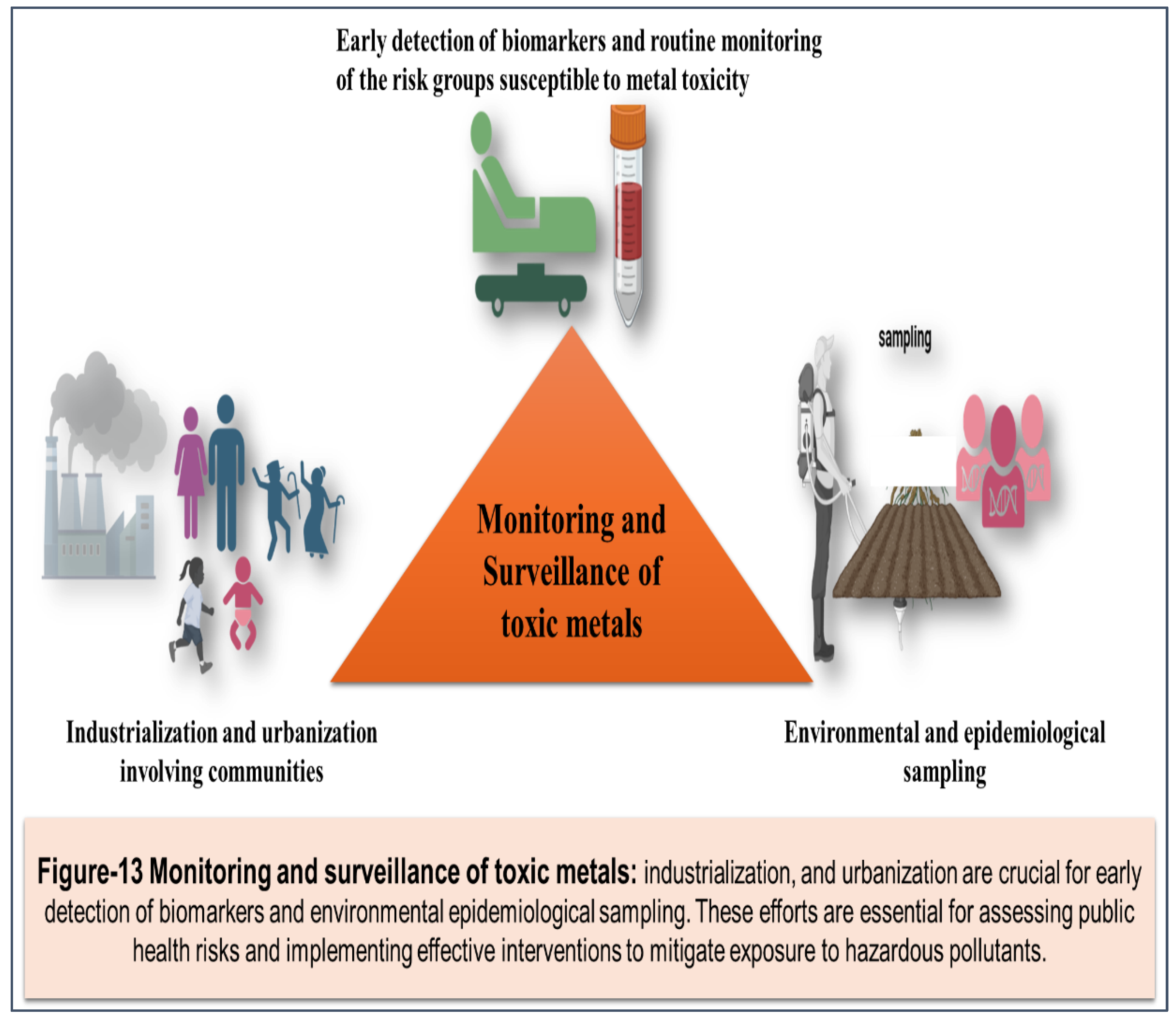
Introduction:
Toxic metal exposure represents a significant public health concern with far-reaching consequences for human health and well-being. [
1] Metals such as lead, mercury, arsenic, cadmium, and others, once considered valuable commodities for industrial and technological purposes, are now recognized for their harmful effects on human health even at low levels of exposure. [
2] The ubiquity of toxic metals in our environment stems from both natural processes and anthropogenic activities. Industrial operations, including mining, smelting, and manufacturing, contribute to the release of metals into the air, water, and soil, leading to environmental contamination. [
3] For instance, lead, a pervasive environmental toxin, has historically been used in lead-based paints, gasoline additives, and plumbing materials, resulting in widespread contamination of soil and water sources. [
4] Similarly, mercury emissions from coal-fired power plants and industrial processes contaminate aquatic ecosystems, leading to bioaccumulation in fish and seafood consumed by humans. Agricultural practices, such as the use of metal-containing pesticides and fertilizers, also contribute to environmental metal contamination, with metals leaching into soil and groundwater. Additionally, consumer products such as electronics, batteries, and cosmetics may contain toxic metals, posing a risk of exposure through direct contact or inhalation of airborne particles. [
5]
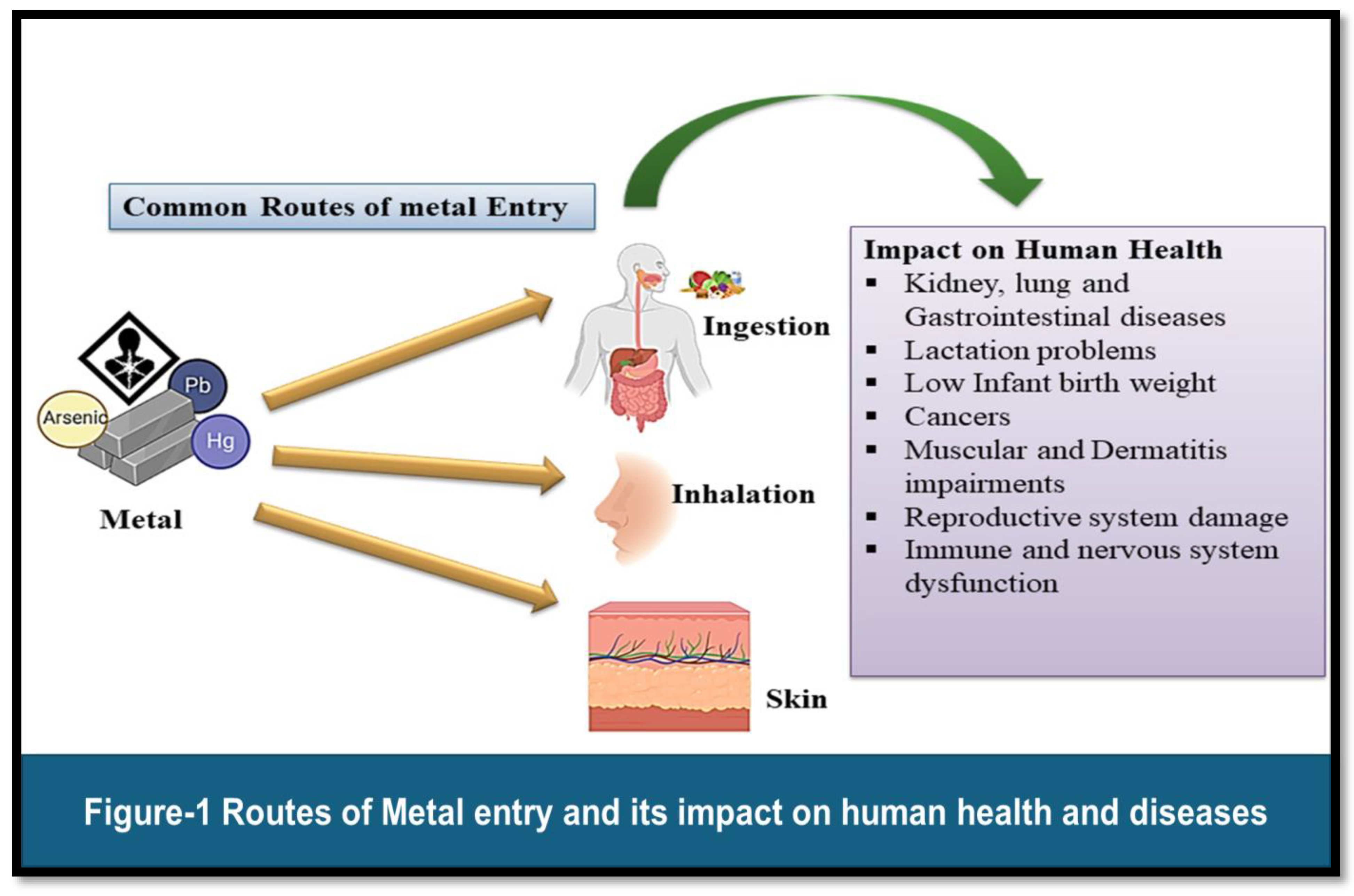
Toxic metals can enter the human body through multiple routes, including inhalation, ingestion, and dermal contact. Inhalation represents a significant route of exposure for metals such as lead, cadmium, and mercury, particularly in occupational settings where workers may be exposed to metal-containing dust, fumes, or vapours. [
6] Industrial processes, including welding, smelting, and soldering, can generate airborne metal particles that are easily inhaled, leading to systemic absorption and distribution within the body. Ingestion of contaminated food and water is another common route of exposure for certain metals, including arsenic and mercury. Arsenic contamination of groundwater, often resulting from natural geological sources or anthropogenic pollution, poses a significant risk to human health, particularly in regions reliant on groundwater for drinking and irrigation purposes. [
7] Similarly, mercury bioaccumulates in fish and seafood, posing a risk of exposure to individuals consuming these contaminated foods. Dermal contact with contaminated soil, water, or consumer products represents a less common but nonetheless significant route of exposure to toxic metals. For example, individuals working in industries involving the handling of metal-containing materials may be at risk of dermal exposure, particularly if proper personal protective equipment is not utilized. Similarly, cosmetic products containing heavy metals such as lead or mercury may pose a risk of dermal absorption upon application to the skin. [
8]
Toxic metals exert their adverse effects on human health through a variety of mechanisms, including oxidative stress, disruption of cellular function, and interference with enzymatic processes. These metals possess the ability to bind to cellular components such as proteins, lipids, and nucleic acids, leading to structural and functional alterations within cells. For instance, lead binds to sulfhydryl groups in proteins, disrupting enzymatic activity and cellular signaling pathways. Oxidative stress represents a common mechanism underlying the toxic effects of many metals, whereby metals generate reactive oxygen species (ROS) and free radicals, leading to oxidative damage to cellular macromolecules such as DNA, proteins, and lipids. [
9] Additionally, certain metals, such as arsenic and cadmium, have been shown to induce epigenetic modifications, altering gene expression patterns and contributing to the development of various diseases. [
10]
The health effects of toxic metal exposure are diverse and can manifest across multiple organ systems, ranging from neurological and cardiovascular disorders to renal dysfunction and carcinogenesis. [
11] Neurological effects, including impaired cognitive function, developmental delays, and behavioral abnormalities, are well-documented consequences of lead and mercury exposure, particularly in children whose developing brains are more susceptible to the neurotoxic effects of these metals. [
12] Cardiovascular effects, such as hypertension, atherosclerosis, and increased risk of cardiovascular disease, have been associated with chronic exposure to metals such as cadmium and lead. [
13] Cadmium, in particular, has been implicated in the development of peripheral arterial disease and endothelial dysfunction, contributing to the pathogenesis of cardiovascular disorders. Renal toxicity represents another significant health concern associated with certain toxic metals, including cadmium and lead. [
14] Chronic exposure to these metals can result in nephrotoxicity, characterized by tubular dysfunction, proteinuria, and ultimately, renal failure. Additionally, certain metals, such as arsenic and cadmium, have been classified as carcinogens by international agencies, with evidence linking them to various cancers, including lung, bladder, and liver cancer. [
15] Toxic metal exposure poses a significant threat to human health, with adverse effects that extend across multiple organ systems. Understanding the sources, routes of exposure, mechanisms of toxicity, and health effects associated with toxic metals is essential for developing effective prevention and intervention strategies to mitigate the risks posed by these environmental contaminants. By addressing the root causes of metal contamination and implementing measures to reduce exposure, we can safeguard public health and promote a healthier environment for current and future generations. This review will provide a comprehensive overview of toxic metal exposure, exploring its sources, routes of exposure, mechanisms of toxicity, and associated health effects, while emphasizing the critical importance of addressing this global health challenge.
Common Toxic Metals and Their Sources:
Toxic metals are ubiquitous environmental contaminants with sources ranging from natural geological processes to anthropogenic activities. Understanding the sources of these metals is crucial for identifying potential exposure pathways and implementing effective mitigation strategies. Various sources of heavy metals include soil erosion, natural weathering of the earth's crust, mining, industrial effluents, urban runoff, sewage discharge, insect or disease control agents applied to crops, and many others. Some common toxic metals, including lead, mercury, arsenic, and cadmium, along with their primary sources of exposure are mentioned here:
Figure 1
Lead: Lead is a highly toxic metal that has been used for centuries in various industrial applications, including lead-based paints, gasoline additives, plumbing materials, and batteries. Lead is a bright silvery metal, slightly bluish in a dry atmosphere. It begins to tarnish on contact with air, thereby forming a complex mixture of compounds, depending on the given conditions. Despite efforts to reduce lead exposure in recent decades, it remains a significant environmental and public health concern, particularly in urban areas with a legacy of lead contamination. [
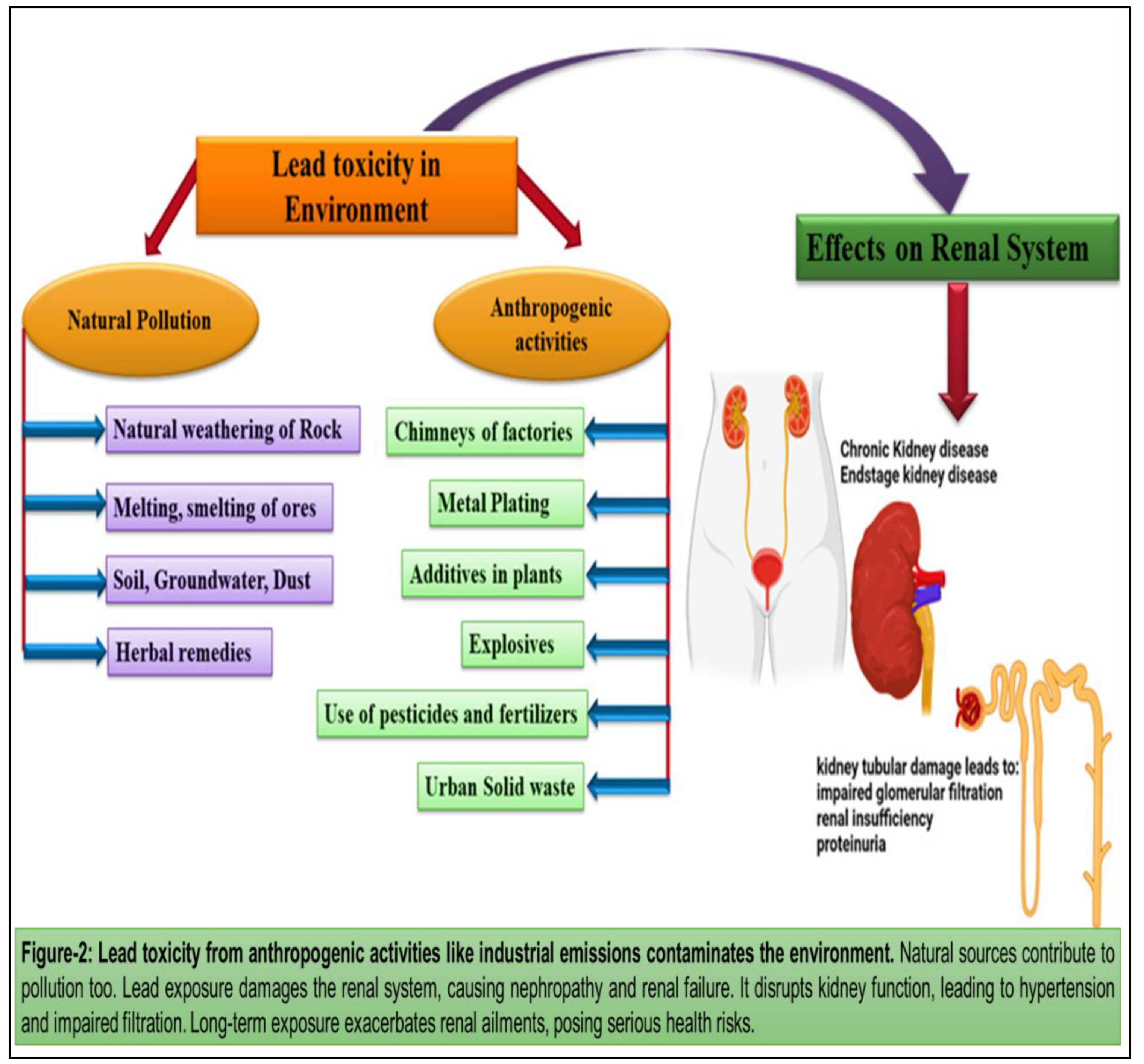
16] Lead toxicity from anthropogenic activities like industrial emissions contaminates the environment. Natural sources contribute to pollution too. Lead exposure damages the renal system, causing nephropathy and renal failure. It disrupts kidney function, leading to hypertension and impaired filtration. Long-term exposure exacerbates renal ailments, posing serious health risks. (
Figure 2) Plants with increased in lead concentration fastens the production of reactive oxygen species (ROS), and lead to the cause of lipid membrane damage that ultimately results in damage of chlorophyll and photosynthetic processes and suppresses the overall growth of the plant [
17]. Research also revealed that lead is capable of inhibiting the growth of tea plant by reducing biomass and debases the quality of tea by changing the quality of its components. [
18] Contamination of lead in soil and dust from deteriorating lead-based paint and leaded gasoline residues continues to pose a risk of exposure, especially in older housing stock and industrial sites. Additionally, lead pipes and plumbing fixtures can leach lead into drinking water, contributing to lead exposure in certain communities. [
19]
Mercury: The metallic mercury metal occurs naturally; it’s a shiny silver-white, odorless liquid and becomes colorless and odorless gas when it is heated. It is very toxic and exceedingly bio-accumulative. Presence of mercury adversely affects the marine environment and hence many studies are directed towards the distribution of mercury in water environment. Major sources of mercury pollution include anthropogenic activities such as agriculture, municipal wastewater discharges, mining, incineration, and discharges of industrial wastewater [
20] Mercury is a potent neurotoxin that exists in various forms, including elemental mercury (found in thermometers and dental amalgams), inorganic mercury compounds (used in industrial processes), and organic mercury compounds (such as methylmercury, found in fish and seafood). [
21] Coal-fired power plants are a major source of mercury emissions, releasing elemental mercury into the atmosphere, where it can be deposited into water bodies and undergo bioaccumulation in aquatic ecosystems. As a result, fish and seafood consumption represent a significant route of exposure to methylmercury, particularly for vulnerable populations such as pregnant women and young children. [
22]
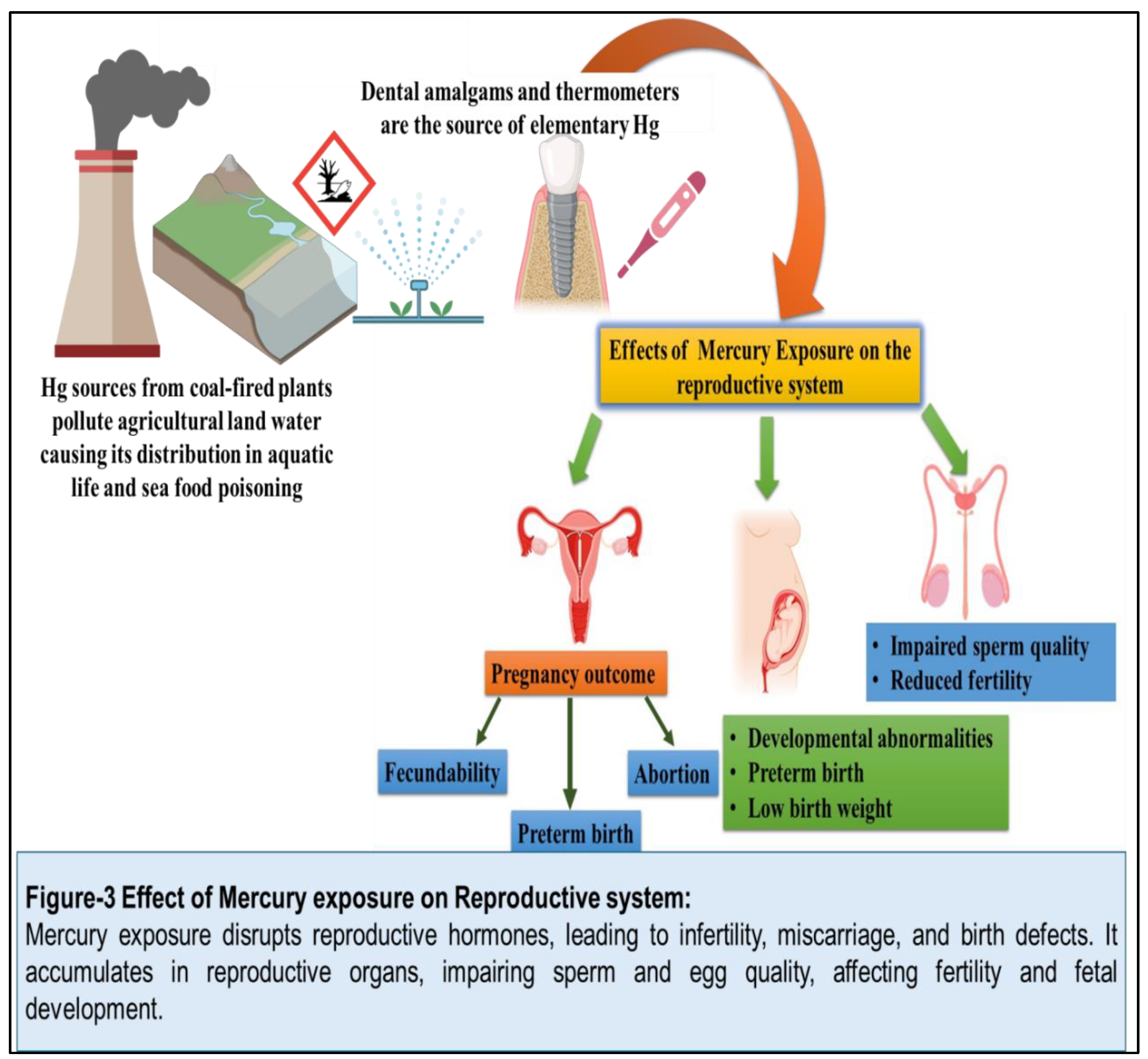
(Figure 3)
Arsenic: Arsenic is one of the most toxic heavy metals causing disquiet from ecological and individual health standpoints. [
23] Arsenic has a semi-metallic property, and it is prominently toxic and carcinogenic, and is extensively available in the form of oxides or sulfides or as a salt of iron, sodium, calcium, copper, etc. [
24]. It is a naturally occurring metalloid found in soil, rocks, and minerals, with both geological and anthropogenic sources of contamination. Inorganic arsenic compounds can leach into groundwater from natural geological formations or be released into the environment through mining, smelting, and agricultural practices. [
25] Arsenic contamination of drinking water is a major public health concern in many regions worldwide, particularly in areas with high levels of naturally occurring arsenic in groundwater. Additionally, arsenic-based pesticides and herbicides were historically used in agriculture, contributing to environmental contamination and human exposure. [
26]
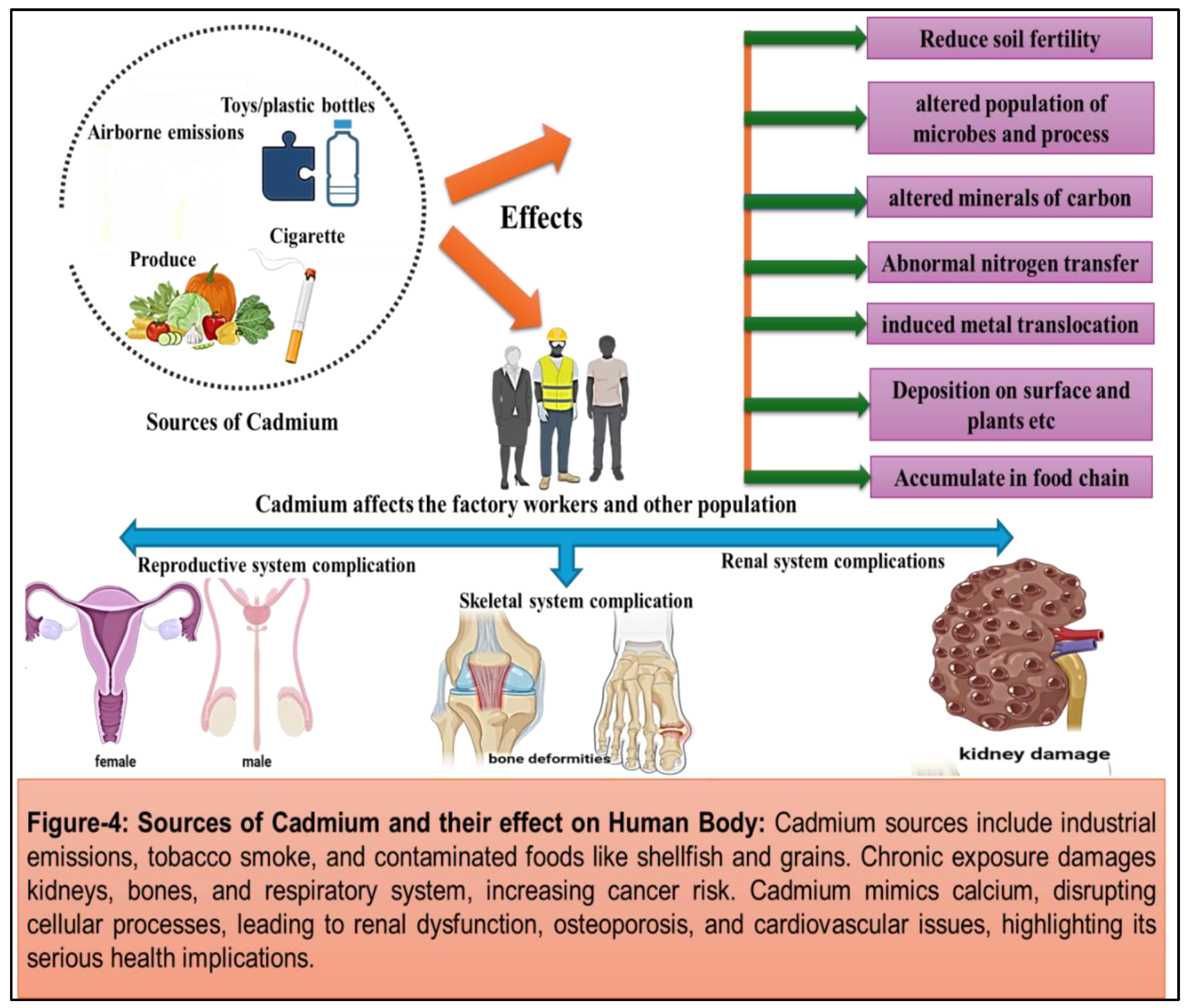
Cadmium: Cadmium is the seventh most toxic heavy metal that is primarily released into the environment through industrial processes such as mining, smelting, and manufacturing. It is a by-product of zinc production which humans or animals get exposed to at work or in the environment. [
27] When this metal gets absorbed by humans, it gets accumulated inside the body throughout the life. Cadmium was first used in World War I as a substitute for tin and in paint industries as a pigment. It is widely used in the production of batteries, coatings of metal, pigments, and in plastics (toys, bottles etc), resulting in the release of cadmium-containing effluents and airborne emissions. Three-fourths of cadmium is used in alkaline batteries as an electrode component, and the remaining part is utilized in coatings, pigments and platings and as a plastic stabilizer. Plants take up these metals which get accumulated in them and concentrate along the food chain, ultimately reaching human body. [
28] In America, more than 500,000 workers get exposed to toxic cadmium every year as per The Agency for Toxic Substances and Disease Registry Board [
29]. Tobacco smoking is another significant source of cadmium exposure, with tobacco plants accumulating cadmium from soil contaminated with cadmium-containing fertilizers. As a result, smokers and individuals exposed to environmental tobacco smoke are at an increased risk of cadmium exposure and associated health effects. [
30] Cadmium is predominantly found in most of the fruits and vegetables due to its high content of soil-to-plant transfer [
31]. It is a highly toxic nonessential heavy metal that is well recognized for its adverse influence on the enzymatic systems of cells, oxidative stress and for inducing nutritional deficiency in plants. [
32]
(Figure 4)
Other Common Toxic Metals: In addition to lead, mercury, arsenic, and cadmium, several other metals have toxicological significance and can pose risks to human health. These include metals such as chromium, nickel, and aluminum, which are used in various industrial processes and consumer products. Chromium, for example, is widely used in stainless steel production and can contaminate soil and water sources near industrial sites. [
33] Nickel is commonly used in alloys, batteries, and electronic devices, with potential exposure occurring through occupational settings and consumer products; it is
strongly associated with high apoptosis rates, and causes pulmonary fibrosis. [
34] Aluminum exposure can occur through food, water, and pharmaceuticals, with concerns regarding its neurotoxic effects and potential links to neurodegenerative diseases. [
35] Common toxic metals pose significant risks to human health and the environment, with sources of exposure ranging from industrial emissions and mining activities to consumer products and agricultural practices. Identifying and mitigating sources of metal contamination is essential for reducing human exposure and preventing adverse health effects. [
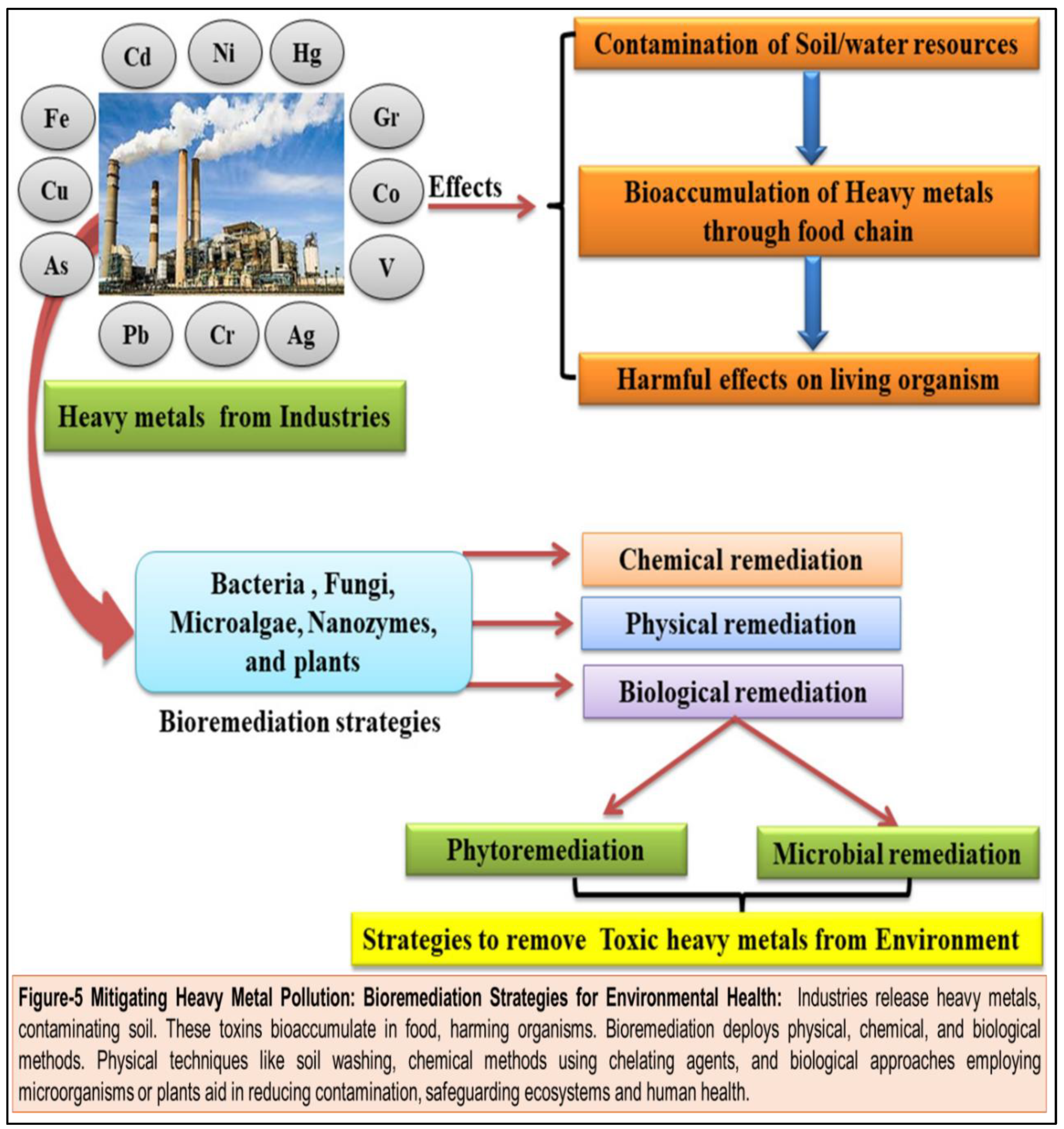
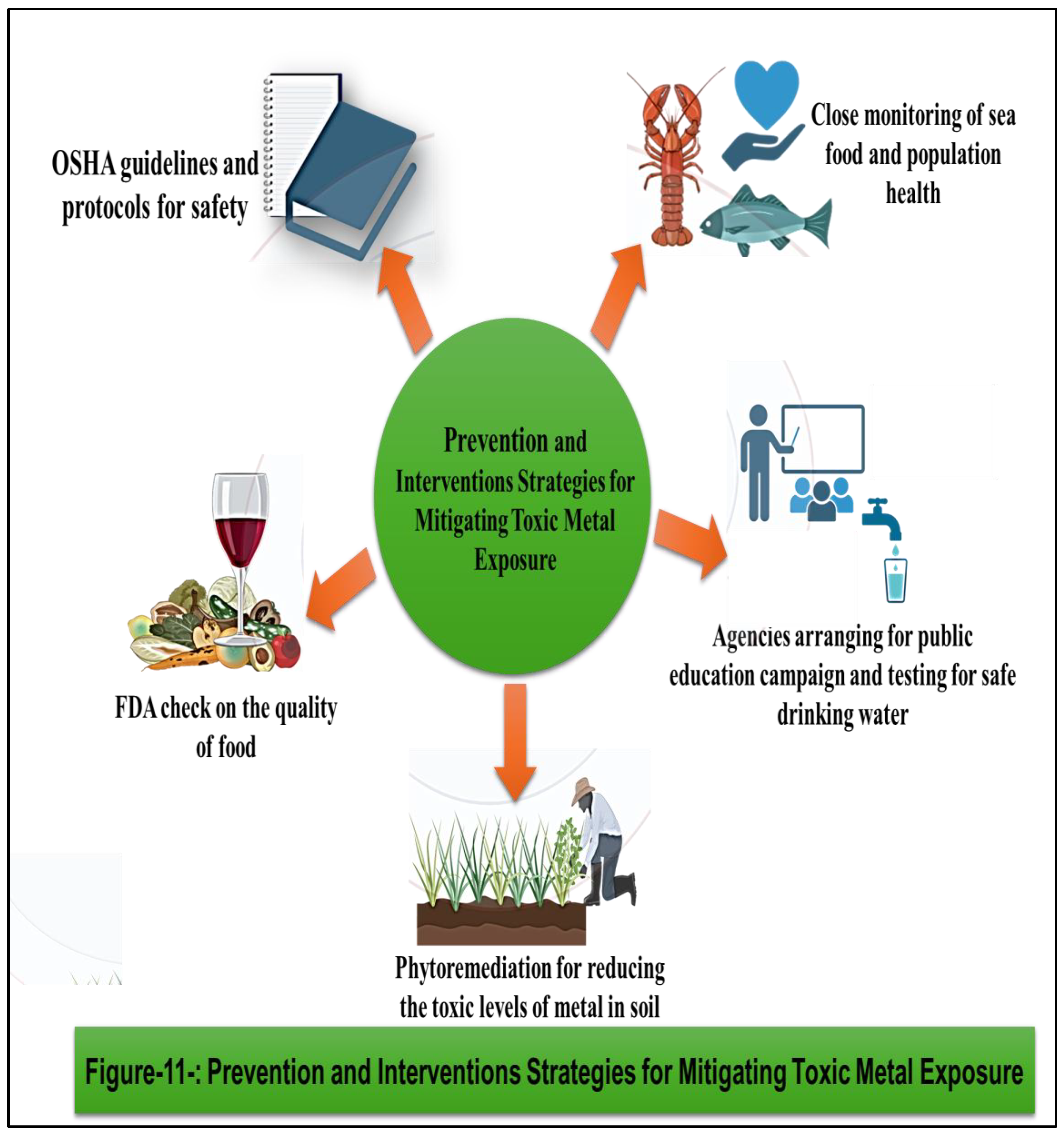
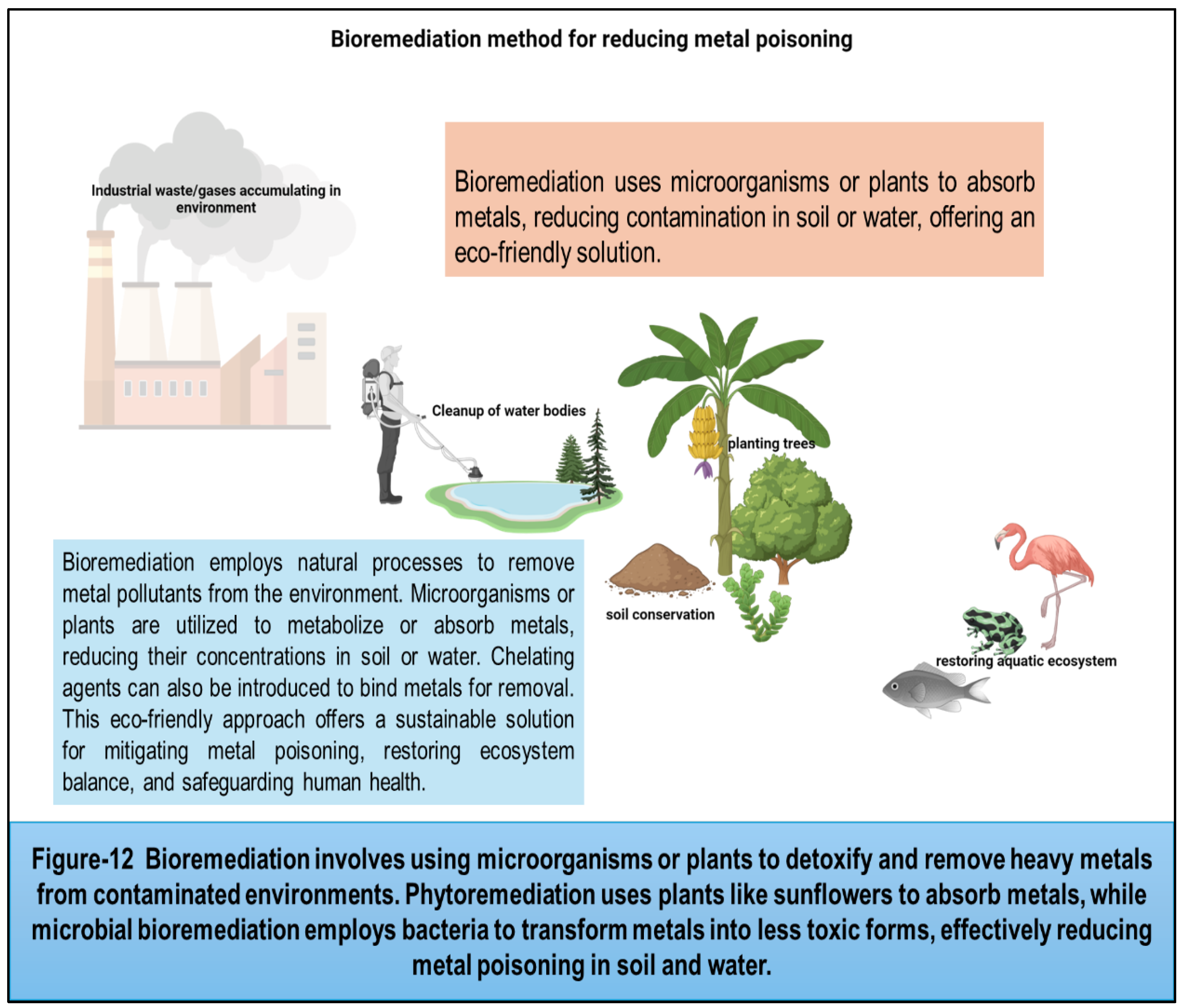
36] Heavy metals discharged by industries contaminate soil, bioaccumulate in the food chain, and harm living organisms. Bioremediation employs physical, chemical, and biological strategies to mitigate this. Physical methods include soil washing or electrokinetic treatment. Chemical methods involve using chelating agents to bind metals for removal. Biological methods utilize microorganisms or plants to metabolize or absorb contaminants. These approaches aid in reducing heavy metal concentrations, restoring ecosystem balance, and safeguarding human health. However, efficacy varies depending on metal type, soil characteristics, and environmental conditions, requiring careful implementation and monitoring for sustainable remediation. Public health interventions, regulatory measures, and pollution control strategies play a crucial role in minimizing metal exposure and safeguarding human health in communities worldwide. By addressing the root causes of metal contamination and promoting sustainable practices, we can mitigate the risks posed by toxic metals and create a safer and healthier environment for current and future generations.
Routes of Exposure to Toxic Metals:
Exposure to toxic metals occurs through various routes, each presenting unique risks to human health. Understanding these pathways is essential for assessing exposure levels, identifying vulnerable populations, and implementing effective preventive measures. This section provides an in-depth exploration of the routes of exposure to toxic metals, including inhalation, ingestion, and dermal contact, along with their associated health risks and mitigation strategies.
1-Inhalation Exposure: Human health is threatened by significant emissions of heavy metals toxicity into the urban environment due to various man made activities. Inhalation is a significant route of exposure to toxic metals, particularly in occupational settings where workers may encounter airborne contaminants during industrial processes such as mining, smelting, welding, and metal fabrication. Inhalable particles containing metals can be generated through mechanical processes, combustion, and thermal decomposition, leading to respiratory exposure and systemic absorption. Similarly, potential health risk of heavy metals in urban population is through ingestion of heavy metals on road dust of < 63-μm diameter, via incidental ingestion, dermal contact, and inhalation exposure routes by children and adults. [
37]
A)-Health Risks by inhalation: Inhaled toxic metals can bypass the body's natural defense mechanisms and enter the bloodstream directly through the lungs, resulting in systemic distribution and potential toxicity to various organs. Chronic exposure to airborne metals such as lead, cadmium, and arsenic has been linked to respiratory disorders, cardiovascular diseases, neurological impairments, and cancer. Additionally, certain metals, such as nickel and chromium, are respiratory sensitizers, capable of inducing allergic reactions and respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD). [
38].
b)-Mitigation Strategies to minimize inhalation: Effective control measures to minimize inhalation exposure include engineering controls such as ventilation systems, local exhaust ventilation, and enclosed processes to capture and remove airborne contaminants at the source. Personal protective equipment (PPE), including respiratory protection such as masks, respirators, and air-supplied helmets, should be provided to workers in high-risk environments. Occupational health and safety regulations, regular monitoring of airborne metal concentrations and employee training programs are essential for reducing the risks associated with inhalation exposure in the workplace. [
39]
2- Health Risks from Ingestion Exposure: Ingested toxic metals can be absorbed through the gastrointestinal tract and distributed throughout the body, where they can accumulate in organs such as the liver, kidneys, and brain, leading to systemic toxicity. Chronic exposure to metals such as lead and cadmium has been associated with neurological impairments, developmental delays, renal dysfunction, cardiovascular diseases, and cancer. Additionally, prenatal exposure to certain metals, such as methylmercury and lead, can result in developmental abnormalities and long-term health effects in infants and children. [
41]
A)- Mitigation Strategies: Preventive measures to reduce ingestion exposure include ensuring the safety and quality of food and drinking water through regulatory standards, monitoring programs, and pollution control measures. Public health interventions, such as education campaigns, dietary guidelines, and nutritional interventions, can help raise awareness about the risks of metal contamination in food and promote healthier dietary habits. Water treatment technologies, such as filtration, ion exchange, and reverse osmosis, can be employed to remove metals from drinking water sources and reduce exposure levels in communities at risk. [
42]
3. Dermal Contact Exposure: Dermal contact represents another route of exposure to toxic metals, particularly through direct skin contact with contaminated soil, water, consumer products, and occupational materials. Metals such as lead, mercury, arsenic, and cadmium can adhere to the skin and be absorbed through the epidermal barrier, leading to systemic distribution and potential health effects.
A)- Health Risks: Dermal exposure to toxic metals can result in localized irritation, dermatitis, and allergic reactions, as well as systemic toxicity depending on the metal and exposure duration. Chronic exposure to metals such as arsenic and cadmium has been associated with skin disorders, including hyperpigmentation, keratosis, and skin cancer. Additionally, occupational exposure to metals such as nickel and chromium can lead to occupational dermatoses, contact dermatitis, and skin sensitization. [
43]
B)- Mitigation Strategies: Preventive measures to reduce dermal contact exposure include wearing appropriate protective clothing, gloves, and footwear in occupational settings where contact with metal-containing materials is likely. Personal hygiene practices, such as handwashing and showering after potential exposure, can help minimize skin contact with contaminated substances. Regulatory measures, such as the restriction of hazardous substances in consumer products and occupational safety standards, are essential for reducing the risks associated with dermal exposure in various settings, exposure to toxic metals occurs through multiple routes, each presenting unique risks to human health. Inhalation, ingestion, and dermal contact are the primary pathways of exposure, with occupational, environmental, and consumer-related activities contributing to human exposure levels. Understanding these routes of exposure is essential for assessing health risks, identifying vulnerable populations, and implementing effective preventive measures to mitigate the adverse effects of metal toxicity. [
44] By addressing the root causes of exposure and implementing targeted interventions, we can reduce the burden of metal-related diseases and create healthier environments for current and future generations. (
Figure 5)
Mechanisms of Toxicity: Understanding How Toxic Metals Impact Human Health
Toxic metals exert their adverse effects on human health through a variety of mechanisms, disrupting normal cellular function and leading to a range of physiological and pathological responses. This section explores the mechanisms by which toxic metals exert their toxicity, including oxidative stress, interference with cellular processes, and disruption of essential biochemical pathways.
1. Toxic metals induce Oxidative Stress:
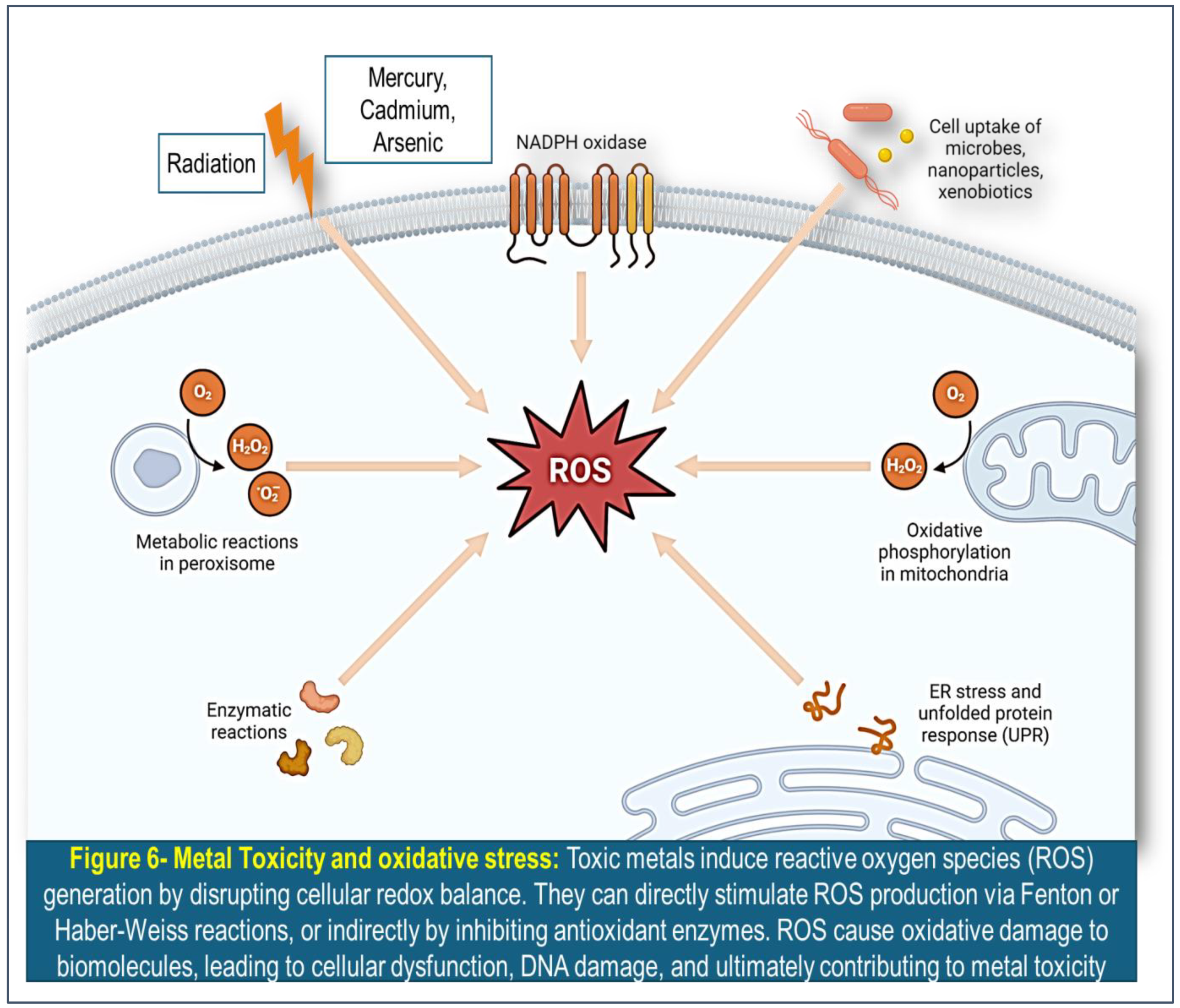
Oxidative stress induced by toxic metals is a significant mechanism underlying their toxicity in biological systems. Toxic metals such as lead, mercury, cadmium, and arsenic have been shown to promote oxidative stress, leading to damage of cellular components including lipids, proteins, and DNA. Toxic metals induce reactive oxygen species (ROS) generation by disrupting cellular redox balance. They can directly stimulate ROS production via Fenton or Haber-Weiss reactions, or indirectly by inhibiting antioxidant enzymes. ROS cause oxidative damage to biomolecules, leading to cellular dysfunction, DNA damage, and ultimately contributing to metal toxicity.
(Figure 6) This oxidative damage can contribute to various adverse health effects, ranging from neurotoxicity and cardiovascular disease to cancer and reproductive dysfunction. [
45] One of the primary ways toxic metals induce oxidative stress is through the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Metals such as cadmium and arsenic can directly generate ROS through redox cycling reactions, while others like lead and mercury can disrupt cellular antioxidant defenses, leading to increased ROS production. [
46] ROS and RNS are highly reactive molecules that can oxidize cellular macromolecules, resulting in cellular dysfunction and tissue damage. Furthermore, toxic metals can impair the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). [
47]
These enzymes play crucial roles in scavenging ROS and maintaining cellular redox homeostasis. For instance, lead has been shown to inhibit SOD and GPx activity, leading to increased oxidative stress and cellular damage. Mercury can also bind to thiol groups in antioxidant enzymes, rendering them inactive and further exacerbating oxidative stress. The mitochondria, known as the powerhouse of the cell, are particularly susceptible to oxidative damage induced by toxic metals. [
48] Mitochondrial dysfunction resulting from oxidative stress can disrupt energy production and lead to the generation of more ROS, creating a vicious cycle of oxidative damage. This can have profound effects on cellular function, especially in highly metabolically active tissues such as the brain and heart. Accumulating evidence suggests that oxidative stress induced by toxic metals plays a key role in the development of various diseases. For example, chronic exposure to lead has been linked to neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease, partly due to its ability to induce oxidative stress and neuronal damage. [
49] Similarly, mercury exposure has been associated with cardiovascular diseases, with oxidative stress playing a central role in endothelial dysfunction and atherosclerosis. Oxidative stress is a critical mechanism underlying the toxicity of toxic metals in biological systems. By promoting the generation of ROS and impairing antioxidant defenses, these metals can induce oxidative damage to cellular components, leading to a wide range of adverse health effects. [
50]
Excessive ROS production can damage cellular macromolecules such as DNA, proteins, and lipids, leading to oxidative damage, inflammation, and cell death. Chronic oxidative stress has been implicated in the pathogenesis of various diseases, including neurodegenerative disorders, cardiovascular diseases, renal dysfunction, and cancer. Additionally, oxidative stress induced by prenatal exposure to toxic metals can result in developmental abnormalities and long-term health effects in infants and children. [
51] Strategies to mitigate oxidative stress associated with metal toxicity include enhancing antioxidant defenses through dietary supplementation, promoting healthy lifestyle habits such as regular exercise and balanced nutrition, and reducing exposure to environmental pollutants and pro-oxidant factors. Antioxidant compounds such as vitamins C and E, glutathione, and polyphenols have been shown to mitigate the adverse effects of oxidative stress and protect against metal-induced toxicity in experimental studies. [
50]
3. Disruption of Essential Biochemical Pathways:
Toxic metals can disrupt essential biochemical pathways within cells, leading to metabolic dysregulation and functional impairment of vital organs and systems. Metals such as arsenic, cadmium, and mercury can interfere with enzymatic processes, metal ion homeostasis, and cellular energy metabolism, leading to a cascade of deleterious effects. Disruption of essential biochemical pathways can lead to metabolic disorders, endocrine disruption, and organ dysfunction. For example, cadmium-induced inhibition of metallothionein synthesis and zinc ion binding can lead to renal tubular dysfunction and impaired renal clearance of toxicants. [
55] Mercury-induced inhibition of mitochondrial respiration and ATP synthesis can impair cellular energy metabolism and contribute to neurological deficits and cognitive impairments. [
56]
Addressing the disruption of essential biochemical pathways associated with metal toxicity requires targeted interventions aimed at restoring metabolic homeostasis, enhancing detoxification processes, and promoting organ function. Strategies such as chelation therapy, which involves the administration of chelating agents to bind and remove toxic metals from the body, may be employed to mitigate the adverse effects of metal-induced metabolic dysfunction. [
57] Additionally, nutritional interventions aimed at replenishing essential nutrients and cofactors may help support cellular function and mitigate the toxic effects of metal exposure. Toxic metals exert their adverse effects on human health through a variety of mechanisms, including oxidative stress, interference with cellular processes, and disruption of essential biochemical pathways. Understanding these mechanisms is essential for elucidating the pathogenesis of metal-induced toxicity and developing targeted interventions to mitigate the adverse effects of metal exposure. By addressing the underlying mechanisms of toxicity and implementing preventive measures to reduce exposure levels, we can minimize the burden of metal-related diseases and promote human health and well-being.
Health Effects from Toxic Metals: Understanding the Risks and Implications
Adverse effects of toxic metals can manifest across multiple organ systems and physiological processes. Understanding the health effects associated with exposure to these metals is essential for assessing risk, identifying vulnerable populations, and implementing preventive measures. This section provides an overview of the health effects associated with exposure to common toxic metals, including lead, mercury, arsenic, and cadmium, along with their physiological mechanisms and implications for human health.
Lead: Exposure to toxic metals, such as lead, poses significant health risks, with deleterious effects on various organ systems and physiological processes. Understanding the mechanisms underlying lead toxicity is essential for elucidating its health effects and developing strategies to mitigate its adverse impact on human health. Lead exerts its toxic effects primarily by interfering with essential biological processes at the cellular and molecular levels. One of the primary mechanisms of lead toxicity involves its ability to mimic essential divalent cations, particularly calcium (Ca2+), and interfere with critical cellular functions reliant on calcium signaling. [
58] Lead ions can infiltrate calcium channels and disrupt intracellular calcium homeostasis, leading to dysregulation of cellular signaling pathways and impaired neurotransmitter release. Moreover, lead interferes with the activity of enzymes and proteins crucial for cellular function and metabolism. Lead ions can bind to sulfhydryl groups on enzymes, inhibiting their activity and disrupting essential biochemical pathways. For instance, lead inhibits the activity of delta-aminolevulinic acid dehydratase (ALAD), an enzyme involved in heme synthesis, leading to the accumulation of toxic intermediates and impairing red blood cell function. [
59]
Another mechanism by which lead exerts its toxic effects is through the generation of oxidative stress and the production of reactive oxygen species (ROS). Lead-induced oxidative stress results from the imbalance between the production of ROS and the antioxidant defense mechanisms, leading to oxidative damage to lipids, proteins, and DNA. This oxidative damage can disrupt cellular membranes, compromise mitochondrial function, and induce inflammation, contributing to tissue injury and organ dysfunction. Furthermore, lead disrupts the integrity of the blood-brain barrier (BBB) and facilitates its entry into the central nervous system (CNS), where it exerts neurotoxic effects. Once in the brain, lead interferes with neurotransmitter release, impairs synaptic function, and disrupts neuronal signaling pathways. These neurotoxic effects can manifest as cognitive deficits, behavioral abnormalities, learning disabilities, and developmental delays, particularly in children, whose developing brains are more vulnerable to lead exposure. Lead exposure also adversely affects the cardiovascular system, contributing to hypertension, atherosclerosis, and cardiovascular disease. Lead-induced oxidative stress and inflammation promote endothelial dysfunction, impair vascular tone regulation, and promote the formation of atherosclerotic plaques, increasing the risk of heart attacks and strokes. [
60] Lead toxicity stems from its ability to disrupt essential cellular processes, interfere with enzyme function, induce oxidative stress, and exert neurotoxic effects. These mechanisms underlie the diverse health effects associated with lead exposure, including neurological impairment, cardiovascular disease, developmental abnormalities, and reproductive dysfunction. Recognizing the mechanisms of lead toxicity is critical for implementing effective preventive measures, such as reducing environmental lead exposure, implementing public health interventions, and promoting early detection and treatment of lead poisoning to mitigate its adverse health effects and protect public health. Lead exposure in children has been associated with developmental delays, learning disabilities, and reduced IQ scores. The neurotoxic effects of lead are thought to result from its ability to interfere with synaptic transmission, disrupt neurotransmitter function, and impair neuronal connectivity in the developing brain. (61,62) Chronic lead exposure has been linked to hypertension, increased risk of cardiovascular disease, and adverse effects on cardiac function. [
63] Lead-induced hypertension is thought to result from alterations in vascular tone, renal function, and the renin-angiotensin system, leading to increased peripheral resistance and elevated blood pressure. [
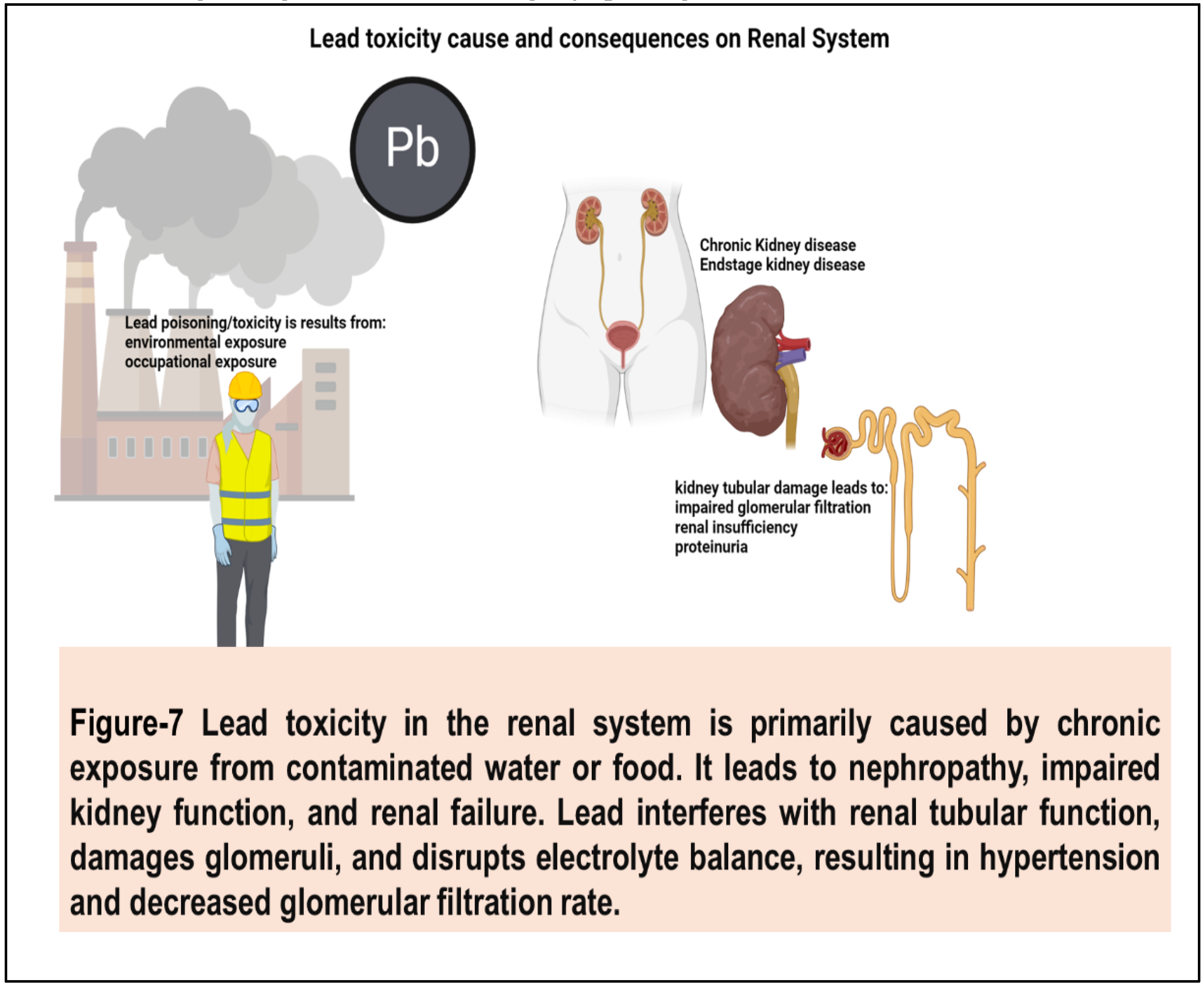
64] Lead is known to accumulate in the kidneys and interfere with renal function, leading to tubular damage, proteinuria, and impaired glomerular filtration. Chronic lead exposure has been associated with chronic kidney disease, renal insufficiency, and end-stage renal disease, particularly in adults with occupational or environmental exposure. (65,66)
(Figure 7) Lead toxicity adversely affects the heart by increasing blood pressure, causing arrhythmias, and impairing cardiac function. Neurologically, it damages the brain, leading to developmental delays, cognitive impairment, and behavioral disorders. These effects stem from lead's interference with enzyme function, neurotransmitter signaling, and cellular integrity, posing serious health risks.
2. Mercury: Mercury, a toxic metal found in various environmental sources, including air, water, and soil, poses significant health risks to human populations worldwide. Understanding the mechanisms by which mercury exerts its toxic effects is essential for elucidating its health impacts and developing strategies to mitigate exposure and prevent adverse outcomes. One of the primary mechanisms of mercury toxicity involves its ability to bind to sulfhydryl groups (-SH) on proteins and enzymes, disrupting their structure and function. Mercury forms stable complexes with sulfhydryl-containing molecules, such as cysteine residues in proteins, leading to inhibition of enzyme activity and impairment of critical biological processes. This interference with enzymatic function can disrupt essential biochemical pathways, including those involved in cellular metabolism, antioxidant defense, and neurotransmitter regulation. [
67] Moreover, mercury disrupts cellular membrane integrity and function, leading to increased permeability and altered membrane fluidity. Mercury ions can incorporate into cell membranes, interfering with lipid bilayer structure and compromising membrane integrity. This disruption of membrane function can impair cellular signaling, ion transport, and nutrient uptake, contributing to cellular dysfunction and tissue damage. Another mechanism by which mercury exerts its toxic effects is through the generation of oxidative stress and the production of reactive oxygen species (ROS). Mercury-induced oxidative stress results from the imbalance between ROS production and antioxidant defense mechanisms, leading to oxidative damage to lipids, proteins, and DNA. This oxidative damage can disrupt cellular function, promote inflammation, and contribute to tissue injury and organ dysfunction. [
68]
Furthermore, mercury has a high affinity for binding to sulfur-containing molecules, such as glutathione (GSH), an essential antioxidant molecule. Mercury binds to GSH, depleting cellular antioxidant reserves and impairing the cell's ability to neutralize ROS and protect against oxidative damage. [
69] This depletion of antioxidants further exacerbates oxidative stress and contributes to cellular injury and dysfunction. In addition to its direct effects on cellular function and oxidative stress, mercury also exerts neurotoxic effects by interfering with neurotransmitter regulation and synaptic function. [
70] Mercury disrupts neurotransmitter release and uptake, impairs synaptic transmission, and alters neuronal signaling pathways. [
71] These neurotoxic effects can manifest as cognitive deficits, memory impairment, and behavioral abnormalities, particularly in developing fetuses and young children, whose developing nervous systems are more vulnerable to mercury exposure. [
72] Mercury toxicity arises from its ability to disrupt essential cellular processes, impair enzymatic function, induce oxidative stress, and exert neurotoxic effects. The diverse health effects associated with mercury exposure, including neurological impairment, cardiovascular disease, immune dysfunction, and reproductive abnormalities cause deleterious effects on human body. Methylmercury is a potent neurotoxin that can cross the blood-brain barrier and accumulate in the central nervous system, leading to neurodevelopmental deficits, cognitive impairments, and motor dysfunction. [
73] Prenatal exposure to methylmercury through maternal consumption of contaminated fish has been linked to adverse effects on fetal brain development, resulting in reduced cognitive function and increased risk of neurodevelopmental disorders such as autism and attention deficit hyperactivity disorder
(ADHD). [72] Chronic exposure to mercury has been associated with cardiovascular disease, including hypertension, myocardial infarction, and arrhythmias. Mercury-induced endothelial dysfunction, oxidative stress, and inflammation are thought to contribute to the pathogenesis of cardiovascular disorders, leading to impaired vascular function and increased risk of adverse cardiovascular events. [
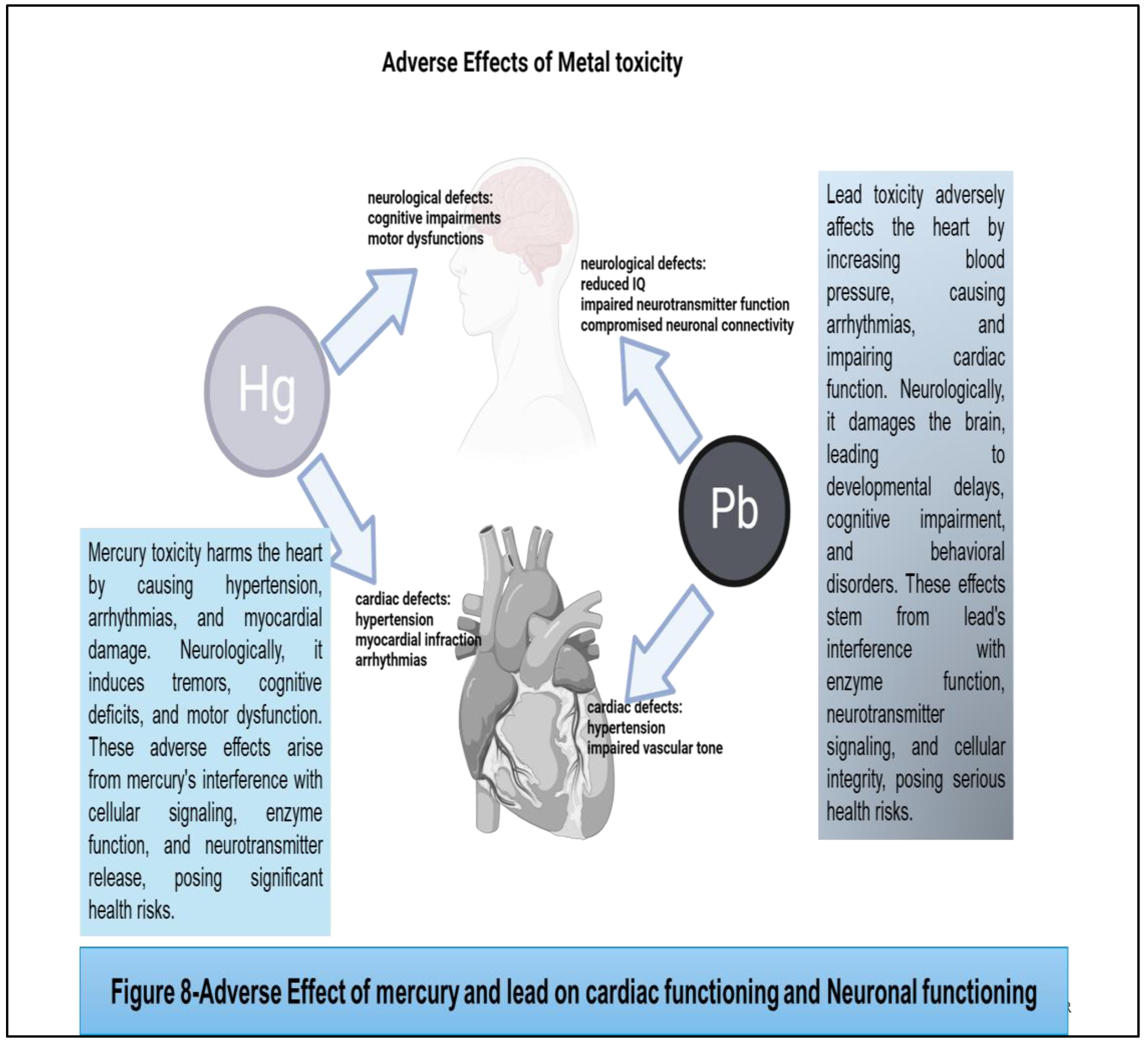
74] Mercury toxicity harms the heart by causing hypertension, arrhythmias, and myocardial damage. Neurologically, it induces tremors, cognitive deficits, and motor dysfunction. These adverse effects arise from mercury's interference with cellular signaling, enzyme function, and neurotransmitter release, posing significant health risks
. (Figure 8) Mercury exposure has been linked to reproductive toxicity, including adverse effects on fertility, pregnancy outcomes, and fetal development. Mercury can accumulate in the placenta and fetal tissues, leading to developmental abnormalities, preterm birth, and low birth weight. Additionally, mercury exposure has been associated with impaired sperm quality and reduced fertility in men. [
75]
3. Arsenic: Arsenic, a naturally occurring toxic metalloid found in soil, water, and various industrial products, poses significant health risks to human populations worldwide. Understanding the mechanisms underlying arsenic toxicity is essential for elucidating its health impacts and developing strategies to mitigate exposure and prevent adverse outcomes. Arsenic exerts its toxic effects primarily through its ability to interfere with essential biological processes at the cellular and molecular levels. One of the primary mechanisms of arsenic toxicity involves its interaction with cellular thiols, such as glutathione (GSH), which plays a crucial role in maintaining cellular redox balance and detoxification. Arsenic binds to sulfhydryl groups (-SH) on proteins and enzymes, disrupting their structure and function.[
76] This interference with enzymatic activity can impair critical biochemical pathways, including those involved in cellular metabolism, DNA repair, and antioxidant defense mechanisms. Moreover, arsenic disrupts cellular signaling pathways and gene expression by interacting with transcription factors and modulating gene expression patterns. [
77] Arsenic can interfere with the activity of transcription factors such as NF-κB and AP-1, which regulate the expression of genes involved in inflammation, cell proliferation, and apoptosis. Dysregulation of these signaling pathways can contribute to oxidative stress, inflammation, and cellular damage, leading to tissue injury and organ dysfunction. [
77] Another mechanism by which arsenic exerts its toxic effects is through the generation of reactive oxygen species (ROS) and induction of oxidative stress. Arsenic exposure leads to the production of ROS, including superoxide anion (O2•−) and hydrogen peroxide (H2O2), which can cause oxidative damage to lipids, proteins, and DNA. This oxidative damage disrupts cellular function, impairs mitochondrial respiration, and activates stress-responsive signaling pathways, leading to cellular injury and apoptosis. [
78] Furthermore, arsenic interferes with DNA repair mechanisms and promotes the formation of DNA adducts and chromosomal abnormalities. Arsenic exposure can induce DNA strand breaks, cross-linking, and base modifications, leading to genomic instability and increased susceptibility to mutations and carcinogenesis. Chronic exposure to arsenic has been associated with an elevated risk of various cancers, including skin, lung, bladder, and liver cancer, due to its genotoxic effects. In addition to its direct effects on cellular function and oxidative stress, arsenic also disrupts immune function and promotes inflammation. [
79] Arsenic exposure can impair immune cell function, alter cytokine production, and suppress immune responses, leading to increased susceptibility to infections and autoimmune diseases. Chronic inflammation associated with arsenic exposure contributes to tissue damage, organ dysfunction, and the development of chronic diseases. [
80] Toxicity arises from its ability to interfere with essential cellular processes, disrupt signaling pathways, induce oxidative stress, and promote genotoxicity and inflammation. Arsenic is classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC), with evidence linking arsenic exposure to various cancers, including skin, lung, bladder, and liver cancer. Chronic arsenic exposure has been associated with increased cancer incidence, particularly in regions with high levels of arsenic contamination in groundwater. [
81] Arsenic exposure has also been linked to cardiovascular disease, including hypertension, atherosclerosis, and peripheral vascular disease. Arsenic-induced endothelial dysfunction, oxidative stress, and inflammation are thought to contribute to the pathogenesis of cardiovascular disorders, leading to impaired vascular function and increased risk of adverse cardiovascular events. [
82] Exposure of arsenic can lead to dermatological effects, including hyperpigmentation, keratosis, and skin cancer. Arsenic-induced skin lesions, known as arsenical keratoses, are a hallmark feature of chronic arsenic poisoning and can progress to squamous cell carcinoma in severe cases. [
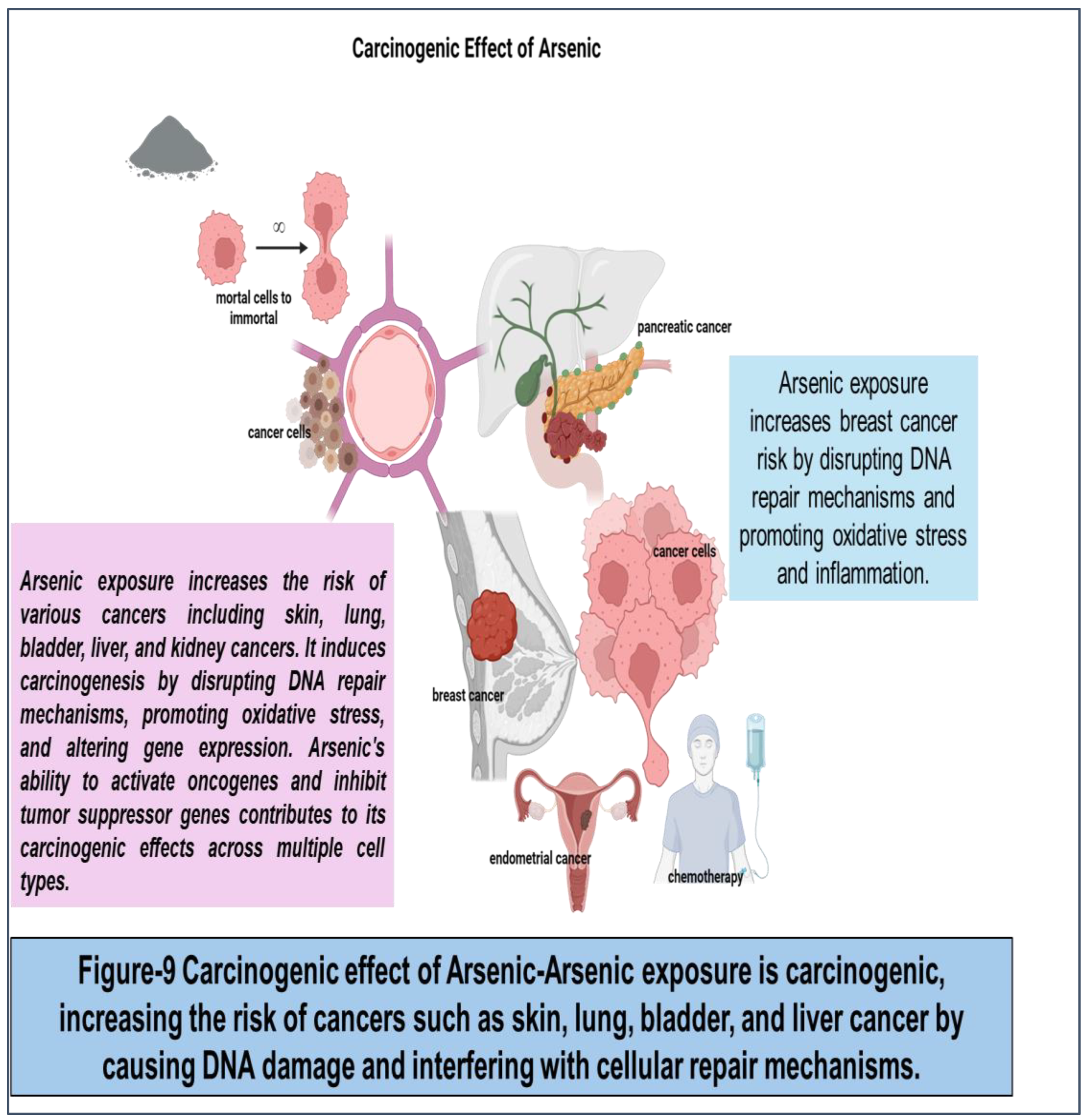
83] Arsenic exposure increases the risk of various cancers including skin, lung, bladder, liver, and kidney cancers. It induces carcinogenesis by disrupting DNA repair mechanisms, promoting oxidative stress, and altering gene expression. Arsenic's ability to activate oncogenes and inhibit tumor suppressor genes contributes to its carcinogenic effects across multiple cell types. Arsenic exposure increases breast cancer risk by disrupting DNA repair mechanisms and promoting oxidative stress and inflammation.
(Figure 9)
4. Cadmium: Cadmium, a heavy metal widely distributed in the environment due to industrial activities and natural processes, poses significant health risks to human populations worldwide. Understanding the mechanisms underlying cadmium toxicity is essential for elucidating its health impacts and developing strategies to mitigate exposure and prevent adverse outcomes. Cadmium exerts its toxic effects primarily through its ability to interfere with essential biological processes at the cellular and molecular levels. One of the primary mechanisms of cadmium toxicity involves its high affinity for sulfhydryl groups (-SH) on proteins and enzymes, leading to the disruption of their structure and function. [
84] Cadmium ions bind to sulfhydryl-containing molecules, such as cysteine residues in proteins, altering their conformation and inhibiting enzymatic activity. This interference with enzymatic function can impair critical biochemical pathways, including those involved in cellular metabolism, DNA repair, and antioxidant defense mechanisms. Moreover, cadmium disrupts cellular signaling pathways and gene expression by activating stress-responsive signaling cascades and modulating the activity of transcription factors. Cadmium exposure induces the activation of mitogen-activated protein kinases (MAPKs), such as p38 MAPK and c-Jun N-terminal kinase (JNK), which regulate gene expression patterns associated with inflammation, apoptosis, and oxidative stress. [
85] Dysregulation of these signaling pathways can lead to the production of reactive oxygen species (ROS), activation of pro-inflammatory cytokines, and promotion of cellular damage and apoptosis. [
85]
Another mechanism by which cadmium exerts its toxic effects is through the induction of oxidative stress and the generation of ROS. Cadmium exposure leads to the production of ROS, including superoxide anion (O2•−) and hydroxyl radicals (•OH), which can cause oxidative damage to lipids, proteins, and DNA. This oxidative damage disrupts cellular function, impairs mitochondrial respiration, and activates stress-responsive signaling pathways, leading to cellular injury and apoptosis. [
86] Furthermore, cadmium interferes with DNA repair mechanisms and promotes the formation of DNA adducts and chromosomal abnormalities. Cadmium exposure can induce DNA strand breaks, cross-linking, and base modifications, leading to genomic instability and increased susceptibility to mutations and carcinogenesis. [
87] Chronic exposure to cadmium has been associated with an elevated risk of various cancers, including lung, prostate, and kidney cancer, due to its genotoxic effects. In addition to its direct effects on cellular function and oxidative stress, cadmium disrupts immune function and promotes inflammation. [
87] Cadmium exposure can impair immune cell function, alter cytokine production, and suppress immune responses, leading to increased susceptibility to infections and autoimmune diseases. [
88] Chronic inflammation associated with cadmium exposure contributes to tissue damage, organ dysfunction, and the development of chronic diseases. Cadmium is known to accumulate in the kidneys and interfere with renal function, leading to tubular damage, proteinuria, and impaired glomerular filtration.
Chronic cadmium exposure has been associated with chronic kidney disease, renal insufficiency, and end-stage renal disease, particularly in individuals with occupational or environmental exposure. [
89] Cadmium exposure has been linked to osteotoxicity, including decreased bone mineral density, osteoporosis, and increased risk of fractures. Cadmium-induced bone damage is thought to result from its ability to interfere with calcium metabolism, disrupt osteoblast function, and stimulate osteoclast activity, leading to bone resorption and skeletal fragility. [
90] It has also been associated with reproductive toxicity, including adverse effects on fertility, pregnancy outcomes, and fetal development. Cadmium can accumulate in the testes and ovaries, leading to impaired sperm quality, reduced fertility, and increased risk of adverse pregnancy outcomes such as spontaneous abortion and low birth weight. [
91] Exposure to toxic metals such as lead, mercury, arsenic, and cadmium can have profound effects on human health, with adverse effects that can manifest across multiple organ systems and physiological processes. Cadmium toxicity arises from its ability to interfere with essential cellular processes, disrupt signaling pathways, induce oxidative stress, and promote genotoxicity and inflammation. These mechanisms underlie the diverse health effects associated with cadmium exposure, including cancer, cardiovascular disease, neurotoxicity, and immune dysfunction.
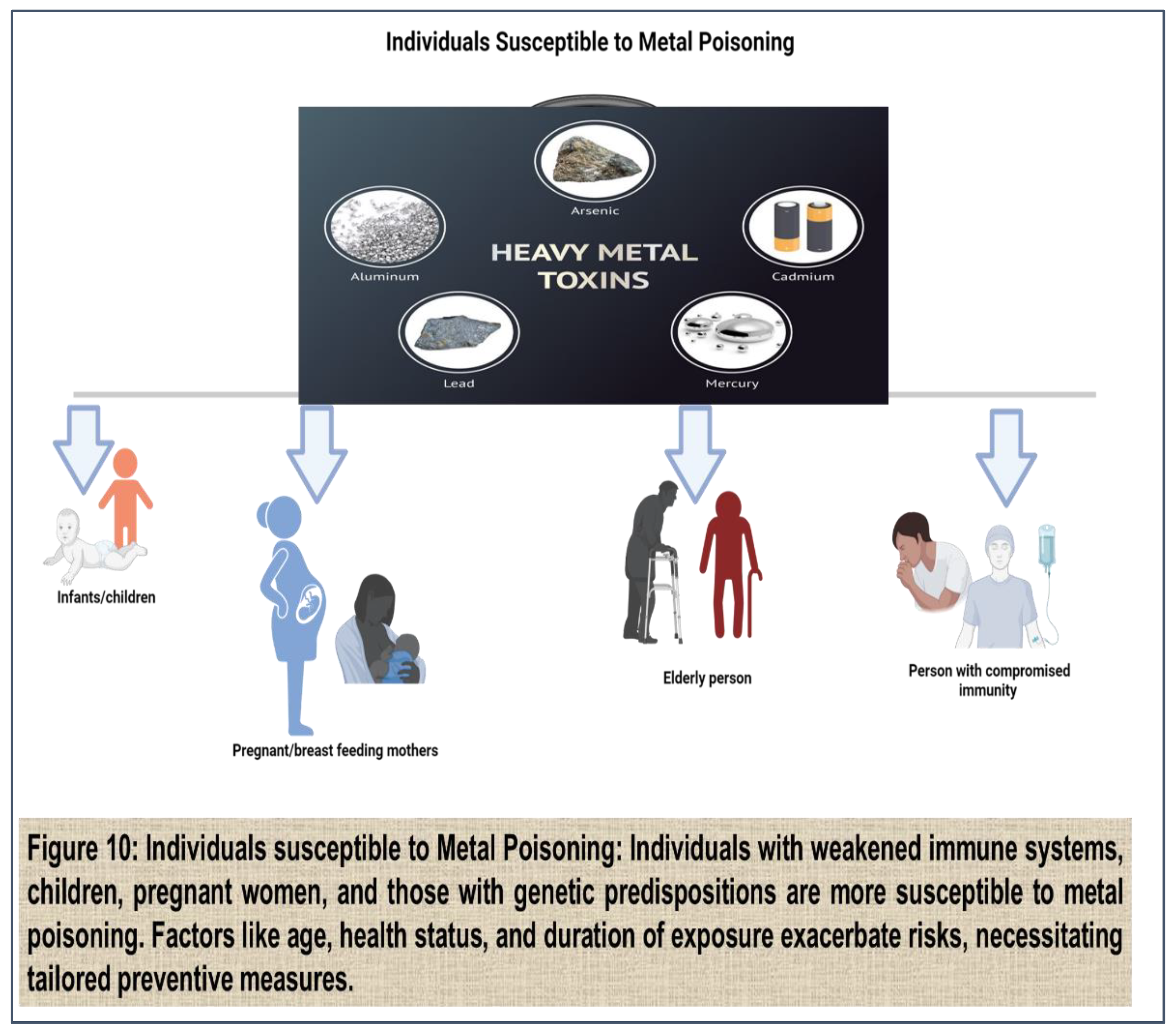
Vulnerable Populations from Toxic metal exposure:
Exposure to toxic metals can affect individuals of all ages and backgrounds, but certain populations are particularly vulnerable to the adverse health effects associated with metal exposure. Vulnerable populations may include children, pregnant women, the elderly, individuals with pre-existing health conditions, and marginalized communities.
(Figure 10)
1. Children: Children are especially susceptible to the adverse effects of toxic metals due to their developing organ systems, higher rates of exposure, and increased sensitivity to environmental toxins. Lead exposure, in particular, poses significant risks to children's health and development, as even low levels of lead exposure can impair cognitive function, learning ability, and behavior. Children may be exposed to lead through lead-based paint in older housing, contaminated soil and dust, lead pipes, and certain consumer products such as toys and cosmetics. [
92] Prenatal exposure to toxic metals can also have lifelong implications for children's health and development. Maternal exposure to metals such as mercury and arsenic during pregnancy can lead to developmental abnormalities, neurological deficits, and increased risk of neurodevelopmental disorders in offspring. Additionally, exposure to metals such as cadmium and lead during critical periods of fetal development can result in adverse pregnancy outcomes, including preterm birth, low birth weight, and developmental delays. (93,94)
2. Pregnant Women: Pregnant women are particularly vulnerable to the adverse effects of toxic metals due to the potential for transplacental transfer of metals to the developing fetus. [
95] Exposure to metals such as mercury, lead, and arsenic during pregnancy can have serious consequences for maternal and fetal health, including increased risk of miscarriage, preterm birth, and birth defects. Additionally, prenatal exposure to toxic metals has been linked to long-term health effects in offspring, including cognitive impairments, behavioral disorders, and developmental delays. (96,97) Certain cultural practices and dietary habits may also increase pregnant women's risk of metal exposure. For example, consumption of certain types of fish and seafood high in mercury, such as swordfish and shark, can lead to elevated mercury levels in pregnant women and their unborn babies. [
98] Similarly, exposure to lead through contaminated water, soil, or occupational settings can pose risks to maternal and fetal health, highlighting the importance of environmental monitoring and public health interventions to protect vulnerable populations during pregnancy. [
99]
3. Elderly Individuals: Elderly individuals are another vulnerable population at increased risk of adverse health effects from toxic metal exposure. Age-related changes in physiology, including decreased renal function, impaired detoxification pathways, and compromised immune function, can make older adults more susceptible to the toxic effects of metals such as lead, cadmium, and arsenic. [
100] Additionally, comorbidities such as hypertension, diabetes, and cardiovascular disease can exacerbate the health effects of metal exposure in elderly individuals, leading to increased morbidity and mortality. [
101] Chronic exposure to toxic metals over a lifetime can also contribute to the development of age-related diseases and cognitive decline in elderly individuals. For example, lead exposure has been linked to increased risk of neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease, [
101] while cadmium exposure has been associated with decreased bone mineral density and increased risk of osteoporosis and fractures in older adults. [
102]
4. Individuals with Pre-existing Health Conditions: Individuals with pre-existing health conditions, such as respiratory disorders, cardiovascular disease, and renal dysfunction, may be at increased risk of adverse health effects from toxic metal exposure. [
103] Metals such as lead, cadmium, and arsenic can exacerbate existing health conditions and increase the risk of disease progression in vulnerable populations. For example, individuals with asthma or chronic obstructive pulmonary disease (COPD) may be more susceptible to the respiratory effects of metal exposure, including exacerbation of symptoms and increased risk of respiratory infections, [
104] similarly, individuals with cardiovascular disease may be at increased risk of adverse cardiovascular events from metal exposure, including hypertension, myocardial infarction, and arrhythmias. [
105] Chronic kidney disease patients are particularly vulnerable to the renal effects of metals such as lead and cadmium, as impaired renal function can lead to decreased clearance of metals and increased accumulation in the body, exacerbating renal dysfunction and increasing the risk of adverse outcomes. [
106]
5. Marginalized Communities: Marginalized communities, including low-income populations, minority groups, and indigenous communities, may face disproportionate exposure to toxic metals due to socioeconomic factors, environmental injustice, and lack of access to resources and healthcare services. Environmental racism and discrimination can contribute to disparities in exposure to environmental pollutants, including toxic metals, leading to higher rates of adverse health effects in marginalized communities. [
107] Industrial facilities, hazardous waste sites, and polluting industries are often located in low-income and minority neighborhoods, leading to increased exposure to environmental contaminants such as lead, mercury, arsenic, and cadmium. Additionally, lack of access to clean drinking water, nutritious food, and healthcare services can exacerbate the health effects of metal exposure in marginalized communities, further widening health disparities and perpetuating cycles of poverty and environmental injustice, [
108] these populations are particularly vulnerable to the adverse health effects associated with exposure to toxic metals, including children, pregnant women, the elderly.
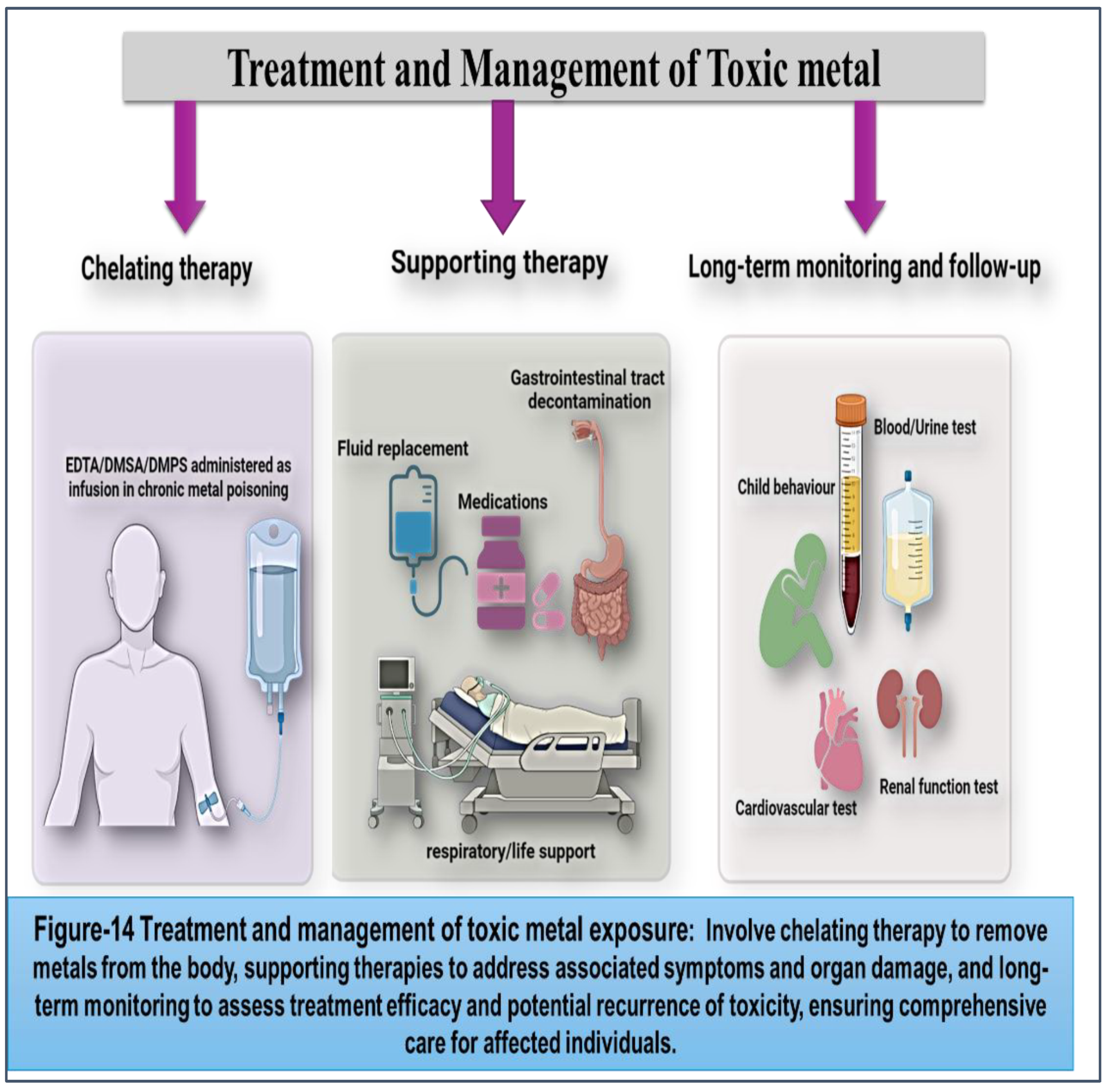
Treatment and Management of Toxic Metal Poisoning: Strategies for Minimizing Health Risks and Promoting Recovery
Toxic metal poisoning poses significant health risks, requiring prompt and effective treatment and management strategies to mitigate adverse effects and promote recovery. While prevention remains the cornerstone of addressing metal exposure, individuals who have been exposed to toxic metals may require medical intervention to address acute toxicity and prevent long-term health consequences.
(Figure 14)
1. Chelation Therapy: Chelation therapy is a medical intervention used to remove toxic metals from the body by administering chelating agents that bind to metal ions and facilitate their excretion through urine or feces. Chelating agents such as dimercaprol, EDTA (ethylene diamine tetra-acetic acid), DMSA (dimercaptosuccinic acid), and DMPS (2,3-dimercapto-1-propanesulfonic acid) are commonly used in clinical practice to treat acute metal poisoning and chronic metal toxicity. Chelation therapy is the primary treatment for lead poisoning, particularly in cases of severe toxicity or symptomatic lead encephalopathy. EDTA and DMSA are FDA-approved chelating agents for lead poisoning, administered intravenously or orally to enhance lead excretion and reduce blood lead levels. Chelation therapy may be indicated in children with blood lead levels ≥45 µg/dL or adults with blood lead levels ≥70 µg/dL, according to CDC guidelines. [
140] Chelation therapy may be considered in cases of acute mercury poisoning or chronic mercury exposure with elevated urine mercury levels. DMPS and DMSA are FDA-approved chelating agents for mercury toxicity, administered orally or intravenously to enhance mercury excretion and reduce body burden. Chelation therapy may be combined with supportive care measures, such as fluid replacement and electrolyte balance, to manage symptoms of mercury toxicity. [
141] Chelation therapy may be used in cases of acute arsenic poisoning or chronic arsenic exposure with elevated urine arsenic levels. Dimercaprol and DMSA are FDA-approved chelating agents for arsenic toxicity, administered intramuscularly or orally to enhance arsenic excretion and reduce body burden. Chelation therapy may be supplemented with supportive measures, such as gastric lavage and activated charcoal, to remove arsenic from the gastrointestinal tract. [
142] Chelation therapy may be considered in cases of acute cadmium poisoning or chronic cadmium exposure with elevated urine cadmium levels. EDTA and DMPS are FDA-approved chelating agents for cadmium toxicity, administered intravenously or orally to enhance cadmium excretion and reduce body burden. Chelation therapy may be combined with supportive care measures, such as respiratory support and hemodialysis, to manage symptoms of cadmium toxicity. [
143]
2. Supportive Care: In addition to chelation therapy, supportive care measures play a crucial role in managing toxic metal poisoning and addressing associated symptoms and complications. Supportive care interventions includes Intravenous fluids, that may be administered to maintain hydration and electrolyte balance in patients with dehydration or electrolyte imbalances resulting from metal poisoning. (144,145) Medications may be prescribed to alleviate symptoms of metal poisoning, such as pain relievers for headache and abdominal pain, antiemetics for nausea and vomiting, and antihypertensives for hypertension. Gastric lavage, activated charcoal, and cathartics may be used to remove ingested metals from the gastrointestinal tract and prevent systemic absorption. Mechanical ventilation may be required in cases of severe respiratory distress or acute respiratory failure resulting from metal fume inhalation or pulmonary edema. [
146]
3. Long-term Monitoring and Follow-up: After initial treatment for toxic metal poisoning, patients may require long-term monitoring and follow-up to assess recovery, monitor residual metal levels, and address potential health effects. Long-term monitoring may involve: Blood and urine tests may be performed periodically to monitor metal levels and assess response to treatment. Serial monitoring of metal biomarkers can help evaluate the effectiveness of chelation therapy and guide adjustments in treatment regimens. [
147] Children exposed to lead or mercury may undergo neurodevelopmental assessment to evaluate cognitive function, learning ability, and behavioral outcomes. Longitudinal follow-up may be recommended to monitor developmental progress and identify any delays or deficits. Individuals exposed to cadmium or arsenic may undergo renal function testing to assess kidney function and detect early signs of renal dysfunction. [
148] Long-term follow-up may be necessary to monitor renal health and identify any deterioration in kidney function.Patients with a history of chronic lead or arsenic exposure may undergo cardiovascular evaluation to assess cardiovascular risk factors, such as hypertension, atherosclerosis, and peripheral vascular disease. Longitudinal monitoring may be recommended to monitor cardiovascular health and manage any complications. [
149] Treatment and management of toxic metal poisoning require a multifaceted approach that encompasses chelation therapy, supportive care, and long-term monitoring. Prompt recognition of metal poisoning, initiation of appropriate treatment, and close follow-up are essential for minimizing health risks, promoting recovery, and preventing long-term complications.
Future Directions in the Management of Toxic Metal Exposure: As we look ahead, several key areas warrant attention and exploration to advance the management of toxic metal exposure and protect public health. These future directions encompass research, technology, policy, and community engagement initiatives aimed at enhancing our understanding of metal toxicity, improving prevention and intervention strategies, and promoting environmental sustainability. [
150] Continued advancements in monitoring technologies, such as sensor-based systems, remote sensing platforms, and portable analytical devices, hold promise for enhancing real-time detection and mapping of metal contamination in air, water, soil, and food sources. These technologies enable rapid response to environmental emergencies, early warning systems for at-risk populations, and targeted interventions to mitigate exposure risks. Tailored interventions based on individual susceptibility factors, genetic predisposition, and biomarker profiles offer opportunities for personalized medicine approaches to the management of metal toxicity. (151,152) Precision medicine strategies, including pharmacogenomics, metabolomics, and molecular imaging techniques, may help identify high-risk individuals, optimize treatment regimens, and improve health outcomes for affected populations. Embracing principles of green chemistry, sustainable manufacturing, and pollution prevention can reduce the environmental burden of toxic metals and minimize exposure risks throughout the product lifecycle. [
153] Innovations in eco-friendly materials, waste management technologies, and clean production processes contribute to a circular economy and promote environmental sustainability while safeguarding human health. Engaging communities, stakeholders, and affected populations in participatory research initiatives fosters collaboration, empowers local decision-making, and promotes environmental justice in addressing metal contamination issues. Community-based participatory research approaches, including citizen science projects, environmental health assessments, and participatory action research, enhance community resilience, build trust, and promote equitable solutions to environmental challenges.
Integrated Risk Assessment Frameworks: Developing integrated risk assessment frameworks that consider cumulative exposures, synergistic effects, and vulnerable populations' susceptibility can improve our understanding of the complex interactions between multiple metals and other environmental stressors. Integrated approaches, such as exposome analysis, systems toxicology, and multi-stressor modeling, enable comprehensive risk assessment and inform evidence-based decision-making for preventive interventions. Strengthening regulatory frameworks, updating exposure guidelines, and implementing evidence-based policies are essential for addressing emerging threats and protecting public health from metal exposure risks. Policy initiatives, such as pollution prevention strategies, green procurement policies, and environmental justice legislation, promote equitable access to clean air, water, and soil, and hold polluters accountable for environmental contamination.
Global Collaboration and Knowledge Sharing: Promoting international collaboration, knowledge sharing, and capacity-building efforts fosters a collective response to global challenges associated with toxic metal exposure. Collaborative initiatives, such as research networks, data-sharing platforms, and international partnerships, facilitate cross-border cooperation, harmonize standards, and strengthen resilience to environmental threats on a global scale. Future efforts to manage toxic metal exposure require a multidisciplinary and collaborative approach that integrates scientific innovation, policy reform, community engagement, and global cooperation. By investing in research, technology, and policy initiatives aimed at preventing exposure, improving treatment outcomes, and promoting environmental sustainability, we can address the complex challenges posed by toxic metal contamination and create healthier, more resilient communities for future generations.
Conclusion:
In conclusion, the management of toxic metal poisoning requires a multi-pronged approach that integrates preventive measures, prompt treatment, and long-term monitoring. Toxic metals such as lead, mercury, arsenic, and cadmium pose significant health risks, with adverse effects ranging from neurological impairment and cardiovascular disease to renal dysfunction and developmental abnormalities. Prevention remains paramount in reducing exposure to these contaminants, with regulatory measures, environmental monitoring, and public health interventions playing critical roles in controlling contamination levels and protecting human health. Once exposure has occurred, timely intervention is essential to mitigate acute toxicity and prevent long-term health consequences. Chelation therapy serves as a cornerstone of treatment for severe cases of metal poisoning, facilitating the removal of toxic metals from the body and reducing body burden. Chelating agents such as EDTA, DMSA, DMPS, and dimercaprol are commonly used in clinical practice to enhance metal excretion and promote recovery. Supportive care measures, including fluid replacement, symptomatic treatment, and gastrointestinal decontamination, complement chelation therapy by addressing associated symptoms and complications. Furthermore, long-term monitoring and follow-up are crucial components of managing toxic metal poisoning, enabling healthcare providers to assess recovery, monitor residual metal levels, and identify potential health effects.
Periodic blood and urine testing, neurodevelopmental assessment, renal function monitoring, and cardiovascular evaluation facilitate ongoing surveillance of affected individuals and guide interventions to optimize health outcomes. By implementing comprehensive management strategies and fostering collaboration between healthcare providers, public health agencies, and community stakeholders, we can minimize the adverse effects of toxic metal exposure and promote health and well-being for affected individuals and communities. However, challenges remain in addressing the complexities of toxic metal poisoning, including resource limitations, data integration issues, emerging contaminants, and the need for community engagement. Continued investment in surveillance infrastructure, capacity-building initiatives, and research efforts is essential for enhancing our understanding of metal exposure risks and developing targeted interventions to address evolving threats. By prioritizing prevention, early intervention, and long-term monitoring, we can mitigate the health risks associated with toxic metal exposure and create healthier environments for current and future generations. In essence, the management of toxic metal poisoning requires a comprehensive and collaborative approach that encompasses prevention, treatment, and ongoing surveillance. By leveraging scientific knowledge, regulatory frameworks, and community partnerships, we can mitigate the adverse effects of metal contamination and safeguard public health for generations to come.