Submitted:
05 December 2024
Posted:
05 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Antibacterial Strategies Applied on Coatings
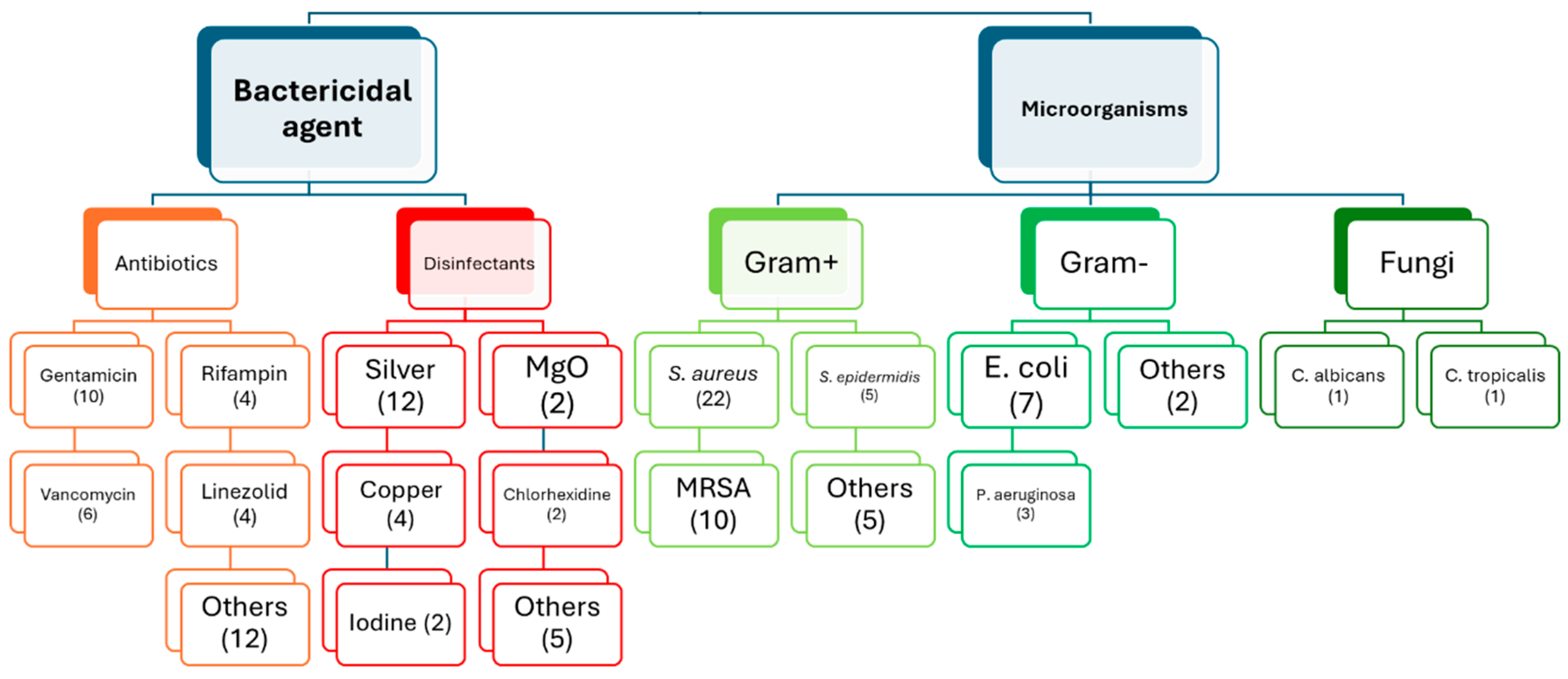
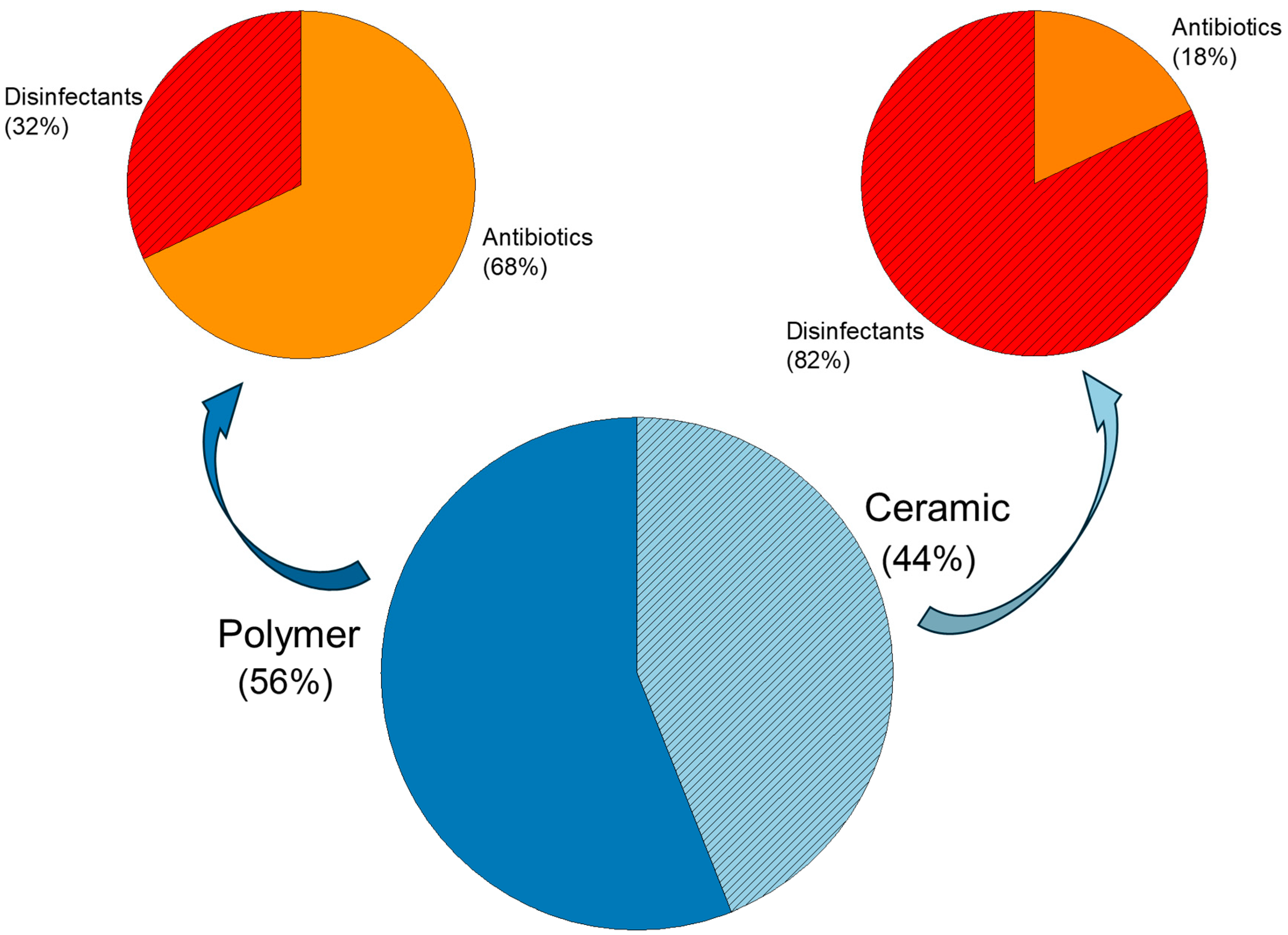
2.1. Use of Different Bactericidal Agents
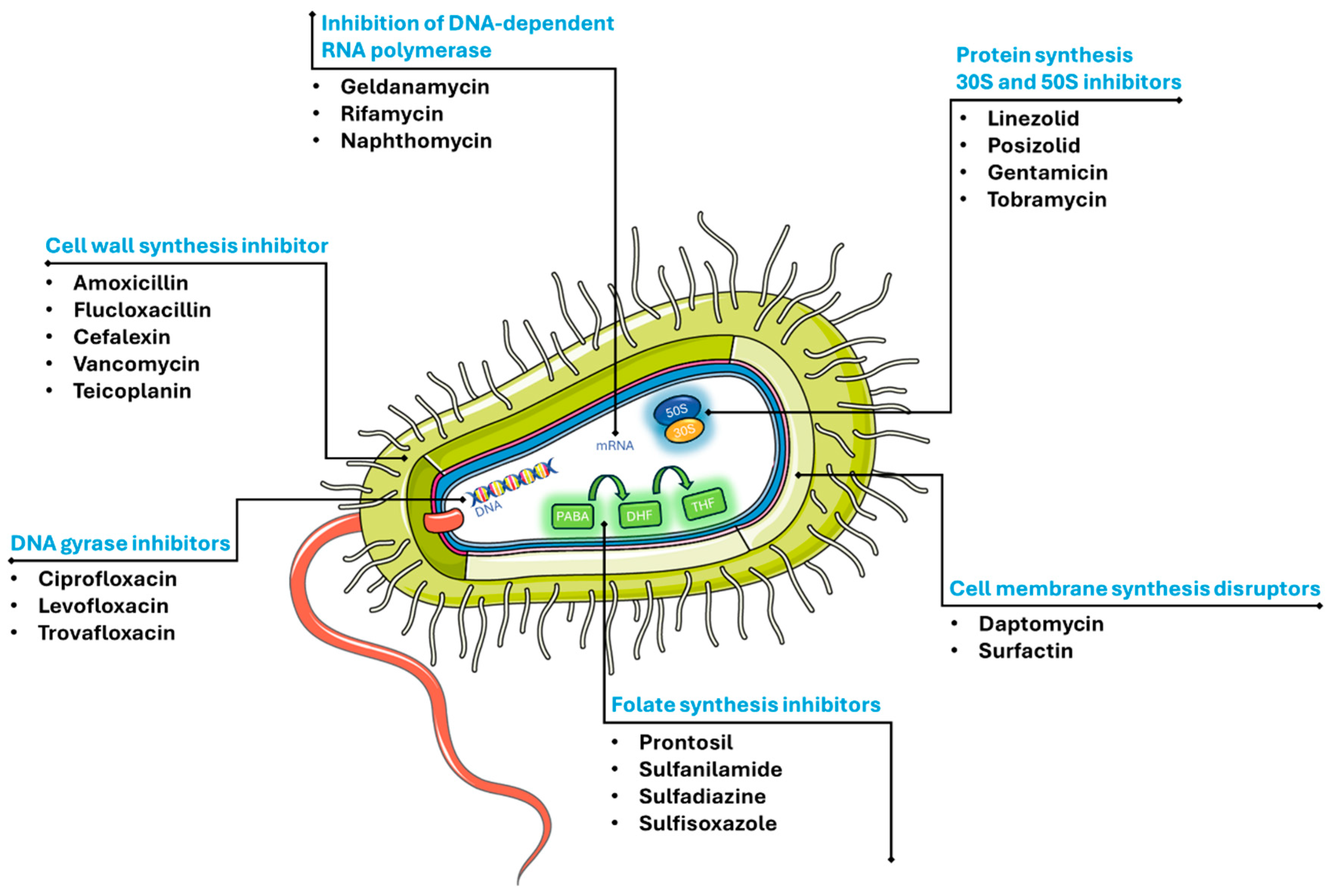
2.1.1. Antibiotics
2.1.2. Disinfectants
3. In-Vitro and In-Vivo Experimental Approaches for Surface-Treated Implants with Antibacterial Properties
3.1. In-Vitro
3.1. In-Vivo
4. Conclusions and Future Prospectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alaee, F.; Angerame, M.; Bradbury, T.; Blackwell, R.; Booth, R.E.; Brekke, A.C.; Courtney, P.M.; Frenkel, T.; Silva, F.R.G.; Heller, S. General assembly, prevention, operating room-surgical technique: proceedings of international consensus on orthopedic infections. The Journal of Arthroplasty 2019, 34, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Osman, K.; Green, G.; Haddad, F.S. The epidemiology of failure in total knee arthroplasty. The Bone & Joint Journal 2016, 98-B, 105–112. [Google Scholar] [CrossRef]
- Irwin, S.; Wang, T.; Bolam, S.M.; Alvares, S.; Swift, S.; Cornish, J.; Williams, D.L.; Ashton, N.N.; Matthews, B.G. Rat model of recalcitrant prosthetic joint infection using biofilm inocula. Journal of Orthopaedic Research 2023, 41, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.C.; Son, M.-S.; Chang, E.T.; Zimmerli, W.; Parvizi, J. Are We Winning or Losing the Battle With Periprosthetic Joint Infection: Trends in Periprosthetic Joint Infection and Mortality Risk for the Medicare Population. The Journal of Arthroplasty 2018, 33, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Barger, J.; Fragomen, A.T.; Rozbruch, S.R. Antibiotic-Coated Interlocking Intramedullary Nail for the Treatment of Long-Bone Osteomyelitis. JBJS Reviews 2017, 5, e5. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef]
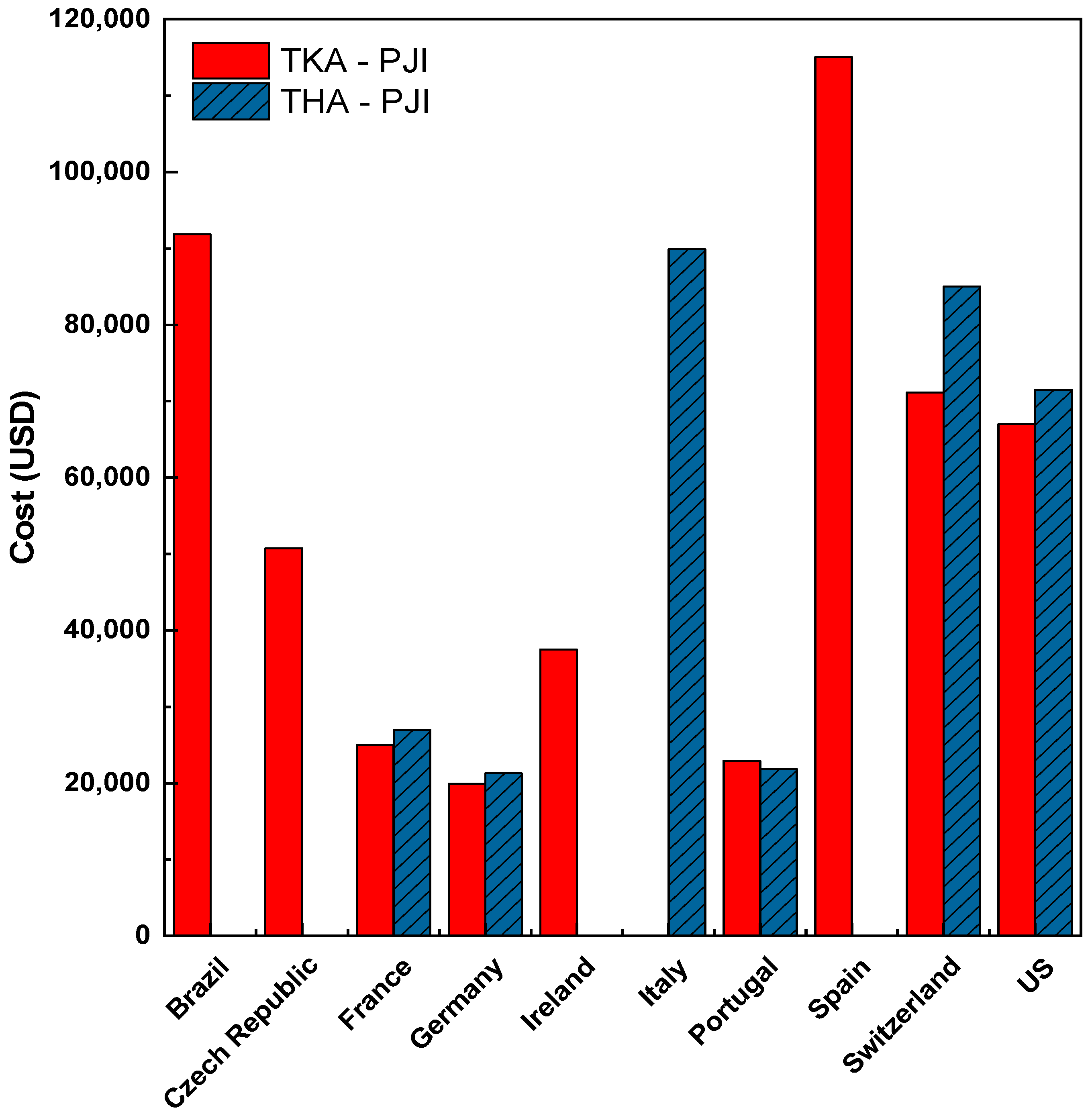
- Szymski, D.; Walter, N.; Hierl, K.; Rupp, M.; Alt, V. Direct Hospital Costs per Case of Periprosthetic Hip and Knee Joint Infections in Europe — A Systematic Review. The Journal of Arthroplasty 2024, 39, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.; Schmier, J.; Ong, K.L.; Zhao, K.; Parvizi, J. Infection Burden for Hip and Knee Arthroplasty in the United States. The Journal of Arthroplasty 2008, 23, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Alotaibi, H.F.; Yergeshov, A.A.; Dang, T.; Abdullin, T.I.; Prokopovich, P. Long acting anti-infection constructs on titanium. Journal of Controlled Release 2020, 326, 91–105. [Google Scholar] [CrossRef]
- Dal-Paz, K.; Oliveira, P.R.D.; Paula, A.P.d.; Emerick, M.C.d.S.; Pécora, J.R.; Lima, A.L.L.M. Economic impact of treatment for surgical site infections in cases of total knee arthroplasty in a tertiary public hospital in Brazil. Brazilian Journal of Infectious Diseases 2010, 14. [Google Scholar]
- Kurtz, S.M.; Higgs, G.B.; Lau, E.; Iorio, R.R.; Courtney, P.M.; Parvizi, J. Hospital Costs for Unsuccessful Two-Stage Revisions for Periprosthetic Joint Infection. The Journal of Arthroplasty 2022, 37, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, R.K.; Mathew, M.; Bowen, E.; James, G.A.; Stulberg, S.D. A Novel Irrigant to Eliminate Planktonic Bacteria and Eradicate Biofilm Superstructure With Persistent Effect During Total Hip Arthroplasty. The Journal of Arthroplasty 2022, 37, S647–S652. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. The Journal of Arthroplasty 2021, 36, 1484–1489.e1483. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Wang, J.; You, Y.; Tan, J.; Shen, H. Distribution characteristics of Staphylococcus spp. in different phases of periprosthetic joint infection: A review (Review). Exp Ther Med 2017, 13, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Zhu, M.; Luey, C.; Young, S.W. Antibiotic resistance in early periprosthetic joint infection. ANZ Journal of Surgery 2016, 86, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Zaatreh, S.; Haffner, D.; Strauss, M.; Dauben, T.; Zamponi, C.; Mittelmeier, W.; Quandt, E.; Kreikemeyer, B.; Bader, R. Thin magnesium layer confirmed as an antibacterial and biocompatible implant coating in a co-culture model. Mol Med Rep 2017, 15, 1624–1630. [Google Scholar] [CrossRef]
- Huotari, K.; Peltola, M.; Jämsen, E. The incidence of late prosthetic joint infections. Acta Orthopaedica 2015, 86, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Al Thaher, Y.; Perni, S.; Prokopovich, P. Nano-carrier based drug delivery systems for sustained antimicrobial agent release from orthopaedic cementous material. Advances in Colloid and Interface Science 2017, 249, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. Journal of Tissue Engineering 2018, 9, 2041731418790694. [Google Scholar] [CrossRef] [PubMed]
- Ständert, V.; Borcherding, K.; Bormann, N.; Schmidmaier, G.; Grunwald, I.; Wildemann, B. Antibiotic-loaded amphora-shaped pores on a titanium implant surface enhance osteointegration and prevent infections. Bioactive Materials 2021, 6, 2331–2345. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama. Journal of Orthopaedic Surgery and Research 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yergeshov, A.A.; Al-Thaher, Y.; Avdokushina, S.; Statsenko, E.; Abdullin, T.I.; Prokopovich, P. Nanocomposite orthopaedic bone cement combining long-acting dual antimicrobial drugs. Biomaterials Advances 2023, 153, 213538. [Google Scholar] [CrossRef]
- Liu, D.; He, C.; Liu, Z.; Xu, W. Gentamicin coating of nanotubular anodized titanium implant reduces implant-related osteomyelitis and enhances bone biocompatibility in rabbits. International Journal of Nanomedicine 2017, 5461–5471. [Google Scholar] [CrossRef]
- Tan, H.; Ao, H.; Ma, R.; Tang, T. Quaternised chitosan-loaded polymethylmethacrylate bone cement: Biomechanical and histological evaluations. Journal of Orthopaedic Translation 2013, 1, 57–66. [Google Scholar] [CrossRef]
- Min, J.; Choi, K.Y.; Dreaden, E.C.; Padera, R.F.; Braatz, R.D.; Spector, M.; Hammond, P.T. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano 2016, 10, 4441–4450. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H.; Yudoh, K.; Hashimoto, M.; Himeda, Y.; Miyoshi, T.; Yoshida, K.; Kano, S. A new antibacterial carrier of hyaluronic acid gel. Journal of Orthopaedic Science 2006, 11, 497–504. [Google Scholar] [CrossRef]
- Alt, V.; Bitschnau, A.; Böhner, F.; Heerich, K.E.; Magesin, E.; Sewing, A.; Pavlidis, T.; Szalay, G.; Heiss, C.; Thormann, U.; et al. Effects of gentamicin and gentamicin–RGD coatings on bone ingrowth and biocompatibility of cementless joint prostheses: An experimental study in rabbits. Acta biomaterialia 2011, 7, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Yang, C.; Chai, H.; Yuan, X.; Liu, W.; Zhang, X. The sulfonated polyetheretherketone with 3D structure modified by two bio-inspired methods shows osteogenic and antibacterial functions. Chemical Engineering Journal 2021, 420, 130059. [Google Scholar] [CrossRef]
- McKenna, P.B.; O’Shea, K.; Masterson, E.L. Two-stage revision of infected hip arthroplasty using a shortened post-operative course of antibiotics. Archives of Orthopaedic and Trauma Surgery 2009, 129, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Guo, G.; Yu, J.; Zhang, X. Antibacterial application of gentamicin–silk protein coating with smart release function on titanium, polyethylene, and Al2O3 materials. Materials Science and Engineering: C 2021, 124, 112069. [Google Scholar] [CrossRef]
- Ashbaugh, A.G.; Jiang, X.; Zheng, J.; Tsai, A.S.; Kim, W.-S.; Thompson, J.M.; Miller, R.J.; Shahbazian, J.H.; Wang, Y.; Dillen, C.A.; et al. Polymeric nanofiber coating with tunable combinatorial antibiotic delivery prevents biofilm-associated infection in vivo. Proceedings of the National Academy of Sciences 2016, 113, E6919–E6928. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, A.I.; Zhu, S.; Hegde, V.; Loftin, A.H.; Ashbaugh, A.G.; Niska, J.A.; Miller, L.S.; Segura, T.; Bernthal, N.M. In Vivo Efficacy of a “Smart” Antimicrobial Implant Coating. JBJS 2016, 98, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Giavaresi, G.; Meani, E.; Sartori, M.; Ferrari, A.; Bellini, D.; Sacchetta, A.C.; Meraner, J.; Sambri, A.; Vocale, C.; Sambri, V.; et al. Efficacy of antibacterial-loaded coating in an in vivo model of acutely highly contaminated implant. International Orthopaedics 2014, 38, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Boot, W.; Vogely, H.C.; Jiao, C.; Nikkels, P.G.; Pouran, B.; van Rijen, M.H.; Ekkelenkamp, M.B.; Hänsch, G.M.; Dhert, W.J.; Gawlitta, D. Prophylaxis of implant-related infections by local release of vancomycin from a hydrogel in rabbits. European cells & materials 2020, 39, 108–120. [Google Scholar] [CrossRef]
- Fang, Y.; Xiao, Y.; Zhao, Y.; Golodok, R.P.; Savich, V.V.; Ilyushchanka, A.P.; Chen, X.; Wang, R. Porous titanium layer co-immobilized with bone morphogenetic protein-2 and vancomycin for biofunctionalization of ultra high molecular weight polyethylene. Materials & Design 2023, 232, 112131. [Google Scholar] [CrossRef]
- Xi, W.; Hegde, V.; Zoller, S.D.; Park, H.Y.; Hart, C.M.; Kondo, T.; Hamad, C.D.; Hu, Y.; Loftin, A.H.; Johansen, D.O.; et al. Point-of-care antimicrobial coating protects orthopaedic implants from bacterial challenge. Nature Communications 2021, 12, 5473. [Google Scholar] [CrossRef]
- Miller, R.J.; Thompson, J.M.; Zheng, J.; Marchitto, M.C.; Archer, N.K.; Pinsker, B.L.; Ortines, R.V.; Jiang, X.; Martin, R.A.; Brown, I.D.; et al. In Vivo Bioluminescence Imaging in a Rabbit Model of Orthopaedic Implant-Associated Infection to Monitor Efficacy of an Antibiotic-Releasing Coating. JBJS 2019, 101. [Google Scholar] [CrossRef] [PubMed]
- Bernthal, N.M.; Stavrakis, A.I.; Billi, F.; Cho, J.S.; Kremen, T.J.; Simon, S.I.; Cheung, A.L.; Finerman, G.A.; Lieberman, J.R.; Adams, J.S.; et al. A Mouse Model of Post-Arthroplasty Staphylococcus aureus Joint Infection to Evaluate In Vivo the Efficacy of Antimicrobial Implant Coatings. PLOS ONE 2010, 5, e12580. [Google Scholar] [CrossRef]
- Kaur, S.; Harjai, K.; Chhibber, S. In Vivo Assessment of Phage and Linezolid Based Implant Coatings for Treatment of Methicillin Resistant S. aureus (MRSA) Mediated Orthopaedic Device Related Infections. PLOS ONE 2016, 11, e0157626. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Wang, S.; Yang, C.; Geng, Z.; Zhang, X. Sponge-inspired sulfonated polyetheretherketone loaded with polydopamine-protected osthole nanoparticles and berberine enhances osteogenic activity and prevents implant-related infections. Chemical Engineering Journal 2022, 437, 135255. [Google Scholar] [CrossRef]
- Kawano, S.; Sonohata, M.; Eto, S.; Kitajima, M.; Mawatari, M. Bone ongrowth of a cementless silver oxide-containing hydroxyapatite-coated antibacterial acetabular socket. Journal of Orthopaedic Science 2019, 24, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Q.; Zhang, Y.; He, H.; Xiong, S.; Chen, P.; Li, C.; Wang, L.; Lu, G.; Xu, Y. A dual-functional PEEK implant coating for anti-bacterial and accelerated osseointegration. Colloids and Surfaces B: Biointerfaces 2023, 224, 113196. [Google Scholar] [CrossRef]
- Zeng, Z.; He, X.; Tan, B.; Dai, C.; Zheng, W. Titanium oxide nanotubes embedded with silver dioxide nanoparticles for Staphylococcus aureus infections after prosthetic joint replacement in animal models. Int. J. Clin. Exp. Med 2018, 11, 7392–7399. [Google Scholar]
- Hashimoto, A.; Sonohata, M.; Kitajima, M.; Kawano, S.; Eto, S.; Mawatari, M. First experience with a thermal-sprayed silver oxide-containing hydroxyapatite coating implant in two-stage total hip arthroplasty for the treatment of septic arthritis with hip osteoarthritis: A case report. International Journal of Surgery Case Reports 2020, 77, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Eto, S.; Kawano, S.; Someya, S.; Miyamoto, H.; Sonohata, M.; Mawatari, M. First Clinical Experience With Thermal-Sprayed Silver Oxide–Containing Hydroxyapatite Coating Implant. The Journal of Arthroplasty 2016, 31, 1498–1503. [Google Scholar] [CrossRef]
- Eto, S.; Miyamoto, H.; Shobuike, T.; Noda, I.; Akiyama, T.; Tsukamoto, M.; Ueno, M.; Someya, S.; Kawano, S.; Sonohata, M.; et al. Silver oxide-containing hydroxyapatite coating supports osteoblast function and enhances implant anchorage strength in rat femur. Journal of Orthopaedic Research 2015, 33, 1391–1397. [Google Scholar] [CrossRef]
- Nakashima, T.; Morimoto, T.; Hashimoto, A.; Kii, S.; Tsukamoto, M.; Miyamoto, H.; Todo, M.; Sonohata, M.; Mawatari, M. Osteoconductivity and neurotoxicity of silver-containing hydroxyapatite coating cage for spinal interbody fusion in rats. JOR SPINE 2023, 6, e1236. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Miyamoto, H.; Yonekura, Y.; Tsukamoto, M.; Ando, Y.; Noda, I.; Sonohata, M.; Mawatari, M. Silver oxide-containing hydroxyapatite coating has in vivo antibacterial activity in the rat tibia. Journal of Orthopaedic Research 2013, 31, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, T.; Miyamoto, H.; Ando, Y.; Noda, I.; Yonekura, Y.; Kawano, S.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. In vivo antibacterial and silver-releasing properties of novel thermal sprayed silver-containing hydroxyapatite coating. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2010, 92B, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Ueno, M.; Fujii, M.; Mawatari, D.; Mawatari, M. Case Series of Silver Oxide–Containing Hydroxyapatite Coating in Antibacterial Cementless Total Hip Arthroplasty: Clinical Results of 50 Cases at 5-Year Follow-Up. Arthroplasty Today 2023, 19, 101067. [Google Scholar] [CrossRef] [PubMed]
- Devlin-Mullin, A.; Todd, N.M.; Golrokhi, Z.; Geng, H.; Konerding, M.A.; Ternan, N.G.; Hunt, J.A.; Potter, R.J.; Sutcliffe, C.; Jones, E.; et al. Atomic Layer Deposition of a Silver Nanolayer on Advanced Titanium Orthopedic Implants Inhibits Bacterial Colonization and Supports Vascularized de Novo Bone Ingrowth. Advanced Healthcare Materials 2017, 6, 1700033. [Google Scholar] [CrossRef]
- Huo, S.; Lyu, Z.; Su, X.; Wang, F.; Liu, J.; Liu, S.; Liu, X.; Bao, X.; Zhang, J.; Zheng, K.; et al. Formation of a novel Cu-containing bioactive glass nano-topography coating with strong bactericidal capability and bone regeneration. Composites Part B: Engineering 2023, 253, 110521. [Google Scholar] [CrossRef]
- Ellenrieder, M.; Haenle, M.; Lenz, R.; Bader, R.; Mittelmeier, W. Titanium-copper-nitride coated spacers for two-stage revision of infected total hip endoprostheses. GMS Krankenhaushygiene interdisziplinar 2011, 6, Doc16. [Google Scholar] [CrossRef] [PubMed]
- Mauerer, A.; Stenglein, S.; Schulz-Drost, S.; Schoerner, C.; Taylor, D.; Krinner, S.; Heidenau, F.; Adler, W.; Forst, R. Antibacterial Effect of a 4x Cu-TiO2 Coating Simulating Acute Periprosthetic Infection—An Animal Model. Molecules 2017, 22, 1042. [Google Scholar] [CrossRef] [PubMed]
- Ciliveri, S.; Bandyopadhyay, A. Enhanced osteogenesis and bactericidal performance of additively manufactured MgO-and Cu-added CpTi for load-bearing implants. IJB 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, C.; Wang, D.; Jiang, H.; Qiao, Y.; Zhang, D.; Zhang, X.; Xu, R.; Liu, C.; Su, J.; et al. Tailoring time-varying alkaline microenvironment on titanium for sequential anti-infection and osseointegration. Chemical Engineering Journal 2022, 431, 133940. [Google Scholar] [CrossRef]
- Shirai, T.; Shimizu, T.; Ohtani, K.; Zen, Y.; Takaya, M.; Tsuchiya, H. Antibacterial iodine-supported titanium implants. Acta biomaterialia 2011, 7, 1928–1933. [Google Scholar] [CrossRef]
- Taga, T.; Kabata, T.; Kajino, Y.; Inoue, D.; Ohmori, T.; Yamamoto, T.; Takagi, T.; Tsuchiya, H. Comparison with the osteoconductivity and bone-bonding ability of the iodine supported titanium, titanium with porous oxide layer and the titanium alloy in the rabbit model. Journal of Orthopaedic Science 2018, 23, 585–591. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Pham, T.X.; Williams, D.L.; Farnsworth, R.W.; Loc-Carrillo, C.M.; Bloebaum, R.D. Model development for determining the efficacy of a combination coating for the prevention of perioperative device related infections: A pilot study. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2013, 101, 1143–1153. [Google Scholar] [CrossRef]
- Yuan, X.; Ouyang, L.; Luo, Y.; Sun, Z.; Yang, C.; Wang, J.; Liu, X.; Zhang, X. Multifunctional sulfonated polyetheretherketone coating with beta-defensin-14 for yielding durable and broad-spectrum antibacterial activity and osseointegration. Acta biomaterialia 2019, 86, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Cui, R.; Mei, L.; Ni, S.; Sun, H.; Zhang, C.; Ni, S. Cytocompatibility and Bone-Formation Potential of Se-Coated 316L Stainless Steel with Nano-Pit Arrays. Journal of Biomedical Nanotechnology 2018, 14, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yu, J.; Xiao, J.; Wang, Y.; Li, Z.; Wang, H. Antibacterial intraosseous implant surface coating that responds to changes in the bacterial microenvironment. Frontiers in Bioengineering and Biotechnology 2023, 10, 1016001. [Google Scholar] [CrossRef] [PubMed]
- Blackburn Jr., W. D.; Alarcón, G.S. Prosthetic joint infections. A role for prophylaxis. Arthritis & Rheumatism 1991, 34, 110–117. [Google Scholar] [CrossRef]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Haas, N.P.; Raschke, M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 2003, 32, 521–531. [Google Scholar] [CrossRef]
- Commission, E.; Centre, J.R.; Sanseverino, I.; Loos, R.; Navarro Cuenca, A.; Marinov, D.; Lettieri, T. State of the art on the contribution of water to antimicrobial resistance; Publications Office: 2018.
- Babutan, I.; Lucaci, A.-D.; Botiz, I. Antimicrobial Polymeric Structures Assembled on Surfaces. Polymers 2021, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Shaygani, H.; Seifi, S.; Shamloo, A.; Golizadeh, M.; Rahnamaee, S.Y.; Alishiri, M.; Ebrahimi, S. Novel bilayer coating on gentamicin-loaded titanium nanotube for orthopedic implants applications. International Journal of Pharmaceutics 2023, 636, 122764. [Google Scholar] [CrossRef]
- Mohan Raj, R.; Priya, P.; Raj, V. Gentamicin-loaded ceramic-biopolymer dual layer coatings on the Ti with improved bioactive and corrosion resistance properties for orthopedic applications. Journal of the mechanical behavior of biomedical materials 2018, 82, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Ballarre, J.; Aydemir, T.; Liverani, L.; Roether, J.A.; Goldmann, W.H.; Boccaccini, A.R. Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: Improving early stage performance of titanium implants. Surface and Coatings Technology 2020, 381, 125138. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y.; Li, D.; Wei, B.; Xu, Y.; Gu, Q.; Wang, L. Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. International Journal of Nanomedicine 2014, 9, 3027–3036. [Google Scholar] [CrossRef]
- Ordikhani, F.; Tamjid, E.; Simchi, A. Characterization and antibacterial performance of electrodeposited chitosan–vancomycin composite coatings for prevention of implant-associated infections. Materials Science and Engineering: C 2014, 41, 240–248. [Google Scholar] [CrossRef]
- Pon-On, W.; Charoenphandhu, N.; Teerapornpuntakit, J.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. In vitro study of vancomycin release and osteoblast-like cell growth on structured calcium phosphate-collagen. Materials Science and Engineering: C 2013, 33, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Thanyaphoo, S.; Kaewsrichan, J. Potential of bone scaffolds containing vancomycin and bone morphogenetic protein-2 in a rat model of osteomyelitis. Asian Biomedicine 2017, 8, 651–658. [Google Scholar] [CrossRef]
- Hirschfeld, J.; Akinoglu, E.M.; Wirtz, D.C.; Hoerauf, A.; Bekeredjian-Ding, I.; Jepsen, S.; Haddouti, E.-M.; Limmer, A.; Giersig, M. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomedicine: Nanotechnology, Biology and Medicine 2017, 13, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Aboltins, C.A.; Page, M.A.; Buising, K.L.; Jenney, A.W.J.; Daffy, J.R.; Choong, P.F.M.; Stanley, P.A. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid. Clinical Microbiology and Infection 2007, 13, 586–591. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Tian, N.; Dong, X.; Cheng, C.-K. Implant Coating Manufactured by Micro-Arc Oxidation and Dip Coating in Resorbable Polylactide for Antimicrobial Applications in Orthopedics. Coatings 2019, 9, 284. [Google Scholar] [CrossRef]
- Kastoris, A.C.; Rafailidis, P.I.; Vouloumanou, E.K.; Gkegkes, I.D.; Falagas, M.E. Synergy of fosfomycin with other antibiotics for Gram-positive and Gram-negative bacteria. European Journal of Clinical Pharmacology 2010, 66, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.S. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. Journal of Antimicrobial Chemotherapy 2011, 66, iv7–iv15. [Google Scholar] [CrossRef] [PubMed]
- Ament, P.W.; Jamshed, N.; Horne, J.P. Linezolid: its role in the treatment of gram-positive, drug-resistant bacterial infections. American family physician 2002, 65, 663–670. [Google Scholar] [PubMed]
- Toirac, B.; Aguilera-Correa, J.J.; Mediero, A.; Esteban, J.; Jiménez-Morales, A. The Antimicrobial Activity of Micron-Thin Sol–Gel Films Loaded with Linezolid and Cefoxitin for Local Prevention of Orthopedic Prosthesis-Related Infections. Gels 2023, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lai, Y.; Zheng, X.; Yang, Y. Facile Fabrication of Linezolid/Strontium Coated Hydroxyapatite/Graphene Oxide Nanocomposite for Osteoporotic Bone Defect. Heliyon 2024. [Google Scholar] [CrossRef]
- Alt, V.; Bitschnau, A.; Österling, J.; Sewing, A.; Meyer, C.; Kraus, R.; Meissner, S.A.; Wenisch, S.; Domann, E.; Schnettler, R. The effects of combined gentamicin–hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials 2006, 27, 4627–4634. [Google Scholar] [CrossRef] [PubMed]
- Miclau, T.; Edin, M.L.; Lester, G.E.; Lindsey, R.W.; Dahners, L.E. Bone Toxicity of Locally Applied Aminoglycosides. Journal of Orthopaedic Trauma 1995, 9, 401–406. [Google Scholar] [CrossRef]
- Edin, M.L.; Miclau, T.; Lester, G.E.; Lindsey, R.W.; Dahners, L.E. Effect of Cefazolin and Vancomycin on Osteoblasts In Vitro. Clinical Orthopaedics and Related Research® 1996, 333, 245–251. [Google Scholar] [CrossRef]
- Isefuku, S.; Joyner, C.J.; Simpson, A.H.R.W. Gentamicin May Have an Adverse Effect on Osteogenesis. Journal of Orthopaedic Trauma 2003, 17, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Duewelhenke, N.; Krut, O.; Eysel, P. Influence on Mitochondria and Cytotoxicity of Different Antibiotics Administered in High Concentrations on Primary Human Osteoblasts and Cell Lines. Antimicrobial Agents and Chemotherapy 2007, 51, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Mouriño, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. Journal of The Royal Society Interface 2012, 9, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Brokesh, A.M.; Gaharwar, A.K. Inorganic Biomaterials for Regenerative Medicine. ACS Applied Materials & Interfaces 2020, 12, 5319–5344. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Barbaro, K.; Kuroda, P.A.B.; Imperatori, L.; De Bonis, A.; Teghil, R.; Curcio, M.; Innocenzi, E.; Grigorieva, V.Y.; Vadalà, G.; et al. Incorporation of Ca, P, Mg, and Zn Elements in Ti-30Nb-5Mo Alloy by Micro-Arc Oxidation for Biomedical Implant Applications: Surface Characterization, Cellular Growth, and Microorganisms' Activity. Coatings 2023, 13, 1577. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, L.; Khalil, A.T.; Ali, N.; Zia, D.; Ali, M.; Shinwari, Z.K. Endophyte-mediated synthesis of silver nanoparticles and their biological applications. Applied Microbiology and Biotechnology 2019, 103, 2551–2569. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioactive Materials 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Burtscher, S.; Krieg, P.; Killinger, A.; Al-Ahmad, A.; Seidenstücker, M.; Latorre, S.H.; Bernstein, A. Thin Degradable Coatings for Optimization of Osteointegration Associated with Simultaneous Infection Prophylaxis. Materials 2019, 12, 3495. [Google Scholar] [CrossRef]
- He, X.; Zhang, X.; Bai, L.; Hang, R.; Huang, X.; Qin, L.; Yao, X.; Tang, B. Antibacterial ability and osteogenic activity of porous Sr/Ag-containing TiO2 coatings. Biomedical Materials 2016, 11, 045008. [Google Scholar] [CrossRef]
- Yang, W.; Shen, C.; Ji, Q.; An, H.; Wang, J.; Liu, Q.; Zhang, Z. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 2009, 20, 085102. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of Biomedical Materials Research 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Barbaro, K.; Kuroda, P.A.B.; De Bonis, A.; Teghil, R.; Monteleone, V.; Imperatori, L.; Ortenzi, M.; Antoniac, I.; Grandini, C.R.; et al. Silver Containing Antimicrobial Coatings on Innovative Ti-30Nb-5Mo β-Alloy Prepared by Micro-Arc Oxidation for Biomedical Implant Applications. Coatings 2024, 14, 214. [Google Scholar] [CrossRef]
- Percival, S.L.; Bowler, P.; Russell, D. Bacterial resistance to silver in wound care. Journal of hospital infection 2005, 60, 1–7. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Grandini, C.R.; Rau, J.V. Comprehensive review of PEO coatings on titanium alloys for biomedical implants. Journal of Materials Research and Technology 2024, 31, 311–328. [Google Scholar] [CrossRef]
- Gosheger, G.; Hardes, J.; Ahrens, H.; Streitburger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Kemper, F.H.; Winkelmann, W.; von Eiff, C. Silver-coated megaendoprostheses in a rabbit model—an analysis of the infection rate and toxicological side effects. Biomaterials 2004, 25, 5547–5556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Wang, X.; Wang, Y.; Hang, R.; Huang, X.; Tang, B.; Chu, P.K. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Materials Science and Engineering: C 2018, 82, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.C.; Barbaro, K.; Kuroda, P.A.B.; De Bonis, A.; Teghil, R.; Krasnyuk, I.I.; Imperatori, L.; Grandini, C.R.; Rau, J.V. Antimicrobial Cu-Doped TiO2 Coatings on the β Ti-30Nb-5Mo Alloy by Micro-Arc Oxidation. Materials 2024, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, X.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Wu, H.; Feng, Q.; Liu, Y. The Cu-containing TiO2 coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids and Surfaces B: Biointerfaces 2018, 170, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Renaudin, G.; Forestier, C.; Nedelec, J.-M.; Descamps, S. Biological properties of copper-doped biomaterials for orthopedic applications: A review of antibacterial, angiogenic and osteogenic aspects. Acta biomaterialia 2020, 117, 21–39. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.H. The roles of ions on bone regeneration. Drug Discovery Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Tan, J.; Wang, D.; Cao, H.; Qiao, Y.; Zhu, H.; Liu, X. Effect of Local Alkaline Microenvironment on the Behaviors of Bacteria and Osteogenic Cells. ACS Applied Materials & Interfaces 2018, 10, 42018–42029. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Z.; Wang, D.; Zhang, X.; Qian, S.; Liu, X. A facile and universal strategy to endow implant materials with antibacterial ability via alkalinity disturbing bacterial respiration. Biomaterials Science 2020, 8, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiology 2014, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Kazemzadeh-Narbat, M.; Hui, Y.; Lu, S.; Ding, C.; Chen, D.D.Y.; Hancock, R.E.W.; Wang, R. Local delivery of antimicrobial peptides using self-organized TiO2 nanotube arrays for peri-implant infections. Journal of Biomedical Materials Research Part A 2012, 100A, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Guastaldi, F.P.S.; Foggi, C.C.d.; Santana, L.C.L.; Vaz, L.G.; Vergani, C.E.; Guastaldi, A.C. Lower Susceptibility of Laser-irradiated Ti-15Mo Surface to Methicillin-resistant <i>Staphylococcus aureus</i> Cells Adhesion. Materials Research 2019, 22. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Wang, Z.; Ren, M.; Wang, X.; Liu, H.; Lin, Q.; Wang, J. Engineering Multifunctional Hydrogel-Integrated 3D Printed Bioactive Prosthetic Interfaces for Osteoporotic Osseointegration. Advanced Healthcare Materials 2022, 11, 2102535. [Google Scholar] [CrossRef]
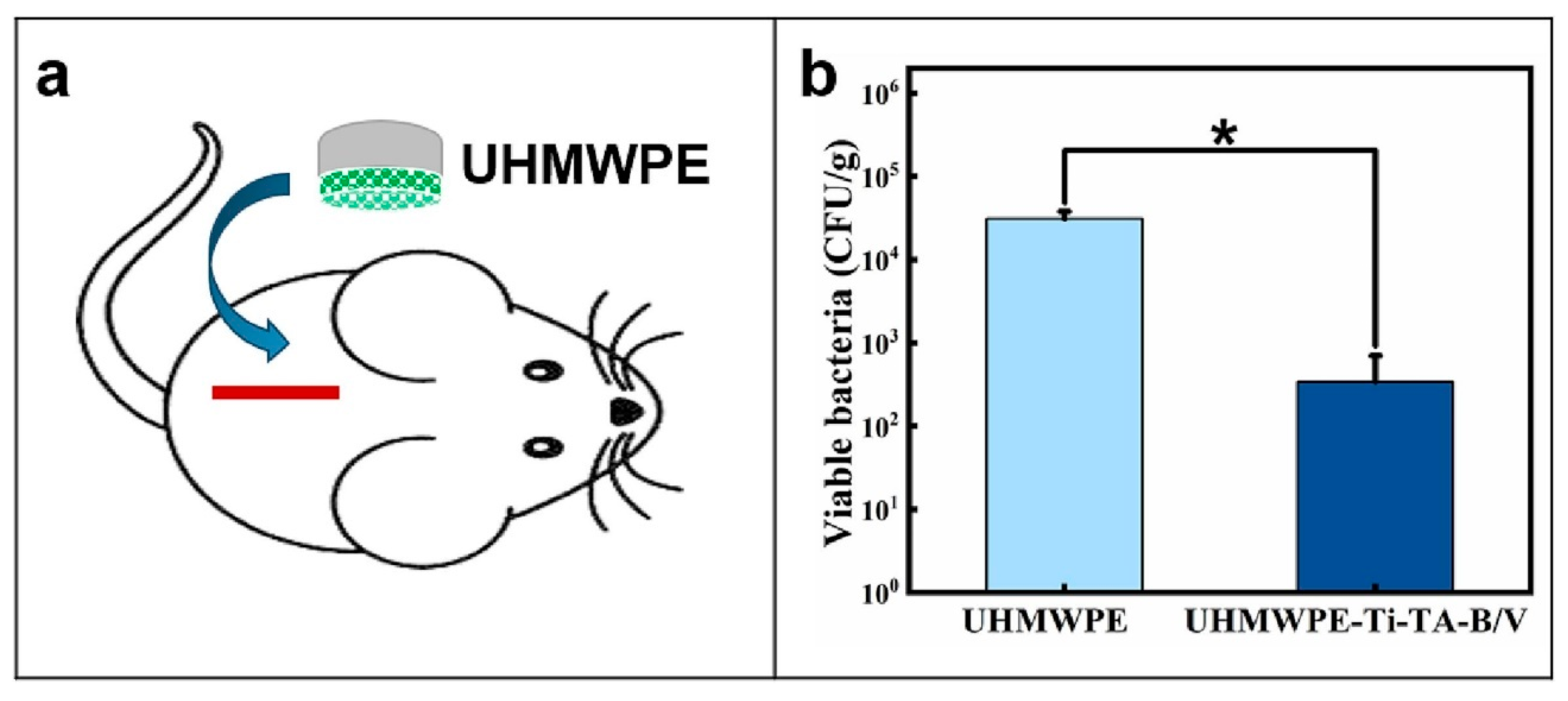

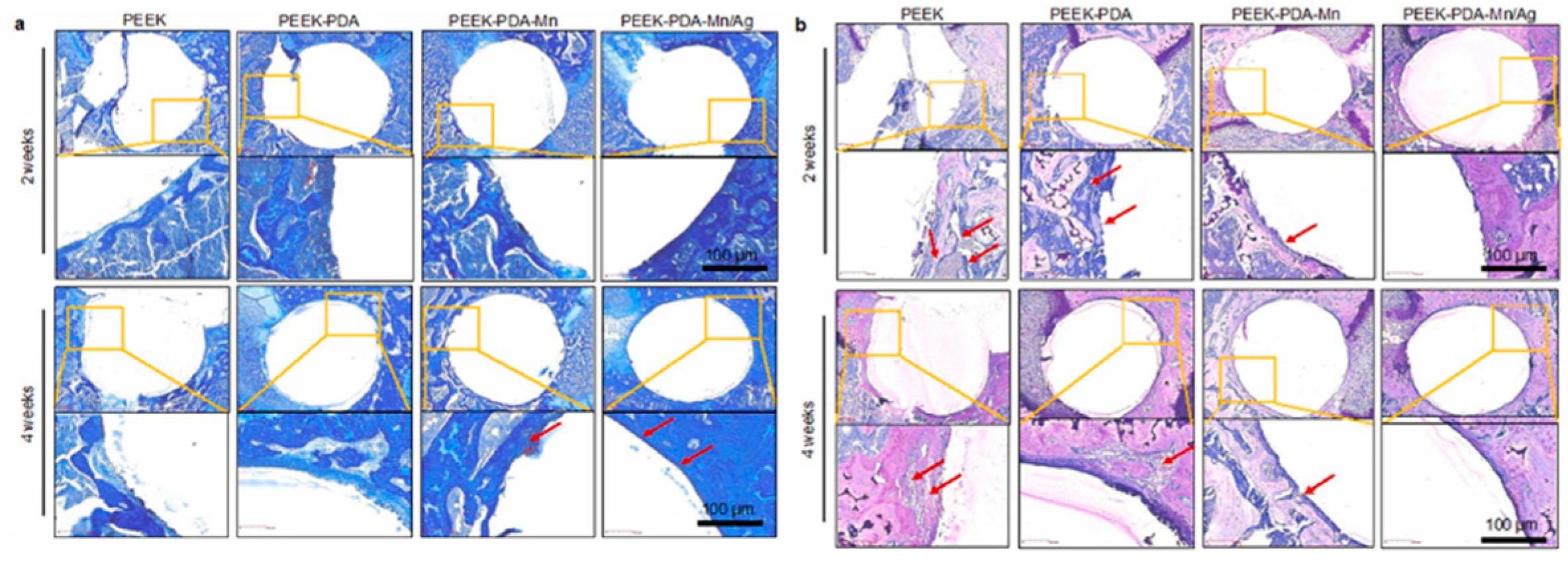

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




