1. Introduction
The studies performed in the following work would not have been available if it weren’t for the preservative qualities of amber. No other type of fossilization could reveal the fine structure of nematodes, hyphal mycelium, parasitic hyphae infecting the body of nematodes and mycophagus bacteria (Poinar & Hess 1985). The preservative qualities of plant resins have been known for centuries, dating back to when since various resins were used by humans as embalming agents, treating wounds and for preventing wine spoilage. The Egyptians realized that resin was the prime embalming agent for the dead since it was an excellent preserver of the skin, much better that the cheaper preservative treatments using salt baths to soak and dry the corpse. Little is known about the types of resin used for embalming. According to Herodotus (1942), myrrh from species of Commiphora was often used by the Egyptians for embalming.
Animals and plants in resin that has become fossilized and hardened, commonly known as amber, represent some of the most exquisitely preserved remains of extinct creatures dating back millions of years. During maturation, some tree resins function as excellent preservatives that maintain the original structure of the inclusions.
The preservative qualities are, in large part, due to the immediate action of antibiotic qualities in the resin that retard or destroy fungi and bacteria on or in cells, tissues and organs of entombed invertebrates, vertebrates and plant remains. The methodology involved in the preservation of amber inclusions involves four major actions: infiltration, fixation, dehydration and polymerization. These can occur simultaneously or in various steps depending on variables presented below. As the resin infiltrates over and throughout the entrapped inclusions, it restricts oxygen and releases antimicrobial compounds that destroy decay-causing microorganisms that could decompose the tissues. The degree of infiltration depends on the size of the inclusion. If it is completely immersed, the thickness of its outer covering and any openings, either natural or produced, may not reach the entire body of the inclusion.
Fixation preserves the organs, tissues and cells of entrapped victims and is accomplished by natural products in the resin, such as various terpenoids and resin acids. Dehydration is accomplished by sugars and terpines in the resin that replace water within the victim’s tissues, resulting in a condition similar to the type of physical preservation known as inert dehydration. In brief, the dehydration processes produce an extreme case of mummification, very similar to a natural embalming process.
After fixation and dehydration, the slow process of polymerization and cross bonding of the molecules begins as the resin hardens. This process also protects the inclusions from environmental factors that occur during the deposition and often re-deposition of the product. During this polymerization process, a semi-fossilized material known as copal is produced after several thousand years. Amber is eventually formed when the state of fossilization of the resin reaches a hardness of between 2-3 on the Moh’s scale, a refractive index between 1.5 and 1.6, a specific gravity between 1.06 and 1.10, and a melting point between 250-300 degrees °C. The process of maturation of resin into amber takes millions of years. Most authenticated amber with identifiable inclusions dates from 15 to 130 million years.
Aside from the use of resins for preserving fossils, resins were also used on wounds to prevent secondary infections and rapid healing. Another antimicrobial use was to prevent wine spoilage. The well-known Greek retsina wines had their origin in ancient times when winne spoilage was a serious problem..The Greeks discovered that when they added pine resin to the wines, preservation was much better. Some Greeks grew accustom to the taste and maintained the process until the present. While the Romans also initially adopted that practice, they never maintained it.
Further studies using the electron microscope to view the tissues in the abdomen of a fly in Baltic amber showed how detailed the preservation was, since it included not only tissue remains but also both extra-cellular and intra-cellular structures (Poinar & Hess, 1985). These detailed structures represented mitochondria, lipid droplets, ribosomes, trachea, tracheoles and muscle fibers. Some mitochrondia were very long and possessed prominent cristae. Most interesting were well-preserved elongate nuclei with dense areas considered to represent chromatin interspersed with less dense areas of nucleoplasm. The authors could not determine what compounds in the original resin were responsible for this remarkable case of mummification that preserved these cellular structures by natural embalming. At any rate, these same compounds allowed visualization of the fungal and bacterial components in the present study.
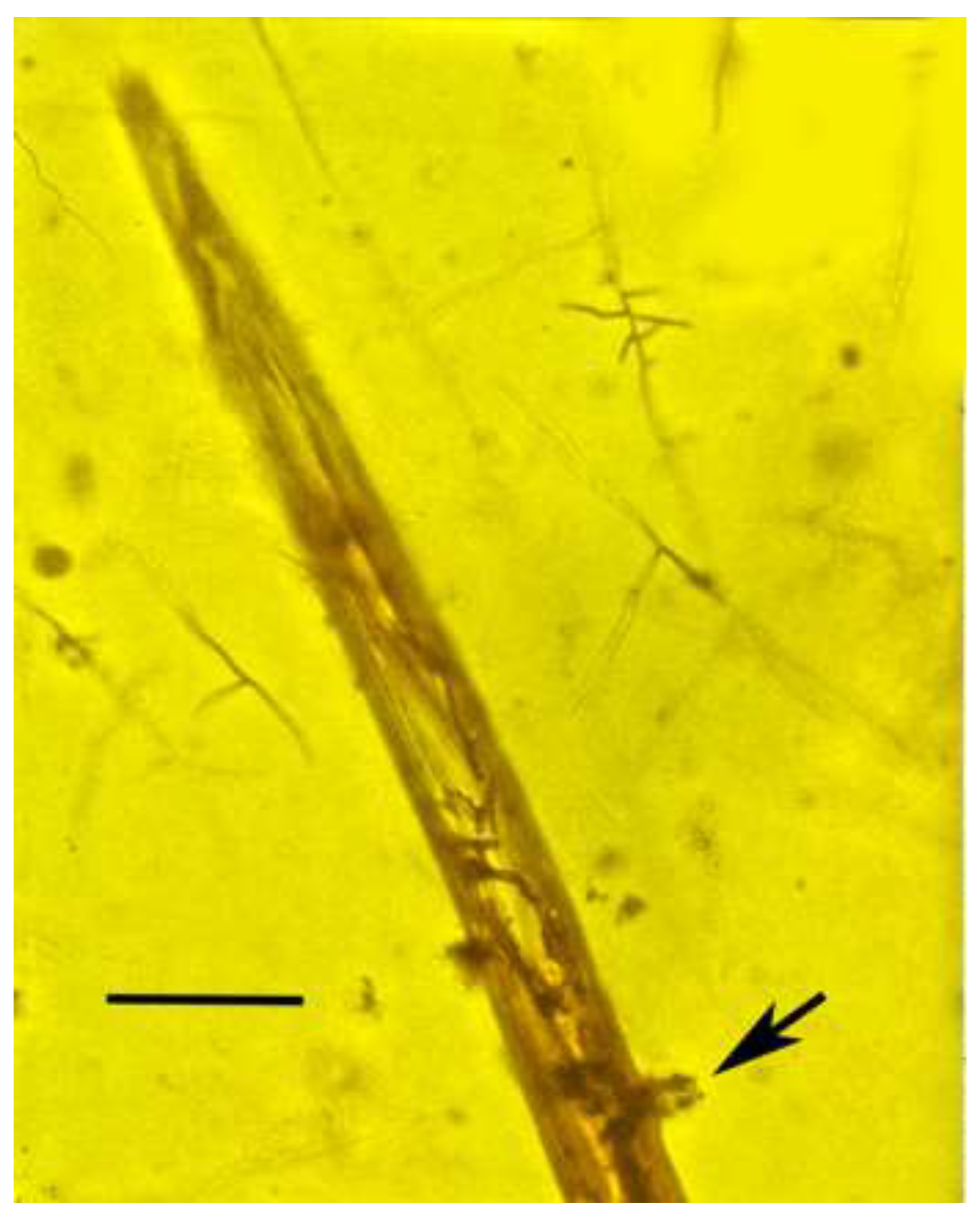
A wide range of fungi attack soil and plant nematodes today, some of which have been found in amber (Taylor et al., 2015), However, in general, fossil fungi are rare and usually difficult or impossible to identify (Turland et al. 2018). A previous study indicated that a free-living filamentous fungus furnished food for a population of the nematode
Oligaphelenchoides atrebora Poinar in Mexican amber (Poinar. 1977)(
Figure 1).
Since the fungus associated with O. atrebora appears to be sterile, it is described below in the form genus Entopeltacites that was erected for specimens in which no reproductive spores are known (Taylor et al., 2015). It is recognized that form genera are an artificial taxonomic category and with their respective species, are erected for taxonomic convenience and are independent of the nomenclatural classification used for extant forms. But form genera and species are important in establishing the presence and associations of fungal lineages at particular geological periods and geographical locations.
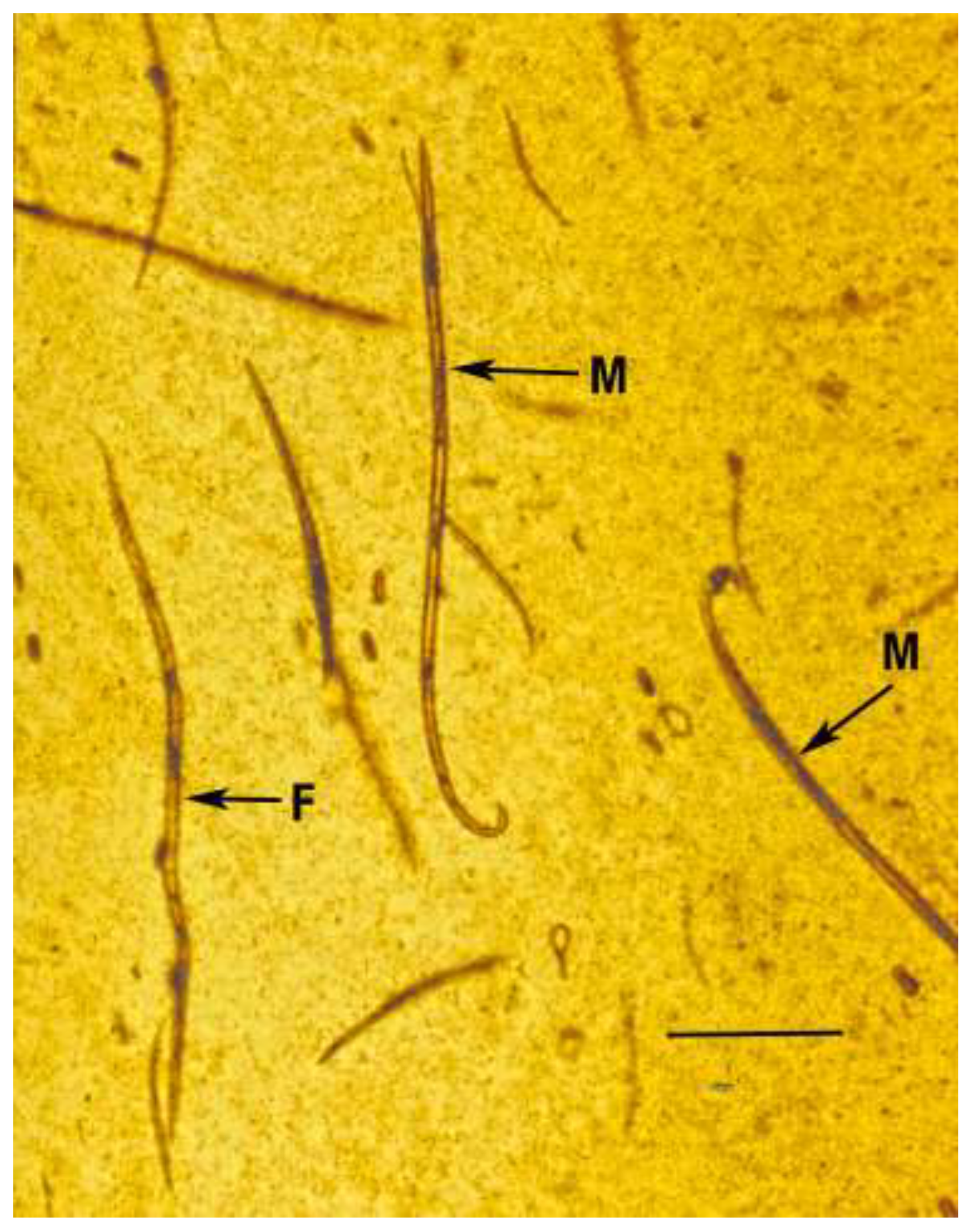
The Mexican amber piece with nematodes actually contains a microcosm involving cells of an angiosperm that presumably were providing nourishment for the filamentous fungus that in turn was providing nourishment to the population of
O. atrebora (males, females, juveniles)(
Figure 2). In addition this piece of amber contains a fungal parasite of the nematode and a spore of that fungal parasite that is infected with a specs of mycophagus bacterium.
2. Materials and Methods
The Mexican amber originated from the Palo-Blanco mine some 22 km of Tapihula, Chiapas, Mexico. mines in the highlands of the Simojovel area in Chiapas, Mexico. Amber from Chiapas, which was produced by Hymenaea mexicana (Fabaceae) (Poinar & Brown 2002), occurs in lignitic beds among sequences of primarily marine calcareous sandstones and silt. The amber is associated with Balumtun Sandstone of Early Miocene and the La Quinta Formation of the Late Oligocene with radiometric ages from 22.5 to 26 million years (Berggren & Van Couvering 1974). Because the amber was secondarily deposited in these marine formations, it may be somewhat older than the above dates. The piece of amber containing O. atrebora is deposited in the Museum of Paleontology at the University of California, Berkeley under accession number B-7053-4. The
The Dominican amber fossil of the sialomorphs originated from La Toca mine in the northern mountain range (Cordillera Septentrional) of the Dominican Republic between Puerto Plata and Santiago (Poinar, 1991; Donnelly, 1988). Amber from mines in this region was produced by Hymenaea protera Poinar (1991)(Fabaceae). Dating of Dominican amber is controversial, with the youngest proposed age of 20-15 mya based on foraminifera (Iturralde-Vincent and MacPhee 1996) and the oldest of 45-30 mya based on coccoliths (Cepek in Schlee 1990). These dates are based on microfossils in the strata containing the amber that is secondarily deposited in turbiditic sandstones of the Upper Eocene to Lower Miocene Mamey Group (Draper et al. 1994). Dilcher et al. (1992) stated that “...the amber casts, from all physical characteristics, were already matured amber at the time of re-deposition into marine basins. Therefore, the age of the amber is greater than Miocene and quite likely is as early as late Eocene”. The issue is further complicated by the discovery of Early Oligocene amber in Puerto Rico and Maastrichtian-Paleocene amber in Jamaica (Iturralde-Vinent, 2001) showing that amber from a range of deposits occurs in the Greater Antilles. Although H. protera is now extinct, its characters are shared by the living specie, Hymenaea verrucosa, in Africa. It is still unknown whether the amphi-Atlantic distribution pattern of Hymenaea was due to oceanic dispersal with the pods floating in the ocean currents or was based on plate tectonics with the genus originating during the period when South America and Africa formed a common land mass. When the continents separated in the late Cretaceous, the population of Hymenaea became disjunct with species remaining on both continents (Poinar, 1991).
The piece of amber containing the sialomorphs is deposited in the California academy of Science under accession #CASG 100155.
Observations and photographs were made with a Nikon SMZ-10 R stereoscopic microscope and a Nikon Optiphot compound microscope with magnifications up to 1000 X.
2.1. Description of Fungus Providing Nourishment for the Nematode. Oligaphelenchoides atrebora
2.1.1. Order: Moniliales
Genus: Entopeltacites (Taylor et al., 2015). Form genus assigned to species with mycelia lacking spores. Form genera provide a convenient way of referring to particular fungal forms that cannot be placed in extant genera and yet provide information about fungal structure, biological interactions and time periods.
2.1.2. Entopeltacites mexicanus sp. nov.
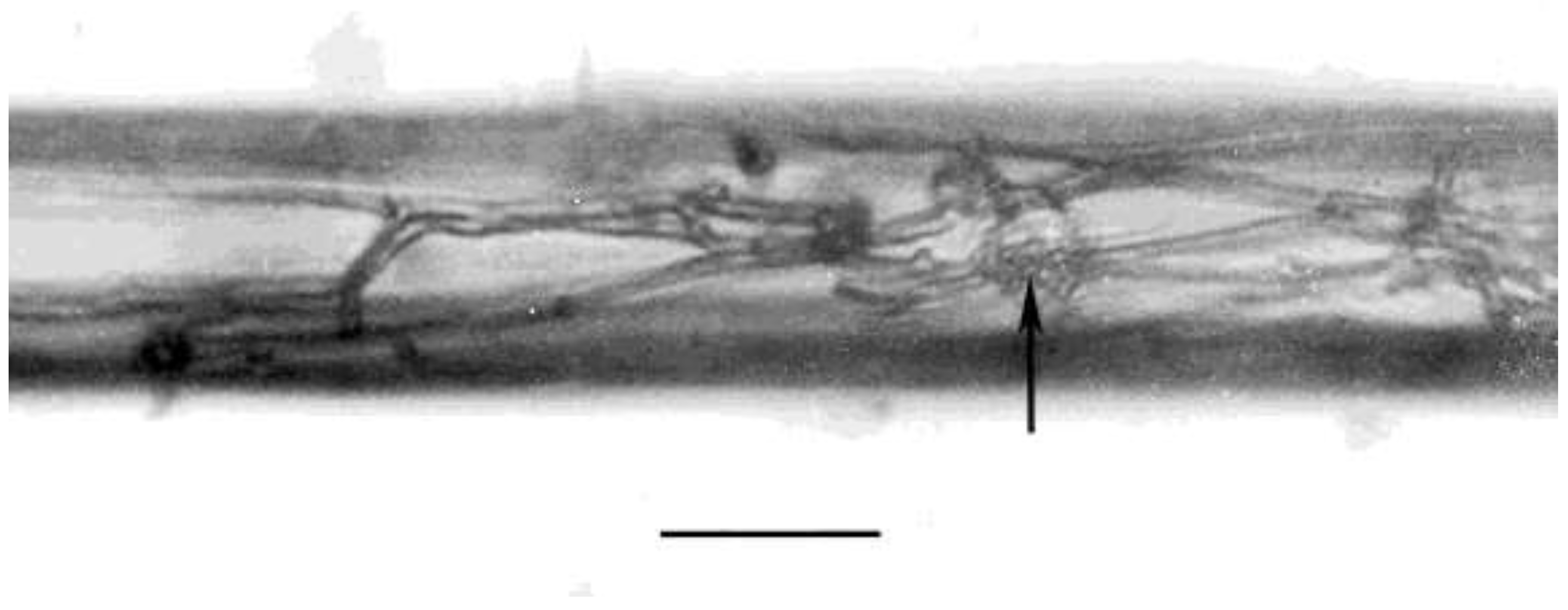
Hyphae septate (
Figure 3). No extensive mycelial development or spores noted in the amber.
Etymology: The specific name indicates the source of the amber.
Type material: Amber block B-7053-4 deposited in the Museum of Paleontology, University of California, Berkeley.
Type locality: Vega de la Campana, Rio San Pedro Valley located about 7 km north of Simojovel, Chipas, Mexico.
Age: Late Oligocene- Early Miocene (22.5 to 26 million years) (Berggren and Van Couvering 1974).
3. Discussion
Extant members of the genus Aphelenchoides, which are very similar in structural features to O. atrebora, are known to develop on fungi in angiosperms. Aphelehchoides ritzema bosi Schwartz, Steiner & Buhrer develops in the intercellular spaces of leaves of chrysanthemum plants and the wide-ranging Aphelehchoides composticola Franklin that develops on a wide range of fungi, is a serious pest of mushrooms (Poinar, 1983).
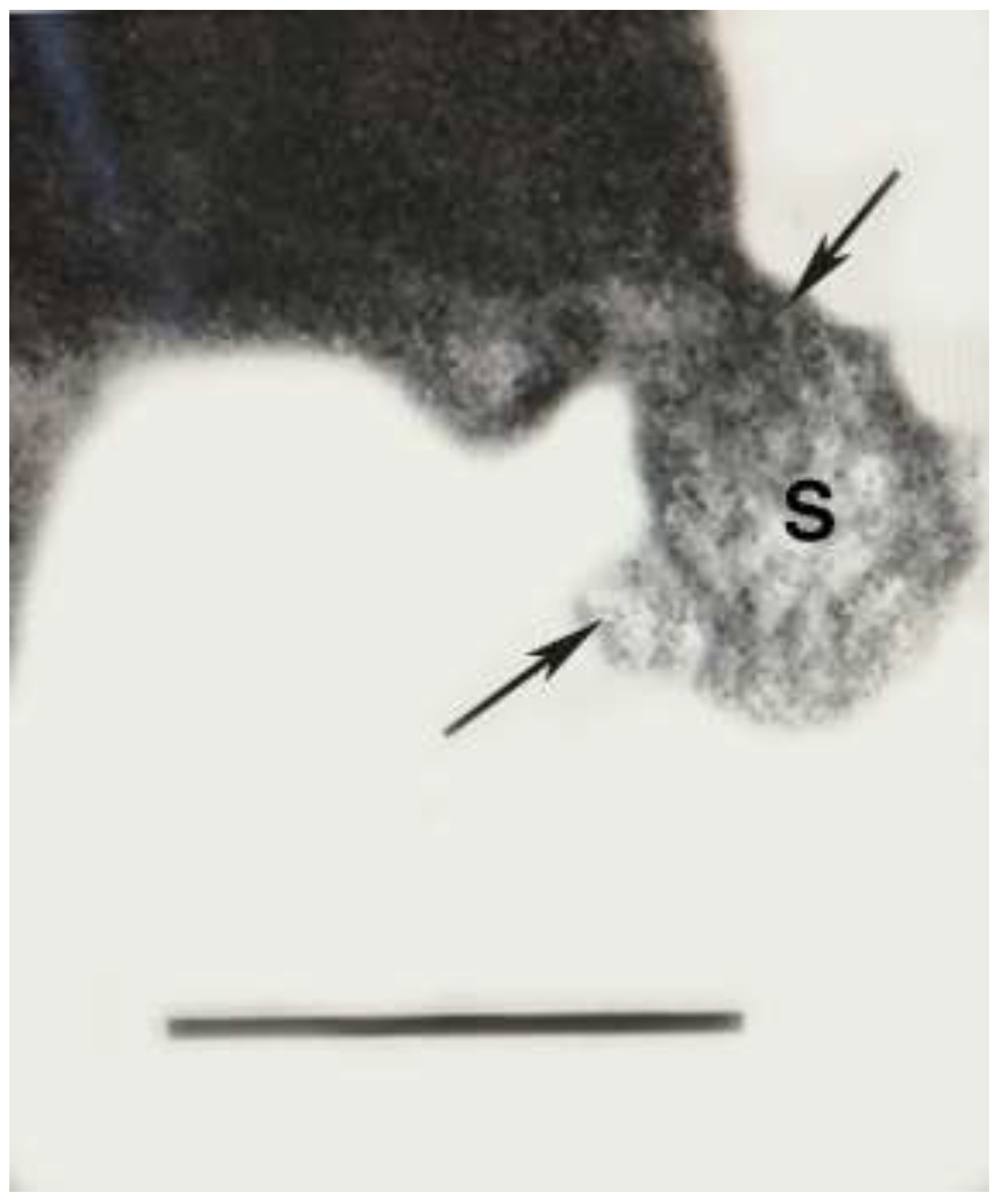
There are 5 separate organisms in this microcosm; 1) deteriorating angiosperm cells that furnish nutrients for the fungus Entopeltacites mexicanus sp. nov. 2) the fungus, Entopeltacites mexicanus sp. nov. that supplies food for the nematode. 3) the nematode, O. atrebora that feeds on the fungus Entopeltacites mexicanus sp. nov. 4) a separate nematophagous fungus infecting the nematode and 5) mycophagous bacteria developing inside a fungal spore of Entopeltacites mexicanus sp. nov.
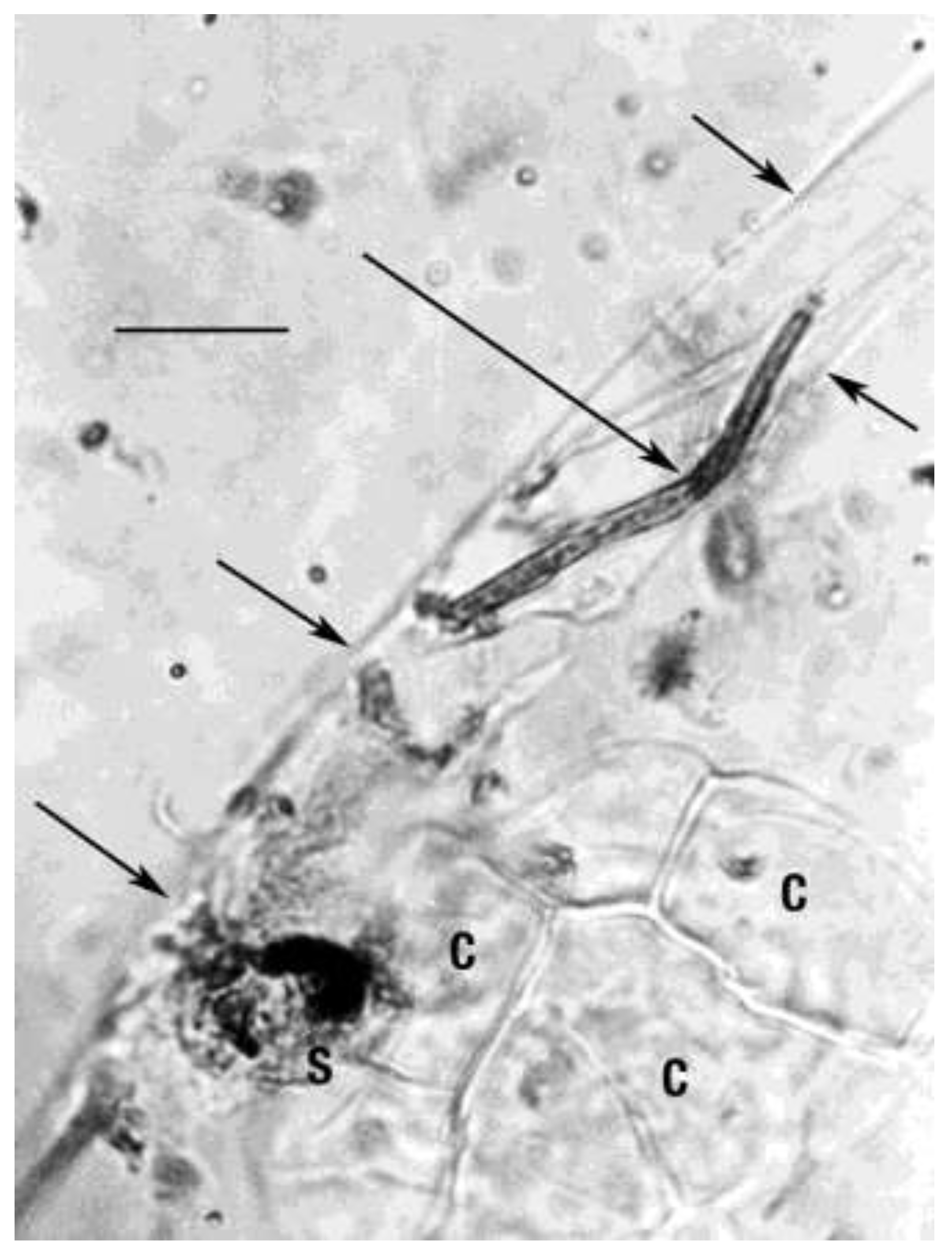
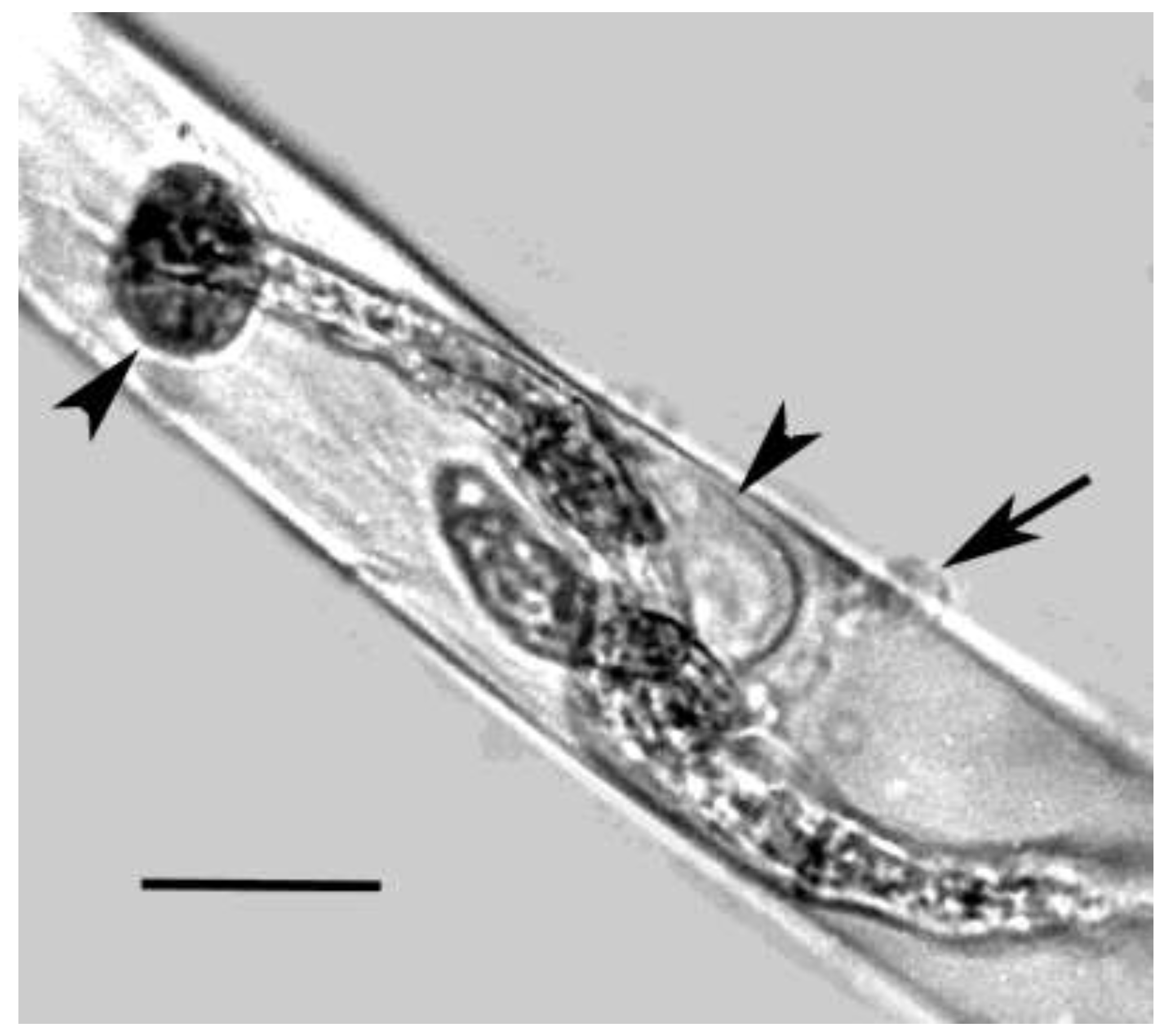
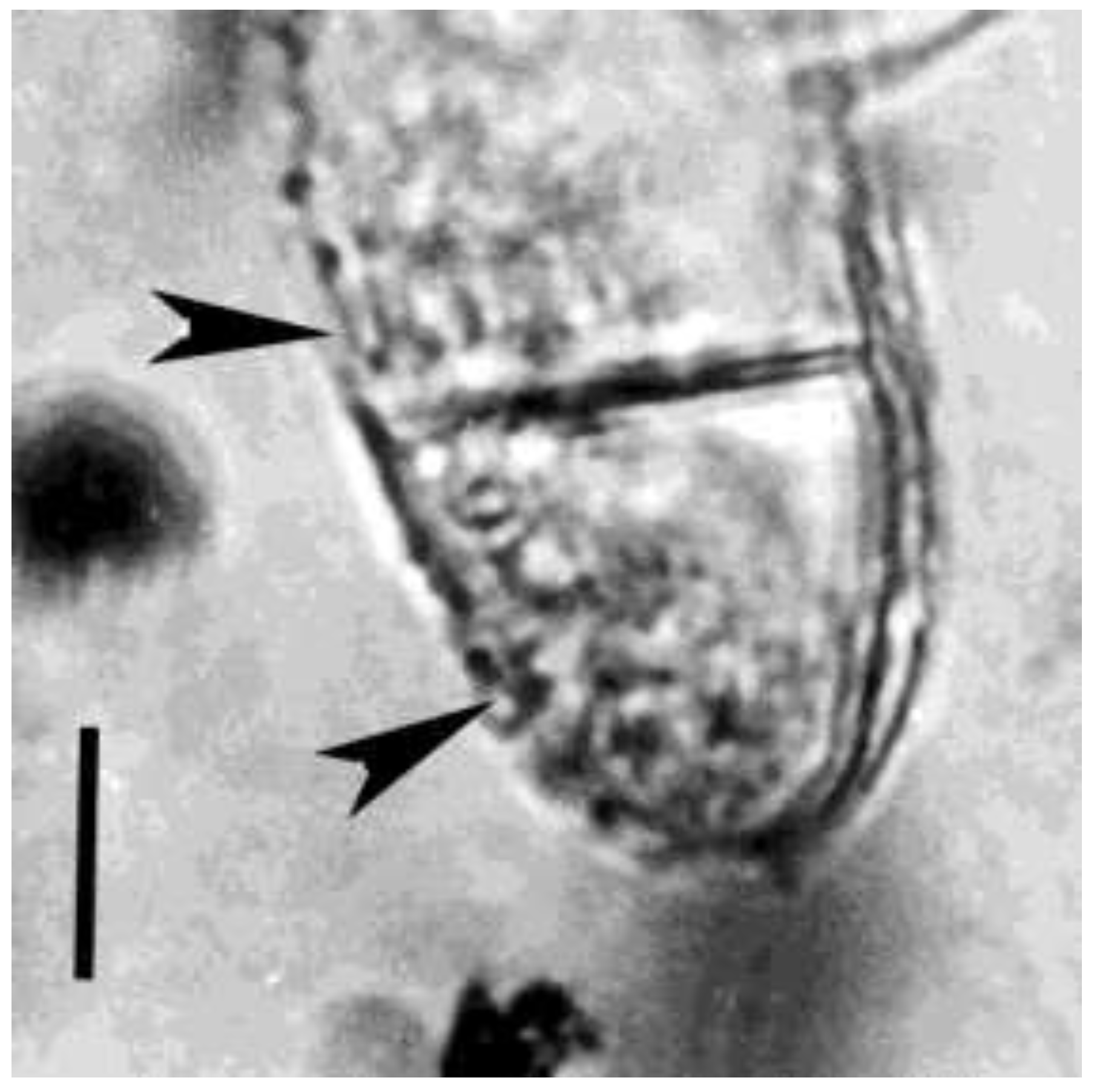
The nematophagous fungus found infecting
O. atrebora is quite interesting (Jansson & Poinar, 1986). Adhesive spores germinating on the surface of eggs produce developing hyphae inside the body
O. atrebora (
Figure 4,
Figure 5 and
Figure 6).
There are two general types of nematophagous fungi. One group is considered nematode-trapping and creates circular rings and knobs that stick to the nematode when it accidently touches them. The second group are nematode parasites that penetrate directly though the body wall to enter the nematode’s body cavity. It is possible that the present parasitic fungus has acquired both of these life styles. It was not possible to align this fungus with any extant fungal group.
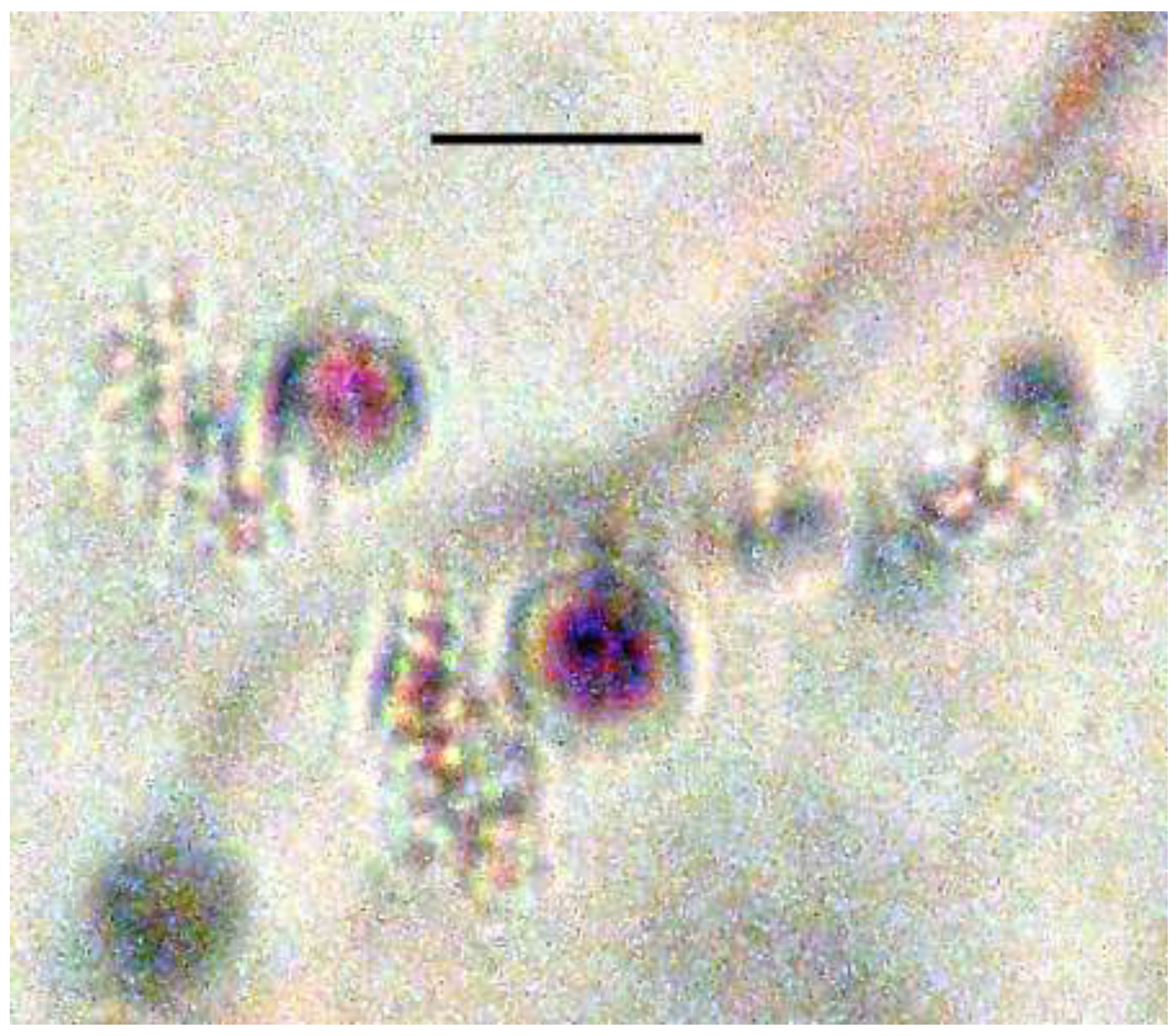
Most interesting were the presence of bacteria cells inside the fungal spore. This is the first fossil case of bacterial parasitism of fungi. The bacteria-infected spore is 3 celled but could also be two celled like members of the fungal genus Isariopsis Fries. that has two or more celled cylindrical to obclavate conidia (Barnett, 1958).
Bacterial mycophagy is defined as bacteria that are able to obtain nutrients from living fungi, thus allowing the conversion of living fungi into living bacteria (Leveau et al., 2010). An example of a present day mycetophagus bacterial genus is Collimonas with three species, C. fungivorans (de Boer et al., 2004), C. arenae (Höppener-Ogawa et al.,2008) and C. pratensis (Höppener-Ogawa et al., 2008) (Leveau et al., 2010).
The genus Collimonas has been recovered from soil environments throughout the world. Soil types with this bacteria included dunes, forests, grasslands, heathlands, tundras, mires and agricultural soils. These included bulk soils, the rhizophere, the mycorrhizosphere, in association with mycorrhizae, the soil layer below lichen mats and the mineral layer (Leveau et al., 2010). Soils with Collimonas were found to be of slight acidity, with the presence of fungi, low nutrient availabilty and limited human disturbance. Antifungal activity against several plant-pathogenic and saprophytic dune soil fungi has been shown for some isolates of Collimonas (Leveau et al., 2010).
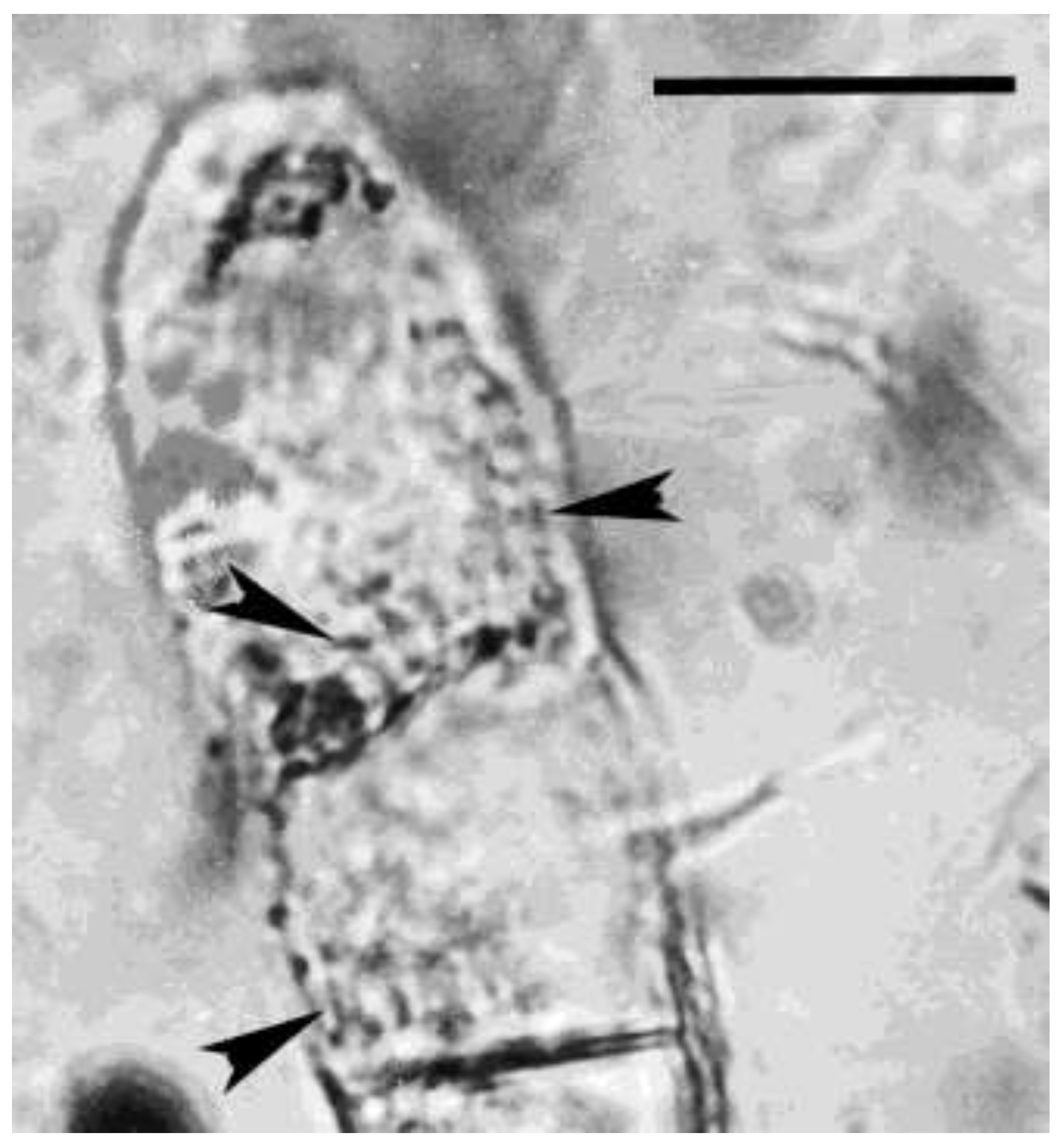
Microscopic observations in some cases showed that groups of
Collimonas cells were present on the tips of fungal hyphae and these tips were often swollen or collapsed. While in the present case, there were bacteria developing in at least two of the three cells of the nematophagous fungal spore infecting
O. atrebora (
Figure 7,
Figure 8 and
Figure 9). It is not possible to determine if the present bacteria inside the fungal spore is a member of the genus
Collimonas, however there are certainly many other genera of bacteria that infect fungi. Many reports have appeared on the presence of mycophagous bacteria in various habitats. Even the idea has been proposed that some of these bacteria could be considered as biological control agents for managing fungal disease in various plants (Leveau et al., 2010).
Since the unknown bacteria infecting the present fungal spore cannot be cultured, it is not possible to determine if they produced a purple-violet pigment as do some of the recent species of cullimonads (Leveau et al., 2010). However other features such as the rod-shaped cells with a length of 1.0-2.0 µm, a width of 0.03- .05 µm and the presence of 1-3 polar flagella (pili) are quite similar to the dimensions of cullimonads, which are 1.0-2.0 µm in length, 0.3- 0.5 µm in width and produce 1-3 polar flagella (pili). In media, most cells of C. fungivorans produced polyphosphate granules about 1 µm in diameter (De Boer et al. 2004). Thus species of mycophagous bacteria will be described in a separate paper.
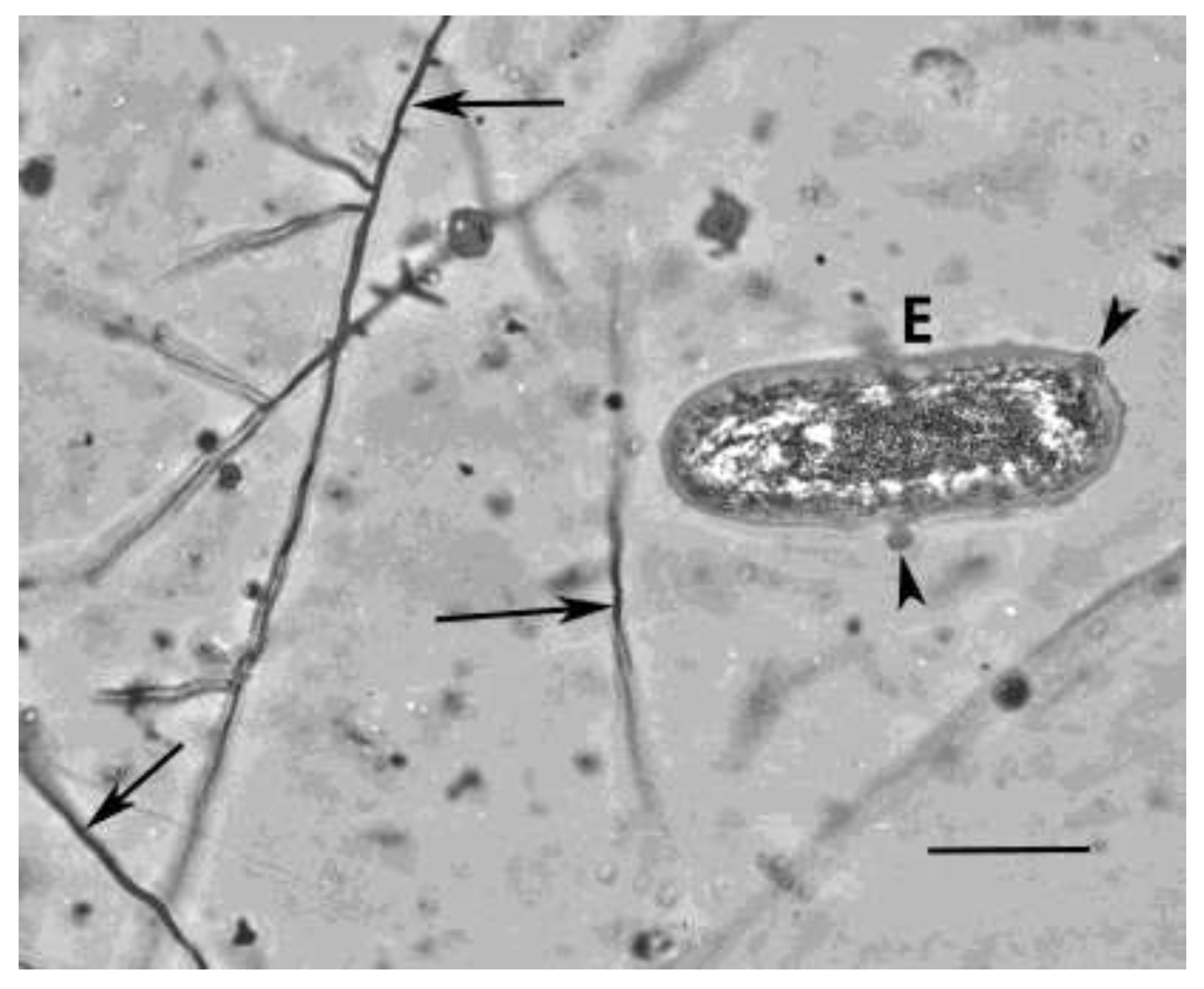
3.1. Fungus Furnishing Food for Sialomorphs
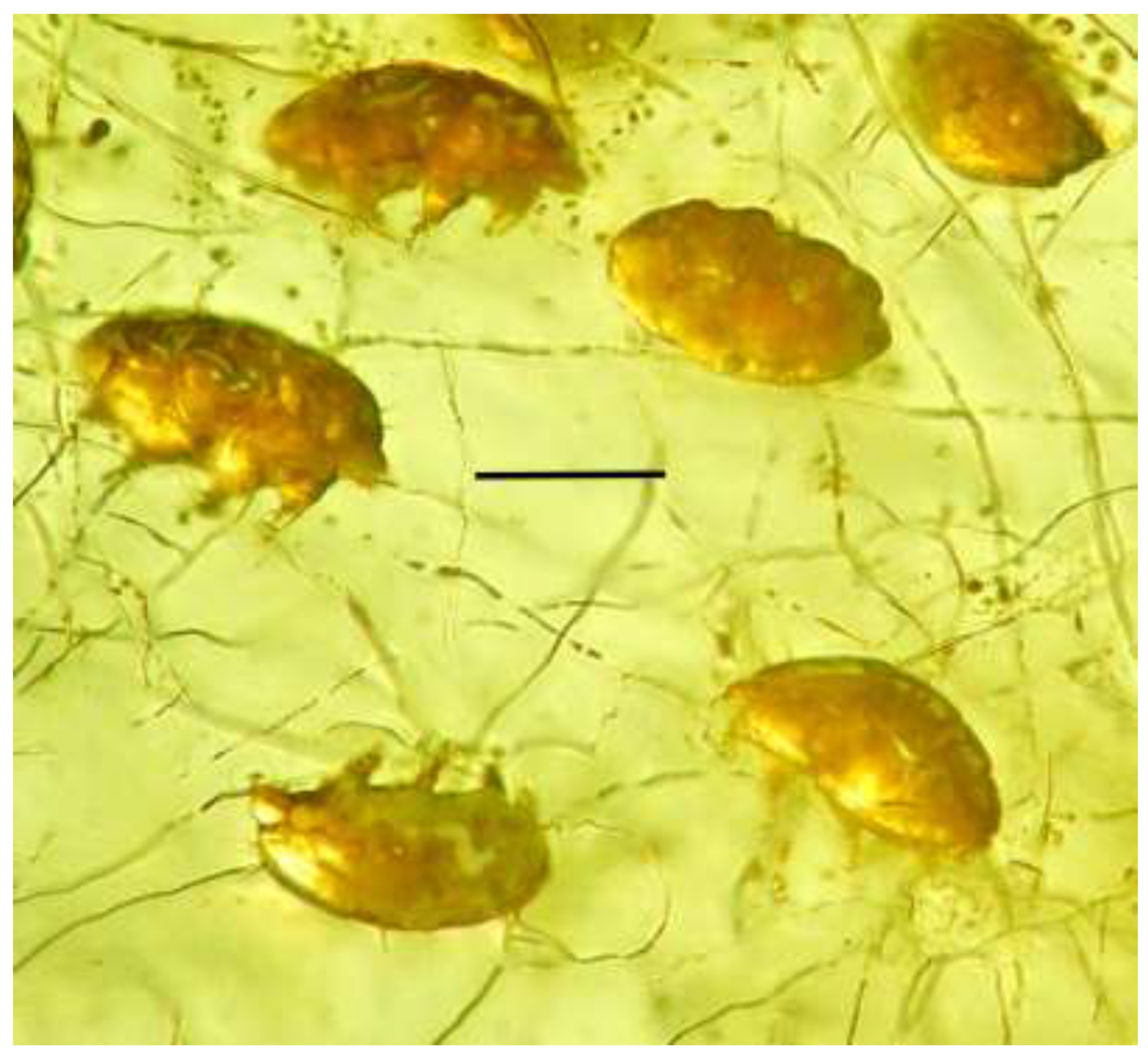
A very minute, rare soil arthropod belonging to the family Sialomorphidae was previously described as
Sialomorpha dominicana in Dominican amber (Poinar & Nelson, 2019). A portion of the original habitat was also preserved, thus providing a microcosm involving nematodes, protozoa and fungi. The population of sialomorphs were closely associated with a fungus described below as
Phagomycetus paleus gen. et sp., nov. This fungus functioned as a food source for
Sialomorpha dominicana (
Figure 10).
3.2. Description of the Fungus That Was Furnishing Food for Sialomophora dominicana
Phagomycetus paleus gen. et sp, nov.
Kingdom: Fungi
Division: Zygomycota C. Moreau 1954
Class: Zygomycetes Winter 1881
Phagomycetus gen. nov.
3.2.1. Type Species: Phagomycetus paleus gen. et sp. nov.
Hyphae septate, mostly vegetative, with certain portions producing Sporangiophores. Sporangiophores short to obsolete, bearing individual sporangia. Sporangia small, globose, dark, thick-walled, ostiolate, Spores hyaline, 1-celled, ovoid, borne in terminal sporangia. Each sporangia bears 12-22 sporangiospores. Sexual stages were not observed.
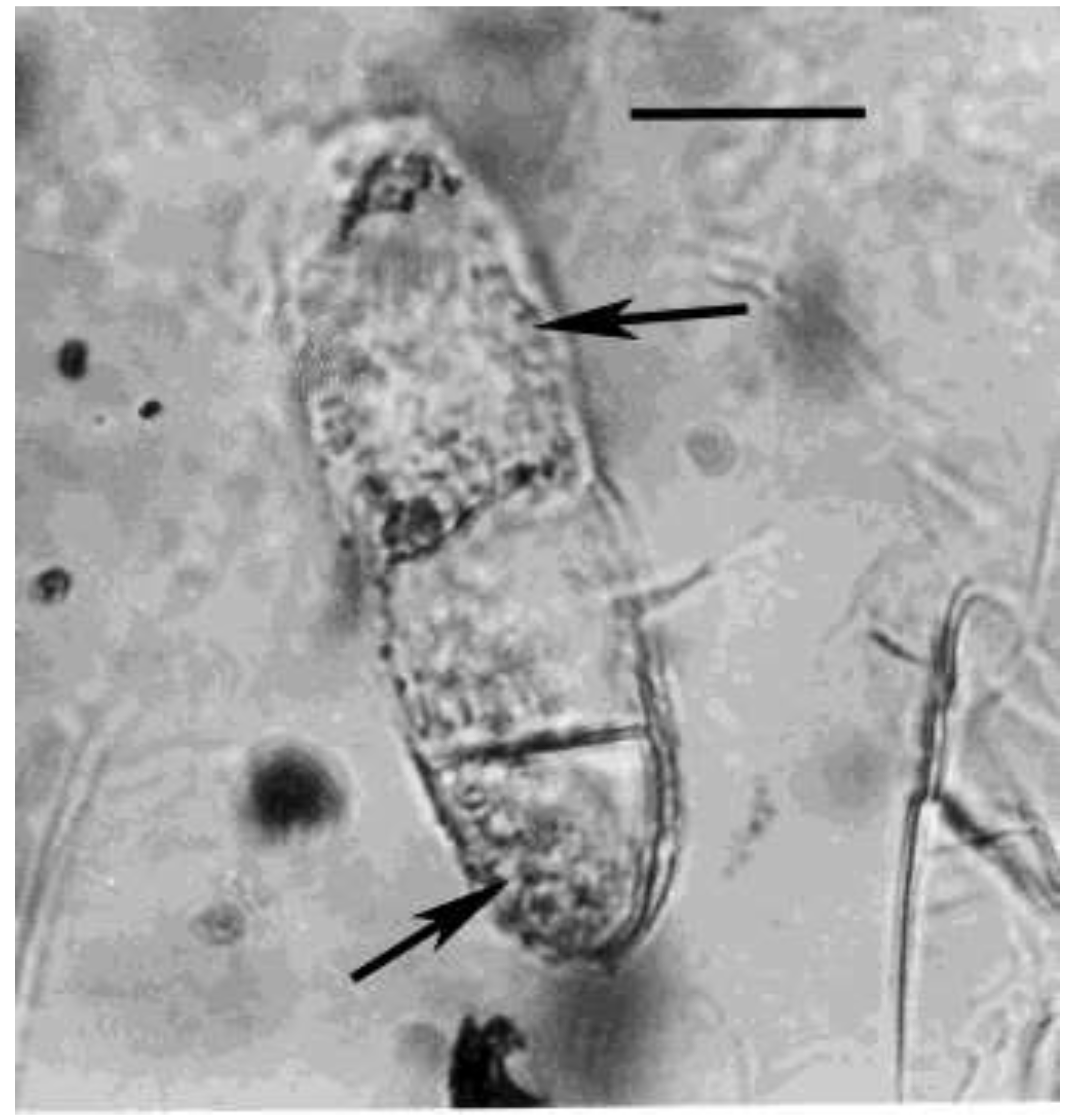
Figure 12.
Sporangium of Phagomycetus paleus gen. et sp, nov. being ingested by Sialomorpha dominicana in Dominican amber. Upper arrow shows extended mandibles of Sialomorpha dominicana, S= sporangium. Lower arrow shows spores extruded from sporangium. Scale bar = 18 µm.
Figure 12.
Sporangium of Phagomycetus paleus gen. et sp, nov. being ingested by Sialomorpha dominicana in Dominican amber. Upper arrow shows extended mandibles of Sialomorpha dominicana, S= sporangium. Lower arrow shows spores extruded from sporangium. Scale bar = 18 µm.
3.2.2. Type species: Phagomycetus paleus sp. nov.
Holotype: The sporangium being held in the mouth of the sialomorph in
Figure 4.
Diagnosis: as for genus (by monotypy).
Etymology: The generic name is taken from the Greek ”phagein” = to eat and the Greek “myketos ” for fungus. The specific epithet is taken from the Greek “palaios” for old.
Type material: Deposited in the California academy of Science under accession #CASG 100155.
Type locality: La Tolca amber mine, Santo Domingo, Dominican Republic.
Age: Miocene or possibly older (Iturralde-Vinent, 2001).
Sialomorpha dominicana was placed among the phyla of animals included within the Ecdysozoa, which is defined as animals that grow by molting their cuticular exoskeleton.
Within the Ecdysosoa, Sialomorpha dominicana would fall in the Panarthropoda, which includes Tardigrades, mites, hexapods and other groups, all of which have representatives that feed on fungi. In fact, the sterile soil fungus, Phagomycetus paleus, that supplies food for Sialomorpha dominicana in Dominican amber could be listed under same name (Phagomycetus paleus) as the fungus nourishing Oligaphelenchoides atrebora in Mexican amber. A fungal diet is not a complete one since some individuals were found feeding on immatures of arthropods.
The examples of Entopeltacites mexicanus with Oligaphelenchoides atrebora in Mexican amber and Phagomycetus paleus with Sialomorpha dominicana in Dominican amber certainly have present day counterparts, including fungal parasites of animals and plants.
It is not known when the lineages of Oligaphelenchoides atrebora and Sialomorpha dominicana originated, how long they continued and if there are still surviving. These organisms show that unique lineages of minute invertebrates with no record of extant descendants existed in the Cenozoic (Poinar 1977; Poinar & Nelson 2019).
Disclosure Statement
No potential conflict of interest was reported by the author.
References
- Barnett, HL. 1958. Illustrated genera of imperfect fungi. Burgess Publishing Company, Minneapolis, Minnesota 218 pp.
- Berggren WA, Van Couvering JAH. 1974. The late Neocene. Biostratigraphy, geochronology and paleoclimatology of the last 15 million years in marine and continental sequences. Palaeogeography, Palaeoclimatology and Palaeoecology 16: 1-216.
- De Boer,W, Leveau, HJL Kowalchuk, JH, Gunnewiek, PJAK. et al. 2004. Collimonas fungivorans gen, nov., sp. nov. a chitinolytic soil bacterium with the ability to grow on living hyphal fungi. Internal Journal of Systematic and Evolutionary Microbiology 54: 587-564.
- Dilcher DL, Herendeen PS, Hueber F. 1992. Fossil Acacia flowers with attached anther glands from Dominican Republic amber. In: P.S. Herendeen and D.L. Dilcher, (eds.) Advances in legume systematics: part 4: the fossil record. The Royal Botanic Gardens, Kew. pp. 33–42.
- Draper G, Mann P, Lewis JF.1994. Hispaniola. In: Donovan S, Jackson TA (eds) Caribbean geology: an introduction. The University of the West Indies Publishers’Association. Kingston, Jamaica, pp. 129–150.
- Herodotus. 1942. The Persian wars. Translated by George Rawlinson. Modern Library, New York. p.156.
- Iturralde-Vinent MA. 2001. Geology of the amber-bearing deposits of the Greater Antilles. Caribbean Journal of Science 37: 141-167.
- Jansson, H-B, Poinar GO Jr. 1986. Some possible fossil nematophagous fungi. Transactions of the British Mycological Society 87: 471-474.
- Leveau, JHJ, Uroz, S., de Boer, W. 2010. The bacterial genus Collimonas: mycophagy, weathering and other adaptive solutions to life in oligotrophic soil environments. Environmental microbiology 12: 281-292. [CrossRef]
- Poinar GO Jr. 1977. Fossil nematodes from Mexican amber. Nematologica 23: 232-238. [CrossRef]
- Poinar GO Jr. 1983. The Natural History of Nematodes. Prentice Hall, INC., Englewood Cliffs, New Jersey, p. 323.
- Poinar GO Jr. 1991. Hymenaea protera sp.n. (Leguminosae: Caesalpinoideae) from Dominican amber has African affinities. Experientia. 47:1075-1082.
- Poinar GO Jr, Hess R. 1985. Preservative qualities of recent and fossil resins: Electron micrograph studies of tissue preserved in Baltic amber. Journal of Baltic studies 16: 222-230.
- Poinar GO Jr, Brown AE. 2002. Hymenaea mexicana sp. nov. (Leguminosae: Caesalpinioideae) from Mexican amber indicates Old World connections. Botanical Journal of the Linnean Society 139: 125–132.
- Poinar, G., Nelson, DR. 2019. A new microinvertebrate with features of mites and tardigrades in Dominican amber. Invertebrate Biology. 138: 1-9. [CrossRef]
- Schlee, D. 1990. Das Bernstein-Kabinett. Stuttgarter Beitrager zur Naturkunde, Ser. C., No. 28, pp. 1-100.
- Taylor AL, Krings, M., Taylor, E.L., 2015. Fossil Fungi. Elsevier, Amsterdam. 382 pp.
- Turland NJ et al. 2018. International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017 (electronic ed.). Glashütten: International Association for Plant Taxonomy.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).