Submitted:
04 December 2024
Posted:
05 December 2024
You are already at the latest version
Abstract
Keywords:
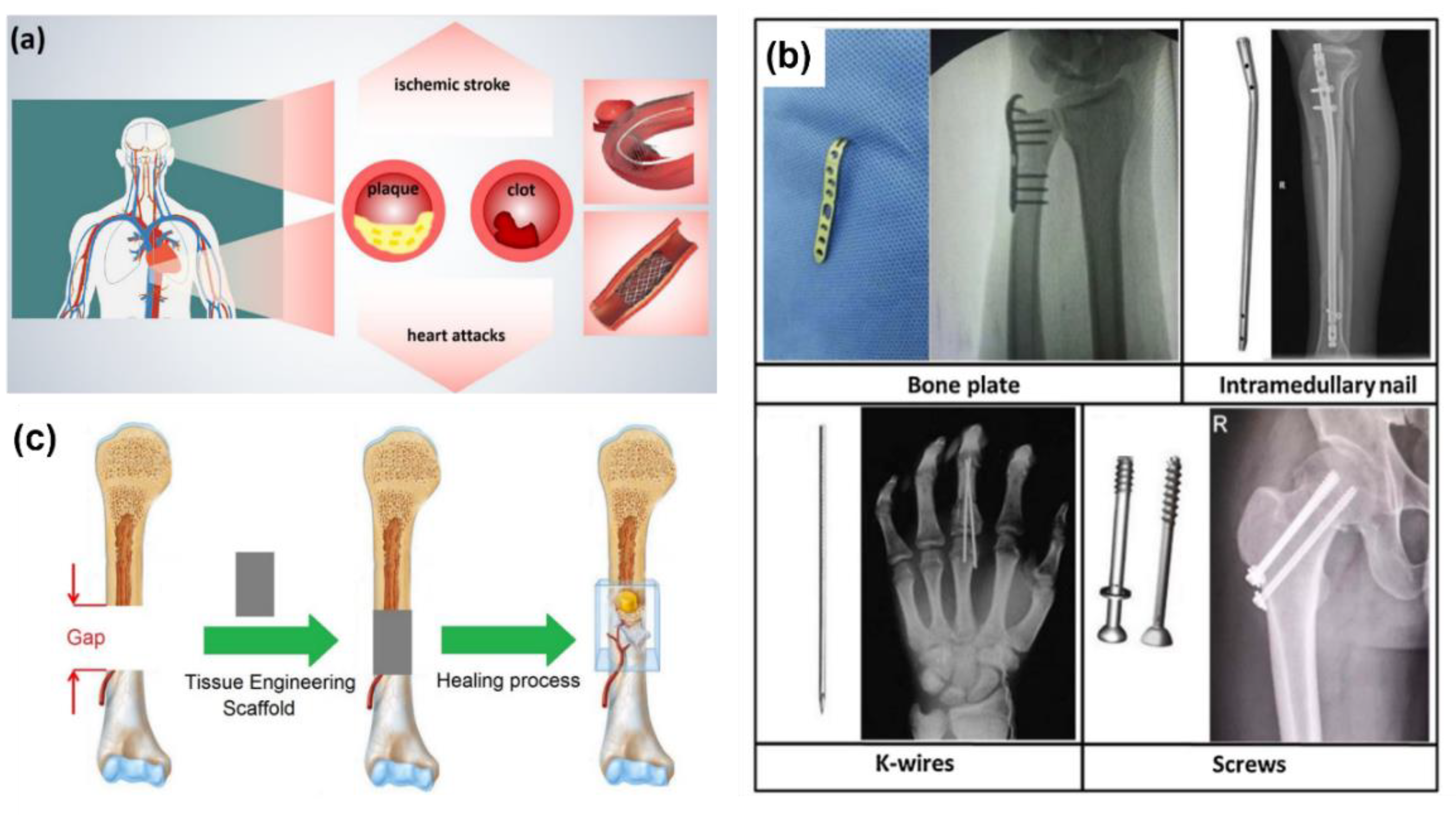
1. Introduction
1.1. Context and Background
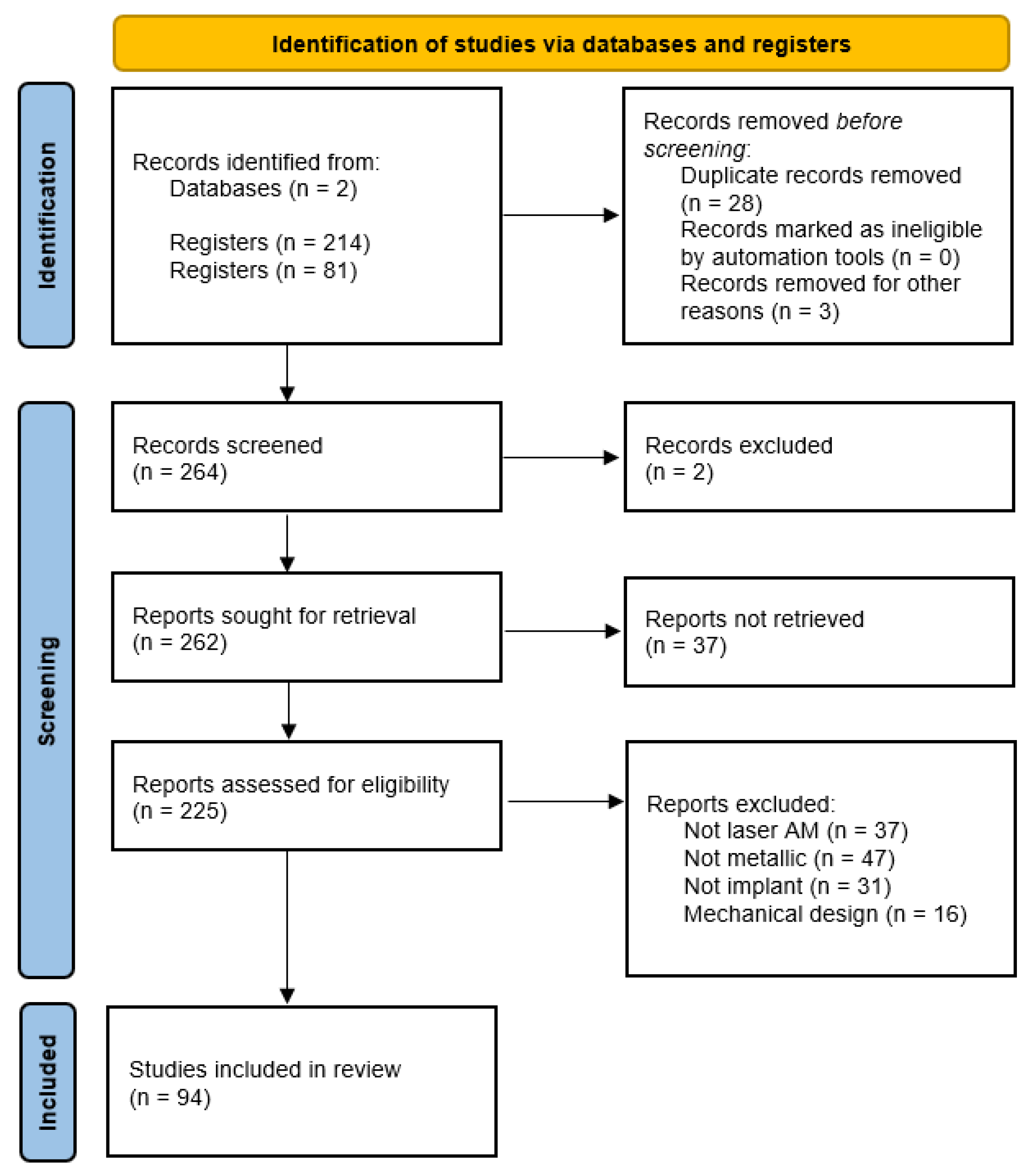
1.2. PRISMA
1.3. Additive Manufacturing – Laser Powder Bed Fusion
2. Additive Manufacturing of Iron Based Alloys
2.1. Linking Processing Parameters and Energy Density to Densification
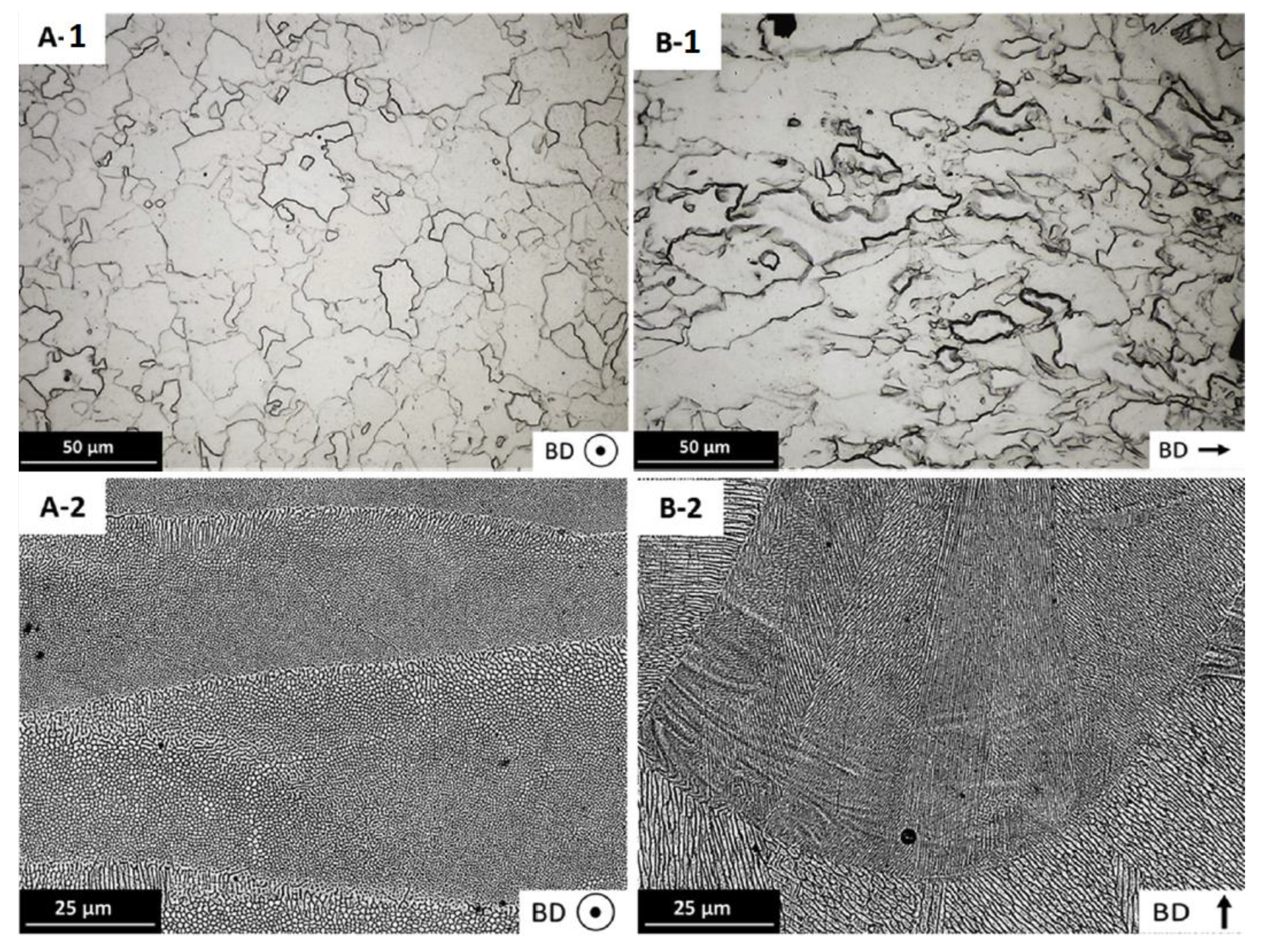
2.2. Influence of Microstructure on the Hardness
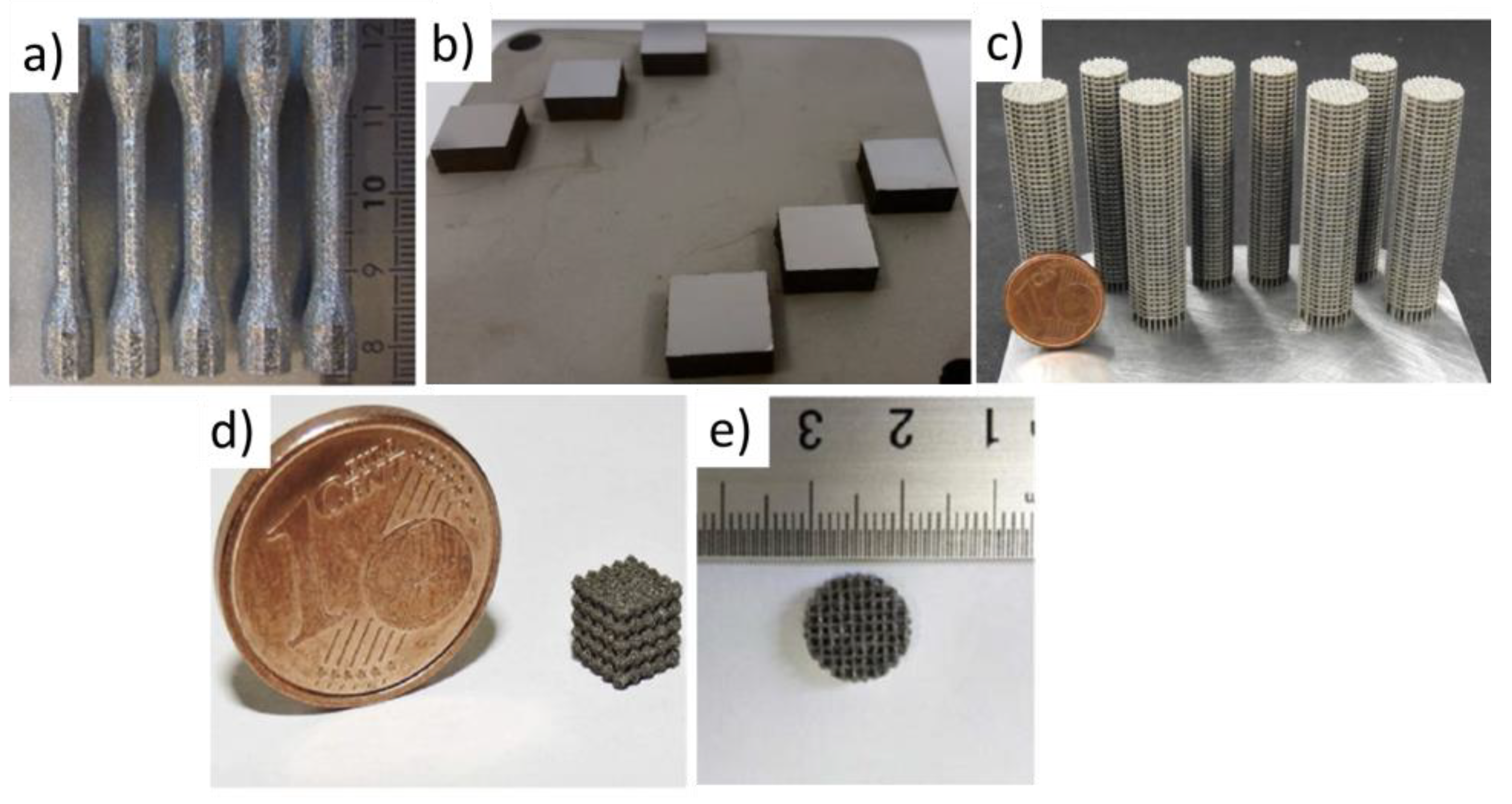
2.3. Mechanical Properties of Dense Structures and Scaffolds
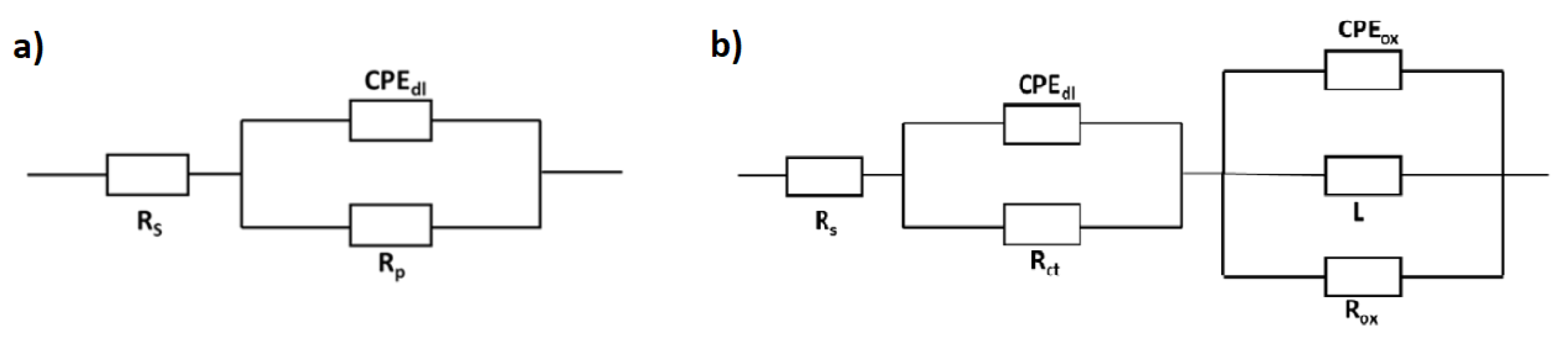
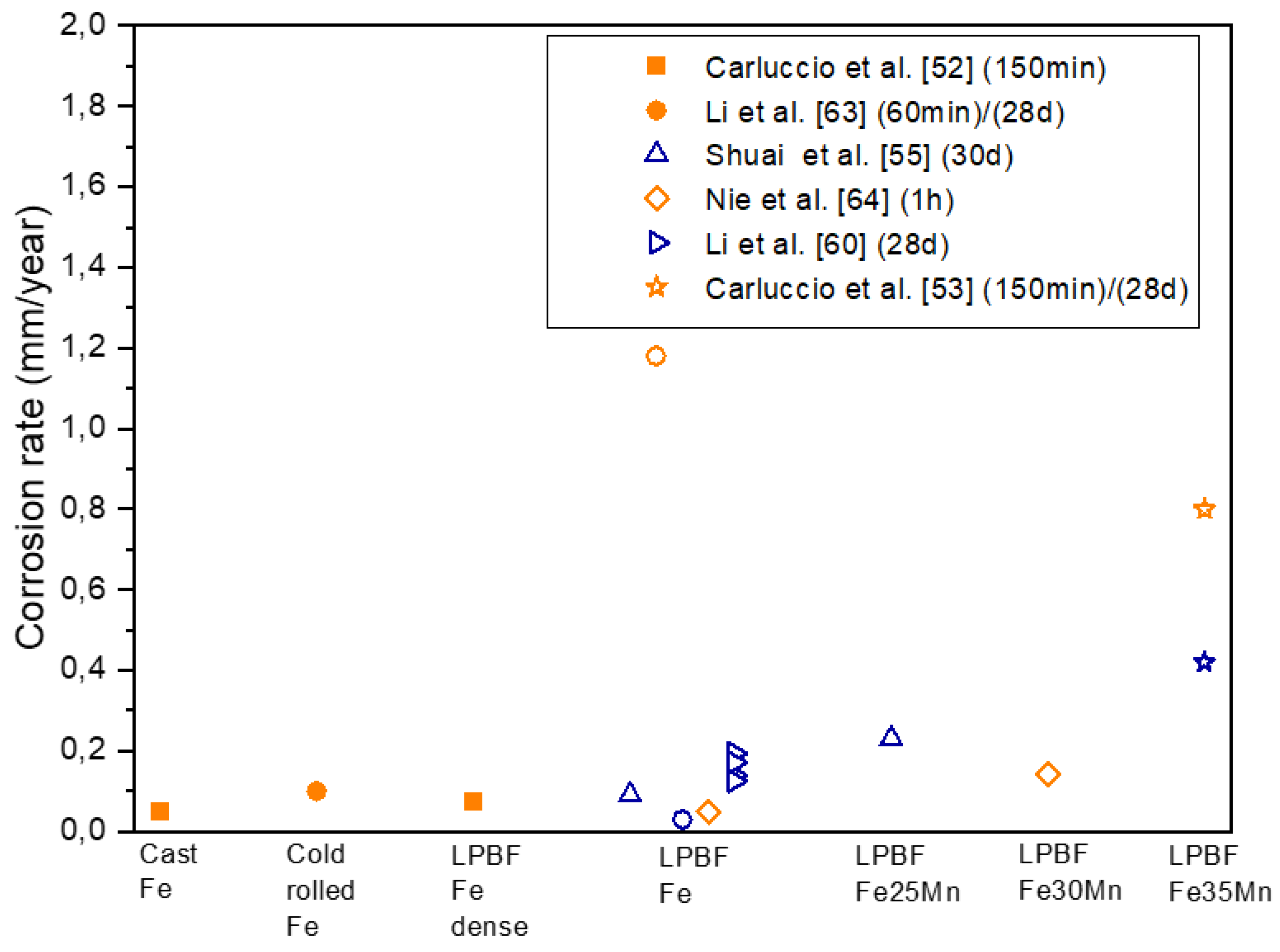
2.4. Corrosion Behavior
2.5. In Vitro Cytocompatibility
2.6. In Vivo Studies
3. Additive Manufacturing of Zn Based Alloys
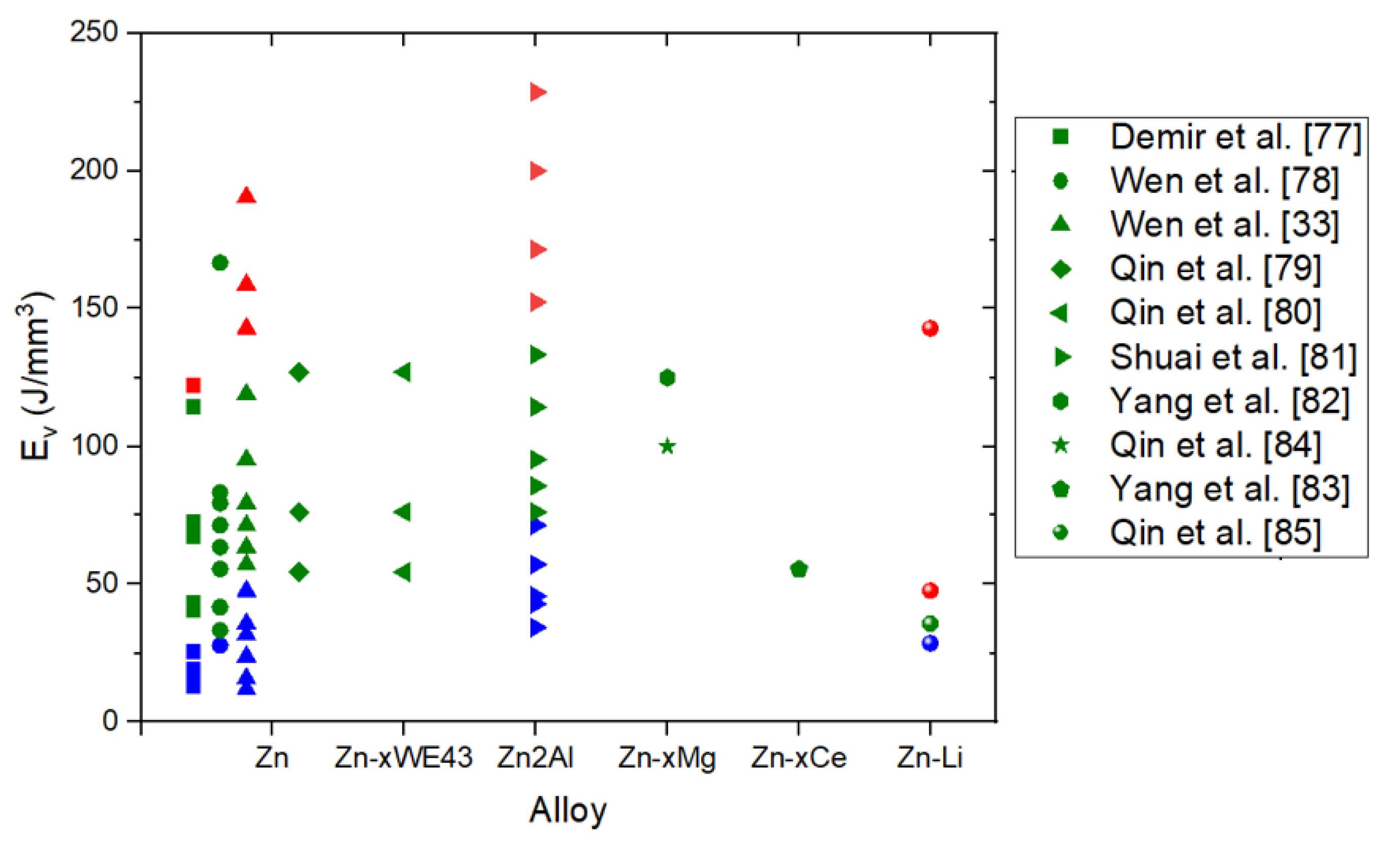
3.1. Linking Processing Parameters and Energy Density to Densification
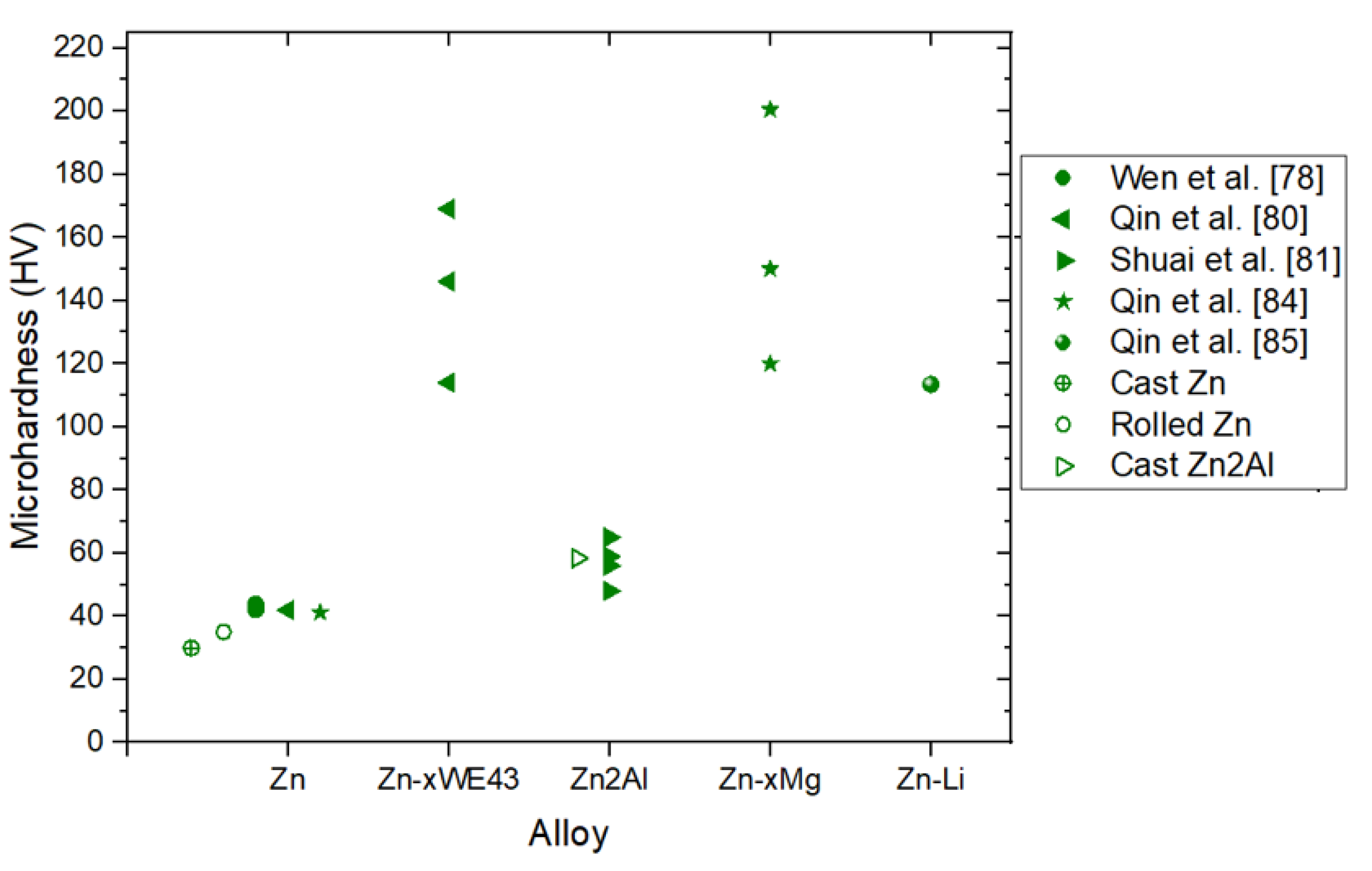
3.2. Influence of the Microstructure on the Hardness
3.3. Mechanical Properties of Dense Structures and Scaffolds
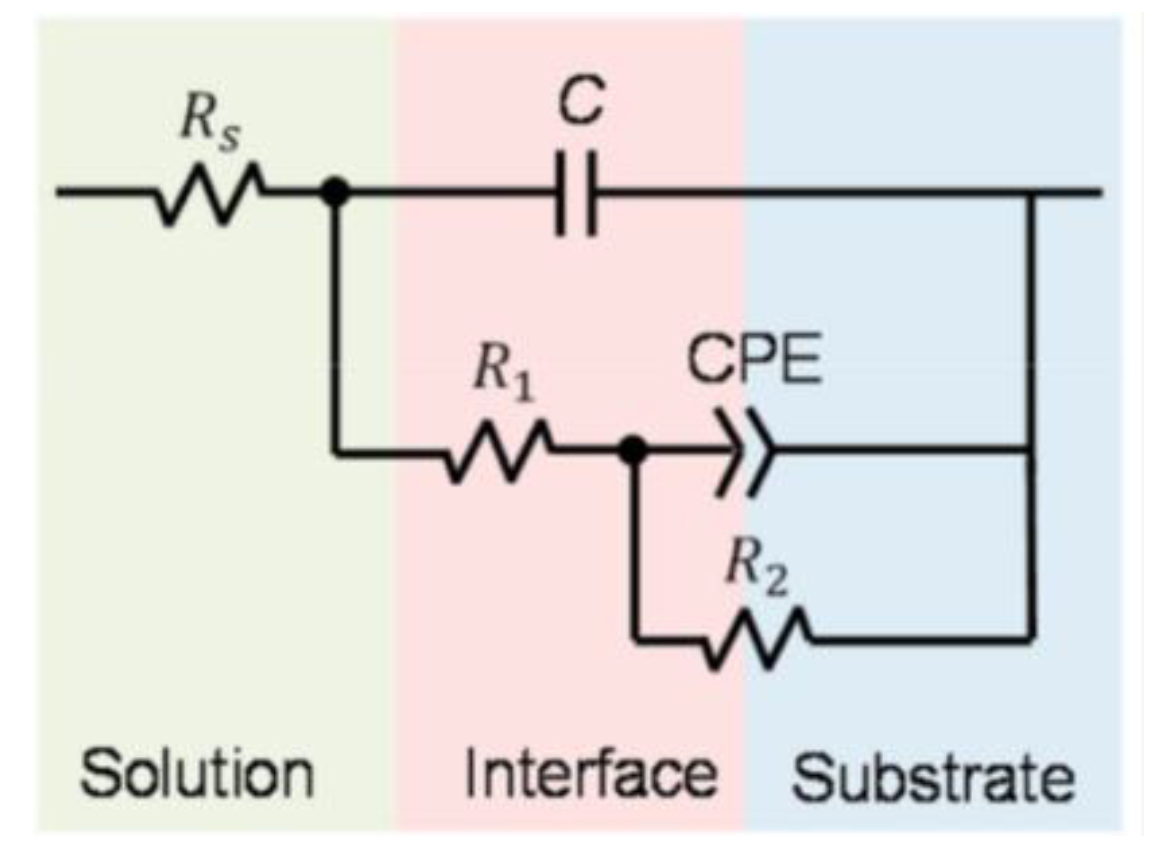
3.4. Corrosion Behavior
3.5. In Vitro Cytocompatibility
3.6. In Vivo Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, Y.F.; Gu, X.N.; Witte, F. , Biodegradable metals, Mater. Sci. Eng. R Reports. 77 (2014) 1–34. [CrossRef]
- Ryu, H.; Seo, M.H.; Rogers, J.A., Bioresorbable Metals for Biomedical Applications: From Mechanical Components to Electronic Devices, Adv. Healthc. Mater. 10 (2021). [CrossRef]
- Han, H.S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.C.; Seok, H.K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. , Current status and outlook on the clinical translation of biodegradable metals, Mater. Today. 23 (2019) 57–71. [CrossRef]
- Cifuentes, S.C.; San-Miguel, V.; Wang, Y.; García-Peñas, A. , Bioresorbable metals for cardiovascular and fracture repair implants, in: Nanohybrids Futur. Mater. Biomed. Appl., Materials Research Foundations, 2020: pp. 134–155. [CrossRef]
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. , Additively manufactured biodegradable porous metals, Acta Biomater. 115 (2020) 29–50. [CrossRef]
- Kimura, T.; Yokoi, H.; Nakagawa, Y.; Tamura, T.; Kaburagi, S.; Sawada, Y.; Sato, Y.; Yokoi, H.; Hamasaki, N.; Nosaka, H.; Nobuyoshi, M. , Three-Year Follow-up after Implantation of Metallic Coronary-Artery Stents, N. Engl. J. Med. 334 (1996) 561–567. [CrossRef]
- Moravej, M.; Mantovani, D., Biodegradable metals for cardiovascular stent application: Interests and new opportunities, Int. J. Mol. Sci. 12 (2011) 4250–4270. [CrossRef]
- Agrawal, C.M.; Haas, K.F.; Leopold, D.A.; Clark, H.G. , Evaluation of poly(L-lactic acid) as a material for intravascular polymeric stents, Biomaterials. 13 (1992) 176–182. [CrossRef]
- Zhou, C.; Li, H.; Yin, Y.; Shi, Z.; Li, T.; Feng, X.; Zhang, J.; Song, C.; Cui, X.; Xu, K.; Zhao, Y.; Hou, W.; Lu, S.; Liu, G.; Li, M.; Ma, J.; Toft, E.; Volinsky, A.A.; Wan, M.; Yao, X.; Wang, C.; Yao, K.; Xu, S.; Lu, H.; Chang, S.; Ge, J.; Wang, L.; Zhang, H. , Long-term in vivo study of biodegradable Zn-Cu stent : A 2-year implantation evaluation in porcine coronary artery, Acta Biomater. 97 (2019) 657–670. [CrossRef]
- Nica, M.; Cretu, B.; Ene, D.; Antoniac, I.; Gheorghita, D.; Ene, R. , Failure Analysis of Retrieved Osteosynthesis Implants, (2020) 1–11.
- Prediger, B.; Mathes, T.; Probst, C.; Pieper, D. , Elective removal vs. retaining of hardware after osteosynthesis in asymptomatic patients — a scoping review, (2020) 1–9.
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. , Three-dimensional ( 3D ) printed scaffold and material selection for bone repair, Acta Biomater. 84 (2019) 16–33. [CrossRef]
- Li, Y.; Shi, Y.; Lu, Y.; Li, X.; Zhou, J.; Zadpoor, A.A.; Wang, L. , Additive manufacturing of vascular stents, Acta Biomater. 167 (2023) 16–37. [CrossRef]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. , Materials evolution of bone plates for internal fixation of bone fractures: A review, J. Mater. Sci. Technol. 36 (2020) 190–208. [CrossRef]
- Barati, D. , Biodegradable Hybrid Tissue Engineering Scaffolds For Reconstruction Of Large Bone Defects, 2016. https://scholarcommons.sc.edu/cgi/viewcontent.cgi?article=4831&context=etd.
- Li, H.; Zheng, Y.; Qin, L. , Progress of biodegradable metals, Prog. Nat. Sci. Mater. Int. 24 (2014) 414–422. [CrossRef]
- Tilton, M.; Lewis, G.S.; Wee, H.B.; Armstrong, A.; Hast, M.W.; Manogharan, G., Additive manufacturing of fracture fixation implants: Design, material characterization, biomechanical modeling and experimentation, Addit. Manuf. 33 (2020) 101137. [CrossRef]
- Milewski, J.O. , Additive Manufacturing of Metals, Springer Cham, 2017.
- Bedmar, J.; Riquelme, A.; Rodrigo, P.; Torres, B.; Rams, J., Comparison of different additive manufacturing methods for 316l stainless steel, Materials (Basel). 14 (2021). [CrossRef]
- Yang, S.W.L.; Hsu, K. , Brian Baughman, Donald Godfrey, Francisco Medina, Mamballykalathil Menon, Additive Manufacturing of Metals: The Technology, Materials, Design and Production, Springer Cham, 2017. [CrossRef]
- Pérez-Ruiz, J.D.; Marin, F.; Martínez, S.; Lamikiz, A.; Urbikain, G.; de Lacalle, L.N.L. , Stiffening near-net-shape functional parts of Inconel 718 LPBF considering material anisotropy and subsequent machining issues, Mech. Syst. Signal Process. 168 (2022) 1–18. [CrossRef]
- Bartolomeu, F.; Fonseca, J.; Peixinho, N.; Alves, N.; Gasik, M.; Silva, F.S.; Miranda, G. , Predicting the output dimensions, porosity and elastic modulus of additive manufactured biomaterial structures targeting orthopedic implants, J. Mech. Behav. Biomed. Mater. 99 (2019) 104–117. [CrossRef]
- Krakhmalev, P.; Yadroitsev, I.; Yadroitsava, I.; de Smidt, O., Functionalization of biomedical Ti6Al4V via in situ alloying by Cu during laser powder bed fusion manufacturing, Materials (Basel). 10 (2017). [CrossRef]
- Gatto, M.L.; Cerqueni, G.; Groppo, R.; Santecchia, E.; Tognoli, E.; Defanti, S.; Mattioli-Belmonte, M.; Mengucci, P., Improved biomechanical behavior of 316L graded scaffolds for bone tissue regeneration produced by laser powder bed fusion, J. Mech. Behav. Biomed. Mater. 144 (2023) 105989. [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; Glanville, J.; Grimshaw, J.M.; Hróbjartsson, A.; Lalu, M.M.; Li, T.; Loder, E.W.; Mayo-Wilson, E.; McDonald, S.; McGuinness, L.A.; Stewart, L.A.; Thomas, J.; Tricco, A.C.; Welch, V.A.; Whiting, P.; Moher, D., The PRISMA 2020 statement: An updated guideline for reporting systematic reviews, BMJ. 372 (2021). [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications, Appl. Phys. Rev. 2 (2015) 41101. [CrossRef]
- Liu, J.H.; Shi, Y.S.; Lu, Z.L.; Xu, Y.; Chen, K.H.; Huang, S.H. , Manufacturing metal parts via indirect SLS of composite elemental powders, Mater. Sci. Eng. A. 444 (2007) 146–152. [CrossRef]
- Maeda, K.; Childs, T.H.C. , Laser sintering (SLS) of hard metal powders for abrasion resistant coatings, J. Mater. Process. Technol. 149 (2004) 609–615. [CrossRef]
- Xu, W. , Direct Additive Manufacturing Techniques for Metal Parts: SLM, EBM, Laser Metal Deposition, Encycl. Mater. Met. Alloy. (2022) 290–318. [CrossRef]
- Zhu, H.; Fu, X.; Fan, S.; Liang, L.; Lin, X.; Ning, Y. The conversion from a Gaussian-like beam to a flat-top beam in the laser hardening processing using a fiber coupled diode laser source, Opt. Laser Technol. 125 (2020) 106028. [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.J. , Lasers in additive manufacturing: A review, Int. J. Precis. Eng. Manuf. - Green Technol. 4 (2017) 307–322. [CrossRef]
- Prabakaran, M.P.; Kannan, G.R. Optimization of CO2 Laser Beam Welding Process Parameters to Attain Maximum Weld Strength in Dissimilar Metals, Mater. Today Proc. 5 (2018) 6607–6616. [CrossRef]
- Wen, P.; Voshage, M.; Jauer, L.; Chen, Y.; Qin, Y.; Poprawe, R.; Schleifenbaum, J.H. , Laser additive manufacturing of Zn metal parts for biodegradable applications: Processing, formation quality and mechanical properties, Mater. Des. 155 (2018) 36–45. [CrossRef]
- Thomas, M.; Baxter, G.J.; Todd, I. , Normalised model-based processing diagrams for additive layer manufacture of engineering alloys, Acta Mater. 108 (2016) 26–35. [CrossRef]
- Li, X.; Liu, Y.; Zhou, Z. , Influence of hatch distance on processing, microstructure and mechanical properties of AlMgScZr alloy fabricated by laser powder bed fusion, J. Manuf. Process. 81 (2022) 78–91. [CrossRef]
- Afrasiabi, M.; Lüthi, C.; Bambach, M.; Wegener, K. Multi-resolution SPH simulation of a laser powder bed fusion additive manufacturing process, Appl. Sci. 11 (2021). [CrossRef]
- Hermawan, H.; Alamdari, H.; Mantovani, D.; Dubé, D. , Iron–manganese: new class of metallic degradable biomaterials prepared by powder metallurgy, Powder Metall. 51 (2008) 38–45. [CrossRef]
- Sikora-jasinska, M.; Chevallier, P.; Turgeon, S.; Paternoster, C.; Mostaed, E.; Vedani, M.; Mantovani, D. , Understanding the e ff ect of the reinforcement addition on corrosion behavior of Fe / Mg 2 Si composites for biodegradable implant applications, Mater. Chem. Phys. 223 (2019) 771–778. [CrossRef]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Acta Biomaterialia Electroformed pure iron as a new biomaterial for degradable stents : In vitro degradation and preliminary cell viability studies q, Acta Biomater. 6 (2010) 1843–1851. [CrossRef]
- Chuan-hao, N.I.; Qiang, X.U.; Fu-chi, W. Grain refinement process of pure iron target under hypervelocity impact, Trans. Nonferrous Met. Soc. China. 21 (2010) 1029–1034. [CrossRef]
- Hermawan, H.; Purnama, A.; Dube, D.; Couet, J.; Mantovani, D. Acta Biomaterialia Fe – Mn alloys for metallic biodegradable stents : Degradation and cell viability studies q, Acta Biomater. 6 (2010) 1852–1860. [CrossRef]
- Drynda, A.; Hassel, T.; Bach, F.W.; Peuster, M. , In vitro and in vivo corrosion properties of new iron–manganese alloys designed for cardiovascular applications, J. Biomed. Mater. Res. Part B Appl. Biomater. 103 (2015) 649–660. [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löf, J.F.; Uggowitzer, P.J. , Degradation performance of biodegradable Fe \ Mn \ C ( \ Pd ) alloys, 33 (2013) 1882–1893. [CrossRef]
- Schinhammer, M.; Gerber, I.; Hänzi, A.C.; Uggowitzer, P.J. , On the cytocompatibility of biodegradable Fe-based alloys, Mater. Sci. Eng. C. 33 (2013) 782–789. [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; Von Schnakenburg, C. , Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta, 27 (2006) 4955–4962. [CrossRef]
- Oliver, A.A.; Sikora-jasinska, M.; Gökhan, A.; Guillory, R.J. , Acta Biomaterialia Recent advances and directions in the development of bioresorbable metallic cardiovascular stents : Insights from recent human and in vivo studies, Acta Biomater. 127 (2021) 1–23. [CrossRef]
- Zheng, J.; Qiu, H.; Tian, Y.; Hu, X.; Luo, T.; Wu, C.; Tian, Y.; Tang, Y.; Song, L.; Li, L.; Xu, L.; Xu, B.; Gao, R. , Preclinical Evaluation of a Novel Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold in Porcine Coronary Artery at 6 Months, 12 (2019). [CrossRef]
- Zheng, J.; Xi, Z.; Li, Y.; Li, J.; Qiu, H.; Hu, X.; Luo, T.; Wu, C.; Wang, X.; Song, L.; Li, L.; Qi, H.; Zhang, G.; Qin, L.; Zhang, W.; Shi, X.; Wang, S.; Zhang, D.; Xu, B.; Gao, R. , Bioactive Materials Long-term safety and absorption assessment of a novel bioresorbable nitrided iron scaffold in porcine coronary artery, 17 (2022) 496–505. [CrossRef]
- Wegener, B.; Sievers, B.; Utzschneider, S.; Müller, P.; Jansson, V.; Rößler, S.; Nies, B.; Stephani, G.; Kieback, B.; Quadbeck, P. Microstructure , cytotoxicity and corrosion of powder-metallurgical iron alloys for biodegradable bone replacement materials, Mater. Sci. Eng. B. 176 (2011) 1789–1796. [CrossRef]
- Yang, C.; Huan, Z.; Wang, X.; Wu, C.; Chang, J. , 3D Printed Fe Scaffolds with HA Nanocoating for Bone Regeneration, ACS Biomater. Sci. Eng. 4 (2018) 608–616. [CrossRef]
- Song, B.; Dong, S.; Deng, S.; Liao, H.; Coddet, C. , Microstructure and tensile properties of iron parts fabricated by selective laser melting, Opt. Laser Technol. 56 (2014) 451–460. [CrossRef]
- Carluccio, D.; Bermingham, M.; Kent, D.; Demir, A.G.; Previtali, B.; Dargusch, M.S. , Comparative Study of Pure Iron Manufactured by Selective Laser Melting, Laser Metal Deposition, and Casting Processes, Adv. Eng. Mater. 21 (2019) 1–9. [CrossRef]
- Carluccio, D.; Xu, C.; Venezuela, J.; Cao, Y.; Kent, D.; Bermingham, M.; Demir, A.G.; Previtali, B.; Ye, Q.; Dargusch, M. , Additively manufactured iron-manganese for biodegradable porous load-bearing bone scaffold applications, Acta Biomater. 103 (2020) 346–360. [CrossRef]
- Carluccio, D.; Demir, A.G.; Caprio, L.; Previtali, B.; Bermingham, M.J.; Dargusch, M.S. , The influence of laser processing parameters on the densification and surface morphology of pure Fe and Fe-35Mn scaffolds produced by selective laser melting, J. Manuf. Process. 40 (2019) 113–121. [CrossRef]
- Shuai, C.; Yang, W.; Yang, Y.; Pan, H.; He, C.; Qi, F.; Xie, D.; Liang, H. Selective laser melted Fe-Mn bone scaffold: Microstructure, corrosion behavior and cell response, Mater. Res. Express. 7 (2019). [CrossRef]
- Palousek, D.; Pantelejev, L.; Zikmund, T.; Koutny, D. Processing of nearly pure iron using 400W selective laser melting – Initial study, MM Sci. J. 2017 (2017) 1738–1743. [CrossRef]
- Song, B.; Dong, S.; Liu, Q.; Liao, H.; Coddet, C. , Vacuum heat treatment of iron parts produced by selective laser melting: Microstructure, residual stress and tensile behavior, Mater. Des. 54 (2014) 727–733. [CrossRef]
- Hansen, N. , Hall – Petch relation and boundary strengthening, 51 (2004) 801–806. [CrossRef]
- Callister, W.D.; Rethwish, D.G. , Materials Science and Engineering an introduction, Wiley, 2014.
- Li, Y.; Jahr, H.; Pavanram, P.; Bobbert, F.S.L.; Puggi, U.; Zhang, X.; Pouran, B.; Leeflang, M.A.; Weinans, H.; Zhou, J.; Zadpoor, A.A. , Acta Biomaterialia Additively manufactured functionally graded biodegradable porous iron, 96 (2019) 646–661. [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. , Mechanical Properties of Trabecular Bone in the Human Mandible: Implications for Dental Implant Treatment Planning and Surgical Placement, J. Oral Maxillofac. Surg. (1999) 700–706. [CrossRef]
- Gabriela, G. , Biodegradable Iron-Based Materials — What Was Done and What More Can Be Done ?, (2021).
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.I.; Leeflang, M.A.; Pouran, B.; Gonzalez-garcia, Y.; Weinans, H.; Mol, J.M.C.; Zhou, J.; Zadpoor, A.A. , Acta Biomaterialia Additively manufactured biodegradable porous iron, 77 (2018) 380–393. [CrossRef]
- Nie, Y.; Chen, G.; Peng, H.; Tang, S.; Zhou, Z.; Pei, F.; Shen, B. , Acta Biomaterialia In vitro and 48 weeks in vivo performances of 3D printed porous Fe-30Mn biodegradable scaffolds, Acta Biomater. 121 (2021) 724–740. [CrossRef]
- Dargusch, M.S.; Dehghan-Manshadi, A.; Shahbazi, M.; Venezuela, J.; Tran, X.; Song, J.; Liu, N.; Xu, C.; Ye, Q.; Wen, C. Exploring the Role of Manganese on the Microstructure, Mechanical Properties, Biodegradability, and Biocompatibility of Porous Iron-Based Scaffolds, ACS Biomater. Sci. Eng. 5 (2019) 1686–1702. [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. , Iron metabolism and toxicity, 202 (2005) 199–211. [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. , Laser and Electron-Beam Powder-Bed Additive Manufacturing of Metallic Implants : A Review on Processes, Materials and Designs, (2016). [CrossRef]
- Falchuk, H. , The Biochemical Basis of Zinc Physiology, 2023.
- Qiao, Y.; Zhang, W.; Tian, P.; Meng, F.; Zhu, H.; Jiang, X.; Liu, X.; Chu, P.K. Biomaterials Stimulation of bone growth following zinc incorporation into biomaterials, Biomaterials. 35 (2014) 6882–6897. [CrossRef]
- Mcclain, C.J. , Antiatherogenic Properties of Zinc : Implications in Endothelial Cell Metabolism, 12 (1996) 711–717.
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents, Adv. Mater. 25 (2013) 2577–2582. [CrossRef]
- Huang, H.; Li, G.; Jia, Q.; Bian, D.; Guan, S.; Kulyasova, O.; Valiev, R.Z.; Rau, J.V.; Zheng, Y. , Acta Biomaterialia Recent advances on the mechanical behavior of zinc based biodegradable metals focusing on the strain softening phenomenon, 152 (2022) 1–18. [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H. , Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr, Sci. Rep. (2015) 1–14. [CrossRef]
- Bowen, P.K.; Guillory, R.J.; Shearier, E.R.; Seitz, J.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. , Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents, Mater. Sci. Eng. C. 56 (2015) 467–472. [CrossRef]
- Lietaert, K.; Baekelant, W.; Thijs, L.; Vleugels, J. , Direct Metal Printing Of Zinc: From Single Laser Tracks To High Density Parts, in: Eur. Congr. Exhib. Powder Merallurgy. Eur. PM Conf. Proc., 2016.
- Montani, M.; Demir, A.G.; Mostaed, E.; Vedani, M.; Previtali, B. , Processability of pure Zn and pure Fe by SLM for biodegradable metallic implant manufacturing, Rapid Prototyp. J. 23 (2017) 514–523. [CrossRef]
- Demir, A.G.; Monguzzi, L.; Previtali, B. , Selective laser melting of pure Zn with high density for biodegradable implant manufacturing, Addit. Manuf. 15 (2017) 20–28. [CrossRef]
- Wen, P.; Jauer, L.; Voshage, M.; Chen, Y.; Poprawe, R.; Henrich, J. , Densi fi cation behavior of pure Zn metal parts produced by selective laser melting for manufacturing biodegradable implants, J. Mater. Process. Tech. 258 (2018) 128–137. [CrossRef]
- Wen, P.; Qin, Y.; Chen, Y.; Voshage, M.; Jauer, L.; Poprawe, R.; Henrich, J. , Journal of Materials Science & Technology Laser additive manufacturing of Zn porous scaffolds : Shielding gas flow, surface quality and densification, J. Mater. Sci. Technol. 35 (2019) 368–376. [CrossRef]
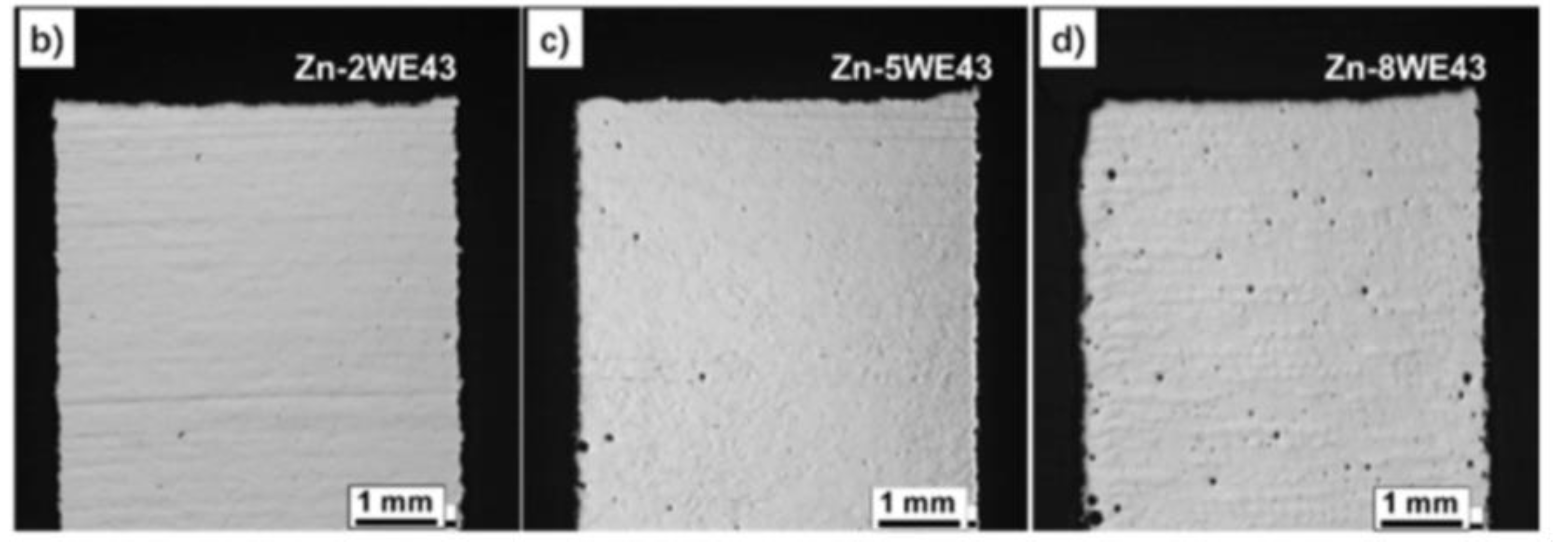
- Qin, Y.; Wen, P.; Voshage, M.; Chen, Y.; Georg, P.; Jauer, L.; Xia, D.; Guo, H.; Zheng, Y.; Henrich, J. , Additive manufacturing of biodegradable Zn-xWE43 porous scaffolds : Formation quality, microstructure and mechanical properties, 181 (2019). [CrossRef]
- Shuai, C.; Cheng, Y.; Yang, Y.; Peng, S.; Yang, W. , Laser additive manufacturing of Zn-2Al part for bone repair : Formability, microstructure and properties, J. Alloys Compd. 798 (2019) 606–615. [CrossRef]
- Yang, Y.; Yang, M.; He, C.; Qi, F.; Wang, D.; Peng, S.; Shuai, C. Rare earth improves strength and creep resistance of additively manufactured Zn implants, Compos. Part B. 216 (2021) 108882. [CrossRef]
- Yang, Y.; Yuan, F.; Gao, C.; Feng, P.; Xue, L.; He, S. , Journal of the Mechanical Behavior of Biomedical Materials A combined strategy to enhance the properties of Zn by laser rapid solidi fi cation and laser alloying, J. Mech. Behav. Biomed. Mater. 82 (2018) 51–60. [CrossRef]
- Qin, Y.; Liu, A.; Guo, H.; Shen, Y.; Wen, P.; Lin, H. , Acta Biomaterialia Additive manufacturing of Zn-Mg alloy porous scaffolds with enhanced osseointegration : In vitro and in vivo studies, 145 (2022) 403–415. [CrossRef]
- Qin, Y.; Yang, H.; Liu, A.; Dai, J.; Wen, P.; Zheng, Y. , Acta Biomaterialia Processing optimization, mechanical properties, corrosion behavior and cytocompatibility of additively manufactured Zn-0. 7Li biodegradable metals, 142 (2022) 388–401. [CrossRef]
- Lietaert, K.; Zadpoor, A.A.; Sonnaert, M.; Schrooten, J.; Weber, L.; Mortensen, A.; Vleugels, J. , Mechanical properties and cytocompatibility of dense and porous Zn produced by laser powder bed fusion for biodegradable implant applications, Acta Biomater. 110 (2020) 289–302. [CrossRef]
- Qin, Y.; Wen, P.; Xia, D.; Guo, H.; Voshage, M.; Jauer, L.; Zheng, Y.; Schleifenbaum, J.H.; Tian, Y. Effect of grain structure on the mechanical properties and in vitro corrosion behavior of additively manufactured pure Zn, Addit. Manuf. 33 (2020) 101134. [CrossRef]
- Shuai, C.; Xue, L.; Gao, C.; Yang, Y.; Peng, S.; Zhang, Y. , Selective laser melting of Zn–Ag alloys for bone repair: microstructure, mechanical properties and degradation behaviour, Virtual Phys. Prototyp. 13 (2018) 146–154. [CrossRef]
- Li, Z.; Shi, Z.Z.; Yan, Y.; Zhang, D.; Yang, K.; Li, H.F.; Zhang, H.; Wang, L.N. Suppression mechanism of initial pitting corrosion of pure Zn by Li alloying, Corros. Sci. 189 (2021) 109564. [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Bobbert, F.S.L.; Kubo, Y.; Leeflang, M.A.; Jahr, H.; Zadpoor, A.A. Additively manufactured functionally graded biodegradable porous zinc, Biomater. Sci. 8 (2020) 2404–2419. [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Taheri, P.; Li, W.; San, H.; Leeflang, M.A.; Mol, J.M.C.; Jahr, H.; Zadpoor, A.A. , Additively manufactured biodegradable porous zinc, Acta Biomater. 101 (2020) 609–623. [CrossRef]
- Xia, D.; Qin, Y.; Guo, H.; Wen, P.; Lin, H.; Voshage, M.; Schleifenbaum, J.H.; Cheng, Y.; Zheng, Y. , Additively manufactured pure zinc porous scaffolds for critical-sized bone defects of rabbit femur, Bioact. Mater. 19 (2023) 12–23. [CrossRef]
- International Organization for Standardization, ISO 10993-5:2009 Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity, Arlington, VA, 2009.
- International Organization for Standardization, ISO 10993-4:2017 Biological Evaluation of Medical Devices – Part 4: Selection of Tests for Interactions with Blood, Arlington, VA, 2017.
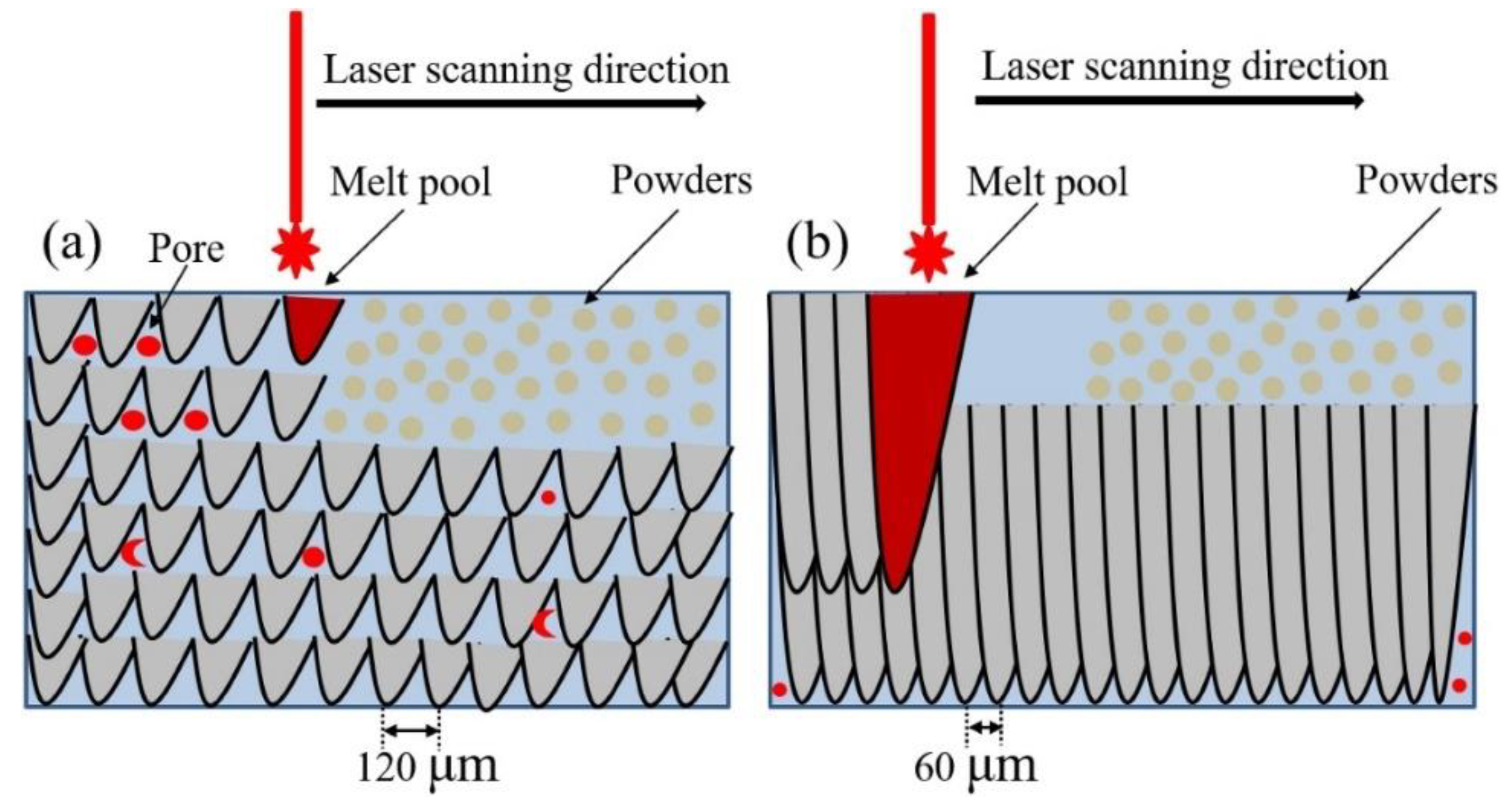
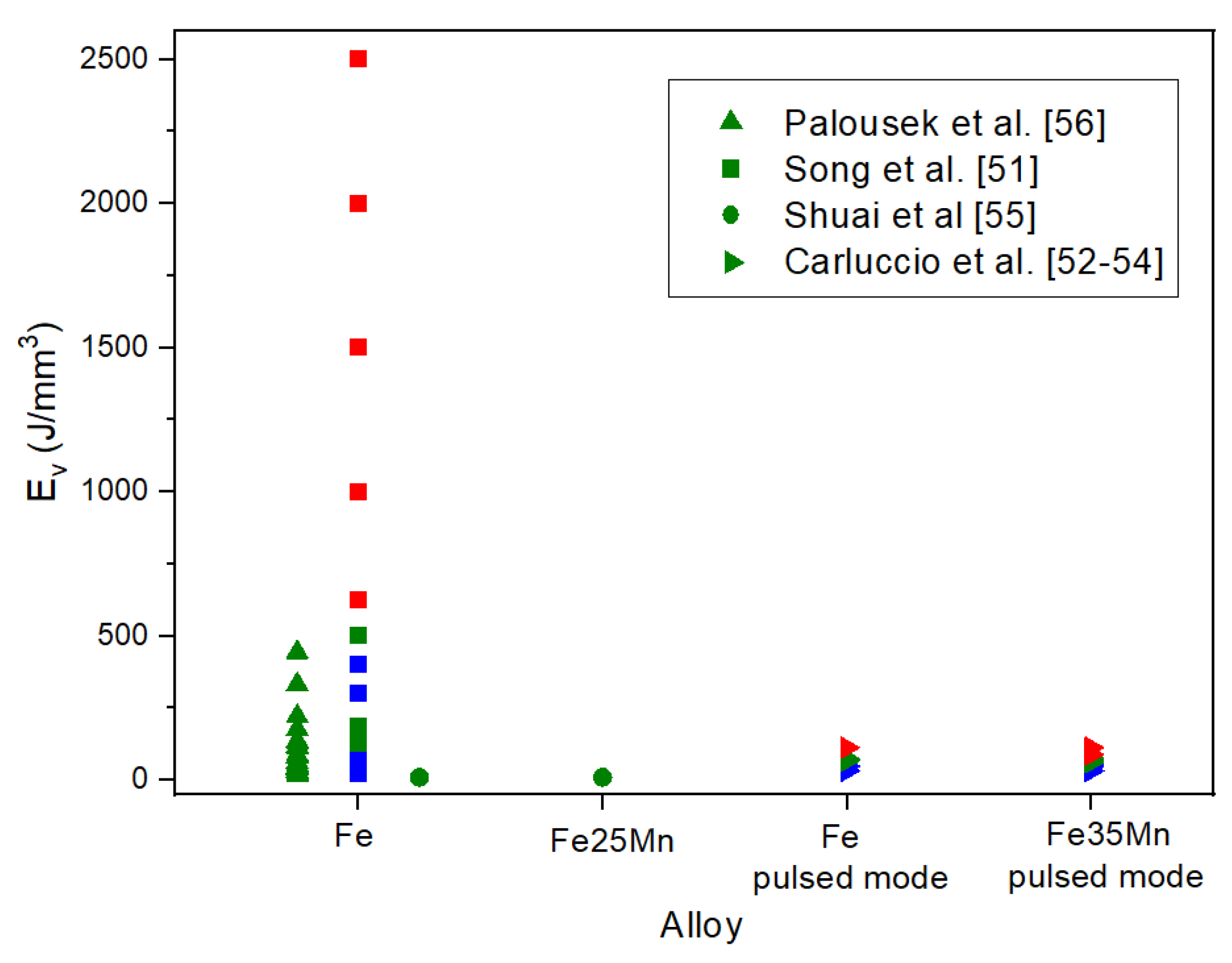
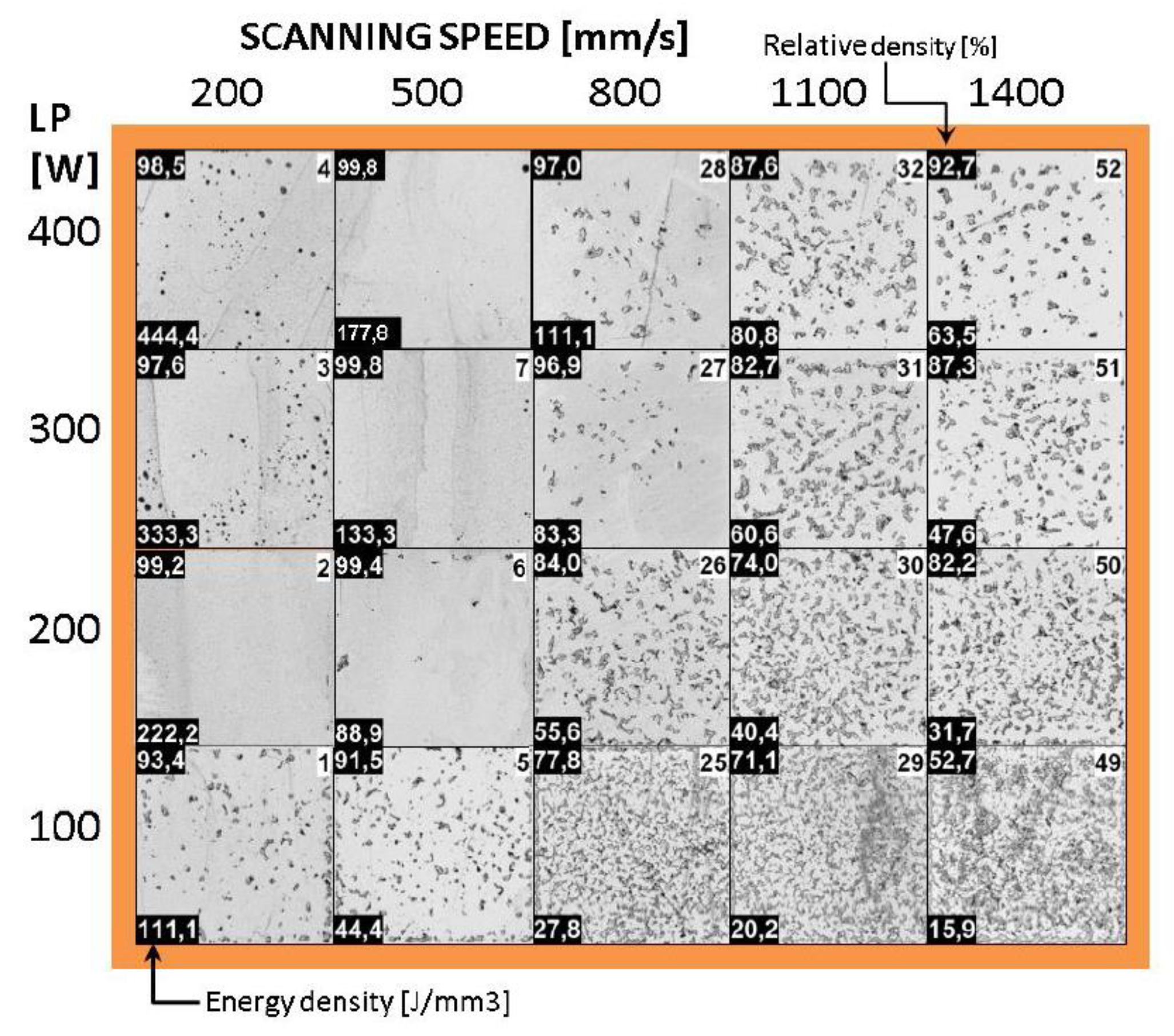
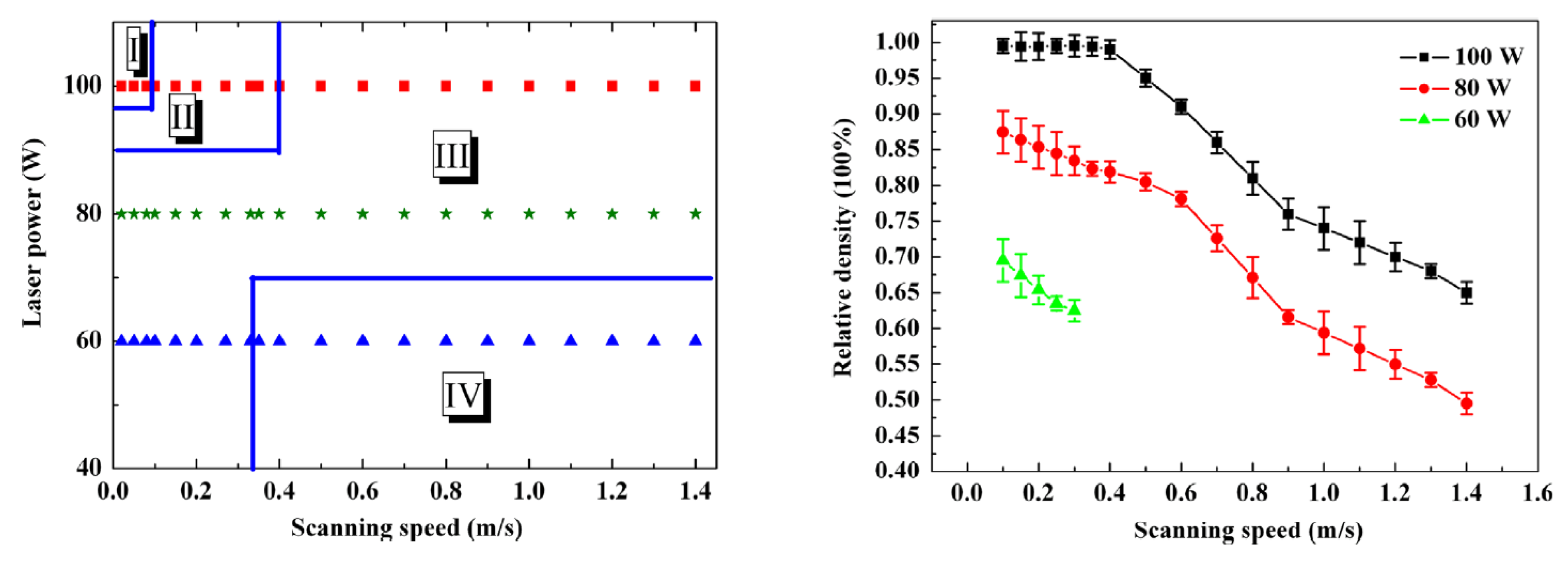



| Amount in human organism (g) | Blood serum level | Daily allowance | Young´s modulus (GPa) | In vitro corrosion rate (mm/year) | |
|---|---|---|---|---|---|
| Fe | 5 | 5 - 17.6 g/L | 10 - 20 mg | 200 | 0.012 |
| Mg | 25 | 1.6 - 2.5 mg/dL | 0.7 g | 41 - 45 | 0.10 ± 0.07 |
| Zn | 2 | 60 - 120 µg/dl | 12 - 15 mg | 96 | 0.08 |
| Properties | Unit | Value | ||
|---|---|---|---|---|
| Zn | Fe | Mg | ||
| Density (20 ˚C) | g/cm3 | 7.14 | 7.874 | 1.74 |
| Melting point | ˚C | 419.5 | 1538 | 650 |
| Boiling point | ˚C | 907 | 2862 | 1091 |
| Heat conductivity (20 ˚C) | W/m·K | 113 | 80 | 158 |
| Heat conductivity (melting point) | W/m·K | 61 | 40 | 78 |
| Specific heat (20 ˚C) | J/kg K | 382 | 444 | 1360 |
| Surface tension (melting point) | mN/m | 782 | 1835 | 559 |
| Viscosity (melting point) | mPa·s | 3.85 | 6.93 | 1.25 |
| Laser absorptivity (powder, 20 ˚C) | % | 70 | 75 | / |
| Human bone | 40-79 | ||
| Material | SLM | Cast | Wrought |
| Pure Fe | 150 ± 6.5 | 130 (Mild steel) | 150 (Mild steel) |
| Fe35Mn | 163 ± 4.0 | n/a | n/a |
| AISI 316L | 245 ± 6.0 | 175 | 220 |
| Pure Zn | 45 ± 5.4 | n/a | 34 ± 2 |
| Pure Mg | 78 ± 8.2 | 30 ± 2 | ± 2 |
| Material | Energy density (J/mm3) | E (GPa) | σ0.2(MPa) | UTS(MPa) | Compressive strength at 20% strain [MPa] |
Ref |
|---|---|---|---|---|---|---|
| Human Cortical Bone | - | 1–35 | 1–20 | 103–140 | [53] | |
| Cast Fe | - | 202.5 ± 6.70 | 157.1 ± 7.7 | 497.8 ± 7.5 | [52] | |
| Cast Fe35Mn | - | 240 | 440 | [53] | ||
| SLM pure Fe | 185 | 205.67 ± 16 | 245.87 ± 17 | 354.27 ± 18 | - | [51] |
| SLM pure Fe | 152 | 208.77 ± 16 | 256.57 ± 17 | 356.67 ± 22 | - | |
| SLM pure Fe | 143 | 210.57 ± 18 | 285.47 ± 20 | 402.77 ± 24 | - | |
| SLM pure Fe | 125 | 215.87 ± 20 | 305.37 ± 22 | 411.57 ± 25 | - | |
| SLM pure Fe | 67 | 199.70 ± 6.70 | 421.1 ± 16 | - | 760.2 ± 6.5 | [52] |
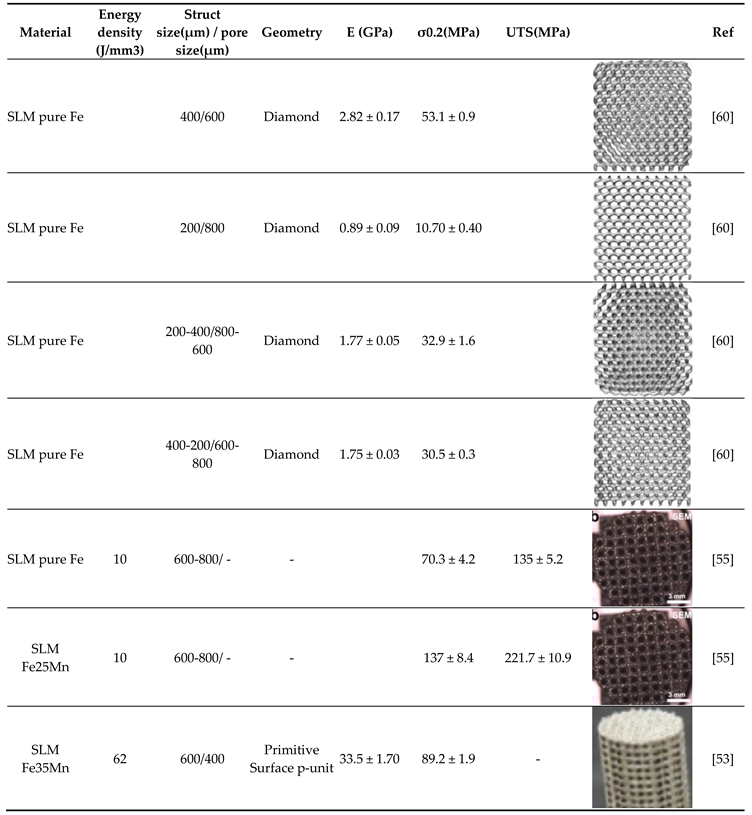 |
| Material | Part | Energy density (J/mm3) | Corrosion test | Conditions | CR (mm/year) | icorr (mA/cm2) | Rp (Ω cm2) |
Ref. |
|---|---|---|---|---|---|---|---|---|
| Cast Fe | - | - | Electrochemical test | OCP: measured for 150 minutes EIS: amplitude of 10mV with scanning frequency between 100 kHz and 10 mHz. Lp: ±0.25 V (vs. SCE) at a scan rate of 0.166mVs-1. |
0.047 ± 0.003 | 4.05 ± 0.3 | 1410 | [52] |
| Col rolled iron | Electrochemical test | OCP: measured for 60 minutes EIS: amplitude of 10mV with scanning frequency between 100 kHz and 10 mHz Lp: -0.3 - +0.5 V (vs. SCE) at a scan rate of 0.5mVs-1 |
0.10 ± 0.01 | 0.0086 ± 0.0009 | - | [63] | ||
| Col rolled iron | Immersion test | Samples immersed for 28 days in r-SBF | - | [63] | ||||
| SLM Pure Fe | Dense | 67 | Electrochemical test | OCP: measured for 150 minutes EIS: amplitude of 10mV with scanning frequency between 100 kHz and 10 mHz. Lp: ±0.25 V (vs. SCE) at a scan rate of 0.166mVs-1. |
0.072 ± 0.001 | 6.2 ± 0.1 | 1035 | [52] |
| SLM Pure Fe | Scaffold | 10 | Electrochemical test | EIS: amplitude of 10mV with scanning frequency between 100 kHz and 10 mHz | - | 0.00738 ± 0.00321 | [55] | |
| SLM Pure Fe | Scaffold | 10 | Immersion test | Samples immersed for 30 days in SBF with a pH of 7.4 | 0.09 ± 0.02 | [55] | ||
| SLM Pure Fe | Scaffold | - | Electrochemical test | OCP: measured for 60 minutes EIS: amplitude of 10mV with scanning frequency between 100 kHz and 10 mHz Lp: -0.3 - +0.5 V (vs. SCE) at a scan rate of 0.5mVs-1 |
1.18 ± 0.22 | 0.1028 ± 0.0192 | - | [63] |
| SLM Pure Fe | Scaffold | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.03 | - | [63] | |
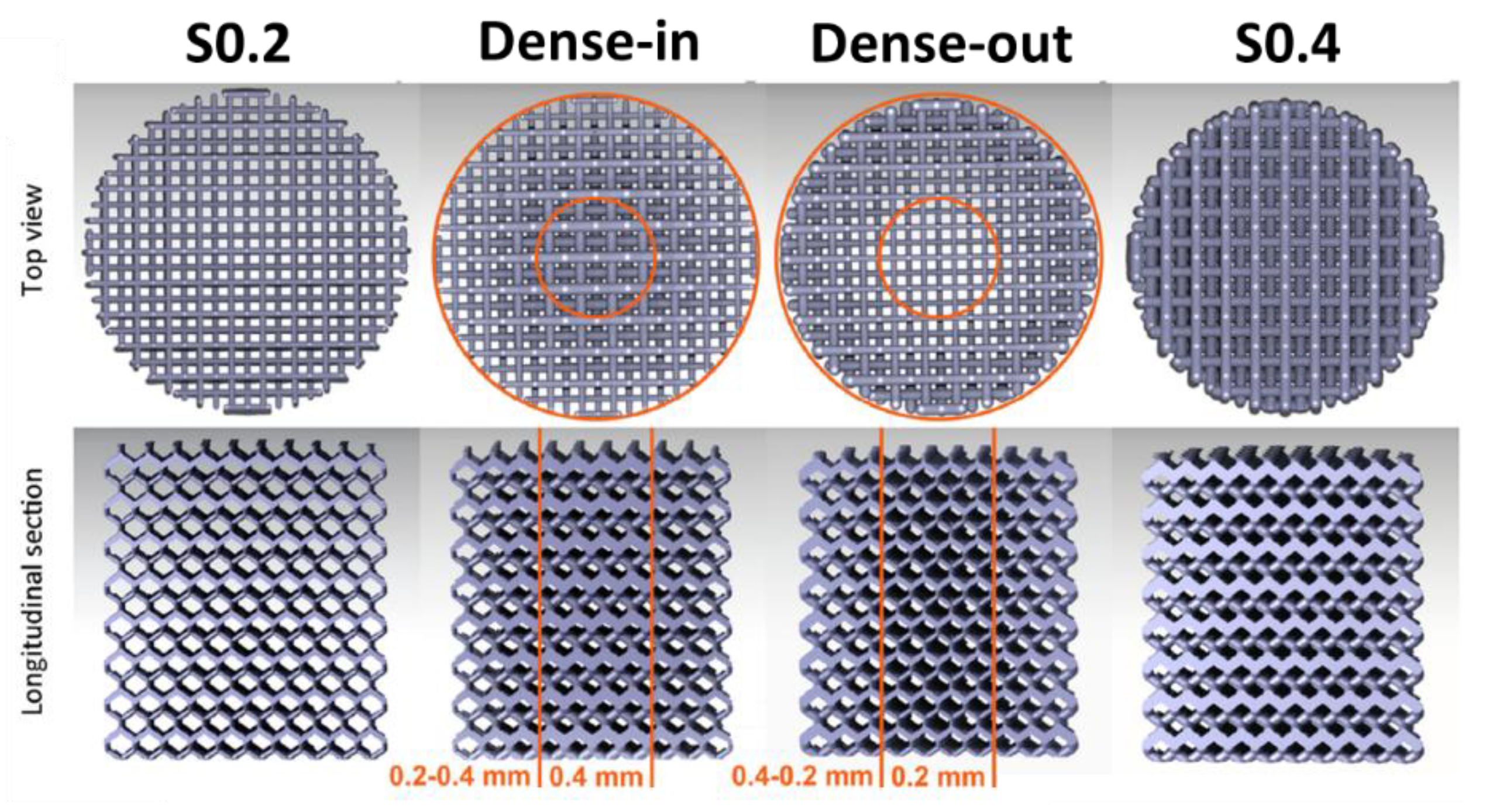
| SLM Pure Fe | Scaffold S0.2 | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.19446 | - | - | [60] |
| SLM Pure Fe | Scaffold (Dense-in) | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.1389 | - | - | [60] |
| SLM Pure Fe | Scaffold (Dense-out) | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.17131 | - | - | [60] |
| SLM Pure Fe | Scaffold S0.4 | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.12501 | - | - | [60] |
| SLM Pure Fe | Scaffold | Electrochemical test | OCP: measured for 60 minutes Lp: -0.2 - +0.5V (vs. SCE) at a scan rate of 0.1mVs-1 |
0.049 | 0.0042 | [64] | ||
| SLM Fe25Mn | Scaffold | 10 | Electrochemical test | EIS: amplitude of 10mV with scanning frequency between 100 kHz and 10 mHz | - | 0.05125 ± 0.00752 | [55] | |
| SLM Fe25Mn | Scaffold | 10 | Immersion test | Samples immersed for 30 days in SBF with a pH of 7.4 | 0.23 ± 0.05 | [55] | ||
| SLM Fe30Mn | Scaffold | Electrochemical test | OCP: measured for 60 minutes Lp: -0.2 - +0.5V (vs. SCE) at a scan rate of 0.1mVs-1 |
0.142 | 0.01191 | [64] | ||
| SLM Fe35Mn | Scaffold | 62 | Electrochemical test | OCP: measured for 150 minutes EIS: amplitude of 10mV with scanning frequency between 100 kHz and 10 mHz Lp: ±0.25 V (vs. SCE) at a scan rate of 0.166mVs-1 |
0.8 | [53] | ||
| SLM Fe35Mn | Scaffold | 62 | Immersion test |
Samples immersed for 28 days in Hank’s balanced salt solution with a pH of 7.4 |
0.42 ± 0.03 | [53] |
| Material | Cell assay |
Cell line | Duration | Results | Ref. |
|---|---|---|---|---|---|
| Fe | Indirect | MG-63 | 3 days | good cytocompatibility, with cells normally grew on the scaffolds | [55] |
| Fe25Mn | Indirect | MG-63 | 3 days | good cytocompatibility, with cells normally grew on the scaffolds | [55] |
| Fe30Mn | Indirect | MC3T3-E1 | 7 days | RGR grade 0. The extract is not cytotoxic. | [64] |
| Fe30Mn | Direct | MC3T3-E1 | 7 days | Seven days co-culture results in many live cells and only a few dead cells | [64] |
| Fe35Mn | Indirect | MC3T3-E1 | 3 days | The scaffold displayed biocompatibility, high viability towards mammalian cells and filopodia on the scaffold indicated that the alloy is suitable for osteoblast adhesion | [53] |
| Fe | Indirect | MG-63 | 3 days | MG-63 viability in extended, long-term extracts (72 h) of iron specimens dropped to below 50%. | [63] |
| Fe | Direct | MG-63 | 1 day | revealed substantial and almost instant cytotoxicity | [63] |
| Material | Shape | Animal | Implantation place | Duration | Results | Reference |
|---|---|---|---|---|---|---|
| Fe30Mn | Scaffold | Rabbit | lateral femoral condyle | 48 weeks | biocompatibility and osseointegration performances in the repair of load-bearing bone defects | [64] |
| Fe35Mn | Scaffold | Rat | Cranium | 4 weeks | the implant integrated with the original bone, and even stimulated bone formation | [53] |
| Material | Ev (J/mm3) | Yield Strength (MPa) | UTS | Elongation | Ref |
|---|---|---|---|---|---|
| Zn | 66.7 J/mm3 | 134 | 10 % | [80] | |
| Zn2WE43 | 298.5 | 1.8 % | |||
| Zn5WE43 | 335.4 | 1 % | |||
| Zn8WE43 | 154.1 | 0.9 % | |||
| Zn | 125 J/mm3 | 43.2 | 61.3 | 1.7 % | [83] |
| Zn1Mg | 74 | 126 | 3.6 % | ||
| Zn2Mg | 117 | 162 | 4.1 % | ||
| Zn3Mg | 152 | 222 | 7.2 % | ||
| Zn4Mg | 132 | 166 | 3.1 % | ||
| Zn2Al | 76.19 J/mm3 | 120 | 170 | 9 % | [81] |
| 95.24 J/mm3 | 135 | 185 | 10 % | ||
| 114.28 J/mm3 | 140 | 190 | 12 % | ||
| 133.33 J/mm3 | 138 | 188 | 11 % | ||
| Zn | 55.55 J/mm3 | 79.9 | 103.6 | 5.10% | [82] |
| Zn1Ce | 140 | 210 | 6% | ||
| Zn2Ce | 180.6 | 247.4 | 7.5% | ||
| Zn3Ce | 182 | 230 | 6.8% | ||
| Zn (Vertical) | 127 J/mm3 (300 mm/s) | 94 MPa | 119 MPa | 2,6% | [87] |
| 76,19 J/mm3 (500 mm/s) |
108 MPa | 130 MPa | 8% | ||
| 54.42 J/mm3 (700 mm/s) | 110.3 MPa | 132 MPa | 7% | ||
| Zn (Horizontal) | 127 J/mm3 (300 mm/s) | 72 MPa | 90 MPa | 2,5% | |
| 54.42 J/mm3 (700 mm/s) | 75 MPa | 92.3 MPa | 5% | ||
| Zn (Vertical) | 39 J/mm3 | 78 MPa | 100 MPa | 10% | [86] |
| Zn (Horizontal) | 55 MPa | 79 MPa | 12% |
| Material | Ev (J/mm3) | Structural porosity / pore size | Geometry | Ultimate Compressive strength (MPa) | Yield strength (MPa) | Elastic Modulus (GPa) |
Ref. |
|---|---|---|---|---|---|---|---|
| Zn | 39 J/mm3 | 73%- / 700 μm | Diamond | 4 | 0.4 | [60] | |
| 69% /Graded pore size 600 – 800 μm | Diamond | 6 | 0.5 | ||||
| 62% / 600 μm | Diamond | 11 | 0.8 | ||||
| Zn | 39 J/mm3 | 20 – 40% | Diamond | 7 – 15 MPa* | [86] | ||
| 22 – 40% | Dodecahedron | 8 – 25 MPa* | |||||
| 25 – 45% | FCC | 10- 50 MPa* | |||||
| 22 – 35% | Kagome | 15 – 50 MPa* | |||||
| 30 – 50% | Octet Truss | 9 – 30 MPa* | |||||
| Zn | 66.7 J/mm3 | 45% / 600 μm | Diamond | 23 | 13 | 0.95 | [80] |
| Zn2WE43 | 60 | 51 | 1.91 | ||||
| Zn5WE43 | 73 | 66 | 2.48 | ||||
| Zn8WE43 | 51 | 51 | 2.54 | ||||
| Zn | 100 J/mm3 | 50 % | Diamond | [84] | |||
| Zn1Mg | 40 MPa | 1.2 | |||||
| Zn2Mg | 35 MPa | 1.3 | |||||
| Zn5Mg | 24 MPa | 1 | |||||
| Zn0.7Li | 35.7 J/mm3 | 80% / 820 μm | Gyroid | 18.2 MPa | 0.298 | [85] |
| Material | Part | Energy density (J/mm3) | Corrosion test | Conditions | CR (mm/year) | icorr (μA/cm2) | Ref. |
|---|---|---|---|---|---|---|---|
| Zn | Dense | 125 | Electrochemical | Samples soaked in SBF at 37ºC to obtain an OCP and polarization curves were recorded. | 0.14* | 9.24 ± 1.21 | [83] |
| Zn1Mg | 0.09* | 5.86 ± 1.42 | |||||
| Zn2Mg | 0.07* | 4.63 ± 0.95 | |||||
| Zn3Mg | 0.05* | 3.62 ± 0.76 | |||||
| Zn4Mg | 0.06* | 3.71 ± 0.87 | |||||
| Zn | Dense | 125 | Immersion test | Samples immersed in SBF at 37ºC during 4 weeks | 0.18 ± 0.03 | [83] | |
| Zn1Mg | 0.14 ± 0.01 | ||||||
| Zn2Mg | 0.13 ± 0.03 | ||||||
| Zn3Mg | 0.10 ± 0.02 | ||||||
| Zn4Mg | 0.11 ± 0.04 | ||||||
| Zn | Dense | Electrochemical | OCP: measured for 90 minutes (SBF). Scanning rate 1 mV/s | 0.12 | 7.76 | [88] | |
| Zn2Ag | 0.08 | 5.01 | |||||
| Zn4Ag | 0.02 | 1.47 | |||||
| Zn6Ag | 0.15 | 9.56 | |||||
| Zn8Ag | 0.21 | 13.94 | |||||
| Zn | Dense | Immersion test | Samples immersed in SBF at 37ºC during 21 days | 0.081 | [88] | ||
| Zn2Ag | 0.086 | ||||||
| Zn4Ag | 0.107 | ||||||
| Zn6Ag | 0.114 | ||||||
| Zn8Ag | 0.133 | ||||||
| Zn2Al | Dense | 95.24 | Electrochemical | Samples immersed in SBF at 37ºC. OCP ± 300 mV | 0.18 * | 11.75 | [81] |
| 114.28 | 0.12 * | 8 | |||||
| 133.33 | 0.10 * | 7.07 | |||||
| Zn2Al | Dense | 95.24 | Immersion test | Samples immersed in SBF at 37 ºC during 14 days | 0.16 | [81] | |
| 114.28 | 0.14 | ||||||
| 133.33 | 0.12 | ||||||
| Zn | Dense | Electrochemical | OCP: Measured for 50 min. Polarization curves obtained from -200 mV to 200 mV at 0.05 mV/s. EIS: 10^-2 - 10^6 Hz with a signal amplitude 10 mV | 0.13* | 9 | [82] | |
| Zn1Ce | 0.12* | 8 | |||||
| Zn2Ce | 0.11* | 7.2 | |||||
| Zn3Ce | 0.10* | 6.9 | |||||
| Zn | Dense | Immersion test | Samples immersed in SBF during 30 days | 0.034 | [82] | ||
| Zn1Ce | 0.027 | ||||||
| Zn2Ce | 0.025 | ||||||
| Zn3Ce | 0.024 | ||||||
| Zn0.7Li | Dense as built | Electrochemical test | EIS: frequency range 10^-2 - 10^5 Hz 10 mV | 1.5* | 101 ± 4.1 | [85] | |
| Dense polished | 0.43* | 28.5 ± 1.6 | |||||
| Scaffold (Porosity 80%) | 1.6* | 111.2 ± 12.2 | |||||
| Zn0.7Li | Dense | Immersion test | Samples immersed in Hank`s solution at 37ºC during 28 days | 0.046 | [85] | ||
| Scaffold (Porosity 80%) | Immersion test | Samples immersed in Hank`s solution at 37ºC during 28 days | 0.035 | ||||
| Zn | Scaffold (Porosity 73%) | Immersion test | Samples immersed in revised simulated body fluid (r-SBF) during 28 days. Static and dynamic tests. Dynamic tests at a flow rate of 0.3 ml/min. | 0.17 (Dynamic), 0.07 (static) | [90] | ||
| Scaffold (Porosity 69%) | 0.14 (Dynamic), 0.06 (static) | ||||||
| Scaffold (Porosity 62%) | 0.13 (Dynamic), 0.07 (static) | ||||||
| Zn | Scaffold (Porosity 62%) | Electrochemical test | r-SBF at 37ºC. Specimen was polarized from -0.2V to +0.5V potential versus OCP at 0.5 mV/s scan rate. | 0.67 ± 0.04 | 45 ± 2 | [91] | |
| Immersion tests | r-SBF at 37ºC. For EIS, the tests were repeated at 1, 2, 7, 14, 21 and 28 days with 10 mV amplitude within a 100 kHz frequency range. | 0.13 mm/y (dynamic), 0.07 mm/y (static) | |||||
| Zn | Scaffold (Porosity 50%) | Electrochemical tests | Sample immersed in Hank´s solution for 1.5h | [84] | |||
| Zn1Mg | 0.20* | 13.5 ± 5.7 | |||||
| Zn2Mg | 0.37* | 24.9 ± 10.6 | |||||
| Zn5Mg | 0.60* | ± 11,3 |
| Material | Cell assay | Cell line | Duration | Medium | Conditions | Results | Ref. |
|---|---|---|---|---|---|---|---|
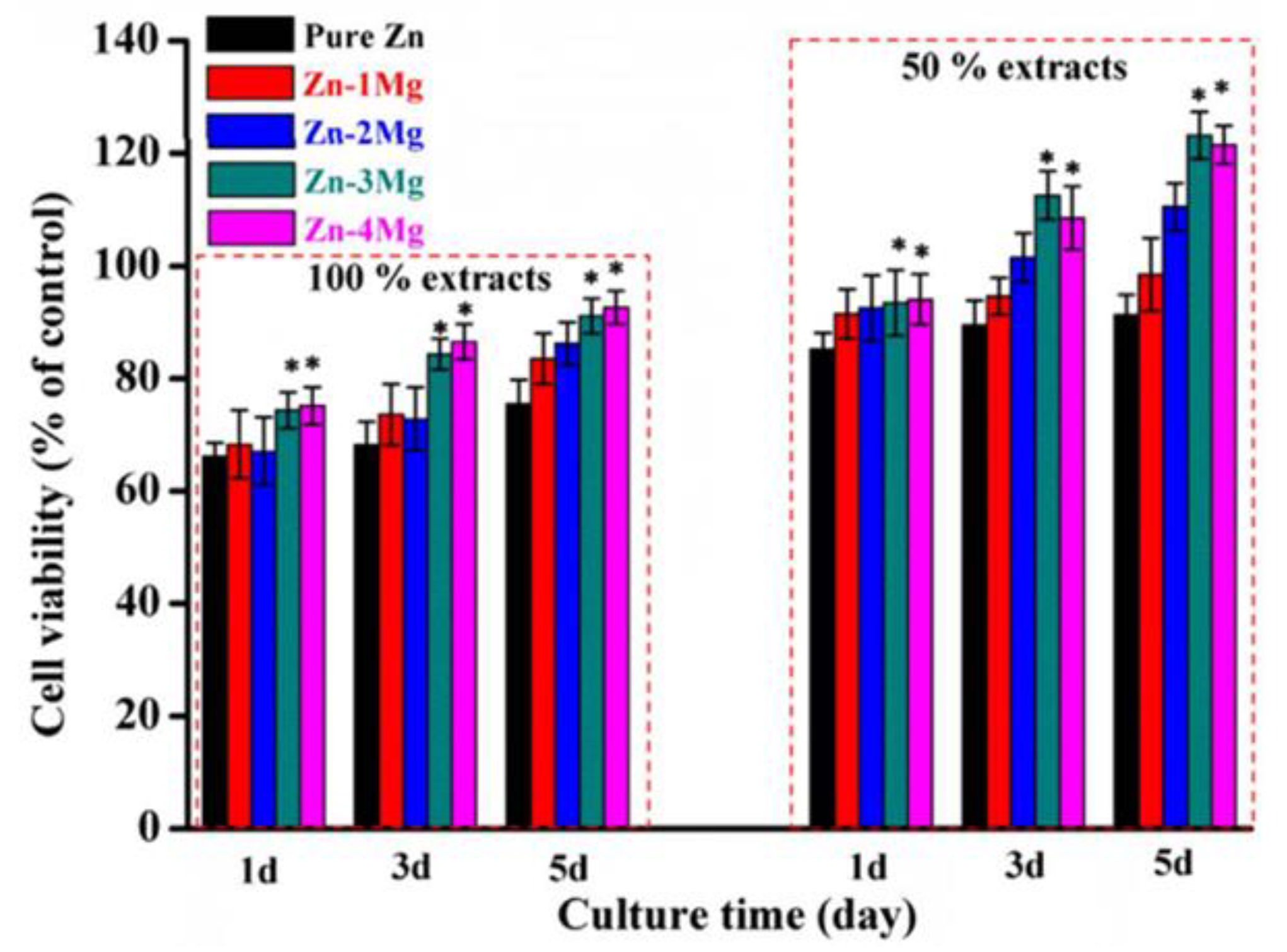
| ZnxMg | Indirect | MG-63 cells | 6h, 1, 3 and 5 days | Dulbecco´s modified eagle medium DMEM + fetal bovine serum. | Dense samples. 100% and 50% extracts. Extracts were prepared at an area to volume ratio of 1.25 cm2/ml. | Good viability in 100% extract and better in 50% extracts. Cell viability increased with increasing exposure time. Mg content increases cell viability. Zn-3Mg best viability. | [83] |
| ZnxCe | Indirect | Human osteosarcoma cells MG-63 | 1, 3 and 7 days | Dulbecco´s Modified Eagle Medium supplemented with 10% fetal bovine serum and antibiotics (100 units/ml penicillun and 100 units/ml streptomycin) | Dense samples. Extracts were prepared at a ratio of 1.25 cm2/ml. | Zn2Ce showed no obvious cell cytotoxicity. Cell viability of Zn2Ce (80.6%) was slightly lower than Zn (83.75%). | [82] |
| Zn0.7Li | Direct | MC3T3-E1 pre-osteoblast | 2 h | Cell suspension was spread all over the surface of samples. | Bulk and porous samples. | Better cell adhesion and viability were found on porous samples in contrast with bulk samples. Cells were shrunk in a spherical shape and separated at the surface of bulk samples. While the cells exhibited a healthier morphology in porous samples. | [85] |
| Zn2Al | Indirect | Human osteosarcoma cells MG-63 | 1, 4 and 7 days | Dulbecco´s modified eagle medium supplemented with 10% fetal bovine serum and antibiotics. | Dense samples. 100% and 50% extracts. Extracts were prepared at a ratio of 1.25 cm2/ml. | 100% extracts reduced the cell viability to 67.5%. Cell viability was higher than 80% for 50% extracts. With culture time the viability of cells increased significantly. | [81] |
| Zn scaffolds |
Direct | Human osteoblast-like cells (MG-63) | 24 h | Dulbecco´s Modified Eagle Medium (DMEM) with 1g/L glucose, 10% fetal calf serum | Most cells were viable (> 70%) and a few exhibited evidence of a compromised cell membrane integrity | [90] | |
| Indirect | 24, 48 and 72 h | Extract 0.2 g Zn/ml for 72 h | After 24h, cell viability in the extracts was higher than 95% for all porous specimens | ||||
| Zn scaffolds | Direct | Human telomerase reverse transcriptase mesenchymal stem cells (hTERT-MSCs) | 14 days | high glucose Dulbecco´s Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% PenStrep | Static seeding and dynamic seeding in a bioreactor | There was no indication of cell attachment and growth for the Zn scaffolds. | [86] |
| Zn scaffolds | Indirect | MC3T3-E1 | 1, 3 and 5 days | α-minimal essential medium with- 10% fetal bovine serum | 100%, 50% and 10% extracts | Viability in 100% extract medium was below 75% after 1 day. Cell viability above 75% after 3 and 5 days. | [92] |
| Zn scaffolds | Direct | Human osteoblasts like cells MG-63 | 24 h | Dulbecco´s Modified Eagle Medium (DMEM) with 1g/L glucose, 10% fetal calf serum | Most of the cells were viable, and results were similar to Ti6Al4V | [91] | |
| Indirect | 24, 48 and 72 h | Extract 0.2 g Zn/ml for 72 h | Cell viability decreased from 95% at 24, 48 h to 85% at 72 h |
| Material | Shape | Animal | Implantation site | Duration | Results | Ref. |
|---|---|---|---|---|---|---|
| Zn | Scaffold | Rabbit | Femur | 24 weeks | Successful osseointegration of the scaffold | [92] |
| Zn and Zn1Mg | Scaffold | Rabbit | Femur | 12 weeks | Osseointegration of Zn1Mg scaffolds. Fibrous connective tissue between bone tissue and Zn scaffold | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).