1. Introduction
Different from the concept of a biological species, the ecological species [
8,
19] emphasizes the species occupying a unique ecological niche, further expanding the concept of a species. The interactions between internal and external factors that promote the differentiation of a biological species can thus be combined to analyze the boundaries between different species. The entire life cycle of a scale insect depends on climatic factors and climate change will affect its life history, physiological characteristics, fecundity, and population dynamics, which in turn will affect their distribution. Models based on climatic niches have been widely used in species distribution studies including animals, plants, and microorganisms. Quantifying the niche differentiation between closer taxa can play a very important role in understanding the pattern of speciation and the dynamics of species evolution. How they react in between and how they avoid “used” seed by chemical markers (meaning of parasitoids), it is real curiosity [
5], but also in this year of no Climate Change, this is the Meteorological-Climate Crisis Devastation Year. Change, are something slow, monitored or predictable. Enough time to study importance of five different parasitoids on
Albizia seeds, within its pods.
As first and corresponding author, dedication of PhD, (started in late 2006) just to spread entomofauna of Amorpha fruticosa L., within particular eco-niches, with emphasis on description of new recorded seed predation on indigo bush in Republic of Serbia and understanding of potentials and priority of biological control measures of suppressing this aggressive invasive plant in our country and region in general. False Indigo bush was first, but latter, in 2012 research about others seed beetles of other five Legumes, together with their parasitoid complexes in Serbia, brought more new and better perspective in native parasitoid complexes “complexity”.
For many years, parasitoids interactions were the center of gigantic discourse. At the end one of them also show pretention to even
Phasheolus vulgaris L., just in 2%, but it was results of several years study. After, that I published more than 90 papers, in country and abroad, from different categories. Five those to one seed beetle, we are in some gambling game - who goanna lost first [
3].
Some of them are really pretty and smart and, why all this -opportunity of a life time occurs. Today we are studying potentially absorption possibilities of
Asine pigmente Albino beech, for heavy metals and other matter toxic for environment. Our discovery is that this phenomenologically and unique warlike organism, which could, or is the future of eco-remediation, as an absorber of various micro and macro elements, as well as heavy metals, studies, known better I could and by borrowing some basic equipment-liquid Nitrogen carried to collect samples from it. No, one thing as much sound fantastic, actually it is not in reality. So, we are lock and load with patience, were waiting some better days. Want to realize where come all fear of Braconid and Eulophid wasps [
24]. They are big, but, no still yet in even Hyper or super parasitoid niche. It should be clear soon. All be for sure, but this year got per four instead per one per two their generation. And, we are expecting most warmer day time to check the flowers of
Albizia julibbrissin.
2. Results
2.1. In Serbia, False Indigo bush seed beetle as introduced insect species was also found to be bivoltine [
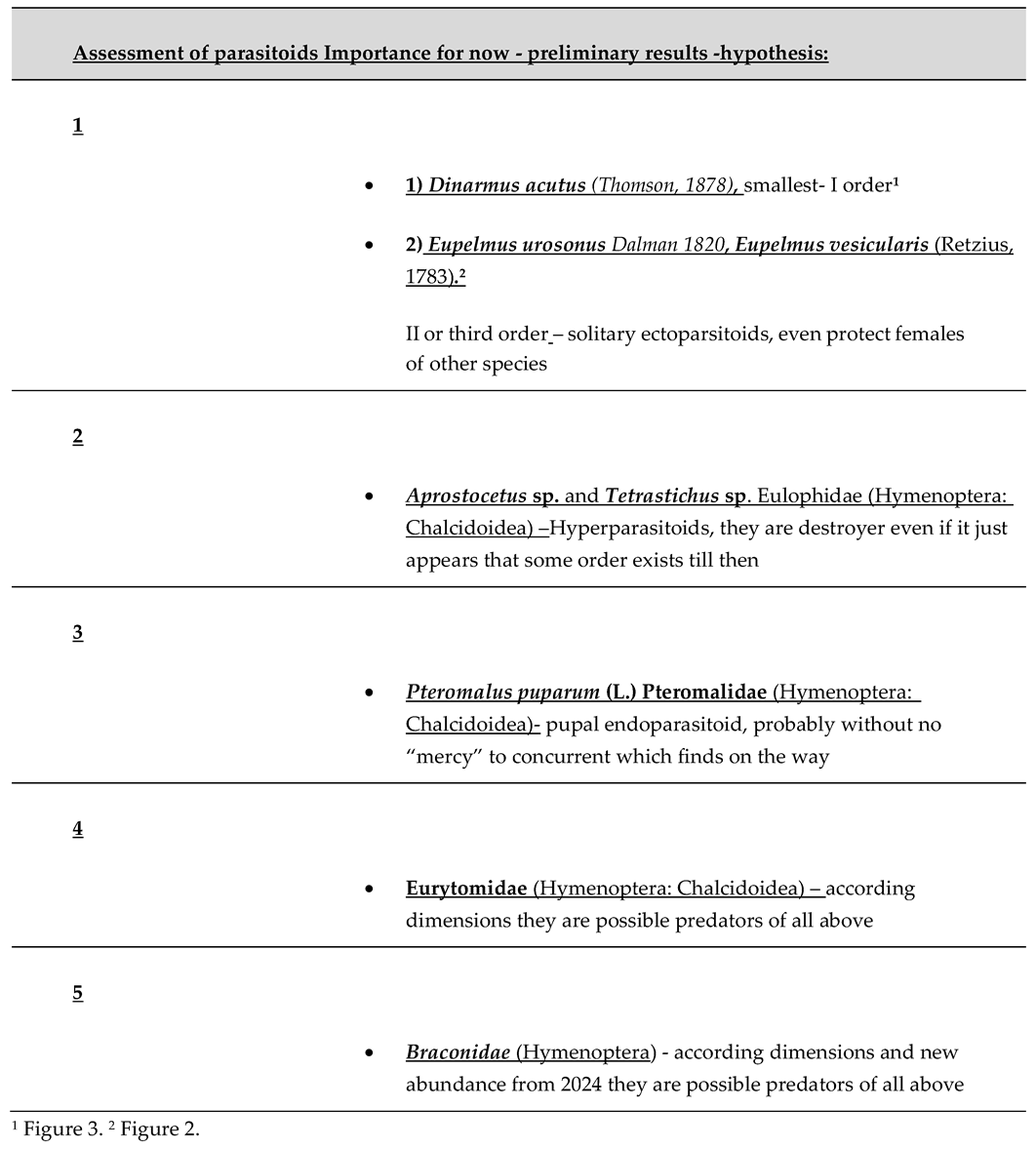
10]. Multiple parasitoid species of the seed beetle are recorded, mostly from Chalcidoideae superfamily. Status of genera from families Hymenoptera: Chalcidoidea; Pteromalidae and Eulophidae after intensive investigations has been trophicly identified. Those are Dinarmus acutus (Thomson, 1878), Eupelmus urosonus Dalman 1820, Eupelmus vesicularis (Retzius, 1783). Nevertheless, they were found by numerous species of Eulophidae (Hymenoptera: Chalcidoidea) as the base, as parasitoids of the second or third order-hyperparasitoids: Eulophid genera Aprostocetus and Tetrastichus. Nothing less important for the abundance of germination of seeds is also mentioned parasitoids that have about 20-25 % of the total number of infested seeds (for B. terrenus and that is pretty high number). In general, average percent of parasitoids in whole number of seeds in experiments is closely to 3%, as mean for all species of weevils. Logically, for infested seeds it varying from 2 % to 15% in maximum cases. Information on the, biology, identity, phenology, distribution and impact of silk tree key parasitoid species in Serbia still need to be investigated in the future.
2.2. New idea that new waive brought. We made our results, especially with Fagus moesiaca, where was more than clear and surprise us, its super-power to be bio radiator, preserving 1,5 to 200-250% of different heavy metals within tissue, usually much healthier in beech. That potential still should be examined with. While they "force" the same eco-niche, they are almost symbionts, when you look at some circumstances: Adults leave seed-pods in the same decades, March and April, in the spring, and September-October-November, in the fall. So, Eurytomid: Chalcids are not herbivores, like superparsitoids (Braconidae: Hymenoptera) – all circle is made of primary, secondary, tertiary, quaternary.
2.3. Generations or species in frame of one introduced pest seed beetle. First it is, it’s of the primary, secondary, and interactive parasitoids, and their synergistic action in suppressing host abundance in this simple model, are facilitated by the use of the negative binomial to distribute parasitoid encounters, and eggs by that among hosts. There’s a lot of to learn about them also, but not in the hurry, reasonable. Knowing that all of them are from superfam. Chalcidoidea, all except bigger and to us non-predictive Braconidae, Hymenoptera)
[4,6,17,21].
Figure 1.
Beetle Bruchidius terrenus after ecclosion, 2017, site Ruma (Original).
Figure 1.
Beetle Bruchidius terrenus after ecclosion, 2017, site Ruma (Original).
Figure 2.
Parasitic wasp family (Hymenoptera: Chalcidoidea: Eupelmidae), Eupelmus vesicularis, Albizia’s Weevil (Original).
Figure 2.
Parasitic wasp family (Hymenoptera: Chalcidoidea: Eupelmidae), Eupelmus vesicularis, Albizia’s Weevil (Original).
Figure 3.
Parasitic wasps family Pteromalide, Dinarmus acutus (Hymenoptera: Chalcidoidea: Pteromalidae) Albicija’s weevil(Original)(Smallest one).
Figure 3.
Parasitic wasps family Pteromalide, Dinarmus acutus (Hymenoptera: Chalcidoidea: Pteromalidae) Albicija’s weevil(Original)(Smallest one).
Figure 4.
Parasitic, wasps fam. Eurytomidae (Hymenoptera: Chalcidoidea; Eurytomidae), by Albizia weevil 2023-2024 (Original) much bigger than else, like “Croc-Wasp”.
Figure 4.
Parasitic, wasps fam. Eurytomidae (Hymenoptera: Chalcidoidea; Eurytomidae), by Albizia weevil 2023-2024 (Original) much bigger than else, like “Croc-Wasp”.
Table 1.
No risk in bio - control, suggestion for proper schedule of parasitoid complex- system or parasitoid on seed beetle during development to adult, with our native taxa: Base of pyramid, or food – Bruchinae larvae or pupa, in seed from second to final stage of Bruchidius terrenus, but neither larvae of Hymenoptera are not safe.
Table 1.
No risk in bio - control, suggestion for proper schedule of parasitoid complex- system or parasitoid on seed beetle during development to adult, with our native taxa: Base of pyramid, or food – Bruchinae larvae or pupa, in seed from second to final stage of Bruchidius terrenus, but neither larvae of Hymenoptera are not safe.
Table 2.
Locations of collection of pods from which the obtained insects appeared.
Table 2.
Locations of collection of pods from which the obtained insects appeared.
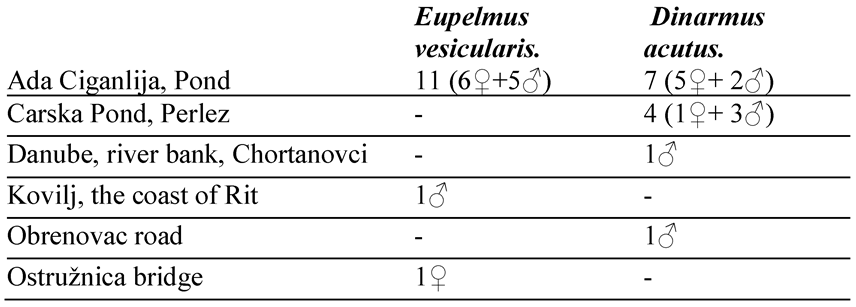
Table 3.
Numbers of several years study results with several parasitoids (4 Chalcids, and one is Braconid) on one seed beetle, Legume, and on one Bruchinae are also came from abroad.
Table 3.
Numbers of several years study results with several parasitoids (4 Chalcids, and one is Braconid) on one seed beetle, Legume, and on one Bruchinae are also came from abroad.
3. Discussion
Parasitoids that are shown and captured in this paper, the first time were recorded and found as
Albizia jullibrisin [
7,
20] weevil parasitoids and they are indigenous species that have adapted to non-native host (host plant as non-native seed beetle) [
12]. Duration of certain developmental stages of
Bruchidius terrenus in laboratory conditions (in days) [
11] were measured by several methodologies (dissection, and one flacon one seed raising, in goal to declare from which “higher” or “first” of parasitoids discovered weevil as host, by order, meaning primary, secondary, tertiary and on which it is goanna appeared first, as host located) [
3]).
That’s opens many questions about all their bionomy and ecology which is in adaptive, denizen state (a). Successful dispersal among habitat patches might prevent local extinctions (spatial rescue effects b).
Coexistence during divergence in symmetry is often overlooked in speciation models, side-lined by focus on growth of associations between and within polygenic traits [
15], assuming symmetries are protected [
5,
7]., each female could choose a mate from anywhere in the habitat, the population could not invade the vacant niche; Diversity-dependence of dispersal: interspecific interactions determine spatial dynamic [
16].
4. Materials and Methods
The biology of these seed pests has been studied in Serbia in detail on over 50 sites in the period 2006-2022, when results on the presence of infested seeds in the total seed of host plant pods were obtained. The research also focused on other 4 representatives of this sub-family Bruchinae, for which there were few references in this country, without suchlike data.
The dissected seeds were inspected with stereo magnifier (Leica Wild M3Z). Breeding of the insects, and monitoring of the duration of certain development stadiums were done in glass flacons or in Petri dishes. Beetles were reared on their natural host seeds in glass containers kept in climate chambers set to reproduce their natural conditions, at 29-32 ◦ C and 50% (±10%) relative humidity (RH) under a12h:12h light: dark cycle. All beetles were fed with 20% saccharine water solution.
During study, and abundance was dissected numerous (1200 per year) seeds within in obvious infested host plant pods. Level of abundance were calculated by in equation used data about percentage of infested VS heathy seed numbers. Species are marked with numbers from 1-5, added after their description data (
Table 1). Presence of parasitoid on seed beetles infesting host plants, their abundance was measured and calculated, until it has been obvious that existed certain abundance of parasitoids presenting risk, accommodated to categories (and capable to reduce infestation of weevils), eventually recognized as obvious influence to further improve biological control. Repeating had been focused more on parasitoid preferences and performances taking into consideration the role of plant domestication (some of them are introduced, some native). Because of artificial selection, cultivated plants are expected to disrupt the coevolutionary history of parasitoids and their hosts. Alterations in plant morphology, physical characteristics, or chemical defenses due to domestication can affect host–parasitoid relationships and impact parasitoid fitness. Theirs review highlights the need for more research on parasitoid ecology and evolution also to better control insect pests.
Simple mathematical tautologic methods were used as a prognose makers (Ratios, ratios with 0) in this meteorological-climate crisis.
5. Conclusions
If N x Q = P is 1: 10 or 1: 100 or 1: 1000, better 0: 50, or 0: 100, 0: 150, in a manner of evolution speed, a next Generations Abundance; or any of those: N Q P, will present the degree of niche overlap between parasitoid species (in %). That number (0 was in calculation) is generally unknown, but seems unlikely to be as minimal as implied by a negative binomial distribution of parasitoid encounters with hosts. Thus, we are currently extending the interactive model, following [
8] and [
9], to determine how variable niche overlap and the presence of they avoid seeds with laid eggs - chemical makers such as this yet have to been proven as a cause - consequently placed females of here three (four at last two years) finding schemes to function (
Figure 5). Almost strictly practice with the hierarchy of for centuries in biological warfare [
10], could theoretically declare a huge part, but even half of all commitment, to from practical training of avoid insects and chemicals, at least white washed family, but not touch, already taken. But, on the other hand, the “thought” make and sound – too, stretched that it would be more than ideal plans in bio-control. All is yet to be examined the degree of niche overlap between parasitoid species is generally unknown, but seems unlikely to be as minimal as implied by a negative binomial distribution of parasitoid encounters with hosts. Different niche overlap and the presence of a host refuge females from parasitism influenced the suitability of interactive parasitoids as candidates for introduction in classical biological control [
11,
12]. That’s opens many questions about all their bionomy and ecology which is in adaptive, denizen state. From more extensive analysis of this simple interactive model, we find that an interactive parasitoid only becomes detrimental to suppression of the equilibrium abundance of the pest as it approaches becoming an obligate hyperparasitoid (a Q N → 0), or as the degree of aggregation becomes insufficient to allow stable coexistence of the two parasitoids (k Q → 1). From this we might conclude that there is minimal risk in introducing interactive parasitoids in classical biological control programs provided there is evidence of strong aggregation in the pattern of attacks among hosts. However, the coexistence of the primary and interactive parasitoids, and their synergistic action in suppressing host abundance in this simple model, are facilitated by the use of the negative binomial to distribute parasitoid encounters among hosts [
23].
As noted by Kakehashi
et al., [
8] the use of the negative binomial in multi-parasitoid-host models implies an independence of niches with little overlap between the two parasitoids (or three likes on
B. terrenus) [
15]. Niche separation favors stability, and allows even minimal additional attack on the primary host (pest) to outweigh the antagonistic effects of the interactive parasitoid on the abundance of the primary parasitoid [
13].
“For Sure Path” could goes through first simulation run of the interactive parasitoid model, showing the host population N in the absence of parasitoids (generations 1-50), after the introduction of a primary parasitoid P (generations 51-100), and the subsequent introduction of an interactive parasitoid Q (generations 101- 150) -model of parameters would look like this
λ = 3, K = 1000, b P = 20, b Q = 1000, ci = 0.5, k is = 0.25, and PN = 0.6, and QN = 0.3,
a QP = 0.8. 1:10, 1:100, 1: 1000, or 0: 50, 0 :100, 0: 150 or Second Option “For Sure” Generations Abundance (also unlikely but eventually).
Is this “For Sure Path”, or probability of accomplishments of event is 0 it also does not mean that alternative evolution direction would not goes by 0: 50, 0: 100, 0: 150
Generations Abundance. N Q P then are the degrees of niche overlap between parasitoid species is generally will exist, but will be unknown, even it seems unlikely to be as minimal as implied by a negative binomial distribution of parasitoid encounters with hosts. Thus, we are currently extending the interactive model, following [
8] and [
10], to determine how variable niche overlap and the presence of a host refuge from parasitism influence the suitability of interactive parasitoids as candidates for introduction in classical biological control (
Figure 5).
Main Conclusion: Since these are not deterministic, but stochastic variables, any prediction without property Prob. calculation is a pointless endeavor.
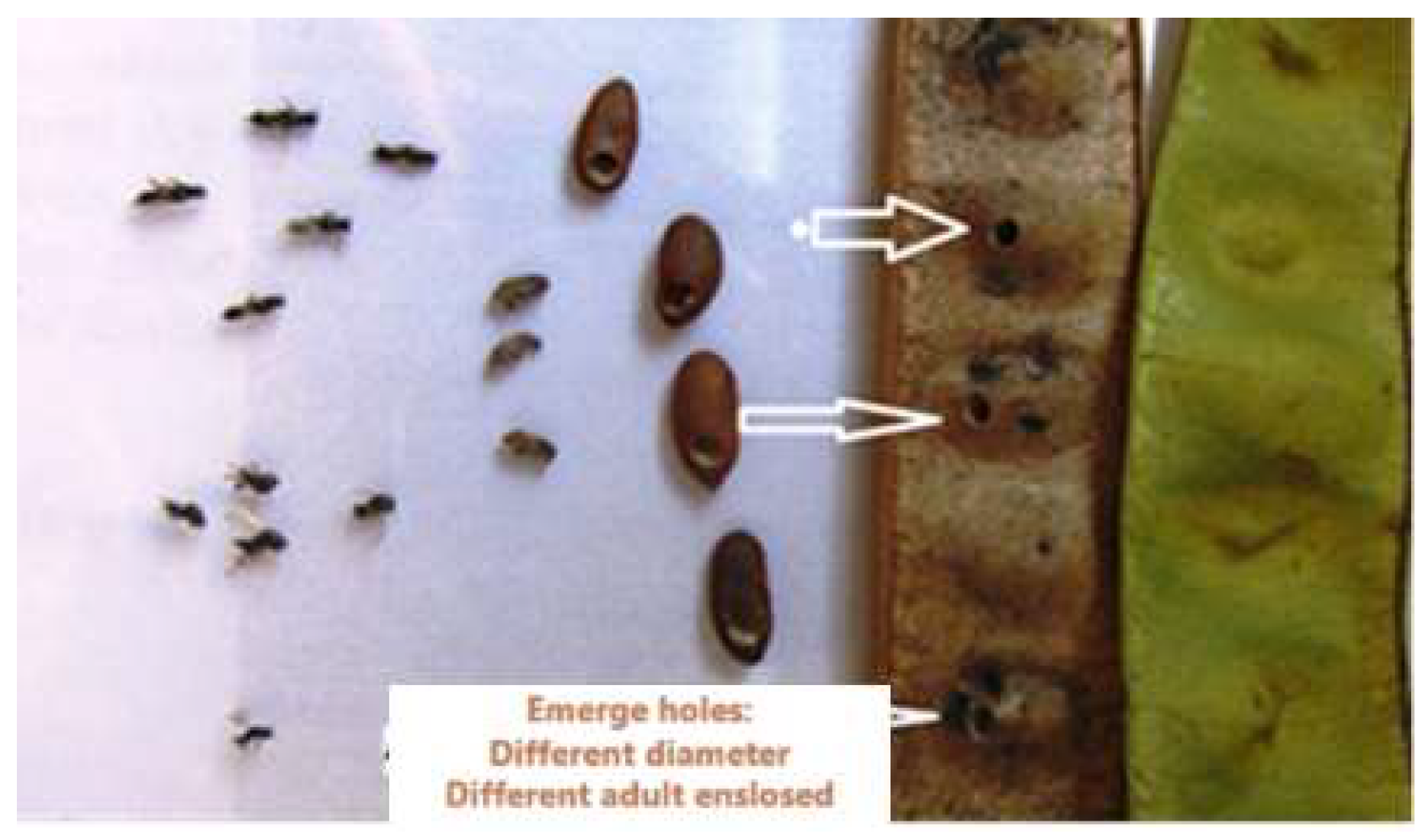
Figure 5.
Adults of Bruchidius terrenus Albicija’s weevil, flew out of the legume pods (seeds) of Albizia jullibrisin collected in Ruma Deteline site, parasitoids, and details in appearance compared of adults and green pods with damage and emerging wholes (Original).
Figure 5.
Adults of Bruchidius terrenus Albicija’s weevil, flew out of the legume pods (seeds) of Albizia jullibrisin collected in Ruma Deteline site, parasitoids, and details in appearance compared of adults and green pods with damage and emerging wholes (Original).
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/ https://www.forest.org.rs/?contact . Research study of native parasitoids niche accomplish in invasive seed infestation: new perspective brought new consequence conclusions: title.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, RGS and MM.; methodology, RGS, MM and SM.; software, GČ BK.; validation LJR and IĐ.; formal analysis ,GČ ĐI.; investigation, RGS DF.; resources RGS DF.; data curation, RGS MM.; writing—original draft preparation, RGS, SM AND DF.; writing—review and editing, MM, ALL.; visualization, All.; supervision, All.; project administration, MM.; funding acquisition, N/A
All authors have read and agreed to the published version of the manuscript.”
Funding
Under full legal awareness of legislative restrictions herby claims that there are no such restrictions needed to be worry about. Manuscript delivered in aim of publishing is original study of above-mentioned authors who are all free to share their knowledge without any kind of conflict of interest, non-breaching any kind of regulatory nor ethic norm, also without need to ask or be given any kind of consent for publication. This statement is given under full material / legal responsibility. For others authors also could admit that not breaching NDA in any part of this research, continuing with also publishing our results in Your honored Journal. This research study is not carrying any sort any financial gaining’s neither for authors nor for their scientific institute where they are all full-time employees. Hence, all declarations regarding conflict of interest, ethics related approvals, are not in any shape or form applicable on this research. In short, authors are completely free to publish their finds as they seem fit to do therefore: Consent for publication are N/A (RELEVENT to)
Data Availability Statement
Has been understood, of course in advance to and authors can deliver metadata if those are needed in any time
Acknowledgments
The study was carried out within projects financed by the Republic of Serbia - Ministry of Education and Science. The registration number of the Agreement for the current year - season 2024 is 451-03-66/2024-03/200027 dated 05.02.2024.
Conflicts of Interest
We are all declaring that there were no conflicts of interest.” Authors must identify and declare any personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. Any role of the funders in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results must be declared in this section. If there is no role, please state “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Mills, N. J. Factors influencing top-down control of insect pest populations in biological control systems. Basic and Applied Ecology (2001) 2: 323-332.
- Медведев Т. С.: Определитеь Насекoмиых Еврoпейскoй Части СССР; Академия Наук СССР, Зooлoгичеий Институт: Тoм III Перепенауатoкрылые; (Identify Insects of the European Part of the USSR; USSR Academy of Sciences, Zoological Institute: Volume III) (1978).
- Aletheia & Atzinger,: The biggest losers: habitat isolation deconstructs complex food webs from top to bottom South African Law Journal, Proceedings B, 2019. The O-antigen mediates differential survival of Salmonella against communities of natural predators. [CrossRef]
- Тряпицэын В.А. Наезники Энциртиды Палеарктики, Ленингра“НаукаЛенинградске Одтеление (The Wasps of En-cyrtidae of the Palearctic, Leningrad, “Science” of Leningrad). (1989).
- Agronomique 147, Rue De La Universiteit, 75007 Paris, Inra Paris, ISBN 2-7380-0343-5; ISSN 1150- 3912, (1978).
- Bonte Dries and Ryser Remo, Philosophical Transactions B. Species interactions and eco-evolutionary dynamics of dispersal: the diversity dependence of dispersal, Philosophical Transactions B. (2024).
- Gagić-Serdar R, Mihajlović Lj., Poduška Z., Djordjević, I., Češljar G., Bilibajkić, S., Stefanović, T. Nevenić R. - Seed Predation in Leguminous Trees and Shrubs: New Invasive seed Beetles (Coleoptera: Chrysomelidae: Bruchinae) To the Serbian Fauna, Agriculture & Forestry, (2014b) Vol. 60 Issue 3: 163-174, Podgorica 163: UDC (UDK) 633.875(497.11).
- Mark A. and Mc Peek: The evolution of passive dispersal versus habitat selection have differing emergent consequences in metacommunities, Published: 24 June 2024.
- Lopatina S.V. and the First other find of Bruchidius terrenus on the territory of Uzbekistan. Available: https://www.researchgate.net/publication/376356717 (PDF) _Lopatina_ SV i_ Pervaa_nahodka dr_Bruchidius_terrenus_na_territorii_Uzbekistana [accessed Aug 15 2024].
- De Graham M. W. R., The Pteromalidae of North- Western Europe (Hymenoptera: Chalcidoidea), Bulletin of British Museum (Natural History), Entomology Supplement I6: LONDON; (1969).
- Bouček* Zdenek, Jean Yves RASPLUS Jean Yves ** Illustrated Key to West-Palearctic Genera of Pteromalidae; *C/O I.I.E or Department of Entomology, Natural History Museum; **Laboratorie De Biositematique De Insecte, Station De Yoologie, Inra F-78029, Versailles Codex, France; Institut National De La Rechearche, (1991).
- Ebadollahi A.: Insecticidal Activity of Essential Oils of Five Aromatic Plants Against Callosobruchus maculatus F. (Coleoptera: Bruchidae) Under Laboratory Conditions, Journal of Essential Oil-Bearing Plants, (2013) Volume 15, Issue 2.
- Jovanović Branislav: Dendrology, University of Belgrade, Textbook, (2000) p. 307-332.
- Kakehashi, Nasayuki, et al. “Niche Overlap of Parasitoids in Host-Parasitoid Systems: Its Consequence to Single Versus Multiple Introduction Controversy in Biological Control.” Journal of Applied Ecology, vol. 21, no. 1, 1984, pp. 115–31. JSTOR, [ Accessed 14 Aug. 2024]. [CrossRef]
- Kishimoto Kiyonori a, Koyama Susumu a b, Akaike Norio a: Synergistic μ-opioid and 5-HT1A presynaptic inhibition of GABA release in rat periaqueductal grey neurons, a Cellular and System Physiology, Graduate School of Medical Sciences, Faculty of Medicine, Kyushu University, Fukuoka (2001), 812-8582, Japan.
- Loni A. & Panahi O. Control of stored grain pest, Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae), using the essential oils isolated from Zingiber officinale (L.) and Mentha pulegium (L.) in laboratory condition, Archives of Phytopathology and Plant Protection, (2015), Volume 48, - Issue 5.
- Hoebeke, E.R., S.G. Wheeler, J.M. Kingsolver & D.L. Stephan. First North American records of the east paleartic seed beetle Bruchidius terrenus (Coleoptera: Chrysomelidae: Bruchinae), a specialist on Mimosa (Albizia julibrissin, Fabaceae). Florida Entomologist (2009) 92(3).
- Kishimoto Kiyonori b Department of Psychosomatic Medicine, Faculty of Medicine, Kyushu University, Fukuoka 812-8582, Japan, Neuropharmacology, (2001) Volume 41, Issue 5, Pages 529-538;
- Gagić Serdar R, Mihajlović Lj, Poduška Z, Ðorðević I, Češljar G, Bilibajkić S, Stefanović T & Nevenić R: New Records of Bruchidius Spermaphagous Species in Albizia julibrissin and Laburnum anagyroides and Their Parasitoid Complex in Serbia. South-east Eurfor (2014a) 5 (2): 163-170. [CrossRef]
- Lindholm, M. Perspectives in Education, Competition and niche partitioning in a floodplain ecosystem: a Cladocera community squeezed between fish and invertebrate predation, 2007.
- Tuda M., Shima K., Johnsons D. C. & Morimoto K. “Acanthoscelides pallidipennis (Motschulsky) (Coleoptera: Bruchidae) feeding in seeds of the introduced legume Amorpha fruticosa, with a new record of its Eupelmus parasitoid in Japan”. Appl. Entomol. Zool. (2001) 36 (3): pp. 269 -276.
- Vukićević E. M. “Rod Albizia L.”, Dekorativna dendrologija, Izd. Šum Fakulteta, Beograd (“Albizia L. genera”, Decorative dendrology, Faculty of Forestry, Belgrade). (1996).
- Тряпицэын В.А. Наезники Энциртиды Палеарктики, Ленингра“НаукаЛенинградске Одтеление (The Wasps of Encyrtidae of the Palearctic, Leningrad, “Science” of Leningrad). (1989).
- Yus Ramos R., Ventura D., Garcнa P.C., Stojanova A. (Sharp, 1886) (Coleoptera: Bruchidae): Primera cita parala Península Ibérica y para Italia, caracterización del imago y primeros datos biológicos // Boletín de la Sociedad Entomológica Aragonesa. (2011) No 48. Р. 253–260.
- Woodcock, Microbiology, Biofilm community succession: A Neutral perspective, 2017.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. ALL AUTHORS AGREED |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).