1. Introduction
Intracranial atherosclerosis is a significant contributor to the global burden of cerebrovascular disease, accounting for a substantial proportion of ischemic strokes worldwide. Epidemiological data suggests that 20 to 40 individuals per 100,000 worldwide experience an ischemic event related to intracranial atherosclerotic disease [
1]. Postmortem examination of intracranial arteries has revealed a high prevalence of severe ICAD, with rates reaching 43% among individuals aged 60 to 69 years, 65% in the 70 to 79 age group, and 80% in those 80 years and older. These findings underscore the increasing burden of ICAD with advancing age [
2].
Atherosclerosis is a progressive disease characterized by the accumulation of lipid deposits within the arterial walls, leading to vessel stenosis and ischemia [
3]. In Kazakhstan, where the prevalence of cardiovascular diseases is notably high, studying the regional burden of intracranial atherosclerosis and its association with comorbid conditions such as hypertension, diabetes, and dyslipidemia is crucial for improving healthcare outcomes [
4]. Understanding the demographic characteristics and treatment approaches for this condition is essential for guiding clinical management and enhancing patient care [
5,
6]. This study aims to comprehensively analyze the demographic characteristics and treatment outcomes of 216 patients with intracranial atherosclerosis treated at a single institution.
2. Materials and Methods
The This retrospective, observational study included 216 patients diagnosed with intracranial atherosclerosis through DSA, CTA, or MRI-MRA, all of whom had a history of stroke or TIA. Patients were eligible for enrollment if they met the following criteria: age between 18 and 80 years, ≥70% intracranial artery stenosis due to atherosclerosis, presentation with a stroke, recurrent symptoms while on medical therapy, poor collateral flow according to CT perfusion or DSA, and more than 7 days after their stroke. The exclusion criteria encompassed non-compliance with prescribed medications, the presence of hemorrhagic infarction within a month before enrollment, and the identification of cardiac embolism sources. These patients were enrolled at a single institution on a non-urgent basis from January 2016 to December 2023.
Table 1 summarizes the baseline clinical features of the participants. The study collected data on patient characteristics such as age, sex, and comorbidities (e.g., diabetes mellitus, hyperlipidemia, hypertension, ischemic heart disease). Additional information included the history of cerebral infarction or TIA, lifestyle factors like smoking, stenosis location and severity, treatment methods, stent type, residual stenosis, periprocedural complications, follow-up duration, in-stent restenosis, and pre-and post-treatment mRS scores. All patients underwent balloon angioplasty followed by stent placement. Prior to stent placement, all participants underwent a regimen of dual antiplatelet therapy consisting of aspirin and clopidogrel for 3 to 5 days. Antiplatelet resistance testing was recommended, and in cases of detected resistance, the regimen was adjusted to include ticagrelor. Following stent implantation, dual antiplatelet therapy alongside statin treatment was maintained for six months. In the absence of antiplatelet resistance, a transition to monotherapy with clopidogrel for 12 months was advised.
2.1. Description of Operation
A femoral artery access with a 6F-8F sheath and guide catheter was obtained, and the flow was carefully inspected for collaterals. Patients received heparinization to an activated clotting time of 250 to 300 seconds, as well as pre-procedural dual antiplatelet therapy. After passing the lesion under roadmap guidance with a microcatheter, using a 300 cm 0.014-inch exchange wire, the balloon was advanced over the exchange wire while stabilizing the wire to prevent movement. A balloon size with a nominal diameter at 6 atmospheres was chosen, approximately 80% of the true luminal diameter or 60% in lesions directly adjacent to angiographically visible perforators. The distal portion of the artery beyond the stenosis is typically 0.5-0.25 millimeters smaller in diameter compared to the normal segment, and the balloon used for angioplasty would be approximately 2 mm in diameter if the artery measures 2.5 mm. Underdilation was advised to avoid arterial dissection, vessel rupture, and the “snowplow effect” of compressed plaque into perforator arteries. Careful monitoring of the distal exchange wire was recommended during stent delivery. The stent’s diameter was selected to match the indicated size on the packaging, inflated to a nominal pressure of 6 atmospheres. If a pressure of 14 atmospheres is specified, the diameter of the stent may be larger, facilitating subsequent post-dilation by approximately 0.25 less than the original stent diameter. Post-stenting balloon dilation within the stent was discouraged unless the residual stenosis remained ≥50% after stenting. Under road map guidance, a micro-guide wire was navigated through the stenotic segment, and a stent was sent to the stenosis for deployment. Patients were monitored in the Neuro Critical Care unit for 24 hours after the procedure, with careful management of blood pressure to minimize the risk of reperfusion hemorrhage.
2.2. Stent Selection
Self-expanding stents are the preferred choice for the middle third-distal part of the basilar artery, as well as the A1, M1, M2, and P1 segments. A preferred option is a 5 mm diameter Credohill stent, which can be placed in an artery with a 1.5 mm diameter. A wall thickness of 2 mm is recommended, although it is worth considering that a smaller vessel diameter may result in a greater radial force. No complications were observed, and in most cases, the stent effectively eliminated the stenosis without the need for angioplasty. When coronary stents are used to treat stenosis in the internal carotid artery, proximal third of the basilar artery, or vertebral artery, the stent diameter should be selected to be approximately 0.25 mm less than the nominal diameter of the target artery.
2.3. Outcome Measures
Patients or their authorized proxies were contacted in person at 3, 6, and 12 months after discharge to collect data on functional status and quality of life. Any death was verified by examining the hospital medical records or local citizen registry. At discharge and 1 year after stroke onset, daily activities were assessed by mRS. Functional dependence was defined as mRS>2.
Table 2 defines the characteristics of outcomes.
2.4. Ethical Consideration
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. The Ethical Committee of the JSC Clinical Hospital provided ethical approval for this study Number 7 of ethical approval for Neurosurgery on December 12th, 2023. In addition, the investigators ensured that the study conforms to the principles of the Declaration of Helsinki 78 (last revised in 2013) and was conducted per the ICH Guideline for Good Clinical Practice.
2.5. Statistical Methods
Statistical calculations were performed using SAS University Edition, version 3.8 (SAS Institute Inc., Cary, NC, USA.). Statistical analysis included a chi-square test the observations were expressed as frequency and percent. Continuous variables were expressed as median with interquartile range (IQR) for non-normally distributed data, assessed with the Shapiro-Wilk test. p<0.05 was considered statistically significant.
3. Results
The cohort had a median age of 63.5 (57-68,6), at the index stroke admission, and 73,7% were male. The mean age of patients gradually increased from 59 years in 2016 to 64 years in 2023, as seen in
Table 1. Of the 216 patients, 98% had a confirmed diagnosis of hypertension, 40,9% were diagnosed with diabetes mellitus, and 28% had dyslipidemia. The incidence of ischemic heart disease was found in 58% of the participants. Of all patients 34% were smokers. The analysis showed a significant association in patients over 75 years old with intracranial stenosis in more than one location (p=0.025), indicating an increased frequency in older patients. Moreover, patients with stenosis occlusion over 70% (p=0.079) and obesity (p=0.064) showed a trend toward significance. In contrast, sex, post-surgery complications, history of smoking, DM, IHD, arterial hypertension, and elevated cholesterol were not significant factors for multi-location ICAS (all p>0.2), as seen in
Table 2. Additionally, patients with multiple comorbidities exhibited more severe stenosis of the intracranial vessels, as measured by imaging techniques.
The target artery distribution was: 54.5% internal carotid artery, 12,3% middle cerebral artery, 21.2% vertebral artery, 10.4% basilar artery, and 1,1% posterior cerebral artery. Patients with vertebrobasilar stenosis accrued a total of 615 hospital days, equating to an average length of stay of 7.1 days, compared to 4.7 days for those with stenosis in the anterior circulation. There was no significant association between stenosis quantity and hospitalization stay length (p=0.214).
The study utilized a variety of stent types, including coronary stents: Ultimaster (Terumo Corporation, Japan) in 135 cases, Acclino flex (Acandis GmbH, Germany) in 67 cases, Resolute Integrity, Onyx (Medtronic Inc., USA) in 19 cases, and self-expandible stents: Neuroform Atlas (Stryker Corporation, USA) in 12 cases, Xience Xpedition (Abbott Vascular, USA) in 11 cases, Orsiro (BIOTRONIK SE & Co., Germany) in 11 cases, Promus PREMIER (Boston Scientific Corporation, USA) in 11 cases, Credo Acandis (GmbH & Co. KG, Pforzheim, Germany) in 8 cases, Solitaire (Medtronic Inc., USA) in 2 cases, BioMime (Meril Life Sciences Pvt. Ltd., India) in 1 case, Supraflex (Sahajanand Medical Technologies Ltd., India) in 1 case, LEO (Balt Extrusion, France) in 1 case.
3.1. Clinical Outcomes
During the 72-hour periprocedural period following the procedure, two deaths from strokes were reported, resulting in a 0.7% periprocedural complication rate. These fatalities occurred in patients with baseline stenosis of 90% or greater in the left vertebral artery. One death was attributed to ischemic complications, while the other resulted from intraventricular tamponade, classified as a hemorrhagic complication.
The study documented various periprocedural complications. Three patients experienced immediate thrombosis of the implanted stent, requiring stent removal. Among these, one patient with 75% basilar artery stenosis who underwent Acclino Flex stenting showed a worsening in modified Rankin Scale (mRS) scores from 3 to 4, while another patient with 95% left vertebral artery stenosis exhibited an mRS increase from 2 to 4 due to pontine ischemia. Additionally, a patient with 70% left vertebral artery stenosis experienced acute intestinal bleeding, leading to a deterioration in mRS scores from 4 to 5.
Clinical deterioration was also noted in a patient with 80–89% right cavernous stenosis who underwent Ultimaster stenting, resulting in an mRS increase from 3 to 5; this patient also had concurrent myocardial issues. Another patient with 70% right vertebral artery stenosis who underwent Neuroform Atlas stenting experienced an mRS score change from 3 to 4, attributed to non-compliance with prescribed medications.
Other complications included one case of stent thrombosis on postoperative day 7 and a patient with right M2 stenosis who initially developed aphasia, which resolved by postoperative day 3. Additionally, one case of sigmoid and transverse sinus thrombosis was reported. Stent-related technical issues were also documented, including stent migration with the Acclino Flex stent and failure to deploy the Promus PREMIER stent due to vessel tortuosity.
The study highlighted a high proportion of mortality among patients with severe basilar artery stenosis who underwent stenting with Acclino Flex or Ultimaster stents. Notably, in three out of four patients who received coronary stents, clinical status worsened, and two out of three mortality cases were observed.
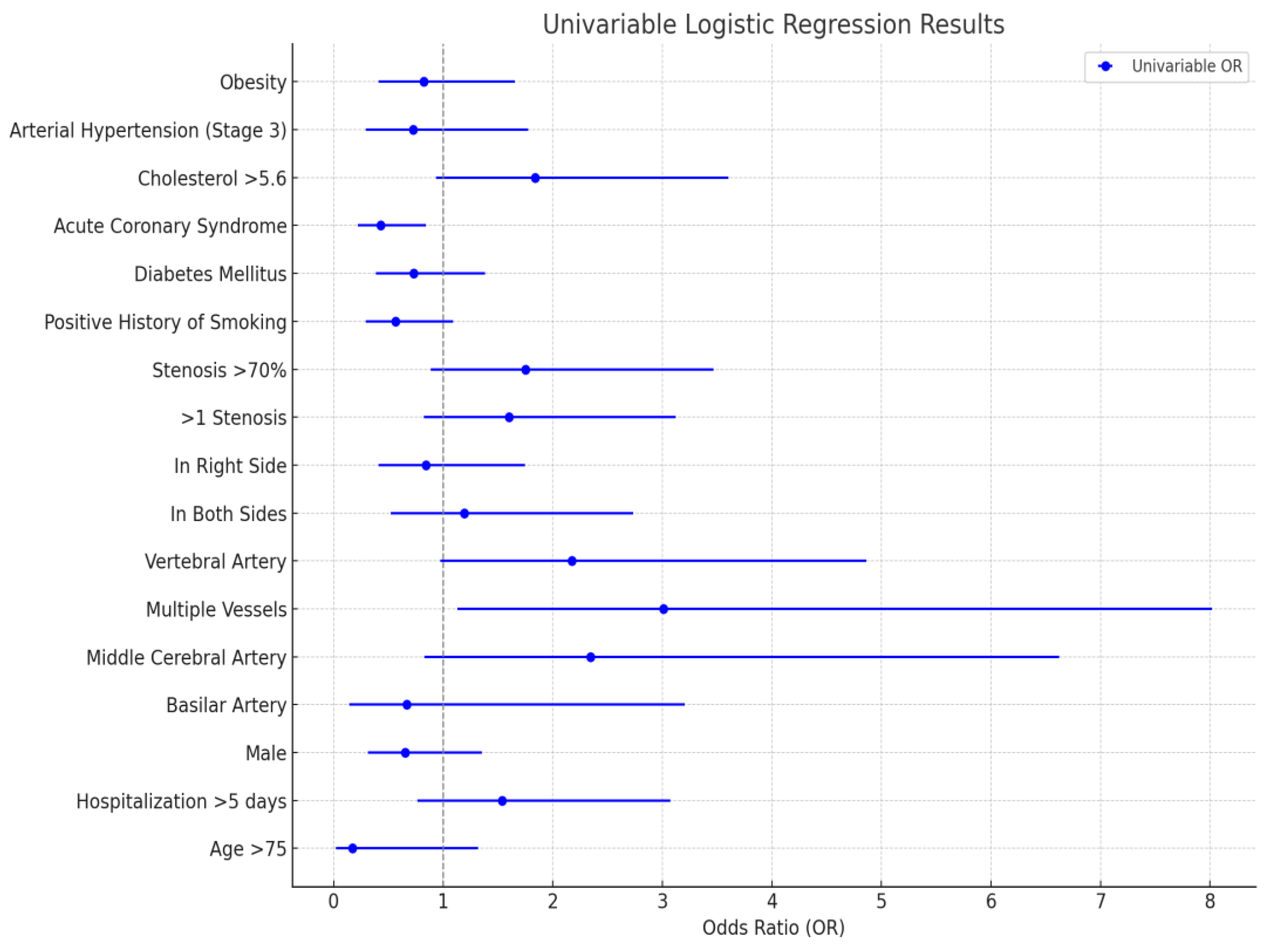
In the univariable analysis (
Figure 1), stenosis in multiple vessels was significantly associated with increased odds (OR = 3.012, 95% CI: 1.131–8.017). Stenosis in the middle cerebral artery (OR = 2.342, 95% CI: 0.828–6.623) and vertebral artery (OR = 2.175, 95% CI: 0.973–4.864) showed trends toward higher odds but lacked statistical significance. Severe stenosis (>70%) and elevated cholesterol (>5.6 mmol/L) also indicated increased odds without significance. Conversely, acute coronary syndrome demonstrated a protective effect, significantly reducing odds (OR = 0.431, 95% CI: 0.220–0.841), while smoking history and age >75 showed trends toward decreased odds but were not significant.
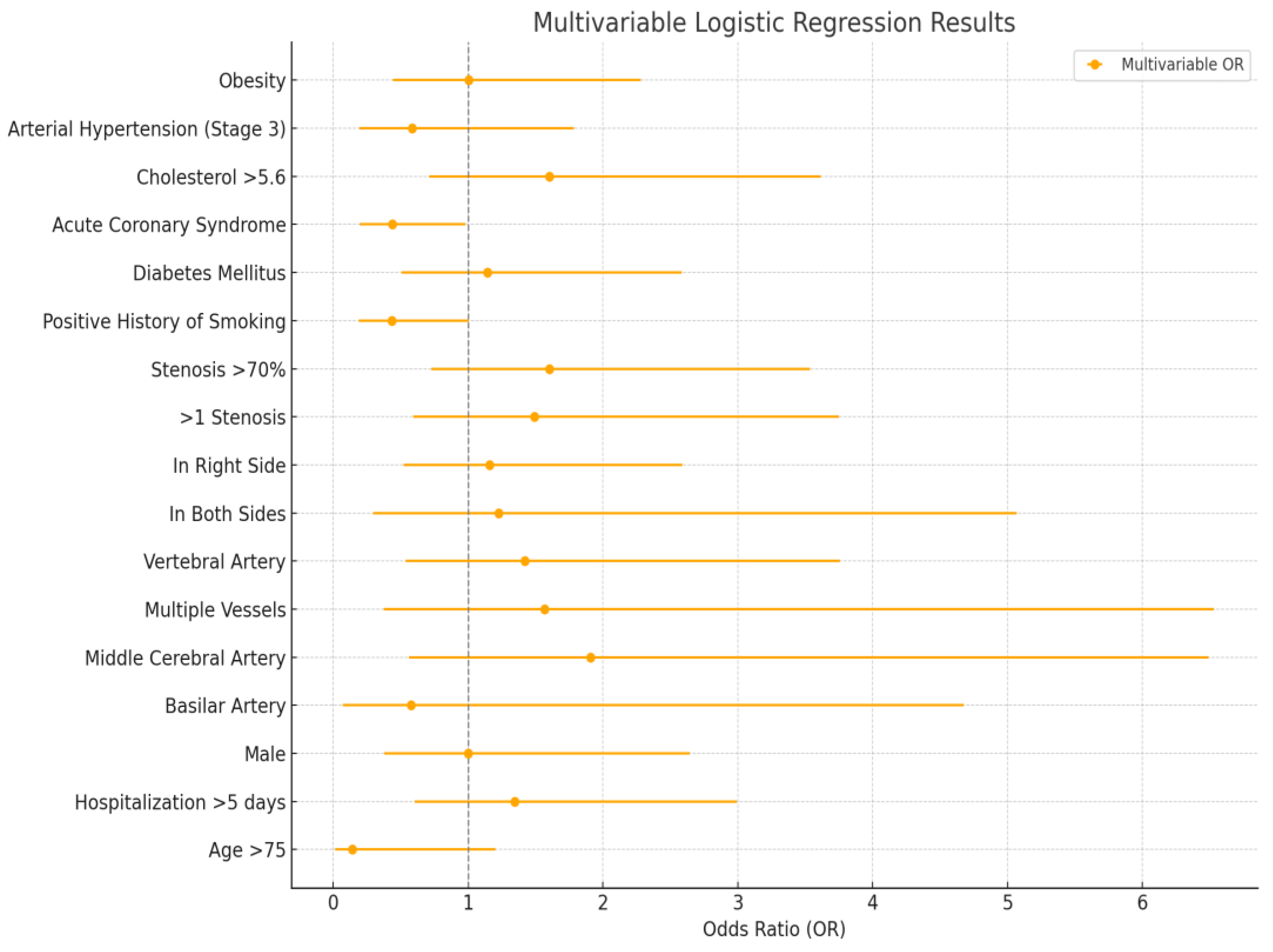
In the multivariable analysis (
Figure 2), the association of multiple vessel stenosis with increased odds was attenuated and lost significance, while the protective effect of acute coronary syndrome remained statistically significant (OR = 0.440). Other factors, including middle cerebral artery stenosis, stenosis severity, elevated cholesterol, age >75, and smoking history, remained consistent with univariable trends but without significance.
3.2. Case-Examples
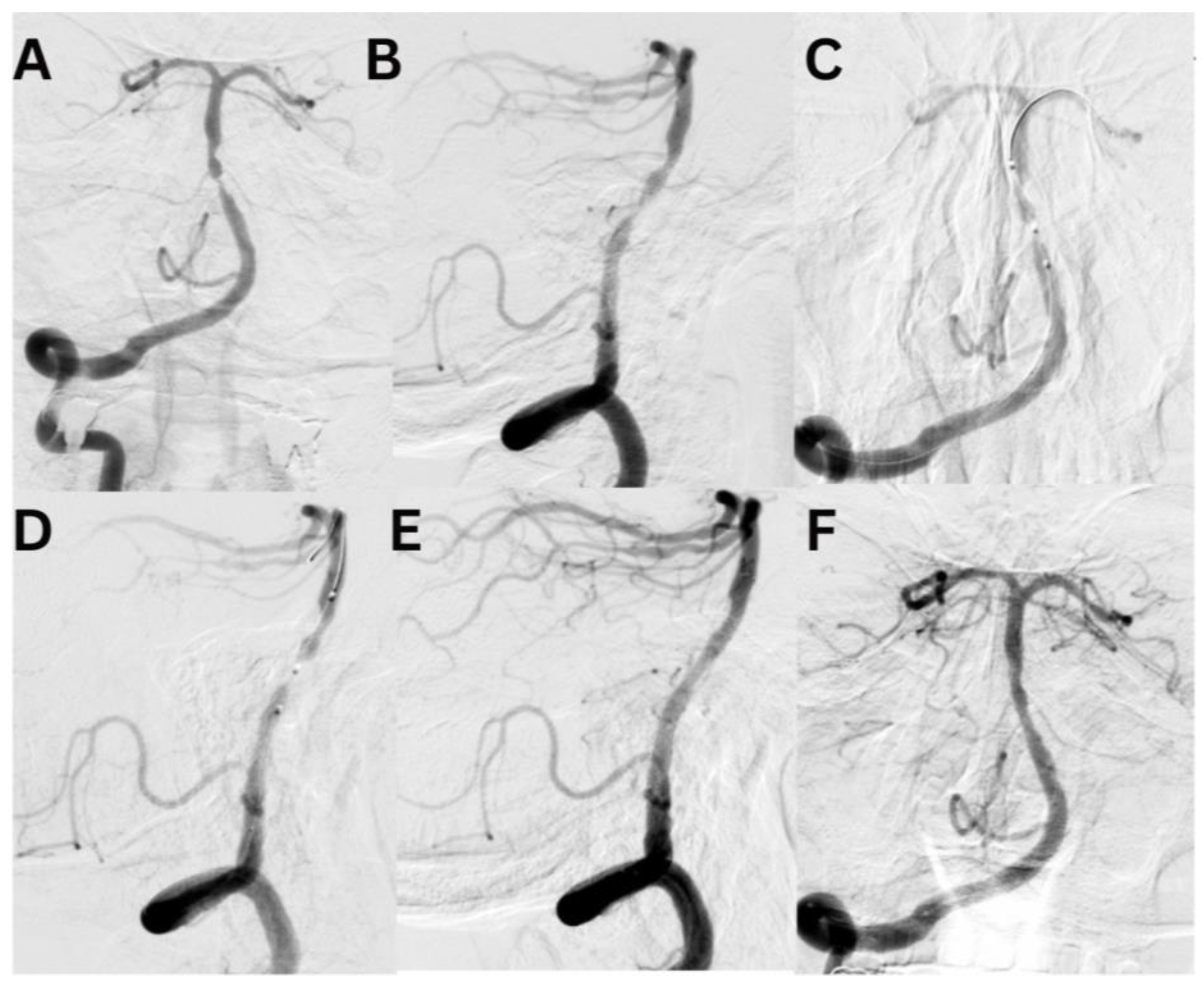
Case #1. A 75-year-old male patient with a history of myocardial infarction, type 2 diabetes mellitus, and hypertension presented with symptoms including slurred speech, temporary followed by full recovery, dizziness, tinnitus, unsteady gait, and lower extremity weakness. According to his wife, these symptoms began one week prior. Further diagnostic imaging with CTA revealed a subocclusion of the basilar artery, moderate stenosis in the V1 segment of the right vertebral artery, and hypoplasia of the left vertebral artery. Neurological examination at the time of admission was unremarkable. The patient was placed on dual antiplatelet therapy, and the VerifyNow test was performed, which detected no resistance. A surgical procedure was performed under local anesthesia. A 7Fr Fubuki guide catheter was inserted into the right vertebral artery, seen in
Figure 3. Under surgical guidance, an ASAHI Sion blue 0.014x180cm microwire was advanced through the stenotic area. A NeuroSpeed 2x8mm catheter balloon was then advanced along the microcatheter, inflated twice for 2 seconds within the stenotic segment, resulting in a residual stenosis of up to 40%. Subsequently, a 5.0x25mm Credo intravascular stent was deployed through the NeuroSpeed catheter balloon. Angiographic control confirmed the elimination of the stenosis, and the arteries and veins were found to be patent. At the 3-month follow-up, the patient reported no complaints, and MRI did not show any new signs of stroke.
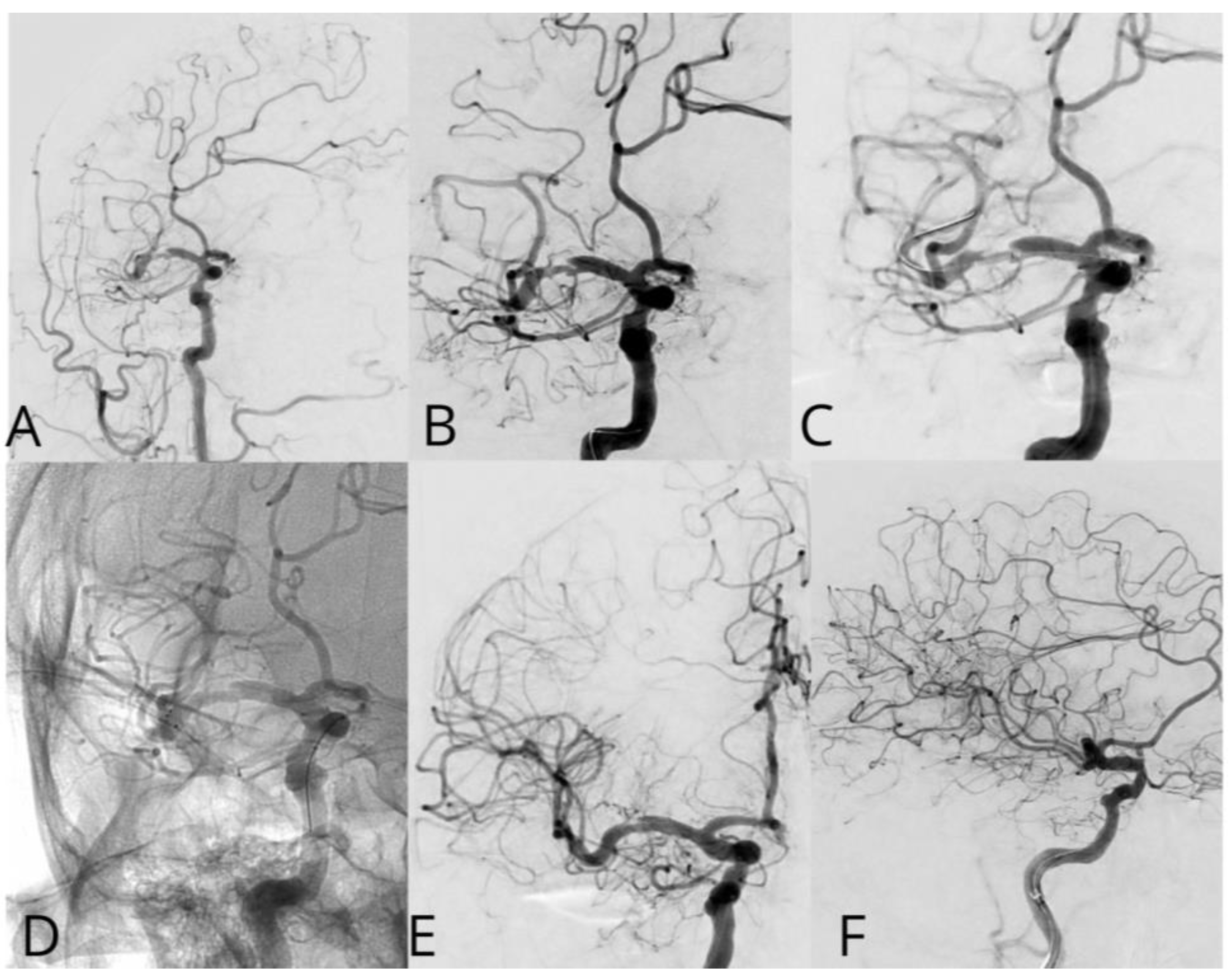
Case #2. A 48-year-old male patient presented with persistent pulsatile sensation in the parietal region, tinnitus, dizziness, blood pressure instability, right-sided weakness, general weakness, decreased work capacity, sleep disturbances, and memory loss. The patient had a history of hypertension for over 10 years and had experienced an ischemic stroke in the left middle cerebral artery territory two months prior, which was managed conservatively. Magnetic resonance imaging revealed signs of chronic ischemia in the right parietal and temporal lobes with cystic-gliotic changes, as well as single foci of hemosiderin deposition in the white matter of the cerebral hemispheres and basal ganglia, likely indicating microhemorrhage in the chronic stage. The patient underwent a comprehensive evaluation by a multidisciplinary team, including a cardiologist, neurologist, and pharmacologist, and was subsequently placed on ticagrelor. The procedure was performed under local anesthesia. An 8 French introducer was placed in the right femoral artery, and 8 French Hyperion catheters were navigated and positioned in the left internal carotid artery, covering the area of dissection and stenosis. DSA revealed a left M1 segment stenosis (
Figure 2A,B). An Asahi Chikai Black 0.014 microwire was then advanced and placed in the M2 segment of the left middle cerebral artery. A dual-lumen coaxial microcatheter, the NeuroSpeed 2.0 x 8.0 mm, was navigated to the stenotic region and inflated twice using an inflator, resulting in a residual stenosis of up to 40%. Next, during the attempt to deploy an Acclino Heal 5.0x20mm stent through a balloon catheter starting from the M1 bifurcation of the left middle cerebral artery, the proximal portion of the stent became fixed within the balloon catheter due to the radiopaque markers. As a result, the balloon microcatheter and the damaged stent were removed (
Figure 2C). Subsequently, a GAMA 17 catheter was navigated through the Asahi Chikai Black 0.014 microwire, and an intravascular Acclino Heal 5.0x20mm stent was reinserted and deployed to eliminate the stenosis (
Figure 2D). The final angiographic evaluation demonstrated a patent left middle cerebral artery without any residual narrowing (
Figure 2E,F).
4. Discussion
Intracranial atherosclerotic stenosis (ICAS) remains a significant cause of ischemic stroke, especially in patients with a high burden of vascular risk factors. Kazakh population with unique epidemiological and clinical characteristics of this region is under-reported in general literature [
4]. The incidence of cerebrovascular disease in Kazakhstan has increased notably, rising from 258.4 cases per 100,000 individuals in 2015 to 433.7 cases per 100,000 in 2020. Additionally, official data suggest an average inpatient mortality rate from stroke of 16.2% within the country, while the average time for patients to reach the hospital following an emergency call is approximately 40 minutes [
7]. This study provides valuable insights into the demographic characteristics, anatomical distribution, and clinical outcomes of ICAS treatment in a single institutional cohort of 216 patients, highlighting the interplay between patient-specific factors and procedural outcomes. The median age of patients in our cohort was 63.5 years, with a gradual increase in mean age over the study period from 59 to 64 years. This aligns with previous studies demonstrating that ICAS is predominantly a disease of older adults. The predominance of males (73.7%) is also consistent with prior reports that ICAS exhibits a male bias, attributed to differences in vascular risk profiles and hormonal influences [
8,
9].
Hypertension (98%), diabetes mellitus (40.9%), and ischemic heart disease (58%) were highly prevalent in our cohort, emphasizing their established role as key risk factors for ICAS. These findings mirror those from the Northern Manhattan Stroke Study and other population-based studies, which consistently identify hypertension as the most prominent risk factor [
10,
11]. Furthermore, our data suggest an association between age over 75 years and multi-location ICAS, as well as a trend toward significance for obesity and stenosis severity, underscoring the contribution of metabolic syndrome to the progression of ICAS [
12].
The distribution of stenosis in our cohort showed the internal carotid artery (ICA) as the most frequently affected vessel (54.5%), followed by the vertebral artery (21.2%) and middle cerebral artery (12.3%). This predominance of anterior circulation stenosis is in line with autopsy studies and imaging analyses, which report ICA and MCA as the most commonly involved vessels in ICAS [
13]. However, patients with posterior circulation stenosis, particularly in the vertebrobasilar territory, exhibited a longer hospital stay (7.1 days on average), reflecting the more severe clinical presentations and poorer outcomes associated with posterior circulation strokes [
13,
14].
Our analysis demonstrated a low periprocedural complication rate of 0.7%, with two ischemic stroke-related deaths within 72 hours. While the rate of complications is comparable to other reports in the literature, such as the SAMMPRIS trial, which reported a 2.5% perioperative stroke or death rate, it is notable that our study also documented unique complications such as stent thrombosis, hemorrhage with ventricular tamponade, and stent migration [
15,
16].
Of particular interest is the finding that worsening outcomes, defined as an mRS score >2, occurred in 4 patients. The basilar artery, vertebral artery, and M1 segment were the most affected sites in patients with severe stenosis, consistent with studies indicating that posterior circulation and high-grade stenosis carry a higher risk of adverse outcomes [
17,
18]. Additionally, the choice of stent did not show a significant association with procedural success or hospital length of stay, supporting findings from prior meta-analyses that suggest procedural technique and patient factors may outweigh stent design in determining outcomes [
19].
Our findings align with and expand upon existing literature by demonstrating that older age, multiple comorbidities, and posterior circulation involvement are associated with worse outcomes in ICAS [
20]. Studies like the Chinese Intracranial Atherosclerosis Study (CICAS) have similarly emphasized the role of vascular risk factors and lesion characteristics in determining stroke risk and prognosis [
21]. However, our results suggest that modern endovascular techniques, coupled with careful patient selection, can achieve favorable outcomes even in patients with severe stenosis.
Stents such as the Pegasus, HPS 4.5 mm, and Credohill 5 mm, which are not coated with cytostatic agents, have a higher rate of restenosis in follow-up angiograms. Coronary stents are preferred as they have demonstrated a lower incidence of restenosis on follow-up angiograms, however some complications might happen.
The existing studies have been limited by small sample sizes, lack of randomization, and inadequate participant selection and blinding. Nevertheless, the natural history of intracranial atherosclerosis indicates that specific subgroups may experience more severe disease progression, and these patient populations may potentially benefit from percutaneous transluminal angioplasty and stenting for long-term reduction of ischemic events, as demonstrated in the WEAVE trial. Furthermore, with the involvement of experienced interventionalists and appropriate patient selection following the on-label usage guidelines, the use of the Wingspan stent for intracranial atherosclerotic disease has exhibited a low periprocedural complication rate and an excellent safety profile. Notably, this represents the largest on-label, multicenter, prospective trial of the Wingspan stent system to date, with the lowest reported complication rate [
22]. The WOVEN study followed up on a group of 129 patients for 1 year after they underwent stenting in accordance with the current guidelines. This patient population was more homogeneous than those in previous studies, and the results showed a relatively low 8.5% rate of stroke and death within 1 year after the stenting procedure [
23].
A recent meta-analysis of four studies including 352 patients who failed medical therapy revealed that patients who underwent stent rescue after failed MT experienced significantly improved rates of favorable clinical outcomes (OR 2.87, 95% CI 1.77-4.66, p<0.001) and lower mortality (OR 0.39, 95% CI 0.16-0.93, p=0.03) at 90 days, without any increased risk of symptomatic intracranial hemorrhage (OR 0.68, 95% CI 0.37-1.27, p=0.23), compared to non-stented patients who failed medical therapy. These findings suggest that in carefully selected patients with symptomatic intracranial stenosis refractory to medical management, endovascular stenting may be a viable option to improve clinical outcomes [
24].
Ischemic heart disease and intracranial atherosclerosis frequently co-occur due to shared risk factors, such as hypertension, hyperlipidemia, diabetes mellitus, and smoking. Studies estimate that up to 20-30% of patients with ICAS also have concurrent coronary artery disease, which is characteristic of IHD [
25].
The most common causes of death in patients with coexisting IHD and ICAS are acute myocardial infarction and large ischemic strokes. Studies suggest that the risk of fatal strokes is higher in patients with ICAS than in those with extracranial carotid stenosis. Moreover, heart failure due to progressive IHD also contributes significantly to mortality in this population [
26].
In our analysis a multivessel involvement was significantly associated with higher odds of the event compared to isolated internal carotid artery involvement. Additionally, our analysis reveals that a history of ischemic heart disease was significantly associated with lower odds of mortality compared to those without ischemic heart disease, a finding consistent in both univariable and multivariable analyses. These results emphasize the importance of considering multivessel involvement as a critical risk factor and highlight the protective association of ischemic heart disease in this context.
This study has certain limitations. First, the participants were from a single treatment center, which may have introduced bias. Second, the retrospective design has inherent limitations. Additionally, the sample size is relatively small, and there was no control group. Nevertheless, the analysis of data from 216 patients presenting with ICAS provides valuable insights, although the conclusions are not definitive. Future large-scale, multi-center, prospective case-control studies are necessary to corroborate the findings demonstrated in the current analysis. This single-center study is limited by its retrospective nature, which may introduce selection bias. Additionally, the small sample size for certain anatomical locations limits the generalizability of findings. Future multicenter, prospective studies are needed to validate these results and explore the role of advanced imaging and novel stent designs in improving ICAS outcomes.
5. Conclusions
This study provides a detailed analysis of the demographic characteristics and treatment outcomes of 216 patients with intracranial atherosclerosis (ICAS) in the Kazakh population. The findings highlight a strong association between ICAS and hypertension, diabetes, and dyslipidemia, with hypertension emerging as the most prevalent comorbidity. Notably, age was identified as a significant factor influencing the presence of multi-location stenosis, while trends for obesity and stenosis severity were observed but not statistically significant. Contrary to expectations, common risk factors such as diabetes mellitus, ischemic heart disease, and hypercholesterolemia did not demonstrate significant associations with multi-location stenosis in this cohort, potentially reflecting unique regional or demographic patterns. These results underscore the necessity of larger-scale research to further investigate genetic, environmental, and lifestyle factors contributing to ICAS in Central Asia.
This study emphasizes the critical importance of managing modifiable risk factors, particularly hypertension and diabetes, to mitigate the progression of ICAS and reduce cerebrovascular risks. The disproportionate impact of posterior circulation stenosis and severe lesions on clinical outcomes calls for more focused strategies in patient management. Comparisons with international literature suggest that advances in endovascular interventions have contributed to improved outcomes globally; however, tailored, region-specific approaches remain essential. Future research should also prioritize assessing long-term functional outcomes and quality of life to optimize treatment strategies for ICAS in the Kazakh population.
Author Contributions
Conceptualization, M.S. and M.B.; methodology, M.A.; software, D.D.; validation, B.T., G.T. and M.S.; formal analysis, S.M.; investigation, M.S.; resources, B.T.; data curation, B.T.; writing—original draft preparation, M.A.; writing—review and editing, M.A.; visualization, M.M.; supervision, M.B.; project administration, G.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of JSC Clinical Hospital provided ethical approval for this study (protocol code 7 and December 12th, 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.”
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qureshi AI, Ziai WC, Yahia AM, Mohammad Y, Sen S, Agarwal P, Zaidat OO, Suarez JI, Wityk RJ. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: a multicenter study. Neurosurgery. 2003;52:1033–1039; discussion 1039.
- Baker AB, Flora GC, Resch JA, Loewenson R. The geographic pathology of atherosclerosis: a review of the literature with some personal observations on cerebral atherosclerosis. J Chronic Dis. 1967;20:685–706. [CrossRef]
- Pahwa R, Jialal I. Atherosclerosis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507799/.
- Bekbossynova, M., Ivanova-Razumova, T., Ablayeva, A., & Oralbekova, Z. (2024). Establishment of an Atherosclerosis and Dyslipidemia Program in Kazakhstan. Mayo Clinic proceedings, 99(11), 1698–1701. [CrossRef]
- Kim, S., & Caplan, L R. (2016, January 1). Non-Atherosclerotic Intracranial Arterial Diseases. Karger Publishers, 179-203. [CrossRef]
- Brutto, V J D., Ortiz, J G., & Biller, J. (2017, July 17). Intracranial Arterial Dolichoectasia. Frontiers Media, 8. [CrossRef]
- Adenova, G., Kausova, G., & Tazhiyeva, A. (2023). Improving multidisciplinary hospital care for acute cerebral circulation disorders in Kazakhstan. Heliyon, 9(8), e18435. [CrossRef]
- Gao, P., Wang, T., Wang, D., Liebeskind, D. S., Shi, H., Li, T., Zhao, Z., Cai, Y., Wu, W., He, W., Yu, J., Zheng, B., Wang, H., Wu, Y., Dmytriw, A. A., Krings, T., Derdeyn, C. P., Jiao, L., & CASSISS Trial Investigators (2022). Effect of Stenting Plus Medical Therapy vs Medical Therapy Alone on Risk of Stroke and Death in Patients With Symptomatic Intracranial Stenosis: The CASSISS Randomized Clinical Trial. JAMA, 328(6), 534–542. [CrossRef]
- Qureshi AI, Feldmann E, Gomez CR, et al. Intracranial atherosclerotic disease: an update. *Ann Neurol*. 2009;66(5):730-738. [CrossRef]
- Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. *Lancet Neurol*. 2013;12(11):1106-1114. [CrossRef]
- Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: the Northern Manhattan Stroke Study. *Stroke*. 1995;26(1):14-20.
- Arenillas JF. Intracranial atherosclerosis: current concepts. *Stroke*. 2011;42(1 Suppl):S20-S23.
- Gupta A, Chugh S. Hypertension, dyslipidemia, and diabetes in older adults with intracranial stenosis. *Clin Neurol Neurosurg*. 2017;157:40-45.
- Mazighi M, Labreuche J, Gongora-Rivera F, et al. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. *Stroke*. 2008;39(4):1142-1147. [CrossRef]
- Caplan LR. Posterior circulation ischemia: then, now, and tomorrow. *Stroke*. 2000;31(8):2011-2023. [CrossRef]
- Akpinar CK, Yilmaz G, Tascilar N, Arsava EM. Intracranial stenosis: clinical and imaging characteristics. *Front Neurol*. 2020;11:1020.
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. *N Engl J Med*. 2011;365(11):993-1003. [CrossRef]
- Jiang WJ, Yu W, Du B, et al. Outcome of patients with ≥70% symptomatic intracranial stenosis after Wingspan stenting. *Stroke*. 2011;42(7):1971-1975. [CrossRef]
- Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. *Neurology*. 1989;39(9):1246-1250.
- Park ST, Jung SY, Kim BJ, et al. Current status of intracranial stenting for atherosclerotic stenosis: a comprehensive review. *J Clin Med*. 2021;10(8):1656.
- Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) study. *Stroke*. 2014;45(3):663-669.
- Alexander, M. J., Zauner, A., Chaloupka, J. C., Baxter, B., Callison, R. C., Gupta, R., Song, S. S., Yu, W., & WEAVE Trial Sites and Interventionalists (2019). WEAVE Trial: Final Results in 152 On-Label Patients. Stroke, 50(4), 889–894. [CrossRef]
- Alexander, M. J., Zauner, A., Gupta, R., Alshekhlee, A., Fraser, J. F., Toth, G., Given, C., Mackenzie, L., Kott, B., Hassan, A. E., Shownkeen, H., Baxter, B. W., Callison, R. C., & Yu, W. (2021). The WOVEN trial: Wingspan One-year Vascular Events and Neurologic Outcomes. Journal of neurointerventional surgery, 13(4), 307–310. [CrossRef]
- Lee, J. S., Lee, S. J., Hong, J. M., Alverne, F. J. A. M., Lima, F. O., & Nogueira, R. G. (2022). Endovascular Treatment of Large Vessel Occlusion Strokes Due to Intracranial Atherosclerotic Disease. Journal of stroke, 24(1), 3–20. [CrossRef]
- Gorelick PB, Wong KS, Bae HJ, Pandey DK. “Large artery intracranial occlusive disease: A large worldwide burden but a relatively neglected frontier.” Stroke. 2008;39(8):2396-2399.
- Bang OY, Lee PH, Joo SY, Lee JS, Jang JY, Huh K. “Frequency and mechanisms of stroke recurrence after cryptogenic stroke.” Annals of Neurology. 2004;56(5):644-652. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).