1. Introduction
Currently, there is a growing industrial demand for rare earth elements (REE), as these elements are now an integral part of the production of many carbon-neutral technologies. Interest has especially increased in secondary sources due to the huge potential for their recycling [
1]. An important difficulty is the separation of rare earth metals from each other. The main methods for extracting REE from both primary and secondary sources are: precipitation, extraction and sorption [
2,
3,
4,
5,
6]. The importance of the efficiency of the technological process for obtaining REE (rare earth metals) is explained by the fact that in recent years the demand for REE has grown rapidly, as they are used in high-tech and environmentally friendly technologies, such as high-performance magnets, rechargeable batteries and low-energy fluorescent lamps [
7]. Therefore, the aim of this study was to determine the conditions of selectivity of intergel systems based on KU-2-8(Na
+):AB-17-8(Cl
-) in relation to praseodymium and neodymium ions in static and dynamic sorption and desorption modes.
2. Materials and Methods
2.1. Equipment
The mass of the sorbents was determined by weighing on a Shimadzu TX423L electronic analytical balance. The residual concentration and concentrations after desorption were determined using a KFK-3 photocolorimeter and atomic emission analysis (Thermo Scientific™ iCAP™ PRO XP ICP-OES).
2.2. Materials
The studies were conducted in solutions of hexahydrate neodymium and praseodymium nitrate (the concentration of Nd3+ and Pr3+ = 30 mg/l each in the mixture and the concentration of Nd3+ and Pr3+ = 100 mg/l when studying the sorption dynamics individually for each ion). Industrial ion exchangers in salt form were used: strongly basic AB-17-8 (Cl-) and strongly acidic KU-2-8 (Na+). To conduct the study, interpolymer systems with different molar ratios of cation exchanger and anion exchanger were composed from these hydrogels. To work with a solution containing both metals, systems with molar ratios of 4:2, 3:3 were selected.
2.3. Experiment
The experiments were carried out at room temperature. Ion-exchange resins KU2-8 (Na+) and AV-17-8 (Cl-) were used in a dried state to study the sorption of neodymium ions. The moisture content in KU-2-8 (Na+) was 17%, and in AV-17-8 (Cl-) 32.34%. The studies of the interpolymer system were carried out in the following order: each ion-exchange resin was placed in dry form in separate polypropylene grids, in different molar ratios (from 6:0 - the initial cation exchanger to 0:6 - the initial anion exchanger). Then the polypropylene grids with ion-exchange resins KU2-8 and AV-17-8 were placed in glasses with solutions of neodymium and praseodymium nitrate (200 ml each), when studying the dynamics of sorption of each metal separately. Exactly the same procedure was performed for a solution of a mixture of these ions. The experiments with each REE separately were conducted in a static mode (without stirring), and aliquots were collected after 1, 6, 24, 48 hours from the start of the sorption process. In the case of the experiment with a mixture of metals, both modes were used: static and dynamic (with active stirring), and aliquots for atomic emission analysis were collected after 48 hours of sorption and 72 hours of desorption. Based on the data obtained on the dynamics of sorption of each REE separately, the molar ratios of cation exchanger and anion exchanger were selected: 4:2, 3:3. The main task was to check the presence of selectivity in these interpolymer systems. Desorption was carried out using nitric acid (2%) for 72 hours.
3. Results and Discussion
3.1. Relevance and Overview of the Problem
The relevance of such searches is confirmed by a huge number of works devoted to the possibilities of extracting REE from solutions obtained by leaching ores, as well as from processed raw materials. Thus, in a review study [
8], the authors confirm that sorption processes of all possible methods are the most relevant and most suitable for the environment. The article lists such modern developments as: polymers with ion imprints, porous organic polymers, chelate ion exchangers, silicate adsorbents (based on covalently and non-covalently bound ligands), metal-organic frameworks, magnetic adsorbents, as well as modified materials from nanocarbon.
However, the extraction and separation of REEs remain expensive and complex, creating numerous environmental problems [
9]. Recently, solvent extraction has become a popular method for recovering REEs from liquid wastes, but its dependence on flammable and toxic organic solvents makes it unsuitable for the treatment of industrial wastewater with low concentrations of rare earth elements. In contrast, adsorption, which binds metal ions to an adsorbent, is more environmentally friendly, reduces the use of solvents and additives, allows for a wider range of concentrations, and is similar in efficiency. Given the stringent environmental regulations, adsorption is commercially superior to solvent extraction [
10]. A variety of adsorbents have been investigated for the recovery of REEs from ores and municipal solid wastes, including activated carbon, carbon nanotubes, dried biomass, nanocomposites, polymeric materials, silica, and zeolites [
11]. The adsorption of REE is influenced by various mechanisms, each of which is driven by specific forces. A special role is played by the mechanisms of chemical coordination between the metal ion and the adsorbent.
The study [
12] devoted to analog materials based on porous sorbents also indicates the advantages of adsorption compared to other methods of extracting target metals. The development of effective porous organic polymers based on carbon, porous silicon and porous metal-organic frameworks are the leading directions today. But the main problem with such materials is the complexity of their manufacture and, therefore, their high cost.
A possible solution to the problems of high cost and complexity of manufacturing highly selective adsorbents may be the use of conventional industrial sparsely cross-linked hydrogels based on the "long-range effect" or, in other words, the "remote interaction effect". The use of cross-linked polyelectrolytes is also justified from an environmental point of view. In this case, there is no need to use toxic and flammable extractants, sorption can be carried out in aqueous solutions, and traditional cationites and anionites can be used repeatedly. The action of this effect is largely associated with the behavioral features of macromolecule charges, and was first studied in [
13,
14,
15,
16].
The phenomenon of remote interaction largely affects the physicochemical properties of hydrogels (both cationites and anionites). The effect can be observed by changing such characteristics of hydrogels in intergel systems of various ratios as: pH of the solution, electrical conductivity, degree of swelling and the amount of sorption of rare earth metal ions. Thus, in the works [
17,
18,
19,
20,
21,
22], the features of sorption of rare earth metals by various polyelectrolyte systems and changes in the properties of cationites and anionites during mutual activation were studied.
3.2. Study of Sorption of Target Metal Ions by Interpolymer Systems
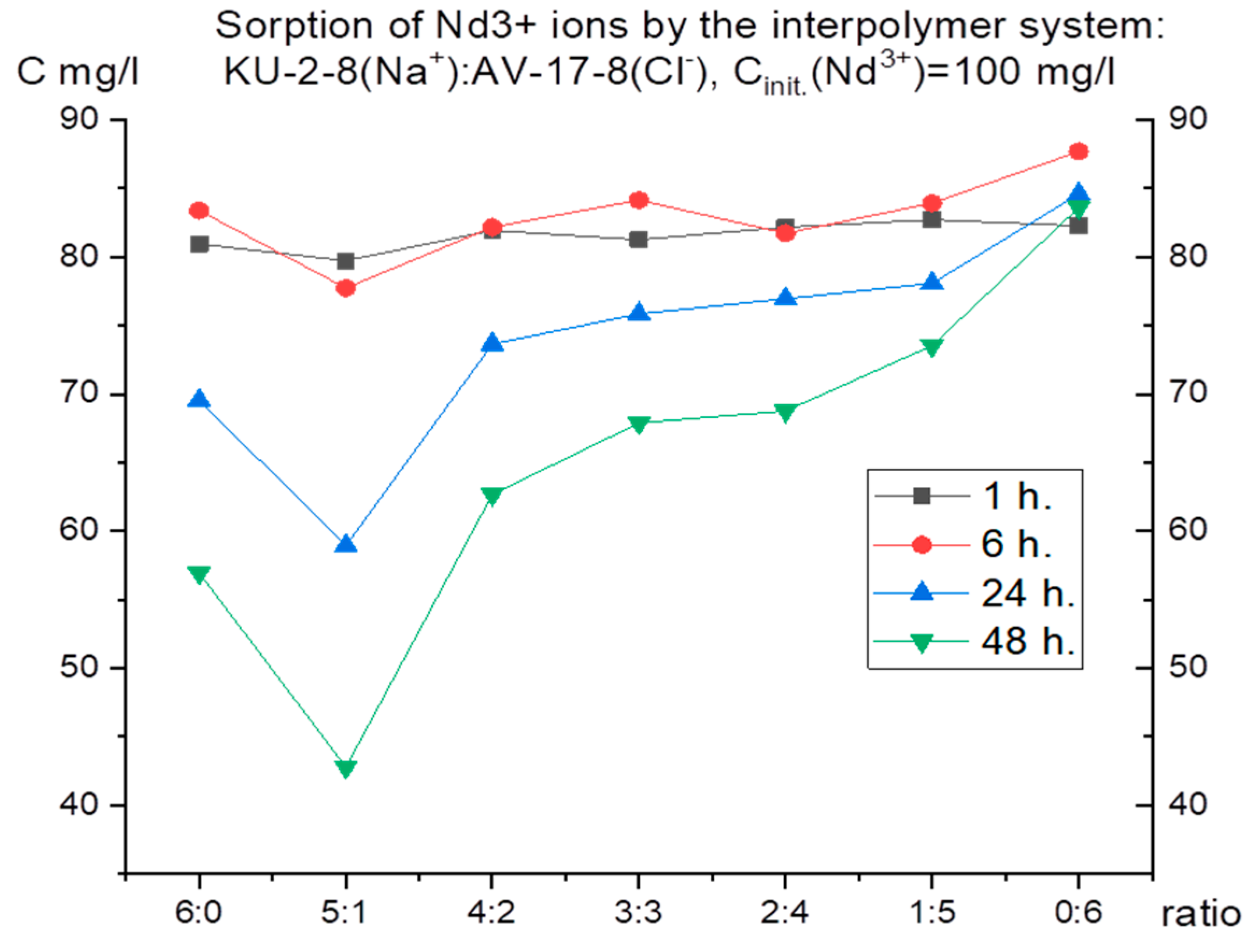
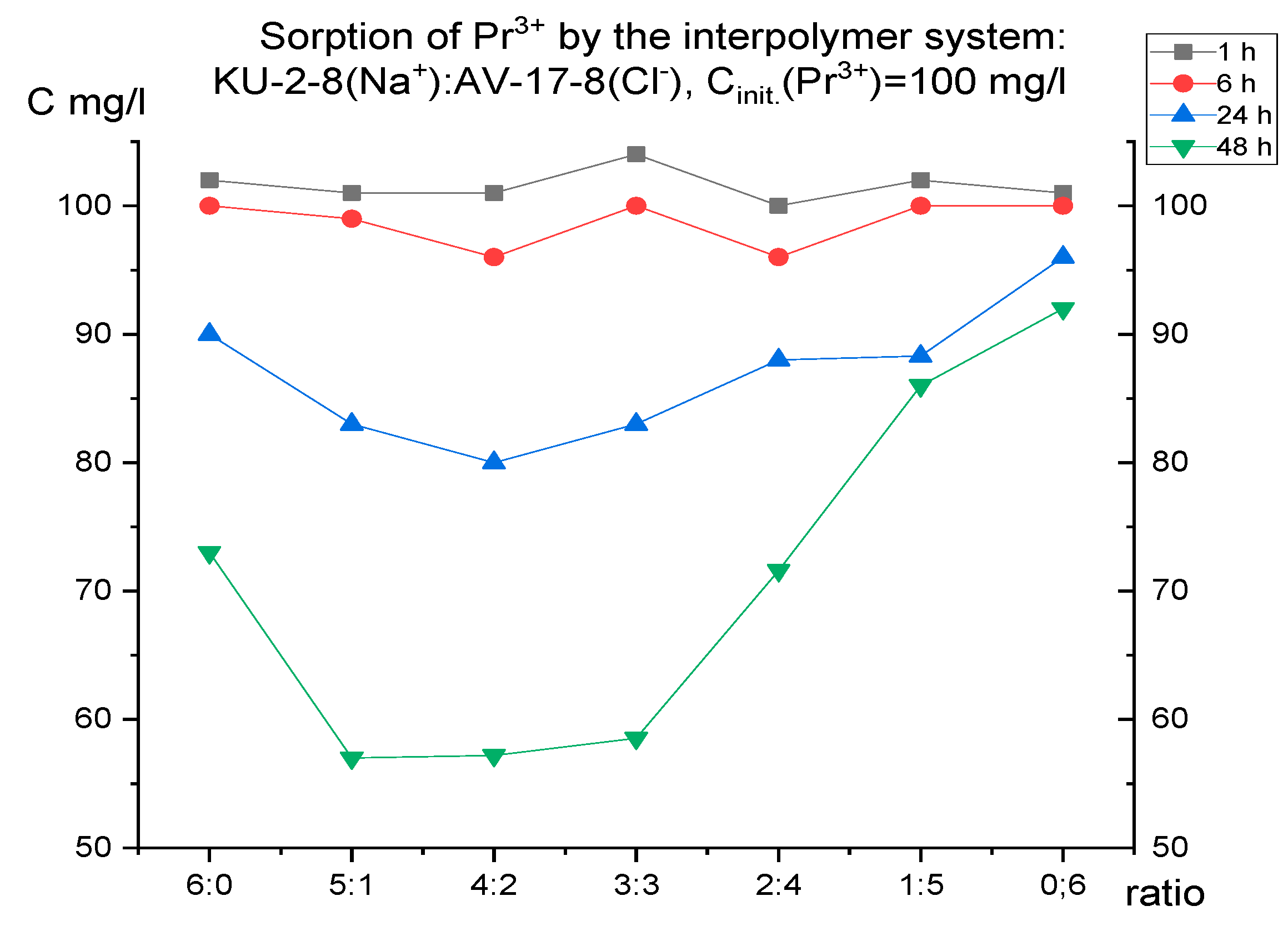
For selective extraction of one of the presented metals, it was necessary to initially establish the features of their individual sorption. For this purpose, the dynamics of absorption were studied separately for 48 hours using model solutions with a concentration of 100 mg/l of each metal (neodymium, praseodymium) (taking aliquots after 1, 6, 24, 48 hours, respectively).
Figure 1 and
Figure 2 present the results of ion sorption at different molar ratios of hydrogels (cation-exchange resin and anion-exchange resin).
It is interesting to note that, despite the close ionic radius, oxidation state and mass, the curves on the graphs of these metals differ. This means that each ion has its own characteristic absorption dynamics by the interpolymer systems KU-2-8 (Na
+): AB-17-8 (Cl
-), which can presumably be used for the selective extraction of one metal in several cycles. Thus, for praseodymium, after 48 hours of sorption, the lowest residual concentrations are observed at PE ratios of 5:1, 4:2 and 3:3. For neodymium, after the same amount of time, the highest absorption is observed mainly only in the ratio of 5:1. Therefore, for further study of the issue of possible selective sorption using these results for each ion separately, interpolymer systems can be selected. Based on the fact that in the case of neodymium there were no peaks of increased sorption in the ratio of 4:2 and 3:3, unlike praseodymium, it is logical to select these molar ratios of hydrogels and not take into account the other systems. Thus, we make an assumption that it is in these systems that we can obtain selectivity with respect to praseodymium ions. To test this idea, molar ratios of cation exchanger and anion exchanger of 4:2 and 3:3 were used in a mixture of neodymium and praseodymium solutions. The initial concentration of each ion was 30 mg/l. In this case, sorption was carried out in two modes: dynamic (with active mixing) and static (without mixing the model solution of the metal mixture). This solution was aimed at testing the possibility of influencing the choice of mode on the selectivity of extraction (since in the case of active mixing, equilibrium is established faster, and even if one of the ions binds to the polymer matrix more energetically favorably, the kinetic factor can negate this small difference).
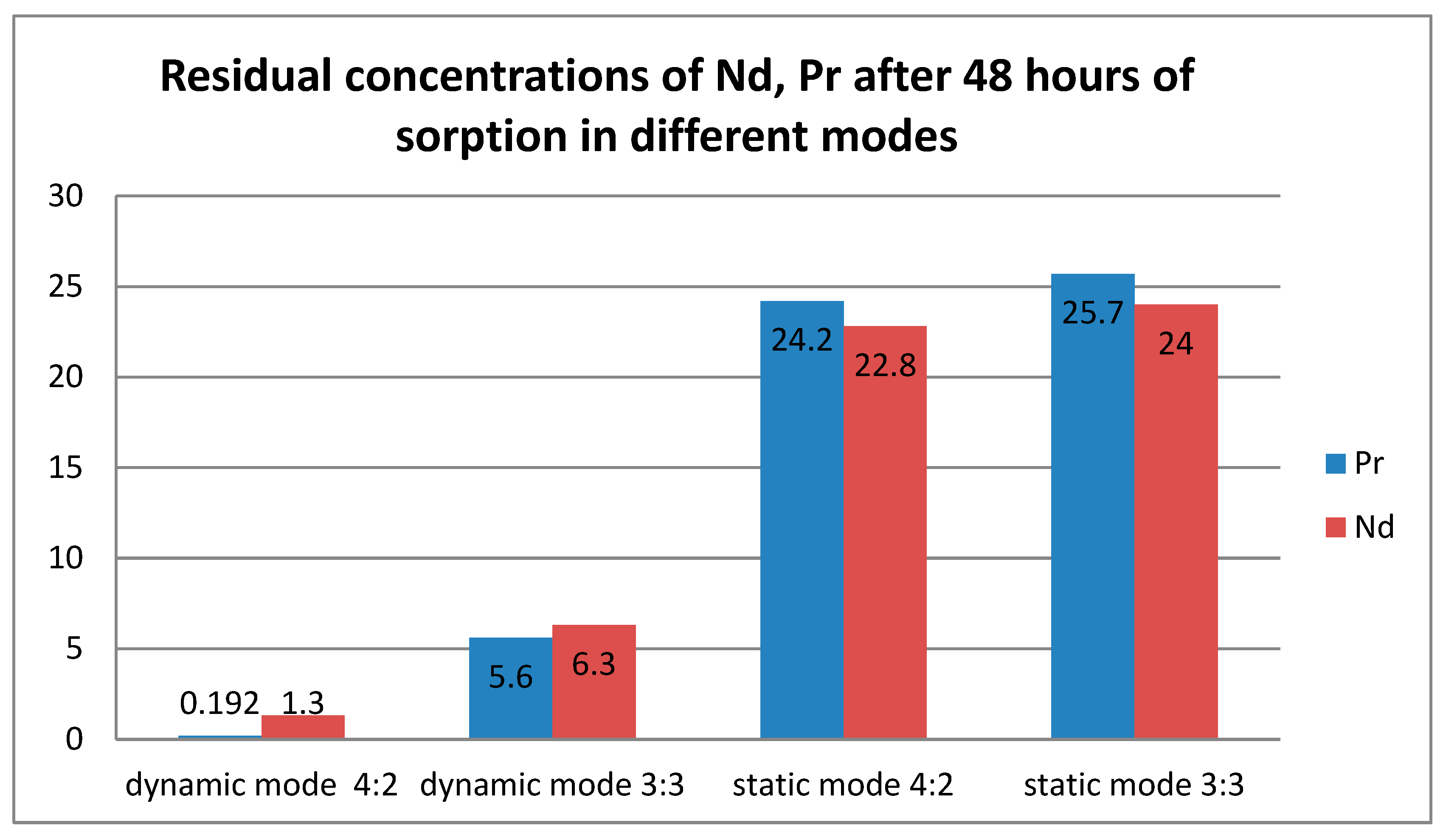
Table 1 presents the results of the residual concentration of the target metals after 48 hours of sorption. As can be seen from the numerical values, with constant mixing, the residual concentration of both metals is significantly lower than in the static mode. Also of interest is the fact of better sorption of Pr
3+ ions in the dynamic mode, and vice versa, better sorption of Nd
3+ in the static mode. This partly contradicts our expectations, since in the case of individual sorption in the ratios of 4:2 and 3:3 in the static mode, we expected higher absorption of praseodymium.
Figure 3 shows the final graph for the residual concentrations of the ions under study.
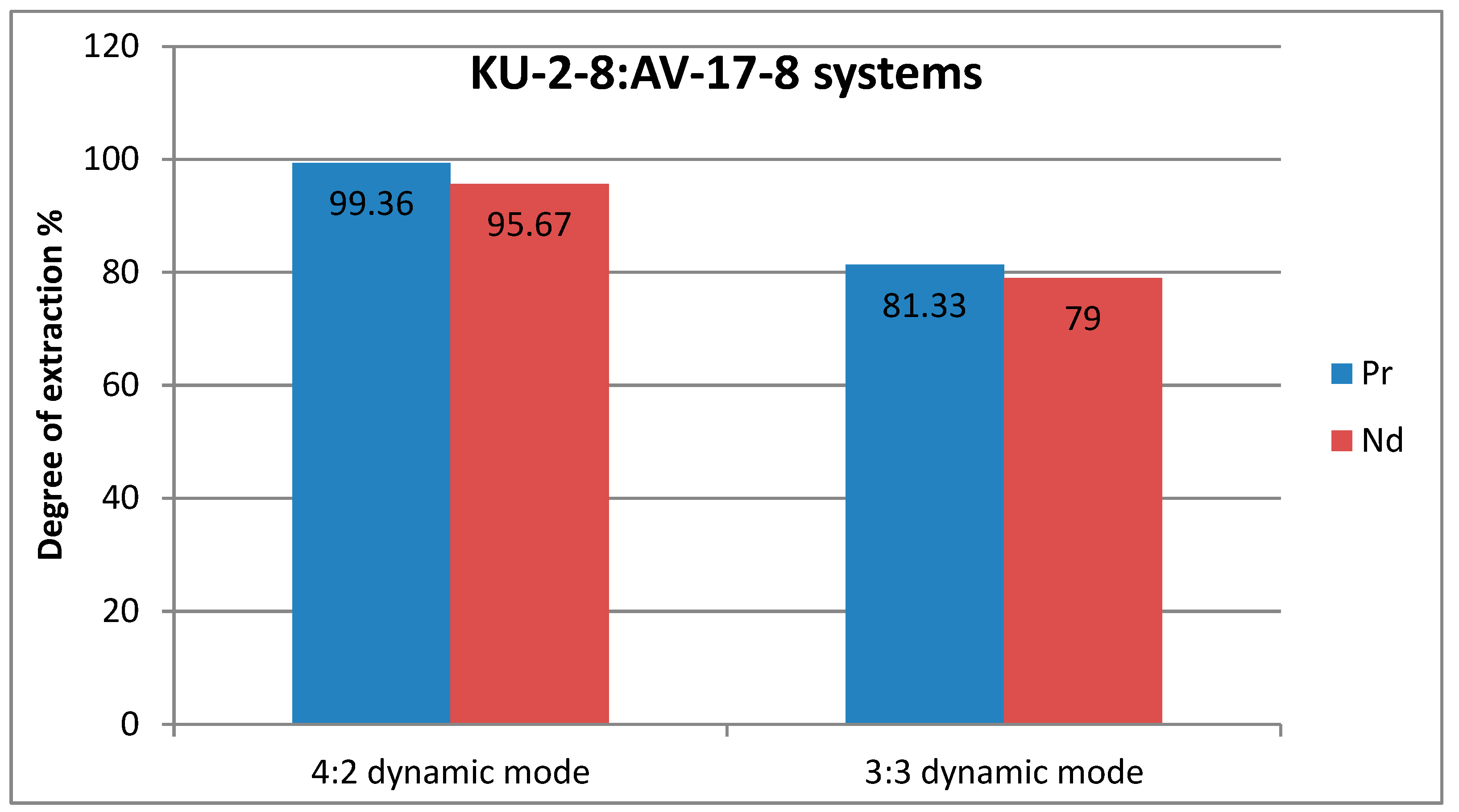
To answer the most important question about the possibility of selectivity, it is necessary to calculate the extraction rates of praseodymium and neodymium and to calculate per 1 mole of polymer, assuming that the anionite does not participate in sorption.
Table 2 presents the results of the extraction rate for each ion. At first glance, it is clear that the greatest difference in the percentage of extraction rate is observed in the 3:3 static mode.
The degree of extraction (sorption) of neodymium and praseodymium ions was calculated using the formula:
where C
init. – initial concentration of neodymium ions in solution, mg/l; С
resid.– residual concentration of neodymium ions in solution, mg/l
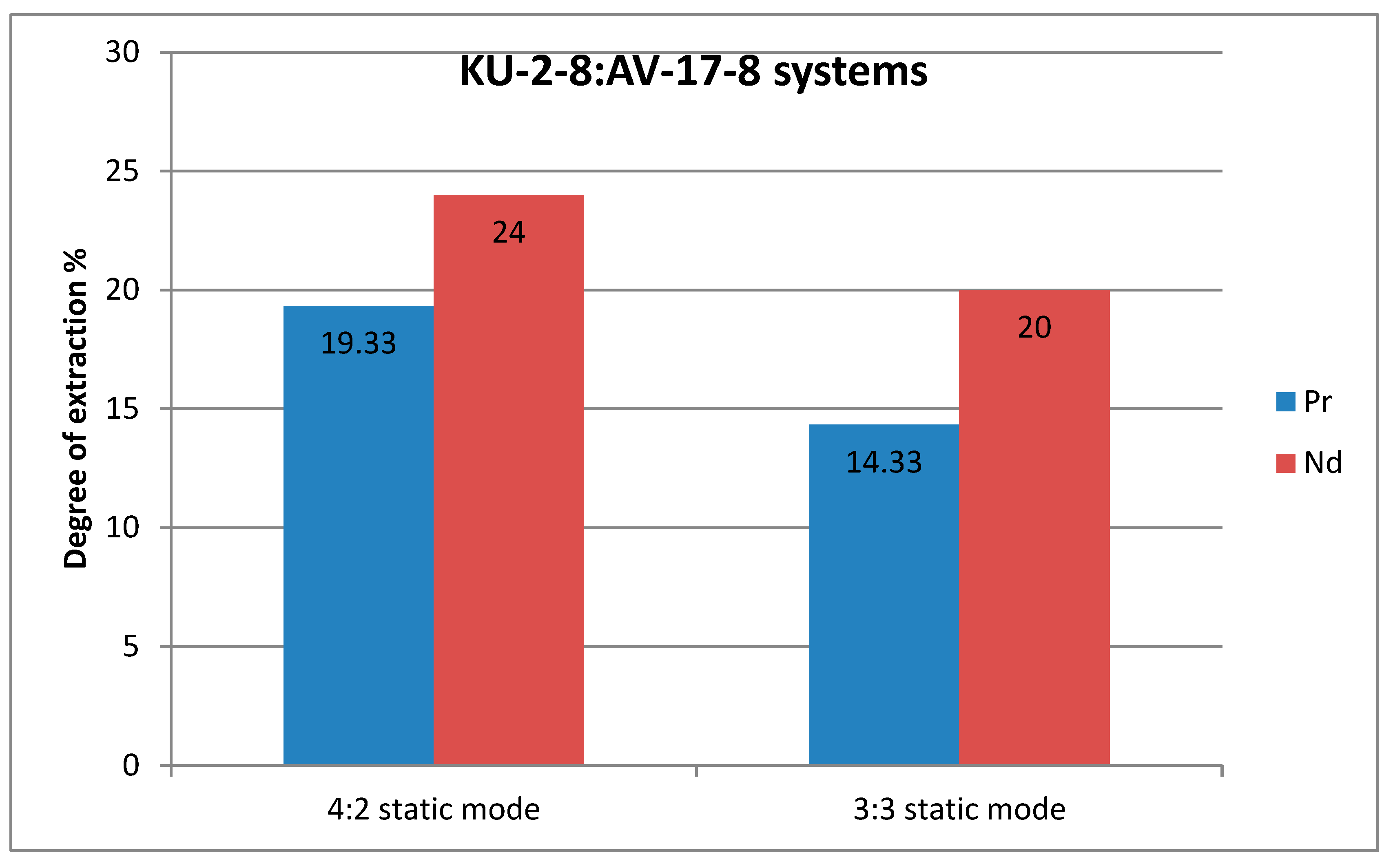
Figure 4 and
Figure 5 show graphs of the degree of extraction, where a higher difference in the values of the degree of extraction in the static mode is clearly visible, which may indicate that despite the rapid establishment of equilibrium with active mixing, it may be more advantageous to carry out the sorption process in a stagnant solution to obtain greater selectivity. In this case, a contradiction arises: the choice of a dynamic mode to accelerate the sorption process and a greater degree of extraction of each metal, or the choice of a static mode with lower indicators of the degree of extraction and a long establishment of equilibrium, but slightly greater selectivity with respect to one of the target ions.
Two other important sorption indices, the total degree of polymer chain binding and the effective dynamic sorption capacity, were calculated to provide a complete picture of the sorption characteristics of each target ion under different interaction conditions. Those results are shown in the
Table 3 and
Table 4.
The total degree of binding of the polymer chain was determined by the following formula:
where ν
sorb. is the amount of sorbed metal, mol; ν is the amount of polymer sample, mol.,
- total degree of binding of the polymer chain. The effective dynamic sorption capacity was calculated using the formula:
where νsorb. is the amount of sorbed metal, mol; msorbent is the mass of the sorbent.
Here we can also observe large differences between the different sorption modes. With constant stirring, the degree of polymer chain binding is four times higher than the same indicator for sorption in a stagnant solution. The same results can be observed for the effective dynamic sorption capacity
3.3. Study of Sorption of Target Metal Ions by Interpolymer Systems
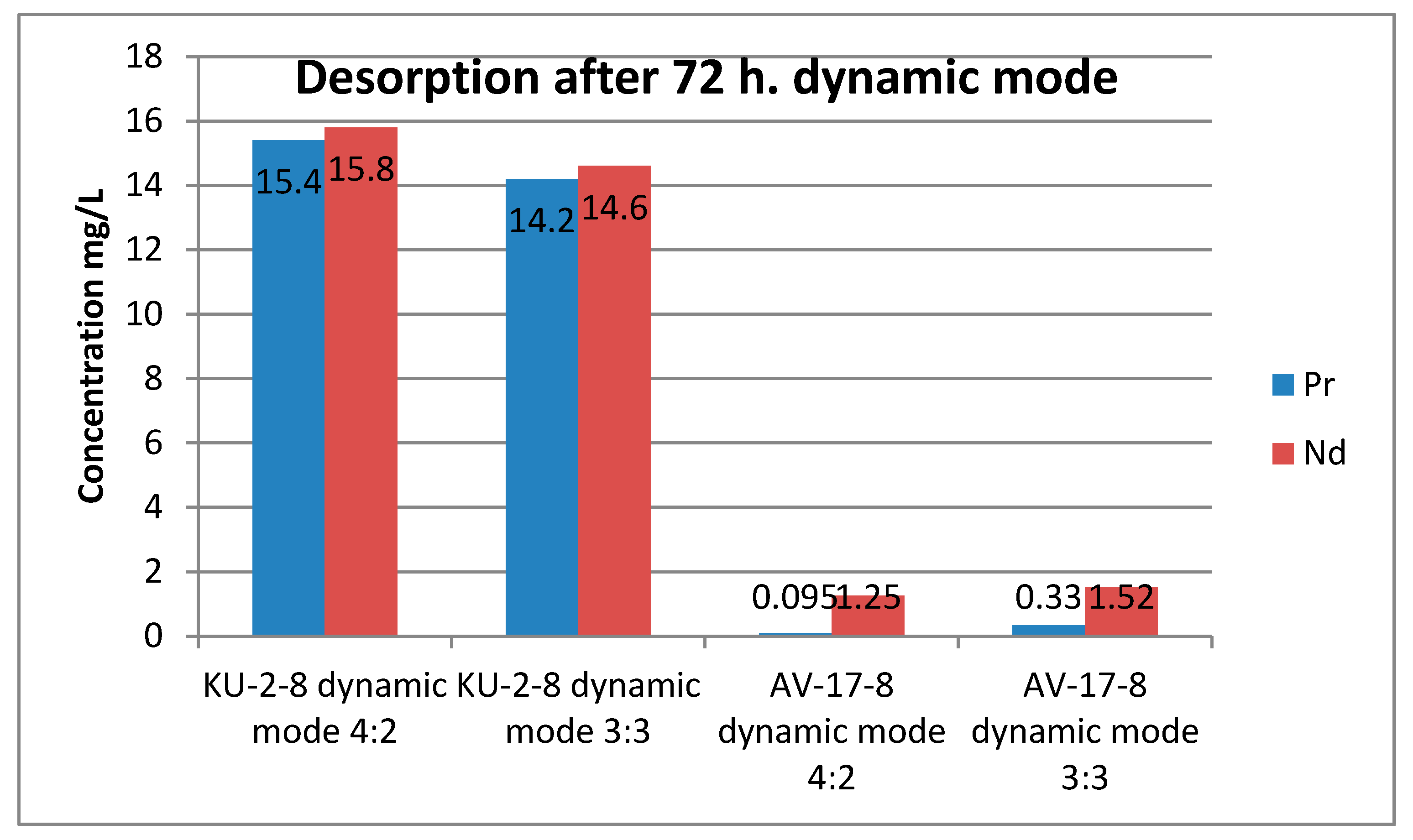
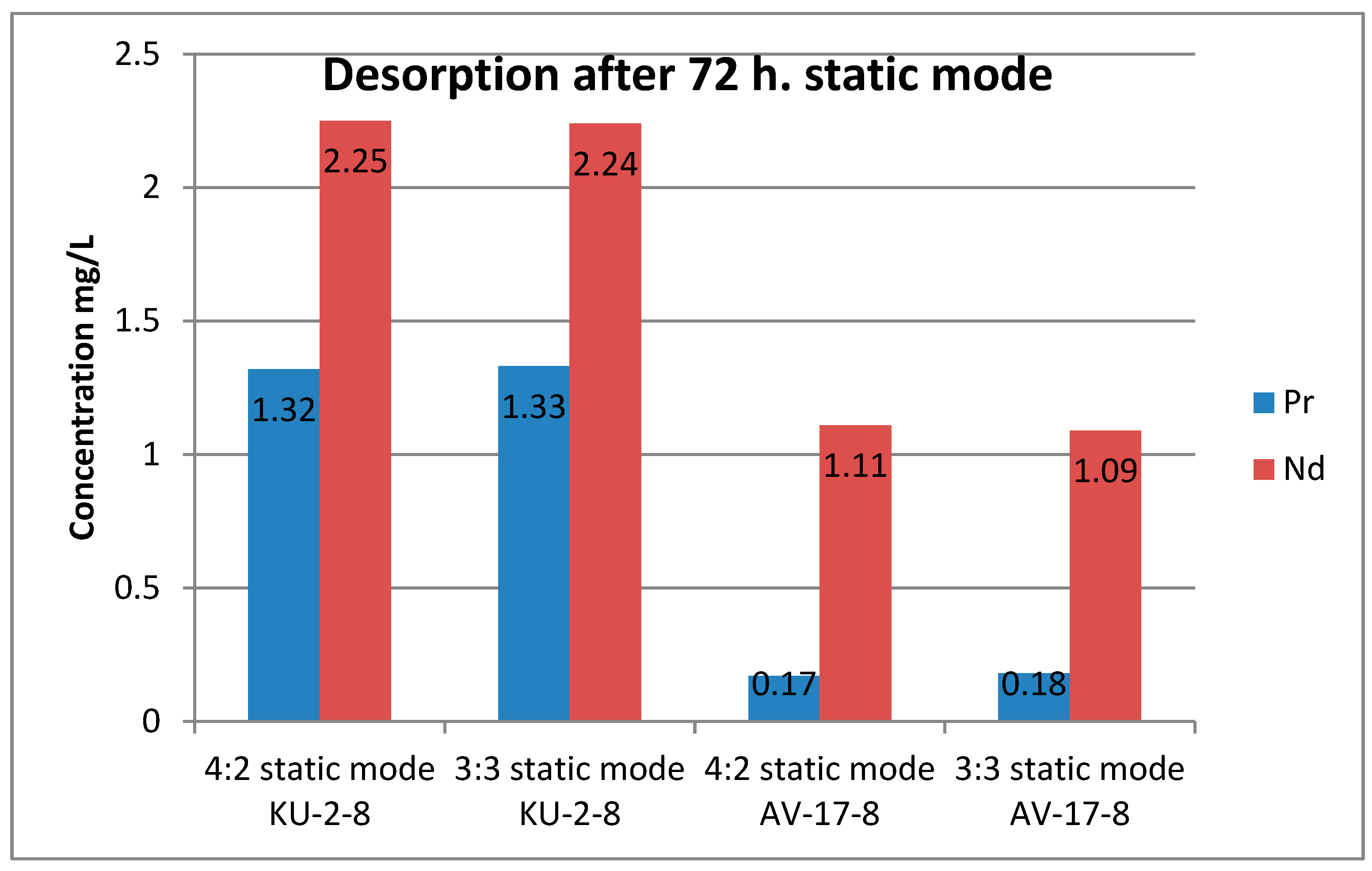
The desorption results presented in
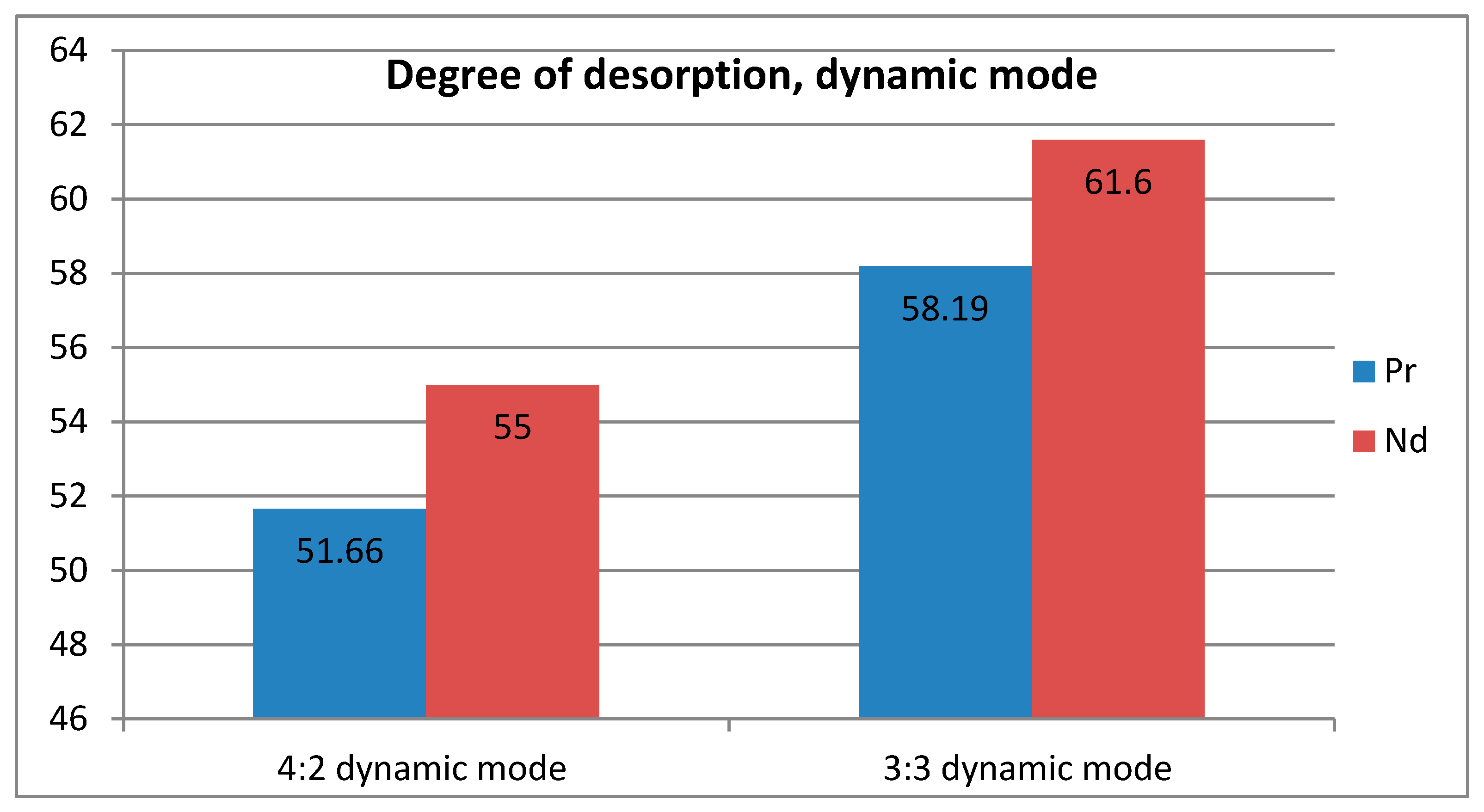
Table 5 are interesting. First of all, considering the results of desorption from the cationite, it is easy to notice that in the dynamic mode it was possible to obtain more of both ions than in the static mode. Moreover, the difference in the ratios of 4:2 and 3:3 is small in both modes. The figure shows a graph of neodymium and praseodymium desorption in the dynamic mode. Regardless of the sorption mode, neodymium was better desorbed from the polymer matrix compared to neodymium.
Table 4 shows the desorption results.
Figure 6 and
Figure 7 show histograms that clearly show the difference in the choice of desorption mode for selectivity.
To get a clearer picture, it is necessary to calculate the degree of desorption in order to operate with percentage ratios. This value was calculated using the following formula:
where ω is the degree of desorption, C
desor. is the concentration of desorbed metal, Csorb is the concentration of absorbed metal. C
sorb. was calculated by subtracting the residual concentration from the initial ion concentration. The results for the degree of desorption for each ion are presented in the
Table 6.
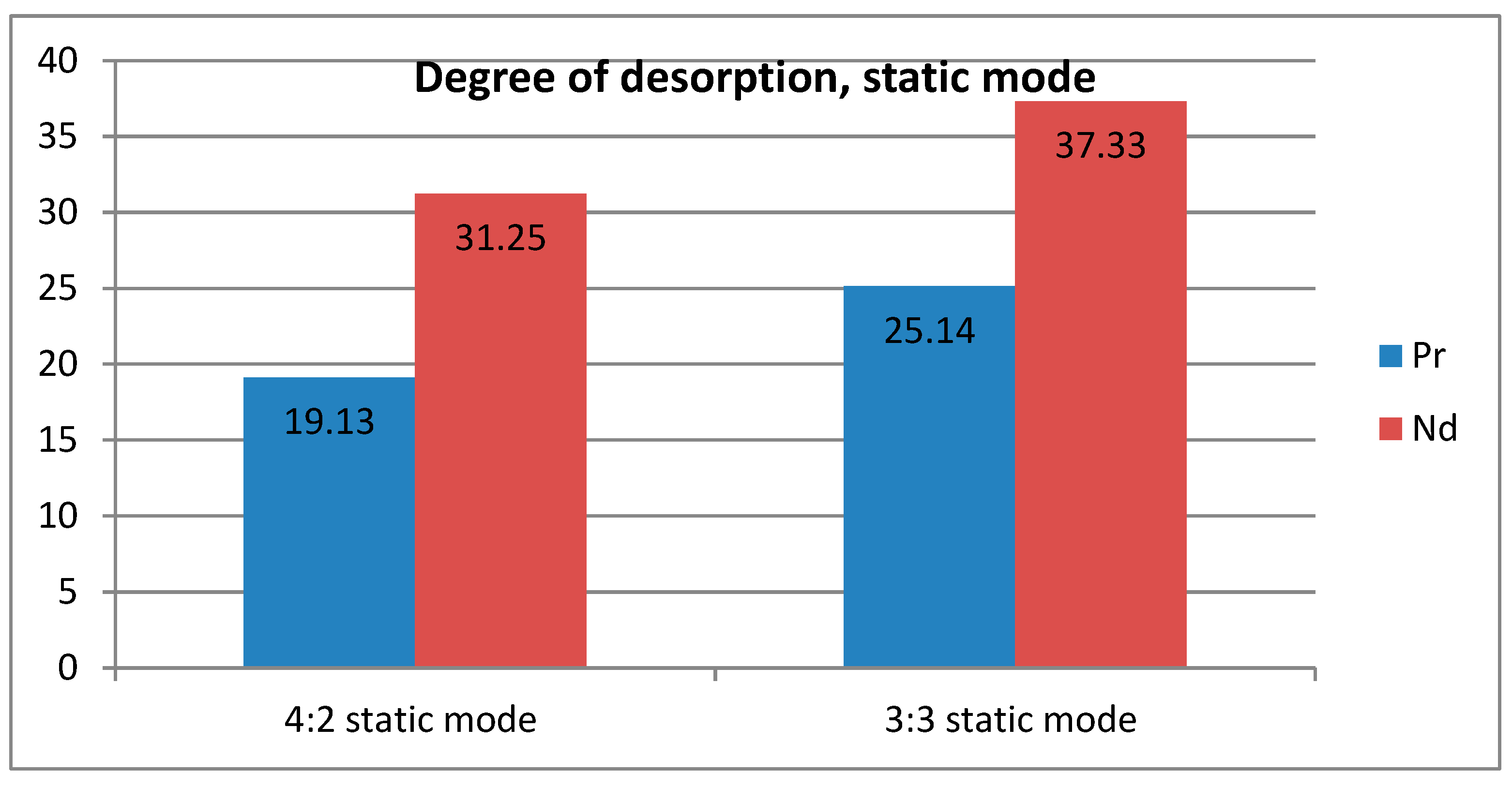
The degree of desorption shows that with active mixing both metals are almost equally extracted from the polymer matrix. In the static mode, a large difference appears between the desorption of the two metals. This leads to the conclusion that the choice of mode can affect the selectivity of both sorption and desorption. In the latter case, this is especially important, since the desired separation can be obtained at this stage (there is no point in selective sorption if it is impossible to obtain the target ion from the sorbent matrix at the next stage).
Figure 8 and
Figure 9 show graphs of the degree of desorption, where the difference between the values of the degree of desorption of two metals in two different modes is clearly visible.
5. Conclusions
Thus, based on the results obtained, several conclusions can be drawn:
1) Interpolymer systems based on KU-2-8 and AV-17-8 are good sorbents of neodymium and praseodymium ions (recovery rates, respectively, 4:2: 95.67% and 99.36%, 3:3: 79% and 81.33 %.
2) It is impossible to predict how this ion will be sorbed in a mixture with another metal based on studying the sorption characteristics of each ion individually from model solutions. For example, according to the initial hypothesis, we suggested that in 4:2 and 3:3 systems in static mode, praseodymium will be better sorbed from a mixture of two ions and neodymium will be worse. This assumption was based on the pattern of their individual sorption from model solutions. As a result of the experiments, the opposite situation was revealed: in the static mode, neodymium ions were better sorbed, and with active mixing, praseodymium ions. This suggests that the process of sorption of ions of similar charge and radius from multicomponent systems is complex.
3) The choice of sorption and desorption modes not only affects the amount of sorbed and desorbed ions, but also the selectivity of the entire process. It was found that without stirring, sorption is slightly more selective towards neodymium. This effect increased during desorption, when also the mode without stirring showed greater selectivity to neodymium.
4) Thus, the choice of conditions for the entire process greatly affects the results obtained. Although the dynamic mode with active mixing showed lower selectivity, the degree of extraction for both ions was much higher compared to sorption and desorption from stagnant solutions. Therefore, a contradiction arises: in statics, to obtain slightly higher selectivity with respect to praseodymium, but to lose significantly in the degree of extraction of both metals, or to obtain high indicators of the degree of extraction and desorption for both metals, but to lose in selectivity.
Author Contributions
Conceptualization and methodology, validation, data curation J.T.; formal analysis, investigation, writing—original draft preparation, visualization K.K.; writing—review and editing, data curation, visualization, Z.M.; supervision, data curation, writing—review and editing, visualization K.Z. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research has been/was/is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR21882220 and BR18574042.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balaram V. Potential future alternative resources for rare earth elements: Opportunities and challenges //Minerals. – 2023. – Т. 13. – №. 3. – С. 425.
- Ivanik S.A., Ilyukhin D.A. Flotation extraction of elemental sulfur from gold-bearing cakes. Journal of Mining Institute. 2020. Vol. 242, p. 202-208. DOI: 10.31897/PMI.2020.2.202. [CrossRef]
- Cui H., Feng X., Sh J., Liu W., Yan N., Rao G., Wang W. A facile process for enhanced rare earth elements separation from dilute solutions using N, N-di(2-ethylhexyl)-diglycolamide grafted polymer resin. Separation and Purification Technology. 2020. Vol. 234. 116096. DOI: 10.1016/j.seppur.2019.116096. [CrossRef]
- Turanov A.N., Karandashev V.K., Baulin V.E., Baulin D.V., Khvostikov V.A. Extraction of Rare-Earth Elements (III) from Nitric Acid Solutions with Diethyl 2-[(Diphenylphosphoryl)methoxy]-5-ethylphenylphosphonate. Russian Journal of Inorganic Chemistry. 2019. Vol. 64(10), p. 1297-1303. DOI: 10.1134/S0036023619100164. [CrossRef]
- Maria L., Cruz A., Carretas J.M., Monteiro B., Galinha C., Gomes S.S., Araújo M.F., Paiva I., Marçalo J., Leal J.P. Improving the selective extraction of lanthanides by using functionalised ionic liquid. Separation and Purification Technology. 2020. Vol. 237. 116354. DOI: 10.1016/j.seppur.2019.116354. [CrossRef]
- Swain N., Mishra S., Acharya M.R. Hydrometallurgical route for recovery and separation of samarium (III) and cobalt (II) from simulated waste solution using tri-n-octyl phosphine oxide – A novel pathway for synthesis of samarium and cobalt oxides nanoparticles. Journal of Alloys and Compounds. 2020. Vol. 815. 152423. DOI: 10.1016/j.jallcom.2019.152423. [CrossRef]
- Balaram V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact //Geoscience Frontiers. – 2019. – Т. 10. – №. 4. – С. 1285-1303.
- Artiushenko O., da Silva R. F., Zaitsev V. Recent advances in functional materials for rare earth recovery: A review //Sustainable Materials and Technologies. – 2023. – С. e00681.
- Charalampides, G.; Vatalis, K.; Karayannis, V.; Baklavaridis, A. Environmental Defects and Economic Impact on Global Market of Rare Earth Metals. IOP Conf. Ser. Mater. Sci. Eng. 2016, 161, 012069. https://doi.org/10.1088/1757-899X/161/1/012069. [CrossRef]
- Chen, Z.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H. Recent Advances in Selective Separation Technologies of Rare Earth Elements: A Review. J. Environ. Chem. Eng. 2022, 10, 107104. https://doi.org/10.1016/j.jece.2021.107104. [CrossRef]
- Mosai, A.K.; Tutu, H. Simultaneous Sorption of Rare Earth Elements (Including Scandium and Yttrium) from Aqueous Solutions Using Zeolite Clinoptilolite: A Column and Speciation Study. Miner. Eng. 2021, 161, 106740. https://doi.org/10.1016/j.mineng.2020.106740. [CrossRef]
- Peng Y. et al. Overview of Functionalized Porous Materials for Rare-Earth Element Separation and Recovery //Molecules. – 2024. – Т. 29. – №. 12. – С. 2824.
- Jumadilov T.K., Ismailova Sh.A. Intergel interactions of three-dimensional structures based on polyacrylic acid and polyethyleneimine // Bulletin of KazNU, ser. chemistry. - 2005. - No. 4 (40). - P. 60-64. 43.
- Ismailova Sh.A., Jumadilov T.K., Bekturov E.A. Interaction of rare-crosslinked polyacrylic acid with nitrogen-containing polymers // Bulletin of KazNTU. - 2004. - No. 3 (41). - P. 186-189.
- Ismailova Sh.A., Jumadilov T.K., Bekturov E.A. Features of complex formation of rare-crosslinked polyacrylic acid with polyacrylamide hydrogel // Izvestiya MES RK NAS RK, ser. chem. - 2004. - No. 4. - P. 80-85.
- Ismailova Sh.A. Features of interaction of three-dimensional structures based on polycarboxylic acids and nitrogen-containing polymers: dissertation for the degree of candidate of chemical sciences:. 02.00.06. – Almaty: ICN MES RK. 2006. – 109 p.
- Kokufuta E., Ogawa K., Miyake M. Polyelectrolyte complex formation between anionic and cationic nanogels in salt-free aqueous solution // Abstr. 6th Int. Symp. Polyelectrolytes, Dresden. - 2006. – Р. 1324-1326.
- Karpushkin E.A., Kechekyan A.S., Zezin A.B. Interpolyelectrolyte reaction between particles of oppositely charged microgels // High-molecular compounds, series B. - 2006. - V. 48, No. 10 - P. 2053-2057.
- Jumadilov T.K. et al. EXTRACTION OF YTTRIUM IONS BY INTERPOLYMER SYSTEMS BASED ON INDUSTRIAL IONITES // Chemical Journal of Kazakhstan. - 2021. - No. 1 (73). - P. 134-141.
- Jumadilov T. K., Kondaurov R. G., Imangazy A. M. COMPARISON OF SORPTION PROPERTIES OF POLYACIDS AND POLYBASES, AS WELL AS INTERGEL SYSTEMS BASED ON THEM IN RELATION TO NEODYMIUM IONS //Chemical Journal of Kazakhstan. – 2019.
- Jumadilov T. et al. Effective sorption of europium ions by interpolymer system based on industrial ion-exchanger resins amberlite IR120 and AB-17-8 //Materials. – 2021. – Т. 14. – №. 14. – С. 3837.
- Jumadilov T. et al. Ion exchange dynamics in cerium nitrate solution regulated by remotely activated industrial ion exchangers //Materials. – 2021. – Т. 14. – №. 13. – С. 3491.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).