Submitted:
06 December 2024
Posted:
06 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Isolation of the Anthoteibinenes
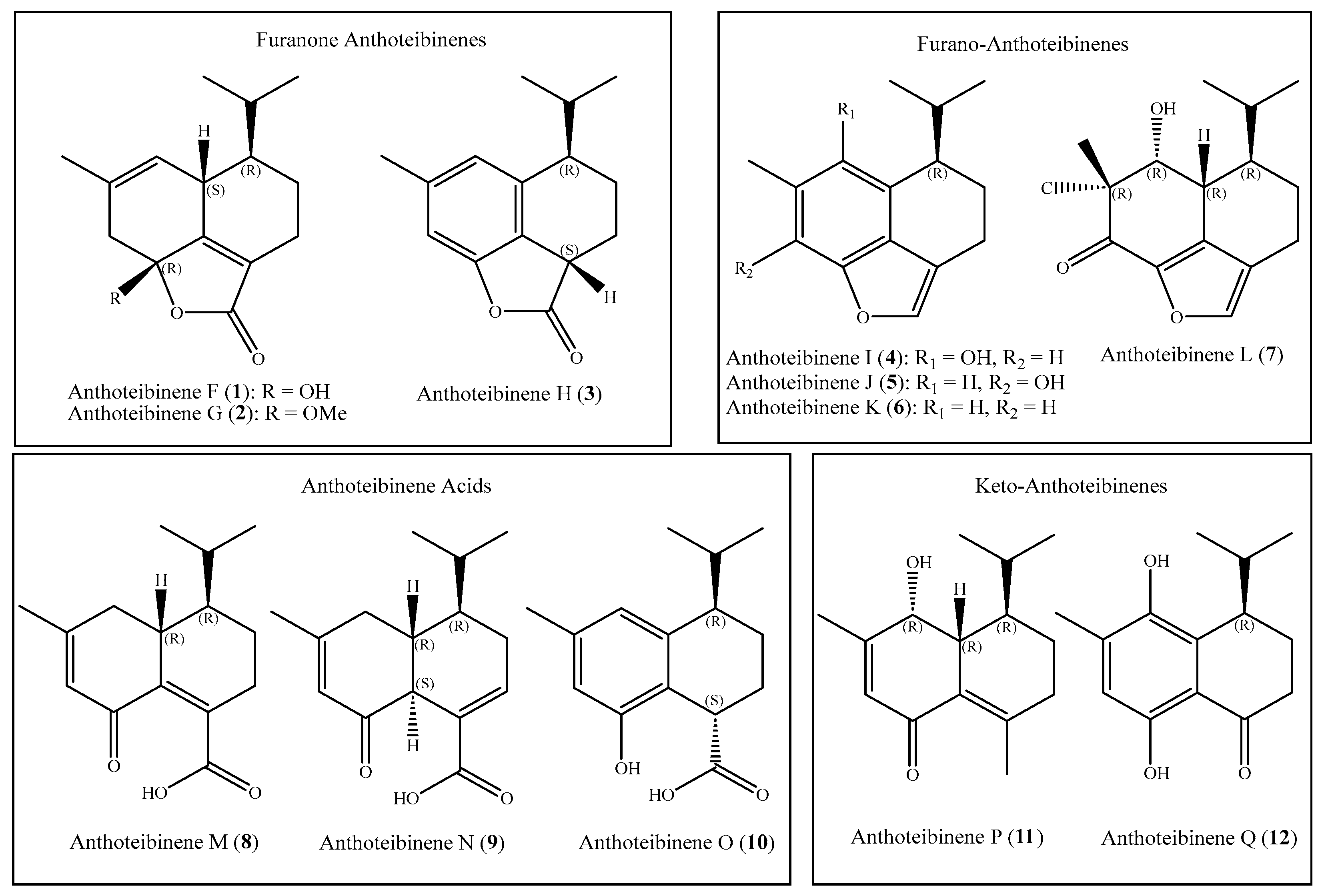
2.1.2. The Furanone Anthoteibinenes (1–3)
2.1.3. The Furano-Anthoteibinenes (4-7)
2.1.4. The Anthoteibinene Acids (8-10)
2.1.5. The Keto-Anthoteibinenes (11, 12)
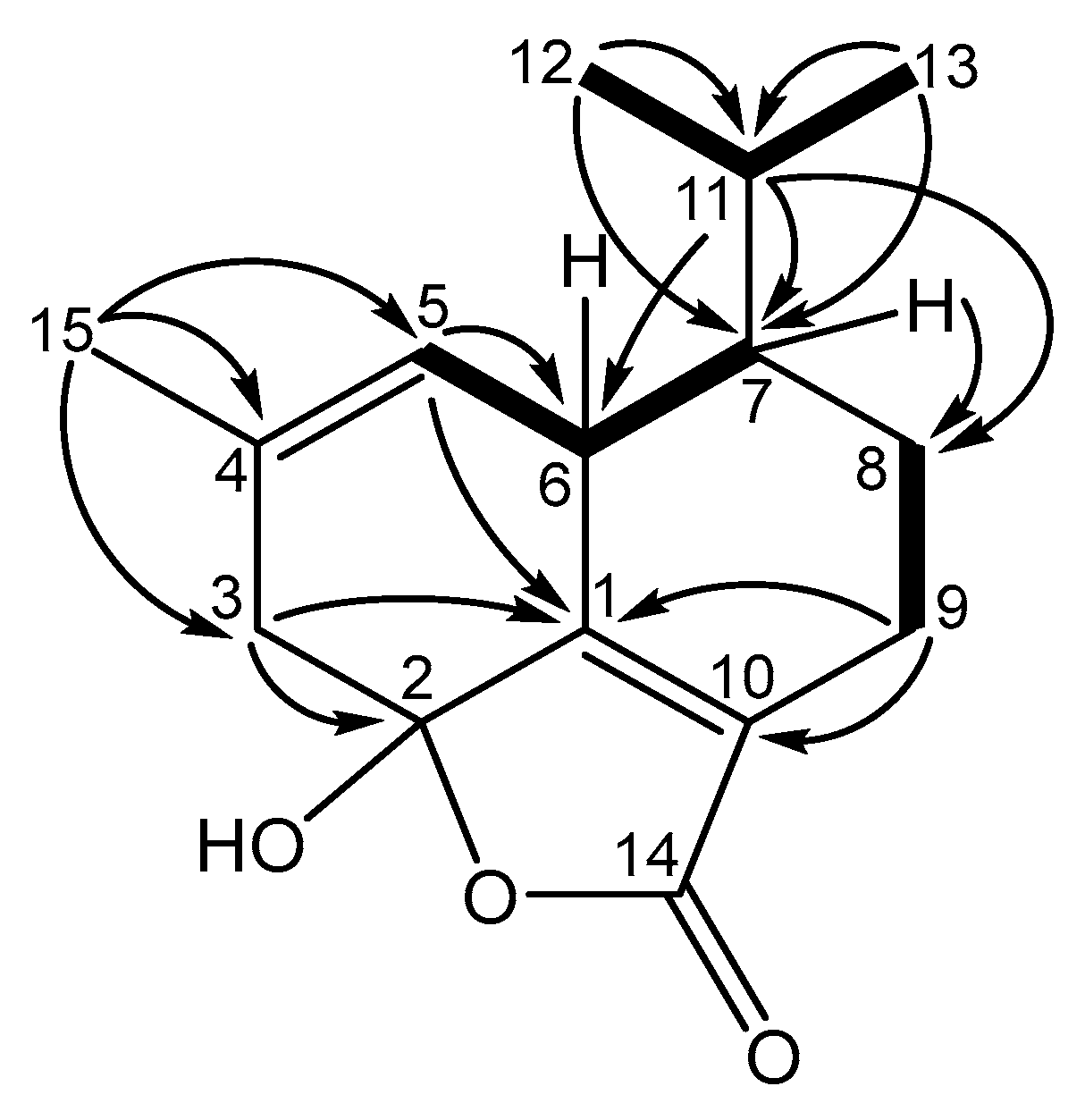
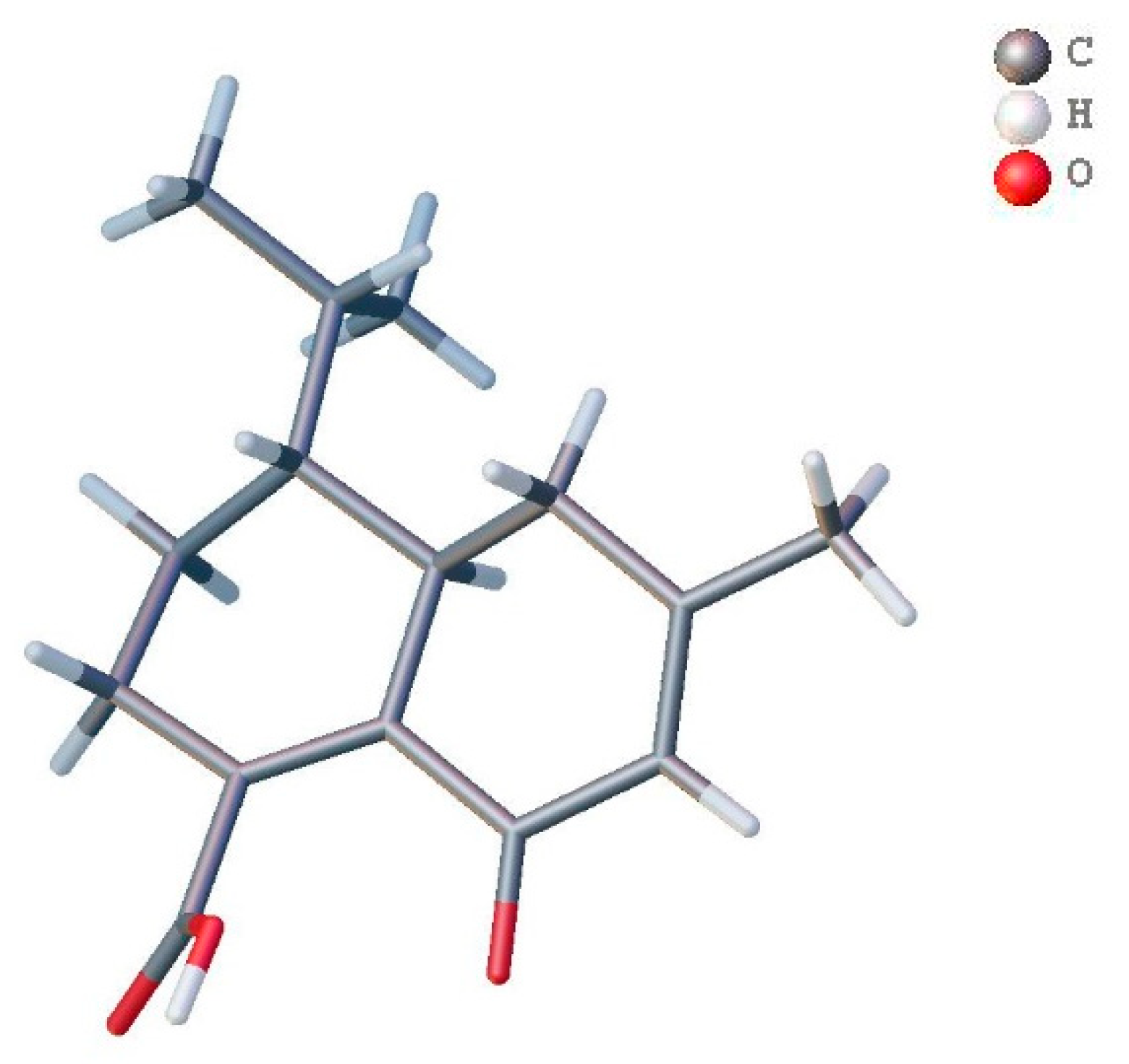
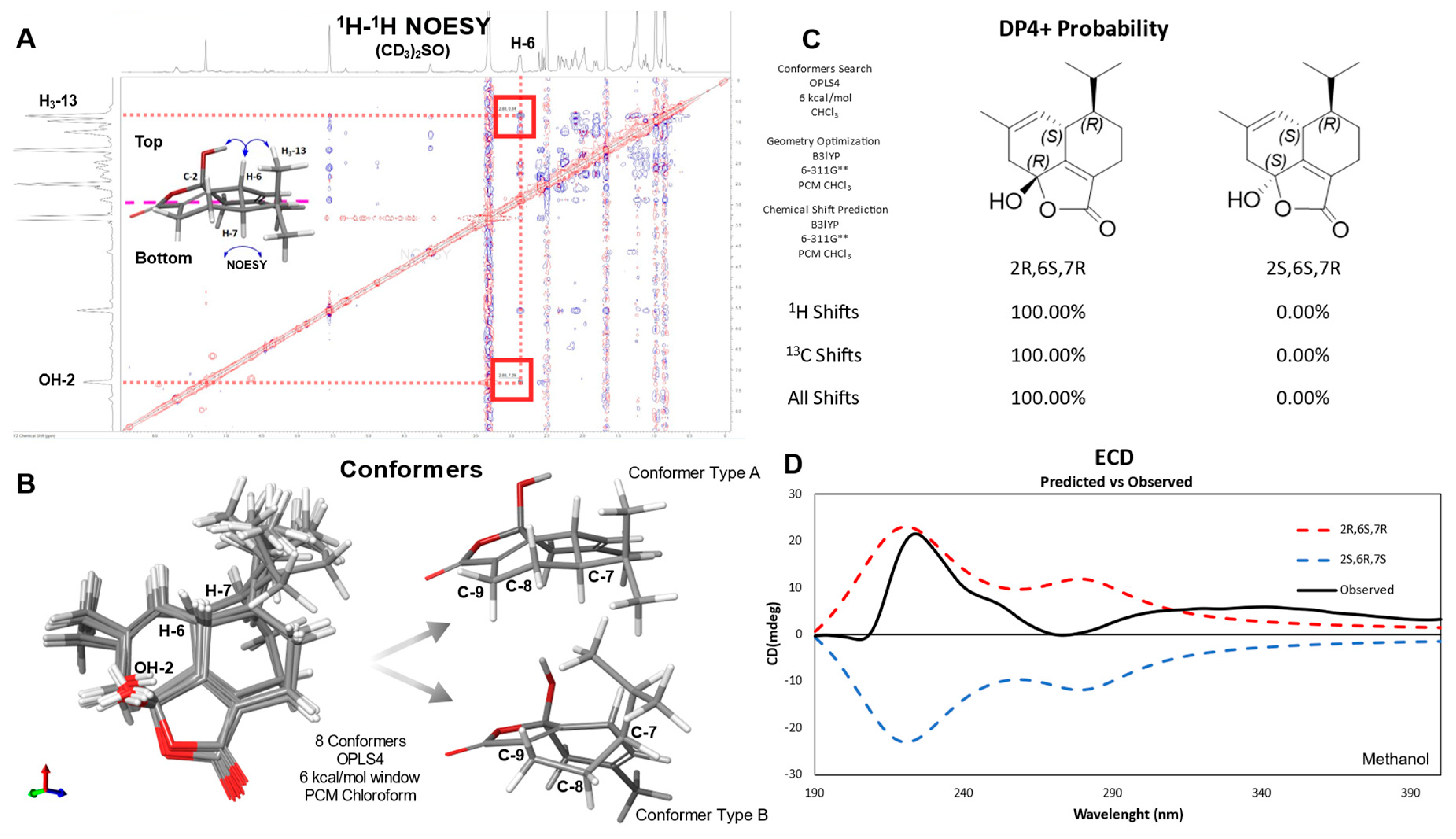
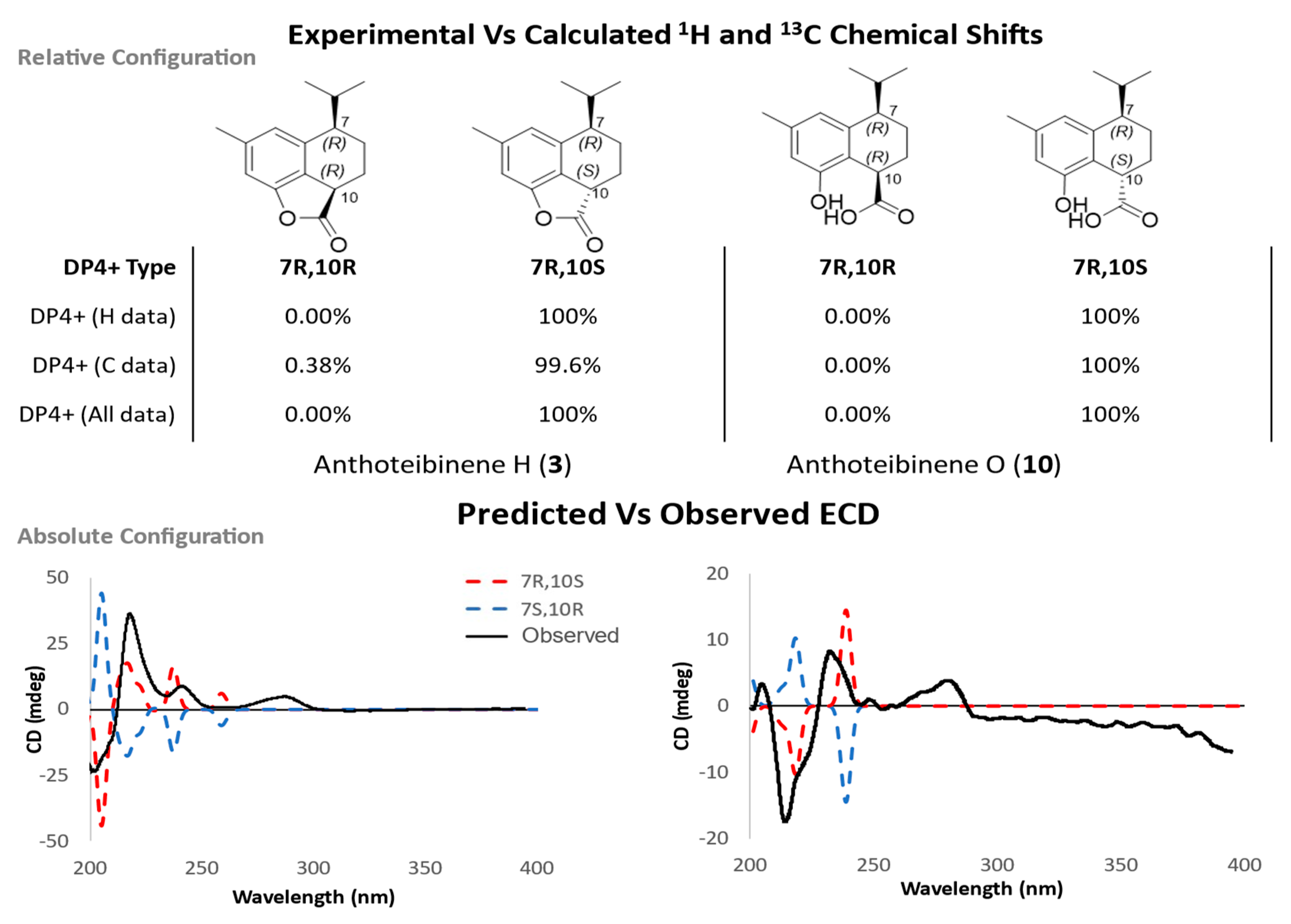
2.1.6. Configurational Analysis
2.1.7. Biological Activity of Anthoteibinenes
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Materials, Extraction, and Isolation
3.3. Spectroscopic Data for the Anthoteibinenes (1-12)
3.4. Antifungal Activity
3.5. Computational Methods
3.5.1. Electronic Circular Dichroism Spectral Predictions
3.5.2. GAIO NMR Predictions
3.6. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Altmann, K.-H. Drugs from the oceans: Marine natural products as leads for drug discovery. Chimia 2017, 71, 646–646. [Google Scholar] [CrossRef]
- Macreadie, P.I.; McLean, D.L.; Thomson, P.G.; Partridge, J.C.; Jones, D.O.B.; Gates, A.R.; Benfield, M.C.; Collin, S.P.; Booth, D.J.; Smith, L.L.; et al. Eyes in the sea: Unlocking the mysteries of the ocean using industrial, remotely operated vehicles (ROVs). Sci. Total Environ. 2018, 634, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef]
- Olsen, S.S.; Afoullouss, S.; Young, R.M.; Johnson, M.; Allcock, A.L.; Teng, M.N.; Tran, K.C.; Baker, B.J. Anthoteibinenes A–E from the Irish Deep-Sea Coral Anthothela grandiflora: An Amination Puzzle. Org. Lett. 2024. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: an improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Bagno, A.; Saielli, G. Addressing the stereochemistry of complex organic molecules by density functional theory-NMR. WIREs Comp. Mol. Sci. 2015, 5, 228–240. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, F.; Menon, A.; Song, J.M.; Espinoza, R.V.; Schultz, P.J.; Garner, A.L.; Tripathi, A. An ECD and NMR/DP4+ computational pipeline for structure revision and elucidation of diphenazine-based natural products. J. Nat. Prod. 2023, 86, 1801–1814. [Google Scholar] [CrossRef]
- Marcarino, M.O.; Cicetti, S.; Zanardi, M.M.; Sarotti, A.M. A critical review on the use of DP4+ in the structural elucidation of natural products: the good, the bad and the ugly. A practical guide. Nat. Prod. Rep. 2022, 39, 58–76. [Google Scholar] [CrossRef]
- Gawel, K.; Langlois, M.; Martins, T.; van der Ent, W.; Tiraboschi, E.; Jacmin, M.; Crawford, A.D.; Esguerra, C.V. Seizing the moment: Zebrafish epilepsy models. Neurosci. Biobehav. Rev. 2020, 116, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tkachev, A.V.; Gur’ev, A.M.; Yusubov, M.S. Acorafuran, a new sesquiterpenoid from Acorus calamus essential oil. Chem. Nat. Compd. 2006, 42, 696–698. [Google Scholar] [CrossRef]
- Tong, X.-G.; Qiu, B.; Luo, G.-F.; Zhang, X.-F.; Cheng, Y.-X. Alkaloids and sesquiterpenoids from Acorus tatarinowii. J. Asian Nat. Prod. Res. 2010, 12, 438–442. [Google Scholar] [CrossRef]
- CLSI. Performance standards for antimicrobial susceptibility testing: Twenty-fifth informational supplement; Clinical and Laboratory Standards Institute: Wayne, PA, 2015; Volume CLSI document M100-S25.
- Bruker APEX4, 2015.9; Bruker AXS Inc: Madison, WI USA, 2022.
- Bruker SAINT, 8.35A; Bruker AXS Inc.: Madison, WI USA, 2016.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. Sec. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G., M. Crystal structure refinement with SHELXL. Acta Crystal. Sec. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

| Position | δC, type | δH, mult. (J in Hz) | gCOSY | gHMBC |
|---|---|---|---|---|
| 1 | 162.0, C | |||
| 2 | 102.0, C | |||
| 3a | 43.4, CH2 | 2.50, br dd (1.3, 17.5) | 3b, 5, 15 | 2, 4, 5 |
| 3b | 2.71, d (17.5) | 3a | 1, 2, 4, 5, 15 | |
| 4 | 131.1, C | |||
| 5 | 120.3, CH | 5.58, br s | 3b, 6, 15 | 1, 3, 6, 15 |
| 6 | 35.5, CH | 3.00, br dq (2.5, 7.9) | 7, 15 | 1, 5, 7 |
| 7 | 45.3, CH | 1.19, dddd (2.4, 2.4, 10.2, 12.4) | 6, 8a, 8b, 11 | 5, 6, 11, 12, 13 |
| 8a | 21.1, CH2 | 1.91, dddd (2.1, 2.1, 5.5, 13.4) | 7, 8b, 9b | 6, 7, 10, 11 |
| 8b | 1.35, dddd (5.3, 12.2, 12.2, 13.1) | 7, 8a, 9a, 9b | 6, 7, 9 | |
| 9a | 20.3, CH2 | 2.42, br dddd (2.0, 2.5, 5.0, 18.0) | 8b, 9b | 1, 7, 10 |
| 9b | 2.09, o/l* | 8a, 8b, 9b | 1, 10 | |
| 10 | 127.1, C | |||
| 11 | 26.8, CH | 2.12, o/l* | 7, 12, 13 | 6, 7, 12, 13 |
| 12 | 21.6, CH3 | 1.03, d (6.9) | 11 | 7, 11, 13 |
| 13 | 15.8, CH3 | 0.89, d (6.9) | 11 | 7, 11, 12 |
| 14 | 170.2, C | |||
| 15 | 23.5, CH3 | 1.77, s | 3b, 5, 6 | 3, 4, 5 |
| OH | 7.30, d (0.7) | 1, 2, 3 |
| Position | 2 | 3 | ||
|---|---|---|---|---|
| δC, type | δH, mult. (J in Hz) | δC, type | δH, mult. (J in Hz) | |
| 1 | 160.6, C | 120.4, C | ||
| 2 | 105.4, C | 153.4, C | ||
| 3a | 42.5, CH2 | 2.73, d (17.4) | 113.7, CH | 6.17, s |
| 3b | 2.41, ddq (1.3, 1.3, 17.3) | |||
| 4 | 131.4, C | 135.9, C | ||
| 5 | 120.2, CH | 5.55, s | 120.8, CH | 6.60, s |
| 6 | 36.1, CH | 2.86, br dq (2.5, 7.9) | 141.7, C | |
| 7 | 45.5, CH | 1.20, dddd (2.2, 2.4, 10.1, 12.4) | 43.3, CH | 2.64, ddd (5.0, 5.5, 6.8) |
| 8a | 21.3, CH2 | 1.93, dddd (2.1, 2.1, 5.5, 13.4) | 21.2, CH2 | 1.94, dddd (3.6, 5.0, 8.5, 13.0) |
| 8b | 1.35, dddd (5.3, 12.2, 12.2, 13.1) | 1.56, dddd (2.8, 6.5, 9.8, 13.0) | ||
| 9a | 20.6, CH2 | 2.48, dddd (2.0, 2.5, 5.0, 18.0) | 24.8, CH2 | 1.74, dddd (3.0, 6.5, 10, 13.0) |
| 9b | 2.13, o/l* | 2.14, o/l* | ||
| 10 | 129.4, C | 37.4, CH | 4.05, t (7.0) | |
| 11 | 26.8, CH | 2.13, o/l* | 30.7, CH | 2.21, octet (6.4) |
| 12 | 21.6, CH3 | 1.03, d (6.9) | 21.7, CH3 | 1.02, d (6.8) |
| 13 | 15.9, CH3 | 0.90, d (6.9) | 18.0, CH3 | 0.76, d (6.8) |
| 14 | 170.1, C | 177.8, C | ||
| 15 | 23.5, CH2 | 1.75, s | 21.2, CH3 | 2.13, s |
| 16 | 50.9, CH3 | 3.22, s | ||
| Position | 4 | 5 | 6 | 7 | ||||
|---|---|---|---|---|---|---|---|---|
| δC, typea | δH, mult. (J in Hz)b | δC, typea | δH, mult. (J in Hz)b | δC, typec | δH, mult. (J in Hz)d | δC, typee | δH, mult. (J in Hz)f | |
| 1 | 125.2, C | 126.7, C | 124.9, C | 140.0, C | ||||
| 2 | 146.8, C | 141.7, C | 152.9, C | 142.0, C | ||||
| 3 | 109.4, CH | 7.05, s | 137.5, C | 108.4, CH | 7.19, s | 177.4, C | ||
| 4 | 123.1, C | 119.9, C | 134.0, C | 69.2, C | ||||
| 5 | 147.0, C | 122.2, C | 6.74, s | 120.6, CH | 6.95, s | 78.6, CH | 4.35, br d (2.9) | |
| 6 | 120.1, C | 125.0, C | 135.1, C | 36.7, C | 3.49, dd (2.8, 11.6) | |||
| 7 | 37.5, CH | 2.98, ddd (3.4, 3.9, 7.8) | 41.9, CH | 2.59, ddd (4.6, 5.8, 6.3) | 42.4, CH | 2.76, o/l* | 39.9, CH | 1.70, dddd (1.9, 1.9, 11.7, 11.7) |
| 8a | 25.7 CH2 | 2.23, o/l* | 25.1 CH2 | 1.86, o/l* | 24.9, CH2 | 1.96, m (2H) | 22.3, CH2 | 2.01, br dddd (1.5, 1.5, 5.9, 13.4) |
| 8b | 1.54, dddd (4.8, 6.0, 12.5, 13.0) | 1.81, o/l* | 1.53, dddd (5.7, 12.6, 12.6, 12.6) | |||||
| 9a | 15.9, CH2 | 2.66, m (2H) | 17.7, CH2 | 2.65, ddd (5.6, 5.8, 16.1) | 17.7, CH2 | 2.88, m | 19.4, CH2 | 2.83, br ddd (<0.5, 5.4, 16.6) |
| 9b | 2.76, ddd (4.9, 8.0, 16.0) | 2.78, o/l* | 2.49, ddd (5.0, 12.1, 16.7) | |||||
| 10 | 116.2, C | 117.1, C | 116.5, C | 123.3, C | ||||
| 11 | 31.1, C | 1.72, octet (7.3) | 29.0, C | 2.03, octet (6.6) | 28.8, CH | 2.17, octet (6.9) | 26.3, CH | 2.09, d sept (2.2, 6.9) |
| 12 | 20.7, CH3 | 0.94, d (6.5) | 21.3, CH3 | 0.97, d (6.8) | 21.2, CH3 | 1.08, d (6.8) | 21.2, CH3 | 1.07, d (6.9) |
| 13 | 21.2, CH3 | 0.91, d (6.7) | 18.9, CH3 | 0.89, d (6.8) | 18.8, CH3 | 0.99, d (6.9) | 15.8, CH3 | 0.97, d (7.0) |
| 14 | 137.6, CH | 7.44, s | 137.1, CH | 7.50, s | 137.3, CH | 7.60, s | 145.2, CH3 | 7.45, s |
| 15 | 17.8, CH3 | 2.25, s* | 16.2, CH3 | 2.24, s | 21.8, CH3 | 2.50, s | 22.8, CH3 | 1.88, s |
| OH | 7.92, s | 9.29, br s | ||||||
| Position | 8 | 9 | 10 | ||||
|---|---|---|---|---|---|---|---|
| δC, typea | δH, mult. (J in Hz)b | δC, typec | δH, mult. (J in Hz)d | δC, typec | δH, mult. (J in Hz)d | ||
| 1 | 137.0, C | 47.1, CH | 3.63, br d (3.9) | 118.6, C | |||
| 2 | 190.6, C | 199.3, C | 153.8, C | ||||
| 3 | 127.0, CH | 6.05, s | 125.6, CH | 5.89, s | 113.9, CH | 6.41, s | |
| 4 | 165.0, C | 159.3, C | 136.9, C | ||||
| 5a | 37.5, CH2 | 2.59, dd (5.3, 17.8)* | 32.2, CH2 | 2.62, br d (20.1) | 121.8, CH | 6.63, s | |
| 5b | 2.18, dd (11.3, 17.4) | 2.48, d (18.5) | |||||
| 6 | 39.3, CH | 2.65, o/l* | 36.8, CH | 2.18, o/l* | 141.8, C | ||
| 7 | 44.9, CH | 1.34, o/l* | 35.1, CH | 1.67, dddd (4.6, 4.6, 11.0, 11.0, ) | 42.8, CH | 2.55, ddd (5.5, 5.5, 5.5) | |
| 8a | 19.9, CH2 | 1.75, br m | 25.2, CH2 | 2.22, o/l* | 20.8, CH2 | 1.87, br m | |
| 8b | 1.31, o/l* | 1.97, o/l* | 1.65, dddd (5.8, 5.8, 5.8, 5.8) | ||||
| 9a | 28.7, CH2 | 2.53, o/l* | 141.4, CH | 7.10, br s | 24.1, CH2 | 1.93, o/l* | |
| 9b | 2.09, br dd (7.4, 9.7) | ||||||
| 10 | 140.4, C | 129.3, C | 40.2, CH | 3.74, t (5.8) | |||
| 11 | 27.1, CH | 1.90, dsept (2.0, 6.7) | 27.1, CH | 1.83, octet (4.8) | 31.6, CH | 2.13, octet (6.2) | |
| 12 | 21.5, CH3 | 1.01, d (6.7) | 20.6, CH3 | 0.89, d (6.9) | 21.8, CH3 | 1.00, d (6.6) | |
| 13 | 16.2, CH3 | 0.82, d (6.9) | 14.1, CH3 | 0.78, d (6.8) | 18.5, CH3 | 0.76, d (6.8) | |
| 14 | 171.4, C | 170.9, C | 181.7, C | ||||
| 15 | 24.5, CH3 | 2.03, s | 24.5, CH3 | 1.99, s* | 21.3, CH3 | 2.19, s | |
| Position | 11 | 12 | ||
|---|---|---|---|---|
| δC, typea | δH, mult. (J in Hz)b | δC, typec | δH, mult. (J in Hz)d | |
| 1 | 125.7, C | 114.6, C | ||
| 2 | 190.6, C | 157.2, C | ||
| 3 | 129.4, CH | 5.94, s | 116.9, CH | 6.65, s |
| 4 | 158.5, C | 135.3, C | ||
| 5 | 68.6, CH | 4.01, br d (2.6) | 143.0, C | |
| 6 | 44.2, CH | 2.59, br ddq (2, 4.5, 10) | 132.6, C | |
| 7 | 39.4, CH | 1.80, dddd (2.5, 2.9, 9.8, 12.5) | 38.1, CH | 2.87, ddd (3.5, 3.5, 9.5) |
| 8a | 19.9, CH2 | 1.72, dddd (3.9, 3.9, 3.9, 12.8) | 33.3, CH2 | 2.29, o/l* |
| 8b | 1.22, dddd (5.5, 11.0, 12.5, 12.5) | 2.04, dddd (4.7, 4.7, 14.0, 14.0) | ||
| 9a | 34.2, CH2 | 2.22, m | 24.7, CH2 | 2.79, ddd (5.8, 14.0, 19.3) |
| 9b | 2.55, ddd (1.6, 5.2, 19.3) | |||
| 10 | 153.1, C | 204.4, C | ||
| 11 | 27.2, CH | 1.95, d sept (2.8, 6.9) | 31.3, CH | 1.87, d sept (6.7, 9.6) |
| 12 | 21.6, CH3 | 1.03, d (6.9) | 21.3, CH3 | 1.10, d (6.5) |
| 13 | 16.2, CH3 | 0.82, d (6.9) | 21.2, CH3 | 0.91, d (6.7) |
| 14 | 22.5, CH3 | 2.10, s* | 17.4, CH3 | 2.29, s* |
| 15 | 22.0, CH3 | 2.10, s* | ||
| OH | 12.21 | |||
| Strain | 5 (µg/mL) | Fluconazole (µg/mL) |
|---|---|---|
| C. albicans MYA-2876 | 9.1 ± 0.64 | 0.25 ± 0.04 |
| C. albicans ATCC-18804 | 7.7 ± 0.71 | 0.29 ± 0.09 |
| C. albicans ATCC-28121 | 7.7 ± 0.79 | 0.61 ± 0.09 |
| C. albicans ATCC-76485 | 8.2 ± 0.78 | 0.92 ± 0.26 |
| C. albicans ATCC-90029 | 7.0 ± 0.81 | 0.19 ± 0.04 |
| C. auris AR0385 | 10.0 ± 1.20 | 0.92 ± 0.26 |
| 1 | iAnthoteibinenes derive their name from a contraction of the genus, Anthothela, with the Irish word for terpene, teibín. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





