Submitted:
04 December 2024
Posted:
05 December 2024
You are already at the latest version
Abstract
Background: Cervical cancer is highly prevalent among women in Amazonas, Brazil, mainly due to late-stage diagnosis, which compromises treatment efficacy and survival rates. This highlights the urgent need for less invasive biomarkers to monitor affected patients. Methods: This study employed real-time PCR targeting the E7 gene of HPV types 16 and 18 to analyze plasma samples from 39 cervical cancer patients treated at the Oncology Control Center Foundation in Amazonas, Brazil. Results: cf-HPV 16 DNA was detected in 54% of samples before treatment. Socioeconomic and behavioral data showed that 46.2% of patients had low educational levels, 77% reported low income, 79.5% experienced early sexual activity onset, and 15.4% had never undergone cytological screening. Recurrence or persistence occurred in 30.8% of cases over 4–33 months of follow-up, with cf-HPV DNA detectable (at any time, pre- or post-treatment) in 75% of these cases. Conclusions: cf-HPV DNA in plasma is a promising biomarker for post-treatment surveillance, facilitating earlier detection of recurrence and proactive interventions. Incorporating this biomarker into clinical protocols could enhance outcomes and survival, particularly in underserved regions like the Amazon, where access to healthcare is limited.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Population Study and Samples
2.2. Biological Samples Collection and Processing
2.3. DNA Extraction
2.4. Human β-actin PCR
2.5. E7 HPV16/HPV18 Type-Specific Quantitative Real-time PCR (qPCR)
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L; Laversanne, M; Soerjomataram, I.; Jemal, A.; Bray,F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 2021, 71(3):209–49. [CrossRef]
- Wild, C.P; Weiderpass,E.; Stewart,B.W. World Cancer Report: Cancer Research for Cancer Prevention. Cancer Control, IARC,2020, 477.
- Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva. Available online: www.inca.gov.br/utero(acessed on march 4, 2024).
- Sousa, G.A.; Viana, J.N.; Souza, C.S.M.; Moysés, R.P.C. Linha de Cuidado do Câncer do Colo do Útero no Amazonas: uma Análise da Prevenção ao Tratamento de Lesões Precursoras. Rev. Bras. de Canc. 2021, 67(3),1-7. [CrossRef]
- Torres, K.L.; Rondon, H.H.M.F.; Martins, T.R.; Martins, S.; Ribeiro, A.; Raiol, T.; Marques, C.P.; Corrêa, F.; Migowski, A.; Minuzzi-Souza, T.T.C.E.; Schiffman, M.; Rodriguez, A.C.; Gage, J.C. Moving towards a strategy to accelerate cervical cancer elimination in a high-burden city—Lessons learned from the Amazon city of Manaus, Brazil. PLoS One, 2021 16(10):e0258539. [CrossRef]
- Torres KL, Mariño JM, Pires Rocha DA, de Mello MB, de Melo Farah HH, Reis RDS, Alves VDCR, Gomes E, Martins TR, Soares AC, de Oliveira CM, Levi JE. Self-sampling coupled to the detection of HPV 16 and 18 E6 protein: A promising option for detection of cervical malignancies in remote areas. PLoS One. 2018 Jul 23;13(7):e0201262. [CrossRef]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana,Z.; Kibiki,G.; Bates,D.O.; Dlamini,Z. Cervical cancer in low and middle-income countries. Oncol Lett 2020, 20(3):2058-2074. [CrossRef]
- Garnelo, L.; Sousa, A.B.L.; Silva, C.O. Health regionalization in Amazonas: Progress and challenges. Ciênc. saúde colet. 2017; 22(4):1225–34. [CrossRef]
- Viana, J.N.; Moysés, R.P.C; Espir, T.T.; Sousa, G.A.; Barcellos, J.F.M.; Alves, M.G.P.M. Social determinants of health and secondary prevention of cervical cancer in the State of Amazonas, Brazil. Med, 2019, 52(2):110-20. [CrossRef]
- INCA. Instituto Nacional de Câncer José Alencar Gomes da Silva. Diretrizes brasileiras para o rastreamento do câncer do colo do útero. Rio de Janeiro 2016, pp114.
- Cervena, K.; Vodicka, P.; Vymetalkova, V. Diagnostic and prognostic impact of cell-free DNA in human cancers: Systematic review. Mut Res - Reviews in Mutation Research, 2019, 781, 100-129. [CrossRef]
- Gu, Y.; Wan, C., Qiu, J.; Cui, Y.; Jiang, T.; Zhuang, Z. Circulating HPV cDNA in the blood as a reliable biomarker for cervical cancer: A meta-analysis. PLoS One. 2020, 15(2). [CrossRef]
- Sabeena, S.; Kuriakose, S.; Damodaran, B.; Ravishankar, N.; Arunkumar, G. Human papillomavirus (HPV) DNA detection in uterine cervix cancer after radiation indicating recurrence: A systematic review and meta-analysis. J Gynecol Oncol. 2020,31(2), 1-11. [CrossRef]
- Campo, F.; Zocchi, J.; Moretto, S.; Mazzola, F.; Petruzzi, G.; Donà, M.G.; Benevolo, M.; Iocca, O.; De Virgilio, A.; Pichi, B.; Manciocco, V.; Pellini, R. Cell-Free Human Papillomavirus-DNA for Monitoring Treatment Response of Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Laryngoscope. 2022,132(3):560-568. [CrossRef]
- Hanna, G.J.; Supplee, J.G.; Kuang, Y.; Mahmood, U.; Lau, C.J.; Haddad, R.I.; Jänne, P.A.; Paweletz, C.P. Plasma HPV cell-free DNA monitoring in advanced HPV-associated oropharyngeal cancer. Ann Oncol. 2018, 29(9):1980-1986. [CrossRef]
- Veo, C.A.R.; Saad, S.S.; Fregnani, J.H.T.G.; Scapulatempo-Neto, C.; Tsunoda, A.T.; Resende, J.C.P.; Lorenzi,A.T.; Mafra,A.;Cinti,C.; Cotrim, I.D.; Rosa, L.A.R.; Oliveira,C.M.; Martins,T.R.; Centrone, C.; Levi, J.E.; Longatto-Filho, A. Clinical characteristics of women diagnosed with carcinoma who tested positive for cervical and anal high-risk human papillomavirus DNA and E6 RNA. Tumour Biol. 2015, 36(7):5399–405. [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; Vandesompele, J.; Wittwer, C.T. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009, 55(4):611-22. [CrossRef]
- Al-Azri,M.H. Delay in cancer diagnosis: Causes and possible solutions. Oman Med J, 2016, 31(5):325-6. [CrossRef]
- Hanna,T.P.; King, W.D.;Thibodeau, S.;Jalink, M.;Paulin, G.A.;Harvey-Jones,E.; O'Sullivan,D.E.; Booth,C.M.;Sullivan,R.;Aggarwal,A. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020, 371, 1-11. [CrossRef]
- de Oliveira, H.M.; Gonçalves, M.J.; Pires, R.O. Characterization of the family health strategy in Amazonas State, Brazil: an analysis of implementation and impact. Cad Saude Publica. 2011, 27(1), 27(1):35-45. [CrossRef]
- Brasil. Ministério da Saúde. Biblioteca Virtual em Saúde. Available online: https://bvsms.saude.gov.br/71-dos-brasileiros-tem-os-servicos-publicos-de-saude-como-referencia/ (acessed on april 4, 2024).
- Thuler, L.C.S.; Bergmann, A.; Casado, L. Perfil das Pacientes com Câncer do Colo do Útero no Brasil, 2000-2009: Estudo de Base Secundária. Rev. Bras. Cancerol, 2012, 58(3): 351-357. [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray,F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020,8(2):e191–203.
- Moysés, R.P.C.; Amaral, G.S.; Nascimento, J.V.; Santos, B.D.; Pereira, M.G. Mulheres Amazônicas com câncer de colo de colo de útero: perfil sociodemográfico e fatores de risco. In: Bases conceituais da saúde. –Eds. Atena: Ponta Grossa -Paraná-Brasil, 2019; v.8, pp 112-123. [CrossRef]
- Musa, J.; Achenbach, C.J.; O'Dwyer, L.C.; Evans, C.T.; McHugh, M.; Hou, L.; Simon, M.A.; Murphy, R.L.; Jordan, N. Effect of cervical cancer education and provider recommendation for screening on screening rates: A systematic review and meta-analysis. PLoS One, 2017, 12(9):e0183924. [CrossRef]
- Gatumo, M.; Gacheri, S.; Sayed, A.R.; Scheibe, A. Women’s knowledge and attitudes related to cervical cancer and cervical cancer screening in Isiolo and Tharaka Nithi counties, Kenya: A cross-sectional study. BMC Cancer. 2018, 18(1):745. [CrossRef]
- Olubodun, T.; Balogun, M.R.; Odeyemi, A.K.; Odukoya, O.O.; Ogunyemi, A.O.; Kanma-Okafor, O.J; Okafor, I.P.; Olubodun, A.B.; Ogundele, O.O.P.; Ogunnowo, B.; Osibogun, A. Barriers and recommendations for a cervical cancer screening program among women in low-resource settings in Lagos Nigeria: a qualitative study. BMC Public Health. 2022, 22(1):1906. [CrossRef]
- Rodrigues, A.N.; Melo, A.C.; Calabrich, A.; Cronemberger, E.; Torres, K.L; Damian,F.; Leal, R.J.D.C.; Azevedo, C.R.A.S.; Fonseca, A.J.; Neron, Y,V.; Nunes, J.S.; Lopes, A.; Thome, F.; Leal, R.; Borges,G.S.; Silva, A.F.; Rodrigues, M.F.; Zaffaroni, F.;Werutsky, G.; Maluf, F.C. Social disparities and patients’ attitudes are associated with lower rates of cervical cancer screening in Brazil: Results of EVITA study (LACOG 0215). Journal of Clinical Oncology.2018, v. 36, 15_suppl. [CrossRef]
- Lucena, L.T.; Za, D.G.; Crispim, P.T.B.; Ferrari, J.O. Fatores que influenciam a realização do exame preventivo do câncer cérvico-uterino em Porto Velho, Estado de Rondônia, Brasil. Rev Pan-Amaz Saude. 2011, vol.2, n.2, pp.45-50. [CrossRef]
- Santos, J.N.; Gomes, R.S. Sentidos e Percepções das Mulheres acerca das Práticas Preventivas do Câncer do Colo do Útero: Revisão Integrativa da Literatura. Rev. Bras. Cancerol, 2022, 68(2):e-031632. [CrossRef]
- Tadesse, S.K. Socio-economic and cultural vulnerabilities to cervical cancer and challenges faced by patients attending care at Tikur Anbessa Hospital: A cross sectional and qualitative study. BMC Womens Health, 2015, 15(1):1–12. [CrossRef]
- Moysés, R.; Marques, I.; Santos, B.D.; Benzaken, A.; Pereira, M.G. Quality of Life in Amazonian Women during Cervical Cancer Treatment: The Moderating Role of Spirituality. Int J Environ Res Public Health. 2023, 20(3):2487. [CrossRef]
- Shrestha, A.D.; Neupane, D.; Vedsted, P.; Kallestrup, P. Cervical cancer prevalence, incidence and mortality in low and middle income countries: A systematic review. Asian Pac J Cancer Prev. 2018. p. 319–24. [CrossRef]
- Mekonnen, A.G.; Mittiku, Y.M. Early-onset of sexual activity as a potential risk of cervical cancer in Africa: A review of literature. PLOS Global Public Health. 2023, 3(3):e0000941. [CrossRef]
- Rozario, S.; Silva, I.F.; Koifman, R.J.; Silva, I.F. Characterization of women with cervical cancer assisted at Inca by histological type. Rev Saude Publica. 2019, 53:88. [CrossRef]
- Östensson, E.; Alder, S.; Elfström, K.M.; Sundström, K.; Zethraeus, N.; Arbyn, M.; Andersson, S. Barriers to and facilitators of compliance with clinic-based cervical cancer screening: Population-based cohort study of women aged 23-60 years. PLoS One. 2015, 10(5). [CrossRef]
- Silva, M.A.S.; Teixiera, E.M.B.; Ferrari, R.A.P.; Cestari, M.E.W.; Cardelli, A.A.M. Factors related to non-adherence to the realization of the Papanicolaou test. Rev Rene, 2015, 16(4), 532–539. [CrossRef]
- Dantas,P.V.J.; Leite, K.N.S.; César,E.S.R.; Silva,S.C.R.; Souza,T.A.; Nascimento, B.B. Women´s knowledge and factors of not adherence to the Pap smear examination. Rev enferm UFPE. 2018;12(3). [CrossRef]
- Aguilar, R.P.; Soares, D.A. Barriers to pap smear: prospects for users and professionals of the Family Health Strategy in Vitória da Conquista-BA . Physis: Rev Saúde Coletiva. 2015 25(2) 359–379. [CrossRef]
- Roque, A. V.; Lima, E.S.; Lopes, G.S. The influence of psychosocial factors on cervical cancer prevention. Braz. J. Develop. 2022, 8(5):41805-19. [CrossRef]
- Korn, A.K.; Muzingwani, L.; O'Bryan, G.; Ensminger, A. Boylan, A.D.; Kafidi, E.L.; Kashali, M.; Ashipala, L.; Nitschke, A.M.; Dziuban, E.J.; Forster, N.; Eckert, L.O.; O'Malley, G. Cervical cancer screening and treatment, HIV infection, and age: Program implementation in seven regions of Namibia. PLoS One. 2022,17(2):e0263920. [CrossRef]
- Clifford GM, Tully S, Franceschi S. Carcinogenicity of Human Papillomavirus (HPV) Types in HIV-Positive Women: A Meta-Analysis From HPV Infection to Cervical Cancer. Clin Infect Dis. 2017, 64(9):1228–1235. [CrossRef]
- Bowden, S.J.; Doulgeraki, T.; Bouras, E.; Markozannes, G.; Athanasiou, A.; Grout-Smith, H.; Kechagias, K.S.; Ellis, L.B.; Zuber, V.; Chadeau-Hyam, M.; Flanagan, J.M.; Tsilidis, K.K.; Kalliala, I.; Kyrgiou, M. Risk factors for human papillomavirus infection, cervical intraepithelial neoplasia and cervical cancer: an umbrella review and follow-up Mendelian randomisation studies. BMC Med. 2023, 21(1):274 . [CrossRef]
- Monteiro,J.C.; Fonseca, R.R.S.; Ferreira, T.C.S.; Rodrigues, L.L.S.; da Silva, A.R.B.; Gomes, S.T.; Silvestre, R.V.D.; Silva, A.N.M.R.; Pamplona, I.; Vallinoto, A.C.R.; Ishak, R.; Machado, L.F.A.Prevalence of High Risk HPV in HIV-Infected Women From Belém, Pará, Amazon Region of Brazil: A Cross-Sectional Study. Front Public Health. 2021. [CrossRef]
- Castle, P.E.; Einstein, M.H.; Sahasrabuddhe, V.V. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA Cancer J Clin. 2021, 71(6):505-526. Epub 2021 Sep 9. [CrossRef]
- Marima, R.; Hull, R.; Lolas, G.; Syrigos, K.N.; Kgoebane-Maseko, M.; Kaufmann, A.M.; Dlamini, Z. The Catastrophic HPV/HIV Dual Viral Oncogenomics in Concert with Dysregulated Alternative Splicing in Cervical Cancer. Int J Mol Sci 2021, 22(18):10115. [CrossRef]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Khalil,A.I.; Baussano, I.; Shah,A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; Bray, F.; Baggaley, R.;Clifford, G.M.; Broutet,N.; Dalal, S. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health 2021. [CrossRef]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.; Shin, H.; Vallejos, C.S.; de Ruiz, P.; Lima, M.A.; Guimera, N.; Clavero, O.; Alejo,M.; Llombart-Bosch, A.; Cheng-Yang, C.; Tatti, S.A.; Kasamatsu, E.; Iljazovic, E.; Odida, M.; Prado, R.; Seoud,M.; Grce, M.; Usubutun, A.; Jain, A.; Suarez, G.A.; Lombardi, L.E.; Banjo, A.; Menéndez, C.; Domingo, E.J.; Velasco, J.; Nessa, A.; Chichareon, S.C.; Qiao, Y.L.; Lerma, E.; Garland, S.M.; Sasagawa, T.; Ferrera, A.; Hammouda, D.; Mariani, L.; Pelayo, A.; Steiner, I.; Oliva, E.; Meijer, C.J.; Al-Jassar, W.F.; Cruz, E.; Wright, T.C.; Puras, A.; Llave, C.L.; Tzardi, M.; Agorastos, T.; Garcia-Barriola, V.; Clavel, C.; Ordi, J.; Andúja,r M.; Castellsagué, X.; Sánchez, G.I.; Nowakowski, A.M. Bornstein, J.; Muñoz, N.; Bosch, F.X. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11(11):1048-56. [CrossRef]
- de Oliveira, C.M.; Fregnani, J.H.T.G.; Carvalho, J.P.; Longatto-Filho, A.; Levi, J.E. Human papillomavirus genotypes distribution in 175 invasive cervical cancer cases from Brazil. BMC Cancer 2013, 13:357.
- Da Silva, R.L.; Da Silva, B. Z.; Bastos, G.R.; Cunha, A.P.A.; Figueiredo, F.V.; De Castro, L.O.; et al. Role of HPV 16 variants among cervical carcinoma samples from Northeastern Brazil. BMC Womens Health. 2020, 20(1):1–11. [CrossRef]
- Santos, M.O.; Lima, F.C.S.; Martins, L.F.L.; Oliveira, J.F.P.; Almeida, L.M.; Cancela, M.C. Estimativa de Incidência de Câncer no Brasil, 2023-2025. Rev Bras Cancerol. 2023;69(1). [CrossRef]
- Garnelo, L.; Parente, R.C.P.; Puchiarelli, M.L.R.; Correia, P.C.; Torres, M.V.; Herkrath, F.J. Barriers to access and organization of primary health care services for rural riverside populations in the Amazon. Int J Equity Health. 2020, 19(1):54. [CrossRef]
- Lopes, V.A.S.; Ribeiro, J.M. Fatores limitadores e facilitadores para o controle do câncer de colo de útero: uma revisão de literatura. Cien Saude Colet. 2019;24(9): 3431–3442. [CrossRef]
- Shimada, T.; Yamaguchi, N.; Nishida, N.; Yamasaki, K.; Miura, K.; Katamine, S.; Masuzaki, H. Human papillomavirus DNA in plasma of patients with HPV16 DNA-positive uterine cervical cancer. Jpn J Clin Oncol. 2010, 40(5):420–4. [CrossRef]
- Jaberipour, M.; Samsami, A.; Sahraiian, F.; Kazerooni, T.; Hashemi, M.; Ghaderi, A.; Habibagahi, M. Elevation of HPV-18 and HPV-16 DNA in the plasma of patients with advanced cervical cancer. Asian Pac J Cancer Prev. 2011, 12(1):163-7. [PubMed]
- Ho,C.M.; Yang, S.S.; Chien, T.;, Huang, S.; Jeng, C.J.; Chang, S.F. Detection and quantitation of human papillomavirus type 16, 18 and 52 DNA in the peripheral blood of cervical cancer patients. Gynecol Oncol. 2005, 99(3):615-21 . [CrossRef]
- Elit, L.; Kennedy, E.B.; Fyles, A.; Metser, U. Follow-up for cervical cancer: A program in evidence-based care systematic review and clinical practice guideline update. Curr Oncol. 2016, 23(2):109-18. [CrossRef]
- Mittelstadt, S.; Kelemen, O.; Admard, J.; Gschwind, A.; Koch, A.; Wörz, S.; Oberlechner, E.; Engler, T.; Bonzheim, I.; Staebler, A.; Weidner, N.; Stubenrauch, F.; Iftner, T.; Riess, O.; Schroeder, C.; Kommoss, S.; Ossowski, S. Detection of circulating cell-free HPV DNA of 13 HPV types for patients with cervical cancer as potential biomarker to monitor therapy response and to detect relapse. Br J Cancer. 2023, 128(11):2097-2103. [CrossRef]
- Cabel, L.; Bonneau, C.; Bernard-Tessier, A.; Héquet, D.; Tran-Perennou, C.; Bataillon, G.; Rouzier, R.; Féron, J.G.; Fourchotte, V.; Le Brun, J.F.; Benoît, C.; Rodrigues, M.; Scher, N.; Minsat, M.; Legrier, M.E.; Bièche, I.; Proudhon, C.; Sastre-Garau, X.; Bidard, F.C.; Jeannot, E. HPV ctDNA detection of high-risk HPV types during chemoradiotherapy for locally advanced cervical cancer. ESMO Open. 2021, 6(3):100154. [CrossRef]
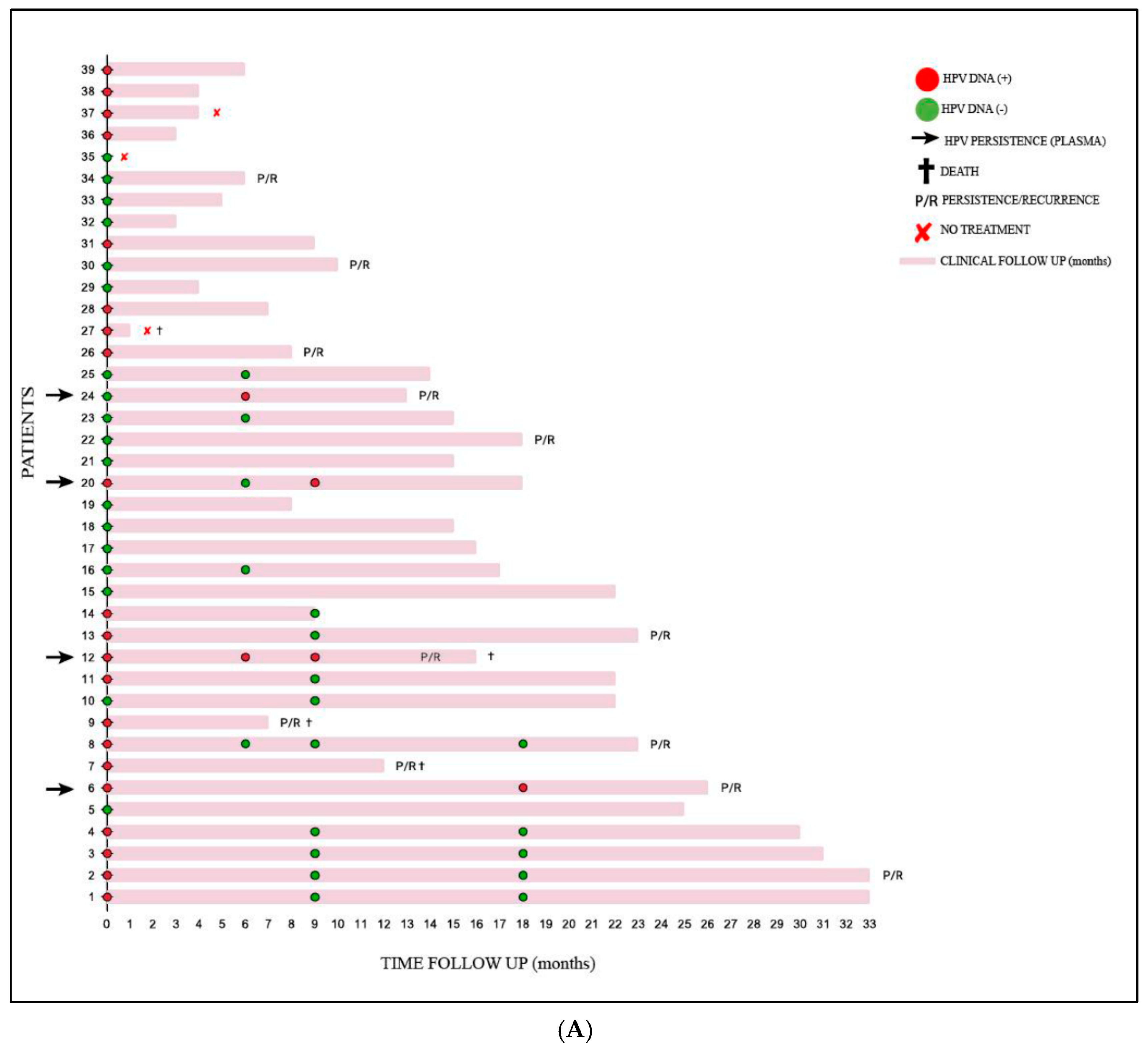
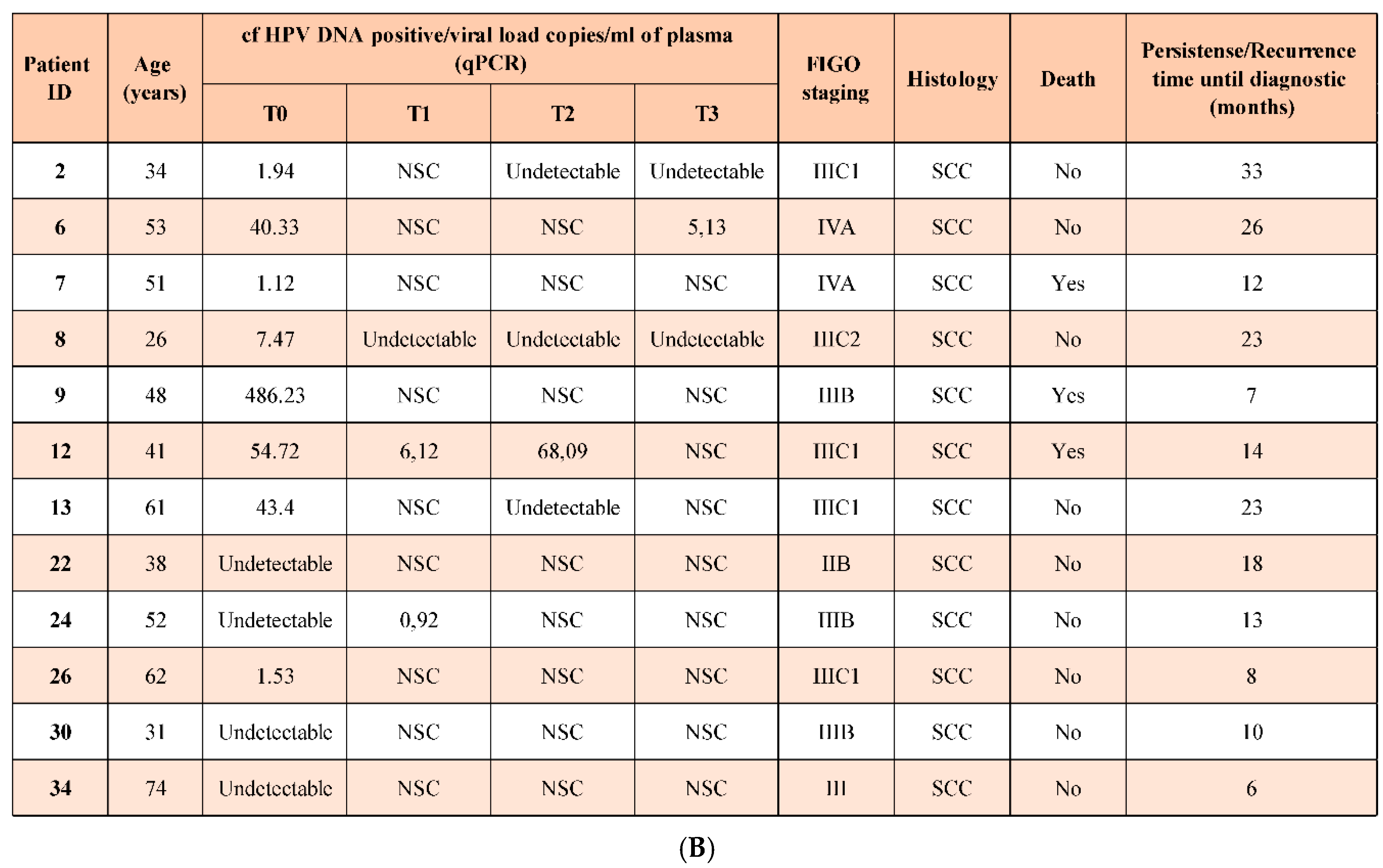
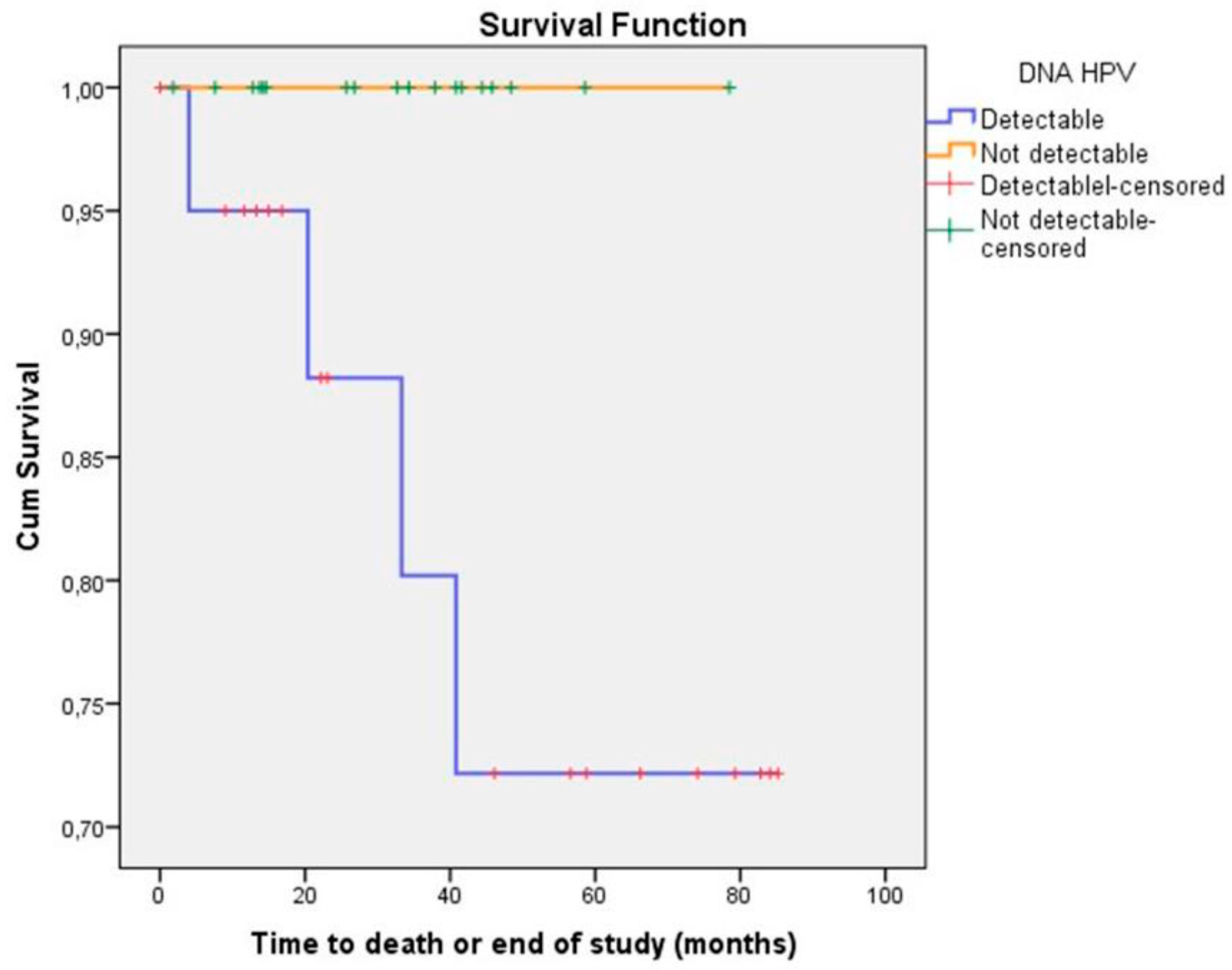
| Variables | n (39) | % |
|---|---|---|
| Age Range | ||
| 21- 40 | 14 | 35.9 |
| 41- 55 | 17 | 43.6 |
| 56- 65 | 2 | 5.1 |
| > 65 | 6 | 15.4 |
| Ethnic group | ||
| White | 3 | 77 |
| Black | 1 | 2.6 |
| Brown | 35 | 89.7 |
| Education level | ||
| Illiterate | 6 | 15.4 |
| Incomplete fundamental | 12 | 30.8 |
| Complete fundamental | 1 | 2.6 |
| Full high school | 15 | 38.5 |
| Incomplete higher education | 1 | 2.6 |
| Complete higher education | 4 | 10.3 |
| Marital status | ||
| Single | 14 | 35.9 |
| Married | 17 | 43.6 |
| Divorced | 2 | 5.1 |
| Widow | 6 | 15.4 |
| Place of birth | ||
| Capital (Manaus) | 9 | 23.1 |
| Interior of Amazon State | 19 | 48.7 |
| Other States of Brazil | 11 | 28.2 |
| Residential history (last five years) | ||
| Capital | 25 | 64.1 |
| Interior of Amazon State | 11 | 28.2 |
| Other States os Brazil | 3 | 7.7 |
| Family income | ||
| No economic income | 15 | 38.5 |
| Until 1 MW | 15 | 38.5 |
| 2 - 3 MW | 8 | 20.5 |
| > 3 MW | 1 | 2.6 |
| Variable | n (39) | % |
|---|---|---|
| Sexual debut (age) | ||
| 12-14 | 12 | 30.8 |
| 15 -17 | 19 | 48.7 |
| From 18 years old | 7 | 17.9 |
| Not informed | 1 | 2.6 |
| Sexual Partners | ||
| Only 1 | 4 | 10.3 |
| 2 - 5 | 24 | 61.5 |
| 6 - 10 | 8 | 20.5 |
| > 10 | 1 | 2.6 |
| Unknown | 2 | 5.1 |
| Condom Use | ||
| Sometimes | 24 | 61.5 |
| Always | 1 | 2.6 |
| Never | 14 | 35.9 |
| Screening by cytology | ||
| Every 6 months | 3 | 7.7 |
| Once per year | 9 | 23.1 |
| 2 in 2 years | 5 | 12.8 |
| Once in More than 3 years | 12 | 30.7 |
| Never | 6 | 15.4 |
| Not informed | 4 | 10.3 |
| History of smoking | ||
| Yes | 15 | 38.5 |
| No | 24 | 61.5 |
| STI | ||
| Yes | 6 | 15.4 |
| No | 23 | 59.0 |
| Don't know | 10 | 25.6 |
| Type of STI | n (6) | % |
| HIV | 1 | 16.7 |
| Syphilis | 1 | 16.7 |
| Unknow | 4 | 66.7 |
| Variables | % | |
|---|---|---|
| Relapse/Persistence | (n=39) | |
| Yes | 12 | 30.8 |
| No | 24 | 61.5 |
| No treatment | 3 | 7.7 |
| FIGO | (n = 12) | % |
| Group A (I/II) | 1 | 2.6 |
| Group B (III/IV) | 11 | 28.2 |
| Outcome | (n=39) | |
| Death | 4 | 10.3 |
| Alive | 35 | 89.7 |
| cf HPV16 DNA | |||||||
|---|---|---|---|---|---|---|---|
| TIME | Patients(n) | % | Detectable | % | Undetectable | % | p* |
| T0 | 39 | 100 | 21 | 53.8 | 18 | 46.2 | |
| T1 | 7 | 17.9 | 2 | 28.6 | 5 | 71.4 | 0.410 |
| T2 | 11 | 28.2 | 2 | 18.2 | 9 | 81.8 | 0.046 |
| T3 | 6 | 15.4 | 1 | 16.7 | 5 | 83.3 | 0.187 |
| Variables | cf HPV16 DNA | n (39) | p* | ||||
|---|---|---|---|---|---|---|---|
| Detectable (n=21) | % | Undetectable (n =18) | % | ||||
| FIGO | 0.041* | ||||||
| Group A | 4 | 19.0 | 9 | 50.0 | 13 | ||
| Group B | 17 | 81.0 | 9 | 50.0 | 26 | ||
| Histology | |||||||
| AC | 2 | 9.5 | 2 | 11.1 | 4 | 0.636 | |
| SCC | 19 | 90.5 | 16 | 88.9 | 35 | ||
| Relapse/Persistence | |||||||
| Yes | 6 | 28.6 | 6 | 33.3 | 12 | 0.491 | |
| No | 15 | 71.4 | 12 | 66.7 | 27 | ||
| Outcome | |||||||
| Death | 4 | 19.0 | 0 | 0.0 | 4 | 0.073 | |
| Survivor | 17 | 81.0 | 18 | 100.0 | 35 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





