinoids such as CBD have attracted attention due to their ability to interact with a wide range of molecular targets within the endocannabinoid system [
27], exhibiting anti-inflammatory, antioxidant, and neuroprotective properties, rendering it a promising candidate for the treatment of several diseases [
28]. Furthermore, studies have demonstrated that CBD can enhance the proliferation, self-renewal, and migration of MSCs [
29,
30].
Cannabinoid receptors CB1 and CB2 are well-characterized components of the endocannabinoid system [
31]. These receptors have been previously identified in mouse embryonic stem cells [
32] and in human AT-MSCs [
33]. In this study, we demonstrate the gene expression of CB1 and CB2 in EqAT-MSCs, suggesting conservation of these receptors across mammalian species [
34]. This finding supports the potential for utilizing synthetic or natural agonists to modulate these receptors, thereby enhancing the anti-inflammatory and immunomodulatory functions of MSCs.
To investigate this potential, EqAT-MSCs were primed with a CBD-rich cannabis extract at concentrations of 5 μM and 7 μM, selected based on prior studies [
22,
35] and initial metabolic activity assessments, which indicated no significant changes up to 7 μM (data not shown). Morphological analysis revealed no structural alterations following priming, consistent with previous findings in human MSCs [
22], indicating that CBD concentrations of 5 μM and 7 μM preserved cellular integrity after 24 hours.
The anti-apoptotic effects of CBD having been previously described in human gingival-derived MSCs [
22] and human adipose-derived mesenchymal stem cells [
27]. However, apoptosis assays in this study revealed no significant differences in EqAT-MSCs primed with 5 μM or 7 μM CBD compared to the control group, suggesting that these concentrations do not impact apoptosis rates in EqAT-MSCs.
CBD is well-known for its anti-inflammatory properties. So, we evaluated the expression of several cytokines after priming of EqAT-MSCs. Although a study reported an increase in the gene expression of IL-1β and IL-6 in human adipose-derived stem cells after priming with CBD at a concentration of 5 μM [
27], we did not observe a significant difference using the same concentration. However, there was a significant decrease in IL-1β and IL-6 after priming at a concentration of 7 µM. This can be explained by the fact that CBD exerts complex effects on the regulation of inflammatory cytokine expression through multiple cellular mechanisms. This phytocannabinoid can inhibit the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, a key regulator of inflammation, thereby reducing the expression of inflammatory cytokines [
36,
37]. Additionally, CBD can modulate the activity of the NOD-like receptor pyrin domain-containing 3 inflammasome (NLRP3 inflammasome), which is involved in the activation of caspase-1 and the subsequent release of inflammatory cytokines [
22,
38]. These findings suggest that priming with CBD-rich cannabis extract at a concentration of 7 μM may enhance the immunomodulatory capacity of EqAT-MSCs.
IL-10 is a key cytokine in immune regulation that plays an essential role in modulating inflammatory processes [
39]. We observed a significant increase in IL-10 gene expression after priming of EqAT-MSCs with CBD at a concentration of 5 μM. This result contrasts with a previous study that used the same concentration to stimulate human adipose-derived mesenchymal stem cells and reported a decrease in the expression of this cytokine [
27]. IL-10 can suppress antigen-presenting cells, inhibit T-cell proliferation, and promote the activity of regulatory T (Treg) cells [
40,
41]. The increase in IL-10 is of great interest in regenerative medicine due to its anti-inflammatory and regulatory properties
Despite previous reports demonstrating that CBD reduces TNF-α and IFN-γ
in vitro in equine peripheral blood mononuclear cells [
42] and IFN-γ
in vitro after stimulation of human adipose-derived mesenchymal stem cells [
27] and
in vivo in senior horses [
43], we identified a significant increase in the expression of both cytokines after priming with CBD-rich cannabis extract at a concentration of 5 µM. Even though TNF-α and IFN-γ are often associated with harmful effects, they can be beneficial in specific contexts. TNF-α is a key cytokine in the inflammatory response, essential for defending against infections [
44] and IFN-γ is crucial in cell-mediated immunity, coordinating various antimicrobial functions, inducing antiviral responses, and exhibiting antiproliferative effects on cancer cells [
45].
This study is limited by the absence of cytokine protein analysis and the lack of assessment of the secretory activity of equine EqAT-MSCs following priming with various concentrations of CBD-rich cannabis extract.
4. Materials and Methods
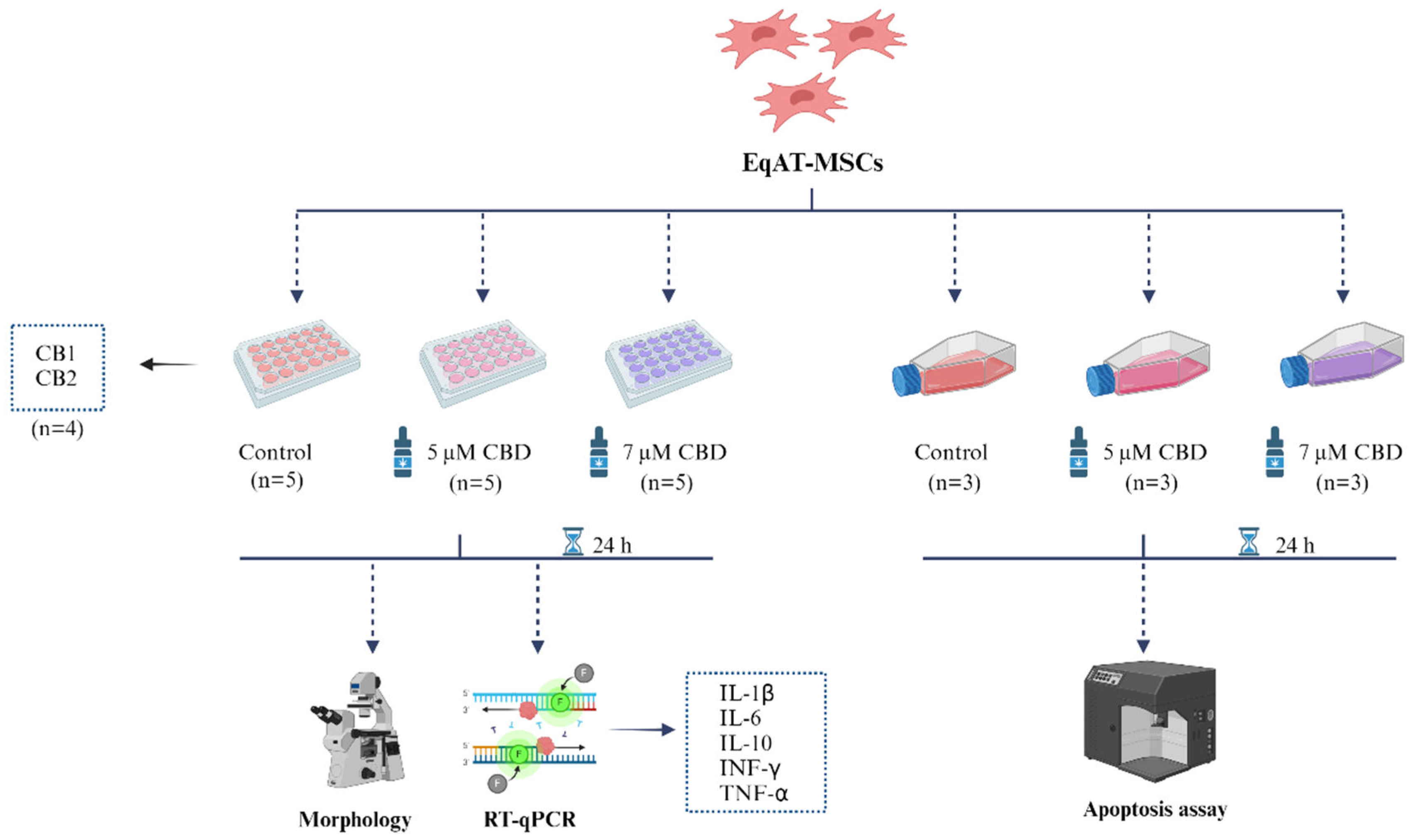
4.1. Experimental design
The EqAT-MSCs were thawed, cultured in complete medium containing 90% Dulbecco’s modified Eagle’s medium DMEM/F12, 10% fetal bovine serum (FBS) (both from LGC Biotecnologia, São Paulo, Brazil), 1% penicillin/streptomycin and 0.5% amphotericin B (both from Gibco, Grand Island, New York, USA). The cells were primed with cannabidiol (CBD)-rich cannabis extract for 24 hours.
Eq-ATMSCs (P3) were divided into three groups: the control group (complete culture medium), the group primed with 5 µM of CBD-rich cannabis extract + complete culture medium, and the group primed with 7 µM of CBD-rich cannabis extract + complete culture medium. After 24 hours of priming, morphological evaluation, gene expression analysis of cytokines such as interleukin 1 beta (IL-1β), interleukin 6 (IL-6), interleukin 10 (IL-10), interferon gamma (INF-γ), and tumor necrosis factor alpha (TNF-α) were performed on five samples using RT-qPCR. Apoptosis assays were conducted on three samples using annexin and 7-AAD (
Figure 5). Additionally, the expression of cannabinoid receptors 1 (CB1) and 2 (CB2) was evaluated in naïve EqAT-MSCs (P2–P5) using four samples per passage.
4.2. EqAT-MSCs
The EqAT-MSCs (P2-P5) from healthy horses were obtained from the MSCs bank of the Center for Translational Research in Regenerative Medicine - School of Veterinary Medicine and Animal Science - São Paulo State University. These MSCs were previously characterized, demonstrating potential for osteogenic, adipogenic, and chondrogenic differentiation, as well as expressing the surface markers CD44, CD90, and CD105, and not expressing CD34 and MHC-II [
46].
4.3. CBD-Rich Cannabis Extract
The full-spectrum Cannabis sativa extract, predominantly rich in CBD, used in this study was supplied by the Maria Flor Cannabis Association (Marília, São Paulo, Brazil). The purity of the CBD-rich cannabis extract was analyzed using High-Performance Liquid Chromatography (HPLC) by DALL Soluções Analíticas (Curitiba, Paraná, Brazil), revealing 28.12% CBD and 0.80% tetrahydrocannabinol (THC). The extract was diluted in dimethyl sulfoxide (DMSO) at a 1:1 ratio, filtered, and subsequently diluted in DMEM to achieve concentrations of 5 µM and 7 µM, which were utilized in this study.
4.4. EqAT-MSCs primed with CBD-rich cannabis extract
The EqAT-MSCs were thawed and seeded at a density of 5 x 104 cells per well in 24-well plates (Kasvi, São José do Pinhais, Paraná, Brazil), in duplicate for gene expression analysis, using 0.5 mL of complete medium containing 90% DMEM/F12, 10% FBS (both from LGC Biotecnologia, São Paulo, Brazil), 1% penicillin/streptomycin, and 0.5% amphotericin B (both from Gibco, Grand Island, New York, USA). The MSCs were incubated at 37.5°C in a humidified atmosphere containing 95% air and 5% CO2. After 24 hours of culture, the supernatant was discarded and the culture medium was replaced for each experimental group: the control group (complete culture medium), group primed with 5 µM of CBD-rich cannabis extract + complete culture medium and group primed with 7 µM of CBD-rich cannabis extract + complete culture medium. The priming was carried out for 24 hours.
For apoptosis assessment, EqAT-MSCs were seeded at the same density in 25 cm² culture flasks (Kasvi, São José do Pinhais, Paraná, Brazil), with 5 ml of complete medium. The cells were cultured under the same conditions mentioned earlier until reaching 70-80% confluence. Afterward, the culture medium was replaced, and the EqAT-MSCs were primed with 5 µM and 7 µM concentrations of CBD-rich cannabis extract for 24 hours.
4.5. Morphological Evaluation
The morphology of EqAT-MSCs was analyzed using an inverted microscopy (LEICA DMIRB, Germany). Photomicrographs of unprimed (control group) and EqAT-MSCs primed with CBD-rich cannabis extract at concentrations of 5 µM and 7 µM were captured after 24 hours of priming.
4.6. Apoptosis Assay
The evaluation of apoptosis rate was conducted using the Annexin V-FITC apoptosis detection kit (Invitrogen, USA) and 7-AAD (BD, Biosciences Pharmingen, USA), following the manufacturer's instructions. In brief, EqAT-MSCs were primed for 24 hours with CBD-rich cannabis extract at concentrations of 5 μM and 7 μM. After this period, cells were resuspended in 200 μl of 1x binding buffer and incubated with 5 μl of Annexin V-FITC for 10 minutes at room temperature in the dark. Subsequently, a wash with 200 μl of 1x binding buffer was performed, and cells were resuspended in 400 μl of 1x binding buffer. Finally, 10 μl of 7-AAD was added, and the analysis was carried out on a FACS CaliburTM flow cytometer (BD Becton Dickinson and Company, USA), acquiring at least 10.000 events.
4.7. Gene Expression
The gene expression of cytokines IL-1β, IL-6, IL-10, IFN-γ, and TNF-α were assessed in the control group (P3) and in EqAT-MSCs (P3) primed with CBD-rich cannabis extract at concentrations of 5 µM and 7 µM, using RT-qPCR. Additionally, the gene expression of CB1 and CB2 was evaluated in the control group of EqAT-MSCs across different passages (P2-P5).
After 24 hours of priming, cell lysis was performed using 1 mL of TrizolTM (InvitrogenTM, USA), and the samples were stored at -80 ºC for further analyses. RNA extraction with TRIzol reagent followed the manufacturer’s instructions. Subsequently, RNA was eluted using RNA-free water, quantified, and analyzed by spectrophotometry using the Thermo Scientific NanoDrop 2000 equipment (ThermoFisher Scientific, Wilmington, USA) to determine absorbance ratios at 260/280 nm and 260/230 nm.
For cDNA synthesis, the High-Capacity cDNA Reverse Transcription Kit (Applied BiosystemsTM, Life Technologies Corporation, Carlsbad, USA) was utilized following the manufacturer’s instructions. The reverse transcription process to obtain the cDNA was conducted using the Veriti 96 Well Thermal Cycler (Applied BiosystemsTM, ThermoFisher Scientific), employing the following thermocycling conditions: 10 minutes at 25ºC; 12 minutes at 37ºC; and 5 minutes at 85ºC. The resulting cDNA samples were cryopreserved at – 80 ºC and utilized as templates for PCR reactions.
PCR reactions were performed in duplicate utilizing cDNA generated with PowerUp
TM SYBR
TM Green Master Mix (Applied Biosystems
TM, Life Technologies, Carlsbad, CA, USA), RNA-free water, and equine-specific primers (Thermo Fisher Scientific, São Paulo, Brazil), designed with Primer Express
TM Software v3.0.1 (Applied Biosystems
TM, Thermo Fisher Scientific) (
Table 1).
Samples were evaluated using the following reference genes, beta-actin (ACTB), beta-2-microglobulin (B2M), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and hypoxanthine-guanine phosphoribosyltransferase (HPRT). Real-time polymerase chain reaction (qPCR) was conducted on the QuantStudio
TM 12K Flex Real-Time PCR System (Applied Biosystems
TM, Thermo Fisher Scientific). The relative quantification of the target genes was performed using the ΔΔCt method [
47].
4.8. Statistical Analysis
The variables of relative quantification did not show a normal distribution. Hence, group comparisons were analyzed using the Kruskal-Wallis test (non-parametric), and when significant, medians were compared using Dunn's test. The variables from the apoptosis assay were assessed through a one-way analysis of variance (ANOVA), assuming a normal distribution. For all analyses, group differences were considered statistically significant at p < 0.05. All statistical analyses were conducted using GraphPad Prism version 8, San Diego, USA.
5. Conclusions
Naïve EqAT-MSCs expressed CB1 and CB2 receptors. Priming with a CBD-rich cannabis extract demonstrated immunomodulatory effects, with 5 μM CBD upregulating IL-10, TNF-α, and IFN-γ expression, and 7 μM CBD downregulating IL-1β and IL-6 expression. These findings highlight the potential of CBD-rich extracts to modulate the inflammatory and immunomodulatory profiles of EqAT-MSCs. Further studies are required to elucidate the underlying mechanisms and optimize CBD-based priming strategies for therapeutic applications.
Author Contributions
Conceptualization, L.B, L.F.A.M. and R.M.A; methodology, L.B, L.F.A.M., L.V.O.F., A.M.M.B. and M.C; validation, A.M.M.B., M.C., M.A.G. and R.M.A.; formal analysis, L.B., L.F.A.M. and L.V.O.F.; investigation, L.B, L.F.A.M., L.V.O.F., A.M.M.B., M.C. and R.M.A.; resources, M.A.G. and R.M.A.; data curation, L.B, L.F.A.M., L.V.O.F., A.M.M.B., M.C. and L.V.O.F.; writing—original draft preparation, L.B, L.F.A.M. and L.V.O.F; writing—review and editing, L.V.O.F and R.M.A.; visualization, L.V.O.F.; supervision, R.M.A.; project administration, L.B, L.F.A.M. and R.M.A; funding acquisition, L.B, L.F.A.M. and R.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2022/08919-2 and 2022/16418-3).
Institutional Review Board Statement
This project was approved by the Ethics Committee on the Use of Animals (CEUA) of UNESP in Botucatu, São Paulo, Brazil (n° 000.254).
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for its financial support.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Andrzejewska, A.; Dabrowska, S.; Lukomska, B.; Janowski, M. Mesenchymal stem cells for neurological disorders. Adv. Sci. 2021, 8, 2002944. [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771-2794. [CrossRef]
- Alvites, R.; Branquinho, M.; Sousa, A.C.; Lopes, B.; Sousa, P.; Maurício, A.C. Mesenchymal stem/stromal cells and their paracrine activity—immunomodulation mechanisms and how to influence the therapeutic potential. Pharmaceutics 2022, 14, 381. [CrossRef]
- Chen, P.M.; Liu, K.J.; Hsu, P.J.; Wei, C.F.; Bai, C.H.; Ho, L.J.; Sytwu, H.K.; Yen, B.L. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J. Leukoc. Biol. 2014, 96, 295-303. [CrossRef]
- Maličev, E.; Jazbec, K. An overview of mesenchymal stem cell heterogeneity and concentration. Pharmaceuticals 2024, 17, 350. [CrossRef]
- Smith, R.K.; Garvican, E.R.; Fortier, L.A. The current ‘state of play’of regenerative medicine in horses: what the horse can tell the human. Regen. Med. 2014, 9, 673-685. [CrossRef]
- Ribitsch, I.; Baptista, P.M.; Consiglio, A.L.; Melotti, L.; Patruno, M.; Jenner, F.; Feichter, E.S.; Dutton, L.C.; Connolly, D.J.; Steenbeek, F.G.V.; Dudhia, J.; Penning, L.C. Large animal models in regenerative medicine and tissue engineering: to do or not to do. Front. Bioeng. Biotechnol. 2020, 8, 972. [CrossRef]
- Haider, K.H. Priming mesenchymal stem cells to develop “super stem cells”. World J. Stem Cells 2024, 16, 623. [CrossRef]
- Noronha, N.C.; Mizukami, A.; Oliveira, C.C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem cell Res. Ther. 2019, 10, 131. [CrossRef]
- Al-Mrahleh, M.; Matar, S.; Jafar, H.; Wehaibi, S.; Aslam, N.; Awidi, A. Human Wharton’s jelly-derived mesenchymal stromal cells primed by tumor necrosis factor-α and interferon-γ modulate the innate and adaptive immune cells of type 1 diabetic patients. Front. Immunol. 2021, 12, 732549. [CrossRef]
- Amorim, R.M.; Clark, K.C.; Walker, N.J.; Kumar, P.; Herout, K.; Borjesson, D.L.; Wang, A. Placenta-derived multipotent mesenchymal stromal cells: a promising potential cell-based therapy for canine inflammatory brain disease. Stem Cell Res. Ther. 2020, 11, 304. [CrossRef]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Albareda, J.; Prades, M.; Gosálvez, J.; Roy, R.; Zaragoza, P.; Martín-Burriel, I.; Rodellar, C. Priming equine bone marrow-derived mesenchymal stem cells with proinflammatory cytokines: implications in immunomodulation–immunogenicity balance, cell viability, and differentiation potential. Stem Cells Dev. 2017, 26, 15-24. [CrossRef]
- Mohammadpour, H.; Pourfathollah, A.A.; Zarif, M.N.; Hashemi, S.M. Increasing proliferation of murine adipose tissue-derived mesenchymal stem cells by TNF-α plus IFN-γ. Immunopharmacol. Immunotoxicol. 2016, 38, 68-76. [CrossRef]
- Seo, Y.; Shin, T.H.; Kim, H.S. Current strategies to enhance adipose stem cell function: an update. Int. J. Mol. Sci. 2019, 20, 3827. [CrossRef]
- Ferreira, L.V.O.; Kamura, B.C.; Oliveira, J.P.M.; Chimenes, N.D.; Carvalho, M.; Santos, L.A.; Dias-Melicio, L.A.; Amorim, R.L.; Amorim, R.M. In vitro transdifferentiation potential of equine mesenchymal stem cells into Schwann-like cells. Stem Cells Dev. 2023, 32, 422-432. [CrossRef]
- Yang, Y.; Lee, E.H.; Yang, Z. Hypoxia-conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng. Part B Rev. 2022, 28, 966-977. [CrossRef]
- Iffland, K.; Grotenhermen, F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017, 2, 139-154. [CrossRef]
- Martínez, V.; De-Hond, A.I.; Borrelli, F.; Capasso, R.; Castillo, M.D.; Abalo, R. Cannabidiol and other non-psychoactive cannabinoids for prevention and treatment of gastrointestinal disorders: useful nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [CrossRef]
- Martini, S.; Gemma, A.; Ferrari, M.; Cosentino, M.; Marino, F. Effects of cannabidiol on innate immunity: experimental evidence and clinical relevance. Int. J. Mol. Sci. 2023, 24, 3125. [CrossRef]
- Fellous, T.; Maio, F.; Kalkan, H.; Carannante, B.; Boccella, S.; Petrosino, S.; Maione, S.; Marzo, V.; Iannotti, F.A. Phytocannabinoids promote viability and functional adipogenesis of bone marrow-derived mesenchymal stem cells through different molecular targets. Biochem. Pharmacol. 2020, 175, 113859. [CrossRef]
- Li, L.; Feng, J.; Sun, L.; Xuan, Y.W.; Wen, L.; Li, Y.X.; Yang, S.; Zhu, B.; Tian, X.Y.; Li, S.; Zhao, L.S.; Dang, R.J.; Jiao, T.; Zhang, H.S.; Wen, N. Cannabidiol promotes osteogenic differentiation of bone marrow mesenchymal stem cells in the inflammatory microenvironment via the CB2-dependent p38 MAPK signaling pathway. Int. J. Stem Cells 2022, 15, 405-414. [CrossRef]
- Libro, R.; Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front. Physiol. 2016, 7, 559. [CrossRef]
- Henson, J.D.; Vitetta, L.; Quezada, M.; Hall, S. Enhancing endocannabinoid control of stress with cannabidiol. J. Clin. Med. 2021, 10, 5852. [CrossRef]
- Stasiulewicz, A.; Znajdek, K.; Grudzién, M.; Pawinski, T.; Sulkowska, J.I. A guide to targeting the endocannabinoid system in drug design. Int. J. Mol. Sci. 2020, 21, 2778. [CrossRef]
- Lee, B.C.; Kang, K.S. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res. Ther. 2020, 11, 397. [CrossRef]
- Li, M.; Jiang, Y.; Hou, Q.; Zhao, Y.; Zhong, L.; Fu, X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: current status and future prospects. Stem Cell Res. Ther. 2022, 13, 146. [CrossRef]
- Kowalczuk, A.; Marycz, K.; Garbowska, K.K.; Kornicka, J.; Zadrozny, M.B.; Groborz, S. Cannabidiol (CBD) protects adipose-derived mesenchymal stem cells (ASCs) against endoplasmic reticulum stress development and its complications. Int. J. Environ. Res. Public Health 2022, 19, 10864. [CrossRef]
- Naya, N.M.; Kelly, J.; Corna, G.; Golino, M.; Abbate, A.; Toldo, S. Molecular and cellular mechanisms of action of cannabidiol. Molecules 2023, 28, 5980. [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Scionti, D.; Diomede, F.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani. Cannabidiol activates neuronal precursor genes in human gingival mesenchymal stromal cells. J. Cell. Biochem. 2017, 118, 1531-1546. [CrossRef]
- Schmuhl, E.; Ramer, R.; Salamon, A.; Peters, K.; Hinz, B. Increase of mesenchymal stem cell migration by cannabidiol via activation of p42/44 MAPK. Biochem. Pharmacol. 2014, 87, 489-501. [CrossRef]
- Lu, H.C.; Mackie, K. Review of the endocannabinoid system. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607-615. [CrossRef]
- Bari, M.; Tedesco, M.; Battista, N.; Pasquariello, N.; Pucci, M.; Gasperi, V.; Scaldaferri, M.L.; Farini, D.; Felici, M.; Maccarrone. Characterization of the endocannabinoid system in mouse embryonic stem cells. Stem Cells Dev. 2011, 20, 139-147. [CrossRef]
- Ruhl, T.; Karthaus, N.; Kim, B.S.; Beier, J.P. The endocannabinoid receptors CB1 and CB2 affect the regenerative potential of adipose tissue MSCs. Exp. Cell Res. 2020, 389, 111881. [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21-47. [CrossRef]
- Libro, R.; Diomede, F.; Scionti, D.; Piattelli, A.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol modulates the expression of Alzheimer’s disease-related genes in mesenchymal stem cells. Int. J. Mol. Sci. 2016, 18, 26. [CrossRef]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010, 285, 1616-1626. [CrossRef]
- Ma, H.; Xu, F.; Liu, C.; Seeram, N.P. A network pharmacology approach to identify potential molecular targets for cannabidiol's anti-inflammatory activity. Cannabis and Cannabinoid Res. 2021, 6, 288-299. [CrossRef]
- Liu, C.; Ma, H.; Slitt, A.L.; Seeram, N.P. Inhibitory effect of cannabidiol on the activation of NLRP3 inflammasome is associated with its modulation of the P2X7 receptor in human monocytes. J. Nat. Prod. 2020, 83, 2025-2029. [CrossRef]
- Minshawi, F.; Lanvermann, S.; McKenzie, E.; Jeffery, R.; Couper, K.; Papoutsopoulou, S.; Roers, A.; Muller, W. The generation of an engineered interleukin-10 protein with improved stability and biological function. Front. Immunol. 2020, 11, 1794. [CrossRef]
- Mittal, S.K.; Roche, P.A. Suppression of antigen presentation by IL-10. Curr. Opin. Immunol. 2015, 34, 22-27. [CrossRef]
- Neumann, C.; Scheffold, A.; Rutz, S. Functions and regulation of T cell-derived interleukin-10. Semin. Immunol. 2019, 44, 101344. [CrossRef]
- Turner, S.; Barker, V.D.; Adams, A.A. Effects of cannabidiol on the in vitro lymphocyte pro-inflammatory cytokine production of senior horses. J. Equine Vet. Sci. 2021, 103, 103668. [CrossRef]
- Turner, S.; Knych, H.K.; Adams, A.A. The effects of cannabidiol on immune function and health parameters in senior horses. Vet. Immunol. Immunopathol. 2023, 257, 110549. [CrossRef]
- Li, X.; Köner, H.; Liu, X. Susceptibility to intracellular infections: contributions of TNF to immune defense. Front. Microbiol. 2020, 11,1643. [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64-79. [CrossRef]
- Barberini, D.J.; Freitas, N.P.P.; Magnoni, M.S.; Maia, L.; Listoni, A.J.; Heckler, M.C.; Sudano, M.J.; Golim, M.A.; Landim-Alvarenga, F.C.; Amorim, R.M. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential. Stem Cell Res. Ther. 2014, 5, 25. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402-408. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).