1. Background
Pregnancy complicated by type 1 diabetes has always been a therapeutic and management challenge, with neonatal and maternal outcomes still not comparable to those in the non-diabetic population. Glycaemic control is the most important variable associated with good or poor pregnancy outcomes (1). As a result, the American Diabetes Association (ADA) recommends strict glycaemic targets for pregnant women with type 1 diabetes including an A1C target of <6.5% without significant hypoglycaemia and self-monitoring of both fasting and postprandial blood glucose (SMBG) using an insulin pump or basal bolus insulin (MDI) (2). The use of technology in the management of diabetes is now common practice, and the use of new tools in pregnancy is becoming increasingly necessary. There is strong evidence from the CONCEPT study that MDI plus CGM users compared to pump plus CGM users were more likely to achieve targets A1c and have increase TIR by 24 weeks gestation (3). The international consensus for CGM-derived glycaemic targets states that pregnant women with type 1 diabetes should have >70% ps-TIR (63-140 mg/dL), <25% of time above range (>140 mg/dL) and 5% of time below range (<4% of time <63 mg/dL and <1% of time <54 mg/dL) (4). The strict glycaemic targets required in pregnancy are difficult to achieve (5). The InPenTM is claimed to be the first FDA-cleared and CE-marked smart insulin pen that integrates with real-time CGM via a convenient smartphone app (6). The use of InPen TM paired with CGM (Guardian G4-InPen TM system) during pregnancy has not been reported previously.
2. Research design and methods
We have retrospectively collected the demographics, medical characteristics and pregnancy outcomes of 4 women with type 1 diabetes who were managed with an InPen
TM system during pregnancy. The system includes a reusable pen compatible with insulin lispro and insulin aspart rapid-acting insulin cartridges (7). After the insulin cartridge is installed the device sends real-time data via Bluetooth to an InPen
TM application (app) available. The InPen
TM has additional features such as bolus dose calculator, detection of prime dose versus actual dose, reminder alerts, active insulin on board (IOB) monitoring, and integration with CGM (8). All patients were managed by a team of physicians, dietitians and gynaecologists with expertise in type 1 diabetes management. The study was approved by the institutional ethics committee (ID 5378 –5378_16.10.2024_P) and complied with the Declaration of Helsinki and good clinical practice guidelines. All participants provided written informed consent. HbA1c, continuous glucose monitoring data with recommended pregnancy-specified target ranges, the insulin: carbohydrate ratios and insulin sensitivity factors during each trimester of pregnancy are summarized in table (
Table 1).
Case 1
This is the second pregnancy of a 30-year-old woman with type 1 diabetes since the age of 6. She had non-proliferative diabetic retinopathy since 2011. In the 2018, the patient had tried continuous subcutaneous insulin infusion (CSII) therapy which was discontinued due to discomfort. The first pregnancy was in 2019 and resulted in a dystocic birth at 37 weeks gestation due to altered cardiotocographic tracing, a 3975 g male. During the first pregnancy, the patient was on controlled therapy with MDI and intermittently scanned continuous glucose monitoring (isCGM), and the pregnancy described in this report was unplanned. Prior to conception, she had an A1C of 6.7% and a pre-pregnancy weight of 70 kg (BMI 23.4 kg/m
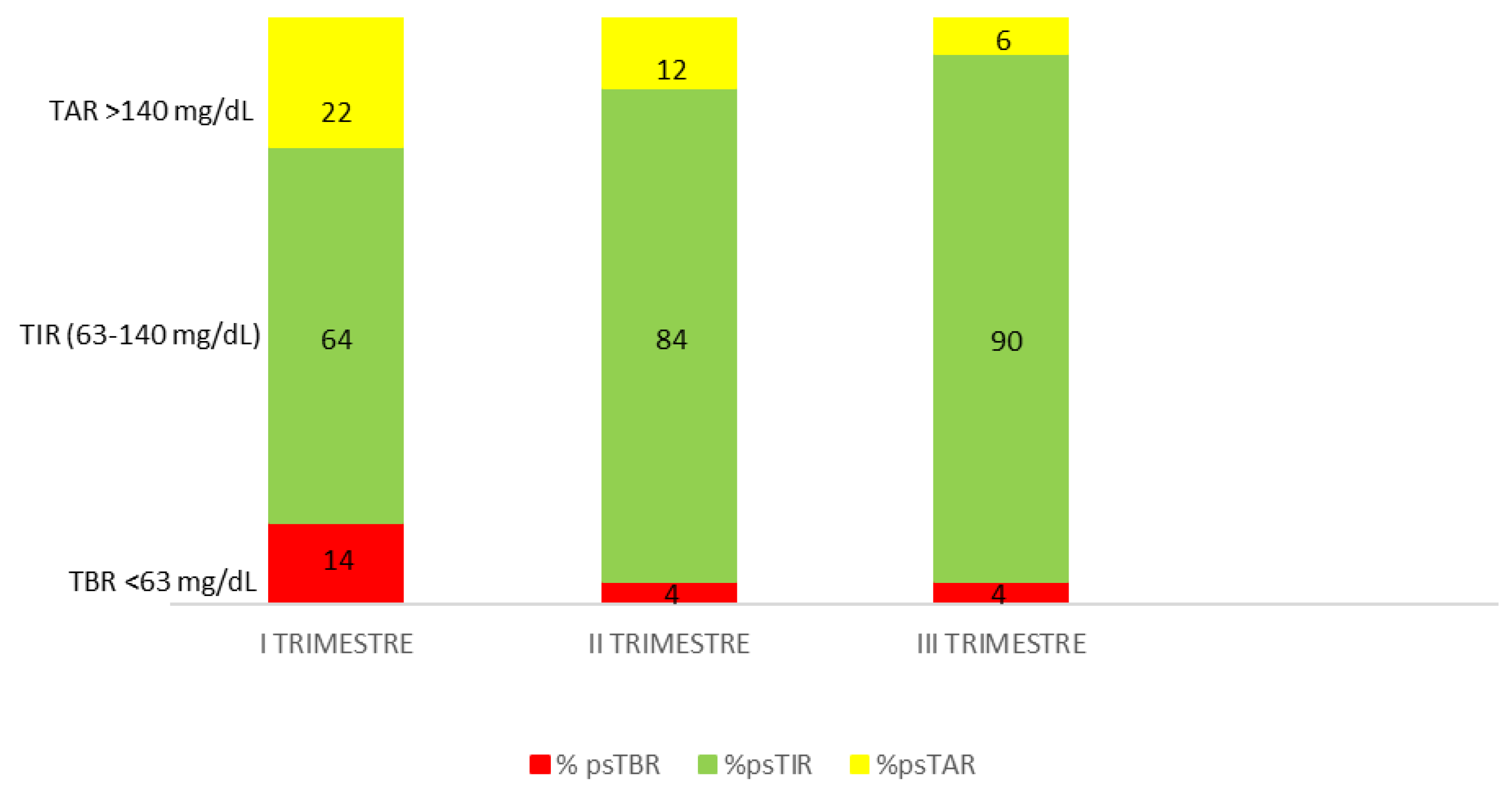
2). She was treated with MDI: insulin aspart with an ins/cho ratio (ICR) of 1/8 at breakfast, 1/7.5 at lunch and 1/10 at dinner before each meal plus insulin glargine at a dose of 9 units nightly at bedtime. She was still monitoring her glucose levels with both SMBG and isCGM. In the month prior to conception, her overall mean sensor glucose was 134 mg/dL with a coefficient of variation (CV) of 41%, indicating unhealthy glycaemic control. The patient was referred to our Centre at 13 weeks' gestation. In the first trimester of pregnancy her isCGM readings were: ps-TIR 68% of the time, ps-TBR <63 mg/dL 6% of the time and <54 mg/dL 2% of the time and ps-TAR 24% of the time. At 15 weeks' gestation, therapy was switched from MDI and isCGM to the InPen
TM integrated with the Guardian 4 CGM system. Pregnancy-specified target and duration of insulin action (IOB) were set up (2.5 hours). The ICR and the insulin sensitivity factors used to calculate dose recommendations were also specified in the system. PS-TIR increases to 74% in the first 2 weeks of InPen
TM use. She noticed an increase in her insulin requirements, with postprandial hyperglycaemia (both morning and afternoon), and the ICR was reduced to 6.5 g in the morning and 5.5 g in the afternoon for more bolus insulin. At 27 weeks' gestation she achieved ps-TIR over 89%, ps-TAR 7%, ps-TBR 4% and GMI 5.9 % without severe hypoglycemia. Time above range of 19% noted during the second trimester was decreased to 7% in the third trimester. The total daily insulin dose was adjusted during the pregnancy. Good control with time below range <5% was maintained in the third trimester (
Figure 1). At the end of pregnancy her gestational weight gain was 14 Kg. The patient gave birth to a male infant at 38 weeks' gestation with a weight of 3980 g and a length of 53 cm (Apgar at 1’ e 5’, 9 and 10 respectively). His size was large for gestational age (LGA) with no congenital malformations; the infant required temporary monitoring for hyperbilirubinemia 3 days after birth which resolved with phototherapy. At 4 weeks postpartum, the infant was healthy and the mother had maintained stable glycaemic control (mean glucose level 115 ± 49 mg/dl, CV 43.0%, GMI 6.1%).
Case 2
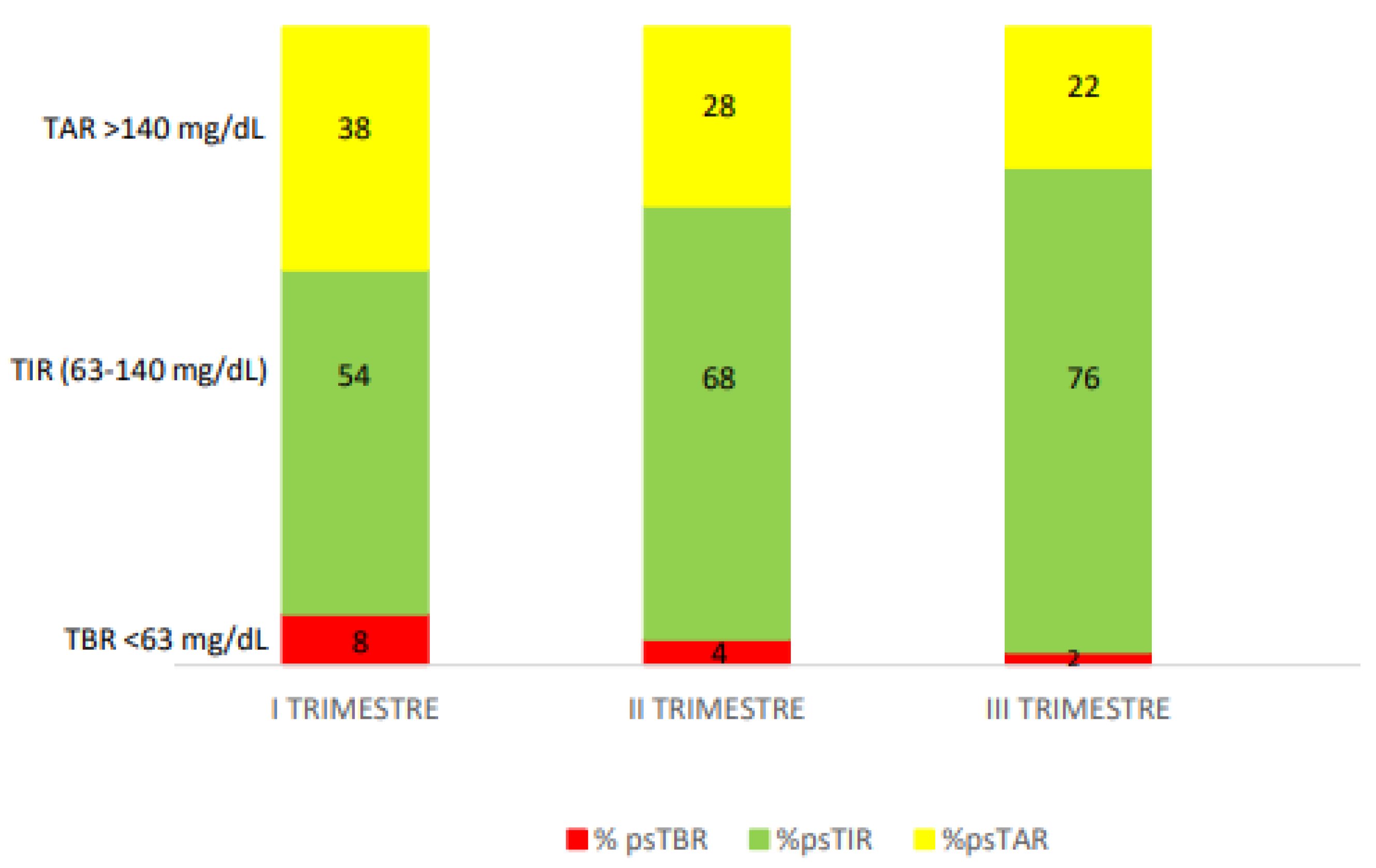
A 36-year-old woman, presented at 8 weeks' gestation in January 2024. She had type 1 diabetes since 2003 and Hashimoto's thyroiditis since 2010. She was treated with MDI and an intermittently scanned glucose meter (isCGM). She did not plan the pregnancy, she was on MDI: insulin aspart 2 units at breakfast, 3 units at lunch and 4 units at dinner and insulin glargine U300 10 units at bedtime. She has no knowledge of CHO counting. Her pre-pregnancy weight was 60 kg, her pre-pregnancy BMI was 21.5 kg/m2, and her HbA1c a month prior to conception was 6.8%. In the first trimester of pregnancy her isCGM readings were: ps-TIR 64% of the time, ps-TBR <63 mg/dL 10% of the time and <54 mg/dL 4% of the time and ps-TAR 22% of the time. At 10 weeks' gestation, therapy was upgraded with InPen
TM integrated with the Guardian 4 CGM system. Pregnancy-specified target and duration of insulin action (IOB) were set up (2.5 hours). She was not able to apply CHO counting method so the dose calculator of the system mobile app was set with fixed doses. Dose calculator of InPen
TM system helped patient in adjusting insulin doses at meals and correct hyperglycaemia, avoiding hypoglycaemia due to excessive correction boluses. At 28 weeks' gestation she achieved ps-TIR over 84%, ps-TAR 12%, ps-TBR 4% and GMI 5.6% with a reduction in hypoglycemic events. The total daily insulin dose was adjusted during the pregnancy. At 37 weeks’ her HbA1c was 5.4% ps-TIR 90%, ps-TAR 6% and ps-TBR 2 + 2% with a overall mean sensor glucose 97 mg/dl and CV 24.5% (
Figure 2). The total daily dose was 29.7 units (15.6 units of total rapid insulin dose and 14.1 units of insulin glargine). At the end of pregnancy her gestational weight gain was 10.5 Kg. The patient gave birth to a female infant at 39+1 weeks' gestation with a weight of 3480 g and a length of 51 cm, appropriated for gestational age (AGA) with no perinatal complications.
Case 3
34-year-old female patient, followed up in another centre, with type 1 diabetes since the age of 14, with a low level of knowledge about the disease. She has been addressed at our facility at 8 weeks gestation in an unplanned pregnancy. She is treated with MDI and isCGM sensor and has always refused insulin pump therapy, with a pre-pregnancy poor metabolic compensation (HbA1c 7.3%) with a weight of 55.5 kg and a pre-pregnancy BMI of 18.7 kg/m2. The patient was treated with rapid-acting insulin analogue glulisine, which was immediately switched to insulin lispro. It was recommended to switch to InPen
TM to simplify the management of insulin therapy and to integrate glucose monitoring data to make it easier for patient to manage her therapy. The total daily dose was 45.8 units distributed 3.5 at breakfast, 1.5 at snack, 8 units at lunch and 8 units at dinner, with 22 units of insulin glargine U100. Pregnancy-specified target and duration of insulin action (IOB) were set up (2.0 hours). She was not able to apply CHO counting method so the dose calculator of the system mobile app was set with fixed doses. Due to low compliance, at the second trimester the time of sensor use was not optimal and ps-TIR was not sufficiently low: 68% and ps-TAR 28%, nonetheless the use of CGM helped in reducing ps-TBR 2 +2%. Dose calculator of InPen
TM system helped patient in adjusting insulin doses at meals and correct hyperglycaemia, avoiding hypoglycaemia due to excessive correction boluses. Sensor glucose profiles helped the patient to promptly identify post-prandial hyperglycaemia dose reminders using the InPen
TM app. Pregnancy specific TIR increased from 68% in the second trimester to 76% in third trimester and CV reduced from 36.3% in first trimester to 30.4% in third trimester (
Figure 3). Especially at third trimester we assisted at an increasing need for rapid acting insulin, due to insulin resistance for pregnancy. The patient attended regular consultations with our dietician to ensure adequate CHO consumption and to limit foods with a high glycaemic index. At the end of the pregnancy, the weight gain was 12 kg since the beginning. At 37 weeks of pregnancy patient was admitted for polyhydramnios (amniotic fluid index 26), a caesarean section was performed and the patient gave birth to a male infant weight of 3680 g and a length of 52 cm, his size was large for gestational age (LGA). At 4 weeks postpartum, the mother had a stable glycaemic control.
Discussion
Efficacy comparisons between new generation systems of technologies versus traditional systems are beginning to provide some interesting data even in pregnancy complicated by type 1 diabetes. Sometimes perceived complexity and patient/provider preference don't allow the use of infuse systems as CSII or HCL. The ADA includes connected insulin pens in the Medical Standards of Care recommendations for insulin therapy as a solution to potentially help patients on injection therapy with dose recall, dose recommendation and dose titration. The ADA note there is no “one-size-fits-all” approach to technology use in diabetes care, and individuals should be supported in technology options that best match their circumstances, desires and needs (2). Therefore, smart insulin pens may have the potential to improve adherence and achieve glycaemic targets in type 1 diabetic pregnancies, which are important for maternal and fetal outcomes. This system uses user-defined settings: personalised insulin to carbohydrate ratios, including duration of insulin action, insulin sensitivity factors and pre-set target glucose range for pregnancy. The ability to automatically process these calculations and the duration of insulin action (IOB) is an extremely useful benefit previously only available to patients using an insulin pump. In addition, the system offers reminders to check blood glucose, missed dose and long-acting insulin dose reminders. Integrated rt-CGM data can provide unuseful overview for healthcare professionals and patients using this system. InPen TM system during unplanned pregnancy with adverse obstetrical or neonatal outcomes can help in optimising glucose control and reducing hypoglycaemia. The bolus calculator of InPen TM system can quickly adapt to the constant changes in rapid-acting insulin that occur during pregnancy. Using the tools provided by the system, the patients were able to manage the patterns of low and high blood glucose and adjust their insulin requirements throughout their pregnancy without severe hypoglycaemia or chetoacidosis. Our patients achieved and exceeded the recommended 70% time in pregnancy glucose range between the second and third trimester of pregnancy. In our experience this new approach can be useful in the management of pregnancies complicated by type 1 diabetes in MDI, thanks to the system's features that add useful tools for optimising insulin therapy in a complex condition such as pregnancy. In conclusion, this brief report reported that the use of this new technological tool in pregnancy complicated by type 1 diabetes must be taken into account considered expecially when planning pregnancy in order to be effective in really improving the outcomes.
Author Contributions
Conceptualization, V.R and A.G.; methodology, V.R.; validation, V.R., A.G. and V.G.; formal analysis, A.C.; resources, E.O.; data curation, Y.P and A.C.; writing—original draft preparation, V.R and A.G.; writing—review and editing, V.R, A.G. and V.G.; supervision, E.O .; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Comitato Etico Territoriale Lombardia 3 (ID 5378 –5378_16.10.2024_P) on 8 August 2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are available within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Inkster ME, Fahey TP, Donnan PT, Leese GP, Mires GJ, Murphy DJ. Poor glycated haemoglobin control and adverse pregnancy outcomes in type 1 and type 2 diabetes mellitus: systematic review of observational studies. BMC Pregnancy Childbirth. 2006 Oct 30;6:30. [CrossRef] [PubMed] [PubMed Central]
- American Diabetes Association American Diabetes Association Diabetes Care. 2023;46(Supplement_1):S1-S284. https://diabetesjournals.org/care/issue/46/Supplement_1.
- Feig DS, Donovan LE, Corcoy R CONCEPTT Collaborative Group. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017 Nov 25;390(10110):2346. [CrossRef] [PubMed] [PubMed Central]
- Battelino T, Danne T, Bergenstal RM Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019 Aug;42(8):1593-1603. [CrossRef] [PubMed]
- Katrien Benhalima, Kaat Beunen, Sarah E Siegelaar, Management of type 1 diabetes in pregnancy: update on lifestyle, pharmacological treatment, and novel technologies for achieving glycaemic targets, The Lancet Diabetes & Endocrinology, Volume 11, Issue 7,2023,Pages 490-508. [CrossRef]
- Food and Drug Administration 2016: https://www.accessdata.fda.gov/cdrh_docs/pdf16/k160629.pdf.
- MacLeod J, Vigersky RA. A Review of Precision Insulin Management With Smart Insulin Pens: Opening Up the Digital Door to People on Insulin Injection Therapy. Journal of Diabetes Science and Technology 2023, 17, 283–289. [CrossRef]
- Bailey TS, Stone JY. A novel pen-based Bluetooth-enabled insulin delivery system with insulin dose tracking and advice. Expert Opin Drug Deliv. 2017 May;14(5):697-703. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).