1. Introduction
In vitro production of bovine embryos has grown to the point where over a million embryos are transferred annually worldwide [
1]. However, improving pregnancy outcomes following embryo transfer (ET) remains a challenge, and depends on the non-invasive assessment of blastocysts [
2]. Around 80% of the approximately 2 million bovine embryos produced in 2022 were created
in vitro [
3]. However, only about 40% of such transferred IVP embryos resulted in live births in 2020 [
4]. The remaining 60% of pregnancy failures can be partly attributed to the transfer of poor-quality embryos, often resulting from inaccurate morphological evaluation using stereomicroscopy, which, currently, remains the primary method used by embryologists to assess the quality of
in vitro produced embryos. This approach is subjective and does not fully evaluate the embryo based on its intrinsic qualities. The current preference for
in vitro embryo production in the cattle industry and the limitations of morphology-based embryo evaluation methods highlight the need for comprehensive studies of pre-implantation bovine embryos to identify viability and quality markers that can more accurately predict post-transfer embryo survival and pregnancy outcomes. The success of conception, proper embryo development, and the birth of healthy offspring are important for both dairy and beef farms [
5].
Currently, three prominent research areas are gaining traction in the field of non-invasive embryo technology assessment: time-lapse microscopy enhanced by AI-based computational modeling [
6]; the examination of extracellular vesicles (EVs) released into the culture media by blastocysts [
7]; and, finally, the exploration of the correlation between metabolomic or proteomic markers in spent culture medium and embryo viability [
8,
9,
10,
11]. The metabolic shifts in the embryo's early development reflect the cellular metabolic processes and the overall health of the embryo [
12]. Thus, the utilization of metabolomic analysis of pre-implantation embryo growth media has demonstrated promising results in cattle and can provide several advantages [
2,
9,
11]. These include reducing the risk of compromising the embryo and its environment, lowering the overall costs of
in vitro production and embryo transfer by enhancing pregnancy rates, and reducing the need for open recipients. Recent research suggests that the metabolite profile of spent culture media may serve as a predictive indicator of pregnancy and birth outcomes in both cattle and humans [
11,
13,
14]. We found [
13] that blastocysts that led to successful embryo implantation exhibited notably elevated levels of methionine sulfoxide, dihydroxyphenylalanine (DOPA), spermidine, acetylcarnitine-to-free-carnitine ratio, C2 + C3-to-free-carnitine ratio, while demonstrating decreased levels of threonine (Thr) and phosphatidylcholine PC ae C30:0 compared to control media. Conversely, in comparison to embryos that did not successfully implant, only DOPA, spermidine, C2/C0, (C2 + C3)/C0, and PC ae C30:0 levels exhibited significant differentiation [
13].
The primary objective of this study was to assess the metabolomic profile of culture media from individual bovine embryos at a range of developmental stages and to investigate its correlation with developmental potential. This included examining outcomes such as morphological blastocyst quality and the ability of embryos to hatch
in vitro, a prerequisite for uterine attachment, and the initiation of placentation. Metabolic signatures have the potential to predict embryo viability and facilitate the development of a non-invasive test to select single embryos for transfer or cryopreservation. We examined the efficacy of targeted metabolomic analysis by utilizing the AbsoluteIDQ® p180 Targeted Metabolomics Kit in conjunction with liquid chromatography-tandem mass spectrometry (LC-MS/MS). This method enables thorough collection of a wide array of molecular content within the culture media of individually cultured bovine embryos [
13]. We hypothesized that the metabolomic profiles of high-quality early blastocysts differ from those of non-viable embryos that reach the blastocyst stage but experience developmental arrest at later stages.
2. Results
The full list of metabolites and their levels by study groups is given in Supplemental
Table S1. The rates of cleavage, blastocyst formation, and hatching observed in our laboratory are presented in Supplemental
Table S2.
2.1. Metabolites Differing in Culture Media of Viable and Non-Viable Blastocysts
In total, 2 metabolites showed highly significant differences (p < 0.001) and 29 exhibited significance (p between 0.05 and 0.001) between the culture media of the viable and non-viable blastocysts and empty controls (
Table 1). The concentrations of Met-SO and acetylornithine (Ac-Orn) in the culture media of degenerating blastocysts were significantly higher than that in the culture media of viable embryos. Asymmetric dimethylarginine (ADMA) and creatinine were undetectable in the empty media (CM) and the culture media of viable or hatched blastocysts but were present in the media of blastocysts that underwent developmental arrest. Kynurenine was detected in the media from non-viable blastocysts and in a small percentage of media from the hatched blastocysts. Viable early blastocysts, however, did not secrete any kynurenine.
Citrulline (Cit) was not detectable in the empty media, nor in culture media of viable blastocysts and hatched blastocysts but was present in the media of blastocysts that underwent developmental arrest. Among acylcarnitines, the uptake of acetylcarnitine (C2), propionylcarnitine (C3), and valerylcarnitine (C5) was higher in viable blastocysts compared to degenerating blastocysts. Similarly, among glycerophospholipids, the uptake of lysophosphatidylcholine C24:0 (LysoPC a C24:0) and phosphatidylcholine C42:4 (PC aa C42:4) was significantly higher in viable blastocysts compared to blastocysts that experienced developmental arrest at a later stage.
2.2. Metabolite Sums and Ratios Differing Between Culture Media of Viable and Non-viable Blastocysts
The summary concentration of metabolites that share a metabolic pathway or ratios of substrates and products of a pathway may be better indicators of metabolic activity than individual metabolites themselves [
2]. The oxidized methionine to methionine ratio (Met-SO/Met) indicated metabolic activity that significantly differentiated the control and viable blastocyst media from the media containing embryos that underwent developmental arrest (
Table 2). The ratio of total hydroxylated sphingomyelins to non-hydroxylated sphingomyelins was significantly lower in the culture media of non-viable blastocysts compared to the culture media from viable embryos, where the ratio resembled that in the control (empty) media.
Elevated ratios of MUFA PC/SFA PC and PUFA PC/SFA PC were observed in the culture media of blastocysts that underwent developmental arrest, while decreases in the concentrations of PC ratios were noted in the culture media of viable and hatched blastocysts compared to the control media. The concentration of hexoses, predominantly glucose, was lower in the culture media from non-viable embryos.
2.3. Metabolites Differing between the Culture Media of Viable Early and Hatched Blastocysts
Hatching is a step closer to successful implantation after surviving the first week of development. Although the zona pellucida, which is shed during hatching, is unlikely to hinder metabolite trafficking, metabolic adjustments may still occur simultaneously. We observed an increase in the concentration of lysophosphatidylcholines (LysoPC a C24:0, LysoPC a C20:4), phosphatidylcholine (PC ae C40:2), putrescine, spermine, spermidine, acylcarnitine C3, and a decrease in the concentrations of Met-SO and LysoPC a C26:0 in the culture media of hatching blastocysts compared to viable early blastocysts.
3. Discussion
3.1. Lipid Metabolism
From the list of metabolites analyzed, various lipids were the most prominent in distinguishing between viable and non-viable or viable and hatching blastocysts. The diverse roles of lipids in early embryo development have been recently reviewed [
15].
Acylcarnitines are intermediates in fatty acid beta oxidation and thus cellular and embryonic energetics [
16,
17]. The results of the current study suggest that an increase in the concentration of short-chain acylcarnitines, such as C2, C3, and C5, in the culture media indicates degenerating embryos. Carnitine esters of short chain fatty acids can originate from the beta oxidation of longer fatty acid residues but also from amino acid catabolism, such as the breakdown of branched-chain amino acids (BCAAs) [
18]. BCAAs play a vital role in embryo development, primarily in the synthesis of other amino acids [
19]. Thus, an excessive concentration of C5 in embryo culture media can be a warning sign of errors in embryo protein synthesis.
We also observed a significant difference in the levels of twelve PCs and a significant difference in one lysoPC in the culture media of viable versus non-viable blastocysts. The concentrations of PCs, with one exception, were lower in the culture media of viable early blastocysts compared to the embryos that underwent developmental arrest. When comparing the individual PC species and sums and ratios it seems that non-viable embryos release more PCs with unsaturated fatty acids. As fundamental structural elements of plasma lipoproteins and cell membranes, phosphatidylcholines play crucial roles in regulating cell function and signaling (25). The unsaturated fatty acid residues can be used for signaling, while saturated fatty acids are an energy reserve of the cells. In this case, the environment of blastocysts with developmental arrest is enriched with unsaturated fatty acid-containing PCs. The findings can be explained by either increased consumption of saturated lipids for energy or the overproduction of monounsaturated lipids.
Blastocyst hatching on day 8 of development was associated with fewer changes in lipid composition. LysoPCs were more likely to appear here, implying more intense lipid catabolism during this period. Free fatty acids, most likely originating from acylglycerols, have been previously identified as potential markers of viability in the later stages of embryo development [
11].
3.2. Monosaccharide Metabolism
We analyzed the concentration of the sum of hexoses in culture media containing vi-able early embryos and blastocysts that underwent developmental arrest. Interestingly, non-viable embryos consumed significant amounts of hexoses. The dominant hexose in the bovine culture media is glucose. During IVM, glucose metabolism, involving glycolysis and the pentose phosphate pathway (PPP), provides substrates essential for ooplasmic integrity and regulates oocyte meiotic maturation [
20]. Glucose serves as a substrate for ATP and NADPH production once biosynthesis gears up after the initial embryo cleavages [
20]. Although glucose is known to adversely affect early-stage embryo development, it serves as a critical energy substrate for embryos at the compacted morula and blastocyst stages. Studies in human pre-implantation embryos suggest that glucose may not be essential for development, but embryos that successfully develop to the blastocyst stage tend to exhibit higher glucose uptake [
21]. In some studies, the level of glucose consumption has been linked to the quality and success of pregnancy [
22]. We assume that the increased uptake of hexoses from culture media by degenerating embryos is indicative of mitochondria's inability to balance ATP supply and demand. Further studies are needed to establish a causal association between the consumption or secretion of hexoses and embryo viability.
3.3. Amino Acid and Derivative Metabolism
Previous studies have demonstrated that the consumption of essential amino acids can vary among embryos. In a recent study on bovine pregnancy prediction [
2], it was discovered that concentrations of glutamine (Glu), proline (Pro), Met, arginine (Arg), lysine (Lys), and Thr in embryo culture media exhibit the highest pregnancy-predictive capability, albeit when assessing a single metabolite at a time. Lechniak et al. [
9] compared various culture setups and observed significant differences in the metabolic pathways of phenylalanine (Phe), tyrosine (Tyr), Met, aspartate (Asp), Arg, Pro, and histidine (His) based on the culture system employed.
In our study, the majority of amino acids showed no significant differences between media containing viable embryos compared to empty control media. This suggests that the consumption and secretion of amino acids by embryos were relatively moderate relative to their levels in the growth media. Yet, three metabolites or metabolite groups related to amino acid metabolism emerged as elevated in non-viable blastocysts.
The first and most significant metabolite was oxidized Met or Met-SO. Met itself is an essential amino acid that has been extensively studied in recent blastocyst quality assessments and embryo implantation studies [
23], it is noteworthy that several studies have reported an increase in the concentration of Met in viable embryos [
24], as well as in the uterine lumen of pregnant animals [
25,
26]. Met oxidation to Met-SO is considered to protect cells from oxidative damage, although a more complex bioregulatory role cannot be excluded [
26,
27]. The twist is, that the levels of Met-SO in the culture media of viable blastocysts, which later progressed to hatching, were the lowest, whereas Met-SO levels rose significantly in the culture media of blastocysts that underwent developmental arrest in later stages. Hence, the antioxidative capacity of viable embryos is sufficient to lower oxidation in the environment, but the oxidation returns at the time of hatching. Previously, we have seen that a mediocre level of oxidative stress and an increase in Met-SO or Met-SO ratio to Met may be beneficial for successful embryo implantation [
13].
The second amino acid with a notable reduction in an environment of viable blastocysts was histidine. It too, is an essential amino acid. The third notable differentiator between culture media of viable and non-viable blastocysts, consistent with the aforementioned studies, is Arg metabolism. Even though Arg itself falls short in significance, an entire group of Arg-related metabolites emerged. ADMA is methylated arginine regulating nitric oxide (NO) generation from Arg. NO is produced when the enzyme nitric oxide synthase (NOS) catalyzes the oxidation of L-arginine to L-citrulline [
28]. It has been discovered that nitric oxide is associated with lower embryo quality and poorer pregnancy outcomes in
in vitro embryo production settings [
29]. Cit in the culture media can be derived from Arg via NOS or from ornithine (Orn) through the breakdown of proline or glutamine/glutamate [
30]. The physiological functions of citrullination in the early embryo remain poorly defined, although the presence of Cit in the culture media has been associated with transcriptional regulation and the DNA damage response [
31].
Cit and Orn together with Arg are intermediates in the most important nitrogen catabolic pathway, the urea cycle. Acetylornithine (Ac-Orn) has been previously found in bo-vine pre-ovulatory follicular fluid [
32]. Ac-Orn is an intermediate in the amino acid synthesis pathway and serves as a precursor to ornithine, which enters the urea cycle. Consequently, Ac-Orn is indirectly linked to the biochemical pathways that produce Arg and Pro [
33]. Non-viable embryos secreted Ac-Orn into the culture media, while viable blastocysts either consumed it slightly or maintained its level the same as in empty culture media.
Creatinine and polyamines are metabolites synthesized from Arg. In contrast to Cit, Orn, or ADMA, which can be reversibly converted to Arg, these are derivatives of Arg that cannot be converted back [
34]. Their upregulation suggests that they are either intentionally synthesized or that there is an excess of Arg that cannot be utilized in more meaningful ways. One can hypothesize that a higher concentration of Arg-related metabolites in the culture media may reflect disrupted development of embryos, dysregulation of polyamine and amino acid production, and a higher rate of embryonic death.
In our previous study on bovine pregnancy prediction, we observed that Thr levels were significantly lower in media from successfully implanted embryos compared to empty control media, however, we did not observe significant differences between the successful and failed implantation groups [
13]. In the current study, no significant differences in Thr levels were observed related to the viability of an early embryo.
3.4. Polyamine Metabolism
Polyamines are vital molecules with numerous roles in embryogenesis. They stabilize DNA and support its transcription, stabilize mRNA and assist in its translation for protein synthesis, promote cell growth, proliferation, and migration, maintain cell membrane stability, bind ATP, regulate ion channel functions, and mediate receptor-ligand interactions [
35]. In the current study, differences in polyamine concentrations in the culture media of viable and non-viable embryos were not significant, although putrescine and spermidine were detected in a few non-viable samples. When comparing the culture media of early embryos to that of hatched embryos, we found a marked increase in the levels of spermidine, spermine, and putrescine as hatching approached, while these metabolites were conspicuously absent in the media of viable early blastocysts. Curiously, spermine appeared exclusively in the culture media of embryos at the hatching stage, underscoring its unique association with this developmental phase. This aligns well with our previous study, in which we found significantly higher spermidine levels in the culture media of embryos that resulted in pregnancies. Specifically, 90.91% of culture media from pregnancy-yielding embryos contained spermidine, compared to only 20% in non-yielding embryos (p = 0.00035) [
13].
4.5. Future Research Directions
Despite various metabolites being proposed as embryo viability markers, available results remain limited, reproducibility is challenging, and their application across thousands of embryo production facilities is impractical. However, this study provides a comprehensive overview of metabolomic biomarkers for selecting viable early embryos from blastocysts undergoing developmental arrest, and describes metabolic changes in the culture media of hatching blastocysts. Further research is needed to develop practical, user-friendly benchtop tests leveraging insights from metabolomics-based studies. In the current study, oocytes for embryo production were obtained from abattoir ovaries, a factor known to influence oocyte quality and blastocyst rates, as indicated in previous studies [
36,
37]. Specifically, many crucial factors affecting oocyte and embryo quality—such as the donor's age, stage of the estrous cycle, nutritional status, genetic potential, and presence of reproductive disorders are often unknown. This aspect could be explored in future studies by running similar experiments using oocytes from live donors, without embryo transfers, to explore hatching outcomes and viable blastocyst development within a controlled laboratory environment, thereby eliminating the influence of the recipients.
4. Materials and Methods
4.1. Media
Serum-free media for all steps of in vitro embryo production, including in vitro maturation, fertilization, and the individual cultivation of embryos, were obtained from IVF Limited T/A IVF Bioscience (Bickland Industrial Park, Falmouth, Cornwall, TR11 4TA, UK). All media for the experiments were selected from the same production batch.
4.2. Experimental Design
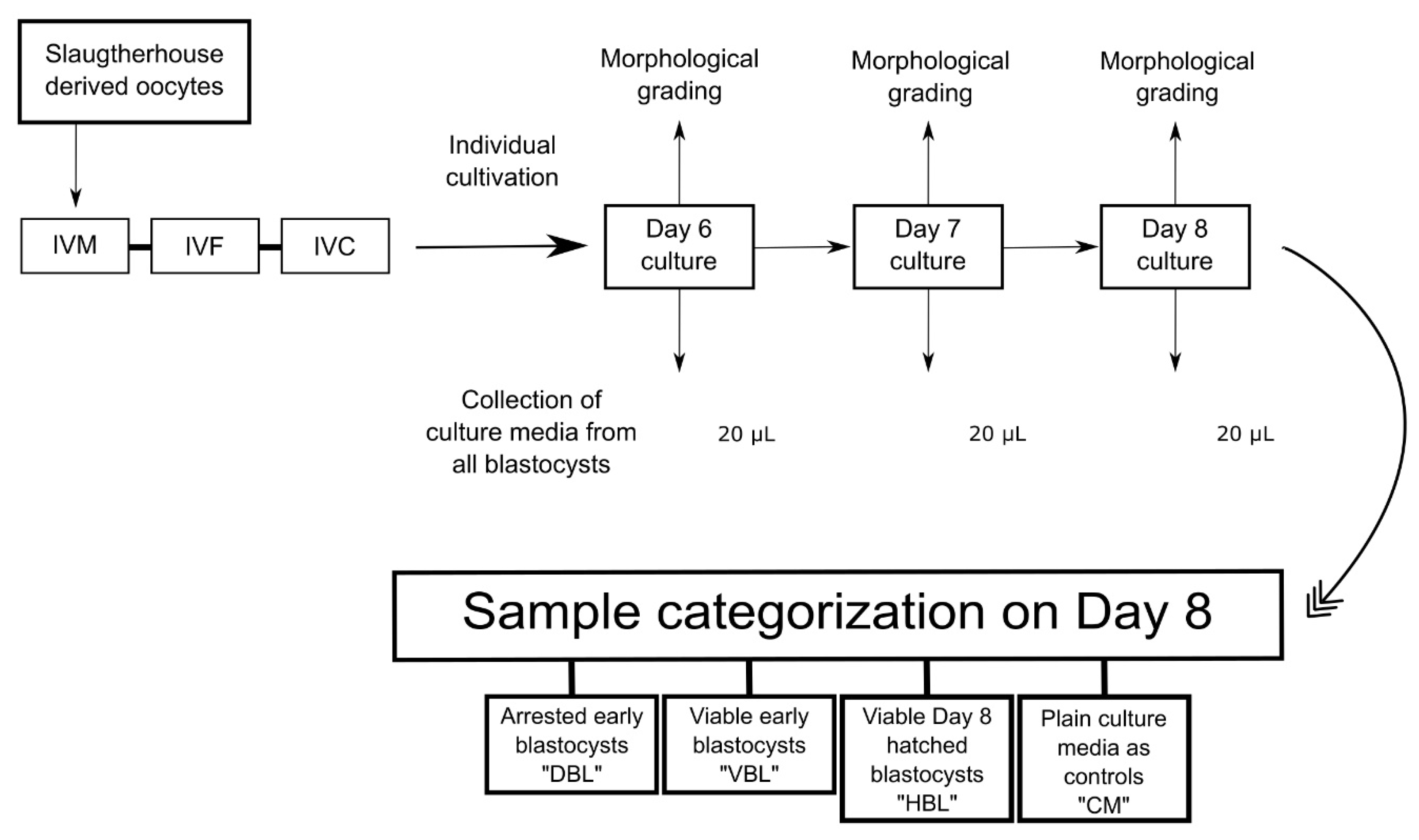
We used bovine embryos produced from slaughterhouse-derived oocytes to compare the metabolic fingerprints of the culture media of viable early blastocysts, hatched blastocysts, and blastocysts that underwent developmental arrest in a later stage. Culture media samples were collected from individually cultured embryos on days 6, 7, and 8 of culture. The samples were derived from IETS Grade 1 early blastocysts that later hatched (VBL; n = 10), IETS Grade 1 day 8 hatched blastocysts (HBL; n = 16), early blastocysts that underwent developmental arrest at later stages (DBL; n = 12), and plain culture media used as controls (CM; n = 5), resulting in a total of 43 samples.
4.3. Oocyte Collection and In vitro Maturation
Ovaries were collected from a local slaughterhouse (HK Scan Estonia Inc., Rakvere, Estonia). Cumulus oocyte complexes (COCs) were aspirated from 2 to 7 mm follicles using an 18-gauge needle. COCs with three or more layers of unexpanded cumulus cells and morphologically bright, evenly granulated cytoplasm were selected for
in vitro maturation [
36]. COCs were cultured in groups of fifty oocytes in 500 µL of BO-IVM medium, incubated at 38.5 °C with 5% CO2 in humidified air for 24 h.
4.4. In vitro Fertilization and Cultivation
In vitro fertilization and cultivation were carried out according to our previous report [
13], with the only modification being that zygotes were cultured for up to 8 days[
13]. In brief, frozen-thawed semen (commercially produced by the Animal Breeders’ Association of Estonia, Keava) from a bull, EHF ZIARD 27481 (ID EE13993023) was used for IVF. The matured oocytes and sperm were co-incubated at a final concentration of approximately 1 × 10
6 motile sperm/mL at 38.5 °C with 5% CO2 in air with maximum humidity. After 18 h, zygotes were denuded and cultured in 60 μL BO-IVC culture medium droplets (one zygote per droplet) overlaid with mineral oil in 90 mm Petri dishes (Sigma-Aldrich, St. Louis, MO, USA) at 38.5 °C under an atmosphere of 5% CO2, 5% O2, and 90% N2 for up to 8 days. Controls were created by placing 60 μL of plain BO-IVC culture media droplets that were never in contact with an embryo under oil, and were kept under the same conditions as zygotes. At 48 h post-IVF, droplets were examined to record embryo cleavage data. All uncleaved oocytes were discarded from the experiment.
4.5. Collection of Media for LC-MS/MS and Categorization of Samples
Culture media samples (20 µL) were collected from droplets of individually cultured blastocysts at days 6, 7, and 8. Morphological assessments were conducted on each embryo at the time of media collection, adapted from previously published work [
38,
39]. Any embryos arrested in development by day 6 were excluded from the experiment. Subsequently, samples were categorized according to their hatching outcomes: “VBL” refers to viable early blastocysts that later progressed to hatching on day 8, “HBL” refers to hatched blastocysts, and “DBL” designates non-viable early blastocysts that developed to the blastocyst stage, but failed to progress to hatching. Samples of plain culture media were collected on day 8 and treated as controls (hereafter referred to as "CM"), following the methodology established in previous research [
40]. All collected media samples were labeled and stored immediately after collection at −20 °C (Figure 1).
4.6. Preparation of Culture Media Samples for LC-MS/MS and Spectrometry
Each 20 µL culture media sample underwent preparation according to the sample preparation protocol of The AbsoluteIDQ® p180 Targeted Metabolomics Kit for Agilent Infinity high-performance liquid chromatography (Agilent, Santa Clara, CA, USA) coupled to the 4500 QTRAP® ion trap mass spectrometer (Sciex, Framingham, MA, USA).
4.7. Statistical Analysis
All statistical analyses were carried out using R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria) [
13]. In brief: all missing values were attributed to being below the detection limit and were replaced with zero. Univariate comparisons were performed for each metabolite or derived index (e.g., metabolite sums or ratios) across the different media samples. For metabolites with more than 30% missing values, the chi-squared test was used to assess whether the metabolite was significantly more frequently detectable in any of the study groups. When fewer than 30% of values were missing, the Shapiro–Wilk test was applied to determine whether the data followed a normal distribution. Based on the results, either ANOVA with Tukey’s honestly significant difference post-hoc test or the Kruskal–Wallis test with Dunn’s
post-hoc test was used. The results were considered statistically highly significant at p < 0.001, and p-values between 0.05 and 0.001 were considered significant.
5. Conclusions
In conclusion, our study affirms the significance of glucose, Met, His, Arg, polyamine, and lipid metabolisms as pivotal processes in blastocyst preimplantation development. Based on our findings and the existing literature, these pathways appear to be the most promising candidates for providing biomarkers to predict blastocyst viability and assess the embryo’s potential for successful implantation. The specific metabolite identified as a biomarker is influenced by experimental conditions and culture media. Although individual metabolites may differ across studies, the core processes likely remain consistent. Undoubtedly, our findings regarding Met-SO, LysoPC a C24:0, Cit, Ac.Orn, PC aa C42:4, ADMA, creatinine, C2, C3, C5, and kynurenine metabolism, and their association with the bovine embryo development potential, warrant careful consideration and further investigation.
Supplementary material
The following supporting information can be downloaded at: Preprints.org, Supplemental Table S1. The list of metabolites measured with the AbsoluteIDQ® p180 Kit that were included in the statistical analyses, along with their corresponding statistical significances.
Author Contributions
Conceptualization, E.T., K.K., and A.G.; methodology, E.T., K.K., and Ü.J.; software, K.K.; validation, E.T. and K.K.; formal analysis, K.K.; investigation, Ü.J.; resources, E.T., K.K., E.N., A.V-S., H.V.; data curation, E.T. and K.K.; writing—original draft preparation, E.T., K.K.; A.G; writing—review and editing, E.T., K.K., A.G., A.V-S., A.K., E.N., H.V. and Ü.J.; visualization, K.K.; supervision, K.K., A.K. and Ü.J.; project administration, E.T; funding acquisition, E.T., K.K., and Ü.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union through the European Regional Development Fund, grant number 616215780014; and by the Estonian Research Council, grant number PRG1665.
Institutional Review Board Statement
Not applicable
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy and/or ethical concerns.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rabel, R.A.C.; Marchioretto, P. V.; Bangert, E.A.; Wilson, K.; Milner, D.J.; Wheeler, M.B. Pre-Implantation Bovine Embryo Evaluation—From Optics to Omics and Beyond. Animals 2023, 13. [Google Scholar] [CrossRef]
- Gimeno, I.; García-Manrique, P.; Carrocera, S.; López-Hidalgo, C.; Valledor, L.; Martín-González, D.; Gómez, E. The Metabolic Signature of in Vitro Produced Bovine Embryos Helps Predict Pregnancy and Birth after Embryo Transfer. Metabolites 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Viana, JHM. 2022 Statistics of Embryo Production and Transfer in Domestic Farm Animals. Embryo Technol. Newsl. 2023 41:4.
- Gómez, E.; Carrocera, S.; Martín, D.; Pérez-Jánez, J.J.; Prendes, J.; Prendes, J.M.; Vázquez, A.; Murillo, A.; Gimeno, I.; Muñoz, M. Efficient One-Step Direct Transfer to Recipients of Thawed Bovine Embryos Cultured in Vitro and Frozen in Chemically Defined Medium. Theriogenology 2020, 146, 39–47. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.; Kaniyamattam, K. A Review of Simulation Analyses of Economics and Genetics for the Use of In-Vitro Produced Embryos and Artificial Insemination in Dairy Herds. Anim Reprod 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Magata, F. Time-Lapse Monitoring Technologies for the Selection of Bovine in Vitro Fertilized Embryos with High Implantation Potential. J Reprod Dev 2023, 69(2), 57–64. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Matsuno, Y.; Fujiwara, H. New Roles for EVs, MiRNA and LncRNA in Bovine Embryo Implantation. Front Vet Sci 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Pallisco, R.; Lazzarino, G.; Bilotta, G.; Marroni, F.; Mangione, R.; Saab, M.W.; Brundo, M.V.; Pittalà, A.; Caruso, G.; Capoccia, E.; et al. Metabolic Signature of Energy Metabolism Alterations and Excess Nitric Oxide Production in Culture Media Correlate with Low Human Embryo Quality and Unsuccessful Pregnancy. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Lechniak, D.; Sell-Kubiak, E.; Warzych, E. The Metabolic Profile of Bovine Blastocysts Is Affected by in Vitro Culture System and the Pattern of First Zygotic Cleavage. Theriogenology 2022, 188, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cheredath, A.; Uppangala, S.; Asha, C.S.; Jijo, A.; Vani Lakshmi, R.; Kumar, P.; Joseph, D.; Nagana, N.G.; Kalthur, G.; Adiga, S.K. Combining Machine Learning with Metabolomic and Embryologic Data Improves Embryo Implantation Prediction. Reproductive Sciences 2023, 30, 984–994. [Google Scholar] [CrossRef]
- Gomez, E.; Canela, N.; Herrero, P.; Cereto, A.; Gimeno, I.; Carrocera, S.; Martin-gonzalez, D.; Murillo, A.; Muñoz, M. Metabolites Secreted by Bovine Embryos in Vitro Predict Pregnancies That the Recipient Plasma Metabolome Cannot, and Vice Versa. Metabolites 2021, 11. [Google Scholar] [CrossRef]
- Lipinska, P.; Pawlak, P.; Warzych, E. Species and Embryo Genome Origin Affect Lipid Droplets in Preimplantation Embryos. Front Cell Dev Biol 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Tsopp, E.; Kilk, K.; Taalberg, E.; Pärn, P.; Viljaste-Seera, A.; Kavak, A.; Jaakma, Ü. Associations of the Single Bovine Embryo Growth Media Metabolome with Successful Pregnancy. Metabolites 2024. [Google Scholar] [CrossRef]
- Cabello-Pinedo, S.; Abdulla, H.; Mas, S.; Fraire, A.; Maroto, B.; Seth-Smith, M.; Escriba, M.; Teruel, J.; Crespo, J.; Munné, S.; et al. Development of a Novel Non-Invasive Metabolomics Assay to Predict Implantation Potential of Human Embryos. Reproductive Sciences 2024. [Google Scholar] [CrossRef] [PubMed]
- Melo-Sterza, F. de A.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under in Vivo, in Vitro, and Stress Conditions. Int J Mol Sci 2021, 22. [Google Scholar]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol Rev 2022, 74, 506–551. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Cashman, K.; Russell, D.L.; Thompson, J.G.; Norman, R.J.; Robker, R.L. Beta-Oxidation Is Essential for Mouse Oocyte Developmental Competence and Early Embryo Development. Biol Reprod 2010, 83, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, H.; Emamgholipour, S.; Hosseinkhani, S.; Arjmand, B.; Rezaei, N.; Dilmaghani-Marand, A.; Ghasemi, E.; Panahi, N.; Dehghanbanadaki, H.; Ghodssi-Ghassemabadi, R.; et al. The Association between Acylcarnitine and Amino Acids Profile and Metabolic Syndrome and Its Components in Iranian Adults: Data from STEPs 2016. Front Endocrinol (Lausanne) 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel Metabolic and Physiological Functions of Branched Chain Amino Acids: A Review. J Anim Sci Biotechnol 2017, 8. [Google Scholar] [CrossRef]
- Wongsrikeao, P.; Otoi, T.; Taniguchi, M.; Karja, N.W.K.; Agung, B.; Nii, M.; Nagai, T. Effects of Hexoses on in Vitro Oocyte Maturation and Embryo Development in Pigs. Theriogenology 2006, 65, 332–343. [Google Scholar] [CrossRef]
- Hufnagel, A.; Grant, I.D.; Aiken, C.E.M. Glucose and Oxygen in the Early Intrauterine Environment and Their Role in Developmental Abnormalities. Semin Cell Dev Biol 2022, 131, 25–34. [Google Scholar] [CrossRef]
- Ferrick, L.; Lee, Y.S.L.; Gardner, D.K. Metabolic Activity of Human Blastocysts Correlates with Their Morphokinetics, Morphological Grade, KIDScore and Artificial Intelligence Ranking. Human Reproduction 2020, 35, 2004–2016. [Google Scholar] [CrossRef]
- Cai, S.; Ye, Q.; Zeng, X.; Yang, G.; Ye, C.; Chen, M.; Yu, H.; Wang, Y.; Wang, G.; Huang, S.; et al. CBS and MAT2A Improve Methionine-Mediated DNA Synthesis through SAMTOR/MTORC1/S6K1/CAD Pathway during Embryo Implantation. Cell Prolif 2021, 54. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kang, J.; Su, J.; Zhang, J.; Zhang, L.; Liu, X.; Zhang, J.; Wang, F.; Lu, Z.; Xing, X.; et al. Methionine Adenosyltransferase 2A Regulates Mouse Zygotic Genome Activation and Morula to Blastocyst Transition. Biol Reprod 2019, 100, 601–617. [Google Scholar] [CrossRef]
- Hugentobler, S.A.; Diskin, M.G.; Leese, H.J.; Humpherson, P.G.; Watson, T.; Sreenan, J.M.; Morris, D.G. Amino Acids in Oviduct and Uterine Fluid and Blood Plasma during the Estrous Cycle in the Bovine. Mol Reprod Dev 2007, 74, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Drazic, A.; Winter, J. The Physiological Role of Reversible Methionine Oxidation. Biochim Biophys Acta Proteins Proteom 2014, 1844, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kim, G.; Liu, C.; Levine, R.L. Transgenic Mice Overexpressing Methionine Sulfoxide Reductase A: Characterization of Embryonic Fibroblasts. Free Radic Biol Med 2010, 49, 641–648. [Google Scholar] [CrossRef]
- Gouge, R.C.; Marshburn, P.; Gordon, B.E.; Nunley, W.; Huet-Hudson, Y.M. Nitric Oxide as a Regulator of Embryonic Development. Biol Reprod 1998, 58, 875–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Wu, M.-Y.; Chen, M.-J.; Chao, K.-H.; Ho, H.-N.; Yang, Y.-S. Nitric Oxide Is Associated with Poor Embryo Quality and Pregnancy Outcome in in Vitro Fertilization Cycles. Fertil Steril. 2004, 82(1), 126–31. [Google Scholar] [CrossRef] [PubMed]
- Tranguch, S.; Steuerwald, N.; Huet-Hudson, Y.M. Nitric Oxide Synthase Production and Nitric Oxide Regulation of Preimplantation Embryo Development. Biol Reprod 2003, 68, 1538–1544. [Google Scholar] [CrossRef]
- Christophorou, M.A.; Castelo-Branco, G.; Halley-Stott, R.P.; Oliveira, C.S.; Loos, R.; Radzisheuskaya, A.; Mowen, K.A.; Bertone, P.; Silva, J.C.R.; Zernicka-Goetz, M.; et al. Citrullination Regulates Pluripotency and Histone H1 Binding to Chromatin. Nature 2014, 507, 104–108. [Google Scholar] [CrossRef]
- Read, C.C.; Edwards, L.; Schrick, N.; Rhinehart, J.D.; Payton, R.R.; Campagna, S.R.; Castro, H.F.; Klabnik, J.L.; Horn, E.J.; Moorey, S.E. Correlation between Pre-Ovulatory Follicle Diameter and Follicular Fluid Metabolome Profiles in Lactating Beef Cows. Metabolites 2021, 11. [Google Scholar] [CrossRef]
- Bovo, S.; Mazzoni, G.; Galimberti, G.; Calò, D.G.; Fanelli, F.; Mezzullo, M.; Schiavo, G.; Manisi, A.; Trevisi, P.; Bosi, P.; et al. Metabolomics Evidences Plasma and Serum Biomarkers Differentiating Two Heavy Pig Breeds. Animal 2016, 10, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Muccini, A.M.; Tran, N.T.; de Guingand, D.L.; Philip, M.; Gatta, P.A.D.; Galinsky, R.; Sherman, L.S.; Kelleher, M.A.; Palmer, K.R.; Berry, M.J.; et al. Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health. Nutrients 2021, 13, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Lenis, Y.Y.; Johnson, G.A.; Wang, X.; Tang, W.W.; Dunlap, K.A.; Satterfield, M.C.; Wu, G.; Hansen, T.R.; Bazer, F.W. Functional Roles of Ornithine Decarboxylase and Arginine Decarboxylase during the Peri-Implantation Period of Pregnancy in Sheep. J Anim Sci Biotechnol 2018, 9. [Google Scholar] [CrossRef]
- Aguila, L.; Treulen, F.; Therrien, J.; Felmer, R.; Valdivia, M.; Smith, L.C. Oocyte Selection for in Vitro Embryo Production in Bovine Species: Noninvasive Approaches for New Challenges of Oocyte Competence. Animals 2020, 10, 1–24. [Google Scholar] [CrossRef]
- Abraham, M.C.; Gustafsson, H.; Ruete, A.; Brandt, Y.C. Breed Influences on in Vitro Development of Abattoir-Derived Bovine Oocytes. Acta Vet Scand 2012, 54, 36. [Google Scholar] [CrossRef]
- Merton, J.S.; Vermeulen, Z.L.; Otter, T.; Mullaart, E.; de Ruigh, L.; Hasler, J.F. Carbon-Activated Gas Filtration during in Vitro Culture Increased Pregnancy Rate Following Transfer of in Vitro-Produced Bovine Embryos. Theriogenology 2007, 67, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Farin, P.W.; Farin, C.E. Transfer of Bovine Embryos Produced In Vivo or In Vitro: Survival and Fetal Development. Biol Reprod 1995, 52, 676–82. [Google Scholar] [CrossRef] [PubMed]
- Yagi, A.; Miyanaga, S.; Shrestha, R.; Takeda, S.; Kobayashi, S.; Chiba, H.; Kamiya, H.; Hui, S.P. A Fatty Acid Profiling Method Using Liquid Chromatography-High Resolution Mass Spectrometry for Improvement of Assisted Reproductive Technology. Clinica Chimica Acta 2016, 456, 100–106. [Google Scholar] [CrossRef]
Table 1.
Metabolites exhibiting significant differences in concentrations (highly significant differences were indicated by p-value in bold). In instances where metabolites were detected in only a limited number of samples, the chi-squared test was employed and the count of samples with detectable concentrations was provided. The Kruskal–Wallis test, coupled with a Dunn post-hoc test, was utilized if not indicated otherwise.
Table 1.
Metabolites exhibiting significant differences in concentrations (highly significant differences were indicated by p-value in bold). In instances where metabolites were detected in only a limited number of samples, the chi-squared test was employed and the count of samples with detectable concentrations was provided. The Kruskal–Wallis test, coupled with a Dunn post-hoc test, was utilized if not indicated otherwise.
| Metabolites |
|
Concentrations in culture media |
| Metabolite |
DBL |
VBL |
HBL |
CM |
p-Value |
| Met-SO (µM) |
0.58 (0.45-4.68) a
|
0.09 (0.059-0.144) b
|
0.34 (0.17-0.53) a
|
0.22 (0.14-0.62) ab
|
0.00012 |
| LysoPC a C24:0 (nM) |
86 ± 32 a
|
45 ± 13 b
|
70 ± 20 a
|
83 ± 5 a
|
0.00075 † |
| LysoPC a C20:4 (nM) |
30 ± 11 ab
|
18 ± 10 a
|
36 ± 17 b
|
15 ± 2 a
|
0.0021 † |
| Cit |
5 (41.67%) a
|
0 (0%) b
|
0 (0%) b
|
0 (0%) b
|
0.0022 # |
| PC.aa.C36.5 (nM) |
3.2 (2-7) a
|
1.7 (1.3-2.0) b
|
1.3 (0.9-2.0) ab
|
2.0 (1.0-2.0) a
|
0.00242 |
| Ac-Orn (µM) |
0.18 (0.14-0.87) a
|
0.12 (0.11-0.12) b
|
0.13 (0.11-0.19) ab
|
0.13 (0.12-0.19) ab
|
0.00437 |
| PC aa C42:4 (nM) |
4 ± 2 a
|
2 ± 1 b
|
3 ± 1 ab
|
2 ± 1 ab
|
0.0062 † |
| Putrescine |
2 (16.67 %) a
|
0 (0 %) a
|
9 (56.25 %) b
|
3 (60 %) a
|
0.0073 # |
| Spermine |
0 (0 %) a
|
0 (0 %) a
|
6 (37.5 %) b
|
0 (0 %) a
|
0.0082 # |
| Spermidine |
4 (33.33 %) ab
|
0 (0 %) a
|
9 (56.25 %) b
|
0 (0 %) a
|
0.0085 # |
| ADMA, Creatinine, Serotonin, t4-OH-Pro, Taurine |
4 (33.33 %) a
|
0 (0 %) b
|
0 (0 %) b
|
0 (0 %) b
|
0.0098 # |
| C2 (µM) |
0.27 (±0.08) a
|
0.195 (±0.024) b
|
0.23 (±0.034) ab
|
0.23 (±0.017) ab
|
0.011 † |
| C3 (nM) |
49 (40-130) a
|
41 (36-43) b
|
50 (44-58) a
|
54 (45-57) ab
|
0.013 |
| C5 (nM) |
60 ±23 a
|
45 ± 7 b
|
45 ± 6 b
|
42 ± 2 ab
|
0.015 † |
| PC aa C34:1 (nM) |
23 (15-96 ) a
|
14 (13-15) ab
|
17 (13-18) ab
|
19 (15-21) ab
|
0.015 |
| PC aa C36:3 (nM) |
7.2 (5.4-9.5) a
|
4.7 (4.0-5.6) b
|
5.0 (3.7-5.6) ab
|
4.1 (4.0-5.2) a
|
0.017 |
| PC ae C42:0 (nM) |
215 ± 12 ab
|
226 ± 11 a
|
211 ± 15 b
|
223 ± 6 ab
|
0.027 † |
| PC ae C40:2 (nM) |
2.7 (2.0-19.5) a
|
1.8 (1.4-2.3) b
|
2.0 (1.8-3.0) b
|
3.0 (3.0-3.0) ab
|
0.027 |
| His (µM) |
53.4 (51.3-55.7) a
|
44.7 (40.6-49.0) b
|
50.4 (45.2-54.4) ab
|
49.5 (48.5-54.7) ab
|
0.028 |
| PC ae C32:2 (nM) |
15 ± 8 a
|
10 ± 3 b
|
11 ± 1 ab
|
10 ± 1 ab
|
0.028 † |
| lysoPC a C26:0 (nM) |
23 ± 10 a
|
26 ± 9 a
|
17 ± 7 b
|
18 ± 4 ab
|
0.029 † |
| PC aa C32:1 (nM) |
9.0 (7.1-61) a
|
5.9 (5.0-6.9) b
|
6.7 (4.5-9.3) ab
|
6.3 (6.0-6.4) ab
|
0.029 |
| PC aa C38:3 (nM) |
4 (1-240) a
|
1.1 (0.9-1.4) b
|
1.7 (1.0-4.0) ab
|
1.2 (1.0-1.4) ab
|
0.0303 |
| PC ae C38:2 (nM) |
4.4 (3.0-24) a
|
2.1 (1.5-2.8) b
|
3.6 (2.7-5.3) ab
|
4.0 (3.5-4.5) ab
|
0.0374 |
| PC ae C44:3 (nM) |
10 (9-11) a
|
13 (11-22) b
|
13 (10-14) ab
|
11 (10-17) ab
|
0.0381 |
| PC ae C38:1 (nM) |
6.0 (3.8-27) a
|
2.8 (2.4-3.4) b
|
3.9 (3.0-5.0) ab
|
3.0 (2.8-3.8) ab
|
0.0423 |
| PC aa C36:2 (nM) |
58 (49-338) a
|
48 (45-50) b
|
50 (47-54) ab
|
55 (54-57) ab
|
0.0453 |
Table 2.
Metabolite sums and ratios exhibiting significant differences (highly significant differences are indicated by p-values in bold). In cases where metabolites were detected in only a few samples, the chi-squared test was employed, providing the count of samples with detectable concentrations. The Kruskal–Wallis test with a Dunn post-hoc test was utilized if not indicated otherwise.
Table 2.
Metabolite sums and ratios exhibiting significant differences (highly significant differences are indicated by p-values in bold). In cases where metabolites were detected in only a few samples, the chi-squared test was employed, providing the count of samples with detectable concentrations. The Kruskal–Wallis test with a Dunn post-hoc test was utilized if not indicated otherwise.
| Metabolites |
|
Concentrations in culture media, μM |
|
| Metabolite |
DBL |
VBL |
HBL |
CM |
p-Value |
| Met-SO/Met |
0.012
(0.009-0.09) a
|
0.0016
(0.001-0.003) b
|
0.006
(0.003-0.011) a
|
0.004
(0.003-0.011) ab
|
6.3 × 10−5 |
| Total SM-OH/SM-non-OH |
0.33±0.16 a
|
0.54±0.11 b
|
0.43±0.13 ab
|
0.57±0.044 b
|
0.001†
|
| C2/C0 |
0.14 (0.13-0.16) a |
0.14 (0.13-0.15) a |
0.16 (0.14-0.18) b |
0.16 (0.16-0.17) b |
0.00448 |
| MUFA PC/SFA PC |
0.37 (0.33-2.14) a
|
0.32 (0.31-0.33) b
|
0.32 (0.29-0.35) ab
|
0.34 (0.31-0.38) a
|
0.00602 |
| Total PC ae |
0.82 (0.80-1.48) ab |
0.82 (0.81-0.84) a |
0.79 (0.78-0.81) b |
0.81 (0.80-0.84) b |
0.0122 |
| Hexoses |
705 (670-830) a
|
891 (838-946) b
|
830 (777-879) ab
|
816 (781-821) ab
|
0.0221 |
| SFA PC |
0.80 (0.76-1.05) ab |
0.81 (0.79-0.82) a |
0.78(0.76-0.79) b |
0.80 (0.79-0.81) ab |
0.0313 |
| PUFA PC/SFA PC |
0.91
(0.86-2.44) a
|
0.84
(0.82-0.86) b
|
0.87
(0.84-0.91) a
|
0.88
(0.84-0.89) a
|
0.0389
|
| Total AC-DC/Total AC |
0.188
(±0.019) a
|
0.202
(±0.006) b
|
0.192
(±0.009) a
|
0.198
(±0.006) a
|
0.041† |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).






