1. Introduction
Edible oils play a crucial role in the human diet, providing essential fatty acids and micronutrients that support overall health [
1]. They are widely used in cooking methods such as deep-frying, grilling, baking, and sautéing [
2]. Among these, deep-frying is particularly common in both domestic kitchens and commercial food establishments, where oils are heated to temperatures as high as 180 °C [
3,
4]. However, when oils are exposed to high heat, typically exceeding their smoke points, they undergo a series of chemical reactions including hydrolysis, oxidation, isomerization, polymerization, and degradation [
5,
6]. These reactions significantly alter the oils’ color, flavor, nutritional properties, and molecular composition.
To reduce costs, cooking oils are often reused in deep-frying for extended periods [
7], which severely degrades their quality. This repeated used leads to the formation of potentially harmful compounds such as polycyclic aromatic hydrocarbons (PAHs) [
8,
9] and aldehydes [
10,
11,
12]. Thermal decomposition of cooking oils initially produces primary lipid oxidation products (LOPs) from unsaturated fatty acids (UFAs), which subsequently degrade into highly toxic aldehydes [
13,
14,
15,
16]. Aldehydes, particularly
α,
β-unsaturated aldehydes, are exceptionally reactive, readily interacting with essential biomolecules like DNA and causing significant damage. Their mutagenic, genotoxic, and carcinogenic effects are well-documented [
17], with additional health risks including cancer, cardiovascular diseases, and neurological disorders [
15,
18].
In addition to deep-frying, improper handling and storage of edible oils exacerbate their degradation through oxidation and related chemical changes [
19]. Factors such as light exposure, temperature, oxygen concentration, water content, and metal contamination affect oil stability and quality. Particularly, sunlight accelerates decomposition via photooxidation. During this process, ultraviolet (UV) and visible light photons interact with unsaturated fatty acids, generating free radicals and reactive oxygen species (ROS). These reactions lead to oxidative degradation, resulting in the formation of primary oxidation products, such as peroxides and secondary oxidation products, including aldehydes and ketones. These degradation products reduce the nutritional values, shelf life and sensory qualities of oils, while also producing potentially toxic substances [
14,
20].
The susceptibility of edible oils to photooxidation depends on their degree of unsaturation and environmental storage conditions. Oils rich in polyunsaturated fatty acids (PUFAs) are particularly vulnerable due to their high reactivity with oxygen and light [
21]. Photooxidation is a free radical chain reaction involving initiation, propagation, and termination steps, where lipid alkyl radicals are generated by the removal of hydrogen atoms from fatty acids or acylglycerols. Factors such as heat, metal catalysts, and light further accelerate this process. Hydrogen atoms adjacent to double bonds, especially those between two double bonds, are most susceptible to oxidation [
14]. Understanding the mechanisms and effects of photooxidation is critical for optimizing storage conditions and developing protective strategies to preserve oil quality.
Repeated heating and improper storage of edible oils pose significant health risks, yet these practices remain widespread. Therefore, understanding how cooking conditions, light exposure, and storage practices affect oil degradation is crucial for mitigating these risks. Advanced analytical techniques are essential for characterizing the molecular changes in oils under these conditions. Among these, proton nuclear magnetic resonance (
1H NMR) spectroscopy is particularly effective. This non-destructive and highly precise method allows for the detection of secondary lipid oxidation products, such as aldehydes and ketones, while simultaneously tracking changes in fatty acid profiles [
22,
23].
Numerous studies highlight the effectiveness of
1H NMR in quantifying the accumulation of toxic aldehydes in heated oils [
10,
11,
24,
25], showing a linear increase in both saturated and unsaturated aldehydes over time. However, one-dimensional (1D)
1H NMR spectroscopy at low magnetic fields has limitations, including poor resolution due to narrow chemical shift dispersion (approximately 10 ppm) and low sensitivity for detecting secondary lipid oxidation products, such as aldehydes.
To address these challenges, this study investigates the effects of thermal and photodegradation on five commonly used edible oils - olive, rapeseed, sunflower, sesame, and peanut, using a high-field 800 MHz NMR instrument equipped with a helium-cooled triple-resonance inverse cryoprobe. This advanced setup offers enhanced resolution and sensitivity compared to conventional analytical technologies, enabling the detection of subtle compositional changes in oils. By analyzing the degradation processes under repeated heating and light exposure, this study aims to elucidate the molecular transformations in these oils. The findings emphasize the need for improved guidelines on the safe use and storage of edible oils to preserve their quality and minimize health risks.
2. Materials and Methods
Five edible oils - olive, rapeseed, sunflower, sesame, and peanut - were purchased from a local supermarket in Germany and used as received. The oils were labeled as follows: extra virgin olive oil, rapeseed oil with omega-3 fatty acids, cold-pressed sunflower oil (native, rich in vitamin E), cold-pressed sesame oil (rich in unsaturated fatty acids), and peanut oil. To prevent light exposure, all oils were purchased in amber-colored bottles and stored in a dark place before and after the experiments. Deuterated chloroform (CDCl3) and 5 mm NMR tubes were obtained from Deutero GmbH, Germany. A Silvercrest® Kitchen Tools mini deep fryer was purchased from a local supermarket in Germany. A MixcMax kitchen thermometer (temperature range: -50 to +300 °C) was used for temperature monitoring. Chicken nuggets were also obtained from a local supermarket in Germany.
2.1. Sunlight Treatment
All five oils - olive, rapeseed, sunflower, sesame, and peanut - were subjected to sunlight exposure experiments. For photodegradation experiments, 60 µL of oil directly from the bottle was dissolved in 540 µL of CDCl3 to a final volume of 600 µL and transferred to 5 mm NMR tubes. The initial 1H NMR spectra of all five oils were recorded immediately as reference spectra (t0). The samples were then placed in bright sunlight at 26 ± 2 °C under moderate to high UV index conditions. 1H NMR spectra were recorded after 3 hours (t1) and 8 hours (t2) of sunlight exposure.
2.2. Deep-Frying
All oils - rapeseed, sunflower, sesame, and peanut - were subjected to deep-frying experiments, except for olive oil. Approximately 1 L of oil was added to the mini deep fryer. Before heating, a 1 mL oil aliquot was taken as the control sample (t0). The fryer temperature was set to 190 °C, monitored using the MixcMax kitchen thermometer. Chicken nuggets were fried intermittently during a 60-minute continuous deep-frying session to mimic commercial fast-food frying practices. Oil samples were taken at 10 minutes (t1) and 60 minutes (t2). Aliquots of 1 mL of oil were transferred into amber-colored vials to prevent light exposure. The oil samples were allowed to cool to room temperature and filtered through 0.2 µm Whatman filters. The filtered oil samples were used to prepare the NMR samples.
2.3. NMR Sample Preparation
For deep-frying experiments, 60 µL of filtered oil was dissolved in 540 µL of CDCl3, resulting in a final volume of 600 µL, and transferred to 5 mm NMR tubes.
2.4. NMR Spectroscopy
NMR spectra were acquired using a Bruker Avance Neo 800 MHz instrument equipped with a helium-cooled 5 mm TCI cryoprobe. Samples were allowed to reach thermal equilibrium before acquisition. Locking, tuning and matching, and shimming were performed for each sample. One-dimensional 1H NMR spectra were recorded using the Bruker standard zg pulse sequence. A total of 64k points were acquired over a 16 kHz spectral width, with an acquisition time of 2 seconds and a relaxation delay of 3 seconds, resulting in a 5-second repetition time. Each spectrum was acquired with 64 scans, requiring 6 minutes per sample. Spectra were processed using an exponential multiplication window function with a 0.3 Hz line-broadening. The residual CDCl3 signal at 7.26 ppm was used as the chemical shift reference. Data acquisition and processing were performed using Topspin 4.4.1 software (Bruker, Germany).
3. Results and Discussion
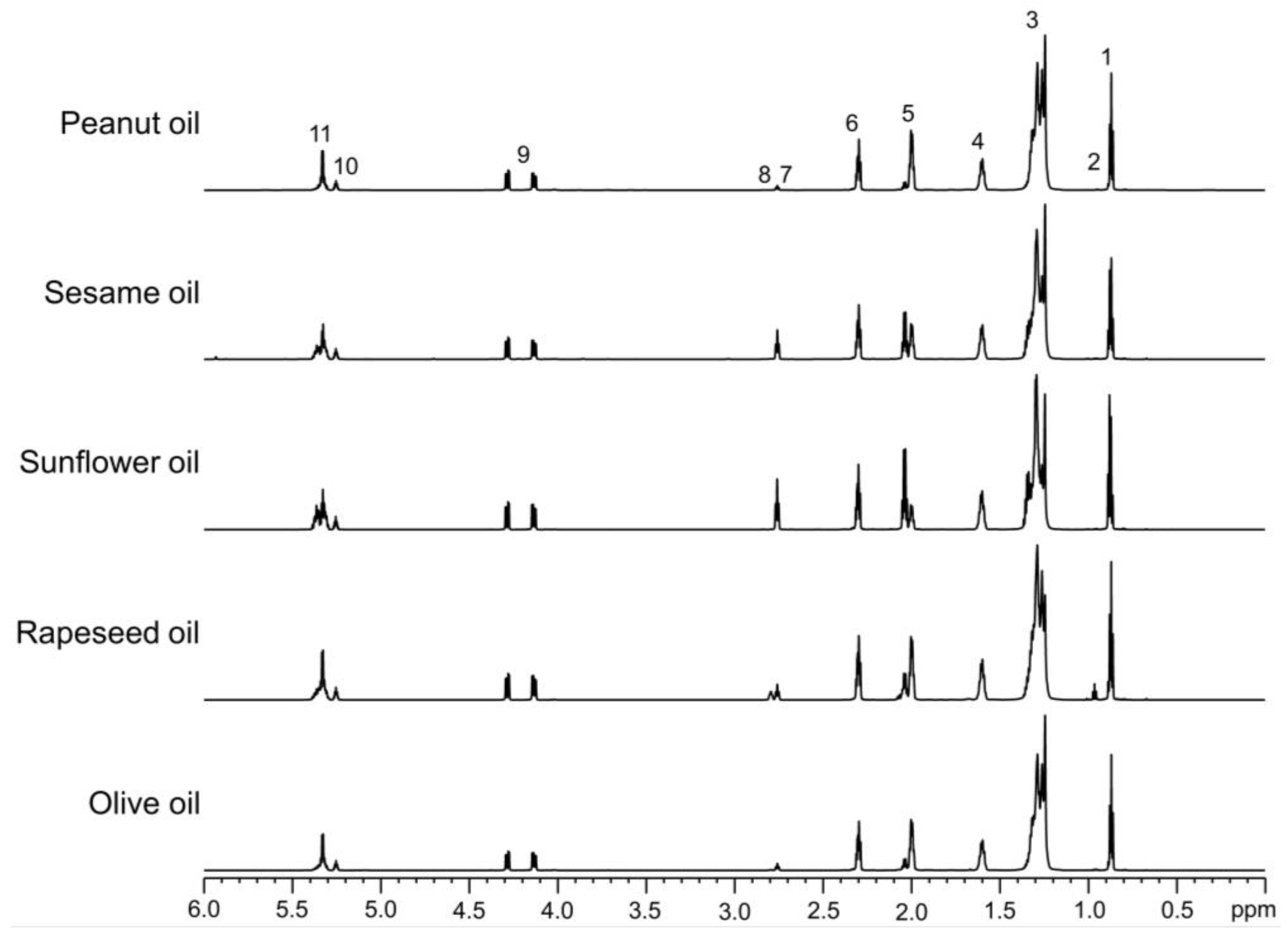
The
1H NMR spectra of olive (O), rapeseed (R), sunflower (SF), sesame (S), and peanut (P) oils display prominent signals corresponding to the protons of triglycerides, the primary lipid components of these oils (
Figure 1). The key structural groups of the oils were assigned based on proton chemical shifts and
J-coupling multiplicity patterns, as previously reported [
24] (
Table 1). While the
1H NMR spectra of all five oils appear broadly similar, closer examination of specific signals provides insights into the relative abundance of saturated and unsaturated fatty acids (FAs), including linoleic and linolenic acids. The integral of the acyl chain methylene group signals in the region of 1.40–1.14 ppm indicates that olive and peanut oils have relatively high levels of saturated FAs, followed by sesame and rapeseed oils, while sunflower oil contains the lowest levels of saturated FAs. Additionally, the integral of the olefinic proton signals in the chemical shift region of 5.41–5.28 ppm reveals that sunflower oil is particularly rich in highly unsaturated FAs, ranking the oils in the order SF > S, R > O, P. This trend aligns well with the reverse order of their degree of saturation. A distinctive feature of rapeseed oil compared to the other oils is the prominent bis-allylic =CH‒CH
2‒CH= signal at 2.79 ppm and the terminal methyl signal at 0.96 ppm, indicating a high abundance of ω-3 FAs. This finding corroborates the information provided on the rapeseed oil label. The intensity of the bis-allylic signal at 2.76 ppm suggests that the proportion of linoleic acid follows the order SF > S > R > O > P. Conversely, the abundance of linolenic acid is highest in rapeseed oil, followed by olive, sesame, sunflower, and peanut oils (R > O > S > SF > P), based on the intensity of the bis-allylic signal at 2.79 ppm. The terminal methyl signal intensity of ω-3 FAs further supports this trend.
3.1. Generation of Primary Lipid Oxidation Products (LOPs) in Oils During Sunlight Irradiation
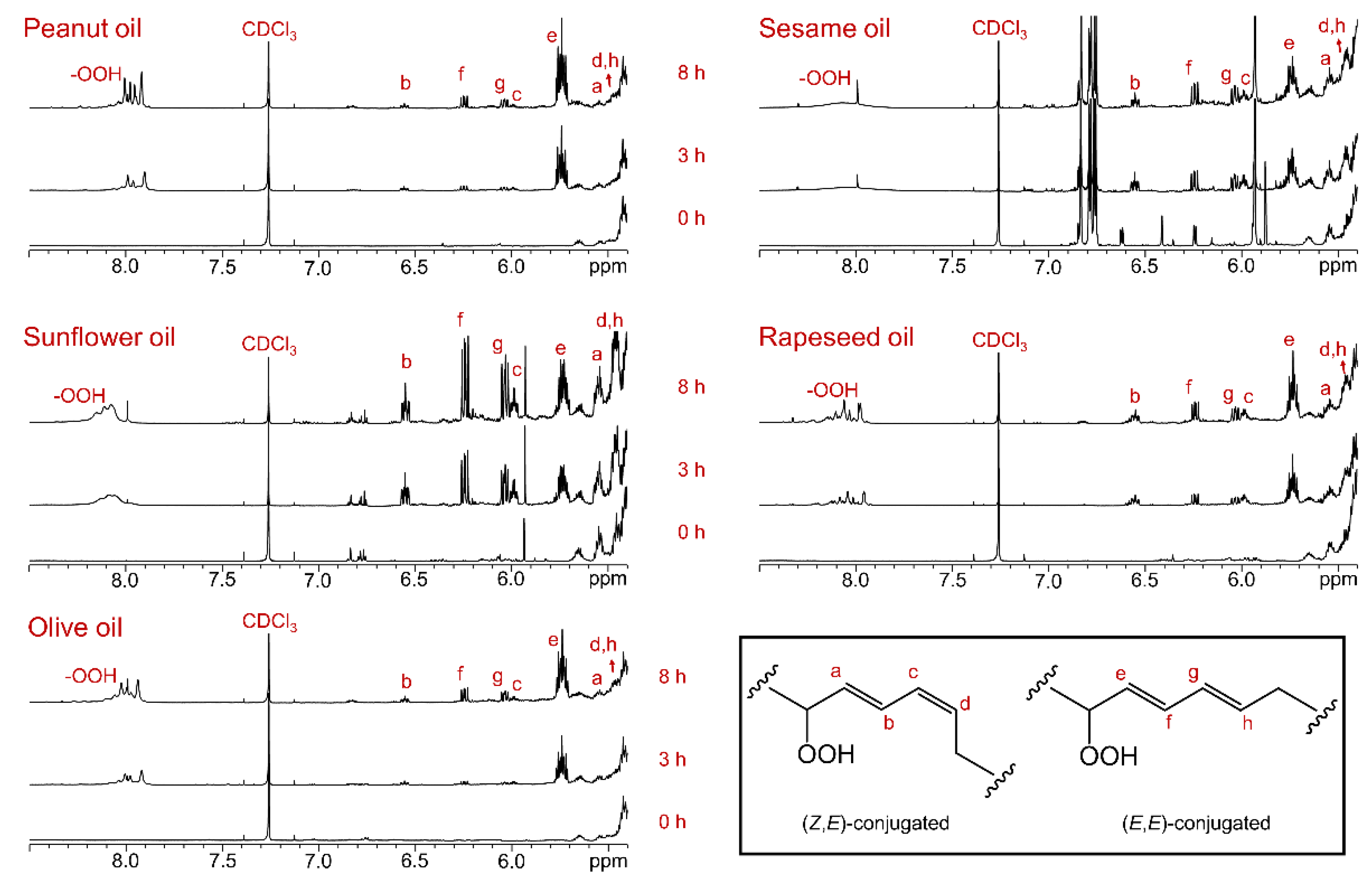
All five oils – olive (O), rapeseed (R), sunflower (SF), sesame (S), and peanut (P) – were exposed to sunlight for 3 and 8 hours at 26 ± 2 °C under moderate to high UV index conditions. The formation of new compounds in these oils was monitored by recording their
1H NMR spectra at specific time intervals. The
1H NMR spectra of the oils at the initial time point (0 hours of sunlight exposure) were used as references to track molecular changes during photooxidation. At 0 hours, no characteristic signals were observed in the chemical shift region of 8.5–5.4 ppm (
Figure 2, 0 h).
After 3 hours of sunlight exposure, new signals appeared in the regions of 6.6–5.4 ppm and 8.5–7.8 ppm, corresponding to olefinic and hydroperoxide protons, respectively (
Figure 2, 3 h). The intensity of these signals increased further after 8 hours of sunlight exposure (
Figure 2, 8 h). In the spectral range of 8.5–7.8 ppm, the residual signal from deuterated chloroform at 7.26 ppm, used as an internal chemical shift reference, was also observed. The broad signals in this region, attributed to hydroperoxide protons, are labeled as -OOH. The characteristic olefinic proton signals, labeled as a–h, were consistent with the assignments shown on the chemical structures (
Figure 2). The initial step of photooxidation in the fatty acid (FA) acyl groups of oils resulted in the formation of primary lipid oxidation products, namely hydroperoxides [
15,
27]. This is evidenced by the broad signals in the 8.5–7.8 ppm region. Based on the chemical shifts and J-coupling patterns of the olefinic protons, the primary oxidation products were identified as
cis,
trans (
Z,
E) and
trans,
trans (
E,
E) conjugated dienes containing hydroperoxides (
Figure 2). The proton signal at 6.55 ppm (dddt, J = 15.4, 11.0, 4, and 0.9 Hz), labeled as “b,” indicated the formation of hydroperoxides with (
Z,
E)-conjugated diene structures. The integration of this olefinic proton signal revealed the relative formation of (
Z,
E)-conjugated diene hydroperoxides in the order: SF > S > R > O > P. Similarly, the integral of the proton signal at 6.24 ppm (dd, J = 15.3 and 10.4 Hz), labeled as “f,” corresponding to (
E,
E)-conjugated diene hydroperoxides, showed the same relative abundance: SF > S > R > O > P.
Figure 2.
Olefinic and hydroperoxide proton regions of the
1H NMR spectra of oils recorded in CDCl
3 using an 800 MHz instrument. Each panel is labeled with the corresponding oil type. The bottom, middle, and top spectra represent oil samples collected after 0, 3, and 8 hours of sunlight exposure, respectively, at 26 ± 2 °C under moderate to high UV index conditions. Compared to untreated control samples, the sunlight-exposed oil samples exhibit the formation of (
Z,
E)- and (
E,
E)-conjugated double bonds associated with hydroperoxides [
24,
26]. The chemical structures of primary lipid oxidation products, such as hydroperoxides, are shown with atom labeling that corresponding to the proton NMR signal assignments.
Figure 2.
Olefinic and hydroperoxide proton regions of the
1H NMR spectra of oils recorded in CDCl
3 using an 800 MHz instrument. Each panel is labeled with the corresponding oil type. The bottom, middle, and top spectra represent oil samples collected after 0, 3, and 8 hours of sunlight exposure, respectively, at 26 ± 2 °C under moderate to high UV index conditions. Compared to untreated control samples, the sunlight-exposed oil samples exhibit the formation of (
Z,
E)- and (
E,
E)-conjugated double bonds associated with hydroperoxides [
24,
26]. The chemical structures of primary lipid oxidation products, such as hydroperoxides, are shown with atom labeling that corresponding to the proton NMR signal assignments.
Table 2.
The chemical shift assignments and multiplicities of olefinic and hydroperoxide proton signals in the
1H NMR spectra of olive, rapeseed, sunflower, sesame, and peanut oils recorded in CDCl
3. The signal letters correspond to those assigned in
Figure 2.
Table 2.
The chemical shift assignments and multiplicities of olefinic and hydroperoxide proton signals in the
1H NMR spectra of olive, rapeseed, sunflower, sesame, and peanut oils recorded in CDCl
3. The signal letters correspond to those assigned in
Figure 2.
| Signal |
Chemical shift (ppm) and multiplicity |
Structural group assignment |
| a |
5.56 (ddd) |
‒CH=CH‒CH=CH‒ |
(Z,E)-conjugated dienes
associated with
hydroperoxides (‒OOH) |
| b |
6.55 (dddt) |
‒CH=CH‒CH=CH‒ |
| c |
5.99 (td) |
‒CH=CH‒CH=CH‒ |
| d,h |
5.49-5.43 (m) |
‒CH=CH‒CH=CH‒ |
|
| e |
5.76-5.70 (m) |
‒CH=CH‒CH=CH‒ |
(E,E)-conjugated dienes
associated with
hydroperoxides (‒OOH) |
| f |
6.24 (dd) |
‒CH=CH‒CH=CH‒ |
| g |
6.03 (dd) |
‒CH=CH‒CH=CH‒ |
| - |
8.50-7.80 (bs) |
‒OOH
|
Hydroperoxide protons |
Overall, sunflower oil demonstrated the highest proportion of hydroperoxides after 8 hours of sunlight exposure, consistent with its high content of unsaturated fatty acids, which are highly prone to photooxidation. Sesame and rapeseed oils followed, exhibiting increased production of conjugated diene hydroperoxides. In contrast, olive and peanut oils produced relatively lower amounts of primary oxidation products, attributable to their higher content of saturated acyl groups in their lipid composition.
3.2. Sunlight-Mediated Formation of Aldehyde Compounds in Oils
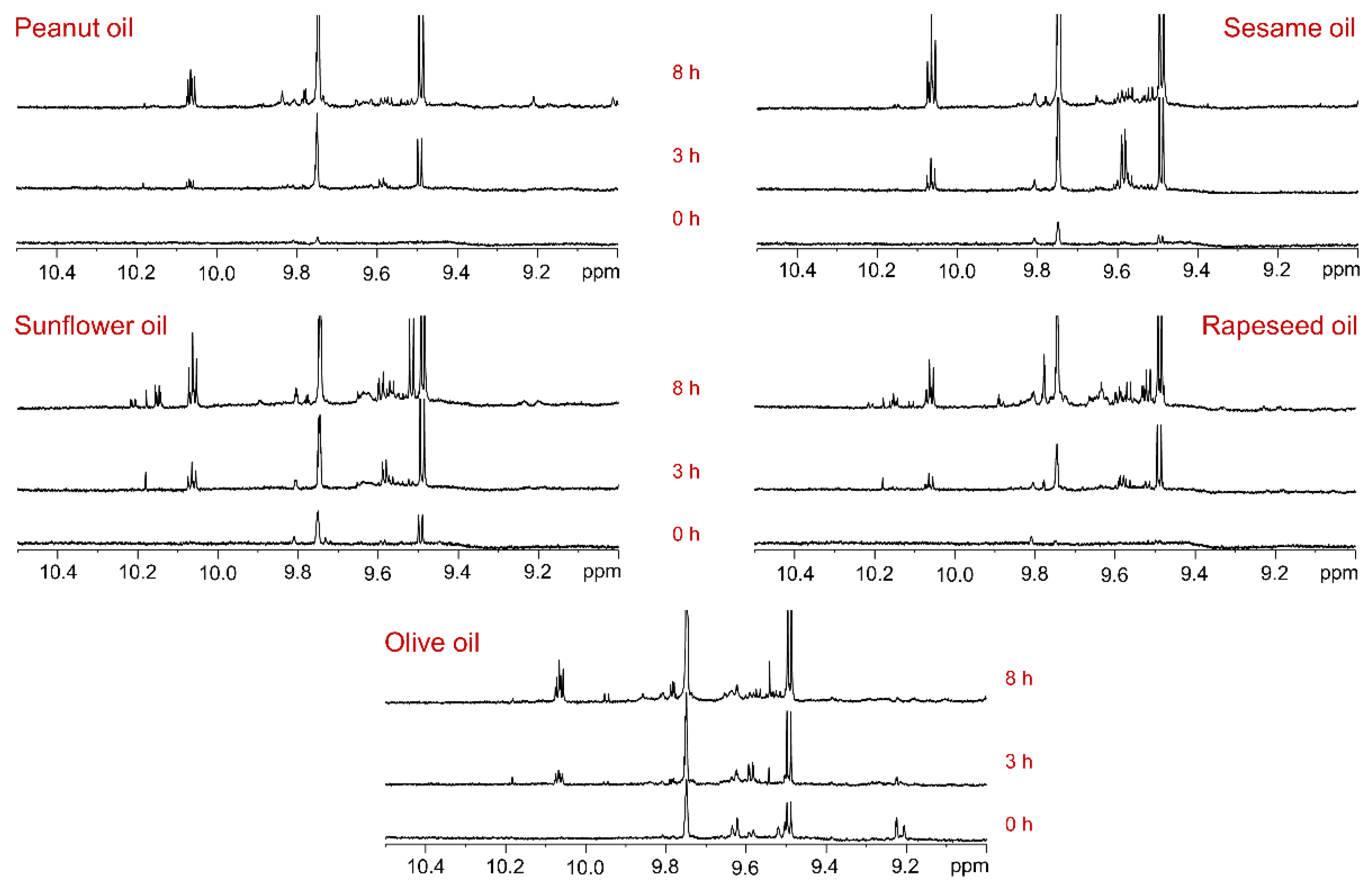
Primary lipid oxidation products, such as hydroperoxides and hydroxides, are highly unstable and degrade further to form secondary oxidation products, including aldehydes, ketones, alcohols, and epoxides. Among these, aldehydes produce characteristic proton signals in the
1H NMR spectral region of 10.5–9.0 ppm, which are well separated from the signals of major acyl and glyceryl groups. At the initial time point (0 hours of sunlight exposure), the
1H NMR spectra of rapeseed, sunflower, sesame, and peanut oils showed negligible aldehyde signals, whereas olive oil exhibited several aldehyde signals (
Figure 2, 0 h). It is noteworthy that the olive oil sample had expired 10 days prior to the photooxidation experiments. The pre-existing aldehyde signals in the olive oil spectrum suggest that degradation due to expiration leads to the formation of aldehydes, indicating potential health risks if consumed after expiration.
These aldehydes, predominantly (
E)-2-alkenals and n-alkanals, were further amplified during photooxidation. After 3 hours of sunlight exposure, new aldehyde proton signals were observed in all oils compared to their respective control spectra (
Figure 3, 3 h). The intensity of these signals increased significantly after 8 hours of sunlight exposure, accompanied by the formation of additional aldehyde compounds (
Figure 2, 8 h). The quantity of aldehydes generated after 8 hours of sunlight exposure was notably higher in oils rich in unsaturated fatty acids, such as sesame, sunflower, and rapeseed oils, compared to olive and peanut oils. The aldehyde proton signal at 9.49 ppm, corresponding to (
E)-2-alkenals, was most intense in sesame oil, followed by sunflower oil. In contrast, it was relatively less intense in olive, rapeseed, and peanut oils. Sunflower oil exhibited the highest quantities of (
E,
E)-2,4-alkadienals, followed by rapeseed and sesame oils, whereas olive and peanut oils contained very low amounts. All oils showed a significant generation of n-alkanals, as indicated by the aldehyde proton signal in the region of 9.76–9.73 ppm. Additionally, 4-oxo-(
E)-2-alkenals and low-molecular-mass short-chain n-alkanals were generated in all oils, as indicated by the aldehyde proton signals in the chemical shift range of 9.81–9.77 ppm. The wide range of chemical shifts observed for the aldehyde proton signals clearly indicates the generation of diverse aldehyde-associated compounds during the light exposure of the oils.
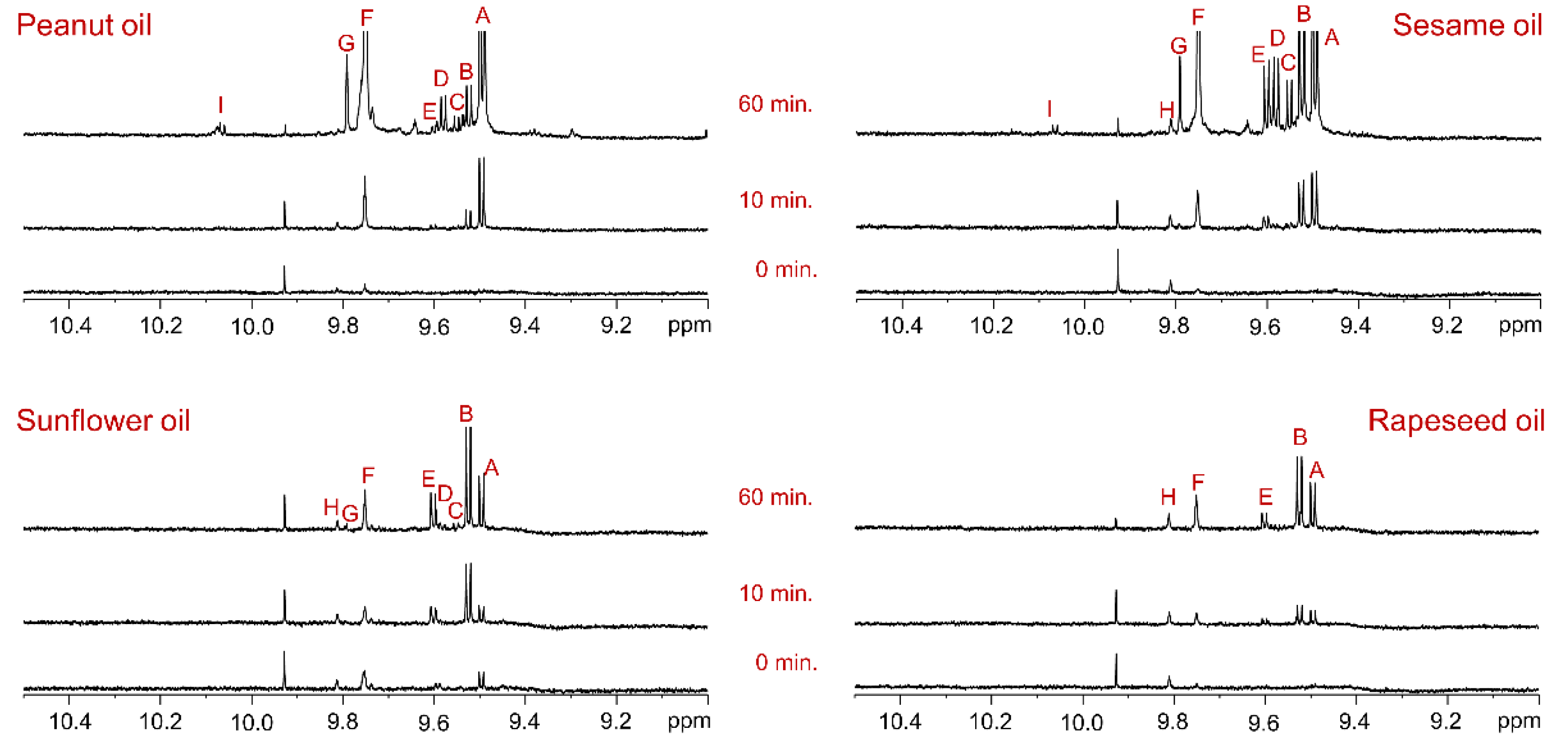
3.3. Generation of Aldehyde Compounds During Deep-Frying of Oils
Rapeseed, sunflower, sesame, and peanut oils were subjected to deep-frying in a commercial mini fryer at 190 ± 5 °C for 60 minutes, with chicken nuggets intermittently fried to simulate commercial food preparation.
Figure 4.
Aldehyde proton region of
1H NMR spectra of oils recorded in CDCl
3 using an 800 MHz instrument. Each panel is labeled with the corresponding oil type. The bottom, middle, and top spectra represent oil samples collected at 0, 10, and 60 minutes of deep-frying, respectively, during intermittent frying of chicken nuggets at 190 ± 5 °C. The signal assignments agree with those given in
Table 3.
Figure 4.
Aldehyde proton region of
1H NMR spectra of oils recorded in CDCl
3 using an 800 MHz instrument. Each panel is labeled with the corresponding oil type. The bottom, middle, and top spectra represent oil samples collected at 0, 10, and 60 minutes of deep-frying, respectively, during intermittent frying of chicken nuggets at 190 ± 5 °C. The signal assignments agree with those given in
Table 3.
The
1H NMR spectra of unheated control oil samples displayed negligible or minimal intensity of aldehyde proton signals. However, even 10 minutes of deep-frying at this high temperature resulted in the formation of new aldehyde compounds, accompanied by an increase in the intensity of pre-existing aldehyde signals. After 60 minutes of continuous deep-frying, the
1H NMR spectra of all four oils showed a significant increase in the intensity of prior aldehyde signals and the generation of numerous new aldehyde compounds. The identified aldehydes included: A: (
E)-2-alkenals, B: (
E,
E)-2,4-alkadienals, C: 4,5-epoxy-(
E)-2-alkenals, D: combined 4-hydroxy/4-hydroperoxy-(
E)-2-alkenals, E: (
Z,
E)-2,4-alkadienals, F: n-alkanals, G: 4-oxo-(
E)-2-alkenals, H: low-molecular-mass short-chain n-alkanals, such as propanal and n-butanal, and I: (
Z)-2-alkenals. These assignments were based on previously reported findings [
10].
Table 3.
The chemical shift assignments and multiplicities of aldehyde signals in the
1H NMR spectra of rapeseed, sunflower, sesame, and peanut oils recorded in CDCl
3. The signal letters correspond to those assigned in
Figure 4.
Table 3.
The chemical shift assignments and multiplicities of aldehyde signals in the
1H NMR spectra of rapeseed, sunflower, sesame, and peanut oils recorded in CDCl
3. The signal letters correspond to those assigned in
Figure 4.
| Signal |
Chemical shift (ppm) and multiplicity |
Structural group assignment |
| A |
9.49 (d, 7.9 Hz) |
‒CHO |
(E)-2-alkenals |
| B |
9.52 (d, 8.0 Hz) |
‒CHO |
(E,E)-2,4-alkadienals |
| C |
9.55 (d, 7.8 Hz) |
‒CHO |
4,5-epoxy-(E)-2-alkenals |
| D |
9.58 (d, 7.8 Hz) |
‒CHO |
Combined 4-hydroxy/4-hydroperoxy-(E)-2-alkenals |
| E |
9.60 (d, 8.0 Hz) |
‒CHO |
(Z,E)-2,4-alkadienals |
| F |
9.75 (t, 1.7 Hz) |
‒CHO |
n-alkanals |
| G |
9.79 (bs) |
‒CHO |
4-oxo-(E)-2-alkenals |
| H |
9.81 (bs) |
‒CHO |
Low-molecular-mass short-chain n-alkanals |
| I |
10.06 (d, 8.1 Hz) |
‒CHO |
(Z)-2-alkenals |
Among the oils, sesame oil exhibited the highest quantities of (
E)-2-alkenals, (
E,
E)-2,4-alkadienals, 4,5-epoxy-(
E)-2-alkenals, combined 4-hydroxy/4-hydroperoxy-(
E)-2-alkenals, (
Z,
E)-2,4-alkadienals, n-alkanals, and 4-oxo-(
E)-2-alkenals. Similarly, sunflower and rapeseed oils demonstrated high abundances of (
E)-2-alkenals, (
E,
E)-2,4-alkadienals, (
Z,
E)-2,4-alkadienals, and n-alkanals. Peanut oil, despite its high saturated fatty acid content, also generated significant levels of (
E)-2-alkenals, (
E,
E)-2,4-alkadienals, combined 4-hydroxy/4-hydroperoxy-(
E)-2-alkenals, n-alkanals, and 4-oxo-(
E)-2-alkenals. These results may be attributed to the unique composition of major and minor constituents in peanut oil and their susceptibility to deep-frying conditions [
24].
These findings underscore that, regardless of oil type or fatty acid composition, all oils are highly susceptible to thermal oxidation processes during prolonged exposure to high temperatures and repeated heating cycles. The presence of toxic aldehyde compounds in freshly used oils, even after a few minutes of deep-frying, suggests that their concentrations could increase several-fold if the oil is continuously heated for hours or days, posing significant health risks. This emphasizes the critical need to limit repeated oil usage to reduce the consumption of harmful oxidation products and ensure food safety.
4. Conclusions
This study highlights the significant oxidative transformations that occur in edible oils during exposure to sunlight and high-temperature deep-frying, providing critical insights into their degradation pathways and the formation of harmful compounds. Using high-field 1H NMR spectroscopy, we identified the generation of primary lipid oxidation products (LOPs), such as hydroperoxides, during sunlight exposure, as well as the subsequent production of diverse secondary oxidation products, including aldehydes and epoxides. These findings underscore the vulnerability of oils, particularly those rich in unsaturated fatty acids, to photooxidation.
Sunflower, sesame, and rapeseed oils demonstrated the highest susceptibility to oxidation, as evidenced by the formation of (Z,E)- and (E,E)-conjugated diene hydroperoxides and various aldehydes after sunlight exposure. In contrast, olive and peanut oils, with higher levels of saturated fatty acids, showed relatively lower oxidation levels. Nevertheless, the presence of pre-existing aldehyde signals in expired olive oil highlights the importance of monitoring oil quality even before environmental stressors are applied.
Deep-frying experiments further confirmed the rapid formation and accumulation of toxic aldehyde compounds, including 4-hydroperoxy-(E)-2-alkenals, (E)-2-alkenals, (E,E)-2,4-alkadienals, 4,5-epoxy-(E)-2-alkenals, 4-hydroxy/4-hydroperoxy-(E)-2-alkenals, and short-chain aldehydes, within minutes of high-temperature cooking. Sesame oil showed the highest aldehyde generation, followed by sunflower, rapeseed, and peanut oils. Despite its high saturated fatty acid content, peanut oil also exhibited substantial oxidation, likely due to its unique composition of minor constituents.
These results emphasize that the type of oil, its fatty acid composition, and exposure conditions play pivotal roles in determining the extent of oxidation and the formation of harmful products. Importantly, the study reveals that even short-term exposure to high temperatures or sunlight can produce toxic oxidation products in significant amounts, posing potential health risks. To mitigate these risks, it is crucial to adopt safer practices, such as limiting the reuse of oils in cooking, using oils with higher oxidative stability for prolonged heating, and avoiding direct sunlight exposure during storage. The findings also underline the importance of proper labeling and monitoring of oils, particularly regarding expiration dates and recommended usage conditions.
This study underscores the urgent need for further research into advanced analytical methods and interventions to improve the oxidative stability of oils, safeguard consumer health, and promote sustainable practices in food preparation and storage.
Author Contributions
Conceptualization, S.P.B.V.; methodology, A.M.F. and S.P.B.V.; formal analysis, A.M.F. and S.P.B.V.; investigation, A.M.F. and S.P.B.V.; resources, S.P.B.V.; data curation, A.M.F. and S.P.B.V.; writing-original draft preparation, review, and editing, A.M.F. and S.P.B.V.; visualization, A.M.F. and S.P.B.V.; supervision, S.P.B.V.; project administration, S.P.B.V. All authors have read and agreed to the submitted version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study will be uploaded to Edmond (
https://doi.org/.............), an open research data repository of the Max Planck Society.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vegetable oils in food technology: Composition, Properties and Uses.
- Fellows, P.J. Food Processing Technology: Principles and Practice; 2022.
- Saguy, I.S.; Dana, D. Integrated approach to deep fat frying: engineering, nutrition, health and consumer aspects. J. Food Eng. 2003, 56, 143–152. [Google Scholar] [CrossRef]
- Berk, Z. Frying, baking, and roasting. In Food Process Engineering and Technology; 2018.
- Li, Y.; Ngadi, M.; Oluka, S. Quality changes in mixtures of hydrogenated and non-hydrogenated oils during frying. J Sci Food Agric 2008, 88, 1518–1523. [Google Scholar] [CrossRef]
- Ng, C.L.; Wehling, R.L.; Cuppett, S.L. Method for determining frying oil degradation by near-infrared spectroscopy. J Agric Food Chem 2007, 55, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Ghazani, S.M.; Marangoni, A.G. Quality and safety of frying oils used in restaurants. Food Research International 2014, 64, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Zhang, J.; Liang, L.; Wen, C.; Li, Y.; Shen, M.; Wu, Y.; He, X.; Liu, G.; et al. Formation, migration, derivation, and generation mechanism of polycyclic aromatic hydrocarbons during frying. Food Chem 2023, 425, 136485. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-K.; Li, K.; Li, X.; Elbadry, S.; Raslan, A.A.; Li, Y.; Mulla, Z.S. ; Asmaa, &; Tahoun, B.M.B.; Rizk El-Ghareeb, W.; et al. Levels of polycyclic aromatic hydrocarbons in edible and fried vegetable oil: a health risk assessment study.
- Moumtaz, S.; Percival, B.C.; Parmar, D.; Grootveld, K.L.; Jansson, P.; Grootveld, M. Toxic aldehyde generation in and food uptake from culinary oils during frying practices: peroxidative resistance of a monounsaturate-rich algae oil. Sci Rep 2019, 9. [Google Scholar] [CrossRef]
- Wann, A.I.; Percival, B.C.; Woodason, K.; Gibson, M.; Vincent, S.; Grootveld, M. Comparative1 h nmr-based chemometric evaluations of the time-dependent generation of aldehydic lipid oxidation products in culinary oils exposed to laboratory-simulated shallow frying episodes: Differential patterns observed for omega-3 fatty acid-containing soybean oils. Foods 2021, 10, 2481. [Google Scholar] [CrossRef]
- Gibson, M.; Percival, B.C.; Edgar, M.; Grootveld, M. Low-Field Benchtop NMR Spectroscopy for Quantification of Aldehydic Lipid Oxidation Products in Culinary Oils during Shallow Frying Episodes. Foods 2023, 12, 1254. [Google Scholar] [CrossRef]
- St. Angelo, A.J. Lipid Oxidation in Foods. Crit Rev Food Sci Nutr 1996, 36. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Saf 2006, 5, 169–186. [Google Scholar] [CrossRef]
- . Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential adverse public health effects afforded by the ingestion of dietary lipid oxidation product toxins: Significance of fried food sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef] [PubMed]
- . Guo, X. yang; Cheng, L. yu; Chang, C.; Jiang, X. ming; Gao, P.; Zhong, W.; Hu, C. rong; He, D. ping; Yin, J. jiao Toxic aldehydes in fried foods: Formation, analysis, and reduction strategies. Food Control 2025, 169, 779S–786S. [Google Scholar] [CrossRef]
- . Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. American Journal of Clinical Nutrition 1993, 57. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Uriarte, P.S. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α,β unsaturated aldehydes. Food Chem 2012, 131. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible Plant Oil: Global Status, Health Issues, and Perspectives. Front Plant Sci 2020, 11. [Google Scholar] [CrossRef]
- . Dodoo, D.; Tulashie, S.K.; Dodoo, T.; Kwaw, F. Assessing the Effects of Sunlight on the Photooxidation of Tropical Oils with Experimental and Computational Approaches. JAOCS, Journal of the American Oil Chemists’ Society 2021, 98, 439–453. [Google Scholar] [CrossRef]
- . Velasco, J.; Dobarganes, C. Oxidative stability of virgin olive oil. European Journal of Lipid Science and Technology 2002, 104. [Google Scholar] [CrossRef]
- . Hatzakis, E. Nuclear Magnetic Resonance (NMR) Spectroscopy in Food Science: A Comprehensive Review. Compr Rev Food Sci Food Saf 2019, 18, 189–220. [Google Scholar] [CrossRef]
- . Marcone, M.F.; Wang, S.; Albabish, W.; Nie, S.; Somnarain, D.; Hill, A. Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Research International 2013, 51, 729–747. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A review of thermo-oxidative degradation of food lipids studied by 1H NMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr Rev Food Sci Food Saf 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Contribution to further understanding of the evolution of sunflower oil submitted to frying temperature in a domestic fryer: Study by1H Nuclear Magnetic Resonance. J Agric Food Chem 2009, 57, 7790–7799. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yang, D.; Gan, L.J.; Zhang, H.; Shin, J.A.; Lee, Y.H.; Jang, Y.S.; Lee, K.T. Effect of positional distribution of Linoleic acid on oxidative stability of triacylglycerol molecules determined by 1H NMR. JAOCS, Journal of the American Oil Chemists’ Society 2015, 92, 157–165. [Google Scholar] [CrossRef]
- Ahmed, R.; Varras, P.C.; Siskos, M.G.; Siddiqui, H.; Choudhary, M.I.; Gerothanassis, I.P. NMR and Computational Studies as Analytical and High-Resolution Structural Tool for Complex Hydroperoxides and Diastereomeric Endo-Hydroperoxides of Fatty Acids in Solution-Exemplified by Methyl Linolenate. Molecules 2020, 25, 4902. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).