Submitted:
29 November 2024
Posted:
29 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Introduction of the Marine Ranching
2.2. Construction of Ecopath Models
2.2.1. Introduction of the Ecopath Model
2.2.2. Survey of the Marine Environment
2.2.3. Functional Group Division
2.2.4. Data Sources of Functional Groups
2.2.5. Model Balancing and Uncertainty
2.3. Construction of Indices System
2.3.1. Description of ENA Indices
2.3.2. Classification of Ecosystem Status Levels
2.3.3. Evaluation of the Ecosystem Status of the Ecosystems
2.4. Evaluation of Ecological Carrying Capacity
2.5. Evaluation of Stock Enhancement Potential and Selection of Stock Enhancement Groups
2.6. Simulation of Stock Enhancement Strategies
2.6.1. Introduction of the Ecosim Model
2.6.2. Construction of Ecosim Model
2.6.3. Simulation Scenario Design
3. Results
3.3. Trophic Structure
3.4. Energy Flow Structure
3.5. Ecosystem Attributes
3.6. Ecosystem Status
3.7. Ecological Carrying Capacity and Stock Enhancement Potential
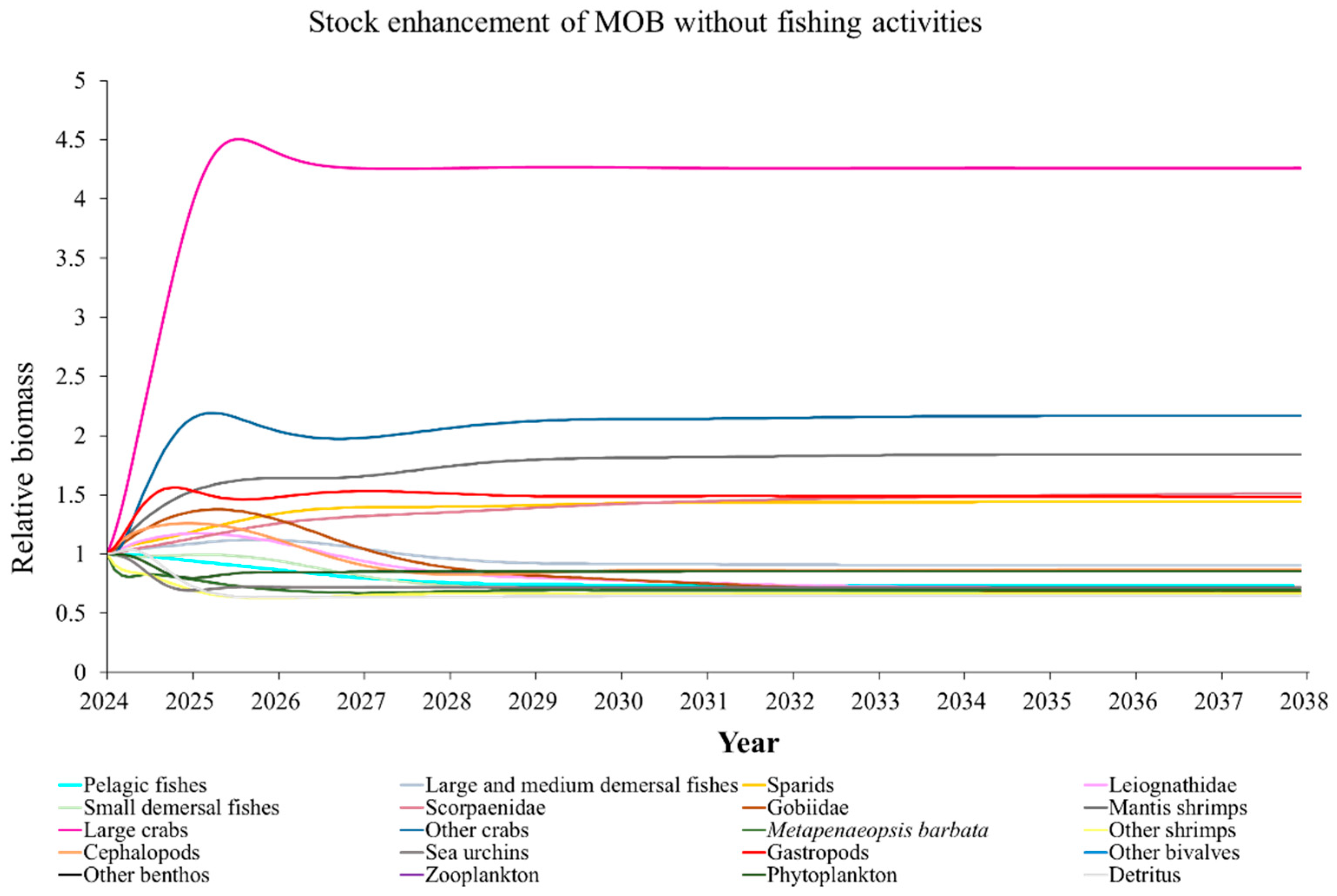
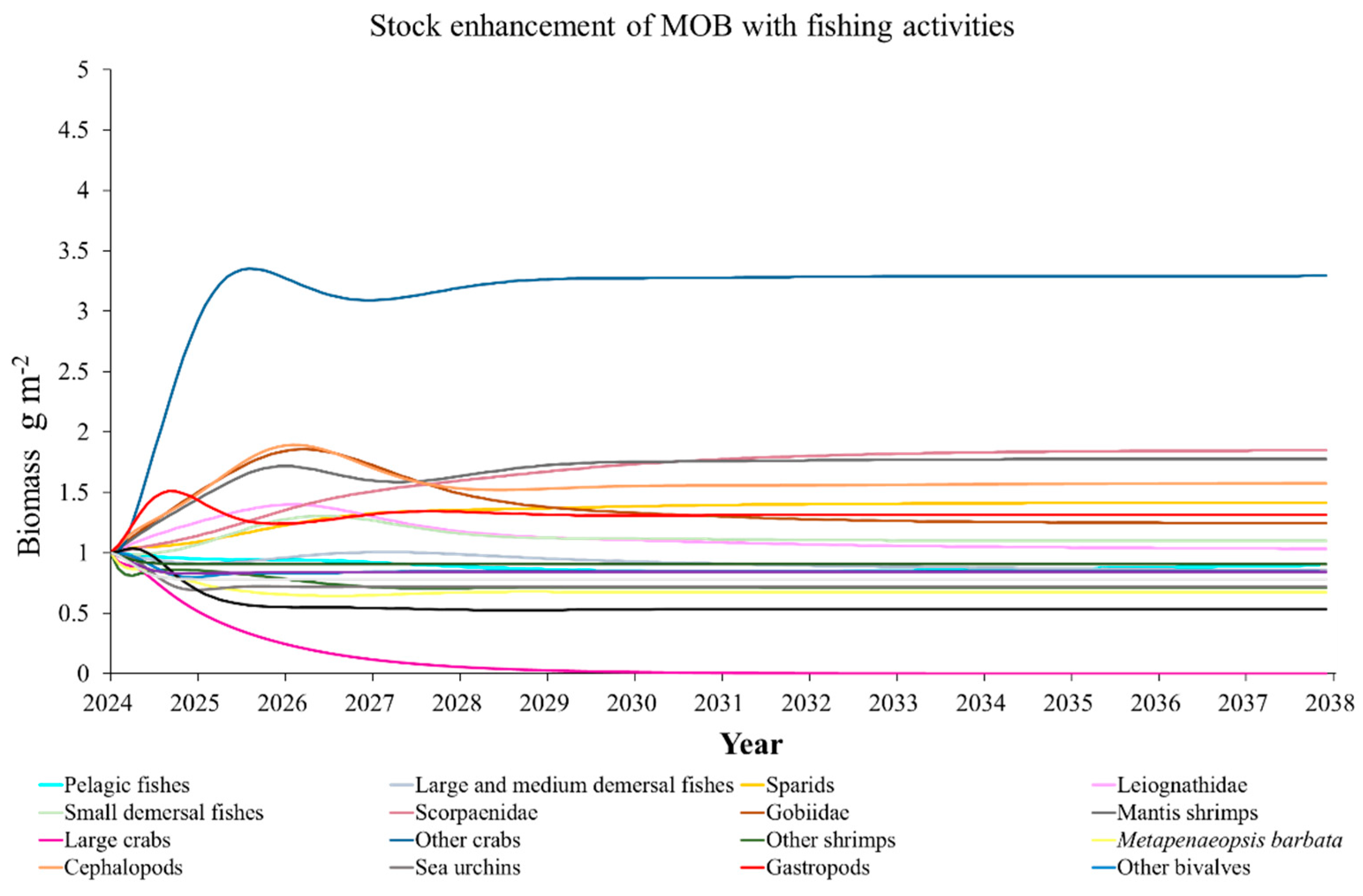
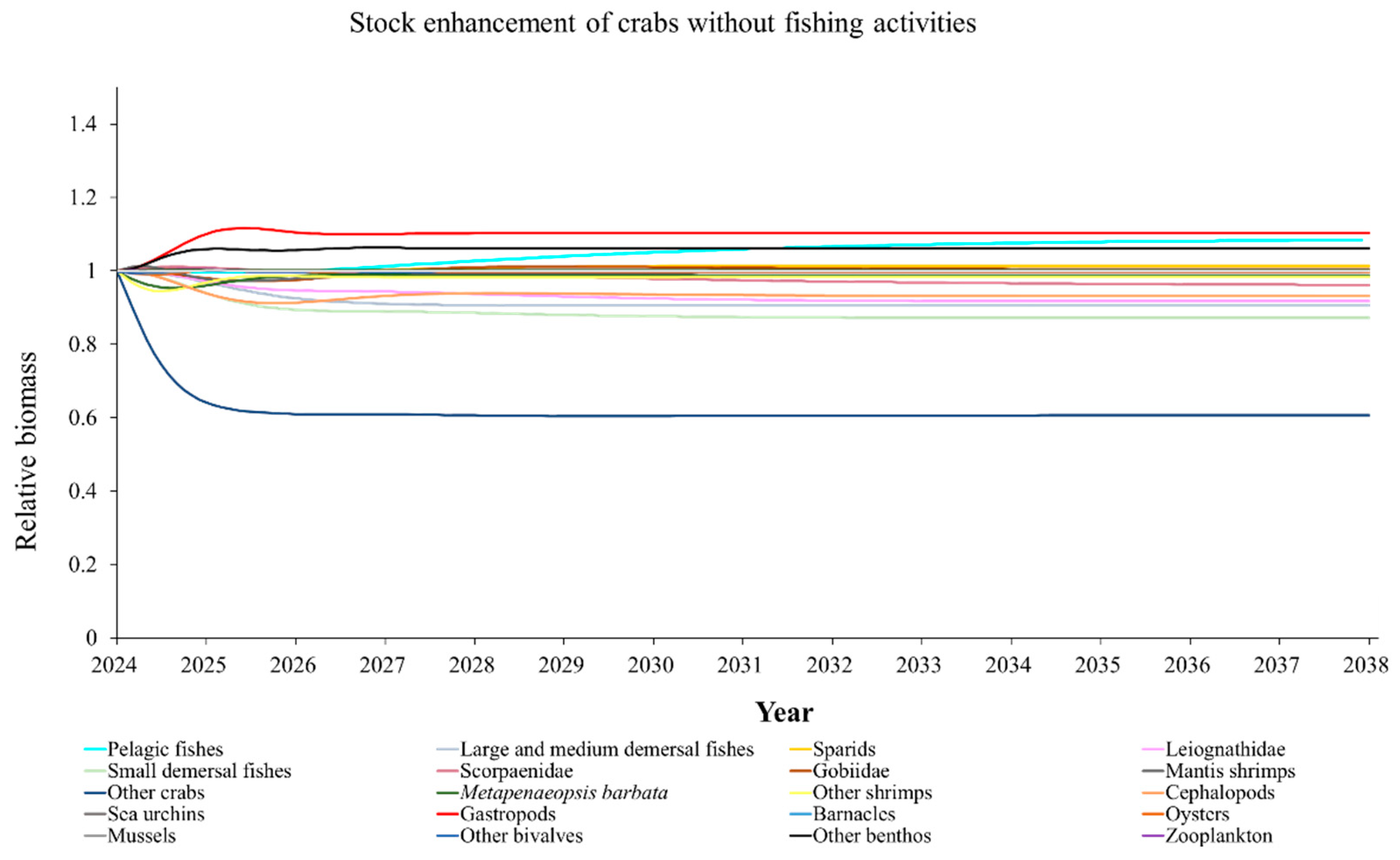
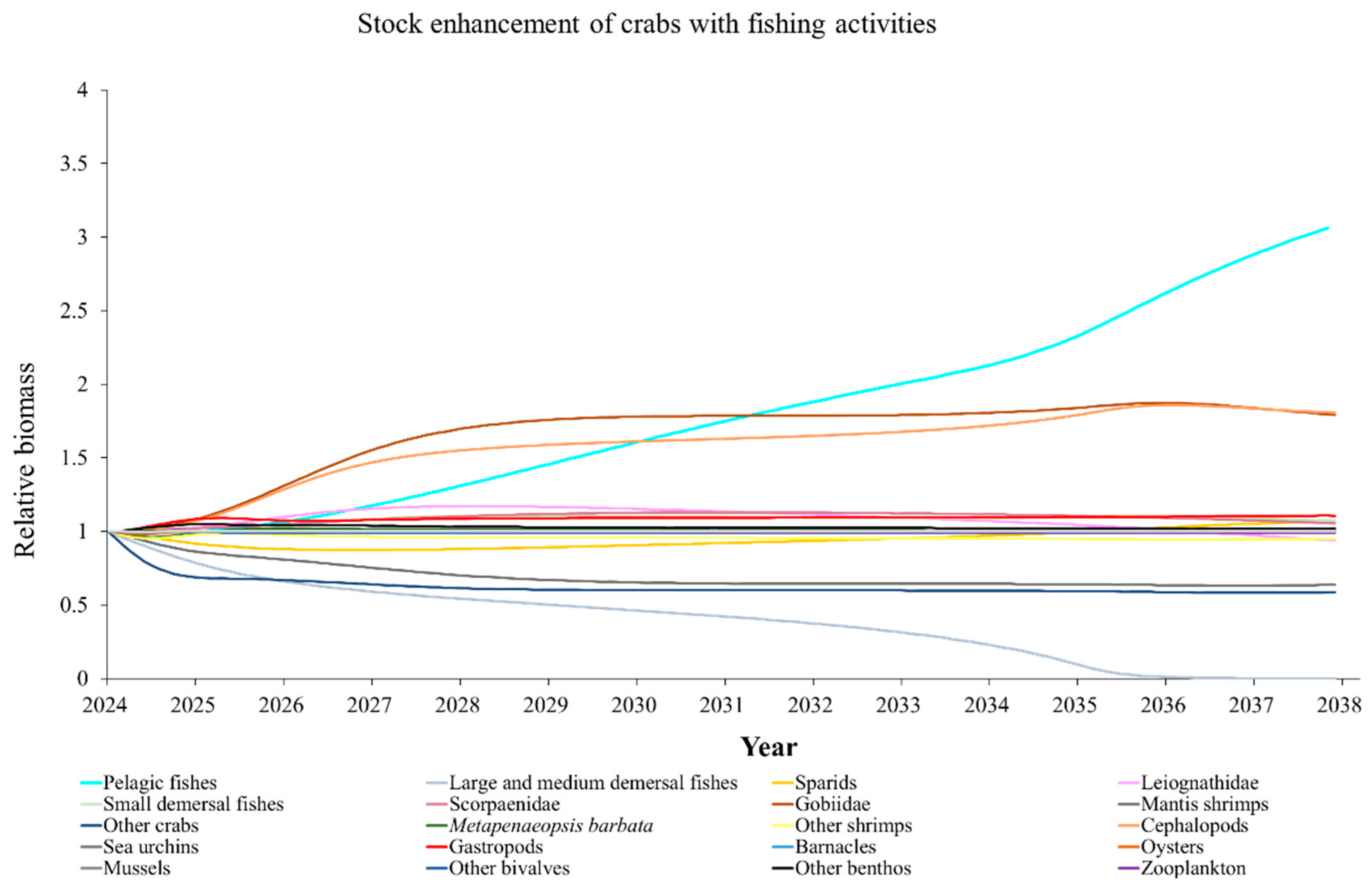
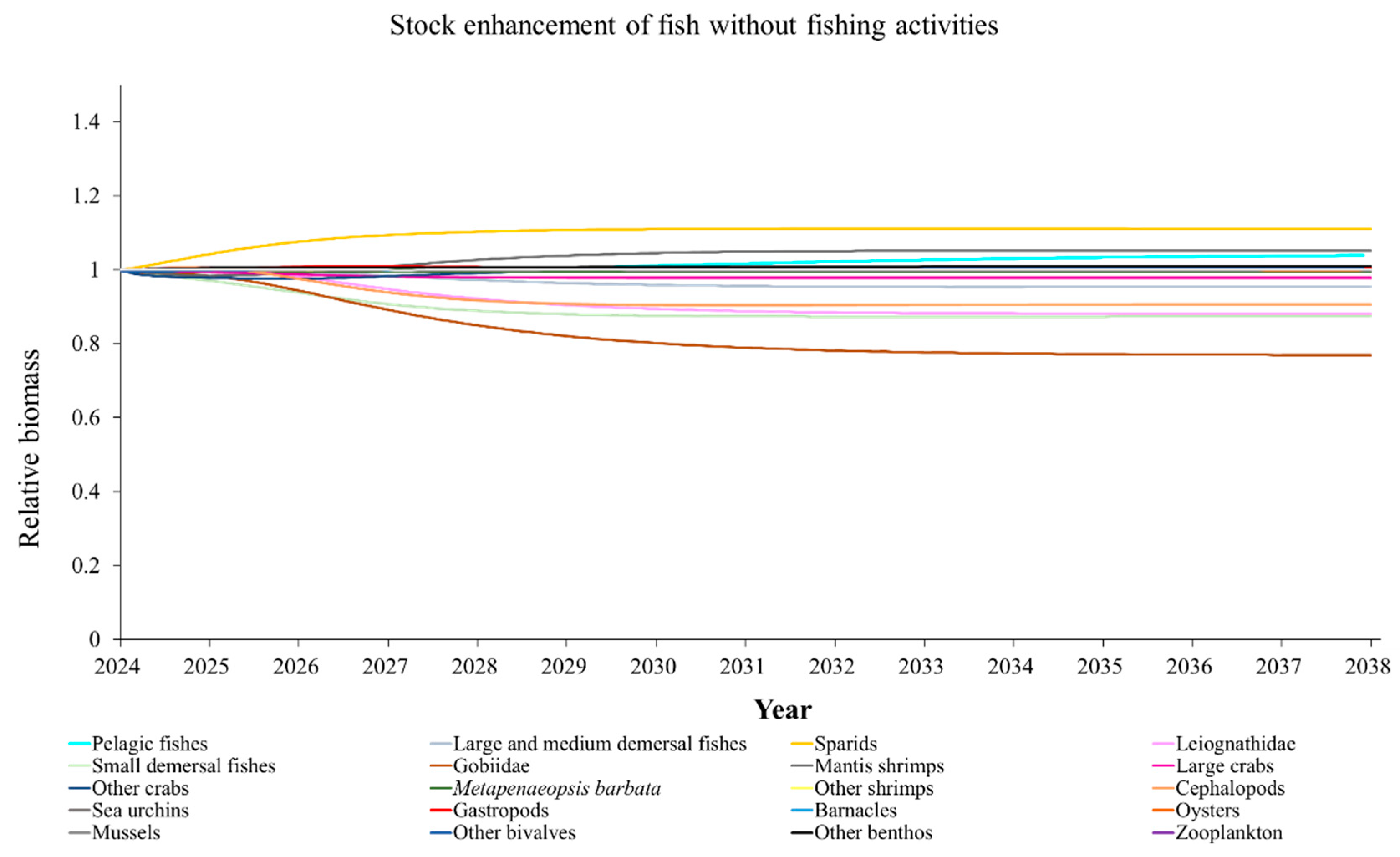
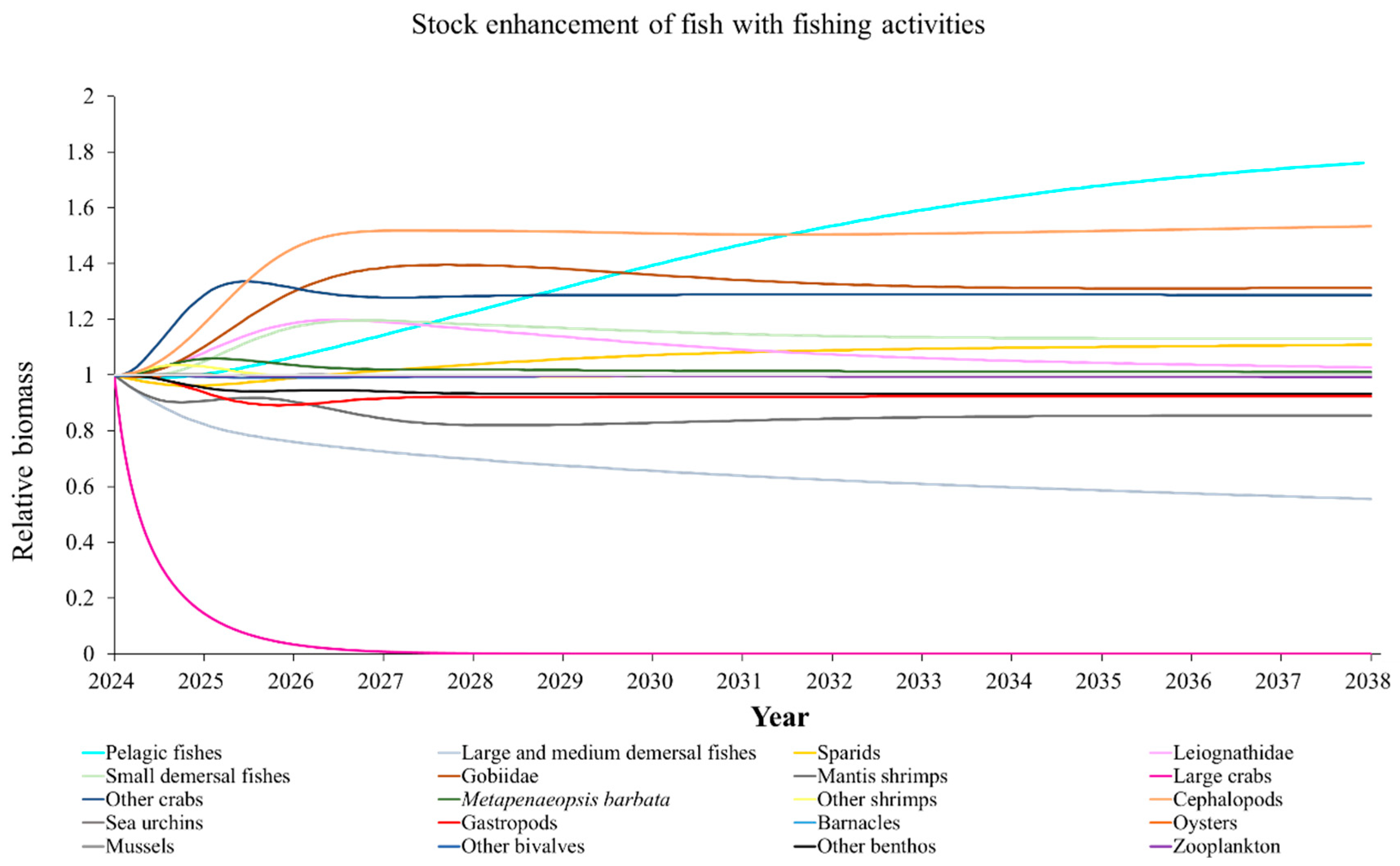
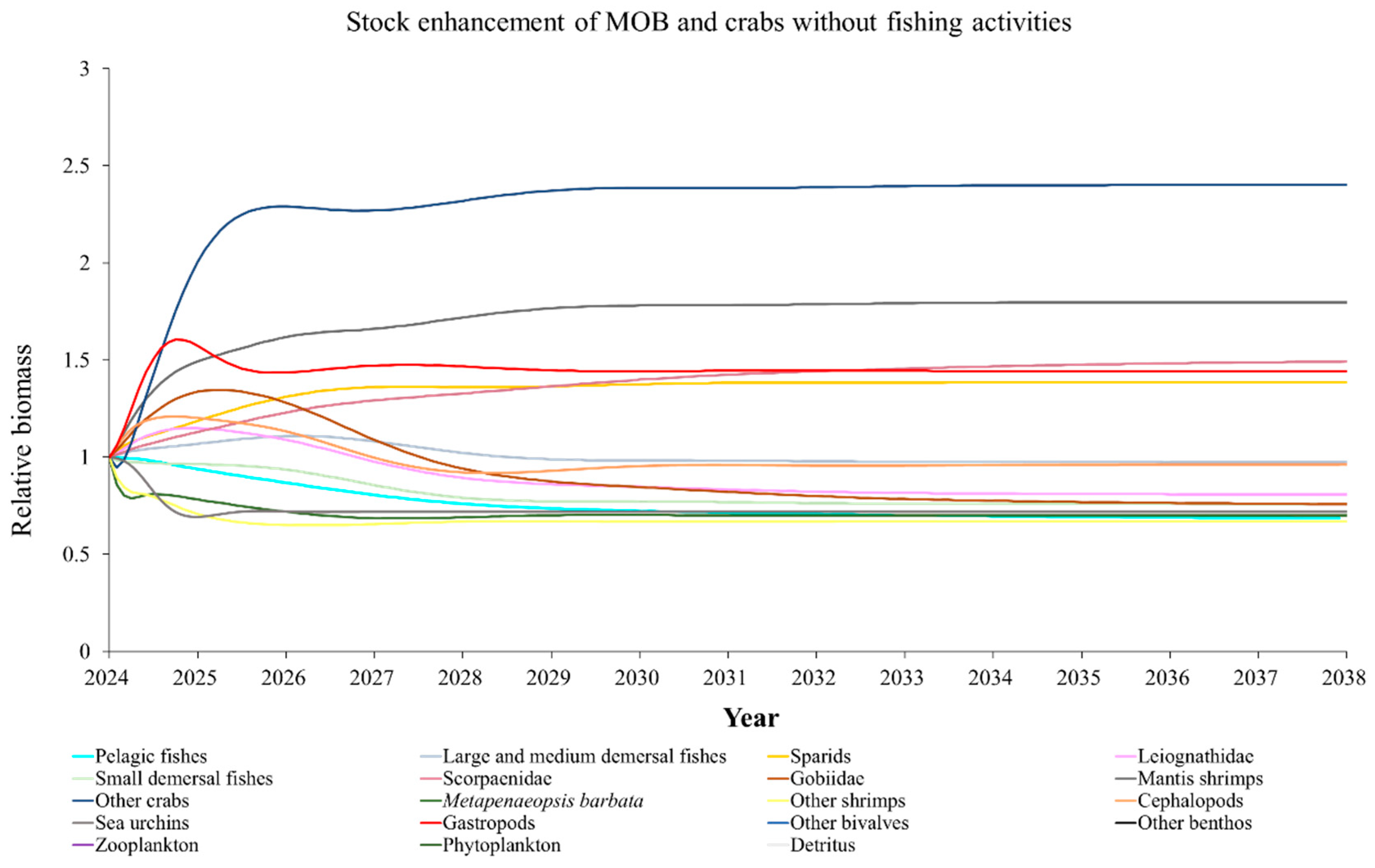
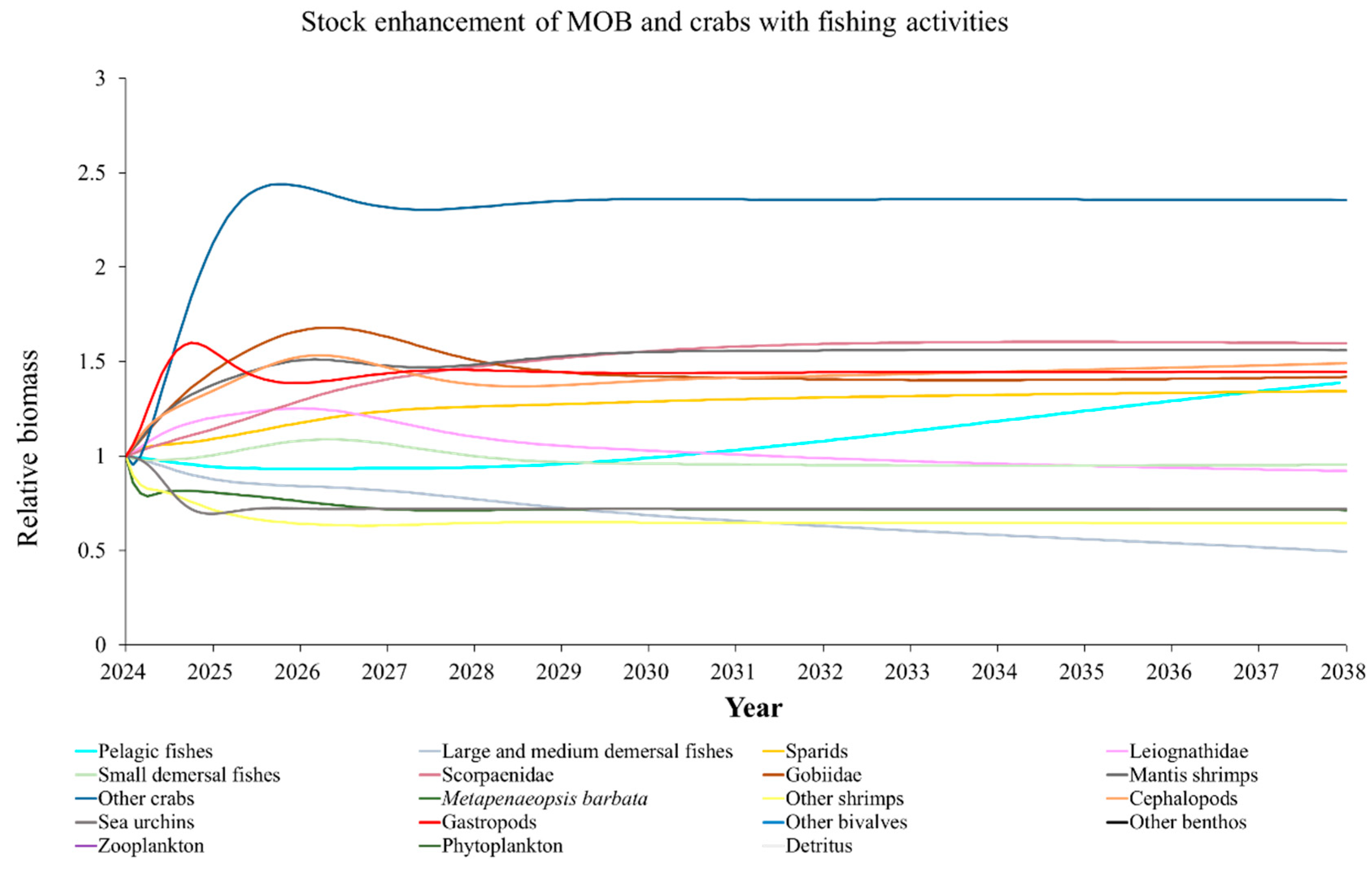
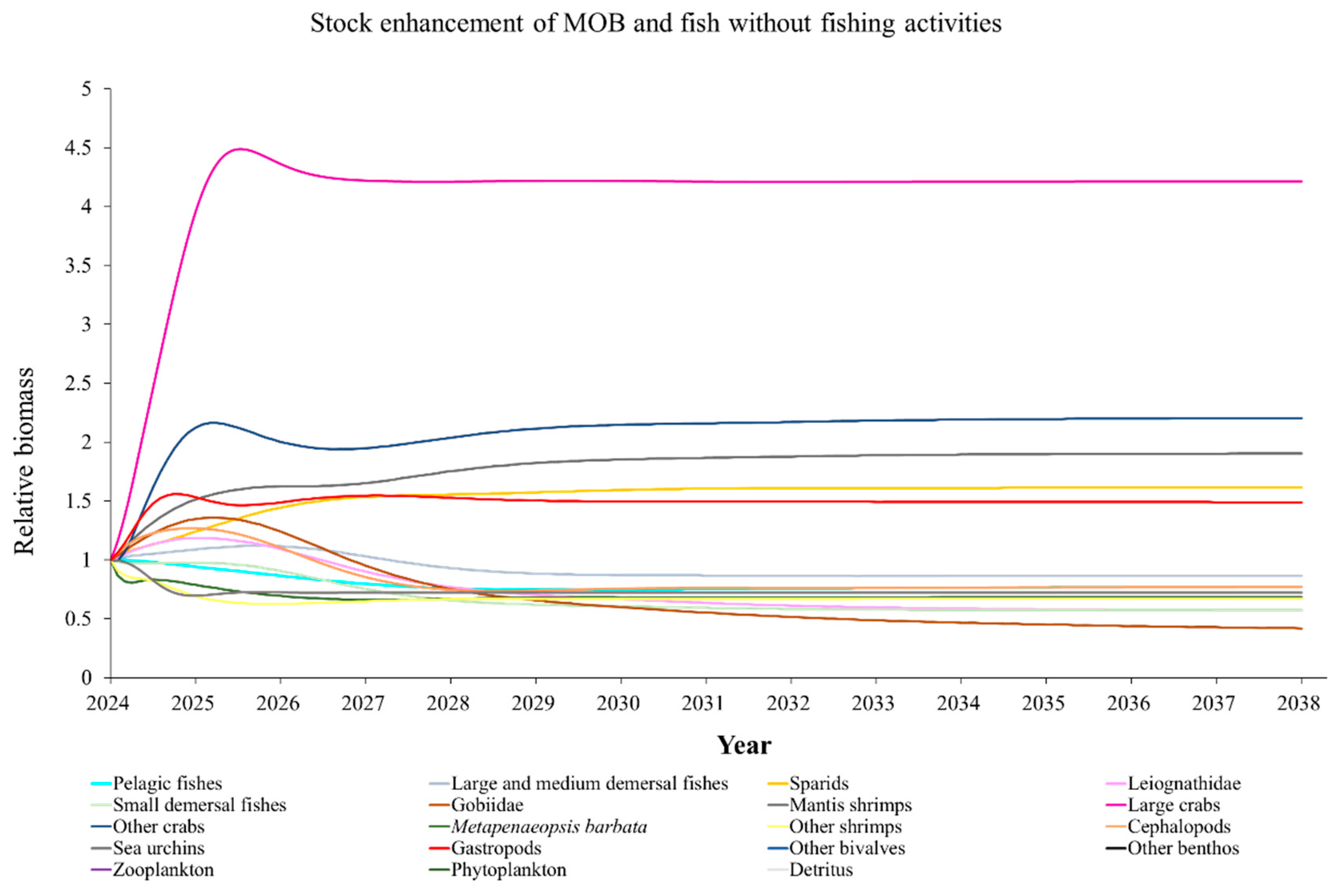
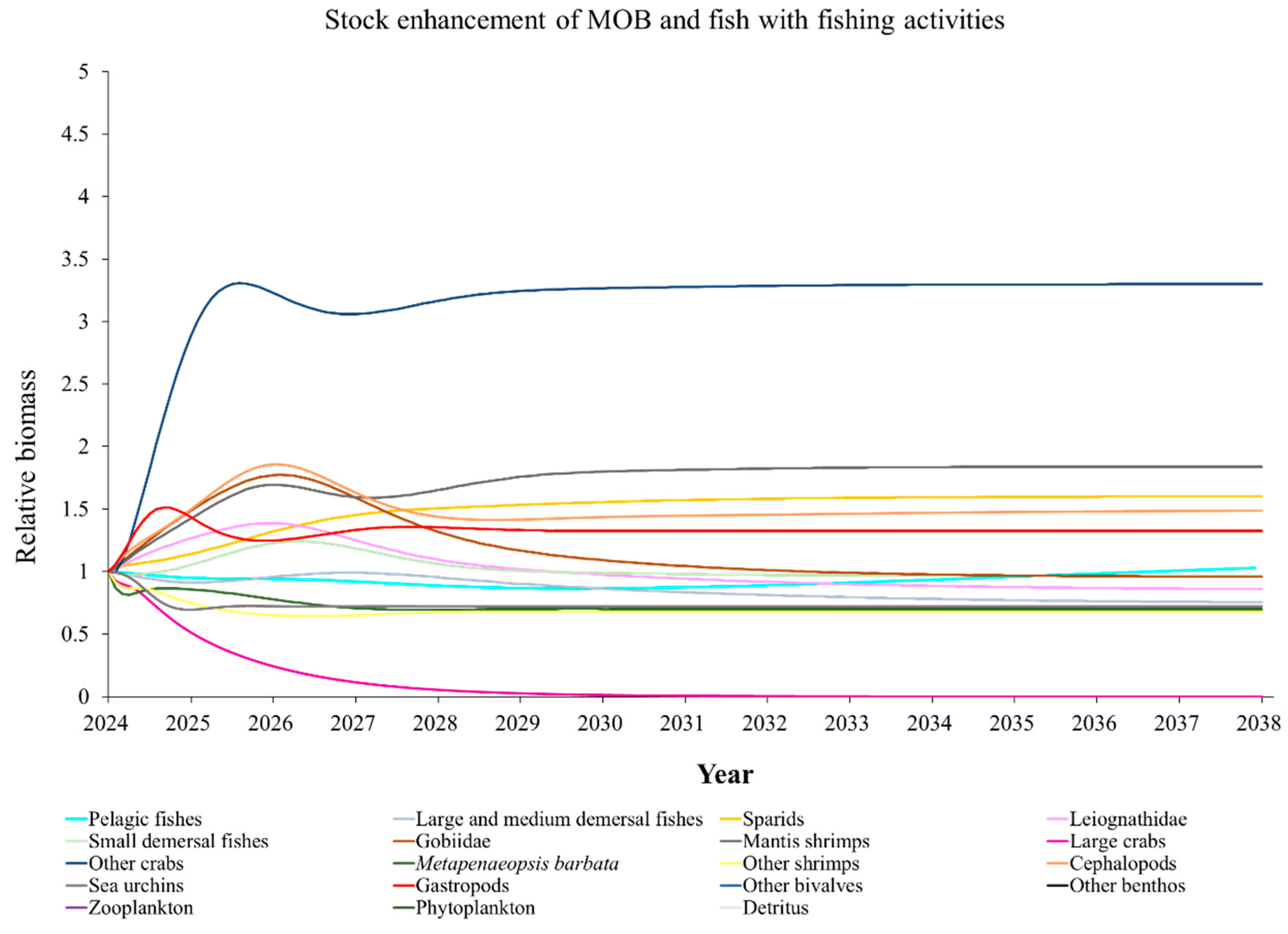
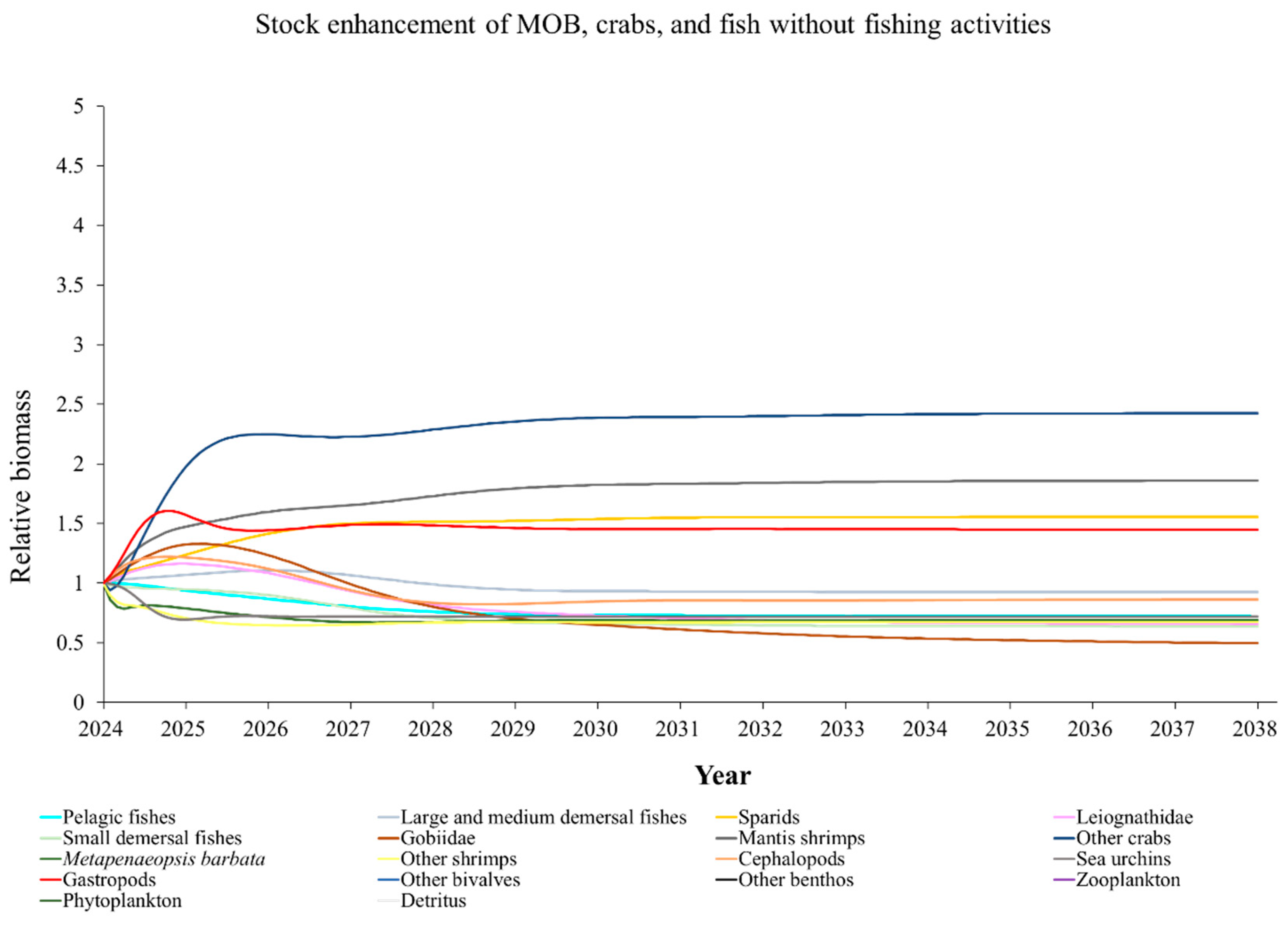
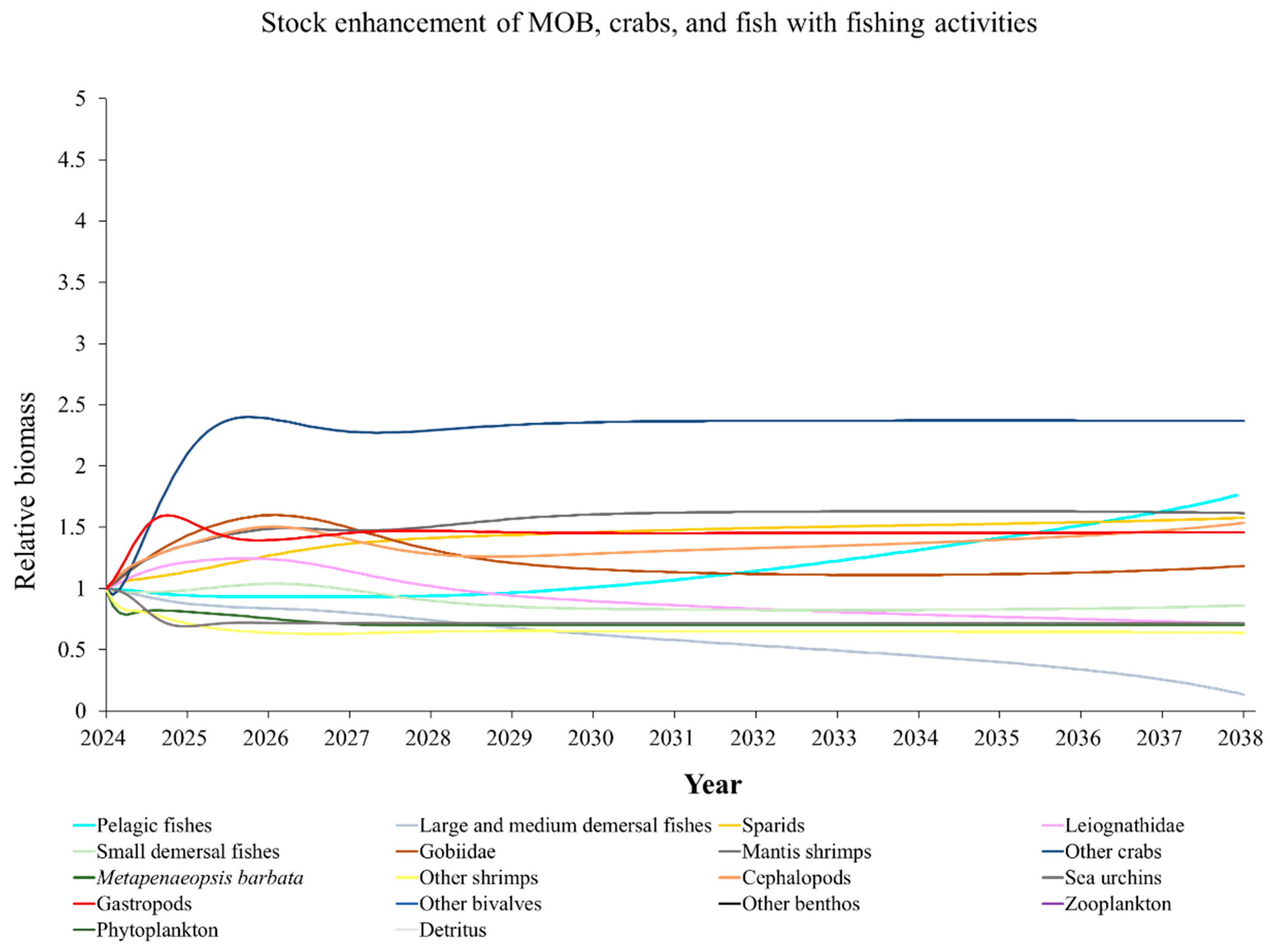
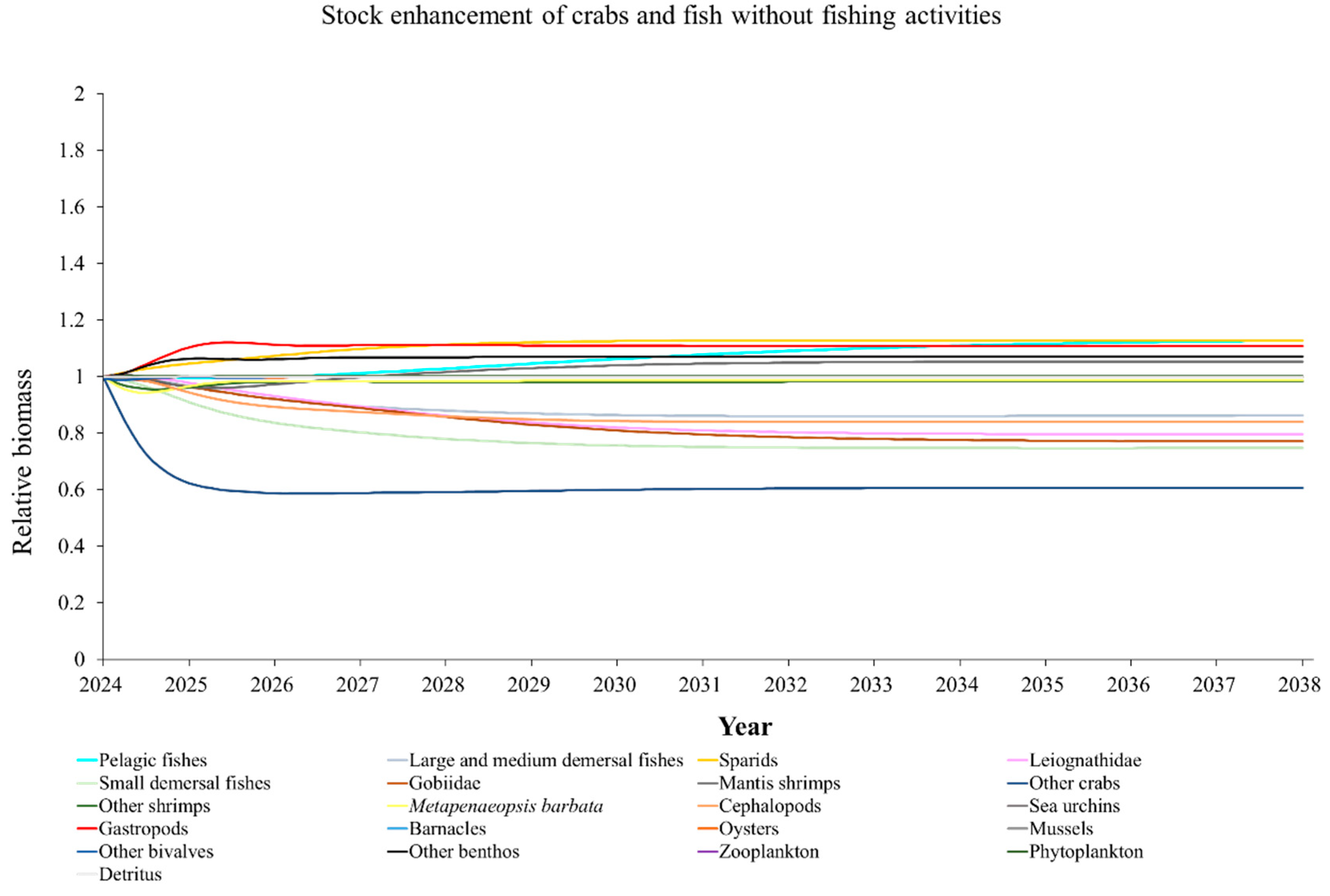
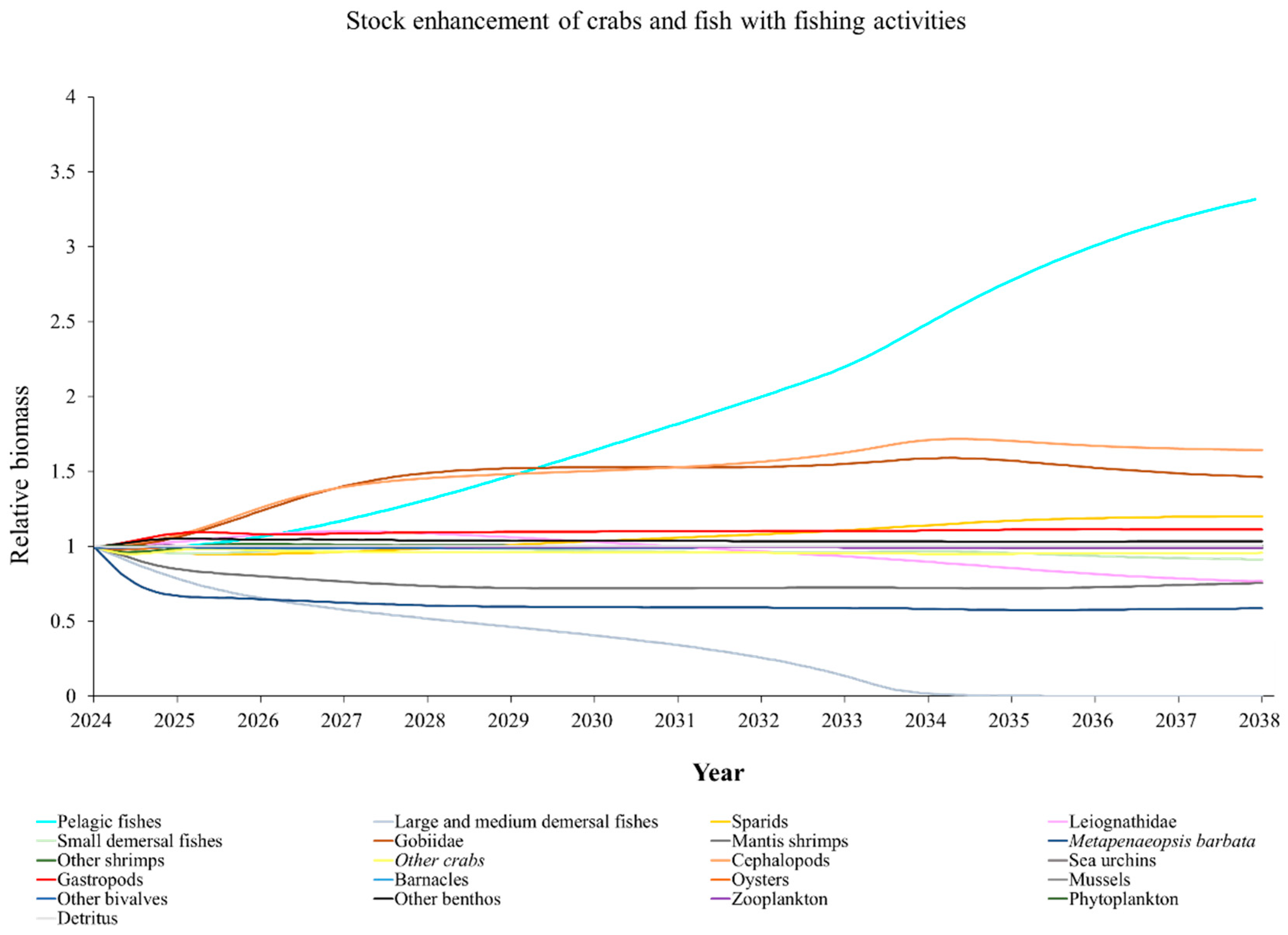
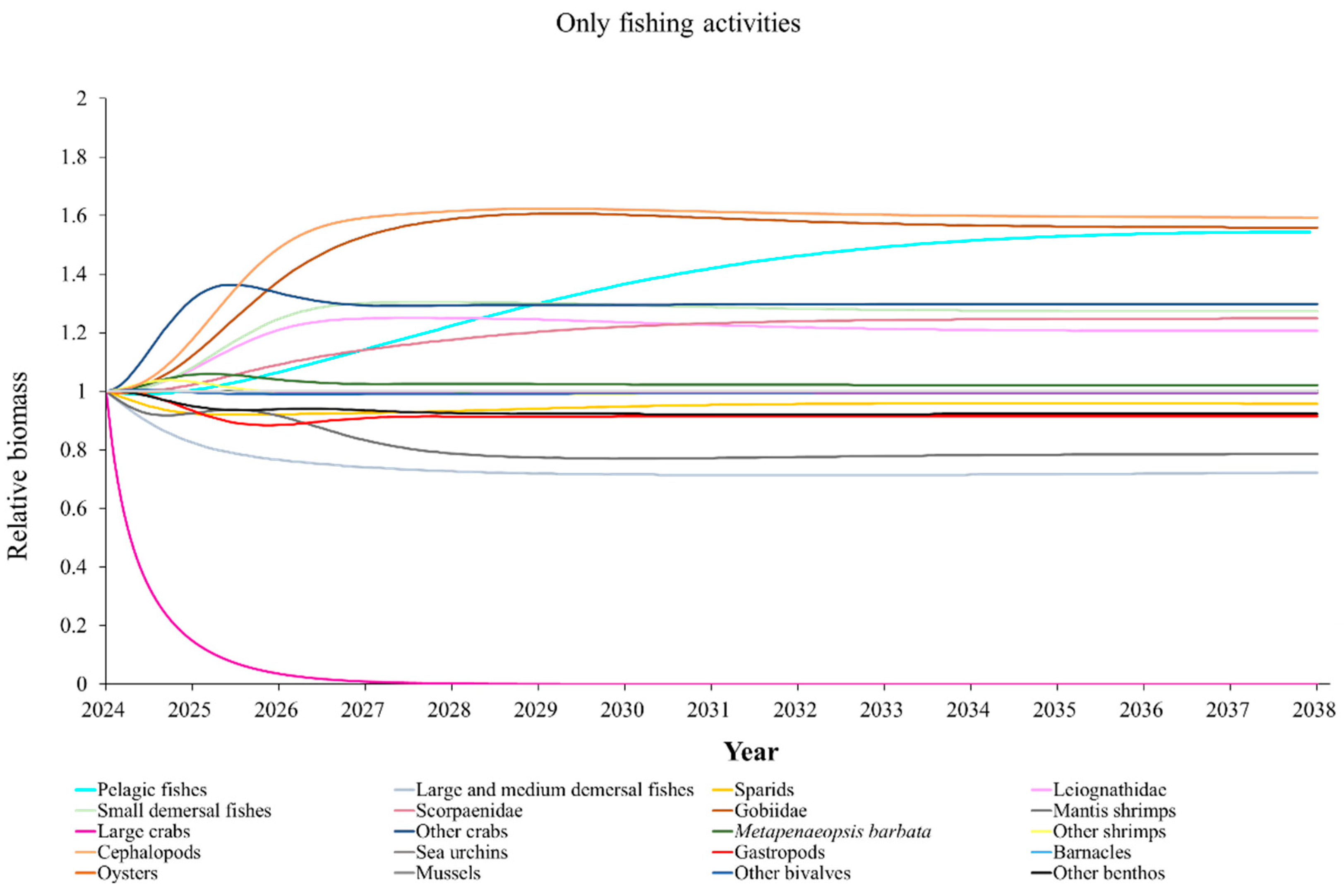
3.8. Ecosystem Dynamics Under Different Stock Enhancement Strategies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Introduction of Ecopath Model
Appendix B. Introduction of the Ecosim model
Appendix C. Introduction of Ecological Network Analysis Indicators
Appendix D. Data Sources for the Ecopath Models of the Marine Ranching and Control Ecosystems
Appendix E. Tables
| Numbers | Functional groups | Maine species |
|---|---|---|
| 1 | Pelagic fishes | Konosirus punctatus, Thryssa dussumieri,Trachinotus ovatus |
| 2 | Large and medium demersal fishes | Sillago sihama, Alepes djedaba, Scatophagus argus, Takifugu alboplumbeus, Saurida elongate, Lagocephalus spadiceus, Ilisha melastoma, Ilisha elongate, Planiliza affinis |
| 3 | Sparids | Plectorhinchus lineatus, Acanthopagrus latus, Evynnis cardinalis, Jaydia lineata |
| 4 | Leiognathidae | Equulites rivulatus, Leiognathus brevirostris, Leiognathus berbis |
| 5 | Small demersal fishes | Pennahia anea, Psenopsis anomala, Callionymus curvicornis, Solea ovata, Johnius belangerii, Johnius fasciatus, Osteomugil strongylocephalus, Sardinella albella, Pennahia macrocephalus |
| 6 | Scorpaenidae | Vespicula trachinoides, Sebastiscus marmoratus |
| 7 | Gobiidae | Cryptocentrus russus, Parachaeturichthys polynema, Amoya caninus, Tridentiger obscurus, Myersina filifer |
| 8 | Mantis shrimps | Oratosquilla oratoria |
| 9 | Large crabs | Charybdis japonica, Portunus trituberculatus, Portunus pelagicus |
| 10 | Other crabs | Pilumnopeus eucratoides, Charybdis helleri, Parthenope Validus, Charybdis acuta, Thalamita sima, Dorippe facchino, Halimede ochtodes |
| 11 | Metapenaeopsis barbata | M. barbata |
| 12 | Other shrimps | Alpheus hoplocheles, Marsupenaeus japonicus, Trachypenaeus curvirostris, Metapenaeus intermedius, Parapenaeopsis hungerfordi Alcock |
| 13 | Cephalopods | Loliolus japonica |
| 14 | Sea urchins | Anthocidaris crassispina, Hemicentrotus pulcherrimus |
| 15 | Gastropods | Patelloida pygmaea, Nassarius semiplicatus, Turritella terebra bacillum, Cellana toreuma, Murex trapa |
| 16 | Barnacle | Amphibalanus reticulatus |
| 17 | Oysters | Crassostrea gigas, Ostrea denselamellosa |
| 18 | Mussels | Perna viridis |
| 19 | Other bivalves | Dosinia aspera, Timoclea scabra, Vepricadium coronatum, Lucina scarlatoi, Clausinella isabelline, Ruditapes variegatus |
| 21 | Other benthos | Anthopleura xanthogrammica |
| 22 | Zooplankton | Paracalanus parvus, Parvocalanus carssirostris, Oithona nana, Corycaeus dahli, Corycaeus affinis |
| 23 | Phytoplankton | Chaetoceros lorenzianus, Eucampia cornuta, Eucampia zoodiacus, Stephanopyxis palmeriana, Chaetoceros constrictus |
| 24 | Detritus | Detritus in water, detritus in sediment |
| Numbers | Functional groups | Maine species |
|---|---|---|
| 1 | Pelagic fishes | Konosirus punctatus |
| 2 | Large and medium demersal fishes | Sillago sihama, Scatophagus argus, Trichiurus japonicus, Nematalosa japonica, Atule mate, Lagocephalus spadiceus, Ilisha elongata |
| 3 | Sparids | Lutjanus erythropterus, Evynnis cardinalis |
| 4 | Leiognathidae | Leiognathus brevirostris, Equulites rivulatus |
| 5 | Small demersal fishes | Pennahia anea, Psenopsis anomala, Callionymus curvicornis, Johnius belangerii, Johnius fasciatus, Osteomugil strongylocephalus |
| 6 | Scorpaenidae | Sebastiscus marmoratus |
| 7 | Gobiidae | Cryptocentrus russus, Trypauchen vagina, Odontamblyopus lacepedii, Parachaeturichthys polynema, Amoya caninus, Myersina filifer |
| 8 | Mantis shrimps | Oratosquilla oratoria |
| 9 | Large crabs | Portunus pelagicus |
| 10 | Other crabs | Charybdis hellerii, Charybdis acuta, Thalamita sima, Pilumnopeus eucratoides |
| 11 | Metapenaeopsis barbata | M. barbata |
| 12 | Other shrimps | Marsupenaeus japonicus, Trachypenaeus curvirostris |
| 13 | Cephalopods | Loliolus japonica |
| 14 | Gastropods | Architectonica perspectiva |
| 15 | Bivalves | Vepricadium coronatum, Trapezium sublaevigatum, Scapharca anomala |
| 16 | Other benthos | |
| 17 | Zooplankton | Paracalanus parvus, Parvocalanus carssirostris, Paracalanus nanus, Corycaeus dahli, Oithona attenuata |
| 18 | Phytoplankton | Chaetoceros constrictus, Thalassionema nitzschioides, Odontella sinensis, Bacteriastrum furcatum Shadbolt, Thalassionema nitzschioides |
| 19 | Detritus | Detritus in water, detritus in sediment |
| Functional groups | B | PB | QB | Diets |
|---|---|---|---|---|
| Pelagic fishes | By trawl nets in non-reef area, SCUBA videos in reef area. Trawl nets near reef were also conducted for correction of video data. | Estimated according to empirical formula [121] | Estimated according to empirical formula [125] | [127,128] |
| Large and medium demersal fishes | Same as pelagic fishes | Estimated according to empirical formula [121] | Estimated according to empirical formula [125] | [129,130,131,132] |
| Sparids | Same as pelagic fishes | Estimated according to empirical formula [121] | Estimated according to empirical formula [125] | [133,134] |
| Leiognathidae | Same as pelagic fishes | Estimated according to empirical formula [121] | Estimated according to empirical formula [125] | [135] |
| Small demersal fishes | Same as pelagic fishes | Estimated according to empirical formula [121] | Estimated according to empirical formula [125] | [16,116,136,137] |
| Scorpaenidae | Same as pelagic fishes | Estimated according to empirical formula [121] | Estimated according to empirical formula [125] | [138] |
| Gobiidae | Same as pelagic fishes | Estimated according to empirical formula [121] | Estimated according to empirical formula [125] | [139,140,141] |
| Mantis shrimps | By trawl nets in non-reef area, cage nets in reef area [32]. Trawl nets and cage nets near reef were also conducted for correction of cage nets data. | Estimated according to empirical formula [122] | By measuring the R/B first [126], then the Q/B was calculated according to: Q/B = P/B + R/B + U/B. U = 0.2Q [58], U/B = 0.2Q/B. | [142] |
| Large crabs | Same as mantis shrimps | Estimated according to empirical formula [122] | Same as mantis shrimps | [143] |
| Other crabs | Same as mantis shrimps | Estimated according to Empirical formula [122] | Same as mantis shrimps | [144] |
| Metapenaeopsis barbata | Same as mantis shrimps | Estimated according to Empirical formula [122] | Same as mantis shrimps | [145] |
| Other shrimps | Same as mantis shrimps | Estimated according to Empirical formula [122] | Same as mantis shrimps | [146] |
| Cephalopods | Same as mantis shrimps | Estimated according to Empirical formula [122] | Same as mantis shrimps | [147] |
| Sea urchins | By SCUBA grasping with a 0.5 × 0.5 m quadrats | Estimated according to Empirical formula [122] | Same as mantis shrimps | [148] |
| Gastropods | Same as sea urchins | Estimated according to Empirical formula [122] | Same as mantis shrimps | [149] |
| Barnacle | By SCUBA grasping with a 0.5 × 0.5 m quadrats, samples were oysters were collected by using a small knife | Estimated according to Empirical formula [122] | Same as mantis shrimps | [150] |
| Oysters | Same as barnacle | Estimated according to Empirical formula [122] | Same as mantis shrimps | [32] |
| Mussels | Same as barnacle | Estimated according to Empirical formula [122] | Same as mantis shrimps | [32] |
| Other bivalves | By collecting the samples with a sediment sampler | Estimated according to Empirical formula [122] | Same as mantis shrimps | [151] |
| Other benthos | By collecting the samples with a sediment sampler | Estimated according to Empirical formula [122] | By measuring the R/B first [126], then the Q/B was calculated according to: Q/B = P/B + R/B + U/B. U = 0.35Q [58], U/B = 0.35Q/B. | [151] |
| Zooplankton | By vertical towing using plankton nets | [123] | Obtained from Duan et al. (2009) [123] | |
| Phytoplankton | Calculated according to Chl a [54] | [124] | ||
| Detritus | Estimated according to empirical formula [83] |
| Numbers | Prey \ predator | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pelagic fishes | 0.02 | ||||||||||||||||||||
| 2 | Large and medium demersal fishes | 0.05 | ||||||||||||||||||||
| 3 | Sparids | 0.01 | ||||||||||||||||||||
| 4 | Leiognathidae | |||||||||||||||||||||
| 5 | Small demersal fishes | 0.02 | 0.023 | 0.02 | 0.03 | 0.02 | ||||||||||||||||
| 6 | Scorpaenidae | 0.001 | ||||||||||||||||||||
| 7 | Gobiidae | 0.015 | 0.02 | 0.01 | 0.01 | |||||||||||||||||
| 8 | Mantis shrimps | 0.04 | 0.01 | |||||||||||||||||||
| 9 | Large crabs | 0.02 | 0.02 | 0.01 | ||||||||||||||||||
| 10 | Other crabs | 0.005 | 0.03 | 0.06 | 0.04 | 0.07 | 0.1 | 0.05 | 0.079 | 0.03 | 0.02 | 0.1 | ||||||||||
| 11 | Metapenaeopsis barbata | 0 | 0.081 | 0.05 | 0.05 | 0.05 | 0.05 | 0.03 | 0.02 | 0.02 | 0.1 | |||||||||||
| 12 | Other shrimps | 0.03 | 0.21 | 0.464 | 0.08 | 0.38 | 0.15 | 0.15 | 0.096 | 0.04 | 0.04 | 0.01 | 0.13 | 0.03 | ||||||||
| 13 | Cephalopods | 0.03 | 0.02 | 0.002 | 0.03 | |||||||||||||||||
| 14 | Sea urchins | 0.1 | ||||||||||||||||||||
| 15 | Gastropods | 0.05 | 0.053 | 0.05 | 0.05 | |||||||||||||||||
| 16 | Barnacle | 0.096 | 0 | 0.05 | ||||||||||||||||||
| 17 | Oysters | 0 | 0.170 | 0.140 | 0.1 | 0.04 | ||||||||||||||||
| 18 | Mussels | 0.09 | 0.11 | 0.17 | 0.17 | 0.16 | 0.544 | 0.561 | 0.1 | 0.04 | 0.04 | 0.07 | 0.03 | |||||||||
| 19 | Other bivalves | 0.28 | 0.13 | 0.31 | 0.27 | 0.215 | 0.013 | 0.014 | 0.05 | 0.07 | 0.17 | 0.05 | 0.03 | |||||||||
| 20 | Other benthos | 0.2 | 0.13 | 0.1 | ||||||||||||||||||
| 21 | Zooplankton | 0.648 | 0.1 | 0.055 | 0.22 | 0.15 | 0.1 | 0.16 | 0.02 | 0.13 | 0.13 | 0.31 | 0.1 | 0.05 | 0.02 | 0.02 | 0.02 | 0.02 | 0.2 | |||
| 22 | Phytoplankton | 0.317 | 0.14 | 0.1 | 0.35 | 0.65 | 0.637 | 0.6 | 0.66 | |||||||||||||
| 23 | Detritus | 0.1 | 0.1 | 0.1 | 0.184 | 0.66 | 0.66 | 0.8 | 0.58 | 0.63 | 0.33 | 0.343 | 0.38 | 0.67 | 0.34 | |||||||
| 24 | Import | 0.515 | 0.1 | 0.1 | 0 | 0.05 | 0.1 | |||||||||||||||
| 25 | Sum | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Numbers | Prey \ predator | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pelagic fishes | 0.008 | ||||||||||||||||
| 2 | Large and medium demersal fishes | 0.05 | ||||||||||||||||
| 3 | Sparids | |||||||||||||||||
| 4 | Leiognathidae | 0.04 | ||||||||||||||||
| 5 | Small demersal fishes | 0.02 | 0.063 | 0.03 | 0.02 | 0.02 | ||||||||||||
| 6 | Scorpaenidae | 0.001 | ||||||||||||||||
| 7 | Gobiidae | 0.015 | 0.092 | 0.06 | 0.01 | |||||||||||||
| 8 | Mantis shrimps | 0.04 | 0.01 | |||||||||||||||
| 9 | Large crabs | 0.02 | 0.02 | 0.01 | 0.08 | |||||||||||||
| 10 | Other crabs | 0.005 | 0.03 | 0.16 | 0.02 | 0.03 | 0.1 | 0.05 | 0.015 | 0.01 | 0.02 | 0.02 | ||||||
| 11 | Metapenaeopsis barbata | 0.17 | 0.02 | 0.02 | 0.1 | 0.02 | 0.03 | 0.0084 | 0.02 | 0.02 | ||||||||
| 12 | Other shrimps | 0.03 | 0.21 | 0.198 | 0.12 | 0.38 | 0.1 | 0.18 | 0.21 | 0.072 | 0.04 | 0.01 | 0.21 | |||||
| 13 | Cephalopods | 0.02 | 0.03 | 0.008 | 0.03 | |||||||||||||
| 14 | Gastropods | 0.047 | 0.05 | 0.05 | ||||||||||||||
| 15 | Bivalves | 0.096 | 0.36 | 0.3 | 0.44 | 0.44 | 0.375 | 0.7 | 0.71 | 0.1 | 0.11 | 0.21 | 0.365 | 0.01 | ||||
| 16 | Other benthos | 0.2 | 0.135 | 0.005 | ||||||||||||||
| 17 | Zooplankton | 0.648 | 0.1 | 0.03 | 0.24 | 0.15 | 0.1 | 0.26 | 0.02 | 0.18 | 0.13 | 0.31 | 0.05 | 0.01 | 0.067 | |||
| 18 | Phytoplankton | 0.317 | 0.14 | 0.6 | 0.66 | |||||||||||||
| 19 | Detritus | 0.1 | 0.1 | 0.1 | 0.21 | 0.66 | 0.71 | 0.58 | 0.39 | 0.923 | 0.34 | |||||||
| 20 | Import | 0.547 | 0.1 | 0.1 | 0.1 | |||||||||||||
| 21 | Sum | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1.0004 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Study area | Time | Ecosystem function | Food web structure | Ecosystem maturity | Data sources | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D/H | A/C | TE | CI | SOI | FCI | FML | TPP/TR | TPP/TB | TB/TST | ||||
| 1 | Tongoy Bay | 1992 | 0.396 | 6.420 | 0.320 | 0.090 | 1.500 | 3.340 | 3.500 | 26.600 | 0.016 | [28,30,32,52,95,101,110,116,137,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221] | |
| 2 | 2002 | 0.318 | 7.130 | 0.320 | 0.110 | 2.900 | 3.490 | 2.500 | 12.200 | 0.034 | |||
| 3 | 2012 | 0.339 | 9.410 | 0.310 | 0.090 | 2.200 | 3.340 | 2.400 | 16.000 | 0.026 | |||
| 4 | Pagasitikos Gulf | 2008 | 0.250 | 1.470 | 9.100 | 0.030 | |||||||
| 5 | The North and Central Gulf of California | 1980s | 1.652 | 0.347 | 22.200 | 0.133 | 0.320 | 6.240 | 2.500 | 16.500 | 0.022 | ||
| 6 | The southeastern Gulf of California | 1994–1997 | 0.322 | 0.290 | 14.500 | 0.200 | 0.200 | 5.200 | 2.344 | 1.947 | 0.039 | ||
| 7 | 2006–2007 | 0.233 | 0.340 | 7.500 | 0.100 | 0.100 | 4.700 | 12.192 | 13.699 | 0.034 | |||
| 8 | Bay of Seine and eastern part of the English Channel | 2007-2013 | 0.520 | 0.173 | 9.160 | 0.030 | |||||||
| 9 | Seine estuary | 1996-2002 | 4.500 | 0.180 | 8.520 | 2.820 | 2.590 | 48.680 | 0.007 | ||||
| 10 | Seine estuary | 1996-2002 | 5.200 | 0.190 | 18.940 | 4.010 | 1.220 | 8.990 | 0.022 | ||||
| 11 | Seine estuary | 1996-2002 | 6.800 | 0.160 | 3.650 | 2.630 | 1.090 | 6.950 | 0.009 | ||||
| 12 | Seine estuary | 1996-2002 | 9.100 | 0.190 | 13.860 | 3.600 | 1.560 | 12.020 | 0.020 | ||||
| 13 | Seine estuary | 1996-2002 | 7.400 | 0.180 | 11.230 | 3.260 | 1.930 | 16.680 | 0.016 | ||||
| 14 | Seine estuary | 1996-2002 | 9.900 | 0.160 | 20.650 | 4.330 | 1.110 | 9.150 | 0.020 | ||||
| 15 | Black Sea | 1960–1969 | 4.275 | 0.070 | 9.400 | 2.660 | 1.630 | 132.000 | 0.003 | ||||
| 16 | Black Sea | 1980–1987 | 4.800 | 0.120 | 4.600 | 2.480 | 2.270 | 90.960 | 0.004 | ||||
| 17 | Black Sea | 1988–1994 | 5.500 | 0.120 | 2.760 | 2.320 | 3.610 | 116.770 | 0.004 | ||||
| 18 | Black Sea | 1995–2000 | 3.675 | 0.120 | 15.010 | 2.940 | 1.160 | 89.850 | 0.004 | ||||
| 19 | North-westem Mediteranean sea | 1999-2003 | 14.300 | 0.190 | 9.120 | 2.750 | 4.890 | 32.000 | |||||
| 20 | Gulf of lions | 2000-2009 | 19.700 | 0.210 | 11.870 | 3.990 | 2.090 | 15.100 | |||||
| 21 | lower coninentalslope of Catalan sea | 2009 | 15.700 | 0.290 | 4.200 | ||||||||
| 22 | North-Central Adriatic Sea | 1990 | 10.000 | 0.190 | 14.700 | 5.410 | 2.730 | 8.820 | |||||
| 23 | North Aegean Sea | 2003–2006 | 17.400 | 0.180 | 14.600 | 3.630 | 2.990 | 16.210 | |||||
| 24 | Greek Ionian Sea | 1998-2006 | 13.100 | 0.360 | 14.330 | 5.850 | 1.830 | 23.250 | |||||
| 25 | Gulf of Gabes | 2000-2005 | 19.240 | 7.350 | 3.050 | 2.410 | 16.750 | ||||||
| 26 | Gulf of Cadiz | 2009 | 14.900 | 0.180 | 3.000 | 2.430 | 3.300 | 39.800 | |||||
| 27 | Jade Bay (German Wadden Sea) | 1935–1937 | 1.800 | 0.349 | 4.960 | 0.432 | 6.100 | 5.130 | |||||
| 28 | 1975–1977 | 1.300 | 0.389 | 2.920 | 0.289 | 4.260 | 3.700 | ||||||
| 29 | 2009 | 4.200 | 0.419 | 4.220 | 0.310 | 7.690 | 3.940 | ||||||
| 30 | Mejillones bay | 2005-2012 | 0.241 | 0.200 | 0.074 | 4.880 | 2.920 | 1.550 | 6.280 | 0.060 | |||
| 31 | Antofagasta bay | 2005-2012 | 0.285 | 0.180 | 0.069 | 6.020 | 2.770 | 1.400 | 7.190 | 0.050 | |||
| 32 | Nigerian coastal waters | 1985 | 12.780 | 0.284 | 0.273 | 2.238 | 9.230 | ||||||
| 33 | 2000 | 11.930 | 0.284 | 0.256 | 2.133 | 9.390 | |||||||
| 34 | Somme Bay | 1998 | 0.350 | 4.000 | 0.250 | 0.009 | 12.200 | 15.509 | 21.816 | 0.012 | |||
| 35 | Kuosheng Bay | 1998-2001 | 2.444 | 6.500 | 0.480 | 0.520 | 32.000 | 4.400 | 1.060 | 40.000 | 0.006 | ||
| 36 | Cadiz Gulf | 2009 | 0.283 | 14.900 | 0.250 | 0.180 | 3.000 | 2.430 | 3.300 | 39.800 | 0.010 | ||
| 37 | Estuary of Sirinhaém River in northeastern Brazil | 2013-2014 | 0.980 | 0.290 | 11.580 | 0.270 | 0.160 | 5.610 | 2.590 | 32.590 | 0.010 | ||
| 38 | Poonthura Estuary | 2016-2020 | 0.240 | 0.150 | 12.450 | 0.350 | 0.380 | 17.940 | 0.460 | 5.210 | 0.020 | ||
| 39 | Estuarine ecosystem around bight of Benin, Nigeria | 0.461 | 0.423 | 6.800 | 0.327 | 0.288 | 1.700 | 2.300 | 6.325 | 82.615 | 0.005 | ||
| 40 | South Catalan Sea | late 1970 | 1.004 | 0.417 | 11.500 | 0.220 | 4.980 | 2.400 | 6.840 | 33.960 | 0.010 | ||
| 41 | South Catalan Sea | mid 1990 | 0.670 | 0.358 | 12.200 | 0.220 | 5.770 | 2.560 | 4.820 | 26.740 | 0.010 | ||
| 42 | South Catalan Sea | early 2000 | 0.664 | 0.411 | 13.300 | 0.200 | 6.220 | 2.410 | 6.710 | 28.980 | 0.010 | ||
| 43 | Gulf of Maine | 1980 | 0.265 | 0.307 | 0.650 | 2.010 | 2.090 | 42.560 | 0.011 | ||||
| 44 | Gulf of Maine | 1990 | 0.265 | 0.290 | 3.630 | 2.310 | 1.760 | 18.940 | 0.023 | ||||
| 45 | A marine protected area on the coast of Sénégal | 2003 | 0.255 | 0.340 | 0.146 | 3.540 | 2.500 | 1.910 | 18.290 | 0.021 | |||
| 46 | 2006-2008 | 0.272 | 0.340 | 0.154 | 3.660 | 2.500 | 2.080 | 18.220 | 0.022 | ||||
| 47 | Venezuela Shelf Ecosystem | 1986-1989 | 0.399 | 6.600 | 0.135 | 2.200 | 4.050 | 27.000 | 0.023 | ||||
| 48 | Eastern Central Pacific Ocean | 1986-1989 | 2.000 | 2.400 | |||||||||
| 49 | Gulf of Mexico | 1986-1989 | 0.391 | 0.195 | 2.100 | 3.030 | 7.000 | 0.015 | |||||
| 50 | British Columbia Shelf | 1991-2007 | 0.401 | 0.140 | 2.030 | 21.100 | 0.180 | ||||||
| 51 | Northern Benguela Upwelling ecosystem | 1991-2007 | 48.500 | 4.220 | 3.500 | ||||||||
| 52 | Terminos Lagoon, Mexico | 1980-1988 | 7.000 | 10.000 | |||||||||
| 53 | Sandy Barrier Lagoon, Taiwan | 1997 | 10.800 | 3.380 | |||||||||
| 54 | Boca Paila Reef, Mexico | 1990-1998 | 0.860 | 13.400 | 1285.000 | 15.600 | |||||||
| 55 | Channel of São Sebastião ecosystems | 1990-1997 | 25.400 | 0.260 | 0.210 | 30.100 | 0.700 | 11.200 | 0.012 | ||||
| 56 | Inner shelf of São Sebastião ecosystems | 1990-1997 | 23.200 | 0.280 | 0.210 | 25.800 | 1.900 | 30.100 | 0.010 | ||||
| 57 | Bengal Bay | 2003 | 0.387 | 5.900 | 0.420 | 0.220 | 10.000 | 2.580 | 1.350 | 14.690 | 0.026 | ||
| 58 | Northern and Central Adriatic Sea | 1990s. | 1.680 | 27.000 | 10.000 | 0.190 | 14.700 | 3.340 | 2.730 | 8.800 | 0.030 | ||
| 59 | Prince Edward Islands marine ecosystem | 1960 | 0.300 | 11.100 | 0.204 | 0.220 | 1.560 | 30.380 | 0.012 | ||||
| 60 | Prince Edward Islands marine ecosystem | 1980 | 0.300 | 11.000 | 0.204 | 0.210 | 1.560 | 30.390 | 0.012 | ||||
| 61 | Prince Edward Islands marine ecosystem | 2000 | 0.300 | 11.000 | 0.204 | 0.200 | 1.560 | 30.440 | 0.012 | ||||
| 62 | Kerguelen lsland marine ecosystem | 2005 | 0.230 | 0.170 | 1.160 | 12.980 | 0.024 | ||||||
| 63 | South Georgia | 2012 | 0.190 | 0.410 | 0.890 | 6.820 | 0.031 | ||||||
| 64 | South Shetlands | 2003 | 0.250 | 0.160 | 2.750 | 53.090 | 0.008 | ||||||
| 65 | Falklands marine ecosystem | 2005 | 0.180 | 0.280 | 12.310 | 83.950 | 0.006 | ||||||
| 66 | Antarctic Peninsula | 2005 | 0.270 | 0.160 | 5.140 | 9.460 | 0.048 | ||||||
| 67 | 2008 | 0.200 | 0.150 | 10.350 | 11.480 | 0.037 | |||||||
| 68 | 2012 | 0.200 | 0.180 | 1.580 | 16.610 | 0.021 | |||||||
| 69 | Southern Plateau, New Zealand | 1989-1996 | 0.160 | 0.290 | 1.490 | 48.560 | 0.006 | ||||||
| 70 | Jurien Bay, Western Australia | 2005-2006 | 9.600 | 0.160 | 0.250 | 1.100 | 2.100 | 0.080 | |||||
| 71 | Eritrean Red Sea | 1997-2005 | 8.600 | 0.460 | 0.210 | 10.760 | 3.640 | 1.100 | 11.950 | 0.023 | |||
| 72 | Subtidal area in Tongoy Bay, Chile | 1971-2001 | 11.500 | 0.200 | 0.140 | 2.610 | 2.400 | 2.700 | 12.220 | 0.034 | |||
| 73 | Western Scotland coast ecosystem | 1997-2003 | 0.290 | 0.180 | 2.540 | 2.060 | 4.510 | 30.610 | 0.013 | ||||
| 74 | Rocky coastal ecosystem Bahia Tortugas, Mexico | 2006-2008 | 0.200 | 0.230 | 0.230 | 1.050 | 1.340 | ||||||
| 75 | Sublittoral community of the Bay of Calvi, Corsica | 1983-1998 | 11.300 | 0.340 | 21.690 | 4.260 | 0.800 | 1.500 | 0.095 | ||||
| 76 | Eastern Bering Sea ecosystem | 1950s | 0.325 | 0.290 | 0.183 | 13.200 | 3.470 | 0.940 | 5.850 | 0.046 | |||
| 77 | 1980s | 0.309 | 0.300 | 0.157 | 11.100 | 3.510 | 0.780 | 4.940 | 0.045 | ||||
| 78 | West Coast of Sabah, Malaysia | 1972 | 0.270 | 0.220 | 2.070 | 19.620 | 0.020 | ||||||
| 79 | West Coast of Sarawak, Malaysia | 1972 | 0.270 | 0.220 | 2.080 | 19.370 | 0.020 | ||||||
| 80 | San Pedro Bay, Leyte, Philippins | 0.450 | 0.290 | 1.390 | 46.810 | 0.008 | |||||||
| 81 | Karnataka Arabian Sea | 1999-2001 | 0.329 | 13.400 | 0.382 | 0.299 | 6.030 | 2.810 | 1.283 | 29.900 | 0.012 | ||
| 82 | northern Benguela upwelling system, Namibia | 1990-1995 | 0.485 | 0.194 | 0.252 | 4.220 | 3.500 | ||||||
| 83 | Tenerife and La Gomera islands marine ecosystem | 2016 | 0.268 | 18.930 | 0.190 | 0.280 | 14.440 | 3.490 | 1.980 | 7.790 | 0.040 | ||
| 84 | Pearl River Delta coastal sea ecosystem | 1997-1999 | 0.237 | 0.327 | 2.867 | 18.134 | 0.017 | ||||||
| 85 | Wangjiadao Islands marine ecosystem | 2019 | 0.633 | 49.100 | 0.240 | 0.180 | 13.890 | 3.550 | 1.650 | ||||
| 86 | Gulf of Ulloa | 1980-2006 | 0.650 | 46.000 | 0.200 | 0.150 | 0.160 | 33.000 | |||||
| 87 | Isla del Coco, Costa Rica, Eastern Tropical Pacific | 2015 | 1.383 | 2.320 | 0.170 | 0.400 | 6.500 | 0.248 | 0.380 | ||||
| 88 | Northern Hangzhou Bay | 2006-2007 | 0.310 | 8.900 | 0.310 | 0.350 | 25.000 | 2.170 | 2.560 | 69.250 | 0.005 | ||
| 89 | Beibu Gulf | 1959-1961 | 0.750 | 0.579 | 7.100 | 0.316 | 0.186 | 1.860 | 1.755 | 1.013 | 28.920 | 0.062 | |
| 90 | 1990s | 0.460 | 0.476 | 9.400 | 0.310 | 0.171 | 0.840 | 1.206 | 2.184 | 54.355 | 0.010 | ||
| 91 | 1997-1999 | 12.200 | 0.333 | 0.319 | 0.840 | 1.206 | 3.182 | 24.547 | 0.018 | ||||
| 92 | Bohai Sea | 1982 | 12.300 | 0.350 | 9.745 | 127.493 | 0.004 | ||||||
| 93 | 1992-1993 | 16.200 | 0.341 | 8.400 | 86.043 | 0.006 | |||||||
| 94 | 2014-2015 | 5.100 | 0.330 | 0.140 | 5.380 | 99.830 | 0.005 | ||||||
| 95 | 2016 | 0.367 | 0.626 | 11.350 | 0.341 | 0.276 | 0.892 | 2.091 | 11.713 | 168.789 | 0.003 | ||
| 96 | Xiangyun Bay | 2019-2020 | 2.036 | 0.268 | 9.160 | 0.240 | 0.161 | 19.810 | 4.071 | 0.748 | 4.257 | 0.038 | |
| 97 | 2019-2020 | 1.240 | 0.319 | 7.570 | 0.247 | 0.130 | 11.810 | 2.883 | 2.657 | 30.734 | |||
| 98 | Northern South China Sea | 1989-1992 | 17.500 | 4.537 | 2.352 | 6.000 | 0.060 | ||||||
| 99 | 1997-2000 | 8.380 | 2.790 | 2.818 | 40.252 | 0.010 | |||||||
| 100 | 2000-2004 | 2.630 | 2.302 | 8.676 | 60.839 | 0.008 | |||||||
| 101 | 2007-2008 | 11.500 | 0.290 | 0.239 | 4.380 | 2.476 | 2.596 | 25.000 | 0.016 | ||||
| 102 | 2015-2016 | 21.940 | 0.313 | 0.325 | 13.680 | 3.775 | 1.005 | 32.190 | 0.008 | ||||
| 103 | Southern East China Sea | 1999-2002 | 0.637 | 12.000 | 0.330 | 0.213 | 4.100 | 2.398 | 3.060 | ||||
| 104 | East China Sea | 1997-2000 | 0.507 | 14.600 | 0.190 | 0.201 | 0.180 | 1.903 | 3.383 | 43.458 | |||
| 105 | South Yellow Sea | 2000-2001 | 0.583 | 0.248 | 8.100 | 0.360 | 0.210 | 9.830 | 1.430 | 41.270 | 0.028 | ||
| 106 | South-west Yellow Sea | 2006-2009 | 0.618 | 13.220 | 0.280 | 0.217 | 3.983 | 2.444 | 2.541 | 50.362 | 0.008 | ||
| 107 | Yangtze River Estuary | 1985-1986 | 0.723 | 12.400 | 0.471 | 0.103 | 9.350 | 2.778 | 1.724 | 31.483 | 0.011 | ||
| 108 | 2000 | 9.400 | 0.449 | 0.256 | 2.215 | 5.293 | 79.021 | 0.006 | |||||
| 109 | 2006 | 9.900 | 0.414 | 0.313 | 0.060 | 2.595 | 1.815 | 41.672 | 0.009 | ||||
| 110 | 2004 | 0.685 | 14.700 | 0.539 | 0.069 | 4.200 | 2.461 | 2.527 | 50.350 | 0.008 | |||
| 111 | 2012 | 0.544 | 9.400 | 0.371 | 0.196 | 5.990 | 2.500 | 2.095 | 67.525 | 0.006 | |||
| 112 | 2016-2017 | 0.073 | 9.300 | 0.345 | 0.321 | 1.245 | 53.402 | 0.007 | |||||
| 113 | 2020 | 0.451 | 9.850 | 0.388 | 0.234 | 3.200 | 31.910 | 0.010 | |||||
| 114 | Haizhou Bay | 2003 | 13.800 | 0.270 | 0.210 | 0.030 | 2.220 | 4.500 | 40.339 | 0.012 | |||
| 115 | 2013 | 7.900 | 0.415 | 0.174 | 0.114 | 2.301 | 1.331 | 44.986 | 0.009 | ||||
| 116 | 2013 | 0.647 | 5.620 | 3.093 | 4.720 | 92.404 | 0.005 | ||||||
| 117 | 2015 | 0.488 | 5.660 | 0.184 | 1.299 | 44.000 | 0.007 | ||||||
| 118 | 2018 | 12.630 | 0.429 | 0.204 | 1.392 | 7.069 | 56.866 | 0.017 | |||||
| 119 | Daya Bay | 2010-2011 | 0.525 | 0.363 | 10.900 | 0.249 | 0.138 | 2.170 | 2.210 | 3.500 | 82.500 | 0.005 | |
| 120 | Laizhou Bay | 2009-2010 | 6.200 | 0.290 | 0.170 | 0.070 | 1.530 | 24.540 | 0.014 | ||||
| 121 | Jiaozhou Bay | 2011 | 0.341 | 14.400 | 0.310 | 0.160 | 2.470 | 2.300 | 3.180 | 30.040 | 0.010 | ||
| 122 | 2015-2016 | 16.350 | 0.248 | 0.116 | 4.269 | 2.436 | 2.518 | 32.873 | 0.012 | ||||
| 123 | Yellow River Estuary | 2012-2013 | 1.163 | 9.700 | 0.300 | 0.150 | 6.160 | 2.470 | 33.300 | 0.012 | |||
| 124 | 2013-2014 | 5.400 | 0.380 | 0.120 | 2.800 | 3.450 | 38.910 | 0.011 | |||||
| 125 | Artificial reef ecosystem near Yantai coast | 2019 | 10.560 | 0.300 | 0.200 | 1.930 | |||||||
| 126 | Artificial reef ecosystem near Li Island | 2014 | 11.700 | 0.320 | 0.140 | 5.460 | 2.690 | 1.820 | 6.600 | 0.060 | |||
| 127 | Artificial reef ecosystem in Laizhou Bay | 2010-2012 | 12.800 | 0.440 | 0.360 | 12.230 | 3.300 | 1.035 | 15.580 | 0.019 | |||
| 128 | Artificial reef ecosystem in Laoshan Bay | 2014-2016 | 10.800 | 0.290 | 0.330 | 20.950 | 4.000 | 1.130 | 13.610 | ||||
| 129 | Gouqi Island marine ecosystem, Shanghai | 2007-2008 | 12.700 | 0.330 | 0.220 | 2.950 | 1.250 | ||||||
| 130 | Artificial reef ecosystem on the west of Furong Island | 2019-2020 | 0.620 | 0.320 | 8.920 | 0.160 | 0.120 | 22.670 | 5.170 | 1.010 | 9.620 | 0.020 | |
| 131 | Artificial reef ecosystem in xiangyun Bay | 2017-2018 | 0.630 | 0.249 | 6.940 | 0.240 | 0.150 | 20.510 | 4.310 | 0.700 | 4.220 | 0.034 | |
| 132 | Oyster-macroalgae reef ecosystem in xiangyun Bay | 2017-2018 | 0.610 | 0.266 | 9.120 | 0.240 | 0.160 | 19.710 | 4.070 | 0.750 | 4.257 | 0.038 | |
| 133 | Artificial reef ecosystem near Dachen island | 2019-2020 | 0.570 | 0.278 | 12.460 | 0.240 | 0.270 | 19.810 | 4.000 | 1.300 | 32.160 | 0.008 | |
| 134 | Artificial reef ecosystem near Wuzhizhou island | 2019-2020 | 0.390 | 0.343 | 12.070 | 0.200 | 0.240 | 16.860 | 5.200 | 2.020 | 33.740 | 0.011 | |
| 135 | Galapagos subtidal rocky reef ecosystem | 1997-2003 | 0.250 | 0.160 | 0.480 | 5.060 | 0.030 | ||||||
| 136 | Qilianyu Islands coral reef ecosystem | 2019 | 0.552 | 26.300 | 0.330 | 0.210 | 3.640 | 2.470 | 0.277 | 3.790 | |||
| 137 | Nanwan Bay coral reef ecosystem | 2001-2003 | 1.400 | 9.900 | 3.500 | 4.400 | |||||||
| 138 | Tampalam Reefs ecosystem, Mexico | 1990-1998 | 1.000 | 13.500 | 1.240 | 14.080 | |||||||
| 139 | Mahahual Reefs ecosystem, Mexico | 1990-1998 | 1.000 | 15.400 | 1.270 | 12.450 | |||||||
Appendix F. Figures
References
- Kang, X.; Zhang, H. Research progress on the evaluation of offshore marine ecosystem service functions and their values. Ocean Development and Management. 2010, 27(05), 60–64. (in Chinese). [Google Scholar]
- Qiu, W. Influence of international non-governmental organizations on the rule of the international law on marine environmental protection: taking an example of IUCN. Master thesis, Wuhan University, Wuhan 2019. (in Chinese). [Google Scholar]
- Field, J.; Hempel, G.; Summerhayes, C.; Scientific Committee on Oceanic Research. International Council of Scientific Unions; Scientific Committee on Problems of the Environment. International Council of Scientific Unions Oceans 2020: Science, Trends, and the Challenge of Sustainability. Isl. Press. 2002. [Google Scholar]
- Sun, X.X.; Yu, R.C.; Hu, Z.Y. Ecological security of coastal ocean and future marine ecosystem management strategies. Bulletin of Chinese Academy of Sciences. 2016, 31(12), 1293–1301, (in Chinese with English abstract). [Google Scholar]
- Yang, Z.Y.; Zhang, D. The high-quality development of Chinese fisheries from the perspective of the all-encompassing approach to food: current situation, problems and improvement strategies. Chinese Fisheries Economics 2023, 41(06), 1–13, (in Chinese with English abstract). [Google Scholar]
- Lin, C.; Yang, H.; Chen, Y.; Jin, X.; Chen, B.; Li, W.; Ren, Z.; Leng, S.; Ding, D. ; Construction and Development of Modern Marine Ranching Academic Review of the 230th Shuangqing Forum. Bulletin of National Natural Science Foundation of China 2021, 35(1), 143–152, (in Chinese with English Abstract). [Google Scholar]
- CCSC. Outline of the 14th Five-Year Plan (2021-2025) for National Economic and Social Development and Long-Range Objectives for 2035. Central Committee of the Communist Party of China & State Council 2021. (in Chinese with English Abstract).
- Chen, Z.; Qiu, Y. Assessment of the food-web structure, energy flows, and system attribute of northern South China Sea ecosystem. Acta Ecologica Sinica. 2020, 30(18), 4855–4865. [Google Scholar]
- Wang, T.; Yin, Z. Exploring Xi Jinping’s View of Marine Ecological Civilization from the Perspective of System Thinking. Modern Communication. 2023, 11, 9–16, (in Chinese with English abstract). [Google Scholar]
- Yang, H.; Huo, D.; Xu, Q. Views on modern marine ranching. Oceanologia et Limnologia Sinica 2016, 47(6), 1069–1074, (in Chinese with English abstract). [Google Scholar]
- Ru, X.; Deng, B.; Feng, Q.; Lin, C.; Zhang, L.; Yang, H. ; Comparison of Marine Ranching Constructions between China and Foreign Countries. Journal of Fisheries of China 2023, 47(11). (in Chinese with English Abstract). [Google Scholar]
- Loneragan, N.R.; Jenkins, G.I.; Taylor, M.D. Marine stock enhancement, restocking, and sea ranching in australia: future directions and a synthesis of two decades of research and development. Rev. Fish. Sci 2013, 21, 222–236. [Google Scholar] [CrossRef]
- Yang, H.; Ru, X. ; Zhang, L; Lin, C. Industrial convergence of marine ranching and offshore wind power: concept and prospect. Bulletin of the Chinese Academy of Sciences 2019a, 34(6), 700–707, (in Chinese with English abstract). [Google Scholar]
- Liu, S.; Zhou, X.; Zeng, C.; Frankstone, T.; Cao, L. Characterizing the Development of Sea Ranching in China. Rev. Rev. Fish Biol. Fish. 2022, 32. [Google Scholar] [CrossRef]
- Seitz, R.D.; Lipcius, R.N.; Knick, K.E.; Seebo, M.S.; Long, W.C.; Brylawski, B.J.; Smith, A. Stock enhancement and carrying capacity of blue crab nursery habitats in Chesapeake Bay. Rev. Fish. Sci. 2008, 16, 329–337. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Tu, Z.; Zhang, P.; Wang, Y.; Gao, T.; Wang, S. Current status and prospect of fisheries resource enhancement in Shandong Province. Chinese Fisheries Economics 2019a, 27(02), 51–58, (in Chinese with English abstract). [Google Scholar]
- Taylor, M.D.; Brennan, N.P.; Lorenzen, K.; Leber, K.M. Generalized predatory impact model: a numerical approach for assessing trophic limits to hatchery releases and controlling related ecological risks. Rev. Fish. Sci. 2013, 21, 341–353. [Google Scholar] [CrossRef]
- Kitada, S. Economic, Ecological and Genetic Impacts of Marine Stock Enhancement and Sea Ranching: A Systematic Review. Fish Fish. 2018, 19(3), 511–532. [Google Scholar] [CrossRef]
- Xu, X.; Tang, W.; Wang, Y. Releasing capacity of Portunus trituberculatus enhancement in Zhoushan fishing ground and Yangtze river estuary fishing ground and their adjacent waters. South China Fisheries Science 2019, 15(3), 126–132, (in Chinese with English abstract). [Google Scholar]
- Tang, Q. On the capacity and its research. Progress in Fishery Sciences. 1996, 02, 1–6, (in Chinese with English abstract). [Google Scholar]
- Yang, H.; Zhang, S.; Zhang, X.; Chen, P.; Tian, T.; Zhang, T. Strategic thinking on the construction of modern marine ranching in China. Journal of Fisheries of China 2019b, 43(4), 1255–1262, (in Chinese with English abstract). [Google Scholar]
- Zhang, Y.Z.; JIiang, R.J.; Liang, J. The evaluation on conservation effect of fishery resources in marine ranching of Ma'an Archipelago area Zhejiang Shengsi. Journal of Zhejiang Ocean University (Natural Science) 2020, 41(05), 466–472, (in Chinese with English abstract). [Google Scholar]
- Yang, C.; Wu, Z.; Liu, H.; Zhang, P.; Li, W.; Zeng, X.; Zhang, X. The fishing strategy of charybdis japonica and rapana venosa and the carrying capacity of Apostichopus japonucus in Zhuwang, Laizhou artificial reef ecosystem based on Ecopath model. Periodical of Ocean University of China 2016, 46(11), 168–177, (in Chinese with English abstract). [Google Scholar]
- Sun, L. Evaluation of artificial reef construction in Shandong province. Doctoral thesis, Ocean University of China, Qingdao 2011. (in Chinese). [Google Scholar]
- Xu, Q.; Zhang, S. ; Site selection evaluation of marine ranching in Zhoushan area based on AHP method. Journal of Shanghai Ocean University 2013, 22(1), 128–133, (in Chinese with English abstract). [Google Scholar]
- Zhao, X.; Sun, W.; Ren, G.; Ma, Y. Ecosystem health evaluation of Haizhou Bay marine ranching. Acta Laser Biology Sinica 2014, 23, 626–632, (in Chinese with English abstract). [Google Scholar]
- Wang, Y.; Li, Y.; Xiao, P.; Shen, S.; Cai, H.; Song, W. Evaluation of Social Effects of Artificial Reefs in the South Bay Islands by AHP. Ocean Dev. Manag. 2019, 36(02), 40–44. [Google Scholar]
- Zhang, R.; Zhang, Q.; Zhao, J.; Wu, Z.; Liu, H.; Shou, L.; Liao, Y.B.; Liu, Q.; Tang, Y.; Zeng, J. Using Ecopath Models to Explore Differences in Ecosystem Characteristics Between an Artificial Reef and a Nearby Natural Reef on the Coast of the North Yellow Sea, China. 2022. [CrossRef]
- Qin, M.; Wang, X.R.; Du, Y.W.; Wan, X.L. Influencing factors of spatial variation of national marine ranching in China. Ocean Coastal Manage. 2021, 199, 12. [Google Scholar] [CrossRef]
- Wu, Z.X.; Zhang, X.M.; Lozano-Montes, H.M.; Loneragan, N.R. Trophic flows, kelp culture and fisheries in the marine ecosystem of an artificial reef zone in the Yellow Sea. Estuar. Coast. Shelf Sci. 2016, 182, 86–97. [Google Scholar] [CrossRef]
- Harrison, S.R. ; M. Comparison of artificial and natural reef productivity in Nantucket Sound, MA, USA. Estuaries Coasts 2020, 43(8), 2092–2105. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Lin, C.G.; Liu, H.; Chen, M.Y.; Zhang, Y.L. Structural and functional improvements of coastal ecosystem based on artificial oyster reef construction in the Bohai Sea, China. Front. Mar. Sci. 2022b, 9, 829557. [Google Scholar] [CrossRef]
- Yuan, Y.; Feng, J.; Xian, W.W.; Zhang, H. Analysis of the ecosystem characteristics and ecological carrying capacity of the main commercial fish in the artificial reef ecosystem in Laizhou Bay using the Ecopath model. Sustainability. 2022a, 14(21), 18. [Google Scholar] [CrossRef]
- Li, Z.P.; Chen, Y.; Wang, G.; Mu, J.D.; Sun, Y.F.; Yu, H.L.; Xu, J.L.; Yan, Y.; Luo, S.Y.; Han, F.Q.; Feng, J.; Pan, Z. Ecological carrying capacity and carbon sequestration potential of bivalve shellfish in marine ranching: A case study in Bohai Bay, China. Front. Mar. Sci. 2023, 10, 1174253. [Google Scholar] [CrossRef]
- Wootton, J.T. Effects of disturbance on species diversity: a multitrophic perspective. The American Naturalist. 1998, 152(6), 803–825. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Ruiz, G.M.; Carlton, J.T.; Grosholz, E.D.; Hines, A.H. Global invasions of marine and estuarine habitats by non-indigenous species: Mechanisms, extent, and consequences. Am. Zool. 1997, 37(6), 621–632. [Google Scholar] [CrossRef]
- Yang, H.; Huo, D.; Xu, Q. Views on modern marine ranching. Oceanologia et Limnologia Sinica 2016, 47(6), 1069–1074, (in Chinese with English abstract). [Google Scholar]
- Zhang, S.; Zhou, X.; Wang, K.; Li, J.; Zhao, J.; Zhao, X.; Guo, Y.; Liu, S.; Cheng, X. Review of marine livestock ecological urbanization hypothesis and marine ranching construction key-technology against blue growth background. Journal of Fisheries of China 2019, 43(1), 81–96, (in Chinese with English abstract). [Google Scholar]
- Baird, D.; Ulanowicz, R.E. ; The seasonal dynamics of the Chesapeake Bay ecosystem. Ecological monographs. 1989, 59(4), 329–364. [Google Scholar] [CrossRef]
- Wulff, J.; Field, K.; Mann, H. Coastal and Estuarine Studies (COASTAL, volume 32). Springer–Verlag, New York. 1989.
- Ulanowicz, R.E. Growth and development: ecosystems phenomenology. Springer–Verlag, New York. 1986.
- Ulanowicz, R.E. Ecology: the ascendant perspective. Columbia University Press, New York. 1997.
- Ulanowicz, E, R. Quantitative methods for ecological network analysis. Computational Biology and Chemistry 2004, 28(5-6), 321–339. [CrossRef]
- Libralato, S. System Omnivory Index. Encycl. Encycl. Ecol. Second Ed. 2008, 3472–3477. [Google Scholar] [CrossRef]
- Biondi, E.; Casavecchia., S.; Pesaresi., S.; Zivkovic., L. Natura 2000 and the Pan-European Ecological Network: a new methodology for data integration[J]. Biodiversity & Conservation 2012, 21(7), 1741–1754. [Google Scholar] [CrossRef]
- Heymans, J.J.; Coll, M.; Libralato, S.; Morissette, L.; Christensen, V. Global patterns in ecological indicators of marine food webs: a modelling approach. PLoS One. 2014, 9(4), 21. [Google Scholar] [CrossRef]
- Safi, G.; Giebels, D.; Larissa Arroyo, N.; Heymans, J.J.; Preciado, I.; Raoux, A.; Schueckel, U.; Tecchio, S.; De Jonge, V.N.; Niquil, N. Vitamine ENA: A framework for the development of ecosystem-based indicators for decision makers. Ocean Coast. Manag. 2019, 174, 116–130. [Google Scholar] [CrossRef]
- De Jonge, V.; Kolkman, M.; Ruijgrok, E.; De Vries, M. The need for new paradigms in integrated socio-economic and ecological coastal policy making, Proceedings of 10th International Wadden Sea Symposium. Ministry of Agriculture, Nature Management and Fisheries. Department North 2003, 247–270. [Google Scholar]
- De Jonge, V.N.; Pinto, R.; Turner, R.K. ; Integrating ecological, economic and social aspects to generate useful management information under the EU Directives'‘ecosystem approach’. Ocean Coastal Management. 2012, 68, 169–188. [Google Scholar] [CrossRef]
- Borrett, S. R.; Freeze, M. A.; Salas, A. K. Equivalence of the realized input and output oriented indirect effects metrics in ecological network analysis[J]. Ecological Modelling 2011, 222. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Ding, L.; Zhang, M.; Xie, H.; Zhang, Q.; Li, S. A preliminary analysis of the ecosystem structure and function of the Yangtze Estuary based on Ecopath model. Journal of Environmental Engineering Technology 2022a, 12(2), 417–425, (in Chinese with English abstract). [Google Scholar]
- Yuan, Y.; Xian, W.; Zhang, H. A review of the ecological carrying capacity of fishery resources in China based on the Ecopath model. Marine Sciences. 2022b, 46(7), 105–119. [Google Scholar]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. Determination of chlorophylls and total carotenoids: spectrophotometric method - sciencedirect. Man. Chem. Biol. Man. Chem. Biol. Methods Seawater Anal. 1984, 101–104. [Google Scholar]
- Willis, T.J.; Babcock, R.C. A baited underwater video system for the determination of relative density of carnivorous reef fish. Marine and Freshwater research. 2000, 51(8), 755–763. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J.; Pauly, D. Ecopath with Ecosim: a user’s guide. Fisheries Centre, University of British Columbia, Vancouver. November 2005 edition, 2005, 154 p.
- Winberg, G.G. Rate of metabolism and food requirements of fishes. Belorussian State University, Minsk. Fisheries Research Board of Canada Translation Series 194 (1960). Biological Station, Nanaimo, BC. 1956.
- Yang, H.S. Monitoring and biocapacity evaluation of marine ranching ecosystem. Science Press, Beijing. 2018 (in Chinese with English abstract).
- Link, J.S. Adding rigor to ecological network models by evaluating a set of pre-balance diagnostics: A plea for PREBAL (vol 221, pg 1582, 2010). Ecol. Model. 2016, 337, 348–349. [Google Scholar] [CrossRef]
- Heymans, J.J.; Coll, M.; Link, J.S.; Mackinson, S.; Steenbeek, J.; Walters, C.; Christensen, V. Best practice in Ecopath with Ecosim food-web models for ecosystem-based management. Ecol. Model. 2016, 331, 173–184. [Google Scholar] [CrossRef]
- Link, J.S. Adding rigor to ecological network models by evaluating a set of pre-balance diagnostics: a plea for PREBAL. Ecol. Model. 2010, 221, 1580–1591. [Google Scholar] [CrossRef]
- Chea, R.; Guo, C.B.; Grenouillet, G.; Lek, S. Toward an ecological understanding of a flood-pulse system lake in a tropical ecosystem: Food web docstructure and ecosystem health. Ecol. Model. 2016, 323, 1–11. [Google Scholar] [CrossRef]
- Ulanowicz, R.E.; Goerner, S.J.; Lietaer, B.; Gomez, R. Quantifying sustainability: resilience, efficiency and the return of information theory. Ecol Complex 2009, 6, 27−36. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, Y.; Qi, Z. Evaluating the ecological state of Chinese lake baiyangdian (BYD) based on ecological network analysis. Ecol. Indic. 2021, 127, 107788. [Google Scholar] [CrossRef]
- Tobor-Kapłon, M., Holtkamp. Evaluation of information indices as indicators of envrionmental stress in terrestial soils. Ecol. Model. 2008, 80–90. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, Y.; Li, J.; Yang, H.; Yu, Y.; Shen, L.; Wu, J. Water quality analysis of the Middle Yangtze River based on fuzzy comprehensive evaluation. Freshwater Fisheries 2021, 51(2), 55–62, (in Chinese with English abstract). [Google Scholar]
- Wu, X.; Hu, F. Analysis of ecological carrying capacity using a fuzzy comprehensive evaluation method. Ecol. Indic. 2020, 113, 106243. [Google Scholar] [CrossRef]
- U.S. GLOBEC. Report on climate change and carrying capacity of the North Pacific ecosystem. U.S. Global Ocean Ecosystem Dynamics Rep. Univ. California, Berkeley. 1996.
- Jiang, W.M.; Gibbs, M.T. Predicting the carrying capacity of bivalve shellfish culture using a steady, linear food web model. Aquaculture 2005, 244(1-4), 171–185. [Google Scholar] [CrossRef]
- Cheung, W.L.; Watson, R.; Pitcher, T. Policy simulation of fisheries in the Hong Kong marine ecosystem. The use of ecosystem models to investigate multispecies management strategies for capture fisheries. The use of ecosystem models to investigate multispecies management strategies for capture fisheries. Fisheries Centre Research Reports. 2002, 10(2), 46–54. [Google Scholar]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: methods, capabilities and limitations. Ecol. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Harvey, C.J. Mediation functions in Ecopath with Ecosim: handle with care. Can. J. Fish. Aquat. 2014, 71, 1020–1029. [Google Scholar] [CrossRef]
- Sadchatheeswaran, S.; Branch, G.M.; Shannon, L.J.; Moloney, C.L.; Robinson, T.B. Modelling changes in trophic and structural impacts of alien ecosystem engineers on a rocky-shore island. Ecol. Model. 2020, 433, 109227. [Google Scholar] [CrossRef]
- Qiu, C.G.; Xu, S.L.; Qi, C.; Lin, S.Z. Studies on early growth and development in Sebastiscus marmoratus with artificial breeding technology. Journal of Ningbo University (NSEE). 2013, 26(4), 17–23, (in Chinese with English abstract). [Google Scholar]
- Yang, C.H.; Liu, Q.; Wang, Y.B. Assessment of the maximum sustainable yield of Portunus Trituberculatus in the northern areas of the East China Sea under the impact of stock enhancement. Oceanologia Et Limnologia Sinica 2022, 53(5), 1219–1224, (in Chinese with English abstract). [Google Scholar]
- Yang, H. Construction of marine ranching in china: reviews and prospects. J. Fish. China 2016, 40(7), 1133–1140, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Boaventura, D.; Moura, A.; Leitao, F.; Carvalho, S.; Cúrdia, J.; Pereira, P.; de Fonseca, L.C.; dos Santos, M.N.; Monteiro, C.C. Macrobenthic colonisation of artificial reefs on the southern coast of Portugal (Ancao, Algarve). Hydrobiologia. 2006, 555, 335–343. [Google Scholar] [CrossRef]
- Walker, S.J.; Schlacher, T.A.; Schlacher-Hoenlinger, M.A. Spatial heterogeneity of epibenthos on artificial reefs: fouling communities in the early stages of colonization on an East Australian shipwreck. Mar. Ecol.-Evol. Persp 2007, 28(4), 435–445. [Google Scholar] [CrossRef]
- Beck, M.; Brumbaugh, R.; Airoldi, L.; Carranza, A.; Coen, L.; Crawford, C.; Defeo, O.; Edgar, G.; Hancock, B.; Kay, M. Shellfish reefs at risk: a global analysis of problems and solutions. Arlington, VA. The Nature Conservancy 2009. [Google Scholar]
- Cresson, P.; Ruitton, S.; Harmelin-Vivien, M. . Artificial reefs do increase secondary biomass production: mechanisms evidenced by stable isotopes. Mar. Ecol.-Prog. Ser. 2014, 509, 15–26. [Google Scholar] [CrossRef]
- Grabowski, J.H.; Brumbaugh, R.D.; Conrad, R.F.; Keeler, A.G.; Opaluch, J.J.; Peterson, C.H.; Piehler, M.F.; Powers, S.P.; Smyth, A.R. Economic Valuation of Ecosystem Services Provided by Oyster Reefs. Bioscience. 2012, 62(10), 900–909. [Google Scholar] [CrossRef]
- Lima, J.S.; Atalah, J.; Sanchez-Jerez, P.; Zalmon, I.R. Evaluating the performance and management of artificial reefs using artificial reef multimetric index (ARMI). Ocean coastal management. 2020, 198, 105350. [Google Scholar] [CrossRef]
- Pauly, D.; Bartz, M.L.S. Improved Construction, Parametrization and Interpretation of Steady-State Ecosystem Models. In Proceedings of the Iclarm Conference. 1993.
- Lindeman, R.L. The trophic-dynamic aspect of ecology. Ecology 1942, 23, 399−418. [Google Scholar] [CrossRef]
- Connell, J.H.; Slatyer, R.O. Slatyer Mechanisms of Succession in Natural Communities and Their Role in Community Stability and Organization. Am. Nat. 1977, 1119–1144. [Google Scholar] [CrossRef]
- Walker, L.R.; Chapin, F.S. ; Interactions among processes controlling successional change. Oikos. 1987, 50, 131–135. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; White, P.S.; Courtney, S.P. The ecology of natural disturbance and patch dynamics. Science. 1985, 230, 434–435. [Google Scholar] [CrossRef]
- Mccann, K.; Hastings, A. Re-evaluating the omnivory–stability relationship in food webs. Proc. R. Soc. Proc. R. Soc. B Biol. Sci. 1997, 264. [Google Scholar] [CrossRef]
- Odum, E.P. Trends expected in stressed ecosystems. BioScience. 1985. [Google Scholar] [CrossRef]
- Scharler, U.M.; Baird, D. A Comparison of selected ecosystem attributes of three south african estuaries with different freshwater inflow regimes, using network analysis. J. Mar. Syst. 2005, 56, 283–308. [Google Scholar] [CrossRef]
- Jin, X. S.; Dou, S. Z.; Shan, X. J.; Wang, Z. Y.; Wang, R. J.; Bian, X. D. Hot spots of frontiers in the research of sustainable yield of Chinese inshore fishery. Prog. Prog. Fish. Sci. 2015, 36(1), 124–131. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres Jr, F. Fishing down marine food webs. Science. 1998, 279(5352), 860–863. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Pauly, D. Fisheries impacts on China's coastal ecosystems: Unmasking a pervasive 'fishing down' effect. PLoS One. 2017, 12(3), 15. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Pauly, D. Masking and unmasking fishing down effects: The Bohai Sea (China) as a case study. Ocean Coastal Manage. 2020, 184, 105033. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Qiu, Y.S.; Jia, X.P. Structure and function of Beibu Gulf ecosystem based on Ecopath model. J. Fish. Sci. China. 2008, 15, 460–468. [Google Scholar] [CrossRef]
- Bohnsack, J.A.; Sutherland, D.L. ; Artificial reef research: a review with recommendations for future priorities. Bulletin of marine science. 1985, 37(1), 11–39. [Google Scholar]
- Nakamura, Makoto. Evolution of artificial fishing reef concepts in Japan. Bulletin of marine. Science. 1985, 37(1), 271–278.
- Liang, Z.; Guo, Z.; Jiang, Z.; Zhu, L.; Sun, L. Construction concept and technology of the marine ranching mode of the whole life history of fishes. Journal of Fisheries of China 2020, 44(7), 1211–1222, (in Chinese with English abstract). [Google Scholar]
- Yang, H.; Ding, D. Marine ranching version 3.0: history, status and prospects. Bulletin of the Chinese Academy of Sciences 2022, 37(6), 832–839, (in Chinese with English abstract). [Google Scholar]
- Odum, E.P. The Strategy of Ecosystem Development. Science. 1969, 164, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, G.; Lobry, J.; Loc’H, F.L.; Bustamante, P.; Certain, G.; Delmas, D.; Dupuy, C.; Hily, C.; Labry, C.; Pape, O.L. Lower Trophic levels and detrital biomass control the bay of Biscay Continental Shelf food web: implications for ecosystem management. Prog. Oceanogr. 2011, 91, 561–575. [Google Scholar] [CrossRef]
- Rooney, N.; Mccann, K.S. Integrating food web diversity, structure and stability. Trends Ecol. Evol. 2012, 27, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, Q.; Hu, C.; Xu, H.; Wang, Y.; Yang, X.; Guo, H. Ecosystem stability of marine ranching and its response to disturbance: research status, issues,and suggestions. Journal of Fisheries of China. 2023, 47(11), 119509, (in Chinese with English abstract). [Google Scholar]
- Laikre, L.; Schwartz, M.K.; Waples, R.S.; Ryman, N. Compromising genetic diversity in the wild: unmonitored large-scale release of plants and animals. Trends in ecology evolution. 2010, 25(9), 520–529. [Google Scholar] [CrossRef]
- Lorenzen, K.; Beveridge, M.C.M.; Mangel, M. Cultured fish: integrative biology and management of domestication and interactions with wild Fish. Biol. Rev. 2012, 87, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.K.; Link, J.S. It is past time to use ecosystem models tactically to support ecosystem-based fisheries management: Case studies using Ecopath with Ecosim in an operational management context. Fish. Fish. 2023, 24(3), 381–406. [Google Scholar] [CrossRef]
- Shui, B.N. Rethinking and optimization of releasing stock enhancement for marine fishery resources: a review. Journal of Dalian Ocean University. 2023, 38(5), 737–743. [Google Scholar]
- Radinger, J.; Matern, S.; Klefoth, T.; Wolter, C.; Feldhege, F.; Monk, C.T.; Arlinghaus, R. Ecosystem-based management outperforms species-focused stocking for enhancing fish populations. Science. 2023, 379(6635), 946–951. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Z.; Wu, Y.; Sun, T.; Liu, H.; Zhao, Y. Ecosystem-based fishery management that accounts for trade-offs between fishing and enhancement stocking: A case study of Juehua island in the Bohai Sea. Ecol. Indic. 2023, 156. [Google Scholar] [CrossRef]
- Hong, X.F.; Chen, Z.Z. ; C.; Zhang, J.; Jiang, Y.E.; J.; Gong, Y.Y.; Cai, Y.C.; Yang, Y.T. Analysis of ecological carrying capacity of reef organisms in Qilianyu Islands based on Ecopath model. Journal of Tropical Oceanography. 2022, 41(01), 15–27, (in Chinese with English abstract). [Google Scholar]
- Yuan, Y.; Xian, W.; Zhang, H. A review of the ecological carrying capacity of fishery resources in China based on the Ecopath model. Marine Sciences. 46(7), 105–119.
- Yang, W.; Zhang, Z.; Wu, Y.; Sun, T.; Liu, H.; Zhao, Y. Ecosystem-based fishery management that accounts for trade-offs between fishing and enhancement stocking: a case study of Juehua Island in the Bohai Sea. Ecol. Indic. 2023, 156. [Google Scholar] [CrossRef]
- Hemraj, D.A.; Bishop, M.; Carstensen, J.; Krause-Jensen, D.; Stæhr, P.A.U.; Russell, B.D. Nature protection must precede restoration. Science. 2024, 383, 158–158. [Google Scholar] [CrossRef] [PubMed]
- Balbar, A.C.; Metaxas, A. The current application of ecological connectivity in the design of marine protected areas. Glob. Ecol. Conserv. 2019, 17, 20. [Google Scholar]
- Ashley, R.; Russell, D.; Swallow, B. The policy terrain in protected area landscapes: challenges for agroforestry in integrated landscape conservation[J]. Biodiversity & Conservation 2006, 15(2), 663–689. [Google Scholar] [CrossRef]
- Yin, J.; Xue, Y.; Li, Y.; Zhang, C.; Xu, B.; Liu, Y.; Ren, Y.; Chen, Y. Evaluating the efficacy of fisheries management strategies in china for achieving multiple objectives under climate change. Ocean Coast. Manag. 2023, 245, 1.1–1.10. [Google Scholar] [CrossRef]
- Ahrens, R.N.M.; Walters, C.J.; Christensen, V. Foraging arena theory. Fish Fish. 2012, 13, 41–59. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J.; Pauly, D.; Forrest, R. Ecopath with Ecosim version 6 user guide. 2008.
- Fath, B.D.; Asmus, H.; Asmus, R.; Baird, D.; Borrett, S.R.; de Jonge, V.N.; Ludovisi, A.; Niquil, N.; Scharler, U.M.; Schückel, U.; Wolff, M. Ecological network analysis metrics: The need for an entire ecosystem approach in management and policy. Ocean Coastal Manage. 2019, 174, 1–14. [Google Scholar] [CrossRef]
- Christensen, V. Ecosystem maturity towards quantification. Ecol. Model. 1995, 77(1), 3–32. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. Ices J. Mar. Sci. 1980, 39(2), 175–192. [Google Scholar] [CrossRef]
- T. A. Collection of empirical relations for use in ecological modelling. Naga. 1999, 22, 24–28.
- Duan, L.J.; Li, S.Y.; Liu, Y.; Jiang, T.; Failler, P. A. Trophic model of the Pearl River Delta coastal ecosystem. Ocean Coast. Manag. 2009, 52, 359–367. [Google Scholar] [CrossRef]
- Wu, Y.C. The temporal and spatial patterns and size-fractioned structure of primary productivity in Beibu Gulf. Doctoral thesis 2008. (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Palomares, M.L.D.; Paulyb, D. Predicting food consumption of fish populations as functions of mortality, food type, morphometrics, temperature and salinity. Marine and Freshwater Research. 1998, 49(5), 447–453. [Google Scholar] [CrossRef]
- Brey, T. Population dynamics in benthic invertebrates. A virtual handbook. Version 01.2 [online]. 2001. Available from http://www. thomas-brey.de/science/virtualhandbook.
- Lv, X.M. Study on the diet composition of juvenile of Liza haematocheila and Konosirus punctatus and its relationship with ambient phytoplankton. Master thesis, Ocean University of Shanghai, Shanghai. 2016(in Chinese with English abstract).
- Jiao, Y.; Chen, D.G.; Liu, Q.; Zhong, C.J.; Zeng, X.Q.; Ren, Y.P. Biological characteristics of some small species in Engraulidae and Clupeidae. Transactions of Oceanology and Limnology. 2001, 25(4), 323–329, (in Chinese with English abstract). [Google Scholar]
- Jiang, R.J.; Xue, L.J.; Zhang, H.L. Zhu, Z.J. Feeding habits of Ilisha Elongata in the East China Sea. Mar. Fish. 2013, 35, 168–175. [Google Scholar]
- Khan, M.A.; Yousuf, K.; Riaz, S. Food and feeding habits of Sillago Sihama (Forsskal, 1775) (Family: Sillaginidae) from Karachi Coast. AkiNik Publ 2014, 1(3), 27–31, (in Chinese with English abstract). [Google Scholar]
- Li, Z.L.; Zhang, W.X.; He, X.B.; Yan, Y.R. Feeding ecology and feeding competition between Decapterus maruadsi and Trachurus japonicus in autumn in the Beibu Gulf, South China Sea. Journal of Guangdong Ocean University 2019, 39(3), 79–86. [Google Scholar]
- Yang, G.; Sun, X.; Hou, X.; Chen, C. Measurement of the trophic level of fish in a coral reef ecosystem using stable isotopes. J. Fish. Sci. China. 2012, 23, 105–115, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhang, Y.M.; Dai, C.T.; Yan, Y.R.; Yang, Y.L.; Lu, H.S. Feeding habits and trophic level of crimson sea bream (Parargyrops edita Tanaka) in the Beibu Gulf. Journal of Fisheries of China. 2014, 38(2), 265–273, (in Chinese with English abstract). [Google Scholar]
- Jin, H.W.; Xue, L.J.; Zhu, Z.J.; Pan, G.L. Feeding Habits of Apogon Lineatusin the East China Sea and Southern Yellow Sea. Mar. Fish 2012, 34(4), 361–370, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Liu, J. Morphological comparison of the feeding apparatus of three co-occurring species of ponyfishes (Leiognathidae). Chinese Journal of Oceanology and Limnology. 1997, 15, 224–226. [Google Scholar] [CrossRef]
- Yang, L.; Cao, W.Q.; Lin, Y.S.; Chen, Y.H.; Lin, Z.J.; Wang, X.H. Preliminary study on feeding habits and trophic niche of nine economic fish species in Beibu Gulf in summer. J. Trop. Oceanogr. 2016, 35(2), 66–75, (in Chinese with English abstract). [Google Scholar]
- Yan, Y.R. Feeding ecology and food relations of the main fishes in the beibu gulf, south china Sea. Doctor thesis, Graduate School of Chinese Academy of Sciences, Beijing. 2010. (in Chinese with English abstract).
- Wang, K.; Li, C.W.; Wang, Z.H.; Zhao, J.; Zhang, S.Y. Feeding habits of the marbled rockfish Sebastiscus Marmoratus in the marine ranching off Ma’an Archipelago, China. Chin. J. Appl. Ecol. 2017, 2321–2326, (in Chinese with English abstract). [Google Scholar]
- Han, D.; Xue, Y.; Ji, Y.; Xu, B.; Ma, Q. Trophic and spatial niche of five gobiid fishes in Jiaozhou Bay. J. Fish. Ences China. 2013, 20, 148–156, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Han, D.Y. Study on feeding ecology of dominate gobiidfishes in Jiaozhou Bay. Master thesis, Ocean University of China, Qingdao. 2013(in Chinese with English abstract).
- Xu, J.W.; Zhang, B.; Zhang, C.L.; Xu, B.D.; Ji, Y.P.; Ren, Y.P.; Xue, Y. Feeding ecology of Amblychaeturichthys hexanema in Haizhou bay based on linear mixed model. Chinese Journal of Applied Ecology 2022, 33(9), 2563–2571, (in Chinese with English abstract). [Google Scholar]
- Ning, J.; Du, F.; Wang, X.; Gu, Y.; Li, Y. Feeding habits of mantis shrimp based on stable isotope analysis. J. Fish. China. 2016a, 40(6), 903–910. [Google Scholar] [CrossRef]
- Ning, J.J.; Du, F.Y.; Li, Y.F. Dietary Composition and trophic position of blue swimmer crab (Portunus Pelagicus)in Honghai Bay. Haiyang Xue bao. 2016b, 8(10), 62–69. [Google Scholar] [CrossRef]
- Huang, M.Z. Study on feeding habitats and nutrient level of four cephalopod specie s from Taiwan Strait and its adjacent areas. Journal of Oceanography in Taiwan Strait 2004, 23(3), 331–340, (in Chinese with English abstract).. [Google Scholar]
- Huang, M.Z. Study on feeding habits and nutrient level of shrimp species from Taiwan Strait and its adjacent sea areas. Journal of Oceanography in Tai wan Strait 2004, 23(4), 481–488, (in Chinese with English abstract).. [Google Scholar]
- Yu, J. ; Chen, P; Feng, X. Food habits and trophic levels for 4 species of economical shrimps in the Pearl River estuary shallow waters. Journal of Southern Agriculture. 2016, 47(5), 736–741, (in Chinese with English abstract).. [Google Scholar]
- Huang, M.Z. Study on feeding habits and nutrient level of four cephalopod species from Taiwan Strait and its adjacent areas. Journal of Oceanography in Tai wan Strait 2004, 23(3), 331–340, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Mo, B.; Qin, C.; Chen, P.; Li, X.; Yuan, H. Feeding habits of the purple sea urchin Heliocidaris Crassispina based on stable carbon and nitrogen isotope analysis. J. Fish. Sci. China 2017, 24(3), 566–575, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Ding, M.M. Diet assessment and gut microbiota of intertidal grazing gastropods. Master thesis, Xiamen University, Xiamen.2019 (in Chinese with English abstract).
- Lu, J.P.; Cai, R.X.; Qian, Z.X. Stomach contents of the several barnacles in Zhoushan waters. In Donghai Marine science; 1996; Volume 14, 1, pp. 28–35, (in Chinese with English abstract). [Google Scholar]
- Xu, Q. Evaluation of food sources of bivalve in seaweed and filter-feeding bivalve polyculture ecosystem. Doctoral thesis, The Institute of Oceanology, Chinese Academy of Sciences, Qingdao. 2007 (in Chinese with English Abstract).
- Abdul, W. O.; Adekoya, E.O. Preliminary Ecopath Model of a tropical coastal estuarine ecosystem around Bight of Benin, Nigeria. Environ. Biol. Fishes. 2016, 99(12), 909–923. [Google Scholar] [CrossRef]
- Adebola, T.; Mutsert, K.D. Spatial simulation of redistribution of fishing effort in Nigerian coastal waters Using Ecospace. Ecosphere. 2019, 10. [Google Scholar] [CrossRef]
- Akoglu, E.; Salihoglu, B.; Libralato, S.; Oguz, T.; Solidoro, C. An indicator-based evaluation of black sea food web dynamics during 1960–2000. J. Mar. Syst. 2014, 134, 113–125. [Google Scholar] [CrossRef]
- Arias-González, J.E.; Nuñez-Lara, E.; González-Salas, C.; Galzin, R. Trophic models for investigation of fishing effect on coral reef ecosystems. Ecol. Model. 2004, 172, 197–212. [Google Scholar] [CrossRef]
- Bella, K.; Sahadevan, P.; Raghavan, R. Ramteke K.K. Sreekanth G.B. Trophic functioning of a small, anthropogenically disturbed, tropical estuary. Mar. Environ. Res. 2023, 192, 1.1–112. [Google Scholar] [CrossRef] [PubMed]
- Bradford-Grieve, J.M.; Probert, P.K.; Nodder, S.D.; Thompson, D.; Hall, J.; Hanchet, S.; Boyd, P.; Zeldis, J.; Baker, A.N.; Best, H.A. Pilot trophic model for subantarctic water over the southern plateau, New Zealand: a low biomass, high transfer efficiency system. J. Exp. Mar. Biol. Ecol. 2003, 289, 223–262. [Google Scholar] [CrossRef]
- Campos, W.L. An Ecosystem Model of San Pedro Bay, Leyte, Philippines: Initial Parameter Estimates. Work. Pap. 2003. [Google Scholar]
- Chen, K.; Meng, Z.; Li, X.; Hu, F.; Du, H.; Liu, L.; Zhu, Y.; Yang, D. Community structure and functional diversity of fishes in Zhelin reservoir,Jiangxi Province. Acta Ecologica Sinica 2022, 42(11), 4592–4602, (in Chinese with English abstract).. [Google Scholar]
- Chen, X.P. A study on the community structure and trophic dynamics of fishery resources in Dachen Island area. Doctoral Thesis, Shanghai Ocean University, Shanghai. 2023(in Chinese with English Abstract).
- Chen, Z.; Qiu, Y. Assessment of the food-web structure, energy flows, and system attribute of northern South China Sea ecosystem. Acta Ecologica Sinica 2010, 30(18), 4855–4865, (in Chinese with English abstract). [Google Scholar]
- Chen, Z.; Qiu, Y.; Jia, X. Mass-balance Ecopath model of Belbu Gulf ecosystem. Ying Yong Sheng Tai Xue Bao. 2006, 17, 1107–1111. [Google Scholar]
- Z. ; Xu, S.; He, P. An ecological model of the artificial ecosystem (Northern Hangzhou Bay, China): analysis of ecosystem structure and fishing impacts. Helgol. Mar. Res. 2011, 65, 217–231. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, S.; Qiu, Y. Using a food-web model to assess the trophic structure and energy flows in Daya Bay, China. Cont. Shelf Res. 2015, 111, 316–326. [Google Scholar] [CrossRef]
- Coll, M.; Palomera, I.; Tudela, S. Decadal Changes in a NW Mediterranean sea food web in relation to fishing exploitation. Ecol. Model. 2009, 220, 2088–2102. [Google Scholar] [CrossRef]
- Coll, M.; Santojanni, A.; Palomera, I.; Tudela, S.; Arneri, E. An ecological model of the northern and central Adriatic Sea: analysis of ecosystem structure and fishing impacts. J. Mar. Syst. 2007, 67, 119–154. [Google Scholar] [CrossRef]
- Colléter, M.; Gascuel, D.; Ecoutin, J.M.; Morais, L.T.D. Modelling trophic flows in ecosystems to assess the efficiency of marine protected area (MPA), a case study on the coast of Senegal. Ecol. Model. 2012, 232, 1–13. [Google Scholar] [CrossRef]
- Corrales, X.; Coll, M.; Tecchio, S.; Bellido, J.M.; Fernández, A.M.; Palomera, I. Ecosystem structure and fishing impacts in the northwestern Mediterranean Sea using a food web model within a comparative approach. J. Mar. Syst. 2015, 148, 183–199. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Keramidas, I.; Tsagarakis, K.; Tsikliras, A.C. Ecosystem models and effort simulations of an Untrawled Gulf in the central Aegean Sea. Front. Mar. Sci. 2019, 6, 648. [Google Scholar] [CrossRef]
- Duan, L. J.; Li, S. Y.; Liu, Y.; Jiang, T.; Failler, P. A trophic model of the Pearl River Delta coastal ecosystem. Ocean & Coastal Management. 2009, 52(7), 359–367. [Google Scholar]
- Fourriére, M.; Alvarado, J.J.; Cortés, J.; Taylor, M.H.; Ayala-Bocos, A.; Azofeifa-Solano, J.C.; Arauz, R.; Heidemeyer, M.; López-Garro, A.; Zanella, I.; Wolff, M. Energy flow structure and role of keystone groups in shallow water environments in Isla Del Coco, Costa Rica, Eastern Tropical Pacific. Ecol. Model. 2019, 396, 74–85. [Google Scholar] [CrossRef]
- Garces, L.R.; Alias, M.; Abu Talib, A.; Mohamad-Norizam, M.; Silvestre, G.T. A trophic model of the coastal fisheries ecosystem off the West Coast of Sabah and Sarawak, Malaysia, Assessment, management and future directions for coastal fisheries in asian countries. WorldFish Center Conference Proceedings 2003, 333–335. [Google Scholar]
- Gurney, L.J.; Pakhomov, E.A.; Christensen, V. An Ecosystem model of the Prince Edward Island Archipelago. Ecol. Model. 2014, 294, 117–136. [Google Scholar] [CrossRef]
- Haggan, N.; Pitcher, T. Ecosystem Simulation Models of Scotland’s West Coast and Sea Lochs. Univ. Br. Columbia Fish. Cent. 2005, 13(4), 1–67. [Google Scholar]
- Han, R.; Chen, Q.; Wang, L.; Tang, X.; Shen, X. Analysis of the ecosystem structure and energy flow of the Yangtze River estuary and adjacent seas,based on the Ecopath model. Acta Ecologica Sinica. 2016, 36(15), 4907–4918. [Google Scholar]
- Hernández-Padilla, J.C.; Zetina-Rejón, M.J.; Arreguín-Sánchez, F.; Del Monte-Luna, P.; Nieto-Navarro, J.T.; Salcido-Guevara, L.A. Structure and function of the southeastern Gulf of California ecosystem during low and high sea surface temperature variability. Reg. Stud. Mar. Sci. 2021, 43, 11. [Google Scholar] [CrossRef]
- Hervé Rybarczyk; Elkaim, B.; Ochs, L.; Loquet, N. Analysis of the trophic network of a macrotidal ecosystem: The Bay of Somme (Eastern Channel). Estuar. Coast. Shelf Sci. 2003, 58, 405–421. [CrossRef]
- Heymans, J.J.; Baird, D. Network Analysis of the Northern Benguela Ecosystem by means of NETWRK and ECOPATH. Ecol. Model. 2000, 131, 97–119. [Google Scholar] [CrossRef]
- Hong, J.; He-Qin, C.; Hai-Gen, X.; Arrequín-Sánchez, F.; Zetina-Rejón, M.J.; Luna, P.D.M.; Le Quesne, W.J. Trophic controls of jellyfish blooms and links with fisheries in the East China Sea. Ecol. Model 2008, 212(3-4). [Google Scholar] [CrossRef]
- Jing, Z.; Shou-yu, Z.; Min, X.U. ; The primary research of the energy flow in Gouqi kelp bed ecosystem. Journal of Shanghai Ocean University. 2010, 19(1), 98–104, (in Chinese with English abstract). [Google Scholar]
- Li, Y.K.; Yu, N.; Chen, L.Q.; Chen, Y.; Feng, D.X. Ecological modeling on structure and functioning of Southern East China Sea ecosystem. Prog. Fish. Sci. 2010, 31, 30–39. [Google Scholar] [CrossRef]
- Lin, H.J.; Shao, K.T.; Kuo, S.R.; Hsieh, H.L.; Wong, S.L.; Chen, I.M.; Lo, W.T.; Hung, J.J. A Trophic Model of a sandy barrier lagoon at Chiku in Southwestern Taiwan. Estuar. Coast. Shelf Sci. 1999, 48, 575–588. [Google Scholar] [CrossRef]
- Lin, Q.; Jin, X.; Guo, X.; Zhang, B. Study on the structure and energy flow of the Yangtze River estuary and adjacent waters ecosystem based on Ecopath Model. J. Hydroecology, 2009, 2, 30–38. [Google Scholar]
- Lin, Q.; Jin, X.; Zhang, B.; Guo, X. Comparative study on the changes of the Bohai Sea ecosystem structure based on Ecopath model between ten years. Acta Ecologica Sinica 2009, 29(7), 3613–3620, (in Chinese with English abstract).. [Google Scholar]
- Lin, Q.; Jin, X.S.; Zhang, B. ; Trophic interactions, ecosystem structure and function in the southern Yellow Sea. Chin. J. Oceanol. Limnol. 2013a, 31(1), 46–58. [Google Scholar] [CrossRef]
- Lin, Q.; Li, X.S.; Li, Z.Y.; Jin, X.S. Ecological Carrying Capacity of Chinese Shrimp stock enhancement in Laizhou Bay of East China based on Ecopath Model. Ying Yong Sheng Tai Xue Bao. J. Appl. Ecol 2013b, 24, 1131–1140, (in Chinese with English abstract).. [Google Scholar]
- Lin, Q.; Shan, X.; Wang, J.; Li, Z. Changes in Chinese Shrimp (Fenneropenaeus chinensis) carrying capacity of the Bohai Sea. Progress in Fishery Sciences. 2018, 39(4), 19–29, (in Chinese with English abstract).. [Google Scholar]
- Lin, Q.; Wang, J.; Li, Z.Y.; Wu, Q. Assessment of ecosystem energy flow and carrying capacity of swimming crab enhancement in the Yellow River Estuary and adjacent waters. Chin. J. Appl. Ecol. 2015, 26, 3523–3531, (in Chinese with English abstract). [Google Scholar]
- Lira, A.; Angelini, R.; Le Loc'h, F.; Menard, F.; Lacerda, C.; Fredou, T.; Fredou, F.L. ; Trophic flow structure of a neotropical estuary in northeastern Brazil and the comparison of ecosystem model indicators of estuaries. J. Mar. Syst. 2018, 182, 31–45. [Google Scholar] [CrossRef]
- Liu, H.; Yang, C.; Zhang, P.; Li, W.; Zhang, X. An Ecopath evaluation of system structure and function for the Laoshan Bay artificial reef zone ecosystem. Acta Ecologica Sinica 2019, 39(11), 3926–3936, (in Chinese with English abstract).. [Google Scholar]
- Liu, P.J.; Shao, K.T.; Jan, R.Q.; Fan, T.Y.; Wong, S.L.; Hwang, J.S.; Chen, J.P.; Chen, C.C.; Lin, H.J. A Trophic model of fringing coral reefs in Nanwan Bay, Southern Taiwan suggests overfishing. Mar. Environ. Res. 2009, 68, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Montes, H.M.; Loneragan, N.R.; Babcock, R.C.; Jackson, K. Using Trophic flows and ecosystem structure to model the effects of fishing in the Jurien Bay Marine Park, Temperate Western Australia. Mar. Freshw. Res. 2011, 62, 421–431. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Z.; Xu, S.; Zhang, J.; Yu, W. Trophic structure and energy flow of continental slope of the northern South China Sea ecosystem. Journal of Fisheries of China 2020, 44(10), 1685–1694, (in Chinese with English abstract).. [Google Scholar]
- Ma, M.; Xu, S.; Xu, Y.; Zhang, K.; Yuan, W.; Chen, Z. Comparative study of Jiaozhou Bay ecosystem based on an Ecopath model. J. Fish. Sci. China 2018, 25, 413–422. [Google Scholar] [CrossRef]
- Manickchand-Heileman, S.; Arreguín-Sánchez, F.; Lara-Domínguez, A.; Soto, L.A. Energy flow and network analysis of Terminos Lagoon, SW Gulf of Mexico. Journal of Fish Biology. 1998, 53, 179–197. [Google Scholar] [CrossRef]
- Monte-Luna, P.D.; Arreguín-Sánchez, F.; Lluch-Belda, D. Marine ecosystem analyses in the gulf of Ulloa, Mexico: BAC meets Ecopath. NCOFISH ecosystem models: transiting from Ecopath to ecospace. Fisheries Centre Research Reports Fisheries Centre, University of British Columbia, Vancouver. 2007, 15(6), 114-133.
- Montero, L.C.; Christensen, V.; Hernández, J.J.C. Simulating trophic impacts of fishing scenarios on two Oceanic Islands using Ecopath with Ecosim. Mar. Environ. Res. 2021, 169, 12. [Google Scholar] [CrossRef]
- Morales-Zárate, M.V.; Lluch-Cota, S.E.; Serviere-Zaragoza, E.; del Próo, S.G. Modeling an exploited rocky coastal ecosystem: Bahia Tortugas, Mexico. Ecol. Model. 2011, 222, 1185–1191. [Google Scholar] [CrossRef]
- Okey, T.A.; Banks, S.; Born, A.R.; Bustamante, R.H.; Calvopiña, M.; Edgar, G.J.; Espinoza, E.; Fariña, J.M.; Garske, L.E.; Reck, G.K.; Salazar, S.; Shepherd, S.; Toral-Granda, V.; Wallem, P. A trophic model of a Galapagos subtidal rocky reef for evaluating fisheries and conservation strategies. Ecol. Model. 2004, 172(2-4), 383–401. [Google Scholar] [CrossRef]
- Ortiz, M.; Berrios, F.; Campos, L.; Uribe, R.; Ramirez, A.; Hermosillo-Núñez, B.; González, J.; Rodriguez-Zaragoza, F. Mass balanced trophic models and short-term dynamical simulations for benthic ecological systems of Mejillones and Antofagasta bays (SE Pacific): Comparative network structure and assessment of human impacts. Ecol. Model. 2015, 309, 153–162. [Google Scholar] [CrossRef]
- Ortiz, M.; Wolff, M. Trophic models of four benthic communities in Tongoy Bay (Chile): Comparative analysis and preliminary assessment of management strategies. J. Exp. Mar. Biol. Ecol. 2002, 268, 205–235. [Google Scholar] [CrossRef]
- Pinnegar, *!!! REPLACE !!!*; Keith, J. Pinnegar; Keith, J. Planktivorous fishes: links between the Mediterranean littoral and pelagic. University of Newcastle upon Tyne, 2000. [Google Scholar]
- Rahman, M.F.; Qun, L.; Shan, X.J.; Chen, Y.L.; Ding, X.S.; Liu, Q. Temporal changes of structure and functioning of the Bohai Sea ecosystem: insights from Ecopath models. Thalass. Thalass. Int. J. Mar. Sci. 2019, 35. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Y.; Xu, B.; Zhang, C.; Ren, Y.; Cheng, Y.; Xue, Y. Ecosystem structure in the Haizhou Bay and adjacent waters based on Ecopath model. Acta Oceanologica Sinica. 2020, 42(6), 101–109, (in Chinese with English abstract).. [Google Scholar]
- Rocha, G.R.A.; Rossi-Wongtschowski, C.L.d.B.; Pires-Vanin, A.M.S.; Soares, L.S.H. Trophic models of São Sebastião channel and continental shelf systems, SE Brasil. Pan-American Journal of Aquatic Sciences. 2007, 2(2), 149–162. [Google Scholar]
- Schückel, U.; Kröncke, I.; Baird, D. Linking long-term changes in trophic structure and function of an intertidal macrobenthic system to eutrophication and climate change using ecological network analysis. Mar. Ecol.-Prog. Ser. 2015, 536, 25–38. [Google Scholar] [CrossRef]
- Steffen, W.; Sanderson, A.; Tyson, P.; Jäger, J.; Matson, P.; Moore, B.; Oldfield, F.; Richardson, K. Reverberations of Change: The Responses of the Earth System to Human Activities. Springer Berl. Heidelb. 2005, 10.1007/b137870, 143–202. [CrossRef]
- Tecchio, S.; Rius, A.T.; Dauvin, J.C.; Lobry, J.; Lassalle, G.; Morin, J.; Bacq, N.; Cachera, M.; Chaalali, A.; Villanueva, M.C. The mosaic of habitats of the Seine Estuary: insights from food-web modelling and network analysis. Ecol. Model. 2015, 312, 91–101. [Google Scholar] [CrossRef]
- Torres, M.; Coll, M.; Heymans, J.J.; Christensen, V.; Sobrino, I. Food-web structure of and fishing impacts on the Gulf of Cadiz Ecosystem (South-Western Spain). Ecol. Model. 2013, 265, 26–44. [Google Scholar] [CrossRef]
- Tsehaye, I.; Nagelkerke, L.A.J. Exploring optimal fishing scenarios for the multispecies artisanal fisheries of eritrea using a trophic Model. Ecol. Model. 2008, 212, 319–333. [Google Scholar] [CrossRef]
- Ullah, Md.H.; Rashed-Un-Nabi, Md.; Al-Mamun, Md.A. Trophic model of the coastal ecosystem of the Bay of Bengal using mass balance Ecopath Model. Ecol. Model. 2012, 225, 82–94. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Zhang, H.; Zhang, S. Ecological carrying capacity of Chinese shrimp stock enhancement in Haizhou Bay of East China based on Ecopath model. Journal of Fishery Sciences of China 2016, 23(4), 965–975, (in Chinese with English abstract). [Google Scholar]
- Wang, W.; Wang, J.; Zuo, P.; Li, Y.; Zou, X. Analysis of Structure and Energy Flow in Southwestern Yellow Sea Ecosystem Based on Ecopath Model. J. Appl. Oceanogr 2019, 38(4), 528–539, (in Chinese with English abstract).. [Google Scholar]
- Wang, X.H.; Li, S.Y.; Peng, R.Y. Establishment and comparative analysis of Ecopath model of ecosystem evolvement in northern continental shelf of South China Sea. Marine Environmental Science. 2009, 28(3), 288–292. [Google Scholar]
- Wang, Y.; Gao, L.; Chen, Y. Assessment of Portunus (Portunus) Trituberculatus (Miers, 1876) stock in the northern East China Sea. Indian J. Fish. 2018. [Google Scholar] [CrossRef]
- Wolff, M. A Trophic model for Tongoy Bay —a system exposed to suspended scallop culture (Northern Chile). J. Exp. Mar. Biol. Ecol. 1994, 182, 149–168. [Google Scholar] [CrossRef]
- Xu, C.; Wang, S.; Zhao, F.; Song, C.; Zhuang, P. Trophic structure and energy flow of the Yangtze estuary ecosystem based on the analysis with Ecopath model. Mar. Fish. 2018, 40(3), 309–318. [Google Scholar]
- Xu, M.; Yang, X.Y.; Song, X.J.; Xu, K.D.; Yang, L.L. Seasonal analysis of artificial oyster reef ecosystems: implications for sustainable fisheries management. Aquac. Int. 2021, 29, 167–192. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, T. ; Fu, X; Zhang, H. A primary study on the energy flow in the ecosystem of fishery ecological restoration area in Haizhou Bay, Lianyungang. Mar. Environ. Sci 2015, 34(1), 42–47, (in Chinese with English abstract). [Google Scholar]
- Zhang, X.; Xian, W. Energy Flow and Network Analysis of the Yangtze Estuary ecosystem during 1985–1986. Mar. Sci. 2016, 40(7), 60–72, (in Chinese with English abstract). [Google Scholar]
- Zhang, Y.; Chen, Y. Modeling and evaluating ecosystem in 1980s and 1990s for American lobster (Homarus Americanus) in the Gulf of Maine. Ecol. Model. 2007, 203, 475–489. [Google Scholar] [CrossRef]
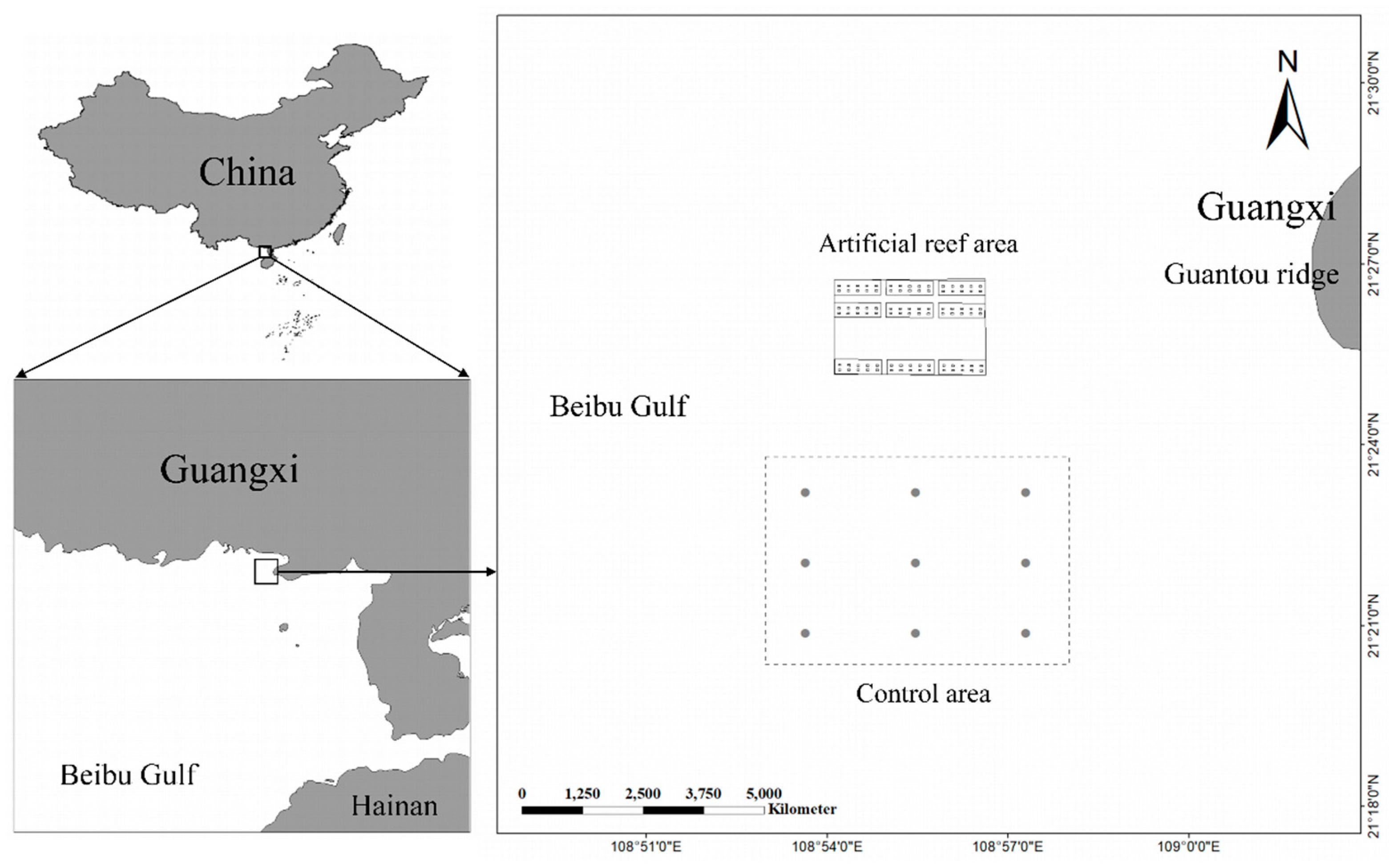

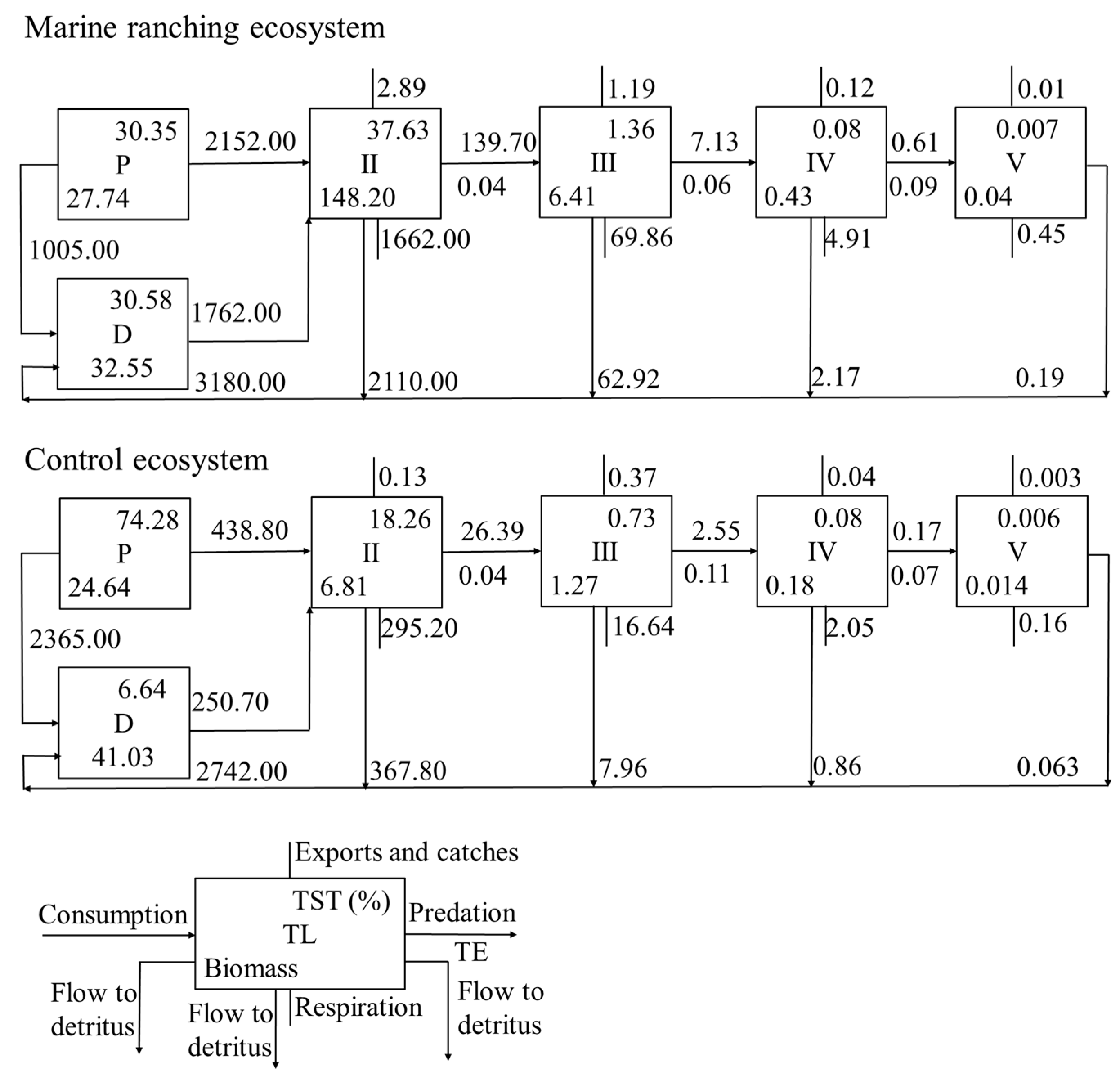
| Overall target | Criterion layers | Indicator tayers | Weight |
|---|---|---|---|
| Ecosystem state | Ecosystem function | D/H | 0.133 |
| 0.4 | A/C | 0.133 | |
| TE | 0.133 | ||
| Food web structure | CI | 0.114 | |
| 0.4 | SOI | 0.114 | |
| FCI | 0.114 | ||
| FML | 0.057 | ||
| Ecosystem maturity | TPP/TR | 0.1 | |
| 0.2 | TPP/TB | 0.05 | |
| TB/TST | 0.05 |
| Indicators | Grades | Indicator types | ||||
|---|---|---|---|---|---|---|
| Poor | Relatively poor | medium | Relatively good | Good | ||
| D/H | 0.073 | 0.414 | 0.540 | 0.631 | 1.209 | Positive |
| TE | 2.920 | 6.800 | 9.400 | 11.500 | 13.236 | Positive |
| A/C | 0.150 | 0.264 | 0.302 | 0.339 | 0.368 | Positive |
| CI | 0.100 | 0.204 | 0.265 | 0.310 | 0.348 | Positive |
| SOI | 0.009 | 0.144 | 0.180 | 0.210 | 0.271 | Positive |
| FCI | 0.650 | 2.800 | 4.980 | 9.400 | 14.700 | Positive |
| FML | 1.206 | 2.336 | 2.568 | 3.193 | 4.000 | Positive |
| TPP/TR | 15.509 | 3.522 | 2.572 | 1.922 | 1.346 | Negtive |
| TPP/TB | 132.000 | 48.656 | 31.964 | 17.338 | 9.110 | Negtive |
| TB/TST | 0.003 | 0.008 | 0.011 | 0.019 | 0.030 | Negtive |
| Functional groups | TL | B (t/km2) | P/B (/a) | Q/B (/a) | EE | Ui |
|---|---|---|---|---|---|---|
| Pelagic fishes | 2.70 | 0.11 | 0.60 | 16.29 | 0.76 | 0.20 |
| Large and medium demersal fishes | 3.42 | 0.18 | 1.16 | 12.82 | 0.77 | 0.20 |
| Sparids | 3.46 | 0.33 | 0.95 | 14.43 | 0.19 | 0.20 |
| Leiognathidae | 2.86 | 0.24 | 2.40 | 14.12 | 0.50 | 0.20 |
| Small demersal fishes | 3.22 | 0.18 | 1.76 | 13.60 | 0.72 | 0.20 |
| Scorpaenidae | 3.27 | 0.02 | 0.97 | 4.00 | 0.25 | 0.20 |
| Gobiidae | 3.22 | 0.07 | 2.32 | 12.96 | 0.86 | 0.20 |
| Mantis shrimps | 3.08 | 0.12 | 5.98 | 30.81 | 0.18 | 0.20 |
| Large crabs | 2.89 | 0.30 | 5.49 | 26.79 | 0.92 | 0.20 |
| Other crabs | 3.13 | 0.30 | 6.82 | 30.35 | 0.75 | 0.20 |
| Metapenaeopsis barbata | 2.33 | 0.52 | 7.89 | 27.61 | 0.31 | 0.20 |
| Other shrimps | 2.37 | 1.50 | 6.68 | 23.92 | 0.63 | 0.20 |
| Cephalopods | 3.28 | 0.07 | 3.81 | 14.83 | 0.80 | 0.20 |
| Sea urchins | 2.10 | 11.43 | 6.38 | 23.60 | 0.01 | 0.20 |
| Gastropods | 2.46 | 0.88 | 5.75 | 23.83 | 0.63 | 0.20 |
| Barnacles | 2.02 | 30.00 | 6.15 | 27.19 | 0.01 | 0.20 |
| Oysters | 2.02 | 21.76 | 4.61 | 20.93 | 0.07 | 0.40 |
| Mussels | 2.02 | 80.40 | 5.06 | 19.34 | 0.05 | 0.40 |
| Other bivalves | 2.02 | 1.58 | 6.20 | 23.46 | 0.74 | 0.40 |
| Other benthos | 2.34 | 0.93 | 6.10 | 21.95 | 0.61 | 0.35 |
| Zooplankton | 2.00 | 4.17 | 32.54 | 192.47 | 0.73 | 0.40 |
| Phytoplankton | 1.00 | 27.74 | 113.82 | 0.68 | ||
| Detritus | 1.00 | 32.55 | 0.55 |
| Functional groups | TL | B (t/km2) | P/B (/a) | Q/B (/a) | EE | Ui |
|---|---|---|---|---|---|---|
| Pelagic fishes | 2.70 | 0.06 | 0.60 | 16.29 | 0.73 | 0.20 |
| Large and medium demersal fishes | 3.35 | 0.18 | 1.30 | 15.18 | 0.78 | 0.20 |
| Sparids | 3.63 | 0.01 | 0.73 | 15.87 | 0.69 | 0.20 |
| Leiognathidae | 2.84 | 0.18 | 2.75 | 17.81 | 0.01 | 0.20 |
| Small demersal fishes | 3.16 | 0.12 | 1.83 | 12.75 | 0.61 | 0.20 |
| Scorpaenidae | 3.30 | 0.00 | 0.97 | 4.00 | 0.16 | 0.20 |
| Gobiidae | 3.16 | 0.07 | 2.30 | 13.92 | 0.41 | 0.20 |
| Mantis shrimps | 3.01 | 0.13 | 5.18 | 31.68 | 0.13 | 0.20 |
| Large crabs | 2.84 | 0.33 | 4.67 | 24.38 | 0.34 | 0.20 |
| Other crabs | 3.09 | 0.11 | 4.84 | 24.90 | 0.92 | 0.20 |
| Metapenaeopsis barbata | 2.31 | 0.06 | 7.40 | 28.04 | 0.91 | 0.20 |
| Other shrimps | 2.37 | 0.58 | 6.77 | 25.85 | 0.96 | 0.20 |
| Cephalopods | 3.25 | 0.07 | 4.58 | 15.82 | 0.36 | 0.20 |
| Gastropods | 2.42 | 0.26 | 5.70 | 23.80 | 0.65 | 0.20 |
| Bivalves | 2.01 | 2.86 | 5.65 | 23.77 | 0.95 | 0.40 |
| Other benthos | 2.08 | 0.11 | 6.10 | 21.95 | 0.89 | 0.35 |
| Zooplankton | 2.00 | 3.13 | 32.39 | 192.29 | 0.07 | 0.40 |
| Phytoplankton | 1.00 | 24.64 | 113.82 | 0.16 | ||
| Detritus | 1.00 | 41.03 | 0.09 |
| Attributes | Marine ranching ecosystem | Control ecosystem | Unit |
|---|---|---|---|
| Total system throughput | 10404.99 | 3777.48 | t/km2/a |
| Total consumption | 4064.63 | 720.83 | t/km2/a |
| Total export | 1422.78 | 0.54 | t/km2/a |
| Total respiration | 1737.31 | 314.03 | t/km2/a |
| Total flow to detritus | 3180.26 | 2742.08 | t/km2/a |
| Total production | 4098.69 | 2932.48 | t/km2/a |
| Total primary production | 3157.37 | 2804.18 | t/km2/a |
| Total biomass | 182.83 | 32.90 | t/km2 |
| Ecosystem | ENA indicators | |||||||||||
| Ecosystem function | Food web structure | Ecosystem maturity | ||||||||||
| D/H | TE | A/C | CI | SOI | FCI | FML | TPP/TR | TPP/TB | TB/TST | |||
| Marine ranching | 0.82 | 5.84 | 0.22 | 0.28 | 0.16 | 12.43 | 3.29 | 1.82 | 17.27 | 0.018 | ||
| Control ecosystem | 0.57 | 6.47 | 0.48 | 0.32 | 0.2 | 2.55 | 2.23 | 8.93 | 85.22 | 0.005 | ||
| Type | Ecosystem | Evaluation grade of ENA indices or ecosystem status | ||||
|---|---|---|---|---|---|---|
| Poor | Relatively poor | medium | Relatively good | Good | ||
| CI | Marine ranching ecosystem | 0 | 0 | 0.67 | 0.33 | 0 |
| Control ecosystem | 0 | 0 | 0 | 0.74 | 0.26 | |
| SOI | Marine ranching ecosystem | 0 | 0.55 | 0.45 | 0 | 0 |
| control ecosystem | 0 | 0 | 0.33 | 0.67 | 0 | |
| FCI | Marine ranching ecosystem | 0 | 0 | 0 | 0.43 | 0.57 |
| control ecosystem | 0.12 | 0.88 | 0 | 0 | 0 | |
| FML | Marine ranching ecosystem | 0 | 0 | 0.12 | 0.88 | 0 |
| control ecosystem | 0.09 | 0.91 | 0 | 0 | 0 | |
| D/H | Marine ranching ecosystem | 0 | 0.32 | 0.68 | 0 | 0 |
| control ecosystem | 0 | 0.4 | 0.6 | 0 | 0 | |
| A/C | Marine ranching ecosystem | 0.42 | 0.58 | 0 | 0 | 0 |
| control ecosystem | 0.03 | 0.97 | 0 | 0 | 0 | |
| TPP/TR | Marine ranching ecosystem | 0 | 0 | 0 | 0.82 | 0.18 |
| control ecosystem | 0.45 | 0.55 | 0 | 0 | 0 | |
| TPP/TB | Marine ranching ecosystem | 0 | 0 | 0 | 0.99 | 0.01 |
| control ecosystem | 0.44 | 0.56 | 0 | 0 | 0 | |
| TB/TST | Marine ranching ecosystem | 0 | 0 | 0.13 | 0.88 | 0 |
| Control ecosystem | 0.6 | 0.4 | 0 | 0 | 0 | |
| TE | Marine ranching ecosystem | 0.25 | 0.75 | 0 | 0 | 0 |
| control ecosystem | 0.09 | 0.91 | 0 | 0 | 0 | |
| Total ecosystem status | Marine ranching ecosystem | 0.08 | 0.26 | 0.23 | 0.35 | 0.09 |
| Control ecosystem | 0.14 | 0.54 | 0.11 | 0.17 | 0.03 | |
| Functional groups | Carrying capacity (t/km²) | |
|---|---|---|
| Marine ranching | Control ecosystem | |
| Pelagic fishes | 2.12 | 0.48 |
| Large and medium demersal fishes | 0.23 | 0.24 |
| Sparids | 0.38 | 0.027 |
| Leiognathidae | 0.9 | 0.26 |
| Small demersal fishes | 0.62 | 0.16 |
| Scorpaenidae | 0.62 | 0.11 |
| Gobiidae | 0.87 | 0.098 |
| Mantis shrimps | 0.34 | 0.12 |
| Large crabs | 0.29 | 0.10 |
| Pelagic fishes | 0.85 | 0.40 |
| Other crabs | 0.86 | 0.135 |
| Metapenaeopsis barbata | 1.6 | 0.40 |
| Other shrimps | 3.1 | 0.95 |
| Cephalopods | 0.34 | 0.12 |
| Gastropods | 1.82 | 0.36 |
| Oysters | 96 | / |
| Mussels | 163 | / |
| Category | Functional groups | Enhancing potential (t/km²) |
|---|---|---|
| TL 3.0-3.5 | Large and medium demersal fishes | 0.05 |
| Sparids | 0.05 | |
| Small demersal fishes | 0.44 | |
| Scorpaenidae | 0.60 | |
| Gobiidae | 0.80 | |
| Cephalopods | 0.27 | |
| Mantis shrimps | 0.17 | |
| Other crabs | 0.56 | |
| TL 2.5-3.0 | Leiognathidae | 0.52 |
| Pelagic fishes | 2.01 | |
| Large crabs | 0.55 | |
| TL 2.0-2.5 | Other shrimps | 1.60 |
| Metapenaeopsis barbata | 1.08 | |
| Gastropods | 0.94 | |
| Oysters | 74.24 | |
| Mussels | 82.60 | |
| Sea urchins | 15.40 |
| Simulation scenarios | ENA indicators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecosystem function | Food web structure | Ecosystem maturity | ||||||||||
| D/H | TE | A/C | CI | SOI | FCI | FML | TPP/TR | TPP/TB | TB/TST | |||
| Only MOB | 0.74 | 5.25 | 0.27 | 0.28 | 0.16 | 20.12 | 3.97 | 1.18 | 10.89 | 0.02 | ||
| MOB+fishing | 0.74 | 6.07 | 0.26 | 0.28 | 0.16 | 19.99 | 3.98 | 1.17 | 10.8 | 0.02 | ||
| Only Crab | 0.73 | 5.14 | 0.22 | 0.28 | 0.15 | 12.45 | 3.3 | 1.82 | 17.24 | 0.02 | ||
| Crab+fishing | / | / | / | / | / | / | / | / | / | / | ||
| Only Fish | 0.73 | 5.45 | 0.22 | 0.28 | 0.13 | 12.44 | 3.29 | 1.81 | 17.22 | 0.02 | ||
| Fish+fishing | / | / | / | / | / | / | / | / | / | / | ||
| MOB+crab | 0.73 | 5.22 | 0.27 | 0.28 | 0.16 | 20.13 | 3.97 | 1.19 | 10.9 | 0.02 | ||
| MOB+crab+fishing | 0.73 | 5.68 | 0.27 | 0.28 | 0.16 | 20.14 | 3.97 | 1.19 | 10.9 | 0.02 | ||
| MOB+fish | 0.73 | 5.48 | 0.27 | 0.28 | 0.14 | 20.11 | 3.97 | 1.18 | 10.87 | 0.02 | ||
| MOB+fish+fishing | / | / | / | / | / | / | / | / | / | / | ||
| Crab+fish | 0.74 | 5.46 | 0.22 | 0.28 | 0.12 | 12.39 | 3.29 | 1.81 | 17.21 | 0.02 | ||
| Crab+fish+fishing | / | / | / | / | / | / | / | / | / | / | ||
| MOB+Crab+fish | 0.74 | 5.44 | 0.27 | 0.28 | 0.14 | 20.12 | 3.97 | 1.19 | 10.88 | 0.02 | ||
| MOB+Crab+fish +fishing | 0.74 | 5.89 | 0.27 | 0.28 | 0.14 | 20.13 | 3.97 | 1.19 | 10.88 | 0.02 | ||
| Only fishing | / | / | / | / | / | / | / | / | / | / | ||
| Simulation scenarios | Poor | Relatively poor | Medium | Relatively good | Good |
|---|---|---|---|---|---|
| Only MOB | 0.05 | 0.29 | 0.22 | 0.08 | 0.36 |
| Only Crab | 0.10 | 0.26 | 0.20 | 0.29 | 0.15 |
| Only Fish | 0.10 | 0.29 | 0.17 | 0.34 | 0.10 |
| MOB+crab | 0.05 | 0.28 | 0.16 | 0.08 | 0.36 |
| MOB+crab+fishing | 0.03 | 0.29 | 0.23 | 0.08 | 0.36 |
| MOB+fish | 0.04 | 0.34 | 0.17 | 0.08 | 0.36 |
| Crab+fish | 0.10 | 0.28 | 0.17 | 0.34 | 0.10 |
| MOB+Crab+fish | 0.04 | 0.34 | 0.17 | 0.08 | 0.36 |
| MOB+Crab+fish +fishing | 0.03 | 0.35 | 0.17 | 0.08 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





