Preprint
Article
Reuse of Activated Carbons from Filters for Water Treatment Derived from the Steam Cycle of a Nuclear Power Plant
Altmetrics
Downloads
18
Views
25
Comments
0
Submitted:
04 December 2024
Posted:
05 December 2024
You are already at the latest version
Alerts
Abstract
Nuclear energy, which is considered clean but not renewable energy, currently has a great impact on the energy mix of different countries. In the case of Spain, it implies over 20% of current energy requirements, pointing out the relevance of nuclear power plants. These plants generate different wastes or products (apart from radioactive) that should be managed. For instance, the activated carbon included in filters (which neutralize isotopes in a possible radioactive leakage) must be periodically replaced. These filters have expiration dates that must be respected. Nevertheless, the activated carbon in these filters might present long service lives, as they have not undergone any adsorption process. Consequently, a considerable and recurring amount of activated carbon can be reused in different processes, even in the same nuclear power plant. The aim of this work was to assess the use of activated carbons (previously included in filters to prevent possible radioactive releases in primary circuits) for water treatment derived from the steam cycle of a nuclear power plant. For this purpose, a regeneration process was carried out, measuring the adsorption efficiency by using ethanolamine, proving that factors such as porosity play an important role in the specific usage of activated carbons.
Keywords:
Subject: Chemistry and Materials Science - Materials Science and Technology
1. Introduction
1.1. Nuclear Power Plants: Current Status
Recently, the development of demanding environmental policies has taken place, requiring lower waste generation from different industrial processes and stricter control of these residues. Initially, many efforts, focused on minimizing the effect of pollutants, have been carried out. However, these measures are not quite effective, as pollution can be transferred to other environments or accumulated, increasing environmental risks.
As a result, the search for alternatives to reduce waste generation can present a more positive impact, selecting the right process to avoid the production of residues with difficult environmental management or, if produced, enhancing these residues by their valorization in other processes, so that their discharge to the environment is the last option. In this sense, terms such as circular economy have gained attention nowadays, where different products and by-products can be reused or recycled, being highly related to green processes[1,2]. This way, green policies promote the implementation of technologies to protect the environment.
Nuclear energy plays an important role in the energy mix of different countries, with much controversy about the sustainability and energy independence of this energy source [3]. Indeed, its scientific interest has increased recently, as observed in the number of publications about this subject [4].
Without a doubt, many different industries are interested in the transition towards this new scenario, including nuclear power plants (NPPs). Specifically, NPPs produce nuclear waste that is considered problematic, including products that are in contact with radioactive materials, that can be managed by following green or circular economic practices [5].
Nowadays, there are more than 400 nuclear power plants operating around the world, distributed according to Figure 1. The number of NPPs can provide an idea about nuclear activity and the subsequent residues that can be generated and must be properly managed.
As observed in this figure, there is a relatively homogeneous distribution of nuclear power plants in the northern hemisphere, where most plants are located, with the United States (exceeding 90 plants), France and China (with over 50 plants each) as the most representative countries. This figure shows how, even though there is a concern about nuclear energy, the number of operating plants, along with projected ones, is considerable and essential for the energy mix of many countries and regions.
Regarding Spain, Figure 2 shows the distribution of NPPs (7 in total), including 8 operating reactors [7]. In this sense, although Spain presents a great potential with renewable sources like solar or wind energy, the energy produced in nuclear power plants represents around 20% of total energy generation in the national electricity system. The NPP selected for this study is located in Almaraz (Extremadura, Spain), with two reactors.
1.2. Use of Activated Carbons in NPPs
An activated carbon is a carbonaceous and porous material obtained through carbonization, taking part in reactions with gas during or after carbonization (optionally with previous addition of chemical products) in order to improve its adsorbent properties. Activated carbons present a disorderly and porous microcrystalline structure, providing an amorphous structure. The space existing between these microcrystals is the cause of porosity in activated carbons. The main requirements of an activated carbon are the following:
- High adsorption capacity and selectivity towards the studied ionic or molecular species.
- Suitable kinetic characteristics for the specific adsorption process, considering the particle size of the activated carbon, adsorbate diffusion and the structure of the bed.
- From an industrial point of view, for filter systems, it is important that activated carbons present long retention times (that is, the adsorbate should be kept in the activated carbon for long periods without undergoing desorption), to reduce the frequency of replacement of these filters.
- A good cost/efficiency ratio, being easily prepared and supplied.
During the operating stage of reactors in NPPs or other facilities, diverse radioactive waste is generated. Due to their potential environmental and health hazard, it is important that these substances be removed through different safety mechanisms [5].
Activated carbon is suitable for its effective use in filters to retain radioactive material produced in different stages in NPPs [8,9,10], with retention efficiencies of radioactive methyl iodide up to 100%. Table 1 shows the minimum requirements for the NPP located in Almaraz (Spain), the subject of study in this work. As inferred from this table, demanding requirements for these filters assure safety in all the stages where activated carbons are used, with the subsequent regular replacement of this material once their service life has expired. In this sense, processes such as aging effect could decrease the effectiveness of activated carbons, especially in this case study, as adsorption of radioiodine could provoke this negative effect. Nevertheless, unless a leakage has taken place, these activated carbons are not normally used as adsorbents, being an interesting alternative for other applications in the same nuclear power plant.
Figure 3 shows the specific use of the activated carbon included in this study. Specifically, the filters are located in the vacuum system of the condenser in Almaraz nuclear power plant, and the objective is the improvement of the thermodynamic yield in the nuclear cycle. For this purpose, a vacuum at the low-pressure turbine outlet is created by removing air and non-condensed gas from steam. Radiation monitors continuously control the flue gas with the aim of detecting radioactive isotopes (in case breakage of steam generator pipes). In this case, flue gas goes through the filter, retaining methyl iodide with an efficiency of 99.27%.
Activated carbons used in these filters are usually microporous, with a homogeneous pore size distribution and high specific surface. These characteristics are highly dependent on different factors, such as the raw material or precursor or the activation process used to prepare the final product, which will be decisive in physical adsorption.
On the other hand, regarding the surface specificity of a solid towards a certain adsorbate, the reactivity of the surface can be modified by adding the suitable impregnation agent.
Among the most popular impregnation agents that are used in activated carbons, there is a wide range of compounds, such as iodides (KI, BaI or PbI), AgNO3, or amino compounds such as triethylenediamine (TEDA) and hexamethylenetetramine (HMTA). Among them, KI and TEDA are the most studied at industrial level to remove elemental iodine and 131ICH3 [11,12,13]. ASTM D3803-91/2022 standard is established for carbon test for 31ICH3 adsorption, specifically for activated carbons that are impregnated with KI or TEDA [14].
On the other hand, thanks to its high capability of neutron adsorption, boron (specifically B-10) is used as a component in neutron shields, to control the safe operation and detect neutrons in nuclear power plants.
The main drawback related to activated carbons in this context is their exposure to moisture during storage or its service life, along with the decrease in retention efficiency of 131ICH3, implying aging effect and, consequently, reducing their service life[15,16]. This effect is related to surface oxidation of activated carbons, increasing their hydrophilicity and the subsequent steam adsorption. Some authors point out that the generation of oxygenates reduces the retention efficiency of 131ICH3, due to the increase in blocked pores due to steam adsorption [17]. In this sense, the reuse of these spent activated carbons could be an interesting point to contribute to circular economy in this context.
1.3. Ethanolamines in NPPs
Thermoelectric plants convert the heat generated in combustion or nuclear fission into steam, which is used in turbines to operate a generator to convert mechanical energy into electricity. After the turbine, a condenser is installed to condensate steam and obtain water, which is placed in the corresponding feeding container to restart the process in the steam boiler. In parallel, the coolant water flows in the condenser, removing heat through a heat exchanger. Nuclear power plants with pressurized water reactors have an additional water circuit, the so-called primary circuit.
All the thermoelectric plants use water for this purpose. In nuclear plants, water also moderates neutrons derived from fission, controlling this nuclear reaction. A thorough chemical analysis of water ensures safety and efficiency of these facilities. Around 50% of unexpected outages are due to the presence of pollutants or the chemical composition of water in water-vapor systems. In many cases, corrosion is a real challenge, and a weak base such as ethanolamine is added to extend the service life of these facilities [18]. Thus, ethanolamine (ETA) is an organic compound that is a primary amine and, at the same time, a primary alcohol. This compound is normally used as a corrosion inhibitor, being a challenge in environmental management due to its low biodegradability, requiring different oxidation processes [19].
1.4. Aim of this Work
Considering the above, the goal of this study was the regeneration of activated carbons from filters devoted to avoiding leak or spills in primary circuits in a nuclear power plant located in Spain. For this purpose, cooling water from the same NPP was used, to remove B and organic compounds in activated carbons to reduce costs. Thus, pH control was carried out to optimize the process, removing B in the shortest possible time.
2. Materials and Methods
The filters selected for this study were provided by Almaraz NPP, located in Extremadura, Spain (39°48′29″ N, 5°41′49″ W). This plant has two reactors, with an open circuit refrigeration system to a reservoir, 1 km away (Arrocampo-Almaraz, 39°49′0″ N, 5°42′0″ W). Its activity started in 1983, and the gross energy production of this plant is 15500 GWh. This NPP has a considerable amount of activated carbon that is used in filters to prevent spills in the primary circuit, that should be replaced due to standards.
The activated carbon collected from this NPP was labeled as ACA. The activated carbon was directly collected without being exposed to radioactive leakages in ventilation filters after its service life. The treatments (washing, oxidation and pyrolysis) that were carried out to pre-treat these activated carbons are described in the following subsections, being characterized and used for adsorption of ethanolamines. Specifically, non-treated samples were labeled as ACAcontrol.
2.1. Boron Removal Through Washing Treatment
For this purpose, two methods have been used. First, an acrylic column with circulation of a HCl solution was used. Second, an immersion method was carried out.
Regarding the first case, activated carbon was sieved and a specific amount (34.5 g) was introduced and packed in the acrylic column. Afterwards, the activated carbon was washed with distilled water to remove the air included in interstices. To fill the volume of the column, glass spheres were added above and below the activated carbon bed, separating these phases with metal mesh.
Figure 4.
Scheme for boron removal in a column.

To start the process, the first step was to pump the specific flow through the column, which was kept constant during the whole experiment at 5 mL·min˗1. Afterwards, different samples were taken to carry out boron determination (explained in further subsections).
Concerning the immersion method, it consisted in adding a certain amount of solid in a fixed volume of HCl solution (with a specific concentration), stirring the mixture until a stationary regime is reached. Due to desorption of B on the adsorbent, changes in the solution will take place, allowing the quantification of desorption in the activated carbon. For these tests, different quantities of adsorbent (0.05 and 0.1 mg) were added in test tubes, adding 10 mL of HCl solution with a certain HCl concentration, which are specified in further subsections. The samples obtained were labeled as ACAHCl washing.
2.2. Oxidation of Activated Carbon
For this test, activated carbon samples (6 g), after washing treatment with HCl solutions, were oxidized by varying the oxidation time between 1.5 and 9.0 h, with a nitric acid solution (7 mol·L˗1), using 60 mL of this solution per gram of activated carbon. The mixture was placed in a Soxhlet apparatus, at the boiling point of the solution, during the corresponding oxidation times. Additionally, the activated carbon was oxidized by using different nitric acid concentrations (from 4 to 7 mol·L˗1) at a constant oxidation time of 1.5 h, following the abovementioned procedure. Finally, the activated carbon was washed with distilled water to remove nitrate content, which is assured by measuring pH in water until pH = 5.5 was reached. Thus, the resulting samples were labeled as ACAoxidation.
2.3. Pyrolysis of Activated Carbon After Washing Treatment with HCl Solution
The experimental facility for ACA oxidation is shown in Figure 5. As observed, a cylindrical reactor (length = 700 mm; external diameter = 42 mm; inner diameter = 36 mm) was used, charging the sample at the top of the reactor, placing it in a basket. Different gas-phase compounds, such as nitrogen (99.999%, Linde, Dublin, Ireland) or steam are introduced in the reactor, whose temperature is kept by using a PID controller, allowing different heating rates. The flue gas is cooled down in a condensation system (two flasks immersed in water with ice) to collect the condensable fraction. The resulting samples are labeled as ACApyrolysis.
2.4. Characterization of Activated Carbons
In this subsection, the main textural and chemical characterization of the activated carbons obtained in previous processes will be described.
2.4.1. Textural Characterization
Nitrogen gas adsorption-desorption isotherms at 77 K were determined by using a semi-automatic equipment (Autosorb-1, Anton Paar, Graz, Austria). The sample (1 g) was previously degasified at 120 ˚C for 12 h. Experimental data of adsorbed nitrogen for each relative pressure P/P0 were analyzed, using the Brunauer-Emmet-Teller method to calculate BET surface area (SBET). Also, Dubinin-Radushkevich method was used to determine micropore volume (Vmi), whereas Gregg and Sing procedure was selected to determine mesopore volume (Vme). Finally, the Sing method was used to determine external surface (Sext), using as a non-porous material a product prepared from olive stone. Inner surface (Sin) was determined by difference (SBET ˗ Sext).
2.4.2. Chemical Characterization
To characterize the surface chemistry of ACA, point of zero charge (PZC) method was used. Thus, the method proposed by Carrot et al. [20] was followed: 0.5 g of activated carbon was weighed, adding 10 mL of NaNO3 solution (0.1 M). The solutions were introduced in a thermostatic bath with a stirring system for 48 h at 25 ˚C. Afterwards, the samples were filtered with Whatman #1 filter paper, measuring pH of the filtered solution with a pHmeter (pH-meter basic 20+, Crison, Barcelona, Spain)
2.5. Boron Determination
Once activated carbon was treated according to the abovementioned methods, the amount of B included in ACA was determined by spectrophotometry (λ = 410 nm) with a previous reaction with azomethine, included in previous standards [21]. The samples were analyzed as follows: 5 ml of samples were taken in a test tube, adding 4 mL of buffer solution and 2 mL of azomethine. The solution was homogenized, and after 2 h, the absorbance at 410 nm was measured in a spectrophotometer (UV-2005, J. P. Selecta, Barcelona, Spain). A calibration line was obtained in order to compare experimental data and determine boron content. Thus, 5 mL of solutions with different B concentrations in deionized water (0, 0.4, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8 and 3.8 mg·L˗1) underwent the same procedure previously explained, showing the calibration curve and the main results in Subsection 3.2.
2.6. Ethanolamine Adsorption Tests in Activated Carbon
Concerning activated carbons that underwent different purification treatments, a final adsorption test with ethanolamine was carried out with the aim of assessing their suitability for this purpose. At this point, a treatment to modify surface chemistry of ACA was carried out to improve the selectivity towards the pollutant of interest (in this case, ethanolamine).
The selected experimental method was immersion, consisting in keeping in contact both adsorbate and ACA in the same solution with constant stirring rates and temperature, to reach the adsorption-desorption equilibrium. Thus, changes in adsorbate concentration will be found, which will be quantified by difference between the initial and stationary states. This way, if different adsorbate solutions (with different concentrations) are tested with the same amount of activated carbon (or vice versa), the corresponding adsorption isotherm can be determined, by representing the adsorbed amount (q, mg·g˗1) versus the concentration of adsorbate in a stationary state (Ce, mg·L˗1).
In these tests, 0.01 (or 0.05 g) of activated carbon were added to test tubes, adding an ethanolamine solution (5, 10 or 20 ppm). These experiments were carried out in a thermostatic bath (Unitronic, Selecta, Barcelona, Spain), with a constant stirring, at 25.0 ± 0.1 ˚C. Once the contact time has finished, the activated carbon was separated from the solution though centrifugation. Ethanolamine concentration was determined through liquid chromatography (Metrohm 850 Professional IC, Metrohm, Herisau, Switzerland) with a Metrosep C6 column. The tests were done by duplicate, with the same starting solution in all cases.
The initial ethanolamine concentrations selected for this experiment were based on the real ethanolamine content in purge water found in Almaraz nuclear power plant, being below 5 ppm. Nevertheless, the concentration range was higher and more restrictive to assess the effectiveness of activated carbons in this case.
3. Results and Discussion
The main characteristics of ACA will be described in the following subsections, before and after undergoing the different treatments explained in Section 2. It should be noted that treatments devoted to surface chemical modification were applied once ACA was washed with HCl solution.
3.1. Characterization of Activated Carbons
Table 2 shows C, H, N and O content in different ACA samples, derived from ultimate analysis.
As inferred from this table, there was a high amount of fixed carbon, which is common in activated carbon obtained from biomass waste. It should be noted that ACA preparation procedure is not known. Nevertheless, according to the literature, the activation of carbons from coconut shell (among other wastes) is generally carried out with different activation agents, such as steam and carbon dioxide [22,23]. From an economic point of view, steam would be a more advantageous option due to its low cost.
Furthermore, the treatments applied to ACA implied some changes in ultimate analysis. Regarding ACAHCl washing and ACAoxidation, there was a decrease in fixed carbon, whereas for ACApyrolysis this amount was increased. This fact could be due to the removal of oxygenated groups due to the thermal treatment at high temperatures. On the other hand, the introduction of oxygenated groups in oxidation processes could imply an increase in oxygen and nitrogen at the expense of fixed carbon.
Thus, the effect of the modification of surface functional groups on adsorption of activated carbons plays an important role currently, and due to the more restrictive policies related to water pollution, there has been an increasing interest in activated carbons with certain functional groups for pollutant removal. In this sense, textural analysis can provide interesting information about this subject, as observed in Figure 6 for nitrogen gas adsorption-desorption isotherms.
As observed in this figure, these curves correspond to Type I, according to the IUPAC classification [24], related to microporous materials. Thus, adsorption mainly takes place at low P/P0 ratios, with a relatively constant adsorbed quantity once the micropores are filled with the adsorbate. Consequently, there is a well-defined plateau, corresponding to monolayer adsorption where the adsorbed quantity is increased with pressure until an upper limit is reached, corresponding to the full covering of the monolayer. It is interesting that, after regeneration processes of ACA, microporosity is recovered, which implies that boron was included in small pores. Subsequently, cleaning the original activated carbon is important to improve its adsorption capacity.
Once the different models (BET, Dubinin-Radushkevich and Greeg and Sing) were applied, the following values related to surface characteristics were obtained. Also, the chemical characterization was carried out, showing PZC (see Table 3).
There were considerable differences between the control sample and the treated ones. Thus, the specific surface of treated samples increased, as well as the rest of textural characteristics, which could make these activated carbon samples suitable for certain applications. It is interesting to note that, as previously explained, the increase in pore volume was mainly due to the presence of micropores. Regarding PZC, it should be noted that, after the corresponding treatments, two activated carbons presented acidic properties (ACAHCl washing and ACAoxidation), whereas ACApyrolysis presented basic properties as in the case of control samples.
3.2. Boron Removal
Firstly, in order to quantify B removal from ACA, its determination in liquid phase was carried out, with the corresponding calibration curve for this purpose, as explained in Section 2 (which is shown in Figure 7).
As observed, an increase in absorbance with B concentration was observed, showing a good adjustment to a linear trend with a coefficient of determination of 0.9997. As a result, this procedure and calibration line were used for the quantification of B removal through the two different methods covered in this study.
3.2.1. Boron Removal in Column
In this case, Figure 8 shows the main findings about B removal from this method, showing B content in eluted samples from the column.
It can be seen that boron is highly desorbed from the activated carbon at an initial stage (during the first 10 h), at the beginning of the contact between the activated carbon and the HCl solution. Comparing the two different concentrations used for this experiment, it should be noted that the lowest concentration used (that is, 0.05 M) would be enough for boron removal from the activated carbon, as the final result in both cases is relatively similar (although slightly higher amounts were removed by using 0.1 M solution, that is, 0.58 mg B per g of ACA). This way, a stationary stage takes place after 10 h, with constant B concentration in the samples analyzed. From then on, HCl solution did not extract B from the activated carbon bed.
According to these data, different kinetic models were considered. Regarding the pseudo-first order model, Lagergren’s first order equation is probably the first known equation applied to the description of adsorption or desorption systems in liquid phase, being highly used currently [25,26]. The equation is shown in Equation 1:
Where q and q1 are the amounts of adsorbed solute per mass unit of activated carbon (at a certain time t and at stationary state, respectively), and k1 is the mass transfer constant. By integrating this equation, for the initial conditions t = 0 and q = 0, Equation 2 is obtained:
In order to obtain adjustment parameters, several authors [27,28,29] linearize Equation 2, obtaining Equation 3:
Thus, for the right use of this expression, it is necessary to know the adsorbed amount in the stationary state (qe).
Concerning the pseudo-second order model, it was proposed by Ho and McKay [30], being expressed as follows (Equation 4):
Integrating with the initial condition (t = 0 and q = 0), Equation 4 is obtained:
Different linearization procedures have been described for a pseudo-second order equation, with Equation 6 being one of the most recurring ones:
In this case, linearized expressions of pseudo-first and pseudo-second reaction orders (Equations 3 and 6, respectively) were used. In most cases, experimental data is better adjusted to a pseudo-second order reaction. A detailed analysis of the procedure used for linearization can explain this fact. It should be considered the fact that the linear adjustment in pseudo-first order requires the knowledge of q value in the stationary stage (qe).
In general, the maximum experimental value obtained can be assumed as qe. The problem related to the mathematical adjustment to the pseudo-first order model is the logarithmic function, ln(qe-q), which tends to infinity with time, as it approaches qe. Therefore, this model does not adjust accurately in long periods of time. In conclusion, linearization of this model is frequently used but not always used properly.
Considering the above, 0-dimensional models were applied to boron desorption in the original activated carbon, by using linear adjustment (Figures 9a and 9b). The kinetic parameters obtained for pseudo-first and pseudo-second order are included in Table 4.
In the light of these figures, the theoretical curves obtained are significantly close to experimental data, which is supported by high determination coefficient in both cases (especially concerning pseudo-second order, with R2 above 0.9945 and 0.9997 at 0.05 and 0.10 M, respectively).
Concerning kinetic parameters (see Table 4), it should be noted that both models present different kinetic constants and quality of adjustment.
3.2.2. Boron Removal Through Immersion Method
Table 5 shows the main results related to this removal method, where different initial concentrations, amounts of ACA and times were used.
As observed in this table, a constant amount of B per gram of activated carbon is extracted regardless of the immersion time.
Therefore, long immersion periods are not necessary, as 5 h seems to be enough to accomplish the maximum boron extraction. Also, low HCl concentrations were enough to remove boron from the activated carbon, as the results obtained in both cases are relatively similar.
This way, if both methods are compared, the immersion of activated carbon removed higher amounts of boron from the samples. However, a complete desorption of B from the surface of activated carbon could not take place, as an amount of B kept on the surface could take part in the equilibrium with desorbed B in HCl solution.
3.3. Ethanolamine Adsorption Tests
Table 6 shows the main results related to the adsorption of ethanolamine on the resulting activated carbon after HCl washing treatment (through the immersion method). Thus, different amounts of activated carbon were put in contact with 10 ml of ethanolamine at different concentrations (from 5 to 20 ppm) for 24 h. It should be noted that the final EA concentration is a concentration range. Accordingly, there were no significative results that support the adsorption effectiveness of the activated carbons tested in this experience, possibly since the pore size was lower than the molecular size of the adsorbate. Also, the surface characteristics of the activated carbon did not play an important role in adsorption process.
In this regard, future studies could be focused on modifying activated carbons with functional groups such as amino or carboxyl groups, which have been shown to enhance adsorption of nitrogen-containing compounds like amines [31].
In addition, the use of alternative adsorbents, such as ion-exchange resins, could present an interesting option. Thus, these products have shown superior adsorption capacities for amines compared to traditional activated carbons, constituting an interesting research line in further works.
4. Conclusions
In this work, a study about the possible reuse of activated carbons located in filters for the steam cycle treatment in a nuclear power plant was carried out. Once the activated carbon underwent a washing treatment, its textural characteristics improved, which is interesting for specific applications after its expiration date. Specifically, the adsorption capacity of this activated carbon was improved after washing treatment.
Regarding boron removal through an acrylic column, the most effective stage was at the beginning of the cycle, that is, when HCl solution comes into direct contact with the activated carbon, reducing the effectiveness to a constant value afterwards. When the immersion method was used, shorter times were required (5 h) compared to the use of the acrylic column (around 24 h), with a higher removal of B per gram of activated carbon.
Even though the activated carbons did not show an effective adsorption of ethanolamine under the current experimental conditions, these results provided valuable insights into the characteristics of the recovered activated carbons from the corresponding filters. Consequently, further studies should be focused on modifying activated carbons, for example, by introducing functional groups such as amino or carboxyl groups, which could improve the adsorption capacity for the compounds covered in this study.
Additionally, the use of alternative adsorbents, such as ion-exchange resins, could be an interesting research point for the future, as they have demonstrated higher effectiveness in adsorbing amines compared to traditional activated carbons. Optimization of experimental parameters, such as pH and temperature, could also play a key role in improving adsorption efficiency. These approaches could be the starting point for the development of more efficient materials devoted to the removal of nitrogen-containing pollutants from aqueous solutions.
Author Contributions
Conceptualization, J.F.G.G. and B.L.C.; methodology, J.F.G.G. and B.L.C.; validation, J.F.G.G. and B.L.C.; formal analysis, B.L.C. and E.M.R.F.; investigation, J.F.G.G., B.L.C. and E.M.R.F.; resources, J.F.G.G.; data curation, B.L.C.; writing—original draft preparation, S.N.; writing—review and editing, B.L.C., E.M.R.F. and S.N.; visualization, B.L.C. and S.N.; supervision, J.F.G.G. and B.L.C.; project administration, J.F.G.G.; funding acquisition, J.F.G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the members of Alcaraz Nuclear Power Plant for their support and the supply of materials for the development of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Y.; Lyu, Y.; Tian, J.; Zhao, J.; Ye, N.; Zhang, Y.; Chen, L. Review of Waste Biorefinery Development towards a Circular Economy: From the Perspective of a Life Cycle Assessment. Renewable and Sustainable Energy Reviews 2021, 139. [Google Scholar] [CrossRef]
- Sharma, N.K.; Govindan, K.; Lai, K.K.; Chen, W.K.; Kumar, V. The Transition from Linear Economy to Circular Economy for Sustainability among SMEs: A Study on Prospects, Impediments, and Prerequisites. Bus Strategy Environ 2021, 30, 1803–1822. [Google Scholar] [CrossRef]
- Gralla, F.; Abson, D.J.; Møller, A.P.; Lang, D.J.; von Wehrden, H. Energy Transitions and National Development Indicators: A Global Review of Nuclear Energy Production. Renewable and Sustainable Energy Reviews 2017, 70, 1251–1265. [Google Scholar] [CrossRef]
- Obregón, L.; Orozco, C.; Camargo, J.; Duarte, J.; Valencia, G. Research Trend on Nuclear Energy from 2008 to 2018: A Bibliometric Analysis. International Journal of Energy Economics and Policy 2019, 9, 542–551. [Google Scholar] [CrossRef]
- Alwaeli, M.; Mannheim, V. Investigation into the Current State of Nuclear Energy and Nuclear Waste Management—A State-of-the-Art Review. Energies (Basel) 2022, 15. [Google Scholar] [CrossRef]
- World Nuclear Energy Status Report World Nuclear Power Reactors 1951-2024.
- Ministerio para la Transición Ecológica y el Reto Demográfico Centrales Nucleares En España.
- Magomedbekov, E.P.; Merkushkin, A.O.; Obruchikov, A. V.; Pokalchuk, V.S. Argon, Krypton and Xenon Adsorption Coefficients on Various Activated Carbons under Dynamic Conditions. J Radioanal Nucl Chem 2022, 331, 1091–1100. [Google Scholar] [CrossRef]
- Matsuo, T.; Nishi, T. Activated Carbon Filter Treatment of Laundry Waste Water in Nuclear Power Plants and Filter Recovery by Heating in Vacuum; 2000; Vol. 38;
- So, J.Y.; Cho, H.R. Thermal Characteristics of Spent Activated Carbon Generated from Air Cleaning Units in Korean Nuclear Power Plants. Nuclear Engineering and Technology 2017, 49, 873–880. [Google Scholar] [CrossRef]
- Seon Choi, B.; Park, G. IL; Hyung Kim, J.; Wook Lee, J.; Kon Ryu, S. Adsorption Equilibrium and Dynamics of Methyl Iodide in a Silver Ion-Exchanged Zeolite Column at High Temperatures; 2001; Vol. 7;
- Won Park, S.; Kook Lee, W.; Moon, H. Adsorption and Desorption of Gaseous Methyl Iodide in a Triethylenediamine-Impregnated Activated Carbon Bed;
- Ml+, $ J; Prmted, M.; Britain, G.; Deitz, V.R. INTERACTION OF RADIOACTIVE IODINE GASEOUS SPECIES WITH NUCLEAR-GRADE ACTIVATED CARBONS; Vol. 25.
- ASTM International Standard Test Method for Nuclear-Grade Activated Carbon.
- Ho, K.; Chun, H.; Lee, H.C.; Lee, Y.; Lee, S.; Jung, H.; Han, B.; Lee, C.H. Design of Highly Efficient Adsorbents for Removal of Gaseous Methyl Iodide Using Tertiary Amine-Impregnated Activated Carbon: Integrated Experimental and First-Principles Approach. Chemical Engineering Journal 2019, 373, 1003–1011. [Google Scholar] [CrossRef]
- Ho, K.; Moon, S.; Lee, H.C.; Hwang, Y.K.; Lee, C.H. Adsorptive Removal of Gaseous Methyl Iodide by Triethylenediamine (TEDA)-Metal Impregnated Activated Carbons under Humid Conditions. J Hazard Mater 2019, 368, 550–559. [Google Scholar] [CrossRef]
- Nacapricha, D.; Taylor, C.G. QUALITY CONTROL OF NUCLEAR CHARCOALS: PARTICLE SIZE EFFECT AND TRAPPING MECHANISM; 1996; Vol. 34;
- Shutikov, A. V.; Ivanov, V.N.; Tyapkov, V.F.; Yerpylyova, S.F.; Bykova, V. V. Introduction of Water Chemistry Regimes with Ethanolamine Metering at Nuclear Power Plants Equipped with VVER-Type Reactors. Thermal Engineering 2008, 55, 402–408. [Google Scholar] [CrossRef]
- Lee, S.D.; Mallampati, S.R.; Lee, B.H. Enhanced Removal of Ethanolamine from Secondary System of Nuclear Power Plant Wastewater by Novel Hybrid Nano Zero-Valent Iron and Pressurized Ozone Initiated Oxidation Process. Environmental Science and Pollution Research 2017, 24, 17769–17778. [Google Scholar] [CrossRef] [PubMed]
- Carrott, P.J.M.; Nabais, J.M.V.; Ribeiro Carrott, M.M.L.; Menéndez, J.A. Thermal Treatments of Activated Carbon Fibres Using a Microwave Furnace. Microporous and Mesoporous Materials 2001, 47, 243–252. [Google Scholar] [CrossRef]
- BOE-A-1982-1323 Orden de 1 de Diciembre de 1981 Por La Que Se Establecen Métodos Oficiales de Análisis de Aguas, Aceites y Grasas, Carne y Productos Carnícos, Fertilizantes, Productos Fitosanitarios, Leche y Productos Lácteos, Productos Orgánicos, Fertilizantes, Suelos y Productos Derivados de La Uva y Similares.
- Sing, K.S.W. Characterization of Porous Materials: Past, Present and Future. In Proceedings of the Colloids and Surfaces A: Physicochemical and Engineering Aspects; Elsevier, July 14 2004; Vol. 241, pp. 3–7.
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Activated Carbons from Lignocellulosic Materials by Chemical and/or Physical Activation: An Overview. Carbon N Y 1992, 30, 1111–1118. [Google Scholar] [CrossRef]
- IUPAC Recommendations for the Characterization of Porous Solids.
- Tseng, R.L.; Wu, F.C.; Juang, R.S. Characteristics and Applications of the Lagergren’s First-Order Equation for Adsorption Kinetics. J Taiwan Inst Chem Eng 2010, 41, 661–669. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical Models of Sorption Kinetics Including a Surface Reaction Mechanism: A Review. Adv Colloid Interface Sci 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Rahman, A.A. Removal of Phenol from Aqueous Solutions by Adsorption onto Activated Carbon Prepared from Biomass Material. J Hazard Mater 2008, 160, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Sivanesan, S. Pseudo Second Order Kinetics and Pseudo Isotherms for Malachite Green onto Activated Carbon: Comparison of Linear and Non-Linear Regression Methods. J Hazard Mater 2006, 136, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of Basic Dye on High-Surface-Area Activated Carbon Prepared from Coconut Husk: Equilibrium, Kinetic and Thermodynamic Studies. J Hazard Mater 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-Second Order Model for Sorption Processes; 1999; Vol. 34;
- Das, D.; Meikap, B.C. Comparison of Adsorption Capacity of Mono-Ethanolamine and Di-Ethanolamine Impregnated Activated Carbon in a Multi-Staged Fluidized Bed Reactor for Carbon-Dioxide Capture. Fuel 2018, 224, 47–56. [Google Scholar] [CrossRef]
Figure 1.
Distribution of nuclear power plants around the world [6].
Figure 1.
Distribution of nuclear power plants around the world [6].

Figure 2.
Distribution of nuclear power plants in Spain, indicating the subject area (Almaraz NPP, in dashed line) [7].
Figure 2.
Distribution of nuclear power plants in Spain, indicating the subject area (Almaraz NPP, in dashed line) [7].
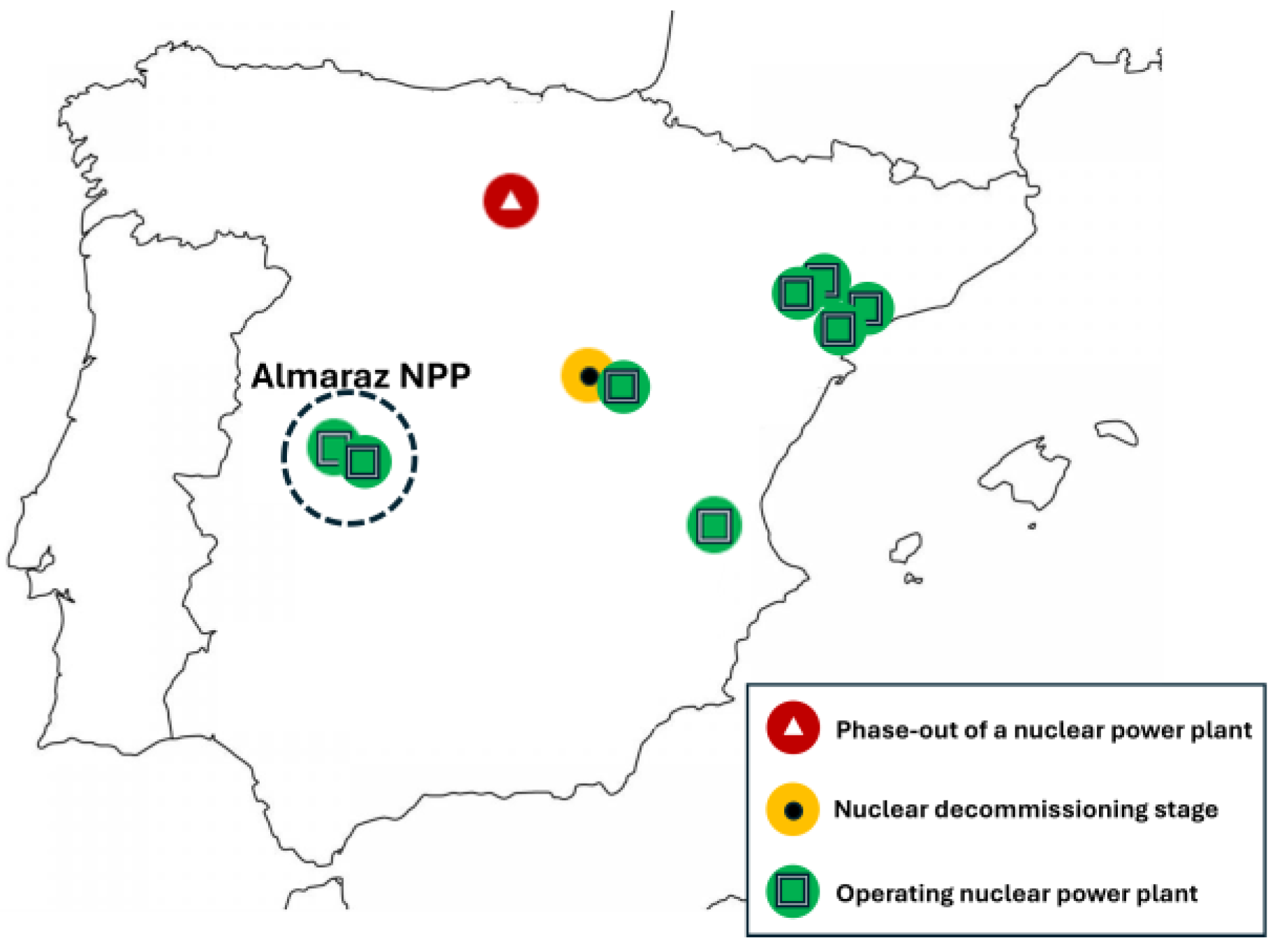
Figure 3.
Example of the role of activated carbon in a nuclear power plant.

Figure 5.
Experimental scheme for pyrolysis of activated carbon.

Figure 6.
Nitrogen gas adsorption-desorption isotherms for different activated carbons.

Figure 7.
Calibration curve for B determination.

Figure 8.
Comparison between different concentrations for B removal.

Figure 9.
Amount of desorbed B over time with different HCl concentrations: a) 0.1 M; b) 0.05 M. Adjustment to pseudo-first and pseudo-second order models.
Figure 9.
Amount of desorbed B over time with different HCl concentrations: a) 0.1 M; b) 0.05 M. Adjustment to pseudo-first and pseudo-second order models.

Table 1.
Different systems in Almaraz NPP where activated carbons are used, and lower limits for their retention efficiency.
Table 1.
Different systems in Almaraz NPP where activated carbons are used, and lower limits for their retention efficiency.
| System | Minimum retention efficiency, % |
|---|---|
| Fuel building | 85.0 |
| Hydrogen purging | 95.0 |
| Safeguard building | 97.5 |
| Control room | 99.5 |
| Auxiliary building | 95.0 |
| Vacuum system of condenser | 99.3 |
| Purging building | 95.0 |
| Pre-accession area | 95.0 |
| Purge in the area | 95.0 |
Table 2.
Ultimate analysis of different activated carbons.
| Activated carbon | C, % | H, % | N, % | O, % |
|---|---|---|---|---|
| ACAcontrol | 78.17 | 0.36 | 0.75 | 20.72 |
| ACAHCl washing | 77.84 | 0.16 | 0.64 | 21.36 |
| ACAoxidation | 76.67 | 0.01 | 1.02 | 22.30 |
| ACApyrolysis | 85.21 | 0.06 | 0.77 | 13.96 |
Table 3.
Textural and chemical analysis of different activated carbons. N2 adsorption at 77 K and point of zero charge.
Table 3.
Textural and chemical analysis of different activated carbons. N2 adsorption at 77 K and point of zero charge.
| Activated carbon | SBET, m2·g-1 | Vmi, cm3·g-1 | Vme, cm3·g-1 | PZC |
|---|---|---|---|---|
| ACAcontrol | 684 | 0.361 | 0.005 | 9.04 |
| ACAHCl washing | 921 | 0.475 | 0.011 | 4.52 |
| ACAoxidation | 893 | 0.464 | 0.002 | 2.60 |
| ACApyrolysis | 934 | 0.485 | 0.009 | 10.24 |
Table 4.
Parameters obtained for pseudo-first and pseudo-second order models in boron desorption in original activated carbon.
Table 4.
Parameters obtained for pseudo-first and pseudo-second order models in boron desorption in original activated carbon.
| Model | Concentration, M | k1, s˗1 | q, mg·g ˗1 | R2 |
|---|---|---|---|---|
| Pseudo-first order | 0.10 | 7.47·10˗5 | 0.37 | 0.9217 |
| 0.05 | 8.54·10˗5 | 0.50 | 0.9722 | |
| Pseudo-second order | 0.10 | 3.89·10˗4 | 0.61 | 0.9997 |
| 0.05 | 2.64·10˗4 | 0.62 | 0.9945 |
Table 5.
B removal over time by using immersion method.
| Initial concentration, M |
Amount of ACA, g |
Time, h |
B, mg |
mgB·gACA˗1 |
|---|---|---|---|---|
| 0.05 | 0.05 | 5 | 0.0402 | 0.80 |
| 10 | 0.0375 | 0.75 | ||
| 15 | 0.0413 | 0.83 | ||
| 20 | 0.0401 | 0.80 | ||
| 25 | 0.0397 | 0.79 | ||
| 0.01 | 5 | 0.0099 | 0.99 | |
| 10 | 0.0094 | 0.94 | ||
| 15 | 0.0110 | 1.10 | ||
| 20 | 0.0100 | 1.00 | ||
| 25 | 0.0095 | 0.95 | ||
| 0.1 | 0.05 | 5 | 0.0352 | 0.70 |
| 10 | 0.0358 | 0.72 | ||
| 15 | 0.0348 | 0.70 | ||
| 20 | 0.0393 | 0.79 | ||
| 25 | 0.0358 | 0.72 | ||
| 0.01 | 5 | 0.0100 | 1.00 | |
| 10 | 0.0102 | 1.02 | ||
| 15 | 0.0093 | 0.93 | ||
| 20 | 0.0092 | 0.92 | ||
| 25 | 0.0120 | 1.20 |
Table 6.
Ethanolamine (EA) content in different kinds and quantities of activated carbons.
| Activated carbon |
ACA amount | Initial EA concentration |
Final EA concentration |
|---|---|---|---|
| g | |||
| ACAHCl washing | 0.01 | 4.86 | 3.15-4.27 |
| 10.48 | 10.42-11.06 | ||
| 18.72 | 16.54-17.11 | ||
| 0.05 | 4.86 | 5.74-5.83 | |
| 10.48 | 12.26-12.44 | ||
| 18.72 | 19.68-20.20 | ||
| ACAOxidation | 0.01 | 4.86 | 4.75-4.92 |
| 10.48 | 10.37-11.15 | ||
| 18.72 | 16.19-17.06 | ||
| 0.05 | 4.86 | 4.44-5.04 | |
| 10.48 | 10.69-11.25 | ||
| 18.72 | 19.27-20.15 | ||
| ACApyrolysis | 0.01 | 4.86 | 4.18-5.25 |
| 10.48 | 10.14-11.36 | ||
| 18.72 | 16.45-17.85 | ||
| 0.05 | 4.86 | 4.44-5.23 | |
| 10.48 | 9.69-11.86 | ||
| 18.72 | 18.55-19.84 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








