1. Introduction
Water-soluble protonated secondary/tertiary diallylammonium polymers (PDAA) are of interest due to their strong antibacterial and antifungal properties [
1,
2,
3]. PDAA polymers have been synthesized in order to obtain non quaternized diallylammonium polymers unlike to well known cationic polymer quaternary poly(N,N-diallyl-N,N-dimethylammonium chloride) (PDADMAC) [
4,
5]. PDADMAC is an adsorbent employed as a flocculent to purify water at wastewater treatment plants and at some production facilities (including paper) to neutralize negatively charged colloid particles and diminish sediment size [
6,
7]. PDAA were first synthesized by radical cyclopolymerization of protonated salts – trifluoroacetates of diallylammonium monomers, secondary (DAATFA) and tertiary (
Scheme 1) [
8,
9].
Presence of protonated ammonium groups in PDAA sufficiently influenced their properties. Polymers PDAA were found to exhibit high nonspecific antimicrobial activity [
1], including rare activity against
M. tuberculosis [
2,
3], in contrast to quaternized polyamines and low molecular weight biocides [
10]. The protonated structure of PDAA was shown to mainly determine the mechanism of its antibacterial action [
3].
Considering possible biomedical application of PDAA, the PDAA cytotoxic properties should attract most attention. The monomeric unit of PDAA is the β,β-methylene substituted pyrrolidinium ring. With that in mind, we may consider PDAA polymer as protonated poly(β,β-k7k ‘methylene pyrrolidine). It is known that pyrrolidine (Pyr) and its C-substituted derivatives are used as scaffold for biologically active compounds (antitumor, antidiabetic, antibacterial etc.) [
11,
12]. Therefore, a problem of cytotoxicity of PDAA polymers and selectivity of their action is the problem of the polymeric nature influence on both cytotoxicity and antimicrobial activity.
Investigations of cytotoxicity, using
in vitro method on cell cultures, are being increasingly used in biochemical and toxicological studies, and are alternative to classical tests on experimental animals [
13,
14]. According to the actual conception of basal toxicity
in vitro, the toxic effect of different substances on a cellular level practically does not depend on a tissue origin of a cell culture because toxicants affect the basal cellular functions and structures which are universal for all types of cells. For that reason, the indexes of toxicity obtained on various mammal cell lines are not much different [
13,
14].
In our first study, we investigated with this method an influence of molecular weight (MW) of polymers in a wide range, including the monomeric unit, on the toxic action of PDAA relative to eukaryotic cells and in parallel on the antimicrobial activity of these polymers relative to a number of bacteria [
15]. Polymers PDAA were shown to exhibit strong cytotoxic action in the MW range (40 – 118)×10
3 g∙mol
-1 that diminishes insignificantly with decreasing polymerization degree. At the same time, Pyr and salt pyrrolidinium trifluoroacetate (PyrTFA, modeling the monomer unit of PDAA) have shown very low toxicity, PyrTFA being less toxic unexpectedly. Based on the study on toxicity and bactericidal activity (relative to
Staphylococcus aureus and
Pseudomonas aeruginosa), it was shown for PDAA polymers with a sufficiently large MW (more than 5×10
4 g⋅mol
-1), that CTD
50 concentrations, at which 50% destruction of the cellular monolayer was observed are an order of magnitude higher than minimal bactericidal concentrations (MBC). Thus, the above-mentioned PDAA polymers have demonstrated rather high selectivity of action due to the strong bactericidal efficiency at those MW and so seem promising [
15].
It can be also distinguished the area of low-molecular-weight (LMW) PDAA (1.8×10
4 g⋅mol
-1 and possibly lower) which are less toxic and rather effective relative to bacteria tested. In the present paper, we focused on study of possible effect of the end groups in LMW polymers on both their toxicity and bactericidal efficiency. With development of controlled radical polymerization method with reversible addition–fragmentation chain transfer (RAFT) mechanism, it became possible to synthesize polymers with variable functional properties due to the introduction of end groups of the RAFT agent [
16,
17,
18]. It allows us to evaluate the effect of the end groups in RAFT-polymers [
19], in particular, on their antimicrobial activity/toxicity [
20]. However, our study of applicability of the RAFT method for radical polymerization of DAATFA with a significant kinetic contribution of the efficient chain transfer to monomer (that noticeably affects molecular weight and polydispersity of the polymers) has shown that choice of water-soluble RAFT agents to control polymerization is extremely limited in this case [
21]. Therefore, in the present work, to obtain the LMW PDAA samples with variable end groups, we elaborated a procedure of free radical polymer synthesis of protonated diallyl monomers in the excess of initiator.
2. Results and Discussion
2.1. Synthesis and Characteristics of Polymers
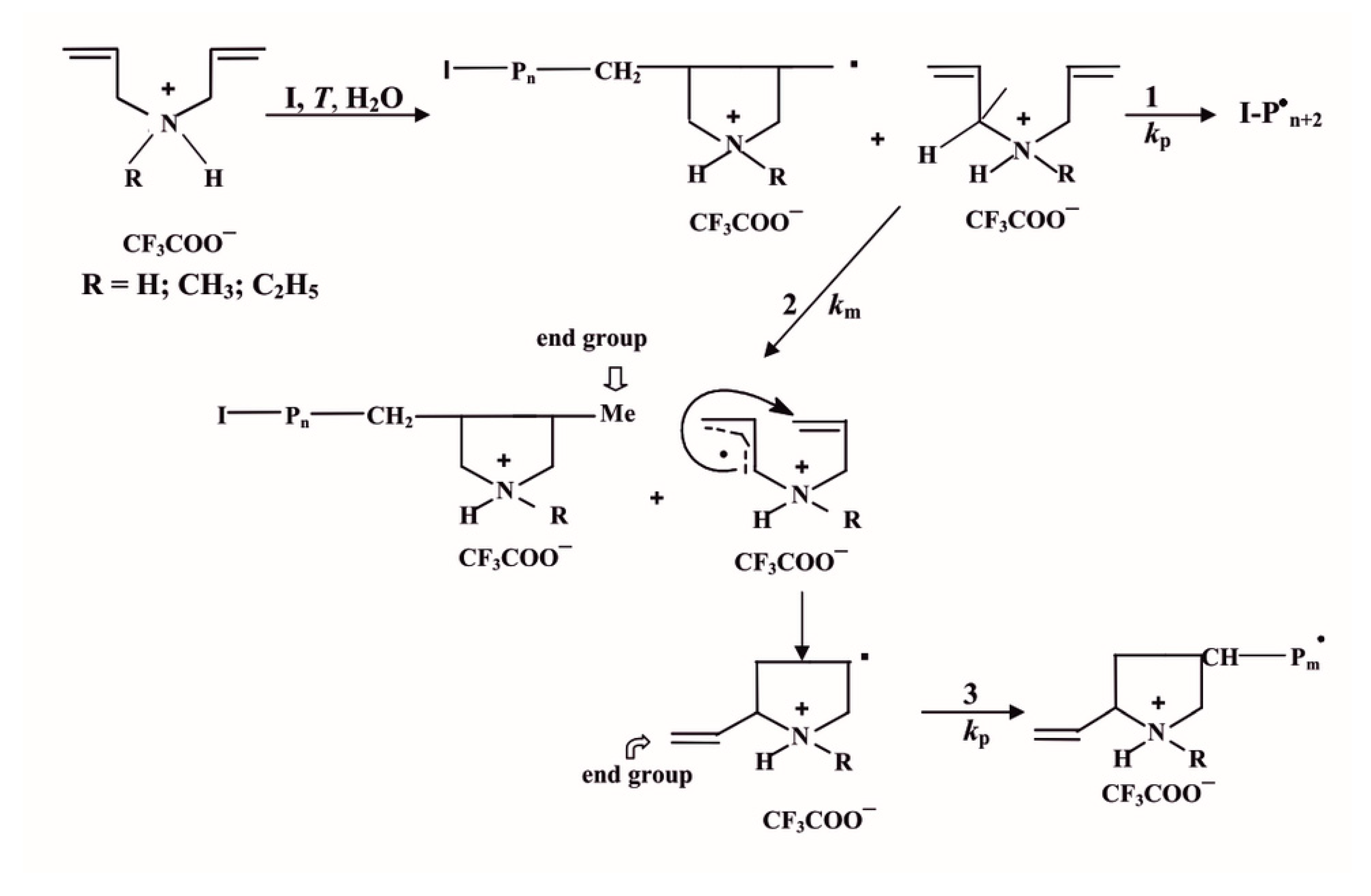
To obtain polymer samples of PDAA with a low degree of polymerization, free radical polymerization of diallyl monomer salt, diallylammonium trifluoroacetate (DAATFA), was carried out in excess of initiators: ammonium persulfate (APS) and 4,4¢-azobis(4-cyanovaleric acid) (ACVA). It was shown using NMR, IR spectroscopy and elemental analysis that in case of the excess of initiator (on the example of APS, [
22]), the characteristic reactions of the effective chain transfer to the monomer (see
Scheme 1) are largely kinetically suppressed by the interactions of macroradicals with the primary radicals of the of the initiator (as it is seen below):
Accordingly, with the excess of initiator and a decrease in the molecular weight of polymers, the relative number of characteristic terminal vinyl groups diminishes, and the end groups formed by the termination of macroradicals by the primary radicals of the initiator become predominant.
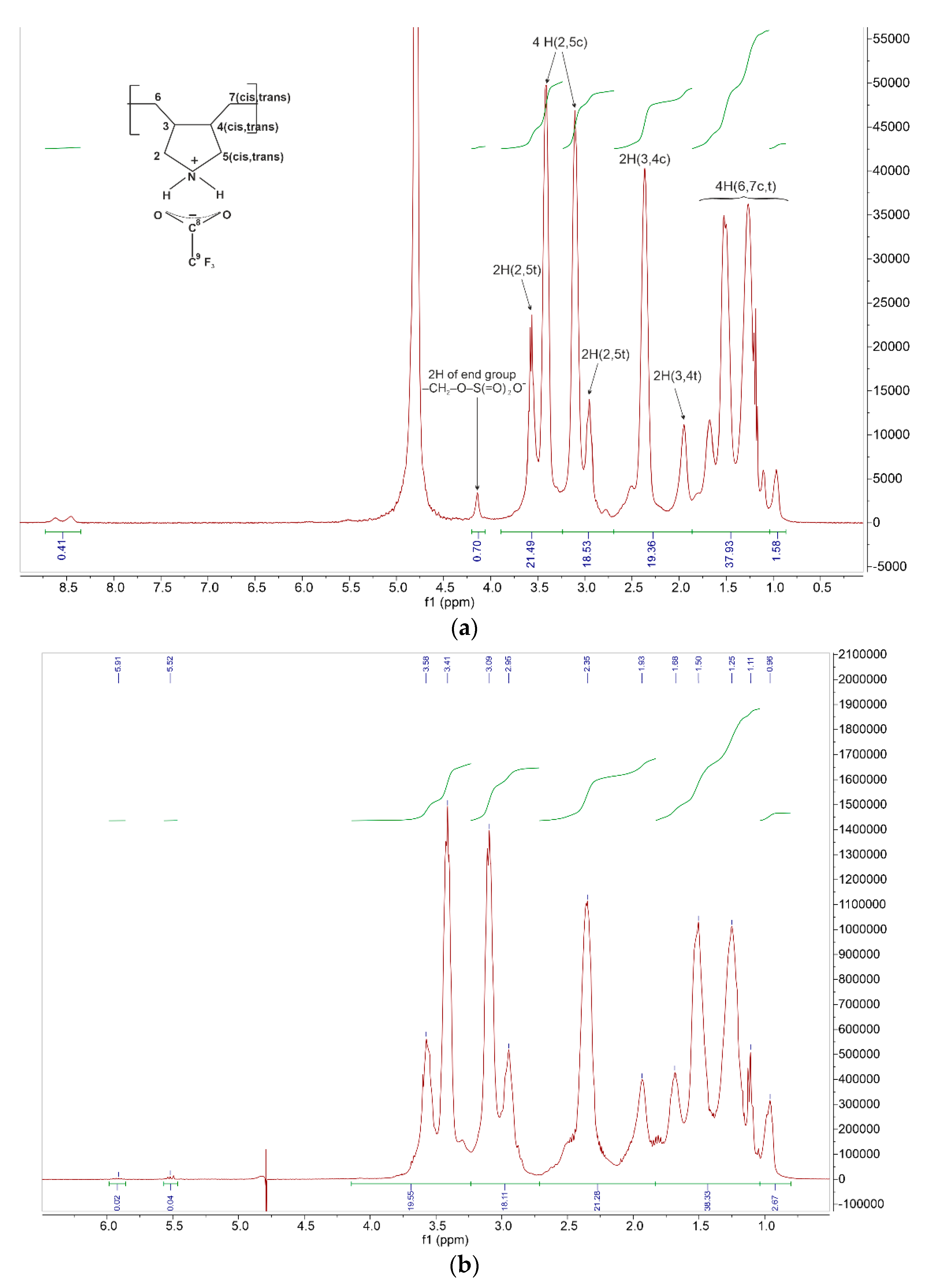
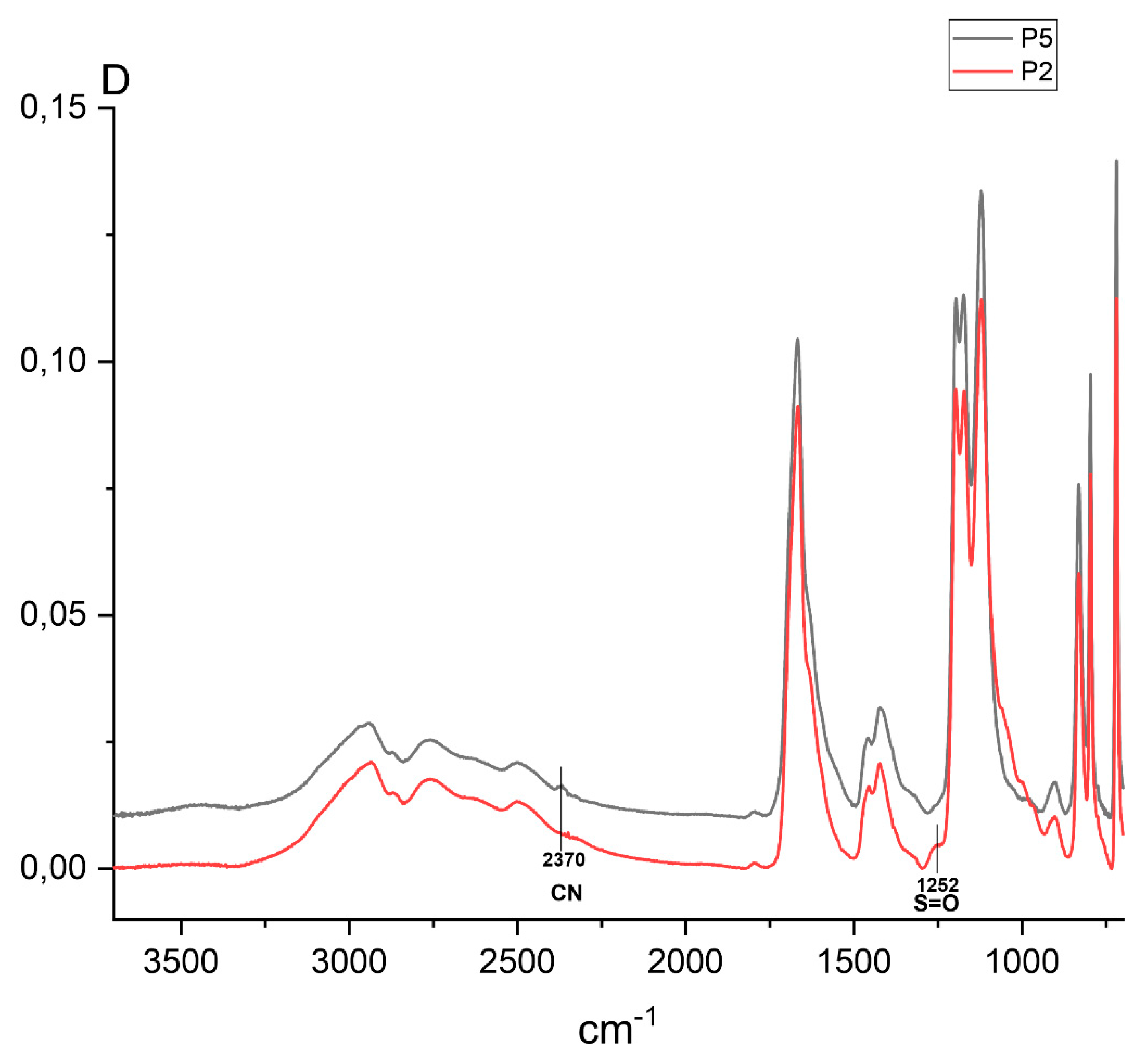
Small (or poorly detected) signals of end vinyl groups and formation of terminal groups of primary radicals of the initiator are confirmed by the NMR
1H and IR-Fourier spectra of the samples prepared with excess of initiators (10
-1 mol⋅l
-1) APS/ACVA at 50ºC/70ºC (
Figure 1 and
Figure 2,
Table 1).
As follows from
Table 1, by varying the initiator concentration and temperature, a number of PDAA polymer samples were obtained in the MW range of (40-12)×10
3 g⋅mol
-1. It is necessary to take into consideration the significant effect of temperature growth on the increase in the probability of chain transfer to the monomer [
8,
9]. An increase in the initiator concentration, as well as the contribution of chain transfer to the monomer with increasing temperature, both lead to a decrease in MW (compare samples P3 and P4,
Table 1).
2.2. Toxicity of Tested Polymers
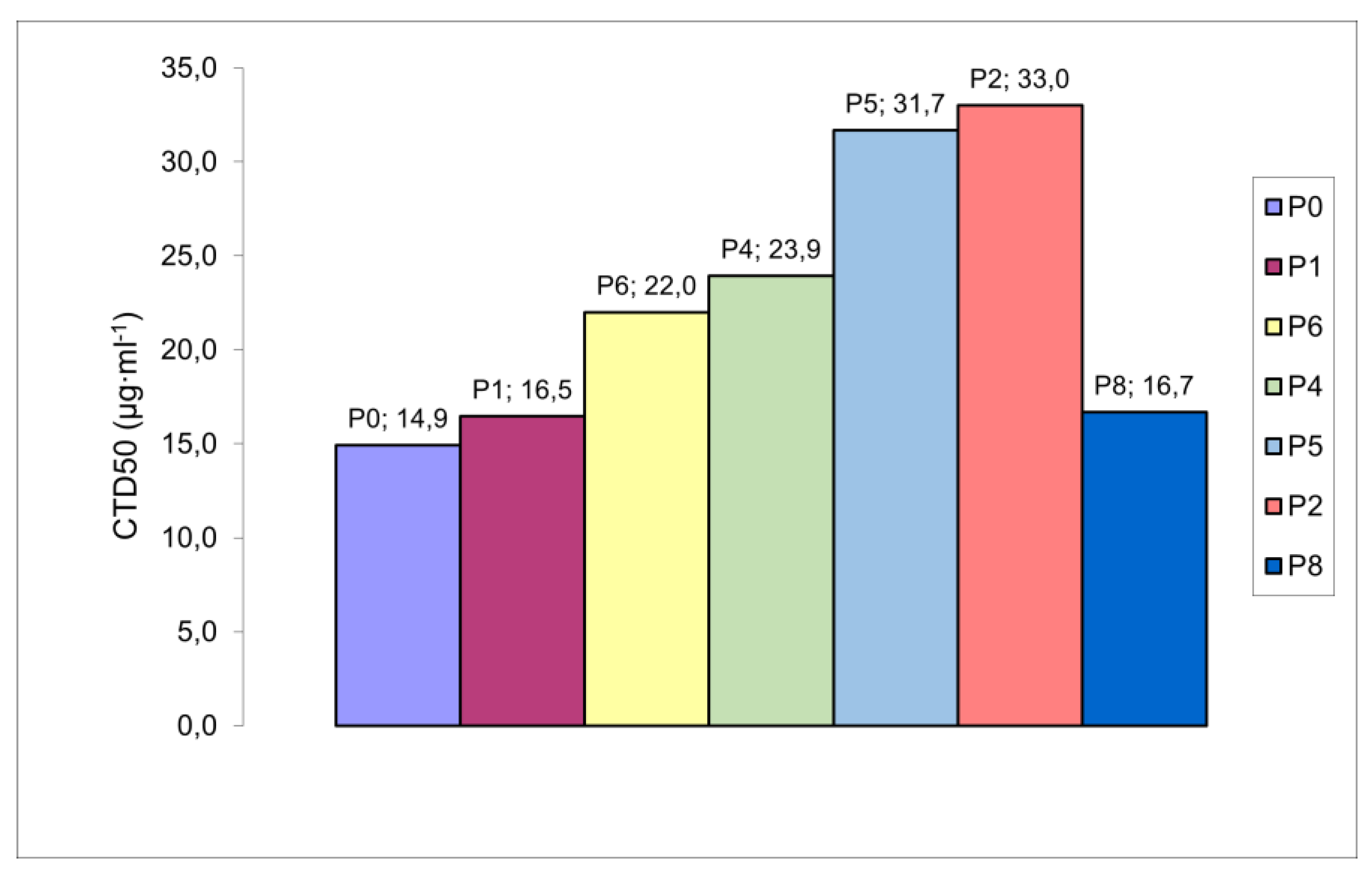
The toxic effects of secondary polydiallylamines PDAATFA with variable MW and different end groups were investigated (
Table 2 и
Figure 3). Analysis of the obtained toxicity data shows that the dependence of toxicity on MW (increase along with MW) is preserved for polymers of medium and small MW. However, comparison of samples with close MW and different terminal groups (P4 and P5), or close toxicity, but different MW and terminal groups (P4 and P6) allows us to conclude that sulfate terminal groups -O-S(=O)
2-O¯ contribute to greater toxicity of the polymer compared to terminal groups -C((C≡N)(CH
3))-(CH
2)
2-COOH.
The high cytotoxicity of RAFT-PDAATFA (sample P8) with the lowest molecular weight from the tested polymers with dithiocarbonyl -S-C(=S)-O-CH
2-CH
3 end group was unexpected. It has shown toxicity comparable to that of samples P0 and P1 with MW five times higher. The effect of the dithiocarbonyl group on PDAATFA toxicity turned out to be more significant than on antimicrobial activity (see
Table 3 and
Figure 3). Thus, in the case of a polymer with a small MW, polar lipophilic group has a strong cytotoxic effect on eukaryotic cells. The result of the strong influence of the end -S-C(=S)-Z (Z = O-CH
2-CH
3) group on cytotoxicity relative to eukaryotic cells is opposite to the data on the weak influence of -S-C(=S)-Z (Z = S(CH
2)
11CH
3, SCH
2CH
3) groups on hemolytic activity (as a measure of toxicity) of the polymethacrylates [
19]. It is related obviously to different mechanisms of a polycation action on eukaryotic cells and its hemolytic action.
Higher toxicity to human malignant cells than to non-transformed kidney cells was expected (
Table 2). The structure and regulation of the actin cytoskeleton in cancer and normal cells differ greatly. As a result, cancer cells are more deformable than normal cells and are approximately 70% softer [
23]. The well-known activity of polycations relative to cancer cells may be due to the easier compressibility of these cells. For studies on variation of toxicity within a series of samples, the results obtained for the A-549 cell line may be applicable. However, for comparison with the bactericidal activity data, toxicity values obtained for the MA-104 cell line seem to be more adequate.
2.3. Bactericidal Activity of Tested Polymers
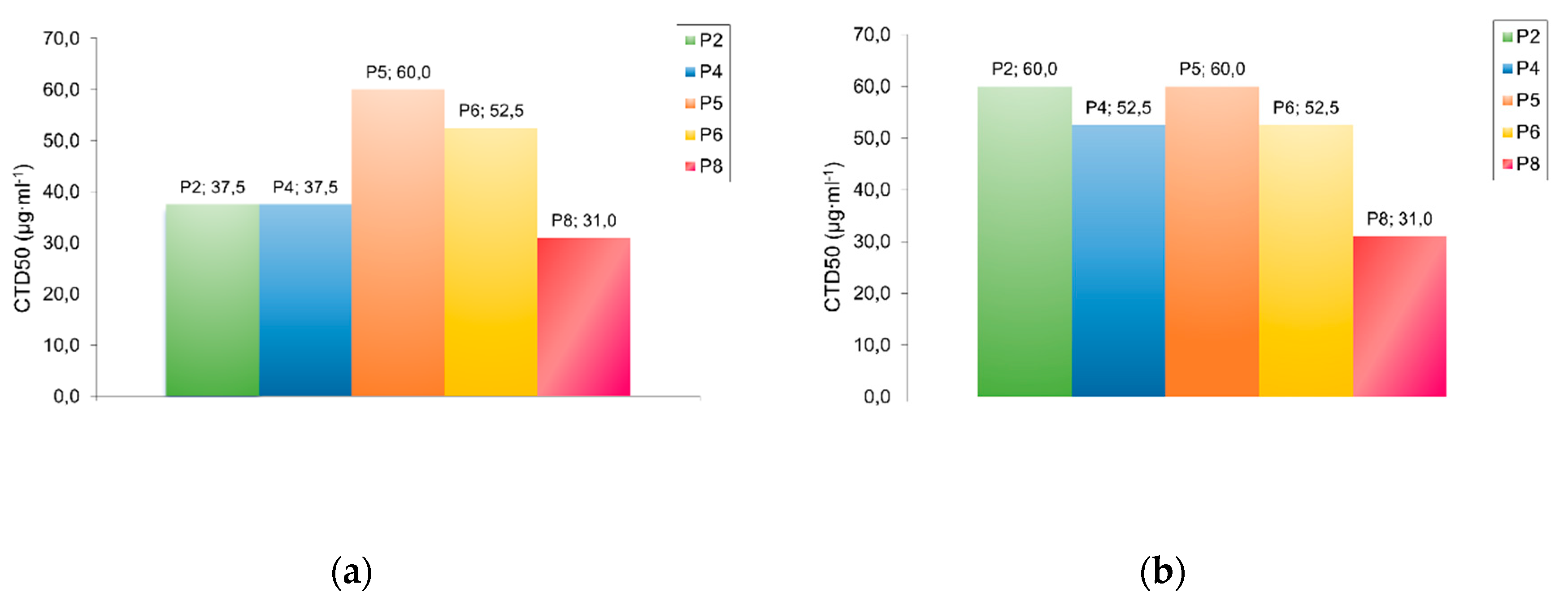
From the data presented in
Table 3 it is evident that PDAATFA with small MWs retain a sufficiently high biocidal activity. Dependence of antibacterial activity of polymers on their MW was discovered by Ikeda [
24], and then was studied for different polymers [
25,
26,
27,
28]. The effect of MW on antibacterial activity of various polymers and synthetic mimics of antibiotic peptides was discussed in detail in the review [
29].The results on the activity of samples with a small MW show a dependence on the nature of the terminal group (
Table 3 and
Figure 4). This is revealed when comparing the MBC of samples P2 and P4 with P5 and P6 against
Staphylococcus aureus. Polymers P2 and P4 with the terminal sulfate group -O-S(=O)
2-O¯ also showed higher toxicity (
Table 2 and
Figure 3) compared to polymers P5 and P6 with the -C((C≡N)(CH
3))-(CH
2)
2-COOH end group. The terminal dithiocarbonyl group of the P8 polymer had the strongest influence on the bactericidal efficiency, significantly enhancing its bactericidal action.
PDAATFA polymers show greater efficiency relative to gram-positive
Staphylococcus aureus than gram-negative
Pseudomonas aeruginosa. It was observed varying efficiency upon investigating PDAATFA of high MW: for
Mw 62×10
3 g⋅mol
-1, MBC = 1.5 ± 0.3 relative to
Staphylococcus aureus, АТСС 6538 Р, while MBC = 125 ± 7.5 relative to
Pseudomonas aeruginosa, АТСС 9027 and MBC = 15 ± 1.8 relative to
Escherichia coli, АТСС 25922 [
1]. McDonnell and Russell noted that, as a whole, gram-negative microorganisms exhibit stronger resistance to antiseptics and disinfectants than gram-positive ones (with the exception of gram-positive mycobacteria) [
30].
Franklin and
Snow related this to the structure of cell walls of these bacteria [
31].
Summarizing the obtained results on the effect of the terminal groups of PDAATFA polymers with a small MW on their bactericidal activity and cytotoxicity relative to eukaryotic cells, we can conclude that the dependence of the activity and, most of all, toxicity on MW is preserved even at a small difference in MW values (~104 g⋅mol-1) in the MW range of (18-35)×103 g⋅mol-1 . Secondly, a clear dependence of the studied properties on the nature of the terminal group is revealed. Sulfate -O-S(=O)2-O¯ end group has a noticeable effect on the bactericidal efficiency and smaller influence on toxicity, while dithiocarbonyl end group -S-C(=S)-O-CH2-CH3 has significant effect on efficiency and especially toxicity, drastically increasing the second. In a whole, based on results obtained and considering toxicity values obtained for the MA-104 cell line, polymers PDAA of small MW seem to be promising as antimicrobial agents for the creation of new transdermal drugs.
3. Materials and Methods
3.1. Materials
Trifluoroacetic acid (TFA, “for synthesis”, ≥99.0%; Merck, Germany), and radical initiators ammonium persulfate (APS, 99+%, for molecular biology, DNAse, RNAse and protease free, Acros, Belgium) and 4,4¢-azobis(4-cyanovaleric acid) (ACVA, 98.0%; Aldrich) were used without additional purification. Diallylamine (DAA) reagents (for synthesis, 97%; Acros; Belgium), and solvents hexane and diethyl ether (“analytically pure”, Khimmed; Russia) were distilled before use. Chromatographically pure DAA: Тb = 111–112°С. 1H NMR (Me2CO-d6): = 3.20 (d, 4 H, 2 α-CH2, J = 5.89 Hz), 5.12 (m, 4Н, 2α-СН2), 5.87 (m, 2Н, 2β-СН).
3.2. Synthesis
The procedures for obtaining trifluoroacetic salts from monomer DAA were described previously [
9,
10]. The structures were confirmed by
1H NMR spectra (characteristic spectrum is given in [
9]).
1H NMR for DAATFA: (Me
2CO-d
6) - 3.71 (d, 4 H, 2 α -CH
2,
J = 6.43 Hz), 5.47 (m, 4Н, 2γ-СН
2), 6.00 (m, 2Н, 2 β-СН).
3.3. DAATFA Polymerization
Polymerization of the DAATFA and DAMATFA was carried out according to the elaborated method [
1,
8,
9]. Aqueous solutions of DAATFA, [M] = 2 mol/L, at several concentrations of the APS initiator, [I] = 2×10
-2, 4×10
-2 and 10
-1 mol/L, and T = 40 and 50ºС were prepared. Example 1: DAATFA (10.575 g, 2 mol/L) was dissolved in a small amount of double distilled water in a pycnometer, then APS (0.57 g, 10
-1 mol/L) was added and the volume was adjusted to 25 ml with double distilled water (pH 2.5 solution). Aqueous solutions of DAATFA, [M] = 2 mol/L, at several concentrations of the ACVA initiator, [I] = 4×10
-2 and 10
-1 mol/L, and T = 70 ºС were prepared. Example 2: DAATFA (10.525 g, 2 mol/L) was dissolved in a small amount of double distilled water in a pycnometer, then ACVA (0.698 g, 10
-1 mol/L) was added and the volume was adjusted to 25 ml with double distilled water (pH 2.5 solution). The ampoule with the solution was degassed by freezing with liquid nitrogen 10-11 times under vacuum down to 5×10
–3 mm Hg, sealed and thermostated at 40 or 50°C. The polymer was isolated in Et
2O, then purified three times by reprecipitation from a solution in MeOH into Et
2O, and dried under vacuum over P
2O
5. The following samples of PDAATFA were obtained: at 50°C for t = 30 h - samples P2 and P3, and at 40°C - sample P4 for 40 h. Samples P5 and P6 were prepared with initiator ACVA at 70°C for 30 h.
3.4. DAATFA RAFT Polymerization
Sample P8 was synthesized in the presence of the RAFT agent xanthate as follows. Radical polymerization of DAATFA was carried out in aqueous solution with initiator ACVA, [M] = 2 mol l
-1, [ACVA] = 5×10
-3 mol l
-1, at the ratio of concentrations [xanthate]/[ACVA] = 3, T = 70°C for 20 h. Sample: DAATFA (10.575 g, 2 mol l
-1) and xanthate (0.068 g, 1.5×10
-2 mol l
-1, corresponding to the [xanthate]/[ACVA] = 3) was dissolved in a small amount of bidistilled water; next, initiator ACVA (0.035 g, 5×10
-3 mol l
-1) and bidistillate were added until the entire volume was 25 ml (pH of solution was 2.5) (see also [
21]).
The conditions of polymerization and characteristics of the samples are listed in
Table 1.
3.5. Measurements
1H and 13C NMR spectra of the synthesized samples were obtained on a Bruker AVANCE III HD spectrometer (400 MHz 1H). IR spectra of PDAATFA samples were recorded in ATR reflection mode (ATR) on an IFS-66 v/s Bruker IR spectrometer (ZnSe crystal, scan 30, range 4000-600 cm-1).
3.6. Determination of Molecular Characteristics of Polymer
The molecular characteristics of the synthesized polymers were determined by hydrodynamic and dynamic light scattering (DLS) methods. The values of the intrinsic viscosity [
η] of the samples in 1 M NaCl (Ostwald viscometer, solvent flow time 70.5 sec) and the translational diffusion coefficients
D0 were determined according to DLS data (Photokor complex, Russia). The viscosity-average molecular weight M
η of the samples was calculated using the Mark-Kuhn-Houwink (M-K-H) relation, which we previously obtained for PDAATFA in 1 M NaCl at 298 K [
32].
In addition, the experimental values of [
η] and
D0 of the synthesized samples were used to calculate their hydrodynamic molecular weight M
Dη according to the equation (3) [
33]:
Here A
0 is the hydrodynamic invariant, T is the absolute temperature,
η0 is the viscosity of the solvent. The value of the hydrodynamic invariant A
0= 3.0×10
-10 erg/K
·mol
1/3, which is included in equation (1), for the homologous series of PDAATFA was determined experimentally in [
32]. The molecular characteristics of the synthesized samples are given in
Table 1. The obtained M
η and M
Dη values correlate well with each other. The methodology of all measurements and formalism are described in detail in [
21,
32].
3.7. Procedure of Toxicity Investigations
In this work, permanent (established) cell lines of eukaryotic cells A-549 (epithelioid line of human lung carcinoma) and MA-104 (epithelioid line of green monkey kidney cells) were used. Cells were grown in the α-MEM cell culture medium (Biolot, St.-Petersburg, Russia) supplemented with 10% calf serum, seeded in 96-well tissue culture plates (Nunc, Denmark) and allowed to grow in the CO
2-incubator at 5% CO
2 until the formation of confluent cellular monolayer (usually 24 h). The medium was discarded and replaced with a solution of tested compounds in serial dilutions in the serum-free α-MEM medium. Cells were further incubated for 24 h or 72 h and their viability was assessed by the MTT (Thiazolyl blue, Sigma, USA) test [
34]. The OD of colored product was measured in ThermoFisher Varioscan Plate Analyzer (Waltham, MA, USA) at 570 nm.
3.8. Mathematical/Statistical Analysis of the Results
Each concentration of a compound under study was tested at least in 4 wells of a culture plate (n=4). Control (intact) cells were represented at n≥4 wells. Each experiment was tripled. CTD50 (50% cytotoxic concentration) – the concentration which provoked 50% destruction of cellular monolayer, was calculated with the software package GraphPadPrism (GraphPadSoftware, SanDiego, California) in the non-linear regression fit: log(inhibitor) vs. response – Variable slope (four parameters).
3.9. Procedure for Antibacterial Activity Research
Standard reference strains used for polymer activity testing were Pseudomonas aeruginosa (ATCC 27853) and Staphylococcus aureus (ATCC 25923) obtained from the State Collection of Pathogenic Microorganisms and Cell Cultures of State Research Center for Applied Microbiology and Biotechnology, Russia. Bacteria were grown in NB medium (Himedia, India) for 20 h. Bacterial inoculums were adjusted with sterile NB medium to a 1 McFarland standard with an organism density of approximately 3×108 colony forming units (CFU)/mL, then the suspension was diluted with NB broth to make a 1:3000 bacterial dilution (1×105 CFU/mL).
3.10. Estimation of Bacterial Viability
Bacteria were then inoculated at a concentration of 105 CFU/mL into 15 mL test tubes containing 2 mL of NB medium (Himedia, India) and polymer aqueous solutions of different concentration prepared by serial dilutions. After 24 hours of incubation at 37°C and 120 r.p.m., the culture from each tube was spread on agar-solidified NB medium by streak seeding method and incubated at 37°C. The viability of bacteria was determined after 2 days (presence or absence of bacterial growth all along the streak), i.e. the minimal bactericidal (killing) concentrations corresponding to each treatment time (MBC100, or MBC) were determined. All experiments were carried out at least 4 times, and the data are reported as the mean values ± ER (experimental errors, which were calculated according to the recommended procedures).
Author Contributions
Conceptualization, total analysis and interpretation, L.M. Timofeeva; polymer synthesis, Yu.A. Simonova and I.V. Eremenko; organic synthesis, M.A. Topchiy; NMR study and analysis, M.P. Filatova; investigation of antibacterial activity and analysis, N.V. Kozobkova and M.O. Shleeva; investigation of cytotoxic effect and analysis, M.Yu. Eropkin; writing, L.M. Timofeeva, M.O. Shleeva and M.Yu. Eropkin; editing, L.M. Timofeeva and I.V. Eremenko.
Funding
This work was supported by the Russian Science Foundation, project no. 23-23-00420. The study of antimicrobial activity was performed within the framework of the State Task for the Federal Research Centre “Fundamentals of Biotechnology” of the Russian Academy of Sciences.
Acknowledgments
The NMR and IR-Fourier measurements were performed using the equipment of the Shared Research Center “Analytical center of deep oil processing and petrochemistry of Topchiev Institute of Petrochemical Synthesis of the Russian Academy of Sciences”. Authors are thankful to Prof. Galina N. Bondarenko for the IR-Fourier analysis and interpretation, and to Dr. Natalia P. Yevlampieva (Saint Petersburg State University) for the determination of molecular characteristics of polymers.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Timofeeva, L.M.; Klescheva, N.A.; Moroz, A.F.; Didenko, L.V. Secondary and tertiary polydiallylammonium salts: novel polymers with high antimicrobial activity. Biomacromolecules 2009, 10, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.M.; Klescheva, N.A.; Shleeva, M.O.; Filatova, M.P.; Simonova, Yu.A.; Ermakov, Yu.A.; Kaprelyants, A.S. Nonquaternary poly(diallylammonium) polymers with different amine structure and their biocidal effect on Mycobacterium tuberculosis and Mycobacterium smegmatis. Appl. Microbiol. Biotechnol. 2015, 99, 2557–2571. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.; Bondarenko, G.; Nikitushkin, V.; Simonova, Y.; Topchiy, M.; Eremenko, I.; Shleeva, M.; Mulyukin, A.; Kaprelyants, A. On the molecular mechanism of nonspecific antimicrobial action of protonated diallylammonium polymers on mycobacterial cells. Europ. Polym. J. 2022, 171, 111214–111229. [Google Scholar] [CrossRef]
- Butler, G.B. Cyclopolymerization and cyclocopolymerization; Marcel Dekker: New York, 1992. [Google Scholar]
- Kabanov, V.A.; Topchiev, D.A. Kinetics and mechanism of radical polymerization of N,N-dialkyl-N,N-diallylammonium halogens. Polym. Sci. USSR, Ser. A. 1988, 30, 667. [Google Scholar] [CrossRef]
- Chang, E.E.; Chiang, P.C.; Tang, W.Y.; Chao, S.H.; Using, H.J. Effects of polyelectrolytes on reduction of model compounds via coagulation. Chemosphere. 2005, 58, 1141–1150. [Google Scholar] [CrossRef]
- Edzwald, J.K. (Ed.) Water Quality and Treatment, 6th Ed; McGraw-Hill: New York, 2011; ISBN 978-0-07-163011-5. [Google Scholar]
- Timofeeva, L.M.; Klescheva, N.A.; Vasilieva, Y.A.; Gromova, G.L.; Timofeeva, G.I.; Filatova, M.P. Mechanism and kinetic features of producing new polymers based on monomers of diallylamine series. Polym. Sci. Ser. A. 2005, 47, 551–565. [Google Scholar]
- Simonova, Y.A.; Filatova, M.P.; Timofeeva, L.M. Radical Polymerization of Protonated Diallylammonium Monomers in Bidistilled Aqueous Solution: Kinetic Study. Polym. Sci., Ser. B. 2018, 60, 445–454. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Control of Hospital Waste. In Book Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier, 2020; pp. 3294–3309. [Google Scholar]
- Li Petri, G.; Raimondi, M.V.; Spano, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Topics in Current Chemistry. 2021, 379, 34–79. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.V.; Listro, R.; Cusimano, M.G.; La Franca, M.; Faddetta, T.; Gallo, G.; Schillaci, D.; Collina, S.; Leonchiks, A.; Barone, G. Pyrrolomycins as antimicrobial agents. Microwave-assisted organic synthesis and insights into their antimicrobial mechanism of action. Bioorg. Med. Chem. 2019, 27, 721–728. [Google Scholar] [CrossRef]
- Seibert, H.; Balls, M.; Fentem, J. Acute toxicity in vitro and the classification and labeling of chemicals. Altern.to Lab. Animals., 1996, 24, 499–510. [Google Scholar] [CrossRef]
- Clemedson, C.; Ekwall, B. Overview of the final MEIC results. I. The in vitro- in vitro evaluation. Toxicol. In Vitro. 1999, 13, 657–663. [Google Scholar] [CrossRef]
- Simonova, Y.A.; Eremenko, I.V.; Topchiy, M.A.; Kozobkova, N.V.; Shleeva, M.O.; Eropkin, M.Yu.; Timofeeva, L.M. Antimicrobial protonated polydiallylamines: how to retain bactericidal efficiency at minimal toxicity. Mendeleev Communications (accepted).
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living-Free radical polymerization by reversible addition–fragmentation chain transfer: the RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Destarac, M.; Bzducha, W.; Taton, D.; Gauthier-Gillaizeau, I.; Zard, S.Z. Xanthates as chain-transfer agents in controlled radical polymerization (MADIX): structural effect of the O-alkyl group. Macromol Rapid Commun 2002, 23, 1049–1054. [Google Scholar] [CrossRef]
- Barner-Kowollik, C. (Ed.) , Handbook of RAFT Polymerization; Wiley-VCHH Verlag Gmbh& Co.: Weinheim, 2008. [Google Scholar]
- Moad, G.; Chong, Y.K.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT polymerization: the synthesis of polymers with defined end-groups. Polymer 2005, 46, 8458–5822. [Google Scholar] [CrossRef]
- Michl, T.D.; Locock, K.E.S.; Stevens, N.E.; Hayball, J.D.; Vasilev, K.; Postma, A.; Qu, Y.; Traven, A.; Haeussler, M.; Meagher, L.; Griesser, H.J. RAFT-derived antimicrobial polymethacrylates: elucidating the impact of end-groups on activity and cytotoxicity. Polym. Chem. 2014, 5, 5813–5822. [Google Scholar] [CrossRef]
- Simonova, Y.A.; Topchiy, M.A.; Filatova, M.P.; Yevlampieva, N.P.; Slyusarenko, M.A.; Bondarenko, G.N.; Asachenko, A.F.; Nechaev, M.S.; Timofeeva, L.M. Impact of the RAFT/MADIX agent on protonated diallylammonium monomer cyclopolymerization with efficient chain transfer to monomer. Eur. Polym. J. 2020, 122, 109363–76. [Google Scholar] [CrossRef]
- Eremenko, I.V.; Simonova, Yu.A.; Ivina, P.D.; Yevlampieva, N.P.; Filatova, M.P.; Bondarenko, G.N.; Kleshcheva, N.A.; and, L.M. Timofeeva, in Book of Abstracts of 9th All-Russian Kargin Conference “Polymers-2024”, Russia, 1-3 July. Moscow, Russia. 2024,140. http://kargin.msu.ru/files/abstracts_2024.pdf.
- Cross, S.E.; Jin, Y.S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nature Nanotechnology 2007, 2, 780–783. [Google Scholar] [CrossRef]
- Ikeda, T.; Hirayama, H.; Yamaguchi, H.; Tazuke, S.; Watanabe, M. Polycationic Biocides with Pendant Active Groups: Molecular Weight Dependence of Antibacterial Activity. Antimicrob. Agents. Chemother. 1986, 30, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- F. Siedenbiedel, J.S. Tiller, Antimicrobial polymers in solution and on surfaces: overview and functional principles, Polymers 4 (2012) 46-71. [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Tang, C.B. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Chen, A.; Peng, H.; Blakey, I.; Whittaker, A.K. Biocidal Polymers: A Mechanistic Overview. Polym. Review 2017, 57, 276–310. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Clinical Microbiology Reviews1999, 12, 147–179.
- Franklin, T.J.; Snow, G.A. Biochemistry and Molecular Biology of Antimicrobial Drug Action; Springer-Verlag: New York, 2005. [Google Scholar]
- Yevlampieva, N.; Vezo, O.; Simonova, Yu.; Timofeeva, L. Protonated Member of Poly(diallylammonium) Family: Hydrodynamic and Conformational Properties. Int. J. Polym. Analysis Character. 2018, 23, 403–414. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers; Plenum Press: NewYork, 1989. [Google Scholar]
- Borenfreund, E.; Babich, H.; Martin-Alguicil, N. Comparison of two in vitro cytotoxicity assays – the neutral red (NR) and tetrazolium (MTT) tests. Toxicol. In Vitro 1988, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).