Submitted:
27 November 2024
Posted:
28 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. NAD+ Metabolism
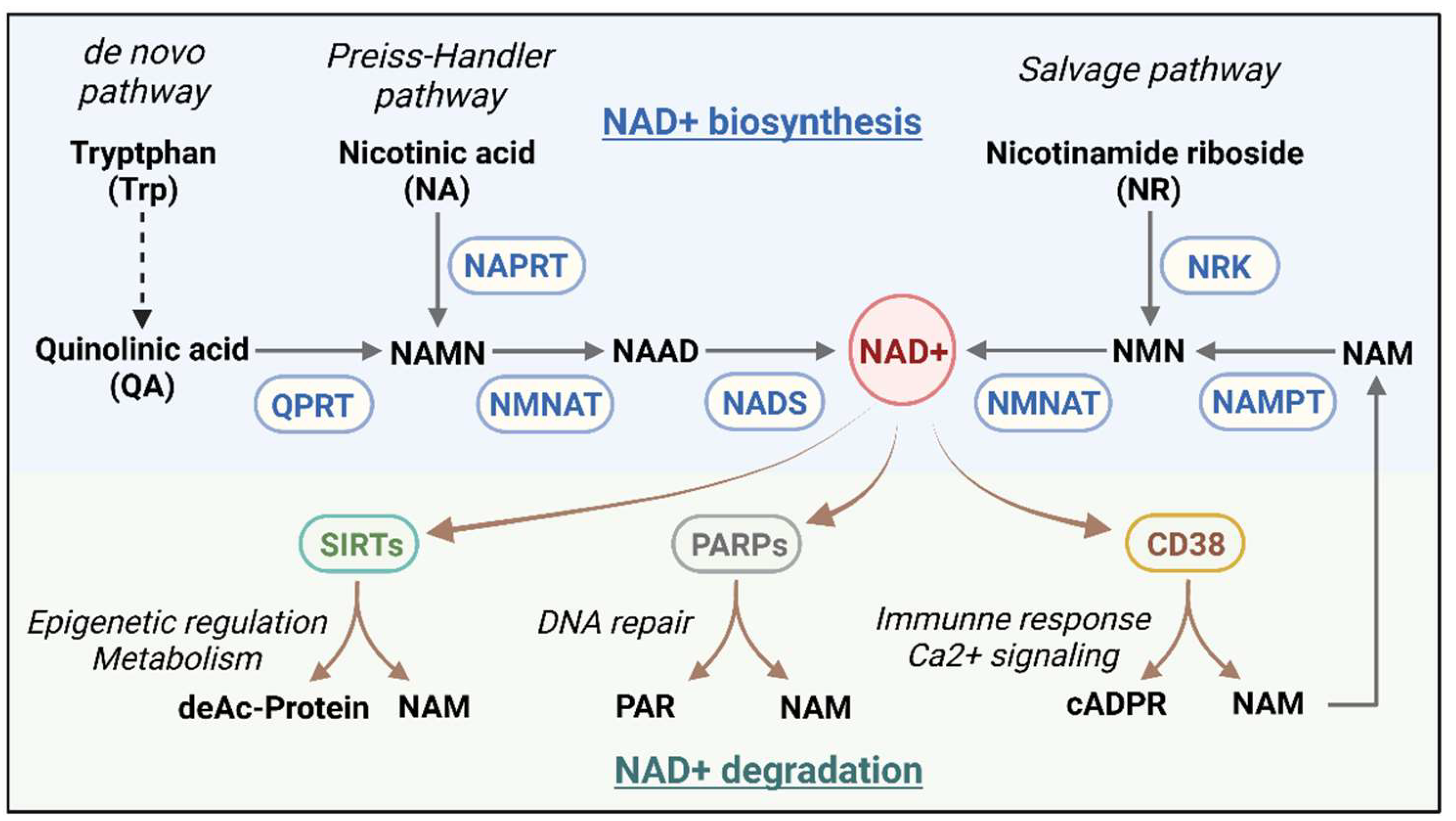
2.1. The NAD+ Metabolic Axis and Its Regulation
2.2. The Importance of NAD+ in Cellular Energy Metabolism and Redox Reactions
2.3. Role of CD38 in Immunoregulation and Cell Signaling
3. NAD+ Metabolism in Infectious and Non-Infectious Diseases
3.1. NAD+ Metabolism in Infectious Diseases
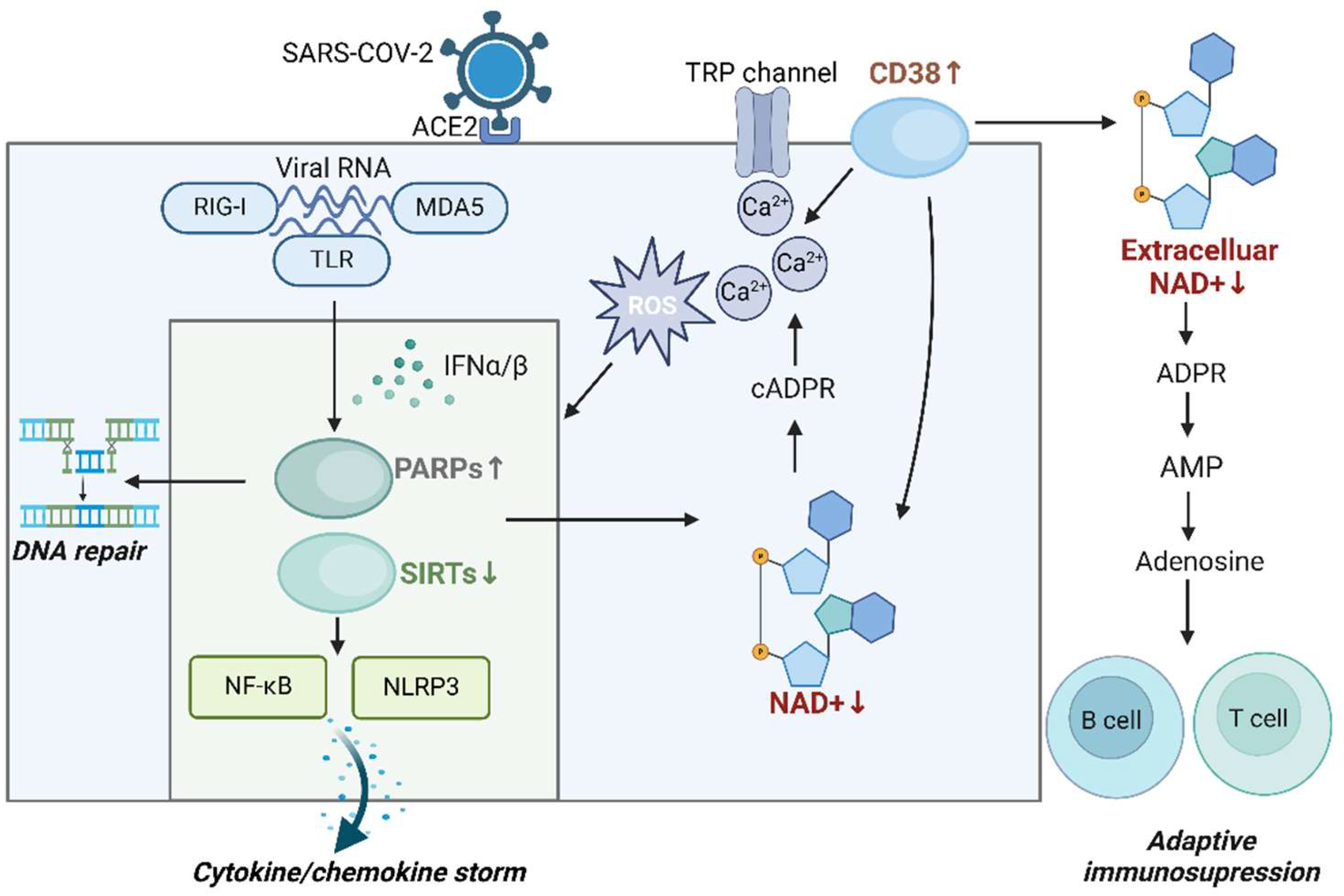
3.2. NAD+ Metabolism in COVID-19
3.3. NAD+ Metabolism in Non-Infectious Diseases
4. NAD+ Metabolism, Aging, and COVID-19
4.1. NAD+ and Aging
4.2. COVID-19, Cellular Senescence, and Aging
5. Potential of Modulating NAD+ Metabolism in COVID-19 Treatment
5.1. Clinical Studies of NAD+ and Its Precursors as Therapeutic Interventions in COVID-19
5.2. Targeting NAD+-Consuming Enzymes for COVID-19 Therapy
5.3. Restoration Strategies and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lan, S.H.; Lai, C.C.; Huang, H.T.; Chang, S.P.; Lu, L.C.; Hsueh, P.R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents 2020, 56(3), 106103. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Fatemi, B.; Karimi Majd, Z.; Minaei, H.; Peikanpour, M.; Anjidani, N.; Taheri, A.; Dastan, F.; Mosaed, R. Efficacy and safety of Tocilizumab in severe and critical COVID-19: A Systematic Review and Meta-Analysis. Expert Rev Clin Immunol 2021, 17(5), 499–511. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Ramanan, A.V.; Kartman, C.E.; de Bono, S.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Saraiva, J.F.K.; Chakladar, S.; Marconi, V.C. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial. Lancet Respir Med 2022, 10(4), 327–336. [Google Scholar] [CrossRef] [PubMed]
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.E.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; de Cassia Pellegrini, R.; Estrada, V.; Som, M.; Cardoso, A.; Chakladar, S.; Crowe, B.; Reis, P.; Zhang, X.; Adams, D.H.; Ely, E.W. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021, 9(12), 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, T.; Zheng, Y.; Xia, Z. Metabolic Reprogramming and Its Regulatory Mechanism in Sepsis-Mediated Inflammation. J Inflamm Res 2023, 16, 1195–1207. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Aschenbrenner, A.C.; Bauer, M.; Bock, C.; Calandra, T.; Gat-Viks, I.; Kyriazopoulou, E.; Lupse, M.; Monneret, G.; Pickkers, P.; Schultze, J.L.; van der Poll, T.; van de Veerdonk, F.L.; Vlaar, A.P.J.; Weis, S.; Wiersinga, W.J.; Netea, M.G. The pathophysiology of sepsis and precision-medicine-based immunotherapy. Nat Immunol 2024, 25(1), 19–28. [Google Scholar] [CrossRef]
- Rudiansyah, M.; Jasim, S.A.; Mohammad Pour, Z.G.; Athar, S.S.; Jeda, A.S.; Doewes, R.I.; Jalil, A.T.; Bokov, D.O.; Mustafa, Y.F.; Noroozbeygi, M.; Karampoor, S.; Mirzaei, R. Coronavirus disease 2019 (COVID-19) update: From metabolic reprogramming to immunometabolism. J Med Virol 2022, 94(10), 4611–4627. [Google Scholar] [CrossRef]
- Cengiz, M.; Borku Uysal, B.; Ikitimur, H.; Ozcan, E.; Islamoğlu, M.S.; Aktepe, E.; Yavuzer, H.; Yavuzer, S. Effect of oral l-Glutamine supplementation on Covid-19 treatment. Clin Nutr Exp 2020, 33, 24–31. [Google Scholar] [CrossRef]
- Gamarra-Morales, Y.; Herrera-Quintana, L.; Molina-López, J.; Vázquez-Lorente, H.; Machado-Casas, J.F.; Castaño-Pérez, J.; Pérez-Villares, J.M.; Planells, E. Response to Intravenous N-Acetylcysteine Supplementation in Critically Ill Patients with COVID-19. Nutrients 2023, 15(9). [Google Scholar] [CrossRef]
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; Wang, T.; Bar-Peled, L.; Zoncu, R.; Straub, C.; Kim, C.; Park, J.; Sabatini, B.L.; Sabatini, D.M. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 347(6218), 188–194. [Google Scholar]
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; Leone, P.M.; Nesci, A.; Paglionico, A.M.; Santoliquido, A.; Santoro, L.; Santucci, L.; Tolusso, B.; Urbani, A.; Marini, F.; Marzetti, E.; Landi, F. Effects of l-Arginine Plus Vitamin C Supplementation on Physical Performance, Endothelial Function, and Persistent Fatigue in Adults with Long COVID: A Single-Blind Randomized Controlled Trial. Nutrients 2022, 14(23). [Google Scholar] [CrossRef] [PubMed]
- Slankamenac, J.; Ranisavljev, M.; Todorovic, N.; Ostojic, J.; Stajer, V.; Ostojic, S.M. Effects of six-month creatine supplementation on patient- and clinician-reported outcomes, and tissue creatine levels in patients with post-COVID-19 fatigue syndrome. Food Sci Nutr 2023, 11(11), 6899–6906. [Google Scholar] [CrossRef] [PubMed]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp Gerontol 2020, 132, 110841. [Google Scholar] [CrossRef] [PubMed]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab 2018, 27(3), 529–547. [Google Scholar] [CrossRef]
- Nielsen, K.N.; Peics, J.; Ma, T.; Karavaeva, I.; Dall, M.; Chubanava, S.; Basse, A.L.; Dmytriyeva, O.; Treebak, J.T.; Gerhart-Hines, Z. NAMPT-mediated NAD(+) biosynthesis is indispensable for adipose tissue plasticity and development of obesity. Mol Metab 2018, 11, 178–188. [Google Scholar] [CrossRef]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 2011, 14(4), 528–536. [Google Scholar] [CrossRef]
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenetics 2016, 8, 61. [Google Scholar] [CrossRef]
- Chalkiadaki, A.; Guarente, L. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer 2015, 15(10), 608–624. [Google Scholar] [CrossRef]
- Schreiber, V.; Dantzer, F.; Ame, J.C.; de Murcia, G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 2006, 7(7), 517–528. [Google Scholar] [CrossRef]
- Guse, A.H. Calcium mobilizing second messengers derived from NAD. Biochim Biophys Acta 2015, 1854(9), 1132–1137. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Peclat, T.R.; Warner, G.M.; Kashyap, S.; Espindola-Netto, J.M.; de Oliveira, G.C.; Gomez, L.S.; Hogan, K.A.; Tarragó, M.G.; Puranik, A.S.; Agorrody, G.; Thompson, K.L.; Dang, K.; Clarke, S.; Childs, B.G.; Kanamori, K.S.; Witte, M.A.; Vidal, P.; Kirkland, A.L.; De Cecco, M.; Chellappa, K.; McReynolds, M.R.; Jankowski, C.; Tchkonia, T.; Kirkland, J.L.; Sedivy, J.M.; van Deursen, J.M.; Baker, D.J.; van Schooten, W.; Rabinowitz, J.D.; Baur, J.A.; Chini, E.N. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD(+) and NMN levels. Nat Metab 2020, 2(11), 1284–1304. [Google Scholar] [CrossRef] [PubMed]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD(+) homeostasis in health and disease. Nat Metab 2020, 2(1), 9–31. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD(+) metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther 2020, 5(1), 227. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem J 2009, 417(1), 1–13. [Google Scholar] [CrossRef]
- Blacker, T.S.; Mann, Z.F.; Gale, J.E.; Ziegler, M.; Bain, A.J.; Szabadkai, G.; Duchen, M.R. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat Commun 2014, 5, 3936. [Google Scholar] [CrossRef]
- Ghisays, F.; Brace, C.S.; Yackly, S.M.; Kwon, H.J.; Mills, K.F.; Kashentseva, E.; Dmitriev, I.P.; Curiel, D.T.; Imai, S.I.; Ellenberger, T. The N-Terminal Domain of SIRT1 Is a Positive Regulator of Endogenous SIRT1-Dependent Deacetylation and Transcriptional Outputs. Cell Rep 2015, 10(10), 1665–1673. [Google Scholar] [CrossRef]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Horio, Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One 2013, 8(9), e73875. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 2005, 280(16), 16456–16460. [Google Scholar] [CrossRef]
- Olmos, Y.; Sánchez-Gómez, F.J.; Wild, B.; García-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid Redox Signal 2013, 19(13), 1507–1521. [Google Scholar] [CrossRef]
- Bause, A.S.; Haigis, M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol 2013, 48(7), 634–639. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 2010, 12(6), 662–667. [Google Scholar] [CrossRef] [PubMed]
- Bryan, S.; Baregzay, B.; Spicer, D.; Singal, P.K.; Khaper, N. Redox-inflammatory synergy in the metabolic syndrome. Can J Physiol Pharmacol 2013, 91(1), 22–30. [Google Scholar] [CrossRef] [PubMed]
- Fritze, C.E.; Verschueren, K.; Strich, R.; Easton Esposito, R. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. Embo j 1997, 16(21), 6495–6509. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem 2012, 287(38), 31633–31640. [Google Scholar] [CrossRef]
- Wei, W.; Graeff, R.; Yue, J. Roles and mechanisms of the CD38/cyclic adenosine diphosphate ribose/Ca(2+) signaling pathway. World J Biol Chem 2014, 5(1), 58–67. [Google Scholar] [CrossRef]
- Skyline, G. Available online: http://rstats.immgen.org/Skyline_microarray/skyline.html.
- Sandoval-Montes, C.; Santos-Argumedo, L. CD38 is expressed selectively during the activation of a subset of mature T cells with reduced proliferation but improved potential to produce cytokines. J Leukoc Biol 2005, 77(4), 513–521. [Google Scholar] [CrossRef]
- Shubinsky, G.; Schlesinger, M. The CD38 lymphocyte differentiation marker: new insight into its ectoenzymatic activity and its role as a signal transducer. Immunity 1997, 7(3), 315–324. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Jin, X.; Liao, Q.; Chen, Z.; Peng, H.; Zhou, Y. CD38: A Significant Regulator of Macrophage Function. Front Oncol 2022, 12, 775649. [Google Scholar] [CrossRef]
- Schneider, M.; Schumacher, V.; Lischke, T.; Lücke, K.; Meyer-Schwesinger, C.; Velden, J.; Koch-Nolte, F.; Mittrücker, H.W. CD38 is expressed on inflammatory cells of the intestine and promotes intestinal inflammation. PLoS One 2015, 10(5), e0126007. [Google Scholar] [CrossRef]
- Schiavoni, I.; Scagnolari, C.; Horenstein, A.L.; Leone, P.; Pierangeli, A.; Malavasi, F.; Ausiello, C.M.; Fedele, G. CD38 modulates respiratory syncytial virus-driven proinflammatory processes in human monocyte-derived dendritic cells. Immunology 2018, 154(1), 122–131. [Google Scholar] [CrossRef]
- Ben Baruch, B.; Blacher, E.; Mantsur, E.; Schwartz, H.; Vaknine, H.; Erez, N.; Stein, R. Stromal CD38 regulates outgrowth of primary melanoma and generation of spontaneous metastasis. Oncotarget 2018, 9(61), 31797–31811. [Google Scholar] [CrossRef]
- Levy, A.; Blacher, E.; Vaknine, H.; Lund, F.E.; Stein, R.; Mayo, L. CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages. Neuro Oncol 2012, 14(8), 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.; Janmaat, M.L.; Mutis, T.; Lammerts van Bueren, J.J.; Ahmadi, T.; Sasser, A.K.; Lokhorst, H.M.; Parren, P.W. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev 2016, 270(1), 95–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Diao, L.; Yang, Y.; Yi, X.; Rodriguez, B.L.; Li, Y.; Villalobos, P.A.; Cascone, T.; Liu, X.; Tan, L.; Lorenzi, P.L.; Huang, A.; Zhao, Q.; Peng, D.; Fradette, J.J.; Peng, D.H.; Ungewiss, C.; Roybal, J.; Tong, P.; Oba, J.; Skoulidis, F.; Peng, W.; Carter, B.W.; Gay, C.M.; Fan, Y.; Class, C.A.; Zhu, J.; Rodriguez-Canales, J.; Kawakami, M.; Byers, L.A.; Woodman, S.E.; Papadimitrakopoulou, V.A.; Dmitrovsky, E.; Wang, J.; Ullrich, S.E.; Wistuba, II; Heymach, J.V.; Qin, F.X.; Gibbons, D.L. CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov 2018, 8(9), 1156–1175. [Google Scholar] [CrossRef] [PubMed]
- Navas, L.E.; Carnero, A. NAD(+) metabolism, stemness, the immune response, and cancer. Signal Transduct Target Ther 2021, 6(1), 2. [Google Scholar] [CrossRef]
- Deaglio, S.; Aydin, S.; Grand, M.M.; Vaisitti, T.; Bergui, L.; D’Arena, G.; Chiorino, G.; Malavasi, F. CD38/CD31 interactions activate genetic pathways leading to proliferation and migration in chronic lymphocytic leukemia cells. Mol Med 2010, 16, (3–4), 87. [Google Scholar] [CrossRef]
- Deaglio, S.; Dianzani, U.; Horenstein, A.L.; Fernández, J.E.; van Kooten, C.; Bragardo, M.; Funaro, A.; Garbarino, G.; Di Virgilio, F.; Banchereau, J.; Malavasi, F. Human CD38 ligand. A 120-KDA protein predominantly expressed on endothelial cells. J Immunol 1996, 156(2), 727–734. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Bracci, C.; Morandi, F.; Malavasi, F. CD38 in Adenosinergic Pathways and Metabolic Re-programming in Human Multiple Myeloma Cells: In-tandem Insights From Basic Science to Therapy. Front Immunol 2019, 10, 760. [Google Scholar] [CrossRef]
- Maluchenko, N.V.; Feofanov, A.V.; Studitsky, V.M. PARP-1-Associated Pathological Processes: Inhibition by Natural Polyphenols. Int J Mol Sci 2021, 22(21), 11441. [Google Scholar] [CrossRef]
- Pacher, P.; Szabo, C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol 2008, 173(1), 2–13. [Google Scholar] [CrossRef]
- Virág, L.; Robaszkiewicz, A.; Rodriguez-Vargas, J.M.; Oliver, F.J. Poly(ADP-ribose) signaling in cell death. Mol Aspects Med 2013, 34(6), 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Michalak, T.I. The Initial Hepatitis B Virus-Hepatocyte Genomic Integrations and Their Role in Hepatocellular Oncogenesis. Int J Mol Sci 2023, 24(19), 14849. [Google Scholar] [CrossRef] [PubMed]
- Rivabene, R.; Straface, E.; Giammarioli, A.M.; Rainaldi, G.; Malorni, W. Combined effect of 3-aminobenzamide and N-acetylcysteine on HIV replication in chronically infected U937 cells. Redox Rep 1997, 3(3), 145–151. [Google Scholar] [CrossRef]
- Rom, S.; Reichenbach, N.L.; Dykstra, H.; Persidsky, Y. The dual action of poly(ADP-ribose) polymerase -1 (PARP-1) inhibition in HIV-1 infection: HIV-1 LTR inhibition and diminution in Rho GTPase activity. Front Microbiol 2015, 6, 878. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Hartman, T.E.; Beites, T.; Kim, J.H.; Eoh, H.; Engelhart, C.A.; Zhu, L.; Wilson, D.J.; Aldrich, C.C.; Ehrt, S.; Rhee, K.Y.; Schnappinger, D. Metabolically distinct roles of NAD synthetase and NAD kinase define the essentiality of NAD and NADP in Mycobacterium tuberculosis. mBio 2023, 14(4), e0034023. [Google Scholar] [CrossRef]
- Thirunavukkarasu, S.; Ahmed, M.; Rosa, B.A.; Boothby, M.; Cho, S.H.; Rangel-Moreno, J.; Mbandi, S.K.; Schreiber, V.; Gupta, A.; Zuniga, J.; Mitreva, M.; Kaushal, D.; Scriba, T.J.; Khader, S.A. Poly(ADP-ribose) polymerase 9 mediates early protection against Mycobacterium tuberculosis infection by regulating type I IFN production. J Clin Invest 2023, 133(12). [Google Scholar] [CrossRef]
- Alqarni, M.H.; Foudah, A.I.; Muharram, M.M.; Labrou, N.E. The Pleiotropic Function of Human Sirtuins as Modulators of Metabolic Pathways and Viral Infections. Cells 2021, 10(2), 460. [Google Scholar] [CrossRef]
- Yu, J.W.; Sun, L.J.; Liu, W.; Zhao, Y.H.; Kang, P.; Yan, B.Z. Hepatitis C virus core protein induces hepatic metabolism disorders through down-regulation of the SIRT1-AMPK signaling pathway. Int J Infect Dis 2013, 17(7), e539-45. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.H.; Tao, Y.; Zhang, Z.Z.; Chen, W.X.; Cai, X.F.; Chen, K.; Ko, B.C.; Song, C.L.; Ran, L.K.; Li, W.Y.; Huang, A.L.; Chen, J. Sirtuin 1 regulates hepatitis B virus transcription and replication by targeting transcription factor AP-1. J Virol 2014, 88(5), 2442–2451. [Google Scholar] [CrossRef]
- He, M.; Gao, S.J. A novel role of SIRT1 in gammaherpesvirus latency and replication. Cell Cycle 2014, 13(21), 3328–3330. [Google Scholar] [CrossRef]
- Li, H.R.; Liu, Q.; Zhu, C.L.; Sun, X.Y.; Sun, C.Y.; Yu, C.M.; Li, P.; Deng, X.M.; Wang, J.F. β-Nicotinamide mononucleotide activates NAD+/SIRT1 pathway and attenuates inflammatory and oxidative responses in the hippocampus regions of septic mice. Redox Biol 2023, 63, 102745. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.; Sedej, S.; Kroemer, G. NAD(+) Metabolism in Cardiac Health, Aging, and Disease. Circulation 2021, 144(22), 1795–1817. [Google Scholar] [CrossRef] [PubMed]
- Smulan, L.J.; Martinez, N.; Kiritsy, M.C.; Kativhu, C.; Cavallo, K.; Sassetti, C.M.; Singhal, A.; Remold, H.G.; Kornfeld, H. Sirtuin 3 Downregulation in Mycobacterium tuberculosis-Infected Macrophages Reprograms Mitochondrial Metabolism and Promotes Cell Death. mBio 2021, 12(1). [Google Scholar] [CrossRef]
- Piedra-Quintero, Z.L.; Wilson, Z.; Nava, P.; Guerau-de-Arellano, M. CD38: An Immunomodulatory Molecule in Inflammation and Autoimmunity. Front Immunol 2020, 11, 597959. [Google Scholar] [CrossRef] [PubMed]
- Montali, I.; Ceccatelli Berti, C.; Morselli, M.; Acerbi, G.; Barili, V.; Pedrazzi, G.; Montanini, B.; Boni, C.; Alfieri, A.; Pesci, M.; Loglio, A.; Degasperi, E.; Borghi, M.; Perbellini, R.; Penna, A.; Laccabue, D.; Rossi, M.; Vecchi, A.; Tiezzi, C.; Reverberi, V.; Boarini, C.; Abbati, G.; Massari, M.; Lampertico, P.; Missale, G.; Ferrari, C.; Fisicaro, P. Deregulated intracellular pathways define novel molecular targets for HBV-specific CD8 T cell reconstitution in chronic hepatitis B. J Hepatol 2023, 79(1), 50–60. [Google Scholar] [CrossRef]
- Rodríguez-Alba, J.C.; Abrego-Peredo, A.; Gallardo-Hernández, C.; Pérez-Lara, J.; Santiago-Cruz, W.; Jiang, W.; Espinosa, E. HIV Disease Progression: Overexpression of the Ectoenzyme CD38 as a Contributory Factor? Bioessays 2019, 41(1), e1800128. [Google Scholar] [CrossRef]
- Dash, S.; Dash, C.; Pandhare, J. Therapeutic Significance of microRNA-Mediated Regulation of PARP-1 in SARS-CoV-2 Infection. Noncoding RNA 2021, 7(4), 60. [Google Scholar] [CrossRef]
- Zheng, M.; Schultz, M.B.; Sinclair, D.A. NAD(+) in COVID-19 and viral infections. Trends Immunol 2022, 43(4), 283–295. [Google Scholar] [CrossRef] [PubMed]
- Horenstein, A.L.; Faini, A.C.; Malavasi, F. CD38 in the age of COVID-19: a medical perspective. Physiol Rev 2021, 101(4), 1457–1486. [Google Scholar] [CrossRef]
- Isman, A.; Nyquist, A.; Strecker, B.; Harinath, G.; Lee, V.; Zhang, X.; Zalzala, S. Low-dose naltrexone and NAD+ for the treatment of patients with persistent fatigue symptoms after COVID-19. Brain Behav Immun Health 2024, 36, 100733. [Google Scholar] [CrossRef]
- Legler, F.; Meyer-Arndt, L.; Mödl, L.; Kedor, C.; Freitag, H.; Stein, E.; Hoppmann, U.; Rust, R.; Wittke, K.; Siebert, N.; Behrens, J.; Thiel, A.; Konietschke, F.; Paul, F.; Scheibenbogen, C.; Bellmann-Strobl, J. Long-term symptom severity and clinical biomarkers in post-COVID-19/chronic fatigue syndrome: results from a prospective observational cohort. EClinicalMedicine 2023, 63, 102146. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Nie, M.; Pang, H.; Wang, B.; Hu, J.; Meng, X.; Li, K.; Ran, X.; Long, Q.; Deng, H.; Chen, N.; Li, S.; Tang, N.; Huang, A.; Hu, Z. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat Commun 2021, 12(1), 1618. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, A.; Mudd, J.C.; Garcia, J.G.N.; Srivastav, S.; Abdel-Mohsen, M.; Palmer, C.; Goldman, A.R.; Kolls, J.K.; Qin, X.; Rappaport, J. SARS-CoV-2 infection dysregulates NAD metabolism. Front Immunol 2023, 14, 1158455. [Google Scholar] [CrossRef]
- Duan, T.; Xing, C.; Chu, J.; Deng, X.; Du, Y.; Liu, X.; Hu, Y.; Qian, C.; Yin, B.; Wang, H.Y.; Wang, R.F. ACE2-dependent and -independent SARS-CoV-2 entries dictate viral replication and inflammatory response during infection. Nat Cell Biol 2024, 26(4), 628–644. [Google Scholar] [CrossRef]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin Microbiol Rev 2021, 34(3).
- Omran, H.M.; Almaliki, M.S. Influence of NAD+ as an ageing-related immunomodulator on COVID 19 infection: A hypothesis. J Infect Public Health 2020, 13(9), 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Wang, L.; Aliyari, S.; Cheng, G. SARS-CoV-2 virus NSP14 Impairs NRF2/HMOX1 activation by targeting Sirtuin 1. Cell Mol Immunol 2022, 19(8), 872–882. [Google Scholar] [CrossRef]
- Walter, M.; Chen, I.P.; Vallejo-Gracia, A.; Kim, I.J.; Bielska, O.; Lam, V.L.; Hayashi, J.M.; Cruz, A.; Shah, S.; Soveg, F.W.; Gross, J.D.; Krogan, N.J.; Jerome, K.R.; Schilling, B.; Ott, M.; Verdin, E. SIRT5 is a proviral factor that interacts with SARS-CoV-2 Nsp14 protein. PLoS Pathog 2022, 18(9), e1010811. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Zhang, H.; Sui, L.; Li, L.; Xu, W.; Du, S.; Hao, P.; Jiang, Y.; Chen, J.; Qu, X.; Tian, M.; Zhao, Y.; Guo, X.; Wang, X.; Song, W.; Song, G.; Wei, Z.; Hou, Z.; Wang, G.; Sun, M.; Li, X.; Lu, H.; Zhuang, X.; Jin, N.; Zhao, Y.; Li, C.; Liao, M. The global succinylation of SARS-CoV-2-infected host cells reveals drug targets. Proc Natl Acad Sci U S A 2022, 119(30), e2123065119. [Google Scholar] [CrossRef] [PubMed]
- Koutsakos, M.; Rowntree, L.C.; Hensen, L.; Chua, B.Y.; van de Sandt, C.E.; Habel, J.R.; Zhang, W.; Jia, X.; Kedzierski, L.; Ashhurst, T.M.; Putri, G.H.; Marsh-Wakefield, F.; Read, M.N.; Edwards, D.N.; Clemens, E.B.; Wong, C.Y.; Mordant, F.L.; Juno, J.A.; Amanat, F.; Audsley, J.; Holmes, N.E.; Gordon, C.L.; Smibert, O.C.; Trubiano, J.A.; Hughes, C.M.; Catton, M.; Denholm, J.T.; Tong, S.Y.C.; Doolan, D.L.; Kotsimbos, T.C.; Jackson, D.C.; Krammer, F.; Godfrey, D.I.; Chung, A.W.; King, N.J.C.; Lewin, S.R.; Wheatley, A.K.; Kent, S.J.; Subbarao, K.; McMahon, J.; Thevarajan, I.; Nguyen, T.H.O.; Cheng, A.C.; Kedzierska, K. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Rep Med 2021, 2(3), 100208. [Google Scholar] [CrossRef]
- Zhuang, M.W.; Cheng, Y.; Zhang, J.; Jiang, X.M.; Wang, L.; Deng, J.; Wang, P.H. Increasing host cellular receptor-angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-nCoV (or SARS-CoV-2) infection. J Med Virol 2020, 92(11), 2693–2701. [Google Scholar] [CrossRef]
- Du, J.; Wei, L.; Li, G.; Hua, M.; Sun, Y.; Wang, D.; Han, K.; Yan, Y.; Song, C.; Song, R.; Zhang, H.; Han, J.; Liu, J.; Kong, Y. Persistent High Percentage of HLA-DR(+)CD38(high) CD8(+) T Cells Associated With Immune Disorder and Disease Severity of COVID-19. Front Immunol 2021, 12, 735125. [Google Scholar] [CrossRef] [PubMed]
- Hogan, K.A.; Chini, C.C.S.; Chini, E.N. The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front Immunol 2019, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu Rev Immunol 2019, 37, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.C.; Viacava, P.R.; Ferreira, R.G.; Damaceno, M.A.; Piñeros, A.R.; Melo, P.H.; Donate, P.B.; Toller-Kawahisa, J.E.; Zoppi, D.; Veras, F.P.; Peres, R.S.; Menezes-Silva, L.; Caetité, D.; Oliveira, A.E.R.; Castro Í, M.S.; Kauffenstein, G.; Nakaya, H.I.; Borges, M.C.; Zamboni, D.S.; Fonseca, D.M.; Paschoal, J.A.R.; Cunha, T.M.; Quesniaux, V.; Linden, J.; Cunha, F.Q.; Ryffel, B.; Alves-Filho, J.C. Sepsis expands a CD39(+) plasmablast population that promotes immunosuppression via adenosine-mediated inhibition of macrophage antimicrobial activity. Immunity 2021, 54(9), 2024–2041.e8. [Google Scholar] [CrossRef]
- Yakymiv, Y.; Marchisio, S.; Ortolan, E.; Bracci, C.; Senetta, R.; Rumore, M.R.; Tampieri, C.; Fia, M.; Ribero, S.; Funaro, A.; Quaglino, P. CD39/CD73 dysregulation and adenosine metabolism contribute to T-cell immunosuppression in patients with Sézary syndrome. Blood 2023, 141(1), 111–116. [Google Scholar] [CrossRef]
- Sica, A.; Colombo, M.P.; Trama, A.; Horn, L.; Garassino, M.C.; Torri, V. Immunometabolic Status of COVID-19 Cancer Patients. Physiol Rev 2020, 100(4), 1839–1850. [Google Scholar] [CrossRef]
- Yu, Y.; Fedele, G.; Celardo, I.; Loh, S.H.Y.; Martins, L.M. Parp mutations protect from mitochondrial toxicity in Alzheimer’s disease. Cell Death & Disease 2021, 12(7). [Google Scholar]
- Hou, Y.; Wei, Y.; Lautrup, S.; Yang, B.; Wang, Y.; Cordonnier, S.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc Natl Acad Sci U S A 2021, 118(37). [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; Mani, C.; Sah, N.; Saamarthy, K.; Young, R.; Reedy, M.B.; Sobol, R.W.; Palle, K. CHK1 inhibitor induced PARylation by targeting PARG causes excessive replication and metabolic stress and overcomes chemoresistance in ovarian cancer. Cell Death Discov 2024, 10(1), 278. [Google Scholar] [CrossRef]
- Lau, C.H.; Seow, K.M.; Chen, K.H. The Molecular Mechanisms of Actions, Effects, and Clinical Implications of PARP Inhibitors in Epithelial Ovarian Cancers: A Systematic Review. Int J Mol Sci 2022, 23(15), 8125. [Google Scholar] [CrossRef]
- Kim, C.; Chen, C.; Yu, Y. Avoid the trap: Targeting PARP1 beyond human malignancy. Cell Chem Biol 2021, 28(4), 456–462. [Google Scholar] [CrossRef] [PubMed]
- Novak, E.A.; Crawford, E.C.; Mentrup, H.L.; Griffith, B.D.; Fletcher, D.M.; Flanagan, M.R.; Schneider, C.; Firek, B.; Rogers, M.B.; Morowitz, M.J.; Piganelli, J.D.; Wang, Q.; Mollen, K.P. Epithelial NAD(+) depletion drives mitochondrial dysfunction and contributes to intestinal inflammation. Front Immunol 2023, 14, 1231700. [Google Scholar] [CrossRef]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; Gademann, K.; Rinsch, C.; Schoonjans, K.; Sauve, A.A.; Auwerx, J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 2012, 15(6), 838–847. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Sun, S.J.; Fu, J.T.; Ouyang, S.X.; Zhao, Q.J.; Su, L.; Ji, Q.X.; Sun, D.Y.; Zhu, J.H.; Zhang, G.Y.; Ma, J.W.; Lan, X.T.; Zhao, Y.; Tong, J.; Li, G.Q.; Shen, F.M.; Wang, P. NAD(+)-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin. Theranostics 2021, 11(9), 4381–4402. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, E.; Suarez-Fueyo, A.; Bradley, S.J.; Mizui, M.; Marin, A.V.; Mulki, L.; Krishfield, S.; Malavasi, F.; Yoon, J.; Sui, S.J.H.; Kyttaris, V.C.; Tsokos, G.C. The CD38/NAD/SIRTUIN1/EZH2 Axis Mitigates Cytotoxic CD8 T Cell Function and Identifies Patients with SLE Prone to Infections. Cell Rep 2020, 30(1), 112–123.e4. [Google Scholar] [CrossRef] [PubMed]
- Vaisitti, T.; Audrito, V.; Serra, S.; Buonincontri, R.; Sociali, G.; Mannino, E.; Pagnani, A.; Zucchetto, A.; Tissino, E.; Vitale, C.; Coscia, M.; Usai, C.; Pepper, C.; Gattei, V.; Bruzzone, S.; Deaglio, S. The enzymatic activities of CD38 enhance CLL growth and trafficking: implications for therapeutic targeting. Leukemia 2015, 29(2), 356–368. [Google Scholar] [CrossRef]
- Becherini, P.; Soncini, D.; Ravera, S.; Gelli, E.; Martinuzzi, C.; Giorgetti, G.; Cagnetta, A.; Guolo, F.; Ivaldi, F.; Miglino, M.; Aquino, S.; Todoerti, K.; Neri, A.; Benzi, A.; Passalacqua, M.; Nencioni, A.; Perrotta, I.; Gallo Cantafio, M.E.; Amodio, N.; De Flora, A.; Bruzzone, S.; Lemoli, R.M.; Cea, M. CD38-Induced Metabolic Dysfunction Primes Multiple Myeloma Cells for NAD(+)-Lowering Agents. Antioxidants (Basel) 2023, 12(2), 494. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Y.; Yang, C.; Zhu, S.; Wang, X.; Zhang, Z.; Deng, H. Decreased NAD Activates STAT3 and Integrin Pathways to Drive Epithelial-Mesenchymal Transition. Mol Cell Proteomics 2018, 17(10), 2005–2017. [Google Scholar] [CrossRef]
- Verdin, E. NAD⁺ in aging, metabolism, and neurodegeneration. Science 2015, 350(6265), 1208–1213. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Zeidler, J.D.; Kashyap, S.; Warner, G.; Chini, E.N. Evolving concepts in NAD(+) metabolism. Cell Metab 2021, 33(6), 1076–1087. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol 2014, 24(8), 464–471. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Bioenergetic origins of complexity and disease. Cold Spring Harb Symp Quant Biol 2011, 76, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.; Kataura, T.; Korsgen, M.E.; Sun, C.; Sarkar, S.; Korolchuk, V.I. The autophagy-NAD axis in longevity and disease. Trends Cell Biol 2023, 33(9), 788–802. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, metabolism, and aging. J Genet Genomics 2022, 49(4), 287–298. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Remuzzi, G.; Benigni, A. Sirtuins in kidney health and disease. Nat Rev Nephrol 2024, 20(5), 313–329. [Google Scholar] [CrossRef]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 1999, 13(19), 2570–2580. [Google Scholar] [CrossRef]
- He, M.; Chiang, H.H.; Luo, H.; Zheng, Z.; Qiao, Q.; Wang, L.; Tan, M.; Ohkubo, R.; Mu, W.C.; Zhao, S.; Wu, H.; Chen, D. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab 2020, 31(3), 580–591.e5. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, X.K.; Lv, S.J.; Wang, H.P.; Liu, Y.; Zhou, J.; Gong, H.; Chen, X.F.; Ren, S.C.; Zhang, H.; Dai, Y.; Cai, H.; Yan, B.; Chen, H.Z.; Tang, X. Sirtuin 2 deficiency aggravates ageing-induced vascular remodelling in humans and mice. Eur Heart J 2023, 44(29), 2746–2759. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; Rocktäschel, P.; Croteau, D.L.; Akbari, M.; Greig, N.H.; Fladby, T.; Nilsen, H.; Cader, M.Z.; Mattson, M.P.; Tavernarakis, N.; Bohr, V.A. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 2019, 22(3), 401–412. [Google Scholar] [CrossRef]
- Membrez, M.; Migliavacca, E.; Christen, S.; Yaku, K.; Trieu, J.; Lee, A.K.; Morandini, F.; Giner, M.P.; Stiner, J.; Makarov, M.V.; Garratt, E.S.; Vasiloglou, M.F.; Chanvillard, L.; Dalbram, E.; Ehrlich, A.M.; Sanchez-Garcia, J.L.; Canto, C.; Karagounis, L.G.; Treebak, J.T.; Migaud, M.E.; Heshmat, R.; Razi, F.; Karnani, N.; Ostovar, A.; Farzadfar, F.; Tay, S.K.H.; Sanders, M.J.; Lillycrop, K.A.; Godfrey, K.M.; Nakagawa, T.; Moco, S.; Koopman, R.; Lynch, G.S.; Sorrentino, V.; Feige, J.N. Trigonelline is an NAD(+) precursor that improves muscle function during ageing and is reduced in human sarcopenia. Nat Metab 2024, 6(3), 433–447. [Google Scholar] [CrossRef]
- Kartsonaki, C.; Baillie, J.K.; Barrio, N.G.; Baruch, J.; Beane, A.; Blumberg, L.; Bozza, F.; Broadley, T.; Burrell, A.; Carson, G.; Citarella, B.W.; Dagens, A.; Dankwa, E.A.; Donnelly, C.A.; Dunning, J.; Elotmani, L.; Escher, M.; Farshait, N.; Goffard, J.C.; Gonçalves, B.P.; Hall, M.; Hashmi, M.; Sim Lim Heng, B.; Ho, A.; Jassat, W.; Pedrera Jiménez, M.; Laouenan, C.; Lissauer, S.; Martin-Loeches, I.; Mentré, F.; Merson, L.; Morton, B.; Munblit, D.; Nekliudov, N.A.; Nichol, A.D.; Singh Oinam, B.C.; Ong, D.; Panda, P.K.; Petrovic, M.; Pritchard, M.G.; Ramakrishnan, N.; Ramos, G.V.; Roger, C.; Sandulescu, O.; Semple, M.G.; Sharma, P.; Sigfrid, L.; Somers, E.C.; Streinu-Cercel, A.; Taccone, F.; Vecham, P.K.; Kumar Tirupakuzhi Vijayaraghavan, B.; Wei, J.; Wils, E.J.; Ci Wong, X.; Horby, P.; Rojek, A.; Olliaro, P.L. Characteristics and outcomes of an international cohort of 600 000 hospitalized patients with COVID-19. Int J Epidemiol 2023, 52(2), 355–376. [Google Scholar] [CrossRef] [PubMed]
- Romero Starke, K.; Reissig, D.; Petereit-Haack, G.; Schmauder, S.; Nienhaus, A.; Seidler, A. The isolated effect of age on the risk of COVID-19 severe outcomes: A systematic review with meta-analysis. BMJ Glob Health 2021, 6(12). [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. Jama 2020, 324(8), 782–793. [Google Scholar] [CrossRef] [PubMed]
- O'Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590(7844), 140–145. [Google Scholar] [CrossRef]
- CDC. https://www.cdc.gov/mmwr/volumes/73/wr/mm7331a1.htm?s_cid=mm7331a1_w.
- Minchella, P.A.; Chanda, D.; Hines, J.Z.; Fwoloshi, S.; Itoh, M.; Kampamba, D.; Chirwa, R.; Sivile, S.; Zyambo, K.D.; Agolory, S.; Mulenga, L.B. Clinical Characteristics and Outcomes of Patients Hospitalized With COVID-19 During the First 4 Waves in Zambia. JAMA Netw Open 2022, 5(12), e2246152. [Google Scholar] [CrossRef] [PubMed]
- Kaml, M.; Weiskirchner, I.; Keller, M.; Luft, T.; Hoster, E.; Hasford, J.; Young, L.; Bartlett, B.; Neuner, C.; Fischer, K.H.; Neuman, B.; Würzner, R.; Grubeck-Loebenstein, B. Booster vaccination in the elderly: their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine 2006, 24(47–48), 6808. [Google Scholar] [CrossRef]
- Yoshikawa, T.T. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis 2000, 30(6), 931–933. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Zarandi, P.K.; Zinatizadeh, M.; Yousefi, M.H.; Amani, J.; Rezaei, N. Efficacy of mRNA, adenoviral vector, and perfusion protein COVID-19 vaccines. Biomed Pharmacother 2022, 146, 112527. [Google Scholar] [CrossRef]
- Franceschi, C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 2007, 65 12 Pt 2, S173–S176. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: molecular mechanisms and diseases. Signal Transduct Target Ther 2023, 8(1), 200. [Google Scholar] [CrossRef]
- Cao, X.; Li, W.; Wang, T.; Ran, D.; Davalos, V.; Planas-Serra, L.; Pujol, A.; Esteller, M.; Wang, X.; Yu, H. Accelerated biological aging in COVID-19 patients. Nat Commun 2022, 13(1), 2135. [Google Scholar] [CrossRef] [PubMed]
- Gioia, U.; Tavella, S.; Martínez-Orellana, P.; Cicio, G.; Colliva, A.; Ceccon, M.; Cabrini, M.; Henriques, A.C.; Fumagalli, V.; Paldino, A.; Presot, E.; Rajasekharan, S.; Iacomino, N.; Pisati, F.; Matti, V.; Sepe, S.; Conte, M.I.; Barozzi, S.; Lavagnino, Z.; Carletti, T.; Volpe, M.C.; Cavalcante, P.; Iannacone, M.; Rampazzo, C.; Bussani, R.; Tripodo, C.; Zacchigna, S.; Marcello, A.; d’Adda di Fagagna, F. SARS-CoV-2 infection induces DNA damage, through CHK1 degradation and impaired 53BP1 recruitment, and cellular senescence. Nat Cell Biol 2023, 25(4), 550–564. [Google Scholar] [CrossRef]
- Tsuji, S.; Minami, S.; Hashimoto, R.; Konishi, Y.; Suzuki, T.; Kondo, T.; Sasai, M.; Torii, S.; Ono, C.; Shichinohe, S.; Sato, S.; Wakita, M.; Okumura, S.; Nakano, S.; Matsudaira, T.; Matsumoto, T.; Kawamoto, S.; Yamamoto, M.; Watanabe, T.; Matsuura, Y.; Takayama, K.; Kobayashi, T.; Okamoto, T.; Hara, E. SARS-CoV-2 infection triggers paracrine senescence and leads to a sustained senescence-associated inflammatory response. Nat Aging 2022, 2(2), 115–124. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Tchkonia, T.; Niedernhofer, L.J.; Robbins, P.D.; Kirkland, J.L.; Lee, S. COVID-19 and cellular senescence. Nat Rev Immunol 2023, 23(4), 251–263. [Google Scholar] [CrossRef]
- Prata, L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin Immunol 2018, 40, 101275. [Google Scholar] [CrossRef]
- Lekva, T.; Ueland, T.; Halvorsen, B.; Murphy, S.L.; Dyrhol-Riise, A.M.; Tveita, A.; Finbråten, A.K.; Mathiessen, A.; Müller, K.E.; Aaløkken, T.M.; Skjønsberg, O.H.; Lerum, T.V.; Aukrust, P.; Dahl, T.B. Markers of cellular senescence is associated with persistent pulmonary pathology after COVID-19 infection. Infect Dis (Lond) 2022, 54(12), 918–923. [Google Scholar] [CrossRef]
- Lieberman, N.A.P.; Peddu, V.; Xie, H.; Shrestha, L.; Huang, M.L.; Mears, M.C.; Cajimat, M.N.; Bente, D.A.; Shi, P.Y.; Bovier, F.; Roychoudhury, P.; Jerome, K.R.; Moscona, A.; Porotto, M.; Greninger, A.L. In vivo antiviral host transcriptional response to SARS-CoV-2 by viral load, sex, and age. PLoS Biol 2020, 18(9), e3000849. [Google Scholar] [CrossRef] [PubMed]
- Heer, C.D.; Sanderson, D.J.; Voth, L.S.; Alhammad, Y.M.O.; Schmidt, M.S.; Trammell, S.A.J.; Perlman, S.; Cohen, M.S.; Fehr, A.R.; Brenner, C. Coronavirus infection and PARP expression dysregulate the NAD metabolome: An actionable component of innate immunity. J Biol Chem 2020, 295(52), 17986–17996. [Google Scholar] [CrossRef]
- Freeberg, K.A.; Ludwig, K.R.; Chonchol, M.; Seals, D.R.; Rossman, M.J. NAD(+)-boosting compounds enhance nitric oxide production and prevent oxidative stress in endothelial cells exposed to plasma from patients with COVID-19. Nitric Oxide 2023, 140–141, 1–7. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Y.; Pang, H.; Ma, T.; Ye, Q.; Chen, Q.; Chen, H.; Hu, Z.; Qin, C.F.; Xu, Z. Treatment of SARS-CoV-2-induced pneumonia with NAD(+) and NMN in two mouse models. Cell Discov 2022, 8(1), 38. [Google Scholar] [CrossRef]
- Huizenga, R. In Dramatic Clinical Improvement in Nine Consecutive Acutely Ill Elderly COVID-19 Patients Treated with a Nicotinamide Mononucleotide Cocktail: A Case Series, 2020; 2020.
- Altay, O.; Arif, M.; Li, X.; Yang, H.; Aydın, M.; Alkurt, G.; Kim, W.; Akyol, D.; Zhang, C.; Dinler-Doganay, G.; Turkez, H.; Shoaie, S.; Nielsen, J.; Borén, J.; Olmuscelik, O.; Doganay, L.; Uhlén, M.; Mardinoglu, A. Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19. Adv Sci (Weinh) 2021, 8(17), e2101222. [Google Scholar] [CrossRef] [PubMed]
- Raines, N.H.; Cheung, M.D.; Wilson, L.S.; Edberg, J.C.; Erdmann, N.B.; Schmaier, A.A.; Berryhill, T.F.; Manickas-Hill, Z.; Li, J.Z.; Yu, X.G.; Agarwal, A.; Barnes, S.; Parikh, S.M. Nicotinamide Adenine Dinucleotide Biosynthetic Impairment and Urinary Metabolomic Alterations Observed in Hospitalized Adults With COVID-19-Related Acute Kidney Injury. Kidney Int Rep 2021, 6(12), 3002–3013. [Google Scholar] [CrossRef]
- Raines, N.H.; Ganatra, S.; Nissaisorakarn, P.; Pandit, A.; Morales, A.; Asnani, A.; Sadrolashrafi, M.; Maheshwari, R.; Patel, R.; Bang, V.; Shreyder, K.; Brar, S.; Singh, A.; Dani, S.S.; Knapp, S.; Poyan Mehr, A.; Brown, R.S.; Zeidel, M.L.; Bhargava, R.; Schlondorff, J.; Steinman, T.I.; Mukamal, K.J.; Parikh, S.M. Niacinamide May Be Associated with Improved Outcomes in COVID-19-Related Acute Kidney Injury: An Observational Study. Kidney360 2021, 2(1), 33–41. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.E.; Jaramillo, S.A.; Jones, A.N.; Vazquez, A.J.; Martz, M.; Versluis, L.M.; Raniere, M.O.; Nunnally, H.E.; Zarn, K.E.; Nottingham, R.; Ng, K.R.; Sahl, J.W.; Wagner, D.M.; Knudsen, S.; Settles, E.W.; Keim, P.; French, C.T. Stenoparib, an Inhibitor of Cellular Poly(ADP-Ribose) Polymerase, Blocks Replication of the SARS-CoV-2 and HCoV-NL63 Human Coronaviruses In Vitro. mBio 2021, 12(1). [Google Scholar] [CrossRef] [PubMed]
- Papp, H.; Tóth, E.; Bóvári-Biri, J.; Bánfai, K.; Juhász, P.; Mahdi, M.; Russo, L.C.; Bajusz, D.; Sipos, A.; Petri, L.; Szalai, T.V.; Kemény, Á.; Madai, M.; Kuczmog, A.; Batta, G.; Mózner, O.; Vaskó, D.; Hirsch, E.; Bohus, P.; Méhes, G.; Tőzsér, J.; Curtin, N.J.; Helyes, Z.; Tóth, A.; Hoch, N.C.; Jakab, F.; Keserű, G.M.; Pongrácz, J.E.; Bai, P. The PARP inhibitor rucaparib blocks SARS-CoV-2 virus binding to cells and the immune reaction in models of COVID-19. Br J Pharmacol 2024. [CrossRef]
- Zarn, K.E.; Jaramillo, S.A.; Zapata, A.R.; Stone, N.E.; Jones, A.N.; Nunnally, H.E.; Settles, E.W.; Ng, K.; Keim, P.S.; Knudsen, S.; Nuijten, P.M.; Tijsma, A.S.L.; French, C.T. Stenoparib, an inhibitor of cellular poly (ADP-ribose) polymerases (PARPs), blocks in vitro replication of SARS-CoV-2 variants. PLoS One 2022, 17(9), e0272916. [Google Scholar] [CrossRef] [PubMed]
- Pasquereau, S.; Nehme, Z.; Haidar Ahmad, S.; Daouad, F.; Van Assche, J.; Wallet, C.; Schwartz, C.; Rohr, O.; Morot-Bizot, S.; Herbein, G. Resveratrol Inhibits HCoV-229E and SARS-CoV-2 Coronavirus Replication In Vitro. Viruses 2021, 13(2), 354. [Google Scholar] [CrossRef]
- Russo, C.; Valle, M.S.; Malaguarnera, L.; Romano, I.R.; Malaguarnera, L. Comparison of Vitamin D and Resveratrol Performances in COVID-19. Nutrients 2023, 15(11), 2639. [Google Scholar] [CrossRef]
- Ramdani, L.H.; Bachari, K. Potential therapeutic effects of Resveratrol against SARS-CoV-2. Acta Virol 2020, 64(3), 276–280. [Google Scholar] [CrossRef]
- McCreary, M.R.; Schnell, P.M.; Rhoda, D.A. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Sci Rep 2022, 12(1), 10978. [Google Scholar] [CrossRef]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J Inflamm (Lond) 2021, 18(1), 3. [Google Scholar] [CrossRef] [PubMed]
- Terao, T.; Naduka, T.; Ikeda, D.; Fukumoto, A.; Kamura, Y.; Kuzume, A.; Tabata, R.; Tsushima, T.; Miura, D.; Narita, K.; Takeuchi, M.; Matsue, K. Depletion of CD38-positive regulatory T cells by anti-CD38 monoclonal antibodies induces a durable response to SARS-CoV-2 vaccination in patients with plasma cell dyscrasia. Br J Haematol 2022, 197(4), 417–421. [Google Scholar] [CrossRef] [PubMed]
- Nahi, H.; Chrobok, M.; Gran, C.; Lund, J.; Gruber, A.; Gahrton, G.; Ljungman, P.; Wagner, A.K.; Alici, E. Infectious complications and NK cell depletion following daratumumab treatment of Multiple Myeloma. PLoS One 2019, 14(2), e0211927. [Google Scholar] [CrossRef]
| Enzymes | Disease | Model | Observations | References |
|---|---|---|---|---|
| PARPs | HBV | HBV-infected HepaRG/ HepG2 | NAD+ consuming and PARP1 recognizes broken dsDNA and promotes DNA repair by (NHEJ) pathways | 53 |
| HIV | HIV infected U937 cells with TNFa. treatment | PARP1 overexpression and NAD+ consuming | 54 | |
| HIV infected MDM with PARP inhibitor treatment | suppression of HIV-1 replication by obstructing HIV-1 LTR activation | 55 | ||
| MTB | Mtb H37Rv wt, NadE-DUC, and PpnK-DUC strains | NAD+ depletion and arrested growth of Mtb | 56 | |
| Parp9–/– mice | increased susceptibility to Mtb infection mediated by type I IFN signaling | 57 | ||
| SIRTs | HCV | HCV-transfected HepG2 cells | decreasing NAD/NADH ratio and the activity of SIRT1, glucose and lipid metabolism disorder | 59 |
| HBV | HBV-transfected HepG2 cells | the upregulation of SIRT1 augmented HBV replication | 60 | |
| KSHV | KSHV-infected PEL cell line BCBL-1 | Low NAD+ level disrupts viral latency by inhibiting SIRT1 function | 61 | |
| Sepsis | Septic mice induced by cecal ligation and puncture (CLP) | Low NAD+ level and NAD+/SIRT1 pathway was inhibited | 62 | |
| MTB | J2 macrophages, BMDM, Sirt3−/− mice | SIRT3 downregulation, increased ROS and cell death | 64 | |
| CD38 | HBV | CD8 T cells from patients with chronic active hepatitis | CD38 overexpression leads to NAD+ depletion and dysregulation of DNA repair mechanisms | 66 |
| HIV | CD8 T cells from HIV patients | Increased CD38 activity promotes NAD+ consumption and exacerbates mitochondrial oxidative stress | 67 | |
| RSV | RSV infected MDDC | The increased production of type I IFNs activates CD38 and CD38/cADPR pathway | 41 |
| Enzymes | Diseases | Models | Observations | References |
|---|---|---|---|---|
| PARPs | AD | Aβ-Arc-expressing flies with PARP mutation | increased NAD+ level and improved mitochondrial function | 68 |
| APP/PS1 mice with NR treatment | Aberrant activation of DNA sensing pathways and the level of neuroinflammation are reduced | 69 | ||
| Ovarian cancer | OC cell lines OVCAR8 and SKOV3 exposed to CHK1 inhibition and PARG inhibitor | increased DNA damage, activation of PARP1/2 and decrease in NAD+ level | 70 | |
| SIRTs | IBD | Mice subjected to experimental colitis | NAD+ depletion, decrease in SIRT1 activity and Mitochondrial dysfunction | 73 |
| T2D | High fat diet induced T2D mice and age-induced diabetic mice | Decrease in NAD+ level, suppression of SIRT1 activity and metabolic complications | 16 | |
| Obesity | High fat diet induced mice | Decrease in NAD+ level, suppression of SIRT1/3 activity and oxidative metabolism | 74 | |
| NAFLD | high-fat diet and methionine/choline-deficient diet induced NAFLD mice with NR treatment | Increase in NAD+ level and SIRT2 activity, reversion of hepatic steatosis and steatohepatitis | 75 | |
| CD38 | SLE | CD8CD38high T cells from patients with SLE | Decrease in MAD+ level and cytotoxicity | 76 |
| CLL | Mec-1/CD38M cells | NAD+ depletion, increase in Ca2+ concentrations and CLL aggressiveness | 77 | |
| MM | MM cell lines with CD38 overexpressing | NAD+ depletion, NAD+ depletion and mitochondrial metabolism reprogramming | 78 | |
| NSCLC/Liver Cancer | CD38+ A549/CD38+ HepG2 | Decrease in NAD+ level, promoting EMT | 79 |
| Trial ID | Interventions | Clinical Phase | Study Type | Results | References |
| NCT04573153 | NR + serine + L-carnitine tartrate + N-acetylcysteine + hydroxychloroquine (CMA) | II/III | Randomized, placebo-controlled | patients using CMAs had the time to complete recovery is significantly shorter, and plasma levels of proteins and metabolites associated with inflammation and antioxidant metabolism are significantly improved. | 135 |
| NCT04407390 | NR 1 g/d | II | Randomized, double-blind, placebo-controlled | No results available yet. | |
| NCT04818216 | NR 1 g/d | II | Randomized, double-blind, placebo-controlled | Patients in the nicotinamide riboside group had higher levels of NAD+ in whole blood. | |
| NCT05175768 | NMN, NMN+L-leucine | Not applicable | Randomized, double-blind, placebo-controlled | No results available yet. | |
| NCT05038488 | MIB-626 | II | Randomized, double-blind, placebo-controlled | No results available yet. | |
| NCT04751604 | Nicotinamide | Not applicable | Randomized, double-blind, placebo-controlled | No results available yet. | |
| NCT04910230 | Nicotinamide | Not applicable | Randomized, double-blind, placebo-controlled | No clinical difference observed between therapy and placebo group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





