Submitted:
19 December 2024
Posted:
20 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. The Role of Mitochondria in the Energy Metabolism of the Brain
1.2. Concept of Mitochondrial Dysfunction
2. Molecular Mechanisms Behind Mitochondrial Dysfunction
2.1. Markers of Mitochondrial Dysfunction
2.2. Mitochondrial Antioxidant System
2.3. The Production of ROS by Mitochondria and the Lack of Mitochondria
2.4. Interaction of ROS and NO with the Mitochondria
2.5. Disorder in the Lipid Layer of Mitochondrial Membranes
3. Genetic and Cellular Factors Involved in Mitochondrial Dysfunction
3.1. Mitochondrial DNA Damage in Mitochondrial Dysfunction
3.2. Ca2+ and Mitochondrial Dysfunction
3.3. Disruption of Native Protein Structure (Unfolded Protein Response – UPR) and Mitochondrial Dysfunction
3.4. HIF-1 and Mitochondrial Dysfunction
3.5. HSP 70 and Mitochondrial Dysfunction
4. Mitochondrial Dysfunction and Apoptosis
5. Possible Strategies for Pharmacocorrection of Mitochondrial Dysfunction
5.1. Idebeon
5.2. Menadione
5.3. Olifen
5.4. Succinic Acid
5.5. Dimefosfon
5.6. NAC (N-Acetylcysteine)
5.7. Emoxypine and Mexidol
5.8. Ethylmethylhydroxypyridine Succinate
5.9. Meldonium
5.10. Thiotriazoline
5.11. Angiolin
5.12. Benzodiazepines
5.13. Estrogens and Selective Estrogen Receptor Modulators.
5.14. Neuropeptides
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cook, A.M.; Jones, G.M.; Hawryluk, G.W.J.; Mailloux, P.; McLaughlin, D.; Papangelou, A.; Samuel, S.; Tokumaru, S.; Venkatasubramanian, C.; Zacko, C.; et al. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocritical Care 2020, 32, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Zissimopoulos, J.M.; Tysinger, B.C.; A, St. Clair, P.; Crimmins, E.M. The Impact of Changes in Population Health and Mortality on Future Prevalence of Alzheimer’s Disease and Other Dementias in the United States. Journals Gerontol. Ser. B 2018, 73, S38–S47. [Google Scholar] [CrossRef] [PubMed]
- Chief Medical Officer’s Annual Report 2023 Health in an Ageing Society.
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress and Mitochondrial Damage in Neurodegenerative Diseases: From Molecular Mechanisms to Targeted Therapies. Oxidative Med. Cell. Longev. 2020, 2020, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. The Complex Interplay between Mitochondria, ROS and Entire Cellular Metabolism. Antioxidants 2022, 11, 1995. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Cherniy, V.I.; Nagornaya, E.A.; Bukhtiyarova, N.V.; Kucherenko, V.I. Neuroprotection and Neuroplasticity. Kiev: Logos, 2015.
- Yang, J.; Guo, Q.; Feng, X.; Liu, Y.; Zhou, Y. Mitochondrial Dysfunction in Cardiovascular Diseases: Potential Targets for Treatment. Front. Cell Dev. Biol. 2022, 10, 841523. [Google Scholar] [CrossRef]
- Weissig, V.; Edeas, M. Targeting Mitochondria 2023 Abstract Book. J. Mitochondria, Plast. Endosymbiosis 2023, 1. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free. Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Murphy, M.P. Mitochondrial Thiols in Antioxidant Protection and Redox Signaling: Distinct Roles for Glutathionylation and Other Thiol Modifications. Antioxidants Redox Signal. 2012, 16, 476–495. [Google Scholar] [CrossRef]
- Aldossary, A.M.; Tawfik, E.A.; Alomary, M.N.; Alsudir, S.A.; Alfahad, A.J.; Alshehri, A.A.; Almughem, F.A.; Mohammed, R.Y.; Alzaydi, M.M. Recent advances in mitochondrial diseases: From molecular insights to therapeutic perspectives. Saudi Pharm. J. 2022, 30, 1065–1078. [Google Scholar] [CrossRef]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: the role of glucose in physiological and pathological brain function. 2013, 36, 587–597. [CrossRef]
- Jain, V.; Langham, M.C.; Wehrli, F.W. MRI Estimation of Global Brain Oxygen Consumption Rate. J Cereb Blood Flow Metab. 2010, 30(9):1598-Epub 2010 Apr Erratum in: J Cereb Blood Flow Metab. 2010, 30(12):Erratum in: J Cereb Blood Flow Metab. 2011, 31(5):1336.
- Raichle, M.E.; Gusnard, D.A. Appraising the Brain's Energy Budget. Proc Natl Acad Sci U S A. 2002, 99(16):10237-9. Epub 2002.
- Watts, M.E.; Pocock, R.; Claudianos, C.; Chowen, J.A.; Arevalo, M.A.; Rosenberger, T.A. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018, 11, 216. [Google Scholar] [CrossRef]
- Steiner, P. Brain Fuel Utilization in the Developing Brain. Ann. Nutr. Metab. 2019, 75, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Mei, S.; Wang, X.; Hu, G.; Lu, M. Focusing on mitochondria in the brain: from biology to therapeutics. Transl. Neurodegener. 2024, 13, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Saab, A.S.; Tzvetavona, I.D.; Trevisiol, A.; Baltan, S.; Dibaj, P.; Kusch, K.; Möbius, W.; Goetze, B.; Jahn, H.M.; Huang, W.; et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 2016, 91, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Qi, G.; Vitali, F.; Shang, Y.; Raikes, A.C.; Wang, T.; Jin, Y.; Brinton, R.D.; Gu, H.; Yin, F. Loss of fatty acid degradation by astrocytic mitochondria triggers neuroinflammation and neurodegeneration. Nat. Metab. 2023, 5, 445–465. [Google Scholar] [CrossRef]
- Thornton, B.; Cohen, B.; Copeland, W.; Maria, B.L. Mitochondrial Disease: Clinical Aspects, Molecular Mechanisms, Translational Science, and Clinical Frontiers. J Child Neurol. 2014, 29(9):1179-207.
- Johri, A.; Beal, M.F. Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef]
- Vincent, A.E.; Ng, Y.S.; White, K.; Davey, T.; Mannella, C.; Falkous, G.; Feeney, C.; Schaefer, A.M.; McFarland, R.; Gorman, G.S.; et al. The Spectrum of Mitochondrial Ultrastructural Defects in Mitochondrial Myopathy. Sci. Rep. 2016, 6, 30610. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Mazur, I.A.; Kucherenko, L.I.; Nagornaya, E.A.; Gorbacheva, S.V.; Bidnenko, A.S. The molecular and ultrastructural aspects of the formation of mitochondrial dysfunction in the modeling of chronic cerebral ischemia: The mitoprotective effects of Angiolin. Neurochem. J. 2016, 10, 131–136. [Google Scholar] [CrossRef]
- Belenichev, I.; Popazova, O.; Bukhtiyarova, N.; Savchenko, D.; Oksenych, V.; Kamyshnyi, O. Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection. Antioxidants 2024, 13, 504. [Google Scholar] [CrossRef]
- Skulachev, V.P.; Vyssokikh, M.Y.; Chernyak, B.V.; Averina, O.A.; Andreev-Andrievskiy, A.A.; Zinovkin, R.A.; Lyamzaev, K.G.; Marey, M.V.; Egorov, M.V.; Frolova, O.J.; et al. Mitochondrion-targeted antioxidant SkQ1 prevents rapid animal death caused by highly diverse shocks. Sci. Rep. 2023, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Di Lisa, F. The Mitochondrial Permeability Transition Pore: Molecular Nature and Role as a Target in Cardioprotection. J Mol Cell Cardiol. 2015, Jan;78:100-6.
- Krasnikov, B.F.; Zorov, D.B.; Antonenko, Y.N.; Zaspa, A.A.; Kulikov, I.V.; Kristal, B.S.; Cooper, A.J.; Brown, A.M. Comparative kinetic analysis reveals that inducer-specific ion release precedes the mitochondrial permeability transition. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2005, 1708, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Gutiérrez-Aguilar, M. The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium 2018, 73, 121–130. [Google Scholar] [CrossRef]
- Park, W.; Wei, S.; Kim, B.-S.; Bae, S.-J.; Chae, Y.C.; Ryu, D.; Ha, K.-T. Diversity and complexity of cell death: a historical review. Exp. Mol. Med. 2023, 55, 1573–1594. [Google Scholar] [CrossRef]
- Orsucci, D.; Ienco, E.C.; Rossi, A.; Siciliano, G.; Mancuso, M. Mitochondrial Syndromes Revisited. J. Clin. Med. 2021, 10, 1249. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. The Link between Oxidative Stress, Redox Status, Bioenergetics and Mitochondria in the Pathophysiology of ALS. Int. J. Mol. Sci. 2021, 22, 6352. [Google Scholar] [CrossRef]
- Valenti, D.; Vacca, R.A. Brain Mitochondrial Bioenergetics in Genetic Neurodevelopmental Disorders: Focus on Down, Rett and Fragile X Syndromes. Int. J. Mol. Sci. 2023, 24, 12488. [Google Scholar] [CrossRef]
- Rahman, M.H.; Suk, K. Mitochondrial Dynamics and Bioenergetic Alteration During Inflammatory Activation of Astrocytes. Front. Aging Neurosci. 2020, 12:614410.
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef]
- Goel, P.; Chakrabarti, S.; Goel, K.; Bhutani, K.; Chopra, T.; Bali, S. Neuronal cell death mechanisms in Alzheimer’s disease: An insight. Front. Mol. Neurosci. 2022, 15, 937133. [Google Scholar] [CrossRef] [PubMed]
- Belenichev, I.; Bukhtiyarova, N.; Ryzhenko, V.; Makyeyeva, L.; Morozova, O.; Oksenych, V.; Kamyshnyi, O. Methodological Approaches to Experimental Evaluation of Neuroprotective Action of Potential Drugs. Int. J. Mol. Sci. 2024, 25, 10475. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen Res. 2013, 8(21):2003-14.
- Siasos, G.; Tsigkou, V.; Kosmopoulos, M.; Theodosiadis, D.; Simantiris, S.; Tagkou, N.M.; Tsimpiktsioglou, A.; Stampouloglou, P.K.; Oikonomou, E.; Mourouzis, K.; et al. Mitochondria and cardiovascular diseases—from pathophysiology to treatment. Ann. Transl. Med. 2018, 6, 256–256. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Faller, D.V. Transcription Regulation by Class III Histone Deacetylases (HDACs)-Sirtuins. Transl Oncogenomics. 2008, 3:53-65.
- Xu, H.; Liu, Y.Y.; Li, L.S.; Liu, Y.S. Sirtuins at the Crossroads Between Mitochondrial Quality Control and Neurodegenerative Diseases: Structure, Regulation, Modifications, and Modulators. Aging Dis. 2023, 14(3):794-824.
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.-R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Khoury, N.; Koronowski, K.B.; Young, J.I.; Perez-Pinzon, M.A. The NAD+-Dependent Family of Sirtuins in Cerebral Ischemia and Preconditioning. Antioxidants Redox Signal. 2018, 28, 691–710. [Google Scholar] [CrossRef]
- Tomizawa, M.; Shinozaki, F.; Fugo, K.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Kishimoto, T.; Ishige, N. Anti-mitochondrial M2 antibody-positive autoimmune hepatitis. Exp. Ther. Med. 2015, 10, 1419–1422. [Google Scholar] [CrossRef]
- Becker, Y.; Loignon, R.-C.; Julien, A.-S.; Marcoux, G.; Allaeys, I.; Lévesque, T.; Rollet-Labelle, E.; Benk-Fortin, H.; Cloutier, N.; Melki, I.; et al. Anti-mitochondrial autoantibodies in systemic lupus erythematosus and their association with disease manifestations. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Liakopoulos, V.; Stefanidis, I. Cytochrome c as a Potentially Clinical Useful Marker of Mitochondrial and Cellular Damage. Front. Immunol. 2016, 7, 279. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Bazylianska, V.; Recanati, M.A.; Fite, A.; Liu, J.; Wan, J.; Mantena, N.; Malek, M.H.; Podgorski, I.; Heath, E.I.; Vaishnav, A.; Edwards, B.F.; Grossman, L.I.; Sanderson, T.H.; Lee, I.; Hüttemann, M. Tissue-Specific Regulation of Cytochrome c by Post-Translational Modifications: Respiration, the Mitochondrial Membrane Potential, ROS, and Apoptosis. FASEB J. 2019, 33(2):1540-1553.
- González-Arzola, K.; Velázquez-Cruz, A.; Guerra-Castellano, A.; Casado-Combreras, M. .; Pérez-Mejías, G.; Díaz-Quintana, A.; Díaz-Moreno, I.; De la Rosa, M.. New moonlighting functions of mitochondrial cytochrome c in the cytoplasm and nucleus. FEBS Lett. 2019, 593, 3101–3119. [Google Scholar] [CrossRef]
- Oh, M.; Kim, S.A.; Yoo, H.J. Higher Lactate Level and Lactate-to-Pyruvate Ratio in Autism Spectrum Disorder. Exp. Neurobiol. 2020, 29, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Shayota, B.J. Biomarkers of mitochondrial disorders. Neurotherapeutics 2024, 21, e00325. [Google Scholar] [CrossRef] [PubMed]
- Hubens, W.; Vallbona-Garcia, A.; de Coo, I.; van Tienen, F.; Webers, C.; Smeets, H.; Gorgels, T. Blood biomarkers for assessment of mitochondrial dysfunction: An expert review. Mitochondrion 2022, 62, 187–204. [Google Scholar] [CrossRef]
- McClintock, C.R.; Mulholland, N.; Krasnodembskaya, A.D. Biomarkers of mitochondrial dysfunction in acute respiratory distress syndrome: A systematic review and meta-analysis. Front. Med. 2022, 9, 1011819. [Google Scholar] [CrossRef]
- Virmani, M.A.; Cirulli, M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef]
- Sharma, S.; Black, S.M. Carnitine Homeostasis, Mitochondrial Function, and Cardiovascular Disease. Drug Discov Today Dis Mech. 2009, 6(1-4):e31-e39.
- McCann, M.R.; De la Rosa, M.V.G.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Liew, G.; Tse, B.; Ho, I.-V.; Joachim, N.; White, A.; Pickford, R.; Maltby, D.; Gopinath, B.; Mitchell, P.; Crossett, B. Acylcarnitine Abnormalities Implicate Mitochondrial Dysfunction in Patients With Neovascular Age-Related Macular Degeneration. Investig. Opthalmology Vis. Sci. 2020, 61, 32–32. [Google Scholar] [CrossRef]
- Nickel, K.; Menke, M.; Endres, D.; Runge, K.; Tucci, S.; Schumann, A.; Domschke, K.; van Elst, L.T.; Maier, S. Altered markers of mitochondrial function in adults with autism spectrum disorder. Autism Res. 2023, 16, 2125–2138. [Google Scholar] [CrossRef]
- Hamilton, R.T.; Walsh, M.E.; Van Remmen, H. Mouse Models of Oxidative Stress Indicate a Role for Modulating Healthy Aging. J Clin Exp Pathol. 2012, 4:005.
- Hibino, M.; Maeki, M.; Tokeshi, M.; Ishitsuka, Y.; Harashima, H.; Yamada, Y. A system that delivers an antioxidant to mitochondria for the treatment of drug-induced liver injury. Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef]
- Chen, T.-H.; Wang, H.-C.; Chang, C.-J.; Lee, S.-Y. Mitochondrial Glutathione in Cellular Redox Homeostasis and Disease Manifestation. Int. J. Mol. Sci. 2024, 25, 1314. [Google Scholar] [CrossRef]
- Ribas, V.; García -Ruiz, C.; Fernández-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int J Mol Med. 2019, 44(1):3-15.
- Hass, D.T.; Barnstable, C.J. Uncoupling proteins in the mitochondrial defense against oxidative stress. Prog. Retin. Eye Res. 2021, 83, 100941–100941. [Google Scholar] [CrossRef] [PubMed]
- Belenichev, I.F.; Odnokoz, O.V.; Pavlov, S.V.; Belenicheva, O.I.; Polyakova, E.N. The neuroprotective activity of tamoxifen and tibolone during glutathione depletion in vitro. Neurochem. J. 2012, 6, 202–212. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cordero, S.; Kirwan, R.; Noguera-Julian, A.; Cardellach, F.; Fortuny, C.; Morén, C. A Mitocentric View of the Main Bacterial and Parasitic Infectious Diseases in the Pediatric Population. Int. J. Mol. Sci. 2021, 22, 3272. [Google Scholar] [CrossRef]
- Han, D.; Williams, E.; Cadenas, E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001, 353, 411–6. [Google Scholar] [CrossRef]
- Ghasemi, M.; Mayasi, Y.; Hannoun, A.; Eslami, S.M.; Carandang, R. Nitric Oxide and Mitochondrial Function in Neurological Diseases. Neuroscience 2018, 376, 48–71. [Google Scholar] [CrossRef]
- Belenichev, I.; Gorbachova, S.; Pavlov, S.; Bukhtiyarova, N.; Puzyrenko, A.; Brek, O. NEUROCHEMICAL STATUS OF NITRIC OXIDE IN THE SETTINGS OF THE NORM, ISHEMIC EVENT OF CENTRAL NERVOUS SYSTEM, AND PHARMACOLOGICAL BN INTERVENTION. . 2021, 169–176. [Google Scholar]
- Mokhtari, B.; Yavari, R.; Badalzadeh, R.; Mahmoodpoor, A. An Overview on Mitochondrial-Based Therapies in Sepsis-Related Myocardial Dysfunction: Mitochondrial Transplantation as a Promising Approach. Can. J. Infect. Dis. Med Microbiol. 2022, 2022, 1–17. [Google Scholar] [CrossRef]
- Martemucci, G.; Portincasa, P.; Di Ciaula, A.; Mariano, M.; Centonze, V.; D’alessandro, A.G. Oxidative stress, aging, antioxidant supplementation and their impact on human health: An overview. Mech. Ageing Dev. 2022, 206, 111707. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P. Kinetics and Mechanisms of Thiol–Disulfide Exchange Covering Direct Substitution and Thiol Oxidation-Mediated Pathways. Antioxidants Redox Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.L.; Subramanian, P.; Bu, G.; Di Polo, A.; Golde, T.E.; Bovenkamp, D.E. Common Features of Neurodegenerative Disease: Exploring the Brain-Eye Connection and Beyond (Part 1): The 2021 Pre-Symposium of the 15th International Conference on Alzheimer's and Parkinson's Diseases. Mol Neurodegener. 2022, 17(1):68.
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Dietz, J.V.; Fox, J.L.; Khalimonchuk, O. Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond. Cells 2021, 10, 2198. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef]
- Norambuena, J.; Flores, R.; Cárdenas, J.P.; Quatrini, R.; Chávez, R.; Levicán, G. Thiol/Disulfide System Plays a Crucial Role in Redox Protection in the Acidophilic Iron-Oxidizing Bacterium Leptospirillum ferriphilum. PLOS ONE 2012, 7, e44576. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Gorbacheva, S.V.; Demchenko, A.V.; Bukhtiyarova, N.V. The thiol-disulfide balance and the nitric oxide system in the brain tissue of rats subjected to experimental acute impairment of cerebral blood flow: The therapeutic effects of nootropic drugs. Neurochem. J. 2014, 8, 24–27. [Google Scholar] [CrossRef]
- Roede, J.R.; Jones, D.P. Reactive species and mitochondrial dysfunction: Mechanistic significance of 4-hydroxynonenal. Environ. Mol. Mutagen. 2010, 51, 380–390. [Google Scholar] [CrossRef]
- Martin, L.J. The mitochondrial permeability transition pore: A molecular target for amyotrophic lateral sclerosis therapy. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2009, 1802, 186–197. [Google Scholar] [CrossRef]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The Mitochondrial Permeability Transition Pore-Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells. 2023, 12(9):1273.
- Zong, L.; Liang, Z. Apoptosis-inducing factor: a mitochondrial protein associated with metabolic diseases—a narrative review. Cardiovasc. Diagn. Ther. 2023, 12, 609–622. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Ademowo, O.S.; Dias, H.K.I.; Burton, D.G.A.; Griffiths, H.R. Lipid (Per)Oxidation in Mitochondria: An Emerging Target in the Ageing Process? Biogerontology. 2017, 18(6):859-879.
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.M.; Pennington, E.R.; Green, W.D.; A Beck, M.; A Brown, D.; Shaikh, S.R. Mechanisms by Which Dietary Fatty Acids Regulate Mitochondrial Structure-Function in Health and Disease. Adv. Nutr. Int. Rev. J. 2018, 9, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, L.; Fernández-Checa, J.C.; de la Rosa, L.C.; Garcia-Ruiz, C. Impact of mitochondrial lipid alterations on liver disease mechanisms and progression. 2024, 3, 382–413. [CrossRef]
- Zhao, J.; Li, J.; Li, G.; Chen, M. The role of mitochondria-associated membranes mediated ROS on NLRP3 inflammasome in cardiovascular diseases. Front. Cardiovasc. Med. 2022, 9, 1059576. [Google Scholar] [CrossRef]
- Lagouge, M.; Larsson, N. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J. Intern. Med. 2013, 273, 529–543. [Google Scholar] [CrossRef]
- Rong, Z.; Tu, P.; Xu, P.; Sun, Y.; Yu, F.; Tu, N.; Guo, L.; Yang, Y. The Mitochondrial Response to DNA Damage. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free. Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M. 8-oxoguanine and 8-oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC–ECD Determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef]
- Weimann, A.; Belling, D.; Poulsen, H.E. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. Nucleic Acids Res. 2002, 30, 7e–7. [Google Scholar] [CrossRef]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2017, 592, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, X. The role of poly(ADP-ribosyl)ation in DNA damage response and cancer chemotherapy. Oncogene 2014, 34, 3349–3356. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Dawson, V. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia–reperfusion. Trends Pharmacol. Sci. 1998, 19, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Burgess, J.T.; O’byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Picard, M.; Shirihai, O.S. Mitochondrial signal transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Moon, D.-O. Calcium’s Role in Orchestrating Cancer Apoptosis: Mitochondrial-Centric Perspective. Int. J. Mol. Sci. 2023, 24, 8982. [Google Scholar] [CrossRef]
- Callens, M.; Kraskovskaya, N.; Derevtsova, K.; Annaert, W.; Bultynck, G.; Bezprozvanny, I.; Vervliet, T. The Role of Bcl-2 Proteins in Modulating Neuronal Ca2+ Signaling in Health and in Alzheimer's Disease. Biochim Biophys Acta Mol Cell Res. 2021, 1868(6):118997.
- Duchen, M.R. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef]
- Negri, S.; Faris, P.; Moccia, F. Reactive Oxygen Species and Endothelial Ca2+ Signaling: Brothers in Arms or Partners in Crime? Int J Mol Sci. 2021, 22(18):9821.
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dalangin, R.; Kim, A.; Campbell, R.E. The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef] [PubMed]
- Zuccolo, E.; Kheder, D.A.; Lim, D.; Perna, A.; Nezza, F.D.; Botta, L.; Scarpellino, G.; Negri, S.; Martinotti, S.; Soda, T.; Forcaia, G.; Riboni, L.; Ranzato, E.; Sancini, G.; Ambrosone, L.; D'Angelo, E.; Guerra, G.; Moccia, F. Glutamate Triggers Intracellular Ca2+ Oscillations and Nitric Oxide Release by Inducing NAADP- and InsP3-Dependent Ca2+ Release in Mouse Brain Endothelial Cells. J Cell Physiol. 2019, 234(4):3538-3554.
- Lee, H.K.; Choi, S.S.; Han, E.J.; Han, K.J.; Suh, H.W. Role of Glutamate Receptors and an On-Going Protein Synthesis in the Regulation of Phosphorylation of Ca2+/Calmodulin-Dependent Protein Kinase II in the CA3 Hippocampal Region in Mice Administered With Kainic Acid Intracerebroventricularly. Neurosci Lett. 2003, 348(2):93-96.
- Zhao, Y.; Xiong, W.; Li, C.; Zhao, R.; Lu, H.; Song, S.; Zhou, Y.; Hu, Y.; Shi, B.; Ge, J. Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 1–42. [Google Scholar] [CrossRef]
- Webster, K.A. Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Futur. Cardiol. 2012, 8, 863–884. [Google Scholar] [CrossRef]
- Nicolson, G.L.; de Mattos, G.F.; Ash, M.; Settineri, R.; Escribá, P.V. Fundamentals of Membrane Lipid Replacement: A Natural Medicine Approach to Repairing Cellular Membranes and Reducing Fatigue, Pain, and Other Symptoms While Restoring Function in Chronic Illnesses and Aging. Membranes 2021, 11, 944. [Google Scholar] [CrossRef]
- Szymański, J.; Janikiewicz, J.; Michalska, B.; Patalas-Krawczyk, P.; Perrone, M.; Ziółkowski, W.; Duszyński, J.; Pinton, P.; Dobrzyń, A.; Więckowski, M.R. Interaction of Mitochondria with the Endoplasmic Reticulum and Plasma Membrane in Calcium Homeostasis, Lipid Trafficking and Mitochondrial Structure. Int. J. Mol. Sci. 2017, 18, 1576. [Google Scholar] [CrossRef]
- Bartoszewska, S.; Collawn, J.F.; Bartoszewski, R. The Role of the Hypoxia-Related Unfolded Protein Response (UPR) in the Tumor Microenvironment. Cancers 2022, 14, 4870. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 1–40. [Google Scholar] [CrossRef]
- Carreras-Sureda, A.; Zhang, X.; Laubry, L.; Brunetti, J.; Koenig, S.; Wang, X.; Castelbou, C.; Hetz, C.; Liu, Y.; Frieden, M.; et al. The ER stress sensor IRE1 interacts with STIM1 to promote store-operated calcium entry, T cell activation, and muscular differentiation. Cell Rep. 2023, 42, 113540. [Google Scholar] [CrossRef]
- Bartoszewska, S.; Collawn, J.F. Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia. Cell. Mol. Biol. Lett. 2020, 25, 18. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, L.; Peng, R. Hypoxia-Inducible Factor 1 and Mitochondria: An Intimate Connection. Biomolecules 2022, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Münch, C. The different axes of the mammalian mitochondrial unfolded protein response. BMC Biol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Shpilka, T.; Haynes, C.M. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 2017, 19, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.G.; Park, C.V.; I Yemm, A.; Kenneth, N.S. PERK/eIF2α signaling inhibits HIF-induced gene expression during the unfolded protein response via YB1-dependent regulation of HIF1α translation. Nucleic Acids Res. 2018, 46, 3878–3890. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.Y.L.G.; Villanueva, C.; Bond, R.A. Hypoxia Inducible Factors as Central Players in the Pathogenesis and Pathophysiology of Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Rivers, R.J.; Meininger, C.J. The Tissue Response to Hypoxia: How Therapeutic Carbon Dioxide Moves the Response toward Homeostasis and Away from Instability. Int. J. Mol. Sci. 2023, 24, 5181. [Google Scholar] [CrossRef]
- Della Rocca, Y.; Fonticoli, L.; Rajan, T.S.; Trubiani, O.; Caputi, S.; Diomede, F.; Pizzicannella, J.; Marconi, G.D. Hypoxia: molecular pathophysiological mechanisms in human diseases. J. Physiol. Biochem. 2022, 78, 739–752. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, W.; Li, C.; Zhao, R.; Lu, H.; Song, S.; Zhou, Y.; Hu, Y.; Shi, B.; Ge, J. Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 1–42. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Y.; Wang, D.; Zhang, X.; Li, Y.; Wang, D.; Liang, Y.; Wang, J.; Zheng, L.; Song, H.; et al. Metabolite and protein shifts in mature erythrocyte under hypoxia. iScience 2024, 27, 109315. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, F.; Yang, H.; Wang, Z. Action Sites and Clinical Application of HIF-1α Inhibitors. Molecules 2022, 27, 3426. [Google Scholar] [CrossRef] [PubMed]
- Belapurkar, R.; Pfisterer, M.; Dreute, J.; Werner, S.; Zukunft, S.; Fleming, I.; Kracht, M.; Schmitz, M.L. A transient increase of HIF-1α during the G1 phase (G1-HIF) ensures cell survival under nutritional stress. Cell Death Dis. 2023, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Qannita, R.A.; Alalami, A.I.; Harb, A.A.; Aleidi, S.M.; Taneera, J.; Abu-Gharbieh, E.; El-Huneidi, W.; Saleh, M.A.; Alzoubi, K.H.; Semreen, M.H.; et al. Targeting Hypoxia-Inducible Factor-1 (HIF-1) in Cancer: Emerging Therapeutic Strategies and Pathway Regulation. Pharmaceuticals 2024, 17, 195. [Google Scholar] [CrossRef]
- Koyasu, S.; Kobayashi, M.; Goto, Y.; Hiraoka, M.; Harada, H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018, 109, 560–571. [Google Scholar] [CrossRef]
- Gaete, D.; Rodriguez, D.; Watts, D.; Sormendi, S.; Chavakis, T.; Wielockx, B. HIF-Prolyl Hydroxylase Domain Proteins (PHDs) in Cancer-Potential Targets for Anti-Tumor Therapy? Cancers (Basel). 2021, 13(5):988.
- Hirota, K. HIF-α Prolyl Hydroxylase Inhibitors and Their Implications for Biomedicine: A Comprehensive Review. Biomedicines 2021, 9, 468. [Google Scholar] [CrossRef]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway and its Potential for Therapeutic Intervention in Malignancy and Ischemia. 2007, 80, 51–60.
- Marsboom, G.; Toth, P.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.-H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-Related Protein 1–Mediated Mitochondrial Mitotic Fission Permits Hyperproliferation of Vascular Smooth Muscle Cells and Offers a Novel Therapeutic Target in Pulmonary Hypertension. Clin. Trans. Res. 2012, 110, 1484–1497. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, L.; Peng, R. Hypoxia-Inducible Factor 1 and Mitochondria: An Intimate Connection. Biomolecules 2022, 13, 50. [Google Scholar] [CrossRef]
- Kirito, K.; Hu, Y.; Komatsu, N. HIF-1 prevents the overproduction of mitochondrial ROS after cytokine stimulation through induction of PDK-Cell Cycle 2009, 8, 2844–2849. [CrossRef]
- Sasabe, E.; Tatemoto, Y.; Li, D.; Yamamoto, T.; Osaki, T. Mechanism of HIF-1alpha-Dependent Suppression of Hypoxia-Induced Apoptosis in Squamous Cell Carcinoma Cells. Cancer Sci. 2005, 96(7):394-402.
- Mialet-Perez, J.; Belaidi, E. Interplay between hypoxia inducible Factor-1 and mitochondria in cardiac diseases. Free. Radic. Biol. Med. 2024, 221, 13–22. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, L.; Peng, R. Hypoxia-Inducible Factor 1 and Mitochondria: An Intimate Connection. Biomolecules 2022, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Belenichev, I.F.; Aliyeva, O.G.; Popazova, O.O.; Bukhtiyarova, N.V. Involvement of heat shock proteins HSP70 in the mechanisms of endogenous neuroprotection: the prospect of using HSP70 modulators. Front. Cell. Neurosci. 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.B.; Krueger-Naug, A.M.; Clarke, D.B.; Arrigo, A.-P.; Currie, R.W. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int. J. Hyperth. 2005, 21, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, M.R.; Gragera, M.; Ochoa-Ibarrola, L.; Quintana-Gallardo, L.; Valpuesta, J.M. Hsp70 – a master regulator in protein degradation. FEBS Lett. 2017, 591, 2648–2660. [Google Scholar] [CrossRef]
- Tsuchida, S.; Arai, Y.; Takahashi, K.A.; Kishida, T.; Terauchi, R.; Honjo, K.; Nakagawa, S.; Inoue, H.; Ikoma, K.; Ueshima, K.; Matsuki, T.; Mazda, O.; Kubo, T. HIF-1α-Induced HSP70 Regulates Anabolic Responses in Articular Chondrocytes Under Hypoxic Conditions. J Orthop Res. 2014, 32(8):975-980.
- Belenichev, I.F.; Kolesnik, Y.M.; Pavlov, S.V.; Sokolik, E.P.; Bukhtiyarova, N.V. Malate-aspartate shunt in neuronal adaptation to ischemic conditions: Molecular-biochemical mechanisms of activation and regulation. Neurochem. J. 2012, 6, 22–28. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Kolesnik, Y.M.; Pavlov, S.V.; Sokolik, E.P.; Bukhtiyarova, N.V. Disturbance of HSP70 chaperone activity is a possible mechanism of mitochondrial dysfunction. Neurochem. J. 2011, 5, 251–256. [Google Scholar] [CrossRef]
- Aliyeva, O.; Belenichev, I.; Popazova, O. Modulation of Hsp70 in the Pharmacological Correction of Nervous System Disorders after Prenatal Hypoxia. International Electronic Conference on Biomedicines. LOCATION OF CONFERENCE, COUNTRYDATE OF CONFERENCE; p. 39.
- Xu, L.; Voloboueva, L.A.; Ouyang, Y.; Emery, J.F.; Giffard, R.G. Overexpression of Mitochondrial Hsp70/Hsp75 in Rat Brain Protects Mitochondria, Reduces Oxidative Stress, and Protects From Focal Ischemia. J Cereb Blood Flow Metab. 2009, 29(2):365-374.
- Zatsepina, O.G.; Evgen’ev, M.B.; Garbuz, D.G. Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection. Cells 2021, 10, 1638. [Google Scholar] [CrossRef]
- Lyon, M.S.; Milligan, C. Extracellular heat shock proteins in neurodegenerative diseases: New perspectives. Neurosci. Lett. 2019, 711, 134462. [Google Scholar] [CrossRef]
- Dudeja, V.; Mujumdar, N.; Phillips, P.; Chugh, R.; Borja–Cacho, D.; Dawra, R.K.; Vickers, S.M.; Saluja, A.K. Heat Shock Protein 70 Inhibits Apoptosis in Cancer Cells Through Simultaneous and Independent Mechanisms. Gastroenterology 2009, 136, 1772–1782. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, G.; Liu, Y.; Zhang, J.; Chen, Y.; Liu, L.; Zhang, G. HSP70 Family in Cancer: Signaling Mechanisms and Therapeutic Advances. Biomolecules 2023, 13, 601. [Google Scholar] [CrossRef]
- Nunes, K.P.; de Oliveira, A.A. HSP70: From Signaling Mechanisms to Therapeutics. Biomolecules. 2023, 13, 1141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Y.; Gorshkov, B.; Haigh, S.; Bordan, Z.; Weintraub, D.; Rudic, R.D.; Chakraborty, T.; Barman, S.A.; Verin, A.D.; et al. Hsp70 Suppresses Mitochondrial Reactive Oxygen Species and Preserves Pulmonary Microvascular Barrier Integrity Following Exposure to Bacterial Toxins. Front. Immunol. 2018, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Bath, S.; Marsden, V.S.; Huang, D.C.S.; Metcalf, D.; Harris, A.W.; Strasser, A. FADD and caspase-8 are required for cytokine-induced proliferation of hemopoietic progenitor cells. Blood 2005, 106, 1581–1589. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Lien, C.-I.; Tseng, Y.-C.; Tu, Y.-F.; Kulczyk, A.W.; Lu, Y.-C.; Wang, Y.-T.; Su, T.-W.; Hsu, L.-C.; Lo, Y.-C.; et al. Deciphering DED assembly mechanisms in FADD-procaspase-8-cFLIP complexes regulating apoptosis. Nat. Commun. 2024, 15, 1–18. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Chen, J.D. Daxx Silencing Sensitizes Cells to Multiple Apoptotic Pathways. Mol. Cell. Biol. 2003, 23, 7108–7121. [Google Scholar] [CrossRef]
- Seyrek, K.; Espe, J.; Reiss, E.; Lavrik, I.N. The Crosstalk of Apoptotic and Non-Apoptotic Signaling in CD95 System. Cells 2024, 13, 1814. [Google Scholar] [CrossRef]
- Kim, J.Y.; Barua, S.; Huang, M.Y.; Park, J.; Yenari, M.A.; Lee, J.E. Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury. Cells 2020, 9, 2020. [Google Scholar] [CrossRef]
- Zhang, K.; Zhai, R.; Xue, T.; Xu, X.; Ren, Y.; Ma, M.; Shi, F.; Wang, H.; Wang, N.; Zhou, F. HSP70 regulates cell proliferation and apoptosis in actinomycin-D-treated lung cancer cells. Transl. Cancer Res. 2020, 9, 1167–1173. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X. Cytochrome c Promotes Caspase-9 Activation by Inducing Nucleotide Binding to Apaf-J. Biol. Chem. 2000, 275, 31199–31203. [Google Scholar] [CrossRef]
- Merlin, J.P.J.; Crous, A.; Abrahamse, H. Combining Photodynamic Therapy and Targeted Drug Delivery Systems: Enhancing Mitochondrial Toxicity for Improved Cancer Outcomes. Int. J. Mol. Sci. 2024, 25, 10796. [Google Scholar] [CrossRef]
- Li, C.-Y.; Lee, J.-S.; Ko, Y.-G.; Kim, J.-I.; Seo, J.-S. Heat Shock Protein 70 Inhibits Apoptosis Downstream of Cytochrome c Release and Upstream of Caspase-3 Activation. J. Biol. Chem. 2000, 275, 25665–25671. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Bratton, S.B. Caspase-9 swings both ways in the apoptosome. Mol. Cell. Oncol. 2016, 4, e1281865. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, L.; Du, F. Novel Small Molecules Relieve Prothymosin Alpha-Mediated Inhibition of Apoptosome Formation by Blocking Its Interaction With Apaf-Biochemistry. 2010, 49(9):1923-1930.
- Leu, J.I.-J.; Barnoud, T.; Zhang, G.; Tian, T.; Wei, Z.; Herlyn, M.; Murphy, M.E.; George, D.L. Inhibition of stress-inducible HSP70 impairs mitochondrial proteostasis and function. Oncotarget 2017, 8, 45656–45669. [Google Scholar] [CrossRef] [PubMed]
- Hussar, P. Apoptosis Regulators Bcl-2 and Caspase-Encyclopedia 2022, 2, 1624–1636. [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The Mitochondrial Permeability Transition Pore-Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells. 2023, 12(9):1273.
- Kinnally, K.W.; Zorov, D.B.; Antonenko, Y.N.; Snyder, S.H.; McEnery, M.W.; Tedeschi, H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc. Natl. Acad. Sci. 1993, 90, 1374–1378. [Google Scholar] [CrossRef]
- Servida, F.; Lecis, D.; Scavullo, C.; Drago, C.; Seneci, P.; Carlo-Stella, C.; Manzoni, L.; Polli, E.; Deliliers, G.L.; Delia, D.; et al. Novel second mitochondria-derived activator of caspases (Smac) mimetic compounds sensitize human leukemic cell lines to conventional chemotherapeutic drug-induced and death receptor-mediated apoptosis. Investig. New Drugs 2010, 29, 1264–1275. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Wan, J.; Morse, P.T.; Zurek, M.P.; Turner, A.A.; Khobeir, A.; Yazdi, N.; Hakim, L.; Liu, J.; Vaishnav, A.; et al. Cytochrome c phosphorylation: Control of mitochondrial electron transport chain flux and apoptosis. Int. J. Biochem. Cell Biol. 2020, 121, 105704–105704. [Google Scholar] [CrossRef]
- Waterhouse, N.J.; Goldstein, J.C.; von Ahsen, O.; Schuler, M.; Newmeyer, D.D.; Green, D.R. Cytochrome C Maintains Mitochondrial Transmembrane Potential and Atp Generation after Outer Mitochondrial Membrane Permeabilization during the Apoptotic Process. J. Cell Biol. 2001, 153, 319–328. [Google Scholar] [CrossRef]
- Barroso, G.; Morshedi, M.; Oehninger, S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum. Reprod. 2000, 15, 1338–1344. [Google Scholar] [CrossRef]
- Ramos, M.F.; Baker, J.; Atzpodien, E.-A.; Bach, U.; Brassard, J.; Cartwright, J.; Farman, C.; Fishman, C.; Jacobsen, M.; Junker-Walker, U.; et al. Nonproliferative and Proliferative Lesions of the Rat and Mouse Special Sense Organs (Ocular eye and glands], Olfactory and Otic). J. Toxicol. Pathol. 2018, 31, 97S–214S. [Google Scholar] [CrossRef]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, A.; Tamrakar, P.; Rosenthal, R.E.; Fiskum, G. Calcium Uptake and Cytochrome c Release From Normal and Ischemic Brain Mitochondria. Neurochem Int. 2018, 117:15-22.
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722–a008722. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Hayashi, T.; Su, T.-P.; Betenbaugh, M.J. Bcl-2 family in inter-organelle modulation of calcium signaling; roles in bioenergetics and cell survival. J. Bioenerg. Biomembr. 2013, 46, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Saddam; Paul, S.K.; Habib, M.A.; Fahim, A.; Mimi, A.; Islam, S.; Paul, B.; Helal, M.U. Emerging biomarkers and potential therapeutics of the BCL-2 protein family: the apoptotic and anti-apoptotic context. Egypt. J. Med Hum. Genet. 2024, 25, 1–28. [Google Scholar] [CrossRef]
- Green, D.R. The Mitochondrial Pathway of Apoptosis Part II: The BCL-2 Protein Family. Cold Spring Harb. Perspect. Biol. 2022, 14, a041046. [Google Scholar] [CrossRef]
- Nguyen, D.; Osterlund, E.; Kale, J.; Andrews, D.W. The C-terminal sequences of Bcl-2 family proteins mediate interactions that regulate cell death. Biochem. J. 2024, 481, 903–922. [Google Scholar] [CrossRef]
- Kantari, C.; Walczak, H. Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2011, 1813, 558–563. [Google Scholar] [CrossRef]
- Bahatyrevich-Kharitonik, B.; Medina-Guzman, R.; Flores-Cortes, A.; García-Cruzado, M.; Kavanagh, E.; Burguillos, M.A. Cell Death Related Proteins Beyond Apoptosis in the CNS. Front. Cell Dev. Biol. 2022, 9, 825747. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Schoeniger, A.; Edlich, F. Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2022, 1869, 119317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Yue, G.; Zhao, Y. Energy Metabolism Disturbance in Migraine: From a Mitochondrial Point of View. Front. Physiol. 2023, 14:1133528.
- ileikytė, J.; Forte, M. The Mitochondrial Permeability Transition in Mitochondrial Disorders. Oxid Med Cell Longev. 2019, 2019:3403075.
- Trindade, D.; Pereira, C.; Chaves, S.R.; Manon, S.; Corte-Real, M.; Sousa, M.J. VDAC regulates AAC-mediated apoptosis and cytochrome c release in yeast. Microb. Cell 2016, 3, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.-S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2006, 1757, 639–647. [Google Scholar] [CrossRef]
- Naumova, N.; Šachl, R. Regulation of Cell Death by Mitochondrial Transport Systems of Calcium and Bcl-2 Proteins. Membranes 2020, 10, 299. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Maes, M.E.; Schlamp, C.L.; Nickells, R.W. BAX to basics: How the BCL2 gene family controls the death of retinal ganglion cells. Prog. Retin. Eye Res. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Kotrasová, V.; Keresztesová, B.; Ondrovičová, G.; Bauer, J.A.; Havalová, H.; Pevala, V.; Kutejová, E.; Kunová, N. Mitochondrial Kinases and the Role of Mitochondrial Protein Phosphorylation in Health and Disease. Life 2021, 11, 82. [Google Scholar] [CrossRef]
- Zeke, A.; Misheva, M.; Reményi, A.; Bogoyevitch, M.A. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol. Mol. Biol. Rev. 2016, 80, 793–835. [Google Scholar] [CrossRef] [PubMed]
- Pahlavani, H.A. Exercise-induced signaling pathways to counteracting cardiac apoptotic processes. Front. Cell Dev. Biol. 2022, 10, 950927. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How Does p53 Induce Apoptosis and How Does This Relate to p53-Mediated Tumour Suppression? Cell Death Differ. 2018, 25(1):104-113.
- la Cour, J.M.; Høj, B.R.; Mollerup, J.; Simon, R.; Sauter, G.; Berchtold, M.W. The apoptosis linked gene ALG-2 is dysregulated in tumors of various origin and contributes to cancer cell viability. Mol. Oncol. 2007, 1, 431–439. [Google Scholar] [CrossRef]
- McCullough, J.; Fisher, R.D.; Whitby, F.G.; Sundquist, W.I.; Hill, C.P. ALIX-CHMP4 interactions in the human ESCRT pathway. Proc. Natl. Acad. Sci. 2008, 105, 7687–7691. [Google Scholar] [CrossRef]
- Zanatta, D.; Betanzos, A.; Azuara-Liceaga, E.; Montaño, S.; Orozco, E. Entamoeba histolytica: EhADH, an Alix Protein, Participates in Several Virulence Events through Its Different Domains. Int. J. Mol. Sci. 2024, 25, 7609. [Google Scholar] [CrossRef]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It is all about energy. Front. Physiol. 2023, 14. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondrial Regulation of Cell Death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706–a008706. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Li, Y.; Rasheed, M.; Liu, J.; Chen, Z.; Deng, Y. Deciphering the Molecular Nexus: An In-Depth Review of Mitochondrial Pathways and Their Role in Cell Death Crosstalk. Cells 2024, 13, 863. [Google Scholar] [CrossRef]
- Bano, D.; Prehn, J.H. Apoptosis-Inducing Factor (AIF) in Physiology and Disease: The Tale of a Repented Natural Born Killer. EBioMedicine 2018, 30, 29–37. [Google Scholar] [CrossRef]
- Flores-Romero, H.; Dadsena, S.; García-Sáez, A.J. Mitochondrial pores at the crossroad between cell death and inflammatory signaling. 2023, 83, 843–856. [CrossRef]
- Nesci, S.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Algieri, C.; Tioli, G.; Lenaz, G. Molecular and Supramolecular Structure of the Mitochondrial Oxidative Phosphorylation System: Implications for Pathology. Life 2021, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Spagnoletta, A.; Oppedisano, F. Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust. Int. J. Mol. Sci. 2023, 24, 6106. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Adameová, A.; Barile, L.; Cabrera-Fuentes, H.A.; Lazou, A.; Pagliaro, P.; Stensløkken, K.; Garcia-Dorado, D.; Action, E.-C.C. Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J. Cell. Mol. Med. 2020, 24, 3795–3806. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, D.J.; Harata, M.; Murphy, E.; Karch, J. Mitochondrial permeability transition pore-dependent necrosis. 2022, 174, 47–55. [CrossRef]
- Javadov, S.; Chapa-Dubocq, X.; Makarov, V. Different approaches to modeling analysis of mitochondrial swelling. Mitochondrion 2018, 38, 58–70. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wei, S.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.-M.; Kim, H.H.; Ha, K.-T.; Park, K.S.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef]
- Miller, M.A.; Zachary, J.F. Mechanisms and Morphology of Cellular Injury, Adaptation, and Death 11 For a glossary of abbreviations and terms used in this chapter see E-Glossary 1-1. Pathol. Basis Vet. Dis. 2017, 2–43.e19. [CrossRef]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Sandai, D.; Zhao, R.; Bai, J.; Wang, Y.; Wang, Y.; Zhang, Z.; Zhang, H.-L.; Song, Z.-J. ATP-induced cell death: a novel hypothesis for osteoporosis. Front. Cell Dev. Biol. 2023, 11, 1324213. [Google Scholar] [CrossRef]
- Imamura, H.; Sakamoto, S.; Yoshida, T.; Matsui, Y.; Penuela, S.; Laird, D.W.; Mizukami, S.; Kikuchi, K.; Kakizuka, A.; Biology, C.; et al. Single-cell dynamics of pannexin-1-facilitated programmed ATP loss during apoptosis. eLife 2020, 9. [Google Scholar] [CrossRef]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-Demiguel, P.; Martinez-Guardado, I.; Dalamitros, A.A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Andreux, P.A.; Houtkooper, R.H.; Auwerx, J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 2013, 12, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Faccenda, D.; Campanella, M. Pharmacological advances in mitochondrial therapy. EBioMedicine 2021, 65, 103244. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Han, Y.; He, Y.; Liu, J.; Wang, Y. Natural compounds targeting mitochondrial dysfunction: emerging therapeutics for target organ damage in hypertension. Front. Pharmacol. 2023, 14, 1209890. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Hannan, M.A.; Rahman, M.A.; Apostolova, N. Editorial: Novel Pharmacological Approaches Targeting Mitochondrial Dysfunction in Diseases. Front. Pharmacol. 2022, 13:1041576.
- Tinker, R.J.; Lim, A.Z.; Stefanetti, R.J.; McFarland, R. Current and Emerging Clinical Treatment in Mitochondrial Disease. Mol. Diagn. Ther. 2021, 25, 181–206. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.E.; Levchenkova, O.S.; Ivantsova, E.N.; Vorobieva, V.V. Mitochondrial Dysfunctions and Antihypoxants. Reviews on Clinical Pharmacology and Drug Therapy. 2019, 17(4):31–42.
- Korski, K.I.; Kubli, D.A.; Wang, B.J.; Khalafalla, F.G.; Monsanto, M.M.; Firouzi, F.; Echeagaray, O.H.; Kim, T.; Adamson, R.M.; Dembitsky, W.P.; et al. Hypoxia Prevents Mitochondrial Dysfunction and Senescence in Human c-Kit+ Cardiac Progenitor Cells. STEM CELLS 2019, 37, 555–567. [Google Scholar] [CrossRef]
- Cuvillier, O.; Ader, I.; Bouquerel, P.; Brizuela, L.; Gstalder, C.; Malavaud, B. Hypoxia, Therapeutic Resistance, and Sphingosine 1-Phosphate. Adv Cancer Res. 2013, 117:117–141.
- Schönenberger, M.J.; Kovacs, W.J. Hypoxia signaling pathways: modulators of oxygen-related organelles. Front. Cell Dev. Biol. 2015, 3, 42. [Google Scholar] [CrossRef]
- Bouhamida, E.; Morciano, G.; Perrone, M.; Kahsay, A.E.; Della Sala, M.; Wieckowski, M.R.; Fiorica, F.; Pinton, P.; Giorgi, C.; Patergnani, S. The Interplay of Hypoxia Signaling on Mitochondrial Dysfunction and Inflammation in Cardiovascular Diseases and Cancer: From Molecular Mechanisms to Therapeutic Approaches. Biology 2022, 11, 300. [Google Scholar] [CrossRef]
- Sifat, A.E.; Nozohouri, S.; Archie, S.R.; Chowdhury, E.A.; Abbruscato, T.J. Brain Energy Metabolism in Ischemic Stroke: Effects of Smoking and Diabetes. Int. J. Mol. Sci. 2022, 23, 8512. [Google Scholar] [CrossRef]
- Martinez, C.S.; Zheng, A.; Xiao, Q. Mitochondrial Reactive Oxygen Species Dysregulation in Heart Failure with Preserved Ejection Fraction: A Fraction of the Whole. Antioxidants 2024, 13, 1330. [Google Scholar] [CrossRef]
- Arnalich-Montiel, A.; Burgos-Santamaría, A.; Pazó-Sayós, L.; Quintana-Villamandos, B. Comprehensive Management of Stroke: From Mechanisms to Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 5252. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Polster, B.M. Idebenone and neuroprotection: antioxidant, pro-oxidant, or electron carrier? J. Bioenerg. Biomembr. 2015, 47, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, M.H.; Schulz, J.B.; Giunti, P. Co-Enzyme Q10 and Idebenone Use in Friedreich's Ataxia. J Neurochem. 2013, 126(Suppl 1):125–141.
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, B.; Villardita, C.; Coppi, R. Idebenone in the treatment of multi-infarct dementia: a randomised, double-blind, placebo controlled multicentre trial. Arch. Gerontol. Geriatr. 1992, 15, 271–278. [Google Scholar] [CrossRef]
- Marigliano, V.; Abate, G.; Barbagallo-Sangiorgi, G.; Bartorelli, L.; Capurso, A.; Cucinotta, D.; Cuzzupoli, M.; Senin, U.; Tammaro, A.E.; Fioravanti, M. Randomized, double-blind, placebo controlled, multicentre study of idebenone in patients suffering from multi-infarct dementia. Arch. Gerontol. Geriatr. 1992, 15, 239–248. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Luo, N.; Chen, F.; Zhou, L.; Niu, M.; Kang, W.; Liu, J. Efficacy of idebenone in the Treatment of iRBD into Synucleinopathies (EITRS): rationale, design, and methodology of a randomized, double-blind, multi-center clinical study. Front. Neurol. 2022, 13, 981249. [Google Scholar] [CrossRef]
- Shneyvays, V.; Leshem, D.; Shmist, Y.; Zinman, T.; Shainberg, A. Effects of menadione and its derivative on cultured cardiomyocytes with mitochondrial disorders. J. Mol. Cell. Cardiol. 2005, 39, 149–158. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Hou, X.; Jacob, C. 1,4-Naphthoquinones: From Oxidative Damage to Cellular and Inter-Cellular Signaling. Molecules 2014, 19, 14902–14918. [Google Scholar] [CrossRef]
- Schmidt, C.A. Prescription drugs and mitochondrial metabolism. Biosci. Rep. 2022, 42. [Google Scholar] [CrossRef]
- Tang, Q.; Xie, Y.; Liu, Y.; Zheng, L. Synthesis of mitochondria-targeted menadione cation derivatives: Inhibiting mitochondrial thioredoxin reductase (TrxR2) and inducing apoptosis in MGC-803 cells. Bioorganic Med. Chem. Lett. 2022, 60, 128586. [Google Scholar] [CrossRef]
- Yaneva, Z.; Ivanova, D.; Toneva, M.; Tzanova, M.; Marutsova, V.; Grozeva, N. Menadione Contribution to the In Vitro Radical Scavenging Potential of Phytochemicals Naringenin and Lignin. Int. J. Mol. Sci. 2023, 24, 16268. [Google Scholar] [CrossRef] [PubMed]
- A Merchant, H. The synergistic combination of trimetazidine, hypoxen and L-carnitine in endurance sports. Br. J. Pharm. 2032, 7. [Google Scholar] [CrossRef]
- Görgens, C.; Möller, T.; Guddat, S.; Svambayev, E.; Geyer, H.; Thomas, A.; Thevis, M. Urinary metabolites indicative of the administration of hypoxen monitored by liquid chromatography–high resolution/accurate mass tandem mass spectrometry. Drug Test. Anal. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K. Extracellular Succinate: A Physiological Messenger and a Pathological Trigger. Int. J. Mol. Sci. 2023, 24, 11165. [Google Scholar] [CrossRef]
- Huang, H.; Li, G.; He, Y.; Chen, J.; Yan, J.; Zhang, Q.; Li, L.; Cai, X. Cellular succinate metabolism and signaling in inflammation: implications for therapeutic intervention. Front. Immunol. 2024, 15, 1404441. [Google Scholar] [CrossRef]
- Zhang, W.; Lang, R. Succinate metabolism: a promising therapeutic target for inflammation, ischemia/reperfusion injury and cancer. Front. Cell Dev. Biol. 2023, 11, 1266973. [Google Scholar] [CrossRef]
- Bielenichev, I.F.; Gorchakova, N.A.; Doroshenko, E.Y.; Samura, I.B.; Ryzhenko, V.P.; Bukhtiiarova, N.V. Use of metabolites, metabolithotropic agents and nutritional supplements in sports and sports medicine: a modern view on the problem. Mod. Med Technol. 2023, 76–88. [Google Scholar] [CrossRef]
- Oguro, H.; Iijima, K.; Takahashi, K.; Nagai, A.; Bokura, H.; Yamaguchi, S.; Kobayashi, S. Successful Treatment with Succinate in a Patient with MELAS. Intern. Med. 2004, 43, 427–431. [Google Scholar] [CrossRef]
- Ehinger, J.K.; Piel, S.; Ford, R.; Karlsson, M.; Sjövall, F.; Frostner, E. .; Morota, S.; Taylor, R.W.; Turnbull, D.; Cornell, C.; et al. Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat. Commun. 2016, 7, 12317. [Google Scholar] [CrossRef]
- Jędrejko, K.; Catlin, O.; Stewart, T.; Muszyńska, B. Mexidol, Cytoflavin, and succinic acid derivatives as antihypoxic, anti-ischemic metabolic modulators, and ergogenic aids in athletes and consideration of their potential as performance enhancing drugs. Drug Test. Anal. 2024. [Google Scholar] [CrossRef]
- Gorchakova, N.; Belenichev, I.; Harnyk, T.; Shumeiko, O.; Klymenko, O. Membranotropic Action of Phytodrugs. Phytotherapy J. 2023, 4:5–10.
- Liu, X.; Zhao, G.; Sun, S.; Fan, C.; Feng, X.; Xiong, P. Biosynthetic Pathway and Metabolic Engineering of Succinic Acid. Front. Bioeng. Biotechnol. 2022, 10, 843887. [Google Scholar] [CrossRef] [PubMed]
- Pedriali, G.; Ramaccini, D.; Bouhamida, E.; Wieckowski, M.R.; Giorgi, C.; Tremoli, E.; Pinton, P. Perspectives on mitochondrial relevance in cardiac ischemia/reperfusion injury. Front. Cell Dev. Biol. 2022, 10, 1082095. [Google Scholar] [CrossRef] [PubMed]
- Genova, M.L.; Lenaz, G. Functional role of mitochondrial respiratory supercomplexes. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2014, 1837, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2021, 23, 141–161. [Google Scholar] [CrossRef]
- Mansilla, N.; Racca, S.; Gras, D.E.; Gonzalez, D.H.; Welchen, E. The Complexity of Mitochondrial Complex IV: An Update of Cytochrome c Oxidase Biogenesis in Plants. Int. J. Mol. Sci. 2018, 19, 662. [Google Scholar] [CrossRef]
- Skulachev, V.P. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Biochem. Biophys. Res. Commun. 2013, 441, 275–279. [Google Scholar] [CrossRef]
- Fock, E.M.; Parnova, R.G. Protective Effect of Mitochondria-Targeted Antioxidants against Inflammatory Response to Lipopolysaccharide Challenge: A Review. Pharmaceutics 2021, 13, 144. [Google Scholar] [CrossRef]
- Skulachev, V.P.; Vyssokikh, M.Y.; Chernyak, B.V.; Averina, O.A.; Andreev-Andrievskiy, A.A.; Zinovkin, R.A.; Lyamzaev, K.G.; Marey, M.V.; Egorov, M.V.; Frolova, O.J.; et al. Mitochondrion-targeted antioxidant SkQ1 prevents rapid animal death caused by highly diverse shocks. Sci. Rep. 2023, 13, 1–15. [Google Scholar] [CrossRef]
- Ivanov, B.; Khorobrykh, S. Participation of Photosynthetic Electron Transport in Production and Scavenging of Reactive Oxygen Species. Antioxidants Redox Signal. 2003, 5, 43–53. [Google Scholar] [CrossRef]
- Silachev, D.N.; Plotnikov, E.Y.; Zorova, L.D.; Pevzner, I.B.; Sumbatyan, N.V.; Korshunova, G.A.; Gulyaev, M.V.; Pirogov, Y.A.; Skulachev, V.P.; Zorov, D.B. Neuroprotective Effects of Mitochondria-Targeted Plastoquinone and Thymoquinone in a Rat Model of Brain Ischemia/Reperfusion Injury. Molecules 2015, 20, 14487–14503. [Google Scholar] [CrossRef]
- Zhou, Z.; Austin, G.L.; Young, L.E.A.; Johnson, L.A.; Sun, R. Mitochondrial Metabolism in Major Neurological Diseases. Cells 2018, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-Demiguel, P.; Martinez-Guardado, I.; Dalamitros, A.A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kükürt, A.; Gelen, V.; Faruk Başer, Ö.; Deveci, H.A.; Karapehlivan, M. Thiols: Role in Oxidative Stress-Related Disorders. IntechOpen. 2021.
- McCarty, M.F.; DiNicolantonio, J.J.; Lerner, A. A Fundamental Role for Oxidants and Intracellular Calcium Signals in Alzheimer’s Pathogenesis—And How a Comprehensive Antioxidant Strategy May Aid Prevention of This Disorder. Int. J. Mol. Sci. 2021, 22, 2140. [Google Scholar] [CrossRef]
- Manda-Handzlik, A.; Bystrzycka, W.; Cieloch, A.; Glodkowska-Mrowka, E.; Jankowska-Steifer, E.; Heropolitanska-Pliszka, E.; Skrobot, A.; Muchowicz, A.; Ciepiela, O.; Wachowska, M.; et al. Nitric oxide and peroxynitrite trigger and enhance release of neutrophil extracellular traps. Cell. Mol. Life Sci. 2019, 77, 3059–3075. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, G.; Godínez-Méndez, L.A.; Fafutis-Morris, M.; Delgado-Rizo, V. Effect of Antioxidant Supplementation on NET Formation Induced by LPS In Vitro: The Roles of Vitamins E and C, Glutathione, and N-Acetyl Cysteine. Int. J. Mol. Sci. 2023, 24:13162.
- Gupta, D.S.; Parab, S.B.; Kaur, G. Promising effects of emoxypine and its succinate derivative in the management of various diseases-with insights on recent patent applications. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100121. [Google Scholar] [CrossRef]
- Peresypkina, A.; Pazhinsky, A.; Danilenko, L.; Lugovskoy, S.; Pokrovskii, M.; Beskhmelnitsyna, E.; Solovev, N.; Pobeda, A.; Korokin, M.; Levkova, E.; et al. Retinoprotective Effect of 2-Ethyl-3-hydroxy-6-methylpyridine Nicotinate. Biology 2020, 9, 45. [Google Scholar] [CrossRef]
- Chekman, I.; Belenichev, I.; Yakovleva, I.; Gorchakova, N.; Buhtiyarova, N.; Morguntsova, S.; Brazhko, O.; Levich, S. Influence of mexidol on early genomic response and morphofunctional parameters of brain cortex sensorimotor zone neurons after arteria carotis communis occlusion. Oxid. Antioxidants Med Sci. 2015, 4, 33–38. [Google Scholar] [CrossRef]
- Parkhomenko, D.; Belenichev, I.; Kuchkovskyi, O.; Ryzhenko, V. Characteristics of HIF-1α and HSP70 mRNA Expression, Level, and Interleukins in Experimental Chronic Generalized Periodontitis. Microrna. 2024, 13(2):132–139.
- Alekseeva, T.G.; Loseva, E.V.; Mering, T.A. The effects of Mexidol on the acquisition of food-related conditioned reflexes and synaptic ultrastructure in field Ca1 of the rat hippocampus after single acoustic stimuli with ultrasonic components. Neurosci. Behav. Physiol. 2005, 35, 363–369. [Google Scholar] [CrossRef]
- Belenichev, I.; Ryzhenko, V.; Popazova, O.; Bukhtiyarova, N.; Gorchakova, N.; Oksenych, V.; Kamyshnyi, O. Optimization of the Search for Neuroprotectors among Bioflavonoids. Pharmaceuticals 2024, 17, 877. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Shevchenko, A.O.; Kyryliuk, A.D.; Siusiuka, V.G. Premature Childbirth: Fundamental and Clinical Aspects. LAP LAMBERT Academic Publishing. 2022, 308 p.
- Panov, A.V.; Mayorov, V.I.; Dikalova, A.E.; Dikalov, S.I. Long-Chain and Medium-Chain Fatty Acids in Energy Metabolism of Murine Kidney Mitochondria. Int. J. Mol. Sci. 2022, 24, 379. [Google Scholar] [CrossRef] [PubMed]
- Popazova, O.; Belenichev, I.; Bukhtiyarova, N.; Ryzhenko, V.; Oksenych, V.; Kamyshnyi, A. Cardioprotective Activity of Pharmacological Agents Affecting NO Production and Bioavailability in the Early Postnatal Period after Intrauterine Hypoxia in Rats. Biomedicines 2023, 11, 2854. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, H.; Yang, W.; Wang, D.; Sui, X.; Sun, Y.; Wang, S.; Yang, Y.; Xiao, Z.; Yang, J.; et al. Novel energy optimizer, meldonium, rapidly restores acute hypobaric hypoxia-induced brain injury by targeting phosphoglycerate kinase Cell Commun. Signal. 2024, 22, 1–17. [Google Scholar] [CrossRef]
- Yang, W.; Lei, X.; Liu, F.; Sui, X.; Yang, Y.; Xiao, Z.; Cui, Z.; Sun, Y.; Yang, J.; Yang, X.; Lin, X.; Bao, Z.; Li, W.; Ma, Y.; Wang, Y.; Luo, Y. Meldonium, as a Potential Neuroprotective Agent, Promotes Neuronal Survival by Protecting Mitochondria in Cerebral Ischemia-Reperfusion Injury. J. Transl. Med. 2024, 22(1):771.
- Bielenichev, I.F.; Vіzіr, V.A.; Mamchur, V.Y.; Kuriata, O.V. Place of tiotriazoline in the gallery of modern metabolitotropic medicines. Zaporozhye Med J. 2019, 118–128. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Feroz, S.; Chekman, I.S.; Nagornaya, E.A.; Gorbacheva, S.V.; Gorchakova, N.A.; et al. Thiol-Disulfide System: Role in Endogenous Cyto- and Organoprotection, Pathways of Pharmacological Modulation. Kyiv: Yuston Publishing House. 2020, 232 p.
- Belenichev, I.F.; Aliyeva, E.G.; Popazova, O.O. Experimental Substantiation of New Target Links in Complex Therapy of Prenatal CNS Damage: Pharmacological Modulation of HSP70-Dependent Mechanisms of Endogenous Neuroprotection. Neurotherapeutics. 2022, 19:1414–1431.
- Popazova, O.; Belenichev, I.; Yadlovskyi, O.; Oksenych, V.; Kamyshnyi, A. Altered Blood Molecular Markers of Cardiovascular Function in Rats after Intrauterine Hypoxia and Drug Therapy. Curr. Issues Mol. Biol. 2023, 45, 8704–8715. [Google Scholar] [CrossRef]
- Belenichev, I.; Kucherenko, L.; Pavlov, S.; Bukhtiyarova, N.; Popazova, O.; Derevianko, N.; Nimenko, G. Therapy of post-COVID-19 syndrome: improving the efficiency and safety of basic metabolic drug treatment with tiazotic acid (thiotriazoline). Pharmacia 2022, 69, 509–516. [Google Scholar] [CrossRef]
- Kamenshchyk, A.; Belenichev, I.; Oksenych, V.; Kamyshnyi, O. Combined Pharmacological Modulation of Translational and Transcriptional Activity Signaling Pathways as a Promising Therapeutic Approach in Children with Myocardial Changes. Biomolecules 2024, 14, 477. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Mazur, I.A.; Abramov, A.V.; Kucherenko, L.I.; Bukhtiyarova, N.V.; Egorov, A.A.; Belenicheva, O.I.; Polyakova, E.N. The endothelium-protective effect of 3-methyl-1,2,4-triazolyl-5-thioacetate (S)-2,6-diaminohexanic acid (lysinium): Effects on the expression of vascular endothelial growth factor (VEGF) and the characteristics of the endotheliocytes of the cerebral vessels of animals with cerebral ischemia. Neurochem. J. 2013, 7, 296–302. [Google Scholar] [CrossRef]
- Belenichev, I.; Kucherenko, L.; Nagornaya, E.; Mazur, I.; Bidnenko, A.; Bukhtiyarova, N.; Avramenko, N. Functional nitric oxide conjugate systems state/restored heart thiols of rats in modeling isadrine-pituitrin's myocardial infarction using metabolite-tropic cardioprotector “Angiolin”. Int. J. Basic Clin. Pharmacol. 2015, 4, 15. [Google Scholar] [CrossRef]
- Aliyeva, O.; Belenichev, I.; Bukhtiyarova, N.; Semenov, D.; Voloshchuk, S. Comparative Assessment of the Effectiveness of HSP70 / HIF-1α System Modulators After Prenatal Hypoxia. Biomed Pharmacol J. 2024, 17(1).
- Belenichev, I.; Aliyeva, O.; Gunina, L.; Bukhtiyarova, N. EVALUATION OF THE EFFICIENCY OF THE NEUROPROTECTIVE DRUGS AFTER PRENATAL HYPOXIA. Fiziolohichnyĭ zhurnal 2023, 69, 43–53. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Aliyeva, E.G.; Kamyshny, O.M.; Bukhtiyarova, N.V.; Ryzhenko, V.P.; Gorchakova, N.O. Pharmacological Modulation of Endogenous Neuroprotection after Experimental Prenatal Hypoxia. Neurochem. J. 2022, 16, 68–75. [Google Scholar] [CrossRef]
- Casellas, P.; Galiegue, S.; Basile, A.S. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem. Int. 2002, 40, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Yaniv, Y.; Nuss, H.B.; Wang, S.; Sollott, S.J. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc. Res. 2009, 83, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Šileikytė, J.; Blachly-Dyson, E.; Sewell, R.; Carpi, A.; Menabò, R.; Di Lisa, F.; Ricchelli, F.; Bernardi, P.; Forte, M. Regulation of the Mitochondrial Permeability Transition Pore by the Outer Membrane Does Not Involve the Peripheral Benzodiazepine Receptor (Translocator Protein of 18 kDa (TSPO)). J. Biol. Chem. 2014, 289, 13769–13781. [Google Scholar] [CrossRef]
- Sarnowska, A.; Beręsewicz, M.; Zabłocka, B.; Domańska-Janik, K. Diazepam neuroprotection in excitotoxic and oxidative stress involves a mitochondrial mechanism additional to the GABAAR and hypothermic effects. Neurochem. Int. 2009, 55, 164–173. [Google Scholar] [CrossRef]
- Sato, K.; Takayama, K.-I.; Inoue, S. Expression and function of estrogen receptors and estrogen-related receptors in the brain and their association with Alzheimer’s disease. Front. Endocrinol. 2023, 14, 1220150. [Google Scholar] [CrossRef]
- Russell, J.K.; Jones, C.K.; Newhouse, P.A. The Role of Estrogen in Brain and Cognitive Aging. Neurotherapeutics 2019, 16, 649–665. [Google Scholar] [CrossRef]
- Devillers, M.M.; Mhaouty-Kodja, S.; Guigon, C.J. Deciphering the Roles & Regulation of Estradiol Signaling during Female Mini-Puberty: Insights from Mouse Models. Int. J. Mol. Sci. 2022, 23, 13695. [Google Scholar] [CrossRef]
- Del Río, J.P.; Molina, S.; Hidalgo-Lanussa, O.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone as Hormonal Therapy and Neuroprotective Agent. Trends Endocrinol. Metab. 2020, 31, 742–759. [Google Scholar] [CrossRef]
- Novick, A.M.; Scott, A.T.; Epperson, C.N.; Schneck, C.D. Neuropsychiatric effects of tamoxifen: Challenges and opportunities. Front. Neuroendocr. 2020, 59, 100869–100869. [Google Scholar] [CrossRef]
- Lee, E.Y.; Sidoryk, M.; Jiang, H.; Yin, Z.; Aschner, M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J. Neurochem. 2009, 110, 530–544. [Google Scholar] [CrossRef]
- Pavlov, S.V.; Belenichev, I.F. Molecular and biochemical aspects of the neuroprotective effect of the selective estrogen receptor modulator tamoxifen in a model of acute cerebral ischemia. Neurochem. J. 2014, 8, 28–32. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Odnokoz, O.V.; Pavlov, S.V.; Belenicheva, O.I.; Polyakova, E.N. The neuroprotective activity of tamoxifen and tibolone during glutathione depletion in vitro. Neurochem. J. 2012, 6, 202–212. [Google Scholar] [CrossRef]
- El Ouaamari, Y.; Bos, J.V.D.; Willekens, B.; Cools, N.; Wens, I. Neurotrophic Factors as Regenerative Therapy for Neurodegenerative Diseases: Current Status, Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 3866. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, Y.; Dai, C.-L.; Baazaoui, N.; Tung, Y.C.; Liu, F.; Iqbal, K. Neurotrophic Treatment Initiated During Early Postnatal Development Prevents the Alzheimer-Like Behavior and Synaptic Dysfunction. J. Alzheimer's Dis. 2021, 82, 631–646. [Google Scholar] [CrossRef]
- Clarke, I.J.; Smith, J.T.; Caraty, A.; Goodman, R.L.; Lehman, M.N. Kisspeptin and seasonality in sheep. Peptides 2008, 30, 154–163. [Google Scholar] [CrossRef]
- Masliah, E.; Diez-Tejedor, E. The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs Today 2012, 48, 3–24. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Pavlov, S.V.; Dunaev, V.V. Neuroprotective Effect of Cerebrocurin in a Model of Acute Cerebral Stroke. Eksp. Klin. Farmakol. 2010, 73(2):6–9.
- Belenichev, I.F.; Aliyeva, O.G.; Bukhtiyarova, N.V.; Popazova, O.O.; Ryzhenko, V.P. Positive Pharmacological Modulation of Hsp70 in Recovery of Brain Energy Metabolism in Various Models of Cerebral Ischemia. International Electronic Conference on Biomolecules. LOCATION OF CONFERENCE, COUNTRYDATE OF CONFERENCE; p. 24.
- Morguntsova, S.A. Neuroprotection Neurotrophic Cerebroprotector Cerebrocurin in Terms of Modeling Acute Stroke. Zaporozhye Med. J. 2014, 16(5).
- Yevtushenko, O.; Yanovskaya, N.; Yevtushenko, S.; Kutyakova, Y.; Omelyanenko, A.; Dubina, S.; Sokhan, D.; Poroshina, Y.; Fomichova, E.; Sachinova, I. 15-Year Experience of Cerebrocurin Use in Combined Therapy in Children with Organic Diseases of the Nervous System. Int. Neurol. J. 2014, (3.65):13–19.
- Belenichev, I.F.; Sokolik, E.P.; Bukhtiarova, N.V.; Levich, S.V. Pharmacological Modulation of Heat Shock Protein 70 (HSP70)—Dependent Mechanisms of Endogenous Neuroprotection in Conditions of Prenatal Chronic Alcoholism by Cerebrocurin and Tiocetam. 2016, 26, 103–108. [CrossRef]
- Repchuk, Y.; Sydorchuk, L.P.; Sydorchuk, A.R.; Fedonyuk, L.Y.; Kamyshnyi, O.; Korovenkova, O.; Plehutsa, I.M.; Dzhuryak, V.S.; Myshkovskii, Y.M.; Iftoda, O.M.; et al. Linkage of blood pressure, obesity and diabetes mellitus with angiotensinogen gene (AGT 704T>C/rs699) polymorphism in hypertensive patients. Bratisl. Lek. Listy 2021, 122, 715–720. [Google Scholar] [CrossRef]
- Kamyshna, I.I.; Pavlovych, L.B.; Maslyanko, V.A.; Kamyshnyi, A.M. Analysis of the transcriptional activity of genes of neuropeptides and their receptors in the blood of patients with thyroid pathology. J. Med. Life 2021, 14, 243–249. [Google Scholar] [CrossRef]
- Lyubomirskaya, E.S.; Kamyshnyi, A.M.; Krut, Y.Y.; A Smiianov, V.; Fedoniuk, L.Y.; Romanyuk, L.B.; Kravets, N.Y.; Mochulska, O.M. SNPs and transcriptional activity of genes of innate and adaptive immunity at the maternal-fetal interface in woman with preterm labour, associated with preterm premature rupture of membranes. Wiad Lek 2020, 73, 25–30. [Google Scholar]
- Halabitska, I.; Petakh, P.; Kamyshna, I.; Oksenych, V.; Kainov, D.E.; Kamyshnyi, O. The interplay of gut microbiota, obesity, and depression: insights and interventions. Cell. Mol. Life Sci. 2024, 81, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Topol, I.; Kamyshny, A. Study of expression of TLR2, TLR4 and transckription factor NF-kB structures of galt of rats in the conditions of the chronic social stress and modulation of structure of intestinal microflora. Georgian Med. News 2013, 225, 115–122. [Google Scholar]
- Dzhuryak, V.; Sydorchuk, L.; Sydorchuk, A.; Kamyshnyi, O.; Kshanovska, A.; Levytska, S.; Knut, R.; Sheremet, M.; Ivashchuk, S.; Petrynych, O.; et al. The cytochrome 11B2 aldosterone synthase gene CYP11B2 (RS1799998) polymorphism associates with chronic kidney disease in hypertensive patients. Biointerface Res. Appl. Chem. 2020, 10, 5406–5411. [Google Scholar] [CrossRef]
- Halabitska, I.; Oksenych, V.; Kamyshnyi, O. Exploring the Efficacy of Alpha-Lipoic Acid in Comorbid Osteoarthritis and Type 2 Diabetes Mellitus. Nutrients 2024, 16, 3349. [Google Scholar] [CrossRef]
- Nosulenko, I.S.; Voskoboynik, O.Y.; Berest, G.G.; Safronyuk, S.L.; Kovalenko, S.I.; Kamyshnyi, O.M.; Polishchuk, N.M.; Sinyak, R.S.; Katsev, A.V. Synthesis and Antimicrobial Activity of 6-Thioxo-6,7-dihydro-2H-[1,2,4]triazino[2,3-c]quinazolin-2-one Derivatives. Sci. Pharm. 2014, 82, 483–500. [Google Scholar] [CrossRef]
- Bilous II, Pavlovych LL, Kamyshnyi AM. Primary hypothyroidism and autoimmune thyroiditis alter the transcriptional activity of genes regulating neurogenesis in the blood of patients. Endocr Regul. 2021; 55(1): 5-15.
- Halabitska, I.; Babinets, L.; Oksenych, V.; Kamyshnyi, O. Diabetes and Osteoarthritis: Exploring the Interactions and Therapeutic Implications of Insulin, Metformin, and GLP-1-Based Interventions. Biomedicines 2024, 12, 1630. [Google Scholar] [CrossRef]
- Bilyi AK, Antypenko LM, Ivchuk VV, Kamyshnyi OM, Polishchuk NM, Kovalenko SI. 2-Heteroaryl-[1,2,4]triazolo[1,5-c]quinazoline-5(6 H)-thiones and Their S-Substituted Derivatives: Synthesis, Spectroscopic Data, and Biological Activity. Chempluschem. 2015; 80(6):980-989.
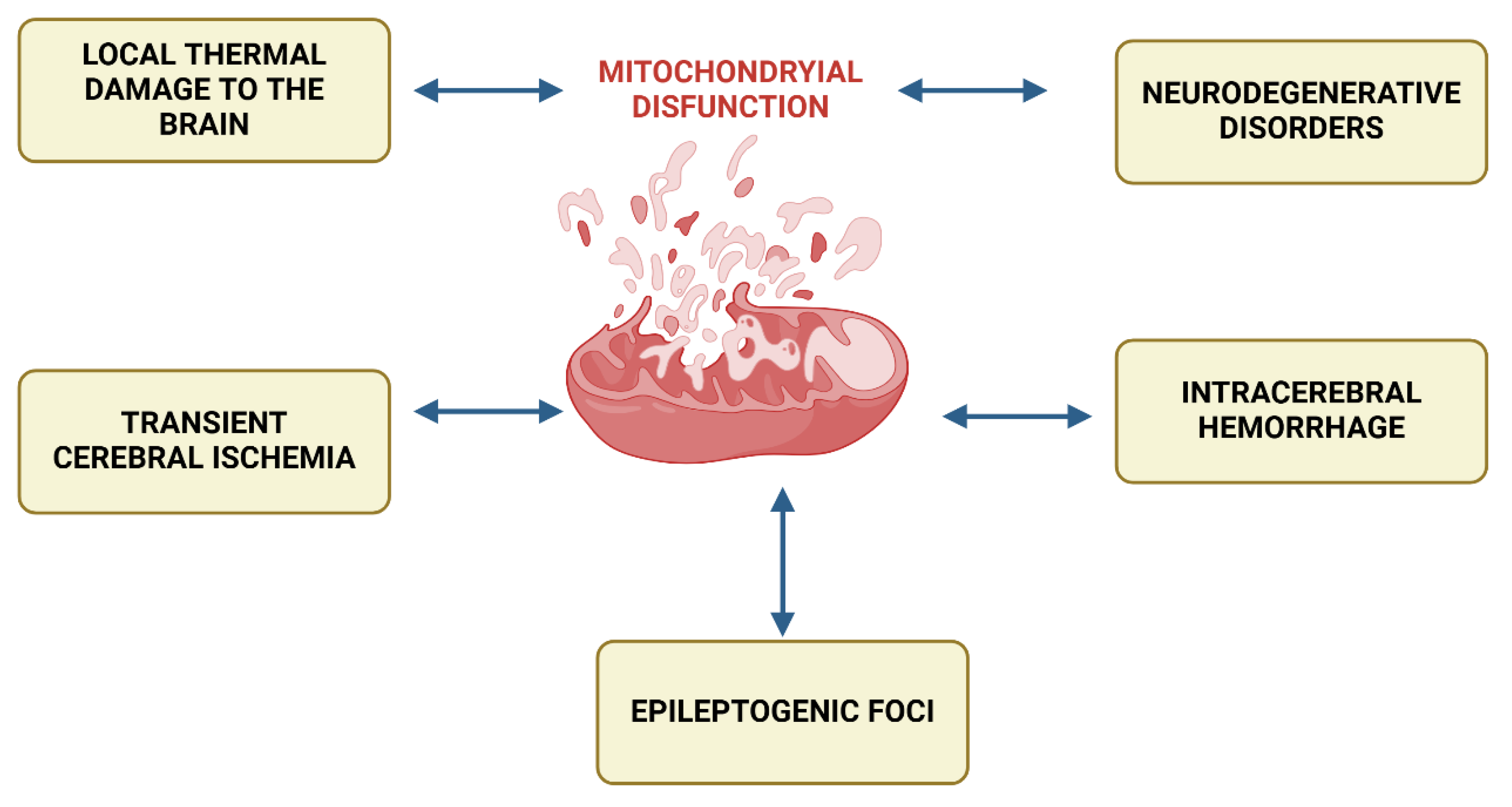
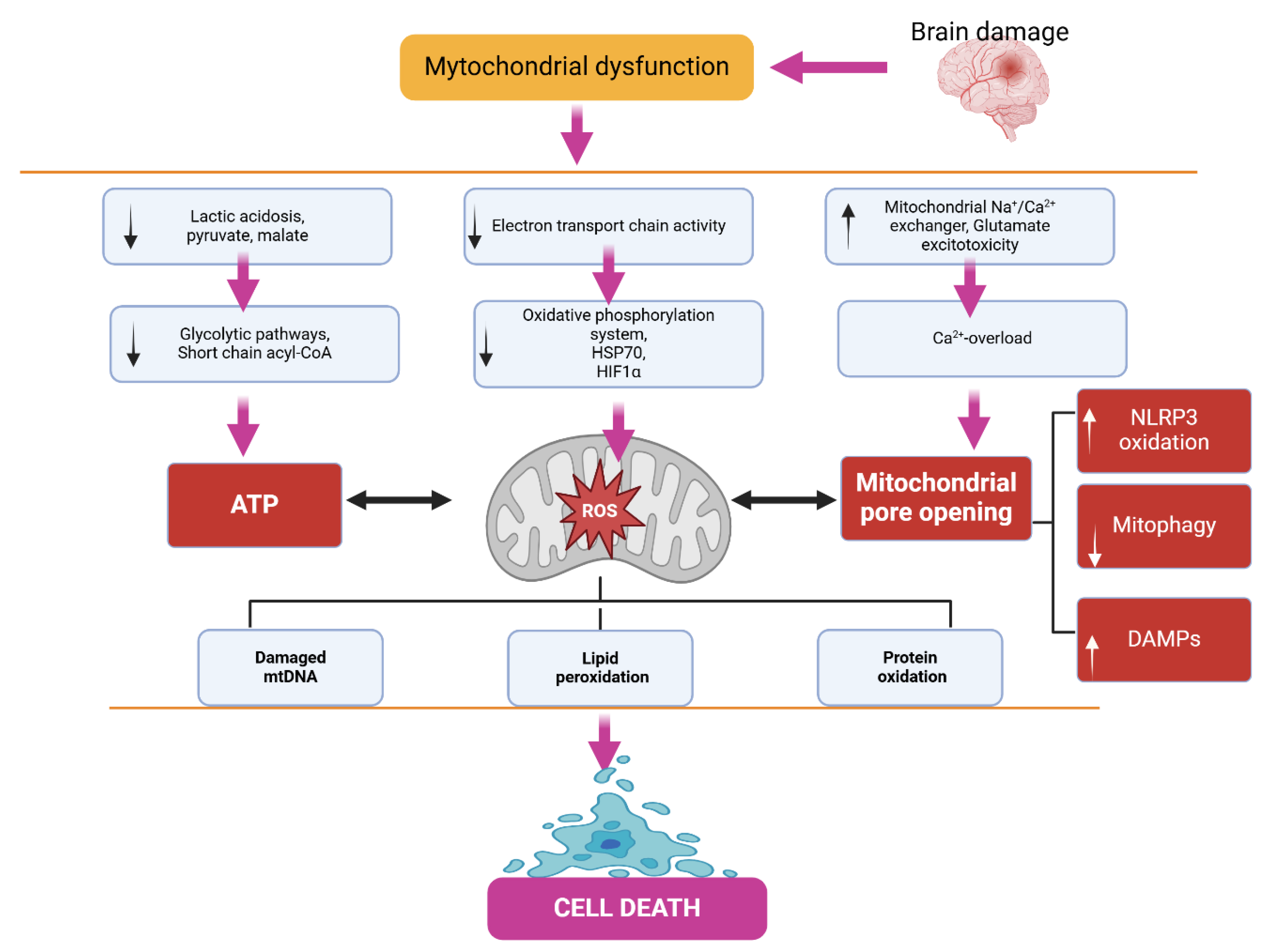
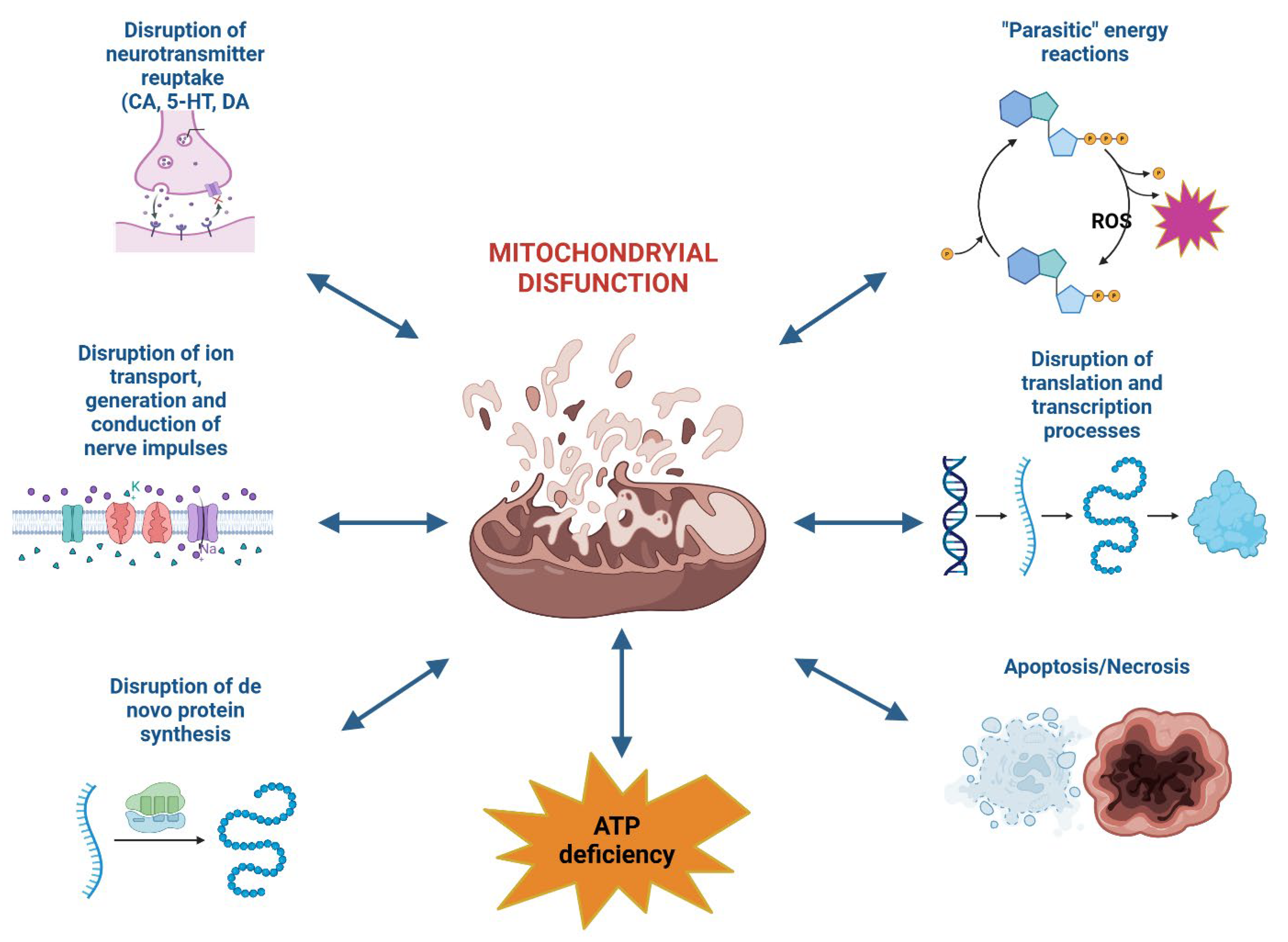
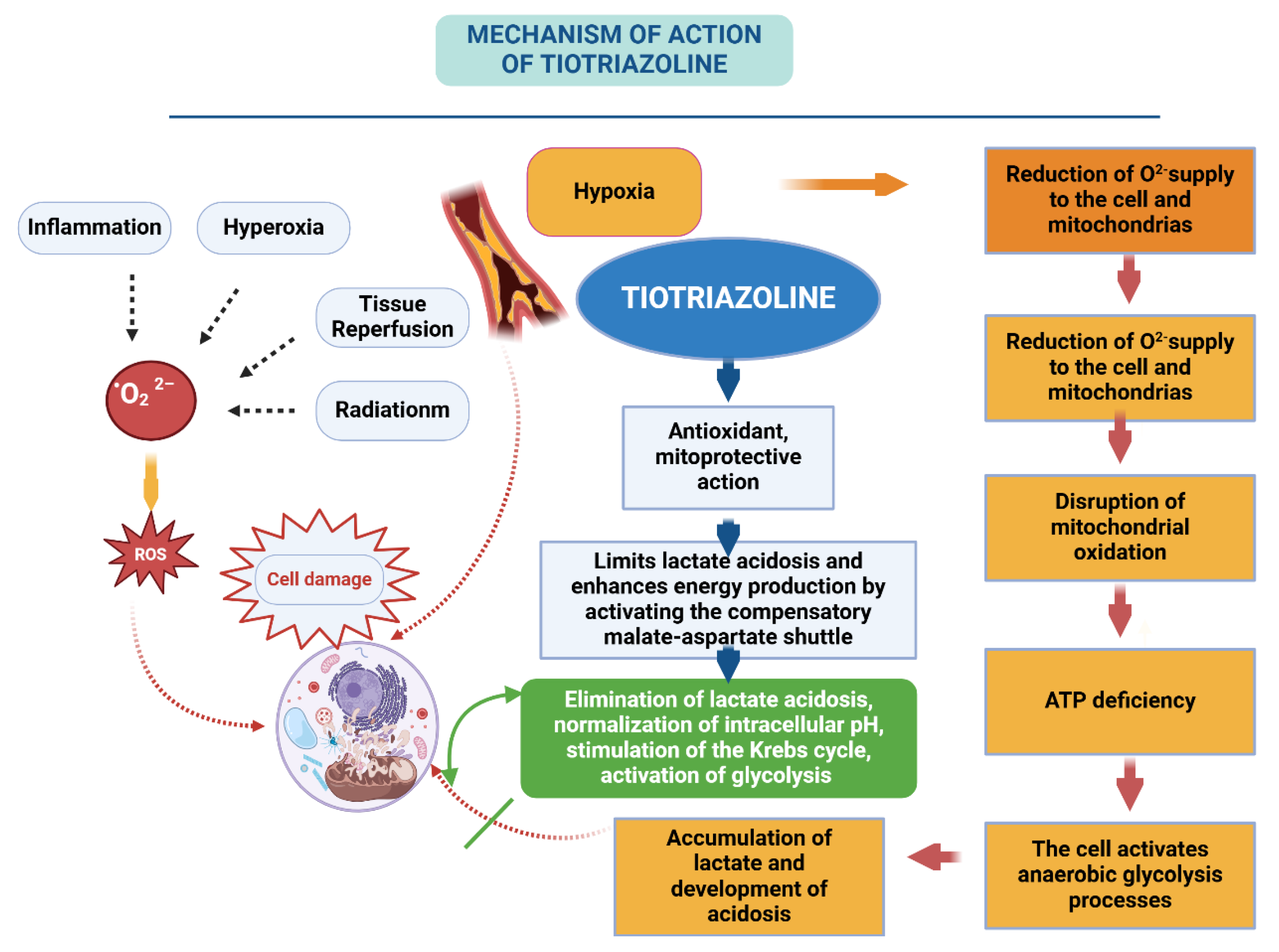
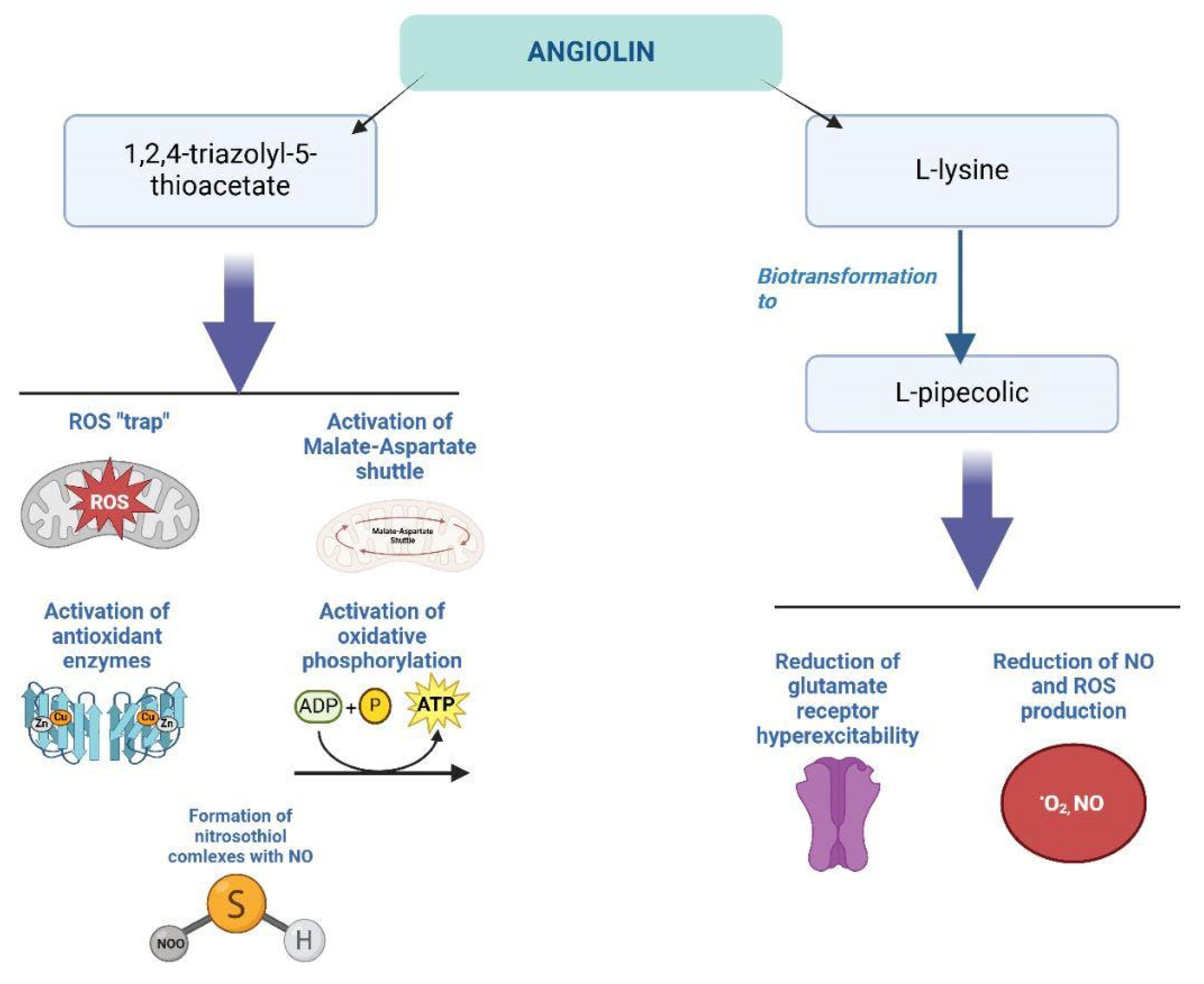
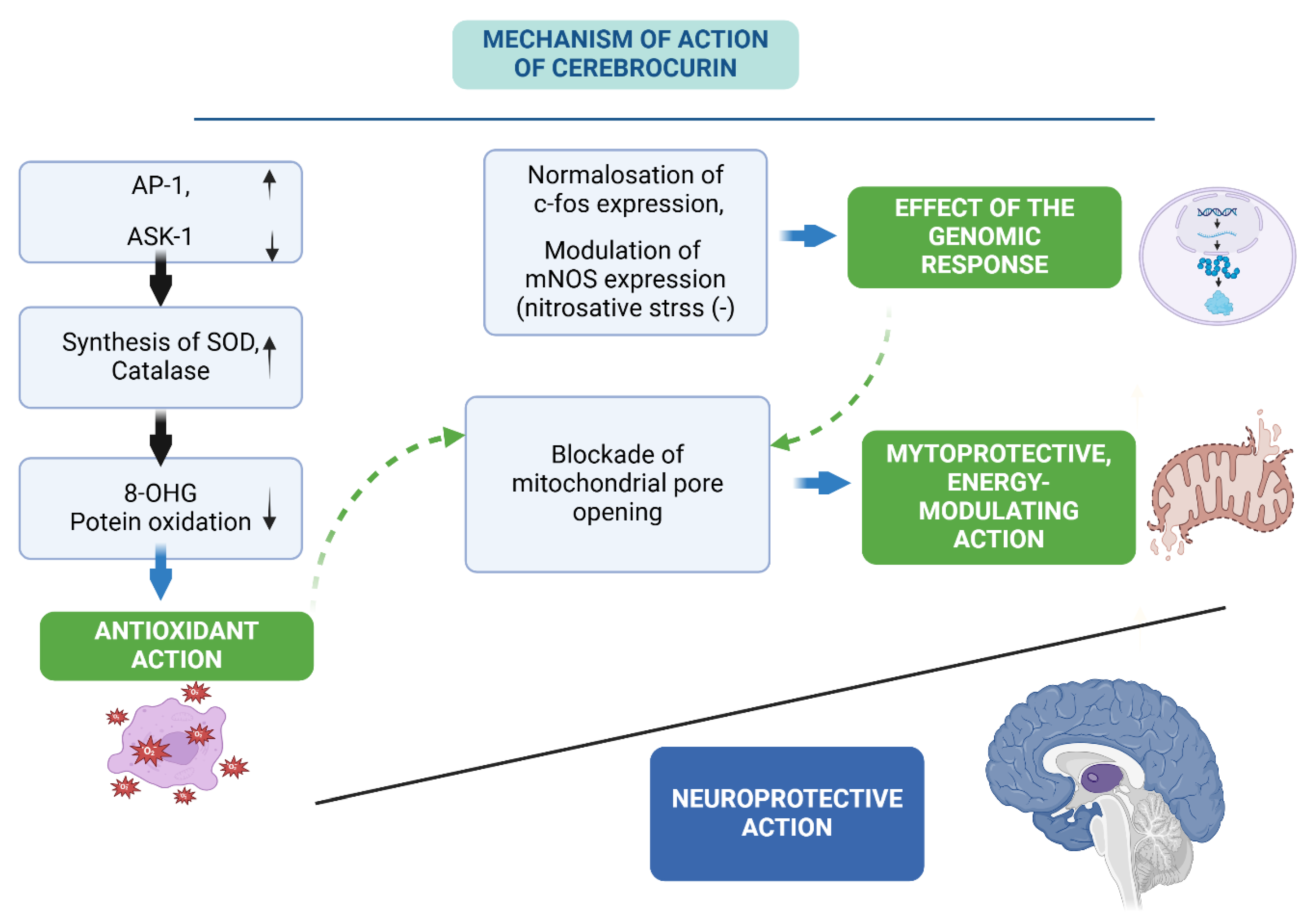
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




