1. Introduction
Neuroendocrine tumors (NETs), first described in the pulmonary tissue, are rare and heterogeneous types of tumors, it could be localized in all organs that present diffuse neuroendocrine system, presenting origin from the endodermal cells, depending on the primitive gut, it being classified in foregut (thymus, esophagus, lung, stomach, duodenum and pancreas), midgut (appendix, ileum, cecum and ascending colon) and hindgut (distal bowel and rectum) [
1,
2,
3]. Pancreatic NETs (P-NETs) are relatively rare type of NET, being classified in insulinoma (I-oma), gastrinoma, Vasoactive Intestinal Peptide Tumors (VIPoma), Pancreatic Polypeptide Secretion Tumors (PPoma), glucagonoma and somatostatinoma [
3,
4].
Severe hypoglycemia is frequently encountered in patients with diabetes treated with insulin or insulin secretagogues, but in patients without diabetes hypoglycemia is unusual and the symptomatology is often misinterpreted [
5,
6]. In this context, the urgent initiation of the clinical, biological and imaging evaluation of the patient is required in order to establish the etiological diagnosis and the optimal therapy to avoid the serious consequences of severe hypoglycemia [
7,
8].
The first step of the etiological diagnosis of hypoglycemia in patients without diabetes involves establishing whether the hypoglycemia is mediated by endogenous hyperinsulinism or other mechanisms. In case of identification of insulin-mediated hypoglycemia, the evaluation of pancreatic neuroendocrine tumors (P-NETs), which represent the most common and worrisome causes of non-diabetic insulin-mediated hypoglycemia, must be considered.
P-NET represents 30% of gastroenteropancreatic neuroendocrine tumors, most of them being non-functional and only 10-30% are functional, secreting one or more hormones or amines that determine suggestive clinical symptomatology [
9].
In order to confirm the etiology of endogenous hyperinsulinemic hypoglycemia, the appropriate pathological diagnosis of P-NET should include the functional evaluation by specific staining for peptide hormones, especially insulin, but also serotonin and other amines depending on the clinical symptoms [
9,
10,
11].
The identification of P-NET as the cause of severe hypoglycemia requires urgent therapeutic intervention for the remission of hypoglycemic episodes and their consequences and to increase the patients' quality of life.
2. Aim
In the current review, we emphasize the pitfalls in the identification and etiological diagnosis of hypoglycemia in nondiabetic individuals. Thus, we present the case of a female patient with small size P-NET, who presented severe hypoglycemia. Our case study also highlights the role of the multidisciplinary team, and especially of the general practitioner, in recognizing the symptoms consistent with hypoglycemia, in evaluating the patient and establishing the positive diagnosis of hypoglycemia and the etiological diagnosis, in order to optimally manage non-diabetic hypoglycemia and also to avoid diagnostic pitfalls.
3. Results
The 57-year-old patient was admitted to the Diabetes Clinic from Craiova Emergency Clinical Hospital, for recurrent episodes of dizziness, fatigue, cold sweat, tremors, changes in behavior, anxiety, sometimes associated with seizures or deterioration of consciousness. The patient did not experience weight gain, palpitations, headache, diarrhea, flushing or itching. The clinical symptoms occurred daily, for about 1 month.
The patient also had history of hospital admissions in the Psychiatric Clinic due to repeated episodes of psychiatric symptoms such as behavioral disorders, psychomotor agitation, anxiety. During the last hospitalization in the Psychiatry Clinic, in the context of loss of consciousness, a capillary blood glucose level of 19 mg/dl was detected.
The patient has no hereditary and personal history of diabetes, multiple endocrine neoplasia syndromes or other significant diseases.
Physical exam indicated overweight (body mass index: 25.55 kg/m2) and abdominal obesity (waist 90 cm), without detecting other significant clinical changes.
Starting from admission in Diabetes Clinic, the patient presented recurrent episodes of hypoglycemia (plasma glucose value being less than 50 mg/dl), unrelated to food intake, accompanied by confusion, dizziness, cold sweats, tremor, unresponsive to the ingestion of carbohydrates, which required the initiation of intravenous boluses of 33% Glucose solution (30-50 ml during episodes of severe hypoglycemia) and continuous intravenous infusion with 10% Glucose solutions (on average 350 g glucose /24 h from glucose solutions administered i.v.). Symptoms of hypoglycemia remitted and glycemic values normalized during continuous intravenous infusion of 10% Glucose solutions, but the trend of glycemia became rapidly decreasing after the interruption of i.v. administration of Glucose, despite the daily intake of 300 g of carbohydrates divided into 6-7 meals. However, two plasma glycemic values ≥200 mg/dl (209 mg/dl, respectively 227 mg/dl) were randomly detected in the absence of i.v. administration of glucose solutions.
The paraclinical evaluation during an episode of hypoglycemia indicated plasma glucose value of 44 mg/dl, insulinemia 16.3 µU/ml (Normal 2.2-24.9 µU/ml), C peptide level 3.72 ng /ml (Normal 1.1-4.4 ng/ml), HbA1c 4.99% (Normal 4–5.6%), absence of urinary ketone bodies and anti-insulin antibodies <0.03U/ml (Normal <0.4 U/ml). HbA1c evaluated 7 months before admission, during routine investigations, was 5.1%.
Based on the paraclinical explorations, other potential causes of hypoglycemia were excluded such as: adrenal insufficiency (ACTH and serum cortisol levels were normal), thyroid dysfunction (normal TSH, FT4, FT3), organ failure (normal kidney and liver function) and sepsis (normal blood counts, normal inflammatory tests) respectively. The ophthalmoscopy, abdominal ultrasound, cranial and chest computed tomography (CT) examination were normal.
Clinical and biochemical data indicating endogenous insulin-mediated hypoglycemia, leading to abdominal imaging investigations searching for P-NET.
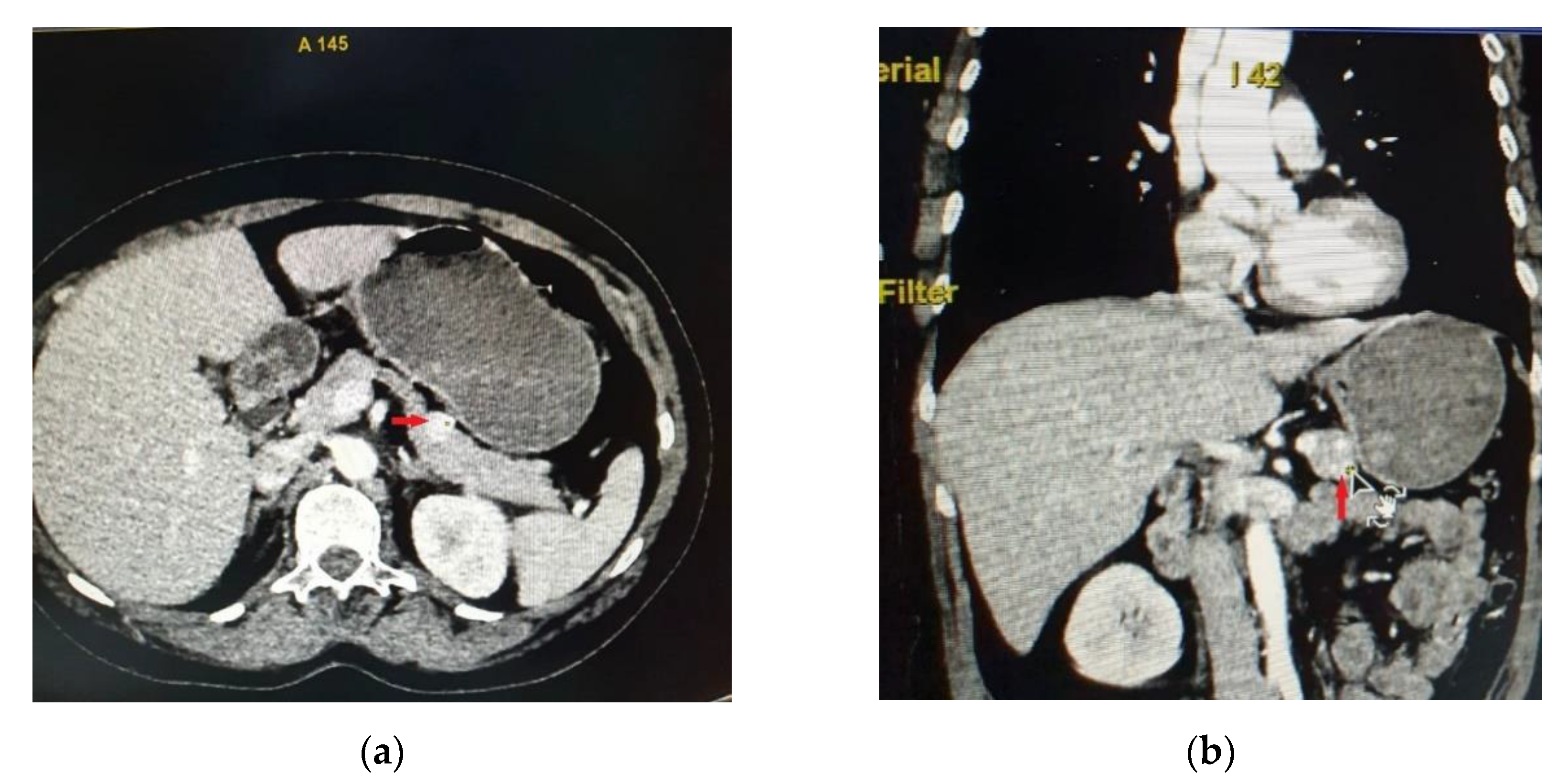
Abdominal CT showed in the anterior part of the pancreatic corporeo-caudal region an isodense rounded tumor measuring 15.3/15 mm, with intense iodophilia that is maintained in the venous phase (
Figure 1). A complementary abdominal magnetic resonance imaging (MRI) was performed, indicating a pancreatic hypervascular tumor that appeared with hypo/isosignal at T1- weighted sequences and slightly hypersignal at T2- weighted sequences, homogeneous in the post-contrast study, with diffusion restrictions in diffusion-weighted imaging sequences correlated with apparent diffusion coefficient maps.
The patient underwent tumor enucleation from the pancreatic corporeo-caudal region. A 1.5 cm, well-defined, yellow-gray, tumor with elastic consistency, was excised (
Figure 2). Visual and palpatory examination of the pancreas revealed no other pancreatic lesions.
Intraoperatively, the capillary glucose trend was monitored at 15-minute intervals, with the adjustment of the intravenous 10% glucose solution infusion rate, based on the evolution of the glycemic profile. Shortly after the excision of the tumor, the glycemic values increased (226 mg/dl), in the absence of intravenous administration of glucose solutions. The postoperative evolution was favorable, without the occurrence of episodes of hypoglycemia. The fasting insulinemia determined on the second postoperative day was 4.1 µU/ml.
The histopathological examination highlighted a fragment of pancreatic tissue showing tumor proliferation consisting of round, large, relatively monomorphic cells, with hyperchromic excentric nuclei and abundant granular cytoplasm with a solid and trabecular pattern, rare mitoses (<2 mitoses/10 HPF) and desmoplastic stroma.
Regarding the immunohistochemical examination, specific staining was performed to confirm the diagnosis of P-NET. Thus, to establish the neuroendocrine origin, staining was performed for chromogranin A and synaptophysin, both reactions being positive. Also, the functional character of the tumor was analyzed, the tumor cells being positive for insulin and negative for glucagon. Ki67 index was analyzed as a tumor proliferation marker, the index being positive in 1% of tumor cells. Immunohistochemical tests supported the diagnosis of P-NET G1, according to WHO 2019 classification for gastroenteropancreatic neuroendocrine neoplasms [
11].
Seven days after the surgical intervention, the patient was discharged asymptomatic and with normal glycemic profiles. Nine months postoperative, the patient is still free of all the previous hypoglycemic symptoms, with normal glycemic values and glycated hemoglobin increased to 6.2% and insulin level was 7.8 μU/mL.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board (Report no 35510/13.08.2024).
The clinical, biological, imaging, histological and immunohistochemical findings support the diagnosis of functional well-differentiated P-NET – insulinoma, confined to the pancreas, grade G1 according to the WHO criteria [
11,
12,
13,
14,
15].
4. Discussion
Hypoglycemia recognition and prompt intervention are a unitary medical challenge, due to the life-threatening risks of hypoglycemia. Hypoglycemia should be suspected if the elements of the Whipple triad are identified in a patient without diabetes: venous blood glucose level <55mg/dl (<3mmol/L), determined at the time of spontaneous development of symptoms, if feasible, the presence of specific signs and symptoms for hypoglycemia and their remission after carbohydrate intake [
16].
In order to establish the etiological diagnosis of hypoglycemia, the anamnesis and clinical examination must take into account the information regarding the main potential causes of hypoglycemia, such as the lifestyle pattern (inanition, type, amount and food sources of ingested carbohydrates, abuse of alcohol or drugs), surreptitious, malicious, accidental or iatrogenic administration of hypoglycemic drugs (insulin, sulfonylureas, etc), personal and hereditary medical history including diseases such as diabetes, cancer, psychiatric diseases, bariatric surgery, critical illness (hepatic, renal or cardiac failure), sepsis, hormone deficiency (glucagon, cortisol, growth hormone), non-beta cell tumors, Familial Endocrine Tumor Syndromes (FETSs) like multiple endocrine neoplasia type 1 or 4, neurofibromatosis type I, tuberous sclerosis complex [
17,
18,
19]. Important to mention is the “paraneoplastic hypoglycemia” which is related to other tumors like hepatocellular carcinomas, hemangiopericytomas, mesotheliomas, fibrosarcoma, gastrointestinal stromal tumors that secret insulin-like hormones, leading to hypoglycemia [
20].
Regardless of the triggering cause of hypoglycemia, the signs and symptoms characteristic of hypoglycemia are the same and are divided into two categories: autonomic (palpitations, tremors, anxiety, pallor, tachycardia, hypertension, cold sweat, imperious hunger, paresthesia) and neuroglycopenic (headache, dizziness, visual impairment, behavioral disturbances, confusion, drowsiness, convulsions, coma) [
5,
6,
21]. Since the initial symptoms would be varied and non-specific, mimicking various cardiovascular, endocrinological, neurological, psychiatric conditions, a multidisciplinary approach in making a detailed differential diagnosis is absolutely necessary for the correct management of hypoglycemia [
21].
The diagnosis of hypoglycemia is performed by determining the plasma levels of glucose, markers of endogenous insulin synthesis and secretion (proinsulin, insulin, C-peptide) and beta-hydroxybutyrate, during a hypoglycemic episode occurring spontaneously or induced by a mixed meal or during a fast of up to 72 h. The biological evaluation should include hormonal profile to identify potential hormonal deficiencies that could justify hypoglycemia and insulin antibodies.
Insulin-mediated hypoglycemia is defined based on glycemia ≤55 mg/dl, insulinemia ≥3 µU/ml, C-peptide levels ≥0.6 ng/ml, proinsulinemia ≥5 pmol/l, β-hydroxybutyrate levels ≤2.7 mmol/l [
13]. Previously, the insulinemia threshold value for the diagnosis of hyperinsulinemic hypoglycemia was 5 µU /ml, but subsequent studies have indicated that in a significant percentage of patients, insulinoma remains undetected if a higher insulinemia cut-off value is used [
22].
The peculiarity of the presented case is the association of neuroglycopenic symptoms of hypoglycemia not preceded by autonomic hypoglycemic symptoms, with values of insulinemia and C peptide higher than the threshold for defining insulin-mediated hypoglycemia, but not so high compared to the severity and persistence of hypoglycemia and refractoriness of hypoglycemic episodes to carbohydrate ingestion and parenteral glucose administration.
The association of severe hypoglycemia with levels of insulinemia and C peptide higher than the diagnostic threshold value of insulin-mediated hypoglycemia, but not significantly increased, can be justified by the challenging coexistence of insulinoma and type 2 diabetes which was masked by insulinoma [
23,
24]. The association of type 2 diabetes in our patient is suggested by the random plasma glucose values higher than 200 mg/dl and also by the occurrence of persistent hyperglycemia after surgical removal of the insulinoma. Persistent hyperglycemia following surgical removal of the insulinoma may suggest an underlying diabetes (the patient had glycemic values >200 mg/dl prior to surgery) but it may also be the consequence of damage to the pancreas during the surgical procedure, which caused secondary hyperglycemia (HbA1c nine months postoperatively was 6.2%) [
24].
Considering that the level of C peptide above 6.1 ng/ml has increased specificity and sensitivity (96% and 100%, respectively), together with the size of the tumor above 2.6 cm in the prediction of malignancy, the value of C peptide in the presented patient can be explained by the benign nature of the insulinoma, confirmed by the pathological examination [
25].
In spite of the large unknown for the pathogenesis of P-NETs, approximately 10% are part of the FETSs [
26,
27,
28] and the treatment is targeted taking account the cellular type and hormonal profile. The risk factors for developing a P-NET are body mass index, smoking, alcohol consumption, genetic factors (-1031 TC and CC genotype distribution of TNF-α promoter polymorphism and IL1β-511 for single-nucleotide polymorphism), family history of cancer (especially of P-NET), first-degree family-history of esophageal cancer, adding of the risk factors other types of cancer, like gallbladder, gastric or ovarian cancer and sarcoma and personal history of chronic pancreatitis [
29,
30,
31,
32,
33,
34,
35,
36,
37].
If P-NETs are suspected as a cause of hypoglycemia, diagnosis and management should be based on key features of neuroendocrine neoplasm represented by proliferative activity, tumor growth rate using response evaluation criteria in solid tumors, locoregional and systemic extent of the disease and somatostatin receptor expression [
9,
11].
The diagnosis of P-NETs includes anamnestic data related to symptomatology suggestive of a certain type of functional P-NET and personal or family history of NETs or conditions frequently associated with NETs, non-invasive imaging evaluation such as CT or MRI and invasive imaging evaluation - endoscopic ultrasonography (EUS). Confirmation of the functional character of P-NET is performed through the immunohistochemical examination of the tumor tissue.
The appropriate pathological (histopathology and immunohistochemistry) diagnosis of P-NET should include the functional evaluation by specific staining for peptide hormones (insulin, serotonin and other amines depending on the clinical symptoms), Chromogranin A and synaptophysin and the establishment of tumor grade by reporting the ki67 index and the mitotic count per 2 mm2 [
9,
10,
11].
I-oma, the most current form of P-NETs, first described in 1927 in a murine model, are the most common and the most frequent functioning endocrine pancreatic tumor [
38]. Also, generally, I-oma’s are benign, solitary, intrapancreatic lesions, with 0,5-2 cm dimension at the moment of diagnosis [
17,
39], but there are sometimes some exceptions. Although the I-oma are typically localized in the pancreatic tissue, it has been described ectopic positions (duodenal wall, spleen, perisplenic tissue, duodenohepatic ligament and surrounding tissue of the pancreas) [
40,
41]. Also, it has been presented rare association like I-oma with pregnancy, diabetes mellitus type 2 and transformation of metastatic non-functioning P-NET into I-oma and until now, eleven giant I-oma (majority with pancreatic tail localization) [
42,
43,
44,
45,
46,
47]. Also, for functional NET, that are hormone secreting tumors, it is necessary to dosage the non-specific (Chromogranin A, Neuron-Specific Enolase, Pancreatic polypeptide, Human Chorionic Gonadotropin and α-fetoprotein) and specific (Serotonin, Gastrin, Insulin, Glucagon, Somatostatin, Vasoactive Intestinal Peptide) neuroendocrine markers, for I-oma being recommended Chromogranin A, Neuron-Specific Enolase and Insulin [
48,
49,
50].
Among the imaging methods for insulinoma detection, CT represents the first line of evaluation with a sensitivity varying between 30-80% depending on the size of the tumor, followed by MRI with a sensitivity of 85-92% for MRI [
51,
52]. Insulinoma detection sensitivity increases significantly to 90% when findings from both MRI and CT are combined [
52].
While MRI is more useful in detecting pancreatic tumors smaller than 2 cm in size, CT is useful in identifying tumor metastasis, but also in detecting a potential association of neuroendocrine neoplasms with non-neuroendocrine neoplasms such as acinar cell carcinoma, adeno-carcinoma, squamous cell carcinoma, an association known as Mixed neuroendocrine/non-neuroendocrine neoplasm (MiNEN) [
11,
15,
51,
52,
53].
However, EUS had higher sensitivity (40-92.6% depending on the location of the tumor) than CT and MRI because of its ability to detect small I-omas, even infracentimetric tumors [
54,
55,
56,
57].
The immunohistochemistry feature is vital for diagnosis, through Chromogranin A, Synaptophysin, insulin and ki67 index [
58,
59]. Islet-1 combined with CDX2 expression is positive in approximately 90% of cases [
60].
Referring to the therapeutic possibilities, there are supportive measures (dietary – glucose rich meals or nocturnal tube feeding for a superior control of hypoglycemia episodes), medical treatment (diazoxide for blocking the ATP-dependent kalium channels of the pancreatic β cells, combined with a thiazide diuretic for fluid retention prevention; somatostatin receptor ligands (SRLs) and sometimes glucocorticoids [
4,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71]. A lot of drugs are used for metastatic or inoperable aggressive forms of I-oma, like SRLs peptide receptor radionuclide therapy, m-TOR (Everolimus) and tyrosine kinase (Sunitinib) inhibitors and also cytotoxic chemotherapy [
4,
61,
66,
72,
73,
74,
75,
76,
77,
78]. For non-functioning P-NETs, is recommended a conservative follow-up therapy [
4,
79].
For reduced dimensions I-oma, endoscopic or surgery (i.e. pancreaticoduodenectomy, debulcking) methods are recommended, with specific peculiarities – depending of grading and functioning or not functioning type [
4,
66,
80,
81,
82]. In liver metastases from I-oma (which could be up to 78% of cases) are recommended surgery resection or methods of embolization or radioablation [
4,
61,
66,
78,
83].
Complications of I-oma are described after surgery (general complication which can appear after an intervention and subphrenic abscess, pancreatic fistula, acute pancreatitis) and in correlation with neuroendocrine tumor disease, like carcinoid crisis and tumor necrosis [
84,
85]. Although, I-oma present a favorable prognosis, surveillance is classic correlated with tumor dimension (≥2cm), metastases, ki-67 proliferation index and age [
58,
86].
5. Conclusions
The presented case highlights the pitfalls in the interpretation of the signs and symptoms of hypoglycemia, especially in the case of people without diabetes, and the need to confirm it by determining the glycemic value at the respective moment, followed by an etiological diagnosis as early as possible in order to correct the underlying cause.
Thus, the management of patients with hyperinsulinemic hypoglycemia secondary to insulinoma involves multidisciplinary collaboration with an important role in recognizing symptoms suggestive of hypoglycemia in a person without diabetes, initiating biological and imaging evaluation, in order to classify, stage the disease and confirm the etiology of endogenous hyperinsulinemic hypoglycemia, establishing the optimal therapeutic option and histopathological confirmation.
The identification of P-NET as the cause of severe hypoglycemia requires urgent therapeutic intervention for the remission of hypoglycemic episodes and their consequences and to increase the patients' quality of life.
Author Contributions
S.G.P.: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. A.L.G.: Funding acquisition, Writing – original draft, Writing – review & editing. C.F.M, A.N.S., R.C.: Data curation, Writing – original draft, Writing – review & editing. C.V., M.M.: Conceptualization, Writing – original draft, Writing – review & editing. A.M.: Funding acquisition, Writing – original draft, Writing – review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: The APC was funded by University of Medicine and Pharmacy of Craiova, Romania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Commission of the Emergency County Clinical Hospital Craiova, Romania (Report no 35510/13.08.2024).” for studies involving humans.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.; Basile, M.L.; Kuga, F.S.; Próspero, J.D.; Paes, R.A.P.; Bernardi, F.D.C. Neuroendocrine tumors: An epidemiological study of 250 cases at a tertiary hospital. Rev Assoc Med Bras (1992) 2017, 63, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Q.; Kar, J.; Verma, A.; Sanghvi, S.; Kaka, N.; Patel, N.; Sethi, Y.; Chopra, H.; Kamal, M.A.; Greig, N.H. A Comprehensive Review on Neuroendocrine Neoplasms: Presentation, Pathophysiology and Management. J Clin Med 2023, 12, 5138. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.W.; Majeed, M.S.; Kirresh, O. Non-Diabetic Hypoglycemia. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK573079/.
- Mete, T.; Cesur, M. Non-diabetic hypoglycemia. Intercont J Int Med 2023, 1, 94–105. [Google Scholar] [CrossRef]
- Elghobashy, M.; Gama, R.; Sulaiman, R.A. Investigation and Causes of Spontaneous (Non-Diabetic) Hypoglycaemia in Adults: Pitfalls to Avoid. Diagnostics 2023, 13, 3275. [Google Scholar] [CrossRef]
- Vezzosi, D.; Bennet, A.; Fauvel, J.; Caron, P. Insulin, C-peptide and proinsulin for the biochemical diagnosis of hypoglycaemia related to endogenous hyperinsulinism. Eur J Endocrinol 2007, 157, 75–83, Erratum in Eur J Endocrinol. 2007, 157, 693. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Kloeppel, G. Pancreatic neuroendocrine neoplasias. In: The WHO Classification of Endocrine Tumors. Lyon, France: IARC Press; 2017.
- WHO Classification of Tumours Editorial Board; Digestive System Tumours, WHO Classification of Tumours. 5th ed. Lyon, France: IARC Press; 2019.
- de Herder, W.W.; Niederle, B.; Scoazec, J.Y.; Pauwels, S.; Kloppel, G.; Falconi, M.; Kwekkeboom, D.J.; Oberg, K.; Eriksson, B.; Wiedenmann, B.; Rindi, G.; O’Toole, D.; Ferone, D.; Frascati Consensus Conference; European Neuroendocrine Tumor Society. Well-differentiated pancreatic tumor/carcinoma: Insulinoma. Neuroendocrinology 2006, 84, 183–188. [Google Scholar] [CrossRef]
- Cryer, P.E.; Axelrod, L.; Grossman, A.B.; Heller, S.R.; Montori, V.M.; Seaquist, E.R.; Service, F.J.; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009, 94, 709–728. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; Reed, N.; Kianmanesh, R.; Jensen, R.T.; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Gama, R.; Teale, J.D.; Marks, V. Best practice No 173: Clinical and laboratory investigation of adult spontaneous hypoglycaemia. J Clin Pathol 2003, 56, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Giusti, F.; Brandi, M.L. Genetic disorders and insulinoma/glucagonoma. Endocr Relat Cancer 2024, 31, e230245. [Google Scholar] [CrossRef]
- Martens, P.; Tits, J. Approach to the patient with spontaneous hypoglycemia. Eur J Intern Med 2014, 25, 415–421. [Google Scholar] [CrossRef]
- Zetu, C.; Popa, S.; Golli, A.L.; Condurache, A.; Munteanu, R. Long-term improvement of dyslipidaemia, hyperuricemia and metabolic syndrome in patients undergoing laparoscopic sleeve gastrectomy. Arch Endocrinol Metab 2021, 64, 704–709. [Google Scholar] [CrossRef]
- Karamanolis, N.N.; Kounatidis, D.; Vallianou, N.G.; Alexandropoulos, K.; Kovlakidi, E.; Kaparou, P.; Karampela, I.; Stratigou, T.; Dalamaga, M. Paraneoplastic hypoglycemia: An overview for optimal clinical guidance. Metabol Open 2024, 23, 100305. [Google Scholar] [CrossRef]
- Rayas, M.S.; Salehi, M. Non-Diabetic Hypoglycemia. [Updated 2024 Jan 27]. In: Feingold KR, Anawalt B, Blackman MR; et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. Available from: https://www.ncbi.nlm.nih.gov/books/NBK355894/.
- Guettier, J.M.; Lungu, A.; Goodling, A.; Cochran, C.; Gorden, P. The role of proinsulin and insulin in the diagnosis of insulinoma: A critical evaluation of the Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013, 98, 4752–4758. [Google Scholar] [CrossRef]
- Ouleghzal, H.; Ziadi, T.; Menfaa, M.; Safi, S. Association of Insulinoma and Type 2 Diabetes Mellitus. Int J Endocrinol Metab 2016, 15, e39439. [Google Scholar] [CrossRef]
- Singbo, J.; Locketz, M.; Ross, I.L. Challenge of coexisting type 2 diabetes mellitus and insulinoma: A case report. J Med Case Rep 2021, 15, 479. [Google Scholar] [CrossRef]
- Queiroz Almeida, M.; Machado, M.C.; Correa-Giannella, M.L.; Giannella-Neto, D.; Albergaria Pereira, M.A. Endogenous hyperinsulinemic hypoglycemia: Diagnostic strategies, predictive features of malignancy and long-term survival. J Endocrinol Invest 2006, 29, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Ro, C.; Chai, W.; Yu, V.E.; Yu, R. Pancreatic neuroendocrine tumors: Biology, diagnosis, and treatment. Chin J Cancer 2013, 32312–32324. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K. Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors). Curr Opin Endocrinol Diabetes Obes 2009, 16, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Antonello, D.; Gobbo, S.; Corbo, V.; Sipos, B.; Lemoine, N.R.; Scarpa, A. Update on the molecular pathogenesis of pancreatic tumors other than common ductal adenocarcinoma. Pancreatology 2009, 9, 25–33. [Google Scholar] [CrossRef]
- Leoncini, E.; Carioli, G.; La Vecchia, C.; Boccia, S.; Rindi, G. Risk factors for neuroendocrine neoplasms: A systematic review and meta-analysis. Ann Oncol 2016, 27, 68–81. [Google Scholar] [CrossRef]
- Hassan, M.M.; Phan, A.; Li, D.; Dagohoy, C.G.; Leary, C.; Yao, J.C. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int J Cancer 2008, 123, 867–873. [Google Scholar] [CrossRef]
- Hassan, M.M.; Phan, A.; Li, D.; Dagohoy, C.G.; Leary, C.; Yao, J.C. Family history of cancer and associated risk of developing neuroendocrine tumors: A case-control study. Cancer Epidemiol Biomarkers Prev. 2008, 17, 959–965. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Bamlet, W.R.; McWilliams, R.R.; Hobday, T.J.; Burch, P.A.; Rabe, K.G.; Petersen, G.M. Risk factors for pancreatic neuroendocrine tumors: A clinic-based case-control study. Pancreas 2014, 43, 1219–1222. [Google Scholar] [CrossRef]
- Cigrovski Berković, M.; Catela Ivković, T.; Marout, J.; Zjačić-Rotkvić, V.; Kapitanović, S. Interleukin 1β gene single-nucleotide polymorphisms and susceptibility to pancreatic neuroendocrine tumors. DNA Cell Biol 2012, 31, 531–536. [Google Scholar] [CrossRef]
- Dinu, I.R.; Popa, S.G.; Moţa, M.; Moţa, E.; Stănciulescu, C.; Ioana, M.; Cruce, M. The association of the rs1049353 polymorphism of the CNR1 gene with hypoadiponectinemia. Rom J Morphol Embryol 2011, 52, 791–795. [Google Scholar]
- Karakaxas, D.; Gazouli, M.; Coker, A.; Agalianos, C.; Papanikolaou, I.S.; Patapis, P.; Liakakos, T.; Dervenis, C. Genetic polymorphisms of inflammatory response gene TNF-α and its influence on sporadic pancreatic neuroendocrine tumors predisposition risk. Med Oncol 2014, 31, 241. [Google Scholar] [CrossRef] [PubMed]
- Hiripi, E.; Bermejo, J.L.; Sundquist, J.; Hemminki, K. Familial gastrointestinal carcinoid tumours and associated cancers. Ann Oncol. 2009, 20, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.X.; Cong, L.; Zhao, Y.P.; Zhang, T.P.; Chen, G. Risk factors for the occurrence of insulinoma: A case-control study. Hepatobiliary Pancreat Dis Int 2013, 12, 324–328. [Google Scholar] [CrossRef]
- Okabayashi, T.; Shima, Y.; Sumiyoshi, T.; Kozuki, A.; Ito, S.; Ogawa, Y.; Kobayashi, M.; Hanazaki, K. Diagnosis and management of insulinoma. World J Gastroenterol 2013, 19, 829–837. [Google Scholar] [CrossRef]
- Tonelli, F.; Giudici, F.; Nesi, G.; Batignani, G.; Brandi, M.L. Operation for insulinomas in multiple endocrine neoplasia type 1: When pancreatoduodenectomy is appropriate. Surgery 2017, 161, 727–734. [Google Scholar] [CrossRef]
- Garg, R.; Memon, S.; Patil, V.; Bandgar, T. Extrapancreatic insulinoma. World J Nucl Med 2020, 19, 162–164. [Google Scholar] [CrossRef]
- Hennings, J.; Garske, U.; Botling, J.; Hellman, P. Malignant insulinoma in ectopic pancreatic tissue. Dig Surg 2005, 22, 377–379. [Google Scholar] [CrossRef]
- Diaz-Sangines, B.P.; Gonzalez-Cofrades, J.; Vazquez-Camacho, E.E.; Malfavon-Farias, M.; Garcia-Lima, L. Insulinoma Management in a Pregnant Woman: A Case Report. Cureus 2023, 15, e34239. [Google Scholar] [CrossRef]
- Dobrindt, E.M.; Mogl, M.; Goretzki, P.E.; Pratschke, J.; Dukaczewska, A.K. Insulinoma in pregnancy (a case presentation and systematic review of the literature). Rare Tumors 2021, 13, 2036361320986647. [Google Scholar] [CrossRef]
- Teixeira, D.; Tavares, R.F.; Rodrigues, A.; Moreira, P. Insulinoma in primary care: A case report. Int Surg J 2023, 10, 1687–1689. [Google Scholar] [CrossRef]
- Nahmias, A.; Grozinsky-Glasberg, S.; Salmon, A.; Gross, D.J. Pancreatic neuroendocrine tumors with transformation to insulinoma: An unusual presentation of a rare disease. Endocrinol Diabetes Metab Case Rep 2015, 150032. [Google Scholar] [CrossRef]
- Buddhavarapu, V.S.; Dhillon, G.; Grewal, H.S.; Soles, B.; Halbur, L.; Surani, S.; Kashyap, R. Transformation of pancreatic nonfunctioning neuroendocrine tumor into metastatic insulinoma: A rare case report. Clin Case Rep 2023, 11, e8152. [Google Scholar] [CrossRef] [PubMed]
- Tarris, G.; Rouland, A.; Guillen, K.; Loffroy, R.; Lariotte, A.C.; Rat, P.; Bouillet, B.; Andrianiaina, H.; Petit, J.M.; Martin, L. Case Report: Giant insulinoma, a very rare tumor causing hypoglycemia. Front Endocrinol (Lausanne) 2023, 14, 1125772. [Google Scholar] [CrossRef] [PubMed]
- Sansone, A.; Lauretta, R.; Vottari, S.; Chiefari, A.; Barnabei, A.; Romanelli, F.; Appetecchia, M. Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors. Cancers (Basel) 2019, 11, 1113. [Google Scholar] [CrossRef] [PubMed]
- Bevere, M.; Masetto, F.; Carazzolo, M.E.; Bettega, A.; Gkountakos, A.; Scarpa, A.; Simbolo, M. An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide. Diagnostics (Basel) 2023, 13, 2820. [Google Scholar] [CrossRef]
- Tsoli, M.; Koumarianou, A.; Angelousi, A.; Kaltsas, G. Established and novel circulating neuroendocrine tumor biomarkers for diagnostic, predictive and prognostic use. Best Pract Res Clin Endocrinol Metab 2023, 37, 101785. [Google Scholar] [CrossRef]
- Matondang, S.; Suwita, B.M.; Budianto, T.; Krisnuhoni, E. Atypical CT and MR imaging of insulinoma: A case report. J Clin Transl Endocrinol Case Reports 2021, 19, 100075. [Google Scholar] [CrossRef]
- Noone, T.C.; Hosey, J.; Firat, Z.; Semelka, R.C. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab 2005, 19, 195–211. [Google Scholar] [CrossRef]
- Uccella, S.; La Rosa, S. Looking into digestive mixed neuroendocrine - nonneuroendocrine neoplasms: Subtypes, prognosis, and predictive factors. Histopathology 2020, 77, 700–717. [Google Scholar] [CrossRef]
- Sotoudehmanesh, R.; Hedayat, A.; Shirazian, N.; Shahraeeni, S.; Ainechi, S.; Zeinali, F.; Kolahdoozan, S. Endoscopic ultrasonography (EUS) in the localization of insulinoma. Endocrine 2007, 31, 238–241. [Google Scholar] [CrossRef]
- Wang, H.; Ba, Y.; Xing, Q.; Du, J.L. Diagnostic value of endoscopic ultrasound for insulinoma localization: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0206099. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Hati, A. Endoscopic ultrasound: A very important tool in detecting small insulinomas. QJM 2022, 115, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, J.; Zhu, J. Diagnostic performance of noninvasive imaging modalities for localization of insulinoma: A meta-analysis. Eur J Radiol 2021, 145, 110016. [Google Scholar] [CrossRef]
- Hackeng, W.M.; Schelhaas, W.; Morsink, F.H.M.; Heidsma, C.M.; van Eeden, S.; Valk, G.D.; Vriens, M.R.; Heaphy, C.M.; Nieveen van Dijkum, E.J.M.; Offerhaus, G.J.A.; Dreijerink, K.M.A.; Brosens, L.A.A. Alternative Lengthening of Telomeres and Differential Expression of Endocrine Transcription Factors Distinguish Metastatic and Non-metastatic Insulinomas. Endocr Pathol 2020, 31, 108–118. [Google Scholar] [CrossRef]
- Gambella, A.; Falco, E.C.; Metovic, J.; Maletta, F.; De Angelis, C.; Maragliano, R.; Uccella, S.; Pacchioni, D.; Papotti, M. Amyloid-Rich Pancreatic Neuroendocrine Tumors: A Potential Diagnostic Pitfall in Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology (EUS-FNAC). Endocr Pathol, 2021, 32, 318–325. [Google Scholar] [CrossRef]
- Graham, R.P.; Shrestha, B.; Caron, B.L.; Smyrk, T.C.; Grogg, K.L.; Lloyd, R.V.; Zhang, L. Islet-1 is a sensitive but not entirely specific marker for pancreatic neuroendocrine neoplasms and their metastases. Am J Surg Pathol 2013, 37, 399–405. [Google Scholar] [CrossRef]
- Hofland, J.; Refardt, J.C.; Feelders, R.A.; Christ, E.; de Herder, W.W. Approach to the Patient: Insulinoma. J Clin Endocrinol Metab 2024, 109, 1109–1118. [Google Scholar] [CrossRef]
- Aida, A.; Noto, H. Diagnosis and Treatment Course of Insulinoma Presenting as Hypoglycemia Unawareness Using a Factory-Calibrated Continuous Glucose Monitoring System. Am J Case Rep 2022, 23, e936723. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Yamada, E.; Matsumoto, S.; Nakajima, Y.; Nobusawa, S.; Yokoo, H.; Sekiguchi, S.; Yoshino, S.; Horiguchi, K.; Ishida, E.; Okada, S.; Yamada, M. A case of insulinoma-induced hypoglycemia managed by Dexcom G4 Platinum. Neuro Endocrinol Lett 2022, 43, 161–166. [Google Scholar]
- Yuan, T.; Liu, S.; Zhu, C.; Dong, Y.; Zhu, H.; Wu, X.; Tang, Y.; Zhao, W. Continuous Glucose Monitoring in Patients With Insulinoma Treated by Endoscopic Ultrasound-Guided Ethanol Injection. Pancreas 2021, 50, 183–188. [Google Scholar] [CrossRef]
- Kawasaki, A.; Suzuki, K.; Miyamoto, M.; Miyamoto, T.; Yanagi, K.; Shimizu, M.; Hirata, K. Disruptive nocturnal behavior due to insulinoma revealed by continuous glucose monitoring. Eur J Neurol 2014, 21, e46–e47. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Watkin, D.; Evans, J.; Yip, V.; Cuthbertson, D.J. Multidisciplinary management of refractory insulinomas. Clin Endocrinol (Oxf) 2018, 88, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Altszuler, N.; Moraru, E.; Hampshire, J. On the mechanism of diazoxide-induced hyperglycemia. Diabetes 1977, 26, 931–935. [Google Scholar] [CrossRef]
- Warren, A.M.; Topliss, D.J.; Hamblin, P.S. Successful medical management of insulinoma with diazoxide for 27 years. Endocrinol Diabetes Metab Case Rep 2020, 20–0132. [Google Scholar] [CrossRef]
- Gill, G.V.; Rauf, O.; MacFarlane, I.A. Diazoxide treatment for insulinoma: A national UK survey. Postgrad Med J 1997, 73, 640–641. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsas, G.A.; Grozinsky-Glasberg, S. Emerging therapies for advanced insulinomas and glucagonomas. Endocr Relat Cancer 2023, 30, e230020. [Google Scholar] [CrossRef]
- Blum, I.; Aderka, D.; Doron, M.; Laron, Z. Suppression of hypoglycemia by DL-propranolol in malignant insulinoma. N Engl J Med 1978, 299, 487. [Google Scholar] [CrossRef]
- Hofland, J.; Falconi, M.; Christ, E.; Castaño, J.P.; Faggiano, A.; Lamarca, A.; Perren, A.; Petrucci, S.; Prasad, V.; Ruszniewski, P.; Thirlwell, C.; Vullierme, M.P.; Welin, S.; Bartsch, D.K. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J Neuroendocrinol 2023, 35, e13318. [Google Scholar] [CrossRef]
- Hofland, J.; Kaltsas, G.; de Herder, W.W. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr Rev 2020, 41, 371–403. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res 2017, 23, 4617–4624. [Google Scholar] [CrossRef]
- Hofland, J.; Brabander, T.; Verburg, F.A.; Feelders, R.A.; de Herder, W.W. Peptide Receptor Radionuclide Therapy. J Clin Endocrinol Metab 2022, 107, 3199–3208. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; Tomassetti, P.; Pavel, M.E.; Hoosen, S.; Haas, T.; Lincy, J.; Lebwohl, D.; Öberg, K.; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; Chen, J.S.; Hörsch, D.; Hammel, P.; Wiedenmann, B.; Van Cutsem, E.; Patyna, S.; Lu, D.R.; Blanckmeister, C.; Chao, R.; Ruszniewski, P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Rinke, A.; Valle, J.W.; Fazio, N.; Caplin, M.; Gorbounova, V.; OConnor, J.; Eriksson, B.; Sorbye, H.; Kulke, M.; Chen, J.; Falkerby, J.; Costa, F.; de Herder, W.; Lombard-Bohas, C.; Pavel, M.; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms. Systemic Therapy 2: Chemotherapy. Neuroendocrinology 2017, 105, 281–294. [Google Scholar] [CrossRef]
- Castellano, D.; Bajetta, E.; Panneerselvam, A.; Saletan, S.; Kocha, W.; O'Dorisio, T.; Anthony, L.B.; Hobday, T.; RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: A subgroup analysis of the phase III RADIANT-2 study. Oncologist 2013, 18, 46–53. [Google Scholar] [CrossRef]
- Howe, J.R.; Merchant, N.B.; Conrad, C.; Keutgen, X.M.; Hallet, J.; Drebin, J.A.; Minter, R.M.; Lairmore, T.C.; Tseng, J.F.; Zeh, H.J.; Libutti, S.K.; Singh, G.; Lee, J.E.; Hope, T.A.; Kim, M.K.; Menda, Y.; Halfdanarson, T.R.; Chan, J.A.; Pommier, R.F. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 1–33. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O'Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; Singh, S.; Salem, R.; Metz, D.C.; Naraev, B.G.; Reidy-Lagunes, D.L.; Howe, J.R.; Pommier, R.F.; Menda, Y.; Chan, J.A. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Sada, A.; McKenzie, T.J.; Vella, A.; Levy, M.J.; Halfdanarson, T.R. Interventional vs surgical procedures in localized/nonmetastatic insulinomas (ablation vs surgery). Endocr Relat Cancer 2023, 30, e220362. [Google Scholar] [CrossRef]
- Habibollahi, P.; Bai, H.X.; Sanampudi, S.; Soulen, M.C.; Dagli, M. Effectiveness of Liver-Directed Therapy for the Management of Intractable Hypoglycemia in Metastatic Insulinoma. Pancreas 2020, 49, 763–767. [Google Scholar] [CrossRef]
- Sakin, A.; Tambas, M.; Secmeler, S.; Can, O.; Arici, S.; Yasar, N.; Geredeli, C.; Demir, C.; Cihan, S. Factors Affecting Survival in Neuroendocrine Tumors: A 15-Year Single Center Experience. Asian Pac J Cancer Prev 2018, 19, 3597–3603. [Google Scholar] [CrossRef]
- Hoskovec, D.; Krška, Z.; Škrha, J.; Klobušický, P.; Dytrych, P. Diagnosis and Surgical Management of Insulinomas-A 23-Year Single-Center Experience. Medicina (Kaunas) 2023, 59, 1423. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.P.R.; Martin, A.J.; Kim, M.K. Neuroendocrine Tumors. Mount Sinai Expert Guides: Oncology, First Edition. 2019 Aug 30. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).