1. Introduction
Rice (
Oryza sativa L.) is a vital staple crop for more than half of the world's population due to its crucial role in global food security. However, the aging process during seed storage results in an annual loss of approximately 3% of rice yield [
1]. On the other hand, the deterioration of seed germination ability due to short seed storability poses a major challenge for high yield and better quality [
2]. Therefore, enhancing seed storability is of paramount importance to guarantee the safety and preservation of grain and rice seeds during natural storage condition.
Seed storability refers to the longevity of seeds after storage. It is regarded as a significant agronomic trait for maintaining seed fitness at post-harvest [
3]. Generally, seed storability is influenced by genetic and environmental factors throughout the stages of plant growth, seed maturation, and post-harvest. Improving the seed storage environment demands substantial resources and labor costs, which is not economically feasible. Nevertheless, leveraging genetic factors through breeding program has proven to be highly effective in enhancing the storability of rice seed and grain. Over the past decade, more than 70 quantitative trait loci (QTLs) associated with seed storability have been identified using QTL location analysis and association mapping approaches under natural or artificial aging conditions [
4]. However, only a few QTLs such as
qSS-9 or
qGP-9,
qSS1, and
qSS3.1 have been precisely mapped [
5,
6,
7]. To date, several genes associated with storage resistance have been successfully cloned. For example, The cloned genes included the lipid peroxidation-involved genes
OsLOX1,
OsLOX2,
OsLOX3 and
OsLOX10 [
1,
8,
9,
10],
OsALDH7,
OsGLYI3 and
OsAKR1 genes responsible for scavenging toxic substances [
11,
12,
13], reactive oxygen species-scavenging genes
OsHSP18.2 and
OsMSRB5 [
14,
15], protein repair genes
OsPIMT1 and
PIMT2 [
16,
17], antioxidant accumulation gene
Rc [
18], and antioxidant enzyme genes
OsCSD2 and
OsCSD2 [
19]. These identified genes have significantly enhanced our understanding of the underlying mechanisms of storage resistance to a certain extent. However, due to the genetic mechanism complexity, QTLs and associated genes of the seed storage mechanism have not been well elucidated.
Metabolomics is primarily used to examine a wide range of small-molecule metabolic intermediates such as lipids, nucleic acids, amino acids, peptides, carbohydrates, organic acids, ketones, aldehydes, amines, steroids, vitamin signaling molecules, hormones, and polyphenols [
20,
21,
22]. Metabolomics can be classified into targeted metabolomics, non-targeted metabolomics, and a novel integrated method known as widely targeted metabolomics [
23]. Widely targeted metabolomic technique offers greater precision than non-targeted analysis [
23,
24,
25]. Presently, metabolomics has emerged as a functional genomics strategy that contributes to dissecting the complex molecular interactions within biological systems [
26].
Usually, the composition and content of accumulated metabolites in newly matured rice seeds have been determined, which directly dictates the subsequent storage capacity under external environmental conditions. In this study, 375 rice core accessions from 47 countries were initially collected and evaluated for their seed storability. Seed storability was observed to show significant variation among four groups (Basmati, Indica, Aus, Japonica), with Basmati rice exhibiting the highest seed storability, followed by Indica rice, Aus rice, and Japonica rice. However, there is also considerable variability in storage tolerance within each group, with the Indica rice group manifesting the largest number of germplasm. Then, a resistant storage pool of five extremely resistant varieties and a sensitive storage pool of five highly sensitive varieties within the Indica rice group were selected subject to the UPLC-MS/MS widely targeted metabolomics analysis to comprehend the metabolic disparities among rice seeds with varying storability. Consequently, a number of differential accumulated metabolites (DAMs) and significant metabolic pathways related to storage stability were identified. These findings will deepen our understanding of the underlying mechanisms of governing rice storability and provide a valuable avenue for improving rice storability through breeding programs.
2. Materials and Methods
2.1. Plant Materials
The rice population consisting of 375 cultivated rice accessions was collected from the 3K Rice Genome (3K-RG) and obtained from the Institute of Crop Sciences, Chinese Academy of Agricultural Sciences in Beijing, China. These accessions were selected as core collections from 47 counties representing major rice-growing regions worldwide (Table S1). These varieties were cultivated in the Fujian Nanfan base, located in Tengqiao Town, Haitang District, Sanya City, Hainan Province.
2.2. Natural Seed Aging Treatment
The Newly harvested rice seeds were stored at room temperature in the Rice Research Institute, Fujian Academy of Agricultural Sciences. Fifty healthy and filled seeds were randomly chosen for treatment after 3 months and 24 months, with three replicates for each sample.
2.3. Seed Germination Test
The treated seeds were sown on two layers of filter paper [
4] and germinated in an incubator set at a temperature of 30°C with a relative humidity of 75% under a light cycle of 14 hours per day for a duration of 14 days. The germination percentage was calculated by dividing the number of germinated seeds by the total number of seeds.
2.4. Sample Preparation and Extraction for Metabolomic Analysis
We selected five Indica rice varieties with extremely resistant storage and extremely sensitive storage, respectively, to form an aging-resistant Indica rice pool (AR) and an aging-sensitive Indica rice pool (AS). Each variety was represented by 50 shelled seeds. The samples were frozen using a vacuum freeze-drier (Scientz-100F), followed by grinding with a mixer mill (MM400, Retsch) and zirconia beads at 30 Hz for 1.5 min until they turned into powder. Lyophilized powder (50 mg) was dissolved in 1.2 ml of a 70% methanol solution, vortexed every 30 min for 30 sec (6 x replicates), and refrigerated overnight at 4℃ to ensure proper dissolution. Subsequently, the samples were centrifuged at 12,000 rpm for 3 min and filtered before UPLC-MS/MS analysis. To assess the repeatability of the measurement process, quality control (QC) samples were prepared by combining all sample extracts, with one QC sample analyzed after every three regular samples.
2.5. Chromatographic Mass Spectrometry Acquisition Conditions
The sample extracts were analyzed using UPLC (Ultra Performance Liquid Chromatography, ExionLC™ AD,
https://sciex.com.cn/) coupled with MS/MS (Tandem mass spectrometry). The liquid phase conditions included a chromatographic column (Agilent SB-C18, 1.8 μm, 2.1 mm x100 mm), and a mobile phase consisting of solvent A (pure water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid). The elution gradient started at 95% A and 5% B for the first 9 min, followed by a linear gradient to reach 5% A and 95% B within that time frame. This composition was maintained for an additional minute before adjusting back to the initial conditions of 95% A and 5% B within another minute, which was then kept constant for the next 4 min. The flow rate was set at a velocity of 0.35 ml per minute, while the column oven temperature was maintained at a constant value of 40℃ throughout the analysis period. An injection volume of precisely two microliters was used in each run.
The effluent from the UPLC system was directed towards an ESI-triple quadrupole linear ion trap (QTRAP)-MS instrument for further analysis. For ESI source operation parameters: the source temperature was set to be stable at 550℃; ion spray voltage (IS) applied positive polarity mode had a value of 5500 V whereas negative polarity mode had -4500 V; GSI (ion source gas I), GSII (ion source gas II) and CUR (curtain gas) were adjusted to 50 psi, 60 psi, 25 psi respectively; CAD (the collision-activated dissociation) was set high. QQQ scans were acquired in MRM mode with nitrogen as collision gas set to medium. DP (declustering potential) and CE (collision energy) for individual MRM (multiple reaction monitoring) transitions were optimized accordingly. A specific set of MRM transitions corresponding to metabolites eluted during each specific period was monitored.
2.6. Multivariate Statistical Analysis
Multivariate statistical analysis was conducted to investigate the accumulation of accession-specific metabolites, employing hierarchical clustering analysis (HCA), Pearson correlation coefficient (PCC), principal component analysis (PCA), and orthogonal partial least squares discriminant analysis (OPLS-DA) on the metabolic data of each sample. Heatmaps with dendrograms were utilized to visually represent the HCA results for both metabolites and samples, while the cor function in R was employed to calculate PCC values for the samples. The heatmap package in R was used for generating heatmaps based on both PCC and HCA outcomes. PCA was performed using GraphPad Prism v9.01 software (GraphPad Software Inc., La Jolla, CA, USA) to display the original state metabolomic data and variables. Prior to HCA and PCA analyses, metabolite data were scaled by unit variance. To compare metabolic characteristics among different rice varieties, an OPLS-DA model was constructed using MetaboAnalystR package in R software. Log2 transformation of metabolite data followed by mean centering was applied before conducting OPLS-DA analysis to improve normality assumptions. A permutation test with 200 permutations was executed to prevent overfitting issues. DAMs screening criteria included a variable importance in projection (VIP) ≥ 1 in the OPLS-DA model, absolute Log2FC (fold change) ≥ 1, and p-value <0.05. Venn diagrams were employed to illustrate the number of DAMs.
2.7. KEGG Annotation and Enrichment Analysis
The different metabolites were annotated using the KEGG compound database (
https://www.kegg.jp/kegg/compound/) and mapped to the KEGG pathway database (
https://www.kegg.jp/kegg/pathway.html) for annotation and enrichment analysis. Metabolite sets enrichment analysis (MSEA) was performed on pathways with significantly regulated metabolites, and their significance was assessed using P-values from hypergeometric tests.
3. Results
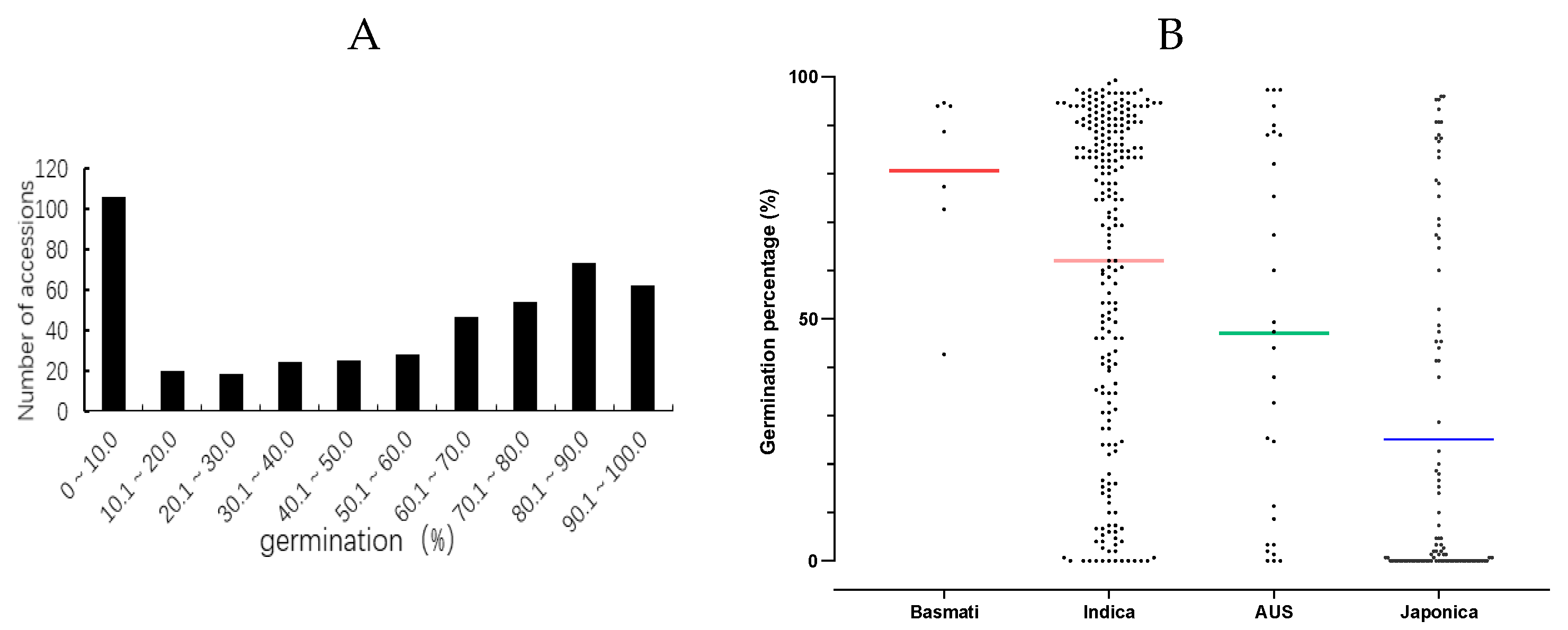
3.1. The Difference in Seed Storability of Global Rice Core Accessions After Natural Aging for 3 Months and Two Years
In this study, we collected a total of 375 rice core collections from 47 different countries (
Table S1), which were categorized into four rice groups (
Basmati,
Indica,
Aus, and
Japonica) [
27]. The seed germination was evaluated after natural aging for 3 months and 24 months in the Fuzhou laboratory, Fujian Province, China to assess the variation in seed storability among global rice core accessions. Following the 3 months of natural aging, the average germination rate was recorded 96.87%, with values ranging from 91.33% to 100% (
Table S2). Significant variations in seed germination percentage were observed within the population after natural aging for 24 months, with values ranging from 0% to 99.33%, and an average of 50.57% (
Table S2,
Figure 1A). The distribution curve of seed germination percentages among the collection of rice core accessions showed a continuity form, indicating the substantial differences with storability among these core accessions. Moreover, there was a notable disparity in seed storability among these four groups (
Figure 1B), with
Basmati exhibiting superior storability followed by
Indica,
Aus, and
Japonica groups, respectively.
3.2. Metabolic Profiling Analysis in AR and AS Pools
To further dissect the disparity and metabolite mechanisms of seed storability in
Indica group popularized in Fujian, China, five
Indica rice varieties exhibiting extreme resistance to storage aging and five extreme sensitivity to storage aging were selected, forming a pool of
Indica varieties with high resistant to aging (AR) and a pool of
Indica varieties with high sensitivity to aging (AS), respectively. As a result, a significant disparity was observed between the five aging-resistant
Indica varieties and the five aging-sensitive
Indica varieties after undergoing natural aging for 24 months. The seed germination percentages of the AR group exceeded 94%, while the AS group exhibited almost negligible germination capability (
Table 1).
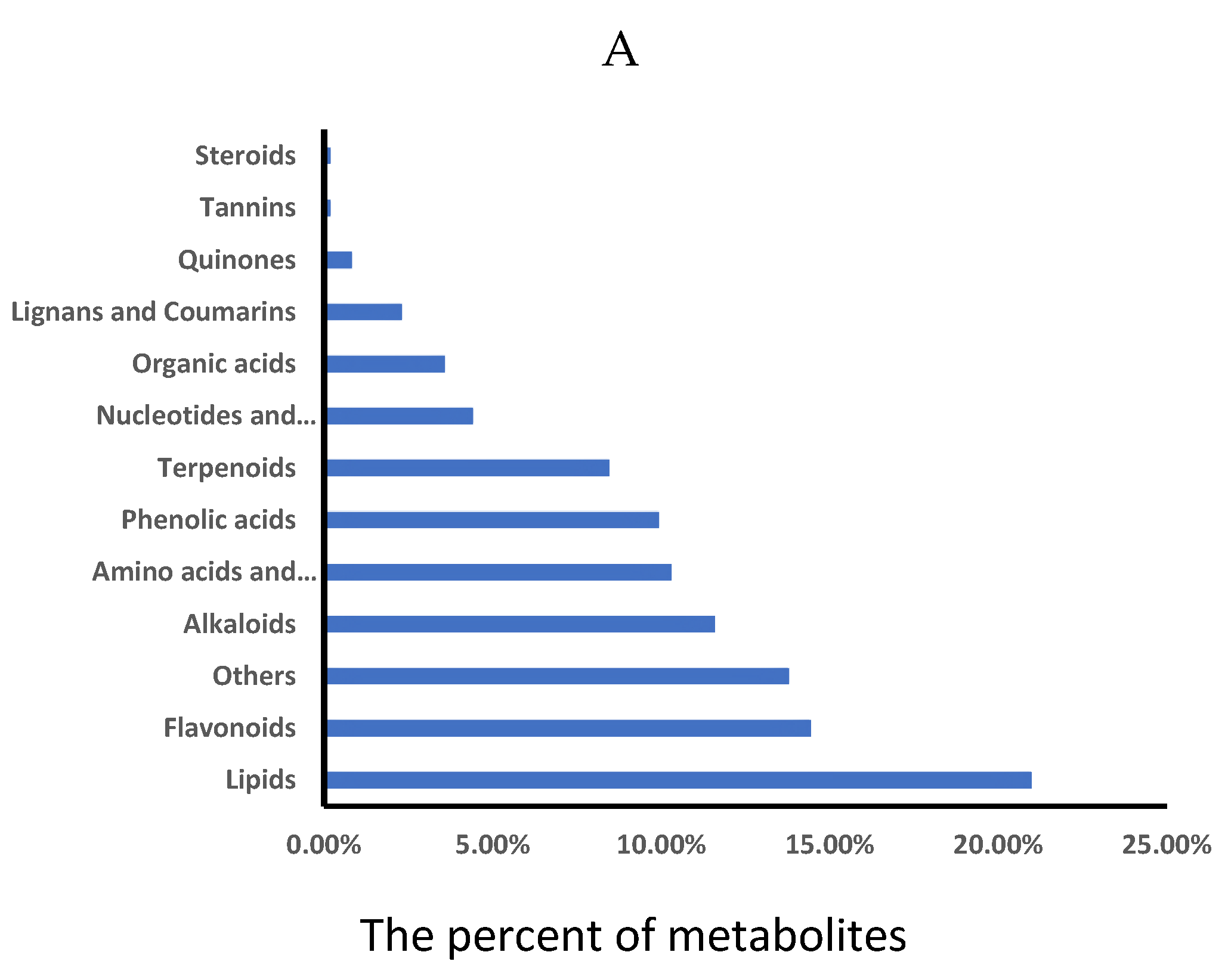
Then, to further elucidate the mechanisms of storage tolerance, comprehensive metabolic profiling of AR and AS groups was analyzed by using a widely targeted metabolite analysis based on UPLC-MS/MS method. The UPLC-MS/MS method enabled to accurately and precisely identify a total of 1098 metabolites, encompassing lipids (20.98%), flavonoids (14.44%), alkaloids (11.59%), amino acids and derivatives (10.30%), phenolic acids (9.94%), terpenoids (8.46%), nucleotides and derivatives (4.42%), organic acids (3.59%), lignans and coumarins (2.30%), quinones (0.83%), tannins (0.18%), as well as others compounds (13.80%) (
Figure 2A,
Table S3).
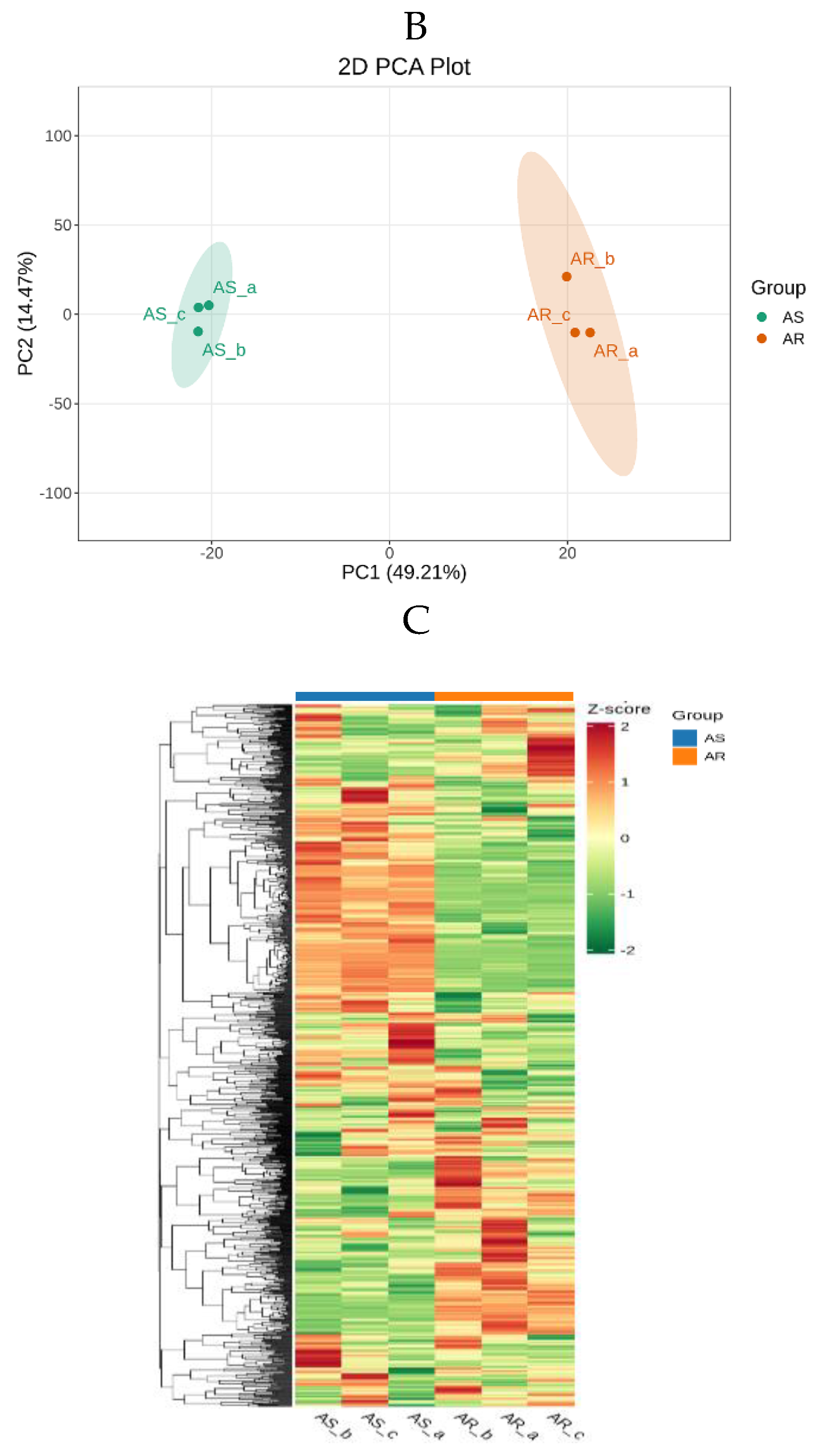
The unsupervised PCA model disclosed that two principal components accounted for 63.68% of total variance, with PC1 contributing to 49.21% and PC2 to 14.47%, respectively. This indicated a significant difference in metabolic profiles between AR and AS groups (
Figure 2B). HCA was carried out based on relative content differences of metabolites. Three biological replicates demonstrated the repeatability and data reliability. The HCA Figure further confirmed significant differences in metabolic profiles observed through PCA analysis (
Figure 2C).
3.3. Identification of Differently Accumulated Metabolites in AR and AS Pools
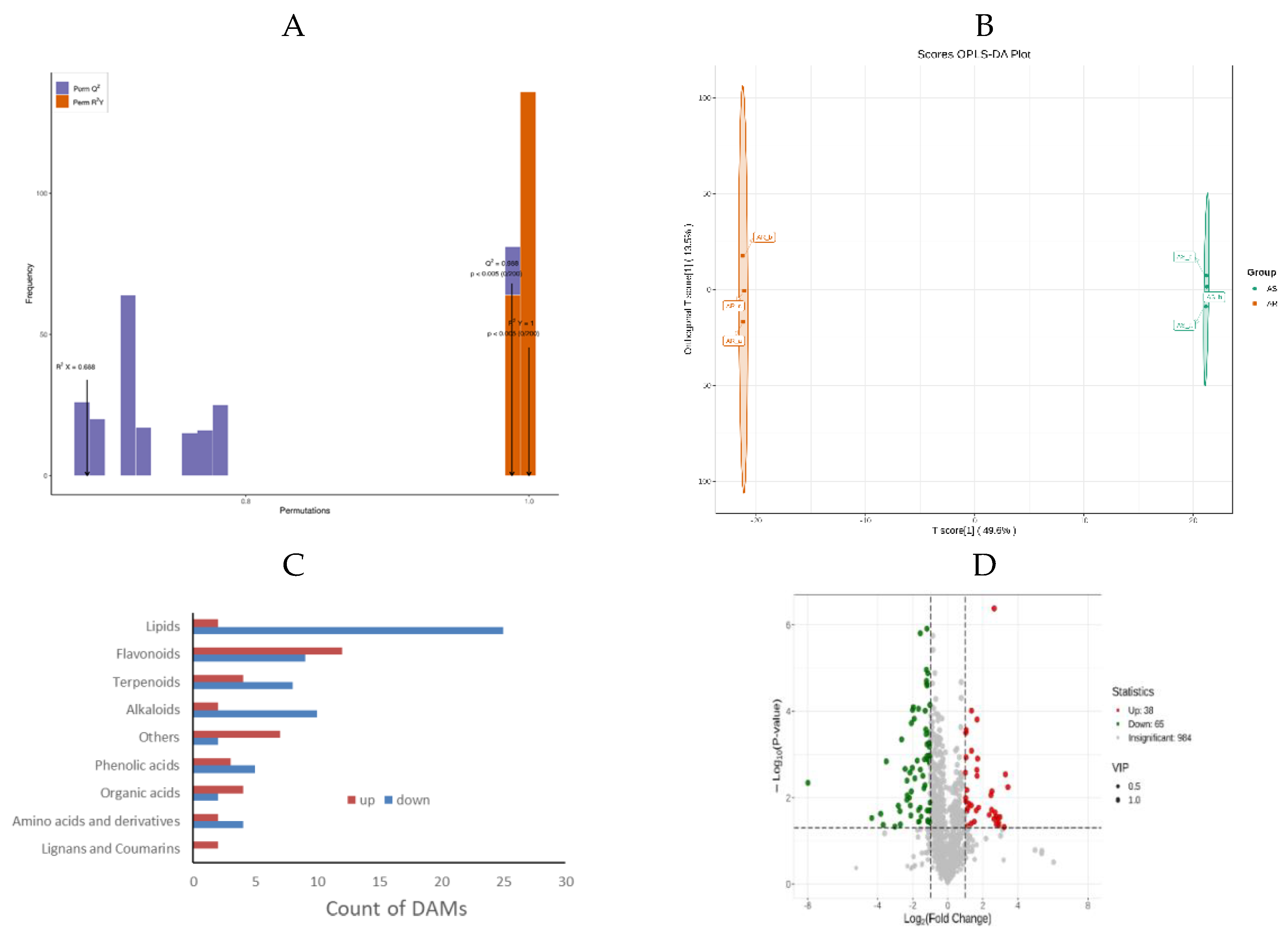
The OPLS-DA model was utilized to identify differently accumulated metabolites (DAMs) by eliminating orthogonal variables that were irrelevant to the classification variables in metabolites, thereby maximizing group discrimination. Pairwise comparisons between AR and AS groups were carried out using the OPLS-DA model. The appropriateness of the OPLS-DA model was verified through 200 alignment experiments, pairwise comparison results showed that both R2Y and Q2 scores exceeded 0.9 (
Figure 3A).
Additionally, the well-distinguished OPLS-DA score plots manifested the differences in the metabolic profiles between AR and AS groups. DAMs were selected based on variable importance in projection (VIP) ≥ 1, fold changes (FC) ≥ 2 or FC ≤ 0.5, and p-value <0.05 as determined by the OPLS-DA model. Volcano plots depicted the screening results (
Figure 3B), revealing a total of 103 significant DAMs between AR and AS groups, with 38 upregulated and 65 down-regulated, respectively (
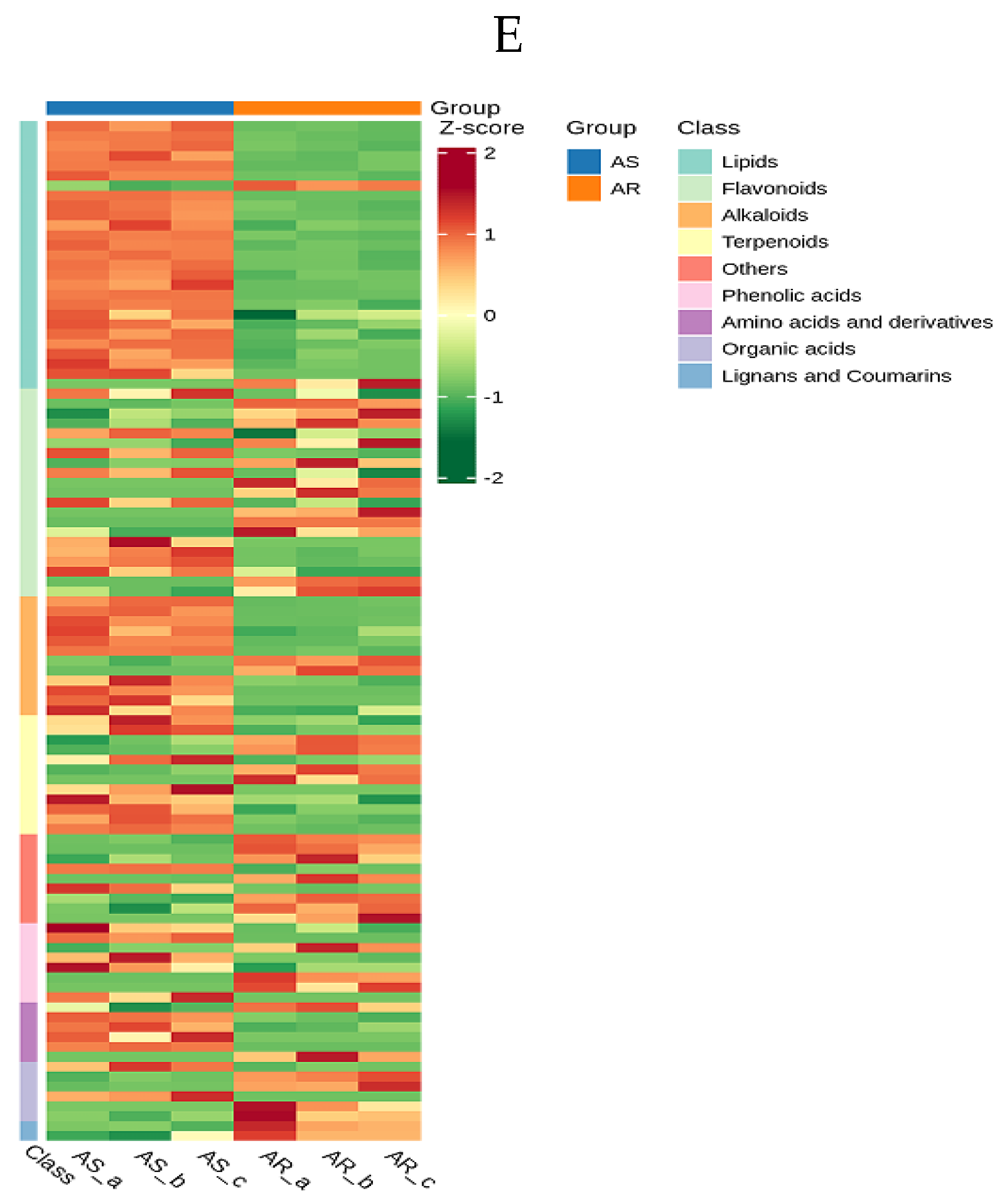
Table S4). An overall cluster heatmap displayed all DAMs for these pools (
Figure 3C). The DAMs were categorized into nine different categories (
Figure 3D), Lipids and alkaloids are the first and fourth categories of DAMs, respectively. 92.6 % of lipids and 83.33 % of alkaloids were down-regulated, indicating that the accumulation of lipids and alkaloids was not favorable for seed storage. As for the second category of DAMs, flavonoids were up-regulated by 57.14 %, indicating that flavonoids had a considerable impact on storage resistance, and most of them were beneficial to storage resistance. Terpenoids constitute the third major group of DAMs, with 66.67% of the metabolites exhibiting down-regulation. On the whole, this observation suggested that these compounds significantly influenced storage resistance, predominantly exerting a negative impact on the seed storage resistance. Organic acids constituted the seventh group of DAMs, with 66.67% of the metabolites upregulated, namely: succinic Acid, methylmalonic acid, isocitric acid-1-O-diglucoside, and D-malic acid. This result also indicated most of them were beneficial to storage resistance. Interestingly, despite being the least abundant DAMs, lignans and coumarins were up-regulated, implying their potential role in positive contribution towards storage tolerance. The effects of metabolites belonging to other categories on storage resistance are more complex and require further exploration.
3.4. KEGG Annotation and Enrichment Analysis of DAMs in AR and AS Pools
The KEGG database functions as a primary public pathway database, facilitating investigations into signal transduction pathways and metabolite accumulation processes [
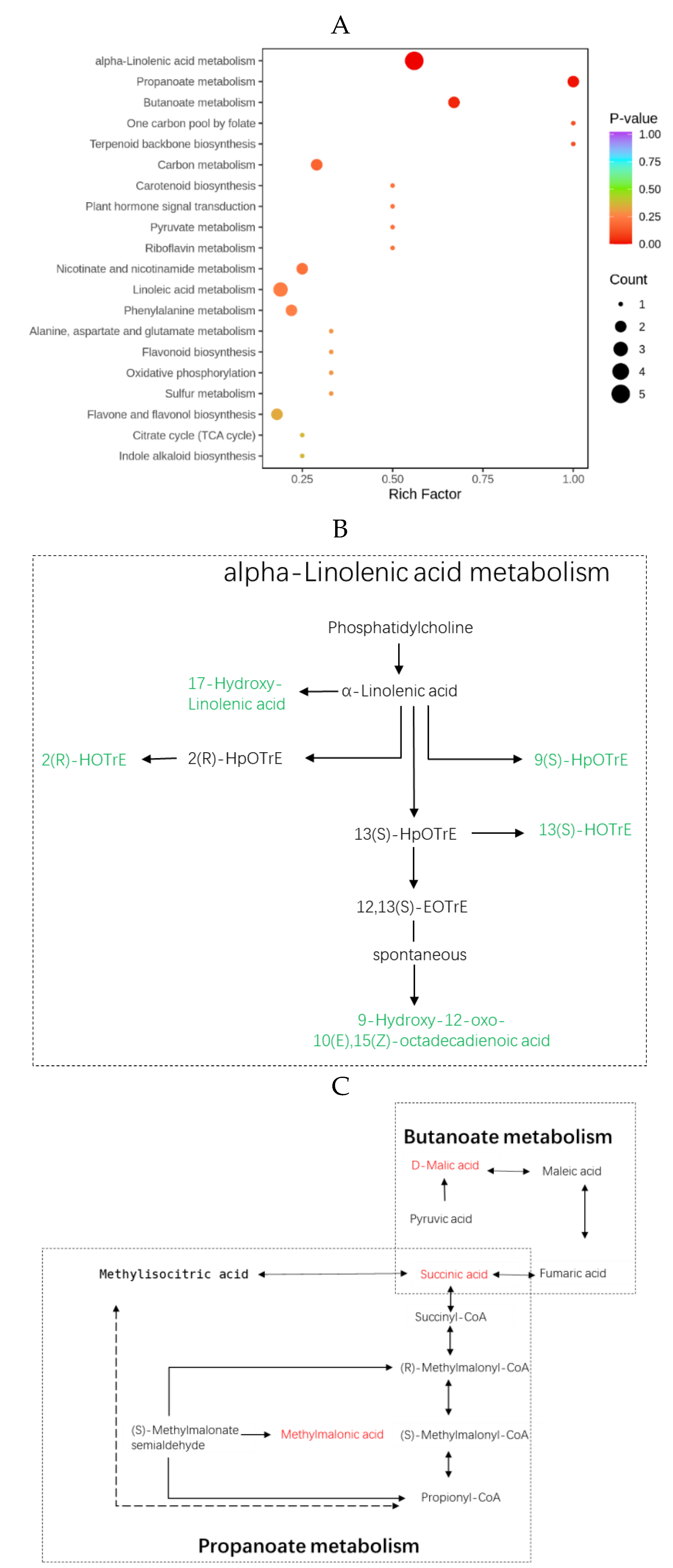
28]. In this study, we annotated and enriched the DAMs by assigning them to distinct KEGG pathways. The enriched DAMs were discovered to be associated with 38 pathways, with major pathways illustrated through bubble plots presented in
Figure 4 and
Table S5. Notably, alpha-linolenic acid metabolism, butanoate metabolism, and propanoate metabolism emerged as three significantly enriched metabolic pathways based on statistical significance criteria set at p-value < 0.05. For the alpha-linolenic acid metabolism pathway, all five metabolites, 17-hydroxy-linolenic acid, 2(R)-HOTrE, 9(S)-HpOTrE, 13(S)-HOTrE, and 9-hydroxy-12-oxo-10(E),15(Z)-octadecadienoic acid, exhibited the down-regulation mode. However, in both butanoate and propanoate metabolism pathways, all three metabolites, succinic acid, D-malic acid, and methylmalonic acid, demonstrated the up-regulation mode. The KEGG enrichment analysis revealed that the inhibition of the linolenic acid metabolic pathway could enhance seed storability, while increased accumulation of organic acids, such as succinic acid, D-malic acid, and methylmalonic acid, in butanoate and propanoate metabolisms was beneficial for seed storage, respectively.
4. Discussion
4.1. There Is a Significant Difference in Metabolite Profiles Between Two Different Storability Indica Rice Pools
Seed deterioration after harvest poses a significant challenge to rice production during natural storage [
29]. Previous studies have explored the alterations in rice metabolites during seed storage with varying storability [
12,
30,
31]. Nevertheless, limited researches have been carried out on the accumulation of initial metabolites in rice with different storage capabilities. In this study, we noticed a considerable disparity in seed storability among
Indica rice groups after 24 months of natural aging. We examined the differences in metabolomes between an extremely resistant storage pool (AR) and a sensitive storage pool (AS) within
Indica rice group. Our results disclosed 103 significantly distinct metabolites with 38 upregulated and 65 down-regulated, respectively. The main differentially abundant metabolites included lipids, flavonoids, alkaloids, and terpenoids. Furthermore, key metabolic pathways such as alpha-linoleic acid metabolism, butanoate, and propanoate metabolism were significantly enriched in the seed storability in the
Indica rice group.
4.2. The Role of Lipids in Seed Storability
The lipid substances, including triacylglycerols (TAGs), diacylglycerols (DAGs), monoacylglycerols (MAGs), and free fatty acids (FFAs), are present in rice seeds. These lipid substances are susceptible to peroxidation, leading to membrane damage and the generation of toxic byproducts [
32]. Lipid peroxidation products such as malondialdehyde and acetaldehyde are closely related to rice storability as they can cause cell damage and intoxication through reactions with macromolecules. Linolenic acid (LNA) and linoleic acid (LA) are significant polyunsaturated fatty acids involved in peroxidation processes that contribute to cell structure breakdown, the formation of cytotoxic products, and the release of volatile decomposition products, resulting in the deterioration of rice seeds [
33]. Our new findings indicated that lipids constituted the primary category of DAMs with a significant down-regulation rate of 92.6%. This suggested that lipid accumulation was detrimental to seed storage. KEGG analysis revealed that alpha-linolenic acid metabolism was the principal metabolite pathway associated with the observations. Notably, all five identified metabolites, 17-hydroxy-linolenic acid, 2(R)-HOTrE, 9(S)-HpOTrE, 13(S)-HOTrE, and 9-hydroxy-12-oxo-10(E), 15(Z)-octadecadienoic acid, showed down-regulation within the context of alpha-linolenic acid metabolism. Consequently, it can be deduced that lipid metabolite accumulation impedes seed storage efficiency.
4.3. The Role of Flavonoids in Seed Storability
Flavonoids, serving as essential antioxidants for growth, development, and breeding while enhancing stress resistance in crops [
34], may function by scavenging ROS to restrict oxidative stress [
35]. Proanthocyanins, a subclass of flavonoids with strong antioxidant activity, accumulation of which could enhance the storability of rice seeds under an elevated partial pressure of oxygen (EPPO) condition [
18]. In this study, a total of 39 different flavonoid metabolites were identified between AR and AS with 23 upregulated and 19 downregulated, respectively. Among the top 20 metabolites analyzed, five upregulated flavonoids were identified: 5,7,5'-trimethoxy-3',4'-methylenedioxyflavonoid; quercetin-3-o-rutinoside (rutin); Jaceosidin-7-O-(6''-O-p-coumaroyl) glucoside-4'-O-glucoside; (Z)-6-O-β-D-glucopranosyl-6,7,3',4'-tetrahydroxyaurone; Hispidulin-8-C-(2''-O-glucosyl) glucoside. Consequently, it can be concluded that the accumulation of flavonoids plays a crucial role in the storability of rice seeds.
4.4. The Role of Organic Acids in Seed Storability
Organic acids are widely utilized as antimicrobial agents within the food industry [
36]. Malic acid, functioning as a food safety reagent, was disclosed to be the most effective antimicrobial acid on diverse pathogen strains [
37,
38]. Succinic acid was also affirmed to possess antimicrobial effects [
39,
40]. In this article, we ascertained that there were six differential metabolites of organic acids, with four of them being upregulated, namely, succinic acid, methylmalonic acid, isocitric acid-1-O-diglucoside, and D-malic acid. These organic acids could potentially inhibit the activity of various microorganisms during rice seed storage, thereby enhancing the storage tolerance of rice seeds. The effect mechanism of organic acids on the storage tolerance of rice remains to be further explored.
4.5. The Role of Lignans and Coumarins in Seed Storability
Lignans consist of two phenylpropanoid units that exhibit C6-C3 linkage at b-b’ position of propyl side chain, showing stereo-selective oxidative coupling and the different substituted patterns of aromatic moieties [
41]. Lariciresinol, belong to lignans, is an antioxidant [
42], antiviral [
43,
44]. Coumarin is a fragrant organic chemical compound of the benzopyrone chemical class that is a natural substance found in many plant species, which exhibits a variety of potent pharmacological activities, including antioxidant, antibacterial, anti-inflammatory, antitumor, and antiviral activities [
30]. Eleutheroside B1, a coumarin compound, has a wide spectrum of anti-human influenza virus efficacy [
45]. In this study, we found that Lariciresinol and Eleutheroside B1 were upregulated in the aging-resistant
Indica pool. Thus, both of these substances could potentially inhibit the activity of various microorganisms during rice seed storage by means of antioxidant, antibacterial, and antiviral activities, thereby enhancing the storage tolerance of rice seeds.
5. Conclusions
We identified the storability of world rice core germplasm stored under natural aging conditions for two years. Subsequently, we conducted a comprehensive widely targeted metabolite analysis of UPLC-MS/MS on two different Indica rice pools with significant seed storability differences and identified 103 DAMs between them. Further analysis found that the aging-resistant rice pool demonstrated higher accumulation levels of flavonoids, terpenoids, phenolic acids, organic acids, lignans, and coumarins while exhibiting lower lipid content and alkaloid levels compared to the aging-sensitive rice pools in mature seeds. KEGG pathway enrichment analysis indicated that the inhibition of the linolenic acid metabolic pathway and increased accumulation of organic acids, such as succinic acid, D-malic acid, and methylmalonic acid within butanoate, and propanoate metabolism, were identified as a beneficial factor for seed storage under natural aging conditions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Table S1: Basic information of 375 rice accessions; Table S2: The average germination rate in the whole population after natural aging 3 and 24 months storage condition; Table S3: Complete list of the measured metabolites in AS vs AR; Table S4: Different accumulated metabolites in AS vs AR; Table S5: Metabolic pathways of differential metabolites in AS vs AR.
Author Contributions
Conceptualization and formal analysis and data curation and writing—original draft preparation, F.W.; data curation, Y.D.W., Y.Z., and W.H.; data curation, writing—review, and editing, Q.C, Y.H.W., and X.L.; validation, J.Z. and G.X.; supervision, H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Fujian Provincial Science and Technology Program (2022R1023009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing, China for providing the 375 cultivated rice accessions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, H.; Wei, Y.; Zhu, Y.; Lian, L.; Xie, H.; Cai, Q.; Chen, Q.; Lin, Z.; Wang, Z.; Xie, H. Antisense suppression of LOX3 gene expression in rice endosperm enhances seed longevity. Plant Biotechnol J. 2015, 13, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Debeaujon, I. Seed longevity: survival and maintenance of high germination ability of dry seeds. C R Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Hang, N. T.; Lin, Q.; Liu, L.; Liu, X.; Liu, S.; Wang, W.; Li, L.; He, N.; Liu, Z.; Jiang, L. Mapping QTLs related to rice seed storability under natural and artificial aging storage conditions. Euphytica. 2015, 203, 673–681. [Google Scholar] [CrossRef]
- Wu, F.; Luo, X.; Wang, L.; Wei, Y.; Li, J.; Xie, H.; Zhang, J.; Xie, G. Genome-wide association study reveals the QTLs for seed storability in world rice core collections. Plants. 2021, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wang, W.; Ren, Y.; Jiang, Y.; Sun, A.; Qian, Y.; Zhang, Y.; He, N.; Hang, N. T.; Liu, Z. Genetic dissection of seed storability using two different populations with a same parent rice cultivar N22. Breed Sci. 2015, 65, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shao, G.; Wang, L.; Wang, Z.; Mao, Y.; Wang, X.; Zhang, X.; Liu, S.; Zhang, H. QTL identification and fine mapping for seed storability in rice (Oryza sativa L.). Euphytica. 2017, 213, 1-12.
- Yuan, Z.; Fan, K.; Xia, L.; Ding, X.; Tian, L.; Sun, W.; He, H.; Yu, S. Genetic dissection of seed storability and validation of candidate gene associated with antioxidant capability in rice (Oryza sativa L.). Int J Mol Sci. 2019, 20, 4442.
- Bai, S.; He, N.; Zhou, L.; Shen, B.; Wu, W.; Liu, X.; Jiang, L.; Wan, J. Knock-down of OsLOX by RNA interference leads to improved seed viability in rice. J Plant Biol. 2015, 58, 293–302. [Google Scholar] [CrossRef]
- Huang, J.; Cai, M.; Long, Q.; Liu, L.; Lin, Q.; Jiang, L.; Chen, S.; Wan, J. OsLOX2, a rice type I lipoxygenase, confers opposite effects on seed germination and longevity. Transgenic Res. 2014, 23, 643–655. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; Zhang, L.; Shi, Y.; Song, Y.; Wang, X.; Cai, Q.; He, W.; Xie, H.; Zhang, J. The lipoxygenase OsLOX10 affects seed longevity and resistance to saline-alkaline stress during rice seedlings. Plant Mol Biol. 2023, 111, 415–428. [Google Scholar] [CrossRef]
- Shin, J.-H.; Kim, S.-R.; An, G. Rice aldehyde dehydrogenase7 is needed for seed maturation and viability. Plant Physiol. 2009, 149, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, W.; Lai, J.; Liu, Q.; Zhang, W.; Chen, Z.; Gao, J.; Song, S.; Liu, J.; Xiao, Y. OsGLYI3, a glyoxalase gene expressed in rice seed, contributes to seed longevity and salt stress tolerance. Plant Physiol Biochem. 2022, 183, 85–95. [Google Scholar] [CrossRef]
- Nisarga, K. N.; Vemanna, R. S.; Kodekallu Chandrashekar, B.; Rao, H.; Vennapusa, A. R.; Narasimaha, A.; Makarla, U.; Basavaiah, M. R. Aldo-ketoreductase 1 (AKR1) improves seed longevity in tobacco and rice by detoxifying reactive cytotoxic compounds generated during ageing. Rice. 2017, 10, 1–12. [Google Scholar]
- Kaur, H.; Petla, B. P.; Kamble, N. U.; Singh, A.; Rao, V.; Salvi, P.; Ghosh, S.; Majee, M. Differentially expressed seed aging responsive heat shock protein OsHSP18. 2 implicates in seed vigor, longevity and improves germination and seedling establishment under abiotic stress. Front Plant Sci. 2015, 6, 713.
- Hazra, A.; Varshney, V.; Verma, P.; Kamble, N. U.; Ghosh, S.; Achary, R. K.; Gautam, S.; Majee, M. Methionine sulfoxide reductase B5 plays a key role in preserving seed vigor and longevity in rice (Oryza sativa). New Phytol. 2022, 236, 1042–1060. [Google Scholar] [CrossRef]
- Petla, B. P.; Kamble, N. U.; Kumar, M.; Verma, P.; Ghosh, S.; Singh, A.; Rao, V.; Salvi, P.; Kaur, H.; Saxena, S. C. Rice PROTEIN l-ISOASPARTYL METHYLTRANSFERASE isoforms differentially accumulate during seed maturation to restrict deleterious isoAsp and reactive oxygen species accumulation and are implicated in seed vigor and longevity. New Phytol. 2016, 211, 627–645. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, H.; Diao, L.; Zhu, Y.; Xie, H.; Cai, Q.; Wu, F.; Wang, Z.; Zhang, J.; Xie, H. Protein repair L-isoaspartyl methyltransferase 1 (PIMT1) in rice improves seed longevity by preserving embryo vigor and viability. Plant Mol Biol. 2015, 89, 475–492. [Google Scholar] [CrossRef]
- Prasad CT, M.; Kodde, J.; Angenent, G. C.; Hay, F. R.; McNally, K. L.; Groot, S. P. Identification of the rice Rc gene as a main regulator of seed survival under dry storage conditions. Plant Cell Environ. 2023, 46, 1962–1980. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yuan, Z.; Yu, Y.; Yu, S.; He, H. OsCSD2 and OsCSD3 enhance seed storability by modulating antioxidant enzymes and abscisic acid in rice. Plants. 2024, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Pramai, P.; Hamid, N. A. A.; Mediani, A.; Maulidiani, M.; Abas, F.; Jiamyangyuen, S. Metabolite profiling, antioxidant, and α-glucosidase inhibitory activities of germinated rice: Nuclear-magnetic-resonance-based metabolomics study. J Food Drug Anal. 2018, 26, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Alseekh, S.; Fernie, A. R.; Luo, J. The structure and function of major plant metabolite modifications. Mol Plant. 2019, 12, 899–919. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; He, L.; Yang, J.; Fernie, A. R.; Luo, J. Natural variance at the interface of plant primary and specialized metabolism. Curr Opin Plant Biol. 2022, 67, 102201. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: application in the study of rice metabolomics. Mol Plant. 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Akiyama, K.; Sakata, A.; Kuwahara, A.; Otsuki, H.; Sakurai, T.; Saito, K.; Hirai, M. Y. Widely targeted metabolomics based on large-scale MS/MS data for elucidating metabolite accumulation patterns in plants. Plant Cell Physiol. 2009, 50, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Cao, G.; Hou, X.; Huang, M.; Du, P.; Tan, T.; Zhang, Y.; Zhou, H.; Liu, X.; Liu, L. Development of a widely targeted volatilomics method for profiling volatilomes in plants. Mol Plant. 2022, 15, 189–202. [Google Scholar] [CrossRef]
- Hall, R.; Beale, M.; Fiehn, O.; Hardy, N.; Sumner, L.; Bino, R., "Plant metabolomics: the missing link in functional genomics strategies," ed: American Society of Plant Biologists, 2002.
- Wang, W.; Mauleon, R.; Hu, Z.; Chebotarov, D.; Tai, S.; Wu, Z.; Li, M.; Zheng, T.; Fuentes, R. R.; Zhang, F. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature. 2018, 557, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fu, H.; Zhou, X.; Chen, Z.; Luo, Y.; Cui, B.; Chen, G.; Liu, J. Comparative proteomic analysis of seed embryo proteins associated with seed storability in rice (Oryza sativa L) during natural aging. Plant Physiol Biochem. 2016, 103, 31–44. [Google Scholar] [CrossRef]
- Yan, S.; Huang, W.; Gao, J.; Fu, H.; Liu, J. Comparative metabolomic analysis of seed metabolites associated with seed storability in rice (Oryza sativa L.) during natural aging. Plant Physiol Biochem. 2018, 127, 590-598.
- Mou, C.; Chen, Y.; Zhang, P.; Tong, Q.; Zhu, Z.; Ma, T.; Wang, P.; Fu, K.; Chen, C.; Huang, Y. Prolongation of seed viability and grain quality in rice by editing OsLOX1 using CRISPR/Cas9. Mol Breed. 2024, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Davies, M. J. The oxidative environment and protein damage. Biochim Biophys Acta. 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Zhou, K.; Luo, Z.; Huang, W.; Liu, Z.; Miao, X.; Tao, S.; Wang, J.; Zhang, J.; Wang, S.; Zeng, X. Biological roles of lipids in rice. Int J Mol Sci. 2024, 25, 9046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, J.; Guo, H.; Yang, C.; Li, Y.; Shen, S.; Zhan, C.; Qu, L.; Liu, X.; Wang, S. OsRLCK160 contributes to flavonoid accumulation and UV-B tolerance by regulating OsbZIP48 in rice. Sci China Life Sci. 2022, 65, 1380–1394. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Rajjou, L.; North, H. M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying alive: molecular aspects of seed longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Coban, H. B. Organic acids as antimicrobial food agents: applications and microbial productions. Bioprocess Biosyst Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef] [PubMed]
- Eswaranandam, S.; Hettiarachchy, N.; Johnson, M. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157: H7, and Salmonella gaminara. J Food Sci. 2004, 69, FMS79–FMS84. [Google Scholar] [CrossRef]
- Gadang, V.; Hettiarachchy, N.; Johnson, M.; Owens, C. Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, malic acid, and EDTA on a turkey frankfurter system. J Food Sci. 2008, 73, M389–M394. [Google Scholar] [CrossRef]
- Cox, N.; Mercuri, A.; Juven, B.; Thomson, J.; Chew, V. Evaluation of succinic acid and heat to improve the microbiological quality of poultry meat. J Food Sci. 1974, 39, 985–987. [Google Scholar] [CrossRef]
- Gao, Z.; Shao, J.; Sun, H.; Zhong, W.; Zhuang, W.; Zhang, Z. Evaluation of different kinds of organic acids and their antibacterial activity in Japanese Apricot fruits. Afr. J. Agric. Res. 2012, 7, 4911–4918. [Google Scholar] [CrossRef]
- Chhillar, H.; Chopra, P.; Ashfaq, M. A. Lignans from linseed (Linum usitatissimum L.) and its allied species: Retrospect, introspect and prospect. Crit Rev Food Sci Nutr. 2021, 61, 2719-2741.
- Bajpai, V. K.; Alam, M. B.; Quan, K. T.; Kwon, K.-R.; Ju, M.-K.; Choi, H.-J.; Lee, J. S.; Yoon, J.-I.; Majumder, R.; Rather, I. A. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci Rep. 2017, 7, 46035. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E. H.; Althagafy, H. S.; Baraka, M. A.; Abd-Alhameed, E. K.; Ibrahim, I. M.; El-Maksoud, A.; Mostafa, S.; Mohamed, N. M.; Ross, S. A. The promising antioxidant effects of lignans: Nrf2 activation comes into view. aunyn Schmiedebergs Arch Pharmacol. 2024, 1–20. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.; Liang, X.; Yang, Z.; Jiang, Z. Transcriptome profiling of influenza A virus-infected lung epithelial (A549) cells with lariciresinol-4-β-D-glucopyranoside treatment. PLoS One. 2017, 12, e0173058. [Google Scholar] [CrossRef]
- Yan, W.; Chen, J.; Wei, Z.; Wang, X.; Zeng, Z.; Tembo, D.; Wang, Y.; Wang, X. Effect of eleutheroside B1 on non-coding RNAs and protein profiles of influenza A virus-infected A549 cells. Int J Mol Med. 2020, 45, 753–768. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).