1. Introduction
Stroke is the leading cause of adult disability in Europe and worldwide, resulting in a significant loss of independence in daily living activities [
1]. The consequences of stroke affect not only the individuals but also their caregivers and families, leading to a significant increase in the socioeconomic burden.
Patients who experience severe strokes often face impairments in both motor and cognitive functions. Among motor impairments, ambulatory functions are often recovered within a few weeks, even after severe hemiplegia, whereas the restoration of upper limb function is extremely difficult [
2,
3]. Furthermore, cognitive functions, such as attention, memory, language, executive functions and spatial awareness are often affected, which can significantly impact the effectiveness of recovery and social participation [
4]. Additionally, patients often experience fatigue, difficulties in swallowing, behavioral disorders, and alterations in emotional functioning such as depression and anxiety. These issues contribute to low levels of subjective well-being, increased psychological distress, and greater dependence on caregivers for daily activities [
5].
The severity of functional consequences after stroke still lacks substantial evidence, although certain topics have begun to emerge in recent years. Among the variables that should be considered, the severity of stroke seems to play a significant role, with notable effects on quality of life that extend beyond functional limitations and mortality outcomes. Understanding the relationship between stroke severity and clinical outcomes is essential for guiding rehabilitative, optimizing patient care, and ensuring the appropriate use of resources [
2,
6]. Nevertheless, among the factors useful in determining stroke severity, the most long-established prognostic indicator is the severity of neurological motor deficits.
Beyond the severity of neurological motor deficits, other clinical factors have emerged in influencing rehabilitative recovery in recent years, such as age, gender, BMI, premorbid conditions, concomitant pathologies etc. [
7]
Scientific literature identifies several negative prognostic factors for rehabilitation outcomes, including the severity of neurological motor deficits, prolonged time between the acute event and the start of rehabilitation, global aphasia, the presence of spatial neglect, and depression [
8].
Beyond these variables, cognitive function impairment also appears to play a significant role in determining both stroke severity and the effectiveness of rehabilitative interventions. The assessment of cognitive function is a significant predictor of overall recovery and social participation when properly evaluated [
9]. Moreover, impairments in memory, language, and praxis appear to influence recovery after stroke [
10]. The lack of recovery in executive functions following rehabilitative interventions also affects motor recovery [
11]. In fact, acquiring motor skills appears to be based on learning mechanisms that are directly dependent on cognitive functions, such as executive functions, which play a fundamental role in determining the success of motor rehabilitation [
4].
To our knowledge, rehabilitation is currently the most effective intervention for promoting recovery in stroke patients [
12], and its effectiveness appears to be influenced by the time lapse between the acute event and the beginning of therapy. In fact, some authors have highlighted that the first three months following stroke onset represent the golden period for functional recovery, due to the cortical plasticity [
13]. The timeline of rehabilitation has generally been divided into three periods: very early rehabilitation, early rehabilitation, and late rehabilitation. Very early rehabilitation is defined as rehabilitative interventions delivered within 24 hours after a stroke [
14]. Early rehabilitation refers to interventions provided within the first two weeks after a stroke, whereas late rehabilitation is defined as rehabilitation delivered after the first two weeks following a stroke [
13].
In this context, very early rehabilitation should be provided with caution, as it may be less effective and potentially inappropriate due to the phenomena associated with the penumbra area and the toxic effect of glutamate in the first 16 hours following stroke onset [
15]. Conversely, early mobilization conducted in the Stroke Unit and fast-tracked for early rehabilitation in the rehabilitative ward is recommended by the majority of clinical practice guidelines, as it is likely to facilitate good recovery outcomes for patients after a stroke [
13]. Early rehabilitation appears to be more effective than late rehabilitation because it is implemented during periods of heightened brain plasticity. Furthermore, early interventions can prevent or reduce secondary damage to the musculoskeletal, cardiovascular, respiratory, and immune systems caused by prolonged bed rest [
16].
Lastly, neuroscientific theories regarding inter-hemispheric bimodal balance and its modification over time suggest a different behavior of inter-hemispheric connectivity in relation to the severity of cerebral lesion [
17].
Despite the division into three phases, the time lapse between the acute event and the beginning of rehabilitation is a continuous variable. In clinical practice it is often influenced more by administrative issues related to the management of the clinical pathway than by demographic or clinical factors. Hence, there is a need for scientific insight into the precise mechanisms underlying the association between the time elapsed since the acute event and other prognostic factors, such as severity and cognitive functions, which are essential for predicting outcomes but remain incompletely understood. Moreover, the variables that appear to correlate with functional outcomes are often considered as independent from one another, rather than as interconnected components of a complex algorithm.
In this study, we aim to further investigate the prognostic roles of stroke severity, cognitive functions, and motor functions, with a specific focus on functional recovery. This will be accomplished by analyzing a cohort of stroke patients categorized according to the timing of neurorehabilitation initiation. We will employ decisional cluster analysis, which has been shown to offer superior accuracy in identifying stroke prognostic factors compared to classical regression and neural network analyses [
18].
2. Materials and Methods
2.1. Participants
This study was a secondary analysis of the data of a total of 386 stroke patients in the subacute phase (discharged from acute wards i.e., Stroke units or neurology wars), who were consecutively admitted from November 2019 to July 2021 at sixteen Italian rehabilitation centers. These patients were enrolled in the observational, prospective, longitudinal, multicentric study named Cognitive and Recovery of Motor functions (CogniReMo). The cohort included both genders (190 males and 129 females), aged between 18 to 90 years, with varying levels of education. Sixty-seven patients dropped out and were transferred to other hospitals due to clinical worsening (26 females and 41 males). The final study group consisted of 319 patients (103 females and 149 males).
Inclusion criteria: All patients with ischemic stroke aged between 18 and 90 years were enrolled. Both males and females of any educational background were admitted.
Exclusion criteria: Patients with hemorrhagic stroke, previous psychiatric or neurological disorders, a prior Barthel Index score lower than 90, and those with a history of drug or alcohol abuse were excluded.
Patients admitted to rehabilitative wards 72 hours before or after 90 days from stroke onset were excluded. Additionally, patients who did not provide informed consent were also excluded
The study was approved by the Ethical Committee of South-East Tuscany under the number 15360. All patients were informed about the aims of the study, and all participants signed the informed consent.
2.2. Procedure
The severity of stroke for each patient enrolled in the study was assessed using the National Institutes of Health Stroke Scale (NIHSS). The locations of brain lesions were classified according to the Bamford classification [
19]. Motor impairment was evaluated with the Fugl-Meyer Lower Limb Motor Scale [
20], while disability was assessed using the Barthel Index [
21]. Cognitive impairment was screened with the Oxford Cognitive Screen (OCS) [
22]. All the assessment scales were administered at both admission and discharge. For each patient, information was collected regarding age, gender, education level, previous Barthel Index scores, and risk factors such as hypertension, diabetes, obesity (BMI), and atrial fibrillation. Data on stroke onset, admission, and discharge dates were also collected. Additionally, information regarding brain lesion localization (according to the Bamford classification), acute phase treatments (fibrinolysis and/or thrombolysis), clinical complications (such as deep vein thrombosis, pulmonary embolism, bladder infection, pulmonary infection, and septicemia), as well as discharge settings, was also gathered.
All the evaluation scales were conducted and scored by members of the patient’s rehabilitation care team.
The baseline examination (T0) was conducted within 48 hours from admission. The follow-up was conducted within 48 hours before discharge.
During the hospital stay all subjects had conventional multidisciplinary stroke rehabilitation treatment. According to the Italian guidelines, conventional treatment for patients admitted at hospital rehabilitation wards is intended as daily treatment for 3 hours per day, 6 days per week. Physiotherapy usually starts within 24 hours from admission and when necessary, training for cognitive rehabilitation, as well as training for swallowing, bowel and bladder functions recovery are added. Additionally, occupational therapy is provided for all patients. Specifically, the physiotherapy training for our sample included exercises aimed at improving trunk control, standing, balance, gait, coordination, and endurance. Cognitive training consisted of exercises designed to improve cognition, attention, memory, spatial awareness, language, praxis, and executive functions. Swallowing treatment was administered for approximately 30 minutes per day, when necessary, along with speech therapy for cases of aphasia and specific psychological interventions for unilateral spatial neglect.
2.3. Measures
All the patients enrolled in the study were assessed by the following rating scales:
The National Institutes of Health Stroke Scale (NIHSS) [
23], designed to evaluate stroke severity. It consists of 11 items, and the total score ranges from a minimum of 0 (indicating normal neurological functioning) to a maximum of 42 (indicating severe neurological damage).
The Oxford Cognitive Screen (OCS) [
22,
24], a screening tool for the rapid and early assessment of cognitive functions. It evaluates five cognitive domains: attention and executive function, language, memory, number processing, and praxis. No scaling corrections are applied to the raw data. The OCS can be administered in about 15 minutes and can be delivered at the bedside whenever necessary. The Italian version of the scale, which includes normative data on a sample of individuals aged 20 to 80 years, was used.
The Fugl-Meyer Assessment (FMA)[
25], provides a quantitative measure of motor impairment in post-stroke hemiplegic patients. It relies on the direct observation of motor performance for each item using a 3-point ordinal scale (0 = cannot perform, 1 = performs partially, and 2 = performs fully). The scale is divided into two sections for assessing upper and lower limb function. The Fugl-Meyer Assessment for the Lower Extremity (FMA-LE) has a maximum score of 34 points, which is based on lower extremity scores (0–28 points) and coordination/speed scores (0–6 points). Motor function, sensory function, passive range of motion, and pain during passive movement are evaluated using the same 3-point ordinal scale. The non-motor domains of the Fugl-Meyer Assessment include Sensation and Joint Passive. Joint Movement and Pain are assessed for both the upper and lower limbs. The Italian version of the scale was utilized in this study.
The modified Barthel Index (mBI)[
25], an ordinal scale used to measure performance and independence in activities of daily living (ADL). It scores ten variables that describe ADL and mobility. A higher score corresponds to greater abilities in functioning independently. Scores range from 0 to 15 based on the level of assistance patients require to perform tasks with the maximum score being 100 points. Higher scores indicate greater autonomy in conducting daily activities.
3. Statistical Analysis
Data are reported as mean ± standard deviation or percentage relative frequencies. Pre- vs. post-rehabilitation scores were compared using non-parametric Wilcoxon test. The alpha level of significance was set at 5% for all the analyses. A cluster analysis was performed utilizing a decisional tree based on the exhaustive CHAID growing model. The minimum number of subjects allowed for parent and child nodes was 20 (6%). The output variable was the Barthel Index (BI) at discharge, according to the aim of this study, the time from stroke at admission was set as the primary decisional variable, whereas 18 other variables recorded at admission have been further included as possible decisional variables such as Age, Gender, Barthel Index, NIHSS, Lower Limb Fugl-Meyer, and the OCS domains: Picture Naming, Semantics, Orientation, Visual Field, Spatial Attention (Broken heart cancellation test score and time), Reading, Number writing, Calculation, Praxis, Verbal Memory, Episodic Memory, Executive Functions.
4. Results
Table 1 presents the demographic and clinical variables of the patients upon admission to the rehabilitation hospitals. At discharge, the mean Barthel Index was 64.6±29.7, showing a significant improvement compared to the score assessed at admission (p<0.001).
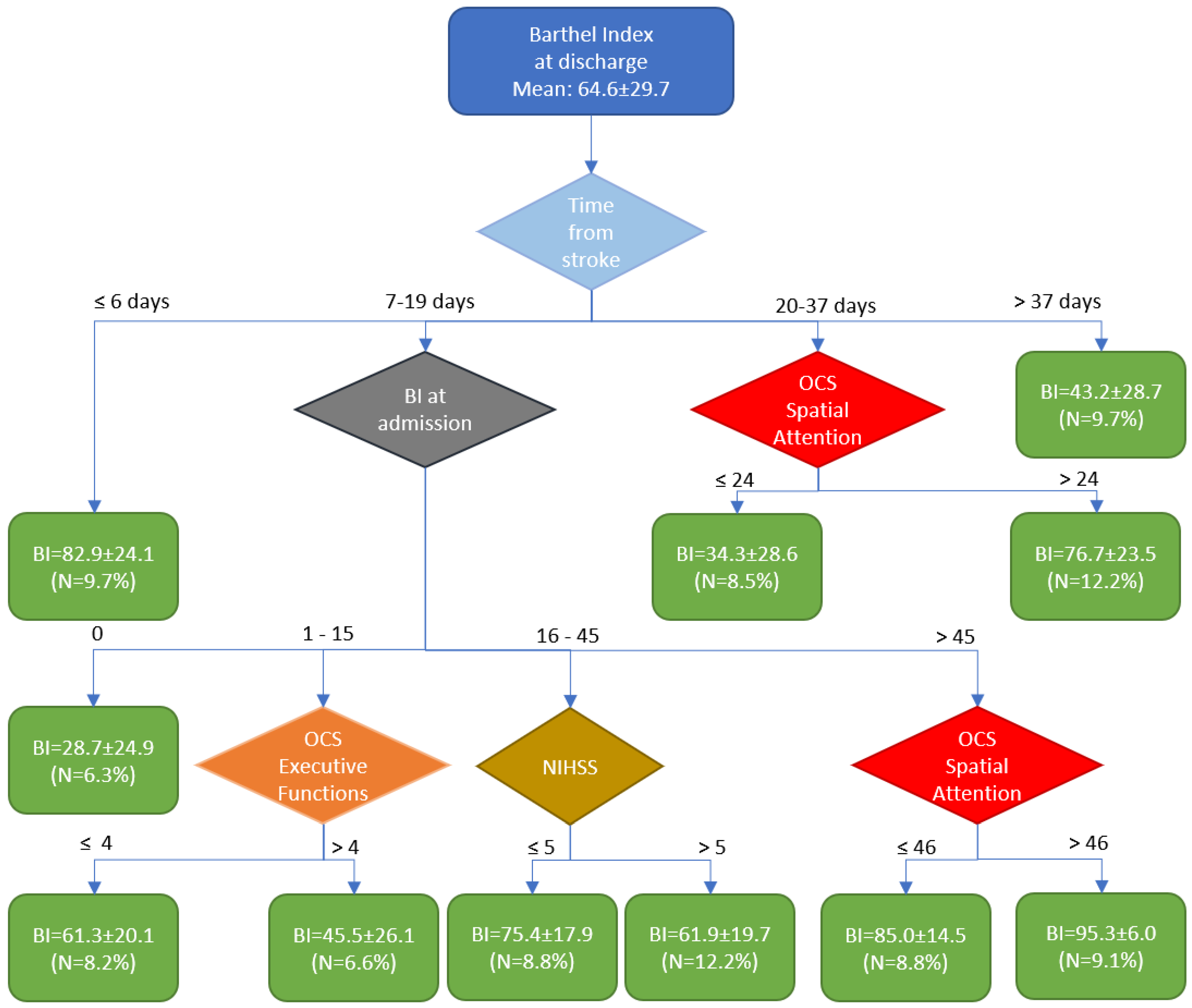
Cluster analysis identified four main time windows. The first cluster was associated with early rehabilitation, which led to a high outcome. (BI=82.9±24.1). The last cluster is associated with a delayed start of rehabilitation (approximately 10% of subjects admitted to the rehabilitation hospital after more than 37 days post-stroke), resulting in a low outcome. (BI=43.2±28.7). Most patients (60%) were admitted to the rehabilitation hospital between 7- and 19-days post-stroke. The Barthel Index (BI) score at admission was the most significant predictor of patient outcomes. Specifically, if the BI was 0 at admission, it increased to an average of 28.7 ± 24.1 by discharge. For patients with a Barthel Index (BI) <15 at discharge, the discriminant factor for achieving an average outcome was unaltered executive functions, resulting in an average BI of 61.3 compared to 45.5. For patients with a BI at admission between 16 and 45, having a NIHSS ≤5 was the key discriminant variable for obtaining a high outcome, with an average BI of 75.4 instead of 61.9. Subjects with a BI at admission >45 were the best responders to therapy, achieving a mean BI at discharge of 85 if they had alterations in spatial attention, and 95.3 if they had no deficits in spatial attention.
The remaining patients (21%) were those admitted to the rehabilitation hospital between the 20th-37th day after the acute event. For these patients, spatial attention was a discriminant variable influencing outcome; those with spatial attention deficits had a poor outcome, with an average Barthel Index (BI) of 34.3, while those without deficits achieved a better outcome, with an average BI of 76.7.
Figure 1.
The figure shows the tree obtained by Cluster Analysis (BI: Barthel Index, NIHSS: National Institute of Health Stroke Severity scale, OCS: Oxford Cognitive Screen, N: the percentage number of subjects included into the cluster; green rectangles are the final clusters, the rhomboids correspond to decisional nodes).
Figure 1.
The figure shows the tree obtained by Cluster Analysis (BI: Barthel Index, NIHSS: National Institute of Health Stroke Severity scale, OCS: Oxford Cognitive Screen, N: the percentage number of subjects included into the cluster; green rectangles are the final clusters, the rhomboids correspond to decisional nodes).
4. Discussion
The aim of the study was to investigate how the timing of rehabilitation onset influences the impact of well-known prognostic factors, such as the severity of neurological deficits and cognitive impairments, on rehabilitative effectiveness.
Our cluster analysis identified four important time windows within the framework of early rehabilitation. These windows revealed different factors that could be considered prognostic indicators of outcomes. Patients who began rehabilitation within one week of the acute event were found to be good responders, regardless of other influencing factors. Similarly, patients who began rehabilitation later than 37 days (approximately 5.5 weeks) were classified as low responders, regardless of other influencing factors. These findings are in accordance with those found by Paolucci et al. (1998) [
8]. Most patients began rehabilitation between 7 and 19 days after the acute event, and their outcomes were influenced by their level of autonomy (measured by the Barthel Index), stroke severity (assessed using the NIHSS), and their executive functions and spatial attention at the time of admission to the rehabilitation hospital. Spatial attention was also a significant factor for patients who started therapy between 20- and 37-days post-stroke. So, four out of the eighteen variables have been identified as discriminants for the outcome. In a previous study [
11], we identified five variables based on linear regression analysis, three of which were also identified by the present cluster analysis: the BI at admission, the OCS-score for executive functions, and the OCS-score for the broken heart cancellation task. The other two variables previously identified were the time taken to complete the OCS broken heart cancellation task (related to spatial attention) and the Lower Limb Fugl-Meyer score. The latter did not enter our model, likely because it was replaced by the NIHSS score.
It is noteworthy that the cluster analysis automatically identified some interesting thresholds for these other variables that aligned with those reported in the scientific literature.
The NIHSS is conventionally divided into minor (0-4 score) and moderate/severe strokes (>4 score). The cluster analysis identified a threshold of 5, which is very close to the conventionally used classification.
Mancuso et al. (2016) [
24] reported that the Executive Functions were altered when the corresponding OCS-score was or ≤ - 4 or ≥ 4. In our analysis we used the absolute values, and the identified threshold was exactly 4.
For Spatial Attention, for the score of heart cancellation task, Mancuso et al. (2016) [
24] reported this equation: score = 46.925 - 0.03 x Age + 0.147 x Education. Considering the mean age and education of our sample (71 years old and 9 years of school) we obtain a cut-off level of 46, that is exactly the threshold found for patients with a BI at admission (> 45) to differentiate between high responders (BI at discharge = 85) and very high responders (95.3). The other threshold identified by the cluster analysis for spatial attention was 24, which is a low value likely indicating a severe deficit in spatial attention, as 57.7% of these patients exhibited neglect.
The most important result of the present work is that it has documented that the prognostic variables of post-stroke functional recovery do not have a fixed weight for all patients, but rather depend on the timing of the initiation of the rehabilitation intervention. This finding is significant as it adds a novel perspective to the discussion surrounding early rehabilitation and, more broadly, the timing of rehabilitation in stroke care to optimize recovery.
This result deserves to be underlined especially considering that the time from the acute event to the beginning of rehabilitation may depend not only on clinical factors, such as the stabilization of the patients’ critical conditions, but also on local administrative issues related to hospitalization procedures in rehabilitation settings.
In fact, we should consider the specific prognostic factors for each time window in which rehabilitation is initiated. This principle aligns with an evaluation of predictive algorithms that adhere to the principles of personalized medicine. Our algorithm is an effective and easily usable tool in daily clinical practice. Unlike the Predicting REcovery Potential PREP [
26] model, which primarily focuses on predicting outcomes related to the upper limb, our algorithm predicts overall patient outcomes. It encompasses essential cognitive aspects relevant to activities of daily living (ADLs) and quality of life. Furthermore, while the PREP model includes neurophysiological factors that may not always be available, our algorithm does not incorporate these aspects, making it more accessible in various clinical settings.
Our study highlighted the importance of prompt and accurate assessment of cognitive functions shortly after a stroke and their effective rehabilitation. Specifically, in patients managed between 7 and 35 days after the event, our algorithm demonstrated that early management (7-19 days) is primarily influenced by the Barthel Index at entry—if it is 0. For patients with a Barthel Index between 1 and 15, outcomes are influenced by executive functions; for those with a Barthel Index between 16 and 45, the severity of the neurological deficit as measured by the NIHSS plays a critical role. Additionally, for patients with a Barthel Index greater than 45, spatial neglect becomes a significant factor.
In patients managed between 20- and 37-days post-stroke, the presence of spatial function deficits is decisive for outcomes. Conversely, both very early and late management are less influenced by the interplay between the timing of onset and cognitive functions. In summary, cognitive functions directly modify the effectiveness of early rehabilitation when patients begin rehabilitation between 20 and 37 days after the stroke. For those starting rehabilitation between 7 and 19 days, cognitive functions play a subordinate role, primarily affecting outcomes based on the Barthel Index.
The findings of this study should be carefully considered considering its limitations. Firstly, the database used to identify the clusters was not verified prospectively with other datasets. Secondly, although the sample size was considerable, some clusters were based on a limited number of subjects. Finally, while the study is multicentric, all participating hospitals were in Italy. This means that clinical management of patients was conducted under Italian laws and guidelines, which may limit the generalizability of the findings to other countries.
In conclusion, the identified algorithm could play a significant role for clinicians in defining appropriate rehabilitation pathways based on the timing of rehabilitation initiation and the severity of motor and cognitive deficits. Furthermore, it may serve as a valuable tool for policymakers in establishing clinical practice guidelines, emphasizing the importance of allocating resources and beds to facilitate the commencement of rehabilitation within a few days following the acute event.
Author Contributions
Conceptualization, M.M. and M.I.; methodology, M.I.; software, M.I.; validation, M.M., M.I. and G.M.; formal analysis, M.M.; investigation, G.M.; data curation, all the authors.; writing—original draft preparation, M.M, G.M., M.I., D.D.B.; writing—review and editing, all the authors; visualization, M.I.; supervision, M.M.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Ethical Committee of the South-East Tuscany with the number 15360.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data will be available upon reasonable request made to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Cogniremo Study Group: Abbruzzese Laura (Arezzo); Alpegiani Federica (Piacenza); Ambiveri Giordano (Piacenza); Antonucci Gabriella (Roma); Bacci Marco (Grosseto); Baldrighi Michela (Piacenza); Baudo Silvia (Verbania); Bariselli Federica (Brescia); Benedetti Adonella (Trevi); Biagioni Clarissa (Grosseto); Bigoni Matteo (Verbania); Cavalli Loredana (Grosseto); Ceriani Francesca (Verbania); Chinosi Elena (Piacenza); Ciancarelli Irene (L’Aquila); Coccia Michela (Ancona); Codazzi Filippo (Piacenza); Costa Tatiana (Sestri Levante-Genova); Damora Alessio (Arezzo); Daturi Lucia (Piacenza); Di Giovanni Rachele (Vercelli); Falcone Giuseppe (Grosseto); Falcone Rosanna (Bari); Fonte Cristina (Verona); Gallinaro Ylenia (Verona); Gambarelli Carmen (Modena); Gamberini Giulia (Vicenza); Guglieri Sara (Piacenza); Grange Erica (Vercelli); Greco Antonino (Bari); Iosa Marco (Roma); Lecis Sabrina (Trevi); Maggi Roberta (Piacenza); Maietti Alessandra (Brescia); Mancuso Mauro (Grosseto); Maruccia Vanessa (L’Aquila); Martinelli Giulia (Arezzo); Matano Alessandro (Roma); Megna Marisa (Bari); Montanari Laura (Piacenza); Mori Massimiliano (Piacenza); Morone Giovanni (L’Aquila); Mulè Chiara (Brescia); Podgorska Aleksandra (Grosseto); Ricci Roberta (Trevi); Rinaldesi Maria Luisa (Macerata); Rizzo Laura Belindo (Bari); Rossi Giulia (Grosseto); Rosso Giuliana (Grosseto); Rubbuoli Barbara (Modena); Russo Livia (Piacenza); Salti Giulia (Arezzo); Scaglia Gian Guido (Piacenza); Secchi Annalisa (Piacenza); Smania Nicola (Verona); Solaro Claudio Marcello (Vercelli); Spaccavento Simona (Bari); Spitali Maria Cristina (Trevi); Stoppini Angelica (Trevi); Strogolo Francesca (Modena); Suranna Valentina (Piacenza); Tognetti Paola (Sestri Levante-Genova); Toniolo Silvia (Piacenza); Varalta Valentina (Verona); Veliaj Altin (Grosseto); Villani Laura (Ancona).
References
- Moore, N.; Reeder, S.; O’Keefe, S.; Alves-Stein, S.; Schneider, E.; Moloney, K.; Lannin, N.A. “I’ve Still Got a Job to Go Back to”: The Importance of Early Vocational Rehabilitation after Stroke. Disability and rehabilitation 2024, 46, 2769–2776. [Google Scholar] [CrossRef]
- Ding, G.-Y.; Xu, J.-H.; He, J.-H.; Nie, Z.-Y. Clinical Scoring Model Based on Age, NIHSS, and Stroke-History Predicts Outcome 3 Months after Acute Ischemic Stroke. Front Neurol 2022, 13, 935150. [Google Scholar] [CrossRef] [PubMed]
- Feys, H.; De Weerdt, W.; Nuyens, G.; van de Winckel, A.; Selz, B.; Kiekens, C. Predicting Motor Recovery of the Upper Limb after Stroke Rehabilitation: Value of a Clinical Examination. Physiother Res Int 2000, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- VanGilder, J.; Hooyman, A.; Peterson, D.; Schaefer, S. Post-Stroke Cognitive Impairments and Responsiveness to Motor Rehabilitation: A Review. Curr Phys Med Rehabil Rep 2020, 8, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Ayasrah, S.; Ahmad, M.; Abuadas, F.; Abu-Snieneh, H.; Basheti, I. Health-Related Quality of Life Among Patients With Stroke: A Cross-Sectional Study. Arch Clin Neuropsychol 2024, 39, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, G.; Ihle-Hansen, H.; Abzhandadze, T.; Reinholdsson, M.; Hansen, H.I.; Sunnerhagen, K.S. Prognostic value of acute National Institutes of Health Stroke Scale Items on disability: a registry study of first-ever stroke in the western part of Sweden. BMJ open 2023, 13, e080007. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Paolucci, S.; Iosa, M. In what daily activities do patients achieve independence after stroke? J Stroke Cerebrovasc Dis 2015, 24, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, S.; Antonucci, G.; Pratesi, L.; Traballesi, M.; Lubich, S.; Grasso, M.G. Functional outcome in stroke inpatient rehabilitation: predicting no, low and high response patients. Cerebrovasc. Dis 1998, 8, 228–234. [Google Scholar] [CrossRef]
- Bisogno, A.L.; Novelletto, L.F.; Zangrossi, A.; De Pellegrin, S.; Facchini, S.; Basile, A.M.; Baracchini, C.; Corbetta, M. The Oxford cognitive screen (OCS) as an acute predictor of long-term functional outcome in a prospective sample of stroke patients. Cortex 2023, 166, 33–42. [Google Scholar] [CrossRef]
- Milosevich, E.T.; Moore, M.J.; Pendlebury, S.T.; Demeyere, N. Domain-specific cognitive impairment 6 months after stroke: the value of early cognitive screening. Int J Stroke 2024, 19, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Marco, I.O.S.A.; Abbruzzese, L.; Matano, A.; Coccia, M.; Baudo, S.; Benedetti, A.; Gambarelli, C.; Spaccavento, S.; Ambiveri, G.; et al. The impact of cognitive function deficits and their recovery on functional outcome in subjects affected by ischemic subacute stroke: Results from the Italian multicenter longitudinal study CogniReMo. European journal of physical and rehabilitation medicine 2023, 59, 284. [Google Scholar] [CrossRef] [PubMed]
- Todhunter-Brown, A.; Baer, G.; Campbell, P.; Choo, P.L.; Forster, A.; Morris, J.; Pomeroy, V.M.; Langhorne, P. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev 2014, 2014, CD001920. [Google Scholar]
- Coleman, E.R.; Moudgal, R.; Lang, K.; Hyacinth, H.I.; Awosika, O.O.; Kissela, B.M.; Feng, W. Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep 2017, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.; Langhorne, P.; Lindley, R.I.; Thrift, A.G.; Ellery, F.; Collier, J.; Churilov, L.; Moodie, M.; Dewey, H.; Donnan, G. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015, 386, 46–55. [Google Scholar]
- Bernhardt, J.; Thuy, M.N.; Collier, J.M.; Legg, L.A. Very early versus delayed mobilisation after stroke. Cochrane Database Syst Rev 2009, 40, e489–e490. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Sun, S.; Zhang, M.; Zhao, Z. A systematic review and meta-analysis of clinical efficacy of early and late rehabilitation interventions for ischemic stroke. BMC neurology 2024, 24, 91. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014, 10, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Morone, G.; Antonucci, G.; Paolucci, S. Prognostic factors in neurorehabilitation of stroke: A comparison among regression, neural network, and cluster analyses. Brain Sciences 2021, 11, 1147. [Google Scholar] [CrossRef]
- Bamford, J.; Sandercock, P.; Dennis, M.; Warlow, C.; Burn, J.J.T.L. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991, 337, 1521–1526. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Fi, M. Functional evaluation: the Barthel index. Md State Med J 1965, 14, 61–65. [Google Scholar]
- Demeyere, N.; Riddoch, M.J.; Slavkova, E.D.; Bickerton, W.L.; Humphreys, G.W. The Oxford Cognitive Screen (OCS): validation of a stroke-specific short cognitive screening tool. Psychological assessment 2015, 27, 883. [Google Scholar] [CrossRef] [PubMed]
- Kwah, L.K.; Diong, J. National institutes of health stroke scale (NIHSS). Journal of physiotherapy 2014, 60, 61. [Google Scholar] [CrossRef]
- Mancuso, M.; Varalta, V.; Sardella, L.; Capitani, D.; Zoccolotti, P.; Antonucci, G.; the Italian OCS Group. Italian normative data for a stroke specific cognitive screening tool: the Oxford Cognitive Screen (OCS). Neurological Sciences 2016, 37, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Viosca, E.; Lafuente, R.; Martínez, J.L.; Almagro, P.L.; Gracia, A.; González, C. Walking recovery after an acute stroke: assessment with a new functional classification and the Barthel Index. Arch Phys Med Rehabil 2005, 86, 1239–1244. [Google Scholar] [CrossRef]
- Stinear, C.M.; Barber, P.A.; Petoe, M.; Anwar, S.; Byblow, W.D. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012, 135, 2527–2535. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).