Submitted:
25 November 2024
Posted:
09 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Sample Testing
2.1. Experimental Methods
2.2. Test Results
3. Numerical Simulation Method
3.1. Calculation Model of Incineration
3.2. Calculation Model of Ash Particles
4. Results and Analysis
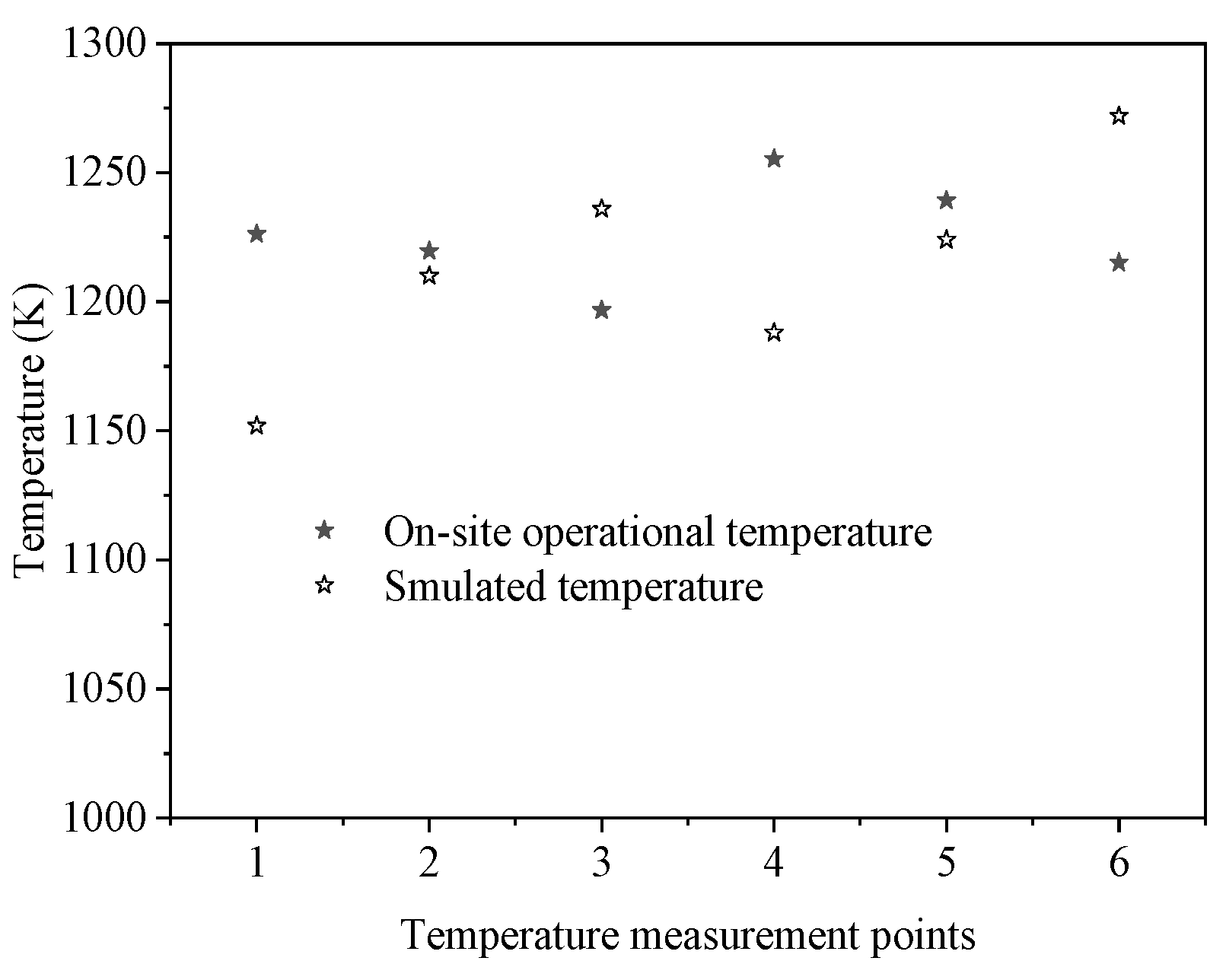
4.1. Calculation Results and Analysis of Gas-Phase Combustion
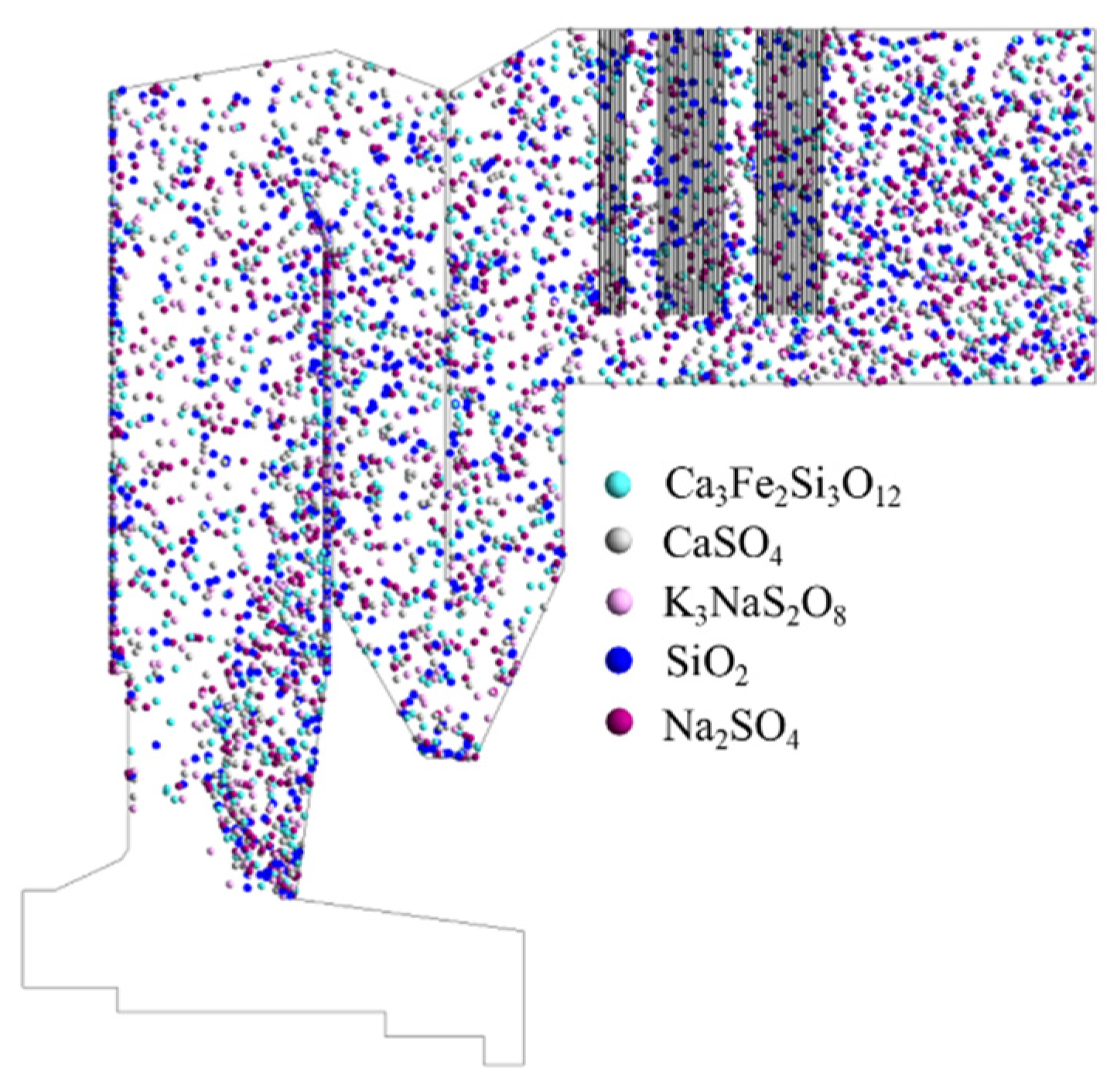
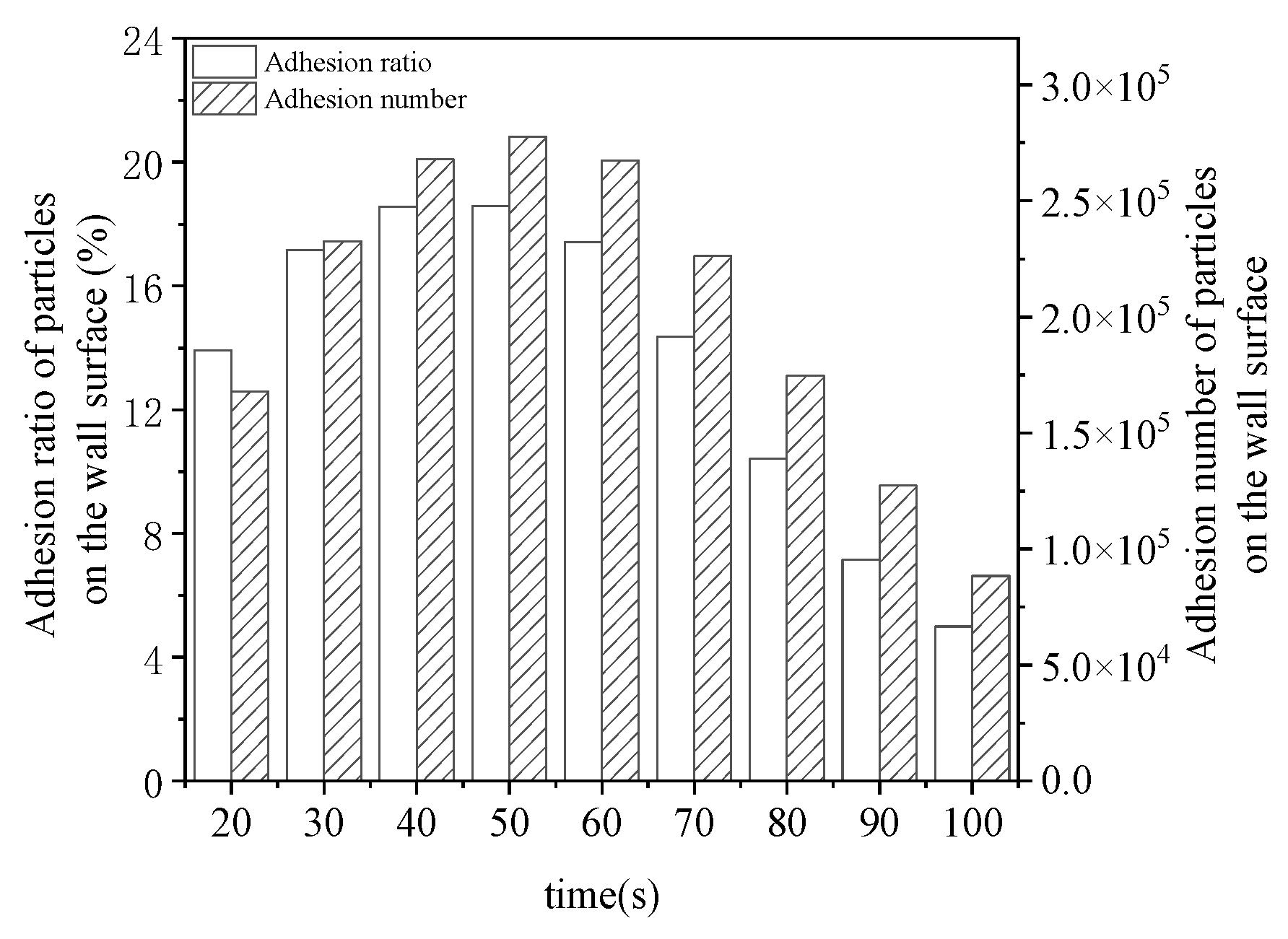
4.2. The motion Trajectory of Ash Particles
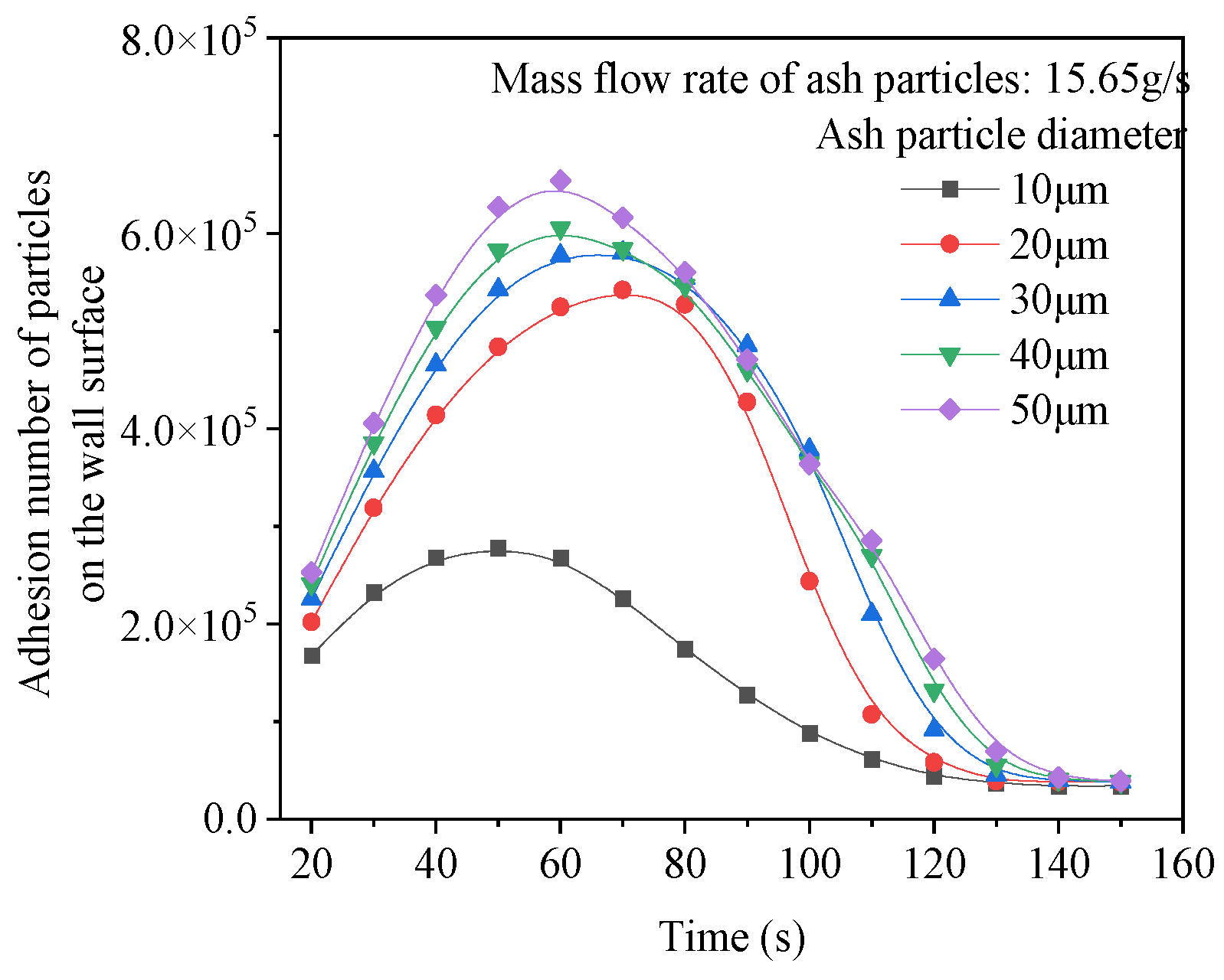
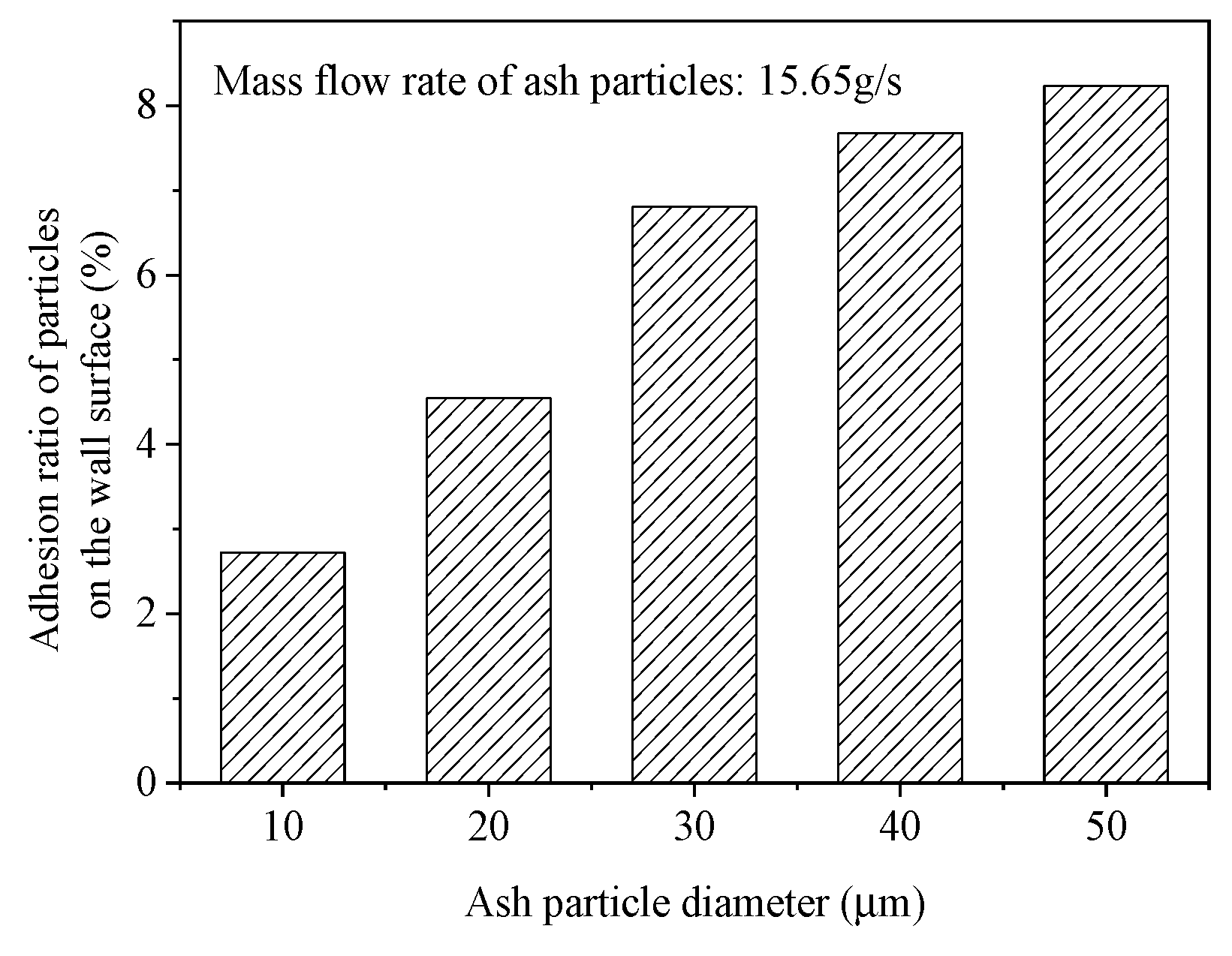
4.3. The Influence of Ash Particle Size
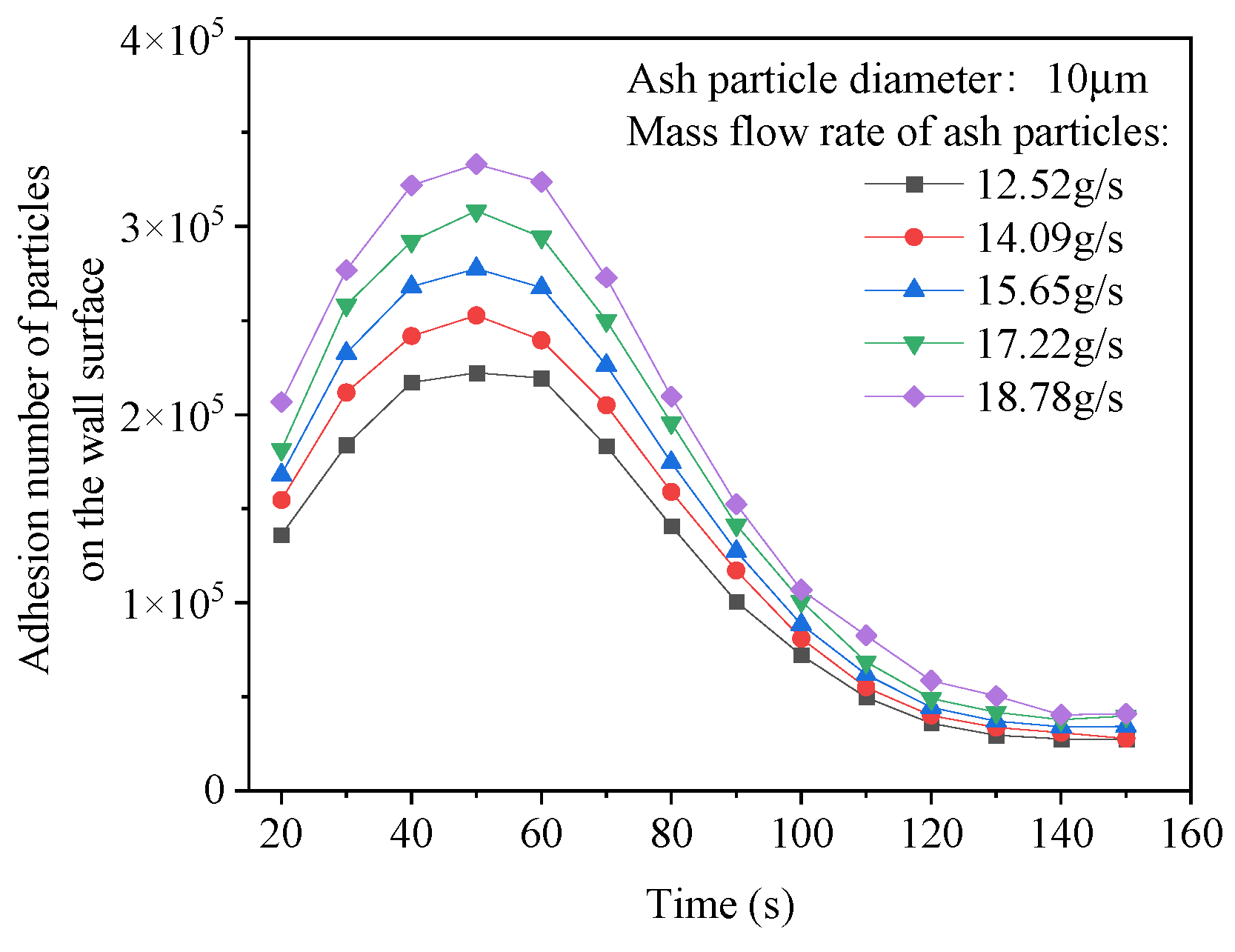
4.4. The Influence of Ash Particle Concentration
5. Conclusions
Acknowledgments
References
- Cheng W, Ju A L. Current Situation and Enlightenment on Incineration Treatment of Domestic Waste in Japan. Environmental Sanitation Engineering, 2019, 27(06): 57-60.
- Zhou D. Rapid development of waste-to-energy incineration in Europe. Chinese and Foreign Energy, 2018, 23(07): 38.
- National Bureau of Statistics. (2024). Retrieved from https://data.stats.gov.cn.
- Zhong D, Zeng K, Li J, et al. Characteristics and evolution of heavy components in bio-oil from the pyrolysis of cellulose, hemicellulose and lignin. Renewable and Sustainable Energy Reviews, 2022, 157: 111989.
- Wang R Q, Bo Rong B, Ma S. et al. Prediction of ash fusion temperatures of municipal solid waste incinerator. Journal of the Energy Institute, 2023,111: 101438.
- Jiang X G, Meng X F, Liu G J. Research status of deposition growth on heat exchange surface of waste incineration system and countermeasures for deposition control. Chemical Industry and Engineering Progress, 2021, 40(S1): 375-385.
- Ma W C, Zhou H, Zhang J K, et al. Behaviour of slagging deposits during coal and biomass co-combustion in a 300 kW down-fired furnace. Energy & Fuels, 2018, 32(4): 4399-4409.
- Walsh P M, Sayre A N, Loehden D O, et al. Deposition of bituminous coal ash on an isolated heat exchanger tube: Effects of coal properties on deposit growth. Progress in Energy and Combustion Science, 1990, 16(4): 327-345.
- Chen L, Yong S Z, Ghoniem A F. Modelling the slag behaviour in three dimensional CFD simulation of a vertically-oriented oxy-coal combustor. Fuel Processing Technology, 2013, 112: 106-117.
- Ni J, Zhou Z, Yu G, et al. Molten slag flow and phase transformation behaviors in a slagging entrained-flow coal gasifier. Industrial & Engineering Chemistry Research, 2010, 49(23): 12302-12310.
- Chen L, Ghoniem A F. Development of a three-dimensional computational slag flow model for coal combustion and gasification. Fuel, 2013, 113: 357-366.
- Wang X H. A review on numerical modeling of ash deposition during the process of pulverized coal combustion. ADVANCES IN MECHANICS, 2005(03): 417-426.
- Li W Y. The Numerical Simulation of Coal Powder Combustion Process and Slagging in Utility Boiler. North China Electric Power University (Beijing), 2003.
- Pintana P, Tippxayawong N. Prediction of Slag Occurrence in a Lignite-Fired Utility Boiler. WSEAS Transactions on Environment and Development, 2014, 10: 202-210.
- Pérez M G, Vakkilainen E, Hyppänen T. 2D dynamic mesh model for deposit shape prediction in boiler banks of recovery boilers with different tube spacing arrangements. Fuel, 2015, 158: 139-151.
- Tang S Z, He Y L, Wang F L, et al. Parametric study on fouling mechanism and heat transfer characteristics of tube bundle heat exchangers for reducing fouling considering the deposition and removal mechanisms. Fuel, 2018, 211: 301-311.
- Fu L, Liu P, Li G. Numerical investigation on ash fouling characteristics of flue gas heat exchanger. Applied Thermal Engineering, 2017, 123: 891-900.
- Wang X H, Zhao D Q, Jiang L Q, et al. The deposition and burning characteristics during slagging co-firing coal and wood: modeling and numerical simulation. Combustion Science and Technology, 2009, 181(5): 710-728.
- Wang J J. Research on Optimization of Slagging Prevention Structure of Municipal Solid Waste Incinerator Based on CFD Numerical Simulation. Zhejiang University, 2022.
- Klasen T, Görner K. The use of CFD for the prediction of problem areas inside a waste incinerator with regard to slagging, fouling and corrosion.
- Ma T, Zhou H Q, Xu F, Chen D Z, et al. Numerical Simulation and Intelligent Prediction of a 500t/d Municipal Solid Waste Incinerator. Energy, 2024, 312: 133646.
- Shuen J S, Solomon A S P, Zhang Q F, et al. Structure of particle-laden jets-Measurements and predictions. AIAA Journal, 1985, 23(3): 396-404.
- Lee F C C, Lockwood F C. Modeling ash deposition in pulverized coal-fired applications. Progress in Energy and Combust Science, 1999, 25(2): 117-132.
- Costen P G, Lockwood F C, Siddique M M. Mathematical modeling of ash deposition in pulverized fuel-fired combustors. Proceedings of the Combustion Institute, 2000, 28(2): 2243-2250.
- Richards G H, Slater P N, Harb J N. Simulation of ash deposit growth in a pulverized coal-fired pilot scale reactor. Energy and Fuels, 1993, 7(6): 774-781.
- Jia T Y, Chen S P, Teng L L, et al. Characteristics and mechanism of slagging in a 500 t/d MSW incinerator, Journal of the Energy Institute, 2024, 114: 101585.


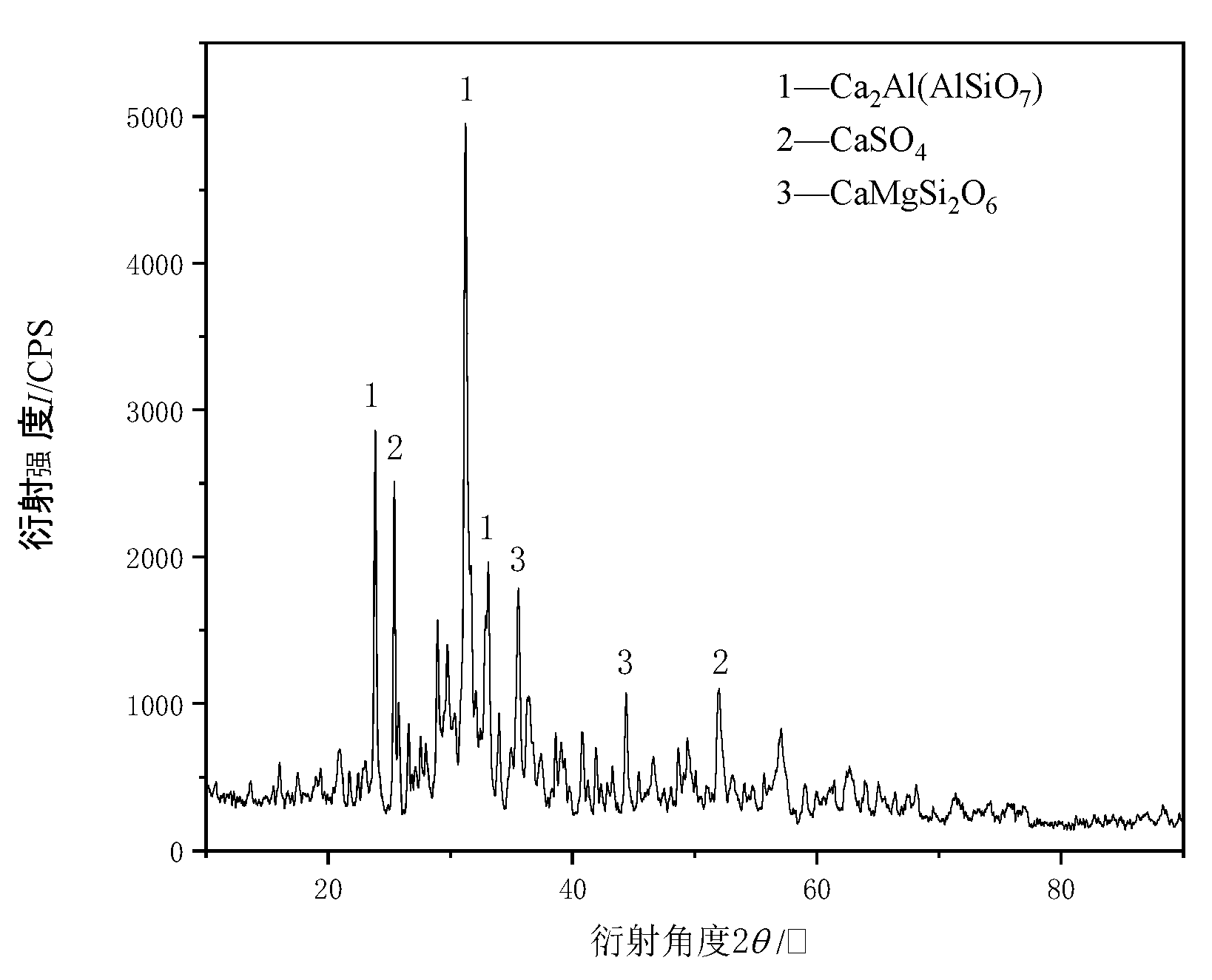
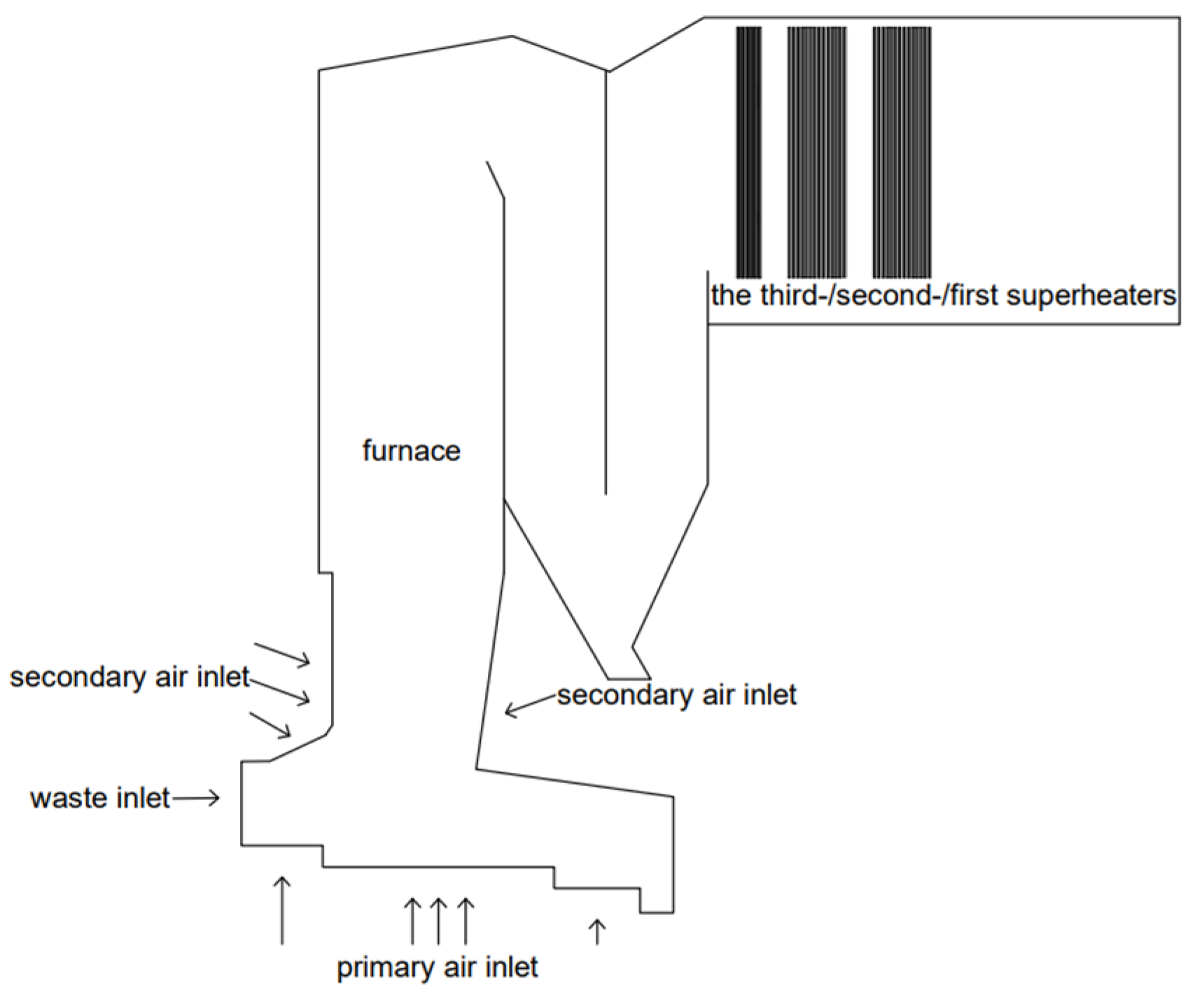
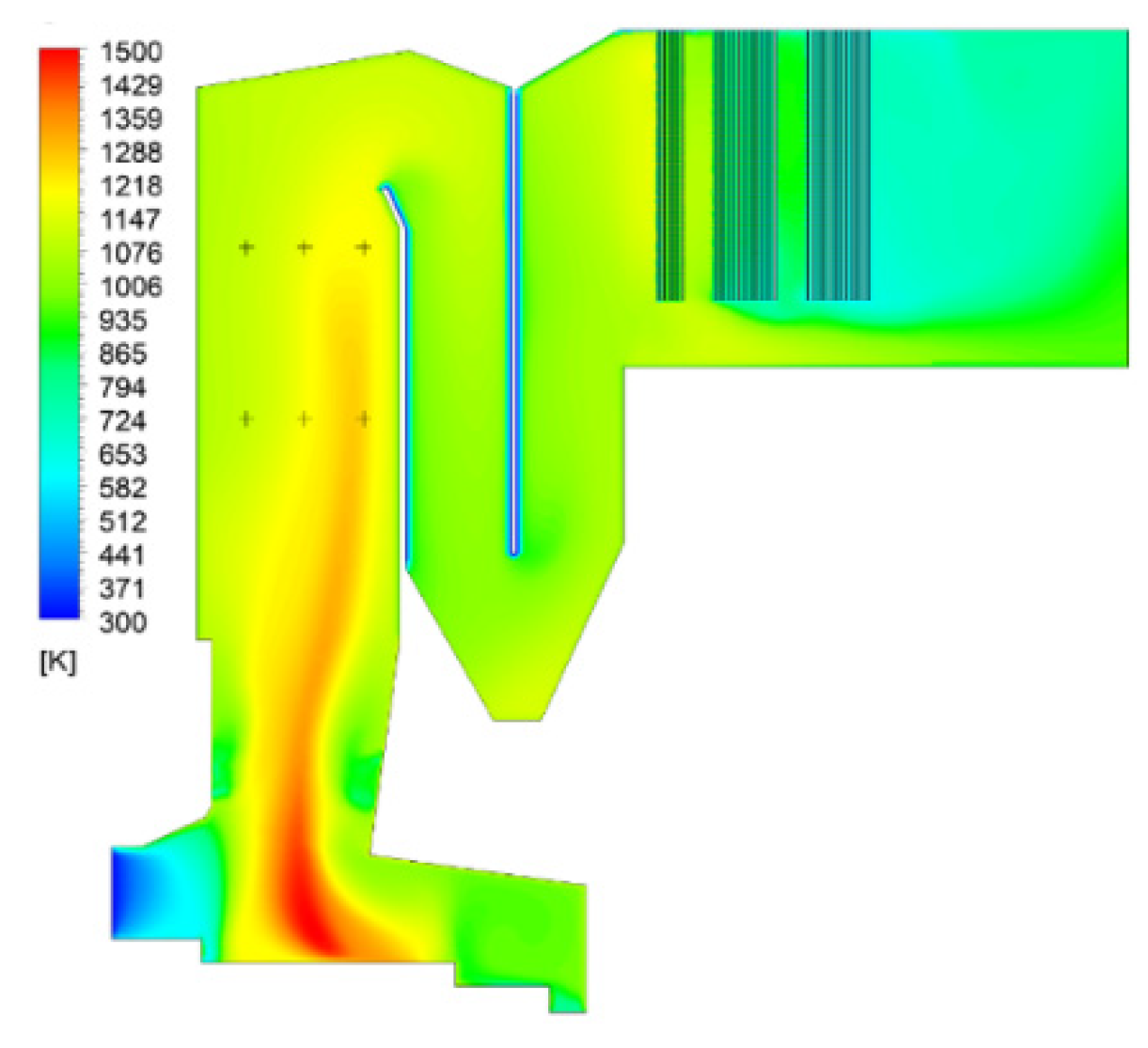
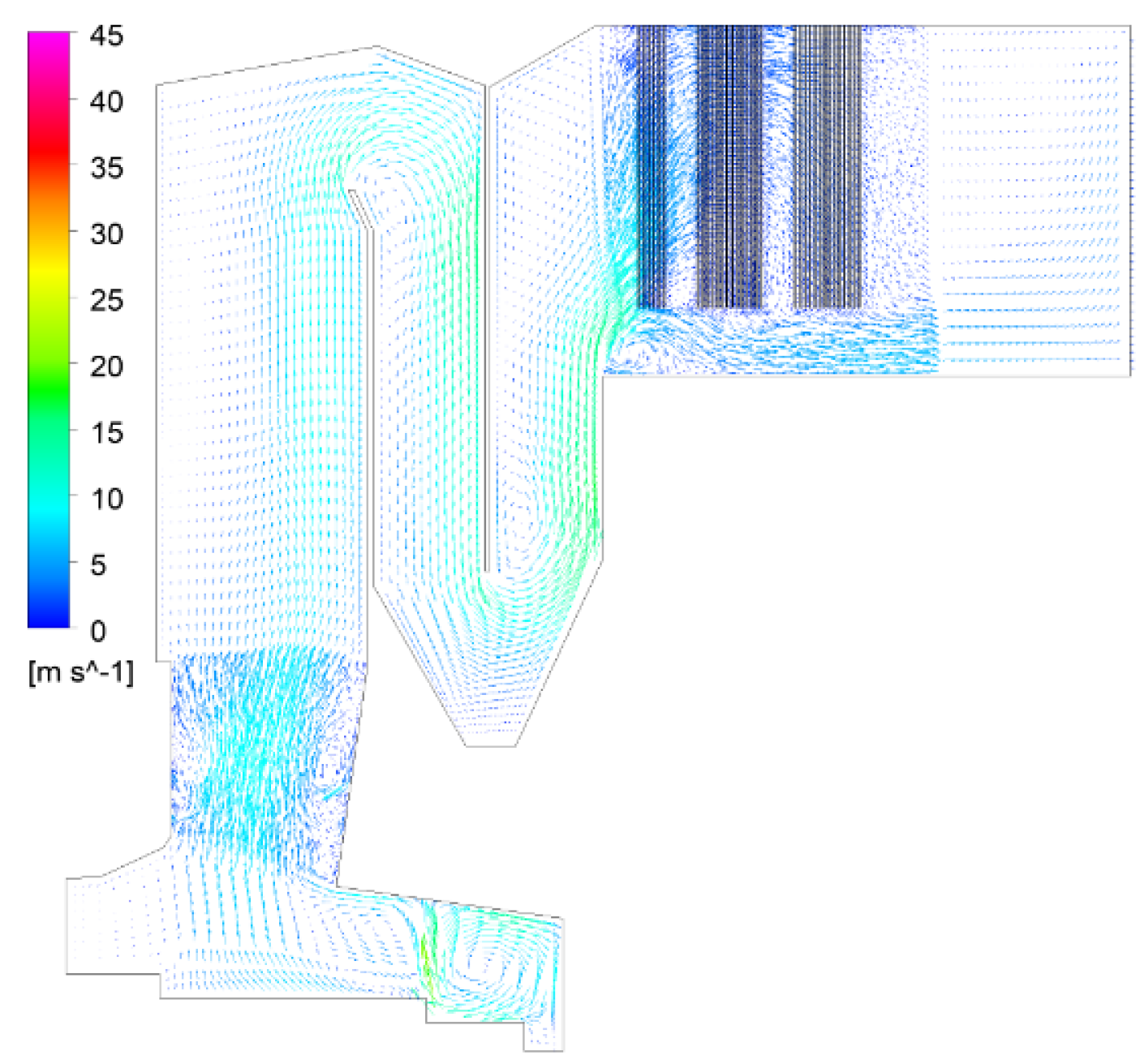


| CaO | SiO2 | SO3 | Fe2O3 | Al2O3 | Na2O | K2O |
|---|---|---|---|---|---|---|
| 25.008 | 17.506 | 14.588 | 13.305 | 7.161 | 7.064 | 4.214 |
| P2O5 | TiO2 | MgO | ZnO | Cl | Cr2O3 | MnO |
| 2.519 | 2.486 | 2.403 | 2.146 | 0.656 | 0.283 | 0.254 |
| CuO | PbO | NiO | SrO | Sb2O3 | ZrO2 | Br |
| 0.094 | 0.084 | 0.07 | 0.05 | 0.042 | 0.041 | 0.016 |
| Deformation temperature (℃) | Softening temperature (℃) | Hemispherical temperature (℃) | Flow temperature (℃) |
|---|---|---|---|
| 1111 | 1113 | 1116 | 1128 |
| Parameter | Value |
|---|---|
| Diameter (m) | 10−5 |
| Temperature (K) | 1090 |
| Mass flow rate (g/s) | 15.65 |
| Velocity (m/s) | 6.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




