1. Introduction
Interstitial cystitis, also known as bladder pain syndrome (IC/BPS), is a chronic condition that leads to pain in the bladder and pelvic area. Over 1 million patients suffer from IC, presenting symptoms such as frequent urination, urgency, and nocturia [
1]. Globally, the prevalence of IC is estimated at 300 cases per 100,000 women, with men affected at approximately 10–20% of the rate seen in women [
2]. Clinically, IC is classified into two types: Hunner-type IC (HIC), characterized by Hunner’s lesions, and non-Hunner IC (NHIC), which occurs without these lesions.
The management of IC generally includes a combination of approaches aimed at alleviating symptoms and enhancing quality of life. Treatments such as glycosaminoglycan (GAG) replenishment and Intravesical treatments with heparin, hyaluronic acid, chondroitin sulfate, bacillus Calmette-Guerin, and botulinum toxin injections have demonstrated effectiveness in reducing symptoms for certain patient groups [
3]. Additionally, newer therapies like low-energy shockwave treatment, platelet-rich plasma, and stem cells are being investigated as adjunctive options for patients with refractory symptoms [
4]. Nonetheless, treatment outcomes for the disease often remain suboptimal [
5].
The etiology of IC remains unknown and is thought to be multifactorial. Previous theories suggest that genetic predisposition, urothelial barrier defects, chronic inflammation, urine toxicity, and neural sensitization may play pivotal roles [
6,
7,
8]. One potential cause of IC may be defective urothelial barrier permeability, which can result from local lesions or systemic inflammation. Another possible contributing factor is immune system dysfunction, which may also play a role in the development of IC [
9]. The human bladder mucosa consists of a single layer of urothelial cells along with various types of immune cells. This urothelial barrier serves to block solutes and bacteria from penetrating the urothelium, thereby preventing immune cell activation. Alterations in several types of immune cells, such as plasma cells [
10] and mast cells [
11], have been noted and are thought to be linked to the development of IC. Proinflammatory cytokines have additionally been detected in the urine of patients with IC [
12]. A prior study demonstrated that the experimental autoimmune cystitis model reproduces increased expression of mast cells and inflammatory cytokines, a process that depends on peptide-induced autoimmunity mediated by CD4+ T cells [
13]. Additionally, the pathophysiological complexity of IC is further reflected in abnormal immune responses and urothelial dysfunction, as evidenced by findings of glycosaminoglycan layer deficiencies and increased urothelial permeability.
The common pathogenesis features of IC include inflammatory fibroblasts, activated CD4+ T cells, neutrophil migration, and activation of autoimmune-related B cells. Currently, research into the pathology and treatment of IC is ongoing, with a focus on identifying biomarkers that could streamline diagnosis and personalize treatment protocols. By characterizing the GEO database analysis and MR, we have access to better understand disease mechanisms and therapeutic targets.
The central objective of this study was to pinpoint DEGs related to IC through the examination of microarray datasets. It also seeks to evaluate the connections and causative functions of these genes in IC pathogenesis using eQTL and MR analyses. Furthermore, GSEA was utilized to investigate relevant functional pathways and mechanisms that underlie the involvement of these genes in IC development. This study seeks to elucidate the molecular basis of IC, identifying potential biomarkers for diagnosis and therapeutic targets.
2. Materials and Methods
2.2. Identification of DEGs
We used R software (version 4.4.1) to read and preprocess the GSE11783 and GSE57560 datasets, applying corrections to each dataset individually. The datasets were then merged, followed by batch correction and differential analysis on a cohort of 9 normal samples and 23 IC samples. DEGs were identified using the “limma” R package, with thresholds set at |log fold change (FC)| > 1 and a false discovery rate (FDR) < 0.05. Volcano plots and heatmaps of DEGs were generated with the “pheatmap” package. Gene expression matrices and annotation files obtained from the GEO database were used for data normalization and standardization. To eliminate batch effects and improve data visualization, PCA was conducted using the “prcomp” function, facilitating the evaluation and validation of key genes distinguishing IC samples from healthy controls.
2.3. GO/KEGG Enrichment Analysis
The “clusterProfiler” R package was employed for GO functional annotation and KEGG pathway enrichment analysis of DEGs to explore potential functional pathways and underlying mechanisms of pathogenesis. The “clusterProfiler” package is an ontology-based tool designed for categorizing biological terms and performing gene cluster enrichment analysis. In our study, a significance threshold of P < 0.05 was applied as the filtering criterion.
2.4. eQTL Data Preprocessing
The summary eQTL data utilized in this study were sourced from the GWAS Catalog (
https://www.ebi.ac.uk/gwas/). The R package “Two-SampleMR” (version 0.6.8) was used to identify SNPs with strong associations (p < 5e-06) as instrumental variables. Linkage disequilibrium was managed by setting the r² threshold to less than 0.001 and specifying a clumping distance of 10,000 kb. SNPs that showed weak associations or did not adequately explain phenotypic variance (F-statistic > 10) were excluded from the analysis.
2.5. Mendelian Randomization Analysis
MR analysis was conducted using the Two-SampleMR software package, applying the inverse variance-weighted (IVW) method to assess the association between specific genes and IC. Additional sensitivity analyses were carried out using MR-Egger, simple mode, weighted median, and weighted mode approaches. Genes were identified as disease-related based on certain criteria. Genes with p < 0.05 in the IVW analysis were initially selected for further investigation. Subsequently, genes were retained based on a consistent direction of the odds ratio among the five methods. Finally, genes showing signs of pleiotropy with p < 0.05 were excluded. Overlapping genes between disease-related genes and the DEGs, co-expressed genes were identified. The analysis involved tests for heterogeneity, pleiotropy, and leave-one-out sensitivity to assess the robustness and reliability of the findings. To illustrate and support these results, scatter plots, forest plots, leave-one-out plots, and funnel plots were created and examined.
2.6. Immune Cell Analysis
The CIBERSORT approach was used to assess the infiltration levels of 22 types of immune cells in IC. This analysis explored the link between co-expressed genes in IC and immune cell infiltration, investigating possible pathways through which co-expressed genes might influence immune cells.
2.7. GSEA Enrichment Analysis
To determine whether functions or pathways linked to co-expressed genes showed enrichment at the top or bottom ranks, indicating trends in upregulation or downregulation, GSEA was performed. Additionally, GSEA was employed to assess the activity levels of relevant functions or pathways within the gene expression dataset. In this analysis, results were deemed statistically significant when the p < 0.05.
2.8. Validation Group Differential Analysis
The dataset GSE11839 was analyzed using R software (version 4.4.1), with preprocessing conducted through the same methods applied to previous datasets. This analysis aimed to validate whether co-expressed genes exhibited differential expression between control and experimental groups, allowing for a comparison of these findings with those observed in our study.
4. Discussion
IC is a complex condition marked by lower urinary tract symptoms, pelvic pain, and considerable psychological distress, all of which contribute to a reduced quality of life. Despite existing diagnostic and therapeutic efforts, outcomes remain suboptimal, emphasizing the need for innovative approaches. This study aimed to deepen understanding of IC’s underlying mechanisms by analyzing datasets from the GEO database, employing MR analysis, and examining immune cell infiltration. Two IC microarray datasets were extracted from GEO for in-depth analysis, allowing the identification of critical DEGs that are likely central to IC’s pathophysiology. GO and KEGG enrichment analyses showed that these DEGs are primarily involved in immune response-activating signaling pathways and cytokine-cytokine receptor interactions, pointing to potential targets for future therapeutic development.
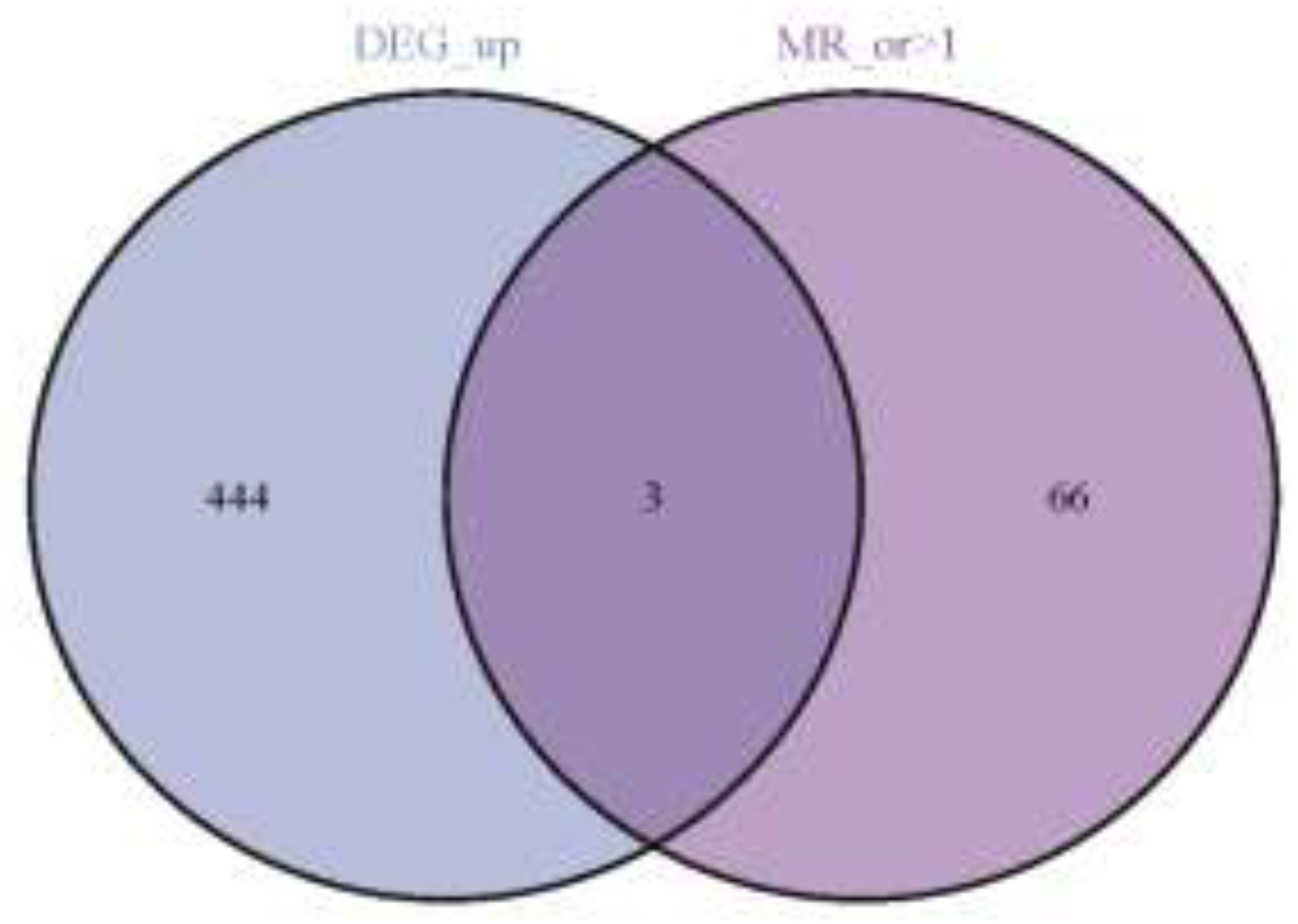
In this study, 447 up-regulated and 326 down-regulated DEGs were intersected with IC-associated genes identified by MR analysis, leading to the identification of three co-expressed target genes (CD38, FPR1, and SLA). The expression patterns of these genes provide new insights into the genetic basis of IC and may reflect key molecular changes in pathogenesis. Additionally, MR analysis indicated that CD38, FPR1, and SLA exhibited risk effects against IC.
CD38: CD38 serves as a multifunctional transmembrane glycoprotein present across various immune cells, such as T cells, B cells, lymphocytes, macrophages, monocytes, and others. CD38 is regarded as a key marker protein for T cell activation, capable of working in tandem with co-stimulatory molecules to drive complete T cell activation [
14]. Upon T cell activation, cellular proliferation is enhanced through the regulation of intracellular proteins or metabolism, accompanied by the secretion of significant quantities of inflammatory cytokines, including IFN-γ and TNF-α. The CD38-NAD (+) axis is instrumental in modulating the chromatin remodeling and reattachment in T cell responses [
15]. Additionally, CD38-catalyzed metabolites, like cADPR, can indirectly elevate Ca
2+ concentrations in T cells by inhibiting the sarcoplasmic endoplasmic reticulum Ca
2+ ATPase, whereas Ca
2+, as essential second messengers, are involved in numerous signaling pathways during T cell activation, thereby further modulating the activation process [
16]. Furthermore, studies have investigated CD38’s role in macrophages, revealing that suppressing CD38 activity in primary inflammatory macrophages disrupts the full production of inflammatory cytokines IL-12 and IL-1β, as well as glycolytic activity. These findings highlight significant role of CD38 in mediating immune responses and inflammation.
CD38 has been observed to be upregulated in specific diseases, indicating its potential to serve as a diagnostic biomarker or therapy target. In systemic lupus erythematosus (SLE), a chronic inflammatory disease, CD38 expression is notably higher in nonclassical monocytes from patients with active SLE compared to those with inactive disease or healthy individuals [
17]. The presence and elevated proportion of CD38+ CD8 T cells serve as a biomarker to monitor the progression of HIV infection toward AIDS [
18]. Gil et al. showed that inhibiting CD38 prevented the decrease in intracellular NAD+/NADH levels and reduced the catabolic activity of chondrocytes under IL-1β stimulation, suggesting that targeting CD38 inhibition may represent a promising therapeutic strategy for treating osteoarthritis [
19]. In our study, we observed upregulation of CD38 in IC patients, which is consistent with the findings of Saha et al [
20].
FPR1: Formyl peptide receptors (FPRs), functioning as pattern recognition receptors (PRRs), are crucial in numerous pathological processes. These receptors, expressed by immune cells, transduce chemotactic signals through coupled receptors, thereby initiating processes such as cell adhesion, migration, tissue repair, angiogenesis, and reactive oxygen species production [
21]. Research findings reveal that neutrophils can undergo FPR1-mediated cell recruitment in a mouse model of bacterial infection [
22]. In a mouse skin wound healing model, FPR1 resulted in rapid neutrophil infiltration, indicating that FPR1 is crucial for the normal healing process of skin wounds [
23]. FPR is also significant in inflammation-associated tumorigenesis. Numerous tumor cells express FPR to stimulate tumor cell proliferation, metastasis, and angiogenesis, possibly through interaction with endogenous ligands released into the microenvironment [
24].
SLA: The SLA gene encodes the SLAP-1 protein, which is expressed in various immune cells, especially at elevated levels in T cells. SLAP-1 functions as an adaptor protein that attenuates T-cell receptor signaling, inhibits activation of the nuclear factor of activated T cells triggered by the T-cell antigen receptor, and contributes to both positive T-cell selection and inhibition of cell division [
25]. Research has demonstrated that SLAP-deficient bone marrow-derived dendritic cells produce lower levels of TNF-α and IL12 in response to LPS stimulation, are unable to effectively stimulate T cells in a mixed lymphocyte response, and show reduced capacity to induce IFN-γ secretion by T cells. [
26]. Therefore, a deficiency in SLAP-1 may weaken immune response, diminishing the production of essential cytokines like IL12, IFN-γ, and TNF-α, which are critical in driving the host’s immune defense [
27].
Applying the CIBERSORT algorithm, we analyzed the distribution of immune cell subpopulations in IC to better understand the role of immune cells in disease pathogenesis. From these results, we examined the relationship between co-expressed genes and immune cell infiltration and explored how these genes uniquely influence immune cell activity. We found that these CD38, FPR1, and SLA were associated with activated CD4 memory T cells, which were elevated in IC, indicating that three co-expressed genes playing a key role in the pathogenesis of IC. Moreover, the biological processes and signaling pathways associated with these three genes we identified, including adaptive immune response, immune receptor activity chemokine signaling pathway, cytokine-cytokine receptor interaction, and JAK stat signaling pathway, suggesting that these three genes participating in the development of IC mainly through immunoregulation. In line with our finding, activated CD4 memory T cells (CD3+ CD4+ HLA-DR+ cells) were found an elevated expression in HIC [
28]. Additionally, previous studies have shown that activated CD4 memory T cells play a key role in inflammatory cells in rheumatoid arthritis, and that the expression of activated CD4 memory T cells are also significantly increased in patients with polymyositis [
29,
30]. These research also consistent with our result.
In summary, this research applied bioinformatics approaches alongside MR to analysis genes connected with IC. We identified three genes with causal roles in the pathogenesis and progression of IC. Furthermore, GSEA indicated that these genes are significantly involved in pathways related to immune signaling. Out finding suggests that the identified genes may represent novel biomarkers and potential treatment targets for IC.
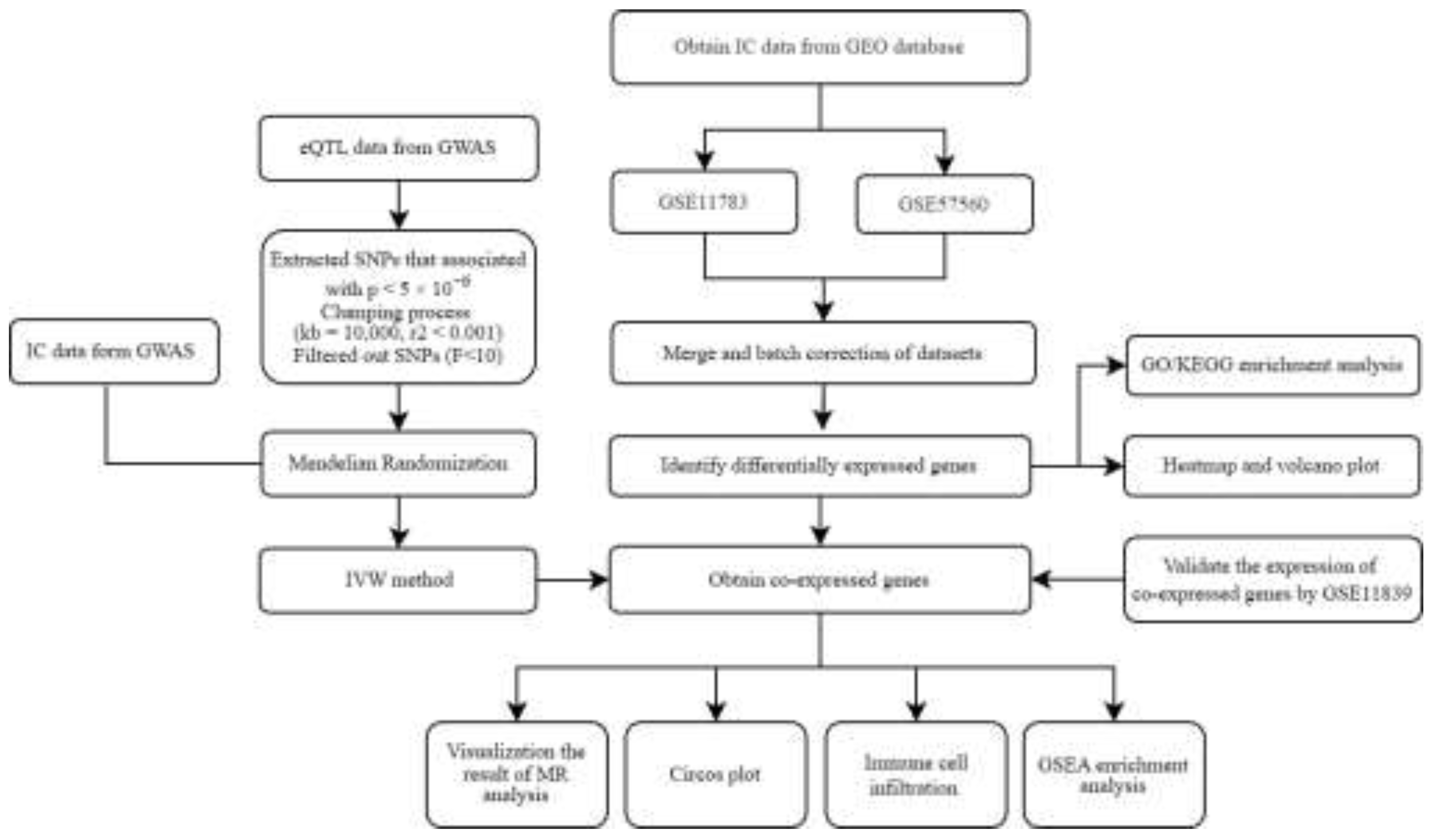
Figure 1.
A flowchart of our research. IC, Interstitial cystitis; GEO, Gene Expression Omnibus; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; eQTL, expression quantitative trait loci; MR, Mendelian randomization; IVW, Inverse Variance-Weighted; GWAS, Genome-Wide Association Studies.
Figure 1.
A flowchart of our research. IC, Interstitial cystitis; GEO, Gene Expression Omnibus; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; eQTL, expression quantitative trait loci; MR, Mendelian randomization; IVW, Inverse Variance-Weighted; GWAS, Genome-Wide Association Studies.
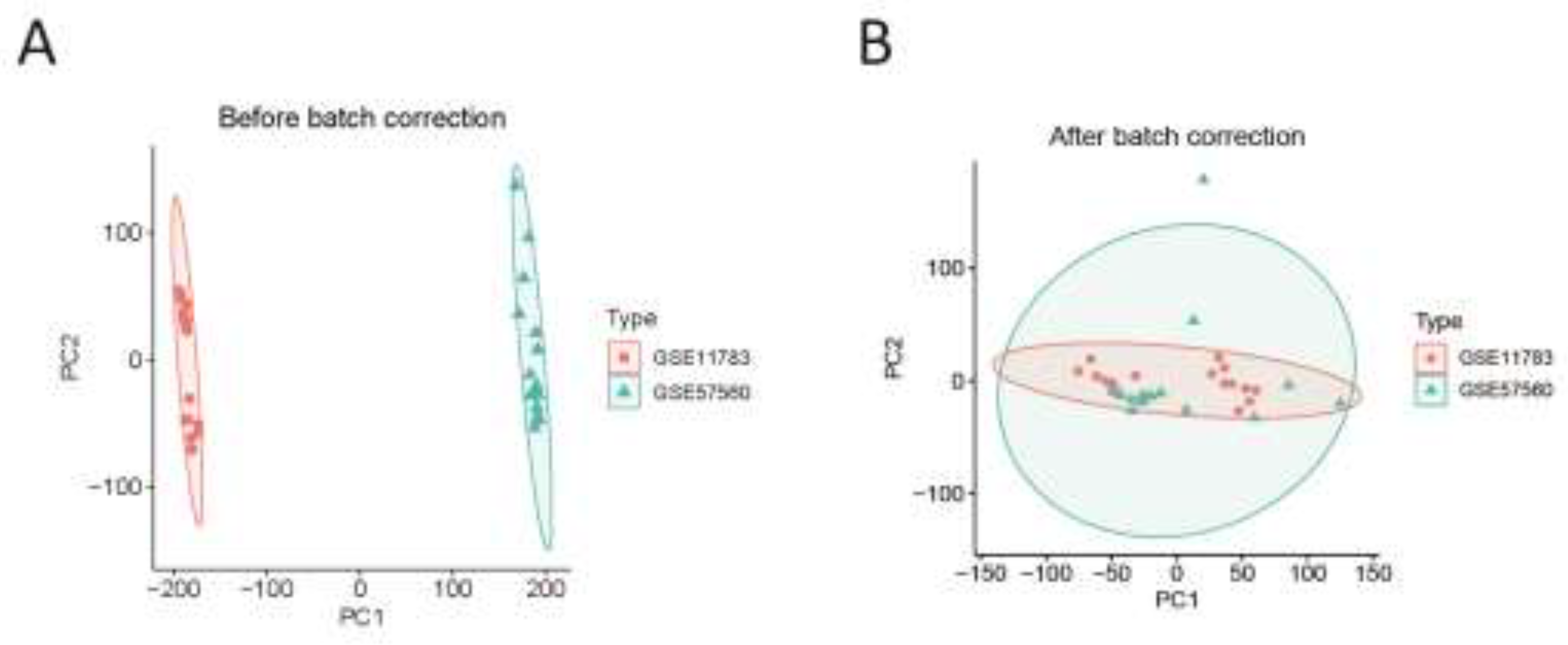
Figure 2.
(A) Two IC gene datasets were obtained from the GEO database. (B) Two samples in the dataset achieved acceptable homogeneity following PCA analysis after batch correction.
Figure 2.
(A) Two IC gene datasets were obtained from the GEO database. (B) Two samples in the dataset achieved acceptable homogeneity following PCA analysis after batch correction.
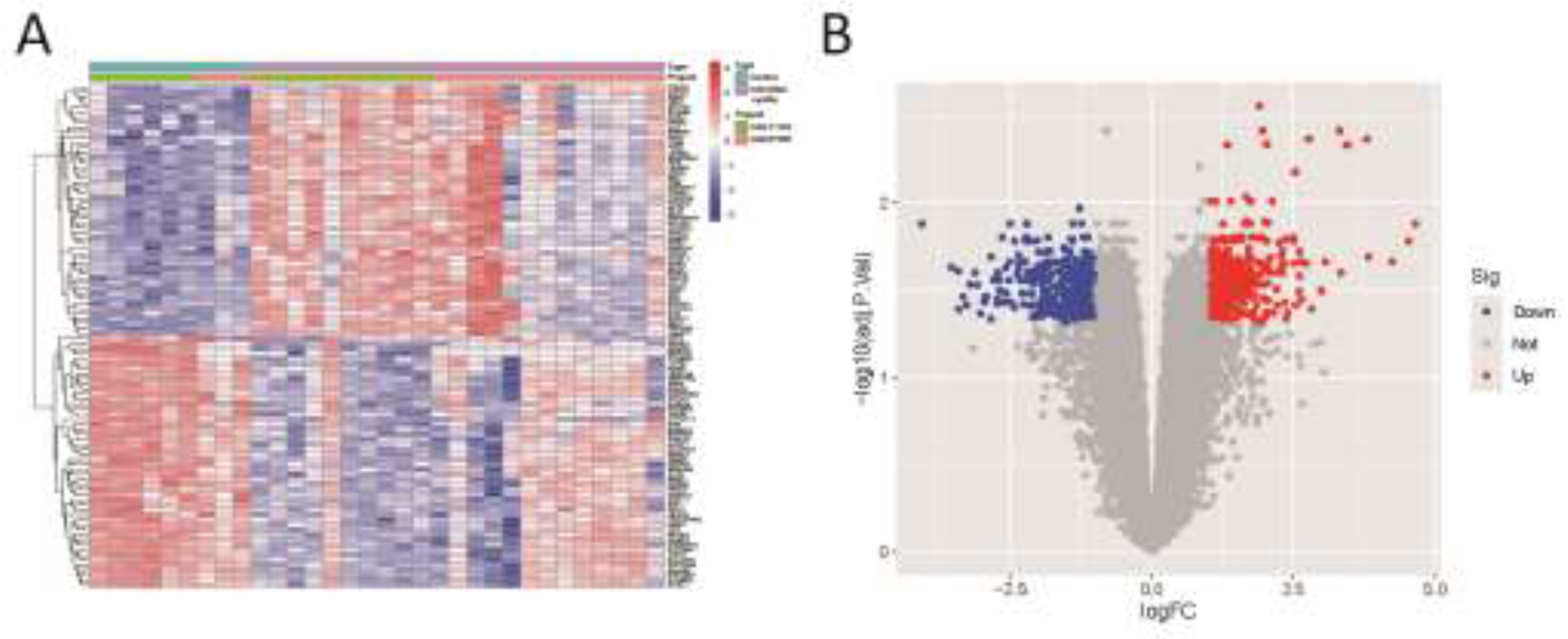
Figure 3.
(A) Heatmap of differential gene expression. (B) Volcano plot.
Figure 3.
(A) Heatmap of differential gene expression. (B) Volcano plot.
Figure 4.
Three up-regulated co-expressed genes.
Figure 4.
Three up-regulated co-expressed genes.
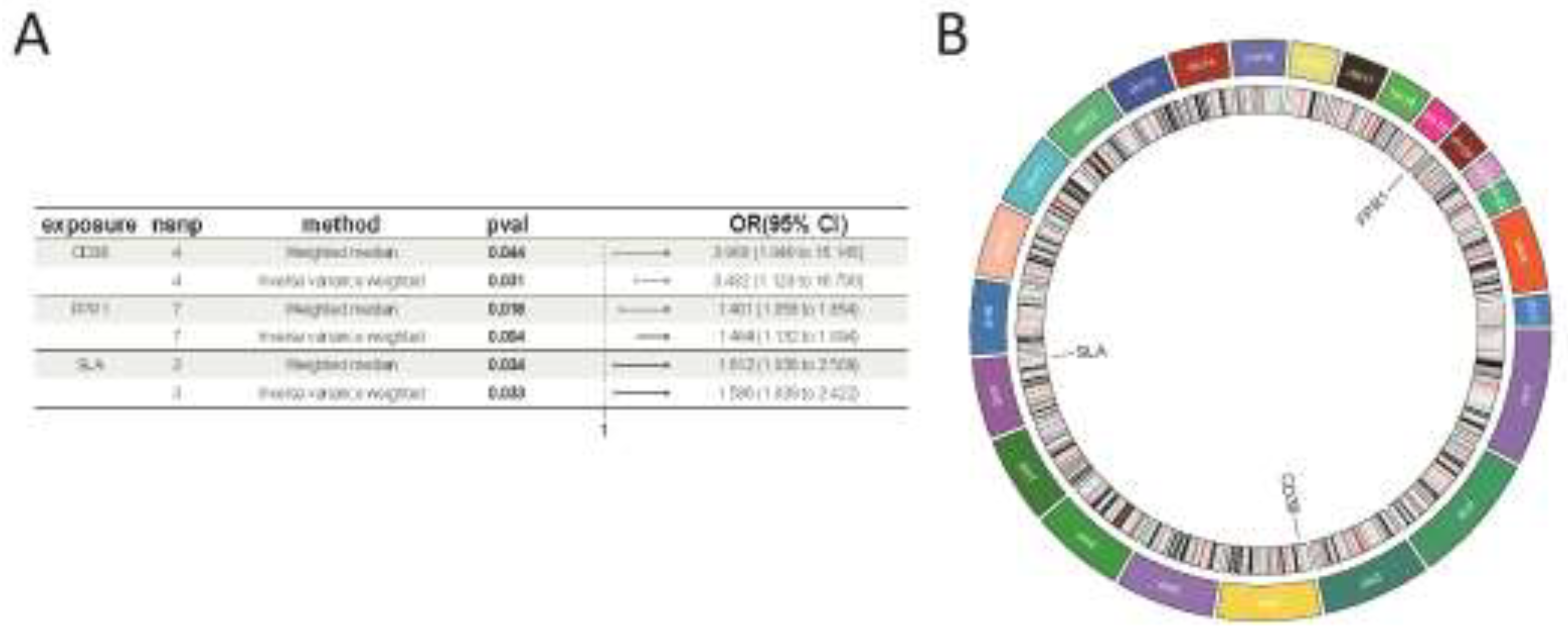
Figure 5.
(A) MR forest plot of co-expressed genes. (B) Circos plot of co-expressed genes.
Figure 5.
(A) MR forest plot of co-expressed genes. (B) Circos plot of co-expressed genes.
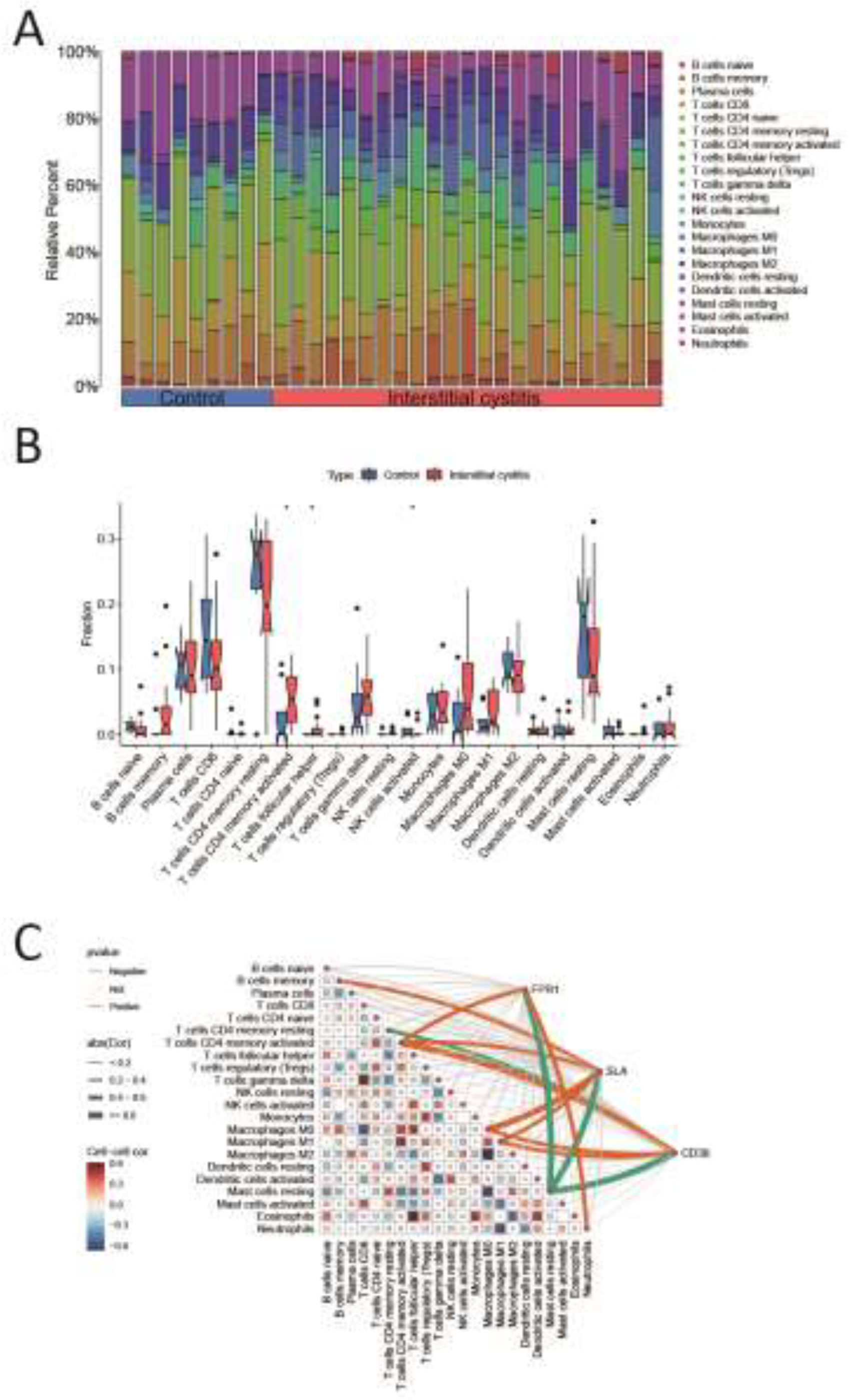
Figure 6.
Analysis of immune cell infiltration on IC. (A) Stacked histogram of the proportions of immune cells between the IC group and the control group. (B) Box plot showing the comparison of 22 types of immune cells between the IC group and the control group. (C) Heatmap showing the correlation between 22 types of immune cells and co-expressed genes. *p<0.05.
Figure 6.
Analysis of immune cell infiltration on IC. (A) Stacked histogram of the proportions of immune cells between the IC group and the control group. (B) Box plot showing the comparison of 22 types of immune cells between the IC group and the control group. (C) Heatmap showing the correlation between 22 types of immune cells and co-expressed genes. *p<0.05.
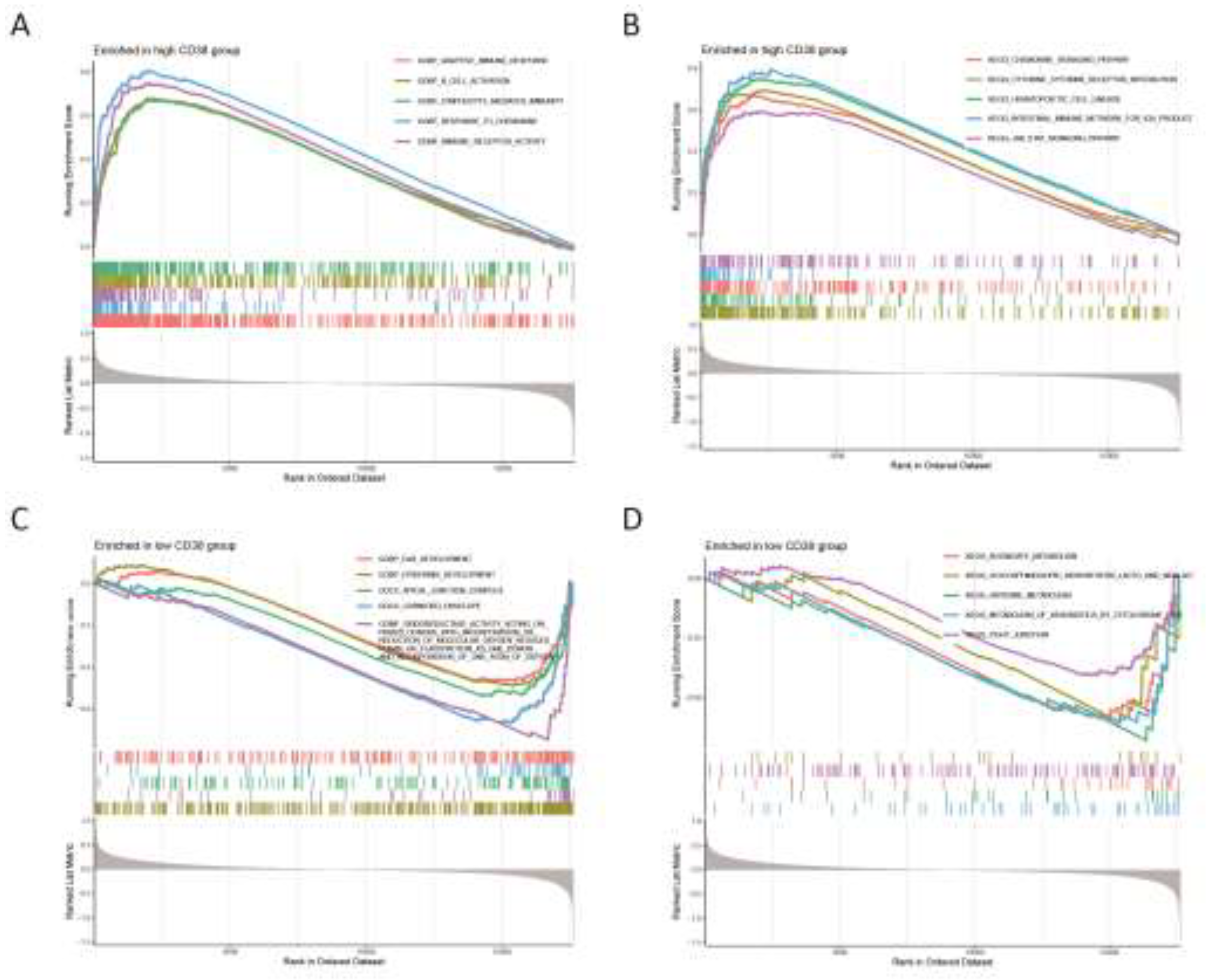
Figure 7.
Gene Set Enrichment Analysis. (A) The top 5 active biological functions in the CD38 high expression group. (B) The top 5 active pathways in the high CD38 expression group. (C) The top 5 active biological functions in the CD38 low expression group. (D) The top 5 active pathways in the low CD38 expression group.
Figure 7.
Gene Set Enrichment Analysis. (A) The top 5 active biological functions in the CD38 high expression group. (B) The top 5 active pathways in the high CD38 expression group. (C) The top 5 active biological functions in the CD38 low expression group. (D) The top 5 active pathways in the low CD38 expression group.
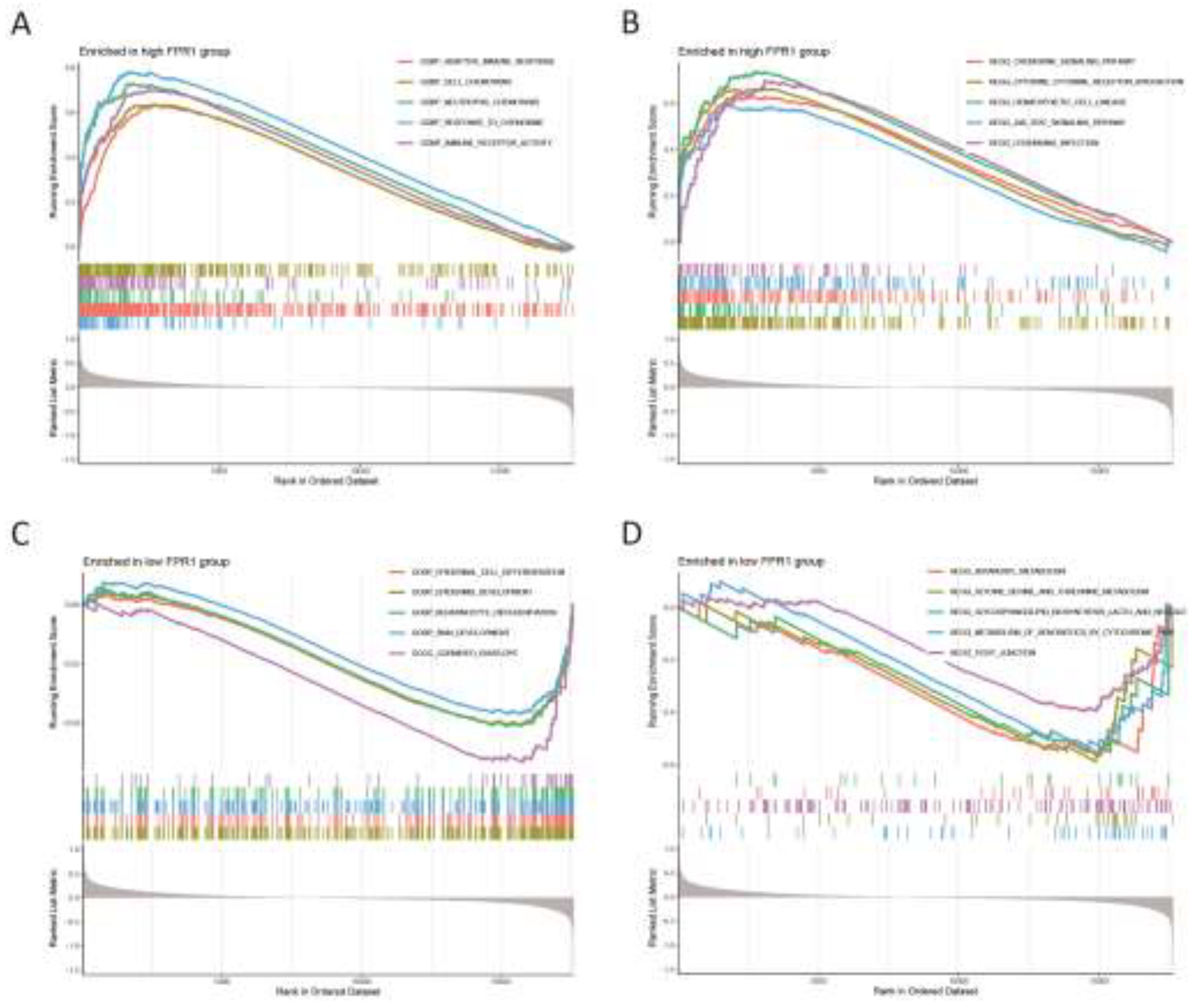
Figure 8.
Gene Set Enrichment Analysis. (A) The top 5 active biological functions in the FPR1 high expression group. (B) The top 5 active pathways in the high FPR1 expression group. (C) The top 5 active biological functions in the FPR1 low expression group. (D) The top 5 active pathways in the low FPR1 expression group.
Figure 8.
Gene Set Enrichment Analysis. (A) The top 5 active biological functions in the FPR1 high expression group. (B) The top 5 active pathways in the high FPR1 expression group. (C) The top 5 active biological functions in the FPR1 low expression group. (D) The top 5 active pathways in the low FPR1 expression group.
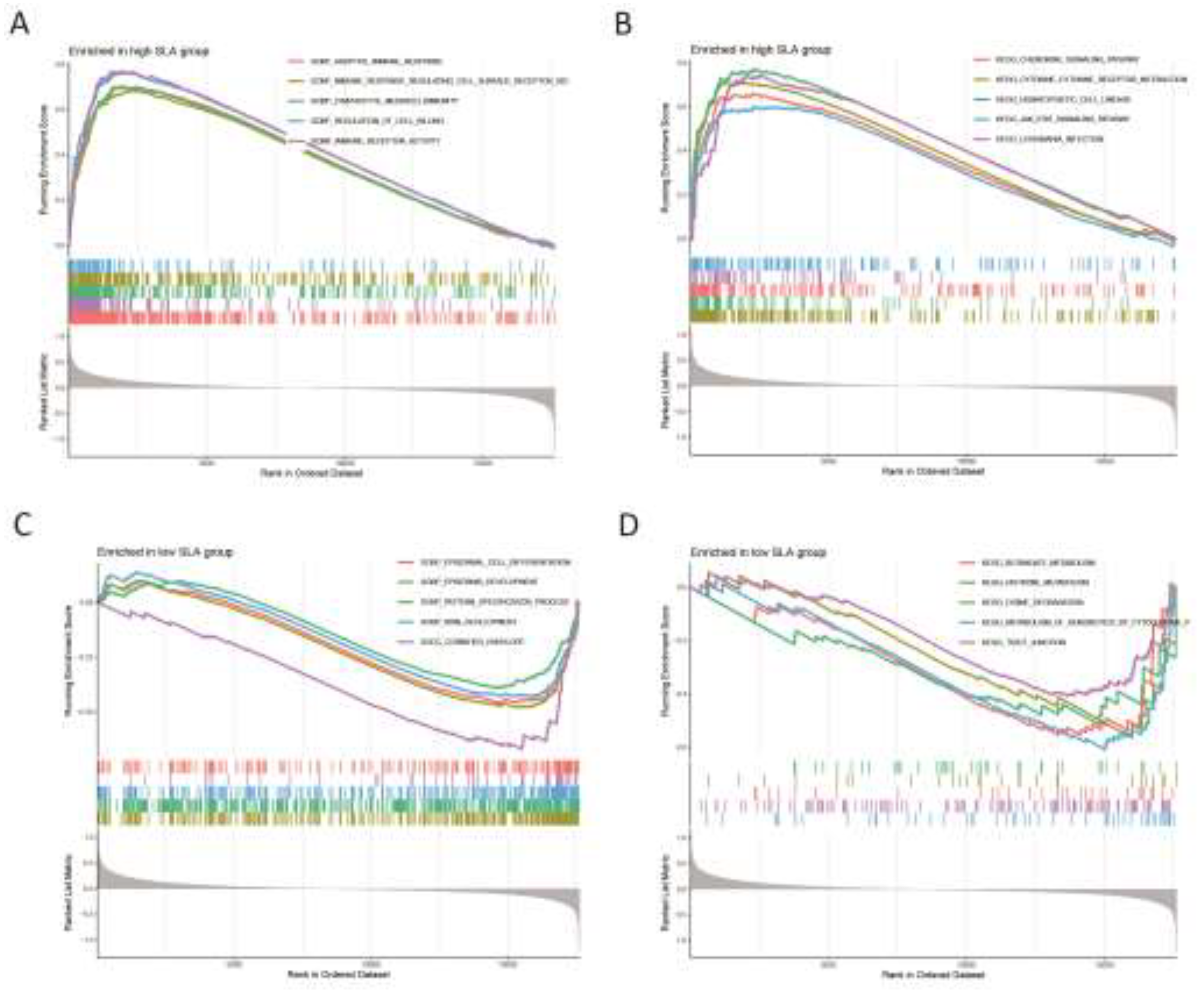
Figure 9.
Gene Set Enrichment Analysis. (A) The top 5 active biological functions in the SLA high expression group. (B) The top 5 active pathways in the high SLA expression group. (C) The top 5 active biological functions in the SLA low expression group. (D) The top 5 active pathways in the low SLA expression group.
Figure 9.
Gene Set Enrichment Analysis. (A) The top 5 active biological functions in the SLA high expression group. (B) The top 5 active pathways in the high SLA expression group. (C) The top 5 active biological functions in the SLA low expression group. (D) The top 5 active pathways in the low SLA expression group.
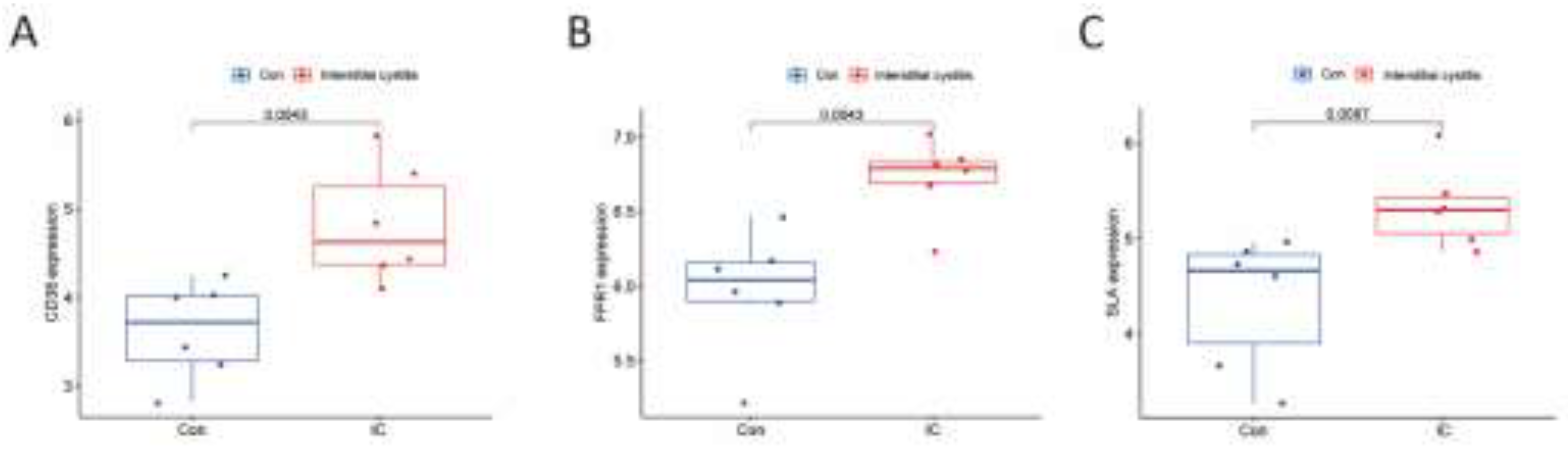
Figure 10.
The expression of CD38 (A), FPR1 (B), and SLA (C) in the validation group. Y-axis shows the relative expression level.
Figure 10.
The expression of CD38 (A), FPR1 (B), and SLA (C) in the validation group. Y-axis shows the relative expression level.