Submitted:
06 December 2024
Posted:
06 December 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Study Population
Clinical Data Collection and Biological Measurements
Prospective Follow-Up
Statistical Analyses
Results
Baseline Characteristics
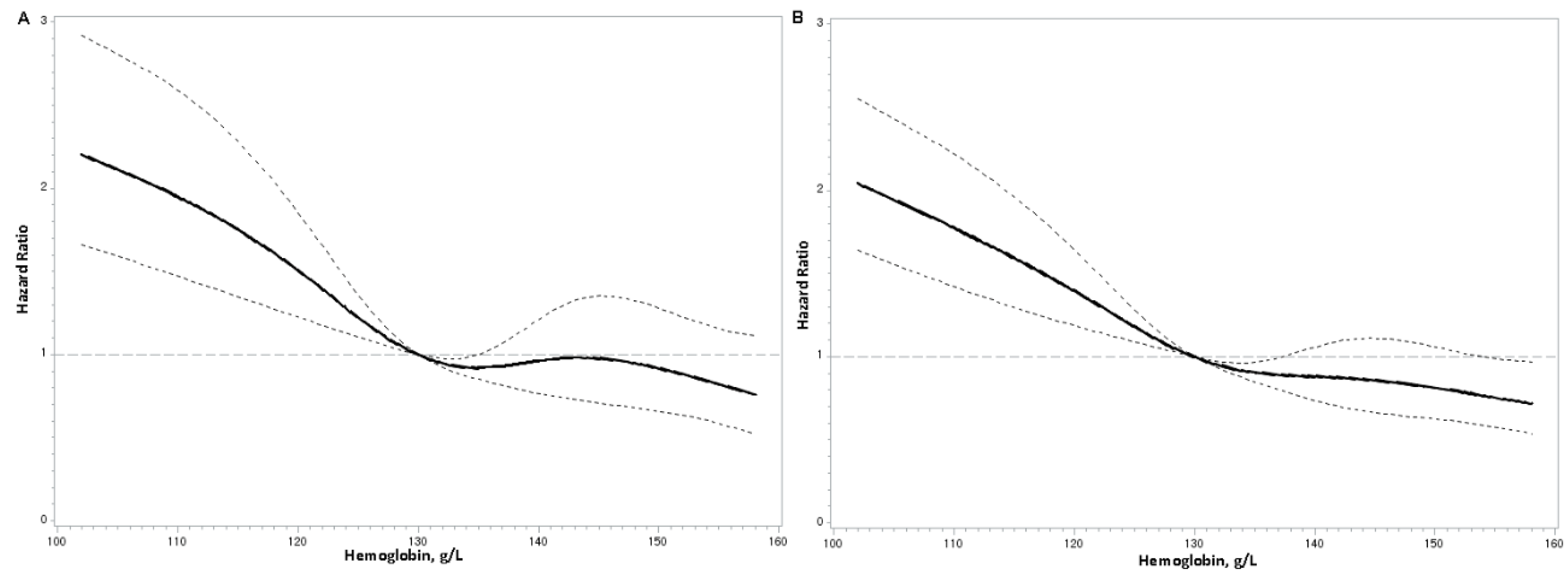
Hemoglobin Concentrations and Mortality Risks
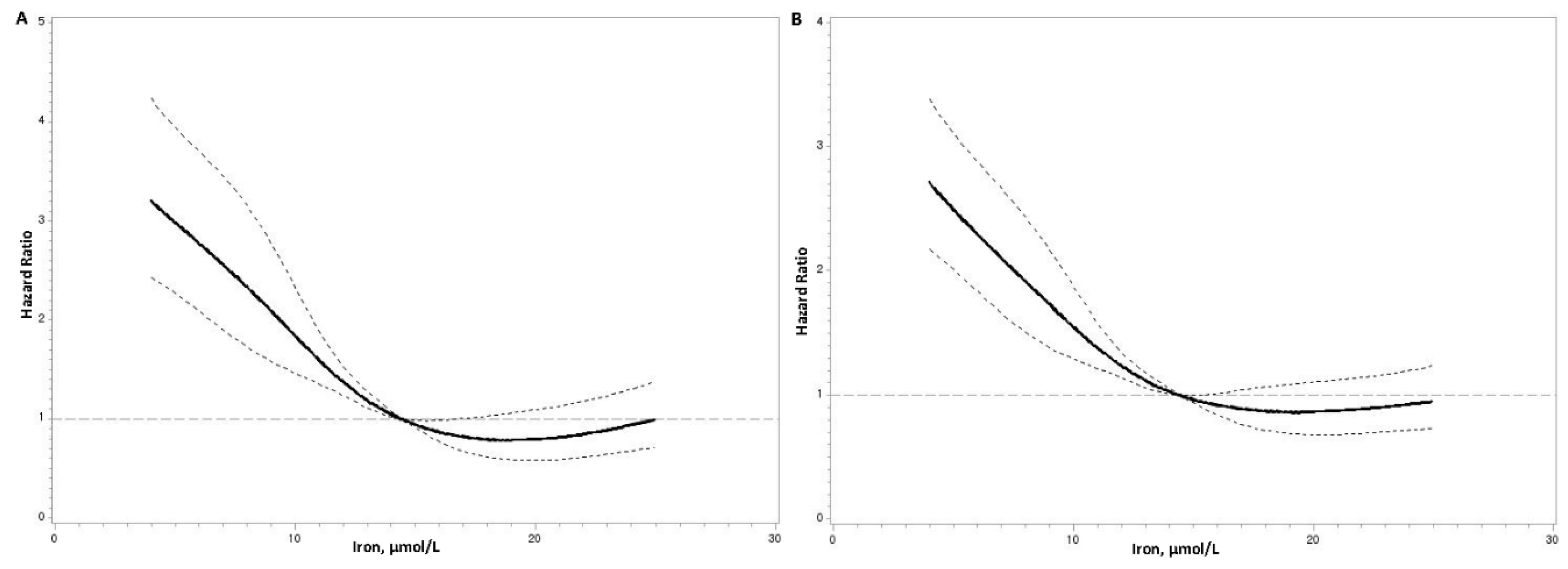
Serum Iron Concentrations and Mortality Risks
Serum Ferritin and Mortality Risks
Serum Transferrin and Mortality Risks
TIBC and Mortality Risks
Transferrin saturation and Mortality Risks
Discussion
Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CVD | Cardiovascular disease |
| CRP | C-reactive protein |
| eGFR | Estimated glomerular filtration rate |
| FPG | Fasting plasma glucose |
| HDL-C | High-density lipoprotein cholesterol |
| HRs | Hazard ratios |
| LDL-C | Low-density lipoprotein cholesterol |
| SBP | Systolic blood pressure |
| SD | standard deviation |
| TC | Total cholesterol |
| TG | Total triglycerides |
| TIBC | Total iron-binding capacity |
References
- Kobayashi, M.; Suhara, T.; Baba, Y.; Kawasaki, N.K.; Higa, J.K.; Matsui, T. Pathological Roles of Iron in Cardiovascular Disease. Curr. Drug Targets 2018, 19, 1068–1076. [CrossRef]
- Bi, Y.; Ajoolabady, A.; Demillard, L.J.; Yu, W.; Hilaire, M.L.; Zhang, Y.; Ren, J. Dysregulation of iron metabolism in cardiovascular diseases: From iron deficiency to iron overload. Biochem. Pharmacol. 2021, 190, 114661. [CrossRef]
- Semenova, Y.; Bjørklund, G.; Butnariu, M.; Peana, M. Iron-related Biomarkers in the Diagnosis and Management of Iron Disorders. Curr. Med. Chem. 2024. [CrossRef]
- Sullivan, J.L. Iron and the sex difference in heart disease risk. Lancet 1981, 1, 1293–1294. [CrossRef]
- Corti, M.-C.; Guralnik, J.M.; Salive, M.E.; Ferrucci, L.; Pahor, M.; Wallace, R.B.; Hennekens, C.H. Serum Iron Level, Coronary Artery Disease, and All-Cause Mortality in Older Men and Women. The American Journal of Cardiology 1997, 79, 120–127. [CrossRef]
- Grammer, T.B.; Kleber, M.E.; Silbernagel, G.; Pilz, S.; Scharnagl, H.; Tomaschitz, A.; König, W.; März, W. Hemoglobin, iron metabolism and angiographic coronary artery disease (The Ludwigshafen Risk and Cardiovascular Health Study). Atherosclerosis 2014, 236, 292–300. [CrossRef]
- Parikh, A.; Natarajan, S.; Lipsitz, S.R.; Katz, S.D. Iron deficiency in community-dwelling US adults with self-reported heart failure in the National Health and Nutrition Examination Survey III: prevalence and associations with anemia and inflammation. Circ. Heart Fail. 2011, 4, 599–606. [CrossRef]
- Bagheri, B.; Shokrzadeh, M.; Mokhberi, V.; Azizi, S.; Khalilian, A.; Akbari, N.; Habibi, V.; Yousefnejad, K.; Tabiban, S.; Nabati, M. Association between Serum Iron and the Severity of Coronary Artery Disease. Int. Cardiovasc. Res. J. 2013, 7, 95–98.
- Salonen, J.T.; Nyyssönen, K.; Korpela, H.; Tuomilehto, J.; Seppänen, R.; Salonen, R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 1992, 86, 803–811. [CrossRef]
- Magnusson, M.K.; Sigfusson, N.; Sigvaldason, H.; Johannesson, G.M.; Magnusson, S.; Thorgeirsson, G. Low iron-binding capacity as a risk factor for myocardial infarction. Circulation 1994, 89, 102–108. [CrossRef]
- Danesh, J.; Appleby, P. Coronary heart disease and iron status: meta-analyses of prospective studies. Circulation 1999, 99, 852–854. [CrossRef]
- Cleland, J.G.F.; Zhang, J.; Pellicori, P.; Dicken, B.; Dierckx, R.; Shoaib, A.; Wong, K.; Rigby, A.; Goode, K.; Clark, A.L. Prevalence and Outcomes of Anemia and Hematinic Deficiencies in Patients With Chronic Heart Failure. JAMA Cardiol. 2016, 1, 539–547. [CrossRef]
- Paterek, A.; Mackiewicz, U.; Mączewski, M. Iron and the heart: A paradigm shift from systemic to cardiomyocyte abnormalities. J. Cell. Physiol. 2019, 234, 21613–21629. [CrossRef]
- Shah, S.V.; Alam, M.G. Role of iron in atherosclerosis. Am. J. Kidney Dis. 2003, 41, S80-3. [CrossRef]
- Tuomainen, T.-P.; Diczfalusy, U.; Kaikkonen, J.; Nyyssönen, K.; Salonen, J.T. Serum ferritin concentration is associated with plasma levels of cholesterol oxidation products in man. Free Radical Biology and Medicine 2003, 35, 922–928. [CrossRef]
- Chen, Q.; Li, Q.; Li, D.; Chen, X.; Liu, Z.; Hu, G.; Wang, J.; Ling, W. Association between liver fibrosis scores and the risk of mortality among patients with coronary artery disease. Atherosclerosis 2020, 299, 45–52. [CrossRef]
- Liu, Y.; Clarke, R.; Bennett, D.A.; Zong, G.; Gan, W. Iron Status and Risk of Heart Disease, Stroke, and Diabetes: A Mendelian Randomization Study in European Adults. J. Am. Heart Assoc. 2024, 13, e031732. [CrossRef]
- Sabatine, M.S.; Morrow, D.A.; Giugliano, R.P.; Burton, P.B.J.; Murphy, S.A.; McCabe, C.H.; Gibson, C.M.; Braunwald, E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 2005, 111, 2042–2049. [CrossRef]
- Lee, G.; Choi, S.; Kim, K.; Yun, J.-M.; Son, J.S.; Jeong, S.-M.; Kim, S.M.; Park, S.M. Association of Hemoglobin Concentration and Its Change With Cardiovascular and All-Cause Mortality. J. Am. Heart Assoc. 2018, 7. [CrossRef]
- Grammer, T.B.; Scharnagl, H.; Dressel, A.; Kleber, M.E.; Silbernagel, G.; Pilz, S.; Tomaschitz, A.; Koenig, W.; Mueller-Myhsok, B.; März, W.; et al. Iron Metabolism, Hepcidin, and Mortality (the Ludwigshafen Risk and Cardiovascular Health Study). Clin. Chem. 2019, 65, 849–861. [CrossRef]
- Gill, D.; Del Greco M, F.; Walker, A.P.; Srai, S.K.S.; Laffan, M.A.; Minelli, C. The Effect of Iron Status on Risk of Coronary Artery Disease: A Mendelian Randomization Study-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1788–1792. [CrossRef]
- Jankowska, E.A.; Wojtas, K.; Kasztura, M.; Mazur, G.; Butrym, A.; Kalicinska, E.; Rybinska, I.; Skiba, J.; Haehling, S. von; Doehner, W.; et al. Bone marrow iron depletion is common in patients with coronary artery disease. Int. J. Cardiol. 2015, 182, 517–522. [CrossRef]
- Kiechl, S.; Willeit, J.; Egger, G.; Poewe, W.; Oberhollenzer, F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation 1997, 96, 3300–3307. [CrossRef]
- Guedes, M.; Muenz, D.G.; Zee, J.; Bieber, B.; Stengel, B.; Massy, Z.A.; Mansencal, N.; Wong, M.M.Y.; Charytan, D.M.; Reichel, H.; et al. Serum Biomarkers of Iron Stores Are Associated with Increased Risk of All-Cause Mortality and Cardiovascular Events in Nondialysis CKD Patients, with or without Anemia. J. Am. Soc. Nephrol. 2021, 32, 2020–2030. [CrossRef]
- Zhang, H.; Zhabyeyev, P.; Wang, S.; Oudit, G.Y. Role of iron metabolism in heart failure: From iron deficiency to iron overload. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1925–1937. [CrossRef]
- Lupu, M.; Tudor, D.; Filip, A. Iron metabolism and cardiovascular disease: Basic to translational purviews and therapeutical approach. Rev. Port. Cardiol. 2022, 41, 1037–1046. [CrossRef]
- Das De, S.; Krishna, S.; Jethwa, A. Iron status and its association with coronary heart disease: systematic review and meta-analysis of prospective studies. Atherosclerosis 2015, 238, 296–303. [CrossRef]
- Duffy, S.J.; Biegelsen, E.S.; Holbrook, M.; Russell, J.D.; Gokce, N.; Keaney, J.F.; Vita, J.A. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation 2001, 103, 2799–2804. [CrossRef]
- Gillum, R.F.; Sempos, C.T.; Makuc, D.M.; Looker, A.C.; Chien, C.Y.; Ingram, D.D. Serum transferrin saturation, stroke incidence, and mortality in women and men. The NHANES I Epidemiologic Followup Study. National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1996, 144, 59–68. [CrossRef]
- Zheng, H.; Cable, R.; Spencer, B.; Votto, N.; Katz, S.D. Iron stores and vascular function in voluntary blood donors. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1577–1583. [CrossRef]
- You, S.-A.; Archacki, S.R.; Angheloiu, G.; Moravec, C.S.; Rao, S.; Kinter, M.; Topol, E.J.; Wang, Q. Proteomic approach to coronary atherosclerosis shows ferritin light chain as a significant marker: evidence consistent with iron hypothesis in atherosclerosis. Physiol. Genomics 2003, 13, 25–30. [CrossRef]
- Baer, D.M.; Tekawa, I.S.; Hurley, L.B. Iron stores are not associated with acute myocardial infarction. Circulation 1994, 89, 2915–2918. [CrossRef]
- Knuiman, M.W.; Divitini, M.L.; Olynyk, J.K.; Cullen, D.J.; Bartholomew, H.C. Serum ferritin and cardiovascular disease: a 17-year follow-up study in Busselton, Western Australia. Am. J. Epidemiol. 2003, 158, 144–149. [CrossRef]
- Sawicki, K.T.; Jesus, A. de; Ardehali, H. Iron Metabolism in Cardiovascular Disease: Physiology, Mechanisms, and Therapeutic Targets. Circ. Res. 2023, 132, 379–396. [CrossRef]
- Sun, Q.; Ma, J.; Rifai, N.; Franco, O.H.; Rexrode, K.M.; Hu, F.B. Excessive body iron stores are not associated with risk of coronary heart disease in women. J. Nutr. 2008, 138, 2436–2441. [CrossRef]
- Carpenter, J.-P.; He, T.; Kirk, P.; Roughton, M.; Anderson, L.J.; Noronha, S.V. de; Sheppard, M.N.; Porter, J.B.; Walker, J.M.; Wood, J.C.; et al. On T2* magnetic resonance and cardiac iron. Circulation 2011, 123, 1519–1528. [CrossRef]
- Haehling, S. von; Jankowska, E.A.; van Veldhuisen, D.J.; Ponikowski, P.; Anker, S.D. Iron deficiency and cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 659–669. [CrossRef]
- Ponikowska, B.; Suchocki, T.; Paleczny, B.; Olesinska, M.; Powierza, S.; Borodulin-Nadzieja, L.; Reczuch, K.; Haehling, S. von; Doehner, W.; Anker, S.D.; et al. Iron status and survival in diabetic patients with coronary artery disease. Diabetes Care 2013, 36, 4147–4156. [CrossRef]
- Syrovatka, P.; Kraml, P.; Potockova, J.; Fialova, L.; Vejrazka, M.; Crkovska, J.; Andel, M. Relationship between increased body iron stores, oxidative stress and insulin resistance in healthy men. Ann. Nutr. Metab. 2009, 54, 268–274. [CrossRef]
| Variables | Total (n=3224) |
Male (n=2052) |
Female (n=1172) |
P value |
|---|---|---|---|---|
| Serum Iron (μmol/L) | 13.1 (9.1–17.5) | 13.2 (9.2–18.2) | 12.9 (9.1–16.5) | 0.001 |
| Hemoglobin (g/L) | 133.0 (121.0–143.0) | 138.0 (127.0–147.0) | 125.0 (115.0–133.0) | <0.001 |
| Age, year | 64.2 (11.1) | 62.4 (11.6) | 67.3 (9.5) | <0.001 |
| Body mass index, kg/m2 | 24.0 (3.4) | 24.0 (3.2) | 24.0 (3.6) | 0.968 |
| Systolic blood pressure, mmHg | 135.4 (22.4) | 133.4 (22.4) | 138.7 (22.0) | <0.001 |
| Diastolic blood pressure, mmHg | 78.1 (12.7) | 78.3 (13.1) | 77.8 (12.0) | 0.301 |
| Fasting plasma glucose, mmol/L | 6.62 (2.84) | 6.55 (2.61) | 6.74 (3.20) | 0.060 |
| Total cholesterol, mmol/L | 4.80 (1.15) | 4.65 (1.12) | 5.05 (1.16) | <0.001 |
| Low-density lipoprotein cholesterol, mmol/L | 2.99 (0.98) | 2.93 (0.97) | 3.09 (1.00) | <0.001 |
| High-density lipoprotein cholesterol, mmol/L | 1.14 (0.31) | 1.08 (0.28) | 1.25 (0.33) | <0.001 |
| Triglycerides, mmol/L | 1.53 (1.09–2.17) | 1.51 (1.07–2.14) | 1.56 (1.12–2.21) | 0.036 |
| C-reactive protein*, mg/L | 3.11 (1.11–11.16) | 3.39 (1.11–13.28) | 2.70 (1.10–8.26) | <0.001 |
| Estimated glomerular filtration rate, ml/min per 1.73 m2, % | <0.001 | |||
| < 60 | 28.7 | 24.5 | 35.8 | |
| ≥ 60 | 71.3 | 75.5 | 64.2 | |
| Duration of coronary artery disease, year | ||||
| First diagnosed coronary artery disease (n = 2114) | - | - | - | - |
| History of coronary artery disease (n = 1110) | 3.00 (0.86–7.16) | 2.34 (0.73–6.34) | 4.00 (1.00–9.80) | <0.001 |
| Smoking status, % | <0.001 | |||
| Never | 60.8 | 41.2 | 95.2 | |
| Current | 29.1 | 43.9 | 3.2 | |
| Past | 10.1 | 15.0 | 1.6 | |
| Alcohol drinking status, % | <0.001 | |||
| Never | 83.9 | 75.3 | 98.7 | |
| Current | 11.7 | 17.8 | 1.1 | |
| Past | 4.4 | 6.8 | 0.2 | |
| Type of coronary artery disease, % | <0.001 | |||
| Acute coronary syndrome (n = 1823) | 56.5 | 61.5 | 47.9 | |
| Chronic coronary artery disease (n = 1401) | 43.5 | 38.5 | 52.1 | |
| History of diseases (Yes), % | ||||
| Hypertension | 76.2 | 74.5 | 79.4 | 0.002 |
| Dyslipidemia | 19.3 | 18.7 | 20.4 | 0.232 |
| Diabetes | 23.7 | 21.7 | 27.0 | 0.001 |
| Use of medication before admission (Yes), % | ||||
| Anti-hypertensive drugs | 48.6 | 44.2 | 57.2 | <0.001 |
| Cholesterol-lowering drugs | 12.3 | 12.5 | 11.8 | 0.668 |
| Anti-diabetic drugs | 16.7 | 14.7 | 20.5 | 0.002 |
| Anti-platelet drugs | 19.9 | 20.7 | 18.2 | 0.202 |
| Hemoglobin levels | |||||||
|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value | Hazard Ratio per 1-SD Increment | P value | |
| Cardiovascular mortality | |||||||
| Model 1 | 1.00 | 0.61 (0.47–0.78) | 0.50 (0.38–0.66) | 0.51 (0.38–0.69) | <0.001 | 0.74 (0.68–0.82) | <0.001 |
| Model 2 | 1.00 | 0.61 (0.47–0.79) | 0.52 (0.39–0.69) | 0.53 (0.39–0.72) | <0.001 | 0.76 (0.68–0.84) | <0.001 |
| Model 3 | 1.00 | 0.64 (0.50–0.83) | 0.58 (0.44–0.77) | 0.62 (0.46–0.85) | <0.001 | 0.81 (0.73–0.90) | <0.001 |
| All–cause mortality | |||||||
| Model 1 | 1.00 | 0.59 (0.48–0.72) | 0.55 (0.45–0.69) | 0.51 (0.41–0.65) | <0.001 | 0.74 (0.68–0.79) | <0.001 |
| Model 2 | 1.00 | 0.61 (0.50–0.75) | 0.60 (0.48–0.75) | 0.56 (0.44–0.72) | <0.001 | 0.77 (0.71–0.83) | <0.001 |
| Model 3 | 1.00 | 0.64 (0.52–0.79) | 0.66 (0.53–0.82) | 0.64 (0.50–0.83) | <0.001 | 0.81 (0.74–0.88) | <0.001 |
| Serum iron levels | |||||||
|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value | Hazard Ratio per 1-SD Increment | P value | |
| Cardiovascularmortality | |||||||
| Model 1 | 1.00 | 0.63 (0.50–0.81) | 0.37 (0.27–0.49) | 0.40 (0.29–0.53) | <0.001 | 0.67 (0.60–0.75) | <0.001 |
| Model 2 | 1.00 | 0.68 (0.53–0.87) | 0.41 (0.30–0.55) | 0.46 (0.34–0.62) | <0.001 | 0.72 (0.64–0.81) | <0.001 |
| Model 3 | 1.00 | 0.72 (0.56–0.92) | 0.44 (0.33–0.59) | 0.51 (0.37–0.69) | <0.001 | 0.76 (0.67–0.85) | <0.001 |
| All–cause mortality | |||||||
| Model 1 | 1.00 | 0.74 (0.61–0.90) | 0.42 (0.33–0.53) | 0.51 (0.41–0.64) | <0.001 | 0.72 (0.65–0.78) | <0.001 |
| Model 2 | 1.00 | 0.79 (0.65–0.97) | 0.47 (0.37–0.59) | 0.59 (0.47–0.75) | <0.001 | 0.77 (0.70–0.84) | <0.001 |
| Model 3 | 1.00 | 0.84 (0.69–1.03) | 0.51 (0.40–0.64) | 0.67 (0.53–0.85) | <0.001 | 0.81 (0.74–0.89) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





