1. Introduction
The aftermath of the most devastating COVID-19 pandemic has brought about several gaps in our understanding of viral-host pathogen interactions. In a short time, the virus spread worldwide, dramatically disrupting what is known about the viral transmission dynamics. However, the massive vaccination campaign efforts of a total of 13.6 billion vaccine doses administered globally brought down the pandemic (WHO coronavirus COVID-19 vaccination data, available online at
https://covid19.who.int/ accessed February 27, 2024) [
1]. This was the most rapid and extensive concerted campaign by companies with state-of-the-art mRNA and DNA products, including Pfizer Biontech, Moderna, and AstraZeneca. Nevertheless, as with all vaccines, increased cases of immune reactions such as mucocutaneous or major bleeding have been reported. Prothrombotic syndrome (PS) is one of the most prominent adverse effects. This has been explained by different names such as vaccine-induced immune thrombotic thrombocytopenia (VITT), thrombosis with thrombocytopenia syndrome (TTS), and vaccine-induced prothrombotic immune thrombocytopenia [
2]. Although a risk of these reactions has been observed, complaints of menstrual disturbances have been reported, particularly in this region.
Despite the success of COVID-19 vaccines, some studies have been undertaken to gauge public opinion on the COVID-19 vaccine induced menstrual disturbances in different geographies. In Columbia, in a total of 950 women aged 18–41 years with normal cycles, in nearly 50% of those met the criteria, significant number reported influence and/or alterations in their menstrual cycle [
3]. Similarly, in a total of 14,153 vaccinated Spanish women (mean age 31.5 ± 9.3 years old), 11,017 (78%) reported menstrual changes, mostly in older ages [
4]. A more profound change was reported among 3972 Young Norwegian women aged 18-30, with 36.7 % cycle disturbance; however, heavier bleeding than usual was reported in 95 % both after the first and second vaccine doses, which did not differ in the vaccine brand[
5]. In the USA, among 545 premenopausal non-pregnant women who received a COVID-19 vaccine, 25% reported predominantly temporary changes in the menstrual cycle[
6]. Furthermore, among 455 Iranian women aged 15-55 years, the prevalence of menstrual disturbances was higher in those with latency and heavy bleeding than in those with other disorders after vaccination. However, while %50 had no complaints, there was increased risk after vaccination, even in menopausal women (>10%)[
7]. In Pakistan, 953 among a total of the 953 students, with a mean age of 20.67±1. 56 years, over half (
n = 512, 53.7%) experienced menstrual cycle abnormalities post-vaccination who also developed anxiety significantly (
P = 0.000)[
8]. Among 4,170 administered, side effects were reported in 85.6% of BNT162b2 and 96.05% of ChAdOx1 vaccines, which were more severe in the former vaccine. However, ChAdOx1 vaccines reported mild, moderate, severe, and critical side effects in 30.13, 28.62, 29.73, and 1.53%, respectively. In contrast, most were mild side effects among the majority of BNT162b2 vaccinees (63.92%)[
9].
Although global human genetic population structure is diverse, the universality of the reactions against common vaccine component(s) implies the need for further post-vaccine studies on components. A recent review of key safety outcomes with the Moderna vaccine included the common effects, association with menstrual cycle, 10-fold higher risk of myocarditis and pericarditis in young males 18-29 and increased titers of anti-plyethylene glycol antibodies. While these were temporary but serious, the Moderna expert opinion suggested that large-scale epidemiological studies with longer follow-up periods are required for the surveillance of rare safety outcomes[
10]. While both vaccines are efficient and safe, reactions were reported to be less frequent with the Pfizer/BioNTech vaccine than with the Moderna COVID-19 vaccine. However, the latter vaccine is easier to transport and store because it is less temperature sensitive[
11]. The universality of vaccine reactions is evident in the vaccine comparisons. Comparisons of T cell, B cell memory, and antibody responses to Moderna mRNA-1273, Pfizer/BioNTech BNT162b2, Janssen Ad26.COV2.S, and Novavax NVX-CoV2373 were performed for six months. One hundred % of individual produced memory CD4
+ T cells, with cTfh and CD4-CTL highly represented after mRNA or NVX-CoV2373 vaccination. mRNA vaccines and Ad26.COV2.S induced comparable CD8
+ T cell frequencies, which were detectable only in 60-67% of subjects at six months. While substantial antibody decline was observed in mRNA vaccines, memory T and B cells were comparatively stable. A unique feature of Ad26.COV2.S (JJ) immunization is the high frequency of the CXCR3
+ memory B cells. These findings imply relevant molecular mimicry in other microbial-pathogen interactions, which is an important confounding factor[
12]. In this regard, the mRNA vaccine performed well and implied minimal reactions over whole-virus vaccines across vaccination phases[
13].
There is ample evidence regarding the relationship between “microbial endocrinology-dysbiosis and menstrual cycle disturbance. Similar to the lower female reproductive tract (FRT), signatures of biomass microbiota exists in the upper FRT [
14,
15,
16,
17,
18] that influence signal transductions through mental health stimulations. In this context, the association between psychological stress and menstrual cycle has been well established. Specifically, lifestyle changes during the COVID-19 pandemic affected the patterns and symptoms of the menstrual cycle in women[
17,
18,
19,
20,
21,
22,
23]. Similarly, there are several reports on the link between mental health stressors and neurogastroentestinal issues such as irritable bowl diseases and irritable bowl syndromes where the exact neurochemicals are involved in eliciting gut-brain axis mis-signalling [
24,
25,
26]. Thus, it is plausible that, similar to the gut and other body microbiome compositions, concerted signaling in upper FRT could results in an inflammatory cascade that manipulates cycle signalling. It is increasingly becoming evident that the array of signals during menstruation is subject to much more complex internal and external senses that regulate the timed patterns of the menstrual cycle. However, there is a paucity of high-quality data regarding the nature of host-viral(or viral components) interactions on reproductive system in women at different geographies owing to the normal local genetic variations in the structure and balance of microbial signatures and the nature of stressors. Since the region has one of the most fascinating stable population structures and a stable microbiome composition, we aimed to study the factors and patterns of disruptive menorrhea post-COVID-19 vaccination.
2. Materials and Methods
2.1. Study Design
This study was a cross-sectional design and used a web-based online public questionnaire sent to different regions of Saudi Arabia. A gap was considered from the time of vaccine administration (December 17, 2020) until the expected significant antibody titer was obtained (Apr to Dec 2021). All responses were recorded after this period including second dose from Jul 2021.
2.2. Sample Size and Sampling Procedure
The sample was a self-administered, non-probability sample of Saudi Arabian social media users. The web-based data collection tool was designed using Google Forms and distributed via social media applications, mainly WhatsApp and Twitter. The invitation letters were sent via WhatsApp groups and posted on Twitter community groups. The invitation letter explained the aim of the study and the approximate time required to complete the questionnaire. Participants were asked to distribute the survey on their social networks. A total of 1467 participants were initially screened from responses; responses before the critical gap period were not considered, and those who had signs of pregnancy or other serious issues made up the actual number 1372.
2.3. Data Collection Tool and Interpretations of Patients’ Responses
The questionnaire comprised of four domains: consent forms, demographic information, health status, and covid-19 vaccine related questions. The questionnaire was prepared in English and was translated into Arabic. Language validity was undertaken by retranslating the Arabic version of the questionnaire into English to ensure that the original meaning of the questions was preserved (back translation). This was done by the authors, who are bilingual speakers of both English and Arabic.
The structure of the questionnaire was designed to obtain detailed information around vaccination and evaluate the patients’ responses to identify stress conditions. However, this being a surveillance study, specific clinical and biomarker assessment of patients for stress condition was out of the scope of this study. Assessment of stress conditions was more accurately estimated by the psychiatric and obgyn specialists coauthors using the “self-reports”. They based this on important triangulated domains vital to the stress including the presence of environmental stressors, respondents psychological and biological reactions to stressors, and the length of time over which the stressor or stress response occurred. Patients’ response to the physical (pandemic/vaccination environmental) stressors and psychological stressors as a response to intense overall fear and emotional problems were accounted for. This included collection of data on different types of mental health problems that are likely to trigger stress and the consequences on menorrhea such as anxiety, fear, emotional problems, and worries.
2.4. Ethics Approval
Ethics approval was provided by the Research Ethics Standing Committee of the University of Hail #UOH 2021-631. All participants were asked to provide consent before participating in the study.
2.5. Statistical Analysis
The updated Statistical Package for Social Sciences software (IBM SPSS; Version 24 SPSS version 23.0, for Windows (SPSS, Inc., Chicago, IL, USA] ) was used for data analysis. Descriptive and stratified analyses were conducted, and absolute numbers, proportions, and graphical distributions are presented. We conducted Chi square statistical tests and showed p-values, where appropriate (p-value <0.05, considered statistically significant). In addition, we have carried out a multivariate logistic regression analysis to identify the risk factors associated with menorrhea disturbances in females.
3. Results
We examined responses of 1372 females who mostly comprised the following groups: 61% (
n=838) were aged 19-29 years, 94% (
n = 1292) were Saudi citizens, 66.4% (
n=911) were graduates, 70%(
n=965) were single, and only 15%(205) had chronic diseases, mostly in the respiratory system (
n=33/205;16%). Most of the respondents (30%) were from the eastern region of KSA, while similar frequencies, i.e., 22.8% (
n=305), 22.7% (
n=303), and 21.6%(n=289), were from Northern, Central, and Western regions of KSA, respectively, and only 5.4% (
n=73) from southern region. Of the respondents, 15% had chronic diseases, and 14% had taken some form of medication. The detailed characteristics are shown in
Table 1.
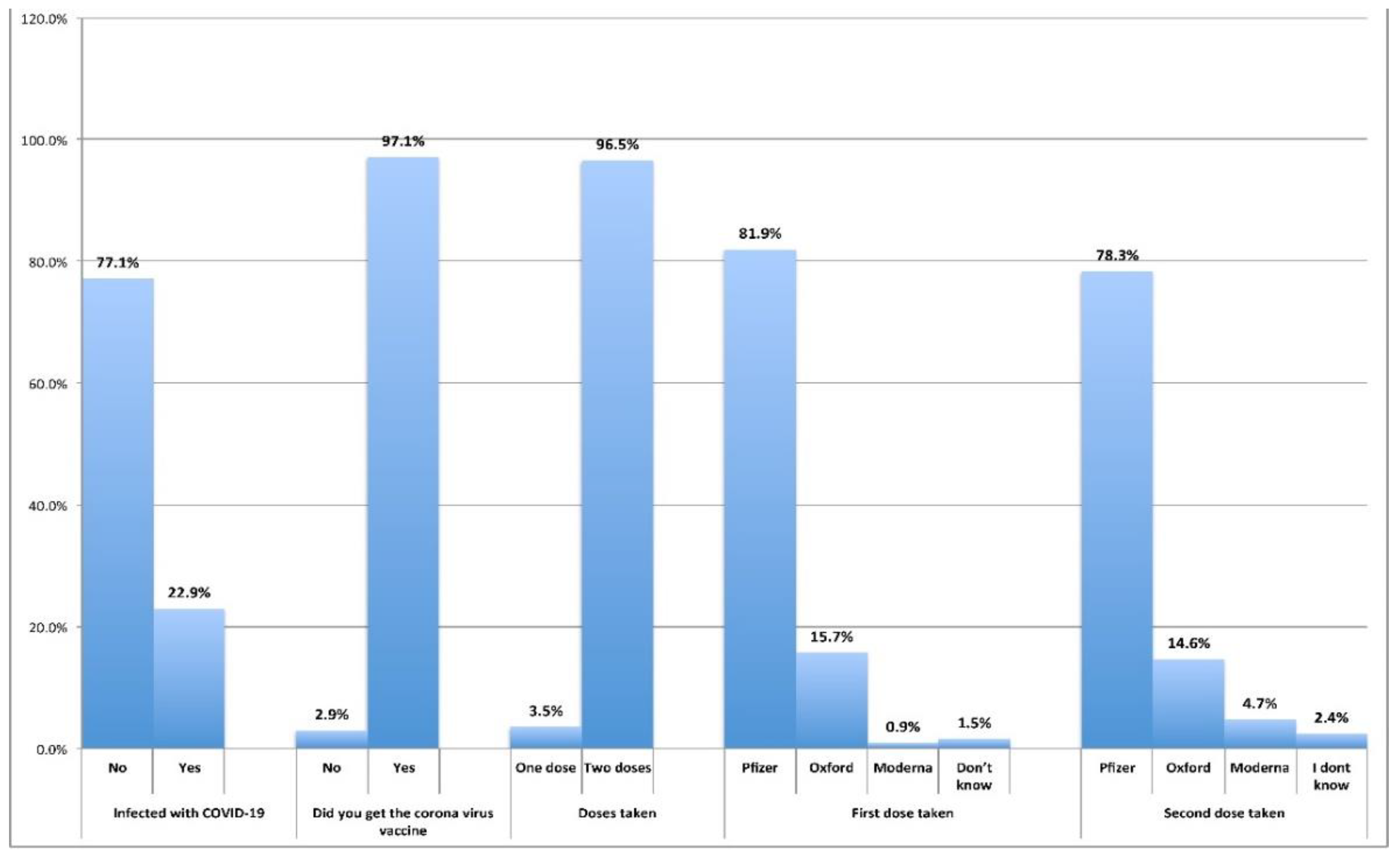
Table 2 shows whether the respondents had SARS-CoV-2 viral infection, the vaccination detail types of vaccines taken, or if they were infected with the virus despite vaccination. Most of the respondents were not infected with acute COVID-19 syndromes (77%) with SARS-CoV-2 before vaccination except 314(23%) who had with similar common but aggravated symptoms. About 96.5% (
n=1285) of the 97% (
n=1332) vaccinated reported taking two doses of the COVID-19 vaccine while only approximately 3.5% (
n=47) took only one dose. No acute COVID syndromes were reported except for the reactions and side effects. The overwhelming majority had taken Pfizer's first and second doses of COVID-19 vaccine (82% and 78, respectively) and the other two vaccines were taken by low percentages of respondents.
Figure 1 and
Table 2 illustrate this information.
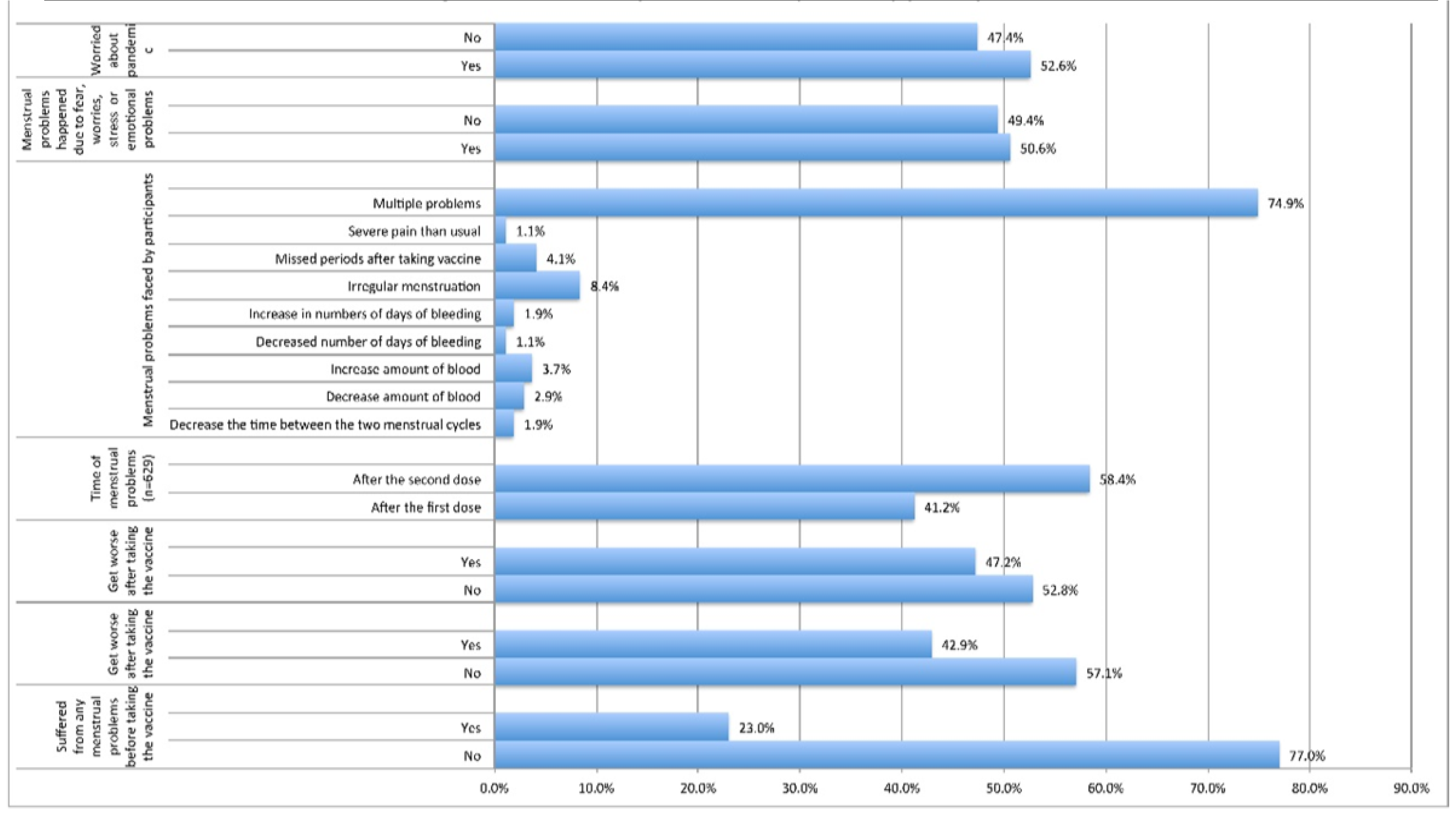
The details of the major issues related to the COVID-19 vaccine administration are shown in
Table 3 and
Figure 2. The overwhelming majority (77%;
n=1057) reported they experienced no menstrual problems before vaccination except 23% (
n=315) who have had issues. Of the latter group, 43% (
n=135/315) reported their menstrual problems worsened after vaccination. However, 47.2%(
n=629) of all respondents experienced specific menstrual problems only after taking the vaccine. Of these, 59% (
n=370) reported the issues increased after the second dose and 41% (
n=259) had them after the first dose. The majority (75%;
n=471) reported occurrence of multiple issues together in their cycles. Independent occurrence of each issue was also surveyed; these were mostly irregularities in cycle times (8.4%;
n=53), followed by approximately 2 to 4% either in decreased days or amount of blood, missed periods, increased blood volume, or increased bleeding days. More importantly, 51% (
n=674) of the participants thought that the menstrual problems were due to fear, stress, and/or emotional problems. A similar percentage of respondents (53%;
n = 701) indicated their worries about the pandemic were their reason [
Table 3,
Figure 2].
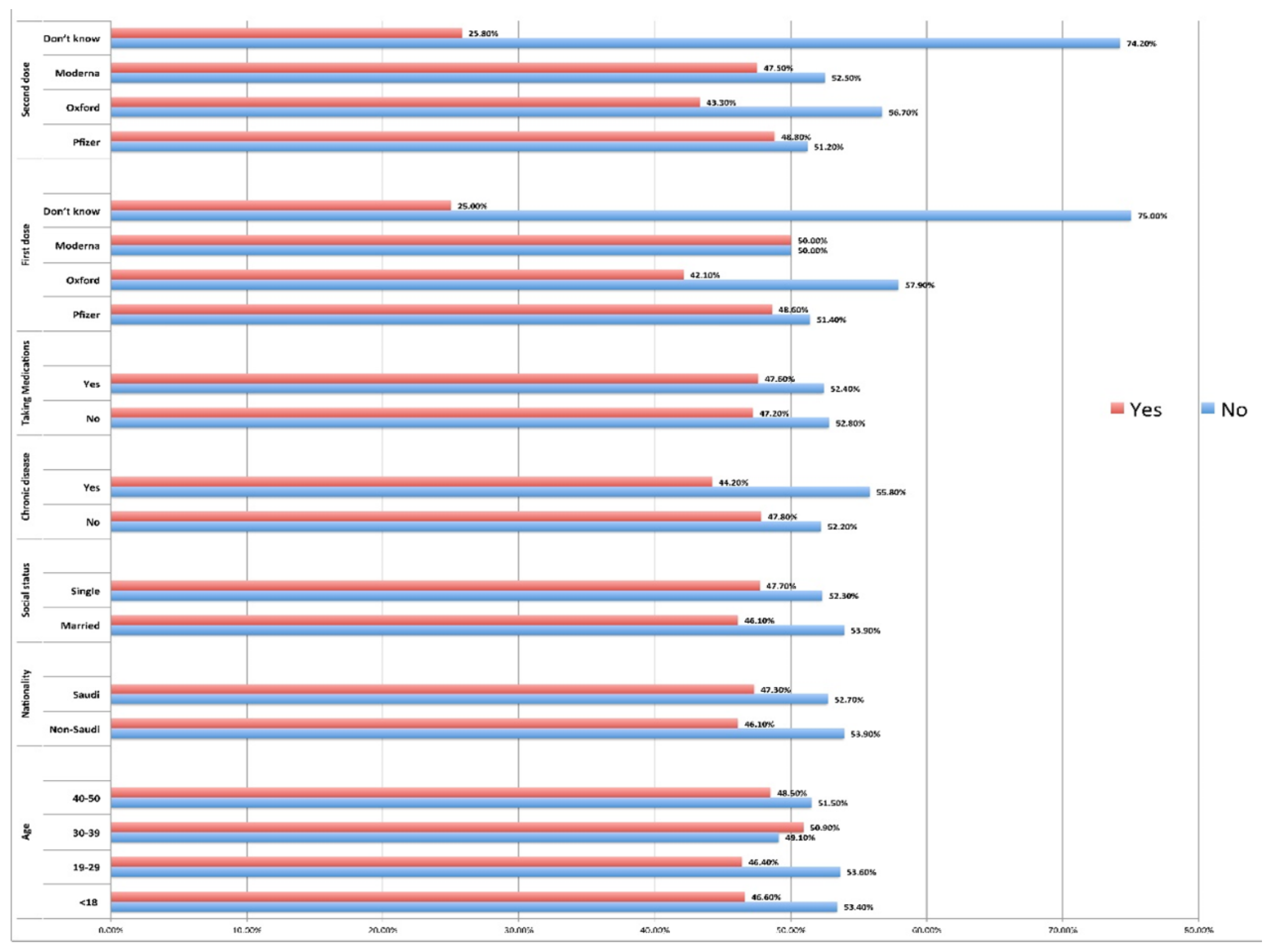
Analysis of the relationship between menstrual problems and age indicated that the difference in proportions between age groups was significant regarding outcomes (menstrual problems) (P =0.05). Although age groups had similar relative frequencies, it was higher in age group 30-39 (51%). No statistically significant differences were observed in menstrual problems between nationalities (P =0.833) or social status (P value =0.592). This study also found that menstrual problems after vaccination did not show any statistically significant association with the presence of chronic diseases (P =0.592) or those who took medications (P =0.913). Participants who received Moderna and Pfizer as the first doses reported more menstrual problems. However, the statistical analysis indicated that there were significant differences in proportions between the study groups (Pfizer, Moderna, Oxford, Don't know) regarding outcomes (menstrual problems) in the first (0.047) as well as in the second dose (0.049). Nevertheless, frequences of mild menstrual problems were higher among females who received both doses of Pfizer and Moderna [
Table 4,
Figure 3].
A multivariate logistic regression analysis was carried out to identify the risk factors associated with menorrhea disturbances in females (Table). The study included various independent variables such as age, nationality, region, education level, employment status, social status, presence of chronic diseases, medication history, vaccination status (type and doses), pre-existing menstrual problems, and emotional factors related to the COVID-19 pandemic. Among these variables, education level and employment status emerged as statistically significant predictors, with postgraduate education [OR=2.11 (0.98-4.72), P = 0.015) and employment [OR=2.18 (0.95-4.86), P = 0.049] being associated with increased odds of menorrhea disturbances. Additionally, Pfizer vaccine [OR = 2.09 (0.96-4.10), P= 0.029] and experiencing menstrual problems after the second vaccine dose [OR=3.21(1.23-5.21), P = 0.030] were also significant predictors. Mental health and emotional factors such as periods affected by fear, worries, and emotional problems other similar issues that triggered stress, were or also associated with increased odds of menorrhea disturbances [OR=1.78 (0.76-3.21), P=0.033]. However, variables such as nationality, region, chronic diseases, medication history, vaccine status, pre-existing menstrual problems, did not show significant associations with menorrhea disturbances.
4. Discussion
The findings of this study provided valuable insights into the impact of vaccination on menstrual health and highlighted the need for further research. We have shown that the major menstrual cycle problems were significantly associated with COVID-19 vaccination (P=0.049) either in those who had (43%) or had no issues prior to vaccination. The evidence became stronger with increasing complaints after the second dose, consistent with other studies[
3]. Examination of menstrual disruptions in the context of diverse factors, including patients’ demographic characteristics, revealed significant findings. For instance, menstrual issues, with emphasis on cycle timing disturbances, were also associated to young women in reproductive age (P =0.0575) who were otherwise healthy. Although SARS-CoV-2 does not adversely affect issues such as ovarian reserve[
27], vigilance has become imperative regarding how the virus disrupts menstrual cycles in young healthy women, in contrast to reported issues in older ages [
28]. These findings are also interesting in the context of the previously reported youth and female resistance to SARS-CoV-2 infections[
29]. However, the notion on women’s resistance to infections is partly due to estrogen, which curtails pro-inflammatory cytokines[
30], albeit distinct evidence exists on immune modulation by uterine microbiome [
14,
31] with further proof of concept from those undergoing hysterectomy[
15].
In the current study, stress was also a major factor implying potential concerted actions through dysbiosis. Majority of the patients reported fear, stress, mental health, worries about the pandemic, and/or emotional problems were the major reasons for their menstrual issues. This is in agreement with the growing evidence on stress- microbiome induced immune-modulations. We and others have previously reported on stress, mental health, and/or anxiety as leading factors in the cycle and immune reaction issues in this and different regions[32,335]. Thus, while the immune response to infections is often envisioned as similar in both sexes, the FRT imparts sex bias differences with increased autoimmune reactions in women[
30]. Thus, there is an association between increased menstrual disturbances, vaccines, and mental health issues. However, the exact mechanism(s) on a potential concerted action is not yet clear.
In this study, the majority of participants had no menstrual problems before vaccination, and in those who had worsened their menstrual problems worsened after vaccination. However, the issues were predominantly multiple transient nonspecific problems that specifically simultaneously occurred, except for the cycle irregularity in the timing, which was the highest common issue. The minor issues ranged from 2 to 4%, either in decreased days or amount of blood, missed periods, increased blood volume, or increased bleeding days. A systematic review represented the most contemporary and largest evidence on the predictors and rates of menstrual problems after COVID-19 vaccination in 78 138 patients. In the aforementioned study, although 52.05% revealed menstrual problems post-COVID-19 vaccination, there were significant heterogeneities, and most studies were unable to report on distinct profiles of menorrhagia (heavy bleeding), oligomenorrhea (irregularities), and dysmenorrhea (pain during period). Furthermore, in three studies, advanced age predicted menstrual problems after the COVID-19 vaccination. Other predictors included smoking, drinking, addiction, history of an unstable family, and pregnancy [
34]. Given that these latter issues are largely irrelevant in women of this region, it is plausible that stress and dysbiosis in the female reproductive tract were inducers. However, despite the similar complexity and structure of neurophysiology and neurochemicals in the reproductive tract and gut-brain axis dysbiosis, the involvement of the former has been overlooked. More importantly, recent studies employing pyrosequencing concluded that the upper FRT is not sterile. Significant differences were found in microbial communities in the endocervix and endometrium; these signatures’ compositions were out of balance in women with menorrhagia versus dysmenorrhea (p = 0.024) [
16]. Thus, “post-acute COVID-19 syndrome”, as dubbed by Meringer and Mehandru (2022) [
35] as well as the post-COVID-19 vaccination syndrome have created important areas for research in the aftermath of SARS-CoV-2 pandemic.The post vaccination menorrhea syndrome in this study is potentially induced by vaccination and the concerted actions of stress and FRT dysbiosis in the absence of major predictors such as smoking, drinking, addiction, history of unstable family, and pregnancy issues in this region. These findings have similar mechanisms of stress induced gut microbiome dysbiosis and the consequences of gastroesophageal and inflammatory bowel syndromes post-COVID-19 (IBS)[1528–323334].
5. Conclusions
The devastating COVID-19 pandemic has created several gaps in host-pathogen interactions during acute as well as long-COVID phases including post-vaccination syndromes. While many syndromes were directly associated to host-viral interaction, the gender-specific mechanism(s) such as to that of menstrual-disturbance post-COVID-19 vaccination has been quite subtle. In this study, we have surveyed and identified major factors and patterns of disruptive menorrhea post-COVID-19. We present statistically significant evidence that menstrual disturbances were associated to COVID-19 vaccinations in healthy, mostly young women of reproductive age. In addition to vaccine, fear, stress, mental health, worries about the pandemic, and emotional problems were the major factors reported. These are consistent with the emerging evidence on the concerted actions of cortisol and dysbiosis on cycle mis-signaling. This is plausible in the absence of significant factors related to old-age, chronic diseases, and lifestyle-behaviors. The findings have significant clinical and public health implications and vaccine improvement strategies. Future vertical studies on cortisol response and uterine-dysbiosis in mis-signaling is warranted.
Author Contributions
Conceptualization, Kamaleldin B Said; Data curation, Khalid F Alshammari, Kamaleldin B Said, Ahmed Aljadani, Arwa Alotaibi, Mohammad Alzughaibi, Fahad . alshammary, Ruba Ahmed, Abdulrahman Alshammari, Turki Alshammari, Hend Alkwai, Mona Shahin, Gamal Eldin Elhussein, Somaia Ibrahim, Fayez Alfouzan, Tarig Mahmoud, Rania Abdalla and Abdelrahim Mohamed; Zaid A. Albayih2, Abuzar A.A.Osman6. Formal analysis, Khalid F Alshammari, Kamaleldin B Said, Ahmed Aljadani, Arwa Alotaibi, Mohammad Alzughaibi, Fahad . alshammary, Ruba Ahmed, Abdulrahman Alshammari, Turki Alshammari, Hend Alkwai, Mona Shahin, Gamal Eldin Elhussein, Somaia Ibrahim, Fayez Alfouzan, Tarig Mahmoud, Rania Abdalla and Abdelrahim Mohamed Zaid A. Albayih2, Abuzar A.A.Osman6; Investigation, Kamaleldin B Said; Methodology, Khalid F Alshammari, Kamaleldin B Said, Ahmed Aljadani, Arwa Alotaibi, Mohammad Alzughaibi, Fahad . alshammary, Ruba Ahmed, Abdulrahman Alshammari, Turki Alshammari, Hend Alkwai, Mona Shahin, Gamal Eldin Elhussein, Somaia Ibrahim, Fayez Alfouzan, Tarig Mahmoud, Rania Abdalla and Abdelrahim Mohamed Zaid A. Albayih2, Abuzar A.A.Osman6; Project administration, Kamaleldin B Said; Resources, Khalid F Alshammari, Kamaleldin B Said, Ahmed Aljadani, Arwa Alotaibi, Mohammad Alzughaibi, Fahad . alshammary, Ruba Ahmed, Abdulrahman Alshammari, Turki Alshammari, Hend Alkwai, Mona Shahin, Gamal Eldin Elhussein, Somaia Ibrahim, Fayez Alfouzan, Tarig Mahmoud, Rania Abdalla and Abdelrahim Mohamed Zaid A. Albayih2, Abuzar A.A.Osman6; Software, Khalid F Alshammari, Kamaleldin B Said, Ahmed Aljadani, Arwa Alotaibi, Mohammad Alzughaibi, Fahad . alshammary, Ruba Ahmed, Abdulrahman Alshammari, Turki Alshammari, Hend Alkwai, Mona Shahin, Gamal Eldin Elhussein, Somaia Ibrahim, Fayez Alfouzan, Tarig Mahmoud, Rania Abdalla and Abdelrahim Mohamed Zaid A. Albayih2, Abuzar A.A.Osman6; Supervision, Kamaleldin B Said; Validation, Khalid F Alshammari, Kamaleldin B Said, Ahmed Aljadani, Arwa Alotaibi, Mohammad Alzughaibi, Fahad . alshammary, Ruba Ahmed, Abdulrahman Alshammari, Turki Alshammari, Hend Alkwai, Mona Shahin, Gamal Eldin Elhussein, Somaia Ibrahim, Fayez Alfouzan, Tarig Mahmoud, Rania Abdalla and Abdelrahim Mohamed Zaid A. Albayih2, Abuzar A.A.Osman6; Visualization, Khalid F Alshammari, Kamaleldin B Said, Ahmed Aljadani, Arwa Alotaibi, Mohammad Alzughaibi, Fahad . alshammary, Ruba Ahmed, Abdulrahman Alshammari, Turki Alshammari, Hend Alkwai, Mona Shahin, Gamal Eldin Elhussein, Somaia Ibrahim, Fayez Alfouzan, Tarig Mahmoud, Rania Abdalla and Abdelrahim Mohamed; Writing – original draft, Kamaleldin B Said; Writing – review & editing, Khalid F Alshammari, Kamaleldin B Said, Ahmed Aljadani, Arwa Alotaibi, Mohammad Alzughaibi, Fahad . alshammary, Ruba Ahmed, Abdulrahman Alshammari, Turki Alshammari, Hend Alkwai, Mona Shahin, Gamal Eldin Elhussein, Somaia Ibrahim, Fayez Alfouzan, Tarig Mahmoud, Rania Abdalla and Abdelrahim Mohamed Zaid A. Albayih2, Abuzar A.A.Osman6. .
Funding
This research received no external funding.
Institutional Review Board Statement
The standard guidelines were followed during this research according to the IRB protocols. The ethical application for this study was reviewed and approved by the Research Ethics Committee (REC at the University of Ha’il (KSA)), dated 22/10/2020 and endorsed by University President letter number Nr. 13675/5/42 dated 08/03/1441 H; for Deanship Project RG20064, REC# H-2022/21-187. The KACST Institutional Review Board (IRB) registration numbers are H-8-L-074 IRB log 2021-11, which also approved the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
no additional data is available elsewhere other than this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- COVID-19 cases | WHO COVID-19 dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 29 February 2024).
- Saudagar, V.; Patil, S.; Goh, S.; Pothiawala, S. Vigilance Regarding Immune Thrombocytopenic Purpura after COVID-19 Vaccine. Ir J Med Sci 2022, 191, 919. [Google Scholar] [CrossRef]
- Rodríguez Quejada, L.; Toro Wills, M. F.; Martínez-Ávila, M. C.; Patiño-Aldana, A. F. Menstrual Cycle Disturbances after COVID-19 Vaccination. Womens Health (Lond) 2022, 18. [Google Scholar] [CrossRef]
- Baena-García, L.; Aparicio, V. A.; Molina-López, A.; Aranda, P.; Cámara-Roca, L.; Ocón-Hernández, O. Premenstrual and Menstrual Changes Reported after COVID-19 Vaccination: The EVA Project. Womens Health (Lond) 2022, 18. [Google Scholar] [CrossRef] [PubMed]
- Trogstad, L.; Laake, I.; Robertson, A. H.; Mjaaland, S.; Caspersen, I. H.; Juvet, L. K.; Magnus, P.; Blix, K.; Feiring, B. Heavy Bleeding and Other Menstrual Disturbances in Young Women after COVID-19 Vaccination. Vaccine 2023, 41, 5271–5282. [Google Scholar] [CrossRef]
- Farland, L. V.; Khan, S. M.; Shilen, A.; Heslin, K. M.; Ishimwe, P.; Allen, A. M.; Herbst-Kralovetz, M. M.; Mahnert, N. D.; Pogreba-Brown, K.; Ernst, K. C.; Jacobs, E. T. COVID-19 Vaccination and Changes in the Menstrual Cycle among Vaccinated Persons. Fertil Steril 2023, 119, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, T.; Feryduni, L.; Fakhraei, M. COVID-19 Vaccine Side Effects on Menstrual Disturbances among Iranian Women. New Microbes New Infect 2023, 53. [Google Scholar] [CrossRef]
- Kareem, R.; Sethi, M. R.; Inayat, S.; Irfan, M. The Effect of COVID-19 Vaccination on the Menstrual Pattern and Mental Health of the Medical Students: A Mixed-Methods Study from a Low and Middle-Income Country. PLoS One 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A. N.; Alotaibi, M. I.; Alqahtani, A. S.; Al Aboud, D.; Abdel-Moneim, A. S. BNT162b2 and ChAdOx1 SARS-CoV-2 Post-Vaccination Side-Effects Among Saudi Vaccinees. Front Med (Lausanne) 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Shabu, A.; Nishtala, P. S. Safety Outcomes Associated with the Moderna COVID-19 Vaccine (MRNA-1273): A Literature Review. Expert Rev Vaccines 2023, 22, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Meo, S. A.; Bukhari, I. A.; Akram, J.; Meo, A. S.; Klonoff, D. C. COVID-19 Vaccines: Comparison of Biological, Pharmacological Characteristics and Adverse Effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci 2021, 25, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mateus, J.; Coelho, C. H.; Dan, J. M.; Moderbacher, C. R.; Gálvez, R. I.; Cortes, F. H.; Grifoni, A.; Tarke, A.; Chang, J.; Escarrega, E. A.; Kim, C.; Goodwin, B.; Bloom, N. I.; Frazier, A.; Weiskopf, D.; Sette, A.; Crotty, S. Humoral and Cellular Immune Memory to Four COVID-19 Vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Kumar, S.; Mishra, G.; Saxena, S. K. Tracking the COVID-19 Vaccines: The Global Landscape. Hum Vaccin Immunother 2023, 19. [Google Scholar] [CrossRef]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R. G. How Uterine Microbiota Might Be Responsible for a Receptive, Fertile Endometrium. Hum Reprod Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Winters, A. D.; Romero, R.; Gervasi, M. T.; Gomez-Lopez, N.; Tran, M. R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S. S.; Hsu, C. D.; Theis, K. R. Does the Endometrial Cavity Have a Molecular Microbial Signature? Sci Rep 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E. S.; Willner, D.; Buttini, M.; Huygens, F. A Role for the Endometrial Microbiome in Dysfunctional Menstrual Bleeding. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology 2018, 111, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.; O’ Keeffe, A.; Phelan, N.; Behan, L. A.; Collier, S.; Hevey, D.; Owens, L. Female Reproductive Health Disturbance Experienced During the COVID-19 Pandemic Correlates With Mental Health Disturbance and Sleep Quality. Front Endocrinol (Lausanne) 2022, 13, 838886. [Google Scholar] [CrossRef] [PubMed]
- Phelan, N.; Behan, L. A.; Owens, L. The Impact of the COVID-19 Pandemic on Women’s Reproductive Health. Front Endocrinol (Lausanne) 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, W.; Al-Maqati, T. N.; Almulhim, M. A.; Alsulmi, E. S.; Alotaibi, J. F.; AlBahrani, S.; Alsuhaibani, O.; Alenezi, E. H.; Albusaili, S.; Alharbi, A.; Alqahtani, A.; Alahmari, F.; Alshahrani, A.; Al Otaibi, D. A.; Alfaifi, A. H.; Madkhali, O. A. Saudi Women’s Perception of the Effect of COVID-19 Infection and Vaccination on Menstrual Cycle Length. Womens Health Rep (New Rochelle) 2024, 5, 495–502. [Google Scholar] [CrossRef]
- Ozimek, N.; Velez, K.; Anvari, H.; Butler, L.; Goldman, K. N.; Woitowich, N. C. Impact of Stress on Menstrual Cyclicity During the Coronavirus Disease 2019 Pandemic: A Survey Study. J Womens Health 2022, 31, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Preethika, A.; Sajeetha Kumari, R.; Anuradha, M.; Mohapatra, S. The Potential Impact of COVID-19 on Women’s Reproductive and Mental Health: A Questionnaire Study. J Obstet Gynaecol (Lahore) 2022, 42, 3328–3335. [Google Scholar] [CrossRef] [PubMed]
- Bruinvels, G.; Blagrove, R. C.; Goldsmith, E.; Shaw, L.; Martin, D.; Piasecki, J. How Lifestyle Changes during the COVID-19 Global Pandemic Affected the Pattern and Symptoms of the Menstrual Cycle. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Barsom, S. H.; Mansfield, P. K.; Koch, P. B.; Gierach, G.; West, S. G. Association between Psychological Stress and Menstrual Cycle Characteristics in Perimenopausal Women. Womens Health Issues 2004, 14, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Haji Seyed Javadi, S. A.; Shafikhani, A. A. Anxiety and Depression in Patients with Gastroesophageal Reflux Disorder. Electron Physician 2017, 9, 5107–5112. [Google Scholar] [CrossRef] [PubMed]
- Sibelli, A.; Chalder, T.; Everitt, H.; Workman, P.; Windgassen, S.; Moss-Morris, R. A Systematic Review with Meta-Analysis of the Role of Anxiety and Depression in Irritable Bowel Syndrome Onset. Psychol Med 2016, 46, 3065–3080. [Google Scholar] [CrossRef]
- Duran-Pinedo, A. E.; Solbiati, J.; Frias-Lopez, J. The Effect of the Stress Hormone Cortisol on the Metatranscriptome of the Oral Microbiome. NPJ Biofilms Microbiomes 2018, 4. [Google Scholar] [CrossRef]
- Yildiz, E.; Timur, B.; Guney, G.; Timur, H. Does the SARS-CoV-2 MRNA Vaccine Damage the Ovarian Reserve? Medicine 2023, 102, E33824. [Google Scholar] [CrossRef]
- Conti, P.; Younes, A. Coronavirus COV-19/SARS-CoV-2 Affects Women Less than Men: Clinical Response to Viral Infection. J Biol Regul Homeost Agents 2020, 34, 339–343. [Google Scholar] [CrossRef]
- Turke, P. W. Five Reasons COVID-19 Is Less Severe in Younger Age-Groups. Evol Med Public Health 2021, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Fish, E. N. The X-Files in Immunity: Sex-Based Differences Predispose Immune Responses. Nat Rev Immunol 2008, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Baker, J. M.; Chase, D. M.; Herbst-Kralovetz, M. M. Uterine Microbiota: Residents, Tourists, or Invaders? Front Immunol 2018, 9. [Google Scholar] [CrossRef]
- Said, K. B.; Al-Otaibi, A.; Aljaloud, L.; Al-Anazi, B.; Alsolami, A.; Alreshidi, F. S. The Frequency and Patterns of Post-COVID-19 Vaccination Syndrome Reveal Initially Mild and Potentially Immunocytopenic Signs in Primarily Young Saudi Women. Vaccines (Basel) 2022, 10, 1015. [Google Scholar] [CrossRef]
- Foster, J. A.; Rinaman, L.; Cryan, J. F. Stress & the Gut-Brain Axis: Regulation by the Microbiome. Neurobiol Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Nazir, M.; Asghar, S.; Rathore, M. A.; Shahzad, A.; Shahid, A.; Ashraf Khan, A.; Malik, A.; Fakhar, T.; Kausar, H.; Malik, J. Menstrual Abnormalities after COVID-19 Vaccines: A Systematic Review. [CrossRef]
- Meringer, H.; Mehandru, S. Gastrointestinal Post-Acute COVID-19 Syndrome. Nat Rev Gastroenterol Hepatol 2022, 19, 345. [Google Scholar] [CrossRef] [PubMed]
- Creed, F. Risk Factors for Self-Reported Irritable Bowel Syndrome With Prior Psychiatric Disorder: The Lifelines Cohort Study. J Neurogastroenterol Motil 2022, 28, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Yarandi, S. S. Overlapping Gastroesophageal Reflux Disease and Irritable Bowel Syndrome: Increased Dysfunctional Symptoms. World J Gastroenterol 2010, 16, 1232. [Google Scholar] [CrossRef] [PubMed]
- Martinson, M. L.; Lapham, J.; Ercin-Swearinger, H.; Teitler, J. O.; Reichman, N. E. Generational Shifts in Young Adult Cardiovascular Health? Millennials and Generation X in the United States and England. J Gerontol B Psychol Sci Soc Sci 2022, 77 (Suppl 2), S177. [Google Scholar] [CrossRef] [PubMed]
- Demir, O.; Sal, H.; Comba, C. Triangle of COVID, Anxiety and Menstrual Cycle. J Obstet Gynaecol (Lahore) 2021, 41, 1257–1261. [Google Scholar] [CrossRef]
- Hunter, P. R. Thrombosis after Covid-19 Vaccination. bmj. British Medical Journal Publishing Group 2021. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).